
A Commercial-Stage Bioelectronic Medicine Company Nasdaq: ECOR Corporate Presentation November 2019 Exhibit 99.1

Forward Looking Statement In addition to historical information, this presentation may contain forward-looking statements with respect to our business, capital resources, strategy and growth reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All forward-looking statements are subject to a number of risks, uncertainties and assumptions, and you should not rely upon forward-looking statements as predictions of future events. All forward-looking statements will be based upon current estimates and expectations about future events and financial and other trends. There is no guarantee that future results, performance or events reflected in the forward-looking statements will be achieved or occur. No person assumes responsibility for the accuracy and completeness of the forward-looking statements, and, except as required by law, no person undertakes any obligation to update any forward-looking statements for any reason after the date of this presentation. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as "anticipates," "believes," "could,” "seeks," "estimates," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements represent our management's beliefs and assumptions only as of the date of the prospectus and are only predictions that may be inaccurate. You should read the Risk Factors set forth in our prospectus and other reports filed from time to time with the Securities and Exchange Commission, which factors may cause our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those or our situation may change in the future. Additionally, in an effort to provide additional information management believes is a useful indicator of operating performance for the fiscal quarter ended September 30, 2019, this presentation contains a financial measure not determined by generally accepted accounting principles (GAAP): Adjusted EBITDA net loss from operations. A reconciliation to the most directly comparable GAAP financial measure of Net Loss from Operations is available on the presentation slide entitled “Adjusted EBITDA Reconciliation.” The rationale for management’s use of non-GAAP information is included in Exhibit 99.1 to the Company’s Form 8-K furnished with the SEC on November 13, 2019.
