
Company Presentation June 2017 Exhibit 99.1

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: references to the development of entrectinib and our other product candidates, including the ability to reduce the size of certain CNS tumors; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs, and the timelines associated with such programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to develop or access companion diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to adequately fund our development programs; our ability to obtain regulatory approvals in order to market any of our product candidates or companion diagnostic tests; references to the potential market size for our products; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2017, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company. A registration statement on Form S-3 has been filed with the Securities and Exchange Commission and declared effective. The offering of these securities will be made only by means of a written prospectus supplement and base prospectus forming part of the effective registration statement relating to the shares. Copies of the prospectus for this offering may be obtained, when available, by contacting J.P. Morgan Securities LLC c/o Broadridge Financial Solutions, 1155 Long Island Avenue, Edgewood, NY 11717, Phone (866) 803-9204 or Piper Jaffray & Co., Attention: Prospectus Department, 800 Nicollet Mall, J12S03, Minneapolis, MN 55402, Phone (800) 747-3924, E-mail at prospectus@pjc.com. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction.
Key Company Highlights Robust pipeline of precision medicine therapies in oncology Entrectinib: Path towards two NDAs in TRK tumor agnostic and ROS1 NSCLC indications in 2018, with anticipated launch in 2019 RXDX-105: Preliminary ORR of 56% (n = 9) in patients with RET fusion-positive solid tumors in Ph 1/1b study RXDX-106: Promising preclinical efficacy as a single agent and combination agent small molecule immunomodulator Integrated approach to Rx/Dx development CAP-accredited, CLIA-certified, QSR-compliant diagnostic lab with multi-modality assays Multiple near-term catalysts to drive value Multiple clinical data readouts and regulatory milestones in next 12 months, while building long-term value for patients and stockholders Experienced leadership team Breadth and depth of expertise in clinical/preclinical development, regulatory affairs, commercial, and other key technical and business disciplines

Leadership Team Brings to Ignyta Experience from Leading Organizations and Institutions Jonathan Lim, M.D. Chairman, CEO & Co-Founder Former Chair, CEO of Eclipse; CEO at Halozyme; McKinsey; NIH post-doc at Harvard/Dana Farber; Surgical resident at NYH-Cornell/Memorial Sloan Kettering; Board member at UC San Diego Moores Cancer Center. Former Senior Director of Business Development at Fate Therapeutics; Director of BD at Halozyme; Marketing at Neurocrine; L.E.K. Consulting; Shire (TKT); Harvard Business School. Former Vice President at TPG Capital; McKinsey; Marshall Scholar at Oxford University; UCLA Medical School; Harvard Business