
Entrectinib and RXDX-105 Update September 11, 2017 Exhibit 99.2

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: references to the development of entrectinib and our other product candidates, including potential differentiating factors; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs, and the timelines associated with such programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; our dependence on third party manufacturers for supply of our product candidates and any approved products; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2017, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.
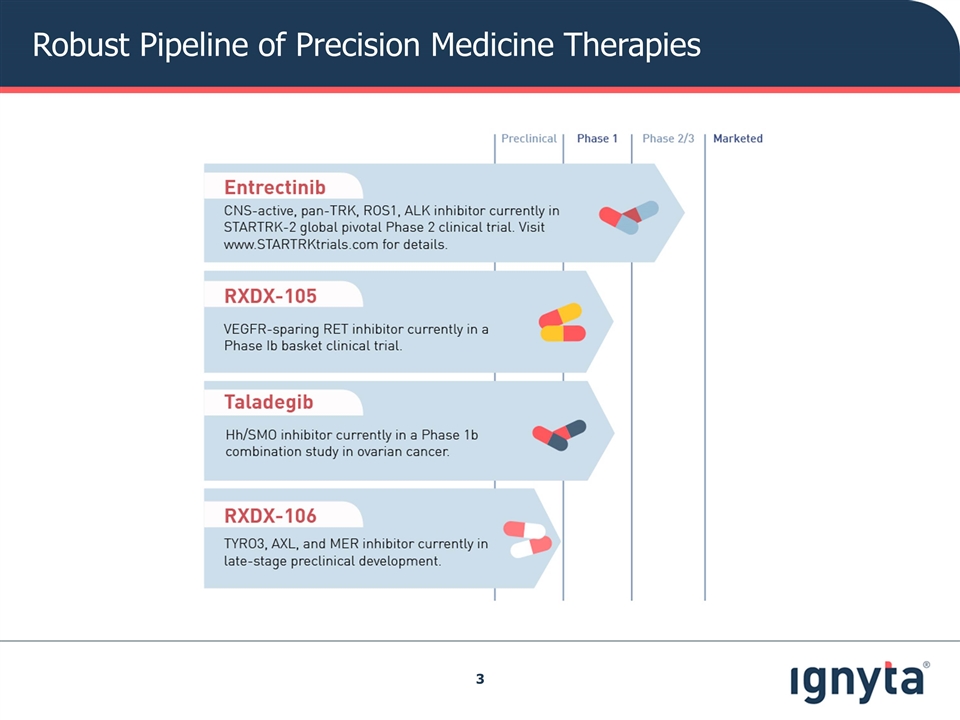
Robust Pipeline of Precision Medicine Therapies
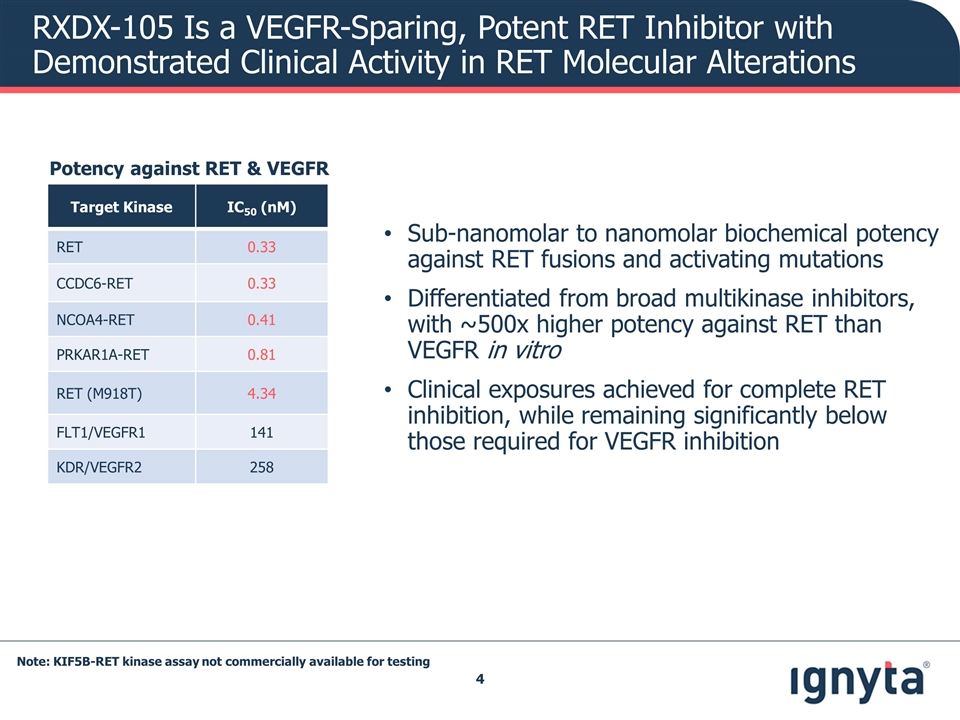
RXDX-105 Is a VEGFR-Sparing, Potent RET Inhibitor with Demonstrated Clinical Activity in RET Molecular Alterations Sub-nanomolar to nanomolar biochemical potency against RET fusions and activating mutations Differentiated from broad multikinase inhibitors, with ~500x higher potency against RET than VEGFR in vitro Clinical exposures achieved for complete RET inhibition, while remaining significantly below those required for VEGFR inhibition Potency against RET & VEGFR Target Kinase IC50 (nM) RET 0.33 CCDC6-RET 0.33 NCOA4-RET 0.41 PRKAR1A-RET 0.81 RET (M918T) 4.34 FLT1/VEGFR1 141 KDR/VEGFR2 258 Note: KIF5B-RET kinase assay not commercially available for testing
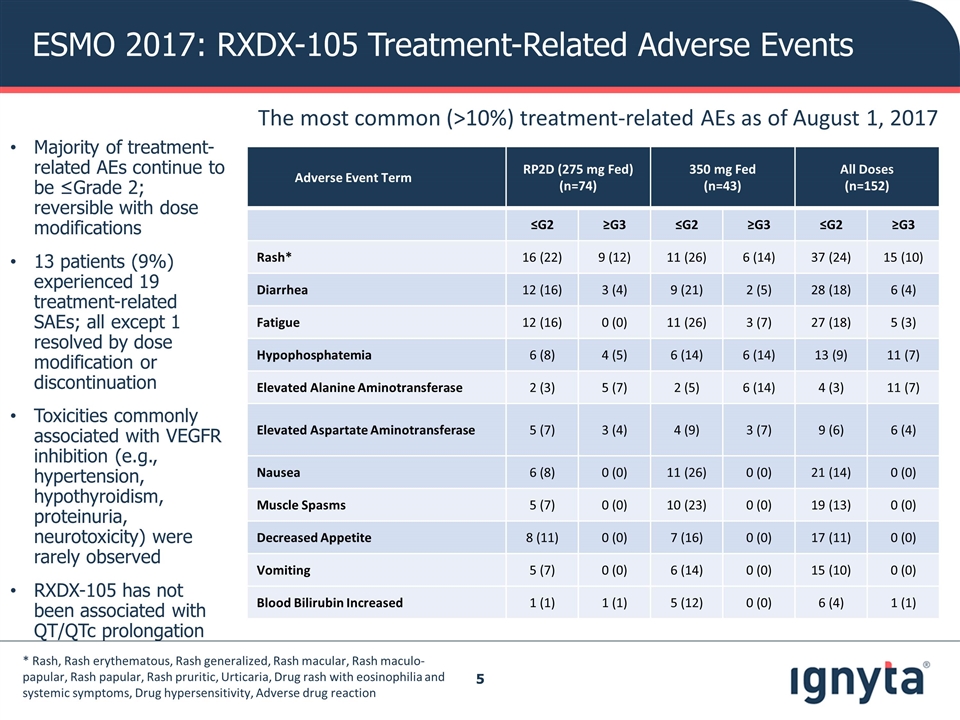
ESMO 2017: RXDX-105 Treatment-Related Adverse Events Adverse Event Term RP2D (275 mg Fed) (n=74) 350 mg Fed (n=43) All Doses (n=152) ≤G2 ≥G3 ≤G2 ≥G3 ≤G2 ≥G3 Rash* 16 (22) 9 (12) 11 (26) 6 (14) 37 (24) 15 (10) Diarrhea 12 (16) 3 (4) 9 (21) 2 (5) 28 (18) 6 (4) Fatigue 12 (16) 0 (0) 11 (26) 3 (7) 27 (18) 5 (3) Hypophosphatemia 6 (8) 4 (5) 6 (14) 6 (14) 13 (9) 11 (7) Elevated Alanine Aminotransferase 2 (3) 5 (7) 2 (5) 6 (14) 4 (3) 11 (7) Elevated Aspartate Aminotransferase 5 (7) 3 (4) 4 (9) 3 (7) 9 (6) 6 (4) Nausea 6 (8) 0 (0) 11 (26) 0 (0) 21 (14) 0 (0) Muscle Spasms 5 (7) 0 (0) 10 (23) 0 (0) 19 (13) 0 (0) Decreased Appetite 8 (11) 0 (0) 7 (16) 0 (0) 17 (11) 0 (0) Vomiting 5 (7) 0 (0) 6 (14) 0 (0) 15 (10) 0 (0) Blood Bilirubin Increased 1 (1) 1 (1) 5 (12) 0 (0) 6 (4) 1 (1) The most common (>10%) treatment-related AEs as of August 1, 2017 * Rash, Rash erythematous, Rash generalized, Rash macular, Rash maculo-papular, Rash papular, Rash pruritic, Urticaria, Drug rash with eosinophilia and systemic symptoms, Drug hypersensitivity, Adverse drug reaction Majority of treatment-related AEs continue to be ≤Grade 2; reversible with dose modifications 13 patients (9%) experienced 19 treatment-related SAEs; all except 1 resolved by dose modification or discontinuation Toxicities commonly associated with VEGFR inhibition (e.g., hypertension, hypothyroidism, proteinuria, neurotoxicity) were rarely observed RXDX-105 has not been associated with QT/QTc prolongation
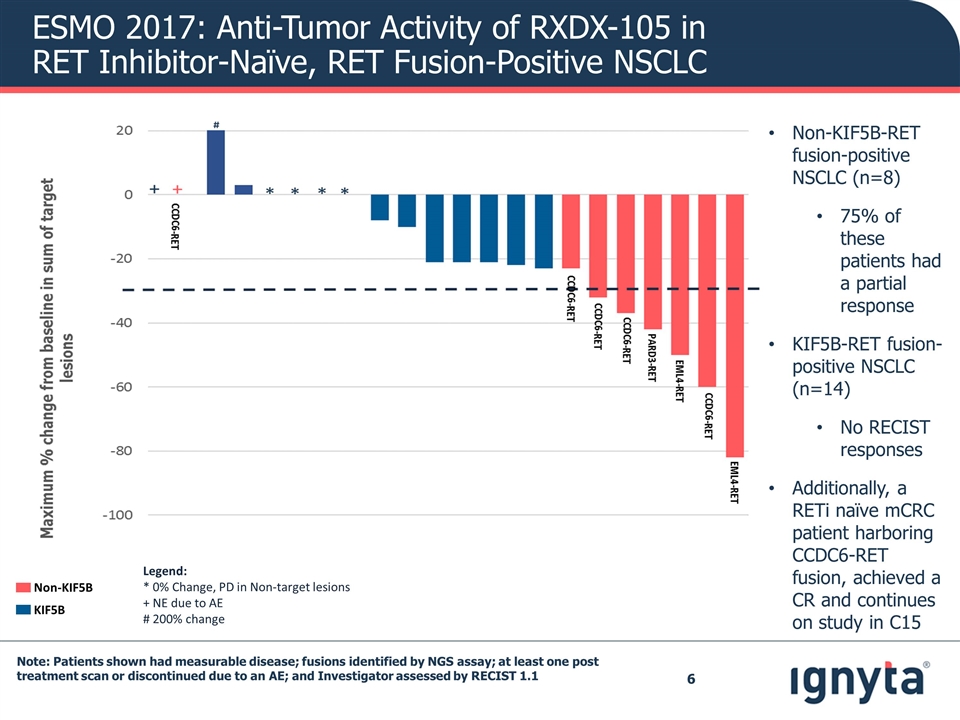
Note: Patients shown had measurable disease; fusions identified by NGS assay; at least one post treatment scan or discontinued due to an AE; and Investigator assessed by RECIST 1.1 ESMO 2017: Anti-Tumor Activity of RXDX-105 in RET Inhibitor-Naïve, RET Fusion-Positive NSCLC Legend: * 0% Change, PD in Non-target lesions + NE due to AE # 200% change Non-KIF5B KIF5B Non-KIF5B-RET fusion-positive NSCLC (n=8) 75% of these patients had a partial response KIF5B-RET fusion-positive NSCLC (n=14) No RECIST responses Additionally, a RETi naïve mCRC patient harboring CCDC6-RET fusion, achieved a CR and continues on study in C15
