
Catalyzing Precision Medicine with Integrated Rx/Dx in Oncology AACR 2016 - Ignyta Investor Reception April 19th, 2016 Exhibit 99.1

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: Ignyta’s corporate and scientific vision and goals, including our ability to reduce the size of tumors and to eradicate residual disease; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to develop or access companion diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to adequately fund our development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2016, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.
Agenda Conversation with a patient Entrectinib program summary Vision and pipeline
Agenda Conversation with a patient Entrectinib program summary Vision and pipeline
Entrectinib Highlights First-in-class and best-in-class Trk inhibitor: most potent in the clinic Best-in-class ROS1 inhibitor; 30-fold more potent than crizotinib Well tolerated: no observed cumulative, renal, hepatic tox, or QTc prolongation Compelling clinical activity in NTRK-, ROS1- and ALK rearranged tumors, across seven different histologies Differentiating CNS activity in brain metastases and primary brain tumors Currently in global basket study designed to be registration-enabling Innovative and strategically enabling companion diagnostic approach 5 Ignyta Goal: entrectinib, the first drug ever approved with a molecular label
History of Entrectinib and NTRK in Oncology Drug Development Ignyta first identified entrectinib in June 2013 Entrectinib was being developed primarily as a 2nd generation ALK inhibitor We noted the Trk activity and hypothesized that Trk fusions would be oncodrivers similar to ALK and ROS At that time, entrectinib and TSR-011 were the only Trk inhibitors in oncology clinical development Trk was a clinically unvalidated target
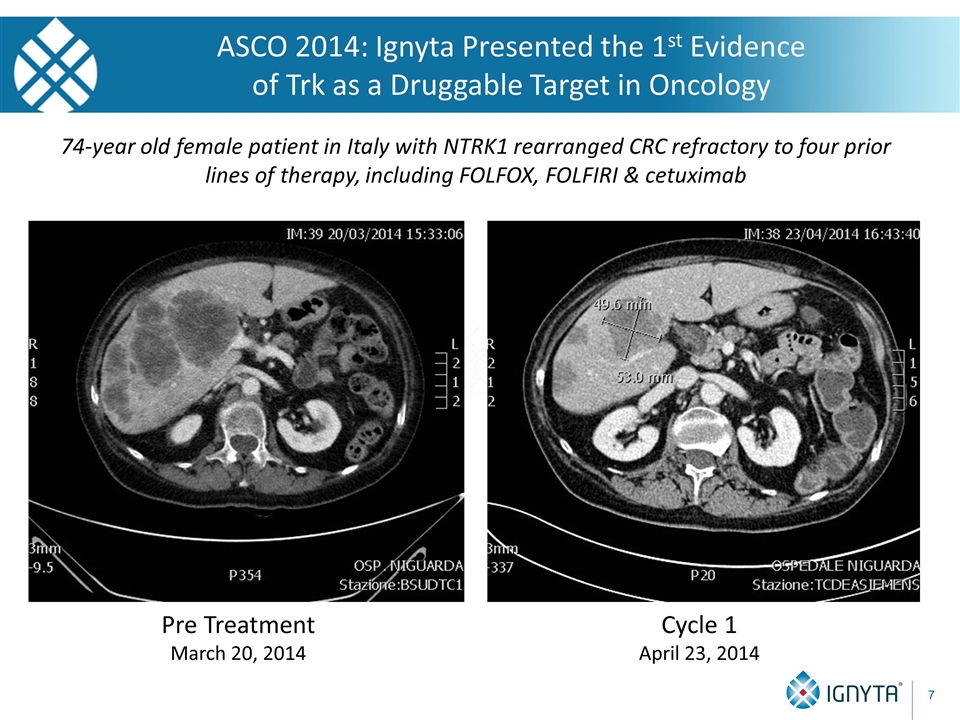
ASCO 2014: Ignyta Presented the 1st Evidence of Trk as a Druggable Target in Oncology Cycle 1 April 23, 2014 Pre Treatment March 20, 2014 74-year old female patient in Italy with NTRK1 rearranged CRC refractory to four prior lines of therapy, including FOLFOX, FOLFIRI & cetuximab
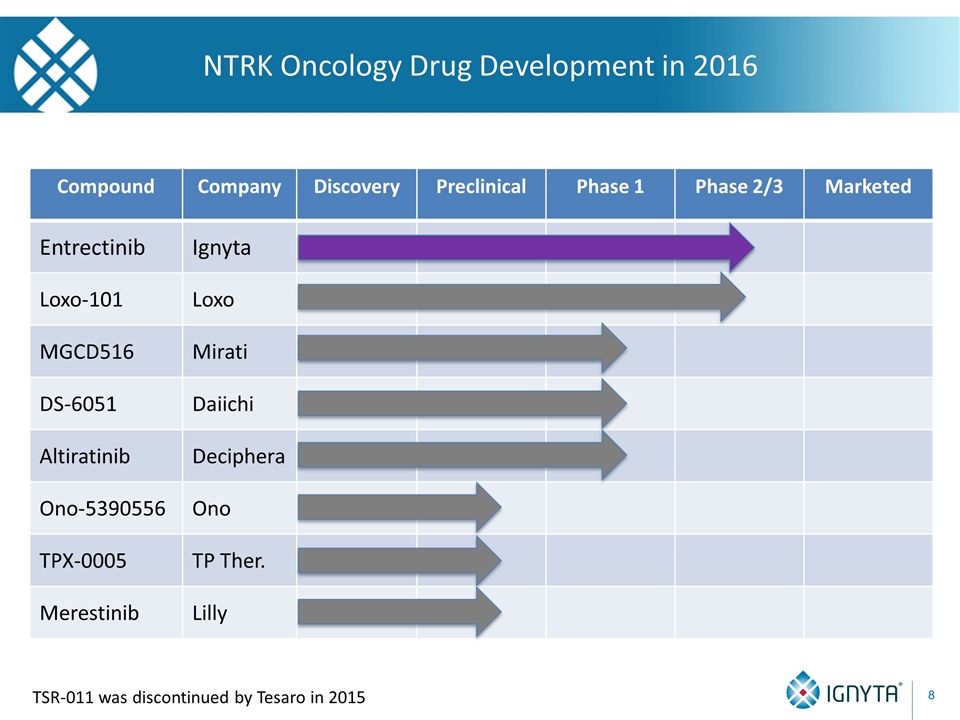
NTRK Oncology Drug Development in 2016 Compound Company Discovery Preclinical Phase 1 Phase 2/3 Marketed Entrectinib Ignyta Loxo-101 Loxo MGCD516 Mirati DS-6051 Daiichi Altiratinib Deciphera Ono-5390556 Ono TPX-0005 TP Ther. Merestinib Lilly TSR-011 was discontinued by Tesaro in 2015

Entrectinib’s and Ignyta’s Winning Proposition The market for entrectinib, particularly Trk, is commercially attractive Entrectinib is well-positioned to be best-in-class for both Trk and ROS1 due to its highest potency, good tolerability and CNS penetration features Ignyta’s in-house Dx capability is a major strategic advantage for rapid study enrollment Entrectinib is well-positioned to be first-in-class for Trk 9

The market for entrectinib, particularly Trk, is commercially attractive
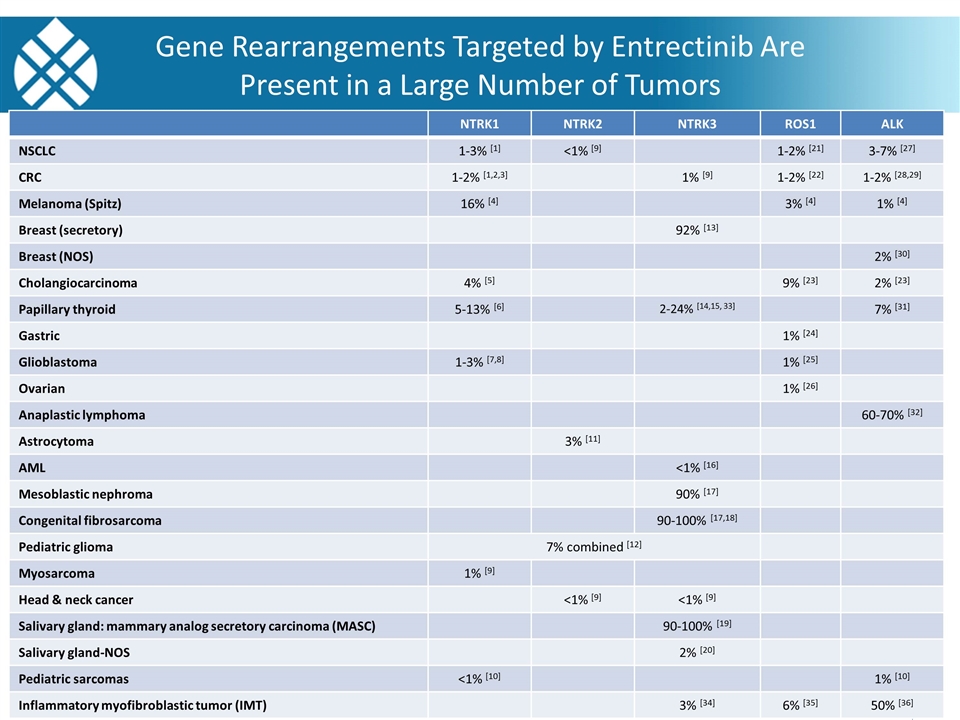
Gene Rearrangements Targeted by Entrectinib Are Present in a Large Number of Tumors Source: Literature and Ignyta analyses; NOS: not otherwise specified NTRK1 NTRK2 NTRK3 ROS1 ALK NSCLC 1-3% [1] <1% [9] 1-2% [21] 3-7% [27] CRC 1-2% [1,2,3] 1% [9] 1-2% [22] 1-2% [28,29] Melanoma (Spitz) 16% [4] 3% [4] 1% [4] Breast (secretory) 92% [13] Breast (NOS) 2% [30] Cholangiocarcinoma 4% [5] 9% [23] 2% [23] Papillary thyroid 5-13% [6] 2-24% [14,15, 33] 7% [31] Gastric 1% [24] Glioblastoma 1-3% [7,8] 1% [25] Ovarian 1% [26] Anaplastic lymphoma 60-70% [32] Astrocytoma 3% [11] AML <1% [16] Mesoblastic nephroma 90% [17] Congenital fibrosarcoma 90-100% [17,18] Pediatric glioma 7% combined [12] Myosarcoma 1% [9] Head & neck cancer <1% [9] <1% [9] Salivary gland: mammary analog secretory carcinoma (MASC) 90-100% [19] Salivary gland-NOS 2% [20] Pediatric sarcomas <1% [10] 1% [10] Inflammatory myofibroblastic tumor (IMT) 3% [34] 6% [35] 50% [36]
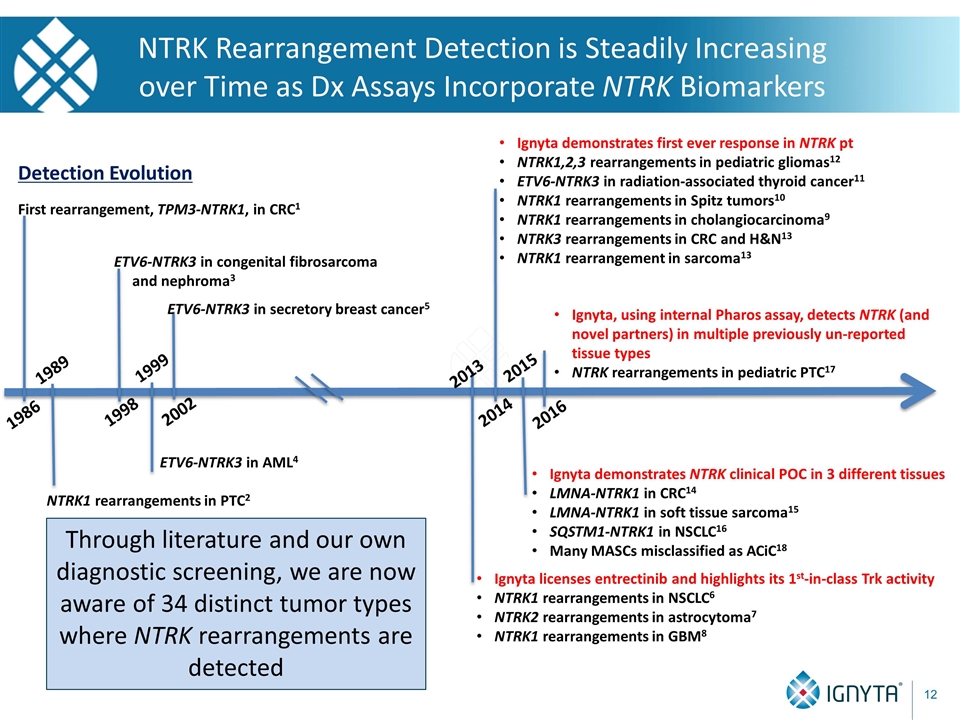
NTRK Rearrangement Detection is Steadily Increasing over Time as Dx Assays Incorporate NTRK Biomarkers 1986 First rearrangement, TPM3-NTRK1, in CRC1 1989 NTRK1 rearrangements in PTC2 1998 ETV6-NTRK3 in congenital fibrosarcoma and nephroma3 1999 ETV6-NTRK3 in AML4 2002 ETV6-NTRK3 in secretory breast cancer5 2013 Ignyta licenses entrectinib and highlights its 1st-in-class Trk activity NTRK1 rearrangements in NSCLC6 NTRK2 rearrangements in astrocytoma7 NTRK1 rearrangements in GBM8 2014 Ignyta demonstrates first ever response in NTRK pt NTRK1,2,3 rearrangements in pediatric gliomas12 ETV6-NTRK3 in radiation-associated thyroid cancer11 NTRK1 rearrangements in Spitz tumors10 NTRK1 rearrangements in cholangiocarcinoma9 NTRK3 rearrangements in CRC and H&N13 NTRK1 rearrangement in sarcoma13 2015 2016 Ignyta, using internal Pharos assay, detects NTRK (and novel partners) in multiple previously un-reported tissue types NTRK rearrangements in pediatric PTC17 Ignyta demonstrates NTRK clinical POC in 3 different tissues LMNA-NTRK1 in CRC14 LMNA-NTRK1 in soft tissue sarcoma15 SQSTM1-NTRK1 in NSCLC16 Many MASCs misclassified as ACiC18 Detection Evolution Through literature and our own diagnostic screening, we are now aware of 34 distinct tumor types where NTRK rearrangements are detected
