
Optimizing Chemotherapy, Advancing Survival February 16, 2021 Exhibit 99.2

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "plan," "anticipate," "estimate," "intend" and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements in this presentation include, but are not limited to, those relating to the therapeutic potential of COSELA™(trilaciclib), rintodestrant and lerociclib, COSELA’s possibility to improve patient outcomes across multiple indications, rintodestrant’s potential to be best-in-class oral SERD, our reliance on partners to develop and commercial licensed products, and the impact of pandemics such as COVID-19 (coronavirus), and are based on the company’s expectations and assumptions as of the date of this presentation. Each of these forward-looking statements involves risks and uncertainties. Factors that may cause the company’s actual results to differ from those expressed or implied in the forward-looking statements in this presentation are discussed in the company’s filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections contained therein and include, but are not limited to, the company’s ability to complete clinical trials for, obtain approvals for and commercialize any of its product candidates; the company’s initial success in ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials; the inherent uncertainties associated with developing new products or technologies and operating as a development-stage company; and market conditions. Rintodestrant and lerociclib are not approved by the FDA. The safety or effectiveness of rintodestrant and lerociclib have not been established by the FDA. Except as required by law, the company assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. Forward-Looking Statements G1TherapeuticsTM and G1Therapeutics logo and COSELATM and COSELA logo are trademarks of G1 Therapeutics, Inc. ©2021 G1 Therapeutics, Inc.
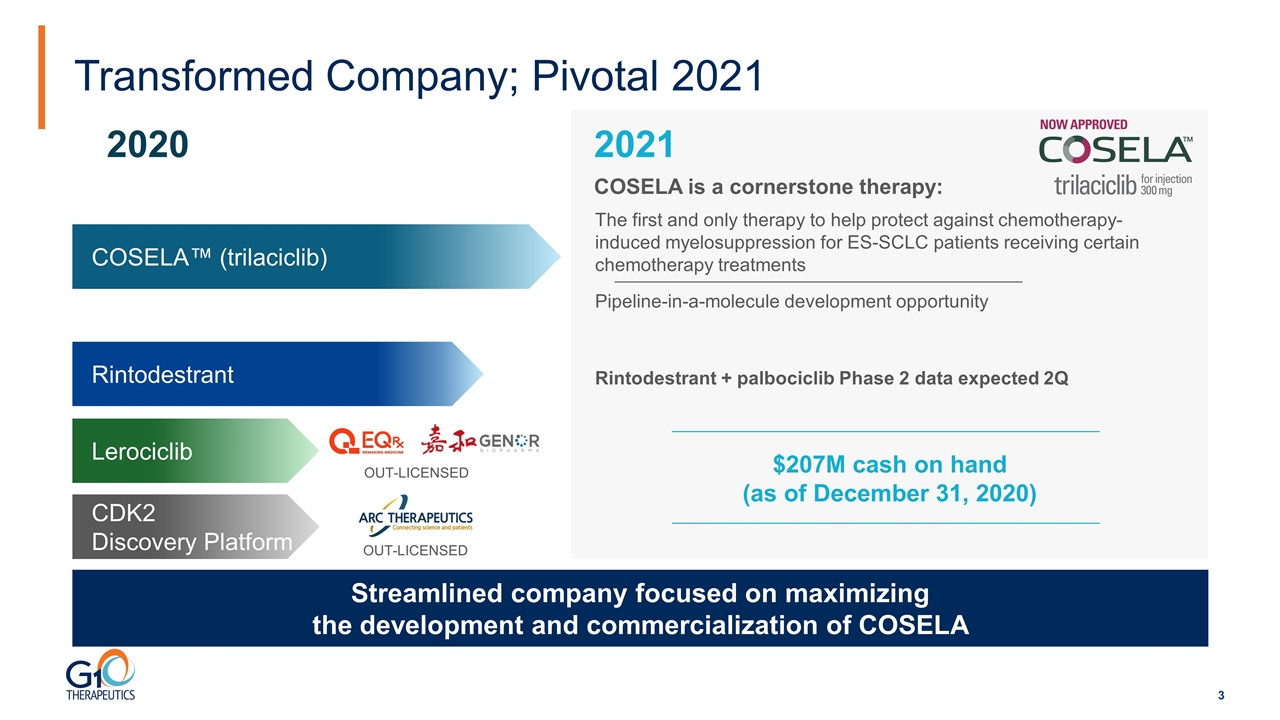
Transformed Company; Pivotal 2021 COSELA™ (trilaciclib) CDK2 Discovery Platform Rintodestrant 2021 2020 Pipeline-in-a-molecule development opportunity Rintodestrant + palbociclib Phase 2 data expected 2Q Lerociclib OUT-LICENSED OUT-LICENSED $207M cash on hand (as of December 31, 2020) Streamlined company focused on maximizing the development and commercialization of COSELA The first and only therapy to help protect against chemotherapy-induced myelosuppression for ES-SCLC patients receiving certain chemotherapy treatments COSELA is a cornerstone therapy:
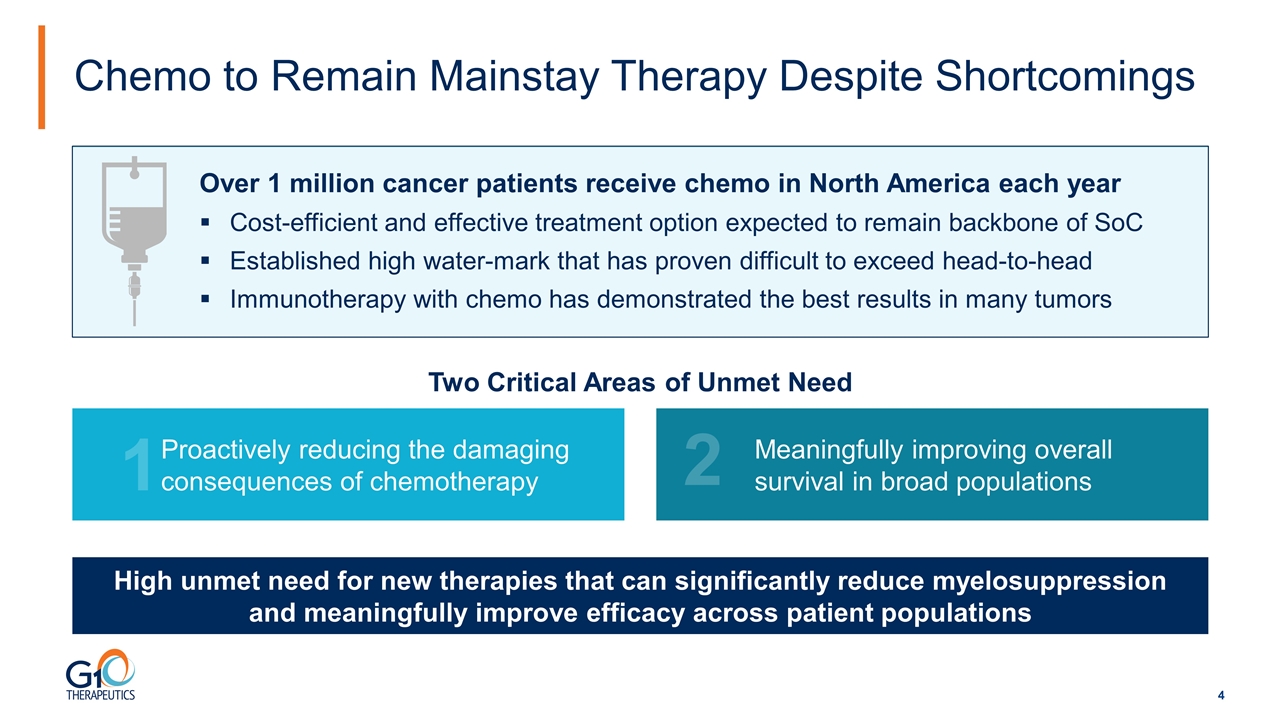
Chemo to Remain Mainstay Therapy Despite Shortcomings Over 1 million cancer patients receive chemo in North America each year Cost-efficient and effective treatment option expected to remain backbone of SoC Established high water-mark that has proven difficult to exceed head-to-head Immunotherapy with chemo has demonstrated the best results in many tumors High unmet need for new therapies that can significantly reduce myelosuppression and meaningfully improve efficacy across patient populations Proactively reducing the damaging consequences of chemotherapy 1 Meaningfully improving overall survival in broad populations 2 Two Critical Areas of Unmet Need
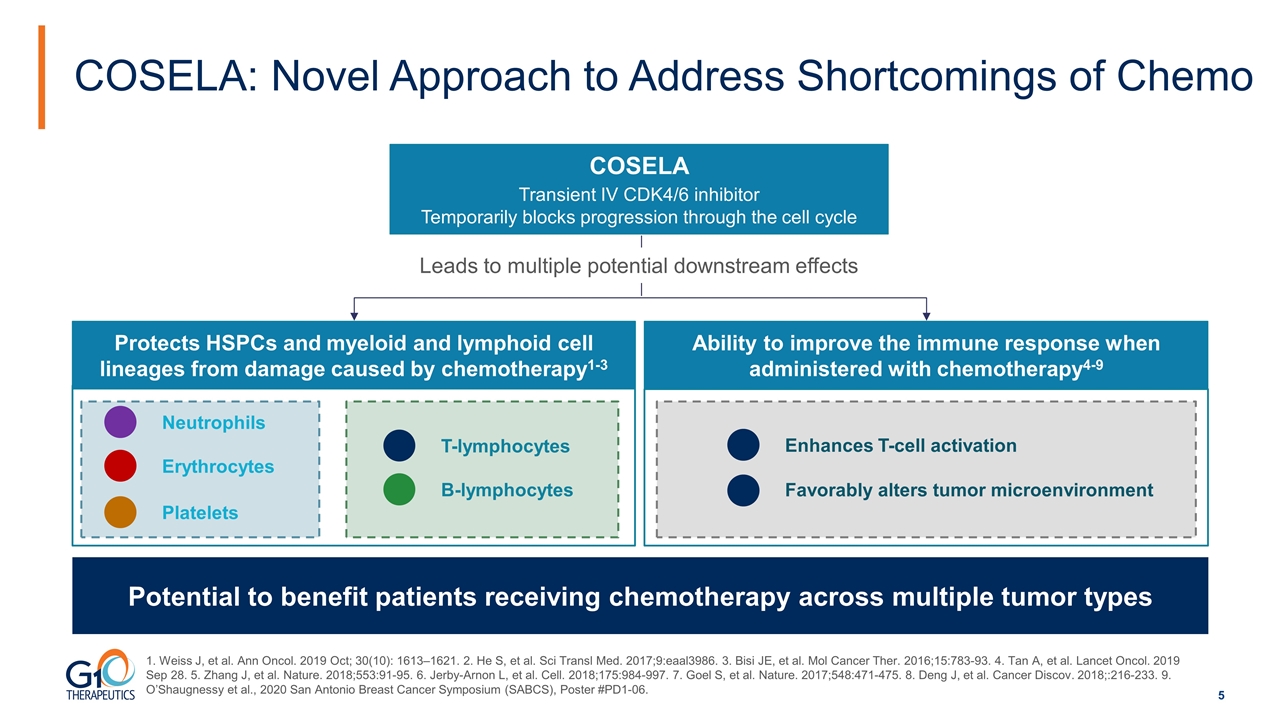
COSELA: Novel Approach to Address Shortcomings of Chemo COSELA Transient IV CDK4/6 inhibitor Temporarily blocks progression through the cell cycle Leads to multiple potential downstream effects Protects HSPCs and myeloid and lymphoid cell lineages from damage caused by chemotherapy1-3 Ability to improve the immune response when administered with chemotherapy4-9 Neutrophils B-lymphocytes T-lymphocytes Erythrocytes Platelets Enhances T-cell activation Favorably alters tumor microenvironment Potential to benefit patients receiving chemotherapy across multiple tumor types 1. Weiss J, et al. Ann Oncol. 2019 Oct; 30(10): 1613–1621. 2. He S, et al. Sci Transl Med. 2017;9:eaal3986. 3. Bisi JE, et al. Mol Cancer Ther. 2016;15:783-93. 4. Tan A, et al. Lancet Oncol. 2019 Sep 28. 5. Zhang J, et al. Nature. 2018;553:91-95. 6. Jerby-Arnon L, et al. Cell. 2018;175:984-997. 7. Goel S, et al. Nature. 2017;548:471-475. 8. Deng J, et al. Cancer Discov. 2018;:216-233. 9. O’Shaugnessy et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PD1-06.
