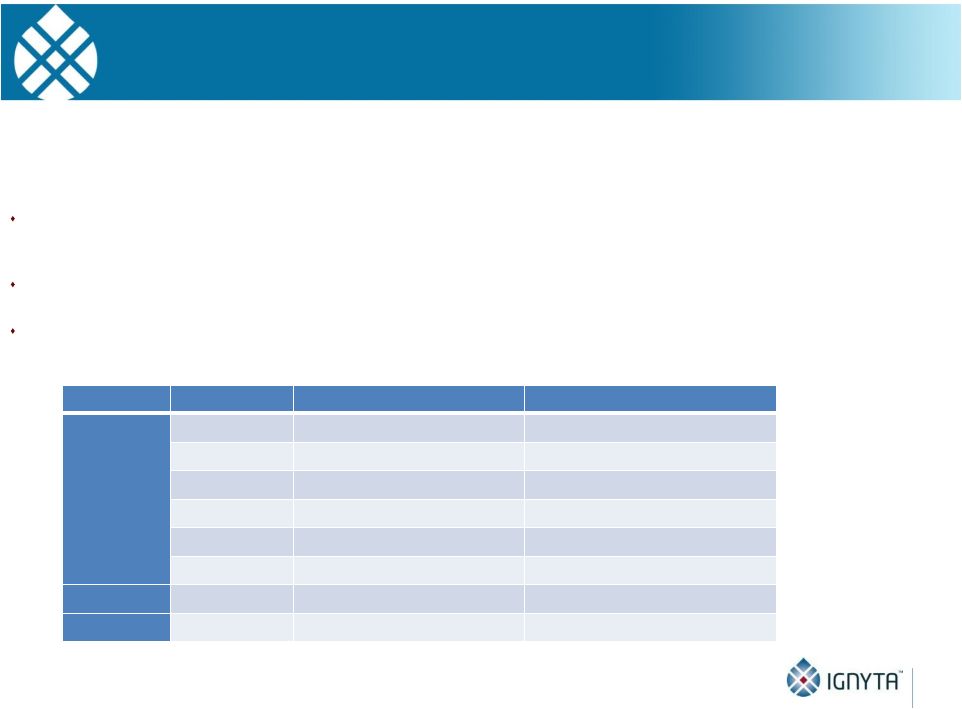
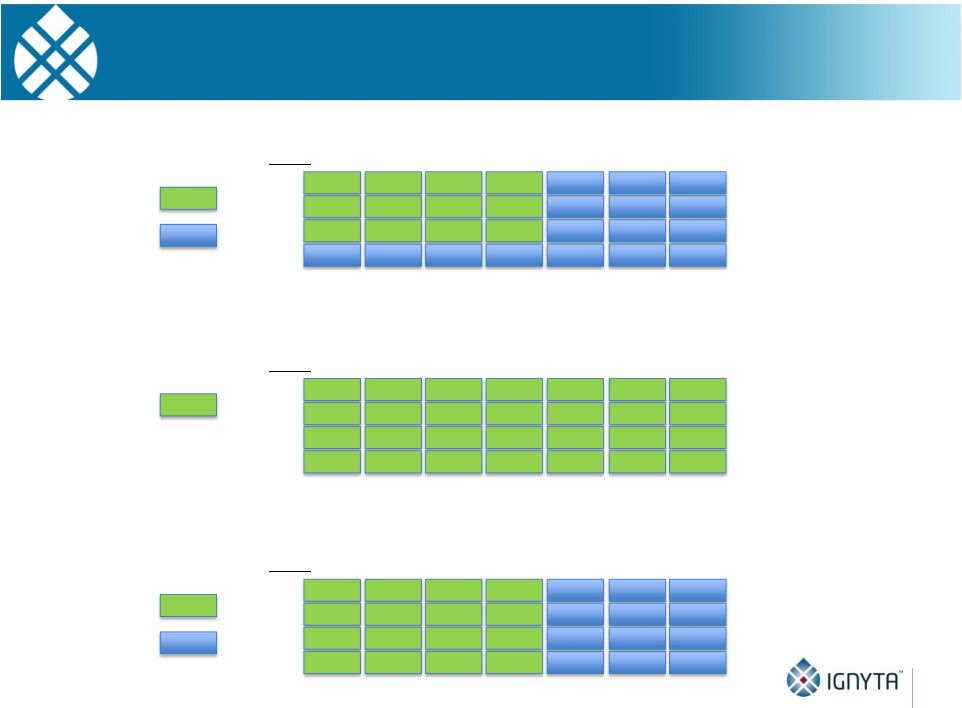
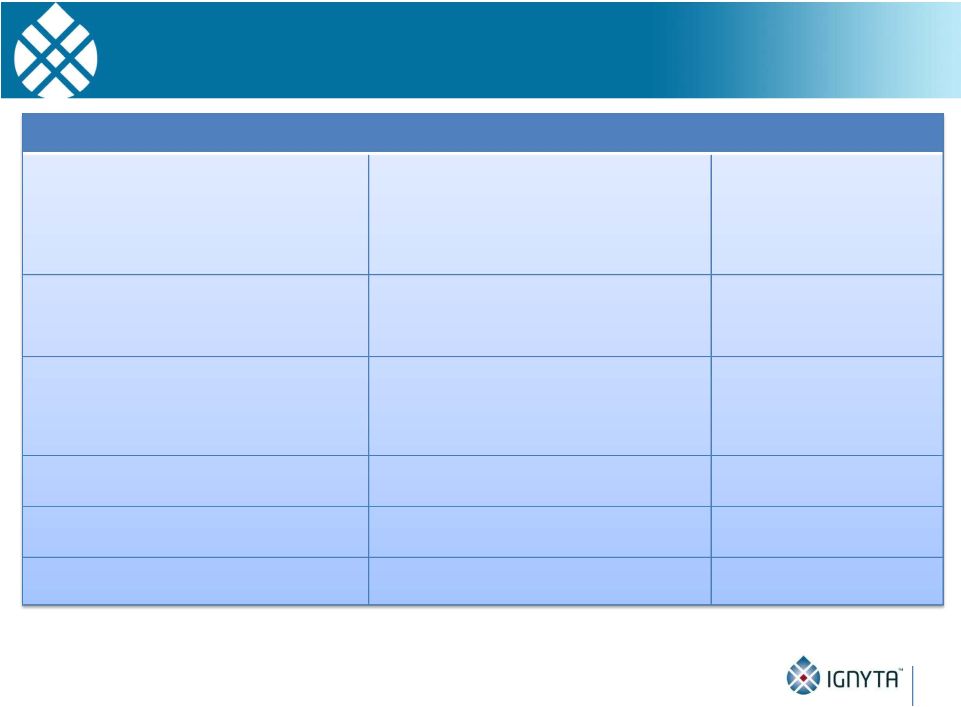
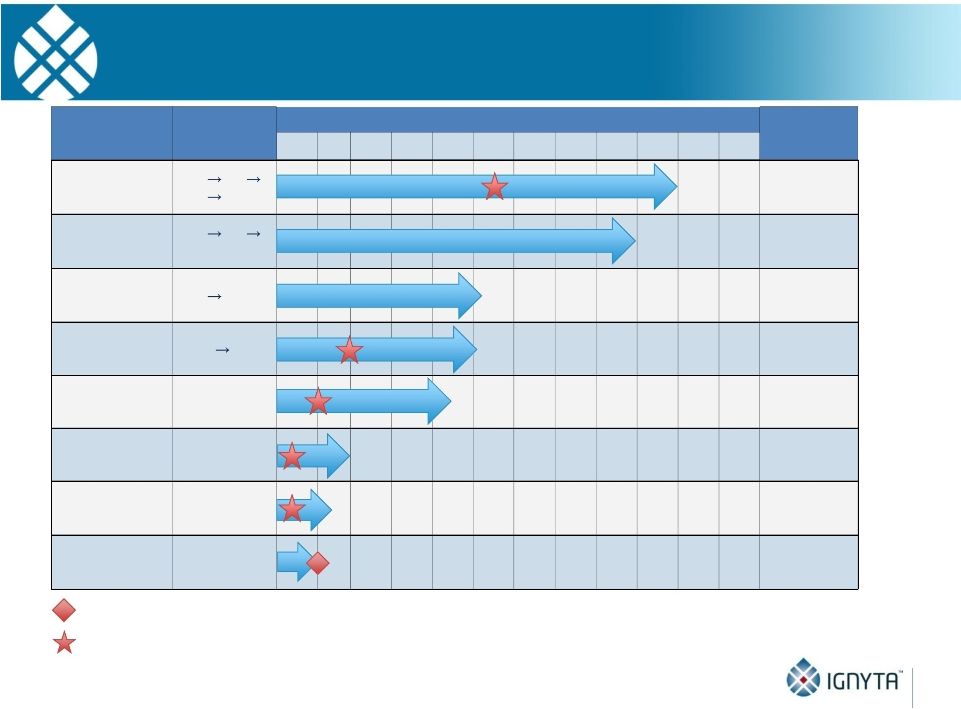
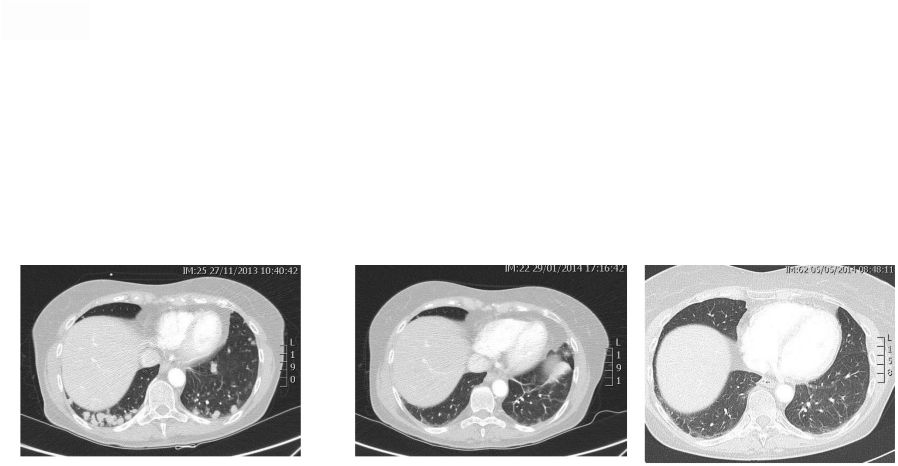
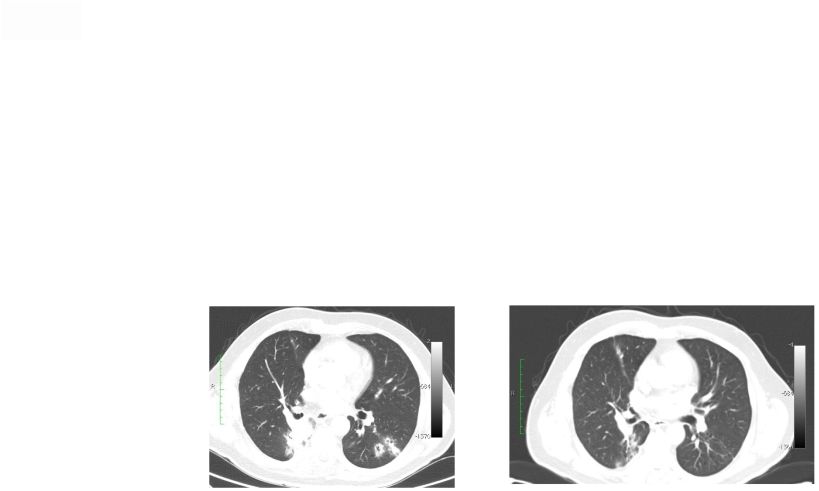
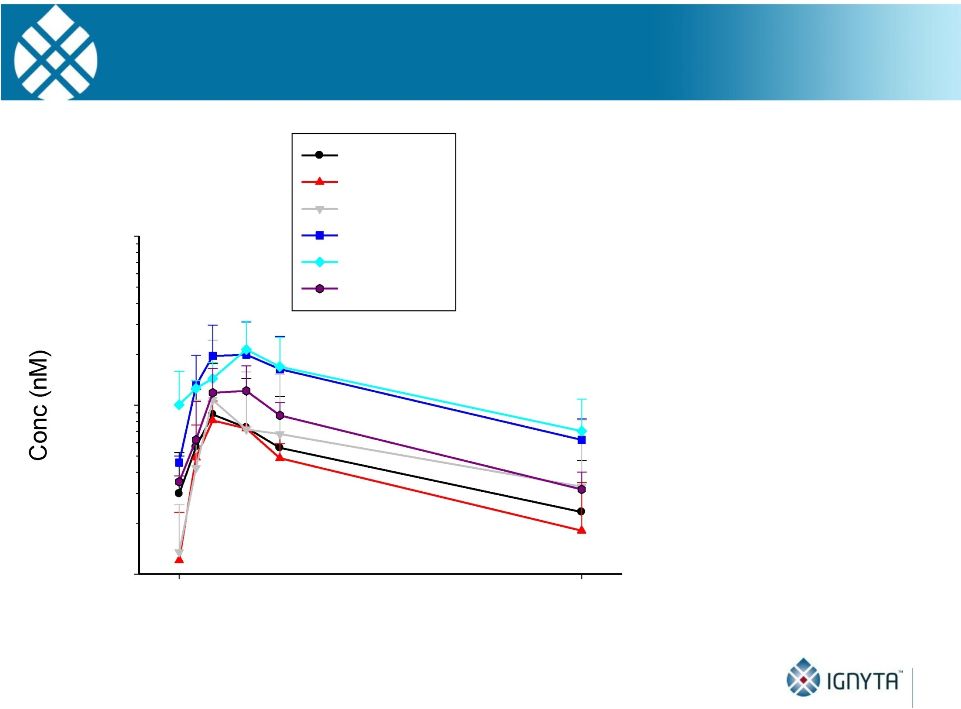
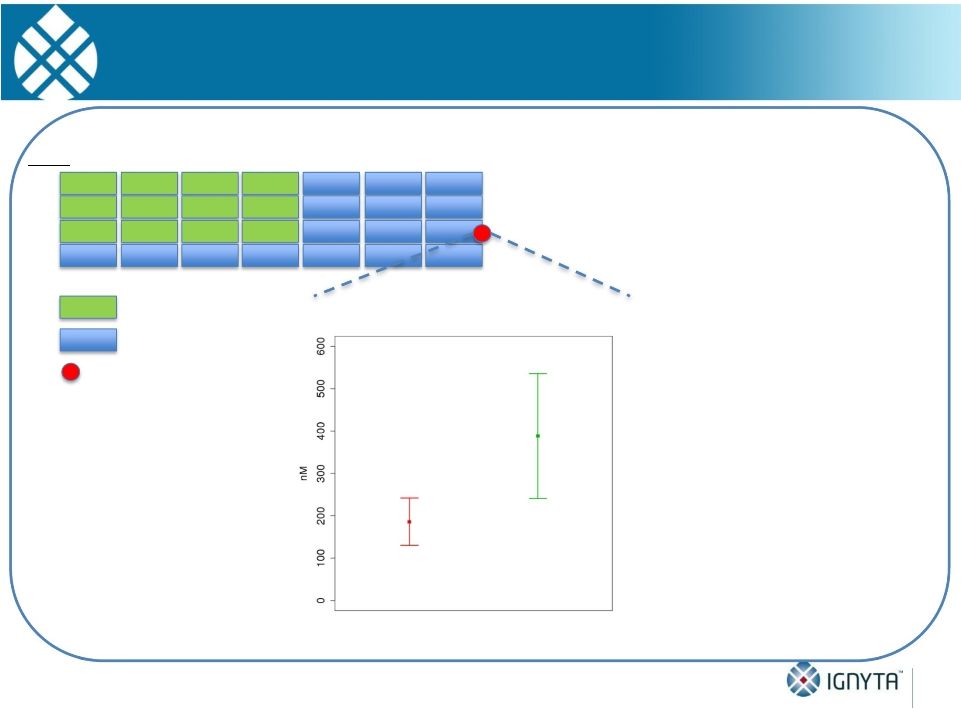
Abstract 2 Background: RXDX-101 is a small molecule inhibitor of TrkA, TrkB, TrkC, ROS1 and ALK, with high potency and selectivity. RXDX-101 demonstrated potent pharmacological activity in preclinical studies and is potentially first-in-class against the Trk family of kinases. This study aims to determine MTD, PK, PD and anti-tumor activity in patients with advanced cancer and applicable molecular alterations. Methods: Phase 1 dose