THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (“SEC”) DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THIS INVESTMENT INVOLVES A DEGREE OF RISK THAT MAY NOT BE SUITABLE FOR ALL PERSONS. ONLY THOSE INVESTORS WHO CAN BEAR THE LOSS OF A SIGNIFICANT PORTION OF THEIR INVESTMENT SHOULD PARTICIPATE IN THE INVESTMENT. (SEE “RISK FACTORS” BELOW.)
AN OFFERING STATEMENT PURSUANT TO REGULATION A RELATING TO THESE SECURITIES HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. INFORMATION CONTAINED IN THIS PRELIMINARY OFFERING CIRCULAR IS SUBJECT TO COMPLETION OR AMENDMENT. THESE SECURITIES MAY NOT BE SOLD NOR MAY OFFERS TO BUY BE ACCEPTED BEFORE THE OFFERING STATEMENT FILED WITH THE COMMISSION IS QUALIFIED. THIS PRELIMINARY OFFERING CIRCULAR SHALL NOT CONSTITUTE AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY NOR MAY THERE BE ANY SALES OF THESE SECURITIES IN ANY STATE IN WHICH SUCH OFFER, SOLICITATION OR SALE WOULD BE UNLAWFUL BEFORE REGISTRATION OR QUALIFICATION UNDER THE LAWS OF ANY SUCH STATE. THE COMPANY MAY ELECT TO SATISFY ITS OBLIGATION TO DELIVER A FINAL OFFERING CIRCULAR BY SENDING YOU A NOTICE WITHIN TWO BUSINESS DAYS AFTER THE COMPLETION OF THE SALE THAT CONTAINS THE URL WHERE THE FINAL OFFERING CIRCULAR OR THE OFFERING STATEMENT IN WHICH SUCH FINAL OFFERING CIRCULAR WAS FILED MAY BE OBTAINED.
THE SECURITIES OFFERED HAVE NOT BEEN APPROVED OR DISAPPROVED BY ANY STATE REGULATORY AUTHORITY NOR HAS ANY STATE REGULATORY AUTHORITY PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THIS OFFERING CIRCULAR. ANY REPRESENTATION TO THE CONTRARY IS UNLAWFUL.
Preliminary Offering Circular
For
McGinley Orthopaedic Innovations, Inc.
A Wyoming Corporation
August 11, 2022
SECURITIES OFFERED : Equity in the form of Shares of common stock
PRICE PER SHARE : $5.25 per Share of Common Stock
MAXIMUM OFFERING AMOUNT : $40,000,000.00
MINIMUM OFFERING AMOUNT : Not Applicable (No Minimum Offering Amount)
MINIMUM INVESTMENT : $100.00
CONTACT INFORMATION :
McGinley Orthopaedic Innovations, Inc.
234 E. 1st St. Suite 242
Casper, WY 82601
(307) 315-6403
Generally, no sale may be made to you in this Offering if the aggregate purchase price you pay is more than ten (10%) percent of the greater of your annual income or net worth. Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, Investors are encouraged to review rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, Investors are encouraged to refer to www.investor.gov.
McGinley Orthopaedic Innovations, Inc. (the “Company” or the “Issuer”) is an orthopedic surgical device/implant innovator and manufacturer based in Casper, Wyoming. The Company was founded as McGinley Orthopaedic Innovations, LLC in 2012. The Company converted to a Wyoming Corporation on May 13, 2022 by filing Articles of Incorporation and a Statement of Conversion with the Wyoming Secretary of State (See Exhibit 2A “Articles of Incorporation and Other Documents”).
The minimum investment amount per Investor is One Hundred Dollars ($100.00), representing Nineteen and Four Hundredths (19.04) Shares at Five Dollars and Twenty-Five Cents ($5.25) per Share. The Company is run by a board of directors, comprised of one (1) director (the “Board” collectively, “Director” when referring to a director). The day-to-day management and investment decisions of the Company are vested in the Board and in the officers of the Company (the “Officers”).
Sales of the Shares pursuant to the Offering will commence immediately upon qualification of the Offering by the Securities and Exchange Commission (the “Effective Date”) and will terminate at the discretion of the Board or twelve (12) months following the Effective Date, whichever is earlier. The maximum amount of the Offering shall not exceed Forty Million Dollars ($40,000,000) in any twelve (12) month period (“Maximum Offering Amount”) in accordance with Tier II of Regulation A as set forth under the Securities Act of 1933, as amended, (“Reg A Tier II” or “Tier II”). The Company intends to offer the Shares described herein on a continuous and ongoing basis pursuant to Rule 251(d)(3)(i)(F). Further, the acceptance of Investor subscriptions, may be briefly paused at times to allow the Company to effectively and accurately process and settle subscriptions that have been received. (See “Terms of the Offering” below.) The Company may increase the Maximum Offering Amount at its sole and absolute discretion, subject to qualification by the SEC of a post-qualification amendment.
Prior to this Offering, there has been no public market for the Shares, and none is expected to develop. The Offering price is arbitrary and does not bear any relationship to the value of the assets of the Company. The Company does not currently have plans to list any Shares on any securities market, however reserves the right to do so in the future. Investing in the Company through the purchase of Shares involves risks, some of which are set forth below. See the section titled “Risk Factors” to read about the factors an Investor should consider prior to purchasing Shares.
Investors who purchase Shares will become shareholders of the Company (“Investors” or “Shareholders” subject to the terms of the Articles of Incorporation and the Bylaws of the Company (see Exhibit 2A “Articles of Incorporation and Other Corporate Documents” and Exhibit 2B “Bylaws”) once the Company deposits the Investor’s investment into the Company’s main operating account.