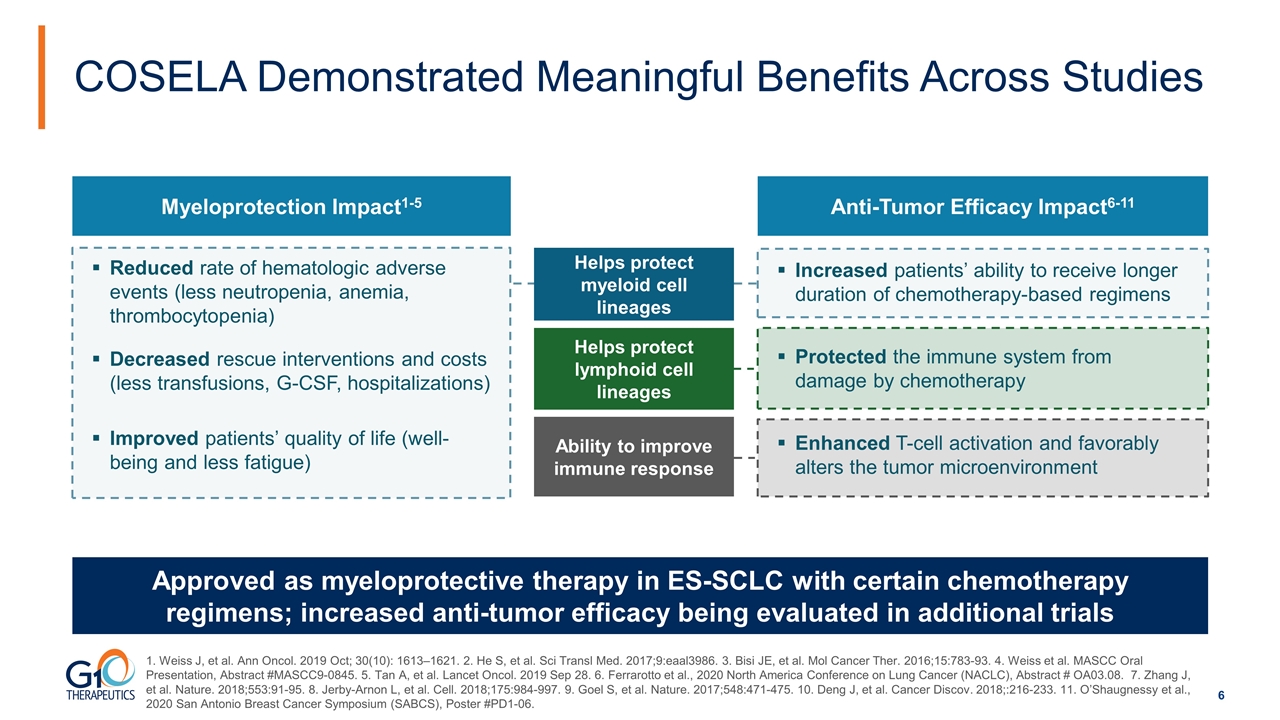
Myeloprotection Impact1-5 Anti-Tumor Efficacy Impact6-11 Reduced rate of hematologic adverse events (less neutropenia, anemia, thrombocytopenia) Increased patients’ ability to receive longer duration of chemotherapy-based regimens Decreased rescue interventions and costs (less transfusions, G-CSF, hospitalizations) Protected the immune system from damage by chemotherapy Improved patients’ quality of life (well-being and less fatigue) Enhanced T-cell activation and favorably alters the tumor microenvironment COSELA Demonstrated Meaningful Benefits Across Studies Approved as myeloprotective therapy in ES-SCLC with certain chemotherapy regimens; increased anti-tumor efficacy being evaluated in additional trials Helps protect myeloid cell lineages Helps protect lymphoid cell lineages Ability to improve immune response 1. Weiss J, et al. Ann Oncol. 2019 Oct; 30(10): 1613–1621. 2. He S, et al. Sci Transl Med. 2017;9:eaal3986. 3. Bisi JE, et al. Mol Cancer Ther. 2016;15:783-93. 4. Weiss et al. MASCC Oral Presentation, Abstract #MASCC9-0845. 5. Tan A, et al. Lancet Oncol. 2019 Sep 28. 6. Ferrarotto et al., 2020 North America Conference on Lung Cancer (NACLC), Abstract # OA03.08. 7. Zhang J, et al. Nature. 2018;553:91-95. 8. Jerby-Arnon L, et al. Cell. 2018;175:984-997. 9. Goel S, et al. Nature. 2017;548:471-475. 10. Deng J, et al. Cancer Discov. 2018;:216-233. 11. O’Shaugnessy et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PD1-06.
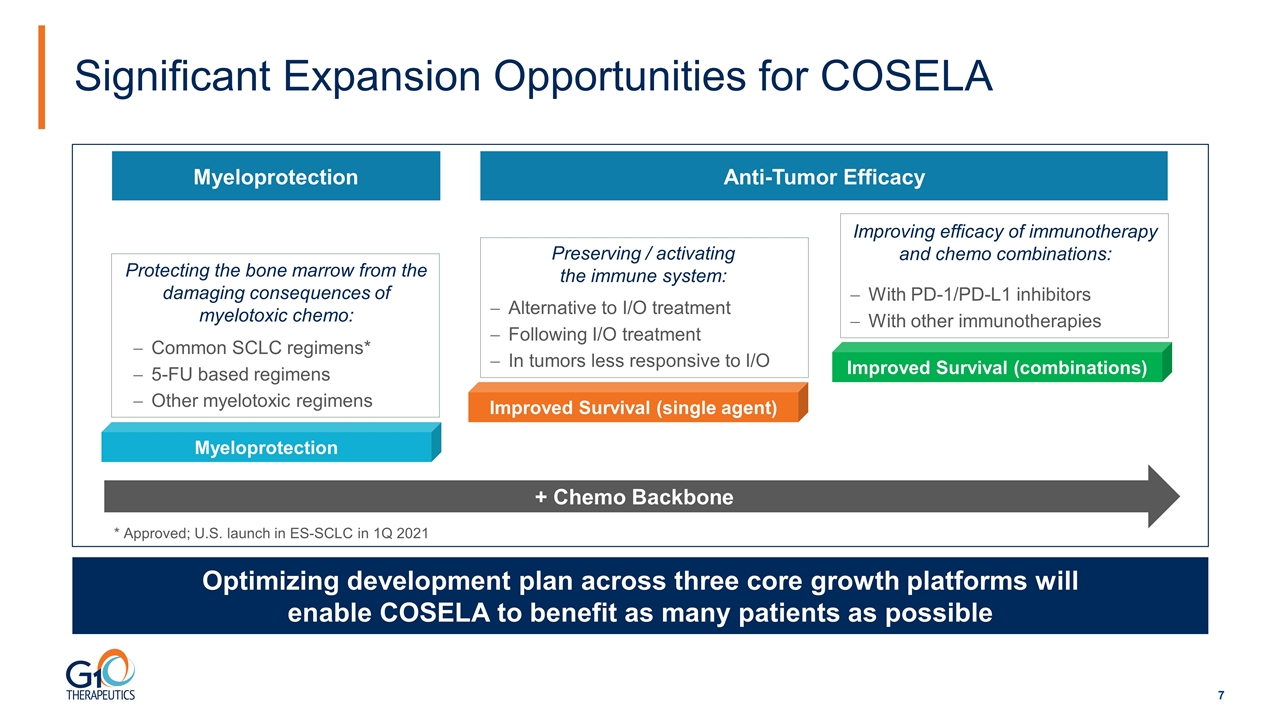
Significant Expansion Opportunities for COSELA Optimizing development plan across three core growth platforms will enable COSELA to benefit as many patients as possible Myeloprotection Improved Survival (single agent) Improved Survival (combinations) Protecting the bone marrow from the damaging consequences of myelotoxic chemo: Preserving / activating the immune system: Improving efficacy of immunotherapy and chemo combinations: Common SCLC regimens* 5-FU based regimens Other myelotoxic regimens Alternative to I/O treatment Following I/O treatment In tumors less responsive to I/O With PD-1/PD-L1 inhibitors With other immunotherapies * Approved; U.S. launch in ES-SCLC in 1Q 2021 Myeloprotection Anti-Tumor Efficacy + Chemo Backbone
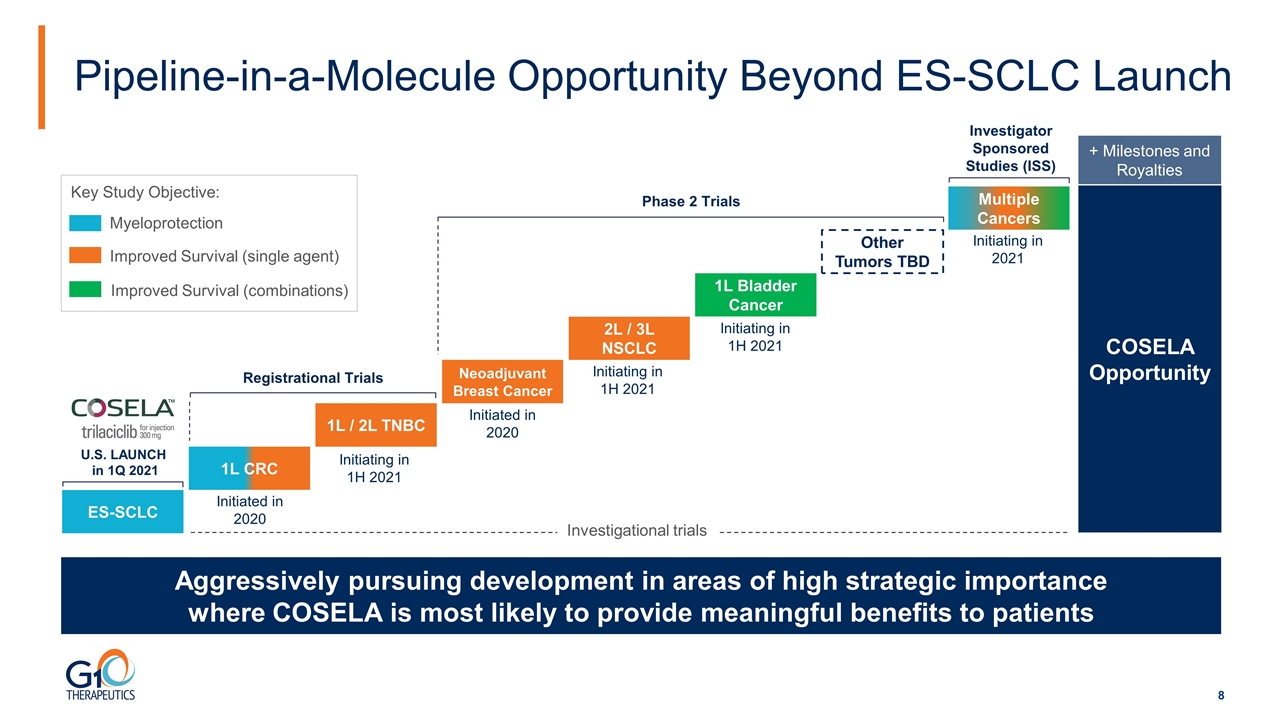
Pipeline-in-a-Molecule Opportunity Beyond ES-SCLC Launch Aggressively pursuing development in areas of high strategic importance where COSELA is most likely to provide meaningful benefits to patients ES-SCLC COSELA Opportunity + Milestones and Royalties Myeloprotection Improved Survival (single agent) Improved Survival (combinations) Key Study Objective: Investigational trials U.S. LAUNCH in 1Q 2021 Initiating in 1H 2021 Initiated in 2020 Initiated in 2020 Initiating in 1H 2021 Initiating in 1H 2021 Initiating in 2021 1L CRC 2L / 3L NSCLC 1L Bladder Cancer Registrational Trials Phase 2 Trials Other Tumors TBD Neoadjuvant Breast Cancer 1L / 2L TNBC Investigator Sponsored Studies (ISS) Multiple Cancers

2021 Key Objectives Focused on successfully launching COSELA in ES-SCLC and accelerating development into other areas where chemotherapy is used Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently

2021 Key Objectives Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently

Approved by U.S. Food and Drug Administration to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer
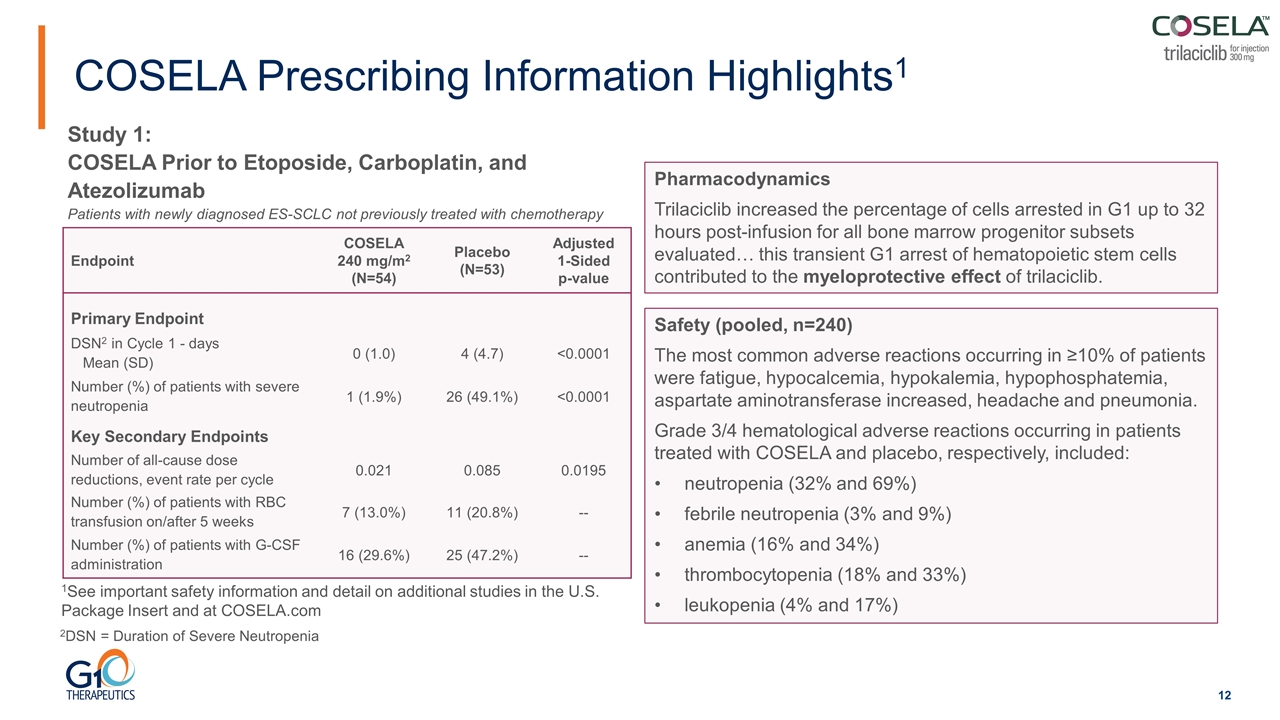