School. Former VP, Product Differentiation/VP, Drug Product Design/VP, Pharmaceutical Development, in addition to numerous other leadership roles at Pfizer. Former General Counsel at Genoptix (a Novartis company); Cooley LLP; Scripps Research Institute; USC Law School; USC Medical School. Former VP of Corporate and Business Development at Foundation Medicine; Exec Dir of BD and Sr. Director of Marketing at Halozyme; IMS Health, Deloitte Consulting; London Business School. Former CMO at Fate; VP, Clin. Dev. at Kalypsys; CMO at Kanisa; VP, Clin. Dev. at Salmedix; Sr. Director of Medical Research at Biogen Idec; MD, MS at Harvard; Residency, MGH; Oncology, Dana Farber. Zachary Hornby Chief Operating Officer Jacob Chacko, M.D. Chief Financial Officer Val Harding, Ph.D. SVP, Chemistry, Mfg. & Controls Christian Kuhlen, M.D. General Counsel Will McCarthy Chief Business Officer Pratik Multani, M.D. Chief Medical Officer
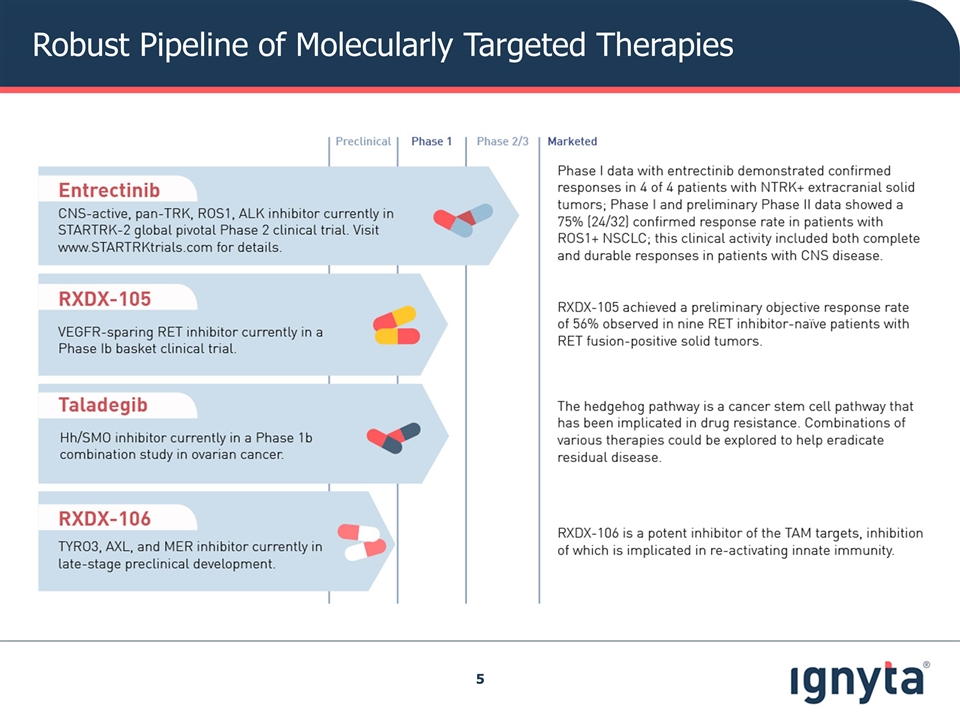
Robust Pipeline of Molecularly Targeted Therapies
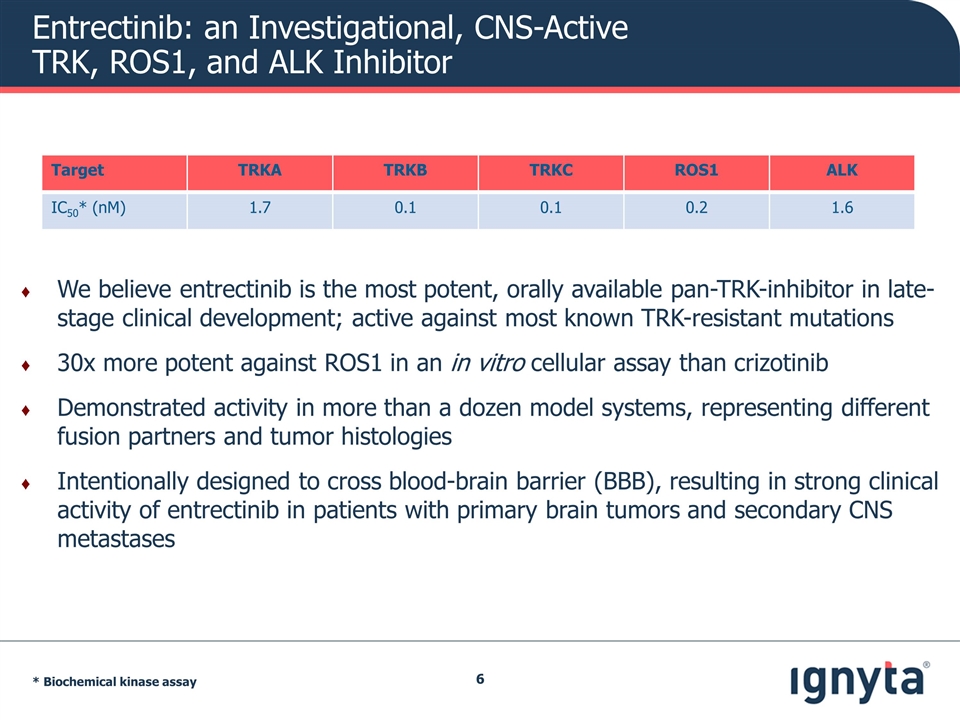
Entrectinib: an Investigational, CNS-Active TRK, ROS1, and ALK Inhibitor We believe entrectinib is the most potent, orally available pan-TRK-inhibitor in late-stage clinical development; active against most known TRK-resistant mutations 30x more potent against ROS1 in an in vitro cellular assay than crizotinib Demonstrated activity in more than a dozen model systems, representing different fusion partners and tumor histologies Intentionally designed to cross blood-brain barrier (BBB), resulting in strong clinical activity of entrectinib in patients with primary brain tumors and secondary CNS metastases Target TRKA TRKB TRKC ROS1 ALK IC50* (nM) 1.7 0.1 0.1 0.2 1.6 * Biochemical kinase assay
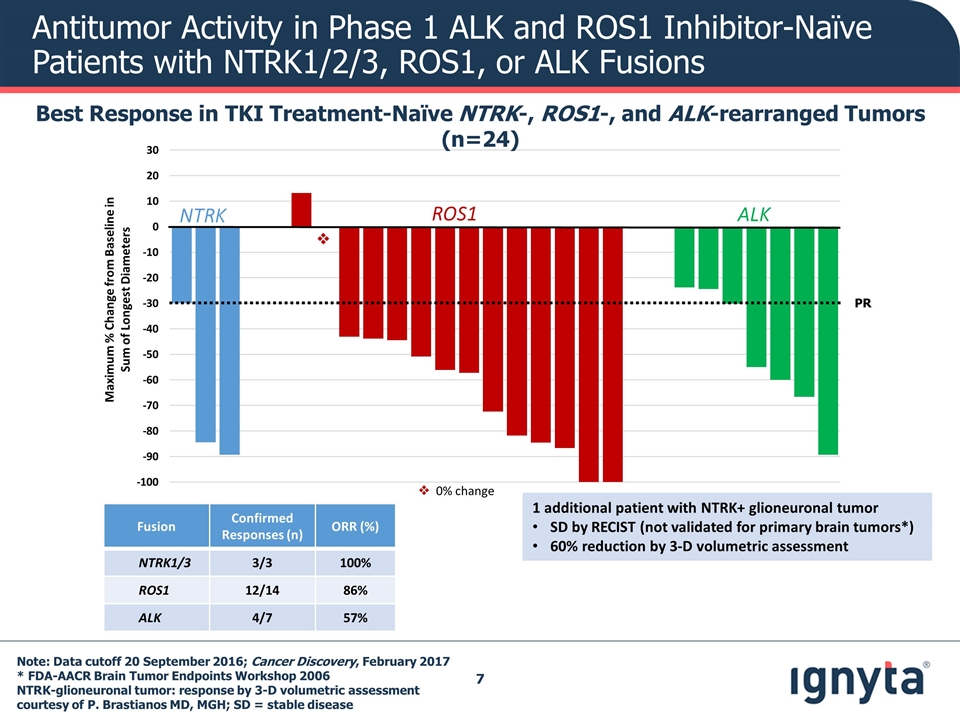
v Maximum % Change from Baseline in Sum of Longest Diameters NTRK ALK ROS1 PR Note: Data cutoff 20 September 2016; Cancer Discovery, February 2017 * FDA-AACR Brain Tumor Endpoints Workshop 2006 NTRK-glioneuronal tumor: response by 3-D volumetric assessment courtesy of P. Brastianos MD, MGH; SD = stable disease Antitumor Activity in Phase 1 ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Fusions Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% ROS1 12/14 86% ALK 4/7 57% v 0% change 1 additional patient with NTRK+ glioneuronal tumor SD by RECIST (not validated for primary brain tumors*) 60% reduction by 3-D volumetric assessment
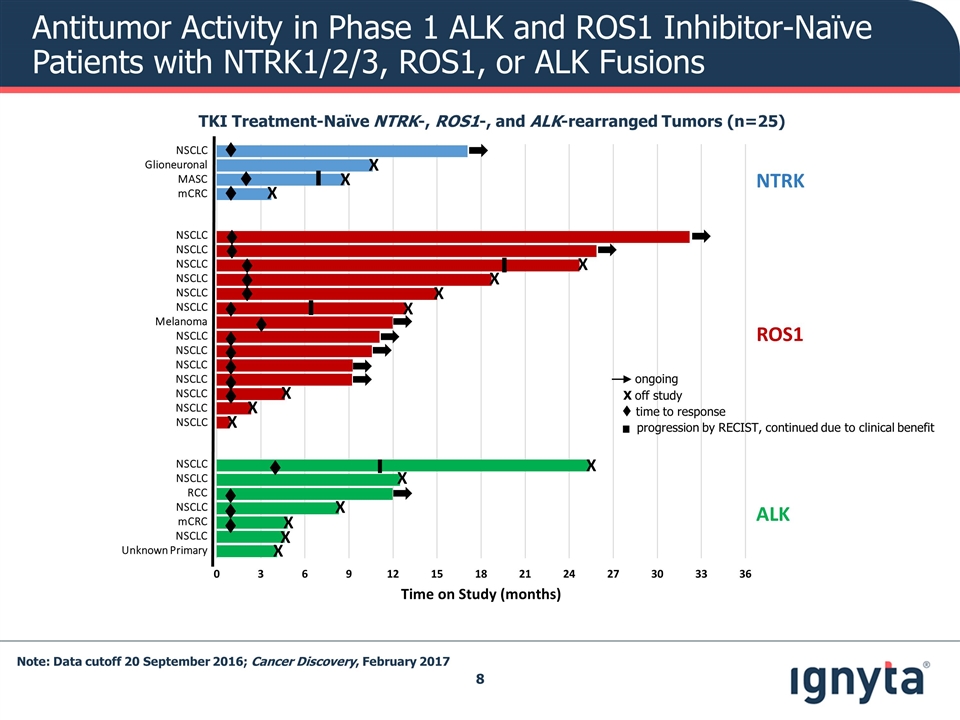
Antitumor Activity in Phase 1 ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Fusions TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=25) t ▌ NSCLC Glioneuronal MASC mCRC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC Melanoma NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC RCC NSCLC mCRC NSCLC Unknown Primary t t t t t t t t t t t t t t ▌ ▌ ▌ t t t t X X X X X X X X X X X X X X X X Time on Study (months) NTRK ALK ROS1 t time to response . ongoing X off study progression by RECIST, continued due to clinical benefit Note: Data cutoff 20 September 2016; Cancer Discovery, February 2017
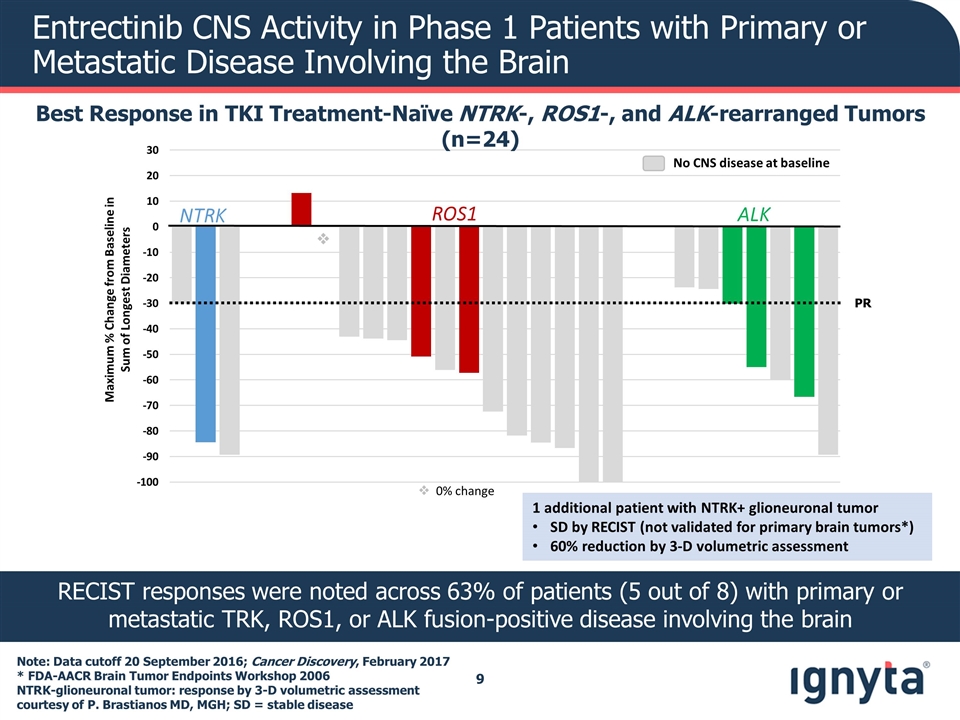
