UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14D-9
Solicitation/Recommendation Statement
under Section 14(d)(4) of the Securities Exchange Act of 1934
IGNYTA, INC.
(Name of Subject Company)
IGNYTA, INC.
(Names of Person(s) Filing Statement)
Common Stock, $0.0001 par value per share
(Title of Class of Securities)
451731103
(CUSIP Number of Class of Securities)
Jonathan E. Lim, M.D.
President and Chief Executive Officer
Ignyta, Inc.
4545 Towne Centre Court
San Diego, California 92121
(858) 255-5959
(Name, address and telephone numbers of person authorized to receive notices and communications
on behalf of the persons filing statement)
with copies to:
Charles K. Ruck
Cheston J. Larson
Michael E. Sullivan
Latham & Watkins LLP
12670 High Bluff Drive
San Diego, California 92130
(858) 523-5400
| ☒ | Check the box if the filing relates solely to preliminary communications made before the commencement of a tender offer. |
On January 9, 2018, the slide presentation set forth below will be presented in various investor meetings at the J.P. Morgan Healthcare Conference by Jonathan E. Lim, M.D., Chairman, President and Chief Executive Officer of Ignyta, Inc. (the “Company”), and Jacob Chacko, M.D., Chief Financial Officer of the Company.

J.P. Morgan Healthcare Conference January 9, 2018

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: references to the development of and path to potential regulatory approval of entrectinib and our other product candidates, including potential differentiating factors; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs, and the corporate milestones and timelines associated with such programs; the potential efficacy of entrectinib and our other product candidates; references to accelerating the Company’s long term vision through a business combination with Roche/Genentech; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; references to the potential market size for our products; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments and changes in our plans to develop and commercialize its product candidates; uncertainties with the timing of a potential business combination with Roche; the effects of disruption caused by a potential business combination with Roche making it more difficult to maintain relationships with employees, collaborators, vendors or other business partners; our dependence on third party manufacturers for supply of our product candidates and any approved products; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs and ability to raise any additional funding to pursue our business and product development plans; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2017, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.

December 21st, 2017, Announcement and Strategic Rationale Transaction Roche and Ignyta announced they have entered into a definitive merger agreement for Roche to fully acquire Ignyta for $27.00 per share in an all-cash transaction Strategic Rationale Ignyta’s focus remains on patients and our long-term vision continues to be to eradicate residual disease – the source of recurrence and relapse – in precisely defined patient populations By combining with Roche/Genentech, Ignyta can accelerate the realization of that vision, since Roche/Genentech is a global leader in oncology and personalized healthcare, with a long and successful track record They were pioneers in this space with the biologics Rituxan and Avastin, and now the more recent targeted small molecules, such as Zelboraf and Alecensa, along with their major presence in the IO space with Tecentriq, an anti-PD-L1 antibody This merger with Roche/Genentech clearly aligns with Ignyta’s development focus and will advance our joint efforts to bring innovative treatments to patients with cancer