Endpoint COSELA 240 mg/m2 (N=54) Placebo (N=53) Adjusted 1‑Sided p‑value Primary Endpoint DSN2 in Cycle 1 - days Mean (SD) 0 (1.0) 4 (4.7) <0.0001 Number (%) of patients with severe neutropenia 1 (1.9%) 26 (49.1%) <0.0001 Key Secondary Endpoints Number of all-cause dose reductions, event rate per cycle 0.021 0.085 0.0195 Number (%) of patients with RBC transfusion on/after 5 weeks 7 (13.0%) 11 (20.8%) -- Number (%) of patients with G‑CSF administration 16 (29.6%) 25 (47.2%) -- COSELA Prescribing Information Highlights1 Safety (pooled, n=240) The most common adverse reactions occurring in ≥10% of patients were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache and pneumonia. Grade 3/4 hematological adverse reactions occurring in patients treated with COSELA and placebo, respectively, included: neutropenia (32% and 69%) febrile neutropenia (3% and 9%) anemia (16% and 34%) thrombocytopenia (18% and 33%) leukopenia (4% and 17%) 1See important safety information and detail on additional studies in the U.S. Package Insert and at COSELA.com Study 1: COSELA Prior to Etoposide, Carboplatin, and Atezolizumab Patients with newly diagnosed ES-SCLC not previously treated with chemotherapy Pharmacodynamics Trilaciclib increased the percentage of cells arrested in G1 up to 32 hours post-infusion for all bone marrow progenitor subsets evaluated… this transient G1 arrest of hematopoietic stem cells contributed to the myeloprotective effect of trilaciclib. 2DSN = Duration of Severe Neutropenia

COSELA Approved: U.S. Launch in 1Q21 This important new treatment is available to the majority of patients with ES-SCLC undergoing chemotherapy in the U.S. Label covers a majority of patients Including those treated with I/O Indicated for broad myelosuppression (vs just neutropenia) Multilineage myeloprotection mechanism All three studies with key endpoints represented 30-minute infusion within 4 hours of chemotherapy; will fit into oncologist practice workflow Identified HCP targets Profiled key accounts Engaged payors Educated leading patient advocacy organizations Executed strategic pricing strategy G1 launch comms plan underway National accounts team reaching key provider networks Communicating approval with targeted payer customers Boehringer Ingelheim field sales team1 notifying priority accounts, initiating customer interaction Clinical nurse educators scheduling in-service meetings MSLs responding to customers G1-to-One pt. support hub launched Broad Coverage of ES-SCLC Pre-Launch Activities Complete Product Launch Ongoing 1Three-year agreement where Boehringer Ingelheim leads sales force engagement initiatives for COSELA in the U.S. for the initial ES-SCLC indication. The agreement does not extend to additional indications.
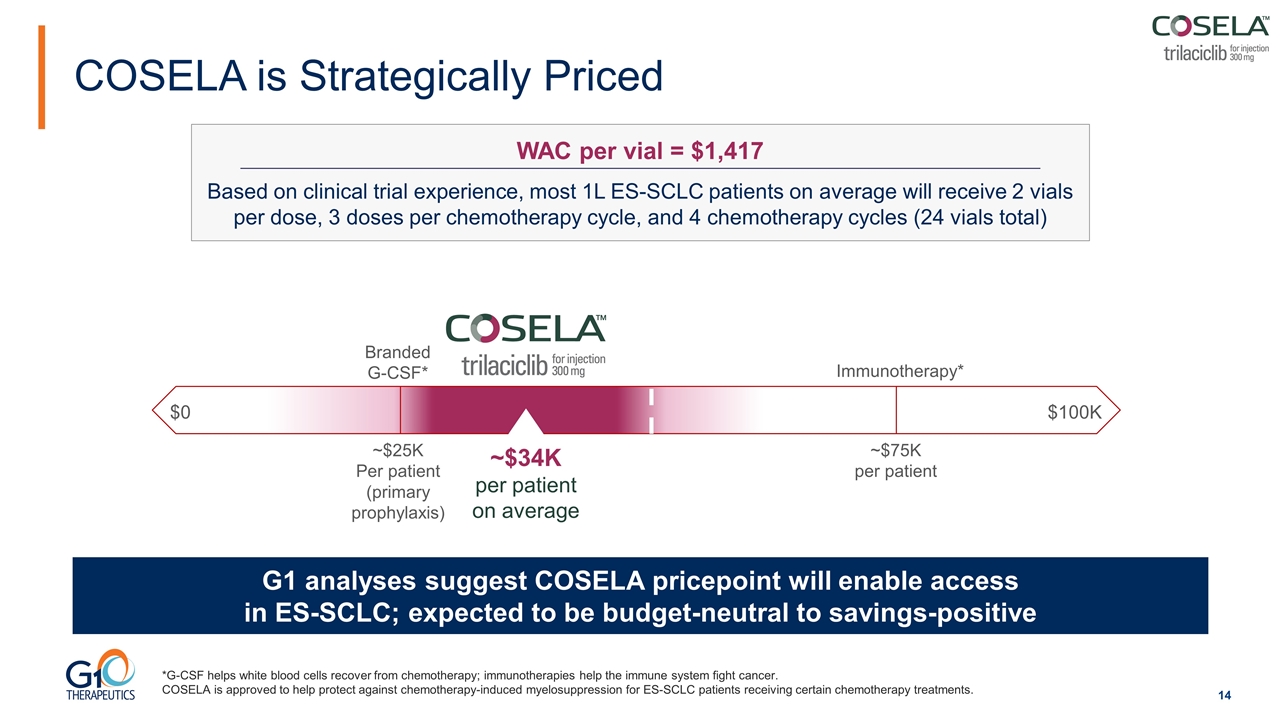
COSELA is Strategically Priced G1 analyses suggest COSELA pricepoint will enable access in ES-SCLC; expected to be budget-neutral to savings-positive Branded G-CSF* Immunotherapy* ~$25K Per patient (primary prophylaxis) ~$75K per patient ~$34K per patient on average WAC per vial = $1,417 Based on clinical trial experience, most 1L ES-SCLC patients on average will receive 2 vials per dose, 3 doses per chemotherapy cycle, and 4 chemotherapy cycles (24 vials total) $0 $100K *G-CSF helps white blood cells recover from chemotherapy; immunotherapies help the immune system fight cancer. COSELA is approved to help protect against chemotherapy-induced myelosuppression for ES-SCLC patients receiving certain chemotherapy treatments.