v Maximum % Change from Baseline in Sum of Longest Diameters NTRK ALK ROS1 PR Entrectinib CNS Activity in Phase 1 Patients with Primary or Metastatic Disease Involving the Brain Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) v 0% change No CNS disease at baseline RECIST responses were noted across 63% of patients (5 out of 8) with primary or metastatic TRK, ROS1, or ALK fusion-positive disease involving the brain Note: Data cutoff 20 September 2016; Cancer Discovery, February 2017 * FDA-AACR Brain Tumor Endpoints Workshop 2006 NTRK-glioneuronal tumor: response by 3-D volumetric assessment courtesy of P. Brastianos MD, MGH; SD = stable disease 1 additional patient with NTRK+ glioneuronal tumor SD by RECIST (not validated for primary brain tumors*) 60% reduction by 3-D volumetric assessment
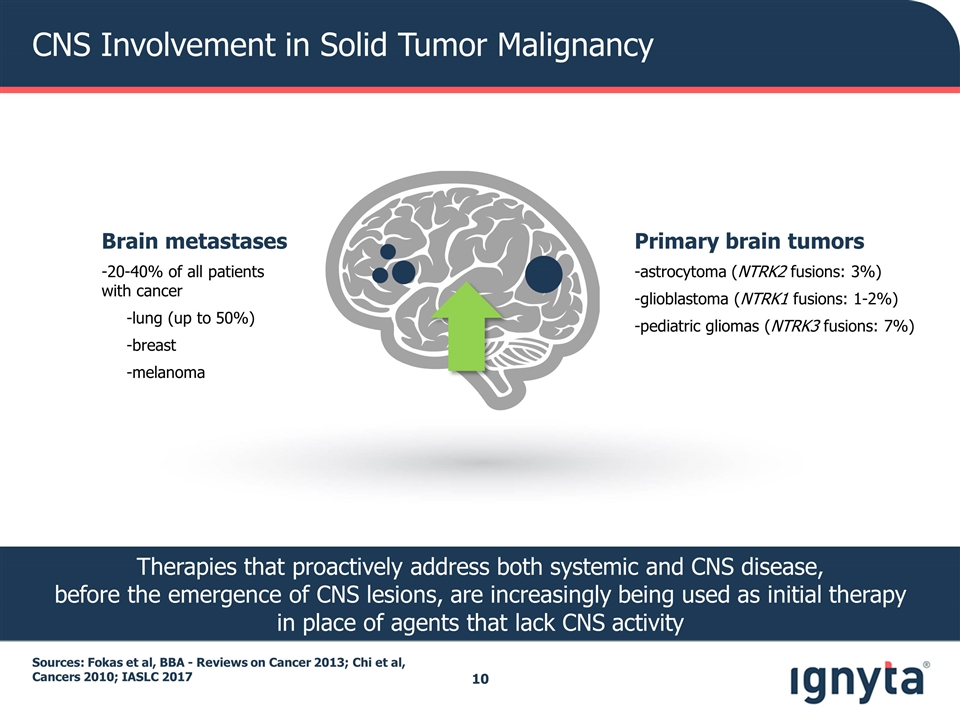
CNS Involvement in Solid Tumor Malignancy Sources: Fokas et al, BBA - Reviews on Cancer 2013; Chi et al, Cancers 2010; IASLC 2017 Brain metastases -20-40% of all patients with cancer -lung (up to 50%) -breast -melanoma Primary brain tumors -astrocytoma (NTRK2 fusions: 3%) -glioblastoma (NTRK1 fusions: 1-2%) -pediatric gliomas (NTRK3 fusions: 7%) Therapies that proactively address both systemic and CNS disease, before the emergence of CNS lesions, are increasingly being used as initial therapy in place of agents that lack CNS activity
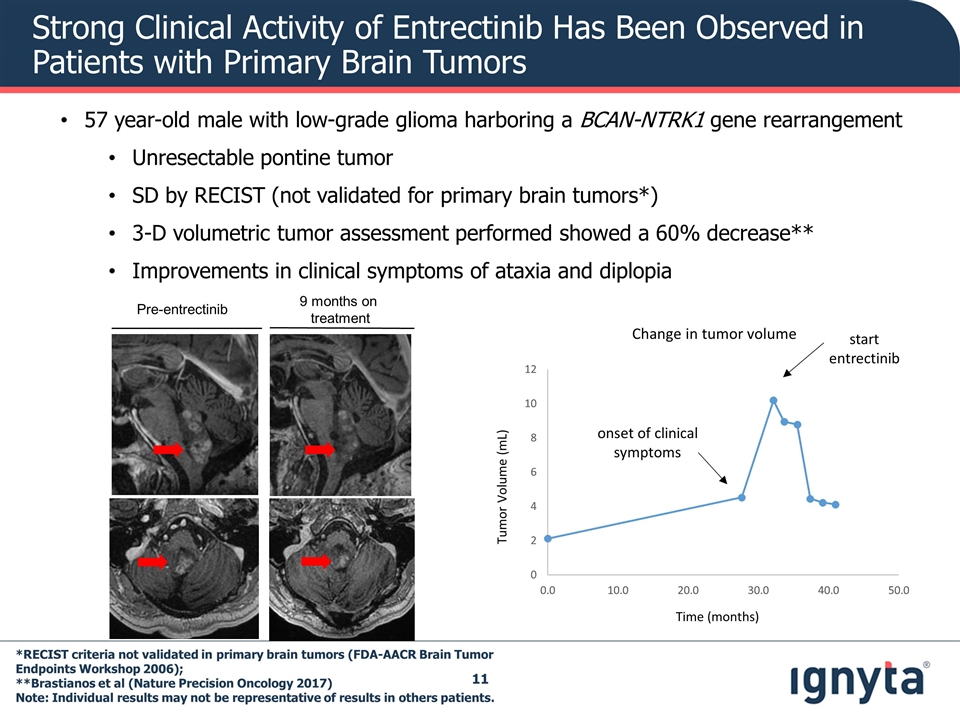
57 year-old male with low-grade glioma harboring a BCAN-NTRK1 gene rearrangement Unresectable pontine tumor SD by RECIST (not validated for primary brain tumors*) 3-D volumetric tumor assessment performed showed a 60% decrease** Improvements in clinical symptoms of ataxia and diplopia *RECIST criteria not validated in primary brain tumors (FDA-AACR Brain Tumor Endpoints Workshop 2006); **Brastianos et al (Nature Precision Oncology 2017) Note: Individual results may not be representative of results in others patients. Change in tumor volume Time (months) Tumor Volume (mL) Pre-entrectinib 9 months on treatment onset of clinical symptoms start entrectinib Strong Clinical Activity of Entrectinib Has Been Observed in Patients with Primary Brain Tumors
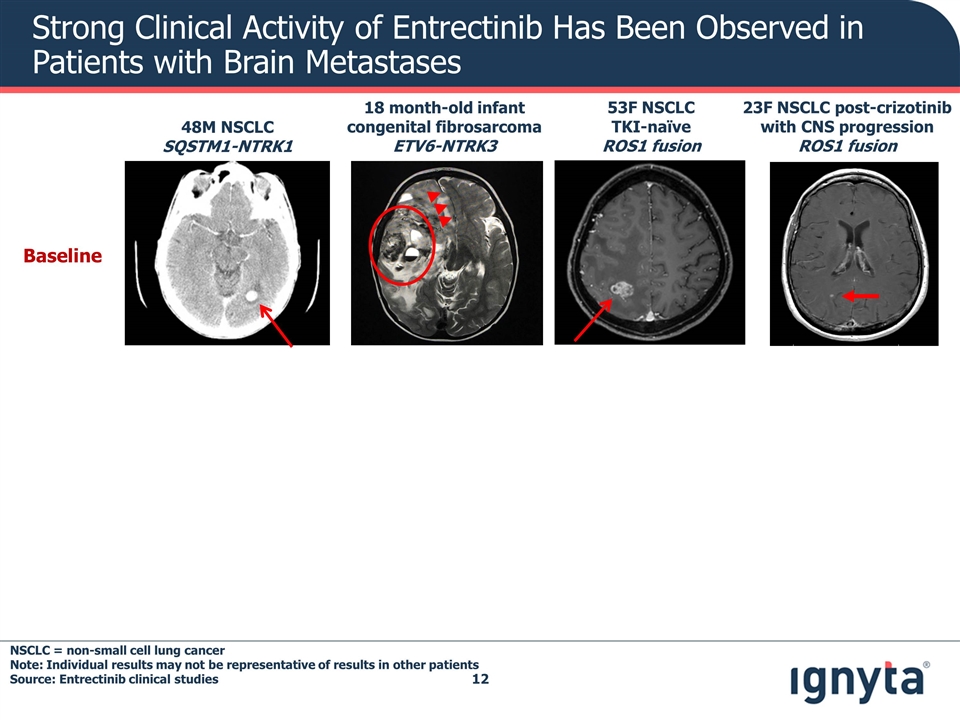
Strong Clinical Activity of Entrectinib Has Been Observed in Patients with Brain Metastases Baseline 48M NSCLC SQSTM1-NTRK1 23F NSCLC post-crizotinib with CNS progression ROS1 fusion 53F NSCLC TKI-naïve ROS1 fusion 18 month-old infant congenital fibrosarcoma ETV6-NTRK3 NSCLC = non-small cell lung cancer Note: Individual results may not be representative of results in other patients Source: Entrectinib clinical studies
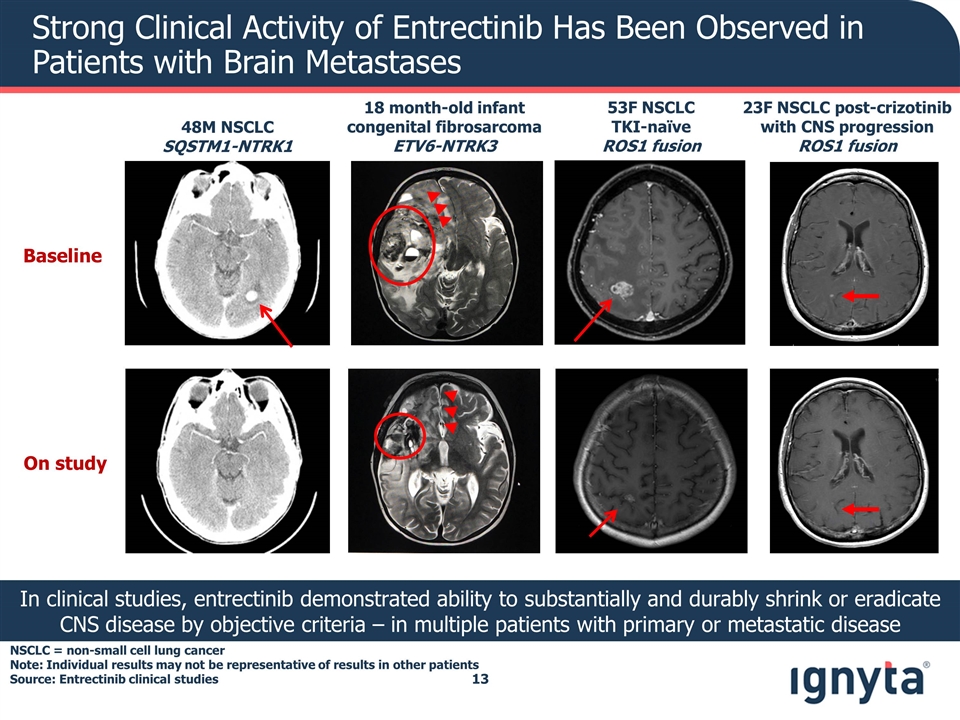
Strong Clinical Activity of Entrectinib Has Been Observed in Patients with Brain Metastases In clinical studies, entrectinib demonstrated ability to substantially and durably shrink or eradicate CNS disease by objective criteria – in multiple patients with primary or metastatic disease Baseline On study 48M NSCLC SQSTM1-NTRK1 23F NSCLC post-crizotinib with CNS progression ROS1 fusion 53F NSCLC TKI-naïve ROS1 fusion 18 month-old infant congenital fibrosarcoma ETV6-NTRK3 NSCLC = non-small cell lung cancer Note: Individual results may not be representative of results in other patients Source: Entrectinib clinical studies