The Director/Officer will receive compensation from the Company as employees (see “Risk Factors” below starting on Page 5 and, “Compensation of Directors and Officers” below). Investing in the Shares is speculative and involves substantial risks, including risk of complete loss. Prospective Investors should purchase these securities only if they can afford a complete loss of their investment (see “Risk Factors” below starting on Page 6) .
As of the date of this Offering Circular, the Company has engaged KoreConX as transfer agent in relation to this Offering. The Company has engaged Wilmington Trust as Escrow Agent for this Offering.
NO PERSON HAS BEEN AUTHORIZED IN CONNECTION WITH THIS OFFERING TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS OTHER THAN THAT INFORMATION AND THOSE REPRESENTATIONS SPECIFICALLY CONTAINED IN THIS OFFERING CIRCULAR; ANY OTHER INFORMATION OR REPRESENTATIONS SHOULD NOT BE RELIED UPON. ANY PROSPECTIVE PURCHASER OF THE SECURITIES WHO RECEIVES ANY OTHER INFORMATION OR REPRESENTATIONS SHOULD CONTACT THE COMPANY IMMEDIATELY TO DETERMINE THE ACCURACY OF SUCH INFORMATION AND REPRESENTATIONS. NEITHER THE DELIVERY OF THIS OFFERING CIRCULAR NOR ANY SALES HEREUNDER SHALL, UNDER ANY CIRCUMSTANCES, CREATE AN IMPLICATION THAT THERE HAS BEEN NO CHANGE IN THE AFFAIRS OF THE COMPANY OR IN THE INFORMATION SET FORTH HEREIN SINCE THE DATE OF THIS OFFERING CIRCULAR SET FORTH ABOVE.
THE INFORMATION CONTAINED IN THIS OFFERING CIRCULAR HAS BEEN SUPPLIED BY THE COMPANY. THIS OFFERING CIRCULAR CONTAINS SUMMARIES OF DOCUMENTS NOT CONTAINED IN THIS OFFERING CIRCULAR, BUT ALL SUCH SUMMARIES ARE QUALIFIED IN THEIR ENTIRETY BY REFERENCES TO THE ACTUAL DOCUMENTS. COPIES OF DOCUMENTS REFERRED TO IN THIS OFFERING CIRCULAR, BUT NOT INCLUDED AS AN EXHIBIT, WILL BE MADE AVAILABLE TO QUALIFIED PROSPECTIVE INVESTORS UPON REQUEST.
RULE 251(D)(3)(I)(F) DISCLOSURE. RULE 251(D)(3)(I)((F) PERMITS REGULATION A OFFERINGS TO CONDUCT ONGOING CONTINUOUS OFFERINGS OF SECURITIES FOR MORE THAN THIRTY (30) DAYS AFTER THE QUALIFICATION DATE IF: (1) THE OFFERING WILL COMMENCE WITHIN TWO (2) DAYS AFTER THE QUALIFICATION DATE; (2) THE OFFERING WILL BE MADE ON A CONTINUOUS AND ONGOING BASIS FOR A PERIOD THAT MAY BE IN EXCESS OF THIRTY (30) DAYS OF THE INITIAL QUALIFICATION DATE; (3) THE OFFERING WILL BE IN AN AMOUNT THAT, AT THE TIME THE OFFERING CIRCULAR IS QUALIFIED, IS REASONABLY EXPECTED TO BE OFFERED AND SOLD WITHIN ONE (1) YEAR FROM THE INITIAL QUALIFICATION DATE; AND (4) THE SECURITIES MAY BE OFFERED AND SOLD ONLY IF NOT MORE THAN THREE (3) YEARS HAVE ELAPSED SINCE THE INITIAL QUALIFICATION DATE OF THE OFFERING, UNLESS A NEW OFFERING CIRCULAR IS SUBMITTED AND FILED BY THE COMPANY PURSUANT TO RULE 251(D) (3)(I)((F) WITH THE SEC COVERING THE REMAINING SECURITIES OFFERED UNDER THE PREVIOUS OFFERING; THEN THE SECURITIES MAY CONTINUE TO BE OFFERED AND SOLD UNTIL THE EARLIER OF THE QUALIFICATION DATE OF THE NEW OFFERING CIRCULAR OR THE ONE HUNDRED EIGHTY (180) CALENDAR DAYS AFTER THE THIRD ANNIVERSARY OF THE INITIAL QUALIFICATION DATE OF THE PRIOR OFFERING CIRCULAR. THE COMPANY INTENDS TO OFFER THE SHARES DESCRIBED HEREIN ON A CONTINUOUS AND ONGOING BASIS PURSUANT TO RULE 251(D)(3)(I)(F). THE COMPANY INTENDS TO COMMENCE THE OFFERING IMMEDIATELY AND NO LATER THAN TWO (2) DAYS FROM THE INITIAL QUALIFICATION DATE. THE COMPANY REASONABLY EXPECTS TO OFFER AND SELL THE SECURITIES STATED IN THIS OFFERING CIRCULAR WITHIN ONE (1) YEAR FROM THE INITIAL QUALIFICATION DATE.
The Company will commence sales of the Shares immediately upon qualification of the Offering by the SEC. The Company approximates that sales will commence during Q3 – 2022.
| | | Price to Public* | | Commissions** | | Proceeds to Other Persons*** | | Proceeds to the Company |
| Amount to be Raised per Share | | $ | 5.25 | | | $ | 0.22 | | | | 1.31 | | | $ | 3.71 | |
| Minimum Investment Amount | | $ | 100 | | | $ | 4.25 | | | $ | 25.00 | | | $ | 68.25 | |
| Minimum Offering Amount | | | N/A | | | | N/A | | | | N/A | | | | N/A | |
| Maximum Offering Amount | | $ | 40,000,000 | | | $ | 1,700,000 | | | $ | 10,000,000 | | | $ | 28,300,000 | |
*The Offering price to Investors was arbitrarily determined by the Board.