G1 to One: Single Source for Access & Affordability Benefits investigation Prior authorization and appeals support Out of pocket assistance Access to PAP for therapy for eligible patients Support for patients getting started on COSELA One-stop hub to ensure excellence in COSELA patient support
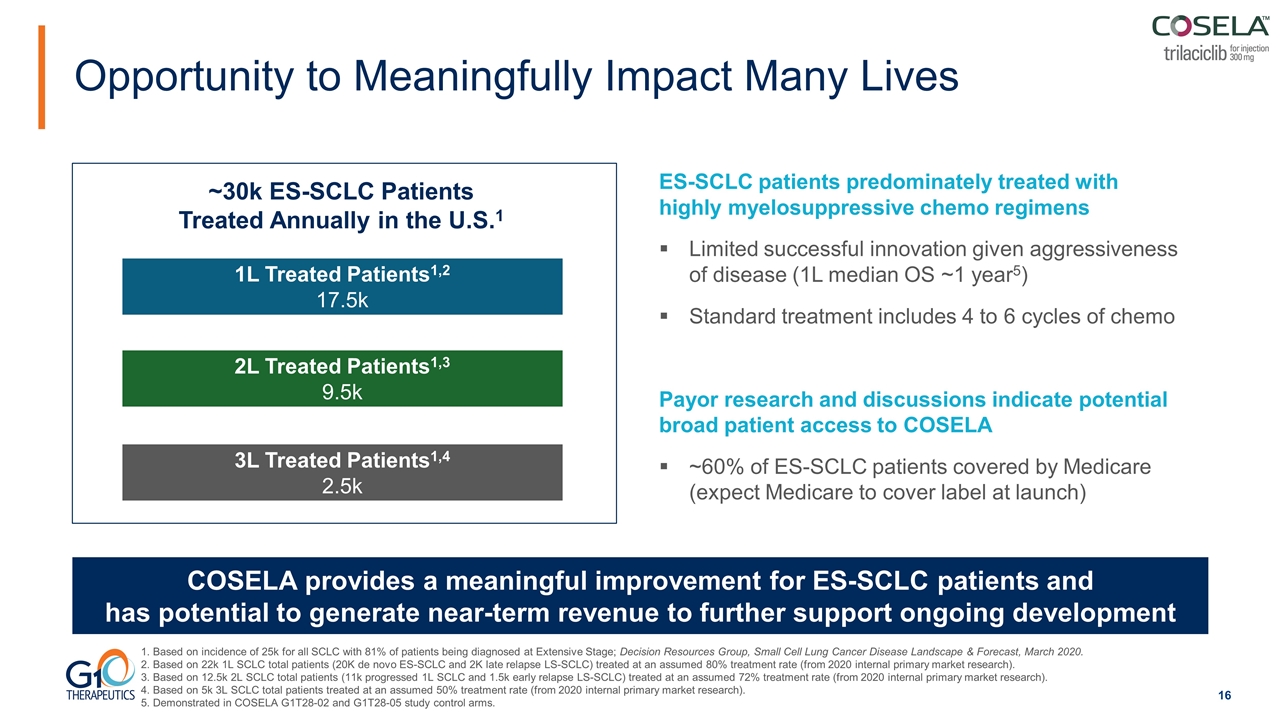
Opportunity to Meaningfully Impact Many Lives Based on incidence of 25k for all SCLC with 81% of patients being diagnosed at Extensive Stage; Decision Resources Group, Small Cell Lung Cancer Disease Landscape & Forecast, March 2020. Based on 22k 1L SCLC total patients (20K de novo ES-SCLC and 2K late relapse LS-SCLC) treated at an assumed 80% treatment rate (from 2020 internal primary market research). Based on 12.5k 2L SCLC total patients (11k progressed 1L SCLC and 1.5k early relapse LS-SCLC) treated at an assumed 72% treatment rate (from 2020 internal primary market research). Based on 5k 3L SCLC total patients treated at an assumed 50% treatment rate (from 2020 internal primary market research). Demonstrated in COSELA G1T28-02 and G1T28-05 study control arms. 2L Treated Patients1,3 9.5k 1L Treated Patients1,2 17.5k 3L Treated Patients1,4 2.5k COSELA provides a meaningful improvement for ES-SCLC patients and has potential to generate near-term revenue to further support ongoing development ~30k ES-SCLC Patients Treated Annually in the U.S.1 ES-SCLC patients predominately treated with highly myelosuppressive chemo regimens Limited successful innovation given aggressiveness of disease (1L median OS ~1 year5) Standard treatment includes 4 to 6 cycles of chemo Payor research and discussions indicate potential broad patient access to COSELA ~60% of ES-SCLC patients covered by Medicare (expect Medicare to cover label at launch)
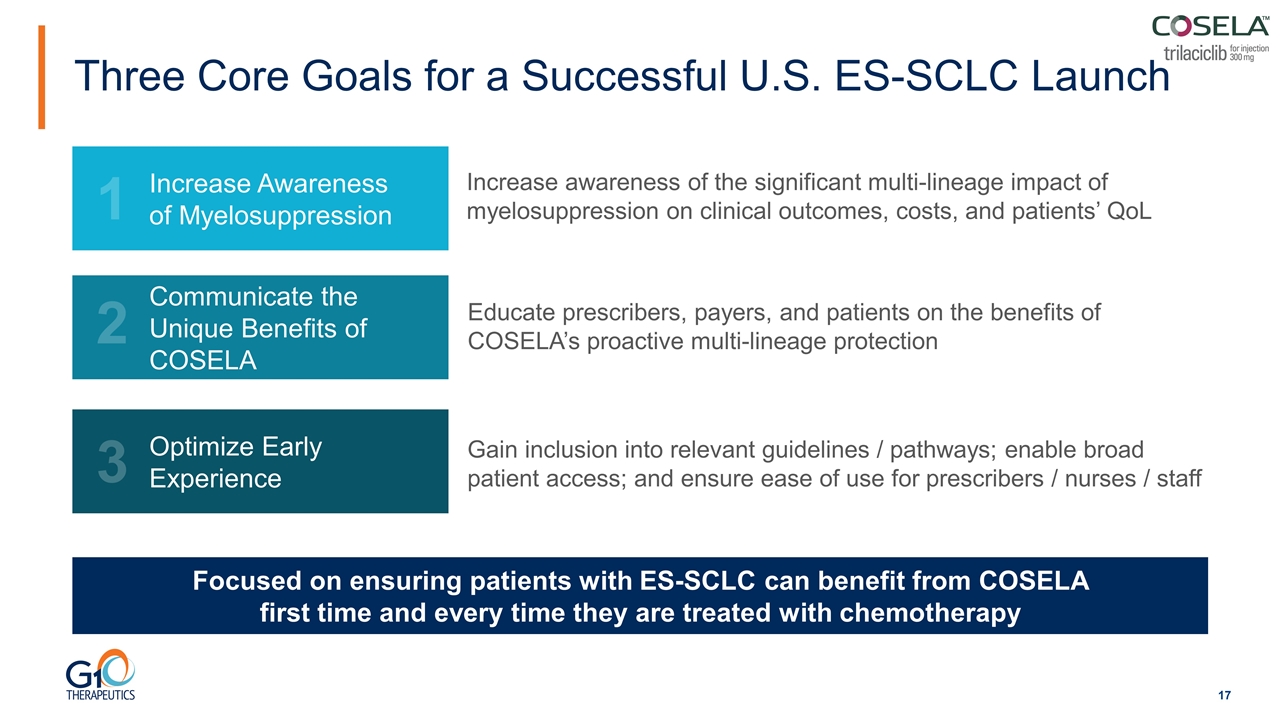
Educate prescribers, payers, and patients on the benefits of COSELA’s proactive multi-lineage protection Gain inclusion into relevant guidelines / pathways; enable broad patient access; and ensure ease of use for prescribers / nurses / staff Increase awareness of the significant multi-lineage impact of myelosuppression on clinical outcomes, costs, and patients’ QoL Increase Awareness of Myelosuppression Communicate the Unique Benefits of COSELA Optimize Early Experience 1 2 3 Focused on ensuring patients with ES-SCLC can benefit from COSELA first time and every time they are treated with chemotherapy Three Core Goals for a Successful U.S. ES-SCLC Launch
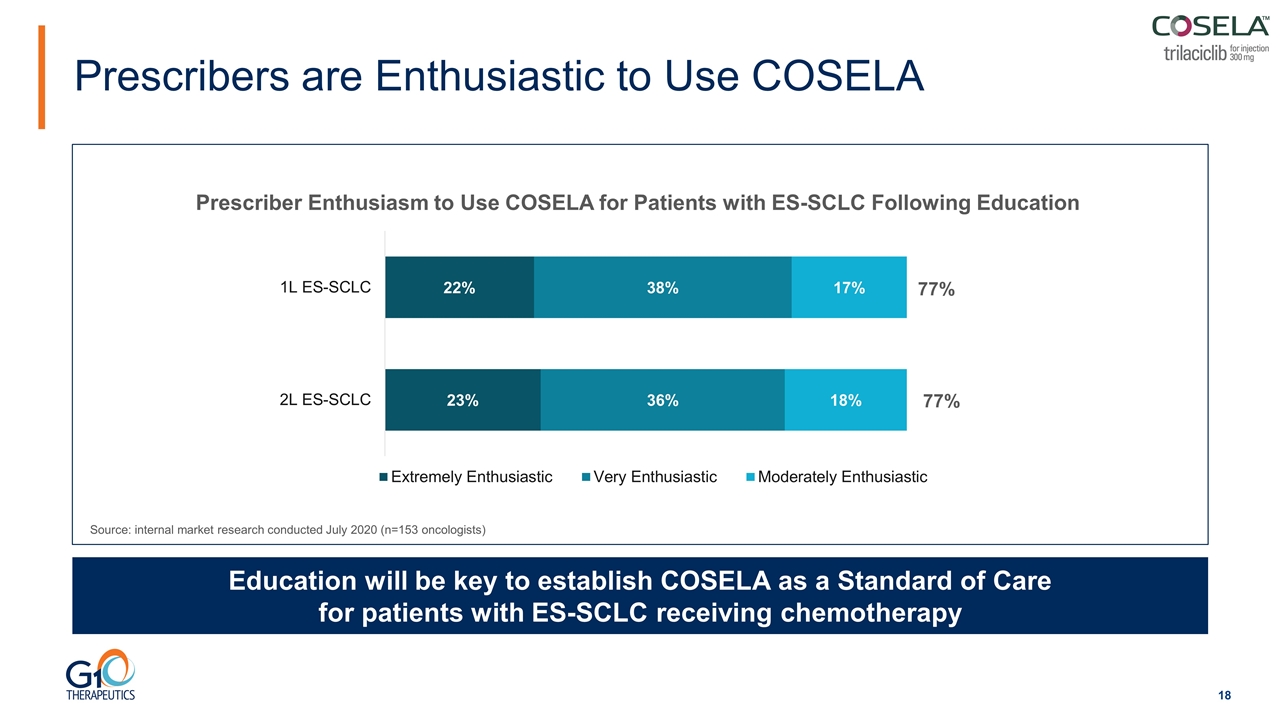
Prescribers are Enthusiastic to Use COSELA Education will be key to establish COSELA as a Standard of Care for patients with ES-SCLC receiving chemotherapy Prescriber Enthusiasm to Use COSELA for Patients with ES-SCLC Following Education Source: internal market research conducted July 2020 (n=153 oncologists) 77% 77%

2021 Key Objectives Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently
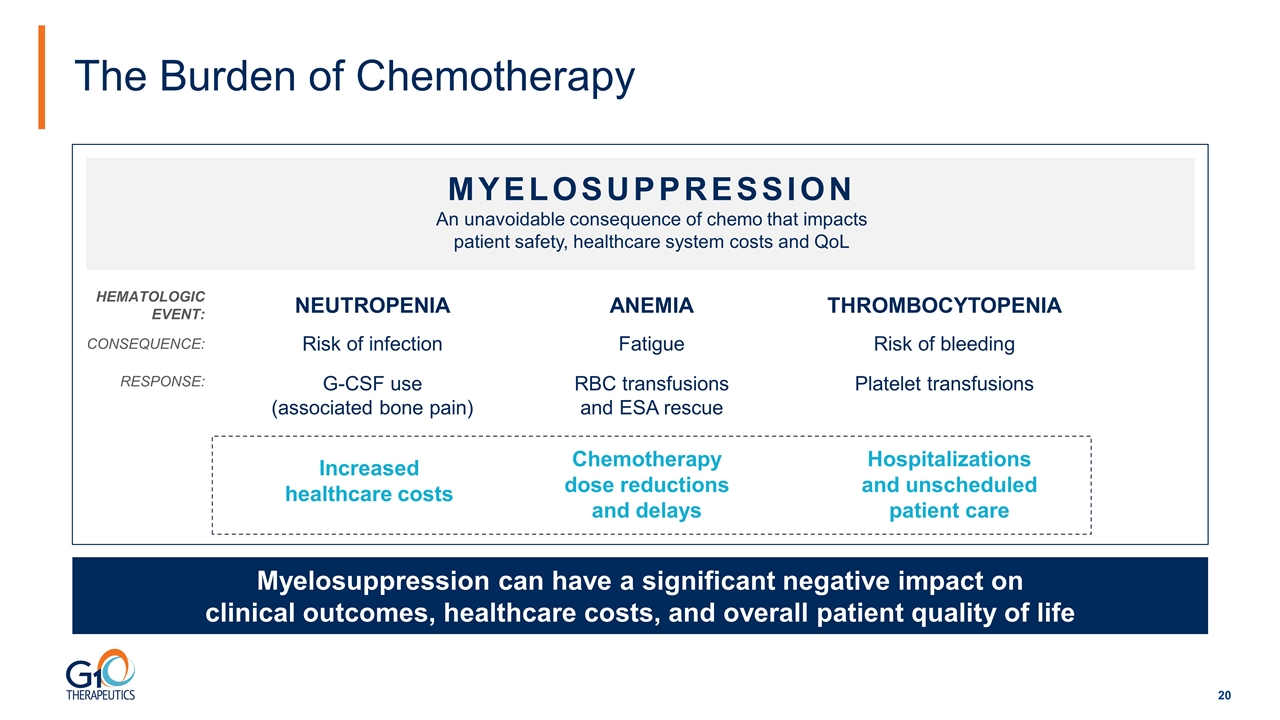