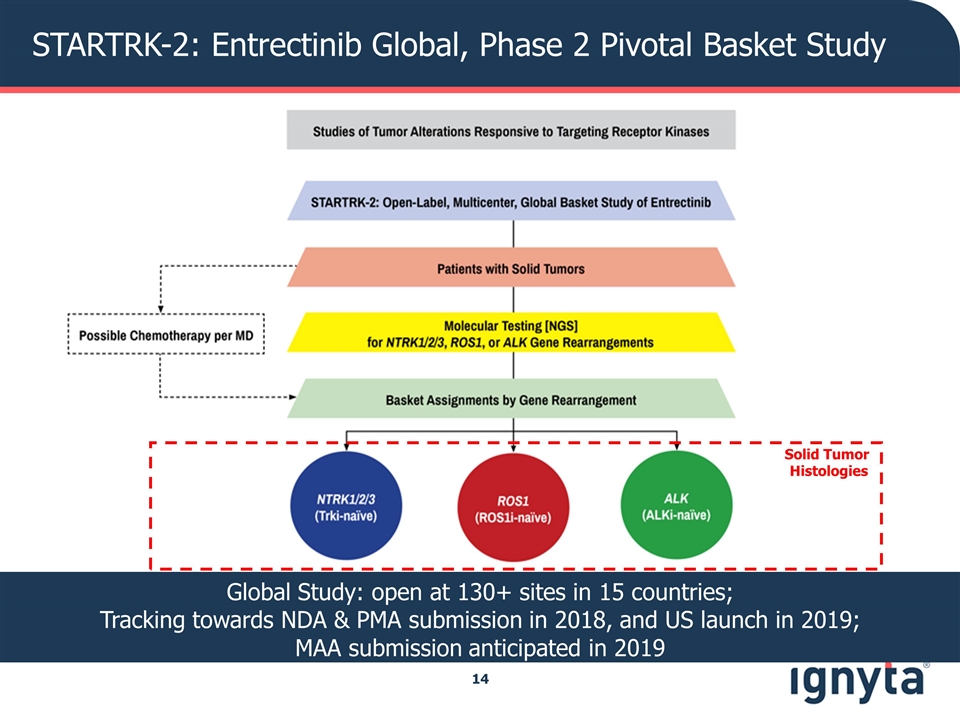
STARTRK-2: Entrectinib Global, Phase 2 Pivotal Basket Study Solid Tumor Histologies Global Study: open at 130+ sites in 15 countries; Tracking towards NDA & PMA submission in 2018, and US launch in 2019; MAA submission anticipated in 2019
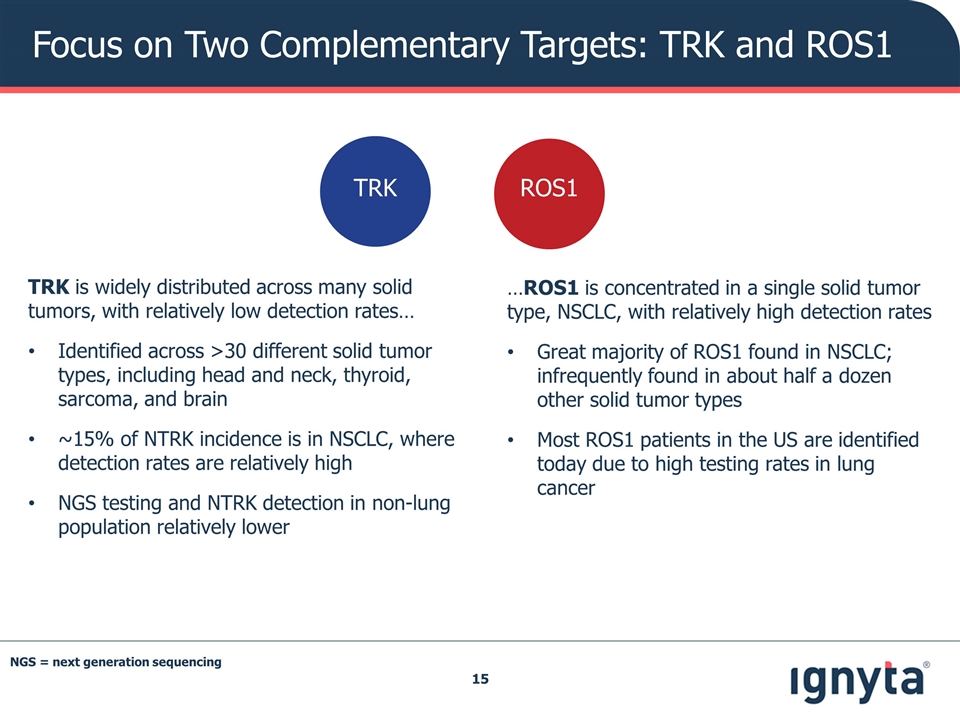
Focus on Two Complementary Targets: TRK and ROS1 TRK is widely distributed across many solid tumors, with relatively low detection rates… Identified across >30 different solid tumor types, including head and neck, thyroid, sarcoma, and brain ~15% of NTRK incidence is in NSCLC, where detection rates are relatively high NGS testing and NTRK detection in non-lung population relatively lower …ROS1 is concentrated in a single solid tumor type, NSCLC, with relatively high detection rates Great majority of ROS1 found in NSCLC; infrequently found in about half a dozen other solid tumor types Most ROS1 patients in the US are identified today due to high testing rates in lung cancer ROS1 TRK NGS = next generation sequencing
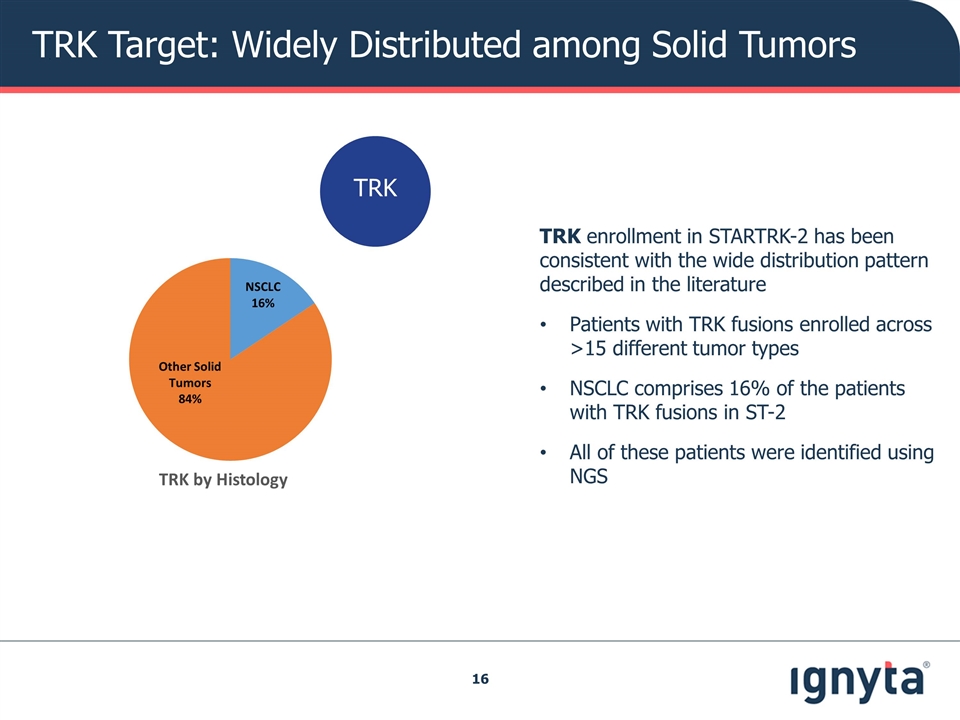
TRK Target: Widely Distributed among Solid Tumors TRK enrollment in STARTRK-2 has been consistent with the wide distribution pattern described in the literature Patients with TRK fusions enrolled across >15 different tumor types NSCLC comprises 16% of the patients with TRK fusions in ST-2 All of these patients were identified using NGS TRK
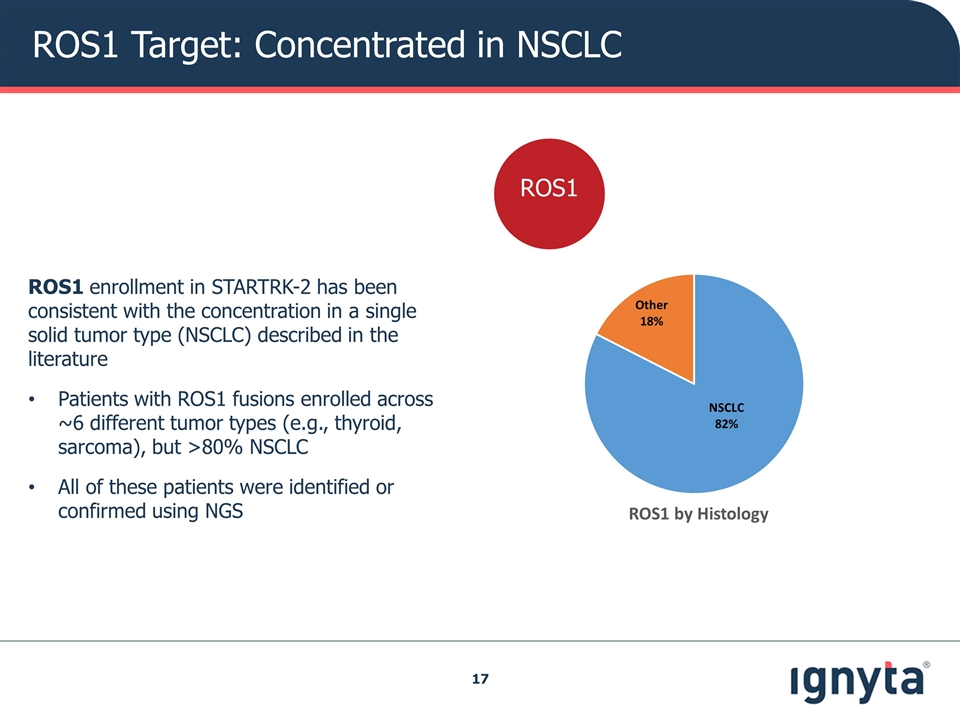
ROS1 Target: Concentrated in NSCLC ROS1 enrollment in STARTRK-2 has been consistent with the concentration in a single solid tumor type (NSCLC) described in the literature Patients with ROS1 fusions enrolled across ~6 different tumor types (e.g., thyroid, sarcoma), but >80% NSCLC All of these patients were identified or confirmed using NGS ROS1
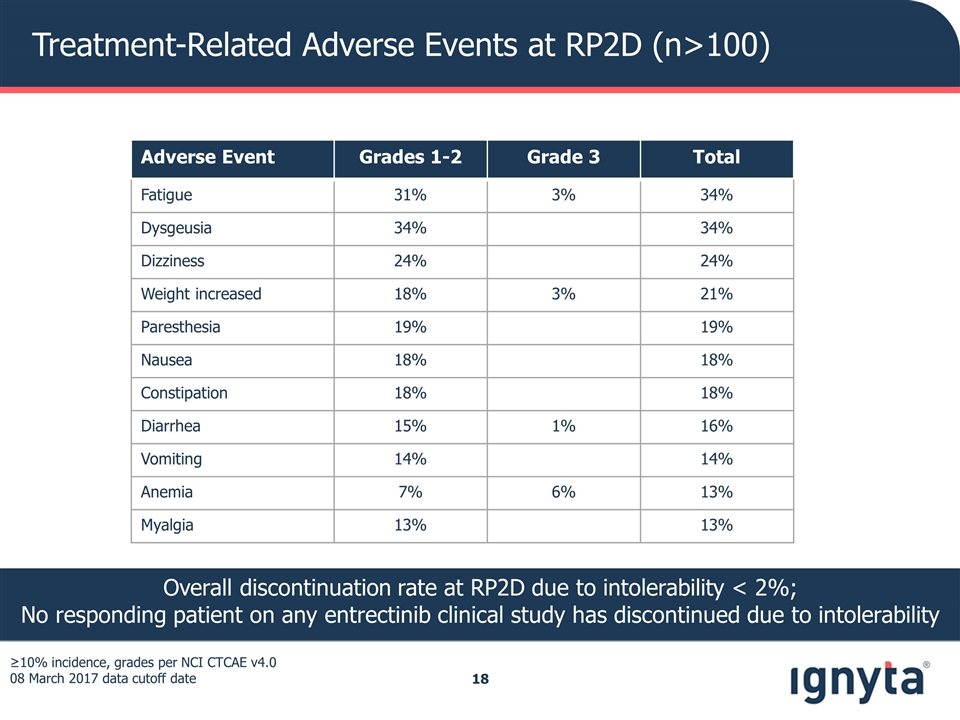
Treatment-Related Adverse Events at RP2D (n>100) ≥10% incidence, grades per NCI CTCAE v4.0 08 March 2017 data cutoff date Overall discontinuation rate at RP2D due to intolerability < 2%; No responding patient on any entrectinib clinical study has discontinued due to intolerability Adverse Event Grades 1-2 Grade 3 Total Fatigue 31% 3% 34% Dysgeusia 34% 34% Dizziness 24% 24% Weight increased 18% 3% 21% Paresthesia 19% 19% Nausea 18% 18% Constipation 18% 18% Diarrhea 15% 1% 16% Vomiting 14% 14% Anemia 7% 6% 13% Myalgia 13% 13%
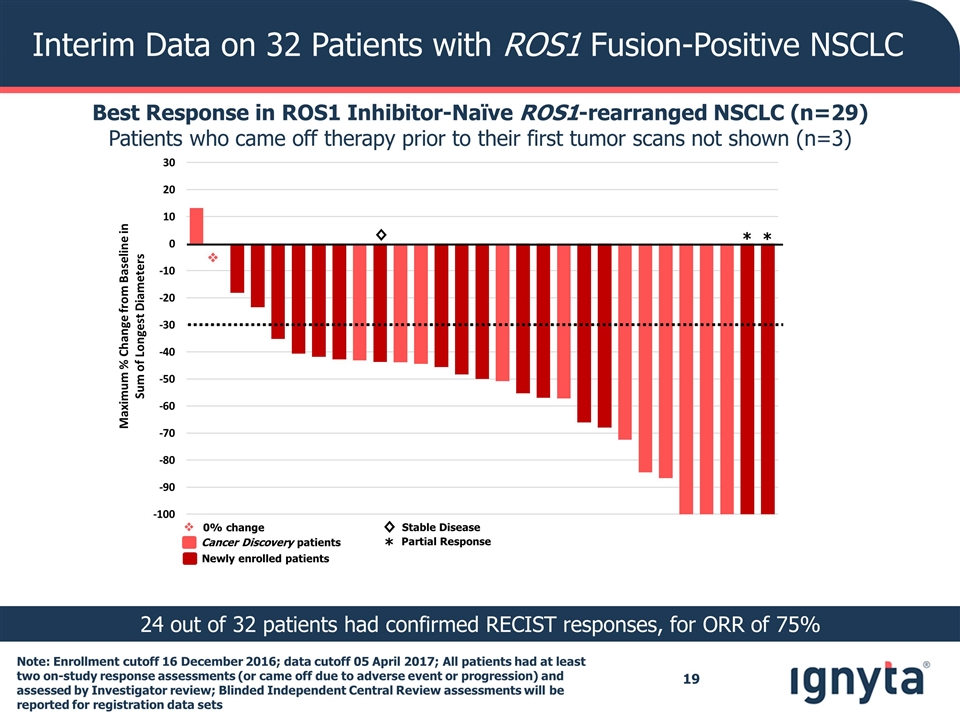
Interim Data on 32 Patients with ROS1 Fusion-Positive NSCLC Best Response in ROS1 Inhibitor-Naïve ROS1-rearranged NSCLC (n=29) Patients who came off therapy prior to their first tumor scans not shown (n=3) 24 out of 32 patients had confirmed RECIST responses, for ORR of 75% v v 0% change Maximum % Change from Baseline in Sum of Longest Diameters Cancer Discovery patients Newly enrolled patients ◊ * * ◊ * Stable Disease Partial Response Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review assessments will be reported for registration data sets
