Exhibit 99.2

79 T.W. Alexander Drive | 4501 Research Commons, Suite 100 | Research Triangle Park, NC 27709
919-213-9835 (P) | 919-741-5830 (F) | www.g1therapeutics.com
November 2017
1

Forward-looking statements
This presentation and the accompanying oral commentary contain “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, known as the PSLRA, that are based on our beliefs and assumptions and on information available to us as of the date of this presentation. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, development plans, regulatory activities, competitive position, potential growth opportunities, use of proceeds and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” “will,” or “would,” or the negative of these terms, or other comparable terminology intended to identify statements about the future.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking
statements. Forward-looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. The risks and uncertainties that we face are described in our most recent filings with the Securities and Exchange Commission.
2
G1 Therapeutics: clinical-stage oncology company
Advancing the validated CDK4/6 inhibitor space
Three wholly owned drug candidates addressing distinct
multi-billion dollar markets
Trilaciclib is first-in-class with compelling clinical data; currently in
four Phase 2 trials
G1T38 has best-in-class potential versus Ibrance, Kisqali and Verzenio
G1T48 (oral SERD) has first/best-in-class potential; on track for 4Q17 IND
Multiple clinical data readouts, value inflection points in 2018
3

Experienced leadership, well financed
Management team
Proven industry leaders with more than 75 years of oncology experience
Mark Velleca, MD, PhD – Chief Executive Officer Raj Malik, MD – Chief Medical Officer Terry Murdock – SVP Development Operations Buck Phillips – CFO, SVP Corporate Development Jay Strum, PhD – Chief Scientific Officer
Board of Directors
Seth Rudnick, MD – board chairman
Fred Eshelman, PharmD – Eshelman Ventures
Glenn P. Muir – audit committee chair Tyrell Rivers, PhD – MedImmune Ventures Christy Shaffer, PhD – Hatteras Venture Partners
Mark Velleca, MD, PhD – CEO
Andrew Witty – former CEO of GSK
Investors
~ $96m private (4Q13, 1Q15, 2Q16)
— Cormorant
— Franklin Templeton
— RA Capital
— Rock Springs Capital
— Tavistock
~ $107m IPO—May 22, 2017
— Nasdaq: GTHX
4
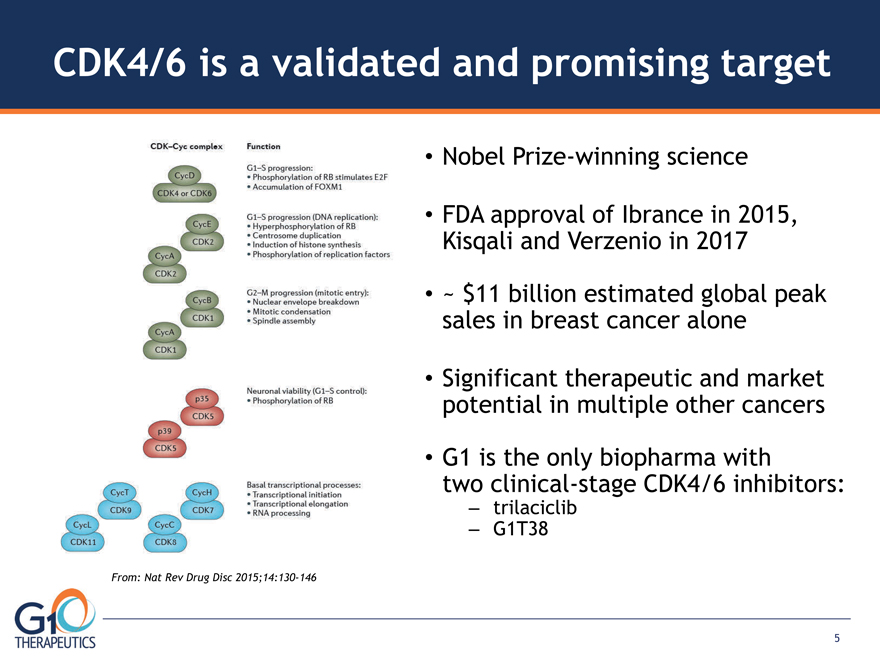
CDK4/6 is a validated and promising target
From: Nat Rev Drug Disc 2015;14:130-146
Nobel Prize-winning science
FDA approval of Ibrance in 2015, Kisqali and Verzenio in 2017
~ $11 billion estimated global peak sales in breast cancer alone
Significant therapeutic and market potential in multiple other cancers
G1 is the only biopharma with two clinical-stage CDK4/6 inhibitors:
– trilaciclib
