Exhibit 99.1
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Company Overview May 2020 EMPOWERING PATIENTS THROUGH KINOME INNOVATION |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "believe,” "expect," "may,“ "plan," "potential," "will," and similar expressions, and are based on Aclaris' current beliefs and expectations. These forward-looking statements include expectations regarding Aclaris’ development of its drug candidates, including the timing for initiation and completion of clinical trials, the availability of data from these trials and the timing of its regulatory submissions related to these trials. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris' reliance on third parties over which it may not always have full control, the uncertainty regarding the COVID-19 pandemic including its impact on the timing of Aclaris’ regulatory and research and development activities, and other risks and uncertainties that are described in the Risk Factors section of Aclaris’ Annual Report on Form 10-K for the year ended December 31, 2019, Aclaris’ Quarterly Report on Form 10-Q for the quarter ended March 31, 2020 and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the “SEC filings" section of the Investors page of Aclaris' website at http://www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this presentation, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Cautionary Note Regarding Forward-Looking Statements 2 |
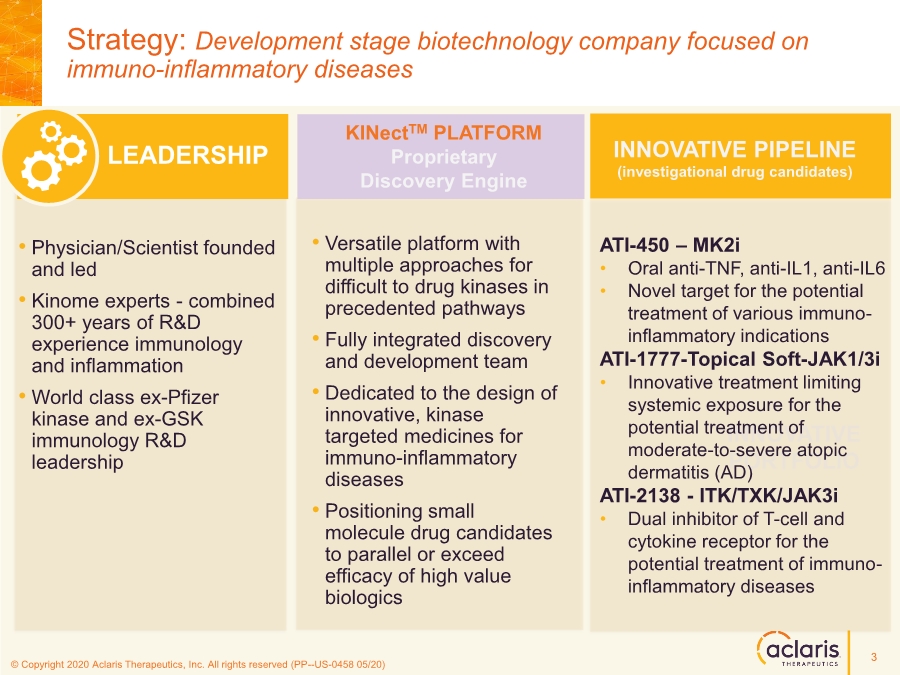
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) LEADERSHIP • Physician/Scientist founded and led • Kinome experts - combined 300+ years of R&D experience immunology and inflammation • World class ex-Pfizer kinase and ex-GSK immunology R&D leadership Strategy: Development stage biotechnology company focused on immuno-inflammatory diseases KINectTM PLATFORM Proprietary Discovery Engine • Versatile platform with multiple approaches for difficult to drug kinases in precedented pathways • Fully integrated discovery and development team • Dedicated to the design of innovative, kinase targeted medicines for immuno-inflammatory diseases • Positioning small molecule drug candidates to parallel or exceed efficacy of high value biologics INNOVATIVE PORTFOLIO INNOVATIVE PIPELINE (investigational drug candidates) ATI-450 – MK2i • Oral anti-TNF, anti-IL1, anti-IL6 • Novel target for the potential treatment of various immuno- inflammatory indications ATI-1777-Topical Soft-JAK1/3i • Innovative treatment limiting systemic exposure for the potential treatment of moderate-to-severe atopic dermatitis (AD) ATI-2138 - ITK/TXK/JAK3i • Dual inhibitor of T-cell and cytokine receptor for the potential treatment of immuno- inflammatory diseases 3 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) R&D Leadership Team Experienced team with deep scientific and operational experience Former VP Research & Global Head, Pfizer Inflammation, co-leader of Pfizer Licensing Team Delivered 8 clinical candidates, 6 INDs and 1 NDA in inflammation and cancer Walter Smith SVP, R&D Jon Jacobsen, PhD VP, Chemistry Former Research Fellow and Director, Pfizer Chemistry >100 publications and patents (15 total on kinases) Project Lead for PFE JAK Program Paul Changelian, PhD VP, Biology Immunologist/drug discovery leader at pharma (Pfizer & biotech) Validated JAK 1/3 as target for transplant/RA/psoriasis, leading to approval of XELJANZ® David R Anderson, PhD Sr. Director, Discovery, Early Development Former research project leader at Pfizer. Director of Chemistry at Mnemosyne, Luc, Cadent. Inventor of 6 clinical candidates and author of 40 peer reviewed publications and patents Gary DeCrescenzo SVP, Pharm R&D Former Exec. Director, Pfizer. Site Head for Medicinal & Structural Chemistry. >100 patents. Co-inventor of multiple drug candidates David Gordon Chief Medical Officer Former SVP, R&D at GSK. Led discovery and development teams in Immuno-Inflammation and Dermatology leading to multiple successful NDAs, including NUCALA® & BENLYSTA® * All trademarks are the property of their respective owners. Former Executive Director, Pfizer Inflammation Research and Leader of Global Kinase Technology Team >95 publications and patents (>30 total on kinases) Joseph Monahan, PhD Exec. VP R&D (Head of Discovery) 4 |