** The Company is not using an underwriter for the sale of Shares. These commissions listed are those for Rialto Markets, a FINRA broker-dealer. Rialto Markets is entitled to 3% on all passive sales of securities as placement agent. If securities are sold through the efforts of Rialto Markets, 8% will be due to Rialto Markets (instead of 3%) up to a maximum of $800,000 – for potential maximum commissions of $1,700,000. The commissions due to Rialto Markets are conditional on the services provided by Rialto Markets with respect to any one sale. See “Plan of Distribution “ below.
*** The Company intends to have selling shareholders as part of this Offering. See “Plan of Distribution” which includes a section named “Selling Shareholders” below.
FORWARD LOOKING STATEMENTS
Investors should not rely on forward-looking statements because they are inherently uncertain. Investors should not rely on forward-looking statements in this Offering Circular. This Offering Circular contains forward-looking statements that involve risks and uncertainties. The use of words such as “anticipated”, “projected”, “forecasted”, “estimated”, “prospective”, “believes”, “expects,” “plans”, “future”, “intends”, “should”, “can”, “could”, “might”, “potential”, “continue”, “may”, “will”, and similar expressions identify these forward-looking statements. Investors should not place undue reliance on these forward-looking statements, which may apply only as of the date of this Offering Circular, and the Company undertakes no obligation to publicly update or revise any forward-looking information, other than as required by applicable law.
TABLE OF CONTENTS
SUMMARY OF THE OFFERING
The following information is only a brief summary of, and is qualified in its entirety by, the detailed information appearing elsewhere in this Offering Circular. This Offering Circular, together with the exhibits attached including, but not limited to, the Articles of Incorporation and Company Bylaws, copies of which are attached hereto as Exhibit 2A and Exhibit 2B, respectively, and should be carefully read in their entirety before any investment decision is made. If there is a conflict between the terms contained in this Offering Circular and these documents, Articles of Incorporation and Bylaws shall prevail and control, and no Investor should rely on any reference herein to the Articles of Incorporation or Bylaws without consulting the actual underlying documents.
| COMPANY INFORMATION | | McGinley Orthopaedic Innovations Inc. with a principal place of business located at 234 E. 1st St. Suite 242, Casper, WY 82601. |
| MANAGEMENT | | The Company is managed by a Board of Directors. The Board is comprised of one (1) Director who also serves as the Company’s one (1) Officer. See “Risk Factors” and “Directors, Officers, and Significant Employees” below. |
| THE OFFERING | | This Offering is the first capital raise by the Issuer under Regulation A. The Company was previously operating as a Wyoming Limited Liability Company. The Company is selling Company equity in the form of Common Stock (the “Common Stock” or the “Shares”) through this Offering. The Company will use the Proceeds of this Offering to scale up its existing operations. See “Use of Proceeds” below |
| SECURITIES BEING OFFERED | | The Shares are being offered at a purchase price of $5.25 per Share. The Minimum Investment Amount for any Investor is $100.00. Upon purchase of the Shares, a Shareholder is granted (i) the right to vote on all matters subject to a Common Stock vote; and (ii) the right to receive dividends or disbursements, when the Board declares such dividends or disbursements. For a summary of the rights granted to Shareholders, see “Description of the Securities” below. |
| COMPENSATION TO DIRECTORS | | The Company pays the Director and Officer’s salary for his role as Director and Officer. For more information on this compensation see “Compensation of the Director and Officers” section below.
The Director, Officer, and employees of the Company will not be compensated through commissions for the sale of the Shares through this Offering. |
| PRIOR EXPERIENCE OF COMPANY MANAGEMENT | | The Director/Officer and significant employees have experience in technology company finance, operations, and manufacturing. See “Directors, Officers, and Significant Employees” below. |
| INVESTOR SUITABILITY STANDARDS | | The Shares will not be sold to any person unless they are a “Qualified Purchaser”. A Qualified Purchaser includes: (1) an “Accredited Investor” as that term is defined in Rule 501(a) of Regulation D promulgated under the Securities Act of 1933 (the “Securities Act”); or (2) all other Investors who meet the investment limitations set forth in Rule 251(d)(2)(i)(C) of Regulation A. Such persons as stated in (2) above must conform with the “Limitations on Investment Amount” as described in this Summary below.
Each person acquiring Shares may be required to represent that he, she, or it is purchasing the Shares for his, her, or its own account for investment purposes and not with a view to resell or distribute the securities.
Each prospective purchaser of Shares may be required to furnish such information or certification as the Company may require in order to determine whether any person or entity purchasing Shares is an Accredited Investor if such is claimed by the Investor.
|
| LIMITATIONS ON INVESTMENT AMOUNT | | For Qualified Purchasers who are Accredited Investors, there is no limitation as to the amount invested through the purchase of Shares. For non-Accredited Investors, the aggregate purchase price paid to the Company for the purchase of the Shares cannot be more than 10% of the greater of the purchaser’s (1) annual income or net worth as determined under Rule 501(a) of Regulation D, if purchaser is a natural person; or (2) revenue or net assets for the purchaser’s most recently completed fiscal year if purchaser is a non-natural person.