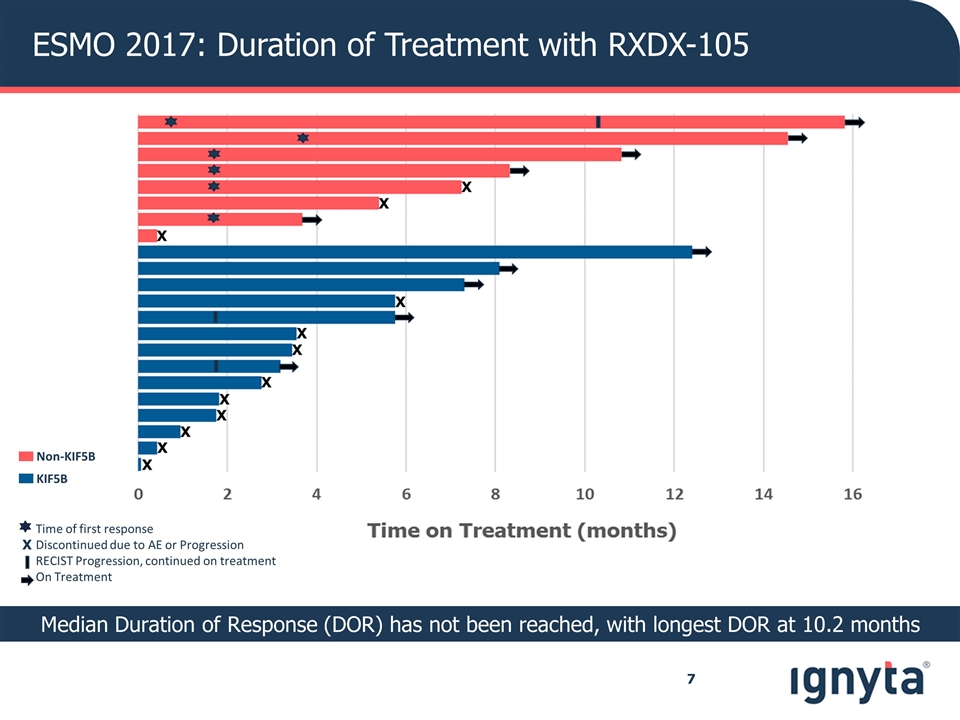
ESMO 2017: Duration of Treatment with RXDX-105 Non-KIF5B KIF5B Median Duration of Response (DOR) has not been reached, with longest DOR at 10.2 months Time of first response Discontinued due to AE or Progression RECIST Progression, continued on treatment On Treatment X

Implications and Next Steps Ignyta has run a robust clinical “experiment” in RET NSCLC and has observed a compelling response rate in an ultra-rare patient population (non-KIF5B-RET fusions) KIF5B-RET fusions show an apparent difference in response profile to RXDX-105 Consistent with previous pooled efficacy evidence of other RET-active agents against KIF5B-RET Will be interesting to monitor the response profiles of earlier stage RET targeting agents in development Wrapping up Phase 1b study (no further enrollment, since critical mass of patients have been enrolled) Discussing program with potential partners, in the context of other business development discussions regarding entrectinib Maintaining development focus on two NDA indications (TRK tissue-agnostic, ROS1 NSCLC) for entrectinib, and bringing RXDX-106 into the clinic
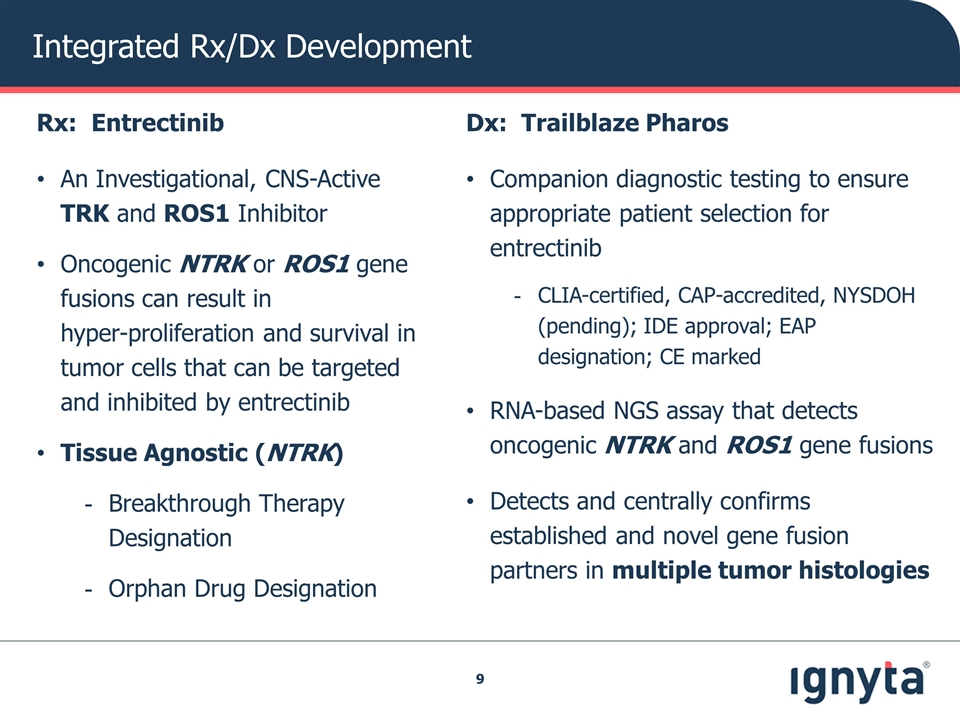
Integrated Rx/Dx Development Rx: Entrectinib An Investigational, CNS-Active TRK and ROS1 Inhibitor Oncogenic NTRK or ROS1 gene fusions can result in hyper-proliferation and survival in tumor cells that can be targeted and inhibited by entrectinib Tissue Agnostic (NTRK) Breakthrough Therapy Designation Orphan Drug Designation Dx: Trailblaze Pharos Companion diagnostic testing to ensure appropriate patient selection for entrectinib