Company Highlights Robust pipeline of precision medicine therapies in oncology Entrectinib: Path towards two NDAs in TRK tissue agnostic and ROS1 NSCLC indications in 2018, with anticipated launch in 2019 RXDX-105: Preliminary ORR of 75% (n = 8) in patients with non-KIF5B-RET fusion-positive NSCLC in Ph 1b study RXDX-106: Promising preclinical activity as a single agent and combination agent small molecule immunomodulator; IND cleared by FDA Dec 2017 Integrated approach to Rx/Dx development CAP-accredited, CLIA-certified, QSR-compliant diagnostic lab with multi-modality assays Multiple near-term value inflection points Multiple clinical data readouts and regulatory milestones in next 12 months Experienced leadership team Breadth and depth of expertise in clinical/preclinical development, regulatory affairs, commercial, and other key technical and business disciplines
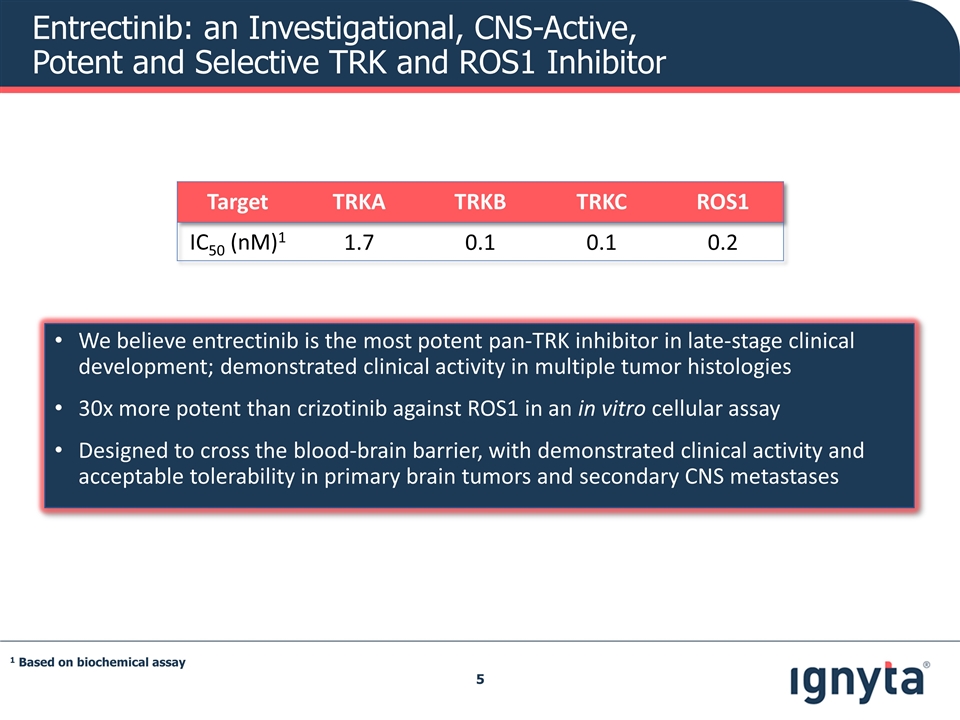
We believe entrectinib is the most potent pan-TRK inhibitor in late-stage clinical development; demonstrated clinical activity in multiple tumor histologies 30x more potent than crizotinib against ROS1 in an in vitro cellular assay Designed to cross the blood-brain barrier, with demonstrated clinical activity and acceptable tolerability in primary brain tumors and secondary CNS metastases Target TRKA TRKB TRKC ROS1 IC50 (nM)1 1.7 0.1 0.1 0.2 1 Based on biochemical assay Entrectinib: an Investigational, CNS-Active, Potent and Selective TRK and ROS1 Inhibitor
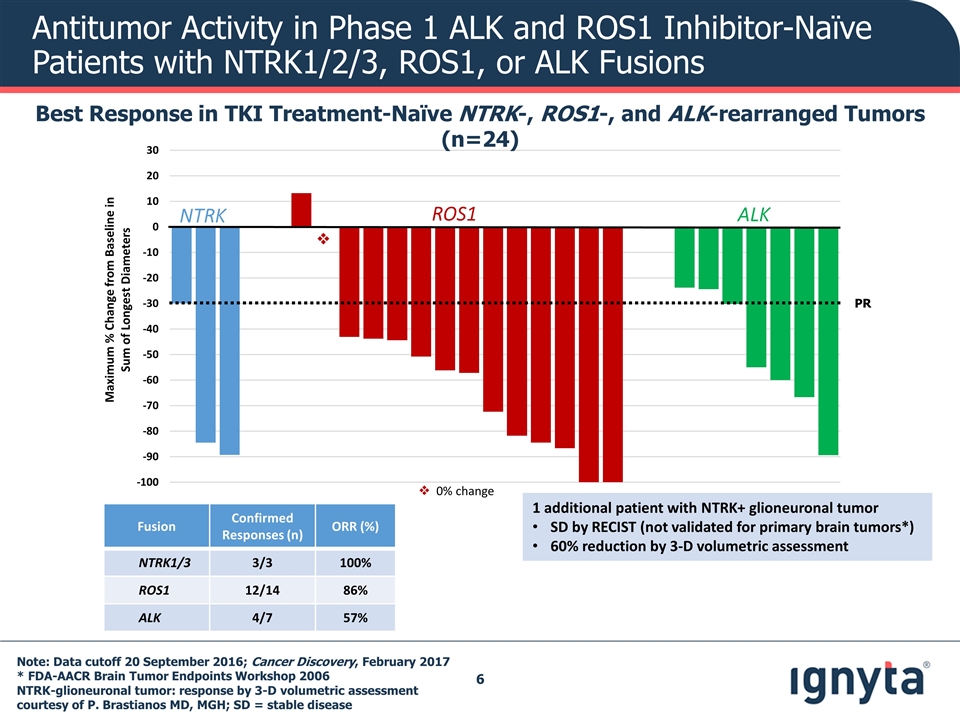
v Maximum % Change from Baseline in Sum of Longest Diameters NTRK ALK ROS1 PR Note: Data cutoff 20 September 2016; Cancer Discovery, February 2017 * FDA-AACR Brain Tumor Endpoints Workshop 2006 NTRK-glioneuronal tumor: response by 3-D volumetric assessment courtesy of P. Brastianos MD, MGH; SD = stable disease Antitumor Activity in Phase 1 ALK and ROS1 Inhibitor-Naïve Patients with NTRK1/2/3, ROS1, or ALK Fusions Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% ROS1 12/14 86% ALK 4/7 57% v 0% change 1 additional patient with NTRK+ glioneuronal tumor SD by RECIST (not validated for primary brain tumors*) 60% reduction by 3-D volumetric assessment
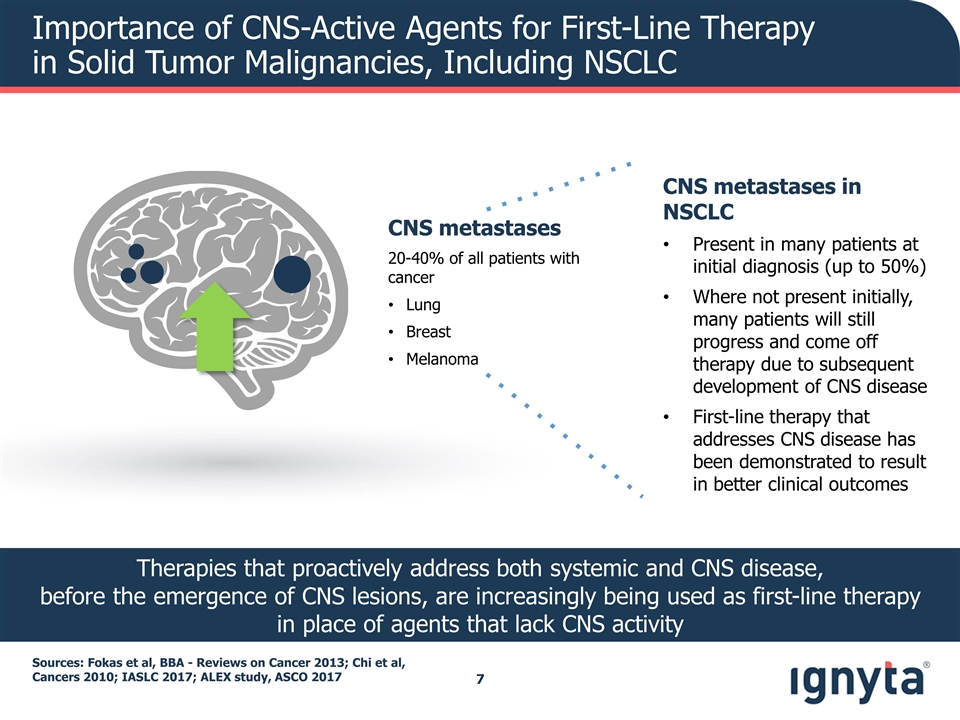
Importance of CNS-Active Agents for First-Line Therapy in Solid Tumor Malignancies, Including NSCLC Sources: Fokas et al, BBA - Reviews on Cancer 2013; Chi et al, Cancers 2010; IASLC 2017; ALEX study, ASCO 2017 CNS metastases in NSCLC Present in many patients at initial diagnosis (up to 50%) Where not present initially, many patients will still progress and come off therapy due to subsequent development of CNS disease First-line therapy that addresses CNS disease has been demonstrated to result in better clinical outcomes Therapies that proactively address both systemic and CNS disease, before the emergence of CNS lesions, are increasingly being used as first-line therapy in place of agents that lack CNS activity CNS metastases 20-40% of all patients with cancer Lung Breast Melanoma
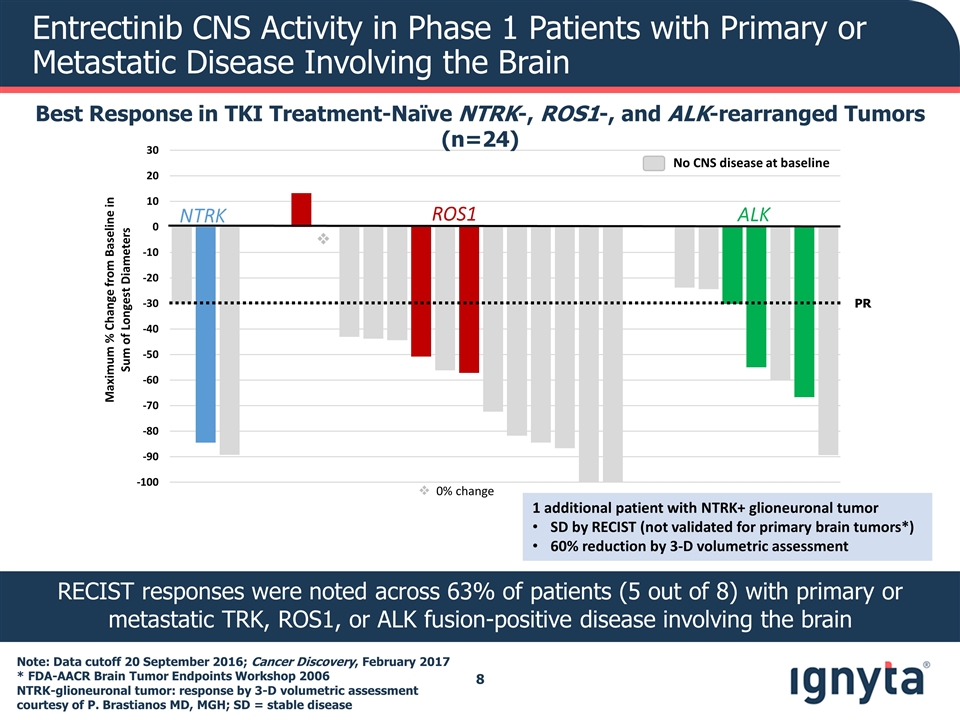
v Maximum % Change from Baseline in Sum of Longest Diameters NTRK ALK ROS1 PR Entrectinib CNS Activity in Phase 1 Patients with Primary or Metastatic Disease Involving the Brain Best Response in TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=24) v 0% change No CNS disease at baseline RECIST responses were noted across 63% of patients (5 out of 8) with primary or metastatic TRK, ROS1, or ALK fusion-positive disease involving the brain Note: Data cutoff 20 September 2016; Cancer Discovery, February 2017 * FDA-AACR Brain Tumor Endpoints Workshop 2006 NTRK-glioneuronal tumor: response by 3-D volumetric assessment courtesy of P. Brastianos MD, MGH; SD = stable disease 1 additional patient with NTRK+ glioneuronal tumor SD by RECIST (not validated for primary brain tumors*) 60% reduction by 3-D volumetric assessment
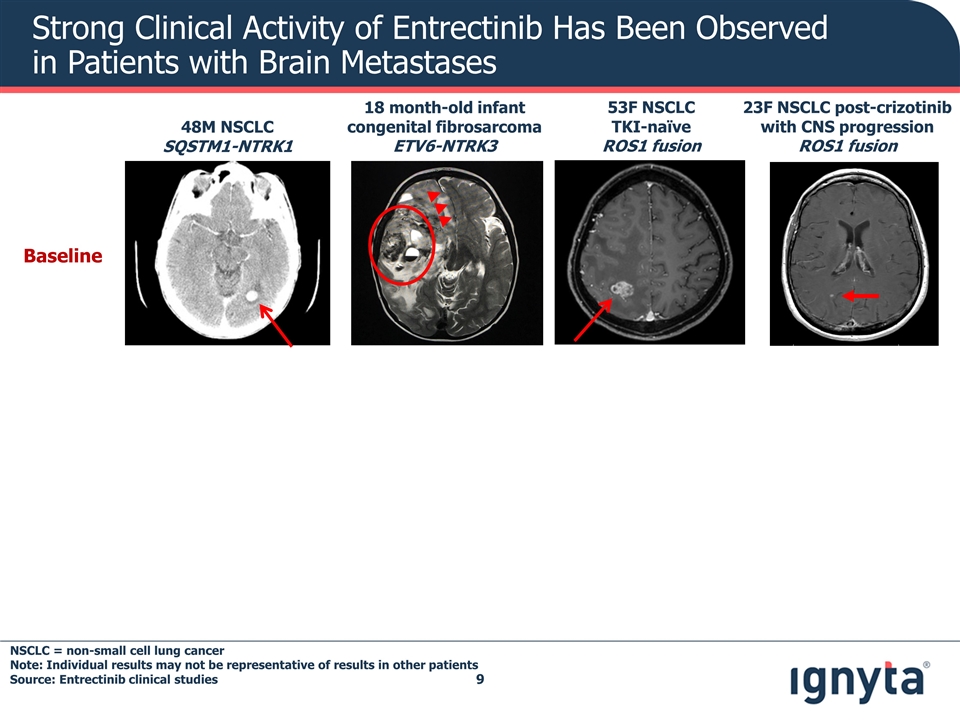