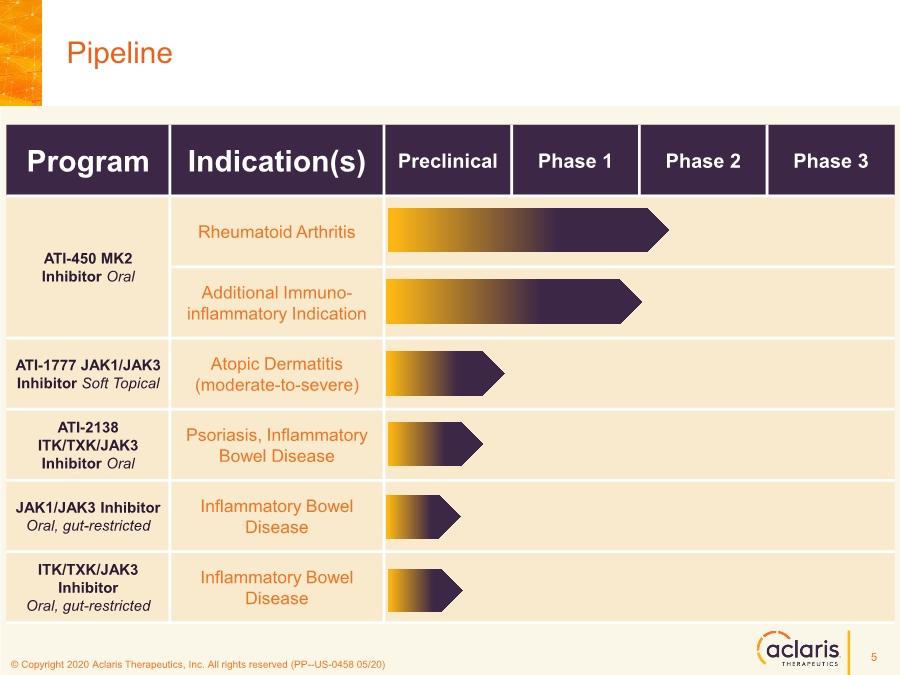
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Pipeline Program Indication(s) Preclinical Phase 1 Phase 2 Phase 3 ATI-450 MK2 Inhibitor Oral Rheumatoid Arthritis Additional Immuno- inflammatory Indication ATI-1777 JAK1/JAK3 Inhibitor Soft Topical Atopic Dermatitis (moderate-to-severe) ATI-2138 ITK/TXK/JAK3 Inhibitor Oral Psoriasis, Inflammatory Bowel Disease JAK1/JAK3 Inhibitor Oral, gut-restricted Inflammatory Bowel Disease ITK/TXK/JAK3 Inhibitor Oral, gut-restricted Inflammatory Bowel Disease 5 |
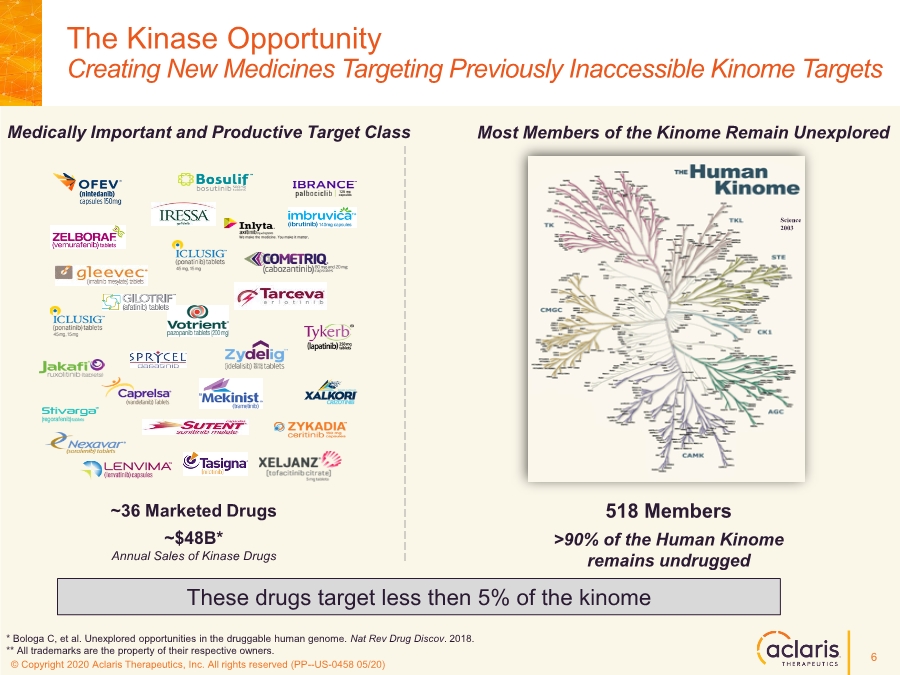
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) The Kinase Opportunity Creating New Medicines Targeting Previously Inaccessible Kinome Targets Science 2003 ~36 Marketed Drugs ~$48B* Annual Sales of Kinase Drugs Medically Important and Productive Target Class Most Members of the Kinome Remain Unexplored 518 Members >90% of the Human Kinome remains undrugged These drugs target less then 5% of the kinome * Bologa C, et al. Unexplored opportunities in the druggable human genome. Nat Rev Drug Discov. 2018. ** All trademarks are the property of their respective owners. 6 |
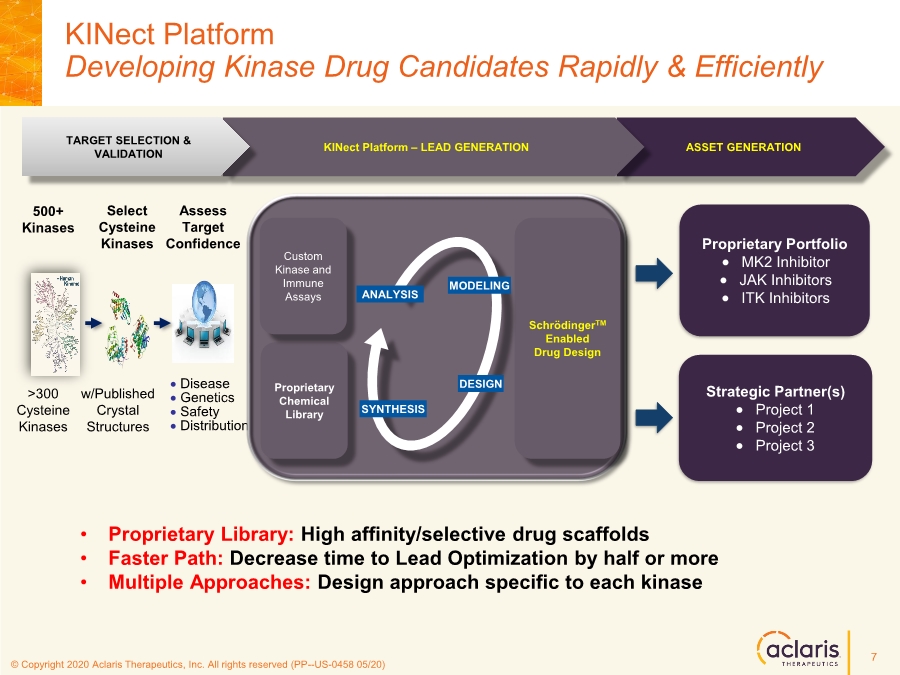
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) KINect Platform Developing Kinase Drug Candidates Rapidly & Efficiently Proprietary Portfolio • MK2 Inhibitor • JAK Inhibitors • ITK Inhibitors Strategic Partner(s) • Project 1 • Project 2 • Project 3 ASSET GENERATION KINect Platform – LEAD GENERATION TARGET SELECTION & VALIDATION >300 Cysteine Kinases 500+ Kinases Select Cysteine Kinases w/Published Crystal Structures Assess Target Confidence • Disease • Genetics • Safety • Distribution • Proprietary Library: High affinity/selective drug scaffolds • Faster Path: Decrease time to Lead Optimization by half or more • Multiple Approaches: Design approach specific to each kinase Proprietary Chemical Library SchrödingerTM Enabled Drug Design Custom Kinase and Immune Assays MODELING DESIGN SYNTHESIS ANALYSIS 7 |
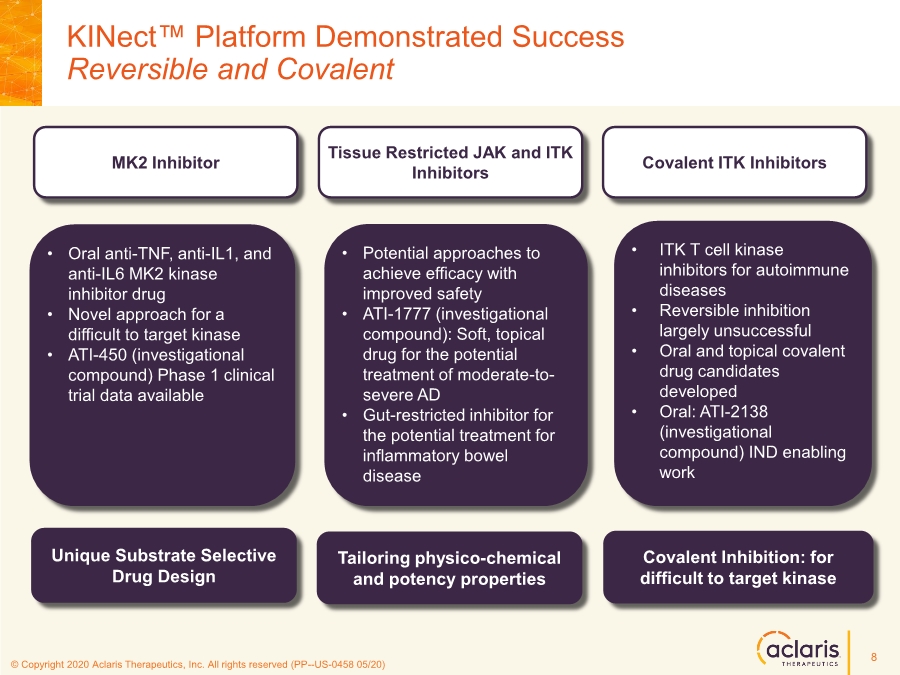
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) KINect™ Platform Demonstrated Success Reversible and Covalent • Oral anti-TNF, anti-IL1, and anti-IL6 MK2 kinase inhibitor drug • Novel approach for a difficult to target kinase • ATI-450 (investigational compound) Phase 1 clinical trial data available • ITK T cell kinase inhibitors for autoimmune diseases • Reversible inhibition largely unsuccessful • Oral and topical covalent drug candidates developed • Oral: ATI-2138 (investigational compound) IND enabling work • Potential approaches to achieve efficacy with improved safety • ATI-1777 (investigational compound): Soft, topical drug for the potential treatment of moderate-to- severe AD • Gut-restricted inhibitor for the potential treatment for inflammatory bowel disease Covalent ITK Inhibitors MK2 Inhibitor Tissue Restricted JAK and ITK Inhibitors Unique Substrate Selective Drug Design Covalent Inhibition: for difficult to target kinase Tailoring physico-chemical and potency properties 8 |
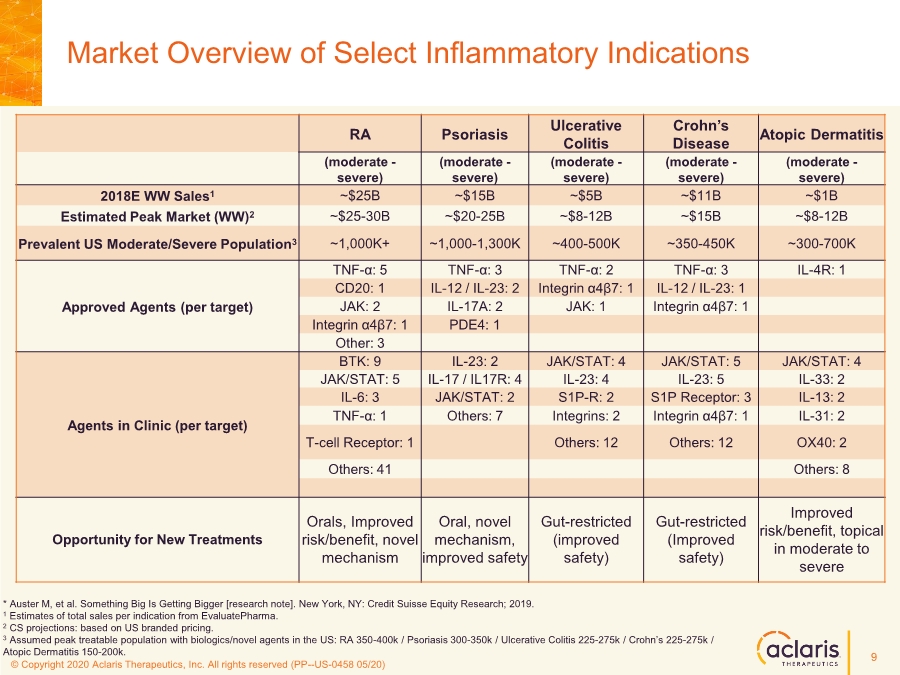
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Market Overview of Select Inflammatory Indications * Auster M, et al. Something Big Is Getting Bigger [research note]. New York, NY: Credit Suisse Equity Research; 2019. 1 Estimates of total sales per indication from EvaluatePharma. 2 CS projections: based on US branded pricing. 