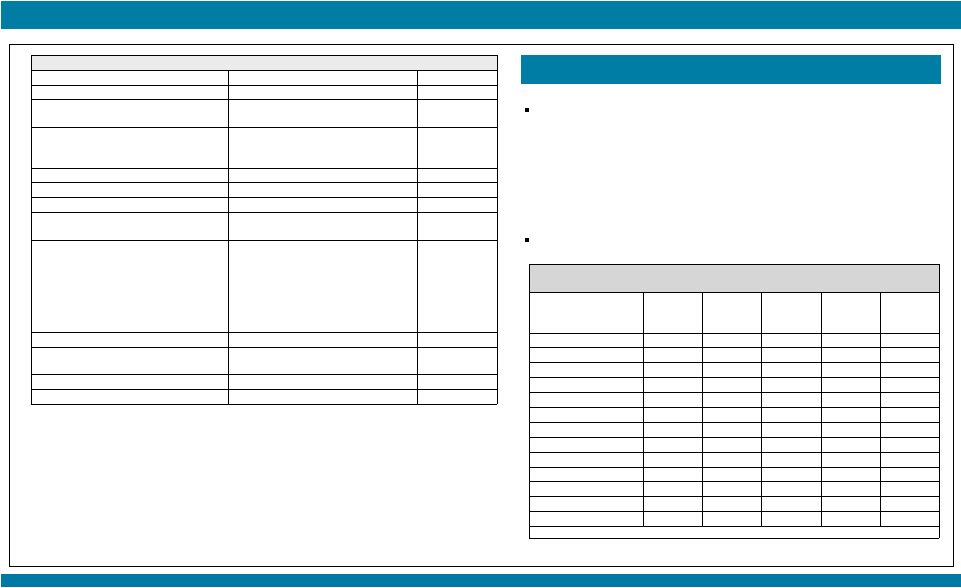
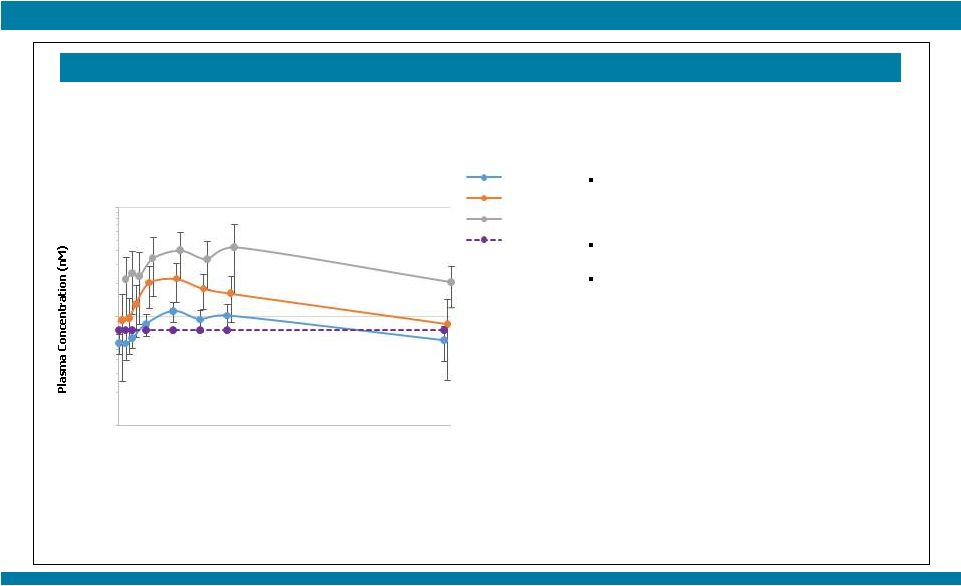
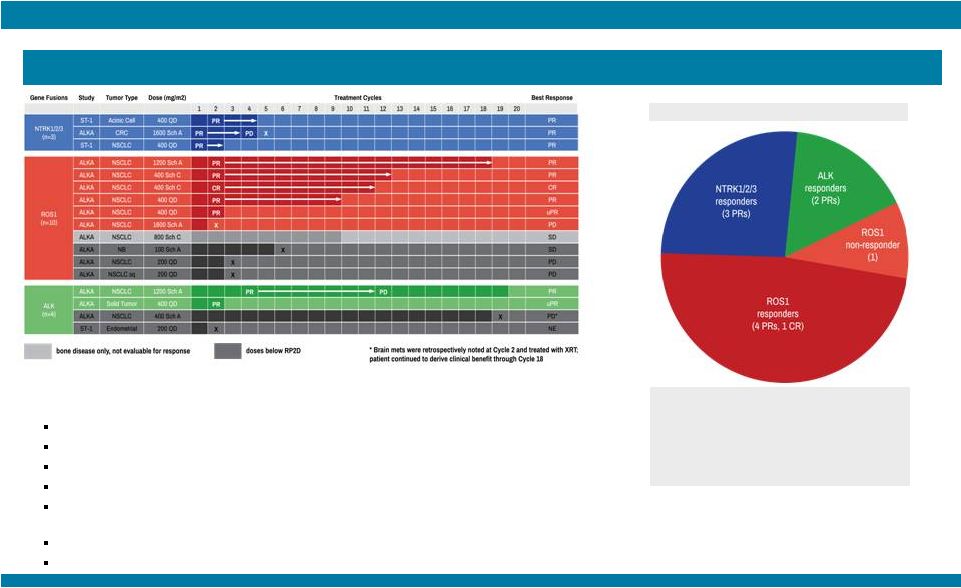
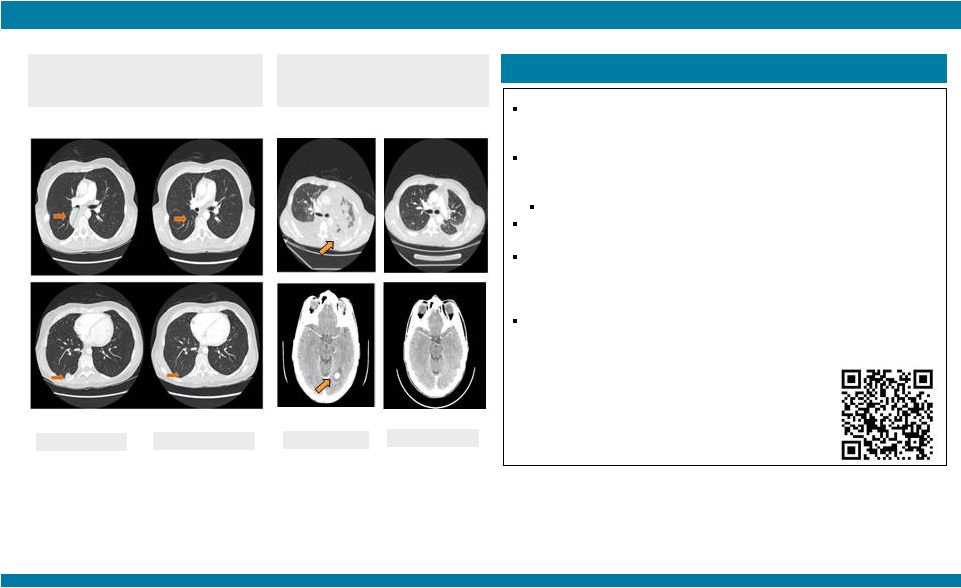
STARTRK-1: Phase 1/2a Study of Entrectinib, an Oral Pan-Trk, ROS1, and ALK Inhibitor, in Patients with Advanced Solid Tumors with Relevant Molecular Alterations Manish R. Patel 1 , Todd M. Bauer², Stephen V. Liu³, Alexander Drilon 4 , Jennifer Wheler 5 , Alice Shaw 6 , Anna Farago 6 , Sai-Hong I. Ou 7 , David Luo 8 , Litain Yeh 8 , Zachary Hornby 8 , Adrian Senderowicz 8 , and Jonathan E. Lim 8 1 Sarah Cannon Research Institute/Florida Cancer Specialists, Sarasota, FL; 2 Sarah Cannon Research Institute/Tennessee Oncology, PLLC, Nashville, TN; 3 Georgetown Lombardi Comprehensive Cancer Center, Washington, D.C.; 4 Memorial Sloan Kettering Cancer Center, New York, NY; 5 The University of Texas MD Anderson Cancer Center, Houston, TX; 6 Massachusetts General Hospital, Boston, MA; 7 Chao Family Comprehensive Cancer Center, University of California, Irvine; 8 Ignyta, Inc., San Diego, CA Abstract #2596 – Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, May 29 – June 2, 