
Entrectinib in ROS1 NSCLC Update Conference Call World Conference on Lung Cancer October 18th, 2017 Exhibit 99.2

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: references to the development of and path to potential regulatory approval of entrectinib and our other product candidates, including potential differentiating factors; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs, and the corporate milestones and timelines associated with such programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; references to the potential market size for our products; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments and changes in our plans to develop and commercialize our product candidates; our dependence on third party manufacturers for supply of our product candidates and any approved products; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs and ability to raise any additional funding to pursue our business and product development plans; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2017, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.

Clinical Context for Unmet Medical Need in ROS1+ NSCLC

Clinical Context for Unmet Medical Need in ROS1+ NSCLC Therapies that proactively address both systemic and CNS disease, before the emergence of CNS lesions, are increasingly being used as first-line therapy in place of agents that lack CNS activity. Currently, there is no CNS-active TKI approved in ROS1+ NSCLC, representing an important unmet medical need There is a strong linkage between the CNS activity of CNS-penetrant compounds and duration of treatment (e.g., median PFS), as evidenced by the experience in ALK+ NSCLC In ROS1+ NSCLC, crizotinib currently is the only approved therapy for this indication, but has low CNS activity; the clinical experience of “real world” durability has been consistent with this low CNS activity In addition to CNS activity, a manageable CNS AE profile is necessary for long-term therapy use
Importance of CNS-Active Agents for First-Line Therapy in Solid Tumor Malignancies, Including NSCLC Sources: Fokas et al, BBA - Reviews on Cancer 2013; Chi et al, Cancers 2010; IASLC 2017; ALEX study, ASCO 2017 CNS metastases in NSCLC Present in many patients at initial diagnosis (up to 50%) Where not present initially, many patients will still progress and come off therapy due to subsequent development of CNS disease First-line therapy that addresses CNS disease has been demonstrated to result in better clinical outcomes Therapies that proactively address both systemic and CNS disease, before the emergence of CNS lesions, are increasingly being used as first-line therapy in place of agents that lack CNS activity CNS metastases 20-40% of all patients with cancer Lung Breast Melanoma
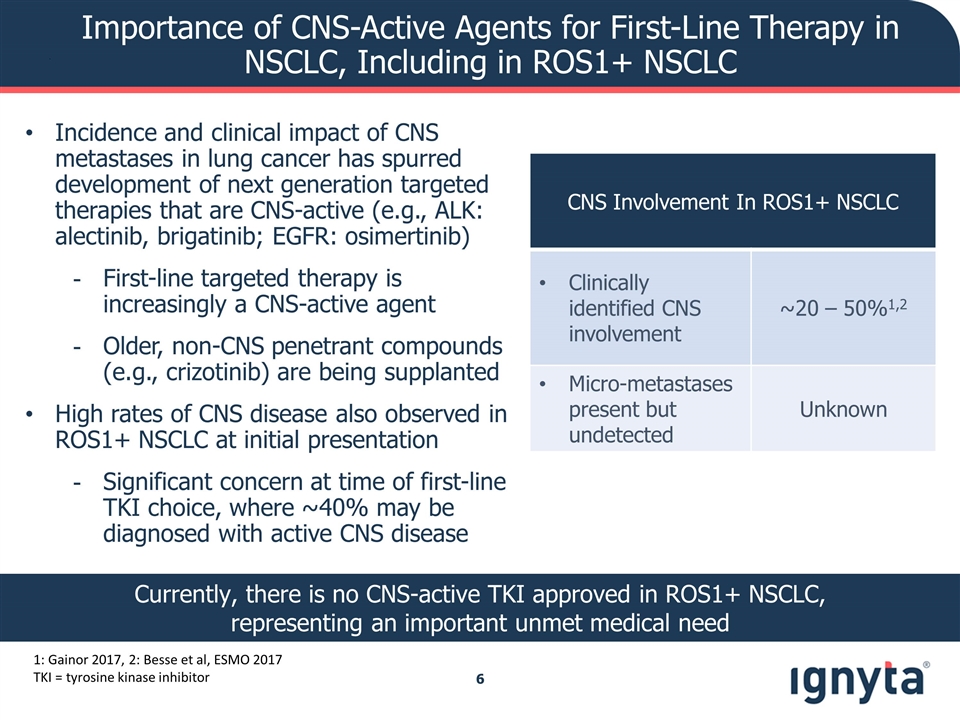
1: Gainor 2017, 2: Besse et al, ESMO 2017 TKI = tyrosine kinase inhibitor Incidence and clinical impact of CNS metastases in lung cancer has spurred development of next generation targeted therapies that are CNS-active (e.g., ALK: alectinib, brigatinib; EGFR: osimertinib) First-line targeted therapy is increasingly a CNS-active agent Older, non-CNS penetrant compounds (e.g., crizotinib) are being supplanted High rates of CNS disease also observed in ROS1+ NSCLC at initial presentation Significant concern at time of first-line TKI choice, where ~40% may be diagnosed with active CNS disease CNS Involvement In ROS1+ NSCLC Clinically identified CNS involvement ~20 – 50%1,2 Micro-metastases present but undetected Unknown Importance of CNS-Active Agents for First-Line Therapy in NSCLC, Including in ROS1+ NSCLC Currently, there is no CNS-active TKI approved in ROS1+ NSCLC, representing an important unmet medical need