3 Assumed peak treatable population with biologics/novel agents in the US: RA 350-400k / Psoriasis 300-350k / Ulcerative Colitis 225-275k / Crohn’s 225-275k / Atopic Dermatitis 150-200k. RA Psoriasis Ulcerative Colitis Crohn’s Disease Atopic Dermatitis (moderate - severe) (moderate - severe) (moderate - severe) (moderate - severe) (moderate - severe) 2018E WW Sales1 ~$25B ~$15B ~$5B ~$11B ~$1B Estimated Peak Market (WW)2 ~$25-30B ~$20-25B ~$8-12B ~$15B ~$8-12B Prevalent US Moderate/Severe Population3 ~1,000K+ ~1,000-1,300K ~400-500K ~350-450K ~300-700K Approved Agents (per target) TNF-α: 5 TNF-α: 3 TNF-α: 2 TNF-α: 3 IL-4R: 1 CD20: 1 IL-12 / IL-23: 2 Integrin α4β7: 1 IL-12 / IL-23: 1 JAK: 2 IL-17A: 2 JAK: 1 Integrin α4β7: 1 Integrin α4β7: 1 PDE4: 1 Other: 3 Agents in Clinic (per target) BTK: 9 IL-23: 2 JAK/STAT: 4 JAK/STAT: 5 JAK/STAT: 4 JAK/STAT: 5 IL-17 / IL17R: 4 IL-23: 4 IL-23: 5 IL-33: 2 IL-6: 3 JAK/STAT: 2 S1P-R: 2 S1P Receptor: 3 IL-13: 2 TNF-α: 1 Others: 7 Integrins: 2 Integrin α4β7: 1 IL-31: 2 T-cell Receptor: 1 Others: 12 Others: 12 OX40: 2 Others: 41 Others: 8 Opportunity for New Treatments Orals, Improved risk/benefit, novel mechanism Oral, novel mechanism, improved safety Gut-restricted (improved safety) Gut-restricted (Improved safety) Improved risk/benefit, topical in moderate to severe 9 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-450: MK2 Inhibitor (Investigational Drug Candidate) 10 |
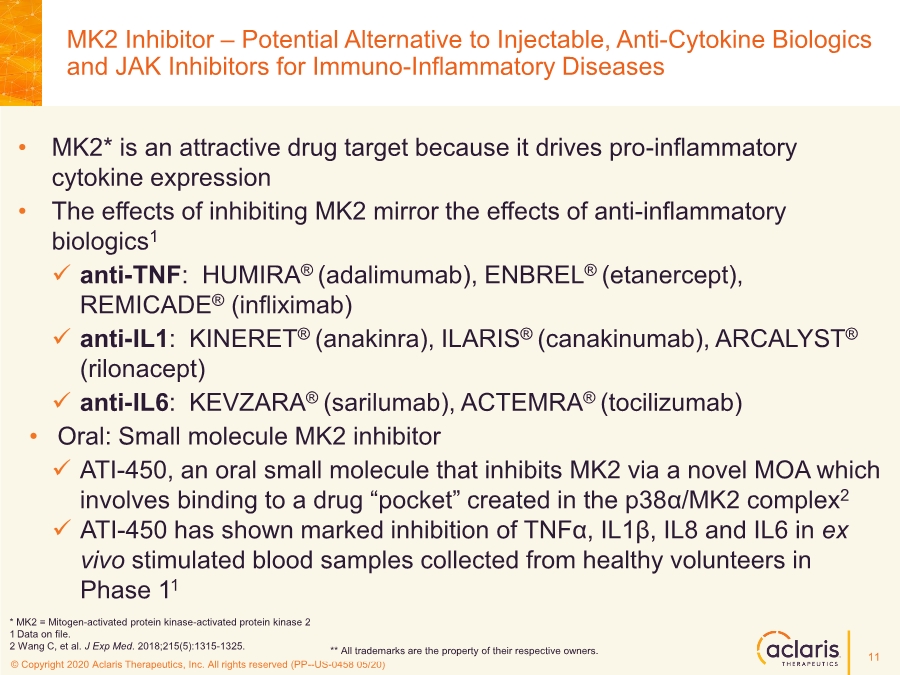
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) MK2 Inhibitor – Potential Alternative to Injectable, Anti-Cytokine Biologics and JAK Inhibitors for Immuno-Inflammatory Diseases • MK2* is an attractive drug target because it drives pro-inflammatory cytokine expression • The effects of inhibiting MK2 mirror the effects of anti-inflammatory biologics1 ✓ anti-TNF: HUMIRA® (adalimumab), ENBREL® (etanercept), REMICADE® (infliximab) ✓ anti-IL1: KINERET® (anakinra), ILARIS® (canakinumab), ARCALYST® (rilonacept) ✓ anti-IL6: KEVZARA® (sarilumab), ACTEMRA® (tocilizumab) • Oral: Small molecule MK2 inhibitor ✓ ATI-450, an oral small molecule that inhibits MK2 via a novel MOA which involves binding to a drug “pocket” created in the p38α/MK2 complex2 ✓ ATI-450 has shown marked inhibition of TNFα, IL1β, IL8 and IL6 in ex vivo stimulated blood samples collected from healthy volunteers in Phase 11 ** All trademarks are the property of their respective owners. * MK2 = Mitogen-activated protein kinase-activated protein kinase 2 1 Data on file. 2 Wang C, et al. J Exp Med. 2018;215(5):1315-1325. 11 |
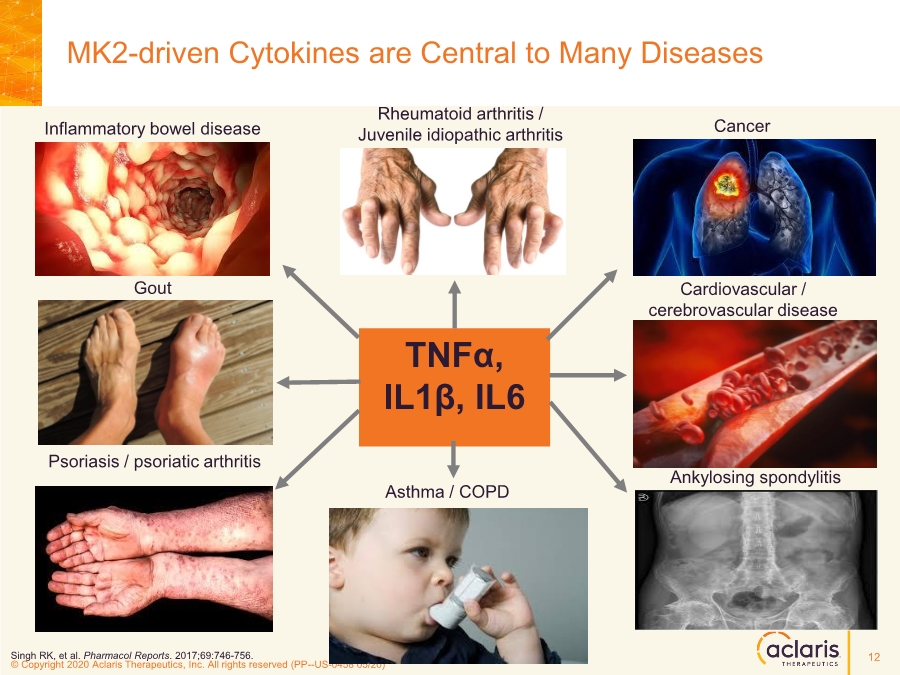
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) MK2-driven Cytokines are Central to Many Diseases TNFα, IL1β, IL6 Psoriasis / psoriatic arthritis Gout Inflammatory bowel disease Rheumatoid arthritis / Juvenile idiopathic arthritis Ankylosing spondylitis Asthma / COPD Cardiovascular / cerebrovascular disease Cancer Singh RK, et al. Pharmacol Reports. 2017;69:746-756. 12 |
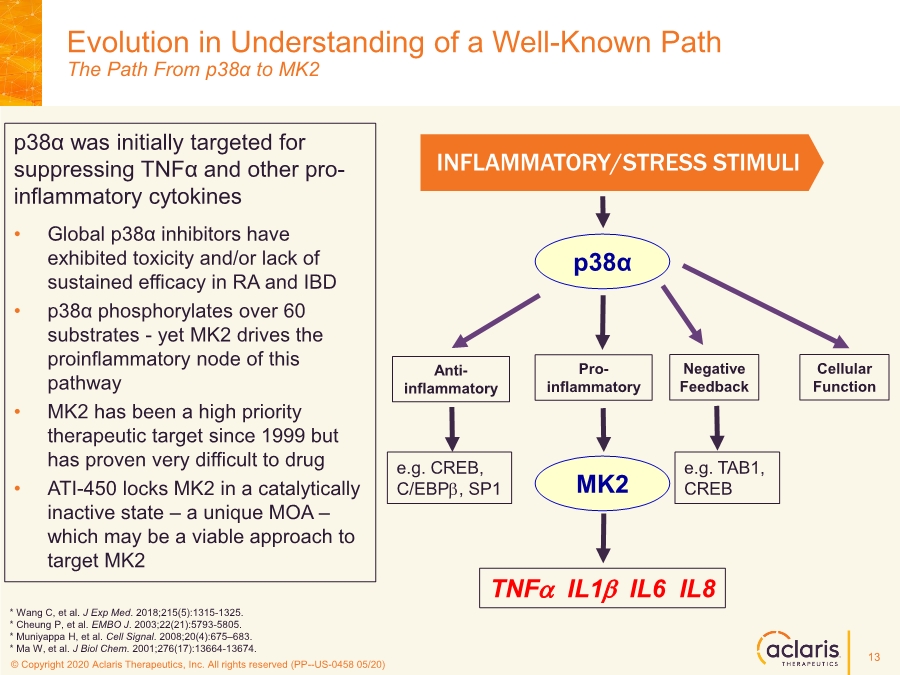
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Evolution in Understanding of a Well-Known Path The Path From p38α to MK2 INFLAMMATORY/STRESS STIMULI p38α Anti- inflammatory Negative Feedback TNFa IL1b IL6 IL8 MK2 Cellular Function Pro- inflammatory p38α was initially targeted for suppressing TNFα and other pro- inflammatory cytokines • Global p38α inhibitors have exhibited toxicity and/or lack of sustained efficacy in RA and IBD • p38α phosphorylates over 60 substrates - yet MK2 drives the proinflammatory node of this pathway • MK2 has been a high priority therapeutic target since 1999 but has proven very difficult to drug • ATI-450 locks MK2 in a catalytically inactive state – a unique MOA – which may be a viable approach to target MK2 e.g. CREB, C/EBPb, SP1 e.g. TAB1, CREB * Wang C, et al. J Exp Med. 2018;215(5):1315-1325. * Cheung P, et al. EMBO J. 2003;22(21):5793-5805. * Muniyappa H, et al. Cell Signal. 2008;20(4):675–683. * Ma W, et al. J Biol Chem. 2001;276(17):13664-13674. 13 |
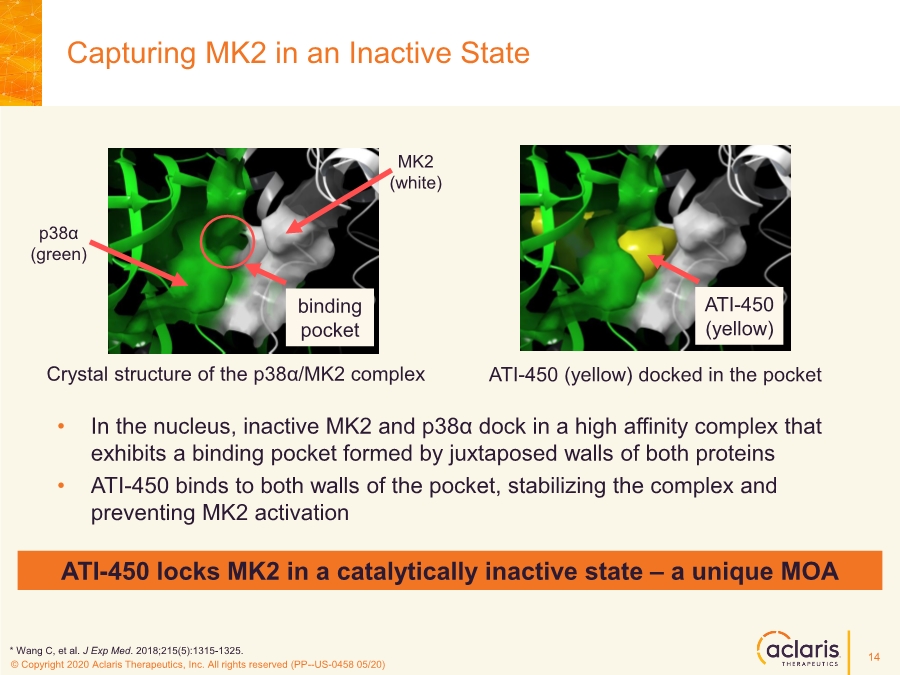
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Capturing MK2 in an Inactive State Crystal structure of the p38α/MK2 complex ATI-450 (yellow) docked in the pocket • In the nucleus, inactive MK2 and p38α dock in a high affinity complex that exhibits a binding pocket formed by juxtaposed walls of both proteins • ATI-450 binds to both walls of the pocket, stabilizing the complex and preventing MK2 activation binding pocket p38α (green) MK2 (white) ATI-450 (yellow) ATI-450 locks MK2 in a catalytically inactive state – a unique MOA * Wang C, et al. J Exp Med. 2018;215(5):1315-1325. 14 |