– G1T38
5
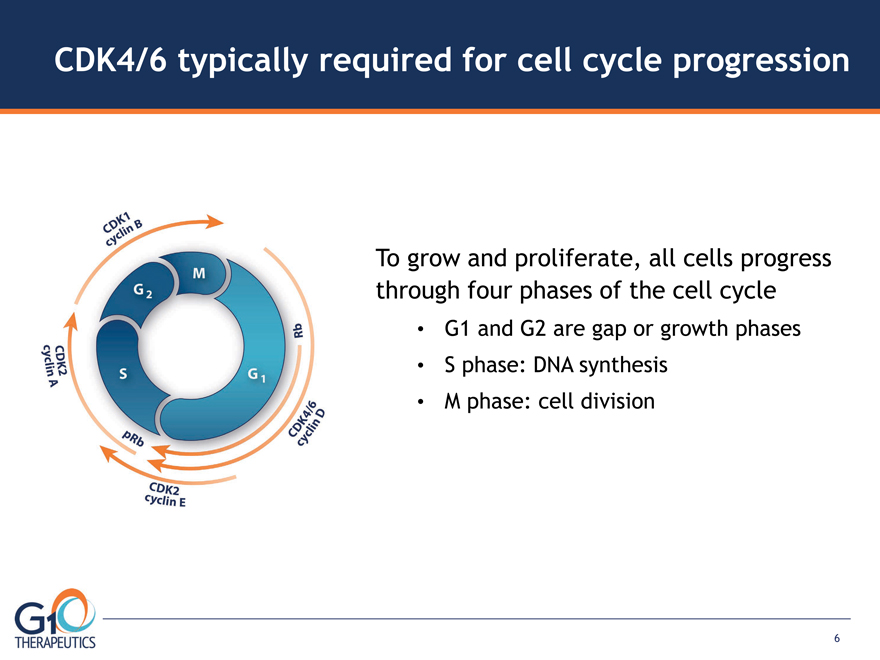
CDK4/6 typically required for cell cycle progression
To grow and proliferate, all cells progress
through four phases of the cell cycle
G1 and G2 are gap or growth phases
S phase: DNA synthesis
M phase: cell division
6
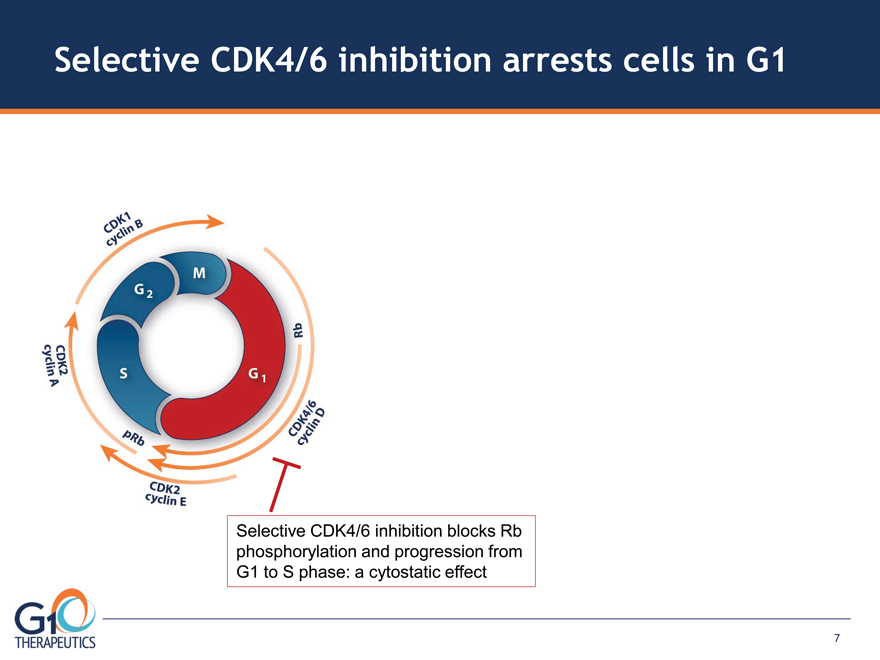
Selective CDK4/6 inhibition arrests cells in G1
Selective CDK4/6 inhibition blocks Rb
phosphorylation and progression from
G1 to S phase: a cytostatic effect
7
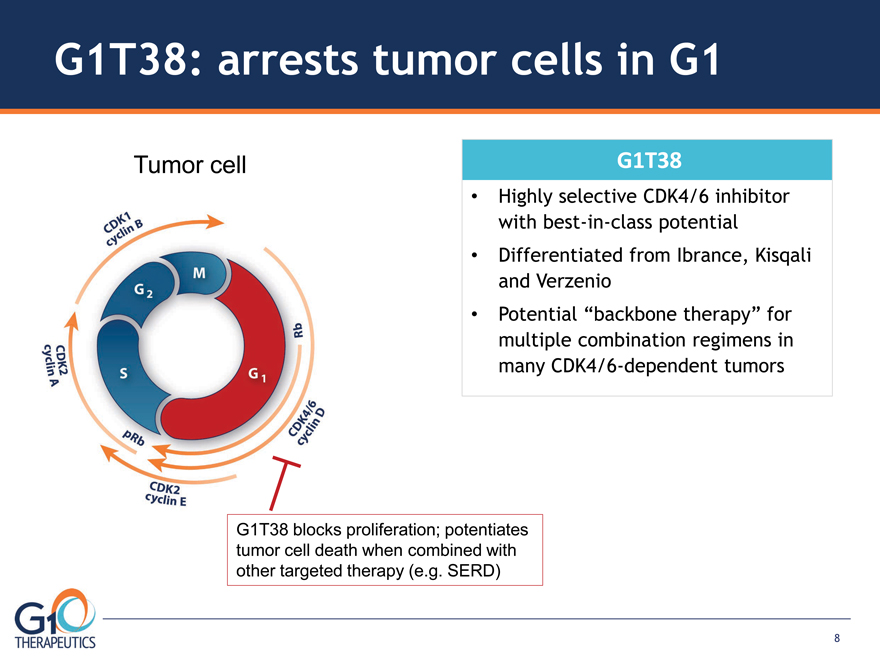
G1T38: arrests tumor cells in G1
G1T38 blocks proliferation; potentiates
tumor cell death when combined with
other targeted therapy (e.g. SERD)
G1T38
Highly selective CDK4/6 inhibitor
with best-in-class potential
Differentiated from Ibrance, Kisqali
and Verzenio
Potential “backbone therapy” for
multiple combination regimens in
many CDK4/6-dependent tumors
Tumor cell
8
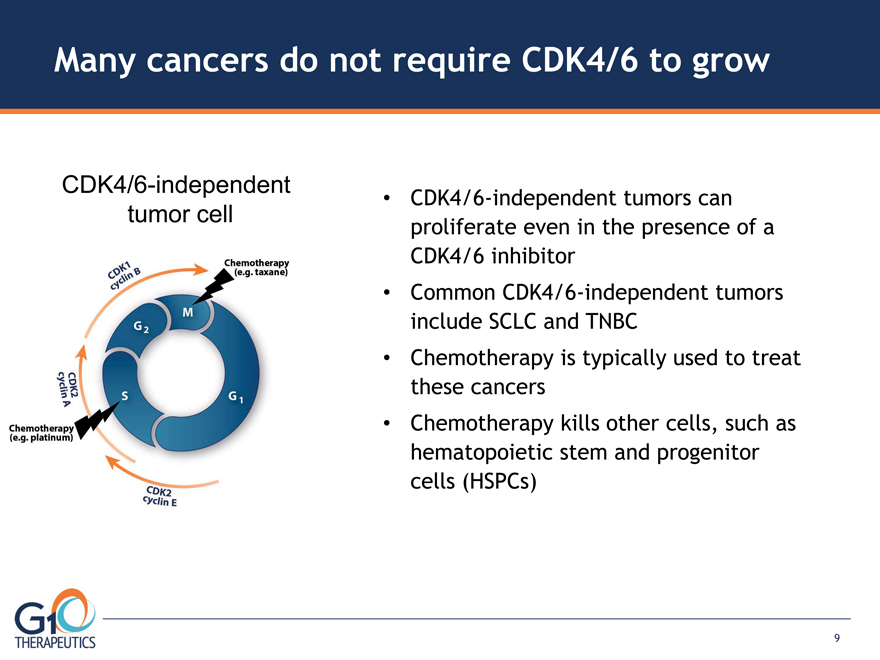
CDK4/6-independent
tumor cell
Many cancers do not require CDK4/6 to grow
CDK4/6-independent tumors can proliferate even in the presence of a CDK4/6 inhibitor Common CDK4/6-independent tumors include SCLC and TNBC
Chemotherapy is typically used to treat these cancers Chemotherapy kills other cells, such as hematopoietic stem and progenitor cells (HSPCs)
9

HSPC HSPCs are the “reservoir” from which
all blood and immune system cells are formed
HSPCs are damaged by chemotherapy, causing
myelosuppression and immunosuppression,
limiting anti-tumor efficacy
CDK4/6-independent tumor cell
Hematopoietic stem and progenitor cells (HSPCs) require CDK4/6
10
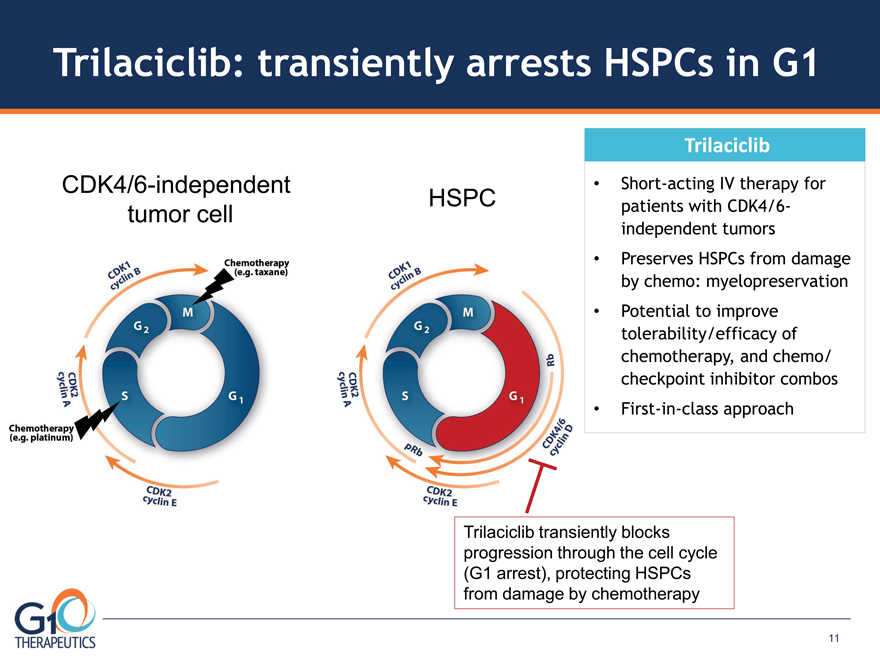
CDK4/6-independent HSPC tumor cell
Trilaciclib: transiently arrests HSPCs in G1
Short-acting IV therapy for patients with CDK4/6-
independent tumors
Preserves HSPCs from damage by chemo: myelopreservation
Potential to improve tolerability/efficacy of chemotherapy, and chemo/
checkpoint inhibitor combos
First-in-class approach Trilaciclib
Trilaciclib transiently blocks progression through the cell cycle
(G1 arrest), protecting HSPCs from damage by chemotherapy
11
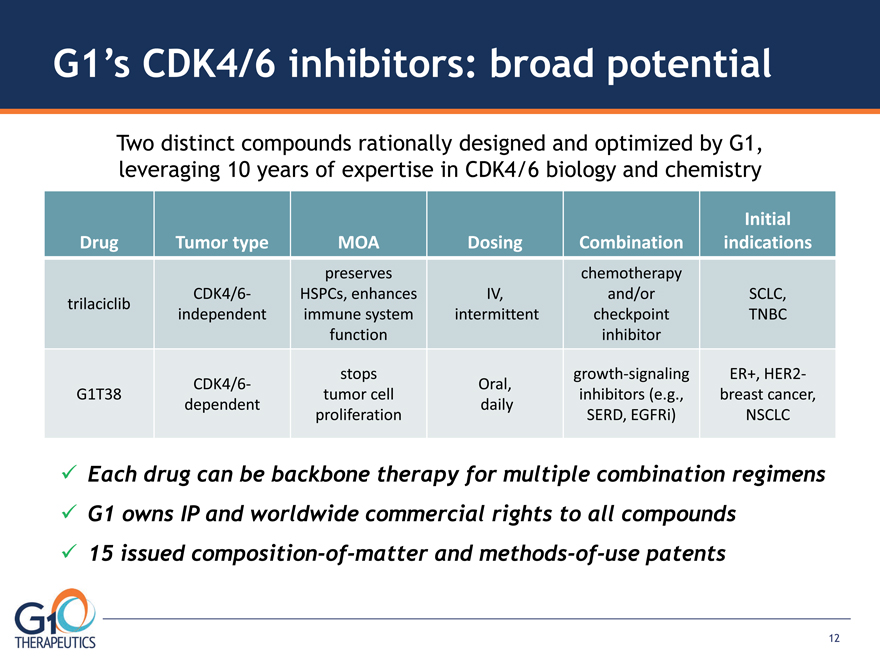
G1’s CDK4/6 inhibitors: broad potential
Drug Tumor type MOA Dosing Combination
Initial indications trilaciclib CDK4/6- independent preserves HSPCs, enhances immune system function
IV, intermittent chemotherapy and/or checkpoint inhibitor SCLC, TNBC G1T38 CDK4/6- dependent
stops tumor cell proliferation Oral, daily growth-signaling inhibitors (e.g., SERD, EGFRi) ER+, HER2-
breast cancer, NSCLC Two distinct compounds rationally designed and optimized by G1, leveraging 10 years of expertise in CDK4/6 biology and chemistry Each drug can be backbone therapy for multiple combination regimens G1 owns IP and worldwide commercial rights to all compounds 15 issued composition-of-matter and methods-of-use patents
