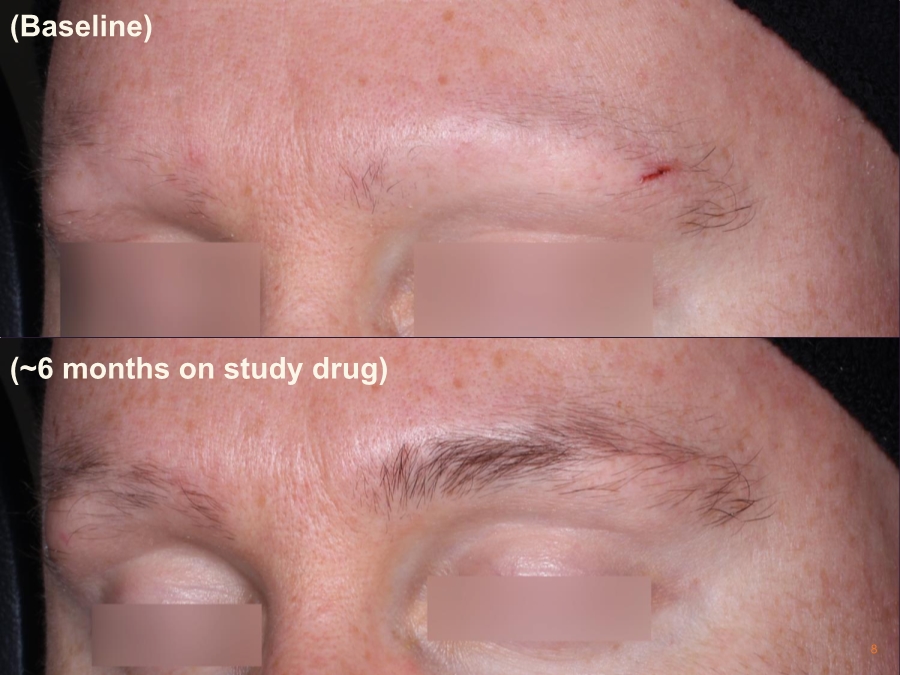
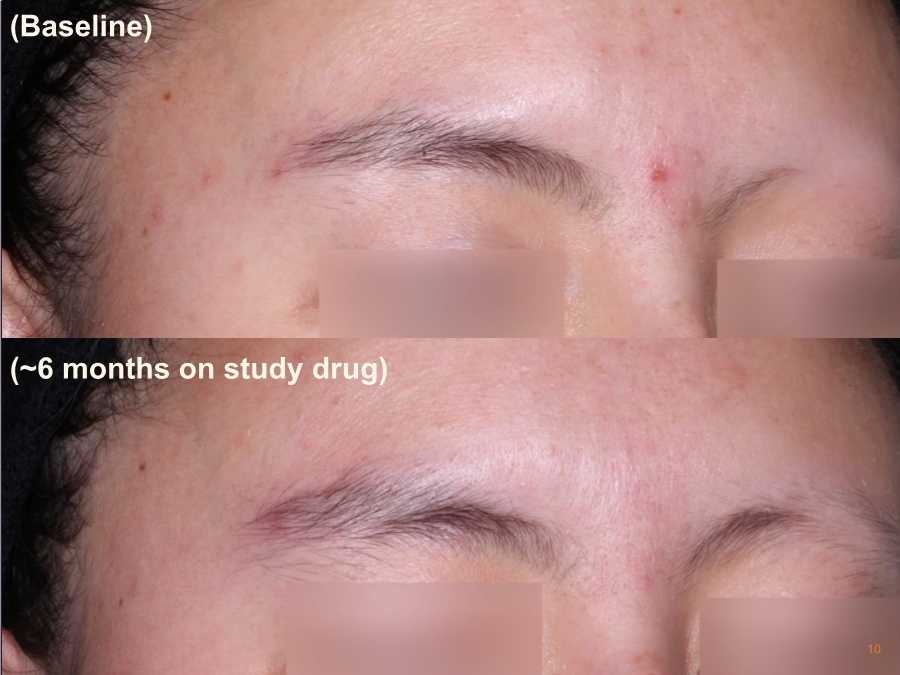
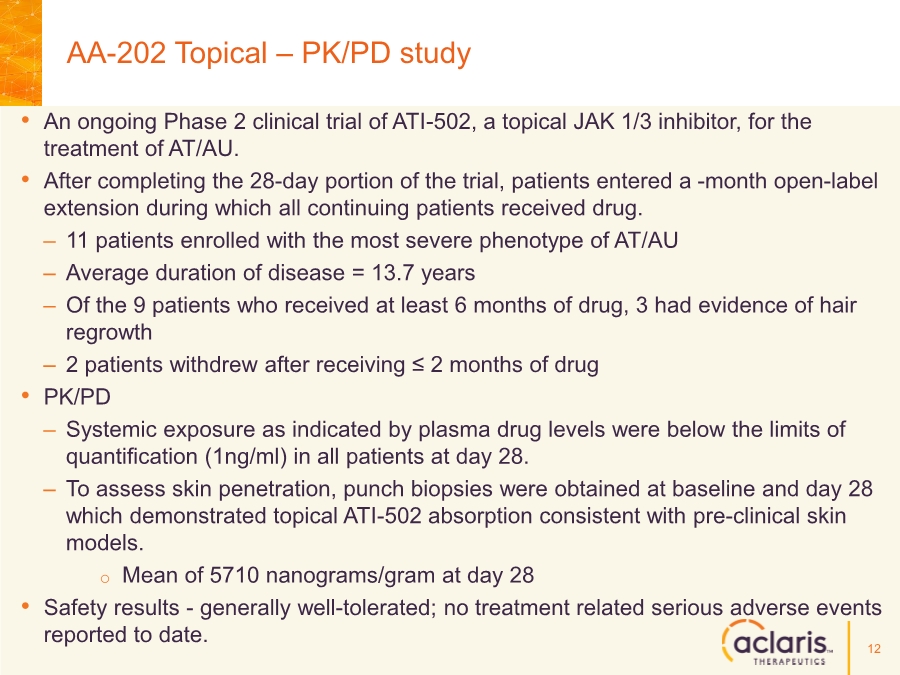
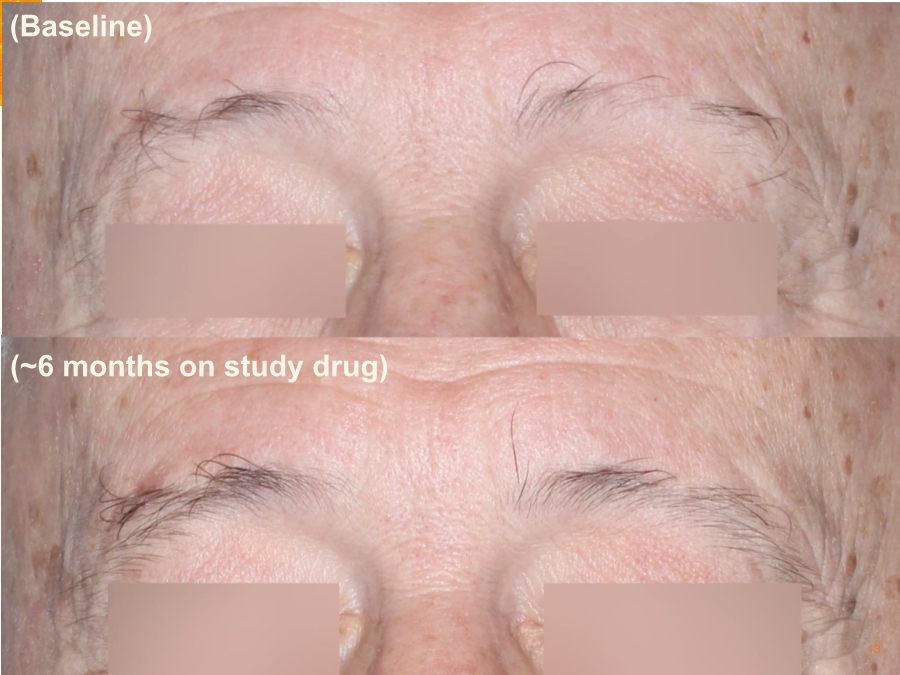
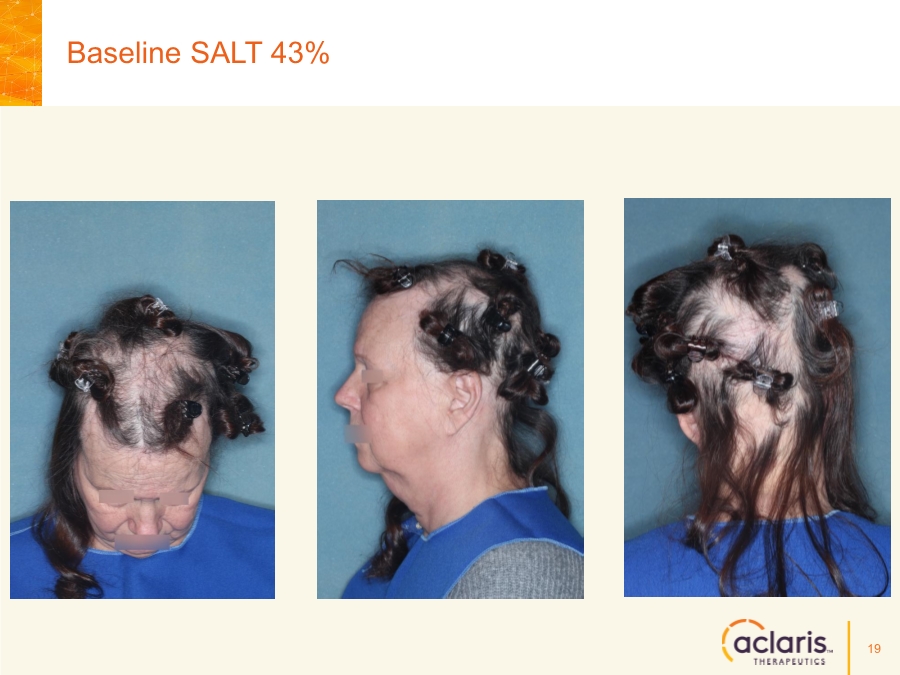
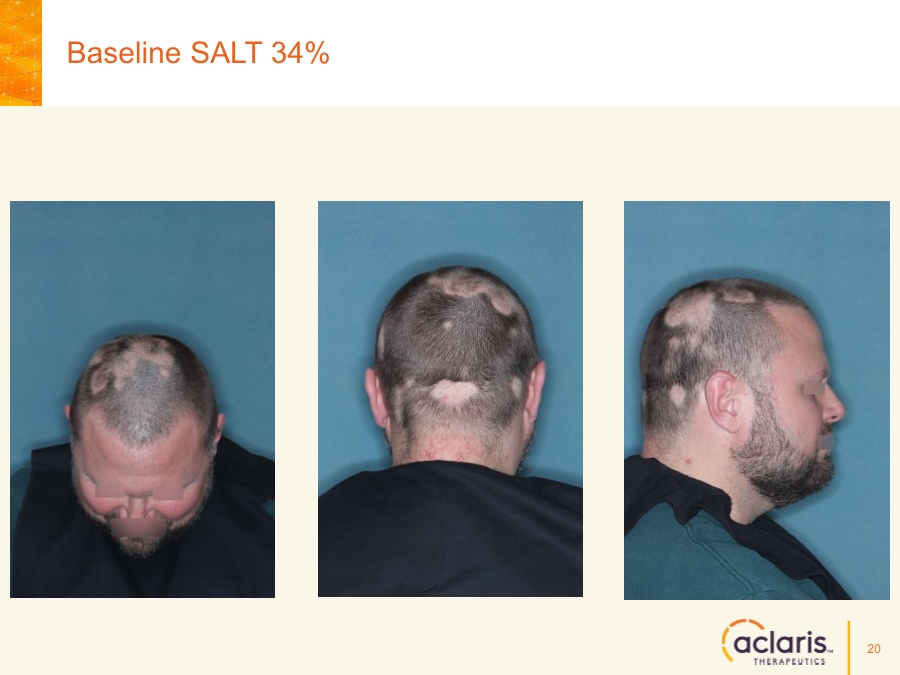
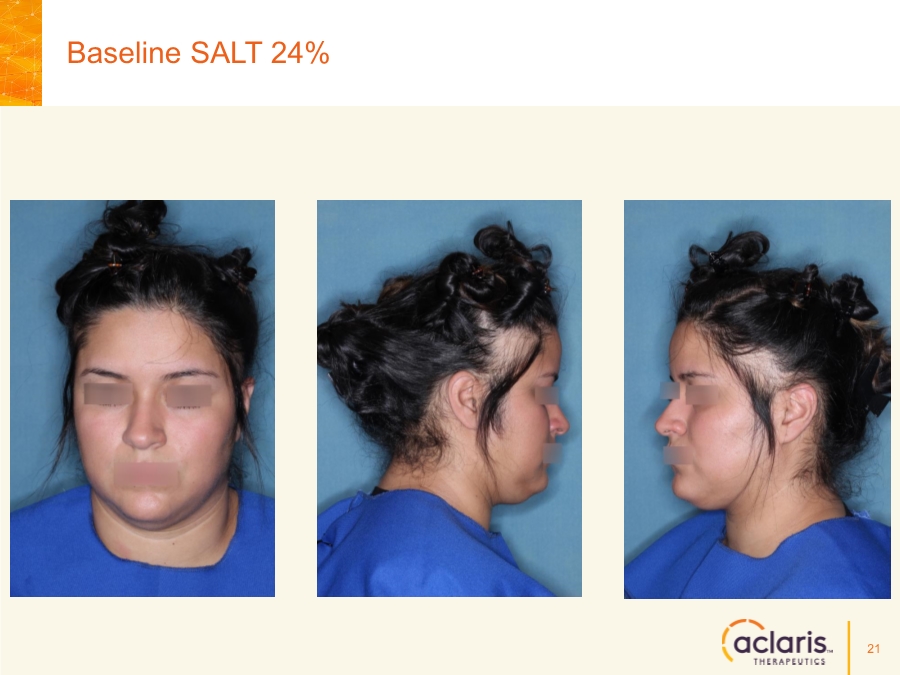
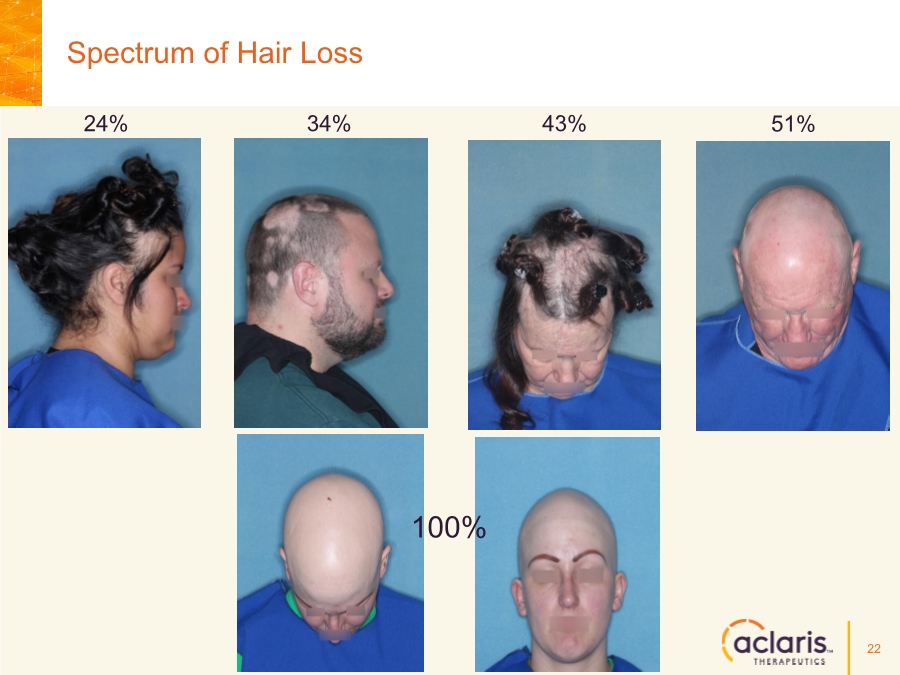
| Cautionary Note Regarding Forward-Looking Statements Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "believe", "expect", "may", "plan," "potential," "will," and similar expressions, and are based on Aclaris’ current beliefs and expectations. These forward-looking statements include expectations regarding Aclaris’ clinical development of its drug candidates, including the timing for initiation and completion of planned clinical trials and the availability of data from these trials. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris’ reliance on third parties over which it may not always have full control, and other risks and uncertainties that are described in the Risk Factors section of Aclaris’ Annual Report on Form 10-K filed for the year ended December 31, 2017, Aclaris’ Quarterly Report on Form 10-Q filed earlier for the quarter ended September 30, 2018 and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the “SEC filings” section of the Investors page of Aclaris’ website at http://www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this presentation, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise. Cautionary Note Regarding Forward-Looking Statements 2 |