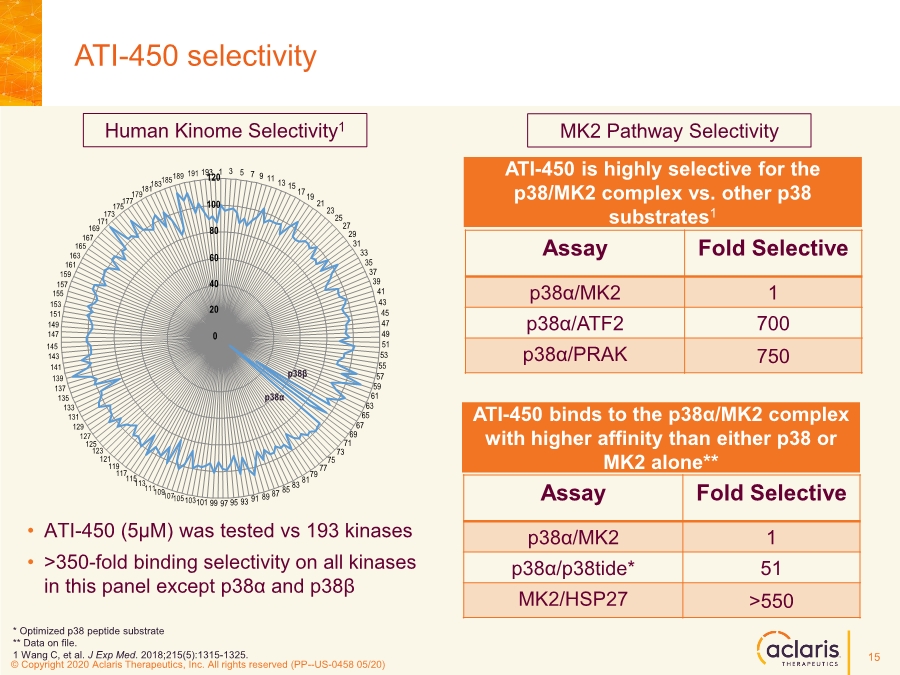
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • ATI-450 (5µM) was tested vs 193 kinases • >350-fold binding selectivity on all kinases in this panel except p38α and p38β ATI-450 selectivity Assay Fold Selective p38α/MK2 1 p38α/p38tide* 51 MK2/HSP27 >550 Human Kinome Selectivity1 MK2 Pathway Selectivity * Optimized p38 peptide substrate ** Data on file. 1 Wang C, et al. J Exp Med. 2018;215(5):1315-1325. ATI-450 is highly selective for the p38/MK2 complex vs. other p38 substrates1 Assay Fold Selective p38α/MK2 1 p38α/ATF2 700 p38α/PRAK 750 ATI-450 binds to the p38α/MK2 complex with higher affinity than either p38 or MK2 alone** 0 20 40 60 80 100 120 1 3 5 7 9 11 13 15 1719 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 95 97 99 101 p38α p38β 103 105 107 109 111 113 115 117 119 121 123 125 127 131 129 133 135 137 139 141 143 145 147 151 149 153 155 157 159 161 163 165 167 171 169 173 175 177 179 181 183185189 191 193 15 |
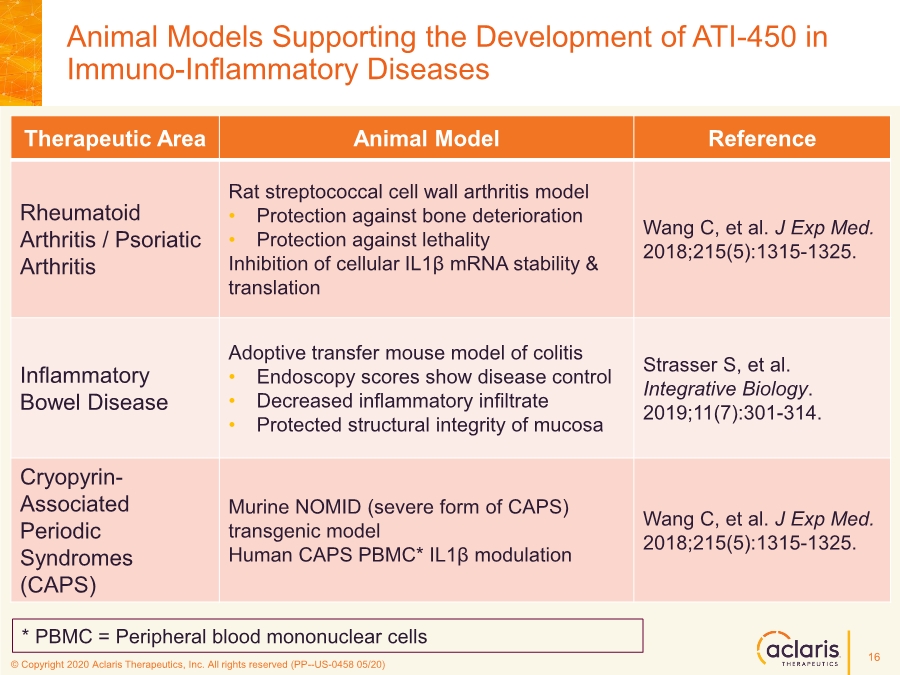
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Animal Models Supporting the Development of ATI-450 in Immuno-Inflammatory Diseases Therapeutic Area Animal Model Reference Rheumatoid Arthritis / Psoriatic Arthritis Rat streptococcal cell wall arthritis model • Protection against bone deterioration • Protection against lethality Inhibition of cellular IL1β mRNA stability & translation Wang C, et al. J Exp Med. 2018;215(5):1315-1325. Inflammatory Bowel Disease Adoptive transfer mouse model of colitis • Endoscopy scores show disease control • Decreased inflammatory infiltrate • Protected structural integrity of mucosa Strasser S, et al. Integrative Biology. 2019;11(7):301-314. Cryopyrin- Associated Periodic Syndromes (CAPS) Murine NOMID (severe form of CAPS) transgenic model Human CAPS PBMC* IL1β modulation Wang C, et al. J Exp Med. 2018;215(5):1315-1325. * PBMC = Peripheral blood mononuclear cells 16 |
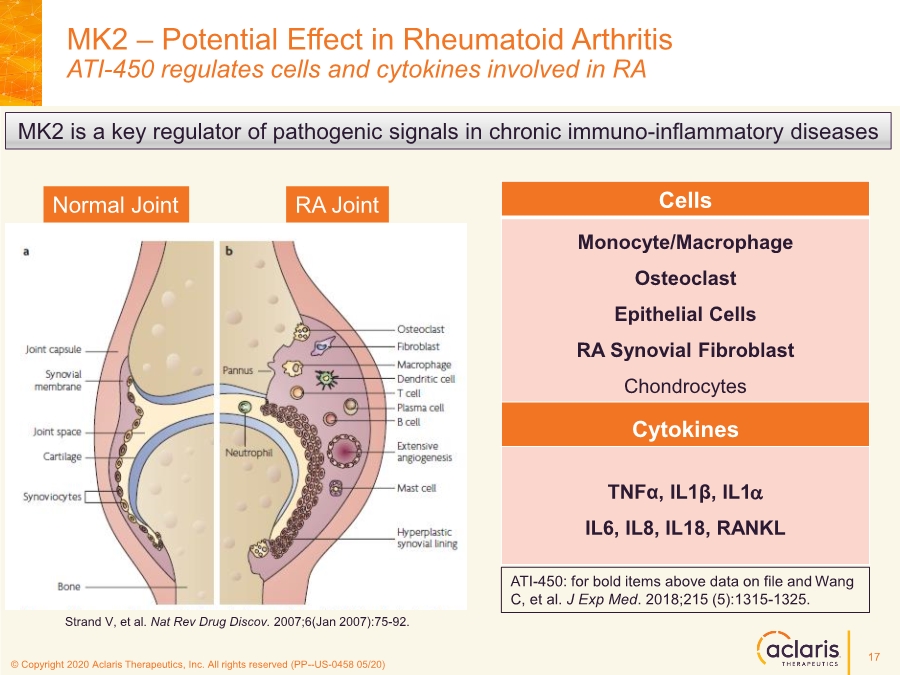
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) MK2 – Potential Effect in Rheumatoid Arthritis ATI-450 regulates cells and cytokines involved in RA MK2 is a key regulator of pathogenic signals in chronic immuno-inflammatory diseases Cells Monocyte/Macrophage Osteoclast Epithelial Cells RA Synovial Fibroblast Chondrocytes Cytokines TNFα, IL1β, IL1a IL6, IL8, IL18, RANKL Normal Joint RA Joint Strand V, et al. Nat Rev Drug Discov. 2007;6(Jan 2007):75-92. ATI-450: for bold items above data on file and Wang C, et al. J Exp Med. 2018;215 (5):1315-1325. 17 |
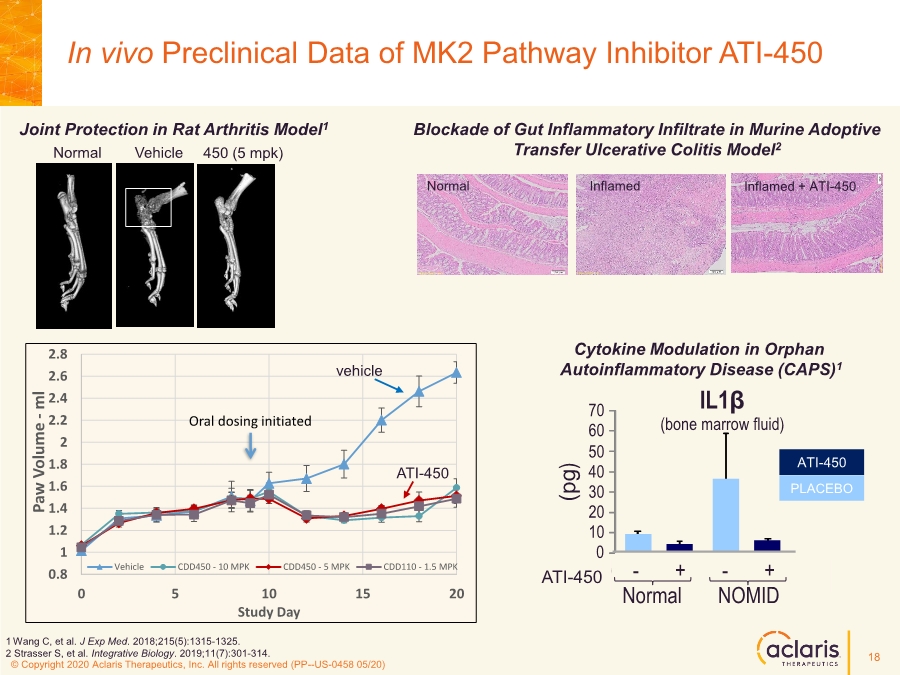
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) In vivo Preclinical Data of MK2 Pathway Inhibitor ATI-450 Normal Vehicle 450 (5 mpk) Joint Protection in Rat Arthritis Model1 Cytokine Modulation in Orphan Autoinflammatory Disease (CAPS)1 Normal Inflamed Inflamed + ATI-450 Blockade of Gut Inflammatory Infiltrate in Murine Adoptive Transfer Ulcerative Colitis Model2 1 Wang C, et al. J Exp Med. 2018;215(5):1315-1325. 2 Strasser S, et al. Integrative Biology. 2019;11(7):301-314. 0.8 1 1.2 1.4 1.6 1.8 2 2.2 2.4 2.6 2.8 0 5 10 15 20 Paw Volume - ml Study Day Vehicle CDD450 - 10 MPK CDD450 - 5 MPK CDD110 - 1.5 MPK Oral dosing initiated vehicle ATI-450 IL1β (bone marrow fluid) CDD-450 - + - + 0 10 20 30 40 50 60 70 ( p g ) ( n g / m l ) 0 0.5 1.0 1.5 2.0 2.5 3.0 Normal NOMID CDD-450 - + - + Normal NOMID IL6 (serum) ( p g ) 0 10 20 30 40 50 60 70 CDD-450 - + - + Normal NOMID IL18 (bone marrow fluid) ATI-450 ATI-450 ATI-450 ATI-450 PLACEBO 18 |
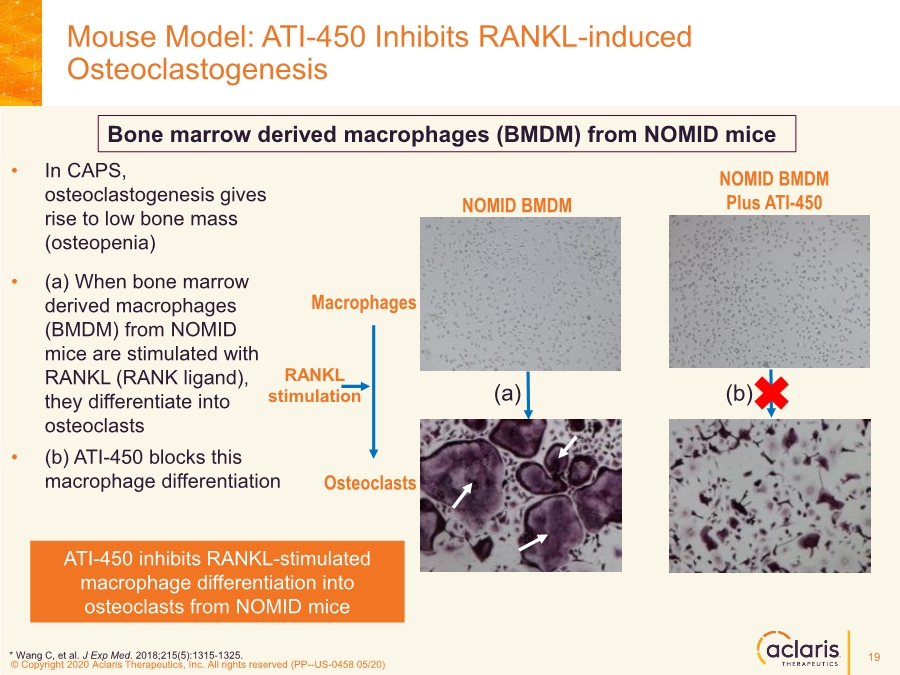