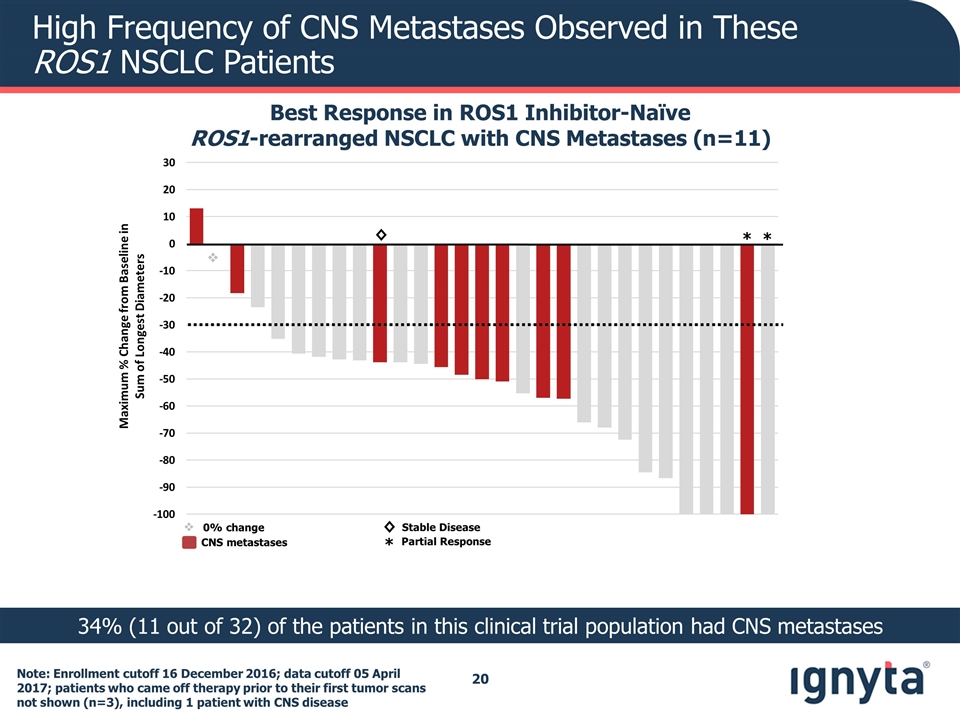
High Frequency of CNS Metastases Observed in These ROS1 NSCLC Patients Best Response in ROS1 Inhibitor-Naïve ROS1-rearranged NSCLC with CNS Metastases (n=11) 34% (11 out of 32) of the patients in this clinical trial population had CNS metastases v Maximum % Change from Baseline in Sum of Longest Diameters ◊ * * CNS metastases ◊ * Stable Disease Partial Response v 0% change Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; patients who came off therapy prior to their first tumor scans not shown (n=3), including 1 patient with CNS disease
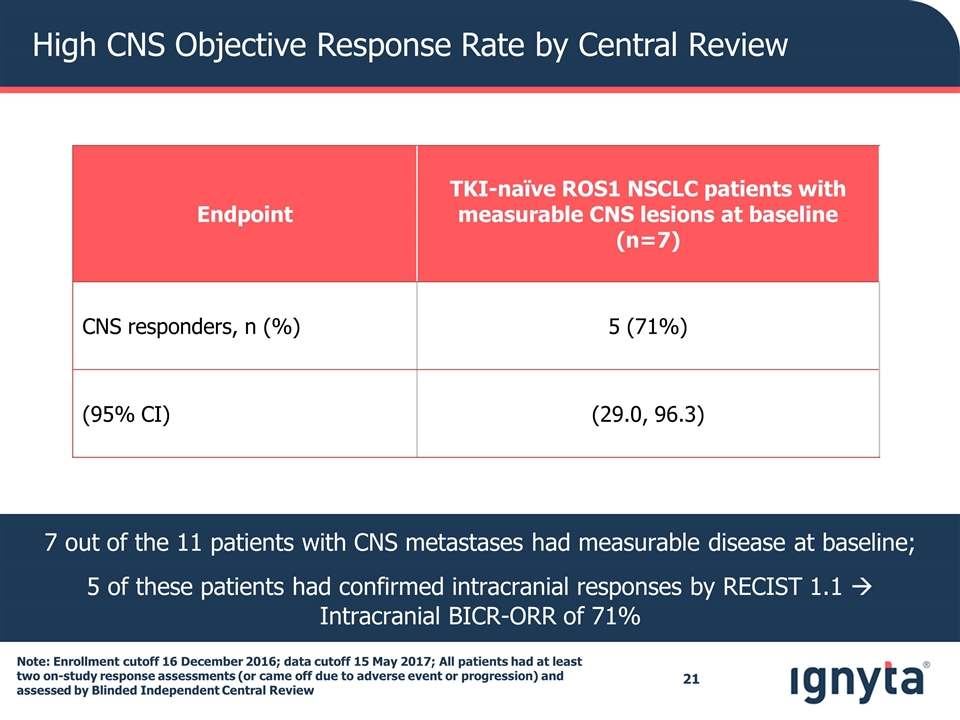
High CNS Objective Response Rate by Central Review Endpoint TKI-naïve ROS1 NSCLC patients with measurable CNS lesions at baseline (n=7) CNS responders, n (%) 5 (71%) (95% CI) (29.0, 96.3) 7 out of the 11 patients with CNS metastases had measurable disease at baseline; 5 of these patients had confirmed intracranial responses by RECIST 1.1 à Intracranial BICR-ORR of 71% Note: Enrollment cutoff 16 December 2016; data cutoff 15 May 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Blinded Independent Central Review
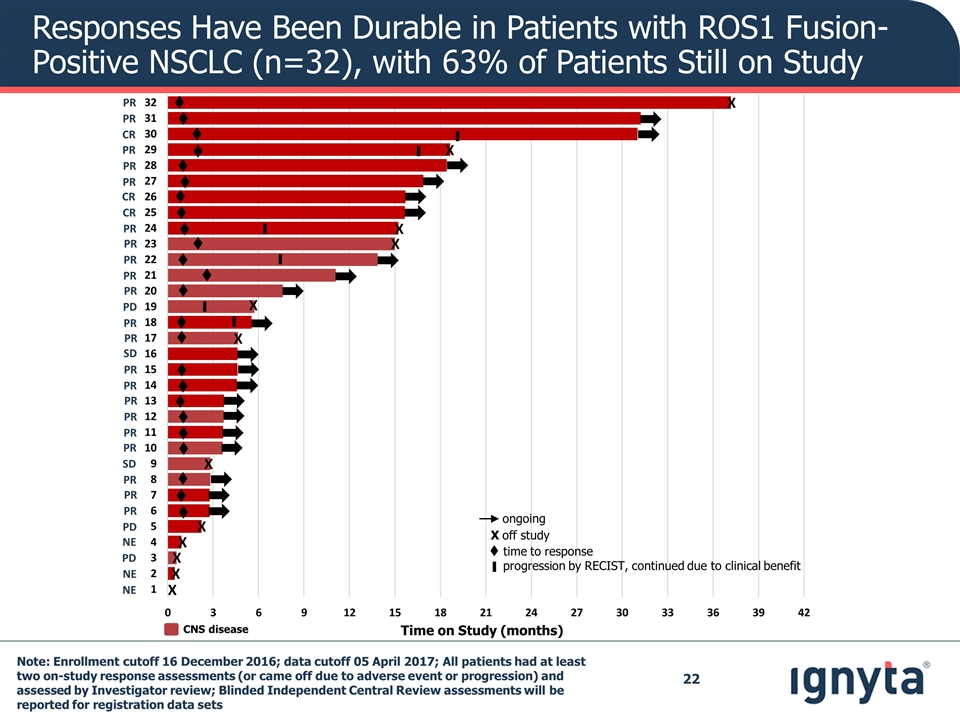
Responses Have Been Durable in Patients with ROS1 Fusion-Positive NSCLC (n=32), with 63% of Patients Still on Study CNS disease Time on Study (months) t X t t t t t t t t t t t t t t t t t t t t t t t X X X X X X X X X X X ▌ ▌ ▌ ▌ ▌ ▌ t time to response ongoing X off study progression by RECIST, continued due to clinical benefit ▌ PR PR CR PR PR PR CR CR PR PR PR PR PR PD PR PR SD PR PR PR PR PR PR SD PR PR PR PD NE PD NE NE Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review assessments will be reported for registration data sets
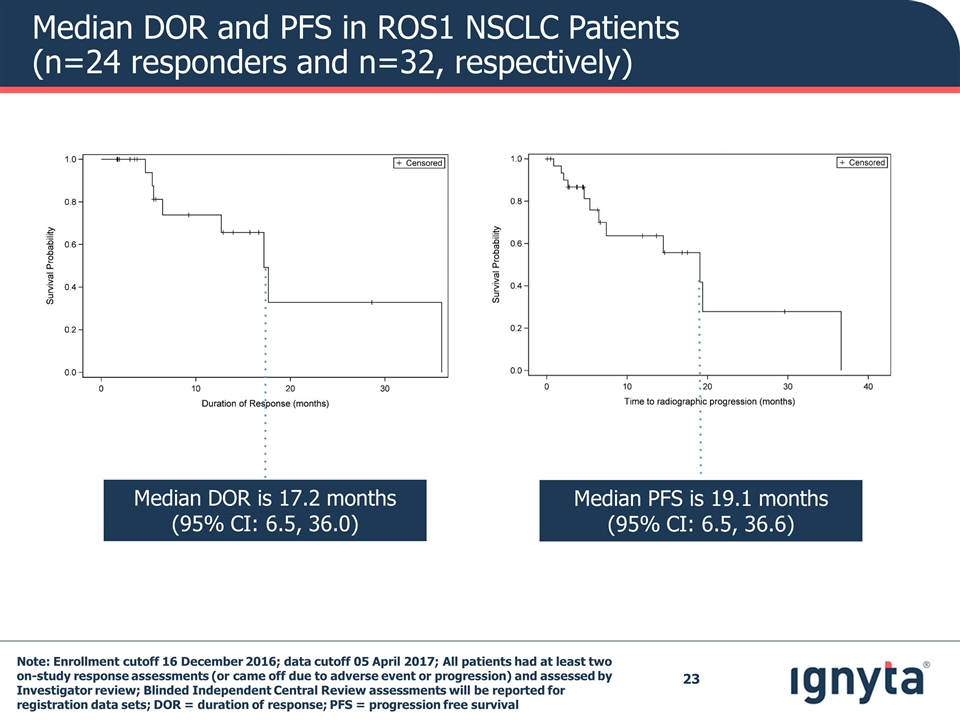
Median DOR and PFS in ROS1 NSCLC Patients (n=24 responders and n=32, respectively) Median PFS is 19.1 months (95% CI: 6.5, 36.6) Median DOR is 17.2 months (95% CI: 6.5, 36.0) Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review assessments will be reported for registration data sets; DOR = duration of response; PFS = progression free survival
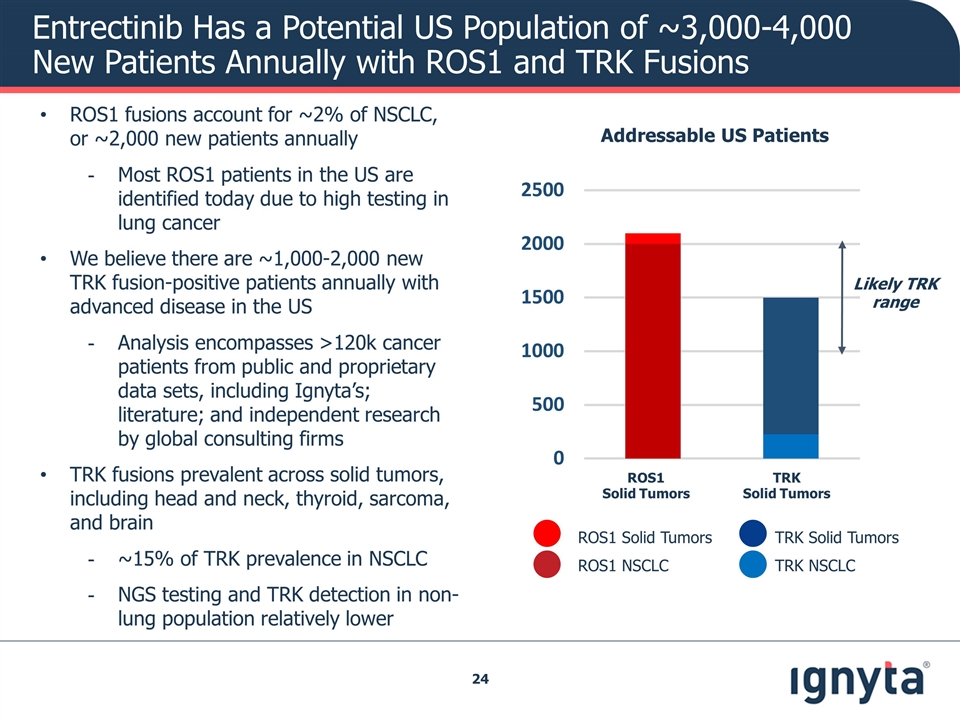
Entrectinib Has a Potential US Population of ~3,000-4,000 New Patients Annually with ROS1 and TRK Fusions Addressable US Patients ROS1 NSCLC ROS1 Solid Tumors TRK NSCLC TRK Solid Tumors ROS1 Solid Tumors TRK Solid Tumors Likely TRK range ROS1 fusions account for ~2% of NSCLC, or ~2,000 new patients annually Most ROS1 patients in the US are identified today due to high testing in lung cancer We believe there are ~1,000-2,000 new TRK fusion-positive patients annually with advanced disease in the US Analysis encompasses >120k cancer patients from public and proprietary data sets, including Ignyta’s; literature; and independent research by global consulting firms TRK fusions prevalent across solid tumors, including head and neck, thyroid, sarcoma, and brain ~15% of TRK prevalence in NSCLC NGS testing and TRK detection in non-lung population relatively lower