electroCore At-a-Glance gammaCore Sapphire™ NASDAQx ECOR Headquarters: Basking Ridge, NJ No. of employees: 50 Market cap: ~$55M (11/14/19) Recent close: $1.85 (11/14/19) Cash & marketable securities (9/30/19): $33.5M Expected cash runway into beginning of 2021
Tony Fiorino, MD Chief Medical Officer 20 years Eric Liebler SVP of Neurology 22 years Michael Ruberio National Sales Director 30 years Iain Strickland Director of Business Development, UK 15 years Daniel Goldberger Chief Executive Officer 35 years Brian Posner Chief Financial Officer 35 years Ardelle Ferris VP of Market Access 30 years Mike Romaniw VP, Operations 30 years Experienced Management Team
Investment Highlights Platform Therapy Large Initial Market Attractive Gross Margins Strong IP Portfolio Revenue stage, proprietary, non-invasive vagus nerve stimulator positioned to unlock the broad potential of bioelectronic medicine Primary headache market opportunity for cluster headache and migraine estimated to be in excess of $4 billion Recurring revenue business model Key patent coverage extends through 2033

1st FDA-cleared non-invasive vagus nerve stimulator Hand-held, simple-to-use, highly targeted therapy gammaCore Sapphire™ Fast acting, comfortable, easy to use option. Initial indications prevention and treatment of cluster headache and acute migraine Recurring revenue model