Strong Clinical Activity of Entrectinib Has Been Observed in Patients with Brain Metastases Baseline 48M NSCLC SQSTM1-NTRK1 23F NSCLC post-crizotinib with CNS progression ROS1 fusion 53F NSCLC TKI-naïve ROS1 fusion 18 month-old infant congenital fibrosarcoma ETV6-NTRK3 NSCLC = non-small cell lung cancer Note: Individual results may not be representative of results in other patients Source: Entrectinib clinical studies
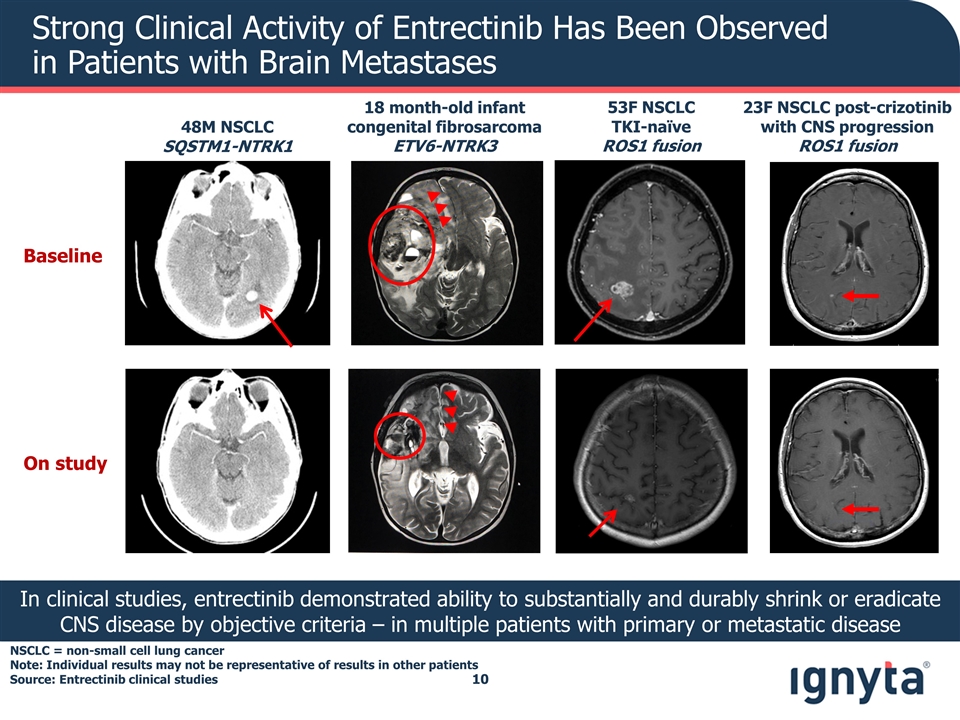
Strong Clinical Activity of Entrectinib Has Been Observed in Patients with Brain Metastases In clinical studies, entrectinib demonstrated ability to substantially and durably shrink or eradicate CNS disease by objective criteria – in multiple patients with primary or metastatic disease Baseline On study 48M NSCLC SQSTM1-NTRK1 23F NSCLC post-crizotinib with CNS progression ROS1 fusion 53F NSCLC TKI-naïve ROS1 fusion 18 month-old infant congenital fibrosarcoma ETV6-NTRK3 NSCLC = non-small cell lung cancer Note: Individual results may not be representative of results in other patients Source: Entrectinib clinical studies
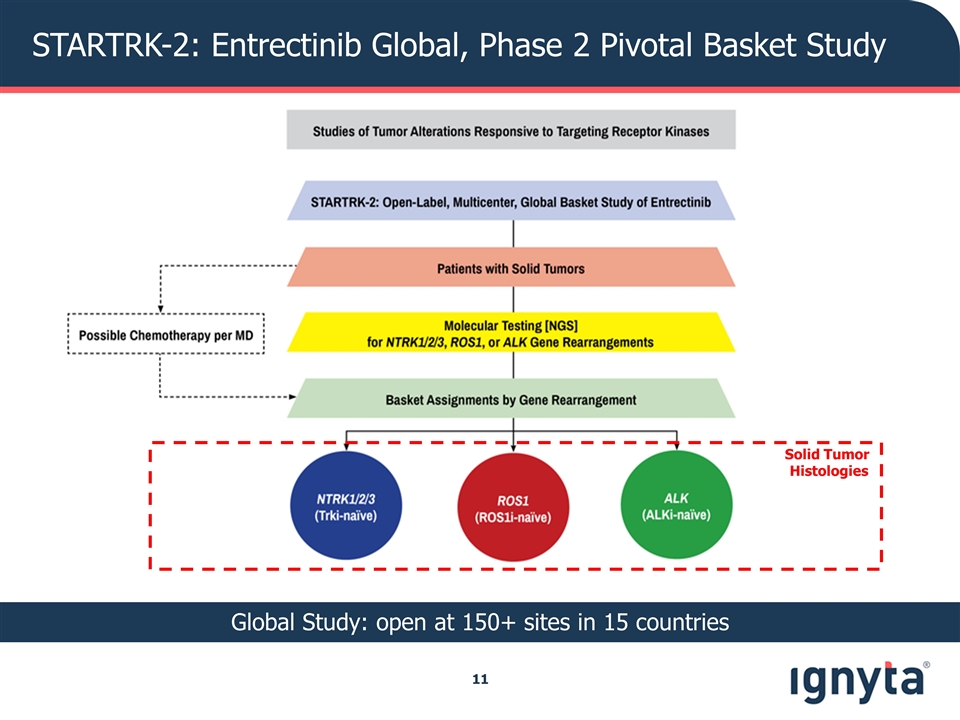
STARTRK-2: Entrectinib Global, Phase 2 Pivotal Basket Study Solid Tumor Histologies Global Study: open at 150+ sites in 15 countries

Entrectinib’s Focus Is on Two Complementary Targets: TRK and ROS1 TRK: widely distributed across many solid tumors, with relatively low detection rates Identified across >30 different solid tumor types, including head and neck, thyroid, sarcoma, brain ~15% of NTRK incidence is in NSCLC, where detection rates are relatively high A number of these TRK driven tumors are associated with CNS metastases Proposed Indication: Entrectinib is indicated for the treatment of NTRK fusion-positive, locally advanced or metastatic solid tumors in adult and pediatric patients who have either progressed following prior therapies or who have no acceptable standard therapies ROS1: concentrated in a single solid tumor type, NSCLC, with relatively high detection rates Most ROS1 found in NSCLC; infrequently found in ~half dozen other solid tumor types Most ROS1 patients in the US are identified today due to high testing rates in lung cancer High incidence of CNS metastases in ROS1+ NSCLC Proposed Indication: Entrectinib is indicated for the treatment of ROS1 fusion-positive, locally advanced or metastatic non-small cell lung cancer (NSCLC) ROS1 TRK

TRK Target: Widely Distributed among Solid Tumors TRK enrollment in STARTRK-2 has been consistent with the wide distribution pattern described in the literature Patients with TRK fusions enrolled across >15 different tumor types NSCLC comprises 16% of the patients with TRK fusions in ST-2 All of these patients were identified using NGS TRK TRK
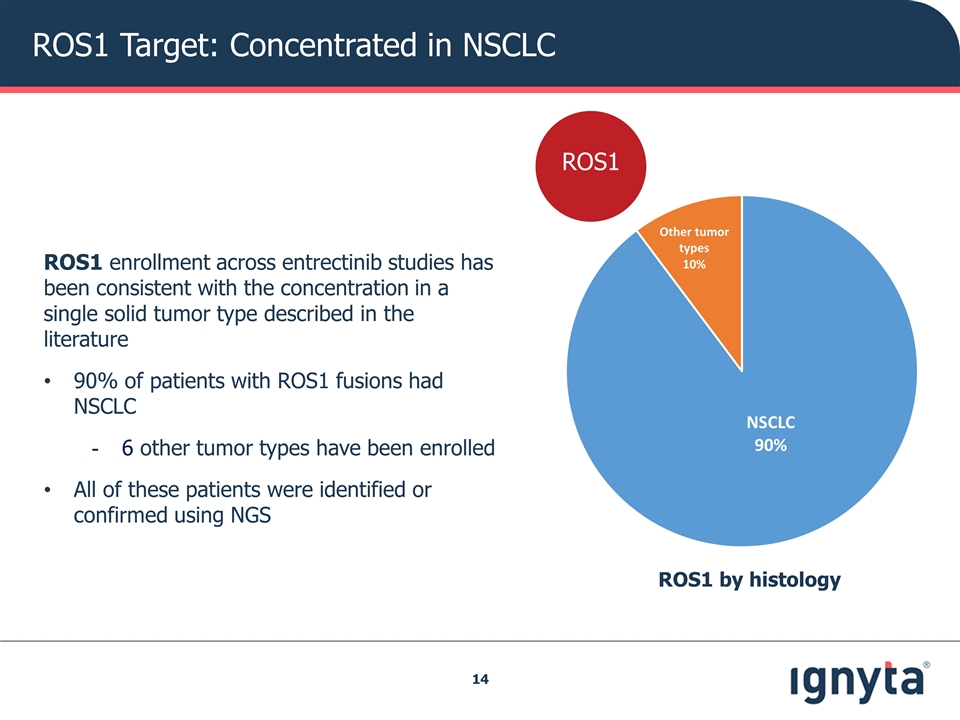
ROS1 Target: Concentrated in NSCLC ROS1 ROS1 by histology ROS1 enrollment across entrectinib studies has been consistent with the concentration in a single solid tumor type described in the literature 90% of patients with ROS1 fusions had NSCLC 6 other tumor types have been enrolled All of these patients were identified or confirmed using NGS ROS1
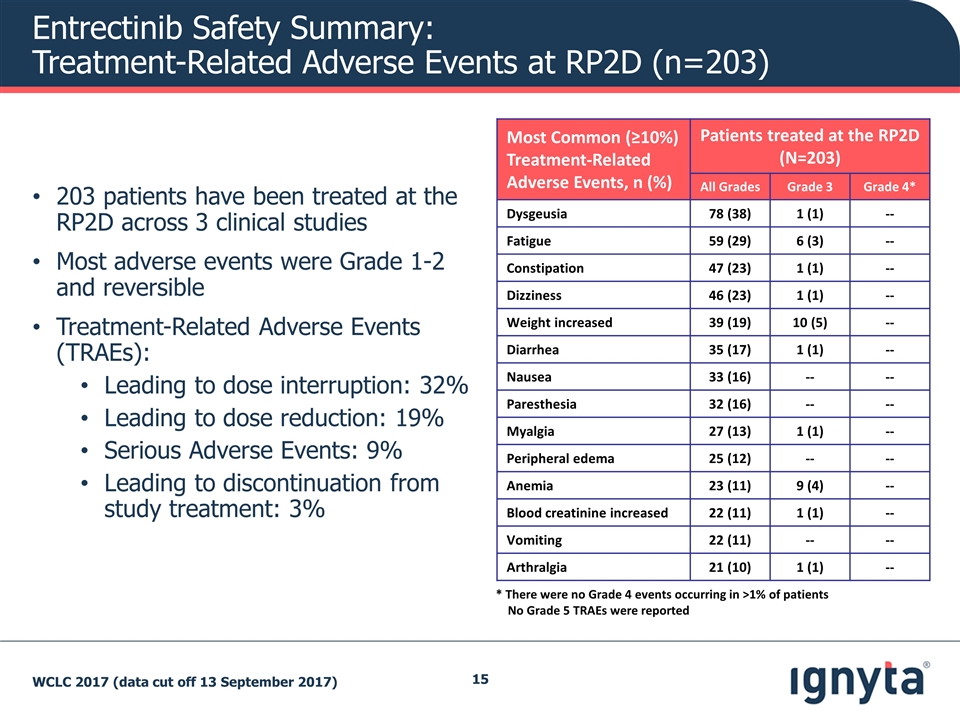
203 patients have been treated at the RP2D across 3 clinical studies Most adverse events were Grade 1-2 and reversible Treatment-Related Adverse Events (TRAEs): Leading to dose interruption: 32% Leading to dose reduction: 19% Serious Adverse Events: 9% Leading to discontinuation from study treatment: 3% Most Common (≥10%) Treatment-Related Adverse Events, n (%) Patients treated at the RP2D (N=203) All Grades Grade 3 Grade 4* Dysgeusia 78 (38) 1 (1) -- Fatigue 59 (29) 6 (3) -- Constipation 47 (23) 1 (1) -- Dizziness 46 (23) 1 (1) -- Weight increased 39 (19) 10 (5) -- Diarrhea 35 (17) 1 (1) -- Nausea 33 (16) -- -- Paresthesia 32 (16) -- -- Myalgia 27 (13) 1 (1) -- Peripheral edema 25 (12) -- -- Anemia 23 (11) 9 (4) -- Blood creatinine increased 22 (11) 1 (1) -- Vomiting 22 (11) -- -- Arthralgia 21 (10) 1 (1) -- * There were no Grade 4 events occurring in >1% of patients No Grade 5 TRAEs were reported WCLC 2017 (data cut off 13 September 2017) Entrectinib Safety Summary: Treatment-Related Adverse Events at RP2D (n=203)