Overview Of Entrectinib Commercial Opportunity US Only NTRK ROS1 ALK Total Addressable patients (US) Target Indication Value proposition Addressable market value (US)**
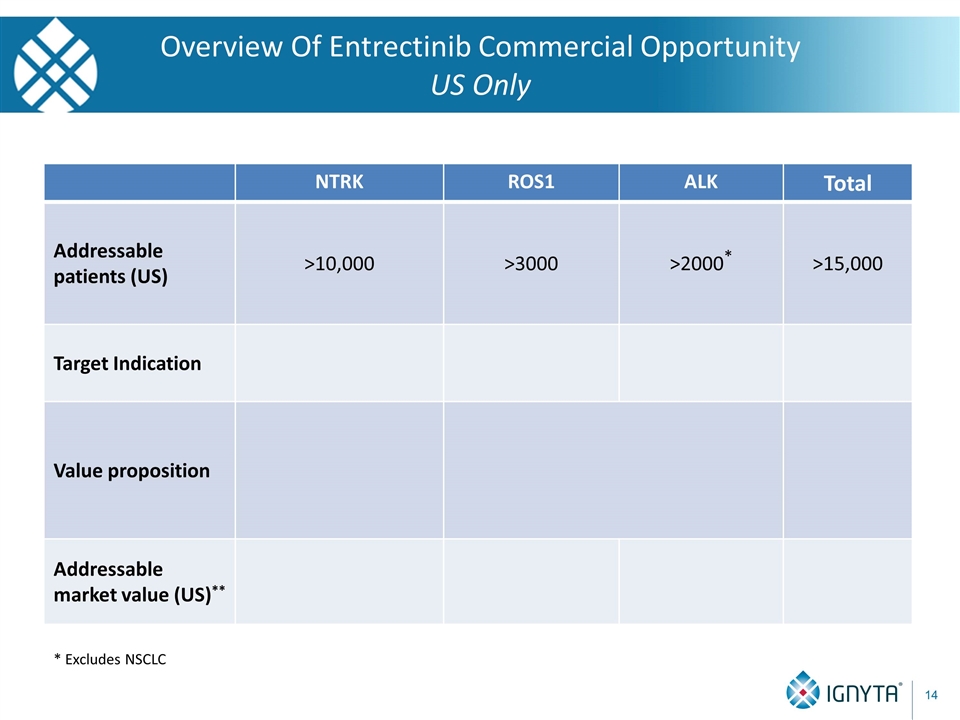
Overview Of Entrectinib Commercial Opportunity US Only * Excludes NSCLC NTRK ROS1 ALK Total Addressable patients (US) >10,000 >3000 >2000* >15,000 Target Indication Value proposition Addressable market value (US)**
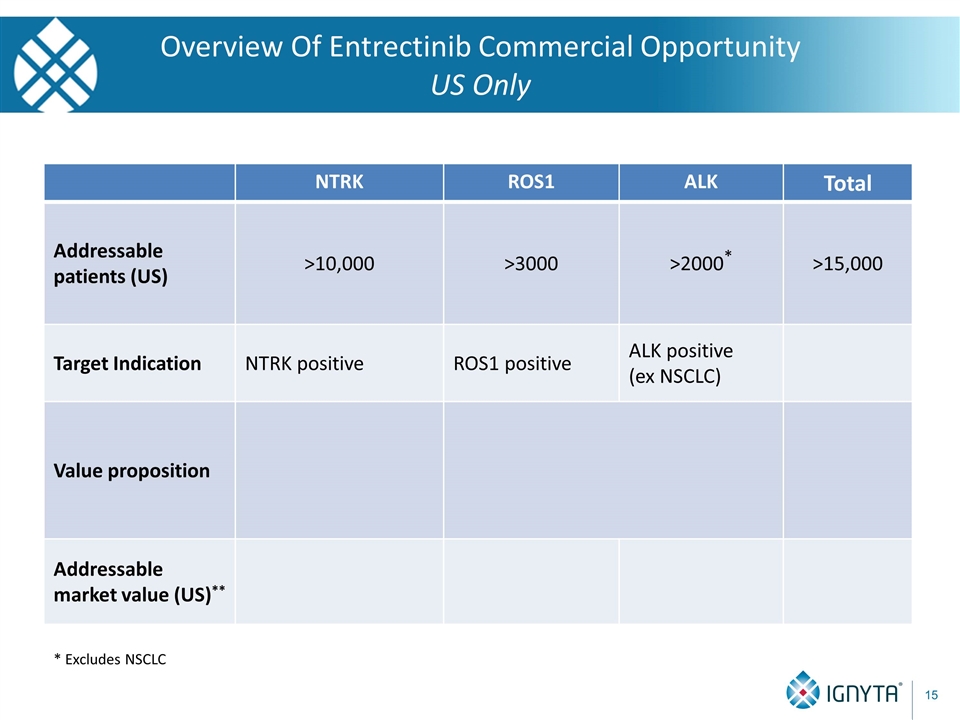
Overview Of Entrectinib Commercial Opportunity US Only * Excludes NSCLC NTRK ROS1 ALK Total Addressable patients (US) >10,000 >3000 >2000* >15,000 Target Indication NTRK positive ROS1 positive ALK positive (ex NSCLC) Value proposition Addressable market value (US)**
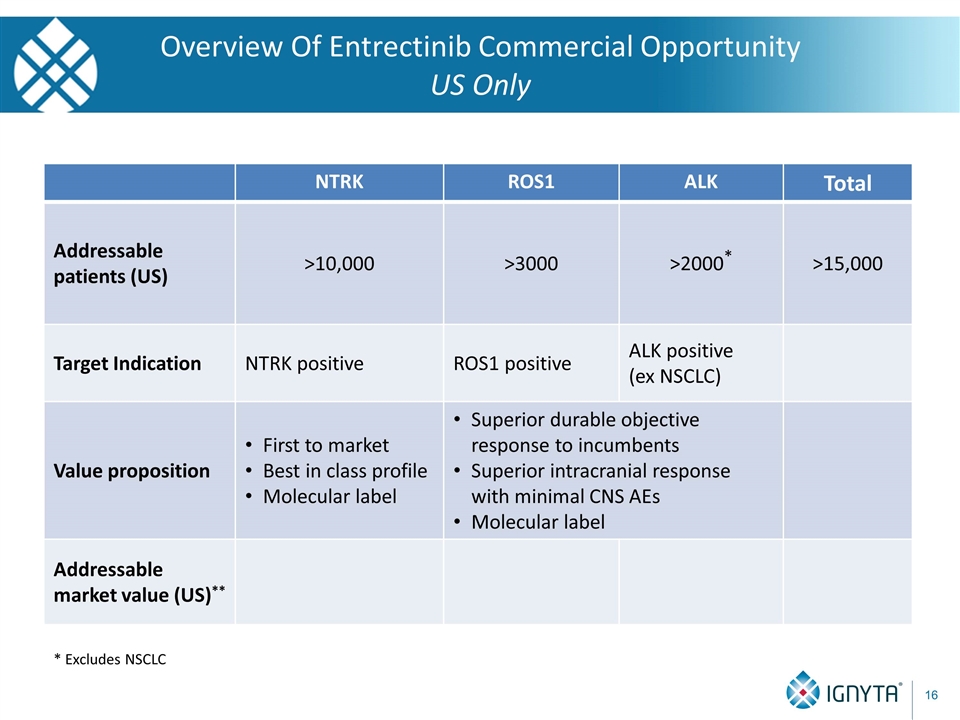
Overview Of Entrectinib Commercial Opportunity US Only * Excludes NSCLC NTRK ROS1 ALK Total Addressable patients (US) >10,000 >3000 >2000* >15,000 Target Indication NTRK positive ROS1 positive ALK positive (ex NSCLC) Value proposition First to market Best in class profile Molecular label Superior durable objective response to incumbents Superior intracranial response with minimal CNS AEs Molecular label Addressable market value (US)**
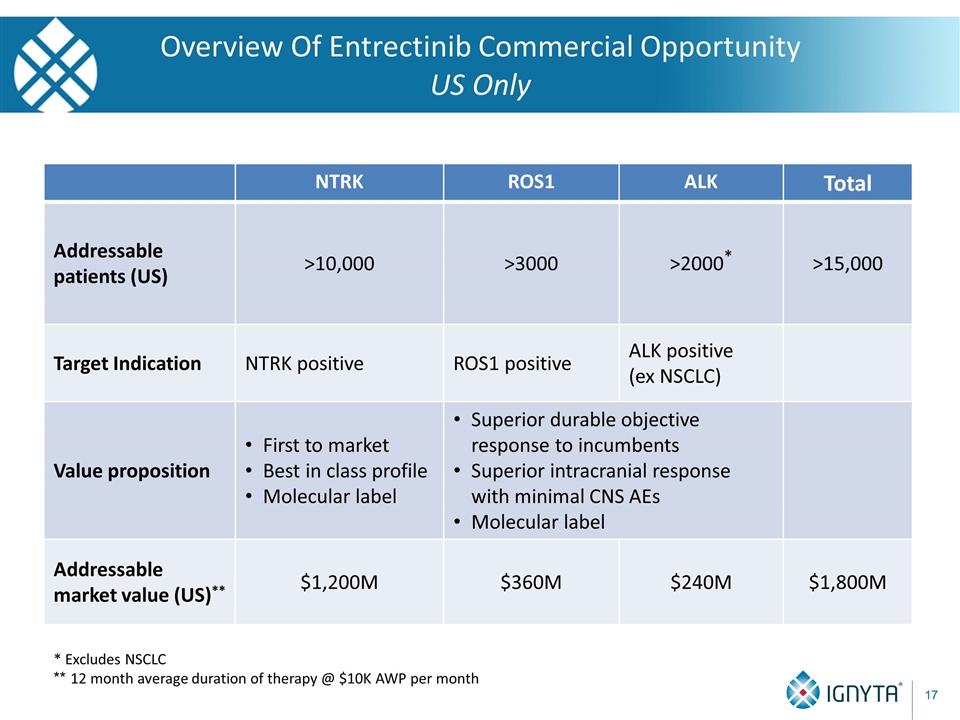
Overview Of Entrectinib Commercial Opportunity US Only NTRK ROS1 ALK Total Addressable patients (US) >10,000 >3000 >2000* >15,000 Target Indication NTRK positive ROS1 positive ALK positive (ex NSCLC) Value proposition First to market Best in class profile Molecular label Superior durable objective response to incumbents Superior intracranial response with minimal CNS AEs Molecular label Addressable market value (US)** $1,200M $360M $240M $1,800M * Excludes NSCLC ** 12 month average duration of therapy @ $10K AWP per month

Entrectinib is well-positioned to be best-in-class for both Trk and ROS1 due to its highest potency, good tolerability and CNS penetration features
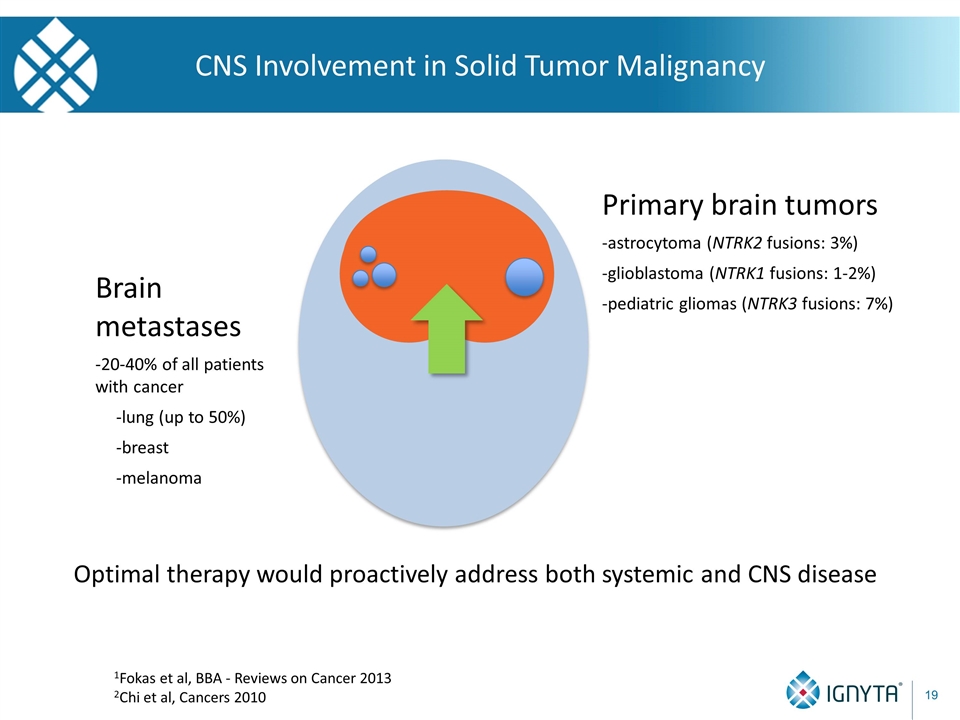
CNS Involvement in Solid Tumor Malignancy 1Fokas et al, BBA - Reviews on Cancer 2013 2Chi et al, Cancers 2010 Brain metastases -20-40% of all patients with cancer -lung (up to 50%) -breast -melanoma Primary brain tumors -astrocytoma (NTRK2 fusions: 3%) -glioblastoma (NTRK1 fusions: 1-2%) -pediatric gliomas (NTRK3 fusions: 7%) Optimal therapy would proactively address both systemic and CNS disease
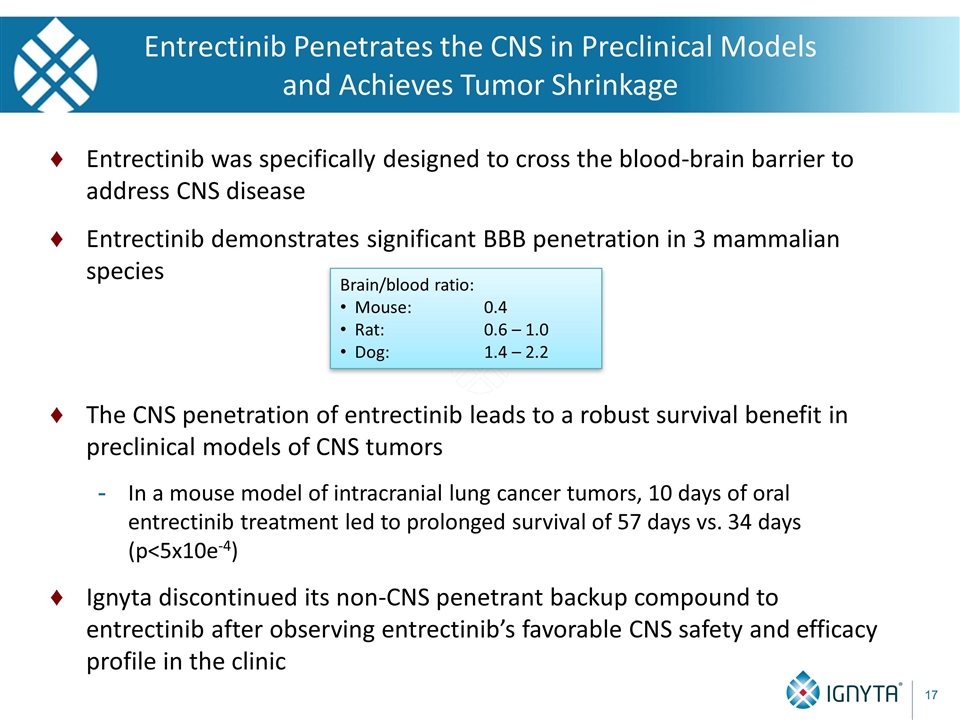
Entrectinib was specifically designed to cross the blood-brain barrier to address CNS disease Entrectinib demonstrates significant BBB penetration in 3 mammalian species The CNS penetration of entrectinib leads to a robust survival benefit in preclinical models of CNS tumors In a mouse model of intracranial lung cancer tumors, 10 days of oral entrectinib treatment led to prolonged survival of 57 days vs. 34 days (p<5x10e-4) Ignyta discontinued its non-CNS penetrant backup compound to entrectinib after observing entrectinib’s favorable CNS safety and efficacy profile in the clinic Entrectinib Penetrates the CNS in Preclinical Models and Achieves Tumor Shrinkage Brain/blood ratio: Mouse: 0.4 Rat: 0.6 – 1.0 Dog: 1.4 – 2.2 17
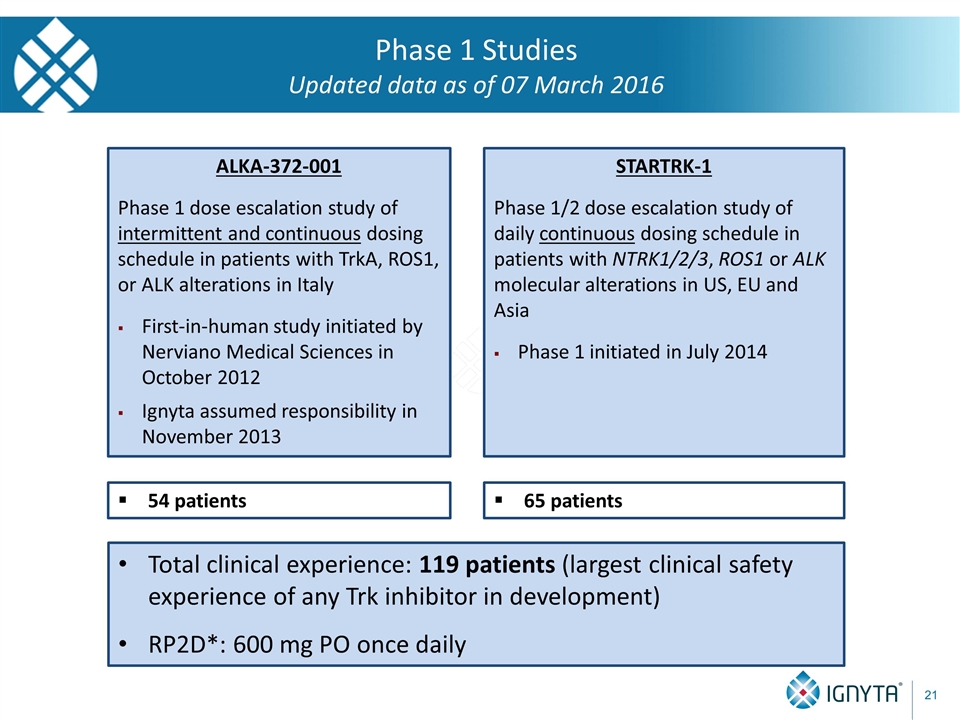
Phase 1 Studies Updated data as of 07 March 2016 STARTRK-1 Phase 1/2 dose escalation study of daily continuous dosing schedule in patients with NTRK1/2/3, ROS1 or ALK molecular alterations in US, EU and Asia Phase 1 initiated in July 2014 * RP2D = Recommended Phase 2 Dose ALKA-372-001 Phase 1 dose escalation study of intermittent and continuous dosing schedule in patients with TrkA, ROS1, or ALK alterations in Italy First-in-human study initiated by Nerviano Medical Sciences in October 2012 Ignyta assumed responsibility in November 2013 54 patients 65 patients Total clinical experience: 119 patients (largest clinical safety experience of any Trk inhibitor in development) RP2D*: 600 mg PO once daily
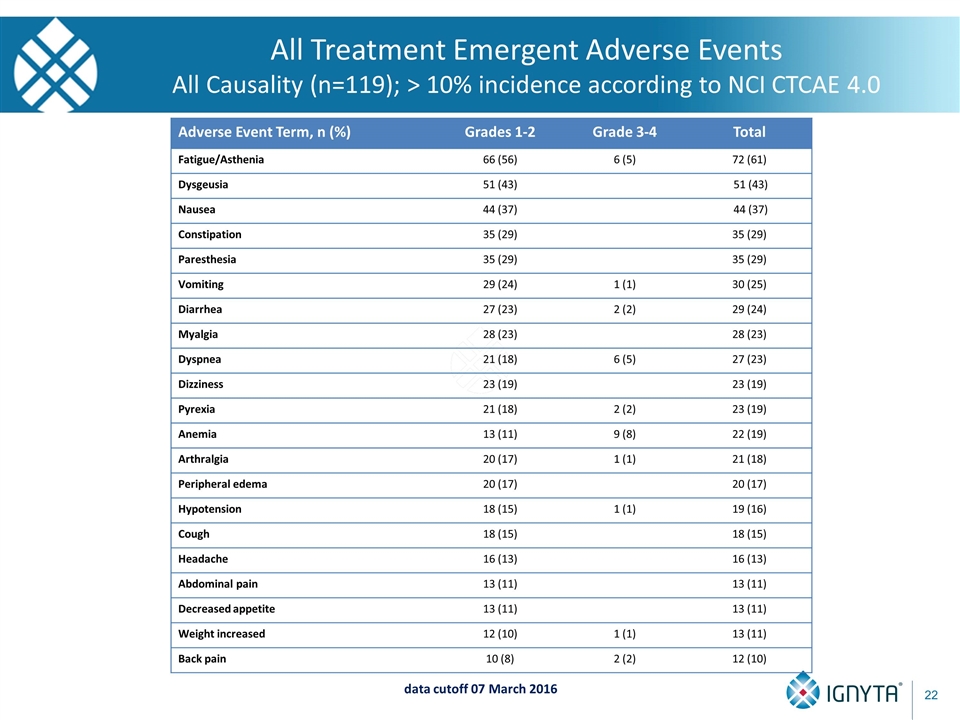
Adverse Event Term, n (%) Grades 1-2 Grade 3-4 Total Fatigue/Asthenia 66 (56) 6 (5) 72 (61) Dysgeusia 51 (43) 51 (43) Nausea 44 (37) 44 (37) Constipation 35 (29) 35 (29) Paresthesia 35 (29) 35 (29) Vomiting 29 (24) 1 (1) 30 (25) Diarrhea 27 (23) 2 (2) 29 (24) Myalgia 28 (23) 28 (23) Dyspnea 21 (18) 6 (5) 27 (23) Dizziness 23 (19) 23 (19) Pyrexia 21 (18) 2 (2) 23 (19) Anemia 13 (11) 9 (8) 22 (19) Arthralgia 20 (17) 1 (1) 21 (18) Peripheral edema 20 (17) 20 (17) Hypotension 18 (15) 1 (1) 19 (16) Cough 18 (15) 18 (15) Headache 16 (13) 16 (13) Abdominal pain 13 (11) 13 (11) Decreased appetite 13 (11) 13 (11) Weight increased 12 (10) 1 (1) 13 (11) Back pain 10 (8) 2 (2) 12 (10) data cutoff 07 March 2016 All Treatment Emergent Adverse Events All Causality (n=119); > 10% incidence according to NCI CTCAE 4.0