Different rules apply to Accredited Investors and non-natural persons. Each Investor should review Rule 251(d)(2)(i)(C) of Regulation A as determined under Rule 501(a) of Regulation D before purchasing the Shares. |
| COMMISSIONS FOR SELLING Shares | | The Shares will be offered and sold directly by the Company, the Board, the Officers, and Company’s employees. No commissions for selling the Shares will be paid to the Company, the Board, the Officers, or the Company’s employees.
The Company is not using an underwriter for the sale of Shares. The commissions listed are those for Rialto Markets, a FINRA broker-dealer. Rialto Markets is entitled to 3% on all passive sales of securities as placement agent. If securities are sold through the efforts of Rialto Markets, 8% will be due to Rialto Markets (instead of 3%) up to a maximum of $800,000 – for potential maximum commissions of $1,700,000. The commissions due to Rialto Markets are conditional on the services provided by Rialto Markets with respect to any one sale. See “Plan of Distribution “ below. |
| NO LIQUIDITY | | There is no public market for the Shares, and none is expected to develop in the near future. Additionally, the Shares will be transferable, in accordance with Federal and state securities laws, and Wyoming law. However, the Shares will not be listed for trading on any exchange or automated quotation system. (See “Description of the Securities” below.) Prospective Investors are urged to consult their own legal advisors with respect to secondary trading of the Shares. See “Risk Factors” below. |
| SELLING SECURITYHOLDERS | | 1,904,761.9 Shares of Common Stock are being offered for the account of Selling Shareholders. This represents 4.21% of the outstanding Shares of Common Stock as of the Date of this Offering Circular. |
| BONUS SHARES | | The Company is offering Bonus Shares as part of this Offering. This includes discounts from 3% to 55% under certain circumstances. See “Plan of Distribution” below. |
| COMPANY EXPENSES | | Except as otherwise provided herein, the Company shall bear all costs and expenses associated with the Offering, the operation of the Company, including, but not limited to, the annual tax preparation of the Company's tax returns, any state and federal income tax due, accounting fees, filing fees, independent audit reports, other costs and expenses, and other advisory fees. |
The Company’s has two primary product lines, the (i) IntelliSense Drill Technologyâ Drill and accessories line of products; and the (ii) Lever Action Plate Systemâ line of products.
The IntelliSense Drill Technology® and accessories will be referred to as the “IntelliSense Drill(s)” or “IntelliSense products”. Please see “Description of the Business” below for a list of what products are included in these terms.
The Lever Action Plate Systemâ line of products will be referred to as “LAPS” or “LAPS products”.
Both “IntelliSense Drill Technology" and “Lever Action Plate System” are registered trademarks owned by the Company. This Offering Circular will not continue to incorporate the “®“ symbol to identify these registered trademarks as such for the sake of simplicity and reserve all rights.
Collectively the IntelliSense products and LAPS products will be referred to collectively as the (the “Products”).
RISK FACTORS
The SEC requires the Company to identify risks that are specific to its business and its financial condition. The Company is still subject to all the same risks that all companies in its business, and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and technological developments (such as hacking and the ability to prevent hacking). Additionally, early-stage companies are inherently riskier than more developed companies. You should consider general risks as well as specific risks when deciding whether to invest.
Risks Related to the Company and Its Business
The Company has not been profitable since its inception.
The Company had net losses of approximately $2,858,000 and $2,200,000 for the years ended December 31, 2021 and 2020, respectively, as well as negative cash flow from operations of approximately $2,055,000 and $1,485,000, respectively. See “Part F/S”, below, and the audit report included therein. There is no assurance that the Company will ever be profitable or generate sufficient revenues to operate its business or pay dividends.
The loss of any member of the Company’s management team or their inability to attract and retain highly skilled scientists, engineers, and salespeople could adversely affect company business.
Company success depends on the skills, experience and performance of key members of the Company’s management team, including the Officer and Director. The individual and collective efforts of the Company’s sole Officer/Director and its significant employees will be essential as the Company continues to develop the Company and Products.
Company research and development and manufacturing operations depend on the Company’s ability to attract and retain highly-skilled employees including scientists, technicians and engineers, marketing managers and salespersons. The Company may not be able to attract or retain a sufficient number of qualified highly-skilled employees in the future due to the competition for qualified personnel in the medical device industry. The Company also faces competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. Recruiting and retention difficulties can limit the ability to support Company. Other than for the Chief Executive Officer, the Company also does not maintain “key person” insurance on any of Company employees.
The Company may not be able to scale the Company’s Products on the anticipated timetable.
The Company’s Products may be difficult to scale to a commercially viable level since it must meet expectations that it is equivalent or superior to traditional orthopedic drills and implants in terms of efficacy, safety, and cost efficiency. The Company will continue to develop and refine the Products and their manufacturing processes to ensure the Products, and future iterations of the Products, meet performance goals and cost targets. The Company may need to perform additional laboratory and clinical trials in the future, and may encounter problems and delays. If future clinical trials reveal technical defects or reveal that the Company’s Products do not meet performance goals and cost targets, the Company’s commercialization schedule could be delayed as the Company attempts to devise solutions to the defects or problems. If the Company is unable to find solutions, the Company’s business may not be as successful.
The Company may not be able to successfully execute the business plan.
In addition to the requirement to successfully develop, manufacture, and market the Products for commercial success, the Company must also raise significant amounts of capital, foster relationships with key suppliers and attract customers. There is no guarantee that the Company will be able to achieve or sustain any of the foregoing within the anticipated timeframe or at all. Even though the Company’s principals are long-time industry professionals, who have been working for some time building relationships in the orthopedics industry, the Company may exceed the budget, encounter obstacles in research and development activities, or be hindered or delayed in implementing the Company’s commercialization plans, any of which could imperil the Company’s ability to secure customer contracts and begin generating significant revenues. In
addition, any such delays or problems may require the Company to secure additional funding over and above what the Company currently anticipates it requires in order to sustain business, which the Company may not be able to raise.