The Burden of Chemotherapy Myelosuppression can have a significant negative impact on clinical outcomes, healthcare costs, and overall patient quality of life An unavoidable consequence of chemo that impacts patient safety, healthcare system costs and QoL MYELOSUPPRESSION Hospitalizations and unscheduled patient care Chemotherapy dose reductions and delays THROMBOCYTOPENIA Risk of bleeding Platelet transfusions ANEMIA Fatigue RBC transfusions and ESA rescue NEUTROPENIA Risk of infection G-CSF use (associated bone pain) Increased healthcare costs HEMATOLOGIC EVENT: CONSEQUENCE: RESPONSE:
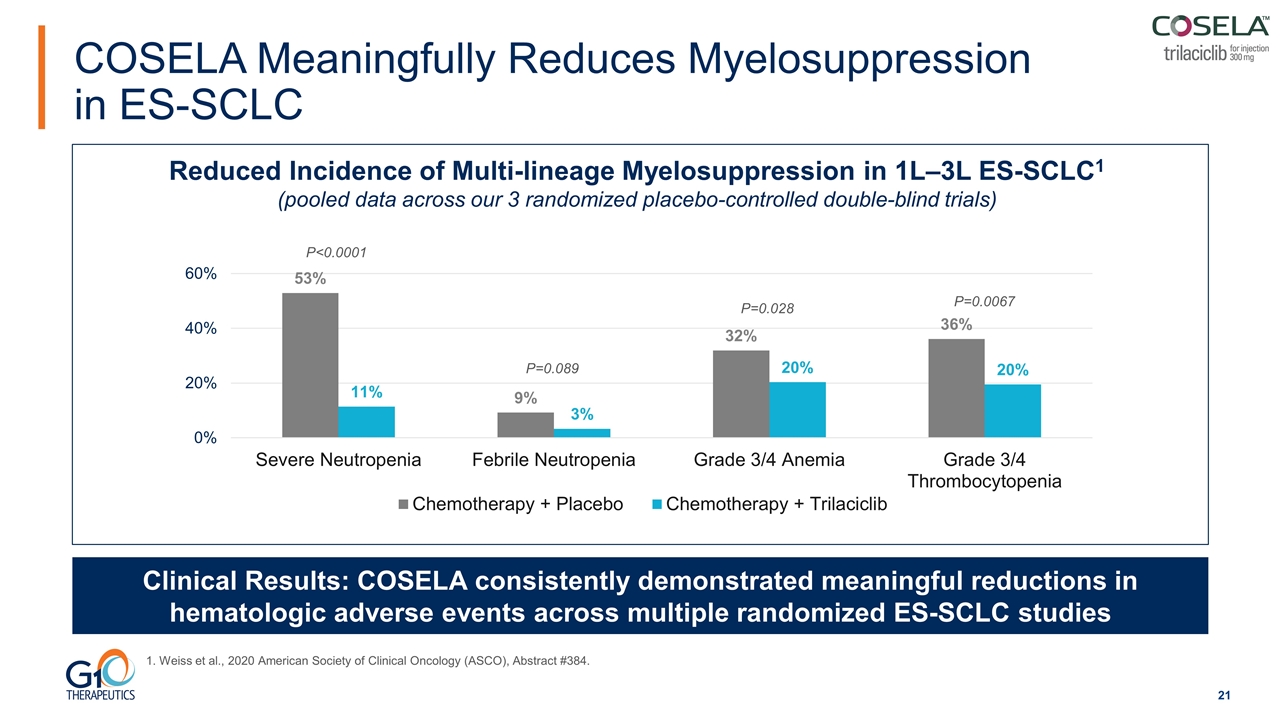
Reduced Incidence of Multi-lineage Myelosuppression in 1L‒3L ES-SCLC1 (pooled data across our 3 randomized placebo-controlled double-blind trials) Clinical Results: COSELA consistently demonstrated meaningful reductions in hematologic adverse events across multiple randomized ES-SCLC studies P<0.0001 P=0.089 P=0.028 P=0.0067 1. Weiss et al., 2020 American Society of Clinical Oncology (ASCO), Abstract #384. COSELA Meaningfully Reduces Myelosuppression in ES-SCLC
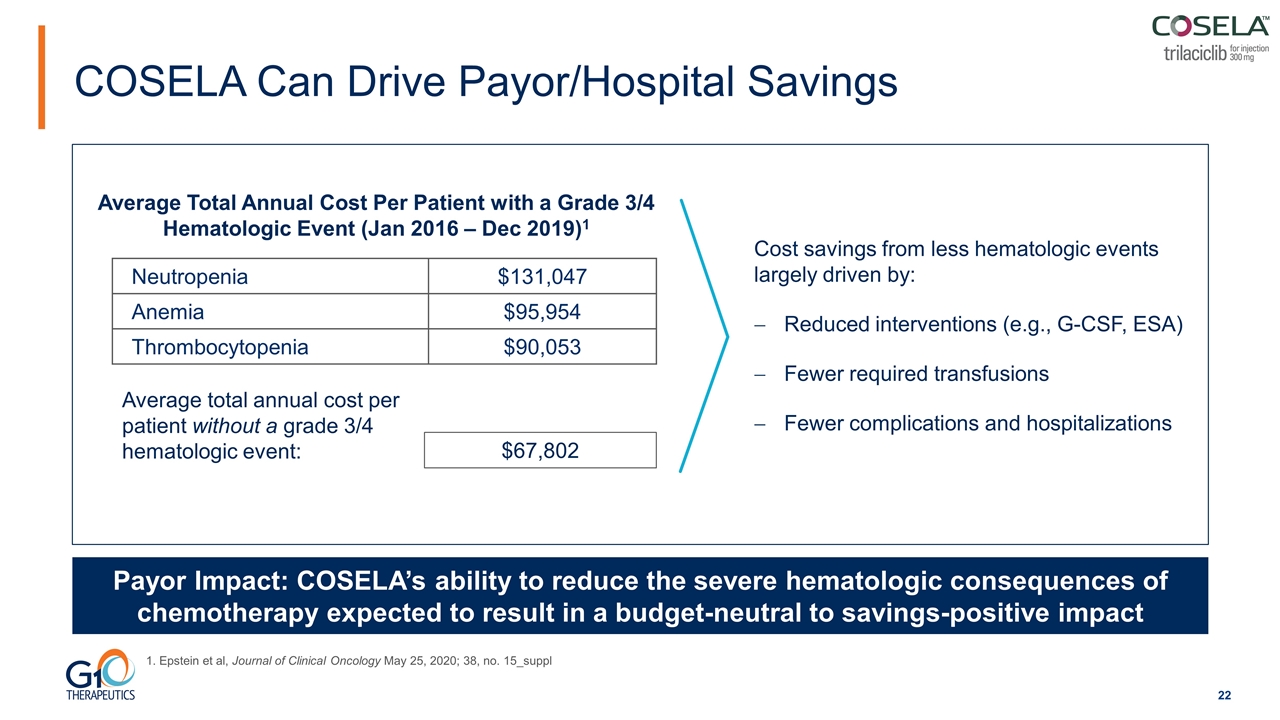
COSELA Can Drive Payor/Hospital Savings Average total annual cost per patient without a grade 3/4 hematologic event: Average Total Annual Cost Per Patient with a Grade 3/4 Hematologic Event (Jan 2016 – Dec 2019)1 Neutropenia $131,047 Anemia $95,954 Thrombocytopenia $90,053 Payor Impact: COSELA’s ability to reduce the severe hematologic consequences of chemotherapy expected to result in a budget-neutral to savings-positive impact $67,802 Cost savings from less hematologic events largely driven by: Reduced interventions (e.g., G-CSF, ESA) Fewer required transfusions Fewer complications and hospitalizations 1. Epstein et al, Journal of Clinical Oncology May 25, 2020; 38, no. 15_suppl
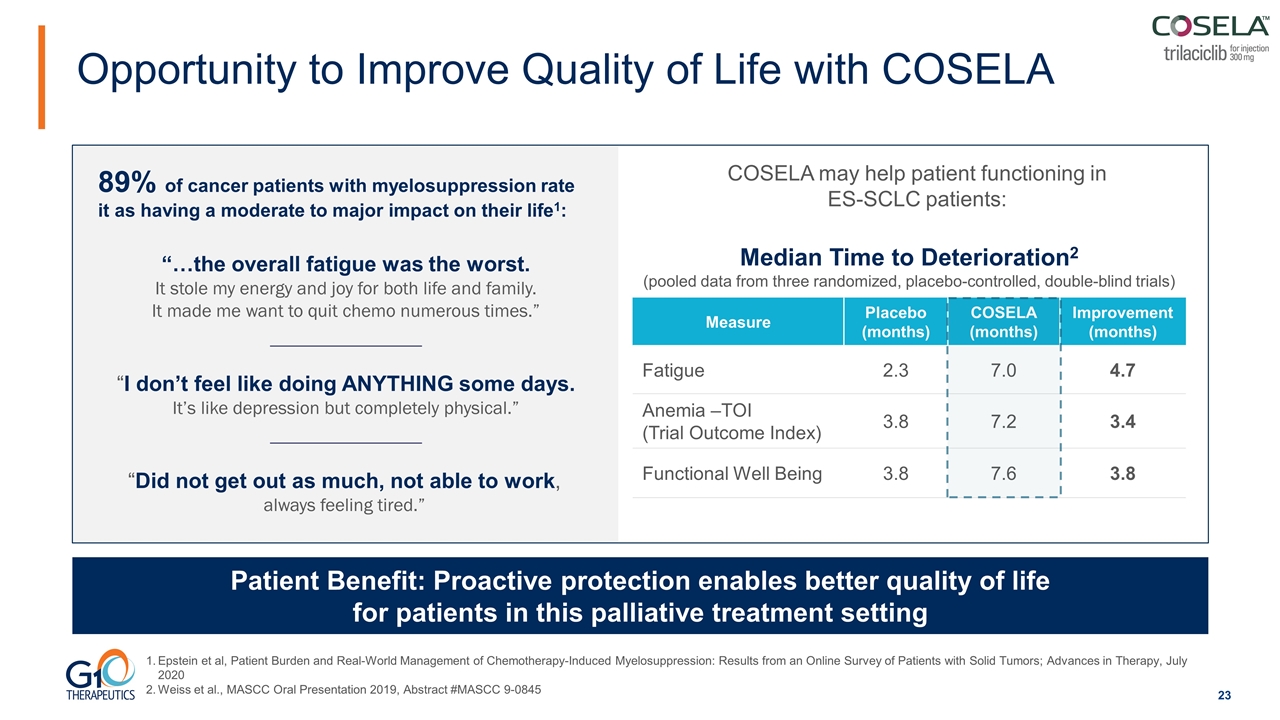
Opportunity to Improve Quality of Life with COSELA Patient Benefit: Proactive protection enables better quality of life for patients in this palliative treatment setting Epstein et al, Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors; Advances in Therapy, July 2020 Weiss et al., MASCC Oral Presentation 2019, Abstract #MASCC 9-0845 “…the overall fatigue was the worst. It stole my energy and joy for both life and family. It made me want to quit chemo numerous times.” “I don’t feel like doing ANYTHING some days. It’s like depression but completely physical.” 89% of cancer patients with myelosuppression rate it as having a moderate to major impact on their life1: Measure Placebo (months) COSELA (months) Improvement (months) Fatigue 2.3 7.0 4.7 Anemia –TOI (Trial Outcome Index) 3.8 7.2 3.4 Functional Well Being 3.8 7.6 3.8 COSELA may help patient functioning in ES-SCLC patients: Median Time to Deterioration2 (pooled data from three randomized, placebo-controlled, double-blind trials) “Did not get out as much, not able to work, always feeling tired.”