2015, Chicago, IL. Thank you to all the patients and their families who participated in this study. Patients who would have qualified for future Phase 2 studies based on fusion, dose, and treatment history; response as per RECIST v1.1 and based upon local assessment. * 10 responses among 11 patients treated at or above the RP2D, leading to a combined 91% response rate; 9 patients remain on study treatment with durable responses for up to 16 cycles Phase 2-eligible patients* (n=11) Among the other 50 non-Phase 2 eligible patients (e.g., non-fusion alterations, ALKi- or ROS1i-resistant), 13 patients (26%) remain on study: RESULTS STARTRK-1 and ALKA-372-001 Studies (n=67) Show Preliminary Antitumor Activity of Entrectinib in ALKi and ROS1i-Naïve Patients (n=17) with NTRK1/2/3, ROS1, or ALK Fusions NTRK1/2/3 SNPs, IHC+, amplifications: n=15 (6 ongoing) ROS1 fusions, ROS1i-resistant: n=3 (1 ongoing) ROS1 amplifications, deletions: n=4 ALK fusions, ALKi-resistant: n=17 (4 ongoing) ALK SNPs, amplifications, deletions: n=7 (2 ongoing; 1 patient with neuroblastoma had a PR for 9 cycles and remains on study) False positives: n=2 No alterations: n=2 |