Exhibit 99.2

CONNECT. INNOVATE. March 2023 Investor Presentation TRANSFORMING BIOSPECIMEN PROCUREMENT An online marketplace for human biospecimens

This presentation may contain forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words . You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward - looking information . Such statements are only predictions and our actual results may differ materially from those anticipated in these forward - looking statements . We believe that it is important to communicate future expectations to investors . However, there may be events in the future that we are not able to accurately predict or control . Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our registration statement and other filings filed with the Securities and Exchange Commission (the "SEC"), including the uncertainties associated with our lack of profitability, our continued capital needs, our lack of a long operating history, our growth strategy, the COVID - 19 pandemic and its continued impact on the business, the uncertain effect of geopolitical developments, our technology development plans, and the regulatory environment in which we operate . We do not assume any obligation to update forward - looking statements as circumstances change . Certain market data information in this presentation is based on management's estimates . We obtained the industry, market and competitive position data used throughout this presentation from internal estimates and research as well as from industry publications and research, surveys and studies conducted by third parties . We believe our estimates to be accurate as of the date of this presentation . However, this information may prove to be inaccurate because of the method by which we obtained some of the data for our estimates or because this information cannot always be verified due to the limits on the availability and reliability of raw data, and the nature of the data gathering process . You may access our SEC filings by visiting the SEC’s website at http : //www . sec . gov . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with us or our affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . Forward - Looking Statements 2 SM0

Investor Highlights 3 >200 Healthcare Provider / Supplier Organizations >450 Customer Organizations 1. Early - stage opportunity with a first mover advantage 2. Unique two - sided marketplace disrupting the biospecimen procurement process 3. Strong revenue growth with a 7 - year CAGR of 48% 4. Growing data asset that’s a key enabler and differentiator