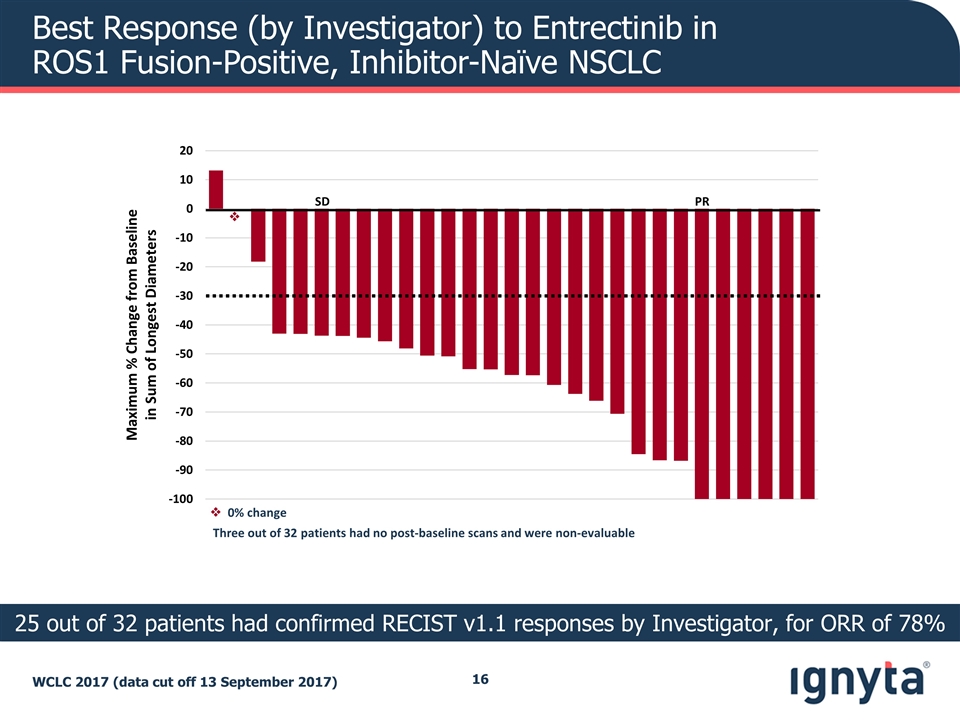
Best Response (by Investigator) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC WCLC 2017 (data cut off 13 September 2017) 25 out of 32 patients had confirmed RECIST v1.1 responses by Investigator, for ORR of 78% Maximum % Change from Baseline in Sum of Longest Diameters PR SD v Three out of 32 patients had no post-baseline scans and were non-evaluable v 0% change
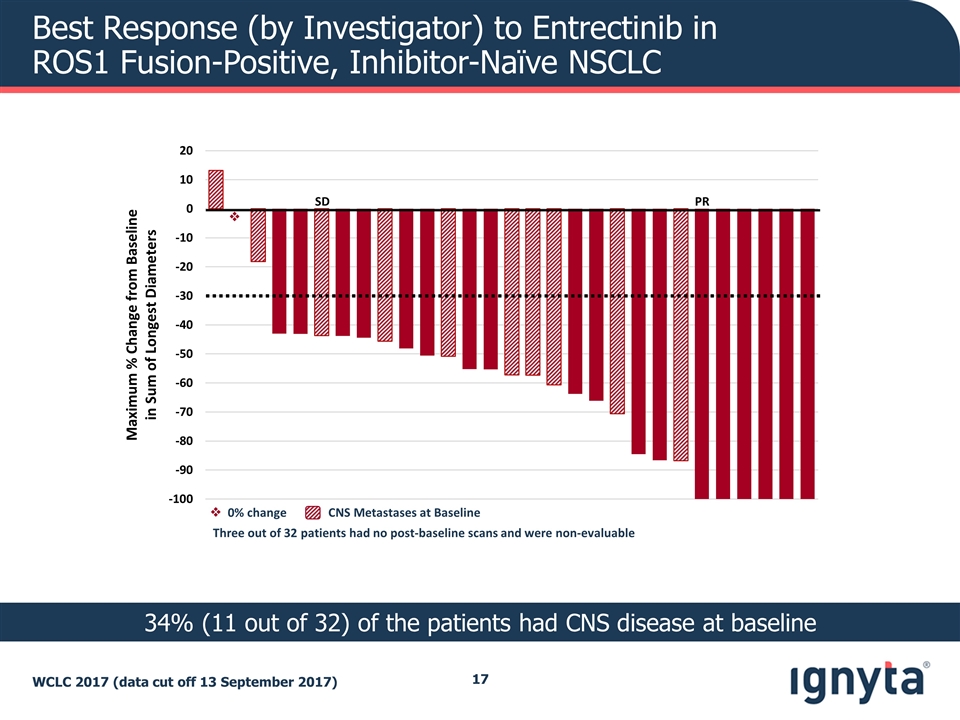
Best Response (by Investigator) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC WCLC 2017 (data cut off 13 September 2017) 34% (11 out of 32) of the patients had CNS disease at baseline Maximum % Change from Baseline in Sum of Longest Diameters PR SD v Three out of 32 patients had no post-baseline scans and were non-evaluable v 0% change CNS Metastases at Baseline
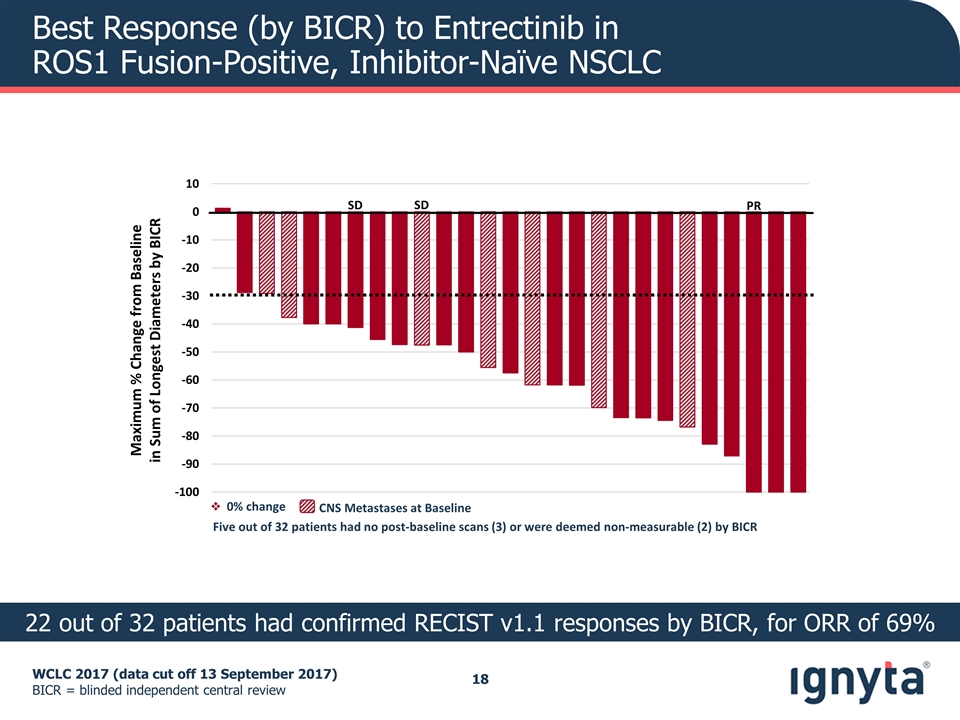
WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Best Response (by BICR) to Entrectinib in ROS1 Fusion-Positive, Inhibitor-Naïve NSCLC 22 out of 32 patients had confirmed RECIST v1.1 responses by BICR, for ORR of 69% Maximum % Change from Baseline in Sum of Longest Diameters by BICR SD PR SD CNS Metastases at Baseline Five out of 32 patients had no post-baseline scans (3) or were deemed non-measurable (2) by BICR v 0% change
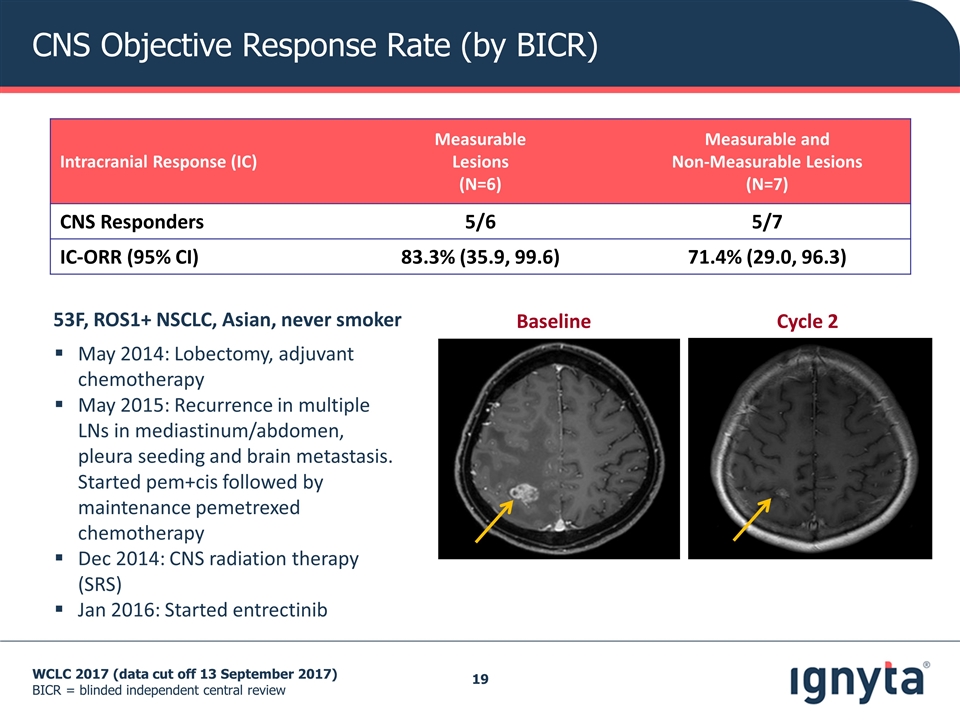
CNS Objective Response Rate (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Intracranial Response (IC) Measurable Lesions (N=6) Measurable and Non-Measurable Lesions (N=7) CNS Responders 5/6 5/7 IC-ORR (95% CI) 83.3% (35.9, 99.6) 71.4% (29.0, 96.3) Baseline Cycle 2 53F, ROS1+ NSCLC, Asian, never smoker May 2014: Lobectomy, adjuvant chemotherapy May 2015: Recurrence in multiple LNs in mediastinum/abdomen, pleura seeding and brain metastasis. Started pem+cis followed by maintenance pemetrexed chemotherapy Dec 2014: CNS radiation therapy (SRS) Jan 2016: Started entrectinib
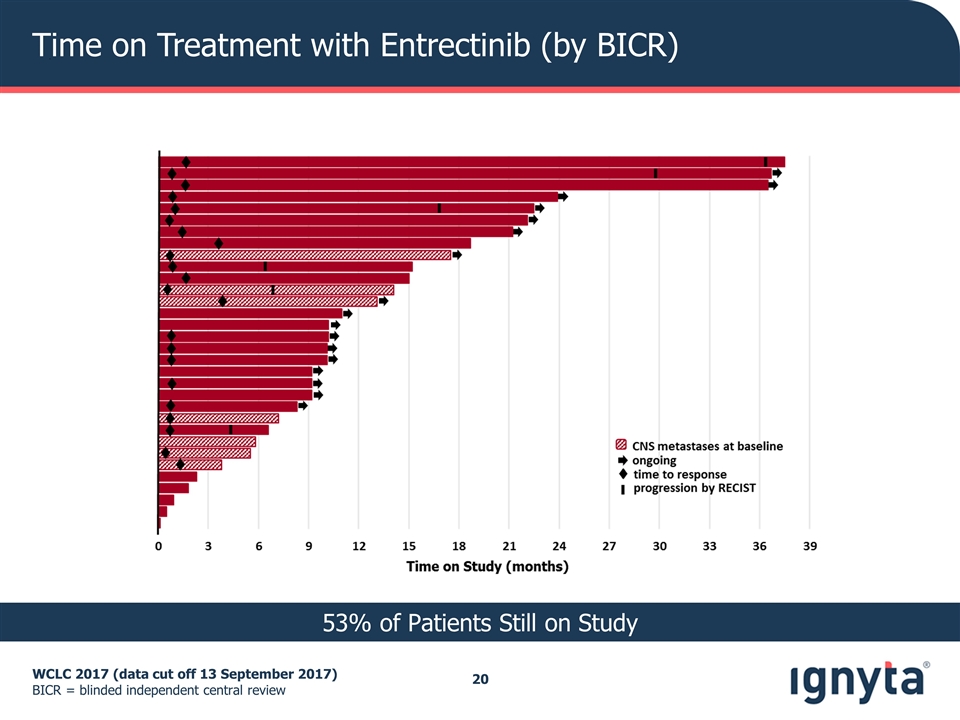
Time on Treatment with Entrectinib (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review 53% of Patients Still on Study
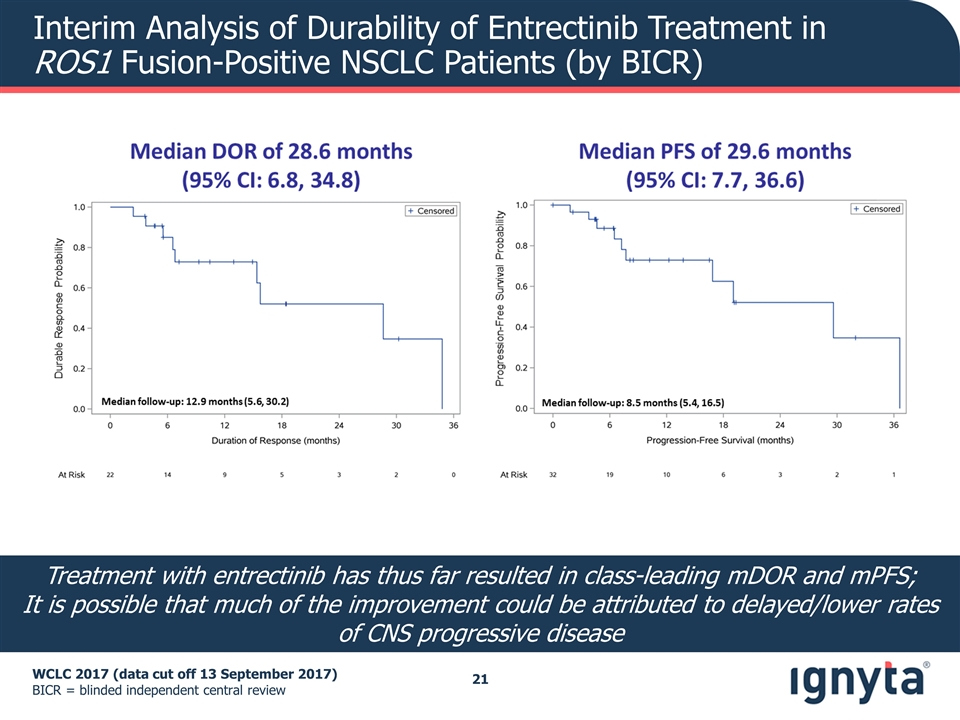
Interim Analysis of Durability of Entrectinib Treatment in ROS1 Fusion-Positive NSCLC Patients (by BICR) WCLC 2017 (data cut off 13 September 2017) BICR = blinded independent central review Treatment with entrectinib has thus far resulted in class-leading mDOR and mPFS; It is possible that much of the improvement could be attributed to delayed/lower rates of CNS progressive disease
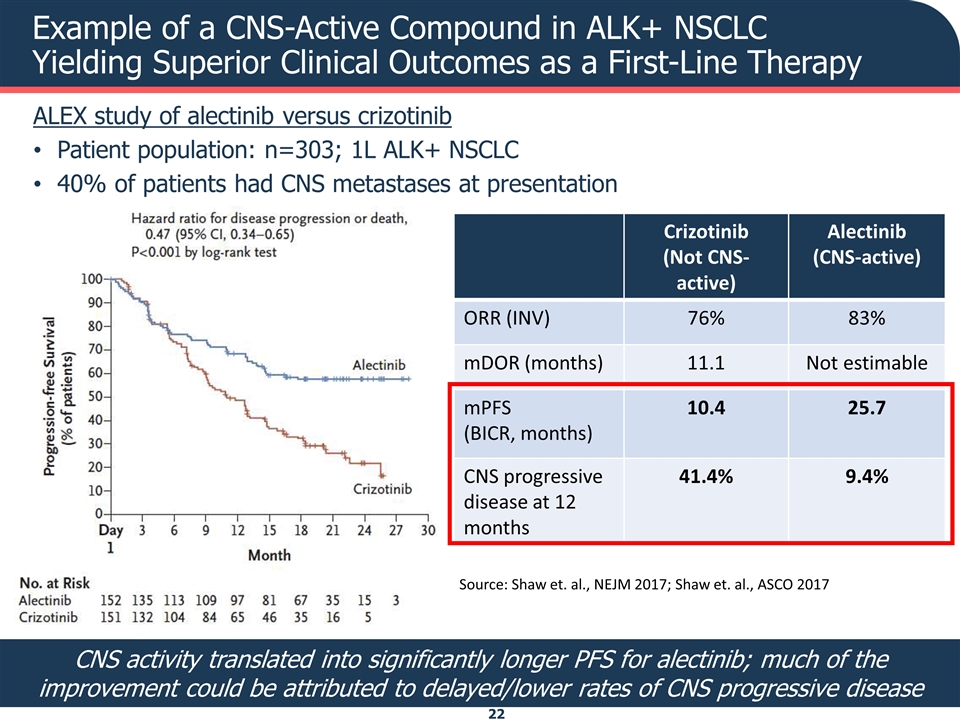
Example of a CNS-Active Compound in ALK+ NSCLC Yielding Superior Clinical Outcomes as a First-Line Therapy ALEX study of alectinib versus crizotinib Patient population: n=303; 1L ALK+ NSCLC 40% of patients had CNS metastases at presentation Crizotinib (Not CNS-active) Alectinib (CNS-active) ORR (INV) 76% 83% mDOR (months) 11.1 Not estimable mPFS (BICR, months) 10.4 25.7 CNS progressive disease at 12 months 41.4% 9.4% CNS activity translated into significantly longer PFS for alectinib; much of the improvement could be attributed to delayed/lower rates of CNS progressive disease Source: Shaw et. al., NEJM 2017; Shaw et. al., ASCO 2017
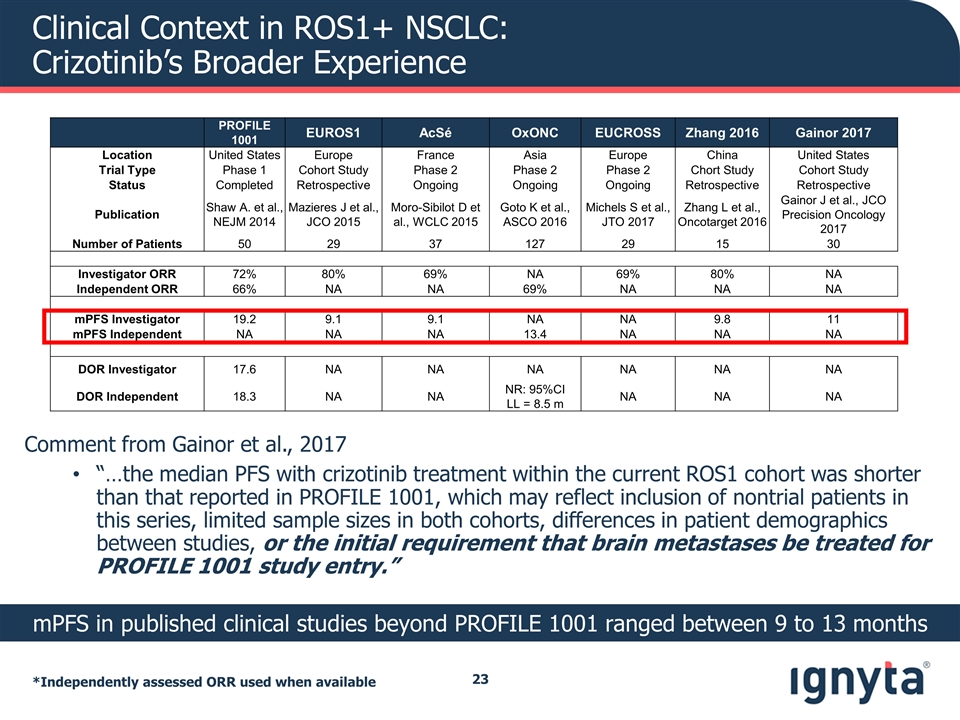