The Company’s Products may not achieve market acceptance for its Products thereby reducing the chance for success.
The Company is in the early stages of selling the Products. Unanticipated events may result in lower sales than anticipated, which could force the Company to limit expenditures on research and development, advertising, and general Company requirements for improving and expanding Product offerings. The Company cannot guarantee market demand or interest in the current Products or future product offerings, which could have a material adverse effect on business, results of operations, and overall financial condition.
The Company has only two main product lines.
The Company’s primary products are the (i) IntelliSense products and (ii) Lever Action Plate System products. These are two distinct products with disparate design and manufacturing paths. The Company may sell or license either or both of these product lines. The Company’s survival in the near term depends on being able to sell these Products to a sufficient number of customers to make a profit. The Company’s current customer base is still small, and the Company will only succeed if it can attract more customers for its primary product and maintain those customers.
The Company has not yet generated profits and it may take a long time for the Company to become profitable.
The Company has not yet generated any profit from the sale of its Products. The Company is working towards having sales of the Products, but the Company anticipates that upon a successful Offering, it will take time to achieve profitability, if at all.
The Company’s ability to raise capital and to commercialize the Products may be materially impacted by the ongoing COVID-19 pandemic.
The Company’s ability to raise capital and to commercialize the Products may be materially impacted by the ongoing COVID-19 pandemic. The full impact on the economy and the capital markets in the U.S. and the rest of the world from the COVID-19 pandemic are uncertain, in terms of both scale and duration. The high level of volatility in the capital markets may make it difficult to raise funds, especially for early stage companies that involve higher risk. If the Company raises sufficient funds to commercialize its Products, the Company may have difficulty securing supplies needed or manufacturing and distribution partners. The impact of social distancing measures and related workforce reductions may negatively impact the ability of suppliers to deliver the Company to the components the Company needs for manufacture or the ability of any of the Company’s potential partners to operate effectively to meet Company requirements. The Company cannot assure an Investor that, should it raise sufficient funds, it will be able to contract with suppliers, manufacturing partners or distribution partners at a level that would allow the Company to achieve profitability, or at all.
If the market chooses to buy competing Products, the Company may fail.
Although the Company believes that its Products will be commercially viable, there is no verification by the marketplace that the Products will be accepted by or purchased by customers at the scale desired by the Company The market may choose existing competitor products to those of the Company. If the market chooses to continue to use competing products, it may be more difficult for the Company to ever become profitable which would be substantially harm the business and, possibly, cause it to fail whereby the Investors could lose their entire investment. In addition to Company dependency on their continued services, Company future success will also depend on the ability to attract and retain additional future key personnel. The Company may face intense competition for such qualified individuals from well-established and better financed competitors. The Company may not be able to attract qualified new employees or retain existing employees, which may have a material adverse effect on the Company’s results of operations and financial condition.
The medical device industry is subject to rapidly changing technology which could make the Products and other products the Company develops obsolete.
The Company’s industry is characterized by rapid technological changes, frequent new product introductions and enhancements and evolving industry standards, all of which could make the Company’s Products obsolete. The Company’s future success will depend on its ability to anticipate and keep pace with the evolving needs of the industry on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of technological and scientific advances. The attractiveness of the Company’s Products partly depends on the ability to continue to improve the Products. Failure to deliver such improvements in the timelines suggested may affect the Company’s business plan and ability to obtain greater market penetration, or otherwise cause the Company to lose market share.
The Company may require additional funding to develop and commercialize the Products. If the Company is unable to secure additional financing on acceptable terms, or at all, the Company may be forced to modify its current business plan or to curtail Company planned operations.
The Company anticipates incurring significant operating losses and using significant funds for marketing the Products, and manufacturing capability scaling. The Company’s existing cash resources are insufficient to finance these operations. Accordingly, the Company will need to secure additional sources of capital to develop its business and the Products as planned. In the event that the Proceeds from this Offering are not sufficient to successfully commercialize the Products, the Company may seek to secure future capital. The Company may seek substantial additional financing through public and/or private financing, which may include equity and/or debt financings, and through other arrangements, including collaborative arrangements. As part of such efforts, the Company may seek loans from certain of their executive Officers and Director(s).
If the Company is unable to secure additional financing in the near term, the Company may be forced to: (1) curtail or abandon the Company’s existing business plans; (2) default on any debt
obligations; (3) file for bankruptcy; (4) seek to sell some or all of Company assets; and/or (4) cease business operations.
If the Company is forced to take any of these steps, the Company’s Common Stock may lose significant value or become worthless.
Any future financing may result in ownership dilution to the Company’s existing Shareholders and may grant rights to future investors more favorable than the rights currently held by the Company’s existing Shareholders.
If the Company raises additional capital by issuing equity, equity-related, or convertible securities, the economic, voting and other rights of the Company’s existing Shareholders may be diluted, and those newly-issued securities may be issued at prices that are at a significant discount to current and/or then fair market value of the Shares. In addition, any such newly issued securities may have rights superior to those of the Company’s Common Stock as offered through this Offering. If the Company obtains additional capital through collaborative arrangements, the Company may be required to relinquish rights to technologies or its Products than the Company might otherwise have or become subject to restrictive covenants that may affect business.