Accelerate scientific discovery via an online marketplace that empowers researchers to instantly search for and gain access to patients, biospecimens, and data across a global network of healthcare providers Our Vision 4
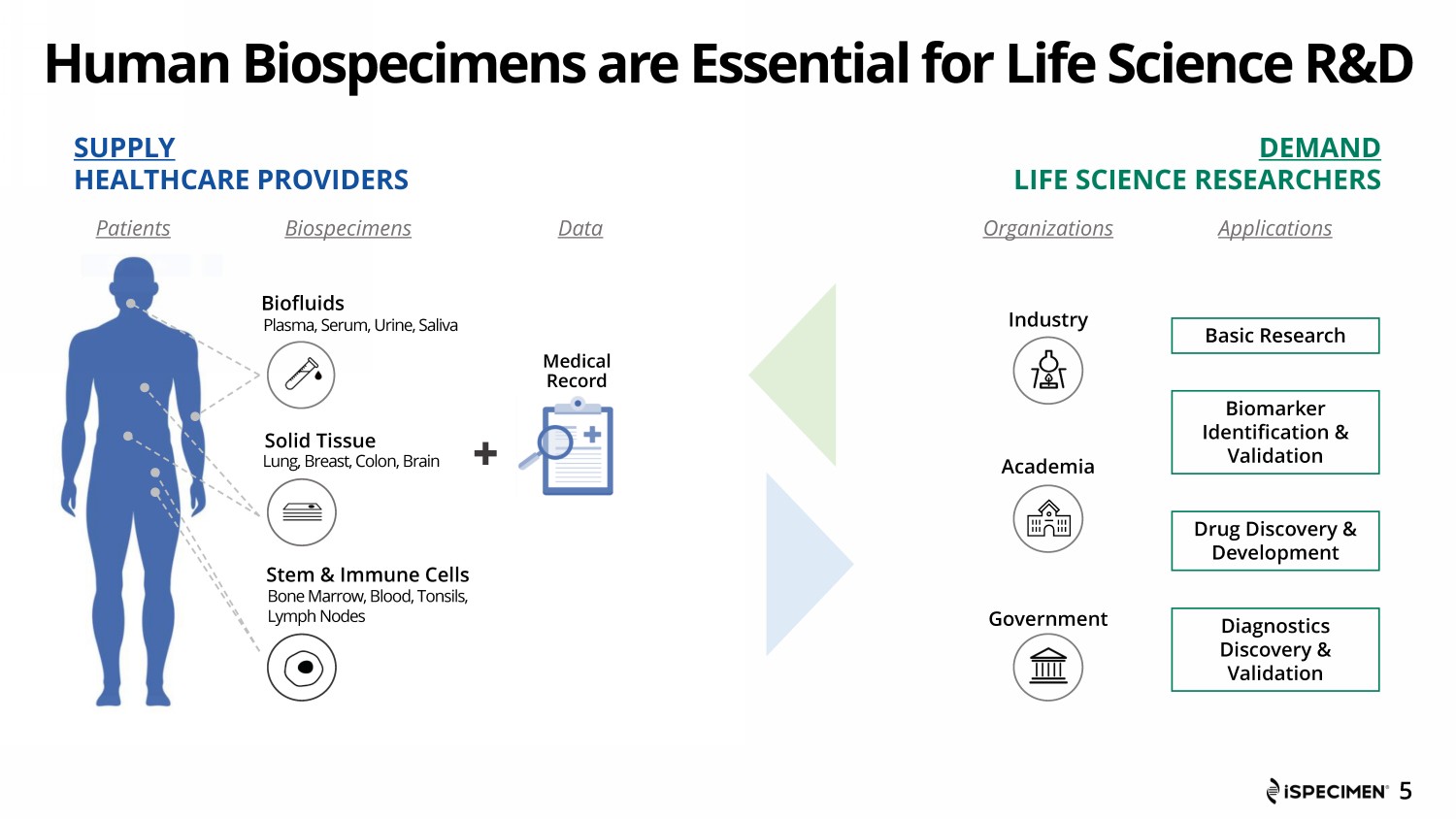
Human Biospecimens are Essential for Life Science R&D Basic Research Biomarker Identification & Validation Drug Discovery & Development Diagnostics Discovery & Validation Biofluids Solid Tissue Stem & Immune Cells Plasma, Serum, Urine, Saliva Industry Academia Government 5 Lung, Breast, Colon, Brain Bone Marrow, Blood, Tonsils, Lymph Nodes Applications Patients SUPPLY HEALTHCARE PROVIDERS DEMAND LIFE SCIENCE RESEARCHERS Biospecimens Data Organizations Medical Record

10 - 15% Annual Growth Fueled by precision and regenerative medicine research 2 Disconnected Market Participants in Need of a Marketplace Solution With an Inefficient Supply Chain Manual processes plus fragmentation make it ripe for an online marketplace solution Large and Inefficient Biospecimen Market $3 - $4 Billion Worldwide yearly spend on human biospecimen procurement 1 6 1, 2 “Sources Cited” page 28 SM0
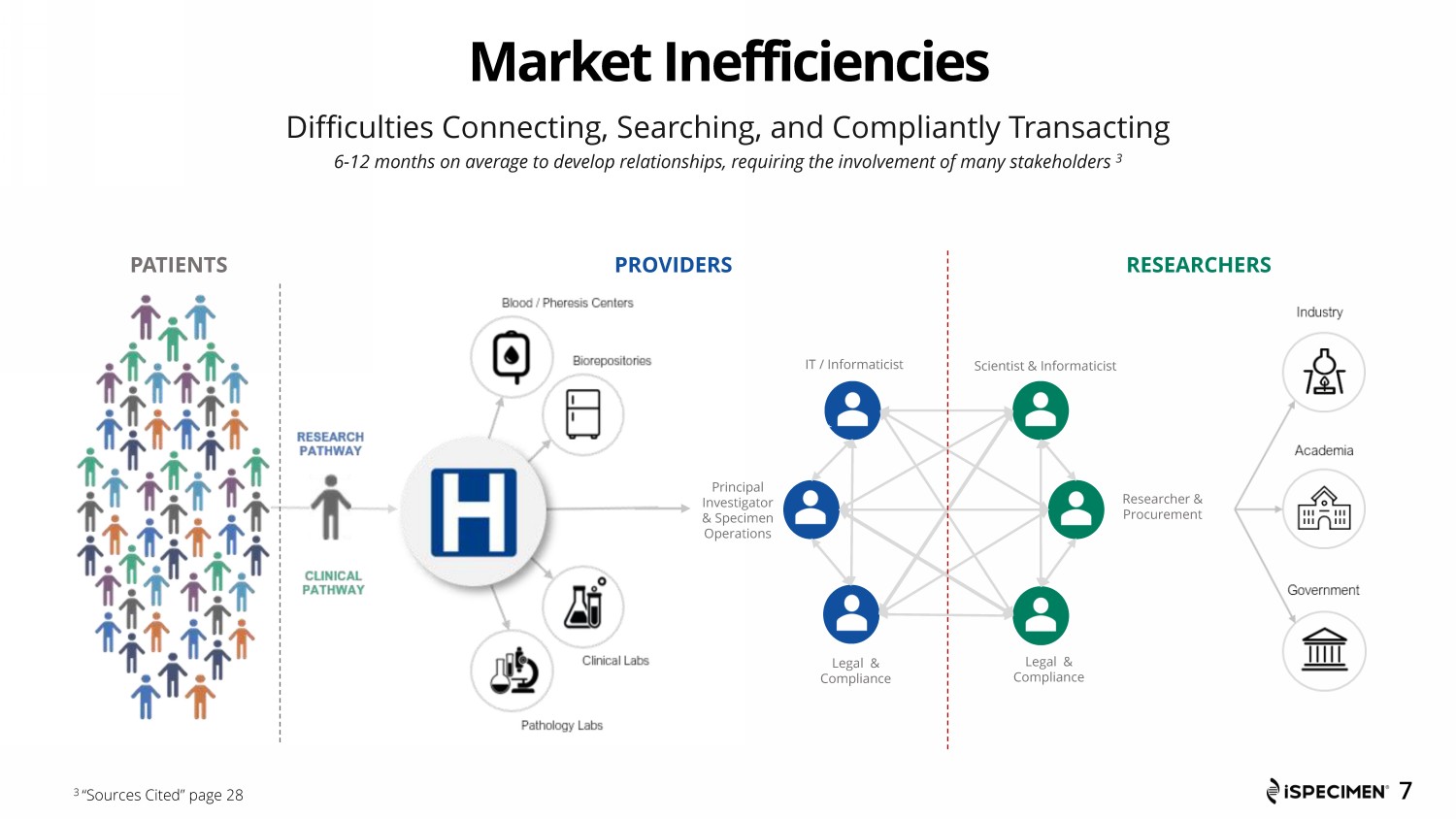
Market Inefficiencies PROVIDER RESEARCHER Scientist / Informaticist Procurem ent Legal & Compliance IT / Informaticist Scientist & Informaticist Legal & Compliance IT / Informaticist Researcher & Procurement Legal & Compliance Principal Investigator & Specimen Operations PATIENTS PROVIDERS RESEARCHERS 7 Difficulties Connecting, Searching, and Compliantly Transacting 6 - 12 months on average to develop relationships, requiring the involvement of many stakeholders 3 3 “Sources Cited” page 28
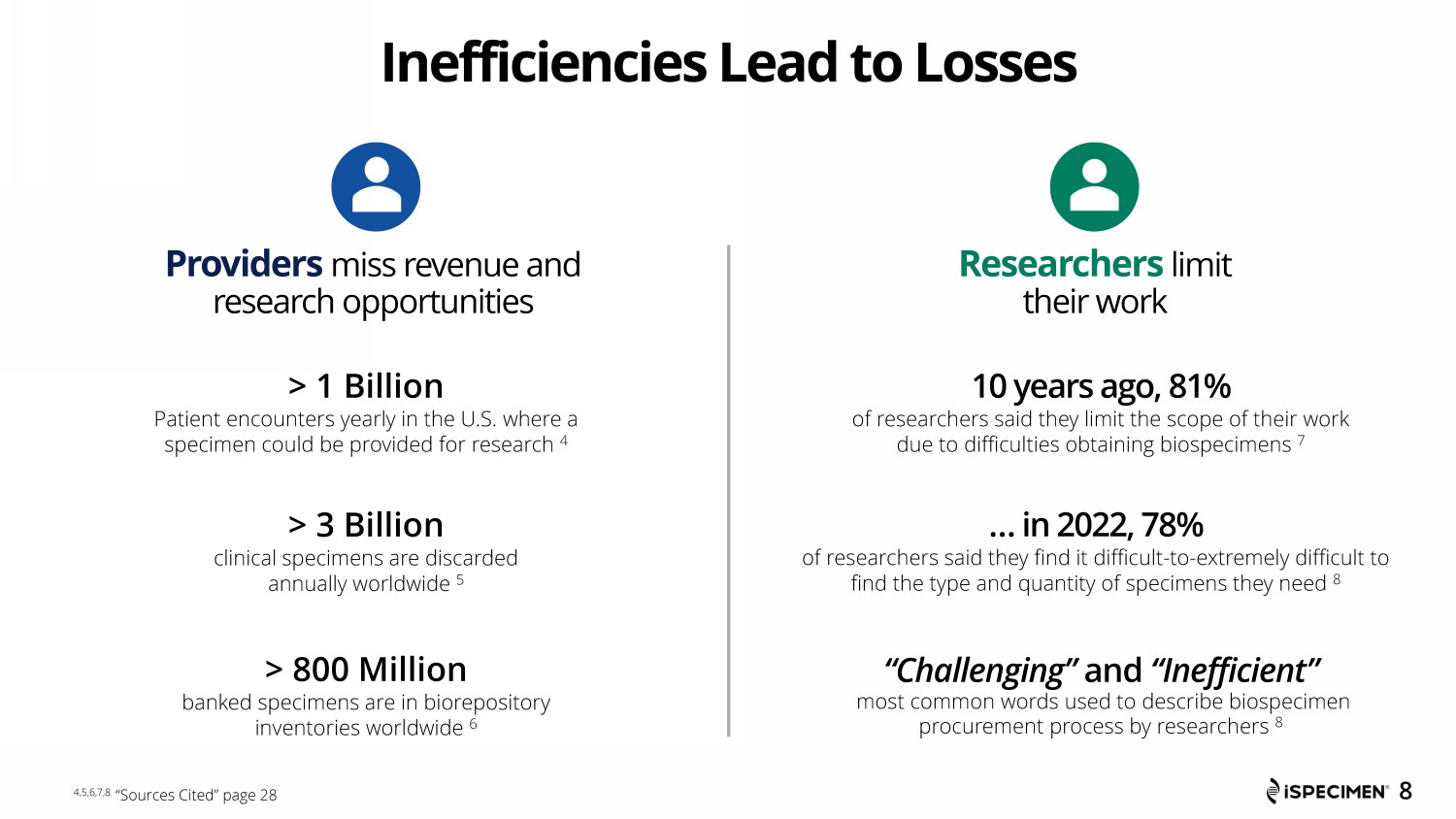
Inefficiencies Lead to Losses Providers miss revenue and research opportunities > 3 Billion clinical specimens are discarded annually worldwide 5 > 1 Billion Patient encounters yearly in the U.S. where a specimen could be provided for research 4 > 800 Million banked specimens are in biorepository inventories worldwide 6 8 Researchers limit their work … in 2022, 78% of researchers said they find it difficult - to - extremely difficult to find the type and quantity of specimens they need 8 “Challenging” and “Inefficient” most common words used to describe biospecimen procurement process by researchers 8 10 years ago, 81% of researchers said they limit the scope of their work due to difficulties obtaining biospecimens 7 4,5,6,7,8 “Sources Cited” page 28