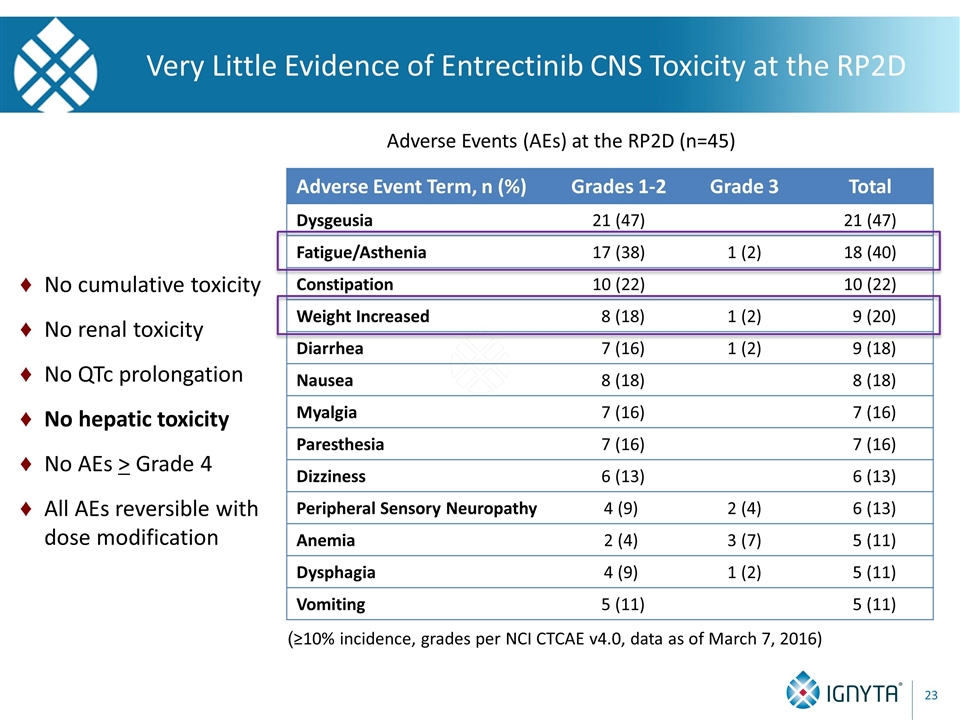
Very Little Evidence of Entrectinib CNS Toxicity at the RP2D (≥10% incidence, grades per NCI CTCAE v4.0, data as of March 7, 2016) Adverse Events (AEs) at the RP2D (n=45) Adverse Event Term, n (%) Grades 1-2 Grade 3 Total Dysgeusia 21 (47) 21 (47) Fatigue/Asthenia 17 (38) 1 (2) 18 (40) Constipation 10 (22) 10 (22) Weight Increased 8 (18) 1 (2) 9 (20) Diarrhea 7 (16) 1 (2) 9 (18) Nausea 8 (18) 8 (18) Myalgia 7 (16) 7 (16) Paresthesia 7 (16) 7 (16) Dizziness 6 (13) 6 (13) Peripheral Sensory Neuropathy 4 (9) 2 (4) 6 (13) Anemia 2 (4) 3 (7) 5 (11) Dysphagia 4 (9) 1 (2) 5 (11) Vomiting 5 (11) 5 (11) No cumulative toxicity No renal toxicity No QTc prolongation No hepatic toxicity No AEs > Grade 4 All AEs reversible with dose modification
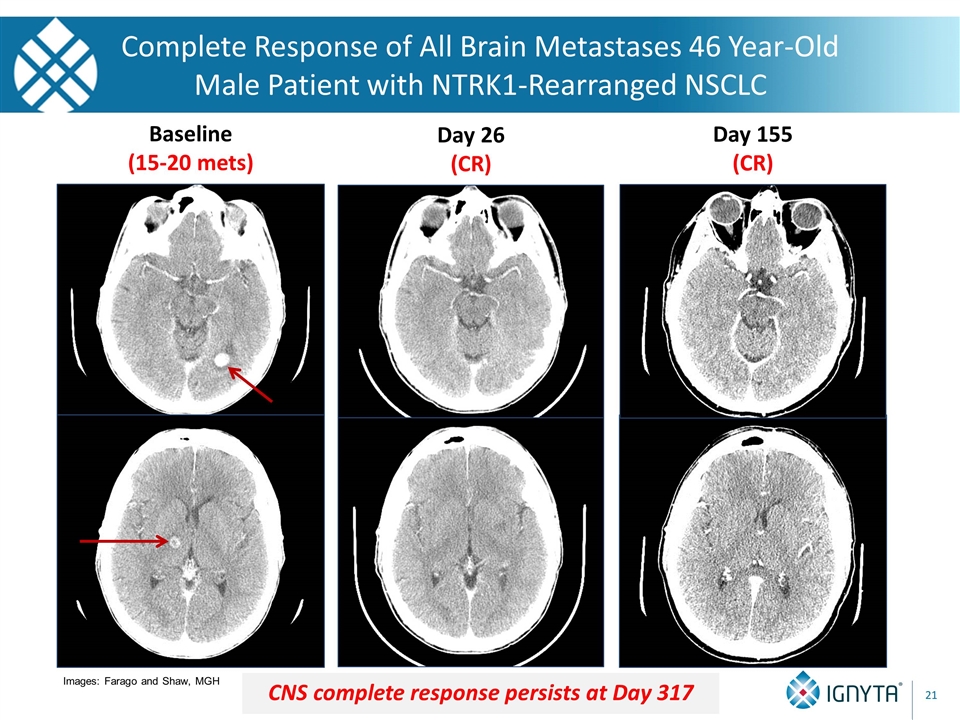
Baseline (15-20 mets) Day 26 (CR) Day 155 (CR) Complete Response of All Brain Metastases 46 Year-Old Male Patient with NTRK1-Rearranged NSCLC CNS complete response persists at Day 317 Images: Farago and Shaw, MGH 21
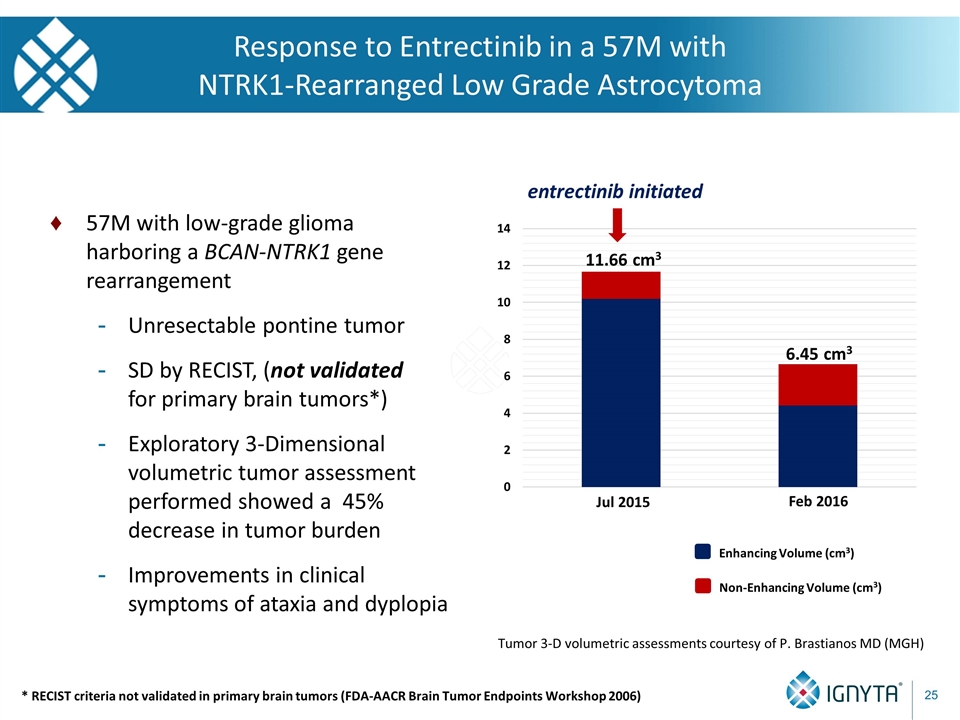
Response to Entrectinib in a 57M with NTRK1-Rearranged Low Grade Astrocytoma 57M with low-grade glioma harboring a BCAN-NTRK1 gene rearrangement Unresectable pontine tumor SD by RECIST, (not validated for primary brain tumors*) Exploratory 3-Dimensional volumetric tumor assessment performed showed a 45% decrease in tumor burden Improvements in clinical symptoms of ataxia and dyplopia entrectinib initiated Non-Enhancing Volume (cm3) Enhancing Volume (cm3) 11.66 cm3 6.45 cm3 Tumor 3-D volumetric assessments courtesy of P. Brastianos MD (MGH) Jul 2015 Feb 2016 * RECIST criteria not validated in primary brain tumors (FDA-AACR Brain Tumor Endpoints Workshop 2006)
20 Month-Old Boy with NTRK3-Rearranged Infantile Fibrosarcoma Metastatic to CNS (Compassionate Use) 20 month-old boy with recurrent, metastatic infantile fibrosarcoma harboring ETV6-NTRK3 gene rearrangement (first detected in Ignyta Diagnostic lab) Presented at birth with left leg mass, requiring through-the-knee amputation At age 4 months, large metastases to left lung identified à 24-weeks of chemotherapy At age 12 months, large right frontal intracranial tumor identified à resected, followed by 5 cycles of salvage chemotherapy Recurrent CNS disease with lesions in the right frontal and temporal lobes, as well as leptomeningeal involvement On physical exam, was very sleepy but responsive to stimuli and had decreased tone and strength in the left arm Baseline head CT showed large tumor mass in the right hemisphere, centering on the right temporal lobe (3.7 x 2.5cm) with massive tumor-related swelling, a 17 mm midline shift, and evidence of transtentorial herniation Due to these radiographic and clinical findings, the patient’s treating physician felt that: “death is likely imminent” Received entrectinib starting February 11th
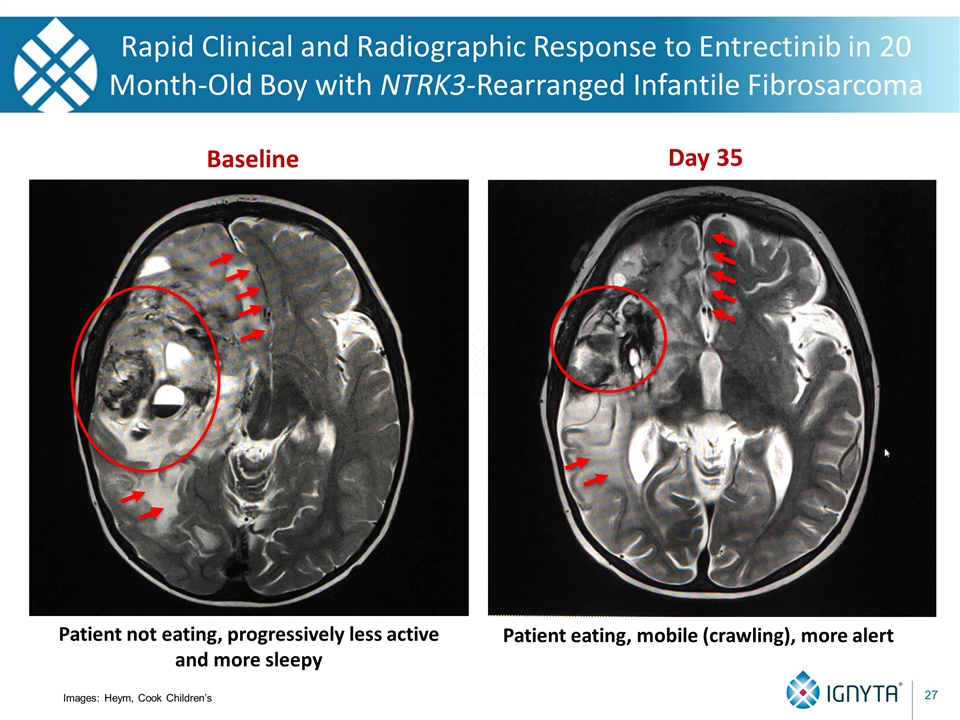
Rapid Clinical and Radiographic Response to Entrectinib in 20 Month-Old Boy with NTRK3-Rearranged Infantile Fibrosarcoma Baseline Day 35 Patient not eating, progressively less active and more sleepy Patient eating, mobile (crawling), more alert Images: Heym, Cook Children’s
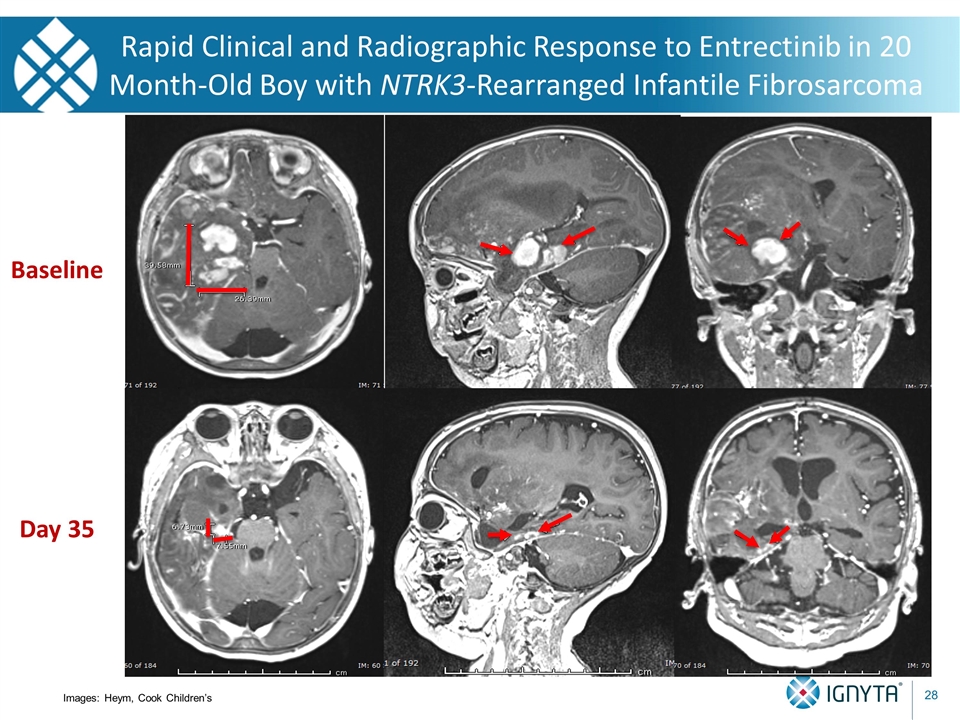
Baseline Day 35 Not certain I’m even measuring the same “stuff”! Rapid Clinical and Radiographic Response to Entrectinib in 20 Month-Old Boy with NTRK3-Rearranged Infantile Fibrosarcoma Images: Heym, Cook Children’s
Physician’s Assessment of 20 Month-Old Boy with NTRK3-Rearranged Infantile Fibrosarcoma Radiologist (Day 35) “Dramatic improvement. Markedly decreased tumor burden. Markedly decreased edema. Markedly decreased midline shift. Resolution of transtentorial herniation; now only mild medialization of RIGHT temporal lobe.” Oncologist (Day 54) “I just saw our boy and he looks amazing. Very active and playful. His left arm is no longer contracted and his strength is improving. He is eating well and really looks as good as he did before his tumor came back…Our miracle continues” 26
Long Term Patient Experience on Entrectinib Defines the Benefit of Its CNS Activity Safety: Patients have been on entrectinib as long as 3+ years 11 patients have been on entrectinib longer than 1 year 19 patients have been on entrectinib longer than 6 months Less than 8% of patients have required dose reduction No entrectinib responder patient has discontinued due to safety or tolerability Efficacy: Mr. Z Astrocytoma patient Baby Y
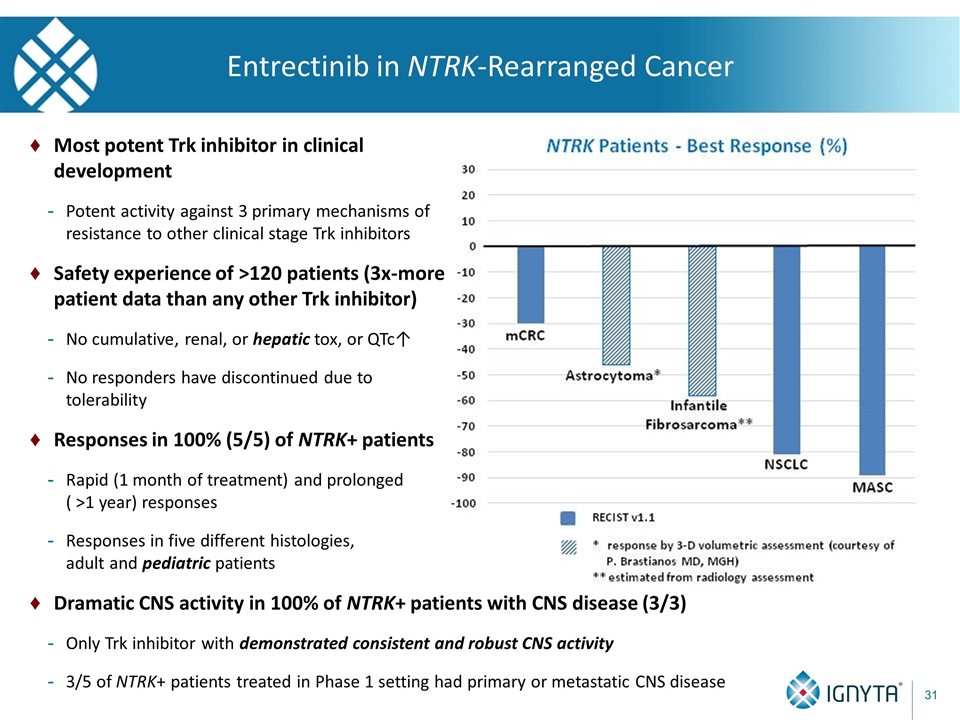