Risks Related to Development and Regulatory Clearance of The Company’s Products
The FDA, other regulatory bodies and other comparable foreign regulatory authorities each have substantial discretion in the clearance process for the Company’s Products and may either refuse to consider the Company’s application(s) for review or may form the opinion after review of the data that the Company’s application is insufficient to grant clearance or authorization for commercial use.
The Company has received the necessary FDA clearances to commercialize its existing Products. However, the FDA and other comparable foreign regulatory authorities each have substantial discretion in the clearance process and may either refuse to consider the Company’s future applications or may form the opinion after review of the Company’s application(s) that the application is insufficient to allow clearance of for human health applications. Clearance procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in United States. Moreover, any clearances that the Company obtains may not cover all of the clinical indications for which the Company will seek clearance, or could contain significant limitations in the form of narrow indications, warnings, precautions or contra-indications with respect to conditions of use. In such an event, the Company’s ability to generate revenues from the Products would be greatly reduced and its business would be harmed.
Failure to obtain clearance to market the Company product for human health applications may limit Company prospects for growth.
The Company may require clearance from the FDA and similar agencies in other countries prior to marketing new products which are under development . The Company will need to establish, to the satisfaction of those organizations, that its new Products are safe for use. Because the Company’s Products are novel and represents a departure from the status quo, the Company cannot assure Investors that the Company will receive clearance.
Risks Related to the Securities and the Offering
There is no current market for the Shares.
There is no formal marketplace for the resale of the Company’s Shares and none is expected to arise for the foreseeable future. Investors should assume that they may not be able to liquidate their investment.
The Offering price of the Company’s Shares was not established on an independent basis; the actual value of an investment may be substantially less than what Investor pays for the securities.
The Company’s Board of Directors established the Offering price of the Company’s Shares on an arbitrary basis. The Offering price of the Shares bears no relationship to the book or asset values or to any other established criteria for valuing Shares. Because the Offering price is not based upon any independent valuation, the Offering price may not be indicative of the proceeds that an Investor would receive upon liquidation. Further, the Offering price may be significantly more than the price at which the Shares would trade if they were to be listed on an exchange or actively traded by broker-dealers.
McGinley Orthopaedic Innovations, Inc. has existing Patents that the Company might not be able to protect.
The Company's most valuable assets are its intellectual property. It holds 71 United States utility patents with approximately 20 pending. It is possible that competitors may attempt to misappropriate or violate intellectual property rights owned by the Company. The Company intends to protect its intellectual property portfolio from such violations, within the constraints of available resources. It is important to note that unforeseeable costs associated with such practices may consume a significant portion of capital, which could negatively affect the Company’s research and development efforts and business, in general. However, the Company has anticipated the possible need to protect its Patents from infringement and therefore has allocated Proceeds from this Offering, in the form of Working Capital, to account for these potential occurrences.
The Company is controlled by Joseph McGinley MD/PhD, Co-Founder and the sole Director and Officer
Dr. Joseph McGinley is the beneficial owner of a majority of the Company’s Shares of Common Stock as of the date of this Offering Circular. Upon a successful Offering (where the Maximum Offering Amount is raised) Dr. Jospeh McGinley will still own a majority of the Company’s voting Shares. Investors in this Offering will not have a majority of voting Shares and therefore will not have the ability to control a vote of the Shareholders without consensus from Dr. Joseph McGinley. Dr. Joseph McGinley, therefore, has complete control as to the direction of the Company. There is a disproportionate reliance on Dr. Joseph McGinley
for the operation of the Company, and therefore a risk that the direction of the Company may change if Dr. McGinley is unable to perform his duties as Director and Officer.
Bonus Shares have a potentially dilutive effect.
As part of this Offering, the Company may offer Bonus Shares to certain investors (See “Plan of Distribution” below). There is the potential that the Company sells the Shares solely through investments subject to the award of Bonus Shares. In the event the Company sells the Shares solely through investments subject to the award of Bonus Shares, the Company could raise gross Proceeds equaling $40,000,000 million or 100% of the Maximum Offering Amount. In the event the Company sells the Shares in amounts less than the total number of Shares offered, the Bonus Shares may still have a dilutive effect to Investors in this Offering.
The Company will not absorb the cost of the issuance of the Bonus Shares; to the extent any are issued, it will not reduce the Proceeds that the Company receives. The Company will issue the Bonus Shares from its authorized Shares. The issuance of these Bonus Shares will have a maximum potential dilutive effect of 50% of the Shares offered for purchase or 3,809,523.81 Bonus Shares, meaning the company is Offering a total of 11,428,571.43 Shares through this Offering.
DILUTION
On May 13, 2022 the Company converted from a Wyoming limited liability company, McGinley Orthopaedic Innovations, LLC, to a Wyoming corporation, McGinley Orthopaedic Innovations, Inc. This conversion was executed through the filing of a Statement of Conversion with the Wyoming Secretary of State (see Exhibit 2A “Articles of Incorporation and Other Corporate Documents”). The Shareholders of the Company received a 1:1 conversion of their LLC Membership Units to Common Stock.
The proportions of ownership by the Director and Officer remained the same as prior to the conversion. However, on various dates additional Shares were accrued but not issued to the Director or Officer within the past calendar year pursuant to the table below:
| NAME | | DATE ACCRUED | | NUMBER OF SHARES ISSUED/ACCRUED | | VALUE OF SHARES ISSUED/ACCRUED |
| Joseph McGinley | | 3/31/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 6/17/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 9/24/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 12/31/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 2/17/2022 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 5/15/2022 | | | 51,282.05 | | | $ | 1.95 | |
| Total Shares Issued/Accrued | | | | | 83,333.35 | | | $ | 162,500.03 | |
All of the Shares above were accrued at the price of $1.95 per Share. When the above persons desire to have the Shares issued, they will be required to pay $1.95 to receive the Shares.