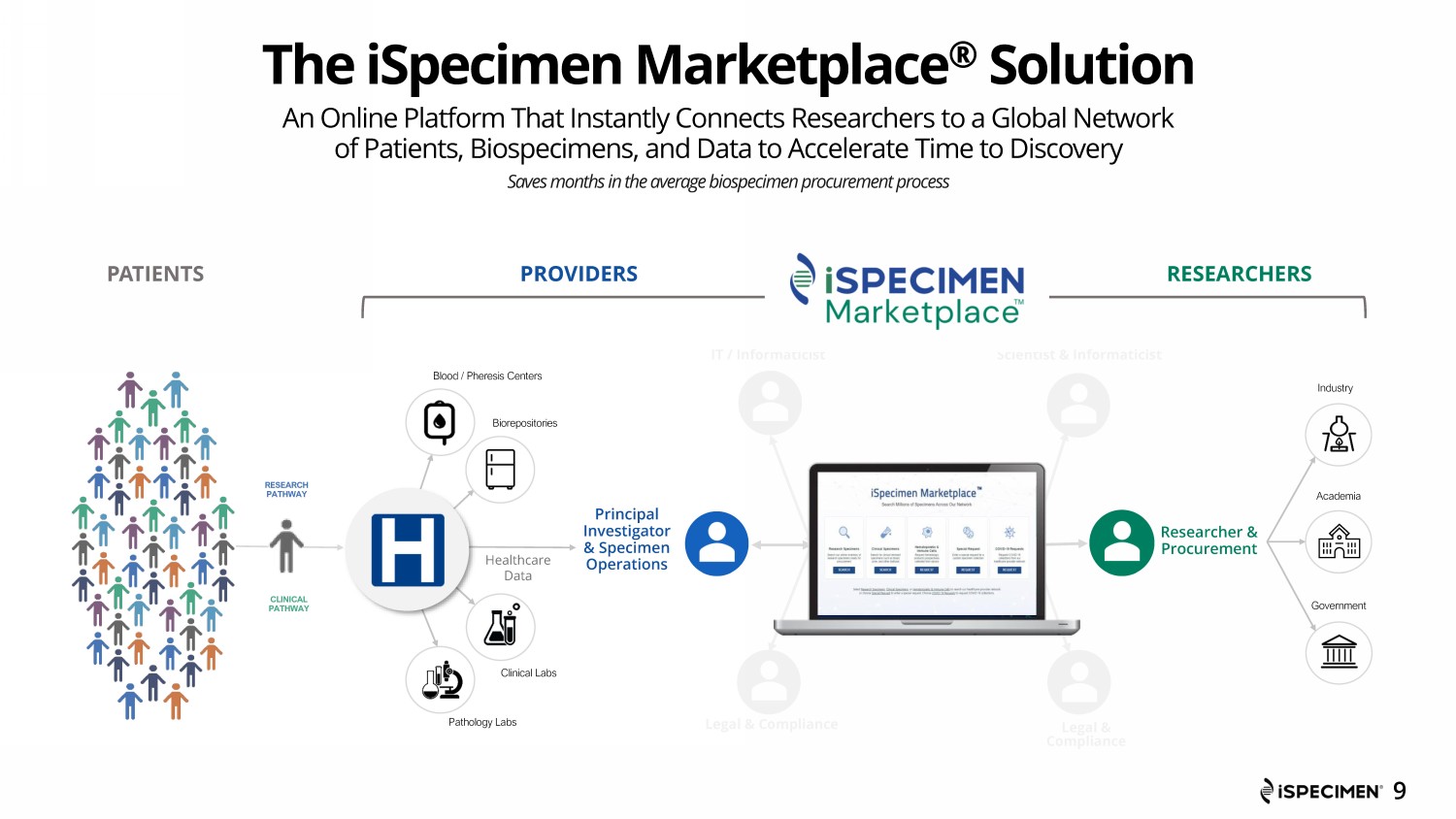
The iSpecimen Marketplace ® Solution 9 PROVIDER Procurement Principal Investigator / Operations IT / Informaticist PATIENTS Legal & Compliance IT / Informaticist Researcher & Procurement Legal & Compliance Principal Investigator & Specimen Operations Scientist & Informaticist Blood / Pheresi s Centers Clinical Labs Biorepositories Pathology Labs RESEARCH PATHWAY CLINICAL PATHWAY Industry Academia Government RESEARCHERS An Online Platform That Instantly Connects Researchers to a Global Network of Patients, Biospecimens, and Data to Accelerate Time to Discovery Saves months in the average biospecimen procurement process PROVIDERS Healthcare Data
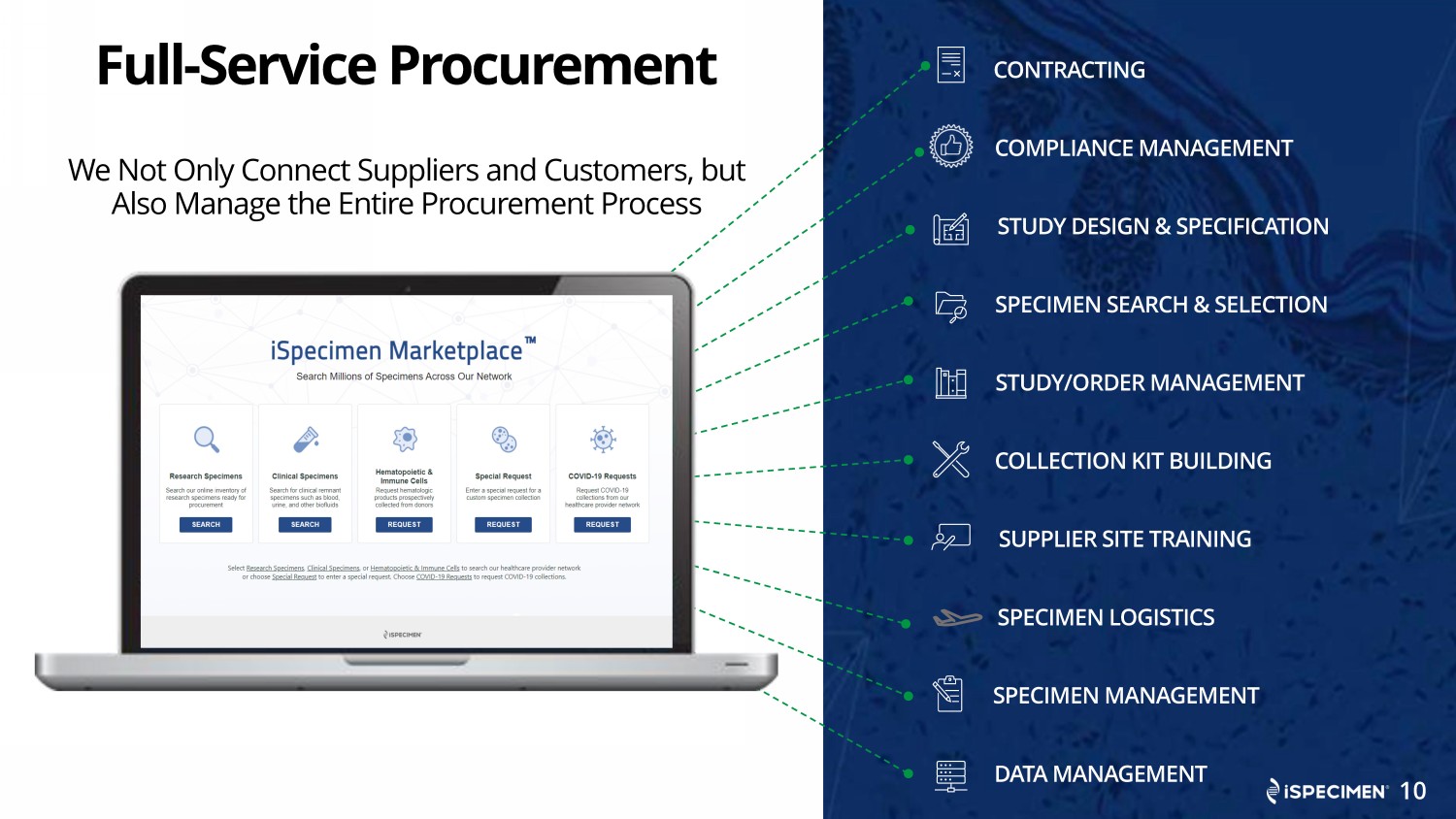
Full - Service Procurement We Not Only Connect Suppliers and Customers, but Also Manage the Entire Procurement Process SPECIMEN SEARCH & SELECTION SPECIMEN LOGISTICS SPECIMEN MANAGEMENT DATA MANAGEMENT STUDY/ORDER MANAGEMENT SUPPLIER SITE TRAINING COLLECTION KIT BUILDING COMPLIANCE MANAGEMENT STUDY DESIGN & SPECIFICATION CONTRACTING 10

Suppliers and Researchers Benefit Advance Discovery Suppor t research mission and advance diagnostic, therapeutic, and vaccine research Increase Revenue Instantly connect to a global network of researchers Ensure Compliance On a platform that protects the privacy and security of patient information Accelerate Research Search for specimens anytime, anywhere, through our easy - to - use online marketplace Save Money Instantly connect to a global network of specimen providers Reduce Risk On a platform that manages contracting and regulatory compliance Providers… Researchers… 11
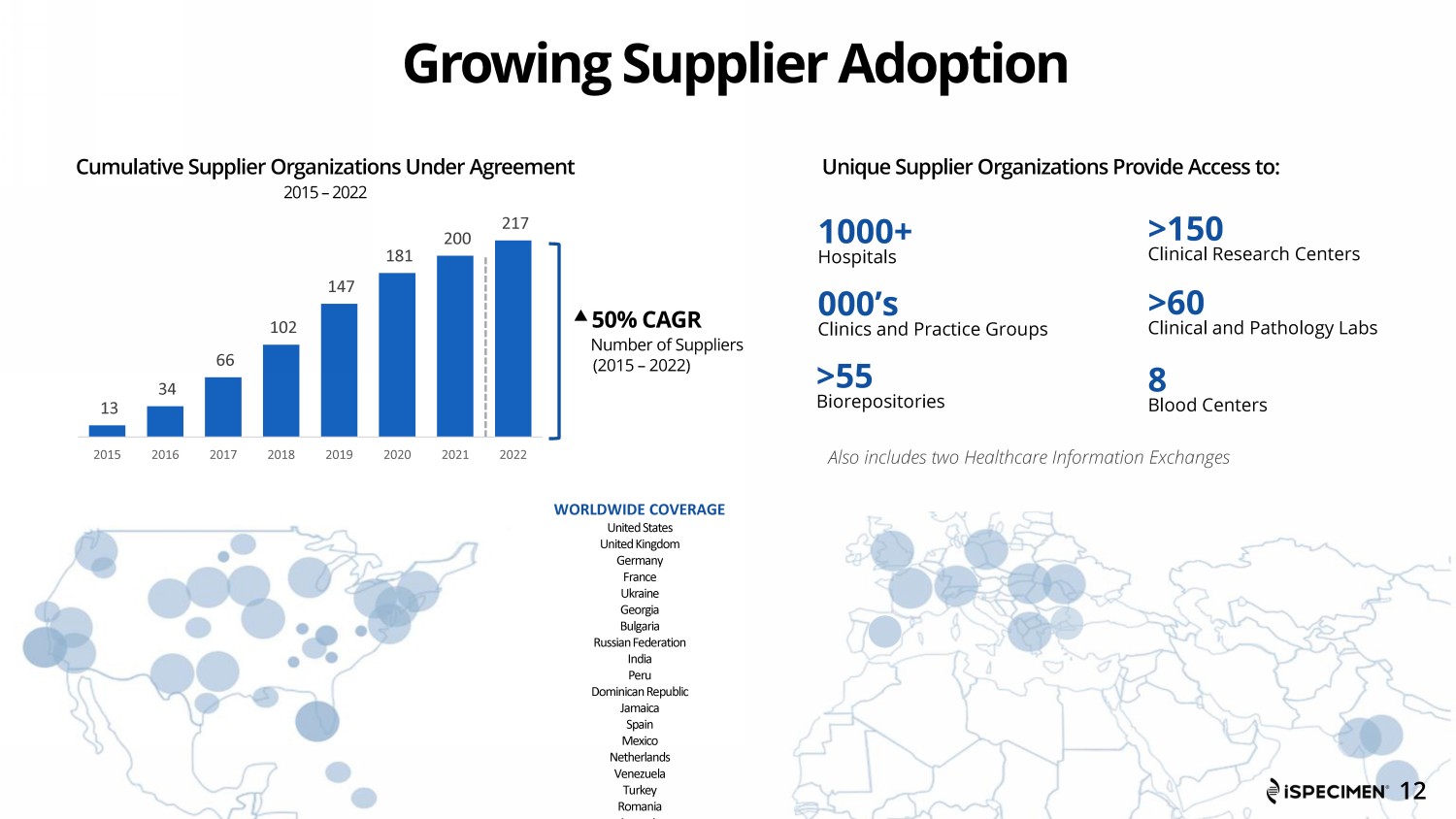
13 34 66 102 147 181 200 217 2015 2016 2017 2018 2019 2020 2021 2022 Growing Supplier Adoption 1000+ Hospitals Also includes two Healthcare Information Exchanges 50% CAGR Number of Suppliers (2015 – 2022) WORLDWIDE COVERAGE United States United Kingdom Germany France Ukraine Georgia Bulgaria Russian Federation India Peru Dominican Republic Jamaica Spain Mexico Netherlands Venezuela Turkey Romania Armenia Cumulative Supplier Organizations Under Agreement 2015 – 2022 Unique Supplier Organizations Provide Access to: 000’s Clinics and Practice Groups >55 Biorepositories >150 Clinical Research Centers >60 Clinical and Pathology Labs 8 Blood Centers 12