Non-Invasive Therapy vs. Drug Alternatives Benefits of gammaCore Drawbacks of Current Drug Treatments Non-systemic Safe and well tolerated Patient self-administered Ability to administer multiple daily doses No known drug interactions Systemic side effects Serious adverse events Potential drug/drug interactions CVD and other contraindications Potential for medication overuse headache
20X 36 million U.S. patients1 Triptans represent 80% of prescribed acute therapies 40% of patients are dissatisfied or unresponsive to triptans2 More than half of insured migraineurs are untreated2 Unmet Need in Migraine & Cluster Headache American Migraine Foundation IMS Pharmetrics Plus. Cephalalgia. 2008 Jun;28(6):614-8. doi: 10.1111/j.1468-2982.2008.01592.x. Epub 2008 Apr 16. MIGRAINE 400,000 U.S. patients3 Up to eight 15-180 min attacks per day Typically occur 4-24 weeks per year gammaCore is FDA-cleared therapy for acute and preventive treatment of Cluster Headache CLUSTER HEADACHE 400,000 U.S. patients3 Up to eight 15-180 min attacks per day Typically occur 4-24 weeks per year gammaCore is FDA-cleared therapy for acute and preventive treatment of Cluster Headache
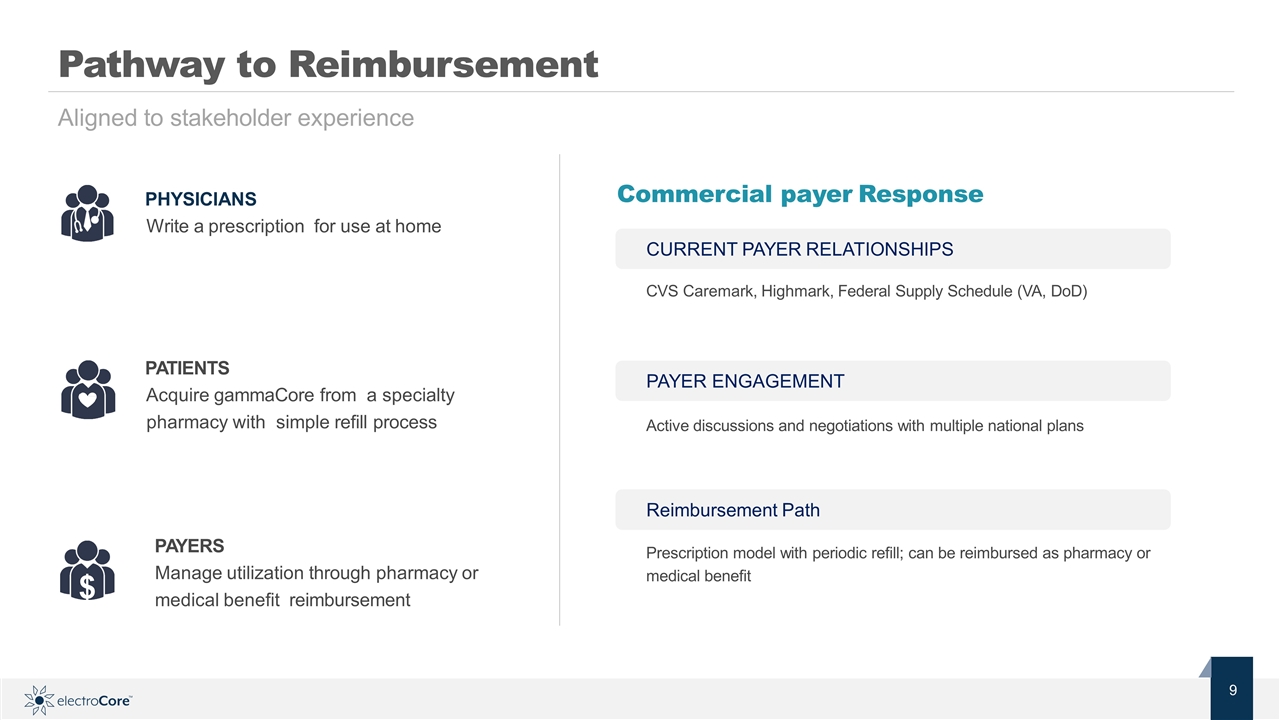
Pathway to Reimbursement PHYSICIANS Write a prescription for use at home PATIENTS Acquire gammaCore from a specialty pharmacy with simple refill process $ Commercial payer Response Aligned to stakeholder experience PAYERS Manage utilization through pharmacy or medical benefit reimbursement CURRENT PAYER RELATIONSHIPS CVS Caremark, Highmark, Federal Supply Schedule (VA, DoD) PAYER ENGAGEMENT Active discussions and negotiations with multiple national plans Reimbursement Path Prescription model with periodic refill; can be reimbursed as pharmacy or medical benefit
Channels With Potential for Revenue Growth Now Driving DOD sales through the Veterans Administration and Military Treatment Facilities sales - started January 15th Pulling through the UK – Innovation Technology Program Award for cluster headache, included in NICE draft guidance started May 1st Workers Comp and Personal Injury through a relationship with Doctor’s Medical, LLC Pursuing additional commercial relationships
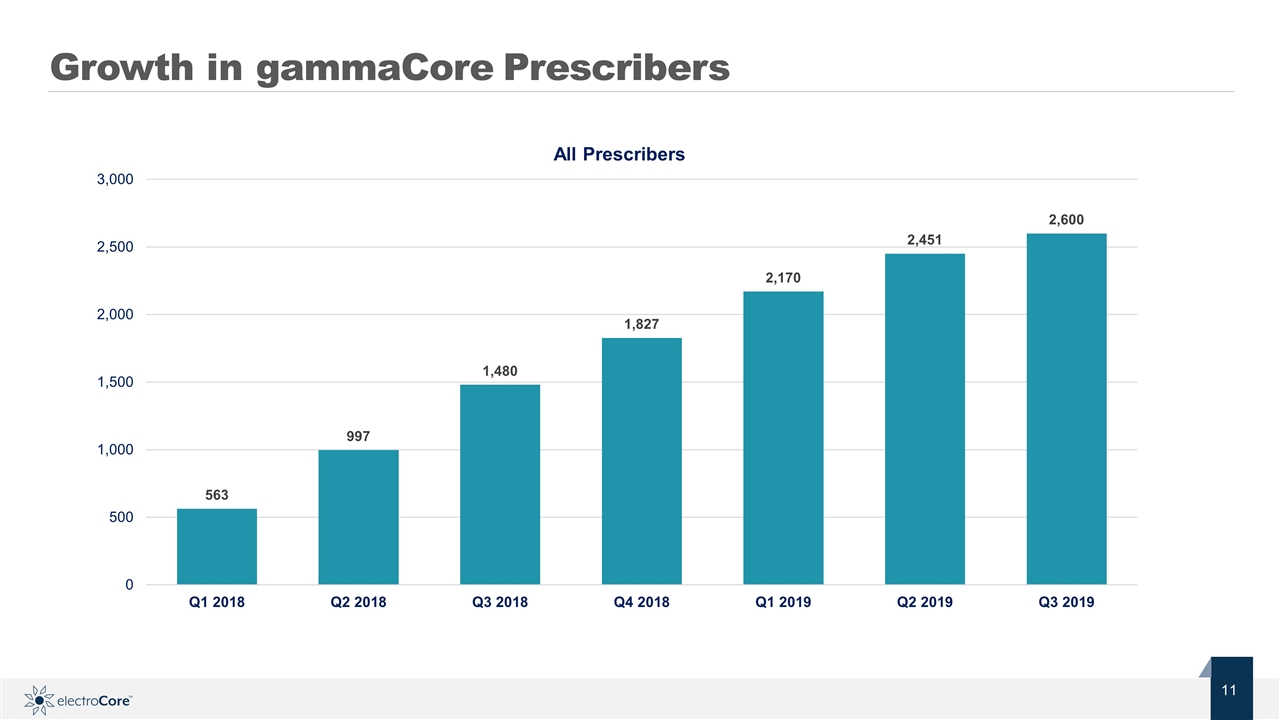
Growth in gammaCore Prescribers
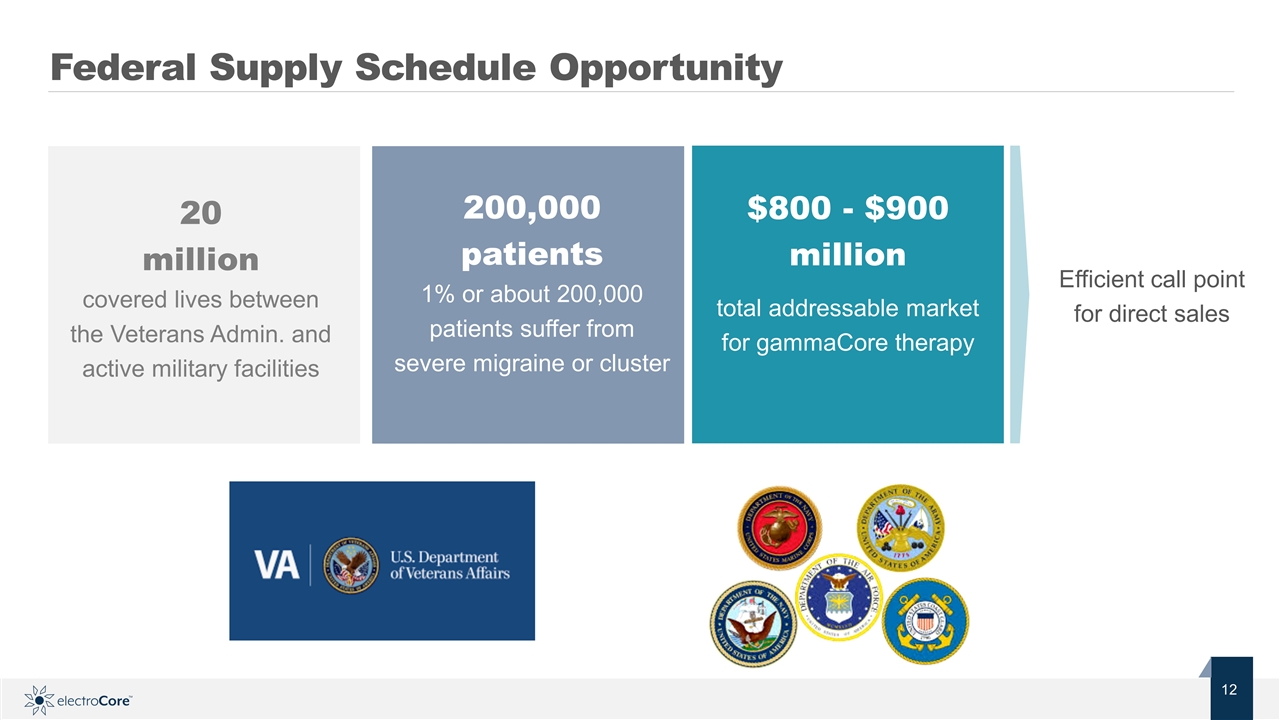