12
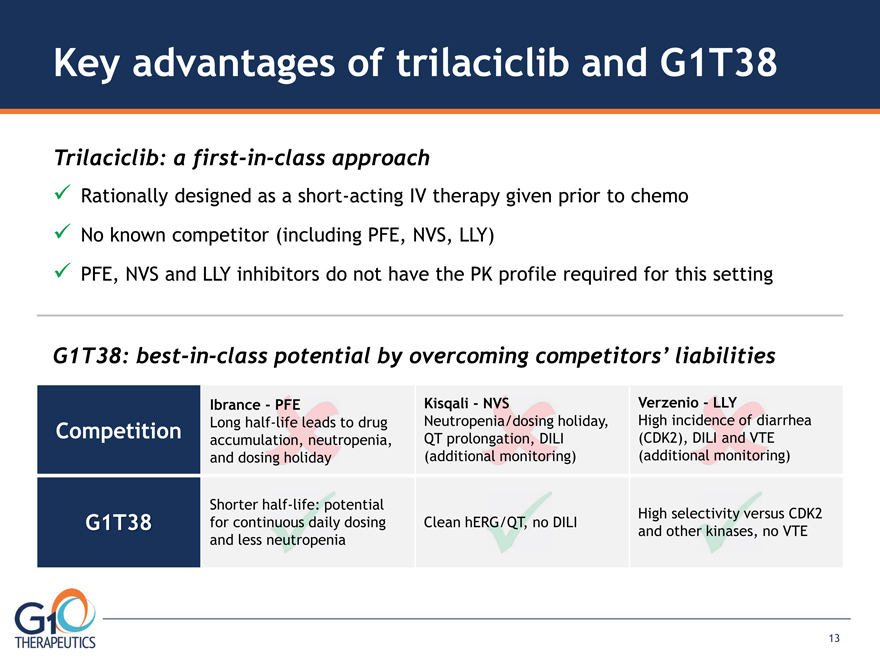
Key advantages of trilaciclib and G1T38 Trilaciclib: a first-in-class approach Rationally designed as a short-acting IV therapy given prior to chemo No known competitor (including PFE, NVS, LLY) PFE, NVS and LLY inhibitors do not have the PK profile required for this setting G1T38: best-in-class potential by overcoming competitors’ liabilities Competition G1T38 Shorter half-life: potential for continuous daily dosing and less neutropenia Clean hERG/QT, no DILI High selectivity versus CDK2 and other kinases, no VTE
Ibrance—PFE Long half-life leads to drug accumulation, neutropenia, and dosing holiday Kisqali—NVS Neutropenia/dosing holiday, QT prolongation, DILI (additional monitoring) Verzenio—LLY High incidence of diarrhea (CDK2), DILI and VTE (additional monitoring) 13
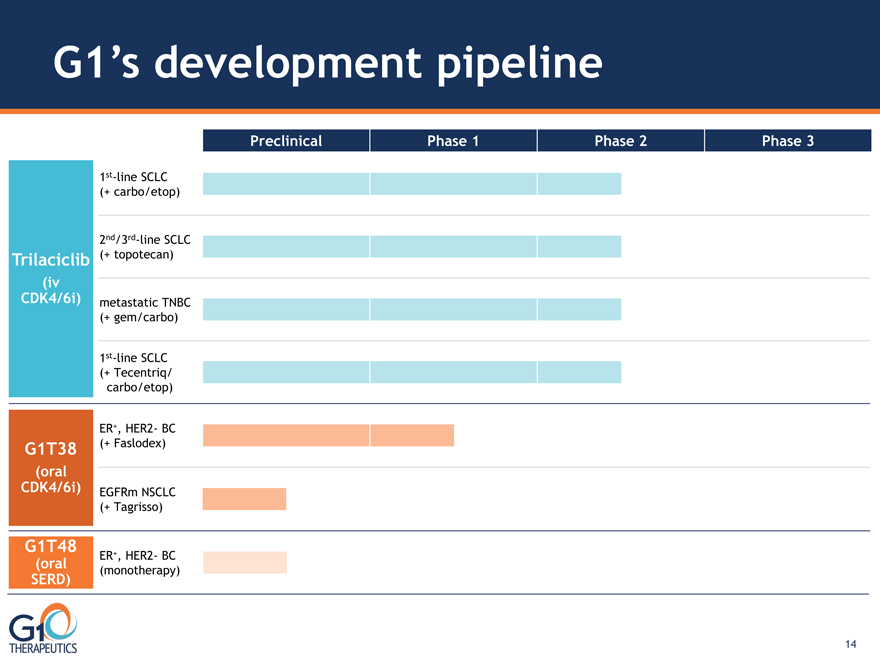
G1’s development pipeline Trilaciclib (iv DK4/6i) G1T38
(oral CDK4/6i)
2nd/3rd-line SCLC
(+ topotecan)
1st-line SCLC
(+ Tecentriq/carbo/etop)
Preclinical Phase 1 Phase 2 Phase 3
ER+, HER2- BC
(monotherapy)
G1T48
(oral
SERD)
ER+, HER2- BC
(+ Faslodex)
EGFRm NSCLC
(+ Tagrisso)
metastatic TNBC
(+ gem/carbo)
1st-line SCLC
(+ carbo/etop)
14
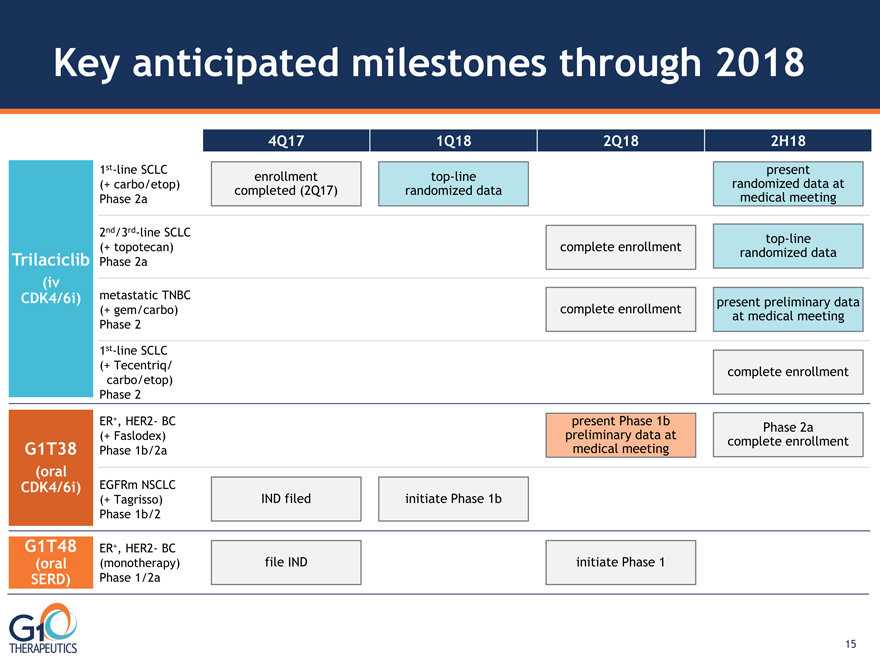
Key anticipated milestones through 2018 Trilaciclib (iv CDK4/6i) G1T38
(oral CDK4/6i) 2nd/3rd-line SCLC (+ topotecan) Phase 2a 1st-line SCLC
(+ Tecentriq/ carbo/etop) Phase 2 4Q17 1Q18 2Q18 2H18 ER+, HER2- BC
(monotherapy) Phase 1/2a G1T48 (oral SERD) ER+, HER2- BC (+ Faslodex)
Phase 1b/2a EGFRm NSCLC (+ Tagrisso) Phase 1b/2 metastatic TNBC
(+ gem/carbo) Phase 2 1st-line SCLC (+ carbo/etop) Phase 2a file IND initiate
Phase 1 IND filed initiate Phase 1b complete enrollment complete enrollment
top-line randomized data complete enrollment present preliminary data at medical meeting
top-line randomized data present randomized data at medical meeting enrollment
completed (2Q17) present Phase 1b preliminary data at medical meeting Phase 2a
complete enrollment 15

TRILACICLIB DEVELOPMENT
PROGRAM
16
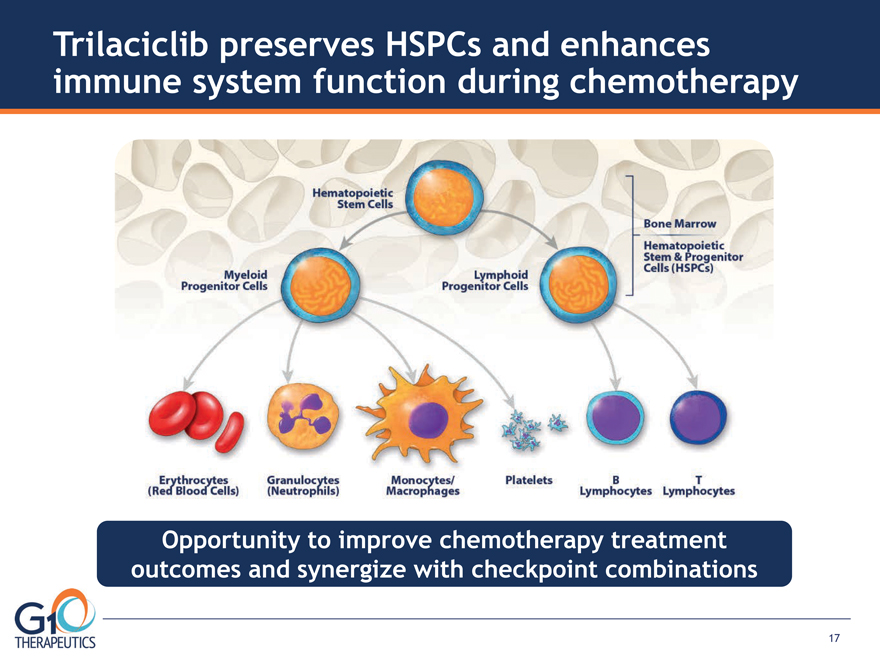
Opportunity to improve chemotherapy treatment
outcomes and synergize with checkpoint combinations
Trilaciclib preserves HSPCs and enhances
immune system function during chemotherapy
17
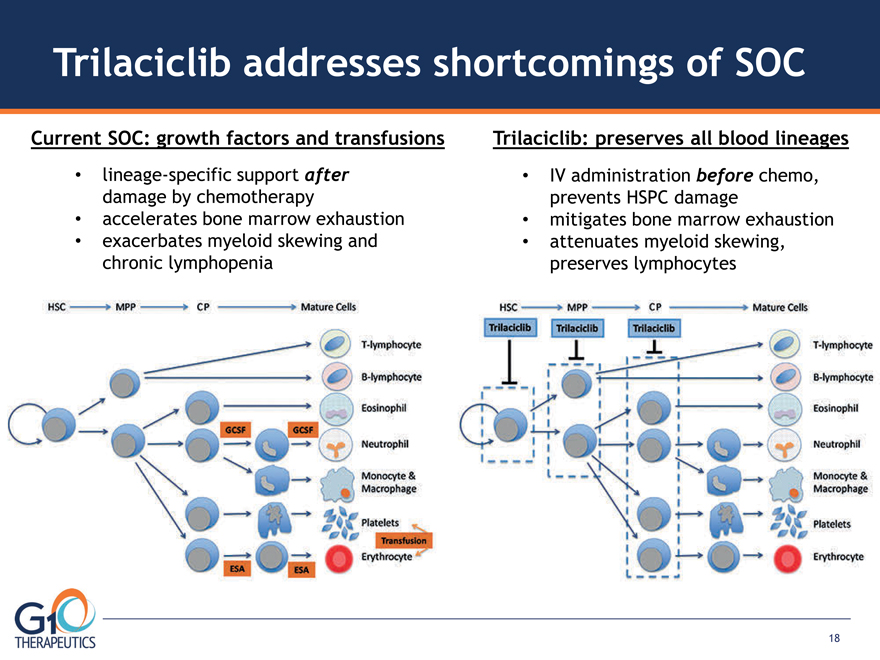
Trilaciclib addresses shortcomings of SOC lineage-specific support after damage by chemotherapy
accelerates bone marrow exhaustion exacerbates myeloid skewing and chronic lymphopenia
Trilaciclib: preserves Current SOC: growth factors and transfusions all blood lineages
IV administration before chemo, prevents HSPC damage mitigates bone marrow exhaustion
attenuates myeloid skewing, preserves lymphocytes 18
Rationally designed product profile