The Company may engage in other financings including future equity raises. In the event the Company sells equity securities subsequent to an Investor’s purchase of Shares through this Offering or future offerings, the Investor’s proportionate ownership of the Company will be diluted.
PLAN OF DISTRIBUTION
The Offering will be made through general solicitation, direct solicitation, and marketing efforts whereby Investors will be directed to the investment website (invest.mcginleyorthopaedics.com) to invest. The Company has engaged Rialto Markets, an independent FINRA broker-dealer to assist with the Share sales in exchange for a 3% commission fee on the aggregate sales. The Offering is conducted on a best-efforts basis. No Commissions or any other renumeration for the Share sales will be provided to the Company, the Directors, any Officer, or any employee of the Company, relying on the safe harbor from broker-dealer registration set forth in Rule 3a4-1 under the Securities Exchange Act of 1934, as amended.
The Company will not limit or restrict the sale of the Shares during this 12-month Offering. No market exists for the Shares and no market is anticipated or intended to exist in the near future, therefore there is no plan to stabilize the market for any securities to be offered.
Directors, Officers, and employees of the Company are primarily engaged in the Company’s business of developing, manufacturing, and selling medical devices, and none of them are, or have ever been, brokers nor dealers of securities. The Directors, Officers, and employees will not be compensated in connection with the sale of securities through this Offering. The Company believes that the Directors, Officers, and employees are associated persons of the Company not deemed to be brokers under Exchange Act Rule 3a4-1 because: (1) no Director, Officer, or employee is subject to a statutory disqualification, as that term is defined in section 3(a)(39) of Exchange Act at the time of their participation; (2) no Director, Officer, or employee will be compensated in connection with his participation by the payment of commissions or by other renumeration based either directly or indirectly on transactions in connection with the sale of securities through this Offering; (3) no Director, Officer, or employee is an associated person of a broker or dealer; (4) the Directors, Officers, and employees primarily perform substantial duties for the Company other than the sale or promotion of securities; (5) no Director, Officer, or employee has acted as a broker or dealer within the preceding twelve months of the date of this Offering Circular; (6) no Director, Officer, or employee will participate in selling this Offering after more than twelve months from the Effective Date of the Offering.
Rialto Markets LLC (“Rialto”) has agreed to act as placement agent to assist in connection with this Offering. Rialto is not purchasing or selling any securities offered by this Offering Circular, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities. However, Rialto has agreed to use their best efforts to arrange for the sale of the Shares offered through this Offering Circular. In addition, Rialto may engage other brokers to sell the securities on their behalf. Rialto will receive compensation for all passive sales of the Shares offered and sold through pursuant to this Offering at a rate of 3% of the gross Proceeds for a maximum of $900,000. The Company may pay Rialto 8% of the gross Proceeds from the sale of up to $10,000,000 in Common Stock resulting from the direct selling efforts of Rialto not to exceed $800,000.
In the event that Rialto’s targeted selling efforts lead to sales of up to $10,000,000 in Shares of Common Stock, Rialto will be entitled to 8% of the gross Proceeds from the sale of such Shares of Common Stock not to exceed $800,000. If Rialto’s efforts lead to all $10,000,000, the maximum commissions to be charged would be $1,700,000. The $1,700,000 is made up of 8% of $10,000,000, or $800,000, and 3% on the remaining $30,000,000 for $900,000. There will not be any commissions charged at a combined 11%.
The Company will also publicly market the Offering using general solicitation through methods that include emails to potential Investors, the internet, social media, and any other means of widespread communication.
The Offering Circular will be furnished to prospective investors via download 24 hours per day, 7 days per week on the Company’s website at invest.mcginleyorthopaedics.com and via of the EDGAR filing system. The following table shows the total discounts and commissions payable to Rialto in connection with this Offering by the Company.
In the event that Rialto’s targeted selling efforts lead to sales up to $10,000,000 in Shares of Common Stock, Rialto will be entitled to 8.0% of the Gross Proceeds from the sale of such shares of Common Stock not to exceed $800,000.
| | | Price Per Share | | Total Offering |
| Public Offering Price | | $ | 5.25 | | | $ | 40,000,000 | |
| Placement Agent Commissions | | $ | 0.22* | | | $ | 1,700,000* | |
| Proceeds, Before Expenses | | $ | 4.90 | | | $ | 38,300,000 | |
*this represents the maximum potential commissions due to Rialto, the commissions actually due may be less than this number conditional on the success of Rialto’s targeted sales efforts.
Other Terms
Rialto has also agreed to perform the following services in exchange for the compensation discussed above:
- Act as lead broker for the Offering, coordinating efforts of parties involved and providing regulatory guidance;
- Manage the back-end process of the Offering Platform technology that Investors use to invest in the Offering;
- Reviewing marketing materials if requested;
- Performing AML/KYC checks on all Investors, and;
- Providing other financial advisory services normal and customary for Regulation A offerings and coordinate with the Company’s registered transfer agent and legal representatives.
In addition to the commissions described above, the Company will also pay $6,500 to Rialto for out-of-pocket accountable expenses paid prior to commencing the Offering. This fee will be used for the purpose of coordinating filings with FINRA (Form 5110). In addition, the Company will pay Rialto $10,000 consulting fee upon the issuance of the FINRA No Objection Letter and a $5,000 Blue Sky filing service fee for managing the filings required for Blue Sky regulations. The Company will forward the fees required for state notice filing fees, estimated to be approximately $13,000. Assuming the full amount of the offering is raised and that Rialto's targeted selling efforts lead to sales of $10,000,000, the Company estimates that the total commissions of the Offering payable by the Company to Rialto will be approximately $1,700,000. Maximum expected out of pocket expenses total $34,500.