CLIA-certified, CAP-accredited, NYSDOH (pending); IDE approval; EAP designation; CE marked RNA-based NGS assay that detects oncogenic NTRK and ROS1 gene fusions Detects and centrally confirms established and novel gene fusion partners in multiple tumor histologies
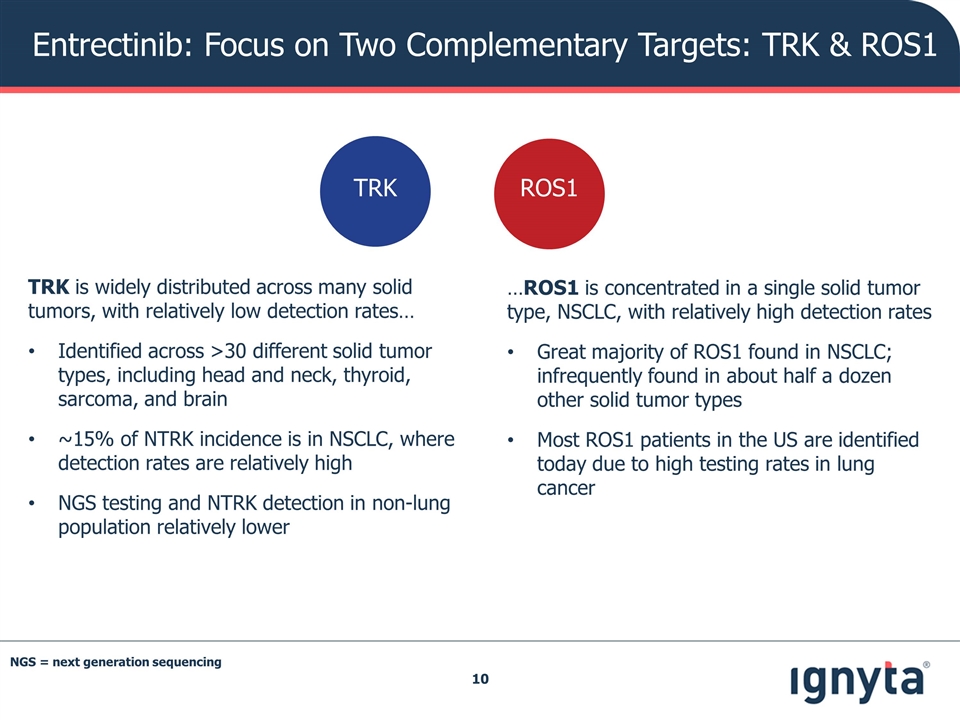
Entrectinib: Focus on Two Complementary Targets: TRK & ROS1 TRK is widely distributed across many solid tumors, with relatively low detection rates… Identified across >30 different solid tumor types, including head and neck, thyroid, sarcoma, and brain ~15% of NTRK incidence is in NSCLC, where detection rates are relatively high NGS testing and NTRK detection in non-lung population relatively lower …ROS1 is concentrated in a single solid tumor type, NSCLC, with relatively high detection rates Great majority of ROS1 found in NSCLC; infrequently found in about half a dozen other solid tumor types Most ROS1 patients in the US are identified today due to high testing rates in lung cancer ROS1 TRK NGS = next generation sequencing

FDA Feedback on Proposed Registration Plan for Entrectinib in “ROS1 Fusion-Positive, Locally Advanced or Metastatic, NSCLC” Based on written feedback from Thoracic Malignancies team that will be responsible for review of the ROS1 NSCLC NDA: Registrational dataset will derive from STARTRK-2, STARTRK-1 and ALKA studies No additional studies were requested Primary endpoint: RECIST v1.1 ORR, based on blinded independent central review Follow-up >12 months required for all responders to assess durability of response Guidance provided for inclusion of CNS efficacy data in entrectinib prescribing information Guidance provided regarding proposed safety database size Based on this FDA