Reduce suppression of all hematopoietic lineages by protecting HSPCs from
damage by chemotherapy (myelopreservation)
Reduce clinically relevant consequences of myelosuppression—e.g. febrile
neutropenia (FN)—and reduce overall cost of care from hospitalizations,
growth factor support, transfusions
Use with multiple chemotherapies in patients with CDK4/6-independent
tumors: SCLC, TNBC, bladder, head and neck, others
Dosing regimen fits with standard clinical practice
– IV infusion of trilaciclib prior to chemotherapy
Potential to increase anti-tumor activity and impact ORR/PFS/OS by:
– enabling maintenance of planned chemotherapy dose and schedule
– enhancing immune system function in context of chemo-mediated tumor cell death
19
Trilaciclib value proposition—base case:
myelopreservation-only, CDK4/6-independent
~ 1 million patients/year receive chemotherapy (US only)
~ 300,000 patients with CDK4/6-independent tumors eligible for trilaciclib
– predominantly CDK4/6-independent: SCLC, TNBC, HPV+ H&N, bladder, cervical, sarcomas
– CDK4/6-independent subsets of: NSCLC, HER2+ BC, uterus, esophagus, CRPC, CRC
Worldwide market potential exceeds several billion dollars annually
– assumes conservative patient capture rate and pricing
Several upside case scenarios with compelling data in hand
– efficacy enhancement
– synergy with checkpoint inhibitor/chemo combos
– use in CDK4/6-dependent tumors
Potential to be “backbone therapy” for multiple chemo regimens and
checkpoint/chemo combinations
20
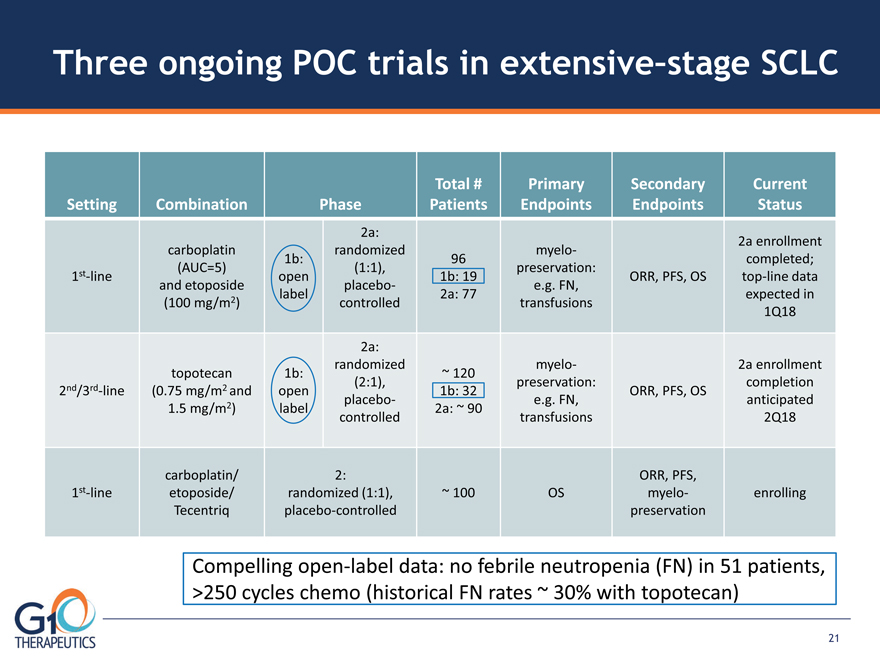
Three ongoing POC trials in extensive–stage SCLC Setting Combination Phase
Total # Patients Primary Endpoints Secondary Endpoints Current Status
1st-line carboplatin (AUC=5) and etoposide (100 mg/m2) 1b: Open label
2a: randomized (1:1), placebocontrolled 96 1b: 19 2a: 77 Myelo preservation: e.g. FN,
transfusions ORR, PFS, OS 2a enrollment completed; top-line data expected in 1Q18 2nd/3rd-line
topotecan (0.75 mg/m2 and 1.5 mg/m2) 1b: open label 2a: randomized
(2:1), placebocontrolled ~ 120 1b: 32 2a: ~ 90 myelopreservation:
e.g. FN, transfusions ORR, PFS, OS 2a enrollment completion
anticipated 2Q18 1st-line carboplatin/ etoposide/ Tecentriq
2: randomized (1:1), placebo-controlled ~ 100 OS ORR, PFS,
myelopreservation enrolling Compelling open-label data: no febrile neutropenia (FN) in 51 patients,
>250 cycles chemo (historical FN rates ~ 30% with topotecan)3 21
1st-line SCLC Phase 1b/2a trial
Trilaciclib + etoposide/carboplatin (EP) in patients with newly
diagnosed extensive-stage SCLC (PS 0-2)
Combination schedule in 21-day cycles
– 30 min IV infusion of trilaciclib prior to EP on days 1-3
Completed open-label Phase 1b trial
– 19 patients enrolled, 17 evaluable for efficacy
– data presented at ASCO 2017 (Rocha Lima et al.)