Opportunity for COSELA to Become Standard of Care in ES-SCLC Heightened awareness of myelosuppression due to the COVID pandemic may further encourage adoption of COSELA as a Standard of Care Clinical Results Meaningfully reduces myelosuppression in ES-SCLC Payer Impact May provide cost savings for system (COSELA expected to be budget neutral or better) Patient Benefits Meaningfully improves the overall quality of life for patients based on patient-reported data

2021 Key Objectives Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently
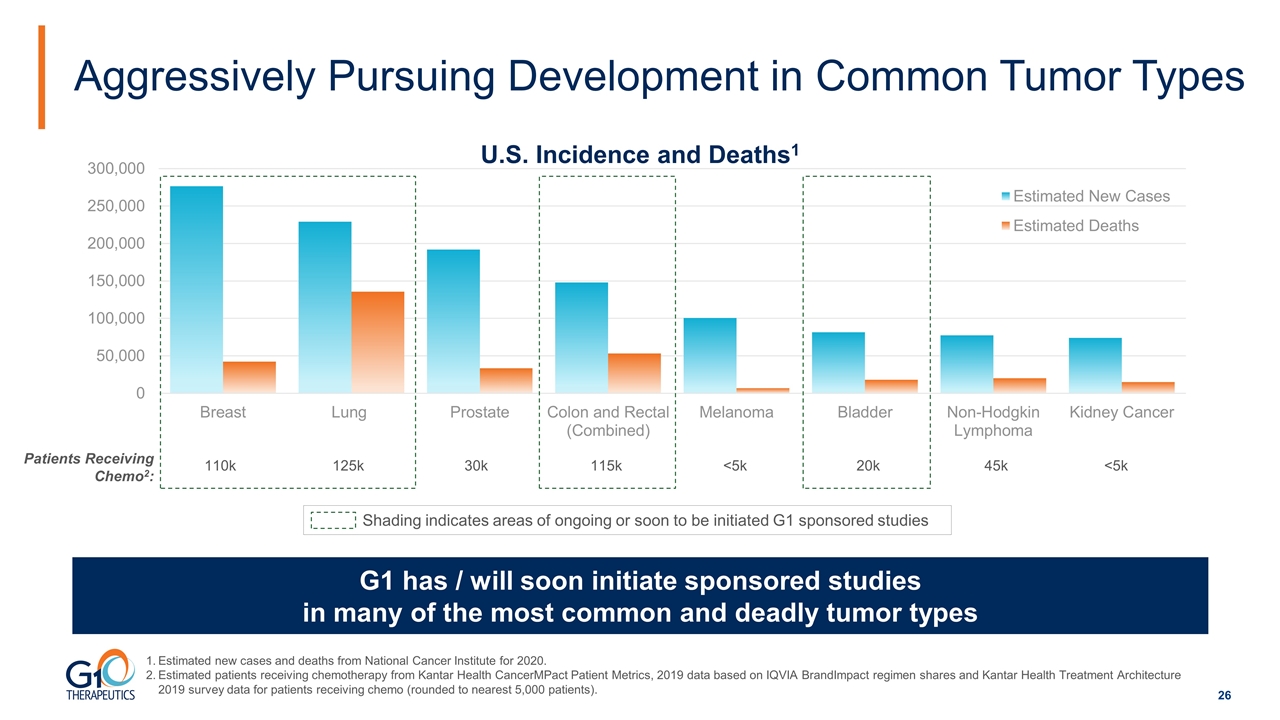
Aggressively Pursuing Development in Common Tumor Types Estimated new cases and deaths from National Cancer Institute for 2020. Estimated patients receiving chemotherapy from Kantar Health CancerMPact Patient Metrics, 2019 data based on IQVIA BrandImpact regimen shares and Kantar Health Treatment Architecture 2019 survey data for patients receiving chemo (rounded to nearest 5,000 patients). G1 has / will soon initiate sponsored studies in many of the most common and deadly tumor types Shading indicates areas of ongoing or soon to be initiated G1 sponsored studies Patients Receiving Chemo2: 110k 125k 30k 115k <5k 20k 45k <5k
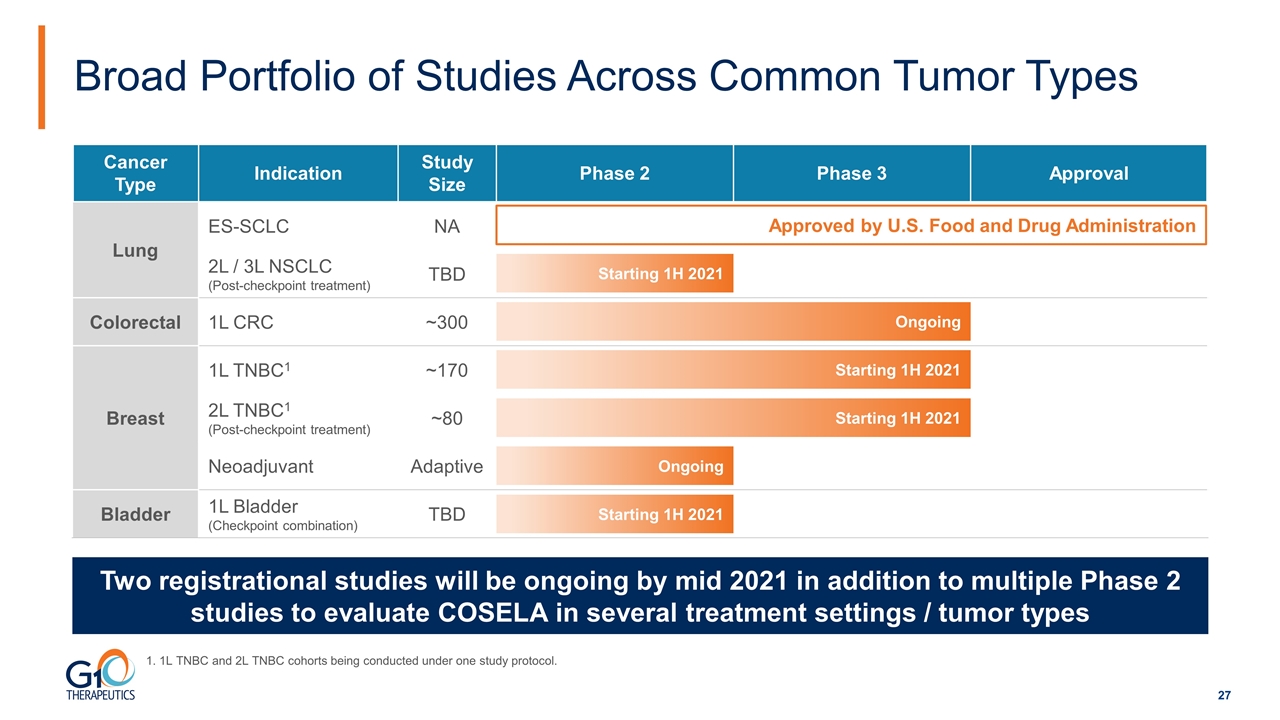
Broad Portfolio of Studies Across Common Tumor Types Two registrational studies will be ongoing by mid 2021 in addition to multiple Phase 2 studies to evaluate COSELA in several treatment settings / tumor types Cancer Type Indication Study Size Phase 2 Phase 3 Approval Lung ES-SCLC NA 2L / 3L NSCLC (Post-checkpoint treatment) TBD Colorectal 1L CRC ~300 Breast 1L TNBC1 ~170 Breast 2L TNBC1 (Post-checkpoint treatment) ~80 Breast Neoadjuvant Adaptive Bladder 1L Bladder (Checkpoint combination) TBD Approved by U.S. Food and Drug Administration Ongoing Starting 1H 2021 Starting 1H 2021 Ongoing Starting 1H 2021 Starting 1H 2021 1. 1L TNBC and 2L TNBC cohorts being conducted under one study protocol.
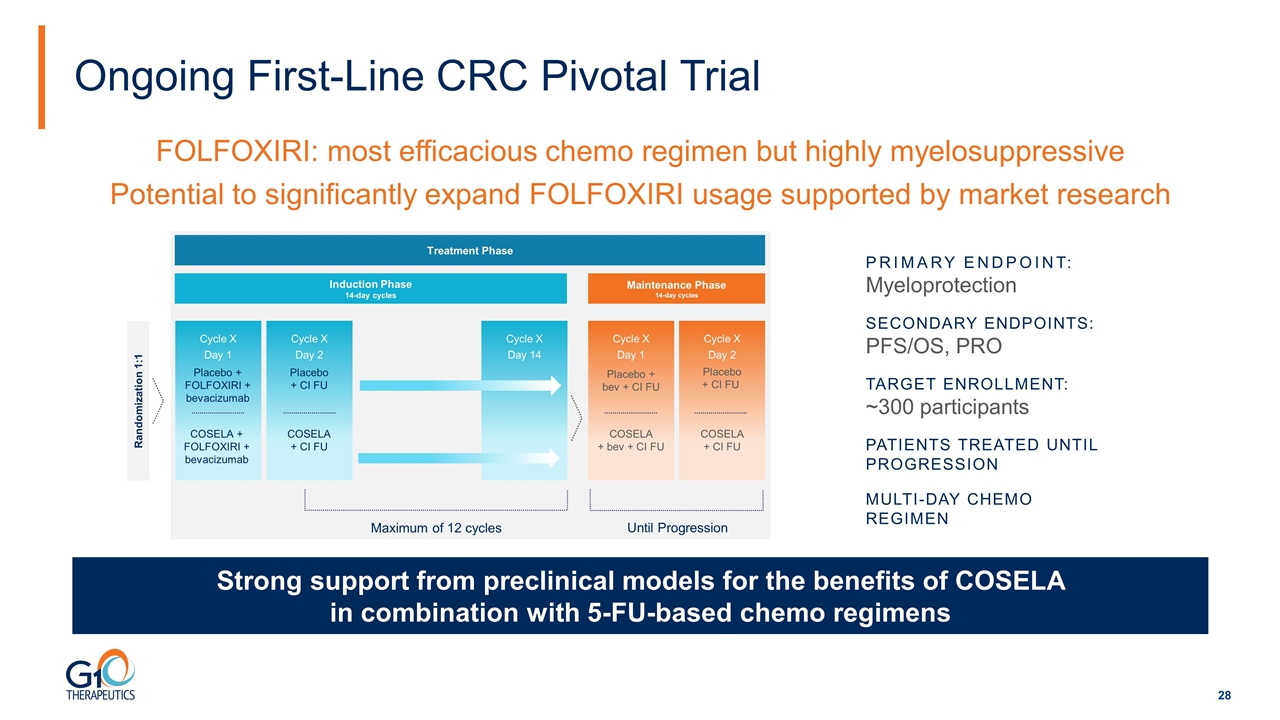
Ongoing First-Line CRC Pivotal Trial Cycle X Day 2 Cycle X Day 1 Cycle X Day 2 Cycle X Day 1 Cycle X Day 14 Randomization 1:1 Placebo + CI FU Treatment Phase Placebo + FOLFOXIRI + bevacizumab COSELA + FOLFOXIRI + bevacizumab COSELA + CI FU Induction Phase 14-day cycles Maintenance Phase 14-day cycles Placebo + bev + CI FU Placebo + CI FU COSELA + bev + CI FU COSELA + CI FU Maximum of 12 cycles PRIMARY ENDPOINT: Myeloprotection SECONDARY ENDPOINTS: PFS/OS, PRO TARGET ENROLLMENT: ~300 participants PATIENTS TREATED UNTIL PROGRESSION MulTi-day Chemo Regimen FOLFOXIRI: most efficacious chemo regimen but highly myelosuppressive Potential to significantly expand FOLFOXIRI usage supported by market research Strong support from preclinical models for the benefits of COSELA in combination with 5-FU-based chemo regimens Until Progression