feedback, Ignyta has completed enrollment of the registration efficacy data set of more than 50 patients that will be included in the ROS1 NSCLC NDA in 2H18 In total, we have treated more than 70 ROS1 fusion-positive NSCLC patients with entrectinib across STARTRK-2, STARTRK-1, and ALKA studies We therefore remain on track to submit an NDA filing in the ROS1 NSCLC indication in 2H18 Data from current studies sufficient for potential ROS1 NSCLC approval

New FDA Feedback on Proposed Registration Plan for Entrectinib in “NTRK Fusion-Positive, Locally Advanced or Metastatic Solid Tumors” Based on new written feedback from the Breakthrough Therapy Designation Cross-Disciplinary Project Team that will be responsible for review of the NTRK Tissue-Agnostic NDA: Registrational dataset will derive from STARTRK-2, STARTRK-1, ALKA, and STARTRK-NG studies No additional studies were requested Primary endpoint: RECIST v1.1 ORR, based on blinded independent central review Follow-up >6 months required for all responders to assess durability of response Guidance provided on the potential structure of the NTRK tissue-agnostic label Guidance provided for inclusion of CNS information in entrectinib prescribing information Established coordination between CDRH for PMA and CDER for NTRK NDA Established procedural mechanism for two concurrent NDA filings (NTRK Tissue-Agnostic and ROS1 NSCLC) Ignyta has now completed enrollment of sufficient numbers of patients necessary to support NDA filings in both the TRK tissue-agnostic and ROS1 NSCLC indications