Randomized, double-blind, placebo-controlled Phase 2a trial ongoing
– enrollment completed in 2Q17 (77 patients, 1:1 randomization)
– top-line data expected in 1Q18 22
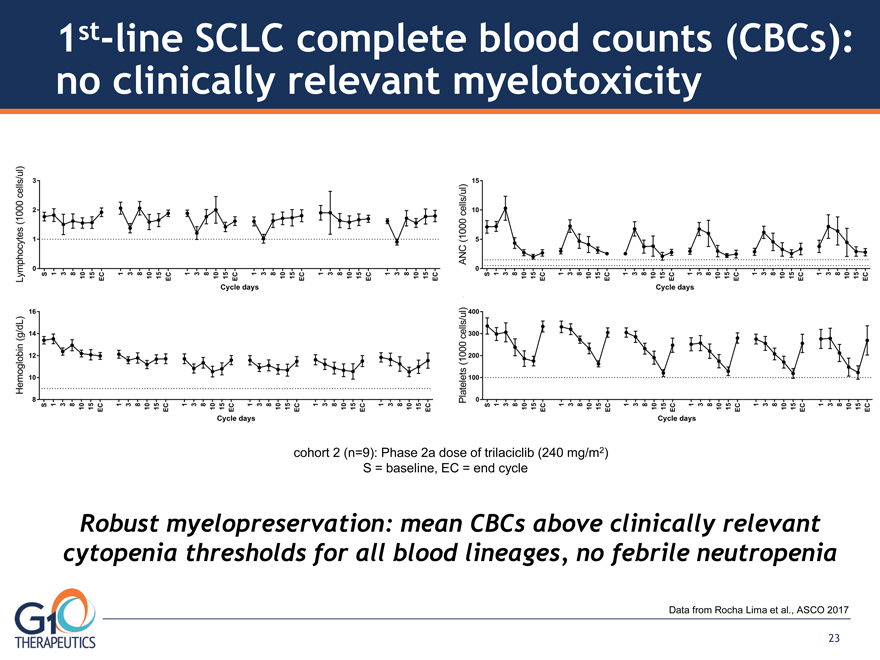
Robust myelopreservation: mean CBCs above clinically relevant
cytopenia thresholds for all blood lineages, no febrile neutropenia
cohort 2 (n=9): Phase 2a dose of trilaciclib (240 mg/m2)
S = baseline, EC = end cycle
1st-line SCLC complete blood counts (CBCs):
no clinically relevant myelotoxicity
Data from Rocha Lima et al., ASCO 2017
23
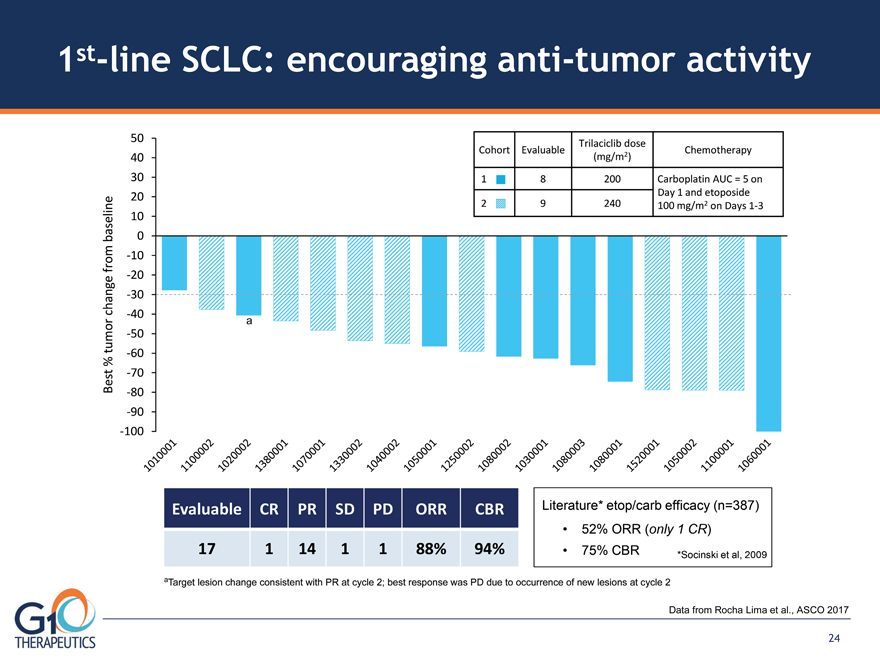
1st-line SCLC: encouraging anti-tumor activity
Data from Rocha Lima et al., ASCO 2017
24
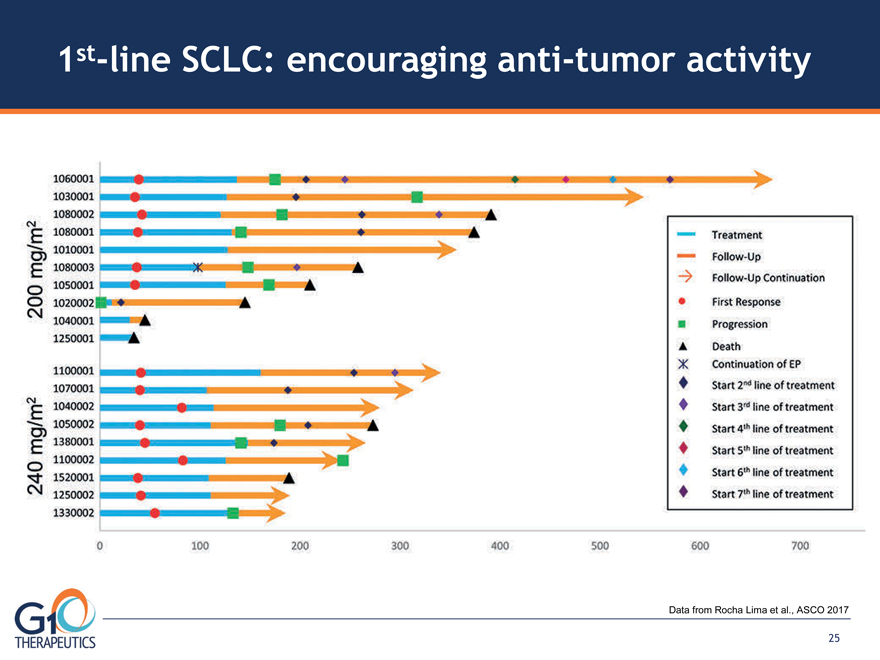
1st-line SCLC: encouraging anti-tumor activity
Data from Rocha Lima et al., ASCO 2017
25
SCLC 1b/2a trials: conclusions and plans Phase 1b data provides strong evidence of myelopreservation in
both first-line and second/third line settings No febrile neutropenia: 51 open-label patients, >250 chemo cycles
Improved overall response vs. historical rates in first-line Both studies in randomized, placebo-controlled Phase 2a
Top-line data from first-line study expected in 1Q18 Potential to move rapidly into pivotal trials 26
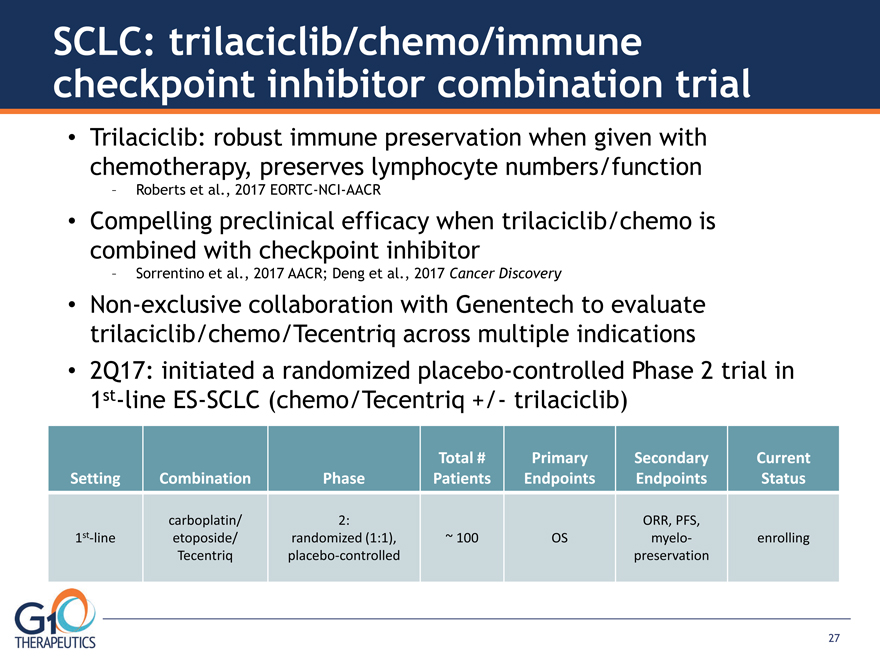
SCLC: trilaciclib/chemo/immune checkpoint inhibitor combination trial Trilaciclib: robust immune preservation when given with
chemotherapy, preserves lymphocyte numbers/function – Roberts et al., 2017 EORTC-NCI-AACR Compelling preclinical efficacy when trilaciclib/chemo is combined with checkpoint inhibitor – Sorrentino et al., 2017 AACR; Deng et al., 2017 Cancer Discovery
Non-exclusive collaboration with Genentech to evaluate trilaciclib/chemo/Tecentriq across multiple indications
2Q17: initiated a randomized placebo-controlled Phase 2 trial in 1st-line ES-SCLC (chemo/Tecentriq +/- trilaciclib) Setting Combination Phase Total # Patients Primary Endpoints Secondary Endpoints Current Status 1st-line carboplatin/
etoposide/ Tecentriq 2: randomized (1:1), placebo-controlled ~ 100 OS ORR, PFS, myelopreservation enrolling 27
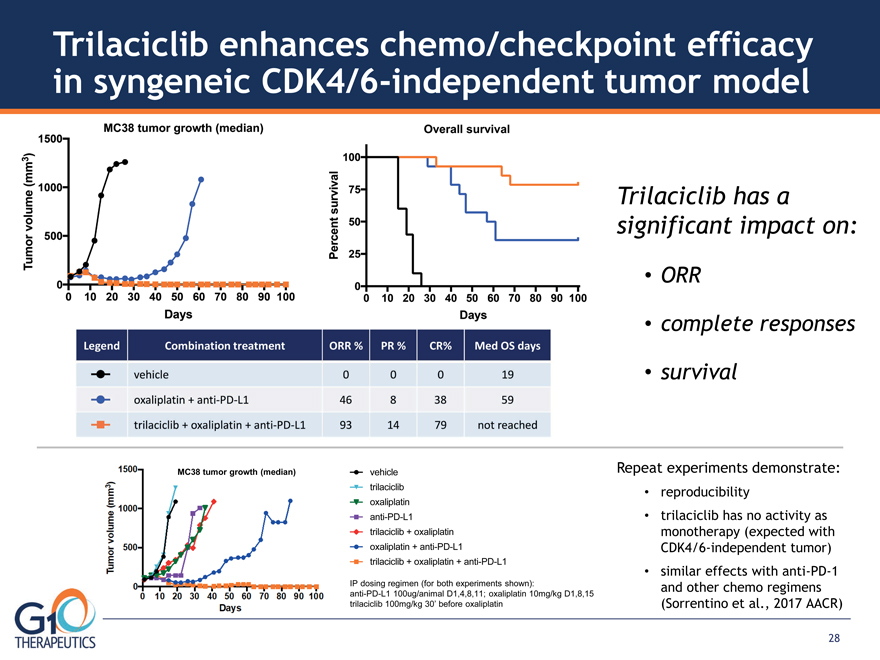
Trilaciclib enhances chemo/checkpoint efficacy in syngeneic CDK4/6-independent tumor model
MC38 tumor growth (median) Trilaciclib has a significant impact on: ORR complete responses
survival Repeat experiments demonstrate: reproducibility trilaciclib has no activity as
monotherapy (expected with CDK4/6-independent tumor) similar effects with anti-PD-1
and other chemo regimens (Sorrentino et al., 2017 AACR) IP dosing regimen (for both experiments shown):
anti-PD-L1 100ug/animal D1,4,8,11; oxaliplatin 10mg/kg D1,8,15 trilaciclib 100mg/kg 30’ before oxaliplatin
vehicle trilaciclib oxaliplatin anti-PD-L1 trilaciclib + oxaliplatin oxaliplatin + anti-PD-L1
trilaciclib + oxaliplatin + anti-PD-L1 28
Expansion to other indications: TNBC
Significant unmet medical need in triple-negative breast cancer (TNBC)
Gemcitabine/carboplatin + trilaciclib in first/second-line metastatic TNBC
~ 90 patient, randomized, open-label Phase 2 trial initiated 1Q17
– primary endpoints: myelopreservation (e.g. FN, transfusions)
– secondary endpoints: ORR, PFS, OS
Immunophenotyping to evaluate trilaciclib effects on T-cells
Expect ~ 20% patients to have CDK4/6-dependent tumors
– pathological confirmation of tumor CDK4/6 status, but enrolling “all comers”