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • In CAPS, osteoclastogenesis gives rise to low bone mass (osteopenia) •(a) When bone marrow derived macrophages (BMDM) from NOMID mice are stimulated with RANKL (RANK ligand), they differentiate into osteoclasts •(b) ATI-450 blocks this macrophage differentiation Mouse Model: ATI-450 Inhibits RANKL-induced Osteoclastogenesis ATI-450 inhibits RANKL-stimulated macrophage differentiation into osteoclasts from NOMID mice * Wang C, et al. J Exp Med. 2018;215(5):1315-1325. RANKL stimulation Macrophages Osteoclasts NOMID BMDM NOMID BMDM Plus ATI-450 (a) (b) Bone marrow derived macrophages (BMDM) from NOMID mice 19 |
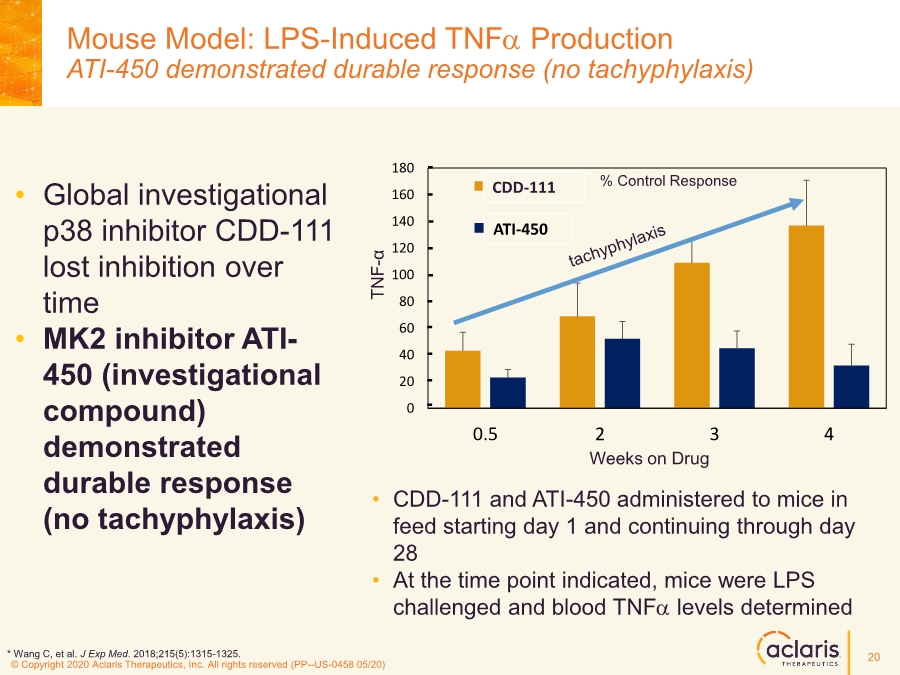
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Mouse Model: LPS-Induced TNFa Production ATI-450 demonstrated durable response (no tachyphylaxis) • CDD-111 and ATI-450 administered to mice in feed starting day 1 and continuing through day 28 • At the time point indicated, mice were LPS challenged and blood TNFa levels determined 0 20 40 60 80 100 120 140 160 180 0.5 2 3 4 CDD111 CDD450 ATI-450 % Control Response Weeks on Drug • Global investigational p38 inhibitor CDD-111 lost inhibition over time • MK2 inhibitor ATI- 450 (investigational compound) demonstrated durable response (no tachyphylaxis) TNF - α CDD-111 * Wang C, et al. J Exp Med. 2018;215(5):1315-1325. 20 |
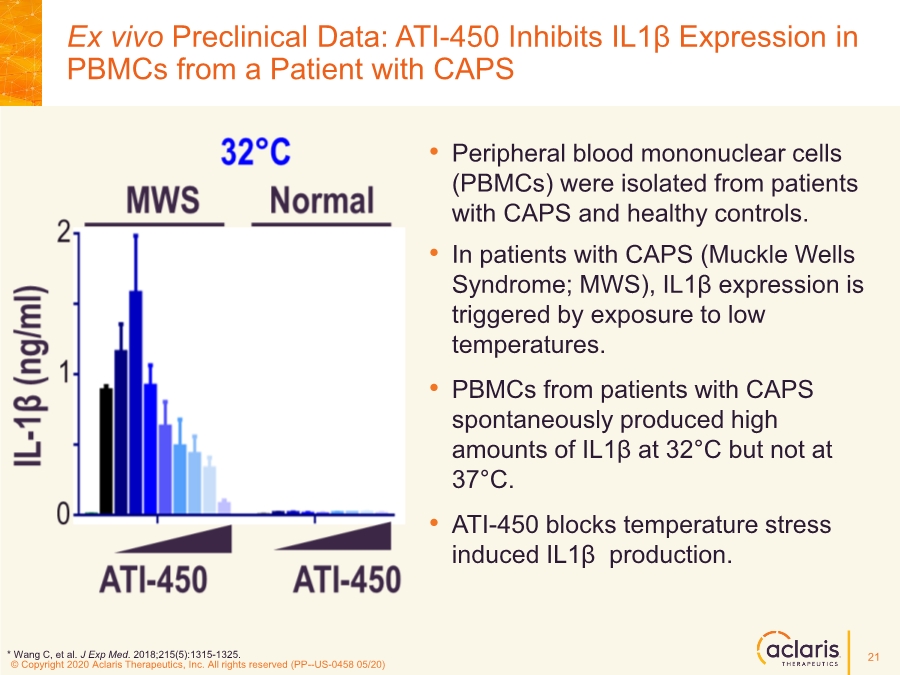
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Ex vivo Preclinical Data: ATI-450 Inhibits IL1β Expression in PBMCs from a Patient with CAPS • Peripheral blood mononuclear cells (PBMCs) were isolated from patients with CAPS and healthy controls. • In patients with CAPS (Muckle Wells Syndrome; MWS), IL1β expression is triggered by exposure to low temperatures. • PBMCs from patients with CAPS spontaneously produced high amounts of IL1β at 32°C but not at 37°C. • ATI-450 blocks temperature stress induced IL1β production. * Wang C, et al. J Exp Med. 2018;215(5):1315-1325. 21 |
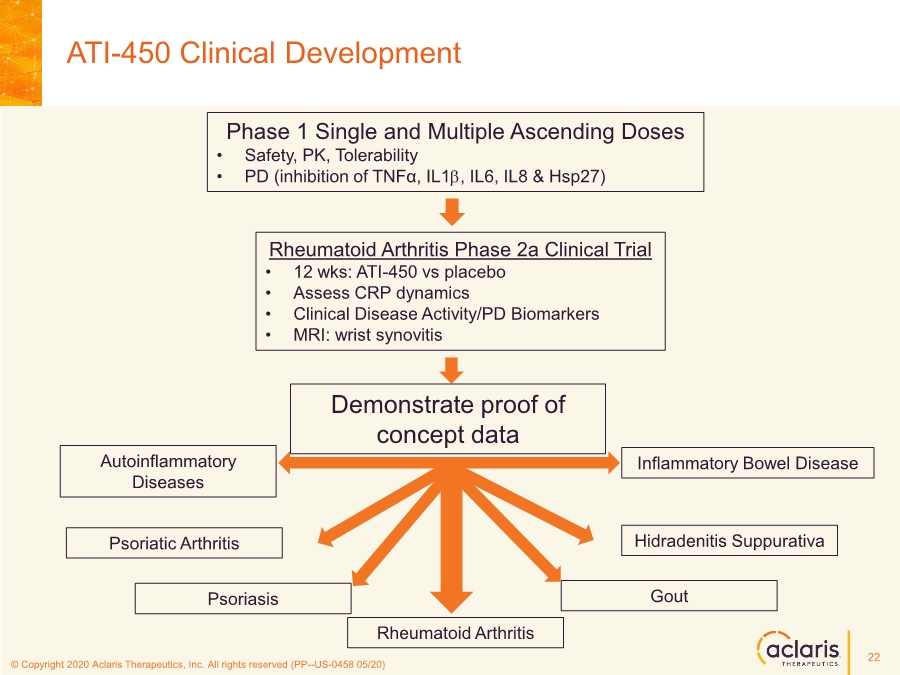
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-450 Clinical Development Rheumatoid Arthritis Phase 2a Clinical Trial • 12 wks: ATI-450 vs placebo • Assess CRP dynamics • Clinical Disease Activity/PD Biomarkers • MRI: wrist synovitis Demonstrate proof of concept data Rheumatoid Arthritis Psoriasis Psoriatic Arthritis Hidradenitis Suppurativa Inflammatory Bowel Disease Gout Autoinflammatory Diseases Phase 1 Single and Multiple Ascending Doses • Safety, PK, Tolerability • PD (inhibition of TNFα, IL1b, IL6, IL8 & Hsp27) 22 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • First-in-human, randomized, observer-blind, placebo- controlled trial ✓Single Ascending Doses and Multiple Ascending Doses (SAD/MAD) • Objectives: ✓Primary • To assess the safety, tolerability, and pharmacokinetics (PK) profile of ATI-450, an investigational oral MK2* inhibitor compound ✓Secondary • To assess the effect of food on the PK of ATI-450 • To explore the pharmacodynamics (PD) of ATI-450 • To evaluate the potential for an interaction with methotrexate ATI-450-PKPD-101 SAD/MAD Phase 1 Trial * MK2 = Mitogen-activated protein kinase-activated protein kinase 2 23 |
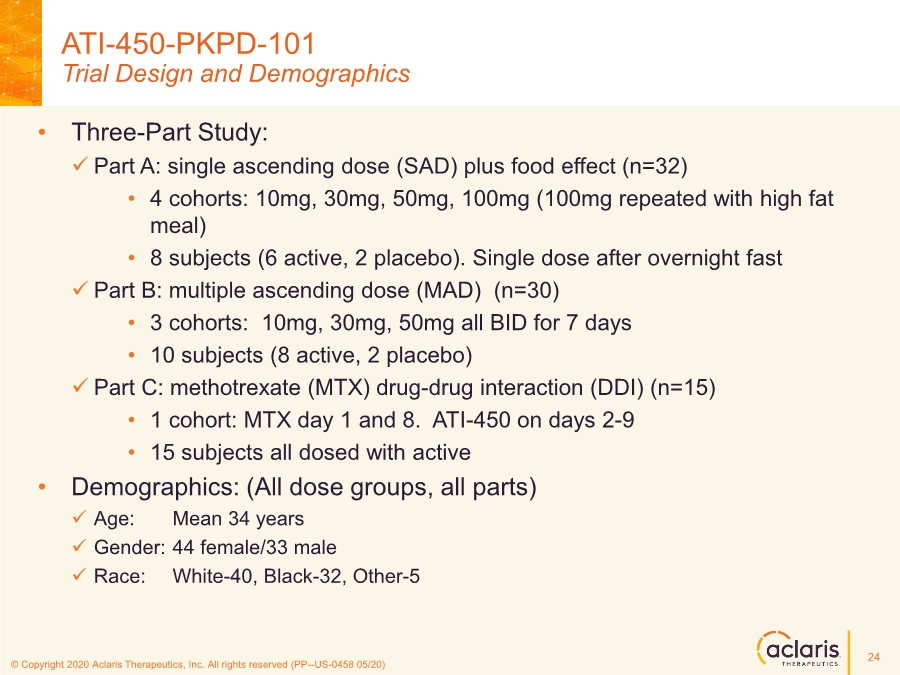
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • Three-Part Study: ✓ Part A: single ascending dose (SAD) plus food effect (n=32) • 4 cohorts: 10mg, 30mg, 50mg, 100mg (100mg repeated with high fat meal) • 8 subjects (6 active, 2 placebo). Single dose after overnight fast ✓ Part B: multiple ascending dose (MAD) (n=30) • 3 cohorts: 10mg, 30mg, 50mg all BID for 7 days • 10 subjects (8 active, 2 placebo) ✓ Part C: methotrexate (MTX) drug-drug interaction (DDI) (n=15) • 1 cohort: MTX day 1 and 8. ATI-450 on days 2-9 • 15 subjects all dosed with active • Demographics: (All dose groups, all parts) ✓ Age: Mean 34 years ✓ Gender: 44 female/33 male ✓ Race: White-40, Black-32, Other-5 ATI-450-PKPD-101 Trial Design and Demographics 24 |
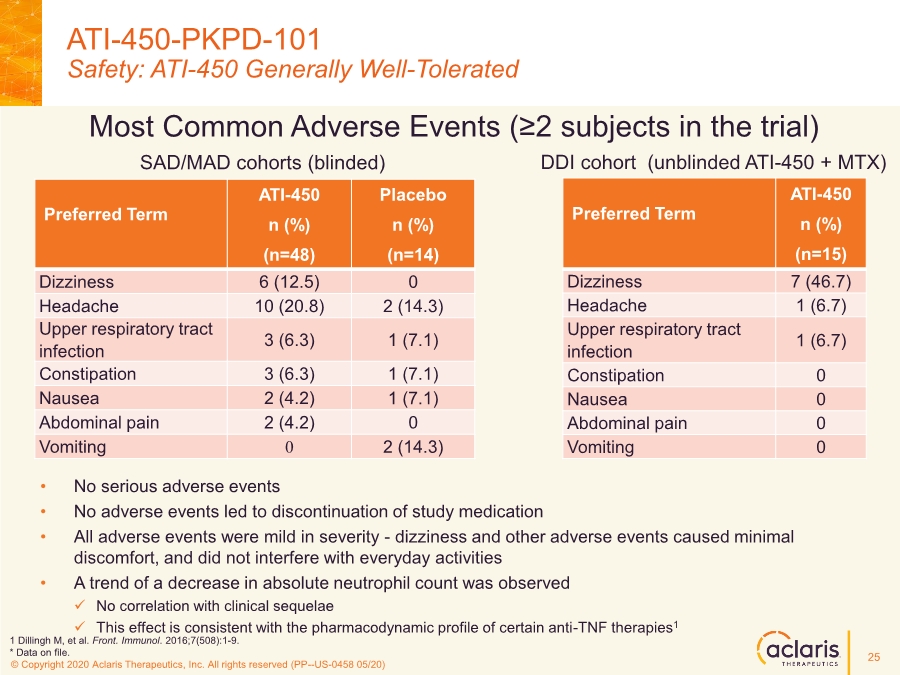