New FDA Feedback on Proposed Registration Plan for Trailblaze Pharos Ignyta believes the optimal way to develop a targeted therapy is in parallel with a companion diagnostic for appropriate patient selection Recently conducted a joint meeting with CDRH and CDER to discuss the companion diagnostic strategy for entrectinib Proposed intended use of Trailblaze Pharos: to be used as an aid in selecting patients with solid tumors that harbor an NTRK1/2/3 gene fusion or in selecting patients with non-small cell lung cancer (NSCLC) that harbor a ROS1 gene fusion for whom entrectinib therapy may be appropriate. Confirmed adequacy of analytical methods plans Confirmed timing of dual NDA and premarket approval (PMA) filings Current PMA filing plan and timeline are tracking with dual NDA filing timelines, and should not be rate-limiting to the potential approval of the drug NDA(s)
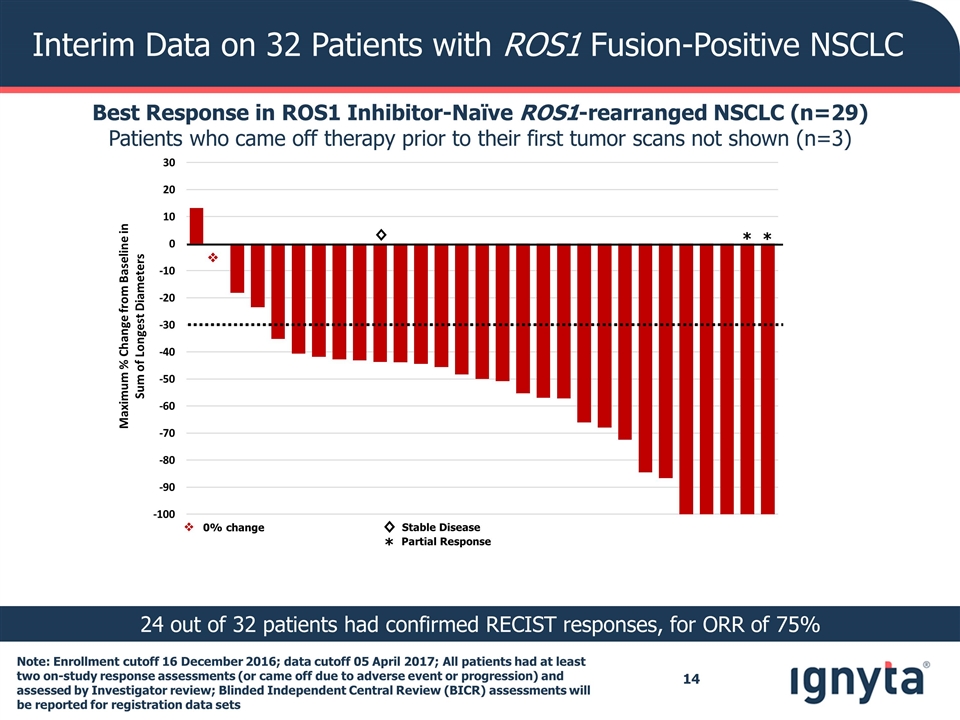
Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review (BICR) assessments will be reported for registration data sets Interim Data on 32 Patients with ROS1 Fusion-Positive NSCLC Best Response in ROS1 Inhibitor-Naïve ROS1-rearranged NSCLC (n=29) Patients who came off therapy prior to their first tumor scans not shown (n=3) 24 out of 32 patients had confirmed RECIST responses, for ORR of 75% v v 0% change Maximum % Change from Baseline in Sum of Longest Diameters ◊ * * ◊ * Stable Disease Partial Response
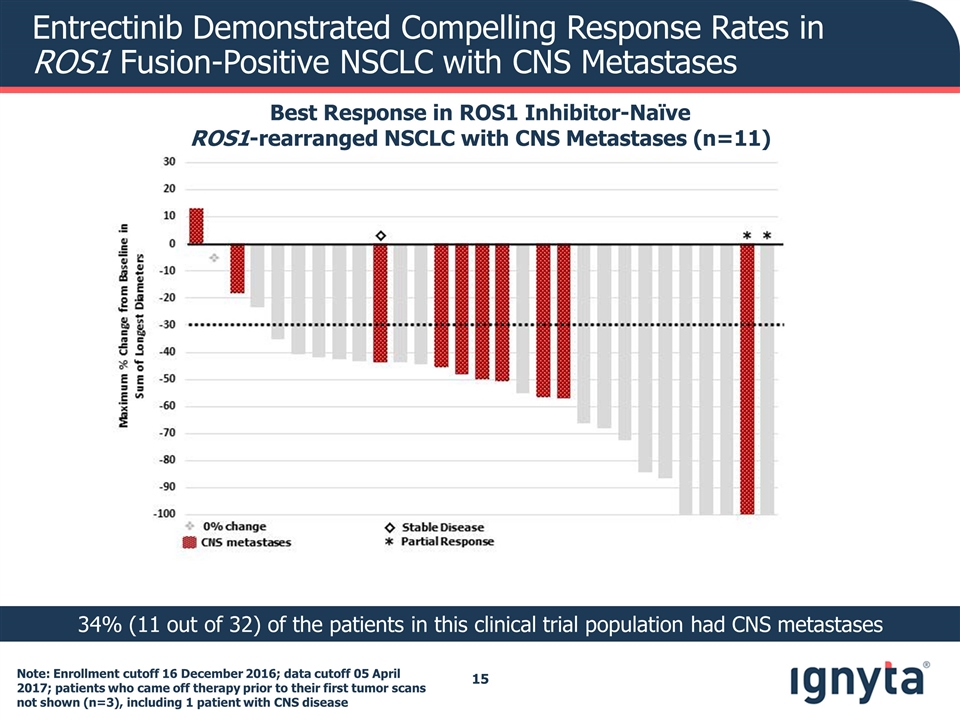
Entrectinib Demonstrated Compelling Response Rates in ROS1 Fusion-Positive NSCLC with CNS Metastases Best Response in ROS1 Inhibitor-Naïve ROS1-rearranged NSCLC with CNS Metastases (n=11) Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; patients who came off therapy prior to their first tumor scans not shown (n=3), including 1 patient with CNS disease 34% (11 out of 32) of the patients in this clinical trial population had CNS metastases