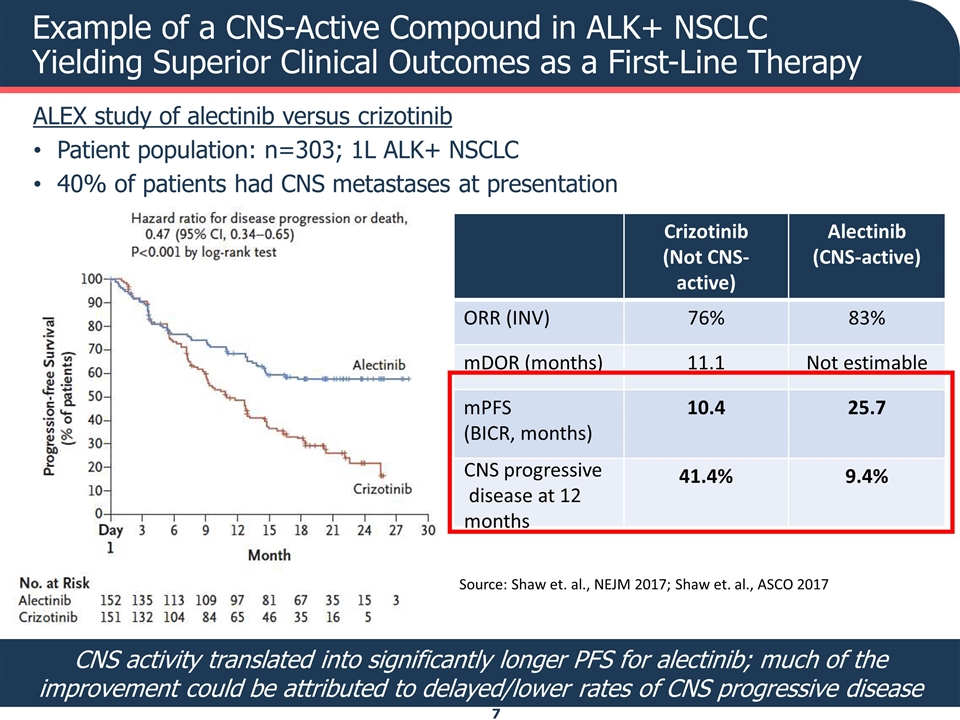
Example of a CNS-Active Compound in ALK+ NSCLC Yielding Superior Clinical Outcomes as a First-Line Therapy ALEX study of alectinib versus crizotinib Patient population: n=303; 1L ALK+ NSCLC 40% of patients had CNS metastases at presentation Crizotinib (Not CNS-active) Alectinib (CNS-active) ORR (INV) 76% 83% mDOR (months) 11.1 Not estimable mPFS (BICR, months) 10.4 25.7 41.4% 9.4% CNS activity translated into significantly longer PFS for alectinib; much of the improvement could be attributed to delayed/lower rates of CNS progressive disease Source: Shaw et. al., NEJM 2017; Shaw et. al., ASCO 2017 CNS progressive disease at 12 months

Nonclinical properties Costa et al, JCO 20111 (Pfizer co-authored publication): Measured plasma and CSF levels of crizotinib in a patient with ALK+ NSCLC metastatic to the brain Low measured CSF-to-plasma ratio: 0.0026 ”In summary, crizotinib seems to penetrate poorly the blood-brain barrier, and this pharmacokinetic property may hinder the anticancer effect of this drug in metastatic brain tumours.” Clinical design of PROFILE 1001 registrational study of crizotinib in ROS1+ NSCLC Shaw et al2 prospective trial as part of multicenter, global Profile 1001 study Single arm cohort in 50 patients with ROS1+ NSCLC No mention of CNS involvement at study entry and no CNS endpoints reported Patients with CNS disease were required to have all brain metastases treated or stable for 2 weeks before study entry Clinical evidence of CNS as one of the most common sites of progression on crizotinib in ROS1+ NSCLC MGH retrospective experience3 Cumulative incidence of CNS progression overall was 34% at 5 years after initial metastatic diagnosis, and 22% in those patients without CNS metastasis at initial presentation 1Costa et al, JCO 2011 2Shaw et al, NEJM 2014 3Gainor et al, 2017 Clinical Context in ROS1+ NSCLC: Approved ROS1+ NSCLC Therapy with Low CNS Activity
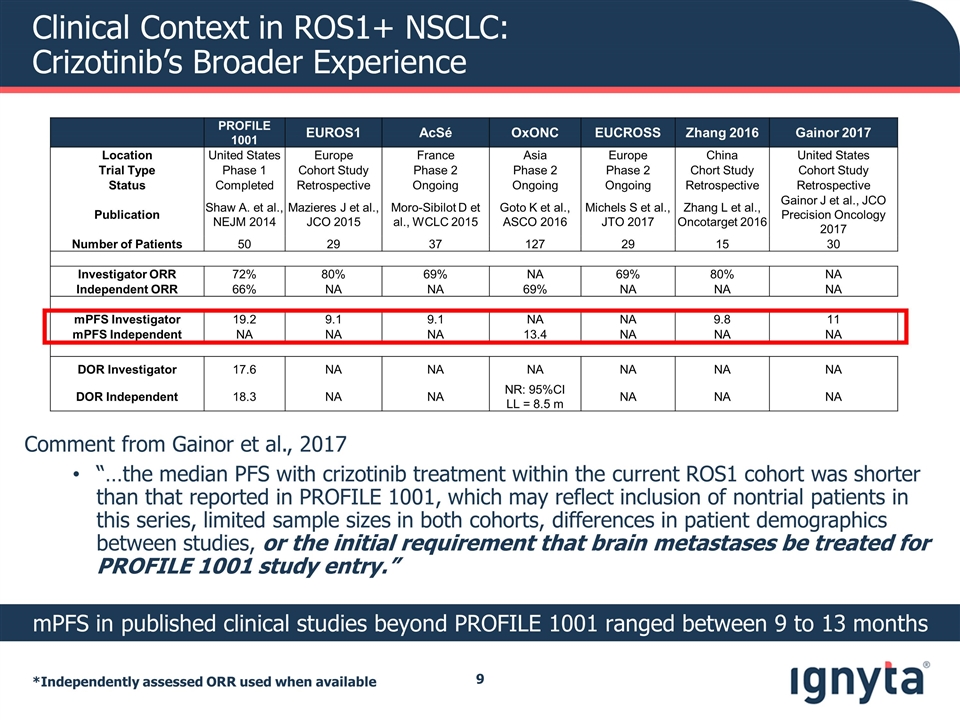
Clinical Context in ROS1+ NSCLC: Crizotinib’s Broader Experience *Independently assessed ORR used when available PROFILE 1001 EUROS1 AcSé OxONC EUCROSS Zhang 2016 Gainor 2017 Location United States Europe France Asia Europe China United States Trial Type Phase 1 Cohort Study Phase 2 Phase 2 Phase 2 Chort Study Cohort Study Status Completed Retrospective Ongoing Ongoing Ongoing Retrospective Retrospective Publication Shaw A. et al., NEJM 2014 Mazieres J et al., JCO 2015 Moro-Sibilot D et al., WCLC 2015 Goto K et al., ASCO 2016 Michels S et al., JTO 2017 Zhang L et al., Oncotarget 2016 Gainor J et al., JCO Precision Oncology 2017 Number of Patients 50 29 37 127 29 15 30 Investigator ORR 72% 80% 69% NA 69% 80% NA Independent ORR 66% NA NA 69% NA NA NA mPFS Investigator 19.2 9.1 9.1 NA NA 9.8 11 mPFS Independent NA NA NA 13.4 NA NA NA DOR Investigator 17.6 NA NA NA NA NA NA DOR Independent 18.3 NA NA NR: 95%CI LL = 8.5 m NA NA NA mPFS in published clinical studies beyond PROFILE 1001 ranged between 9 to 13 months Comment from Gainor et al., 2017 “…the median PFS with crizotinib treatment within the current ROS1 cohort was shorter than that reported in PROFILE 1001, which may reflect inclusion of nontrial patients in this series, limited sample sizes in both cohorts, differences in patient demographics between studies, or the initial requirement that brain metastases be treated for PROFILE 1001 study entry.”