Entrectinib in NTRK-Rearranged Cancer Most potent Trk inhibitor in clinical development Potent activity against 3 primary mechanisms of resistance to other clinical stage Trk inhibitors Safety experience of >120 patients (3x-more patient data than any other Trk inhibitor) No cumulative, renal, or hepatic tox, or QTc↑ No responders have discontinued due to tolerability Responses in 100% (5/5) of NTRK+ patients Rapid (1 month of treatment) and prolonged ( >1 year) responses Responses in five different histologies, adult and pediatric patients Dramatic CNS activity in 100% of NTRK+ patients with CNS disease (3/3) Only Trk inhibitor with demonstrated consistent and robust CNS activity 3/5 of NTRK+ patients treated in Phase 1 setting had primary or metastatic CNS disease
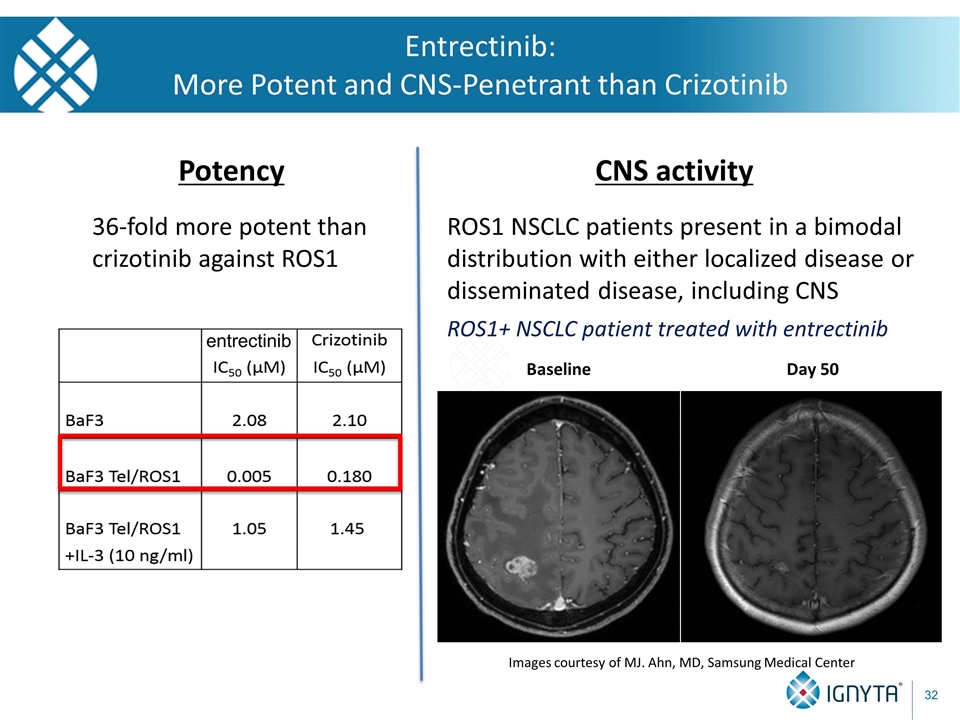
Entrectinib: More Potent and CNS-Penetrant than Crizotinib entrectinib Potency 36-fold more potent than crizotinib against ROS1 CNS activity ROS1 NSCLC patients present in a bimodal distribution with either localized disease or disseminated disease, including CNS ROS1+ NSCLC patient treated with entrectinib Baseline Day 50 Images courtesy of MJ. Ahn, MD, Samsung Medical Center
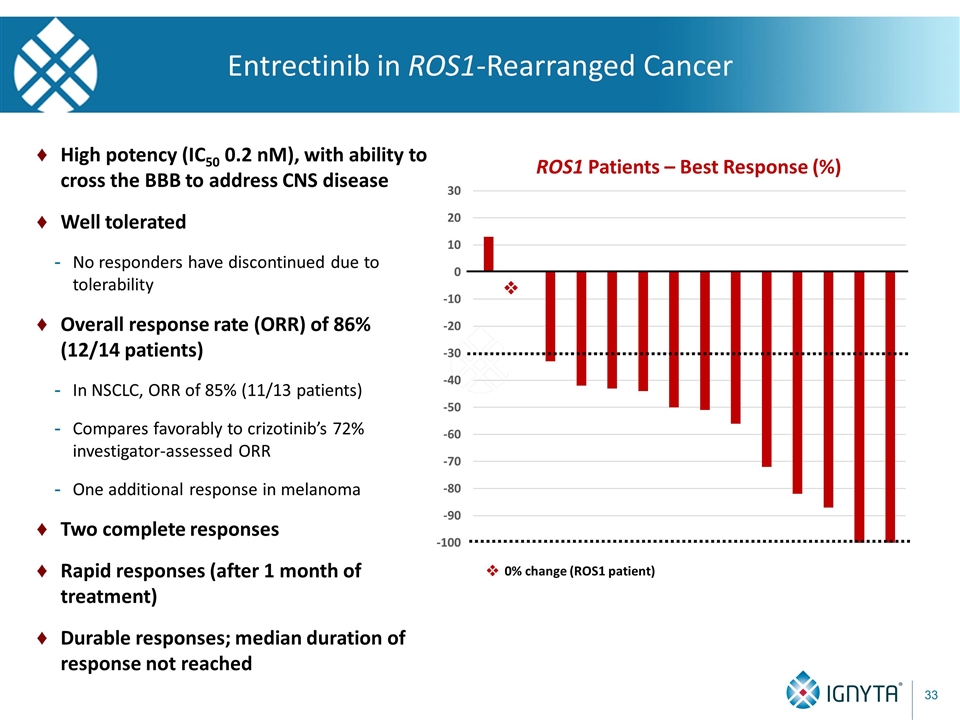
Entrectinib in ROS1-Rearranged Cancer v 0% change (ROS1 patient) v High potency (IC50 0.2 nM), with ability to cross the BBB to address CNS disease Well tolerated No responders have discontinued due to tolerability Overall response rate (ORR) of 86% (12/14 patients) In NSCLC, ORR of 85% (11/13 patients) Compares favorably to crizotinib’s 72% investigator-assessed ORR One additional response in melanoma Two complete responses Rapid responses (after 1 month of treatment) Durable responses; median duration of response not reached
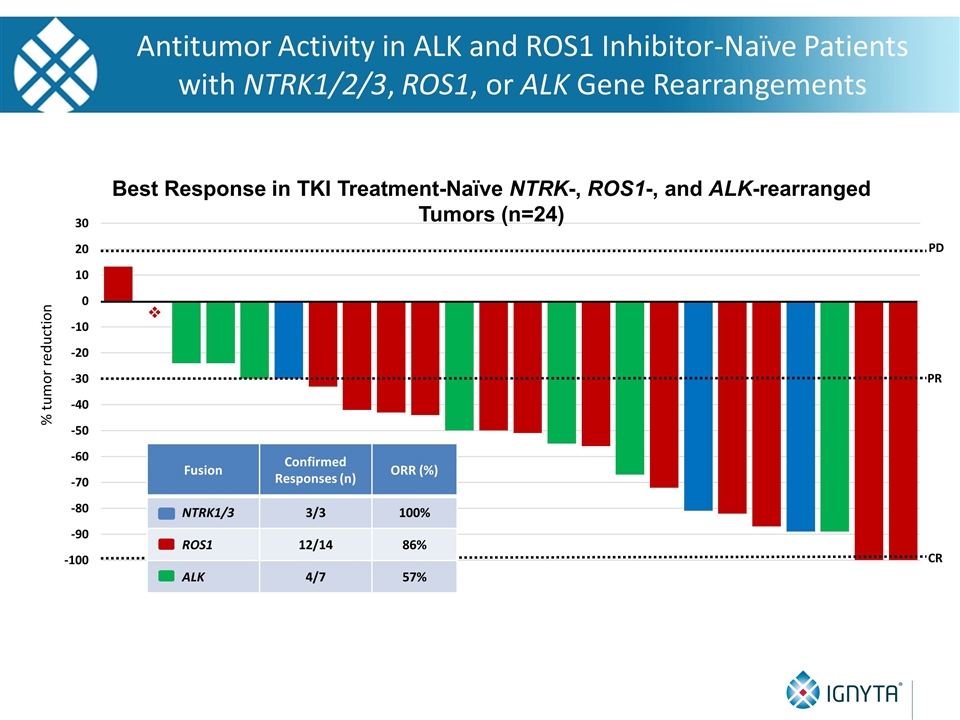
Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% ROS1 12/14 86% ALK 4/7 57% % tumor reduction PD v PR CR Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) Antitumor Activity in ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Gene Rearrangements
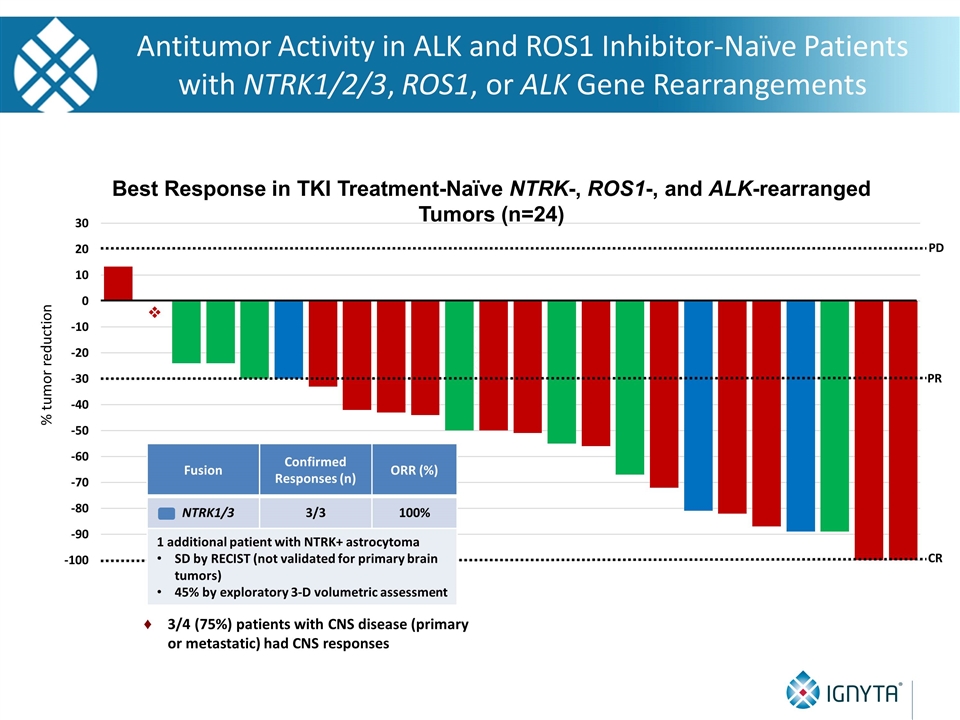
Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% 1 additional patient with NTRK+ astrocytoma SD by RECIST (not validated for primary brain tumors) 45% by exploratory 3-D volumetric assessment % tumor reduction PD v PR CR Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) Antitumor Activity in ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Gene Rearrangements 3/4 (75%) patients with CNS disease (primary or metastatic) had CNS responses
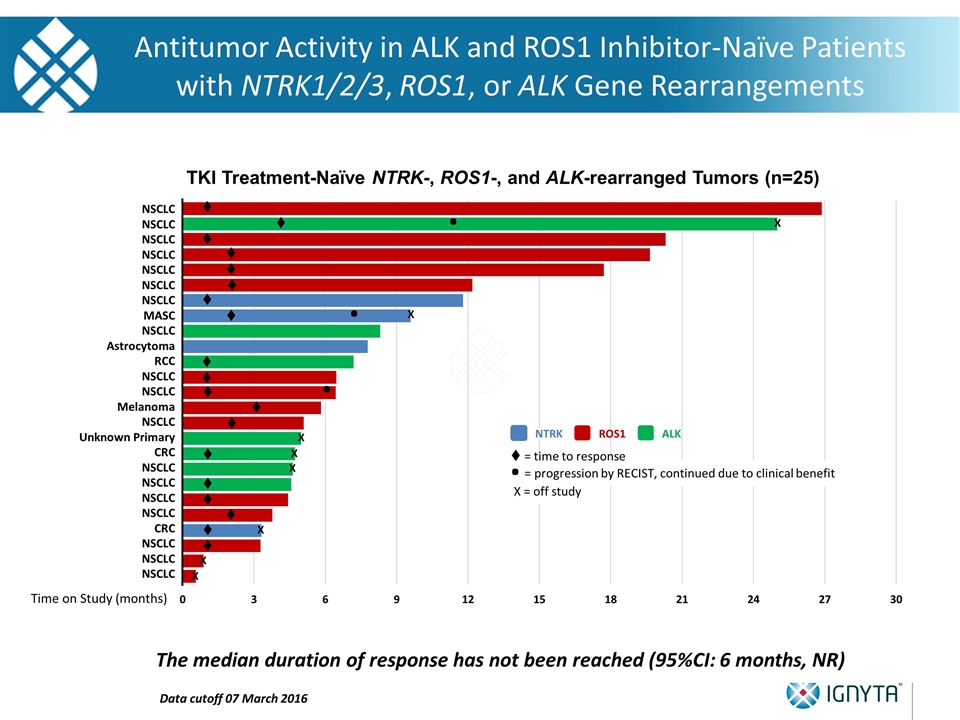
NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC MASC NSCLC Astrocytoma RCC NSCLC NSCLC Melanoma NSCLC Unknown Primary CRC NSCLC NSCLC NSCLC NSCLC CRC NSCLC NSCLC NSCLC Time on Study (months) X X X X X X X X . . X = off study . = progression by RECIST, continued due to clinical benefit NTRK ALK ROS1 . t t t t t t t t t t t t t t t t t t t t = time to response TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=25) . Antitumor Activity in ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Gene Rearrangements The median duration of response has not been reached (95%CI: 6 months, NR) Data cutoff 07 March 2016
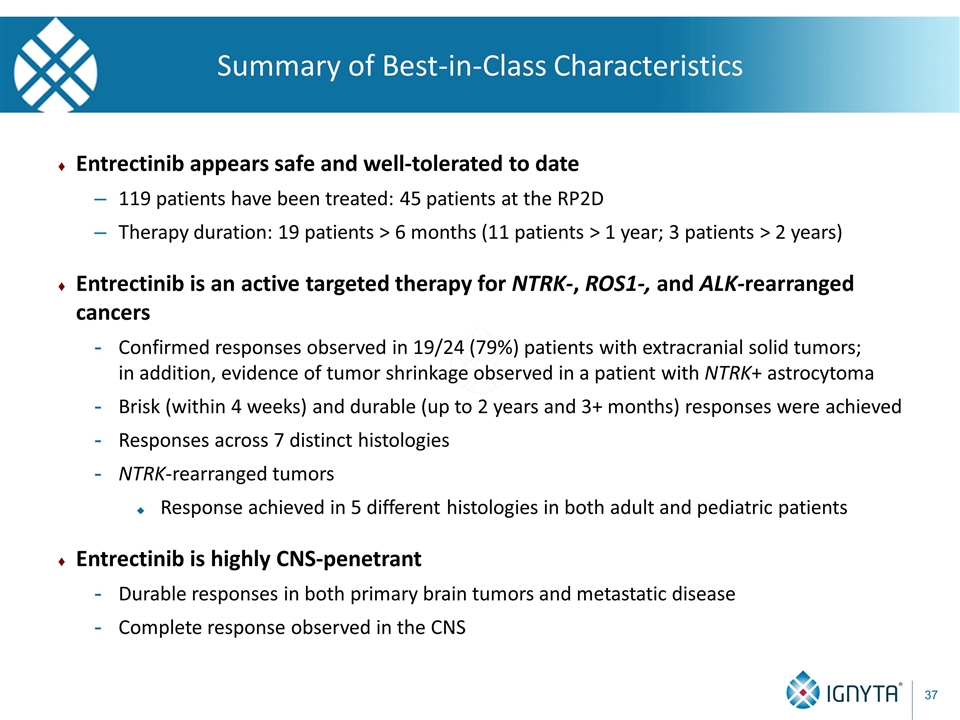
Summary of Best-in-Class Characteristics Entrectinib appears safe and well-tolerated to date 119 patients have been treated: 45 patients at the RP2D Therapy duration: 19 patients > 6 months (11 patients > 1 year; 3 patients > 2 years) Entrectinib is an active targeted therapy for NTRK-, ROS1-, and ALK-rearranged cancers Confirmed responses observed in 19/24 (79%) patients with extracranial solid tumors; in addition, evidence of tumor shrinkage observed in a patient with NTRK+ astrocytoma Brisk (within 4 weeks) and durable (up to 2 years and 3+ months) responses were achieved Responses across 7 distinct histologies NTRK-rearranged tumors Response achieved in 5 different histologies in both adult and pediatric patients Entrectinib is highly CNS-penetrant Durable responses in both primary brain tumors and metastatic disease Complete response observed in the CNS

Ignyta’s in-house Dx capability is a major strategic advantage for rapid study enrollment
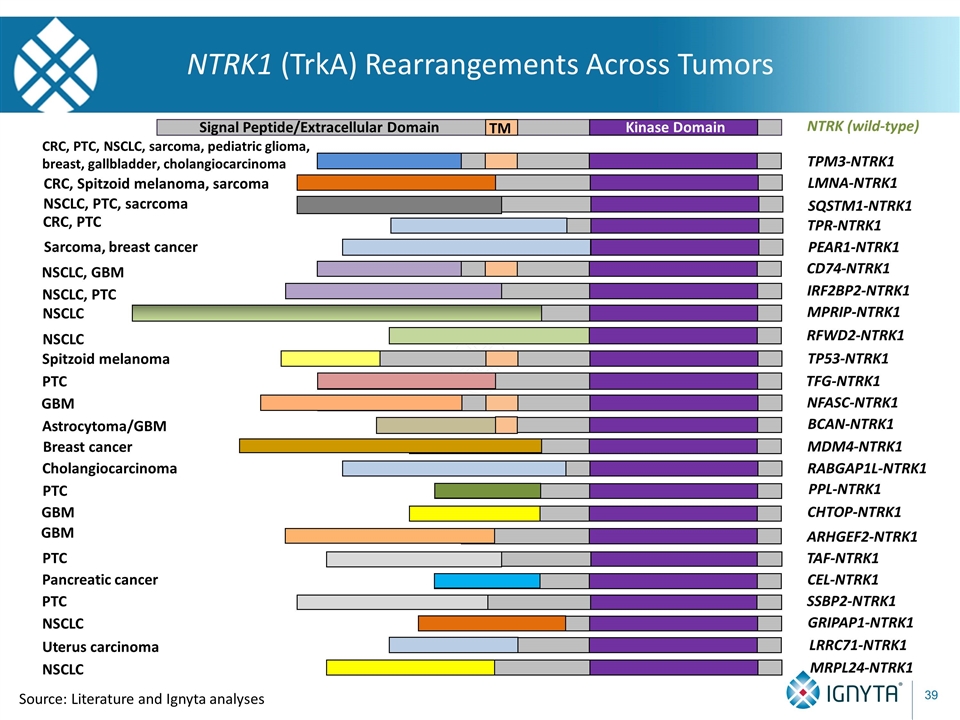
NTRK1 (TrkA) Rearrangements Across Tumors TM Kinase Domain NTRK (wild-type) Signal Peptide/Extracellular Domain MPRIP-NTRK1 NSCLC IRF2BP2-NTRK1 NSCLC, PTC RFWD2-NTRK1 NSCLC TPM3-NTRK1 CRC, PTC, NSCLC, sarcoma, pediatric glioma, breast, gallbladder, cholangiocarcinoma LMNA-NTRK1 CRC, Spitzoid melanoma, sarcoma TP53-NTRK1 Spitzoid melanoma TFG-NTRK1 PTC TPR-NTRK1 CRC, PTC NFASC-NTRK1 GBM PEAR1-NTRK1 Sarcoma, breast cancer SQSTM1-NTRK1 NSCLC, PTC, sacrcoma CD74-NTRK1 NSCLC, GBM BCAN-NTRK1 MDM4-NTRK1 RABGAP1L-NTRK1 PPL-NTRK1 CHTOP-NTRK1 ARHGEF2-NTRK1 TAF-NTRK1 CEL-NTRK1 SSBP2-NTRK1 GRIPAP1-NTRK1 LRRC71-NTRK1 MRPL24-NTRK1 Astrocytoma/GBM Breast cancer Cholangiocarcinoma PTC GBM GBM PTC Pancreatic cancer PTC NSCLC NSCLC Uterus carcinoma Source: Literature and Ignyta analyses
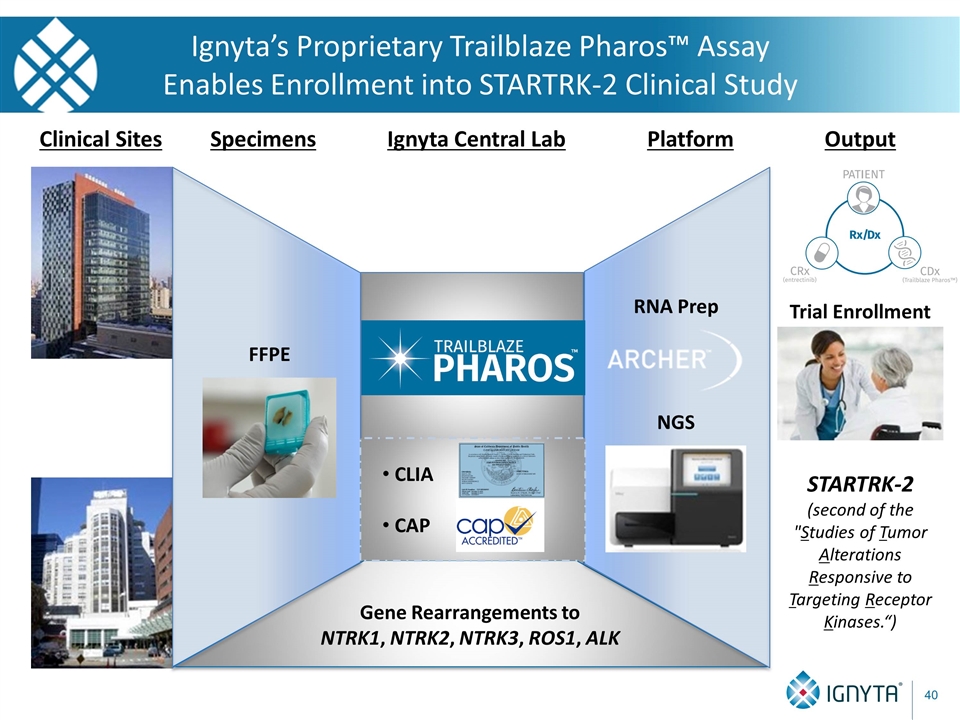