Federal Supply Schedule Opportunity 20 million covered lives between the Veterans Admin. and active military facilities 200,000 patients 1% or about 200,000 patients suffer from severe migraine or cluster $800 - $900 million total addressable market for gammaCore therapy Efficient call point for direct sales
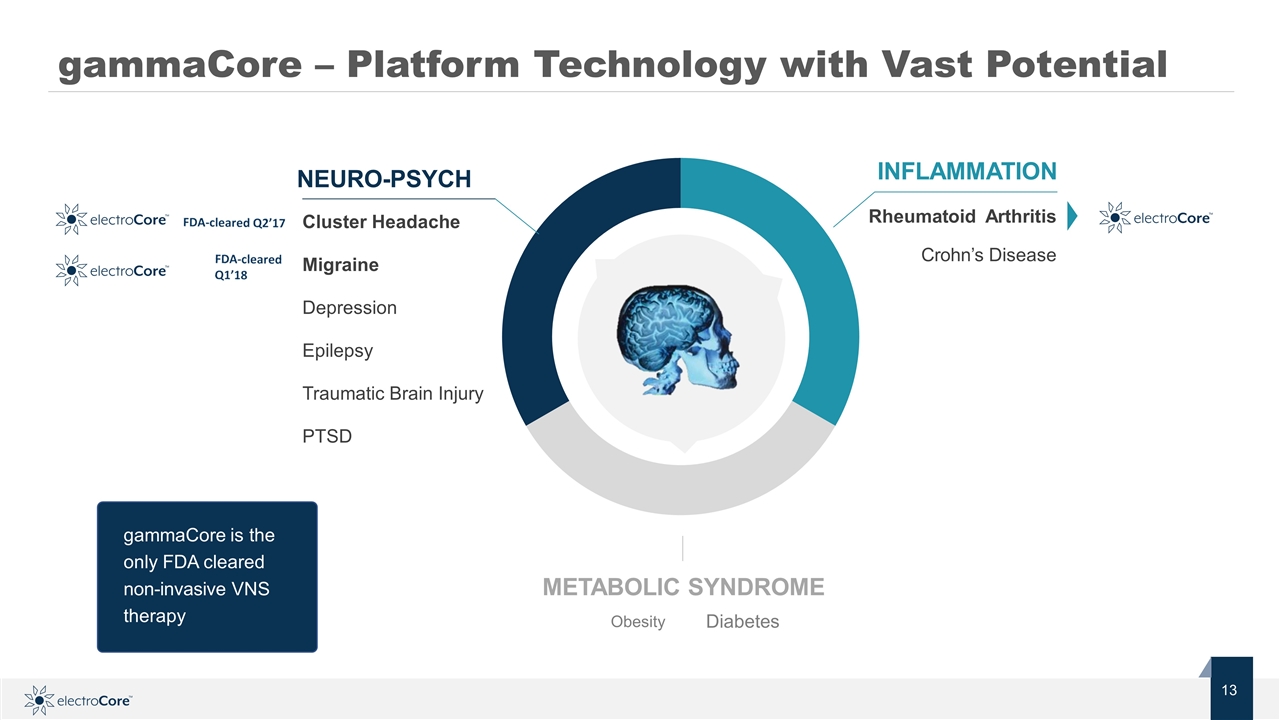
Depression Epilepsy Migraine Obesity Diabetes gammaCore – Platform Technology with Vast Potential gammaCore is the only FDA cleared non-invasive VNS therapy Rheumatoid Arthritis Crohn’s Disease INFLAMMATION METABOLIC SYNDROME Cluster Headache Migraine Depression Epilepsy Traumatic Brain Injury PTSD NEURO-PSYCH
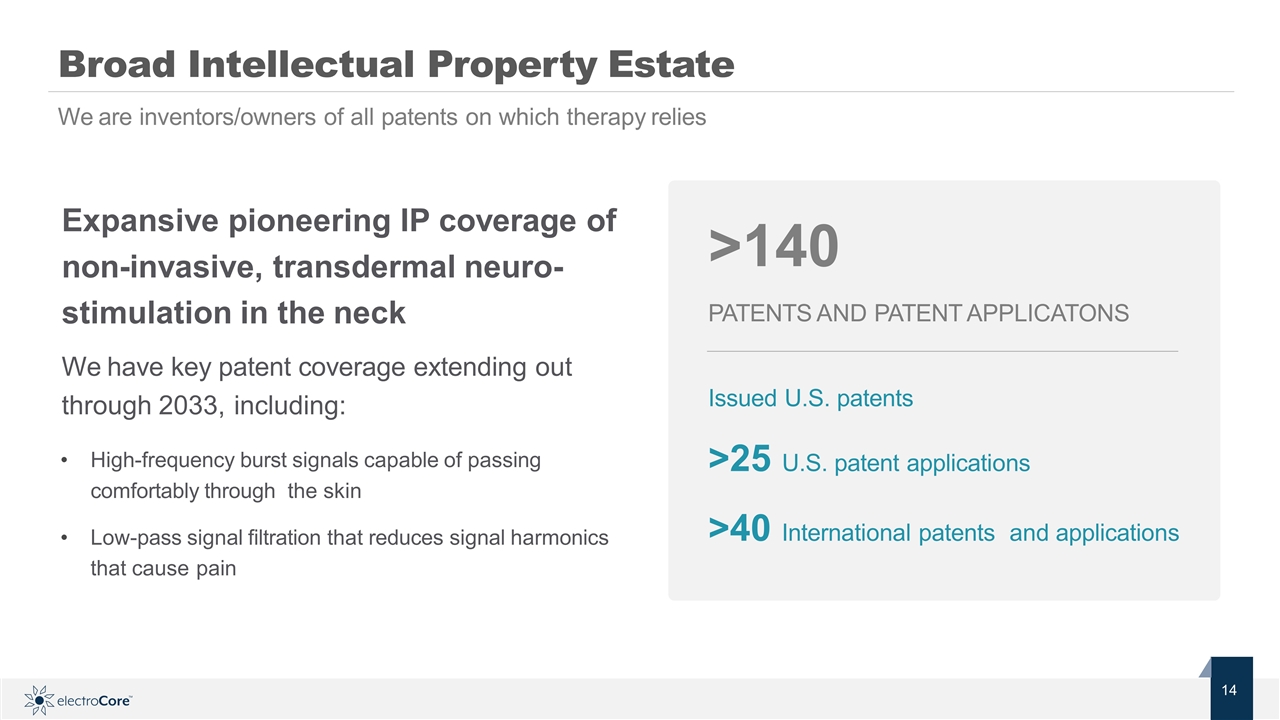
Expansive pioneering IP coverage of non-invasive, transdermal neuro-stimulation in the neck We have key patent coverage extending out through 2033, including: High-frequency burst signals capable of passing comfortably through the skin Low-pass signal filtration that reduces signal harmonics that cause pain Broad Intellectual Property Estate We are inventors/owners of all patents on which therapy relies >140 PATENTS AND PATENT APPLICATONS Issued U.S. patents >25 U.S. patent applications >40 International patents and applications