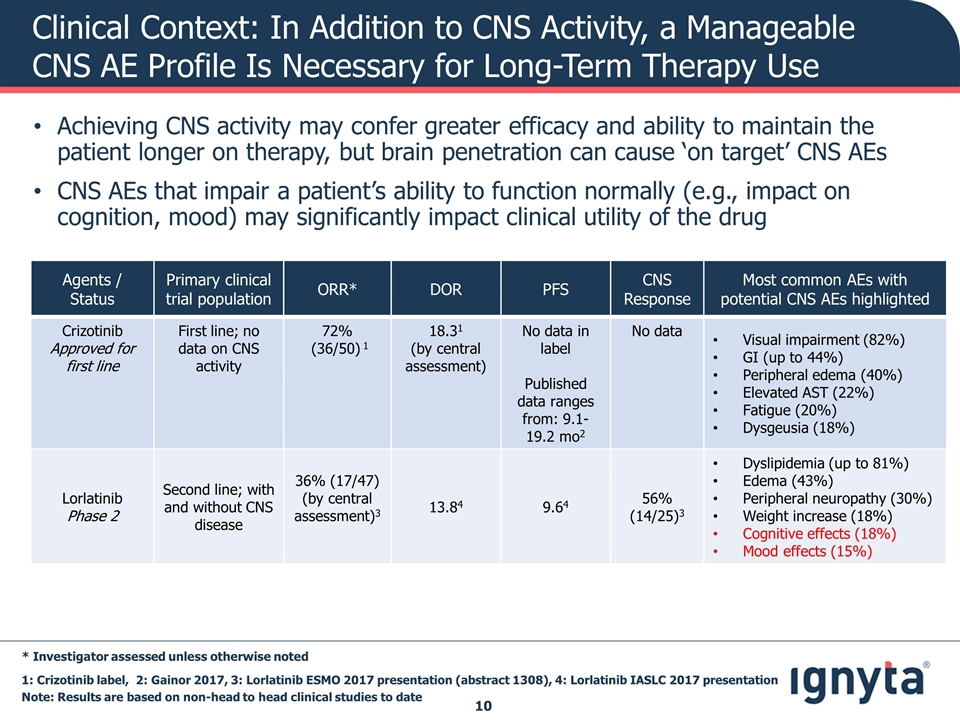
Clinical Context: In Addition to CNS Activity, a Manageable CNS AE Profile Is Necessary for Long-Term Therapy Use Agents / Status Primary clinical trial population ORR* DOR PFS CNS Response Most common AEs with potential CNS AEs highlighted Crizotinib Approved for first line First line; no data on CNS activity 72% (36/50) 1 18.31 (by central assessment) No data in label Published data ranges from: 9.1-19.2 mo2 No data Visual impairment (82%) GI (up to 44%) Peripheral edema (40%) Elevated AST (22%) Fatigue (20%) Dysgeusia (18%) Lorlatinib Phase 2 Second line; with and without CNS disease 36% (17/47) (by central assessment)3 13.84 9.64 56% (14/25)3 Dyslipidemia (up to 81%) Edema (43%) Peripheral neuropathy (30%) Weight increase (18%) Cognitive effects (18%) Mood effects (15%) * Investigator assessed unless otherwise noted Note: Results are based on non-head to head clinical studies to date Achieving CNS activity may confer greater efficacy and ability to maintain the patient longer on therapy, but brain penetration can cause ‘on target’ CNS AEs CNS AEs that impair a patient’s ability to function normally (e.g., impact on cognition, mood) may significantly impact clinical utility of the drug 1: Crizotinib label, 2: Gainor 2017, 3: Lorlatinib ESMO 2017 presentation (abstract 1308), 4: Lorlatinib IASLC 2017 presentation

Clinical Context for Unmet Medical Need in ROS1+ NSCLC Therapies that proactively address both systemic and CNS disease, before the emergence of CNS lesions, are increasingly being used as first-line therapy in place of agents that lack CNS activity. Currently, there is no CNS-active TKI approved in