Entrectinib Is the Only TRK Inhibitor to Have Demonstrated CNS Activity Entrectinib is the only TRK inhibitor with clinical demonstration of CNS activity Entrectinib TRK CNS responses: 100% (3/3, including CNS CR in NSCLC)* “…Loxo-101 was designed to have limited distribution into central nervous system (CNS) tissues.” (Loxo-101 pedsODAC briefing package, June 29, 2016) Loxo-101 CNS responses: 0% (0/4, including NSCLC)** In addition to robust CNS activity, entrectinib has an attractive safety profile Overall discontinuation rate at RP2D due to intolerability < 2% No responding patient discontinuing therapy due to intolerability Entrectinib clinical profile is consistent with positioning as first-line targeted agent for all patients with TRK fusions * Cancer Discovery, February 2017 ** Hong et al, AACR 2016; Schram et al, AACR 2017 Note: Results are based on non-head to head clinical studies to date; pedsODAC = Pediatric Oncologic Drugs Advisory Committee
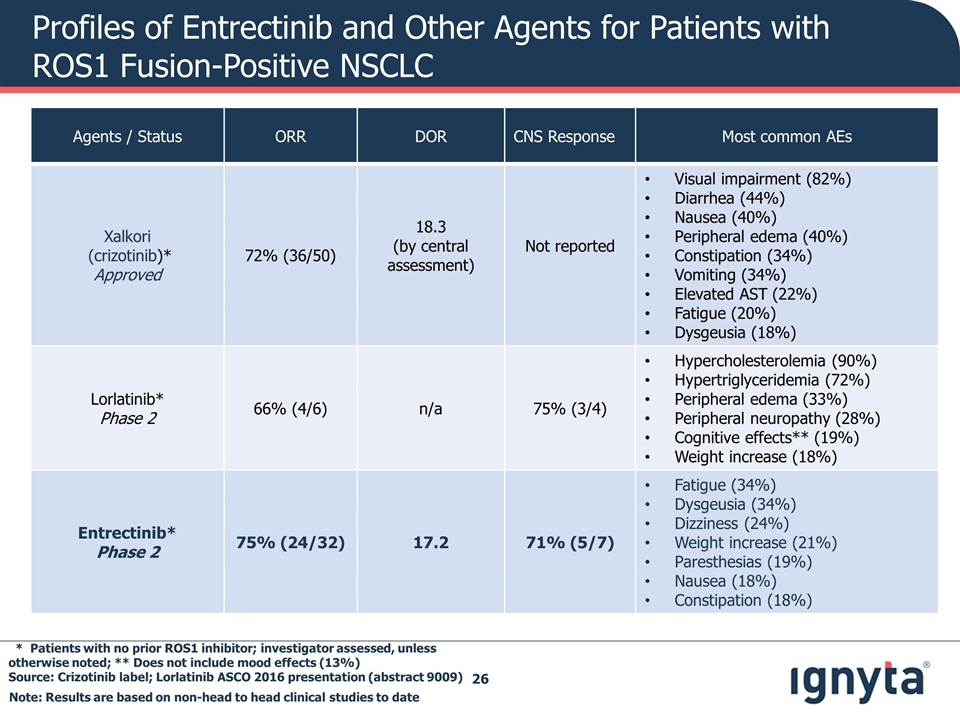
Profiles of Entrectinib and Other Agents for Patients with ROS1 Fusion-Positive NSCLC Agents / Status ORR DOR CNS Response Most common AEs Xalkori (crizotinib)* Approved 72% (36/50) 18.3 (by central assessment) Not reported Visual impairment (82%) Diarrhea (44%) Nausea (40%) Peripheral edema (40%) Constipation (34%) Vomiting (34%) Elevated AST (22%) Fatigue (20%) Dysgeusia (18%) Lorlatinib* Phase 2 66% (4/6) n/a 75% (3/4) Hypercholesterolemia (90%) Hypertriglyceridemia (72%) Peripheral edema (33%) Peripheral neuropathy (28%) Cognitive effects** (19%) Weight increase (18%) Entrectinib* Phase 2 75% (24/32) 17.2 71% (5/7) Fatigue (34%) Dysgeusia (34%) Dizziness (24%) Weight increase (21%) Paresthesias (19%) Nausea (18%) Constipation (18%) * Patients with no prior ROS1 inhibitor; investigator assessed, unless otherwise noted; ** Does not include mood effects (13%) Source: Crizotinib label; Lorlatinib ASCO 2016 presentation (abstract 9009) Note: Results are based on non-head to head clinical studies to date
Entrectinib Clinical Profile Supports Positioning as First-Line Targeted Agent in ROS1 Fusion-Positive NSCLC Competitive response rate and durability Attractive tolerability profile that we expect will support preferred use and extended duration of use Overall discontinuation rate at RP2D due to intolerability < 2% No responding patient discontinuing therapy due to intolerability Potential to address significant morbidity and mortality associated with CNS disease CNS progression must be treated proactively and aggressively with immediate and intensive intervention (e.g., WBRT, gamma knife) CNS metastases may be hard to detect and so may not be caught in time A CNS penetrant drug proactively at first-line may prevent or delay onset of metastases and extend response Clinical profile consistent with positioning as first-line targeted agent for ROS1 patients, regardless of their CNS disease status at presentation
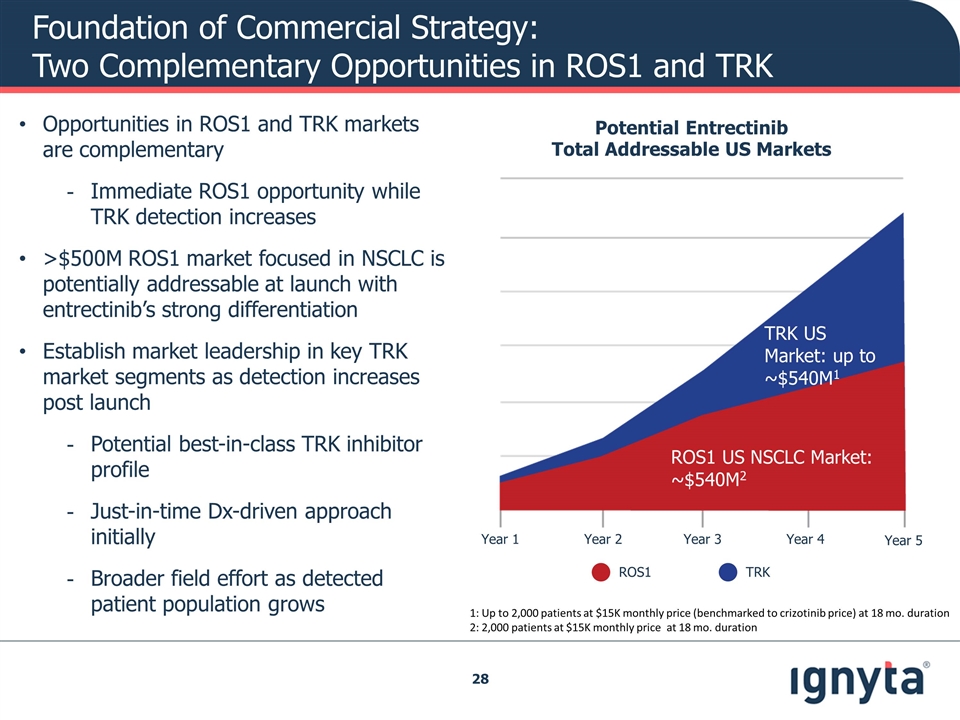