Gene Rearrangements to NTRK1, NTRK2, NTRK3, ROS1, ALK Clinical Sites Specimens Platform Output FFPE NGS Trial Enrollment Ignyta’s Proprietary Trailblaze Pharos™ Assay Enables Enrollment into STARTRK-2 Clinical Study Ignyta Central Lab CLIA CAP RNA Prep STARTRK-2 (second of the "Studies of Tumor Alterations Responsive to Targeting Receptor Kinases.“)
Trailblaze Pharos™ and STARTRK-2 Trailblaze Pharos is one mechanism of identifying patients for enrollment into STARTRK-2 In addition, enrollment is supported by local and commercial testing In fact, Ignyta currently has formal screening relationships in place with five commercial diagnostic entities, supporting enrollment domestically and abroad Nonetheless, >20% of the patients enrolled into STARTRK-2 to date were first detected as having gene rearrangements by Trailblaze Pharos Ignyta has identified NTRK rearrangements in multiple new tissue histologies never previously reported in the literature Our CLIA/CAP lab recently identified two new NTRK patients in different histologies at a single site in just one week We are currently receiving tumor samples for testing from 43 different clinical sites Of the eligible fusion positive samples detected in our lab, >70% have gone on to receive entrectinib

Entrectinib is well-positioned to be first-in-class for Trk
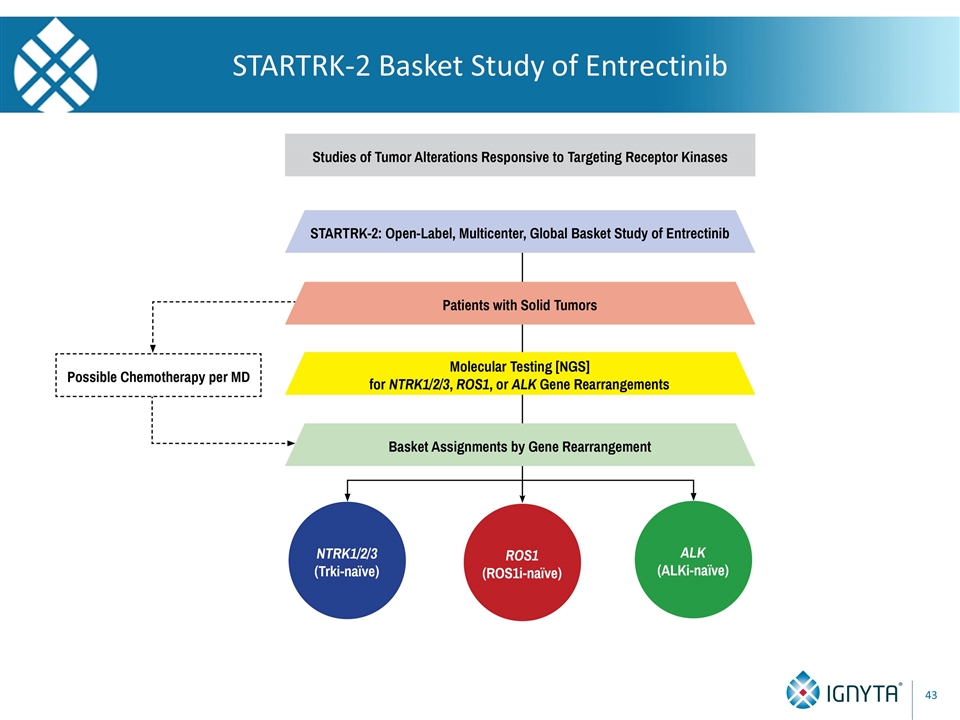
STARTRK-2 Basket Study of Entrectinib

STARTRK-2: Status Update STARTRK-2 An Open-Label, Multicenter, Global Phase 2 Basket Study of Entrectinib for the Treatment of Patients with Locally Advanced or Metastatic Solid Tumors that Harbor NTRK1/2/3, ROS1, or ALK Gene Rearrangements Global Study ~20 countries Approximately 150 Sites including ~40-50 US sites Regulatory approval in 7 countries Nearly 50 clinical sites activated NCT02568267 www.startrktrials.com
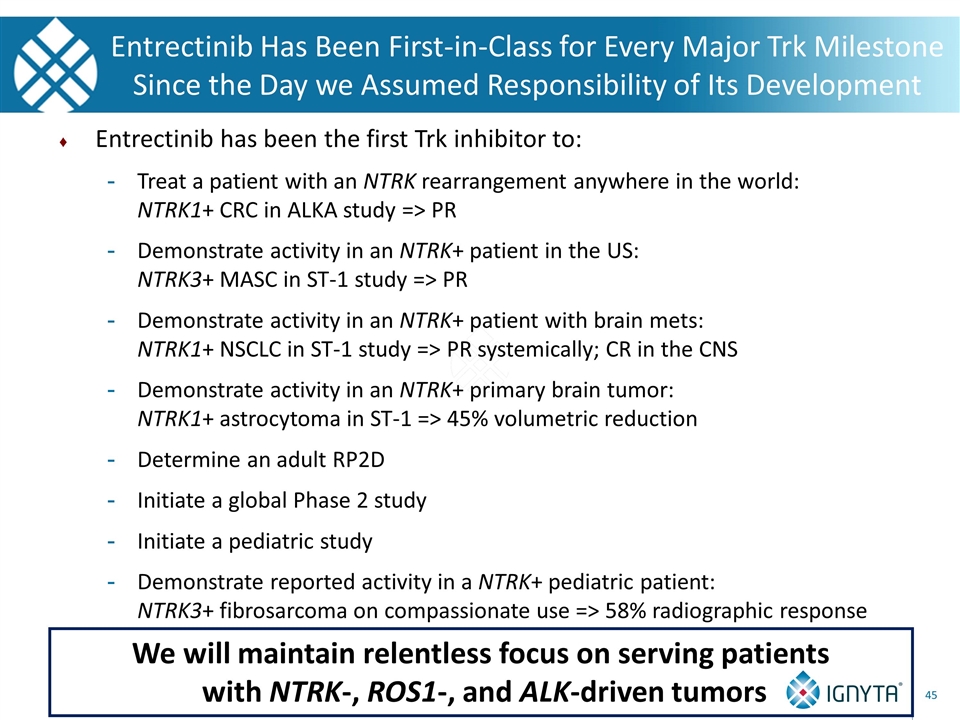
Entrectinib Has Been First-in-Class for Every Major Trk Milestone Since the Day we Assumed Responsibility of Its Development Entrectinib has been the first Trk inhibitor to: Treat a patient with an NTRK rearrangement anywhere in the world: NTRK1+ CRC in ALKA study => PR Demonstrate activity in an NTRK+ patient in the US: NTRK3+ MASC in ST-1 study => PR Demonstrate activity in an NTRK+ patient with brain mets: NTRK1+ NSCLC in ST-1 study => PR systemically; CR in the CNS Demonstrate activity in an NTRK+ primary brain tumor: NTRK1+ astrocytoma in ST-1 => 45% volumetric reduction Determine an adult RP2D Initiate a global Phase 2 study Initiate a pediatric study Demonstrate reported activity in a NTRK+ pediatric patient: NTRK3+ fibrosarcoma on compassionate use => 58% radiographic response We will maintain relentless focus on serving patients with NTRK-, ROS1-, and ALK-driven tumors
Agenda Conversation with a patient Entrectinib program summary Vision and pipeline
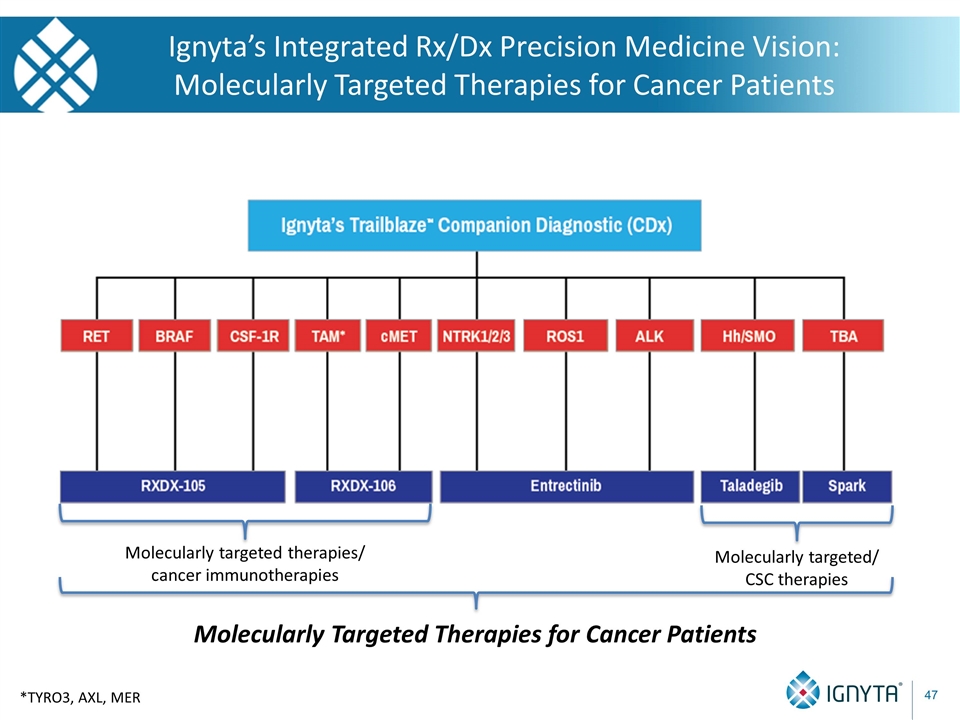
Ignyta’s Integrated Rx/Dx Precision Medicine Vision: Molecularly Targeted Therapies for Cancer Patients *TYRO3, AXL, MER Molecularly targeted therapies/ cancer immunotherapies Molecularly targeted/ CSC therapies Molecularly Targeted Therapies for Cancer Patients
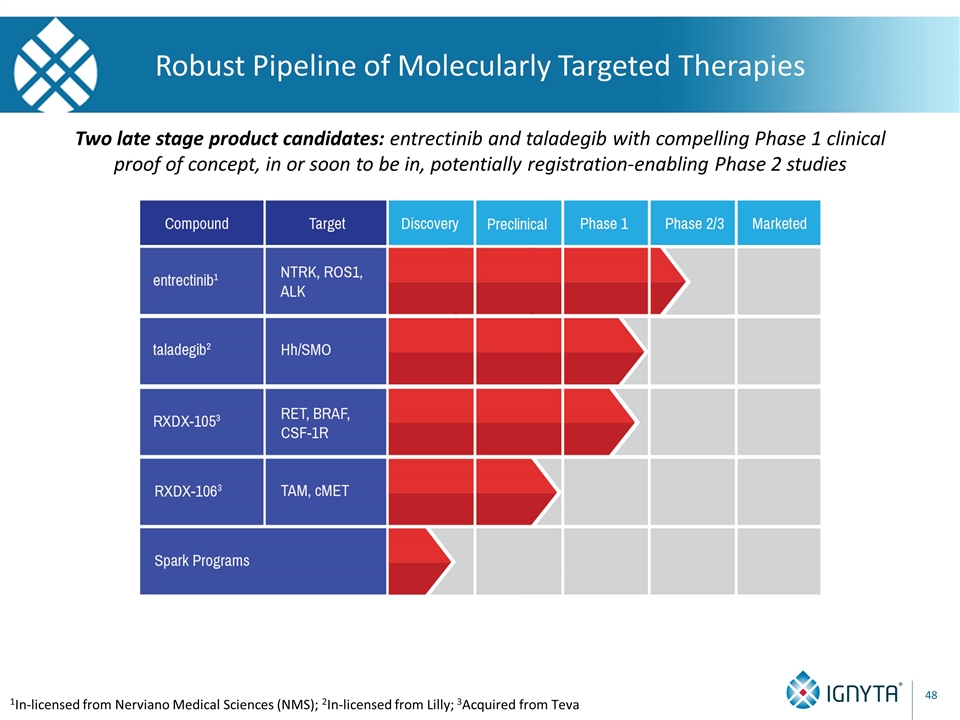
Robust Pipeline of Molecularly Targeted Therapies 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Two late stage product candidates: entrectinib and taladegib with compelling Phase 1 clinical proof of concept, in or soon to be in, potentially registration-enabling Phase 2 studies
Ignyta’s 3 Clinical Programs Are Differentiated, with Compelling POC and Multiple Basket Study Readouts 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva
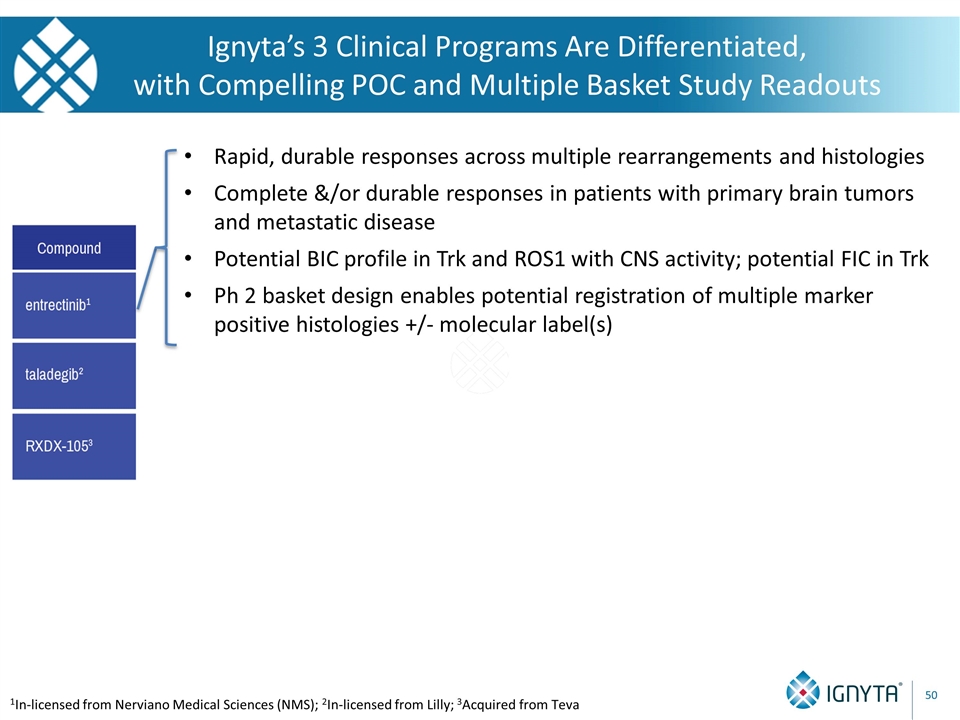
Ignyta’s 3 Clinical Programs Are Differentiated, with Compelling POC and Multiple Basket Study Readouts 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Rapid, durable responses across multiple rearrangements and histologies Complete &/or durable responses in patients with primary brain tumors and metastatic disease Potential BIC profile in Trk and ROS1 with CNS activity; potential FIC in Trk Ph 2 basket design enables potential registration of multiple marker positive histologies +/- molecular label(s)
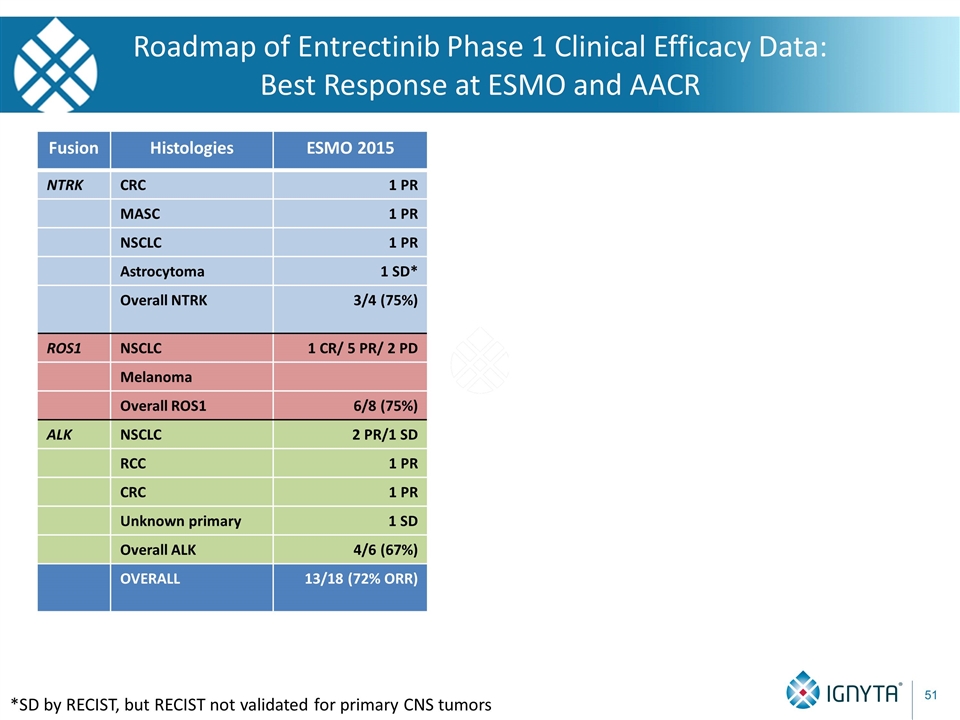
Roadmap of Entrectinib Phase 1 Clinical Efficacy Data: Best Response at ESMO and AACR Fusion Histologies ESMO 2015 NTRK CRC 1 PR MASC 1 PR NSCLC 1 PR Astrocytoma 1 SD* Overall NTRK 3/4 (75%) ROS1 NSCLC 1 CR/ 5 PR/ 2 PD Melanoma Overall ROS1 6/8 (75%) ALK NSCLC 2 PR/1 SD RCC 1 PR CRC 1 PR Unknown primary 1 SD Overall ALK 4/6 (67%) OVERALL 13/18 (72% ORR) *SD by RECIST, but RECIST not validated for primary CNS tumors
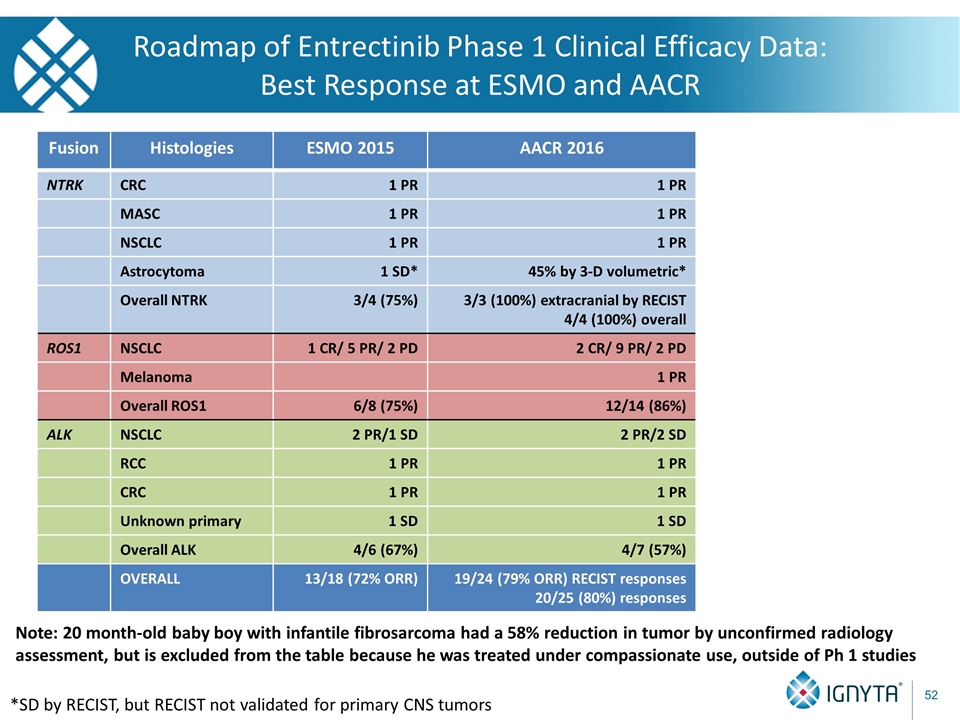