Clinical Context in ROS1+ NSCLC: Crizotinib’s Broader Experience *Independently assessed ORR used when available PROFILE 1001 EUROS1 AcSé OxONC EUCROSS Zhang 2016 Gainor 2017 Location United States Europe France Asia Europe China United States Trial Type Phase 1 Cohort Study Phase 2 Phase 2 Phase 2 Chort Study Cohort Study Status Completed Retrospective Ongoing Ongoing Ongoing Retrospective Retrospective Publication Shaw A. et al., NEJM 2014 Mazieres J et al., JCO 2015 Moro-Sibilot D et al., WCLC 2015 Goto K et al., ASCO 2016 Michels S et al., JTO 2017 Zhang L et al., Oncotarget 2016 Gainor J et al., JCO Precision Oncology 2017 Number of Patients 50 29 37 127 29 15 30 Investigator ORR 72% 80% 69% NA 69% 80% NA Independent ORR 66% NA NA 69% NA NA NA mPFS Investigator 19.2 9.1 9.1 NA NA 9.8 11 mPFS Independent NA NA NA 13.4 NA NA NA DOR Investigator 17.6 NA NA NA NA NA NA DOR Independent 18.3 NA NA NR: 95%CI LL = 8.5 m NA NA NA mPFS in published clinical studies beyond PROFILE 1001 ranged between 9 to 13 months Comment from Gainor et al., 2017 “…the median PFS with crizotinib treatment within the current ROS1 cohort was shorter than that reported in PROFILE 1001, which may reflect inclusion of nontrial patients in this series, limited sample sizes in both cohorts, differences in patient demographics between studies, or the initial requirement that brain metastases be treated for PROFILE 1001 study entry.”
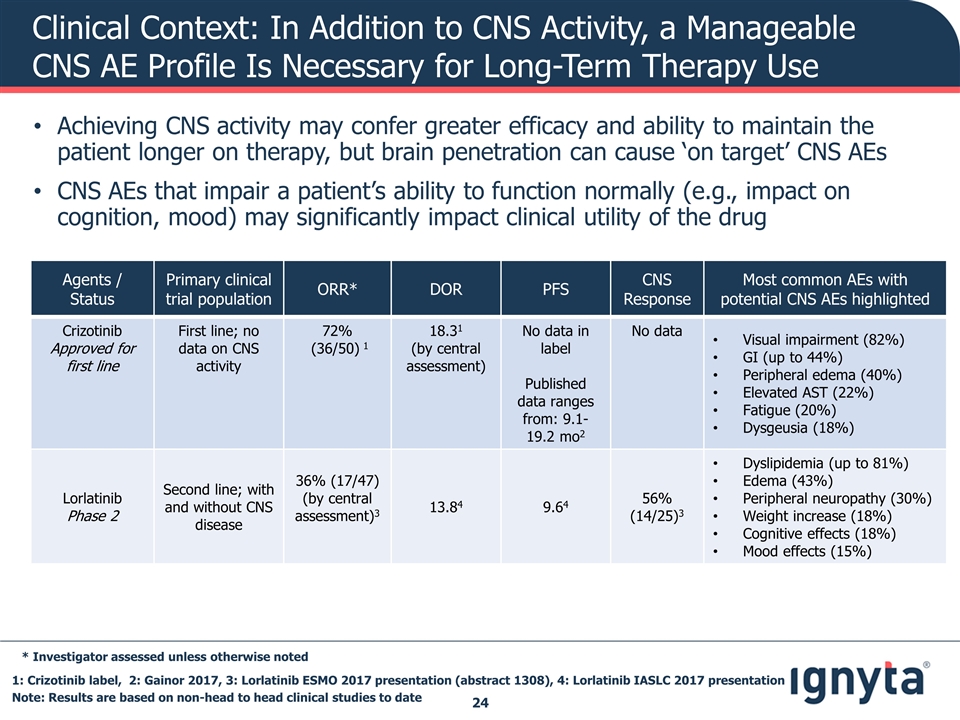
Clinical Context: In Addition to CNS Activity, a Manageable CNS AE Profile Is Necessary for Long-Term Therapy Use 1: Crizotinib label, 2: Gainor 2017, 3: Lorlatinib ESMO 2017 presentation (abstract 1308), 4: Lorlatinib IASLC 2017 presentation Agents / Status Primary clinical trial population ORR* DOR PFS CNS Response Most common AEs with potential CNS AEs highlighted Crizotinib Approved for first line First line; no data on CNS activity 72% (36/50) 1 18.31 (by central assessment) No data in label Published data ranges from: 9.1-19.2 mo2 No data Visual impairment (82%) GI (up to 44%) Peripheral edema (40%) Elevated AST (22%) Fatigue (20%) Dysgeusia (18%) Lorlatinib Phase 2 Second line; with and without CNS disease 36% (17/47) (by central assessment)3 13.84 9.64 56% (14/25)3 Dyslipidemia (up to 81%) Edema (43%) Peripheral neuropathy (30%) Weight increase (18%) Cognitive effects (18%) Mood effects (15%) * Investigator assessed unless otherwise noted Note: Results are based on non-head to head clinical studies to date Achieving CNS activity may confer greater efficacy and ability to maintain the patient longer on therapy, but brain penetration can cause ‘on target’ CNS AEs CNS AEs that impair a patient’s ability to function normally (e.g., impact on cognition, mood) may significantly impact clinical utility of the drug
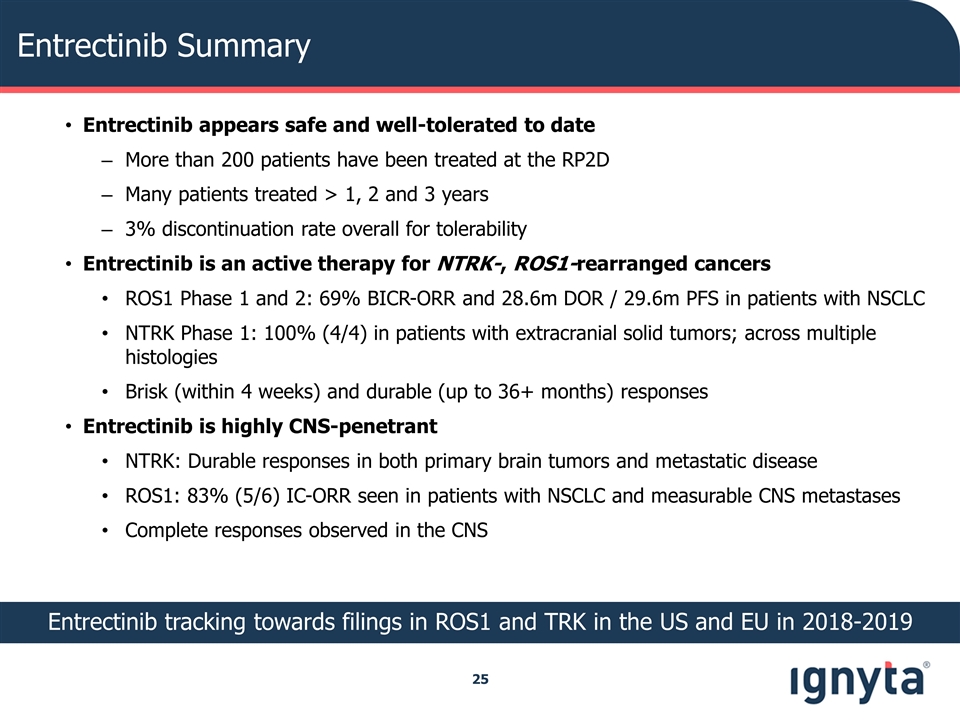
Entrectinib Summary Entrectinib appears safe and well-tolerated to date More than 200 patients have been treated at the RP2D Many patients treated > 1, 2 and 3 years 3% discontinuation rate overall for tolerability Entrectinib is an active therapy for NTRK-, ROS1-rearranged cancers ROS1 Phase 1 and 2: 69% BICR-ORR and 28.6m DOR / 29.6m PFS in patients with NSCLC NTRK Phase 1: 100% (4/4) in patients with extracranial solid tumors; across multiple histologies Brisk (within 4 weeks) and durable (up to 36+ months) responses Entrectinib is highly CNS-penetrant NTRK: Durable responses in both primary brain tumors and metastatic disease ROS1: 83% (5/6) IC-ORR seen in patients with NSCLC and measurable CNS metastases Complete responses observed in the CNS Entrectinib tracking towards filings in ROS1 and TRK in the US and EU in 2018-2019
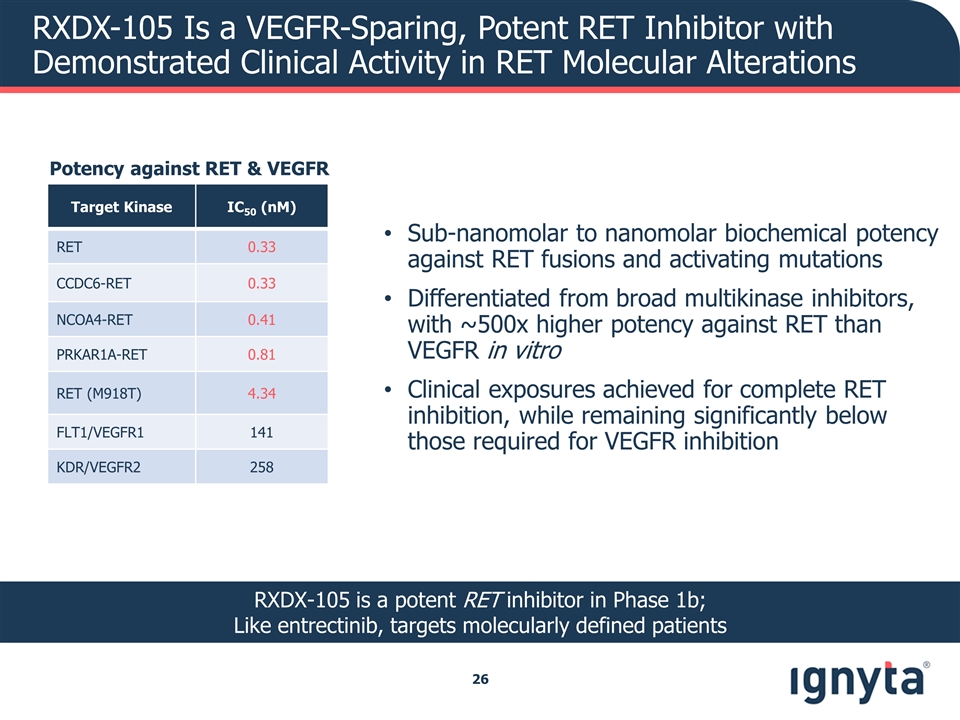
RXDX-105 Is a VEGFR-Sparing, Potent RET Inhibitor with Demonstrated Clinical Activity in RET Molecular Alterations Sub-nanomolar to nanomolar biochemical potency against RET fusions and activating mutations Differentiated from broad multikinase inhibitors, with ~500x higher potency against RET than VEGFR in vitro Clinical exposures achieved for complete RET inhibition, while remaining significantly below those required for VEGFR inhibition Potency against RET & VEGFR Target Kinase IC50 (nM) RET 0.33 CCDC6-RET 0.33 NCOA4-RET 0.41 PRKAR1A-RET 0.81 RET (M918T) 4.34 FLT1/VEGFR1 141 KDR/VEGFR2 258 RXDX-105 is a potent RET inhibitor in Phase 1b; Like entrectinib, targets molecularly defined patients