ROS1+ NSCLC, representing an important unmet medical need There is a strong linkage between the CNS activity of CNS-penetrant compounds and duration of treatment (e.g., median PFS), as evidenced by the experience in ALK+ NSCLC In ROS1+ NSCLC, crizotinib currently is the only approved therapy for this indication, but has low CNS activity; the clinical experience of “real world” durability has been consistent with this low CNS activity In addition to CNS activity, a manageable CNS AE profile is necessary for long-term therapy use

Opportunity for Entrectinib in ROS1+ NSCLC
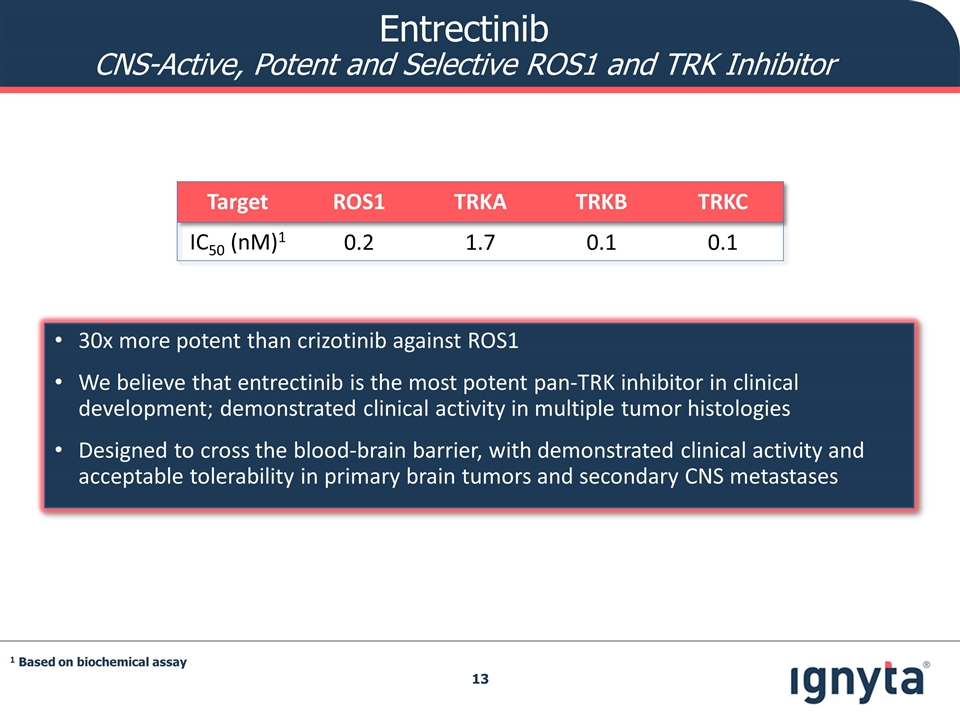
Entrectinib CNS-Active, Potent and Selective ROS1 and TRK Inhibitor 30x more potent than crizotinib against ROS1 We believe that entrectinib is the most potent pan-TRK inhibitor in clinical development; demonstrated clinical activity in multiple tumor histologies Designed to cross the blood-brain barrier, with demonstrated clinical activity and acceptable tolerability in primary brain tumors and secondary CNS metastases Target ROS1 TRKA TRKB TRKC IC50 (nM)1 0.2 1.7 0.1 0.1 1 Based on biochemical assay
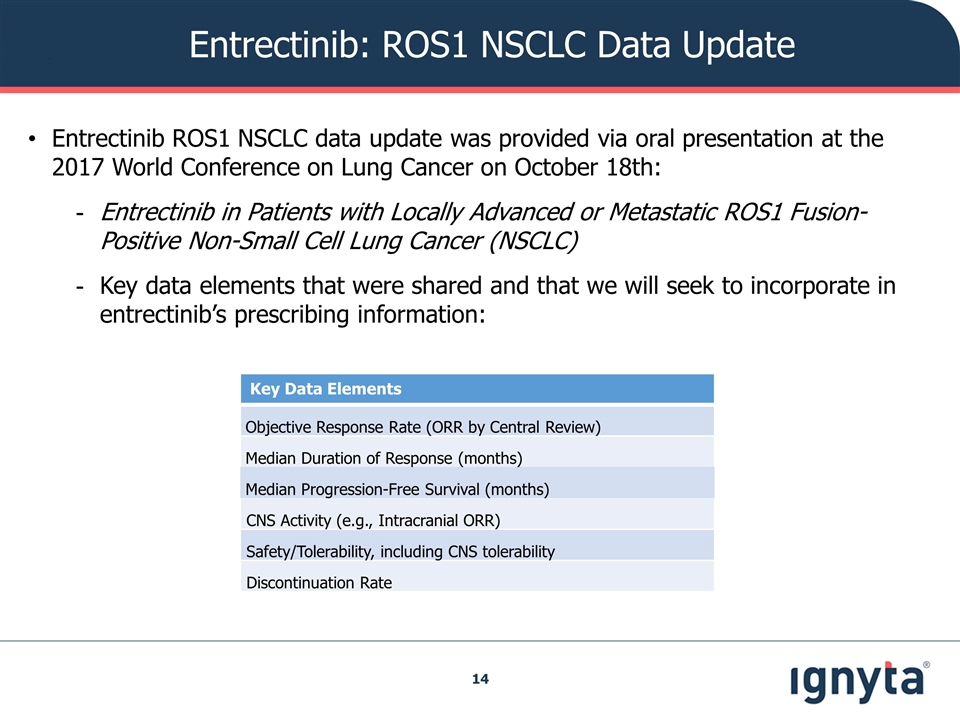
Entrectinib: ROS1 NSCLC Data Update Key Data Elements Objective Response Rate (ORR by Central Review) Median Duration of Response (months) Median Progression-Free Survival (months) CNS Activity (e.g., Intracranial ORR) Safety/Tolerability, including CNS tolerability Discontinuation Rate Entrectinib ROS1 NSCLC data update was provided via oral presentation at the 2017 World Conference on Lung Cancer on October 18th: Entrectinib in Patients with Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small Cell Lung Cancer (NSCLC) Key data elements that were shared and that we will seek to incorporate in entrectinib’s prescribing information:
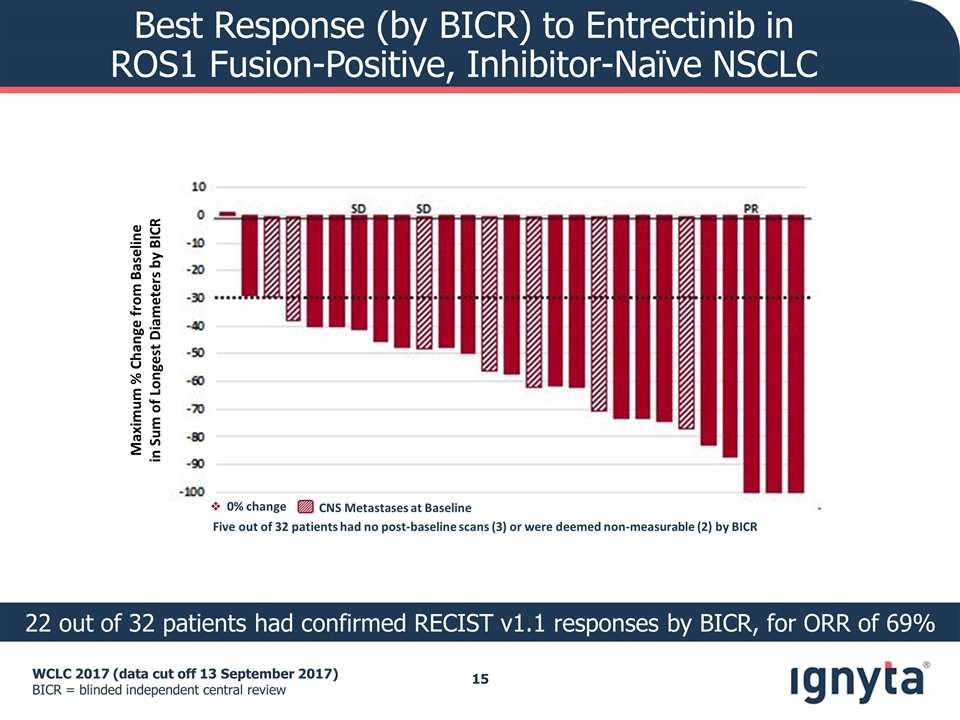
WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Best Response (by BICR) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC 22 out of 32 patients had confirmed RECIST v1.1 responses by BICR, for ORR of 69% SD PR SD Maximum % Change from Baseline in Sum of Longest Diameters by BICR Five out of 32 patients had no post-baseline scans (3) or were deemed non-measurable (2) by BICR CNS Metastases at Baseline v 0% change
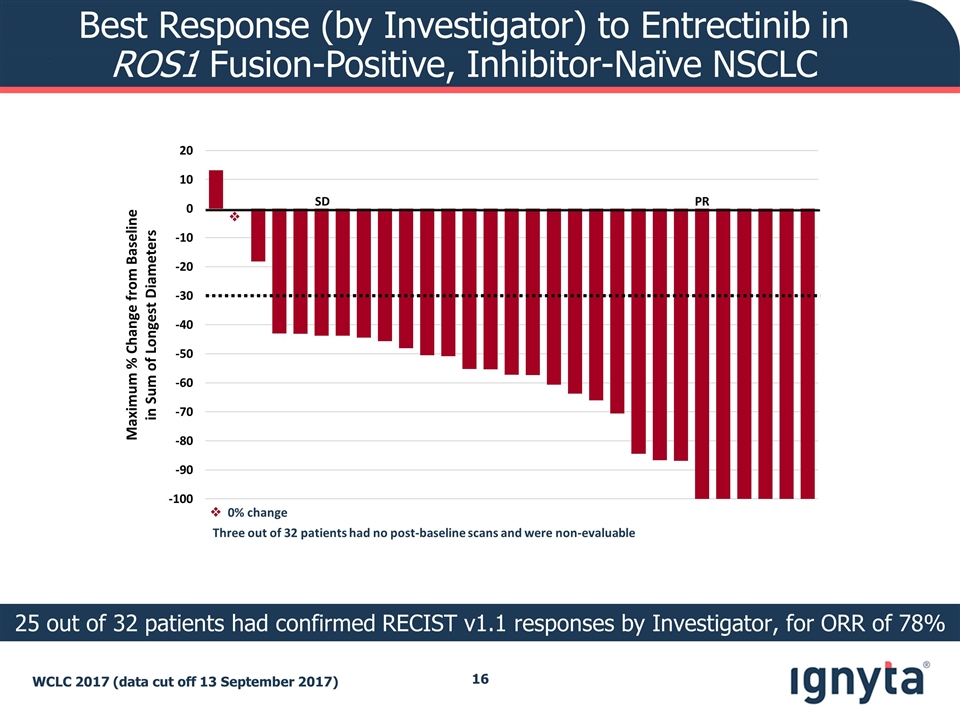
Best Response (by Investigator) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC WCLC 2017 (data cut off 13 September 2017) 25 out of 32 patients had confirmed RECIST v1.1 responses by Investigator, for ORR of 78% Maximum % Change from Baseline in Sum of Longest Diameters PR SD v Three out of 32 patients had no post-baseline scans and were non-evaluable v 0% change
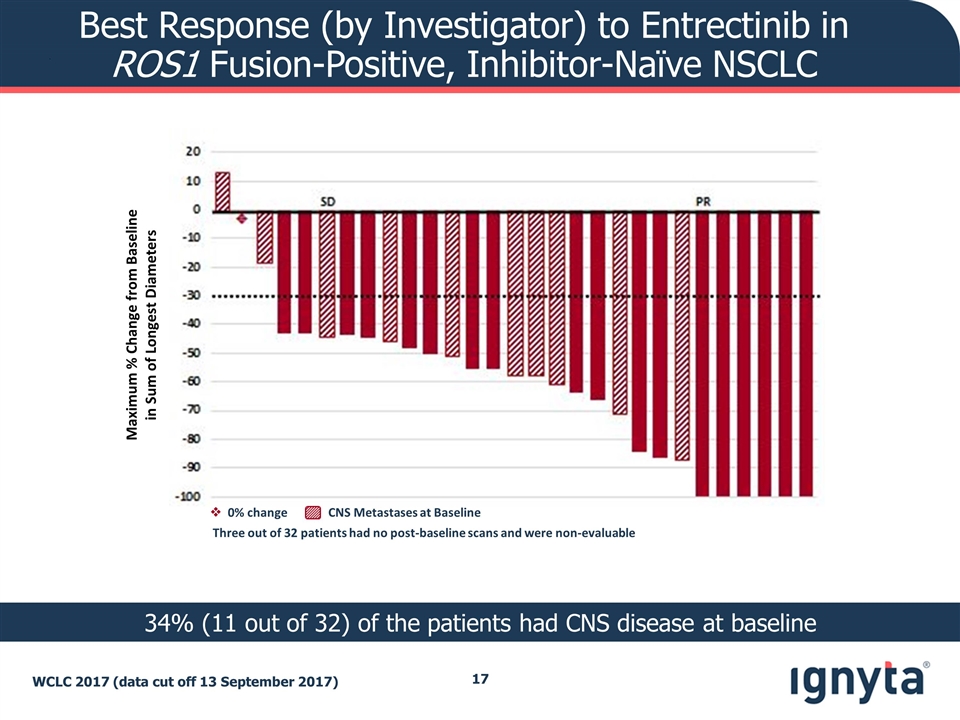
Best Response (by Investigator) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC WCLC 2017 (data cut off 13 September 2017) 34% (11 out of 32) of the patients had CNS disease at baseline Maximum % Change from Baseline in Sum of Longest Diameters Three out of 32 patients had no post-baseline scans and were non-evaluable v 0% change CNS Metastases at Baseline
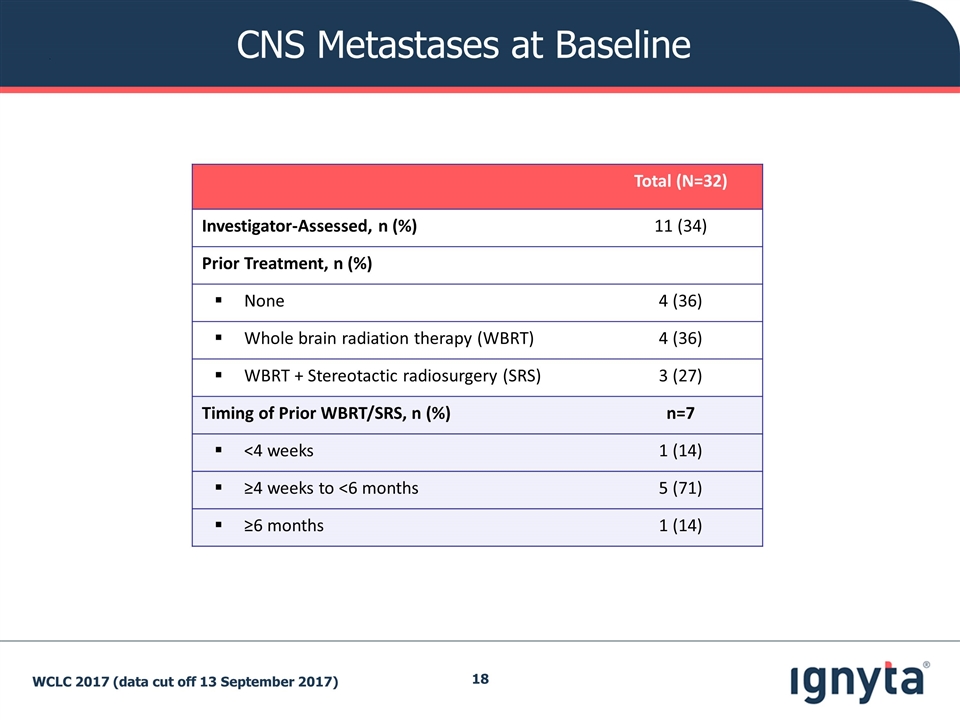
CNS Metastases at Baseline Total (N=32) Investigator-Assessed, n (%) 11 (34) Prior Treatment, n (%) None 4 (36) Whole brain radiation therapy (WBRT) 4 (36) WBRT + Stereotactic radiosurgery (SRS) 3 (27) Timing of Prior WBRT/SRS, n (%) n=7 <4 weeks 1 (14) ≥4 weeks to <6 months 5 (71) ≥6 months 1 (14) WCLC 2017 (data cut off 13 September 2017)