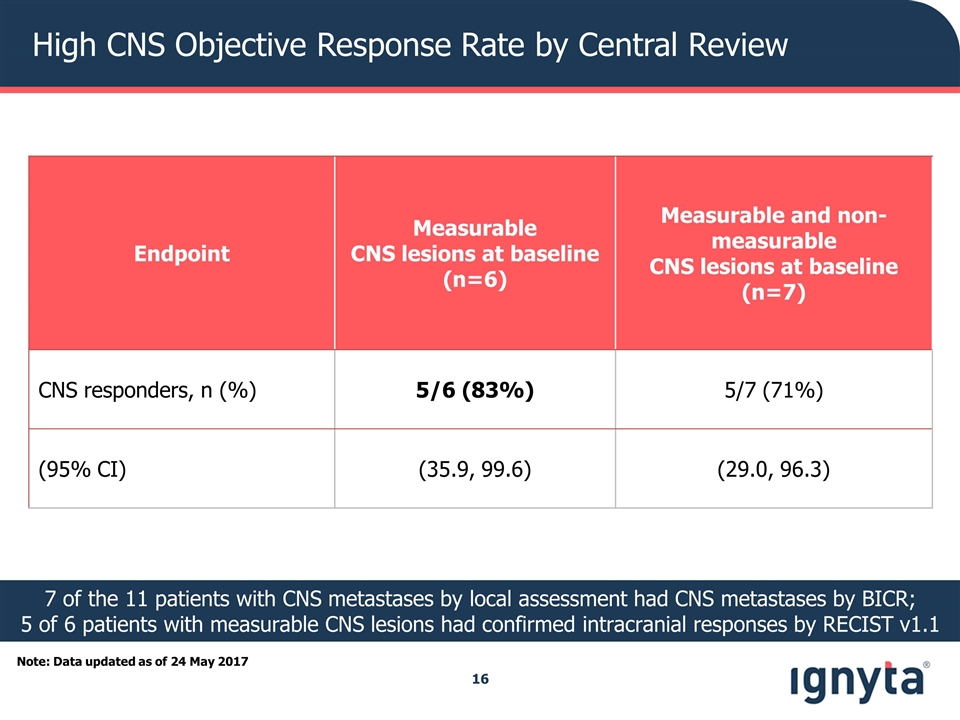
Note: Data updated as of 24 May 2017 Endpoint Measurable CNS lesions at baseline (n=6) Measurable and non-measurable CNS lesions at baseline (n=7) CNS responders, n (%) 5/6 (83%) 5/7 (71%) (95% CI) (35.9, 99.6) (29.0, 96.3) 7 of the 11 patients with CNS metastases by local assessment had CNS metastases by BICR; 5 of 6 patients with measurable CNS lesions had confirmed intracranial responses by RECIST v1.1 High CNS Objective Response Rate by Central Review
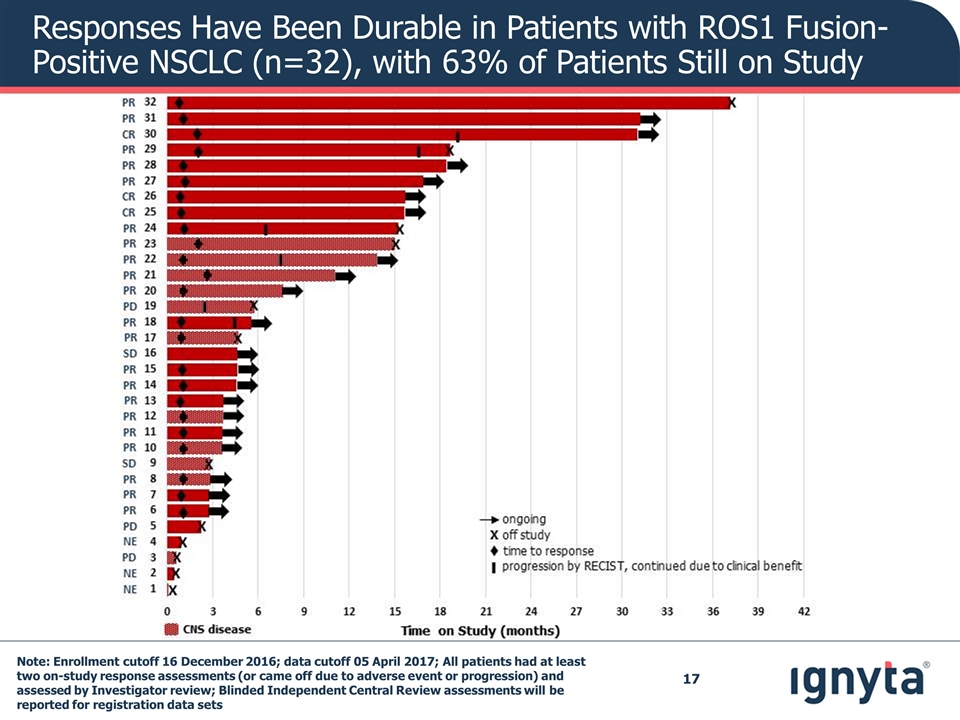
Responses Have Been Durable in Patients with ROS1 Fusion-Positive NSCLC (n=32), with 63% of Patients Still on Study Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review assessments will be reported for registration data sets
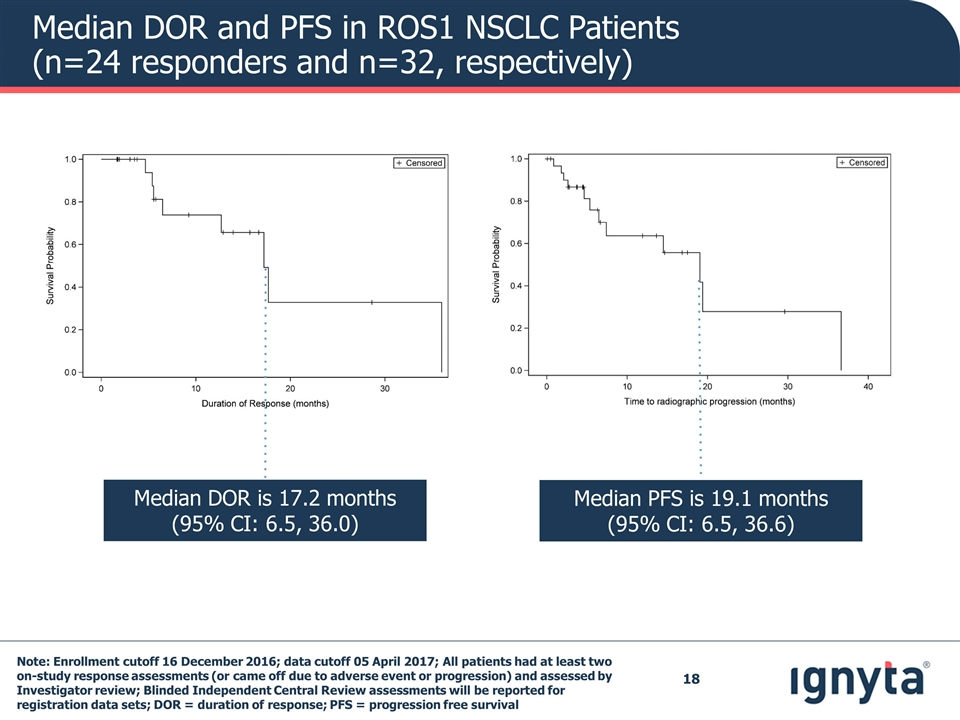
Median DOR and PFS in ROS1 NSCLC Patients (n=24 responders and n=32, respectively) Median PFS is 19.1 months (95% CI: 6.5, 36.6) Median DOR is 17.2 months (95% CI: 6.5, 36.0) Note: Enrollment cutoff 16 December 2016; data cutoff 05 April 2017; All patients had at least two on-study response assessments (or came off due to adverse event or progression) and assessed by Investigator review; Blinded Independent Central Review assessments will be reported for registration data sets; DOR = duration of response; PFS = progression free survival
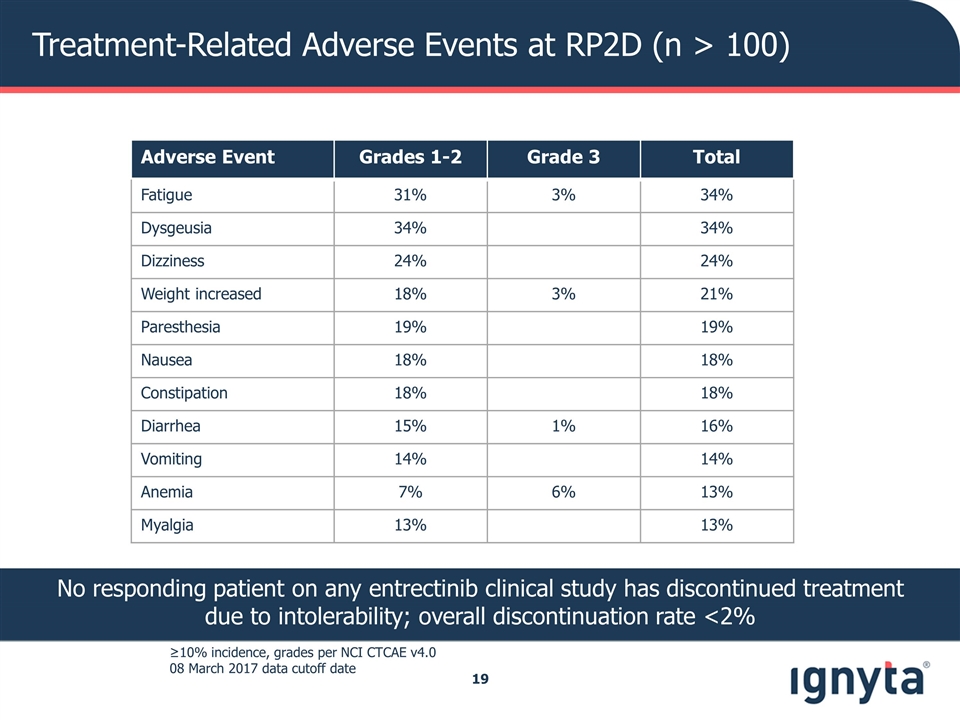
Treatment-Related Adverse Events at RP2D (n > 100) ≥10% incidence, grades per NCI CTCAE v4.0 08 March 2017 data cutoff date No responding patient on any entrectinib clinical study has discontinued treatment due to intolerability; overall discontinuation rate <2% Adverse Event Grades 1-2 Grade 3 Total Fatigue 31% 3% 34% Dysgeusia 34% 34% Dizziness 24% 24% Weight increased 18% 3% 21% Paresthesia 19% 19% Nausea 18% 18% Constipation 18% 18% Diarrhea 15% 1% 16% Vomiting 14% 14% Anemia 7% 6% 13% Myalgia 13% 13%