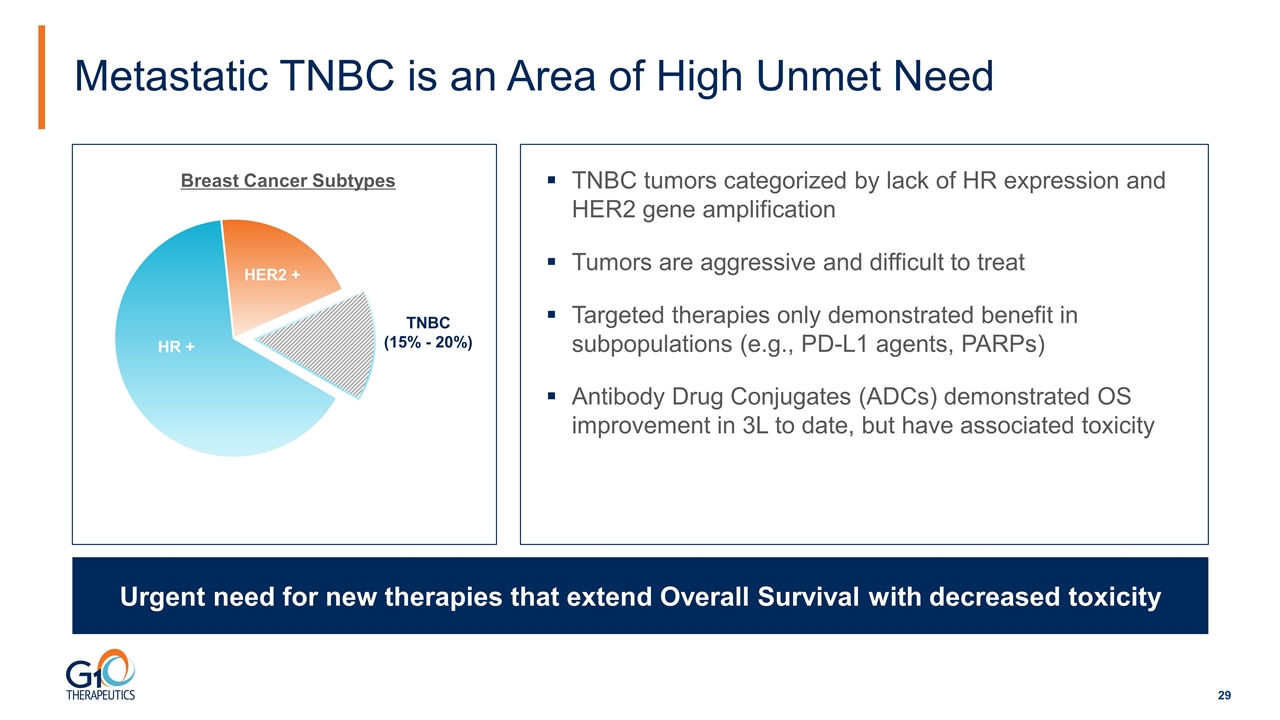
TNBC tumors categorized by lack of HR expression and HER2 gene amplification Tumors are aggressive and difficult to treat Targeted therapies only demonstrated benefit in subpopulations (e.g., PD-L1 agents, PARPs) Antibody Drug Conjugates (ADCs) demonstrated OS improvement in 3L to date, but have associated toxicity Metastatic TNBC is an Area of High Unmet Need Urgent need for new therapies that extend Overall Survival with decreased toxicity TNBC (15% - 20%) Breast Cancer Subtypes
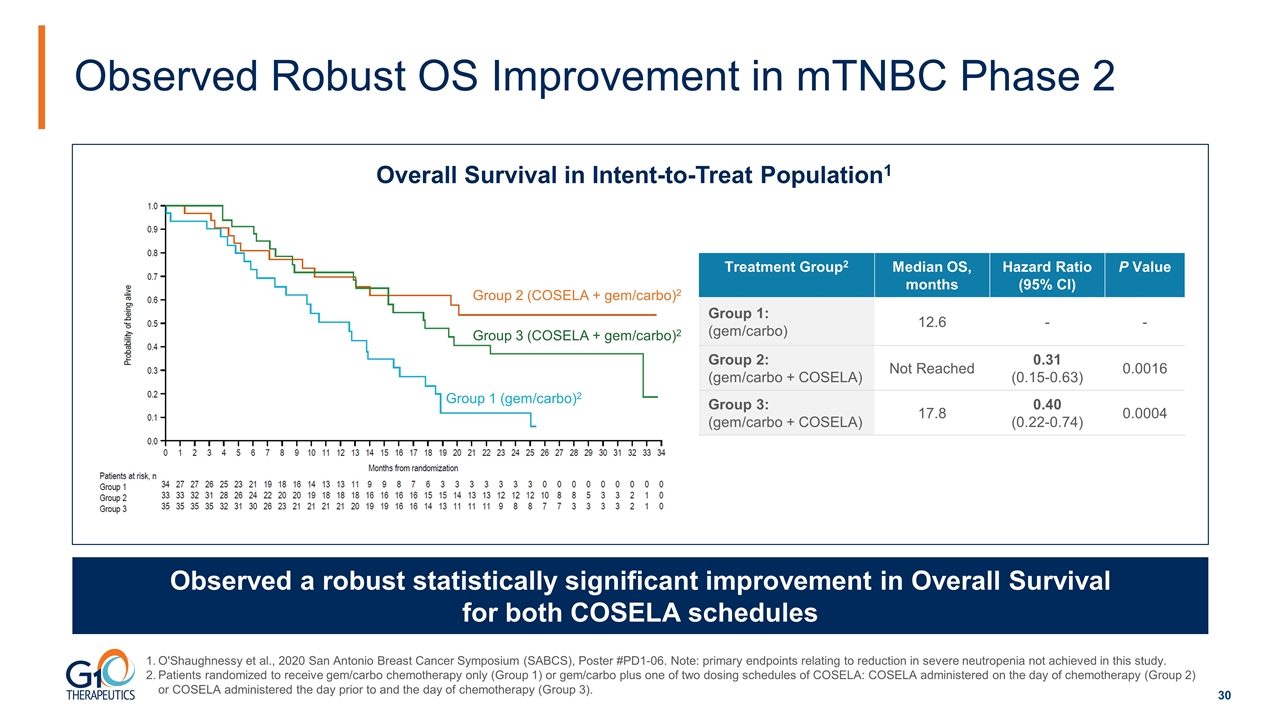
Observed Robust OS Improvement in mTNBC Phase 2 Observed a robust statistically significant improvement in Overall Survival for both COSELA schedules Group 1 (gem/carbo)2 Group 3 (COSELA + gem/carbo)2 Group 2 (COSELA + gem/carbo)2 Treatment Group2 Median OS, months Hazard Ratio (95% CI) P Value Group 1: (gem/carbo) 12.6 - - Group 2: (gem/carbo + COSELA) Not Reached 0.31 (0.15-0.63) 0.0016 Group 3: (gem/carbo + COSELA) 17.8 0.40 (0.22-0.74) 0.0004 Overall Survival in Intent-to-Treat Population1 O'Shaughnessy et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PD1-06. Note: primary endpoints relating to reduction in severe neutropenia not achieved in this study. Patients randomized to receive gem/carbo chemotherapy only (Group 1) or gem/carbo plus one of two dosing schedules of COSELA: COSELA administered on the day of chemotherapy (Group 2) or COSELA administered the day prior to and the day of chemotherapy (Group 3).
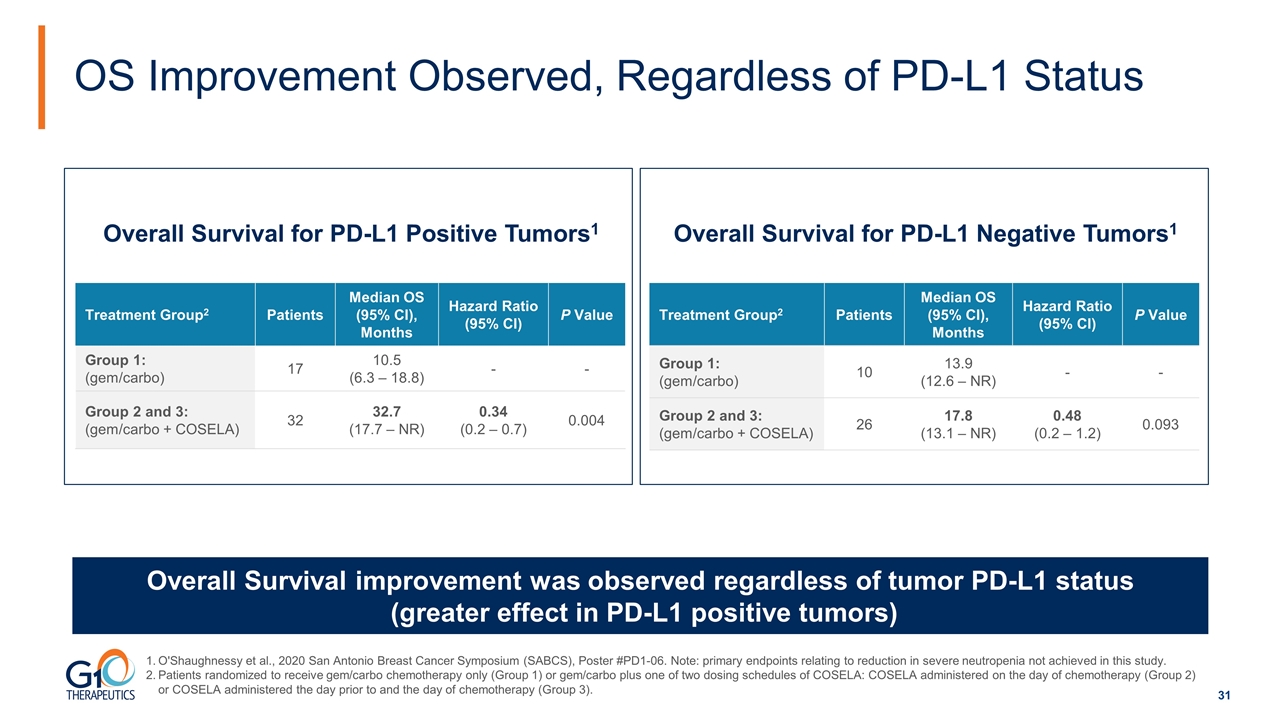
OS Improvement Observed, Regardless of PD-L1 Status Overall Survival improvement was observed regardless of tumor PD-L1 status (greater effect in PD-L1 positive tumors) Overall Survival for PD-L1 Positive Tumors1 Treatment Group2 Patients Median OS (95% CI), Months Hazard Ratio (95% CI) P Value Group 1: (gem/carbo) 17 10.5 (6.3 – 18.8) - - Group 2 and 3: (gem/carbo + COSELA) 32 32.7 (17.7 – NR) 0.34 (0.2 – 0.7) 0.004 Overall Survival for PD-L1 Negative Tumors1 Treatment Group2 Patients Median OS (95% CI), Months Hazard Ratio (95% CI) P Value Group 1: (gem/carbo) 10 13.9 (12.6 – NR) - - Group 2 and 3: (gem/carbo + COSELA) 26 17.8 (13.1 – NR) 0.48 (0.2 – 1.2) 0.093 O'Shaughnessy et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PD1-06. Note: primary endpoints relating to reduction in severe neutropenia not achieved in this study. Patients randomized to receive gem/carbo chemotherapy only (Group 1) or gem/carbo plus one of two dosing schedules of COSELA: COSELA administered on the day of chemotherapy (Group 2) or COSELA administered the day prior to and the day of chemotherapy (Group 3).