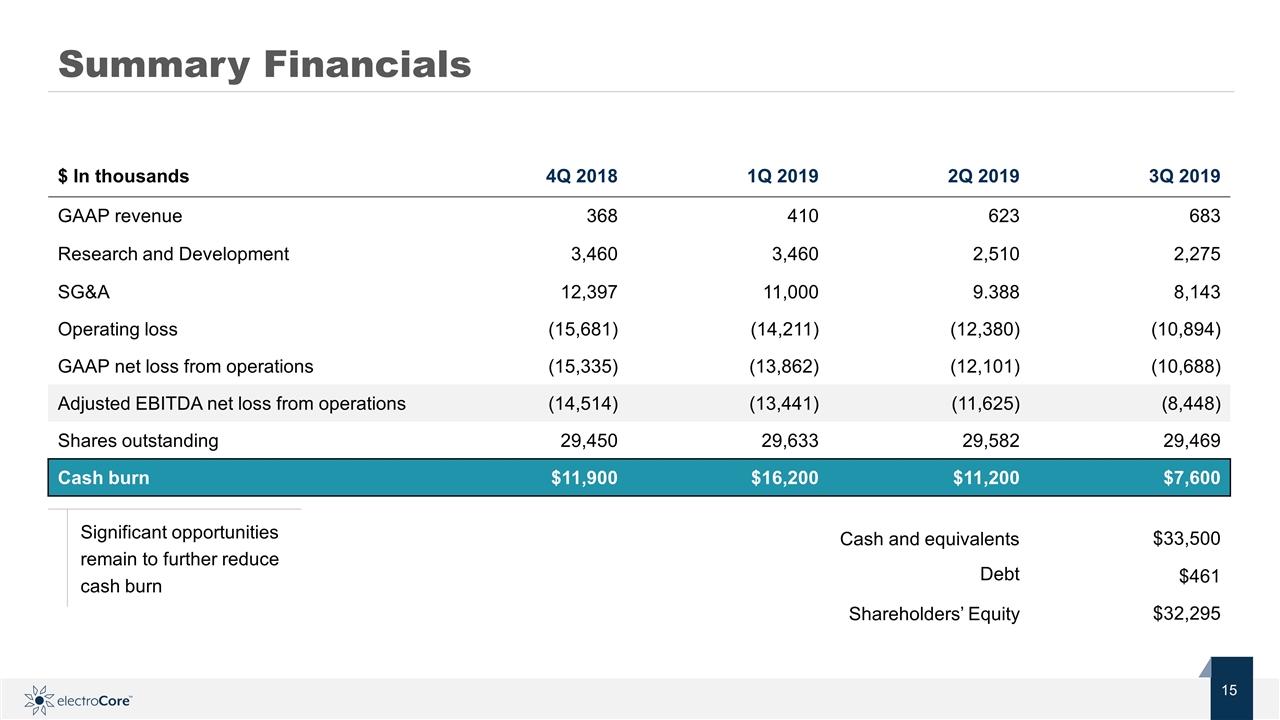
Summary Financials $ In thousands 4Q 2018 1Q 2019 2Q 2019 3Q 2019 GAAP revenue 368 410 623 683 Research and Development 3,460 3,460 2,510 2,275 SG&A 12,397 11,000 9.388 8,143 Operating loss (15,681) (14,211) (12,380) (10,894) GAAP net loss from operations (15,335) (13,862) (12,101) (10,688) Adjusted EBITDA net loss from operations (14,514) (13,441) (11,625) (8,448) Shares outstanding 29,450 29,633 29,582 29,469 Cash burn $11,900 $16,200 $11,200 $7,600 Cash and equivalents $33,500 Debt $461 Shareholders’ Equity $32,295 Significant opportunities remain to further reduce cash burn
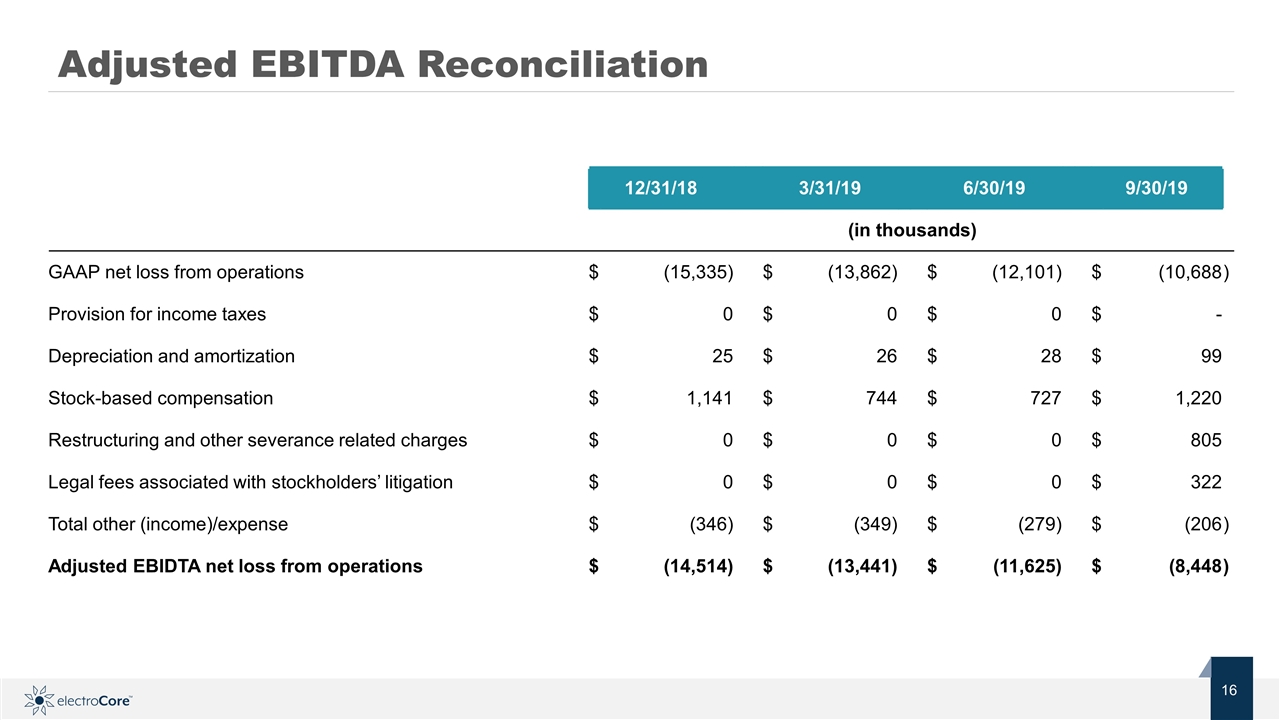
Adjusted EBITDA Reconciliation 12/31/18 3/31/19 6/30/19 9/30/19 (in thousands) GAAP net loss from operations $ (15,335) $ (13,862) $ (12,101) $ (10,688 ) Provision for income taxes $ 0 $ 0 $ 0 $ - Depreciation and amortization $ 25 $ 26 $ 28 $ 99 Stock-based compensation $ 1,141 $ 744 $ 727 $ 1,220 Restructuring and other severance related charges $ 0 $ 0 $ 0 $ 805 Legal fees associated with stockholders’ litigation $ 0 $ 0 $ 0 $ 322 Total other (income)/expense $ (346) $ (349) $ (279) $ (206 ) Adjusted EBIDTA net loss from operations $ (14,514) $ (13,441) $ (11,625) $ (8,448 )