Roadmap of Entrectinib Phase 1 Clinical Efficacy Data: Best Response at ESMO and AACR Fusion Histologies ESMO 2015 AACR 2016 NTRK CRC 1 PR 1 PR MASC 1 PR 1 PR NSCLC 1 PR 1 PR Astrocytoma 1 SD* 45% by 3-D volumetric* Overall NTRK 3/4 (75%) 3/3 (100%) extracranial by RECIST 4/4 (100%) overall ROS1 NSCLC 1 CR/ 5 PR/ 2 PD 2 CR/ 9 PR/ 2 PD Melanoma 1 PR Overall ROS1 6/8 (75%) 12/14 (86%) ALK NSCLC 2 PR/1 SD 2 PR/2 SD RCC 1 PR 1 PR CRC 1 PR 1 PR Unknown primary 1 SD 1 SD Overall ALK 4/6 (67%) 4/7 (57%) OVERALL 13/18 (72% ORR) 19/24 (79% ORR) RECIST responses 20/25 (80%) responses Note: 20 month-old baby boy with infantile fibrosarcoma had a 58% reduction in tumor by unconfirmed radiology assessment, but is excluded from the table because he was treated under compassionate use, outside of Ph 1 studies *SD by RECIST, but RECIST not validated for primary CNS tumors
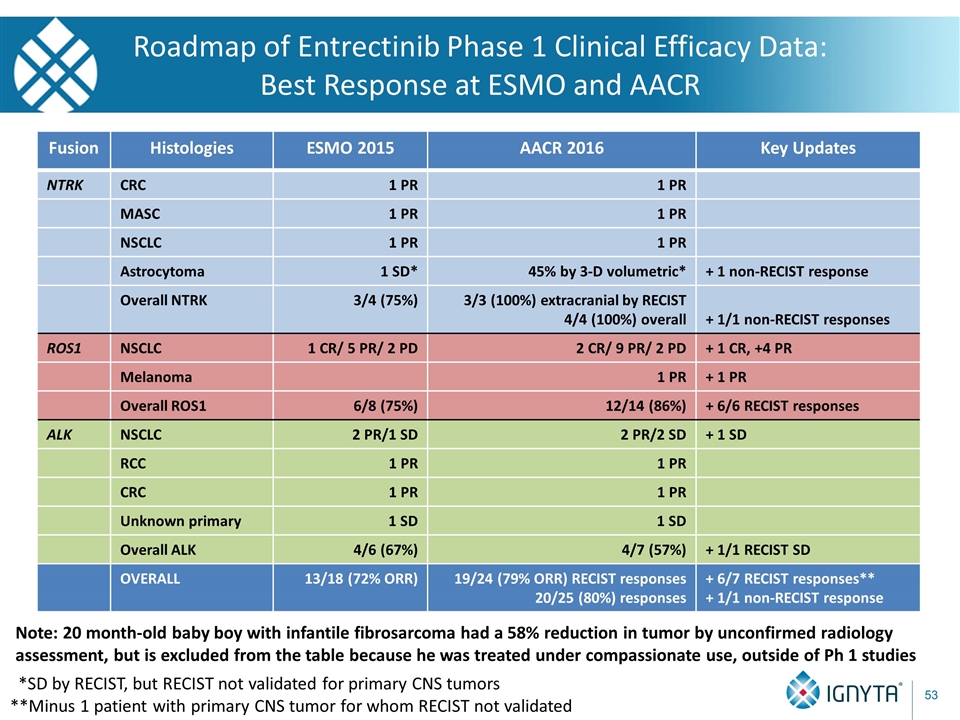
Roadmap of Entrectinib Phase 1 Clinical Efficacy Data: Best Response at ESMO and AACR Fusion Histologies ESMO 2015 AACR 2016 Key Updates NTRK CRC 1 PR 1 PR MASC 1 PR 1 PR NSCLC 1 PR 1 PR Astrocytoma 1 SD* 45% by 3-D volumetric* + 1 non-RECIST response Overall NTRK 3/4 (75%) 3/3 (100%) extracranial by RECIST 4/4 (100%) overall + 1/1 non-RECIST responses ROS1 NSCLC 1 CR/ 5 PR/ 2 PD 2 CR/ 9 PR/ 2 PD + 1 CR, +4 PR Melanoma 1 PR + 1 PR Overall ROS1 6/8 (75%) 12/14 (86%) + 6/6 RECIST responses ALK NSCLC 2 PR/1 SD 2 PR/2 SD + 1 SD RCC 1 PR 1 PR CRC 1 PR 1 PR Unknown primary 1 SD 1 SD Overall ALK 4/6 (67%) 4/7 (57%) + 1/1 RECIST SD OVERALL 13/18 (72% ORR) 19/24 (79% ORR) RECIST responses 20/25 (80%) responses + 6/7 RECIST responses** + 1/1 non-RECIST response Note: 20 month-old baby boy with infantile fibrosarcoma had a 58% reduction in tumor by unconfirmed radiology assessment, but is excluded from the table because he was treated under compassionate use, outside of Ph 1 studies *SD by RECIST, but RECIST not validated for primary CNS tumors **Minus 1 patient with primary CNS tumor for whom RECIST not validated
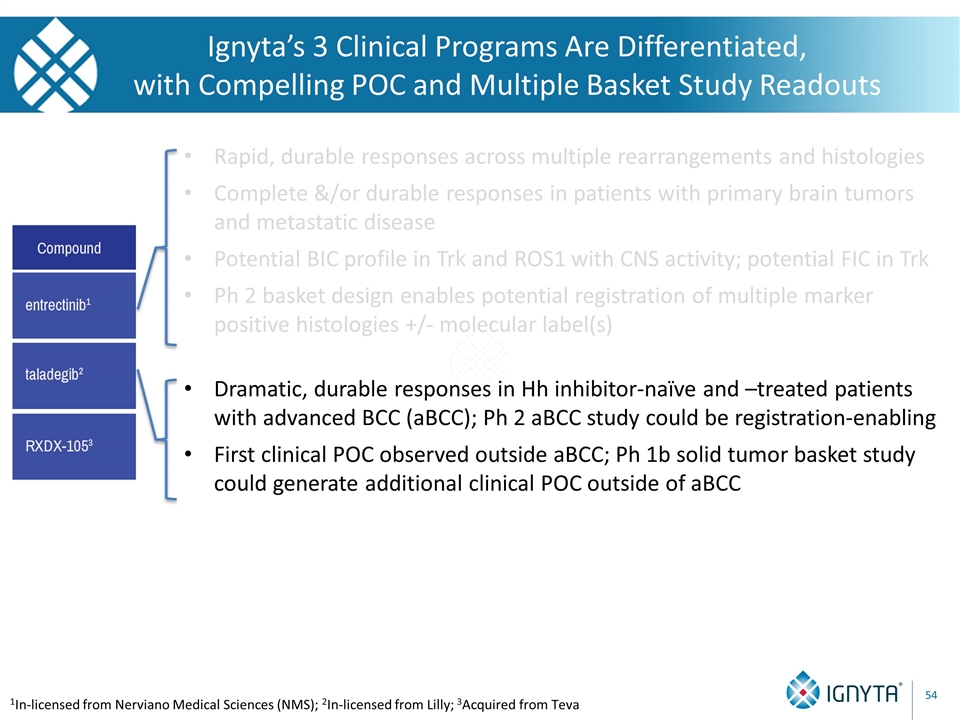
Ignyta’s 3 Clinical Programs Are Differentiated, with Compelling POC and Multiple Basket Study Readouts 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Rapid, durable responses across multiple rearrangements and histologies Complete &/or durable responses in patients with primary brain tumors and metastatic disease Potential BIC profile in Trk and ROS1 with CNS activity; potential FIC in Trk Ph 2 basket design enables potential registration of multiple marker positive histologies +/- molecular label(s) Dramatic, durable responses in Hh inhibitor-naïve and –treated patients with advanced BCC (aBCC); Ph 2 aBCC study could be registration-enabling First clinical POC observed outside aBCC; Ph 1b solid tumor basket study could generate additional clinical POC outside of aBCC
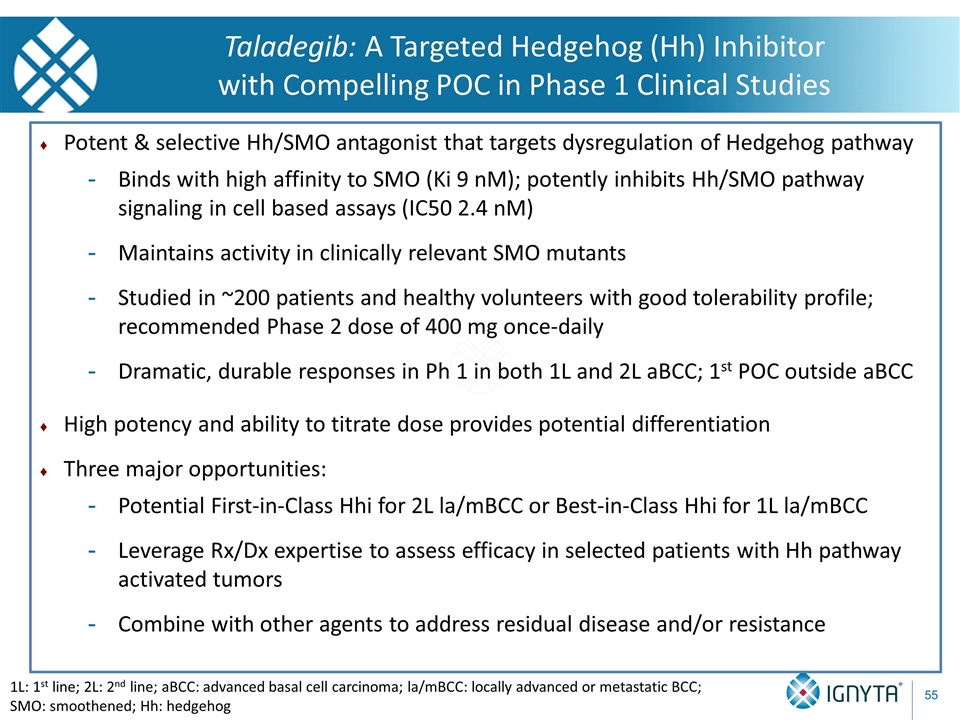
Potent & selective Hh/SMO antagonist that targets dysregulation of Hedgehog pathway Binds with high affinity to SMO (Ki 9 nM); potently inhibits Hh/SMO pathway signaling in cell based assays (IC50 2.4 nM) Maintains activity in clinically relevant SMO mutants Studied in ~200 patients and healthy volunteers with good tolerability profile; recommended Phase 2 dose of 400 mg once-daily Dramatic, durable responses in Ph 1 in both 1L and 2L aBCC; 1st POC outside aBCC High potency and ability to titrate dose provides potential differentiation Three major opportunities: Potential First-in-Class Hhi for 2L la/mBCC or Best-in-Class Hhi for 1L la/mBCC Leverage Rx/Dx expertise to assess efficacy in selected patients with Hh pathway activated tumors Combine with other agents to address residual disease and/or resistance Taladegib: A Targeted Hedgehog (Hh) Inhibitor with Compelling POC in Phase 1 Clinical Studies 1L: 1st line; 2L: 2nd line; aBCC: advanced basal cell carcinoma; la/mBCC: locally advanced or metastatic BCC; SMO: smoothened; Hh: hedgehog
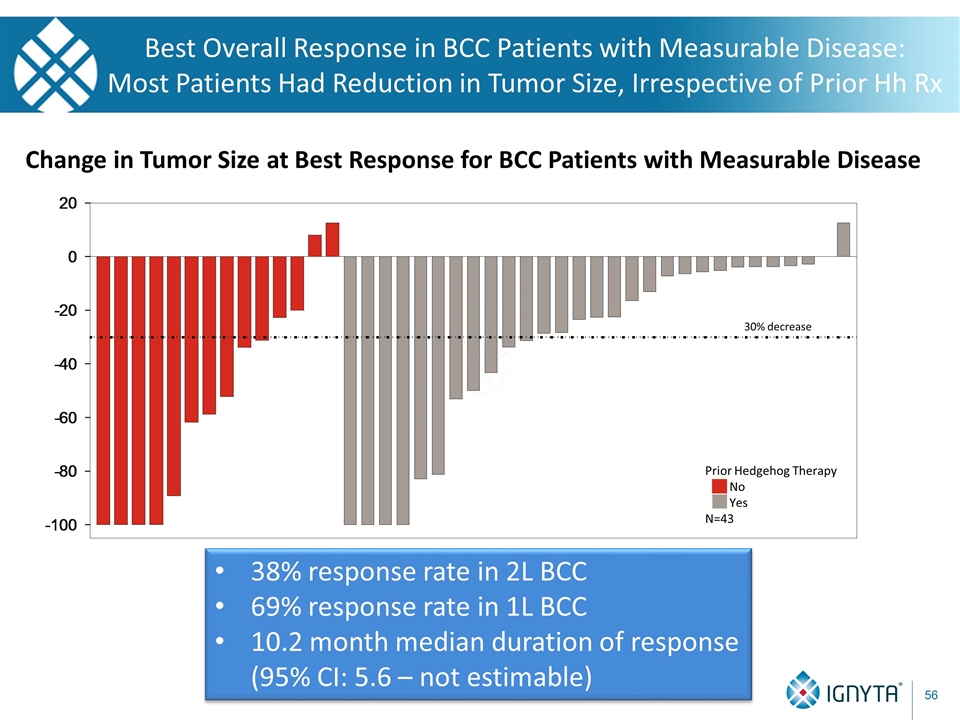
Best Overall Response in BCC Patients with Measurable Disease: Most Patients Had Reduction in Tumor Size, Irrespective of Prior Hh Rx Change in Tumor Size at Best Response for BCC Patients with Measurable Disease 30% decrease Prior Hedgehog Therapy No Yes N=43 38% response rate in 2L BCC 69% response rate in 1L BCC 10.2 month median duration of response (95% CI: 5.6 – not estimable)
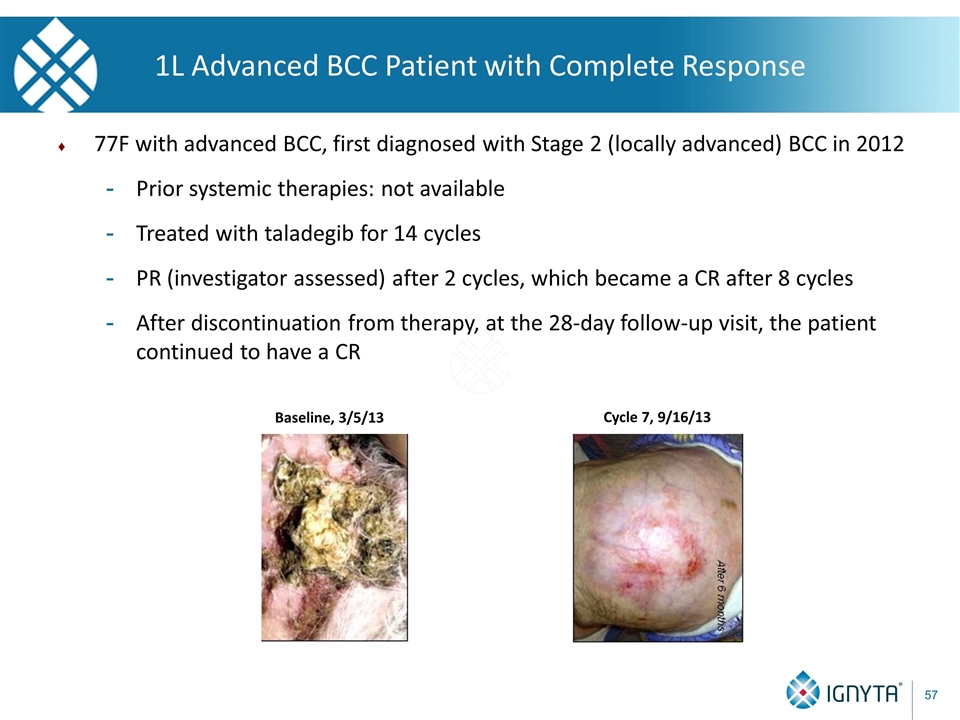
1L Advanced BCC Patient with Complete Response 77F with advanced BCC, first diagnosed with Stage 2 (locally advanced) BCC in 2012 Prior systemic therapies: not available Treated with taladegib for 14 cycles PR (investigator assessed) after 2 cycles, which became a CR after 8 cycles After discontinuation from therapy, at the 28-day follow-up visit, the patient continued to have a CR Baseline, 3/5/13 Cycle 7, 9/16/13
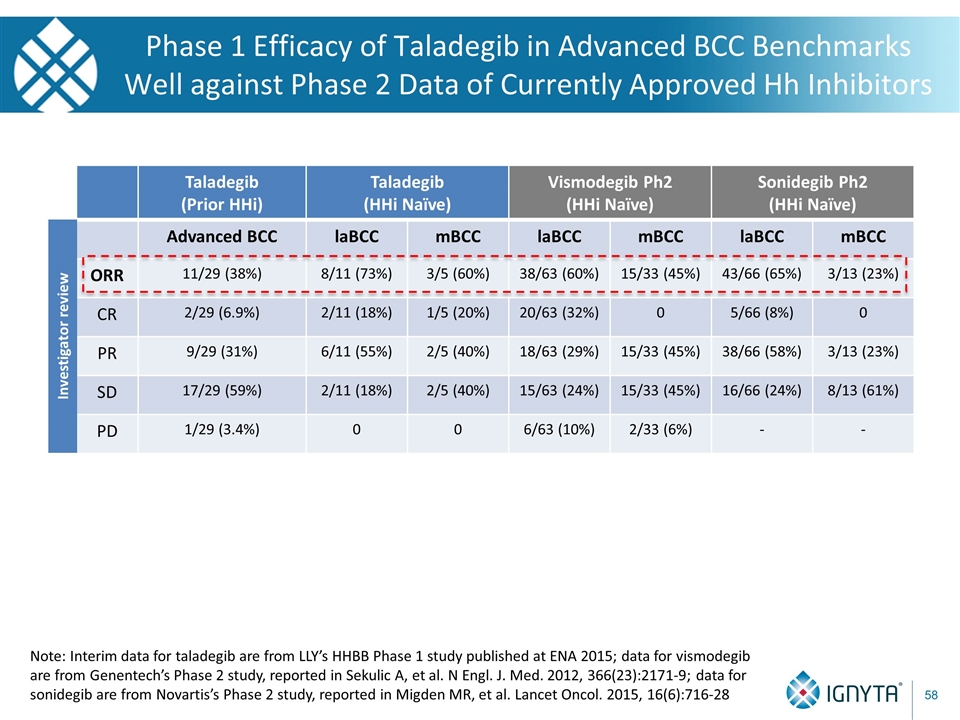