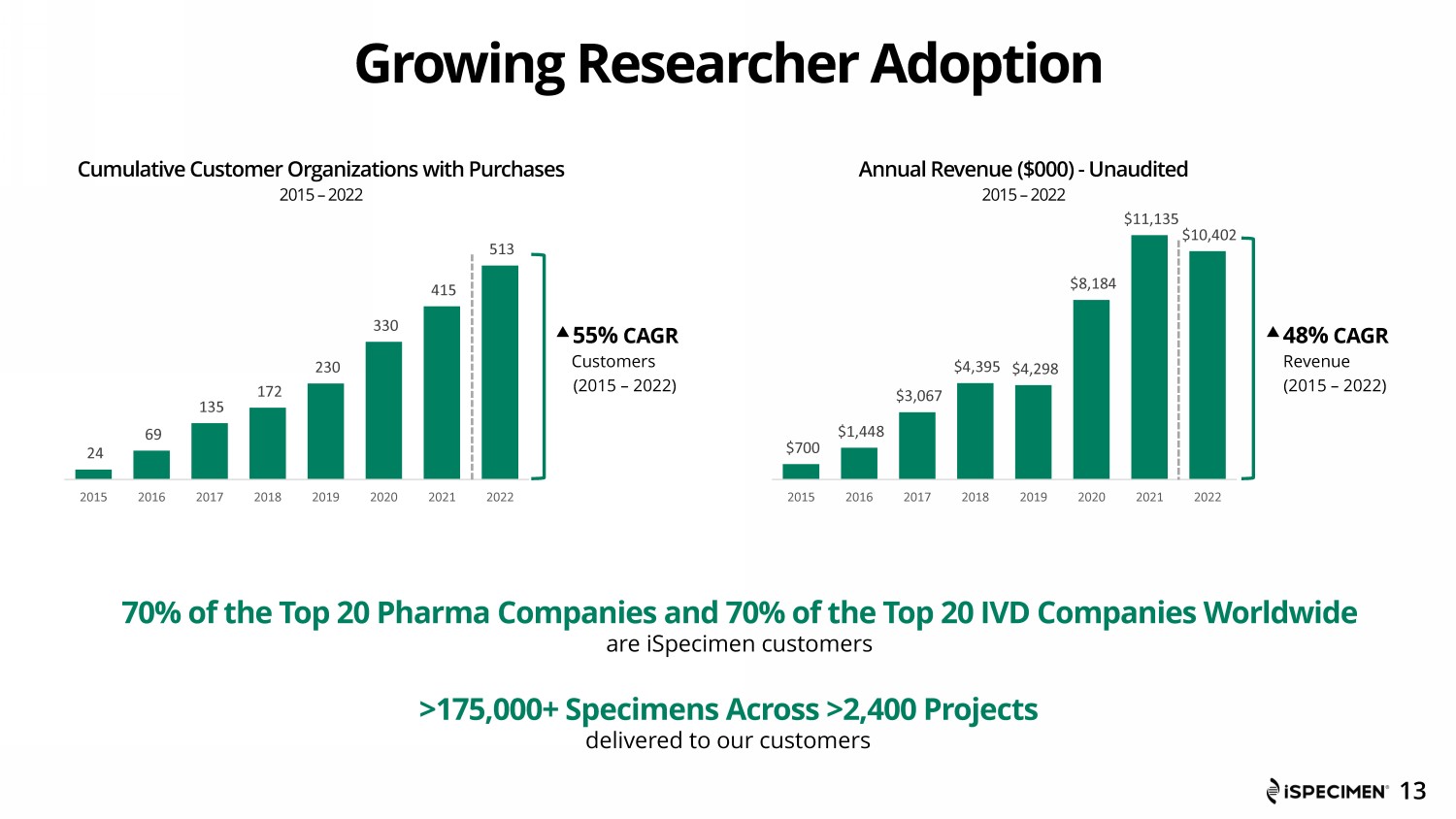
$700 $1,448 $3,067 $4,395 $4,298 $8,184 $11,135 $10,402 2015 2016 2017 2018 2019 2020 2021 2022 Cumulative Customer Organizations with Purchases 2015 – 2022 55% CAGR Customers (2015 – 2022) Annual Revenue ($000) - Unaudited 2015 – 2022 48% CAGR Revenue (2015 – 2022) Growing Researcher Adoption 70% of the Top 20 Pharma Companies and 70% of the Top 20 IVD Companies Worldwide are iSpecimen customers are iSpecimen customers >175,000+ Specimens Across >2,400 Projects delivered to our customers 13 24 69 135 172 230 330 415 513 2015 2016 2017 2018 2019 2020 2021 2022
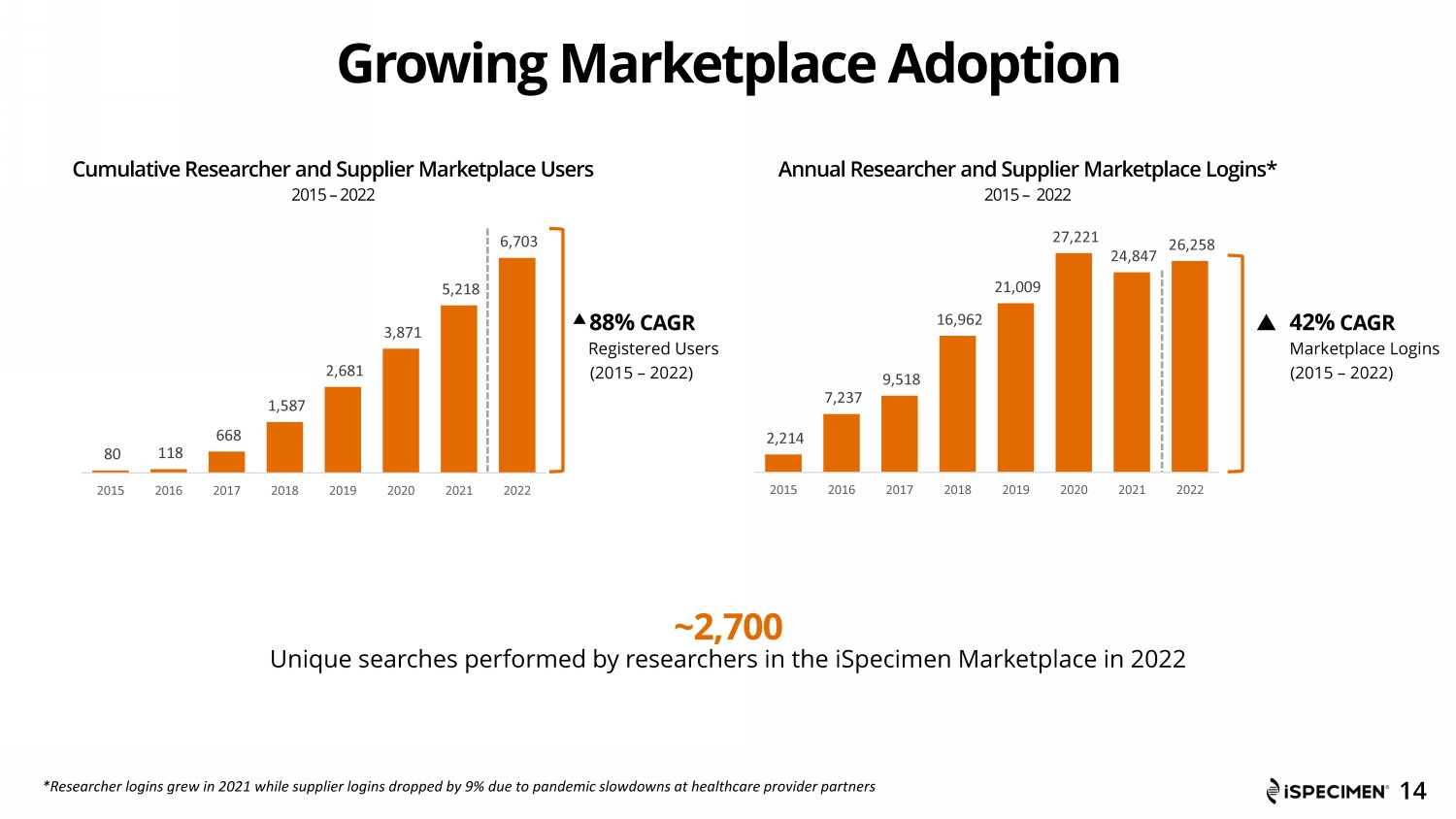
80 118 668 1,587 2,681 3,871 5,218 6,703 2015 2016 2017 2018 2019 2020 2021 2022 Cumulative Researcher and Supplier Marketplace Users 2015 – 2022 88% CAGR Registered Users (2015 – 2022) Growing Marketplace Adoption ~2,700 Unique searches performed by researchers in the iSpecimen Marketplace in 2022 14 Annual Researcher and Supplier Marketplace Logins* 2015 – 2022 42% CAGR Marketplace Logins (2015 – 2022) 2,214 7,237 9,518 16,962 21,009 27,221 24,847 26,258 2015 2016 2017 2018 2019 2020 2021 2022 *Researcher logins grew in 2021 while supplier logins dropped by 9% due to pandemic slowdowns at healthcare provider partners
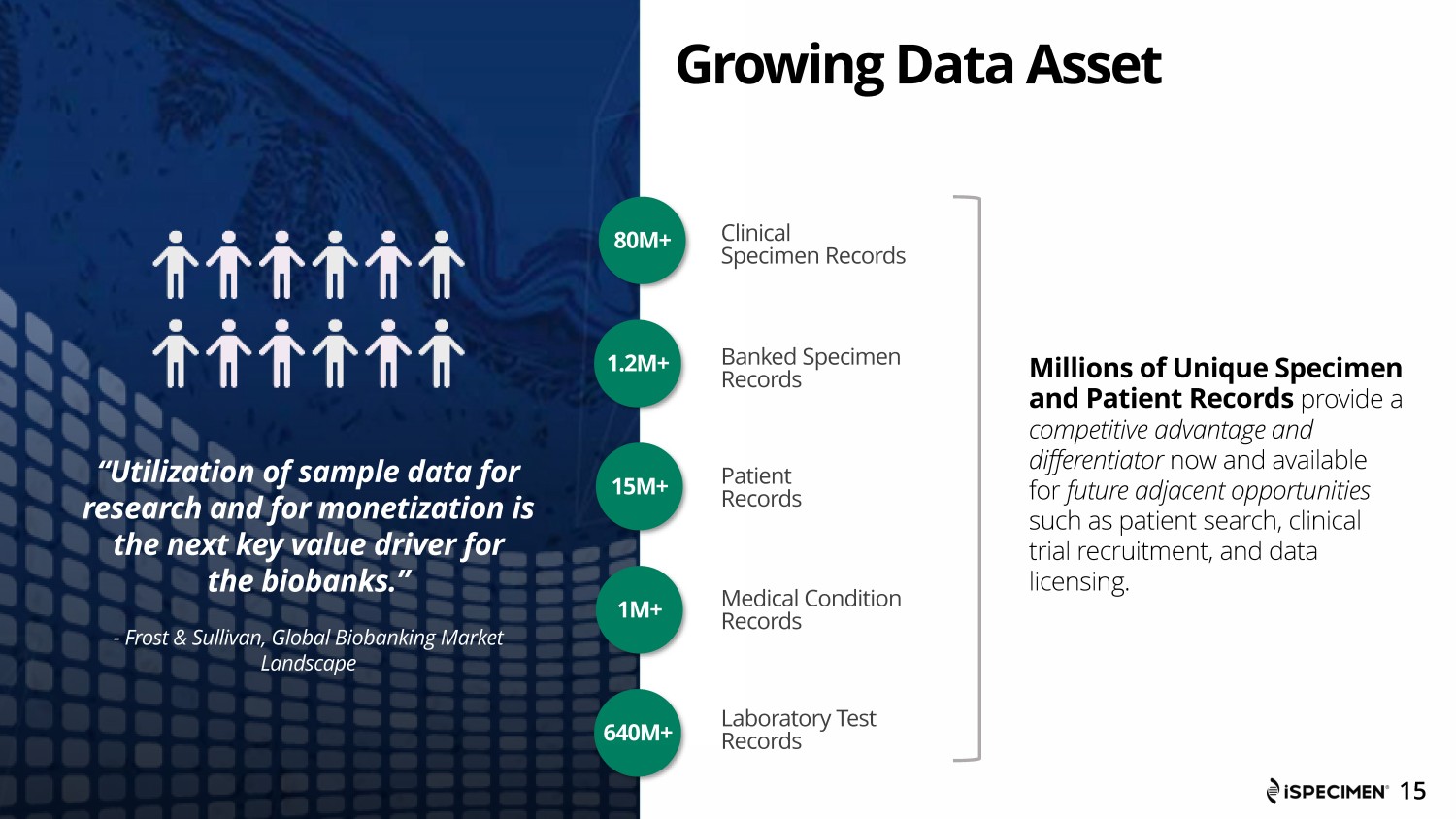
“Utilization of sample data for research and for monetization is the next key value driver for the biobanks.” - Frost & Sullivan, Global Biobanking Market Landscape Growing Data Asset Banked Specimen Records Patient Records Medical Condition Records Clinical Specimen Records Laboratory Test Records Millions of Unique Specimen and Patient Records provide a competitive advantage and differentiator now and available for future adjacent opportunities such as patient search, clinical trial recruitment, and data licensing. 80M+ 15 1.2M+ 15 M+ 1M+ 640M+
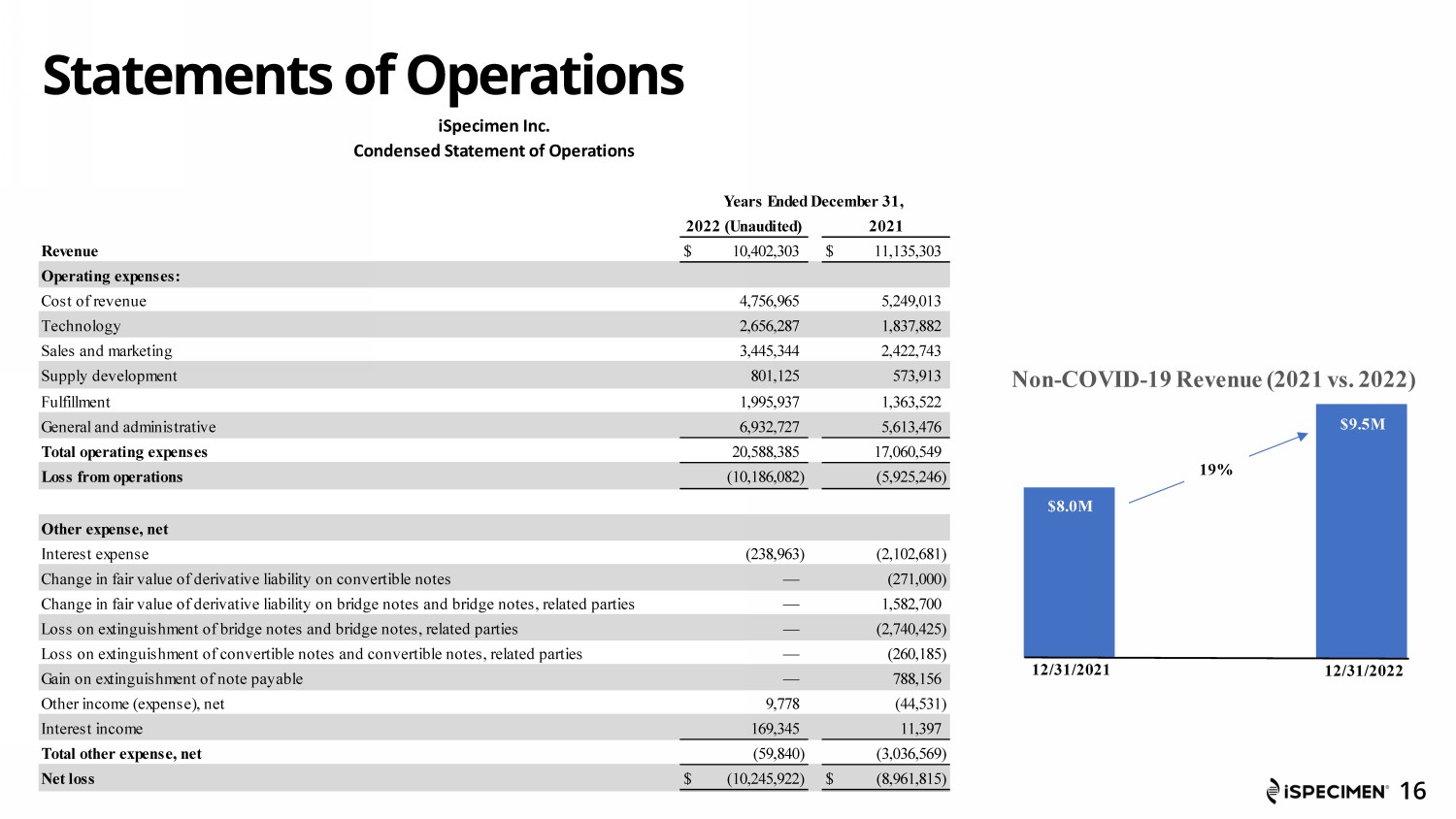
Statements of Operations 16 iSpecimen Inc. Condensed Statement of Operations Years Ended December 31, 2022 (Unaudited) 2021 Revenue $ 10,402,303 $ 11,135,303 Operating expenses: Cost of revenue 4,756,965 5,249,013 Technology 2,656,287 1,837,882 Sales and marketing 3,445,344 2,422,743 Supply development 801,125 573,913 Fulfillment 1,995,937 1,363,522 General and administrative 6,932,727 5,613,476 Total operating expenses 20,588,385 17,060,549 Loss from operations (10,186,082) (5,925,246) Other expense, net Interest expense (238,963) (2,102,681) Change in fair value of derivative liability on convertible notes — (271,000) Change in fair value of derivative liability on bridge notes and bridge notes, related parties — 1,582,700 Loss on extinguishment of bridge notes and bridge notes, related parties — (2,740,425) Loss on extinguishment of convertible notes and convertible notes, related parties — (260,185) Gain on extinguishment of note payable — 788,156 Other income (expense), net 9,778 (44,531) Interest income 169,345 11,397 Total other expense, net (59,840) (3,036,569) Net loss $ (10,245,922) $ (8,961,815) Non - COVID - 19 Revenue (2021 vs. 2022) 12/31/2021 12/31/2022 $8.0M $9.5M 19% SM0
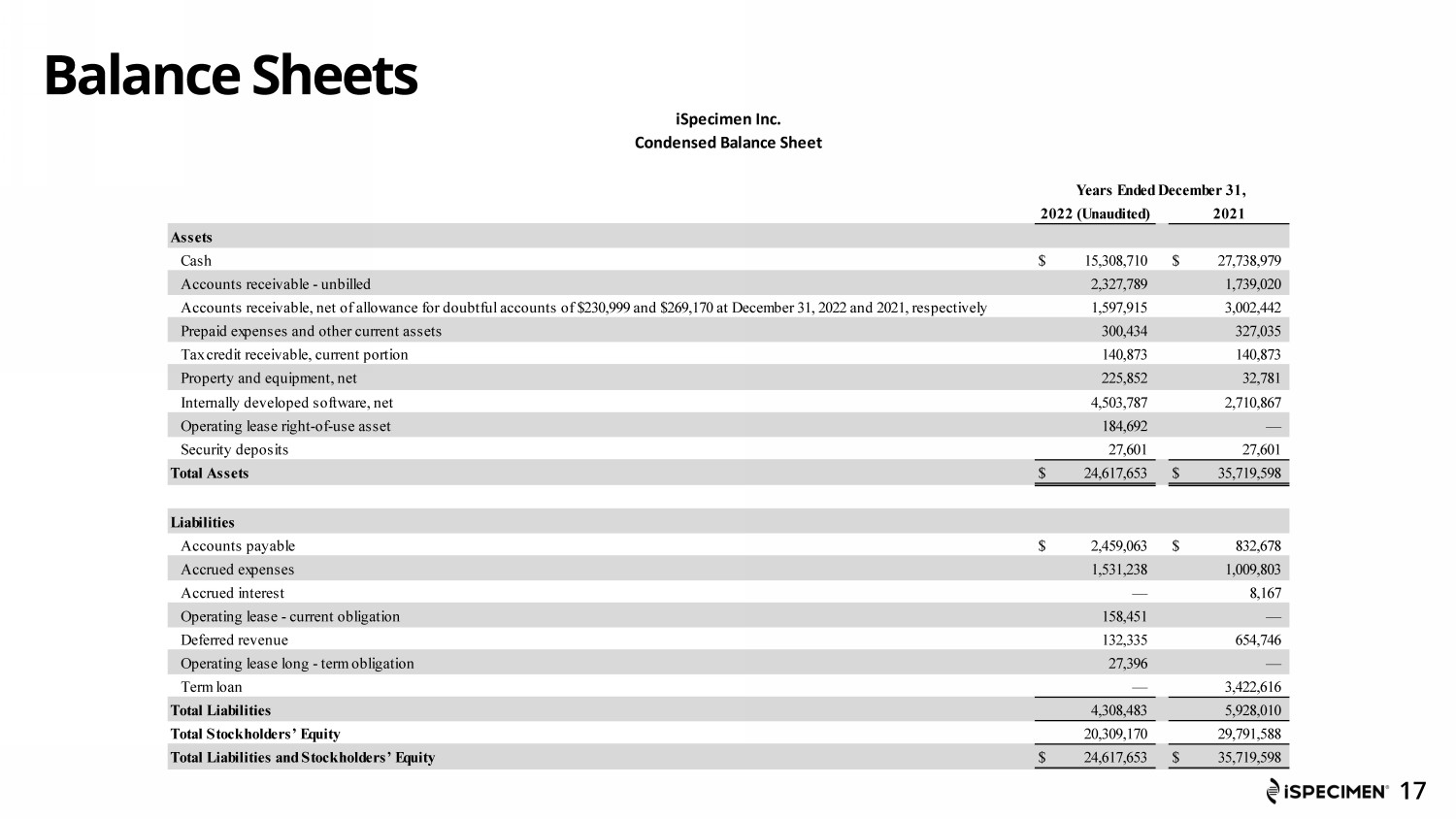
Balance Sheets 17 iSpecimen Inc. Condensed Balance Sheet Years Ended December 31, 2022 (Unaudited) 2021 Assets Cash $ 15,308,710 $ 27,738,979 Accounts receivable - unbilled 2,327,789 1,739,020 Accounts receivable, net of allowance for doubtful accounts of $230,999 and $269,170 at December 31, 2022 and 2021, respectively 1,597,915 3,002,442 Prepaid expenses and other current assets 300,434 327,035 Tax credit receivable, current portion 140,873 140,873 Property and equipment, net 225,852 32,781 Internally developed software, net 4,503,787 2,710,867 Operating lease right-of-use asset 184,692 — Security deposits 27,601 27,601 Total Assets $ 24,617,653 $ 35,719,598 Liabilities Accounts payable $ 2,459,063 $ 832,678 Accrued expenses 1,531,238 1,009,803 Accrued interest — 8,167 Operating lease - current obligation 158,451 — Deferred revenue 132,335 654,746 Operating lease long - term obligation 27,396 — Term loan — 3,422,616 Total Liabilities 4,308,483 5,928,010 Total Stockholders’ Equity 20,309,170 29,791,588 Total Liabilities and Stockholders’ Equity $ 24,617,653 $ 35,719,598
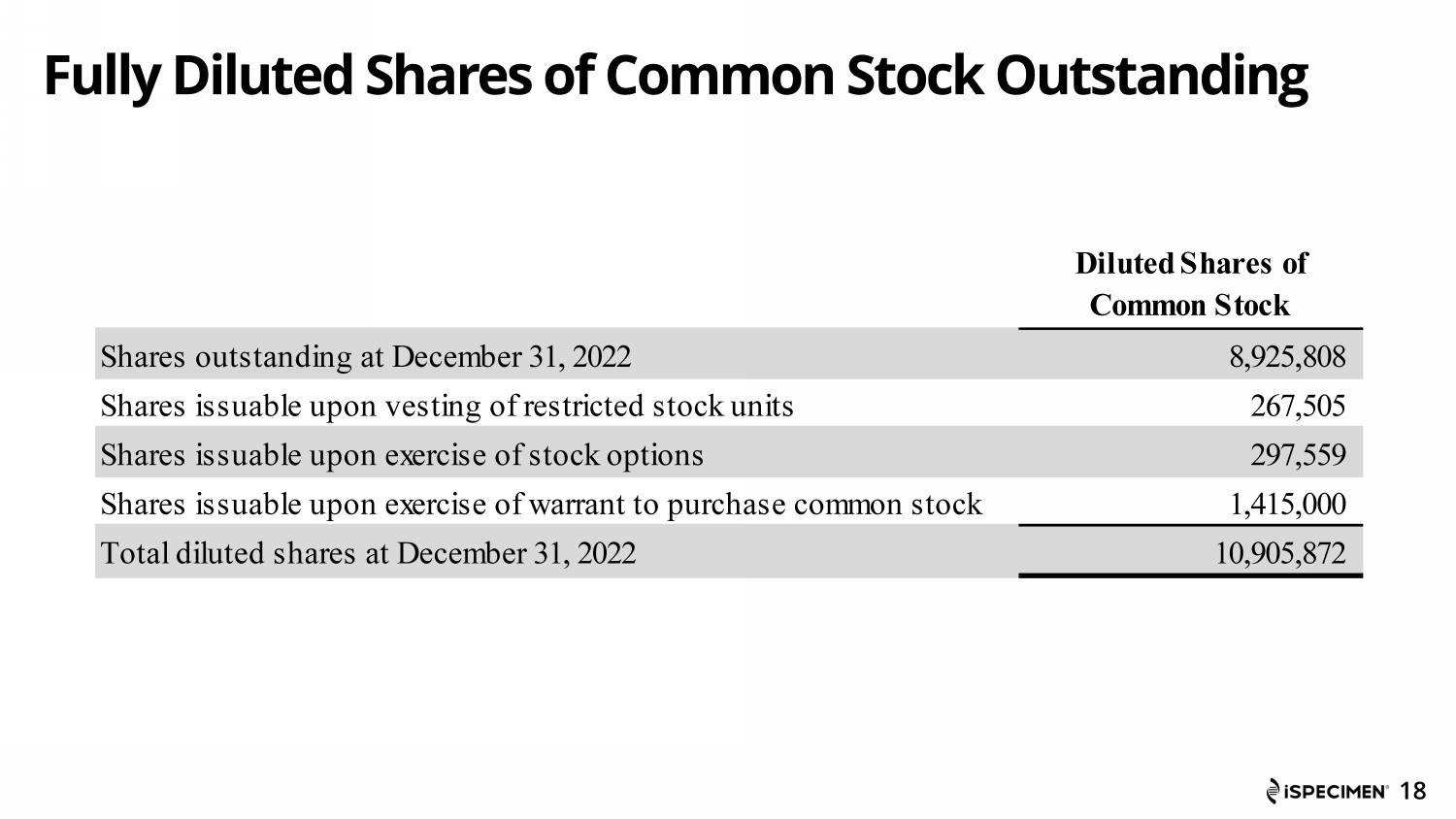
Fully Diluted Shares of Common Stock Outstanding 18 Diluted Shares of Common Stock Shares outstanding at December 31, 2022 8,925,808 Shares issuable upon vesting of restricted stock units 267,505 Shares issuable upon exercise of stock options 297,559 Shares issuable upon exercise of warrant to purchase common stock 1,415,000 Total diluted shares at December 31, 2022 10,905,872