Foundation of Commercial Strategy: Two Complementary Opportunities in ROS1 and TRK Opportunities in ROS1 and TRK markets are complementary Immediate ROS1 opportunity while TRK detection increases >$500M ROS1 market focused in NSCLC is potentially addressable at launch with entrectinib’s strong differentiation Establish market leadership in key TRK market segments as detection increases post launch Potential best-in-class TRK inhibitor profile Just-in-time Dx-driven approach initially Broader field effort as detected patient population grows Potential Entrectinib Total Addressable US Markets ROS1 US NSCLC Market: ~$540M2 1: Up to 2,000 patients at $15K monthly price (benchmarked to crizotinib price) at 18 mo. duration 2: 2,000 patients at $15K monthly price at 18 mo. duration TRK US Market: up to ~$540M1 Year 1 Year 2 Year 3 Year 4 Year 5 ROS1 TRK
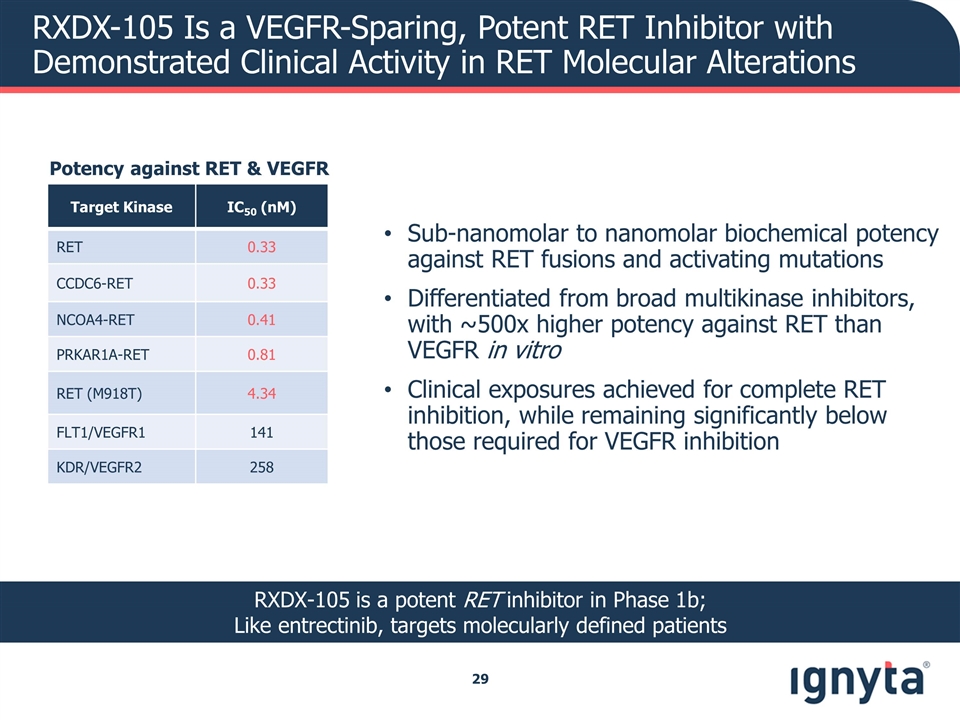
RXDX-105 Is a VEGFR-Sparing, Potent RET Inhibitor with Demonstrated Clinical Activity in RET Molecular Alterations Sub-nanomolar to nanomolar biochemical potency against RET fusions and activating mutations Differentiated from broad multikinase inhibitors, with ~500x higher potency against RET than VEGFR in vitro Clinical exposures achieved for complete RET inhibition, while remaining significantly below those required for VEGFR inhibition Potency against RET & VEGFR Target Kinase IC50 (nM) RET 0.33 CCDC6-RET 0.33 NCOA4-RET 0.41 PRKAR1A-RET 0.81 RET (M918T) 4.34 FLT1/VEGFR1 141 KDR/VEGFR2 258 RXDX-105 is a potent RET inhibitor in Phase 1b; Like entrectinib, targets molecularly defined patients
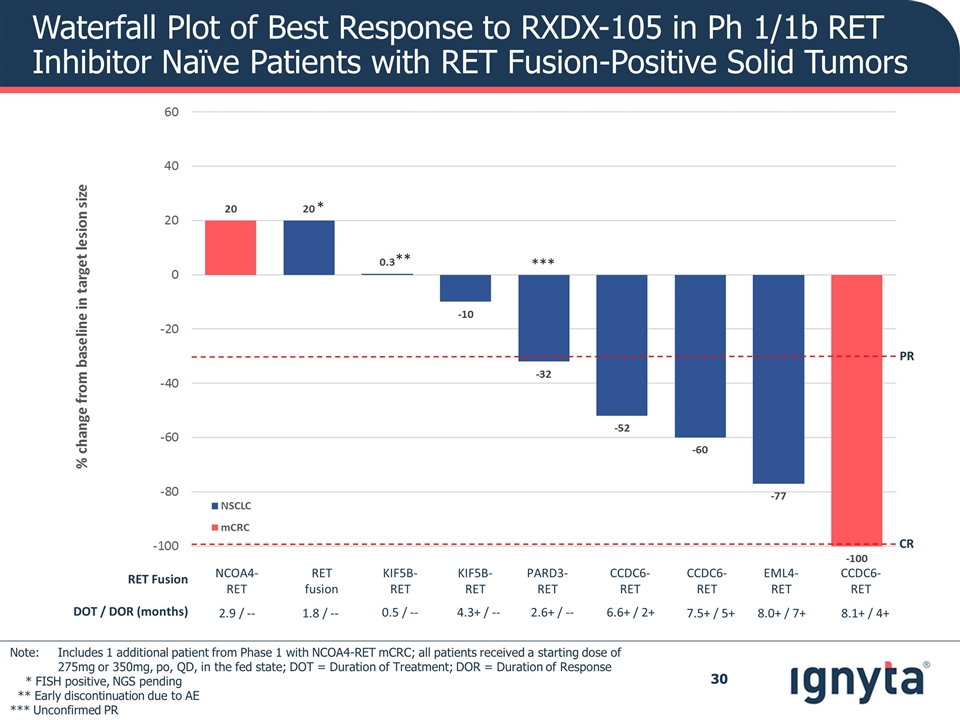
Waterfall Plot of Best Response to RXDX-105 in Ph 1/1b RET Inhibitor Naïve Patients with RET Fusion-Positive Solid Tumors * ** RET Fusion NCOA4-RET RET fusion KIF5B-RET KIF5B-RET PARD3-RET CCDC6-RET CCDC6-RET EML4-RET CCDC6-RET DOT / DOR (months) 2.9 / -- 1.8 / -- 0.5 / -- 4.3+ / -- 2.6+ / -- 6.6+ / 2+ 7.5+ / 5+ 8.0+ / 7+ 8.1+ / 4+ *** PR CR Note: Includes 1 additional patient from Phase 1 with NCOA4-RET mCRC; all patients received a starting dose of 275mg or 350mg, po, QD, in the fed state; DOT = Duration of Treatment; DOR = Duration of Response * FISH positive, NGS pending ** Early discontinuation due to AE *** Unconfirmed PR
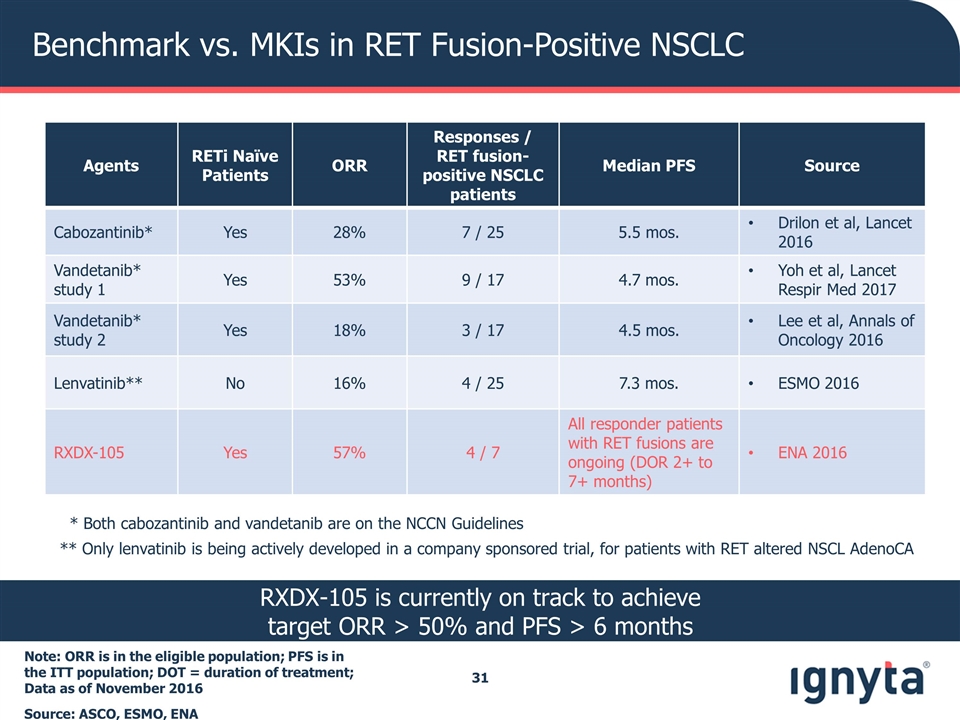
Benchmark vs. MKIs in RET Fusion-Positive NSCLC Note: ORR is in the eligible population; PFS is in the ITT population; DOT = duration of treatment; Data as of November 2016 Source: ASCO, ESMO, ENA Agents RETi Naïve Patients ORR Responses / RET fusion-positive NSCLC patients Median PFS Source Cabozantinib* Yes 28% 7 / 25 5.5 mos. Drilon et al, Lancet 2016 Vandetanib* study 1 Yes 53% 9 / 17 4.7 mos. Yoh et al, Lancet Respir Med 2017 Vandetanib* study 2 Yes 18% 3 / 17 4.5 mos. Lee et al, Annals of Oncology 2016 Lenvatinib** No 16% 4 / 25 7.3 mos. ESMO 2016 RXDX-105 Yes 57% 4 / 7 All responder patients with RET fusions are ongoing (DOR 2+ to 7+ months) ENA 2016 * Both cabozantinib and vandetanib are on the NCCN Guidelines ** Only lenvatinib is being actively developed in a company sponsored trial, for patients with RET altered NSCL AdenoCA RXDX-105 is currently on track to achieve target ORR > 50% and PFS > 6 months
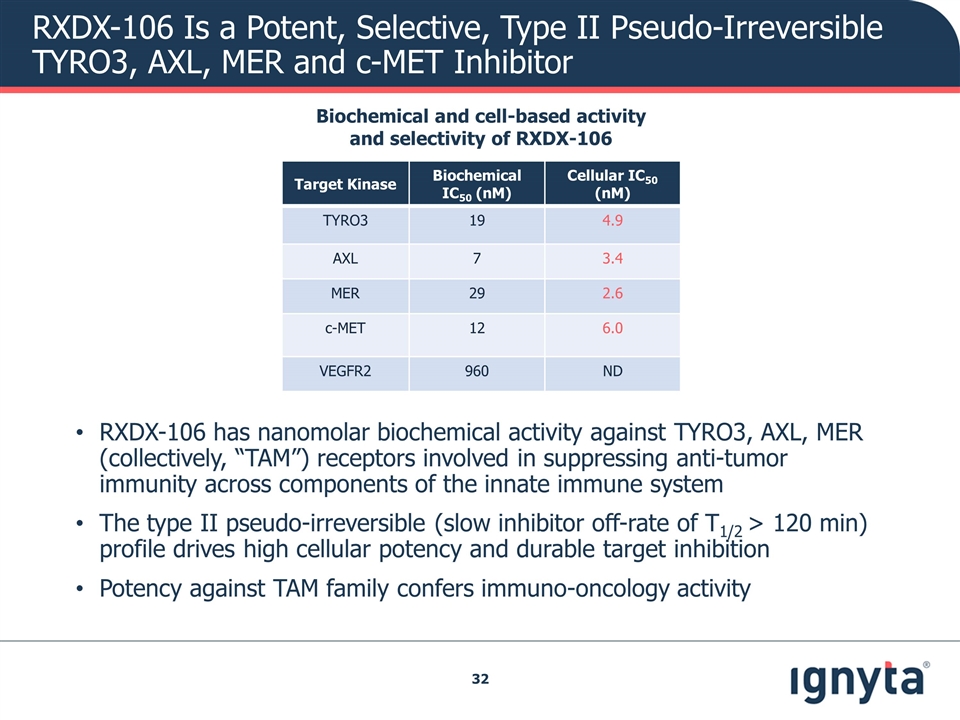