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Most Common Adverse Events (≥2 subjects in the trial) • No serious adverse events • No adverse events led to discontinuation of study medication • All adverse events were mild in severity - dizziness and other adverse events caused minimal discomfort, and did not interfere with everyday activities • A trend of a decrease in absolute neutrophil count was observed ✓ No correlation with clinical sequelae ✓ This effect is consistent with the pharmacodynamic profile of certain anti-TNF therapies1 ATI-450-PKPD-101 Safety: ATI-450 Generally Well-Tolerated Preferred Term ATI-450 n (%) (n=48) Placebo n (%) (n=14) Dizziness 6 (12.5) 0 Headache 10 (20.8) 2 (14.3) Upper respiratory tract infection 3 (6.3) 1 (7.1) Constipation 3 (6.3) 1 (7.1) Nausea 2 (4.2) 1 (7.1) Abdominal pain 2 (4.2) 0 Vomiting 0 2 (14.3) Preferred Term ATI-450 n (%) (n=15) Dizziness 7 (46.7) Headache 1 (6.7) Upper respiratory tract infection 1 (6.7) Constipation 0 Nausea 0 Abdominal pain 0 Vomiting 0 SAD/MAD cohorts (blinded) DDI cohort (unblinded ATI-450 + MTX) 1 Dillingh M, et al. Front. Immunol. 2016;7(508):1-9. * Data on file. 25 |
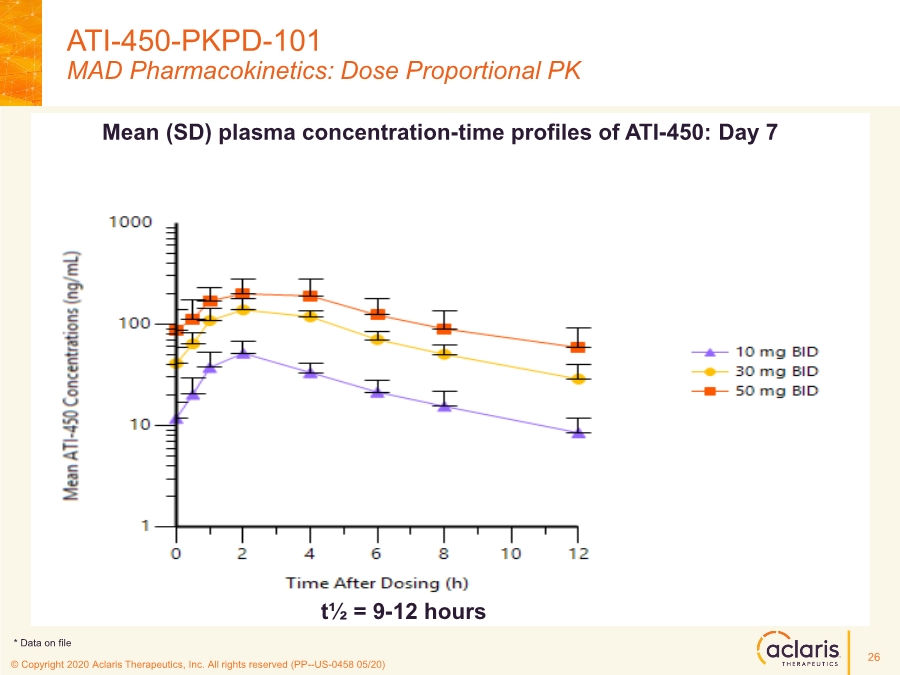
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-450-PKPD-101 MAD Pharmacokinetics: Dose Proportional PK 26 Mean (SD) plasma concentration-time profiles of ATI-450: Day 7 t½ = 9-12 hours * Data on file |
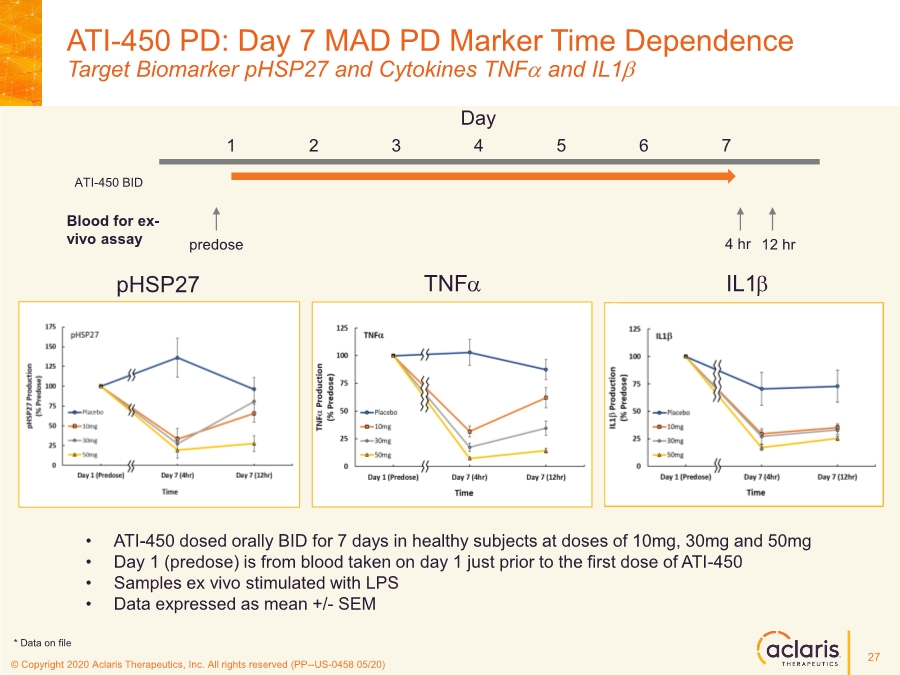
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-450 PD: Day 7 MAD PD Marker Time Dependence Target Biomarker pHSP27 and Cytokines TNFa and IL1b • ATI-450 dosed orally BID for 7 days in healthy subjects at doses of 10mg, 30mg and 50mg • Day 1 (predose) is from blood taken on day 1 just prior to the first dose of ATI-450 • Samples ex vivo stimulated with LPS • Data expressed as mean +/- SEM TNFa IL1b pHSP27 Day 1 2 3 4 5 6 7 ATI-450 BID Blood for ex- vivo assay predose 4 hr 12 hr 27 * Data on file |
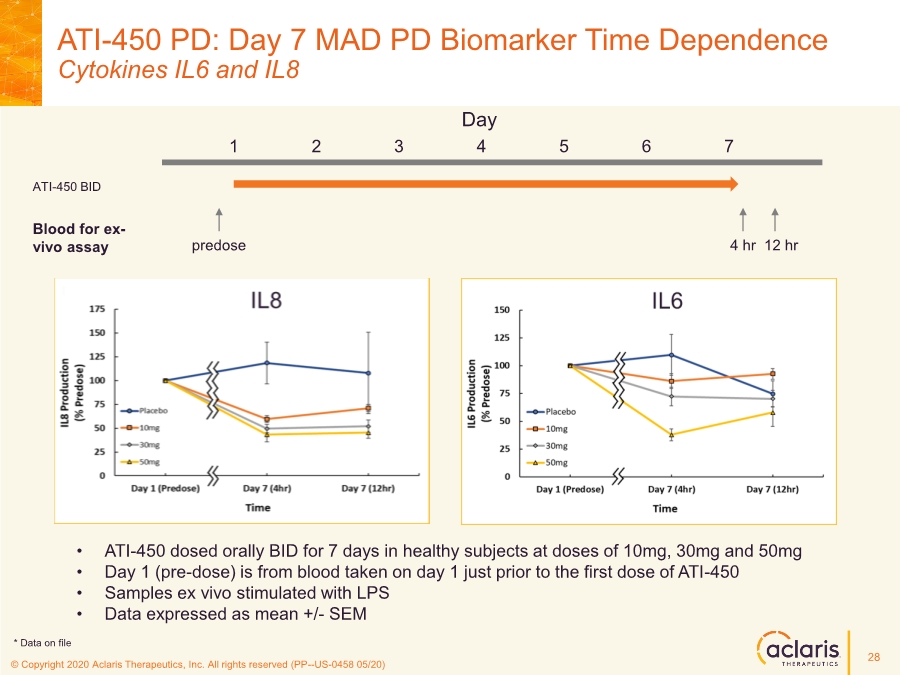
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-450 PD: Day 7 MAD PD Biomarker Time Dependence Cytokines IL6 and IL8 • ATI-450 dosed orally BID for 7 days in healthy subjects at doses of 10mg, 30mg and 50mg • Day 1 (pre-dose) is from blood taken on day 1 just prior to the first dose of ATI-450 • Samples ex vivo stimulated with LPS • Data expressed as mean +/- SEM Day 1 2 3 4 5 6 7 predose 4 hr 12 hr ATI-450 BID Blood for ex- vivo assay 28 * Data on file |
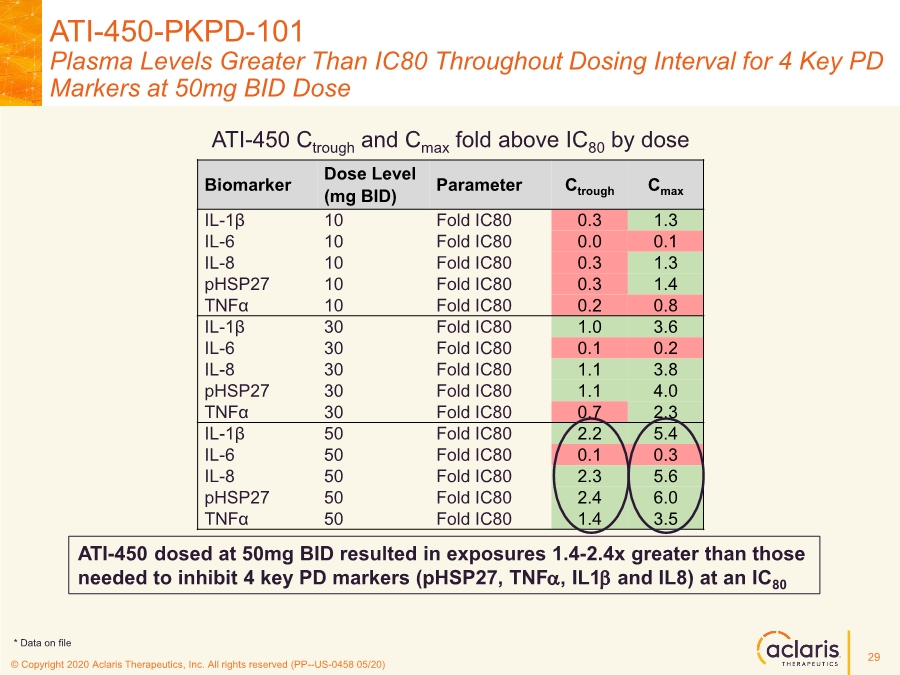
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Biomarker Dose Level (mg BID) Parameter Ctrough Cmax IL-1β 10 Fold IC80 0.3 1.3 IL-6 10 Fold IC80 0.0 0.1 IL-8 10 Fold IC80 0.3 1.3 pHSP27 10 Fold IC80 0.3 1.4 TNFα 10 Fold IC80 0.2 0.8 IL-1β 30 Fold IC80 1.0 3.6 IL-6 30 Fold IC80 0.1 0.2 IL-8 30 Fold IC80 1.1 3.8 pHSP27 30 Fold IC80 1.1 4.0 TNFα 30 Fold IC80 0.7 2.3 IL-1β 50 Fold IC80 2.2 5.4 IL-6 50 Fold IC80 0.1 0.3 IL-8 50 Fold IC80 2.3 5.6 pHSP27 50 Fold IC80 2.4 6.0 TNFα 50 Fold IC80 1.4 3.5 ATI-450-PKPD-101 Plasma Levels Greater Than IC80 Throughout Dosing Interval for 4 Key PD Markers at 50mg BID Dose ATI-450 dosed at 50mg BID resulted in exposures 1.4-2.4x greater than those needed to inhibit 4 key PD markers (pHSP27, TNFa, IL1b and IL8) at an IC80 ATI-450 Ctrough and Cmax fold above IC80 by dose 29 * Data on file |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • Rheumatoid Arthritis Trial ✓PD/safety study with early look at efficacy given small patient numbers • A Phase 2a, Randomized, Investigator and Patient-blind, Sponsor-unblinded, Parallel Group, Placebo-controlled Study of ATI-450 Plus Methotrexate (MTX) vs MTX Alone in Patients With Moderate to Severe Active Rheumatoid Arthritis ✓Topline data will consist of: • Safety and tolerability • Assess CRP dynamics • Clinical Disease Activity/PD Biomarkers • MRI: wrist synovitis • Descriptive efficacy statistics ATI-450-RA-201 Phase 2a Trial 30 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • Discovered an approach to drug the target ✓ Lock MK2 in a catalytically inactive state – a unique MOA ✓ Multiple relevant inflammatory cytokines impacted • Potential alternative for numerous diseases currently treated by biologics and JAK inhibitors ✓ Robust efficacy in a range of