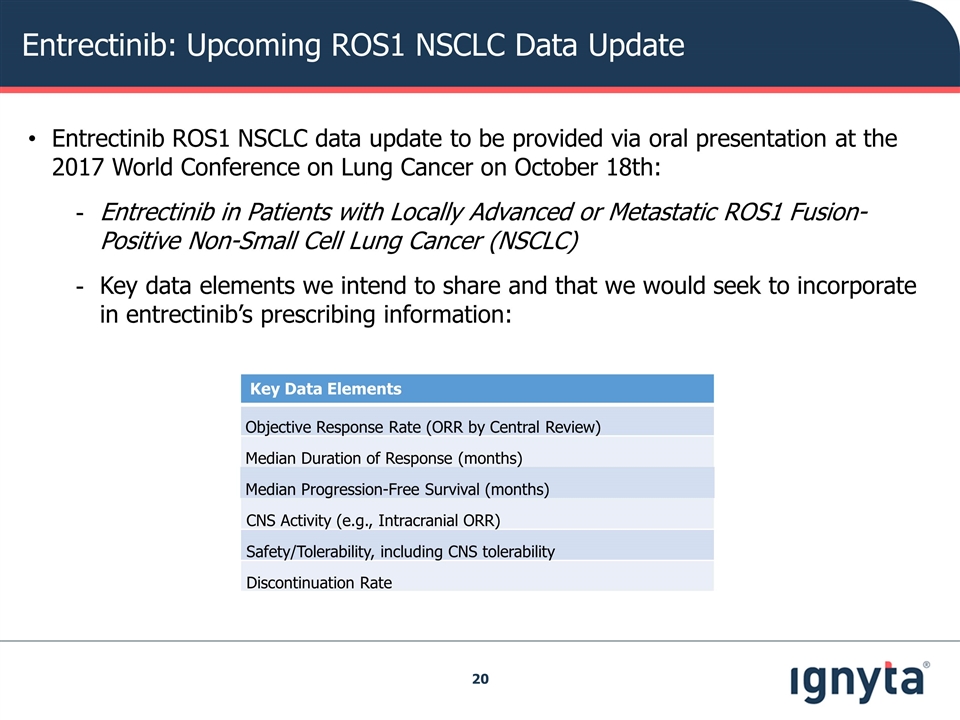
Entrectinib: Upcoming ROS1 NSCLC Data Update Key Data Elements Objective Response Rate (ORR by Central Review) Median Duration of Response (months) Median Progression-Free Survival (months) CNS Activity (e.g., Intracranial ORR) Safety/Tolerability, including CNS tolerability Discontinuation Rate Entrectinib ROS1 NSCLC data update to be provided via oral presentation at the 2017 World Conference on Lung Cancer on October 18th: Entrectinib in Patients with Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small Cell Lung Cancer (NSCLC) Key data elements we intend to share and that we would seek to incorporate in entrectinib’s prescribing information:

2017 & 2018 Anticipated Corporate Milestones & Clinical Updates 2030 Vision Entrectinib STARTRK-1/ALKA Ph 1 data update, 1Q17 STARTRK-2 interim data (after consultation with FDA) + entrectinib commercial roadmap, 2Q17 Complete TRK and ROS1 enrollment of STARTRK-2, 3Q17 Announce interim ROS1 NSCLC data from STARTRK-2, 4Q17 Announce top-line NDA registration-enabling data (after consultation with FDA), 1H18 Submit TRK Tissue-Agnostic and ROS1 NSCLC NDAs (and PMA) with US FDA, 2018 Pipeline RXDX-105 Ph 1b basket study data update, ESMO 2017 RXDX-106 Ph 1 trial initiation, 2H17 RXDX-106 preliminary Ph 1 data, 2H18 2011 – 2015 Advance clinical pipeline 2016 – 2020 Commercialize Rx/Dx products 2021 – 2025 Scale pipeline revenue 2026 – 2030 Drive sustainable profitability Leading Precision Medicine Company that Eradicates Residual Disease ✔ ✔ ✔ ✔




