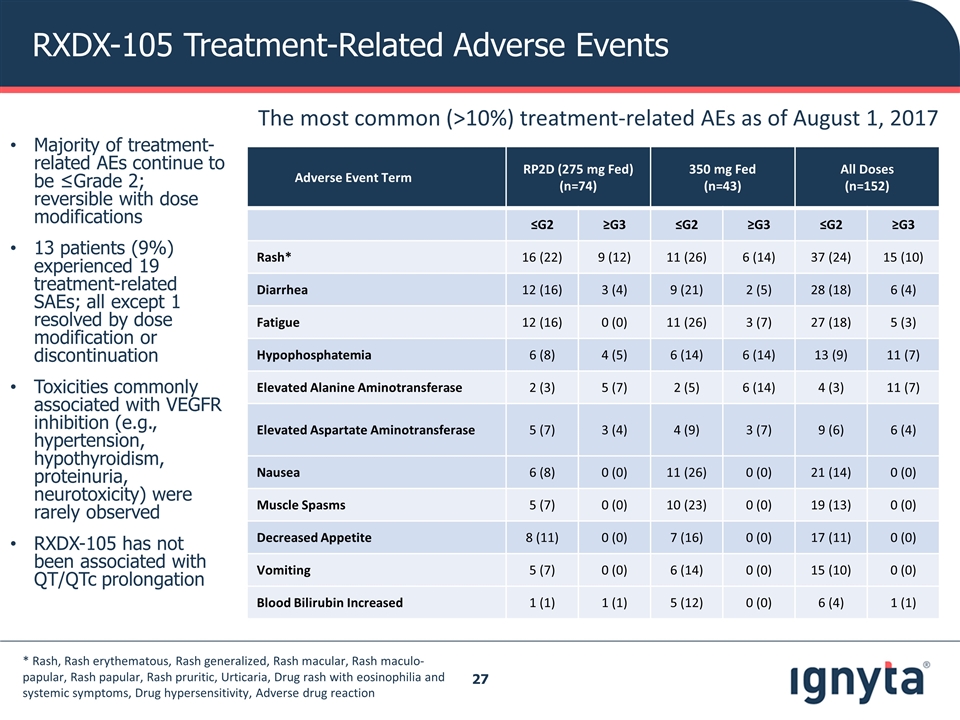
RXDX-105 Treatment-Related Adverse Events Adverse Event Term RP2D (275 mg Fed) (n=74) 350 mg Fed (n=43) All Doses (n=152) ≤G2 ≥G3 ≤G2 ≥G3 ≤G2 ≥G3 Rash* 16 (22) 9 (12) 11 (26) 6 (14) 37 (24) 15 (10) Diarrhea 12 (16) 3 (4) 9 (21) 2 (5) 28 (18) 6 (4) Fatigue 12 (16) 0 (0) 11 (26) 3 (7) 27 (18) 5 (3) Hypophosphatemia 6 (8) 4 (5) 6 (14) 6 (14) 13 (9) 11 (7) Elevated Alanine Aminotransferase 2 (3) 5 (7) 2 (5) 6 (14) 4 (3) 11 (7) Elevated Aspartate Aminotransferase 5 (7) 3 (4) 4 (9) 3 (7) 9 (6) 6 (4) Nausea 6 (8) 0 (0) 11 (26) 0 (0) 21 (14) 0 (0) Muscle Spasms 5 (7) 0 (0) 10 (23) 0 (0) 19 (13) 0 (0) Decreased Appetite 8 (11) 0 (0) 7 (16) 0 (0) 17 (11) 0 (0) Vomiting 5 (7) 0 (0) 6 (14) 0 (0) 15 (10) 0 (0) Blood Bilirubin Increased 1 (1) 1 (1) 5 (12) 0 (0) 6 (4) 1 (1) The most common (>10%) treatment-related AEs as of August 1, 2017 * Rash, Rash erythematous, Rash generalized, Rash macular, Rash maculo-papular, Rash papular, Rash pruritic, Urticaria, Drug rash with eosinophilia and systemic symptoms, Drug hypersensitivity, Adverse drug reaction Majority of treatment-related AEs continue to be ≤Grade 2; reversible with dose modifications 13 patients (9%) experienced 19 treatment-related SAEs; all except 1 resolved by dose modification or discontinuation Toxicities commonly associated with VEGFR inhibition (e.g., hypertension, hypothyroidism, proteinuria, neurotoxicity) were rarely observed RXDX-105 has not been associated with QT/QTc prolongation
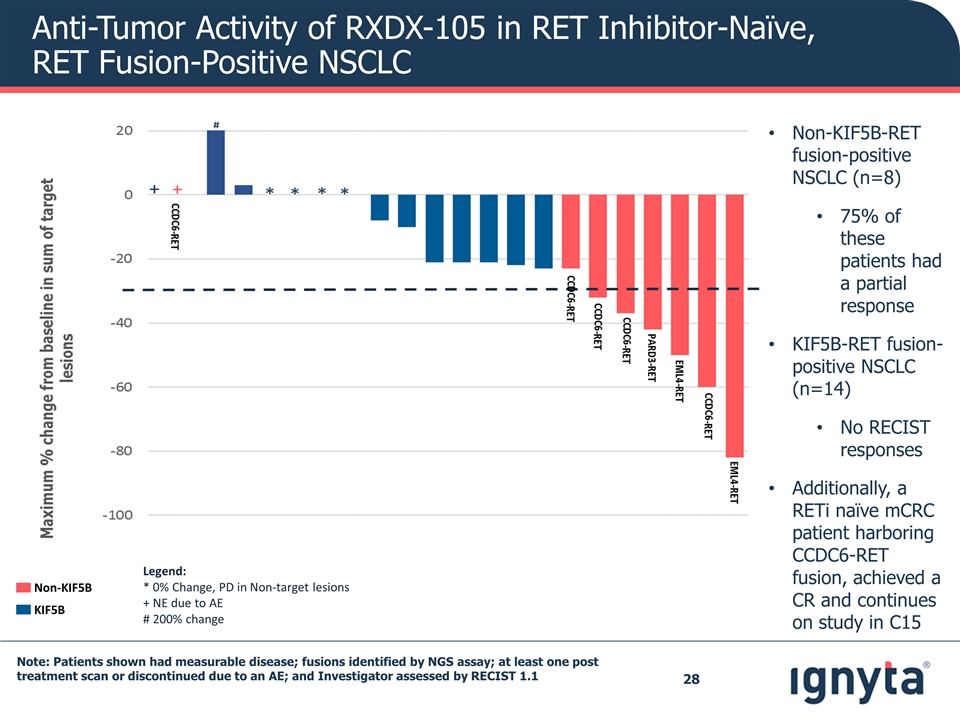
Note: Patients shown had measurable disease; fusions identified by NGS assay; at least one post treatment scan or discontinued due to an AE; and Investigator assessed by RECIST 1.1 Anti-Tumor Activity of RXDX-105 in RET Inhibitor-Naïve, RET Fusion-Positive NSCLC Legend: * 0% Change, PD in Non-target lesions + NE due to AE # 200% change Non-KIF5B KIF5B Non-KIF5B-RET fusion-positive NSCLC (n=8) 75% of these patients had a partial response KIF5B-RET fusion-positive NSCLC (n=14) No RECIST responses Additionally, a RETi naïve mCRC patient harboring CCDC6-RET fusion, achieved a CR and continues on study in C15
RXDX-106: A Novel Small Molecule Immunomodulator with Potent Anti-Tumor Activity Alone and in Combination Functions to restore and enhance overall immune function by overcoming the immunosuppressive tumor microenvironment by potent, selective inhibition of TYRO3, AXL, and MER (TAM) receptors Slow off rate leads to high potency and durable target inhibition Mechanism of action demonstrates enhanced innate and adaptive anti-tumor immunity Demonstrates immune-mediated in vivo activity in preclinical models as a single agent, as well as potentiating effect with checkpoint inhibitors in vivo Significant commercial opportunity to achieve single agent or combination agent efficacy in patients with cancer who are refractory to checkpoint inhibitors
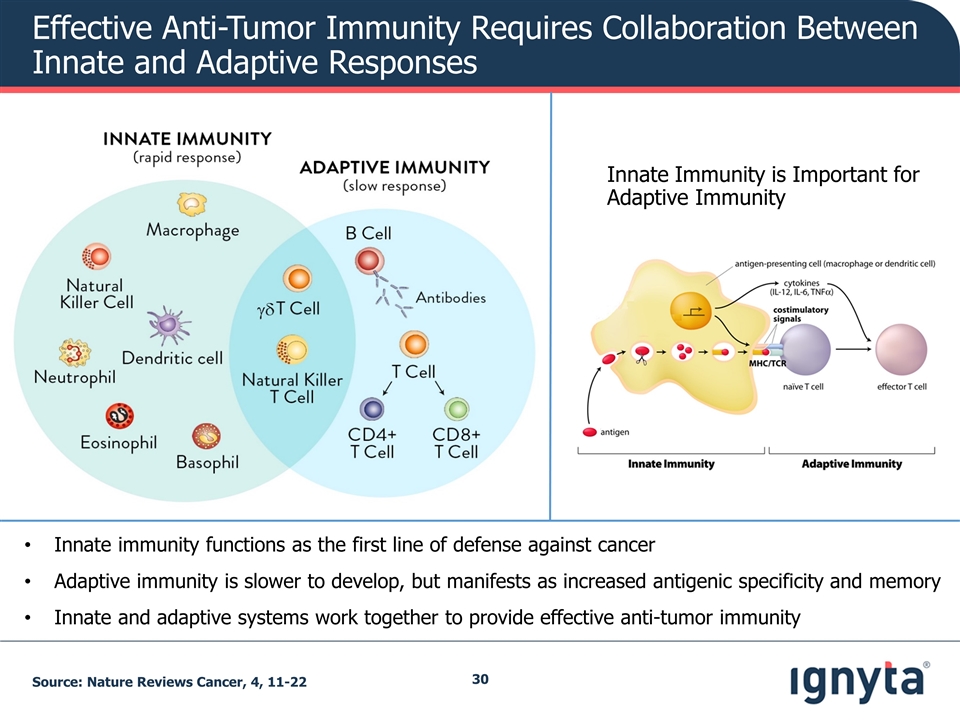
Effective Anti-Tumor Immunity Requires Collaboration Between Innate and Adaptive Responses Source: Nature Reviews Cancer, 4, 11-22 Innate Immunity is Important for Adaptive Immunity Innate immunity functions as the first line of defense against cancer Adaptive immunity is slower to develop, but manifests as increased antigenic specificity and memory Innate and adaptive systems work together to provide effective anti-tumor immunity
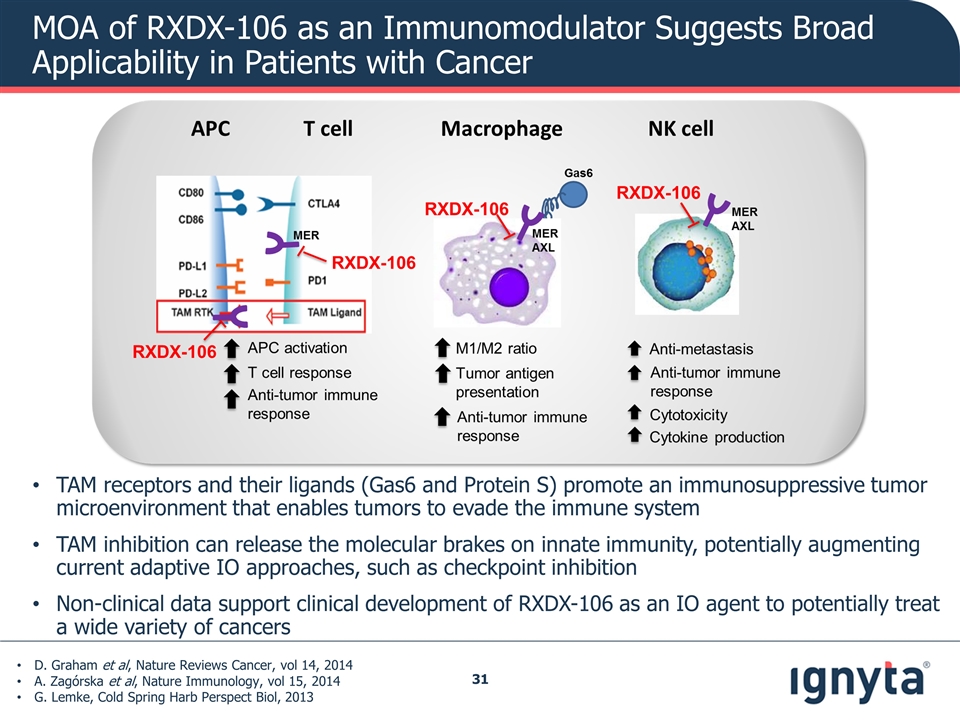