APPENDIX
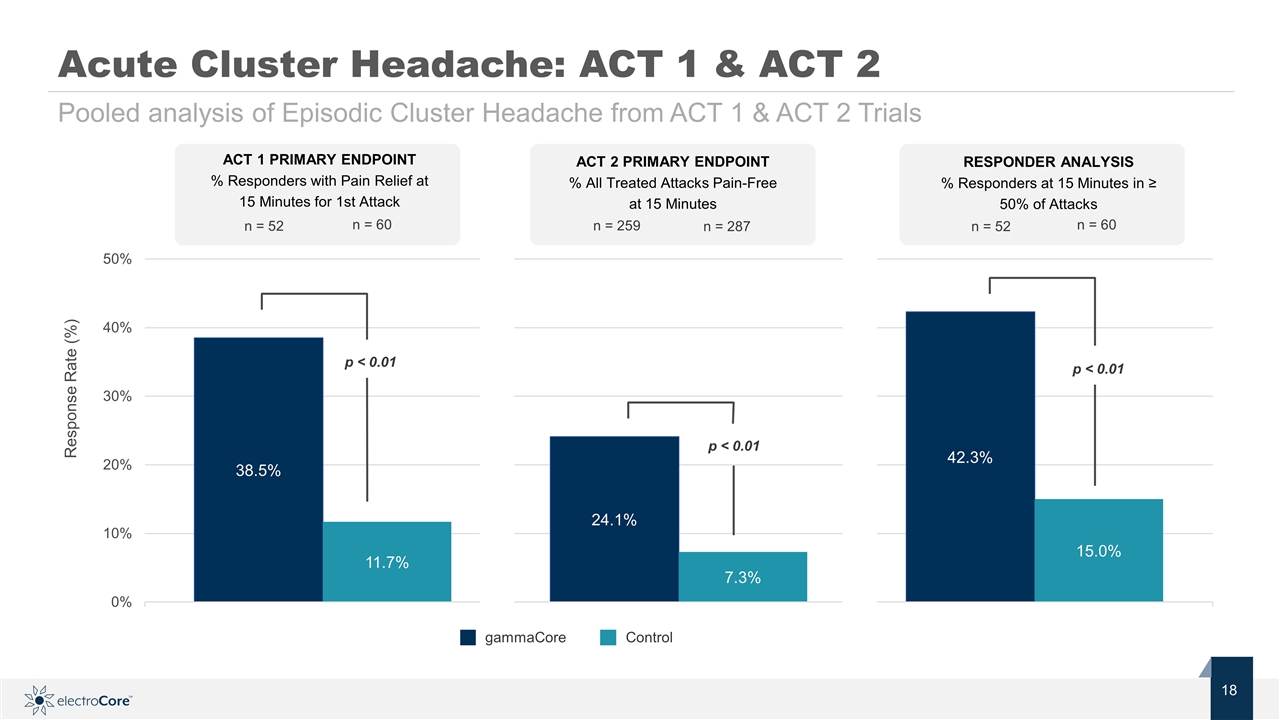
n = 52 n = 60 ACT 1 PRIMARY ENDPOINT % Responders with Pain Relief at 15 Minutes for 1st Attack Pooled analysis of Episodic Cluster Headache from ACT 1 & ACT 2 Trials p < 0.01 p < 0.01 p < 0.01 Acute Cluster Headache: ACT 1 & ACT 2 Response Rate (%) Control gammaCore n = 52 n = 60 RESPONDER ANALYSIS % Responders at 15 Minutes in ≥ 50% of Attacks n = 259 n = 287 ACT 2 PRIMARY ENDPOINT % All Treated Attacks Pain-Free at 15 Minutes
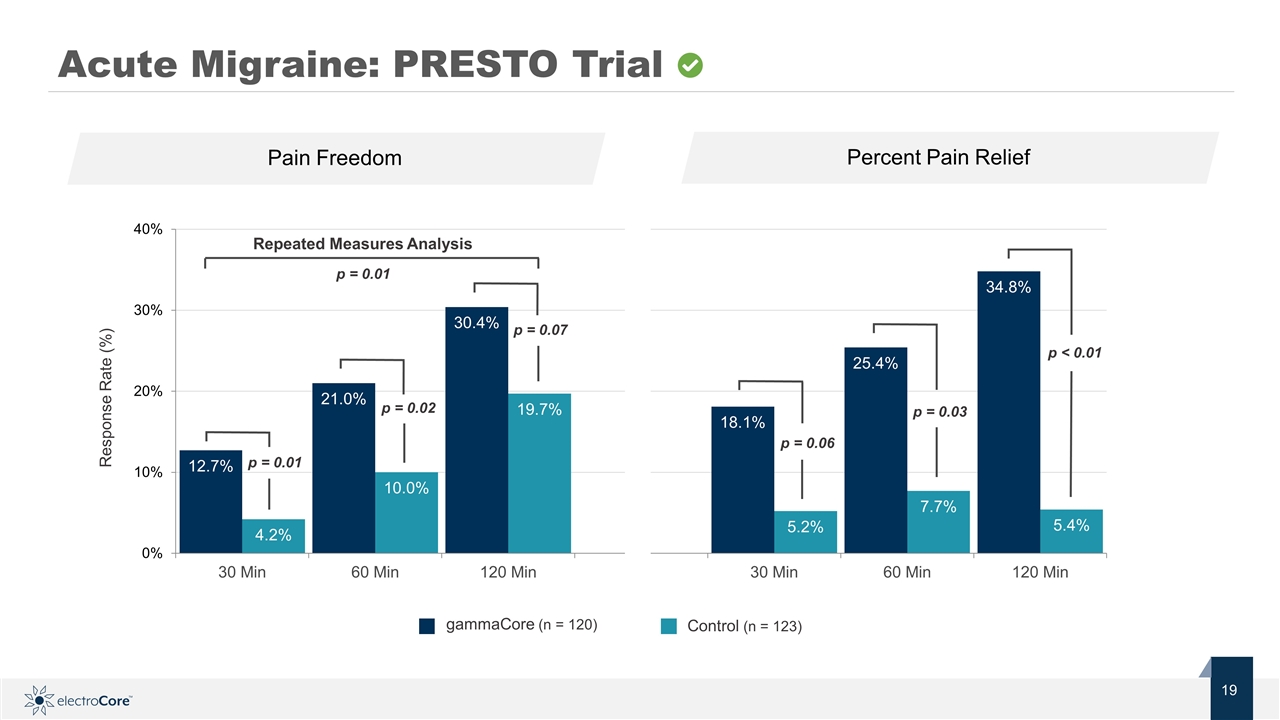
Response Rate (%) Repeated Measures Analysis p = 0.01 gammaCore (n = 120) Acute Migraine: PRESTO Trial p = 0.02 p = 0.01 p = 0.07 p < 0.01 p = 0.06 p = 0.03 Pain Freedom Percent Pain Relief Control (n = 123)
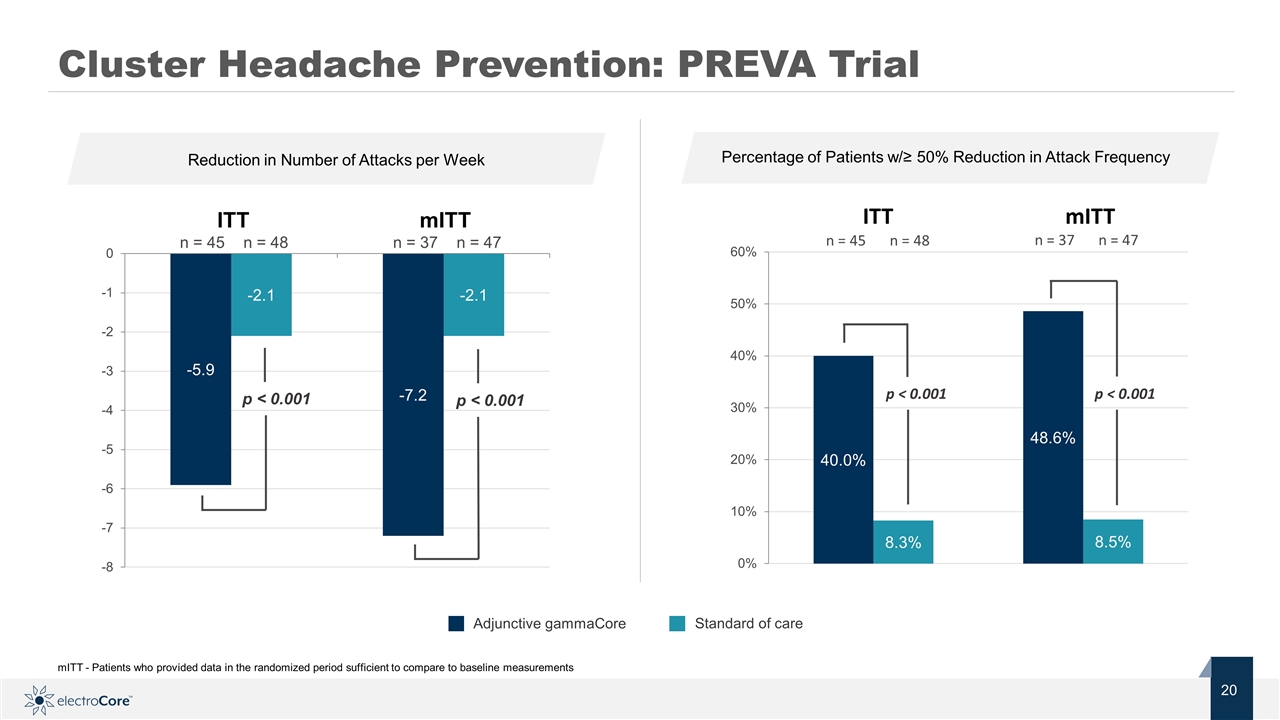
Standard of care Adjunctive gammaCore Cluster Headache Prevention: PREVA Trial n = 45 n = 48 ITT mITT n = 37 n = 47 p < 0.001 p < 0.001 p < 0.001 p < 0.001 n = 45 n = 48 n = 37 n = 47 ITT mITT mITT - Patients who provided data in the randomized period sufficient to compare to baseline measurements Reduction in Number of Attacks per Week Percentage of Patients w/≥ 50% Reduction in Attack Frequency



