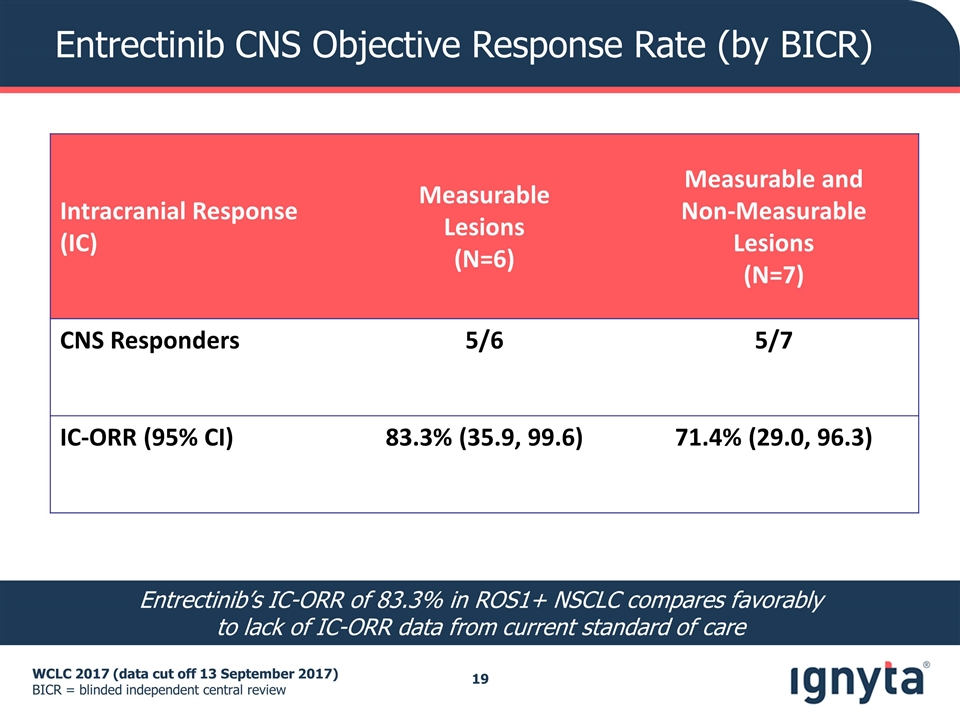
Entrectinib CNS Objective Response Rate (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Intracranial Response (IC) Measurable Lesions (N=6) Measurable and Non-Measurable Lesions (N=7) CNS Responders 5/6 5/7 IC-ORR (95% CI) 83.3% (35.9, 99.6) 71.4% (29.0, 96.3) Entrectinib’s IC-ORR of 83.3% in ROS1+ NSCLC compares favorably to lack of IC-ORR data from current standard of care
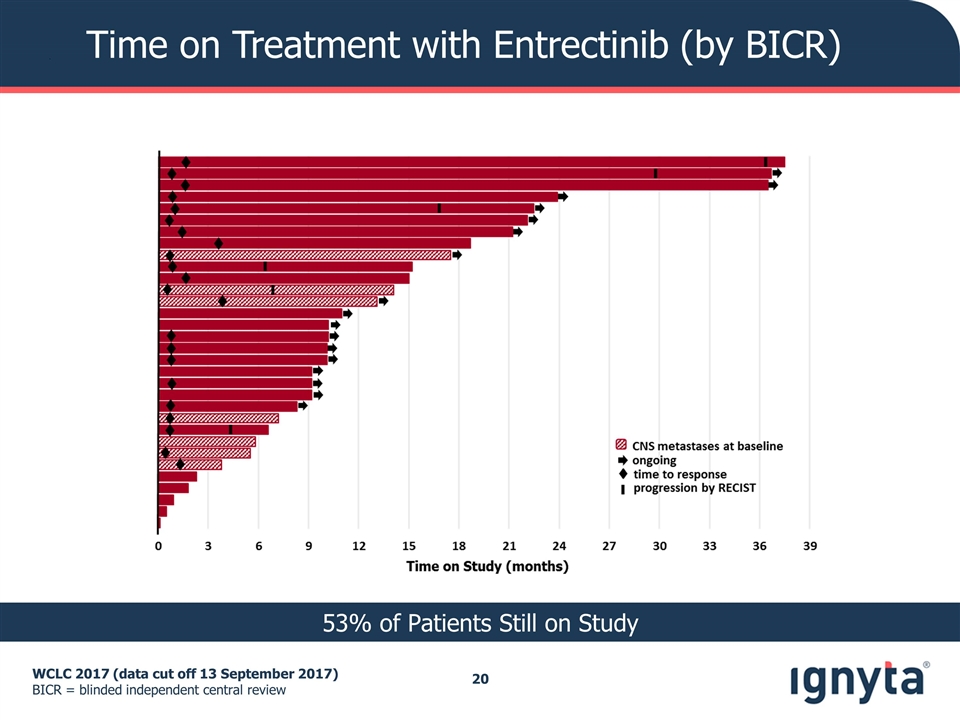
Time on Treatment with Entrectinib (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review 53% of Patients Still on Study
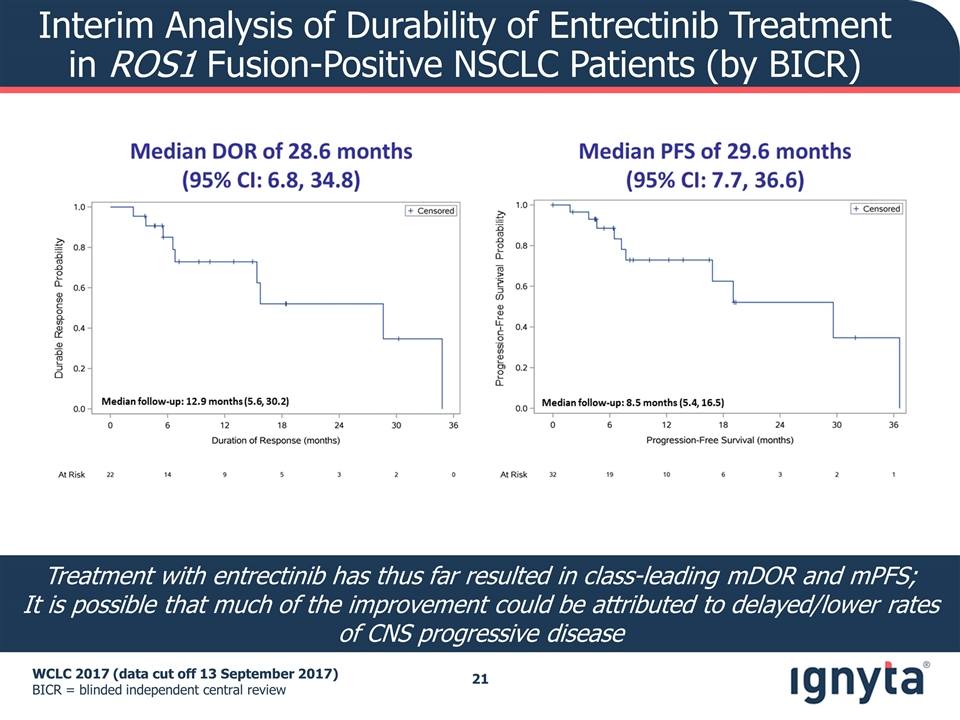
Interim Analysis of Durability of Entrectinib Treatment in ROS1 Fusion-Positive NSCLC Patients (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Treatment with entrectinib has thus far resulted in class-leading mDOR and mPFS; It is possible that much of the improvement could be attributed to delayed/lower rates of CNS progressive disease
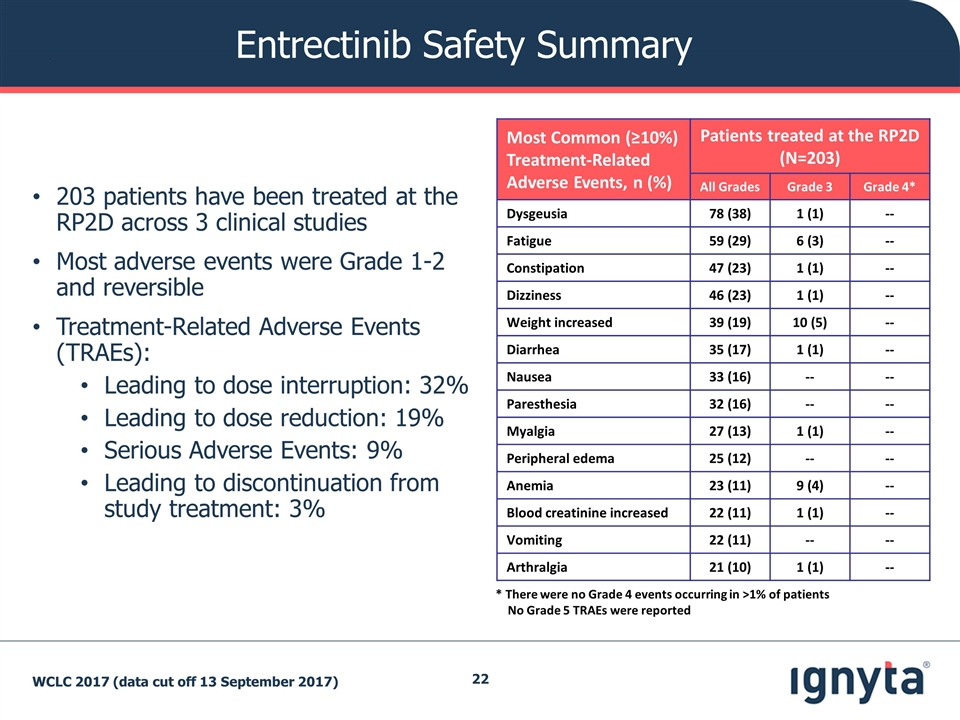
Entrectinib Safety Summary 203 patients have been treated at the RP2D across 3 clinical studies Most adverse events were Grade 1-2 and reversible Treatment-Related Adverse Events (TRAEs): Leading to dose interruption: 32% Leading to dose reduction: 19% Serious Adverse Events: 9% Leading to discontinuation from study treatment: 3% Most Common (≥10%) Treatment-Related Adverse Events, n (%) Patients treated at the RP2D (N=203) All Grades Grade 3 Grade 4* Dysgeusia 78 (38) 1 (1) -- Fatigue 59 (29) 6 (3) -- Constipation 47 (23) 1 (1) -- Dizziness 46 (23) 1 (1) -- Weight increased 39 (19) 10 (5) -- Diarrhea 35 (17) 1 (1) -- Nausea 33 (16) -- -- Paresthesia 32 (16) -- -- Myalgia 27 (13) 1 (1) -- Peripheral edema 25 (12) -- -- Anemia 23 (11) 9 (4) -- Blood creatinine increased 22 (11) 1 (1) -- Vomiting 22 (11) -- -- Arthralgia 21 (10) 1 (1) -- * There were no Grade 4 events occurring in >1% of patients No Grade 5 TRAEs were reported WCLC 2017 (data cut off 13 September 2017)
Opportunity for Entrectinib to Potentially Address Unmet Medical Need in ROS1+ NSCLC Based on the interim efficacy (inclusive of CNS activity) and safety data of entrectinib as a potential first-line targeted therapy in ROS1+ NSCLC, we believe that entrectinib fulfills an important unmet medical need in this indication Competitive ORR Compelling CNS activity Significant durability as measured by mDOR and mPFS Compelling overall safety/tolerability, including good CNS tolerability This profile could position entrectinib as a potential best-in-class first-line targeted therapy option for patients with ROS1+ NSCLC

ROS1 Market Represents a Highly Attractive Commercial Target Population of ~2,000 ROS1+ patients in the US Similar incidence rate yields ~3,100 ROS1+ patients in the EU Assumes 24 month duration of therapy based on 29.6 months PFS reported at the IASLC 18th World Conference on Lung Cancer Total US + EU TAM ~$1.3B Total Addressable Market ($M)1 ~$720M ~$560M 1: US market value based on crizotinib US price of ~$15k per month; EU value based on 50% of US crizotinib price