MOA of RXDX-106 as an Immunomodulator Suggests Broad Applicability in Patients with Cancer D. Graham et al, Nature Reviews Cancer, vol 14, 2014 A. Zagórska et al, Nature Immunology, vol 15, 2014 G. Lemke, Cold Spring Harb Perspect Biol, 2013 TAM receptors and their ligands (Gas6 and Protein S) promote an immunosuppressive tumor microenvironment that enables tumors to evade the immune system TAM inhibition can release the molecular brakes on innate immunity, potentially augmenting current adaptive IO approaches, such as checkpoint inhibition Non-clinical data support clinical development of RXDX-106 as an IO agent to potentially treat a wide variety of cancers
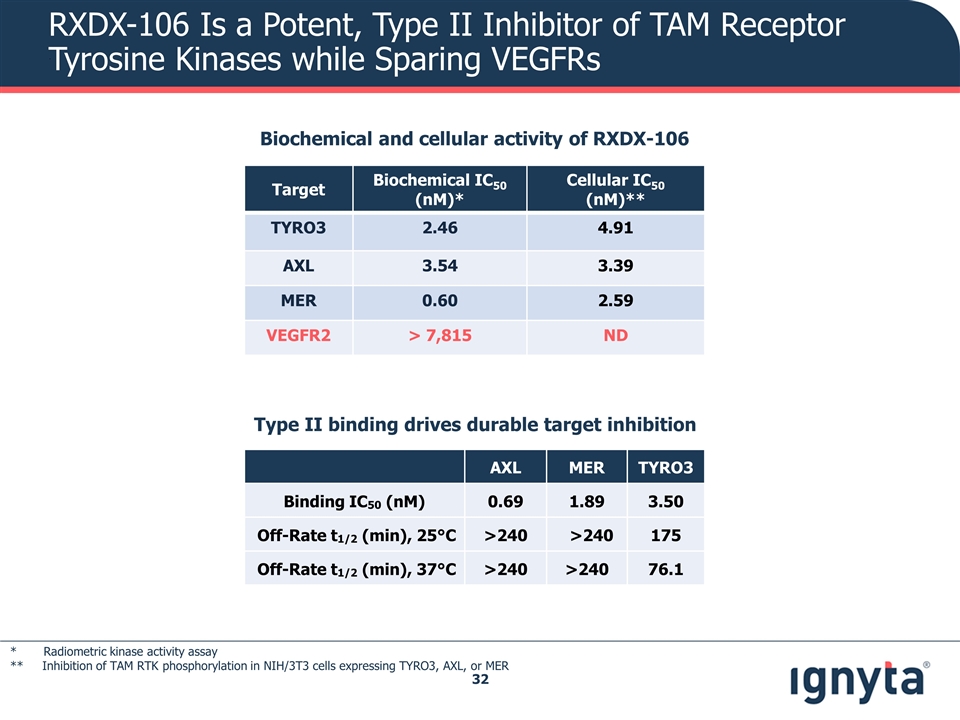
RXDX-106 Is a Potent, Type II Inhibitor of TAM Receptor Tyrosine Kinases while Sparing VEGFRs Target Biochemical IC50 (nM)* Cellular IC50 (nM)** TYRO3 2.46 4.91 AXL 3.54 3.39 MER 0.60 2.59 VEGFR2 > 7,815 ND Type II binding drives durable target inhibition Biochemical and cellular activity of RXDX-106 * Radiometric kinase activity assay ** Inhibition of TAM RTK phosphorylation in NIH/3T3 cells expressing TYRO3, AXL, or MER AXL MER TYRO3 Binding IC50 (nM) 0.69 1.89 3.50 Off-Rate t1/2 (min), 25°C >240 >240 175 Off-Rate t1/2 (min), 37°C >240 >240 76.1
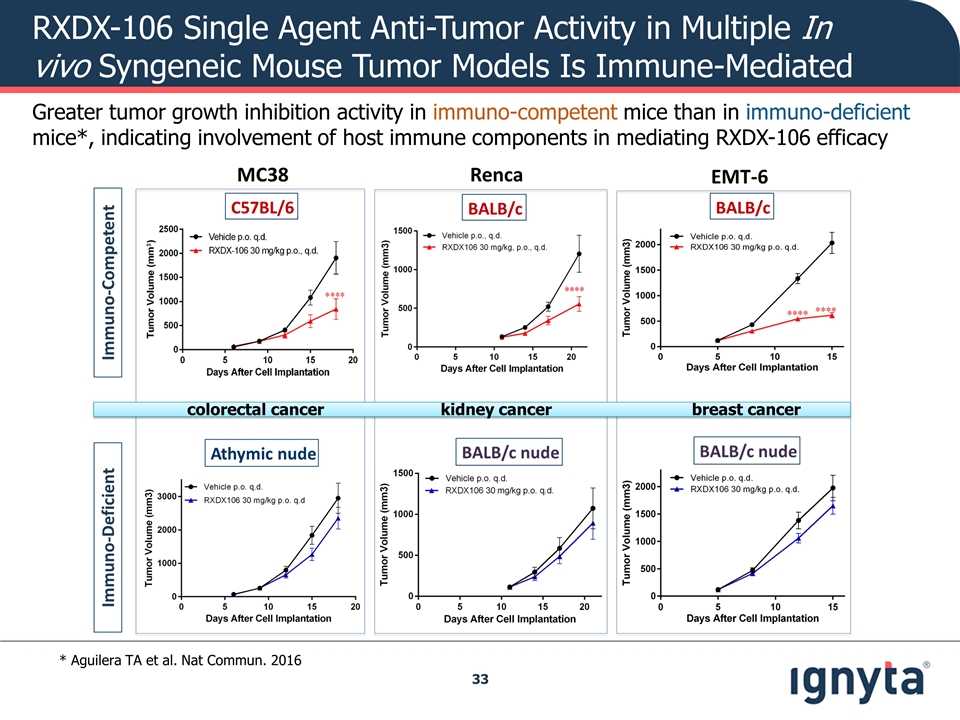
Greater tumor growth inhibition activity in immuno-competent mice than in immuno-deficient mice*, indicating involvement of host immune components in mediating RXDX-106 efficacy * Aguilera TA et al. Nat Commun. 2016 colorectal cancer kidney cancer breast cancer RXDX-106 Single Agent Anti-Tumor Activity in Multiple In vivo Syngeneic Mouse Tumor Models Is Immune-Mediated
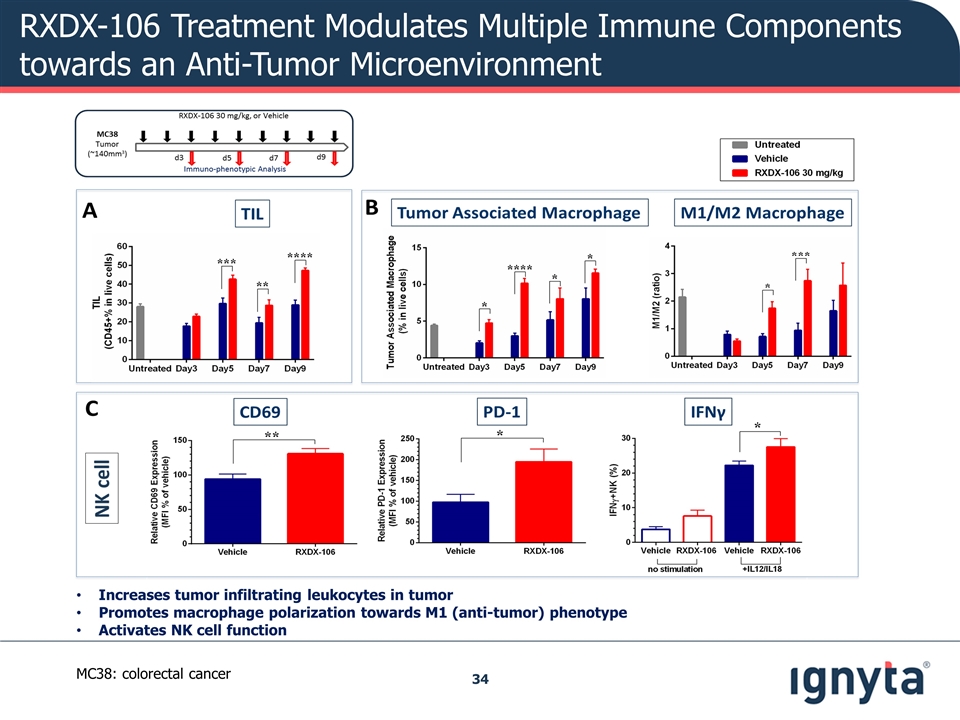
RXDX-106 Treatment Modulates Multiple Immune Components towards an Anti-Tumor Microenvironment Increases tumor infiltrating leukocytes in tumor Promotes macrophage polarization towards M1 (anti-tumor) phenotype Activates NK cell function MC38: colorectal cancer
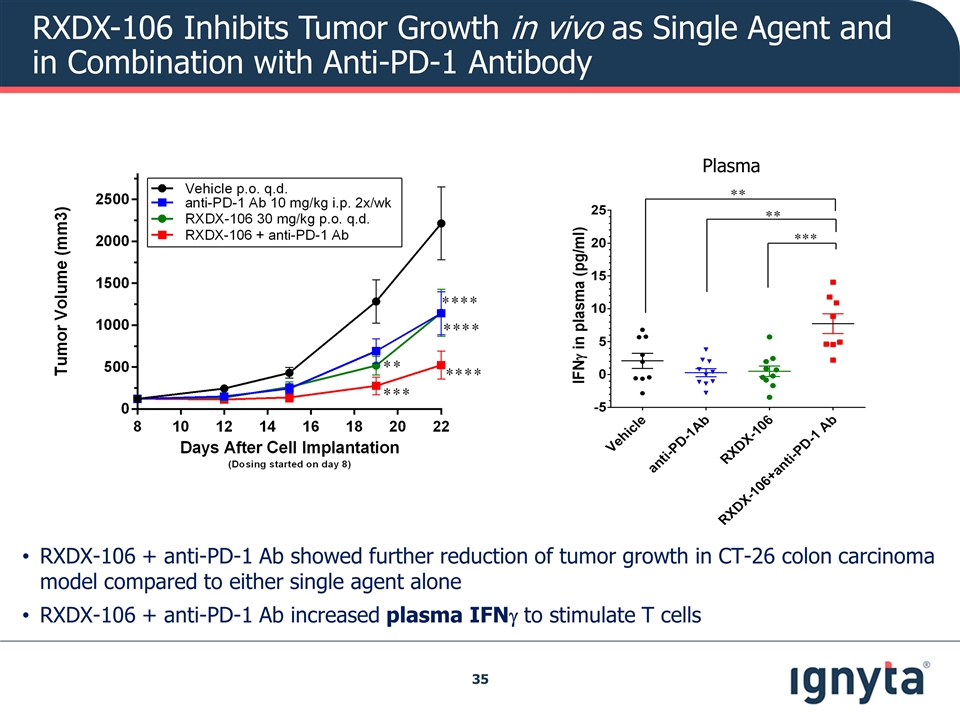
RXDX-106 + anti-PD-1 Ab showed further reduction of tumor growth in CT-26 colon carcinoma model compared to either single agent alone RXDX-106 + anti-PD-1 Ab increased plasma IFNg to stimulate T cells Plasma RXDX-106 Inhibits Tumor Growth in vivo as Single Agent and in Combination with Anti-PD-1 Antibody
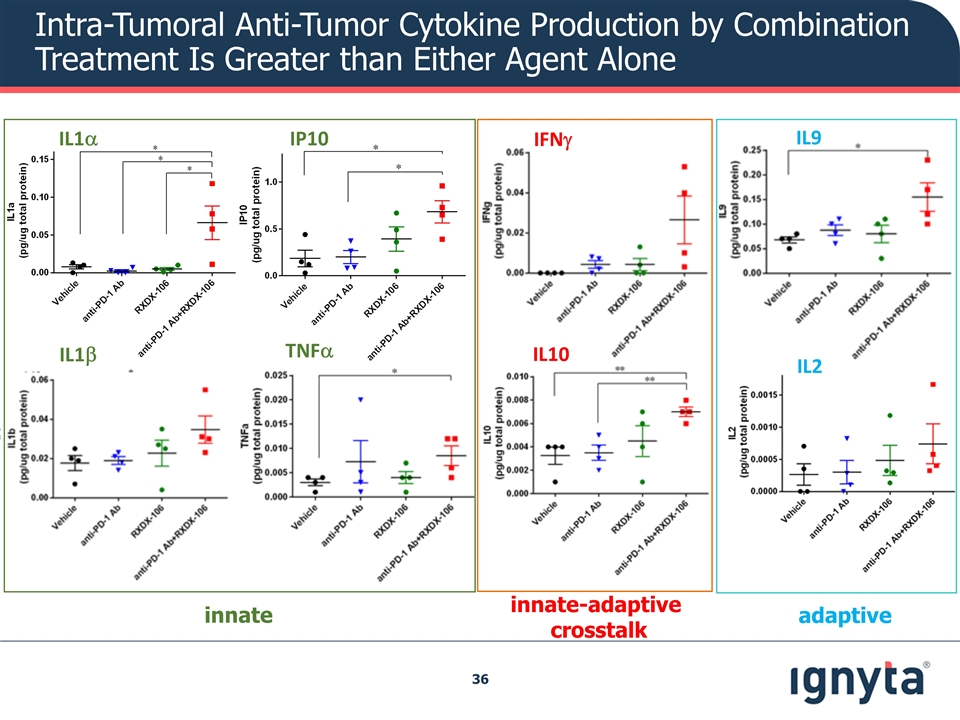
Intra-Tumoral Anti-Tumor Cytokine Production by Combination Treatment Is Greater than Either Agent Alone IL1a IP10 IL1b TNFa IFNg IL10 IL2 IL9 adaptive innate innate-adaptive crosstalk
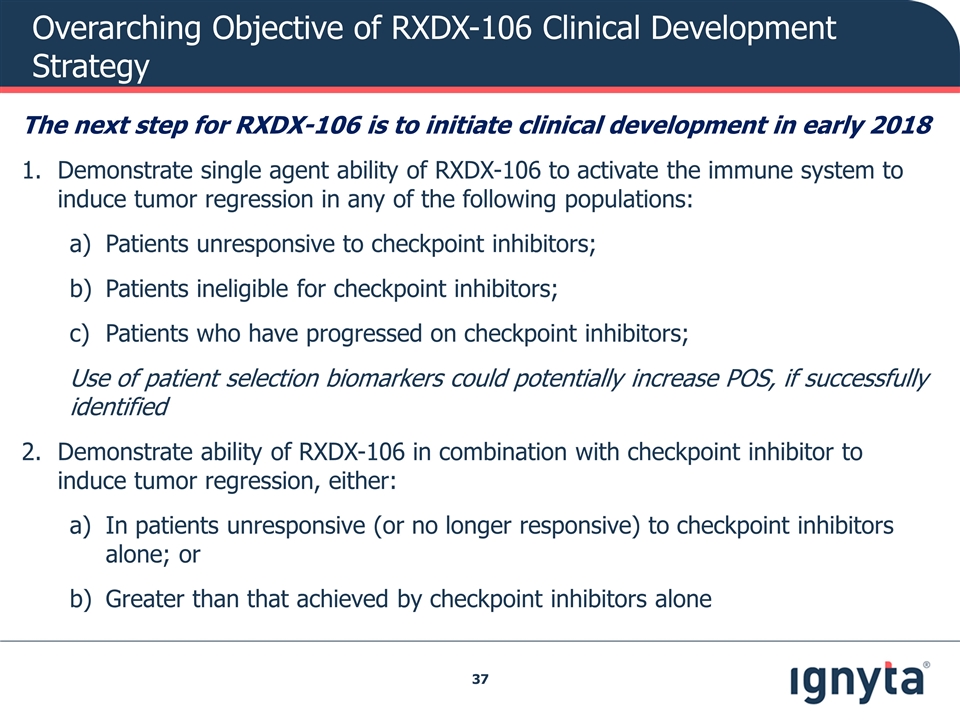
Overarching Objective of RXDX-106 Clinical Development Strategy The next step for RXDX-106 is to initiate clinical development in early 2018 Demonstrate single agent ability of RXDX-106 to activate the immune system to induce tumor regression in any of the following populations: Patients unresponsive to checkpoint inhibitors; Patients ineligible for checkpoint inhibitors; Patients who have progressed on checkpoint inhibitors; Use of patient selection biomarkers could potentially increase POS, if successfully identified Demonstrate ability of RXDX-106 in combination with checkpoint inhibitor to induce tumor regression, either: In patients unresponsive (or no longer responsive) to checkpoint inhibitors alone; or Greater than that achieved by checkpoint inhibitors alone
Company Highlights Robust pipeline of precision medicine therapies in oncology Entrectinib: Path towards two NDAs in TRK tissue agnostic and ROS1 NSCLC indications in 2018, with anticipated launch in 2019 RXDX-105: Preliminary ORR of 75% (n = 8) in patients with non-KIF5B-RET fusion-positive NSCLC in Ph 1b study RXDX-106: Promising preclinical activity as a single agent and combination agent small molecule immunomodulator; IND cleared by FDA Dec 2017 Integrated approach to Rx/Dx development CAP-accredited, CLIA-certified, QSR-compliant diagnostic lab with multi-modality assays Multiple near-term value inflection points Multiple clinical data readouts and regulatory milestones in next 12 months Experienced leadership team Breadth and depth of expertise in clinical/preclinical development, regulatory affairs, commercial, and other key technical and business disciplines