– will correlate response rate with CDK4/6 status to determine applicability of
approach in broader population (i.e. CDK4/6-dependent tumors)
29

G1T38 DEVELOPMENT
PROGRAM
30
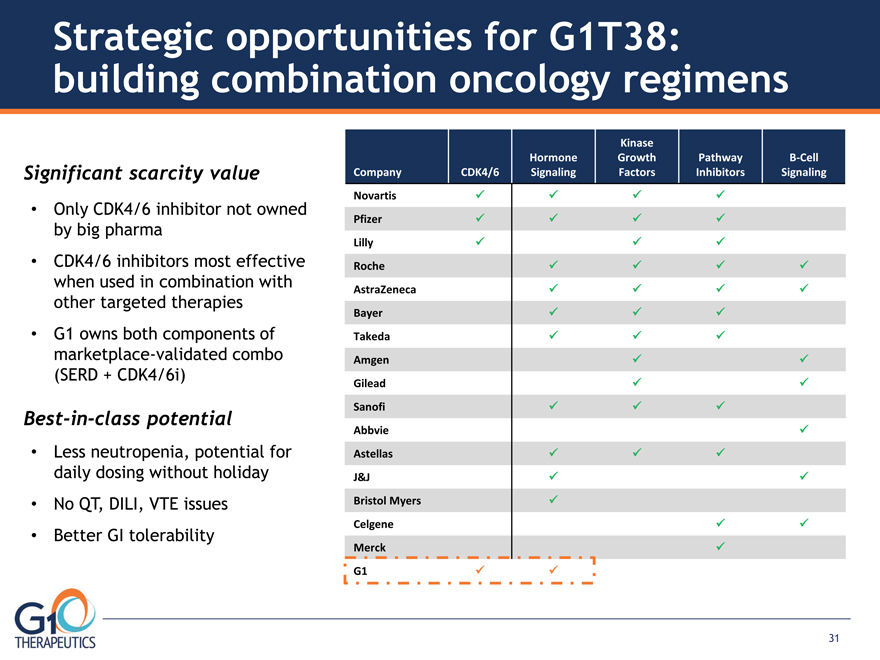
Company CDK4/6 Hormone Signaling Kinase Growth Factors Pathway Inhibitors
B-Cell Signaling Novartis Pfizer Lilly Roche AstraZeneca Bayer Takeda
Amgen Gilead Sanofi Abbvie Astellas J&J Bristol Myers Celgene
Merck G1 Only CDK4/6 inhibitor not owned by big pharma CDK4/6 inhibitors most effective
when used in combination with other targeted therapies G1 owns both components of
marketplace-validated combo (SERD + CDK4/6i) Less neutropenia, potential for
daily dosing without holiday No QT, DILI, VTE issues Better GI tolerability
Best-in-class potential Strategic opportunities for G1T38: building combination oncology regimens
Significant scarcity value
31

2017 publications
– Molecular Cancer Research (Stice et al., preclinical data in prostate cancer)
– Oncotarget (Bisi et al., preclinical data in breast cancer and NSCLC) Extensive preclinical validation and differentiation
– head-to-head studies with Ibrance (Bisi et al., 2017 Oncotarget) Drug well-tolerated in 75 subject Phase 1a single-dose HNV trial
– no DLTs or grade 3/4 AEs; no QT, DILI, VTE concerns
– differentiated PK profile: shorter t1/2, larger Vd than Ibrance, Kisqali Encouraging early results in Phase 1b/2a breast cancer trial (in
combination with Faslodex) with continuous dosing of G1T38
– pharmacodynamic activity observed at first dose level
– well-tolerated GI profile; no liver or CV AEs
– plan to present preliminary Phase 1b data in 2Q18 G1T38 differentiation: robust preclinical package and encouraging initial clinical data 32

G1T38: backbone therapy for multiple combination regimens—potential development paths
Cancer Indication/Line G1T38 with: Rationale Breast HR+/HER2- 1L G1T48
Approval of palbociclib with fulvestrant; extension of mPFS from 4.6 to 9.5 months (PALOMA-3, Turner et
al 2015, Cristofanilli et al 2016). G1T38 + G1T48 enhances efficacy and extends time to resistance in
preclinical models (Wardell et al AACR 2017). HR-/HER2+ TDM1 failures > 3L trastuzumab
+ lapatinib CDK4/6 inhibitors re-sensitize HER2+ cancers to HER2 blockade (Goel et al 2016, Malumbres 2016,
Witkiewicz et al 2014). G1T38 + lapatinib/trastuzumab enhances efficacy and extends time to resistance
in preclinical models. NSCLC EGFRm: 1L EGFRi CDK4/6 + EGFR inhibition overcomes resistance in preclinical
models (Zhou et al 2016). G1T38 + EGFRi enhances efficacy and extends time to resistance in NSCLC
EGFRT790M murine EGFRm: EGFRi model (Bisi et al 2017). failures, 2L osimertinib ALKi failures, 2L alectinib ALK and CDK4/6 inhibition demonstrates synergy in preclinical models (Wood et al 2016). KRASm, > 2L MEKi MEKi + CDK4/6i has significant anti-KRAS–mutant NSCLC activity (Tao et al 2016, LeBlanc et al 2016). Prostate CRPC 1L/2L AR-blocker Prostate tumor cells are highly dependent on cyclin D for cellular proliferation (Balk et al 2008). G1T38 demonstrates robust preclinical efficacy in CRPC models (Stice et al 2017). Lymphoma MCL, MZL, CLL, FL, DLBCL; 2L BTKi CDK4 activation drives BTKi resistance; CDK4/6i sensitizes MCL to ibrutinib (Chiron et al 2014). Positive clinical data in palbociclib/ibrutinib MCL study (Martin et al 2016).