Tracy Curley | Chief Executive Officer, Chief Financial Officer and Treasurer Ms . Curley brings three decades of experience in public accounting and corporate finance for both publicly traded companies and emerging companies like iSpecimen and joined iSpecimen in August 2020 . She came to iSpecimen after over a decade with national accounting firms such as CohnReznick where she focused on serving clients in the middle markets . During her time as a partner in public accounting firms, she was responsible for creating and leading teams to provide audit and consulting services to a growing clientele of private and public emerging growth companies primarily in the technology and life sciences industries . Ms . Curley received her Master of Accountancy and Bachelor of Science in Business Administration with a concentration in accounting from Kansas State University . She also attended the United States Military Academy . She is a certified public accountant licensed in the Commonwealth of Massachusetts . Benjamin Bielak | Chief Information Officer Mr . Bielak has been serving as our Chief Information Officer since June 2018 . He served as the Chief Information Officer at GNS Healthcare, a leading casual machine learning product and services company, from January 2017 to May 2018 and as Director of Academic Technology at Harvard University, from February 2015 to January 2017 . Prior to his work at GNS and Harvard, Mr . Bielak was the Chief Information Officer at Dovetail Health from November 2006 to April 2014 . He previously held roles as Manager of Development and Integration at Boston Medical Center and Senior Manager of Technology at Sapient, a global services company, from December 1997 to July 2005 . Mr . Bielak holds a Masters of Business Administration degree from Bentley University, where his studies focused on change management, and a master’s degree from Boston University in computer science . David Wages, M . D . , PHD | Chief Medical Officer Mr . Wages has been serving as our Chief Medical Officer since October 2018 . Prior to joining iSpecimen , David served as Senior Medical Director at Quintiles, the world’s largest clinical research organization, where he supported both large pharmaceutical companies as well as smaller biotech organizations in a wide variety of clinical oncology programs . David has worked in both start - up biotechs and large pharma . David began his career working on the clinical development of novel drug - device combinations for blood decontamination at Cerus Corporation . He then worked on clinical trials at Wyeth that led to