inflammation and mouse cancer models1,2 • Phase 1 SAD/MAD Data* ✓ Generally well-tolerated at all doses ✓ Dose proportional pharmacokinetics and a half-life supporting BID, and potentially QD, dosing ✓ Inhibits key cytokines and biomarkers in a dose-dependent way • Proof of concept Phase 2a trial in RA underway ✓ To assess safety and tolerability ✓ To demonstrate clear pharmacodynamic effect and no tachyphylaxis ✓ To demonstrate early signs of efficacy in a well understood disease • Phase 2a trial for an additional immuno-inflammatory indication being planned MK2 inhibitor ATI-450 Summary 1 Murali B, et al. Cancer Res. 2018;78(19):1-13. 2 Wang C, et al. J Exp Med. 2018;215(5):1315-1325. * Data on file 31 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-1777 (Topical Soft-JAK Inhibitor) (Investigational Drug Candidate) 32 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • Atopic dermatitis (AD) is a disease of unknown origin that usually starts in early infancy and is typified by pruritus, eczematous lesions, xerosis (dry skin), and lichenification on the skin (thickening of the skin and increase in skin markings).1 ✓ Large and growing market – Projected to be $8-12 billion at peak (moderate-to-severe AD)2 ✓ Unmet need for effective and safe topical treatment for AD ✓ Systemic and topical JAK inhibition has demonstrated promising results in clinical trials for treating pruritus and inflammation in AD3 ✓ In AD, a compromised skin barrier means that a topically dosed JAK inhibitor might result in pharmacologically active systemic drug levels • Topical soft-JAK inhibitor has potential to achieve efficacy with improved safety ✓ Achieve efficacy in skin while minimizing systemic JAK inhibitor toxicity ✓ JAK1/3 selectivity minimizes JAK2 toxicities • Topical formulations being optimized into a differentiated, patient-friendly emollient formulation (topical spray vs cream/ointment) • First in human studies planned for second half 2020 in moderate-to-severe AD ATI-1777 (Topical Soft-JAK Inhibitor) Novel approach for moderate to severe Atopic Dermatitis 1 https://emedicine.medscape.com/article/1049085-overview. Last accessed 11-1-19. 2 Auster M, et al. Something Big Is Getting Bigger [research note]. Credit Suisse Equity Research; 2019. 3 Shreberk-Hassidim R, et al. J Am Acad Dermatol. 2017;Apr;76(4):745-753. 33 |
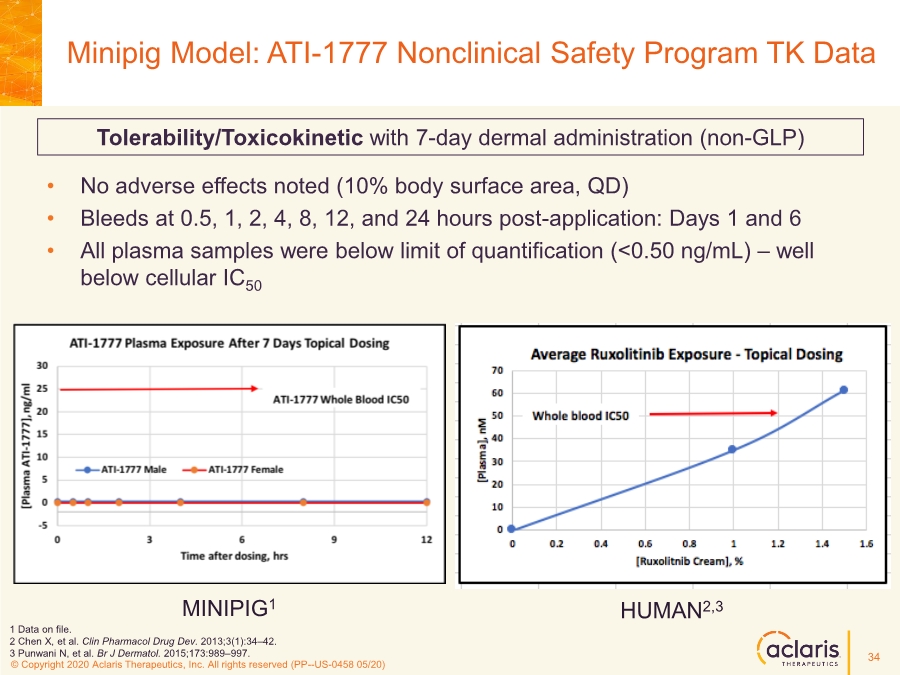
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • No adverse effects noted (10% body surface area, QD) • Bleeds at 0.5, 1, 2, 4, 8, 12, and 24 hours post-application: Days 1 and 6 • All plasma samples were below limit of quantification (<0.50 ng/mL) – well below cellular IC50 Minipig Model: ATI-1777 Nonclinical Safety Program TK Data Tolerability/Toxicokinetic with 7-day dermal administration (non-GLP) 1 Data on file. 2 Chen X, et al. Clin Pharmacol Drug Dev. 2013;3(1):34–42. 3 Punwani N, et al. Br J Dermatol. 2015;173:989–997. HUMAN2,3 MINIPIG1 34 |
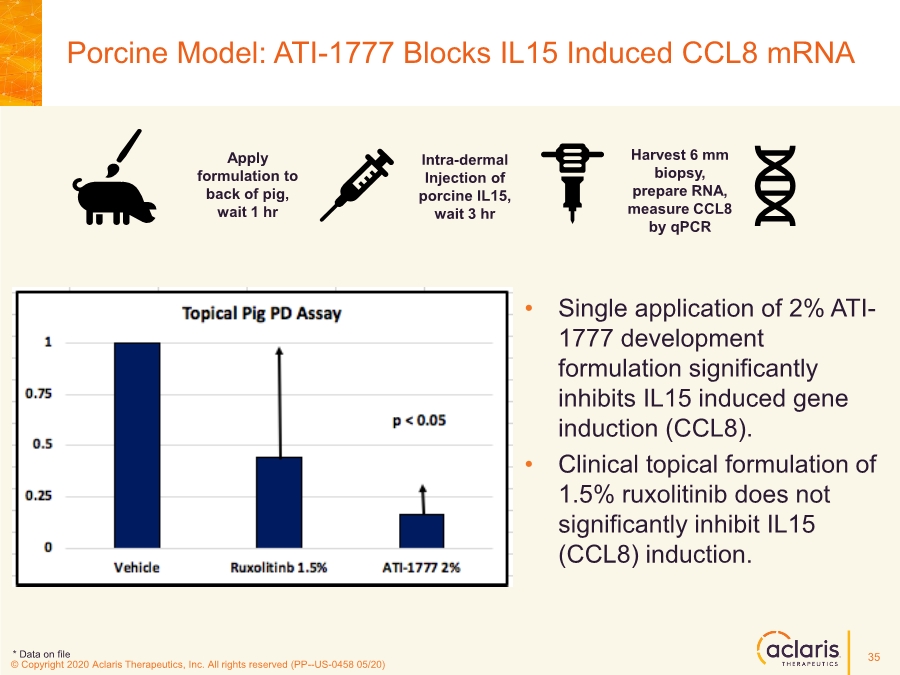
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • Single application of 2% ATI- 1777 development formulation significantly inhibits IL15 induced gene induction (CCL8). • Clinical topical formulation of 1.5% ruxolitinib does not significantly inhibit IL15 (CCL8) induction. Porcine Model: ATI-1777 Blocks IL15 Induced CCL8 mRNA Apply formulation to back of pig, wait 1 hr Intra-dermal Injection of porcine IL15, wait 3 hr Harvest 6 mm biopsy, prepare RNA, measure CCL8 by qPCR * Data on file 35 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Formulate a topical therapy for atopic dermatitis which meets the medical, aesthetic and compliance needs of patients and physicians ATI-1777: Topical Soft-JAK Inhibitor to Target Moderate-to- Severe AD • Designed to be: •“Soft” drug to minimize the potential for systemic immunosuppression • JAK1/3 selective to minimize JAK2 inhibition toxicity • Delivered in a patient-friendly formulation to clearly differentiate it from other topical therapies Approach • Plan to study in patients with moderate-to-severe AD • IND-enabling preclinical safety program initiated • Next key milestone: First In Human - 2H2020 Status 36 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-2138 (ITK/TXK/JAK3 Inhibitor) (Investigational Drug Candidate) 37 |