Summary of Entrectinib Opportunity in ROS1+ NSCLC WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Based on interim analysis presented at WCLC 2017, entrectinib demonstrates potential best-in-class profile as an investigational first-line targeted agent in ROS1+ NSCLC: Clinically meaningful benefit in ROS1+ NSCLC BICR-ORR of 69%, including 2 complete responses Investigator-assessed ORR of 78% Compelling CNS activity Intracranial BICR-ORR of 83% in patients presenting with measurable CNS disease at baseline Durable benefit in ROS1+ NSCLC Median BICR-DOR of 29 months and median BICR-PFS of 30 months Well tolerated, including in the CNS, in over 200 patients treated at the RP2D Mostly Grade 1-2 reversible treatment-related adverse events 3% of patients treated at RP2D discontinued entrectinib due to treatment-related adverse events New Drug Application for entrectinib in ROS1+ NSCLC anticipated in 2H18 Opportunity to address >5,000 patients globally in ROS1+ NSCLC

2017 & 2018 Anticipated Corporate Milestones & Clinical Updates 2030 Vision Entrectinib STARTRK-1/ALKA Ph 1 data update, 1Q17 STARTRK-2 interim data (after consultation with FDA) + entrectinib commercial roadmap, 2Q17 Complete TRK and ROS1 enrollment of STARTRK-2, 3Q17 Announce interim ROS1 NSCLC data from STARTRK-2, 4Q17 Announce top-line NDA registration-enabling data, 1H18 Submit TRK Tissue-Agnostic and ROS1 NSCLC NDAs (and PMA) with US FDA, 2018 Pipeline RXDX-105 Ph 1b basket study data update, 3Q17 RXDX-106 IND filing, 4Q17 RXDX-106 preliminary Ph 1 data, 2H18 2011 – 2015 Advance clinical pipeline 2016 – 2020 Commercialize Rx/Dx products 2021 – 2025 Scale pipeline revenue 2026 – 2030 Drive sustainable profitability Leading Precision Medicine Company that Eradicates Residual Disease ✔ ✔ ✔ ✔ ✔

