escalation in patients with advanced solid tumors. RXDX-101 was dosed orally once/day in a 4 day on, 3 day off schedule for 3 weeks, followed by a 7 day rest period, in continuous 28-day cycles (Schedule A); once/day in continuous 28-day cycles (Schedule B); and once/day in a 4 day on, 3 day off schedule without interruption in continuous 28-day cycles (Schedule C). Minimum of 3 patients were enrolled at each dose level. Endpoints include safety, PK, and tumor response by RECIST. Results: 19 patients have been treated at 6 dose levels (100, 200, 400, 800,1200 and 1600 mg/m ) in Schedule A; 3 patients at one dose level (200 mg/m ) in Schedule B; and 3 patients at one dose level (400 mg/m ) in Schedule C. RXDX-101 has been well tolerated to date; the MTD has not been reached. No DLT has been seen at any dose level in any dosing schedule. The most common AEs (mainly grade 1-2) considered possibly treatment-related included paresthesia, nausea, diarrhea, asthenia, myalgia, vomiting, arthralgia, and dysgeusia. There was one possibly treatment-related grade 3 AE of asthenia. No treatment-related serious adverse events have been reported. A patient with colorectal carcinoma (TrkA+) had a PR and progressed after cycle 4. One patient with NSCLC (ROS1+) has achieved CR, and three patients with NSCLC (2 ROS1+; 1 ALK+) have achieved PRs, and all remain on treatment. An additional patient with neuroblastoma (ALK+) has a PR and is currently in cycle 21. Two patients have had prolonged stabilization of their disease: a patient with NSCLC (ALK+) in cycle 19 who remains on treatment and a patient with pancreatic cancer (ROS1+) who progressed in cycle 11. PK analysis shows maximum concentrations of RXDX-101 were generally achieved within 2 to 4 hours following dosing and exposure increased in an approximate dose proportional manner up to doses of 800 mg/m with minimal accumulation following multiple doses. Mean plasma half-life was 17 to 44 hours and steady state reached within 4 days. Conclusions: RXDX-101 intermittent and continuous dosing schedules have been well tolerated in patients with advanced solid tumors. Early responses in patients with 3 different relevant molecular alterations are promising. A global phase I/II trial was recently initiated. 2 2 2 2 |