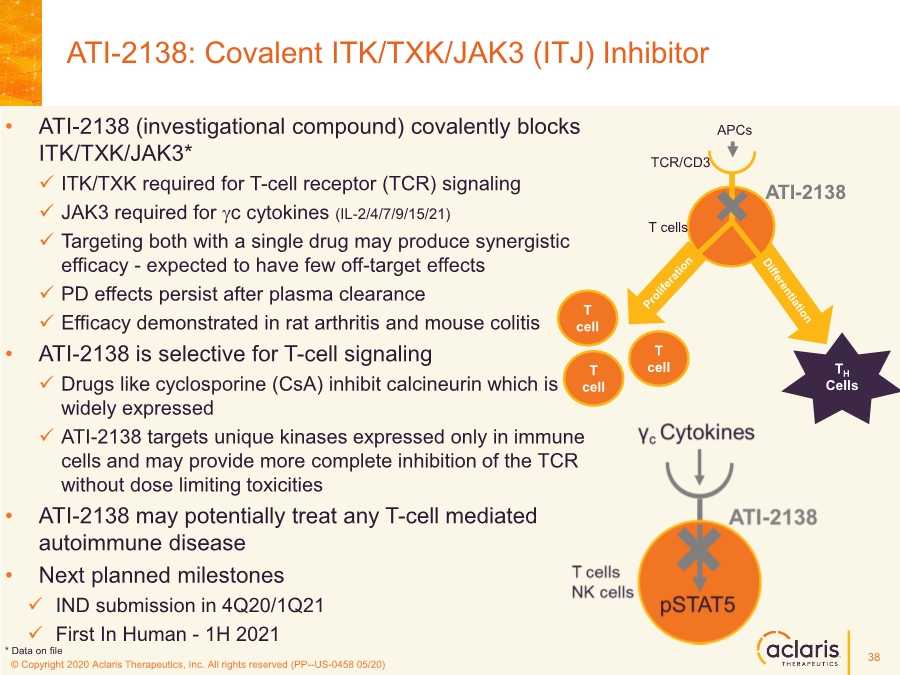
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) • ATI-2138 (investigational compound) covalently blocks ITK/TXK/JAK3* ✓ ITK/TXK required for T-cell receptor (TCR) signaling ✓ JAK3 required for gc cytokines (IL-2/4/7/9/15/21) ✓ Targeting both with a single drug may produce synergistic efficacy - expected to have few off-target effects ✓ PD effects persist after plasma clearance ✓ Efficacy demonstrated in rat arthritis and mouse colitis • ATI-2138 is selective for T-cell signaling ✓ Drugs like cyclosporine (CsA) inhibit calcineurin which is widely expressed ✓ ATI-2138 targets unique kinases expressed only in immune cells and may provide more complete inhibition of the TCR without dose limiting toxicities • ATI-2138 may potentially treat any T-cell mediated autoimmune disease • Next planned milestones ✓ IND submission in 4Q20/1Q21 ✓ First In Human - 1H 2021 ATI-2138: Covalent ITK/TXK/JAK3 (ITJ) Inhibitor TH Cells TCR/CD3 T cell T cell T cells T cell ATI-2138 APCs * Data on file 38 |
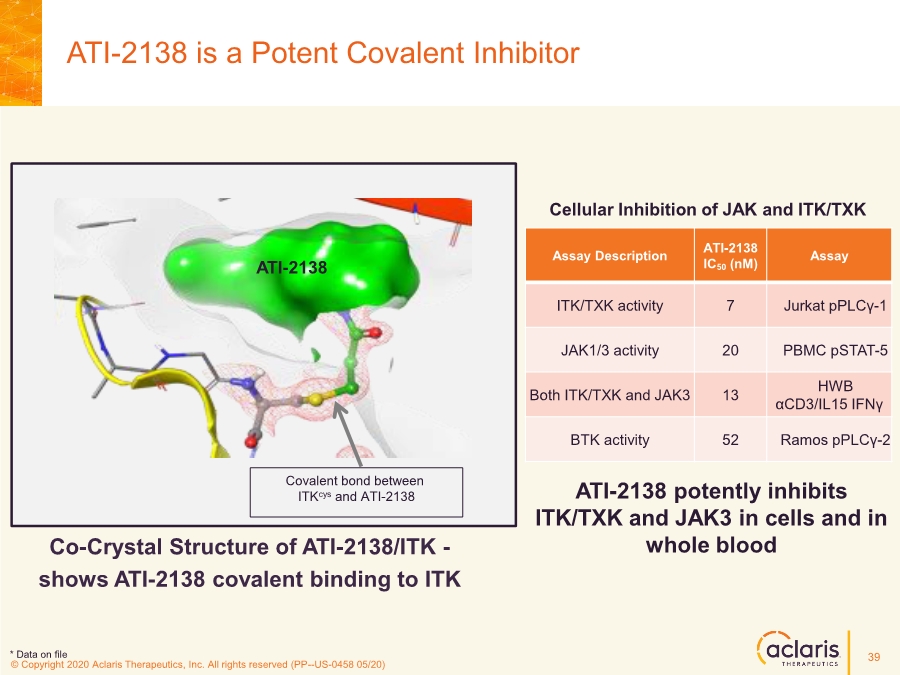
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-2138 is a Potent Covalent Inhibitor ATI-2138 potently inhibits ITK/TXK and JAK3 in cells and in whole blood Assay Description ATI-2138 IC50 (nM) Assay ITK/TXK activity 7 Jurkat pPLCγ-1 JAK1/3 activity 20 PBMC pSTAT-5 Both ITK/TXK and JAK3 13 HWB αCD3/IL15 IFNγ BTK activity 52 Ramos pPLCγ-2 Cellular Inhibition of JAK and ITK/TXK Co-Crystal Structure of ATI-2138/ITK - shows ATI-2138 covalent binding to ITK Covalent Bond between ATI-2138 and ITK ATI-2138 Covalent bond between ITKcys and ATI-2138 * Data on file 39 |
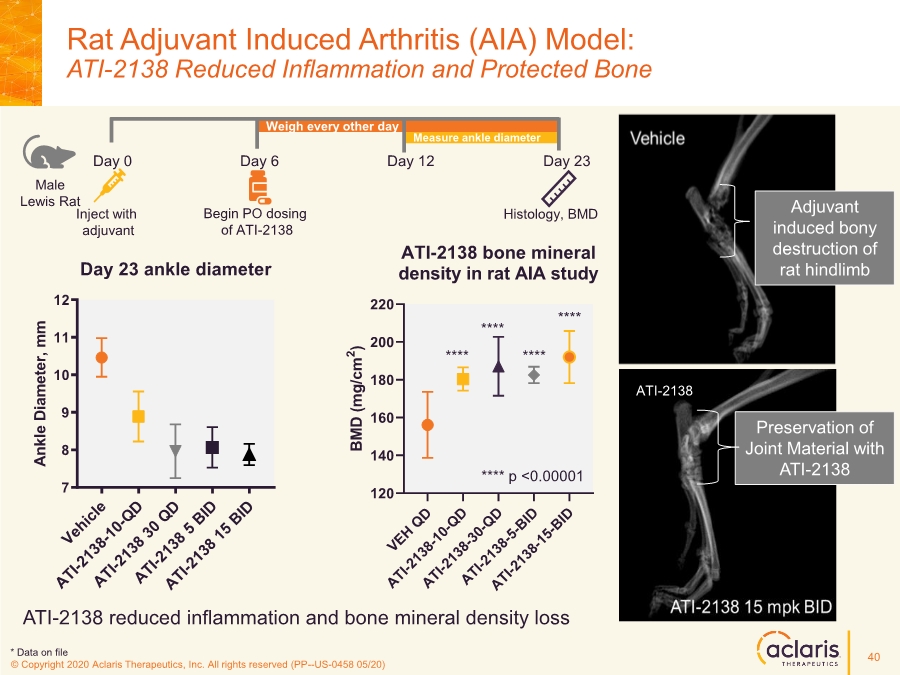
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) ATI-2138 reduced inflammation and bone mineral density loss Rat Adjuvant Induced Arthritis (AIA) Model: ATI-2138 Reduced Inflammation and Protected Bone VEH QD ATI-2138-10-QD ATI-2138-30-QD ATI-2138-5-BID ATI-2138-15-BID 120 140 160 180 200 220 ATI-2138 bone mineral density in rat AIA study B M D ( m g / c m 2 ) **** **** **** **** **** p <0.00001 Adjuvant induced bony destruction of rat hindlimb Preservation of Joint Material with ATI-2138 * Data on file ATI-2138 Vehicle ATI-2138-10-QD ATI-2138 30 QD ATI-2138 5 BID ATI-2138 15 BID 7 8 9 10 11 12 A n k l e D i a m e t e r , m m Day 23 ankle diameter Measure ankle diameter Weigh every other day Male Lewis Rat Day 0 Inject with adjuvant Day 6 Begin PO dosing of ATI-2138 Day 12 Day 23 Histology, BMD 40 |
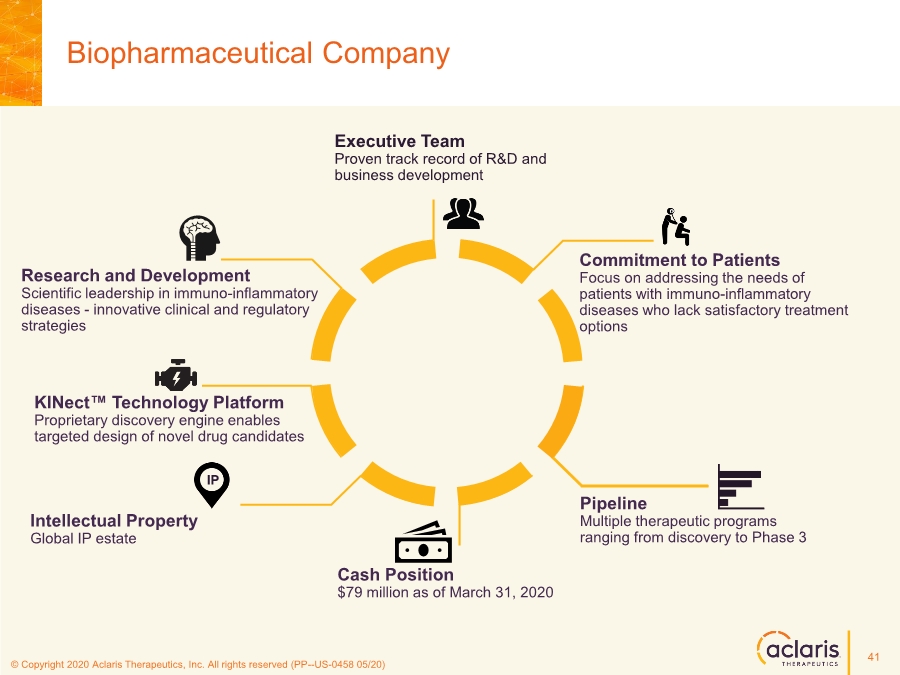
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Biopharmaceutical Company Cash Position $79 million as of March 31, 2020 Commitment to Patients Focus on addressing the needs of patients with immuno-inflammatory diseases who lack satisfactory treatment options Research and Development Scientific leadership in immuno-inflammatory diseases - innovative clinical and regulatory strategies Executive Team Proven track record of R&D and business development Pipeline Multiple therapeutic programs ranging from discovery to Phase 3 Intellectual Property Global IP estate KINect™ Technology Platform Proprietary discovery engine enables targeted design of novel drug candidates IP 41 |
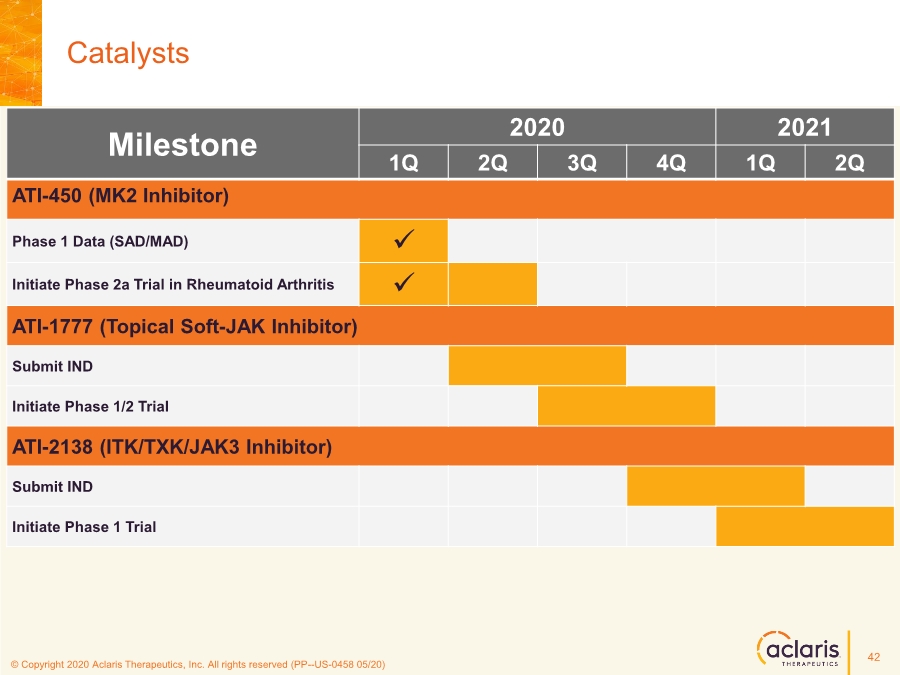
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) Catalysts Milestone 2020 2021 1Q 2Q 3Q 4Q 1Q 2Q ATI-450 (MK2 Inhibitor) Phase 1 Data (SAD/MAD) ✓ Initiate Phase 2a Trial in Rheumatoid Arthritis ✓ ATI-1777 (Topical Soft-JAK Inhibitor) Submit IND Initiate Phase 1/2 Trial ATI-2138 (ITK/TXK/JAK3 Inhibitor) Submit IND Initiate Phase 1 Trial 42 |
| © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (XX) © Copyright 2020 Aclaris Therapeutics, Inc. All rights reserved (PP--US-0458 05/20) THANK YOU EMPOWERING PATIENTS THROUGH KINOME INNOVATION |