RXDX-106 Is a Potent, Selective, Type II Pseudo-Irreversible TYRO3, AXL, MER and c-MET Inhibitor Target Kinase Biochemical IC50 (nM) Cellular IC50 (nM) TYRO3 19 4.9 AXL 7 3.4 MER 29 2.6 c-MET 12 6.0 VEGFR2 960 ND RXDX-106 has nanomolar biochemical activity against TYRO3, AXL, MER (collectively, “TAM”) receptors involved in suppressing anti-tumor immunity across components of the innate immune system The type II pseudo-irreversible (slow inhibitor off-rate of T1/2 > 120 min) profile drives high cellular potency and durable target inhibition Potency against TAM family confers immuno-oncology activity Biochemical and cell-based activity and selectivity of RXDX-106
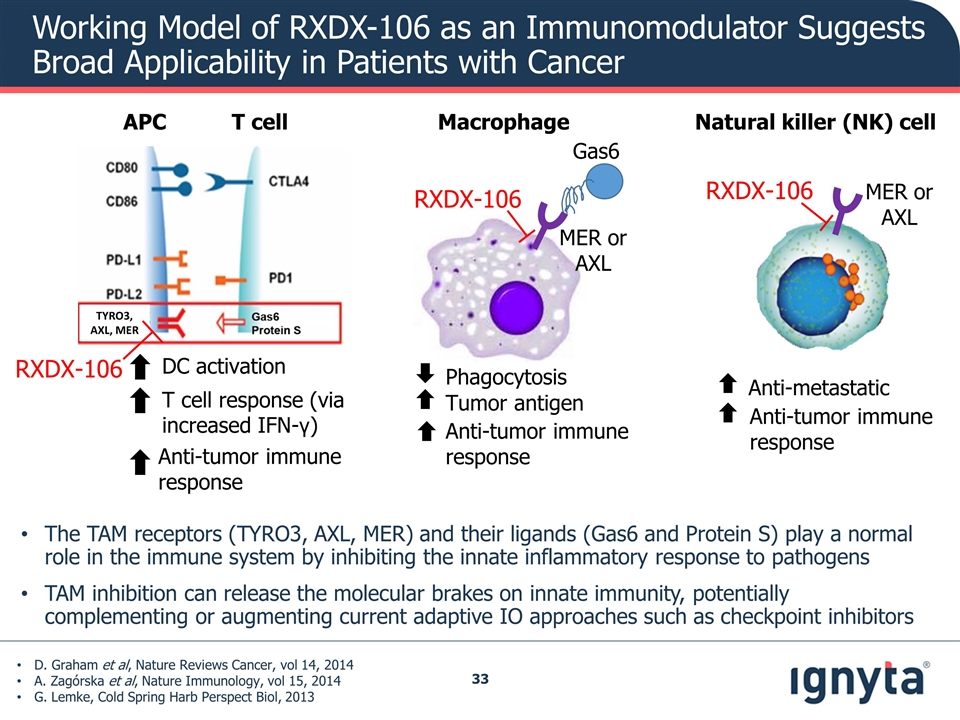
Working Model of RXDX-106 as an Immunomodulator Suggests Broad Applicability in Patients with Cancer Macrophage Natural killer (NK) cell APC T cell T cell response (via increased IFN-γ) Anti-tumor immune response DC activation Phagocytosis Tumor antigen Anti-tumor immune response Anti-tumor immune response Anti-metastatic Gas6 MER or AXL MER or AXL RXDX-106 RXDX-106 D. Graham et al, Nature Reviews Cancer, vol 14, 2014 A. Zagórska et al, Nature Immunology, vol 15, 2014 G. Lemke, Cold Spring Harb Perspect Biol, 2013 The TAM receptors (TYRO3, AXL, MER) and their ligands (Gas6 and Protein S) play a normal role in the immune system by inhibiting the innate inflammatory response to pathogens TAM inhibition can release the molecular brakes on innate immunity, potentially complementing or augmenting current adaptive IO approaches such as checkpoint inhibitors TYRO3, AXL, MER RXDX-106
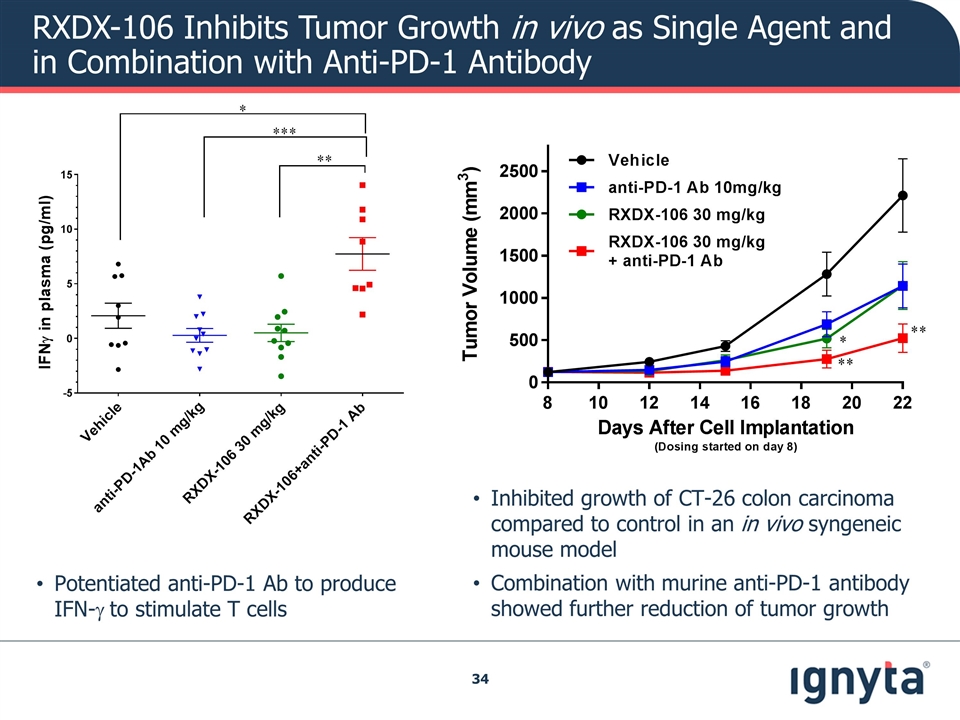
RXDX-106 Inhibits Tumor Growth in vivo as Single Agent and in Combination with Anti-PD-1 Antibody Inhibited growth of CT-26 colon carcinoma compared to control in an in vivo syngeneic mouse model Combination with murine anti-PD-1 antibody showed further reduction of tumor growth Potentiated anti-PD-1 Ab to produce IFN-g to stimulate T cells
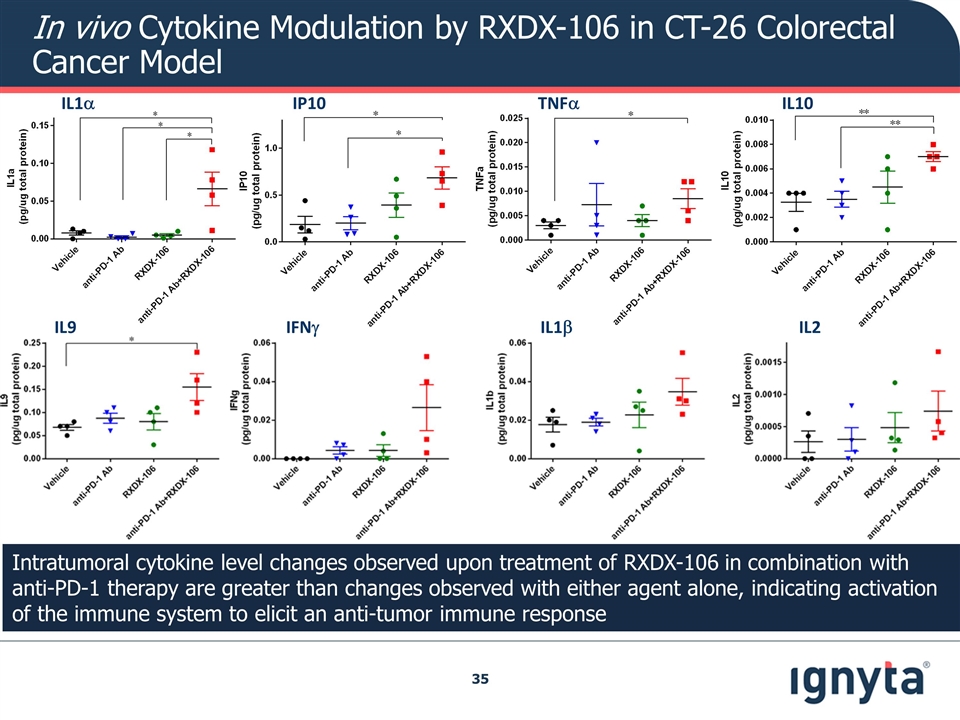
In vivo Cytokine Modulation by RXDX-106 in CT-26 Colorectal Cancer Model IL1a IP10 TNFa IL10 IL9 IFNg IL1b IL2 Intratumoral cytokine level changes observed upon treatment of RXDX-106 in combination with anti-PD-1 therapy are greater than changes observed with either agent alone, indicating activation of the immune system to elicit an anti-tumor immune response

2017 & 2018 Anticipated Corporate Milestones & Clinical Updates 2030 Vision Entrectinib STARTRK-1/ALKA Ph 1 data update, 1Q17 STARTRK-2 interim data (after consultation with FDA) + entrectinib commercial roadmap, 2Q17 Complete TRK and ROS1 enrollment of STARTRK-2, 3Q17 Announce top-line NDA registration-enabling data (after consultation with FDA), 1H18 Submit NDAs (and PMA) with US FDA, 2018 Pipeline RXDX-105 Ph 1b basket study data update, 2H17 RXDX-106 Ph 1 trial initiation, 2H17 RXDX-106 preliminary Ph 1 data, 2H18 2011 – 2015 Advance clinical pipeline 2016 – 2020 Commercialize Rx/Dx products 2021 – 2025 Scale pipeline revenue 2026 – 2030 Drive sustainable profitability Leading Precision Medicine Company that Eradicates Residual Disease ✔ ✔



