Melanoma BRAFm 1L MEKi + RAFi CDK4/6i enhances efficacy and extends time to resistance in preclinical models (Harris et al AACR 2017). MEKi + CDK4/6i are synergistic in BRAFm melanoma (Teh et al 2016). RASm basket
CRC, pancreatic, cholangiocarcinoma MEKi CDK4/6 inhibitors enhance the efficacy of MEK inhibitors in KRASm tumors including CRC (Pek et al 2017, Ziemke et al 2015) and PDAC (Franco et al 2014, Franco et al 2016). GIST GIST; 3L after
imatinib, sunitinib regorafenib GIST features CDKN2A loss/CCND1 amp (Yang et al 2008), which correlate with CDK4/6 inhibitor
sensitivity. CDK4/6i is efficacious in imatinib resistant preclinical models (Eilers et al 2015). 33

G1T48 DEVELOPMENT
PROGRAM
34

G1T48: oral SERD, strong strategic fit Ibrance approved only in combination with an anti-estrogen
– first letrozole (aromatase inhibitor), then Faslodex (IM SERD) Multiple companies with oral SERDs in early development
G1T48 provides a competitive advantage by controlling economics of a combination G1T38/48 regimen
– stand-alone opportunity in early stage disease Compelling preclinical data package (Wardell et al., 2017 AACR)
– more potent than Faslodex On track for IND filing in December 2017 35
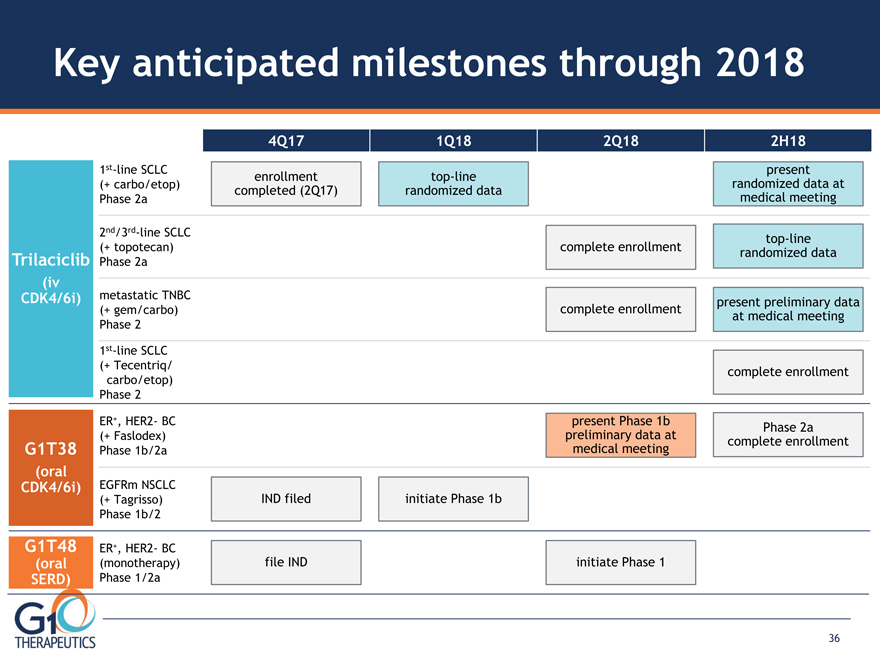
Key anticipated milestones through 2018 Trilaciclib (iv CDK4/6i) G1T38 (oral CDK4/6i) 2nd/3rd-line SCLC
(+ topotecan) Phase 2a 1st-line SCLC (+ Tecentriq/ carbo/etop) Phase 2 4Q17 1Q18 2Q18 2H18 ER+, HER2- BC
(monotherapy) Phase 1/2a G1T48 (oral SERD) ER+, HER2- BC (+ Faslodex) Phase 1b/2a EGFRm NSCLC (+ Tagrisso)
Phase 1b/2 metastatic TNBC (+ gem/carbo) Phase 2 1st-line SCLC (+ carbo/etop) Phase 2a file IND initiate Phase 1 IND filed initiate Phase 1b complete enrollment complete enrollment top-line randomized data complete enrollment present preliminary data
at medical meeting top-line randomized data present randomized data at medical meeting enrollment
completed (2Q17) present Phase 1b preliminary data at medical meeting Phase 2a complete enrollment 36
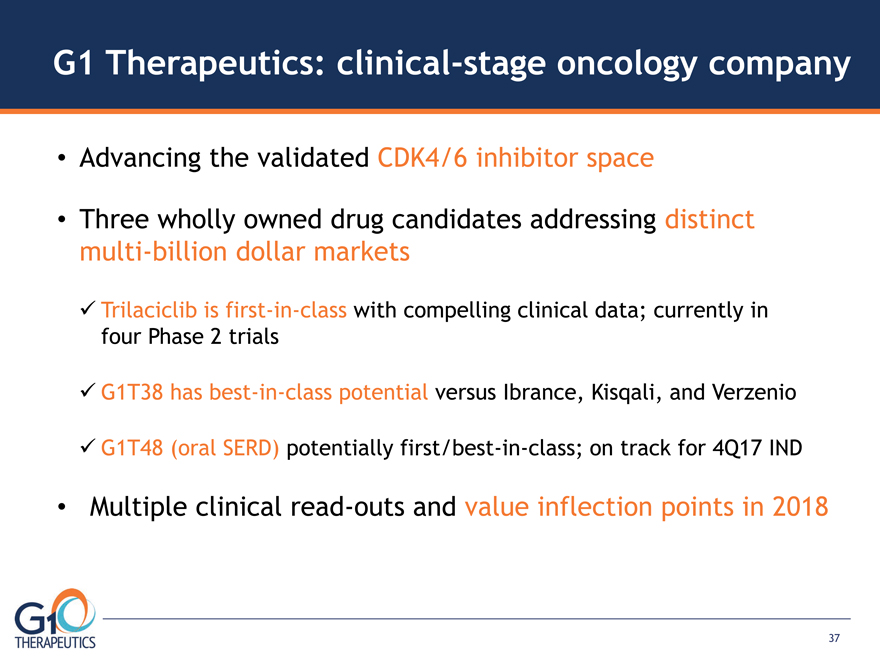
G1 Therapeutics: clinical-stage oncology company Advancing the validated CDK4/6 inhibitor space
Three wholly owned drug candidates addressing distinct multi-billion dollar markets Trilaciclib is first-in-class with compelling clinical data; currently in four Phase 2 trials G1T38 has best-in-class potential versus Ibrance, Kisqali, and Verzenio
G1T48 (oral SERD) potentially first/best-in-class; on track for 4Q17 IND Multiple clinical read-outs and value inflection points in 2018 37

APPENDIX: TRILACICLIB
38
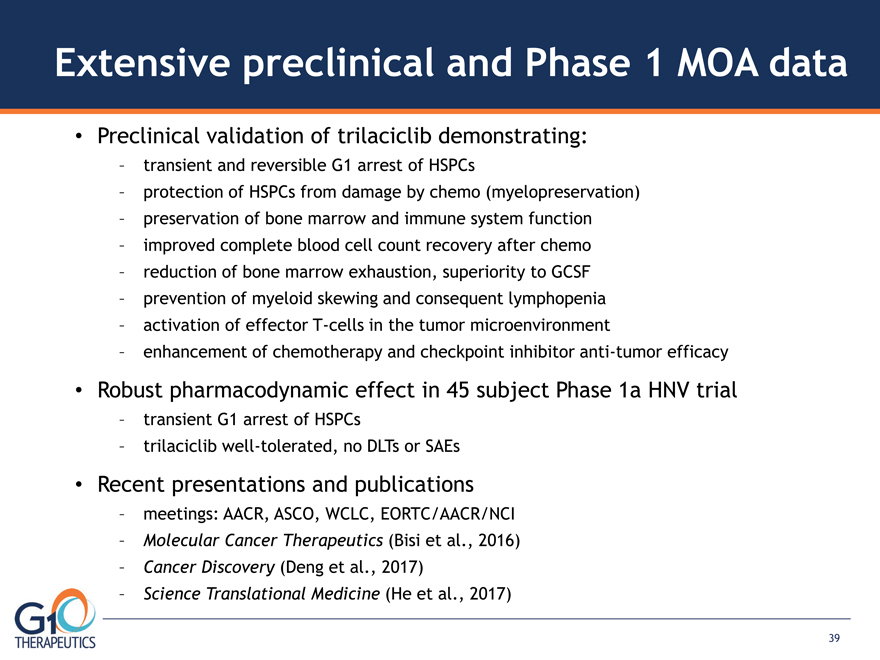
Extensive preclinical and Phase 1 MOA data Preclinical validation of trilaciclib demonstrating:
– transient and reversible G1 arrest of HSPCs – protection of HSPCs from damage by chemo (myelopreservation)
– preservation of bone marrow and immune system function – improved complete blood cell count recovery after chemo
– reduction of bone marrow exhaustion, superiority to GCSF – prevention of myeloid skewing and consequent lymphopenia
– activation of effector T-cells in the tumor microenvironment – enhancement of chemotherapy and checkpoint inhibitor anti-tumor efficacy Robust pharmacodynamic effect in 45 subject Phase 1a HNV trial
– transient G1 arrest of HSPCs – trilaciclib well-tolerated, no DLTs or SAEs Recent presentations and publications