the development of Xyntha ®, a drug used to treat hemophilia . After a brief stint assisting institutional investors on healthcare technology opportunities at Leerink - Swann’s Medacorp , David then moved on to ARIAD Pharmaceuticals where he turned to cancer research, working on ARIAD’s mTOR inhibitor for solid tumors as well as initiating ARIAD’s phase I trial for chronic myeloid leukemia drug, Iclusig ® . David next joined EMD Serono where he worked on phase I - III trials of immune - agents for oncology . David holds an MD and PhD from the University of Virginia School of Medicine where he focused on the basic science of oncology . He then completed a residency at the University of California, San Francisco and a fellowship in hematopathology at Boston’s Beth Israel hospital . Management Team 19
Investor Summary 20 >200 Healthcare Provider / Supplier Organizations >450 Customer Organizations 1. $3B - $4B global biospecimen market growing at 10 - 15% per year 2. Early - stage opportunity with a first mover advantage 3. Unique two - sided marketplace disrupting the biospecimen procurement process 4. Strong revenue growth with a 7 - year CAGR of 48% 5. Growing data asset that’s a key enabler and differentiator

CONNECT. INNOVATE. Sign up for a free iSpecimen Marketplace account at www.ispecimen.com CONTACT US iSpecimen Inc. 450 Bedford Street Lexington, MA 02420 investors@ispecimen.com ispecimen.com

Appendix 22
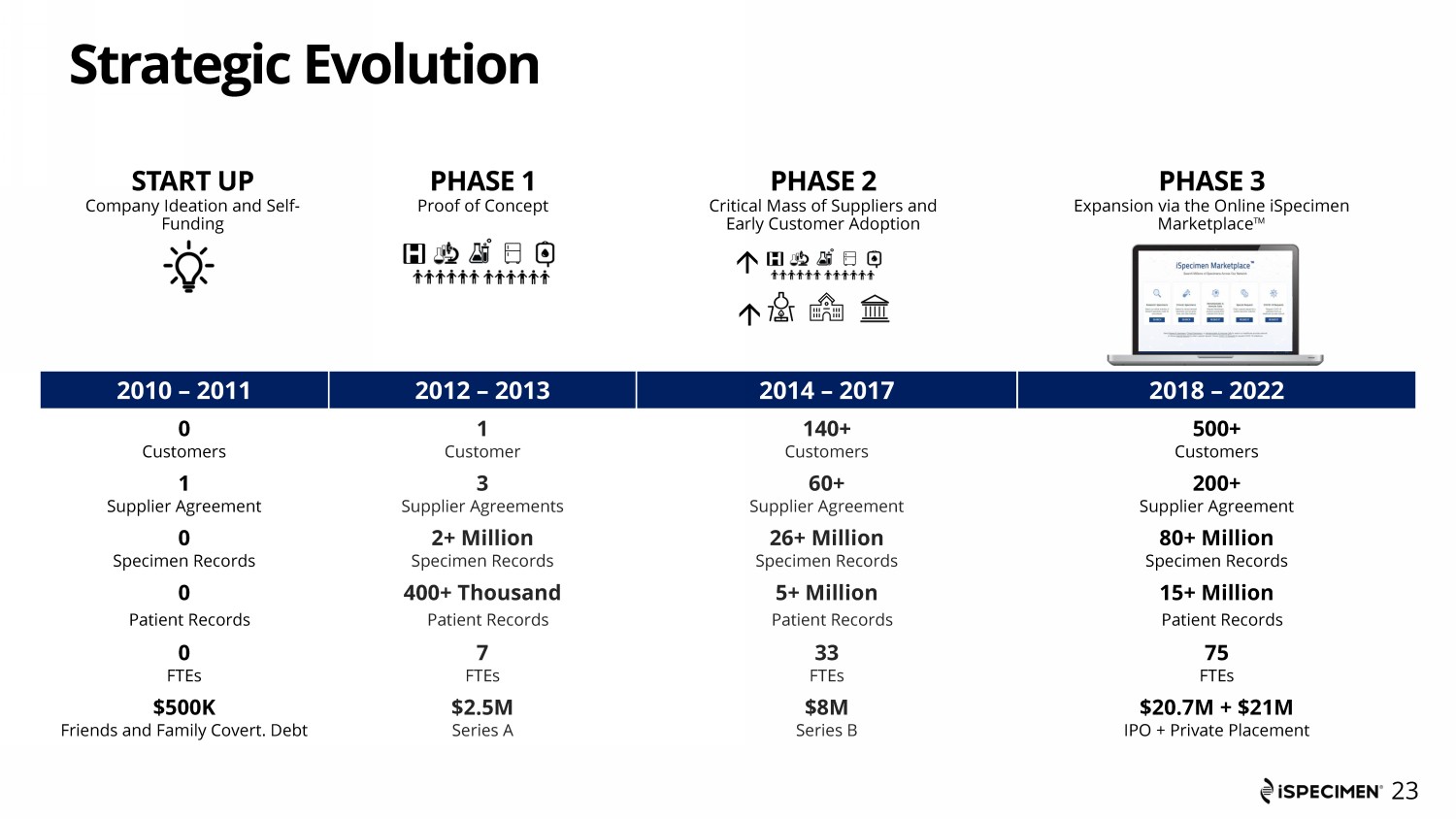
Strategic Evolution PHASE 3 Expansion via the Online iSpecimen Marketplace Ƞ START UP Company Ideation and Self - Funding PHASE 1 Proof of Concept PHASE 2 Critical Mass of Suppliers and Early Customer Adoption 2010 – 2011 2012 – 2013 2014 – 2017 2018 – 2022 0 Customers 1 Supplier Agreement 0 Specimen Records 0 Patient Records 0 FTEs $500K Friends and Family Covert. Debt 1 Customer 3 Supplier Agreements 2+ Million Specimen Records 400+ Thousand Patient Records 7 FTEs $2.5M Series A 140+ Customers 60+ Supplier Agreement 26+ Million Specimen Records 5+ Million Patient Records 33 FTEs $8M Series B 500+ Customers 200+ Supplier Agreement 80+ Million Specimen Records 15+ Million Patient Records 75 FTEs $20.7M + $21M IPO + Private Placement 23

iSpecimen Marketplace UI/UX Click Search to start searching for specimens 24
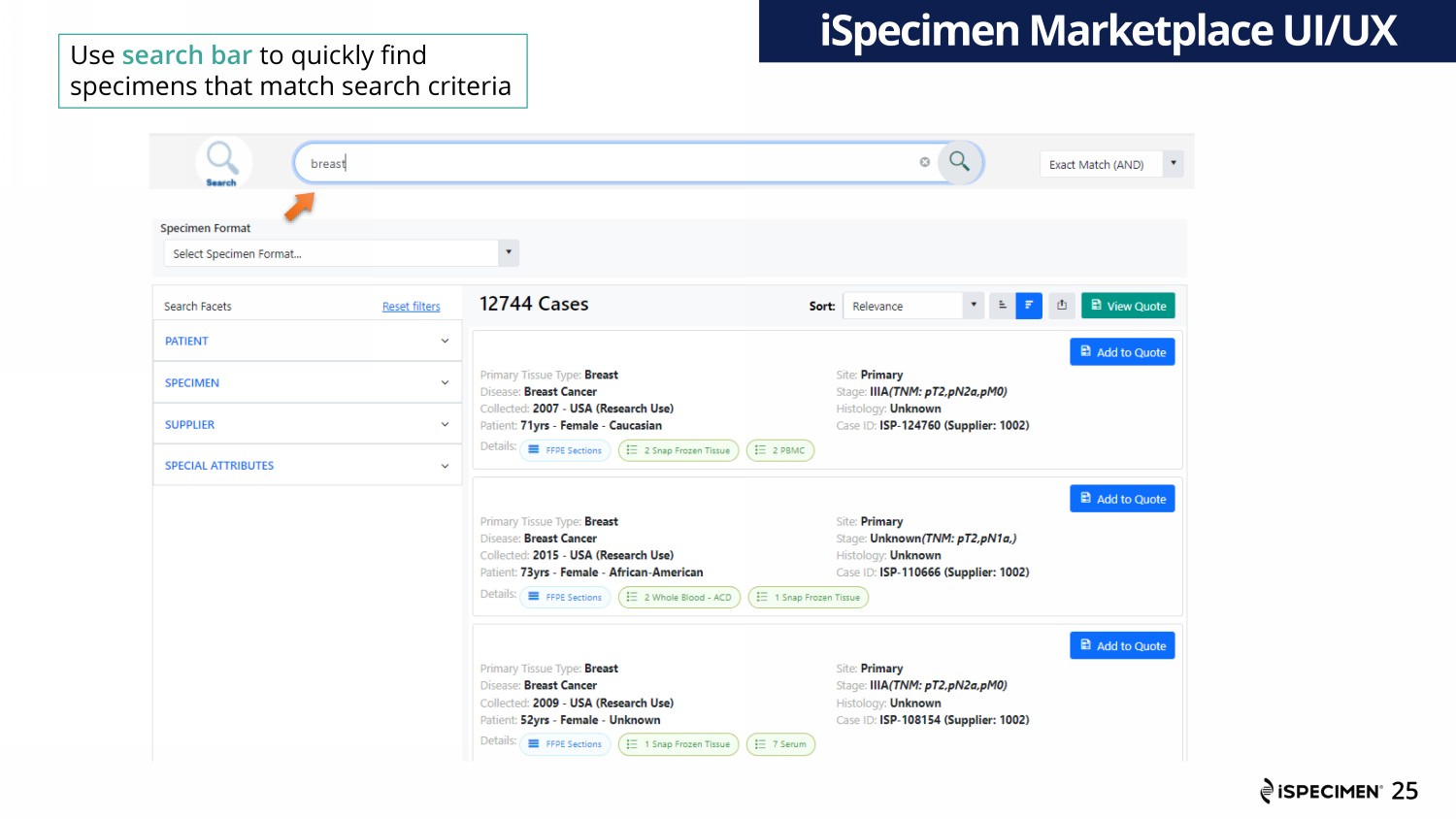
25 iSpecimen Marketplace UI/UX Use search bar to quickly find specimens that match search criteria