Additional Information and Where to Find It
The tender offer for the outstanding shares of common stock of the Company (the “Tender Offer”) has not yet commenced. This communication is not an offer to buy nor a solicitation of an offer to sell any securities of Ignyta, Inc. (“Ignyta”). The solicitation and the offer to buy shares of Ignyta’s common stock will only be made pursuant to a tender offer statement on Schedule TO, including an offer to purchase, a letter of transmittal and other related materials that Roche Holdings, Inc. and Abingdon Acquisition Corp. intend to file with the Securities and Exchange Commission (the “SEC”). In addition, Ignyta will file with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the Tender Offer. Once filed, investors will be able to obtain the tender offer statement on Schedule TO, the offer to purchase, the Solicitation/Recommendation Statement of Ignyta on Schedule 14D-9 and related materials with respect to the Tender Offer and the merger, free of charge at the website of the SEC at www.sec.gov, and from the information agent named in the tender offer materials. Investors may also obtain, at no charge, any such documents filed with or furnished to the SEC by Ignyta under the “Investors” section of Ignyta’s website at www.ignyta.com. INVESTORS AND SECURITY HOLDERS ARE ADVISED TO READ THESE DOCUMENTS WHEN THEY BECOME AVAILABLE, INCLUDING THE SOLICITATION/RECOMMENDATION STATEMENT OF IGNYTA AND ANY AMENDMENTS THERETO, AS WELL AS ANY OTHER DOCUMENTS RELATING TO THE TENDER OFFER AND THE MERGER THAT ARE FILED WITH THE SEC, CAREFULLY AND IN THEIR ENTIRETY PRIOR TO MAKING ANY DECISIONS WITH RESPECT TO WHETHER TO TENDER THEIR SHARES INTO THE TENDER OFFER BECAUSE THEY CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE TENDER OFFER.
Forward-Looking Statements
The statements included above that are not a description of historical facts are forward-looking statements. Words or phrases such as “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would” or similar expressions are intended to identify forward-looking statements. These forward-looking statements include without limitation statements regarding the planned completion of the transactions contemplated by the Agreement and Plan of Merger dated as of December 21, 2017 by and among Ignyta, Inc., Roche Holdings, Inc. and Abingdon Acquisition Corp. Risks and uncertainties that could cause results to differ from expectations include: uncertainties as to the timing of the Tender Offer and the merger; uncertainties as to the percentage of Ignyta stockholders tendering their shares in the Tender Offer; the possibility that competing offers will be made; the possibility that various closing conditions for the Tender Offer or the merger may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the merger; the effects of disruption caused by the transaction making it more difficult to maintain relationships with employees, collaborators, vendors and other business partners; the risk that stockholder litigation in connection with the Tender Offer or the merger may result in significant costs of defense, indemnification and liability; and risks and uncertainties pertaining to Ignyta’s business, including the risks and uncertainties detailed in Ignyta’s public periodic filings with the SEC, as well as the tender offer materials to be filed by Roche Holdings, Inc. and Abingdon Acquisition Corp. and the Solicitation/Recommendation Statement to be filed by Ignyta in connection with the Tender Offer.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Ignyta undertakes no obligation to revise or update these statements to reflect events or circumstances after the date hereof, except as required by law. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.





