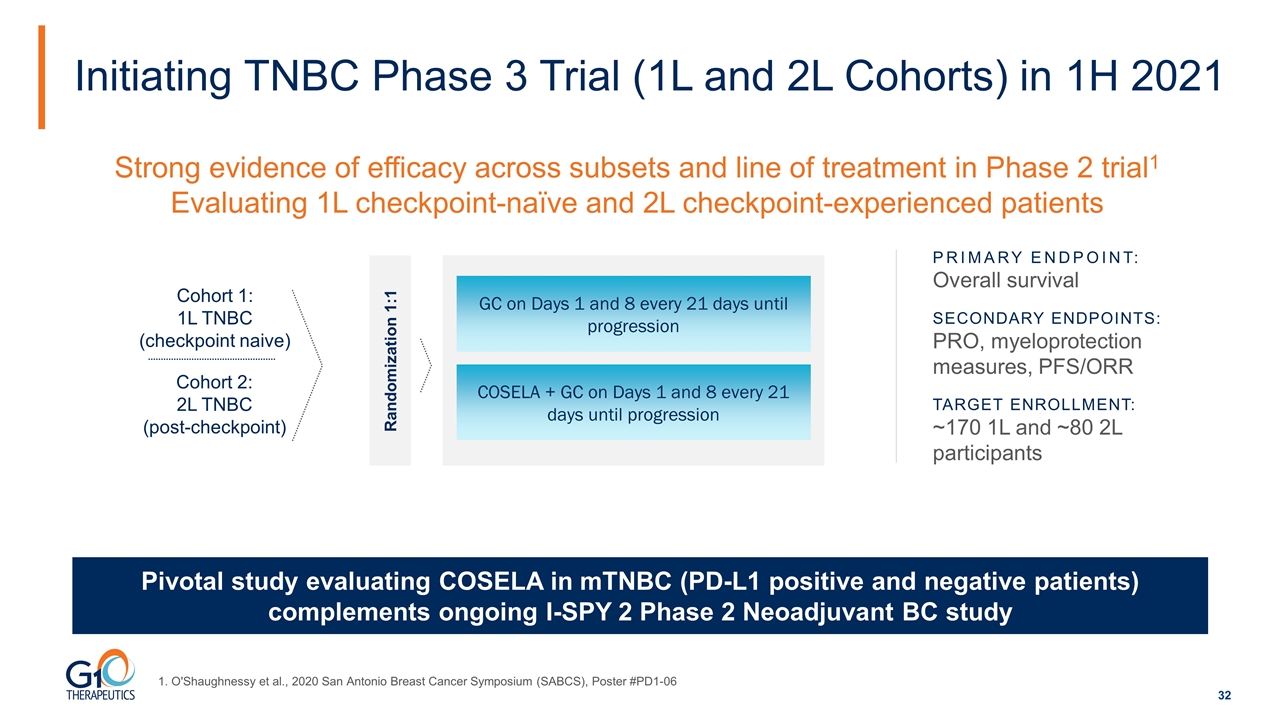
Initiating TNBC Phase 3 Trial (1L and 2L Cohorts) in 1H 2021 Pivotal study evaluating COSELA in mTNBC (PD-L1 positive and negative patients) complements ongoing I-SPY 2 Phase 2 Neoadjuvant BC study GC on Days 1 and 8 every 21 days until progression COSELA + GC on Days 1 and 8 every 21 days until progression Cohort 2: 2L TNBC (post-checkpoint) Cohort 1: 1L TNBC (checkpoint naive) Strong evidence of efficacy across subsets and line of treatment in Phase 2 trial1 Evaluating 1L checkpoint-naïve and 2L checkpoint-experienced patients Randomization 1:1 PRIMARY ENDPOINT: Overall survival SECONDARY ENDPOINTS: PRO, myeloprotection measures, PFS/ORR TARGET ENROLLMENT: ~170 1L and ~80 2L participants 1. O'Shaughnessy et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PD1-06
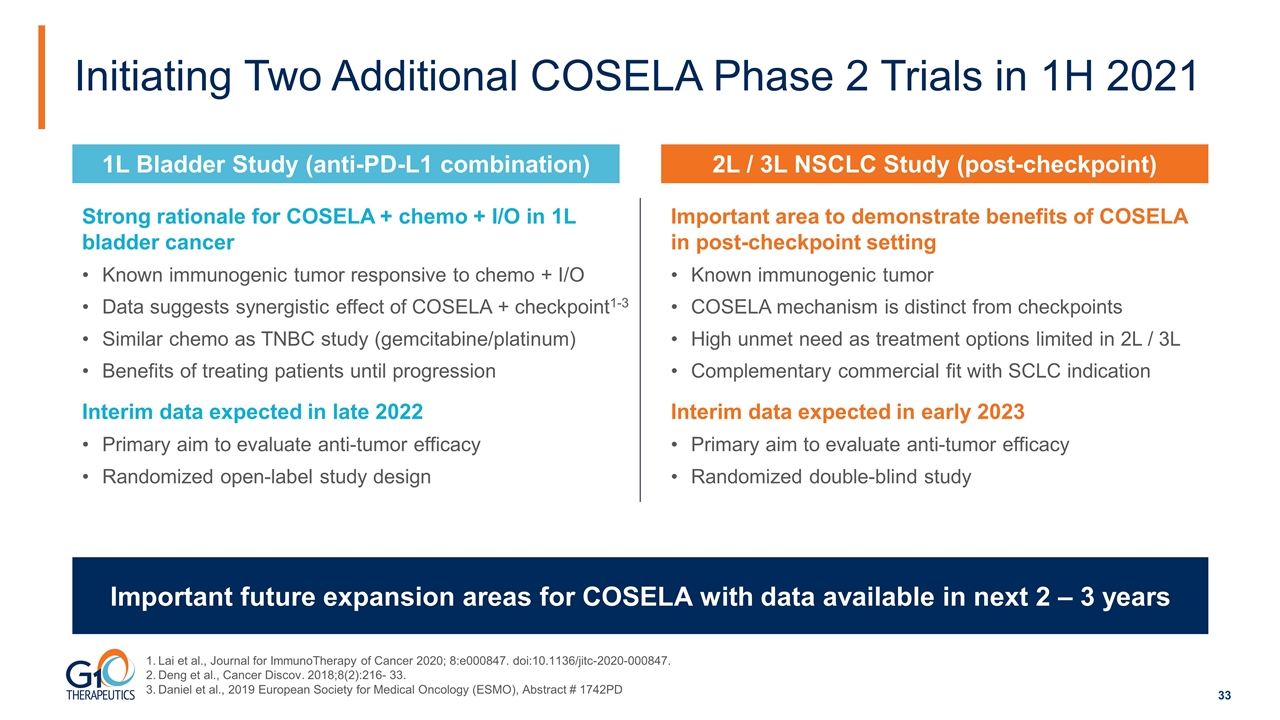
Initiating Two Additional COSELA Phase 2 Trials in 1H 2021 Important future expansion areas for COSELA with data available in next 2 – 3 years Strong rationale for COSELA + chemo + I/O in 1L bladder cancer Known immunogenic tumor responsive to chemo + I/O Data suggests synergistic effect of COSELA + checkpoint1-3 Similar chemo as TNBC study (gemcitabine/platinum) Benefits of treating patients until progression Interim data expected in late 2022 Primary aim to evaluate anti-tumor efficacy Randomized open-label study design 2L / 3L NSCLC Study (post-checkpoint) 1L Bladder Study (anti-PD-L1 combination) Important area to demonstrate benefits of COSELA in post-checkpoint setting Known immunogenic tumor COSELA mechanism is distinct from checkpoints High unmet need as treatment options limited in 2L / 3L Complementary commercial fit with SCLC indication Interim data expected in early 2023 Primary aim to evaluate anti-tumor efficacy Randomized double-blind study Lai et al., Journal for ImmunoTherapy of Cancer 2020; 8:e000847. doi:10.1136/jitc-2020-000847. Deng et al., Cancer Discov. 2018;8(2):216- 33. Daniel et al., 2019 European Society for Medical Oncology (ESMO), Abstract # 1742PD

2021 Key Objectives Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently
Rintodestrant Demonstrated a Favorable Oral SERD* Profile in Clinical Trials Next steps will be evaluated following data readout expected in 2Q21 * SERD = Selective Estrogen Receptor Degrader 40-patient Phase 2 combination trial with CDK4/6 inhibitor palbociclib ongoing; data in 2Q21 Phase 2 combination data will be important to help secure partner to fund Phase 3 investment Fulvestrant is currently only SERD available Proven approach but painful intramuscular injections limit use to 2L and preclude use in earlier lines of therapy An oral SERD has potential to move into earlier lines of ER-positive breast cancer therapy Rintodestrant monotherapy Phase 1b findings to date: Favorable tolerability - AEs mostly Grade 1 or Grade 2 Strong ER target engagement/occupancy with evidence of anti-tumor activity in heavily pre-treated patients 1. Aftimos et al., 2020 San Antonio Breast Cancer Symposium (SABCS), Poster #PS12-04

2021 Key Objectives Obtain U.S. approval for ES-SCLC and successfully launch COSELA in 1Q Establish COSELA as Standard of Care for ES-SCLC patients in the U.S. Maximize long-term value of COSELA by executing robust development plan Evaluate partnership options for rintodestrant following combination data readout in 2Q Continue managing investor capital efficiently

~$207M cash at year-end 2020 provides runway into second half of 2022 Efficiently executing plan with lean organization of ~125 FTEs Utilizing capital efficient promotion arrangement with Boehringer Ingelheim for COSELA U.S. launch in SCLC Expect to leverage co-development opportunities with partner Simcere for potential cost and timing efficiencies Access to debt facility up to $100M total ($20M drawn to date) Potential future milestones (up to $486M) and royalties from licensing agreements Continue to Efficiently Manage Capital Efficiently managing capital with a lean organization and benefiting from existing partnership arrangements
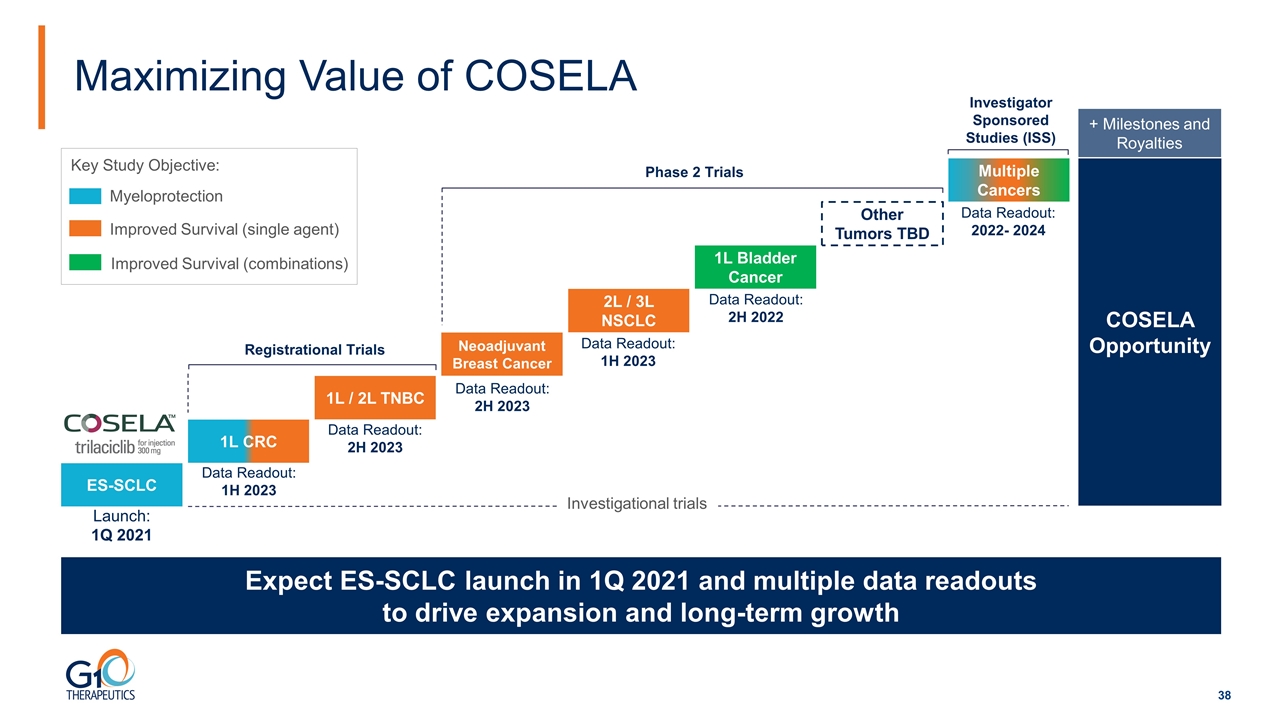
Maximizing Value of COSELA Expect ES-SCLC launch in 1Q 2021 and multiple data readouts to drive expansion and long-term growth ES-SCLC 1L CRC 2L / 3L NSCLC 1L Bladder Cancer Registrational Trials Phase 2 Trials Other Tumors TBD COSELA Opportunity + Milestones and Royalties Neoadjuvant Breast Cancer Myeloprotection Improved Survival (single agent) Improved Survival (combinations) 1L / 2L TNBC Data Readout: 1H 2023 Data Readout: 2H 2023 Data Readout: 1H 2023 Data Readout: 2H 2023 Data Readout: 2H 2022 Key Study Objective: Launch: 1Q 2021 Investigational trials Data Readout: 2022- 2024 Investigator Sponsored Studies (ISS) Multiple Cancers





