– meetings: AACR, ASCO, WCLC, EORTC/AACR/NCI – Molecular Cancer Therapeutics (Bisi et al., 2016)
– Cancer Discovery (Deng et al., 2017) – Science Translational Medicine (He et al., 2017) 39
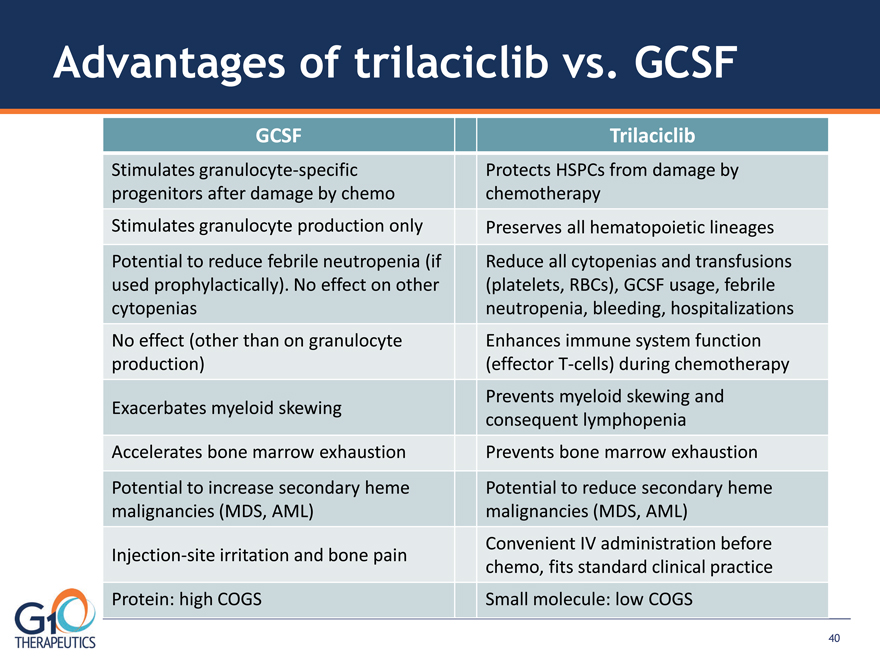
Advantages of trilaciclib vs. GCSF GCSF Trilaciclib Stimulates granulocyte-specific
progenitors after damage by chemo Protects HSPCs from damage by
chemotherapy Stimulates granulocyte production only Preserves all hematopoietic lineages
Potential to reduce febrile neutropenia (if used prophylactically). No effect on other
cytopenias Reduce all cytopenias and transfusions (platelets, RBCs), GCSF usage, febrile neutropenia, bleeding, hospitalizations
No effect (other than on granulocyte production) Enhances immune system function (effector T-cells) during chemotherapy Exacerbates myeloid skewing Prevents myeloid skewing and consequent lymphopenia
Accelerates bone marrow exhaustion Prevents bone marrow exhaustion Potential to increase secondary heme
malignancies (MDS, AML) Potential to reduce secondary heme malignancies (MDS, AML) Injection-site irritation and bone pain Convenient IV administration before chemo, fits standard clinical practice Protein: high COGS Small molecule: low COGS
40
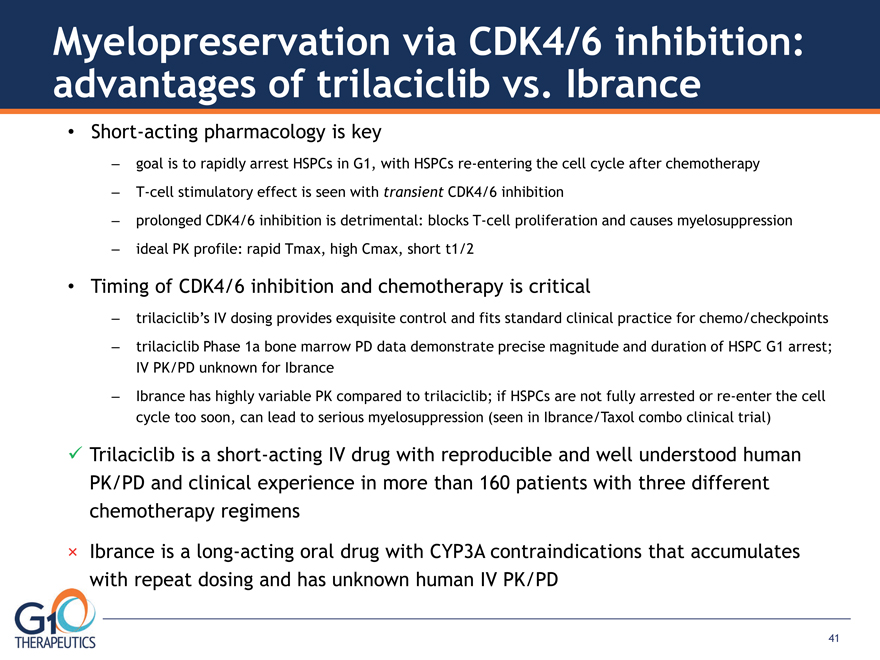
Short-acting pharmacology is key
– goal is to rapidly arrest HSPCs in G1, with HSPCs re-entering the cell cycle after chemotherapy – T-cell stimulatory effect is seen with transient CDK4/6 inhibition – prolonged CDK4/6 inhibition is detrimental: blocks T-cell proliferation and causes myelosuppression – ideal PK profile: rapid Tmax, high Cmax, short t1/2
Timing of CDK4/6 inhibition and chemotherapy is critical – trilaciclib’s IV dosing provides exquisite control and fits standard clinical practice for chemo/checkpoints – trilaciclib Phase 1a bone marrow PD data demonstrate precise magnitude and duration of HSPC G1 arrest;
IV PK/PD unknown for Ibrance
– Ibrance has highly variable PK compared to trilaciclib; if HSPCs are not fully arrested or re-enter the cell cycle too soon, can lead to serious myelosuppression (seen in Ibrance/Taxol combo clinical trial) Trilaciclib is a short-acting IV drug with reproducible and well understood human PK/PD and clinical experience in more than 160 patients with three different chemotherapy regimens
× Ibrance is a long-acting oral drug with CYP3A contraindications that accumulates with repeat dosing and has unknown human IV PK/PD Myelopreservation via CDK4/6 inhibition: advantages of trilaciclib vs. Ibrance 41
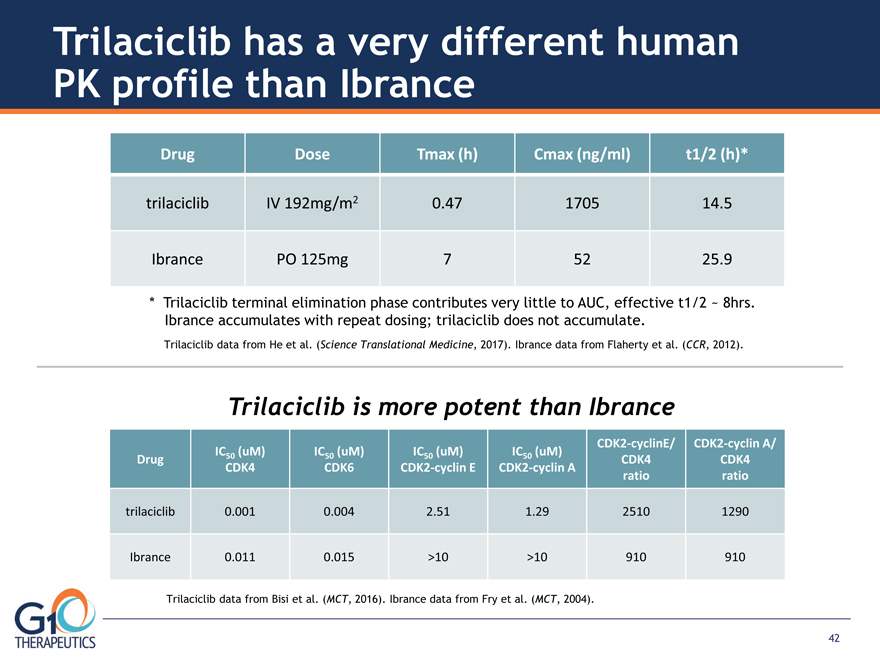
Drug IC50 (uM) CDK4 IC50 (uM) CDK6 IC50 (uM) CDK2-cyclin E IC50 (uM) CDK2-cyclin A CDK2-cyclinE/
CDK4 ratio CDK2-cyclin A/ CDK4 ratio trilaciclib 0.001 0.004 2.51 1.29 2510 1290 Ibrance 0.011 0.015 >10 >10 910 910
Trilaciclib has a very different human PK profile than Ibrance Drug Dose Tmax (h) Cmax (ng/ml) t1/2 (h)*
trilaciclib IV 192mg/m2 0.47 1705 14.5 Ibrance PO 125mg 7 52 25.9 * Trilaciclib terminal elimination phase contributes very little to AUC, effective t1/2 ~ 8hrs. Ibrance accumulates with repeat dosing; trilaciclib does not accumulate.
Trilaciclib data from He et al. (Science Translational Medicine, 2017). Ibrance data from Flaherty et al. (CCR, 2012).
Trilaciclib is more potent than Ibrance Trilaciclib data from Bisi et al. (MCT, 2016). Ibrance data from Fry et al. (MCT, 2004).
42









