Phase 1 Efficacy of Taladegib in Advanced BCC Benchmarks Well against Phase 2 Data of Currently Approved Hh Inhibitors Taladegib (Prior HHi) Taladegib (HHi Naïve) Vismodegib Ph2 (HHi Naïve) Sonidegib Ph2 (HHi Naïve) Advanced BCC laBCC mBCC laBCC mBCC laBCC mBCC ORR 11/29 (38%) 8/11 (73%) 3/5 (60%) 38/63 (60%) 15/33 (45%) 43/66 (65%) 3/13 (23%) CR 2/29 (6.9%) 2/11 (18%) 1/5 (20%) 20/63 (32%) 0 5/66 (8%) 0 PR 9/29 (31%) 6/11 (55%) 2/5 (40%) 18/63 (29%) 15/33 (45%) 38/66 (58%) 3/13 (23%) SD 17/29 (59%) 2/11 (18%) 2/5 (40%) 15/63 (24%) 15/33 (45%) 16/66 (24%) 8/13 (61%) PD 1/29 (3.4%) 0 0 6/63 (10%) 2/33 (6%) - - Investigator review Note: Interim data for taladegib are from LLY’s HHBB Phase 1 study published at ENA 2015; data for vismodegib are from Genentech’s Phase 2 study, reported in Sekulic A, et al. N Engl. J. Med. 2012, 366(23):2171-9; data for sonidegib are from Novartis’s Phase 2 study, reported in Migden MR, et al. Lancet Oncol. 2015, 16(6):716-28
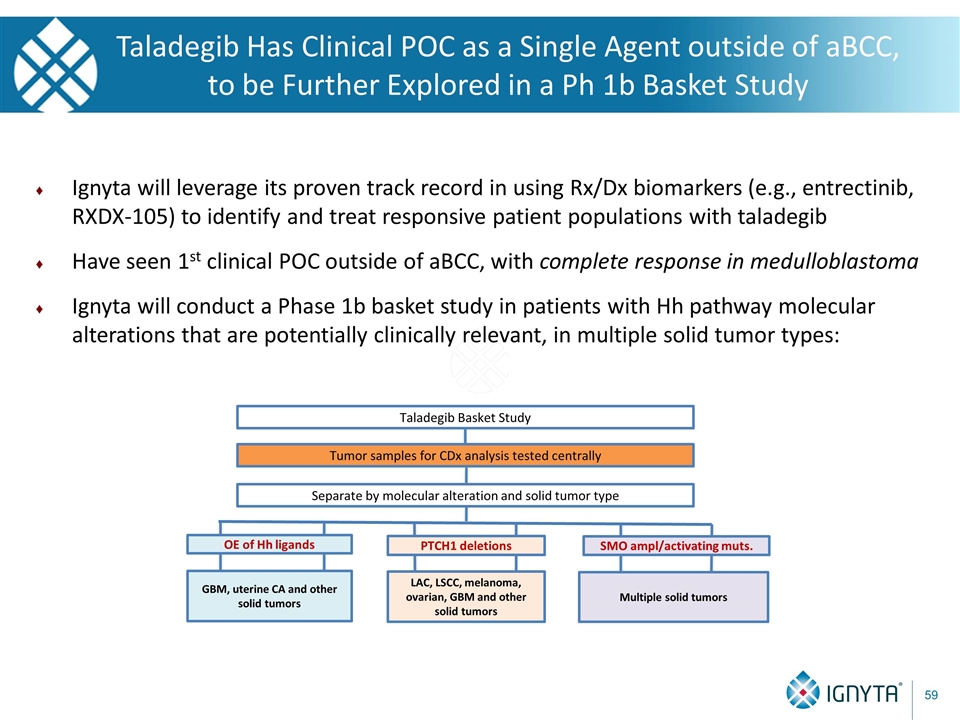
Taladegib Has Clinical POC as a Single Agent outside of aBCC, to be Further Explored in a Ph 1b Basket Study Ignyta will leverage its proven track record in using Rx/Dx biomarkers (e.g., entrectinib, RXDX-105) to identify and treat responsive patient populations with taladegib Have seen 1st clinical POC outside of aBCC, with complete response in medulloblastoma Ignyta will conduct a Phase 1b basket study in patients with Hh pathway molecular alterations that are potentially clinically relevant, in multiple solid tumor types: Tumor samples for CDx analysis tested centrally Separate by molecular alteration and solid tumor type LAC, LSCC, melanoma, ovarian, GBM and other solid tumors Taladegib Basket Study Multiple solid tumors PTCH1 deletions SMO ampl/activating muts. GBM, uterine CA and other solid tumors OE of Hh ligands
Ignyta’s 3 Clinical Programs Are Differentiated, with Compelling POC and Multiple Basket Study Readouts 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Rapid, durable responses across multiple rearrangements and histologies Complete &/or durable responses in patients with primary brain tumors and metastatic disease Potential BIC profile in Trk and ROS1 with CNS activity; potential FIC in Trk Ph 2 basket design enables potential registration of multiple marker positive histologies +/- molecular label(s) Dramatic, durable responses in Hh inhibitor-naïve and –treated patients with advanced BCC (aBCC); Ph 2 aBCC study could be registration-enabling First clinical POC observed outside aBCC; Ph 1b solid tumor basket study could generate additional clinical POC outside of aBCC Durable RECIST and rapid clinical responses observed in NSCLC, highlighting initial evidence of clinical activity Ph 1b basket design enables further exploration of efficacy in RET-, BRAF- and NSCLC baskets; RP2D selected
RXDX-105: A Multikinase Inhibitor with Potent Activity Against Such Targets as RET and BRAF, with Demonstrated Clinical POC Has demonstrated profound and unique-in-class efficacy in cancer xenograft and PDX models harboring RET fusions or mutant BRAF; strong binding affinity (Kd < 15nM) to other kinase targets such as PDGFR, VEGFR2 and EGFR Recommended Phase 2 dose selected; Phase 1b basket study initiated in 1Q16 Target indications RET+ NSCLC, mCRC, and/or other solid tumors BRAF+ locally advanced or metastatic NSCLC; upside in BRAF+ metastatic colorectal cancer (mCRC) with EGFR feedback activation Multikinase activity could potentially be leveraged for broader targeting of NSCLC (adenocarcinoma and/or squamous)
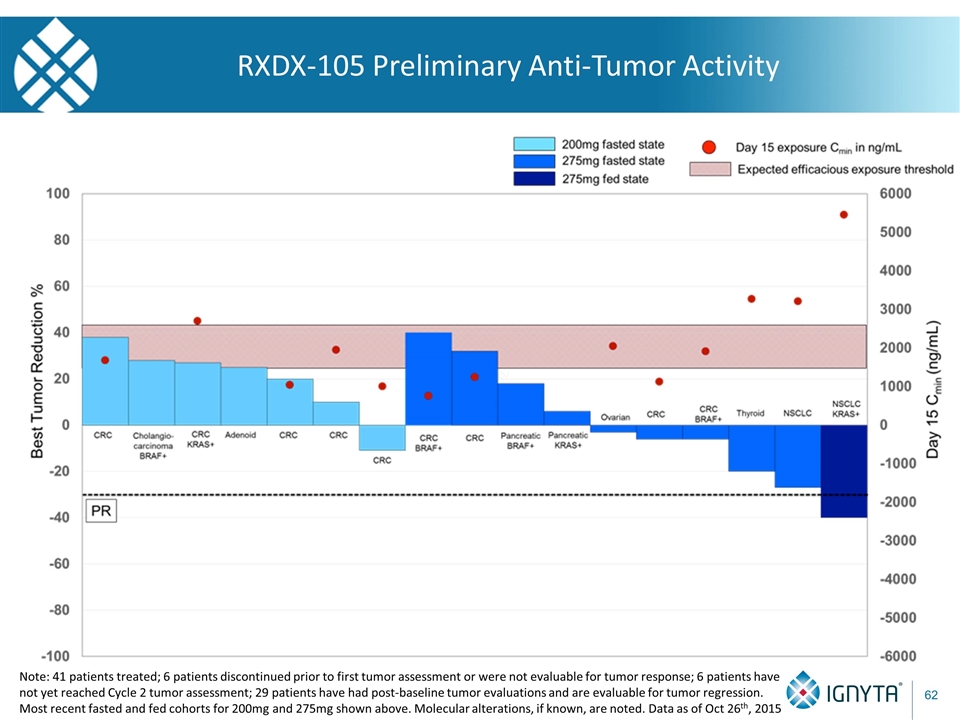
RXDX-105 Preliminary Anti-Tumor Activity Note: 41 patients treated; 6 patients discontinued prior to first tumor assessment or were not evaluable for tumor response; 6 patients have not yet reached Cycle 2 tumor assessment; 29 patients have had post-baseline tumor evaluations and are evaluable for tumor regression. Most recent fasted and fed cohorts for 200mg and 275mg shown above. Molecular alterations, if known, are noted. Data as of Oct 26th, 2015
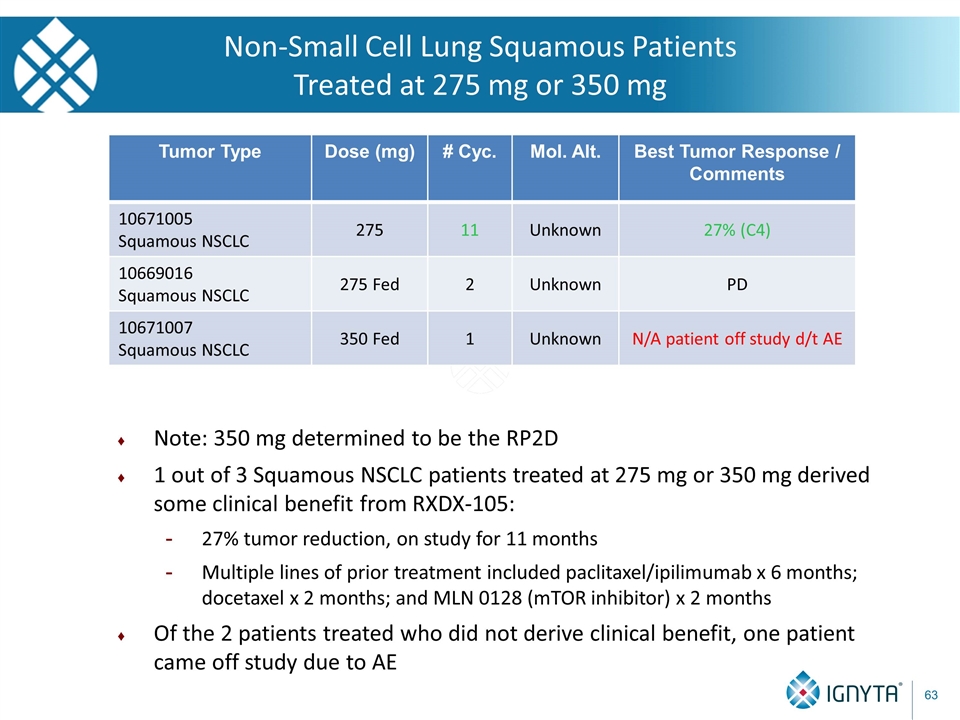
Tumor Type Dose (mg) # Cyc. Mol. Alt. Best Tumor Response / Comments 10671005 Squamous NSCLC 275 11 Unknown 27% (C4) 10669016 Squamous NSCLC 275 Fed 2 Unknown PD 10671007 Squamous NSCLC 350 Fed 1 Unknown N/A patient off study d/t AE Note: 350 mg determined to be the RP2D 1 out of 3 Squamous NSCLC patients treated at 275 mg or 350 mg derived some clinical benefit from RXDX-105: 27% tumor reduction, on study for 11 months Multiple lines of prior treatment included paclitaxel/ipilimumab x 6 months; docetaxel x 2 months; and MLN 0128 (mTOR inhibitor) x 2 months Of the 2 patients treated who did not derive clinical benefit, one patient came off study due to AE Non-Small Cell Lung Squamous Patients Treated at 275 mg or 350 mg
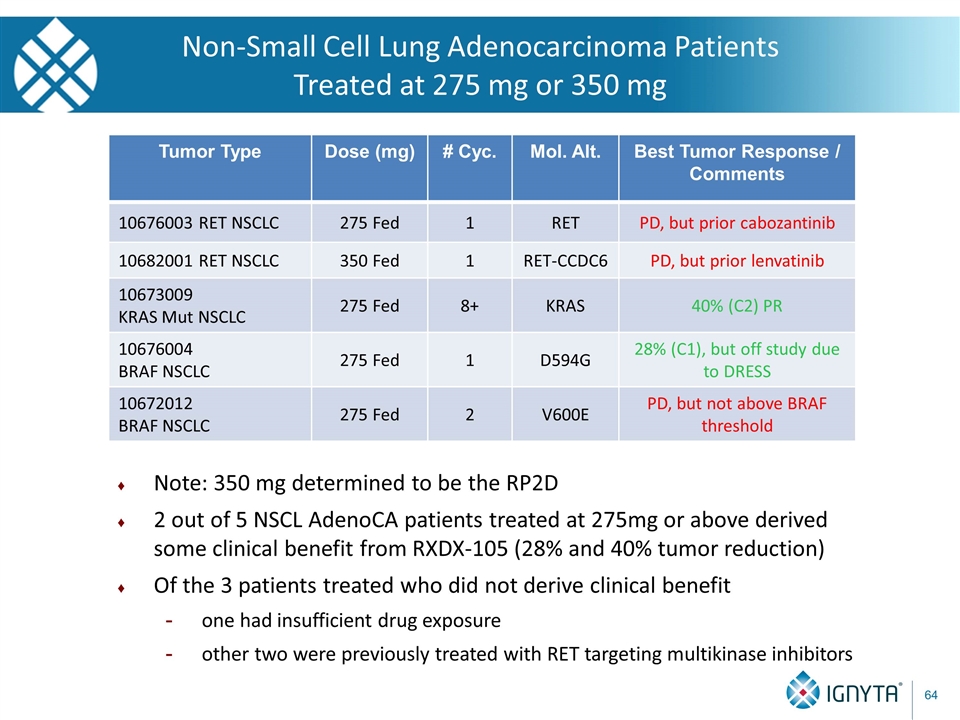
Non-Small Cell Lung Adenocarcinoma Patients Treated at 275 mg or 350 mg Tumor Type Dose (mg) # Cyc. Mol. Alt. Best Tumor Response / Comments 10676003 RET NSCLC 275 Fed 1 RET PD, but prior cabozantinib 10682001 RET NSCLC 350 Fed 1 RET-CCDC6 PD, but prior lenvatinib 10673009 KRAS Mut NSCLC 275 Fed 8+ KRAS 40% (C2) PR 10676004 BRAF NSCLC 275 Fed 1 D594G 28% (C1), but off study due to DRESS 10672012 BRAF NSCLC 275 Fed 2 V600E PD, but not above BRAF threshold Note: 350 mg determined to be the RP2D 2 out of 5 NSCL AdenoCA patients treated at 275mg or above derived some clinical benefit from RXDX-105 (28% and 40% tumor reduction) Of the 3 patients treated who did not derive clinical benefit one had insufficient drug exposure other two were previously treated with RET targeting multikinase inhibitors