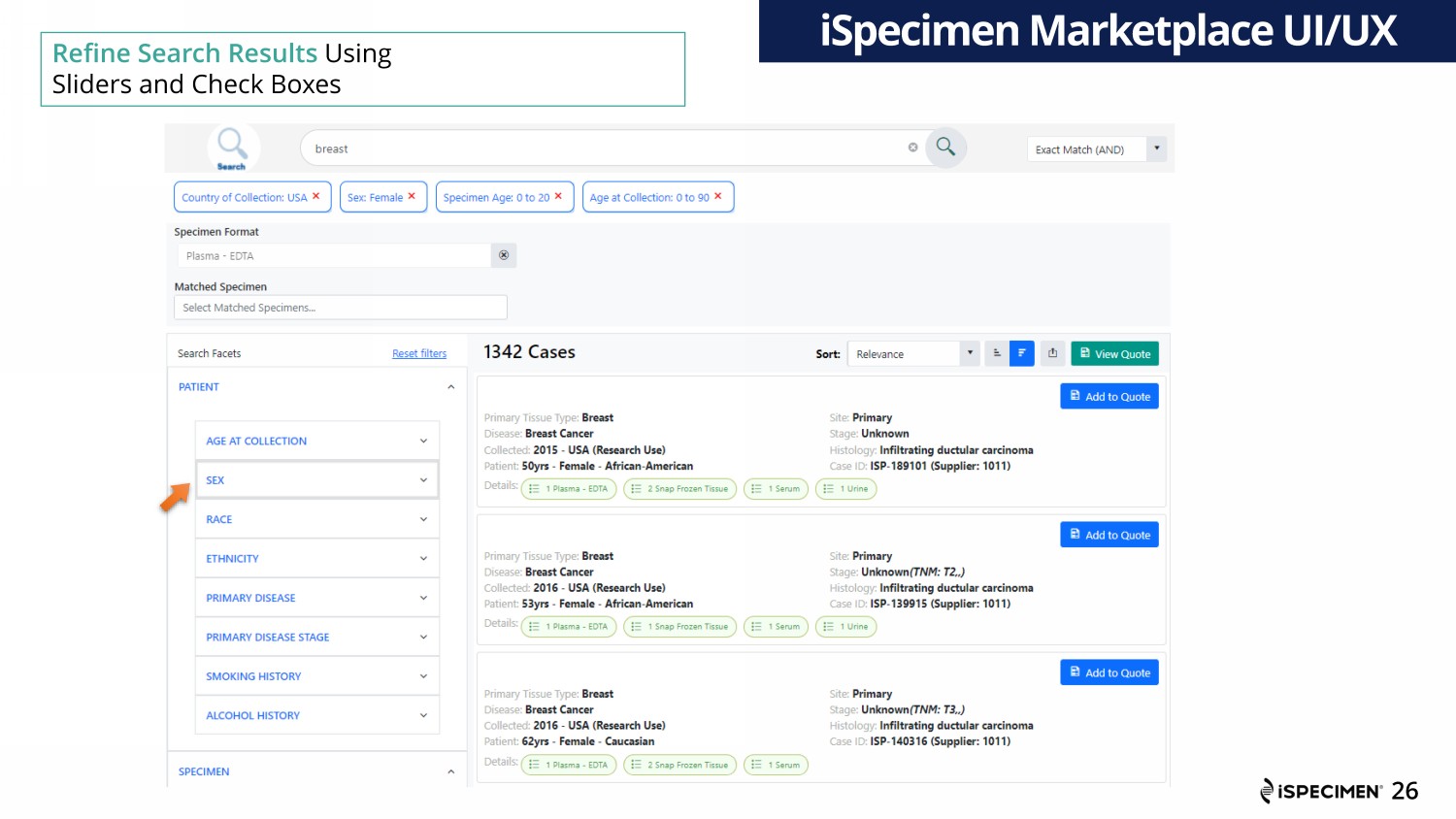
Refine Search Results Using Sliders and Check Boxes iSpecimen Marketplace UI/UX 26
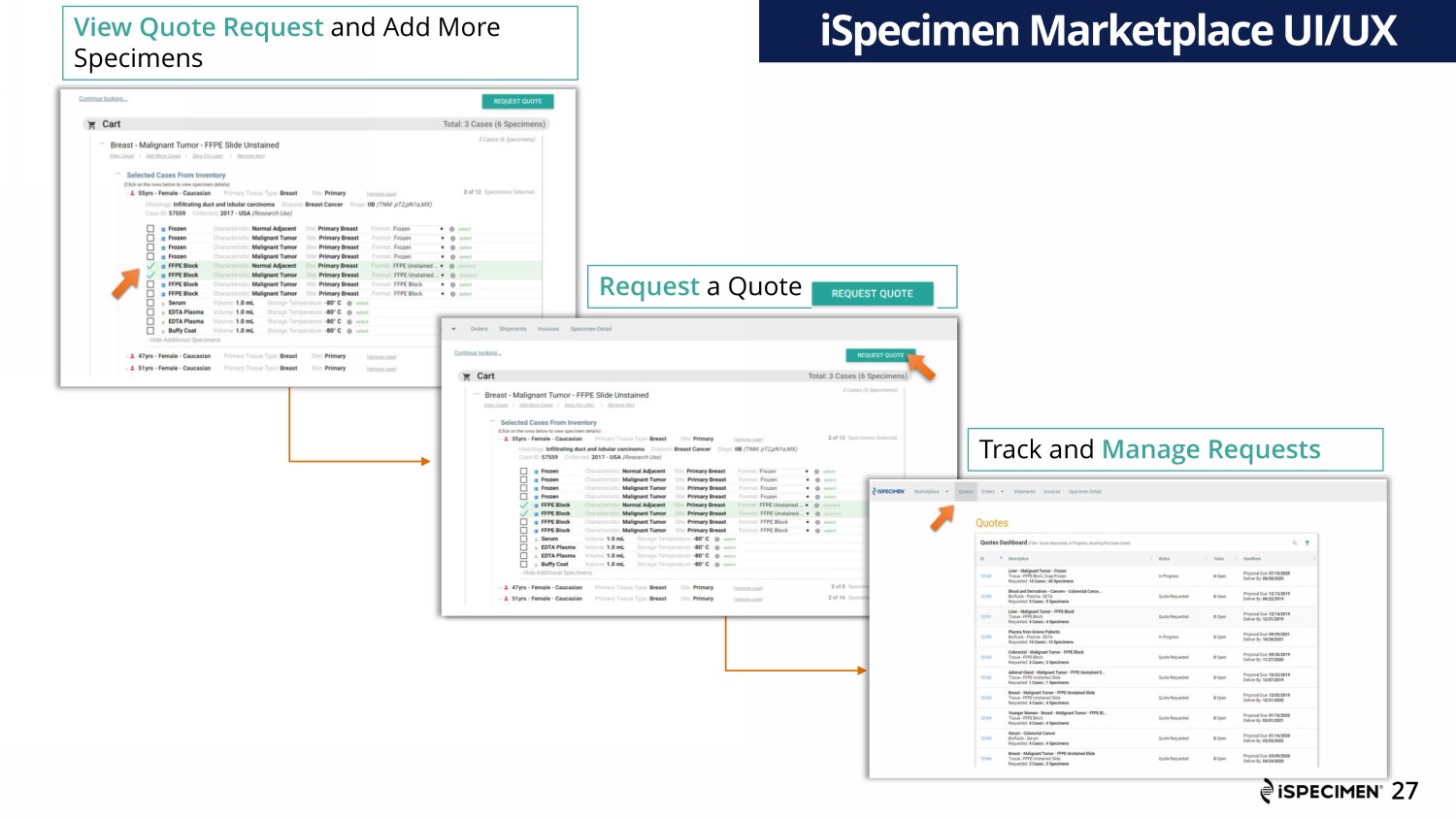
View Quote Request and Add More Specimens Request a Quote iSpecimen Marketplace UI/UX 27 Track and Manage Requests

28 Page Note Source Cited 7 1 iSpecimen estimates based on In Vitro Diagnostics Market Size, Share & Trends Analysis Report By Product, By Application, By Technology (Immunochemistry, Molecular Diagnostics) By End - use, By Region, And Segment Forecasts, 2021 – 2027. Grandview Research, January 2021; and World Preview 2020,Outlook to 2026. EvaluatePharma, July 2020. 7 2 Global Marketing Insights, Precision Medicine Market Size By Technology (Big Data Analytics, Bioinformatics, Gene Sequencing, Drug Discovery, Companion Diagnostics), By Application (Oncology, Immunology, CNS, Respiratory), By End - use (Pharmaceutical Companies, Diagnostic Companies, Healthcare IT companies), Industry Analysis Report, Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2020 - 2026, Feb. 2020. and Regenerative Medicine Market Size, Share and Industry Analysis by Product (Cell Therapy, Gene Therapy, Tissue Engineering, Platelet Rich Plasma), By Application (Orthopaedics, Wound Care, Oncology), By Distribution Channel (Hospitals, Clinics) & Re gio nal Forecast, 2019 – 2026. Fortune Business Insight, 2019. 8 3 Frost & Sullivan, Global Biobanking Market Landscape. May 20, 2020, page 357 . 9 4 Centers for Disease Control, https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf 9 5 iSpecimen estimates based upon American Association of Clinical Chemistry US lab test data cited at https://www.360dx.com/research - funding/aacc - calls - congress - fund - clinical - lab - training - programs, 2020; and Clinical Lab Services, Global Market Trajectory and Analytics; Global Industry Analysts report, 2020. 9 6 iSpecimen estimate based upon Henderson, G.E., Cadigan, R.J., Edwards, T.P. et al. Characterizing biobank organizations in th e U .S.: results from a national survey, 2013; and Puchois, Comprehensive Biomarker Discovery and Validation for Clinical Application, 2013. 9 7 Holly A. Massett et al. Assessing the need for a standardized cancer HUman Biobank (caHUB): findings from a national survey w ith cancer researchers; JNCI Monographs, Volume 2011, Issue 42, June 2011. 9 8 Specimen Independent Researcher Survey, 2022. Sources Cited





