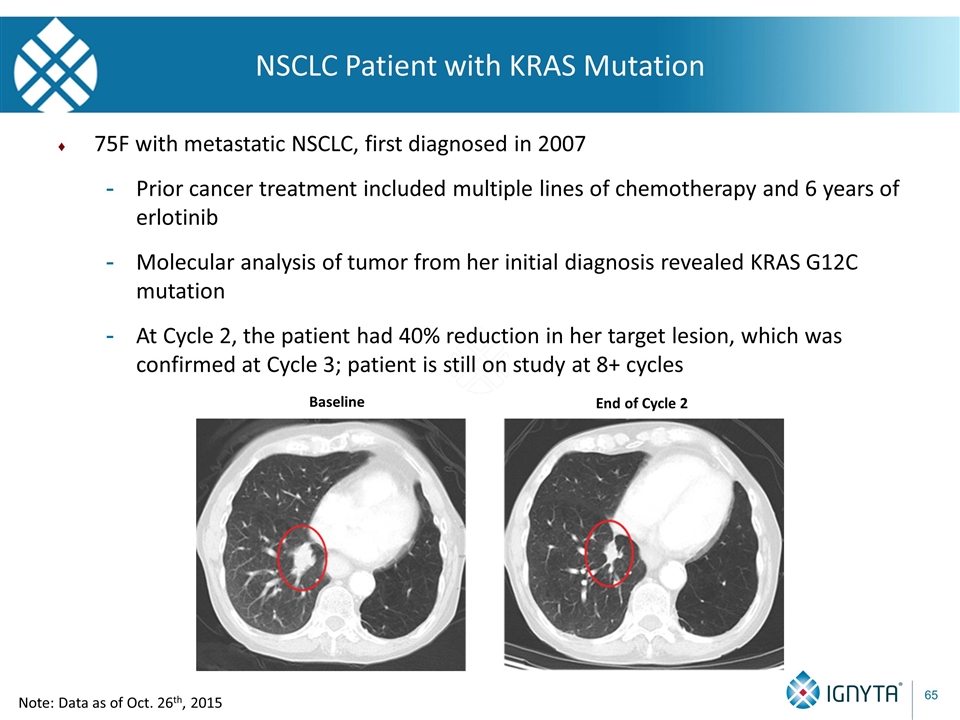
NSCLC Patient with KRAS Mutation 75F with metastatic NSCLC, first diagnosed in 2007 Prior cancer treatment included multiple lines of chemotherapy and 6 years of erlotinib Molecular analysis of tumor from her initial diagnosis revealed KRAS G12C mutation At Cycle 2, the patient had 40% reduction in her target lesion, which was confirmed at Cycle 3; patient is still on study at 8+ cycles Baseline End of Cycle 2 Note: Data as of Oct. 26th, 2015
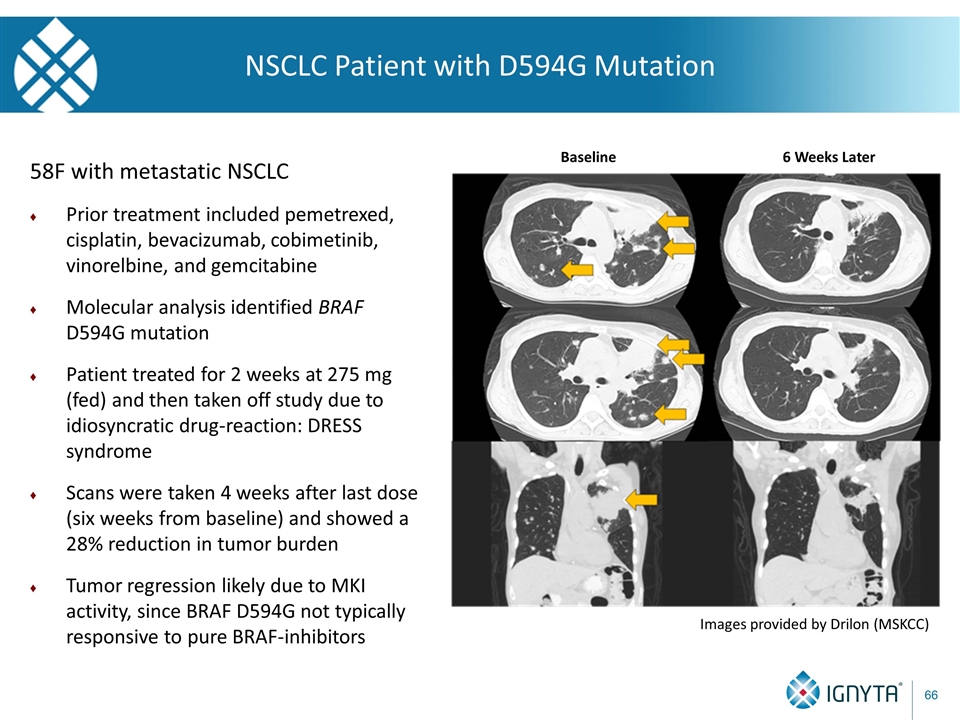
58F with metastatic NSCLC Prior treatment included pemetrexed, cisplatin, bevacizumab, cobimetinib, vinorelbine, and gemcitabine Molecular analysis identified BRAF D594G mutation Patient treated for 2 weeks at 275 mg (fed) and then taken off study due to idiosyncratic drug-reaction: DRESS syndrome Scans were taken 4 weeks after last dose (six weeks from baseline) and showed a 28% reduction in tumor burden Tumor regression likely due to MKI activity, since BRAF D594G not typically responsive to pure BRAF-inhibitors Images provided by Drilon (MSKCC) NSCLC Patient with D594G Mutation Baseline 6 Weeks Later
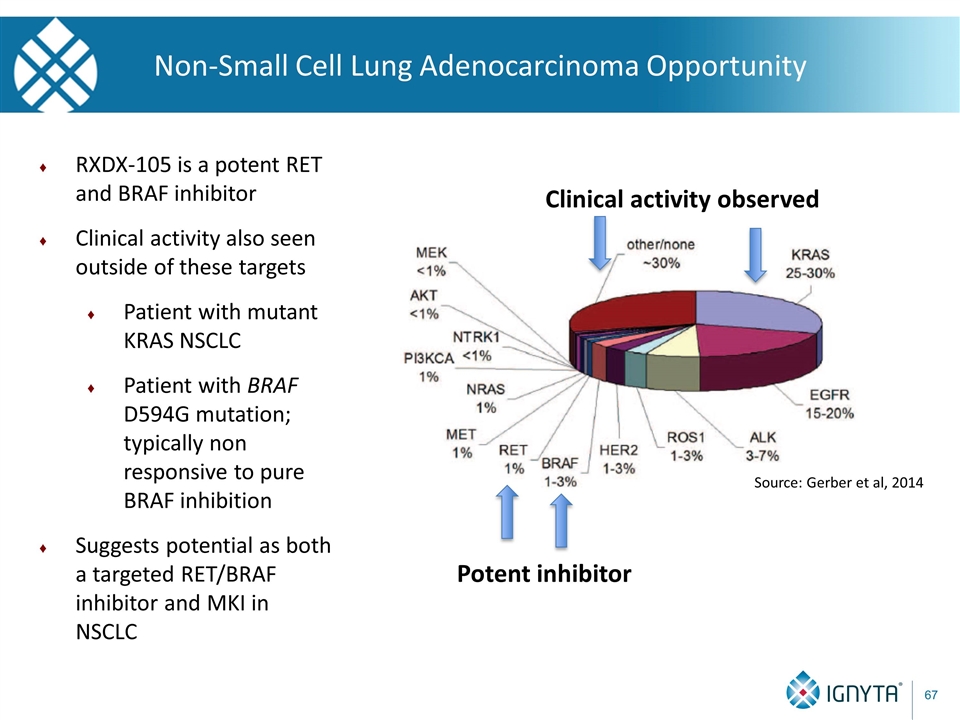
Non-Small Cell Lung Adenocarcinoma Opportunity Potent inhibitor Clinical activity observed RXDX-105 is a potent RET and BRAF inhibitor Clinical activity also seen outside of these targets Patient with mutant KRAS NSCLC Patient with BRAF D594G mutation; typically non responsive to pure BRAF inhibition Suggests potential as both a targeted RET/BRAF inhibitor and MKI in NSCLC Source: Gerber et al, 2014
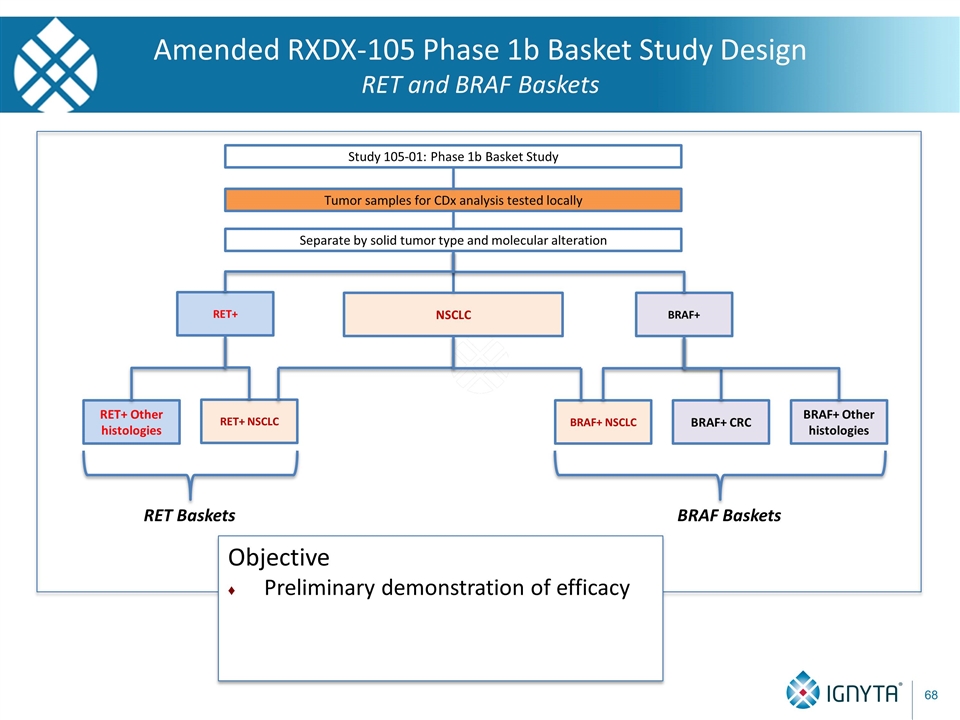
Tumor samples for CDx analysis tested locally Separate by solid tumor type and molecular alteration Study 105-01: Phase 1b Basket Study BRAF+ NSCLC RET+ BRAF+ NSCLC BRAF+ CRC BRAF+ Other histologies RET+ Other histologies RET Baskets Amended RXDX-105 Phase 1b Basket Study Design RET and BRAF Baskets RET+ NSCLC BRAF Baskets Objective Preliminary demonstration of efficacy
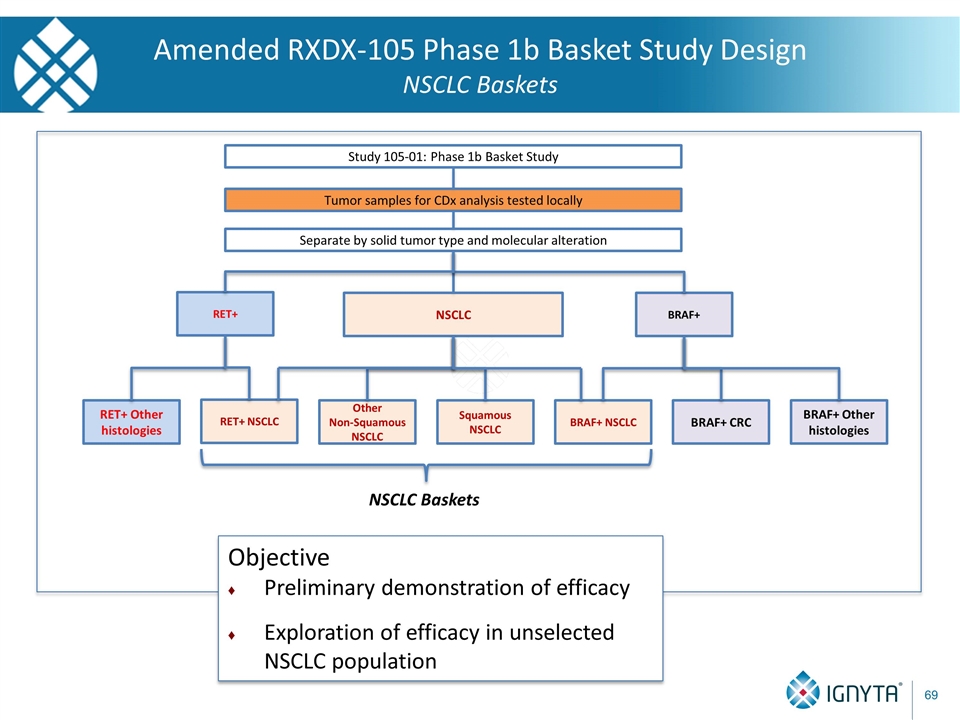
Tumor samples for CDx analysis tested locally Separate by solid tumor type and molecular alteration Study 105-01: Phase 1b Basket Study BRAF+ NSCLC RET+ BRAF+ NSCLC BRAF+ CRC BRAF+ Other histologies RET+ Other histologies Other Non-Squamous NSCLC Squamous NSCLC NSCLC Baskets RET+ NSCLC Amended RXDX-105 Phase 1b Basket Study Design NSCLC Baskets Objective Preliminary demonstration of efficacy Exploration of efficacy in unselected NSCLC population
Ignyta Has 3 Differentiated Programs with Compelling Clinical POC and Multiple Basket Study Readouts 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Rapid, durable responses (80%) across multiple rearrangements and histologies Complete &/or durable responses in primary brain tumors and metastatic disease 5/5 Trk tumor regression, including 3/3 Trk CNS tumor regression Ph 2 basket design enables potential registration of multiple marker positive histologies +/- molecular label(s) Dramatic, durable responses in both Hh inhibitor-naïve (69% ORR) and –treated (38% ORR) patients with advanced BCC (aBCC) First clinical POC outside aBCC with CR in medulloblastoma Ph 1b solid tumor basket study could generate additional clinical POC outside of BCC; Ph 2 aBCC study could be potentially registration-enabling Durable RECIST and rapid clinical responses observed in NSCLC, highlighting initial evidence of clinical activity: 40% PR in patient with mKRAS+ NSCLC who continues on study at 8+ mos. 28% tumor reduction in patient with mD594G NSCLC after 2 weeks of therapy Ph 1b basket design enables further exploration of efficacy in RET-, BRAF- and NSCLC baskets; RP2D selected

Investment Thesis Leading precision medicine company in oncology: Aspirational goal is to eradicate residual disease in precisely defined patient populations by 2030 Integrated approach to Rx/Dx development: Internal Dx allows Ignyta to illuminate the molecular drivers of cancer and quickly advance the most appropriate molecularly targeted therapies to address them Two late stage product candidates: entrectinib and taladegib with compelling Phase 1 clinical proof of concept, in or soon to be in, potentially registration-enabling Phase 2 studies Pipeline with multiple shots on goal: Molecularly targeted first-in-class and best-in-class product candidates in clinical development utilizing basket study designs enable Ignyta to generate multiple clinical data readouts in next 12 months, while building long-term value for patients and shareholders






































