Selling Shareholders
| Selling Shareholders | | Common Shares Held Prior to Offering | | Common Shares Offered for Sale | | Common Shares Held After Offering |
| Achreja, Jivan Singh | | | 148,470.35 | | | | 113,917.72 | | | | 34,552.64 | |
| Anderson, David | | | 25,641.03 | | | | 20,512.82 | | | | 5,128.21 | |
| Becker, Garry | | | 13,661.20 | | | | 1,400.00 | | | | 12,261.20 | |
| Bishop, David F. | | | 38,461.54 | | | | 10,000.00 | | | | 28,461.54 | |
| Biswal, Sandip | | | 191,380.16 | | | | 38,276.03 | | | | 153,104.13 | |
| Brookstein, David | | | 15,337.42 | | | | 12,269.94 | | | | 3,067.48 | |
| Brus, Cary and Karen | | | 110,000.00 | | | | 40,000.00 | | | | 70,000.00 | |
| Chan , Bryan | | | 10,000.00 | | | | 8,000.00 | | | | 2,000.00 | |
| Connor , Kathleen | | | 70,333.69 | | | | 5,000.00 | | | | 65,333.69 | |
| Cook, Thomas | | | 10,000.00 | | | | 5,000.00 | | | | 5,000.00 | |
| Cross Jr., Gregory H. | | | 15,337.42 | | | | 13,803.68 | | | | 1,533.74 | |
| Cubin, Eric | | | 137,356.42 | | | | 120,888.54 | | | | 16,467.88 | |
| Cubin, Jonna | | | 47,500.00 | | | | 42,750.00 | | | | 4,750.00 | |
| Cundy, Bradley A. | | | 10,928.96 | | | | 8,743.17 | | | | 2,185.79 | |
| Davis, Tad | | | 111,336.01 | | | | 30,000.00 | | | | 81,336.01 | |
| D'elia, Joshua D. | | | 32,269.94 | | | | 29,042.95 | | | | 3,226.99 | |
| DeVore, Robert | | | 27,322.40 | | | | 10,000.00 | | | | 17,322.40 | |
| Domsic, James D. | | | 46,283.54 | | | | 25,000.00 | | | | 21,283.54 | |
| Domsic, Joseph J. | | | 35,875.38 | | | | 15,000.00 | | | | 20,875.38 | |
| Duerloo, Brian | | | 34,013.60 | | | | 15,000.00 | | | | 19,013.60 | |
| Edwards, Bill | | | 13,661.20 | | | | 6,830.60 | | | | 6,830.60 | |
| Ekis, James L. | | | 12,269.94 | | | | 6,000.00 | | | | 6,269.94 | |
| Ellis, Dennis | | | 13,661.20 | | | | 10,928.96 | | | | 2,732.24 | |
| Emery, Richard | | | 21,857.92 | | | | 17,486.34 | | | | 4,371.58 | |
| Finch, Mark L. | | | 17,006.80 | | | | 1,700.00 | | | | 15,306.80 | |
| Flaherty, Michael | | | 486,072.16 | | | | 125,000.00 | | | | 361,072.16 | |
| French, Dan | | | 10,000.00 | | | | 9,000.00 | | | | 1,000.00 | |
| Gillem, Michael T. | | | 41,821.72 | | | | 20,910.86 | | | | 20,910.86 | |
| Giriech, Michael | | | 12,269.94 | | | | 11,042.95 | | | | 1,226.99 | |
| Goodreau, Randall G. | | | 15,337.42 | | | | 7,500.00 | | | | 7,837.42 | |
| Graham, Travis | | | 15,337.42 | | | | 13,803.68 | | | | 1,533.74 | |
| Grunfeld, Robert | | | 26,455.03 | | | | 21,164.02 | | | | 5,291.01 | |
| Hays, Michael | | | 22,354.50 | | | | 11,628.84 | | | | 10,725.66 | |
| Healey, Nicholas | | | 22,269.94 | | | | 5,567.49 | | | | 16,702.45 | |
| Higgins, Patrick | | | 163,934.43 | | | | 131,147.54 | | | | 32,786.89 | |
| Hogan, Brian | | | 15,337.42 | | | | 13,803.68 | | | | 1,533.74 | |
Selling Shareholders (continued)
| Selling Shareholders | | | Common Shares Held Prior to Offering | | | | Common Shares Offered For Sale | | | | Common Shares Held After Offering | |
| Hogan, John F. | | | 82,344.23 | | | | 25,000.00 | | | | 57,344.23 | |
| Ivanov, Oleg | | | 50,000.00 | | | | 45,000.00 | | | | 5,000.00 | |
| Jackson, Lisa | | | 10,000.00 | | | | 9,000.00 | | | | 1,000.00 | |
| King , Jackie | | | 17,006.80 | | | | 15,306.12 | | | | 1,700.68 | |
| Kozin M.D., William | | | 50,674.85 | | | | 10,000.00 | | | | 40,674.85 | |
| Larsen , Dr. Aaron | | | 20,000.00 | | | | 4,000.00 | | | | 16,000.00 | |
| Larsen , Jerrold L. | | | 50,000.00 | | | | 15,000.00 | | | | 35,000.00 | |
| Larsen , Nicholas | | | 20,000.00 | | | | 5,000.00 | | | | 15,000.00 | |
| Larsen , Ryan | | | 20,000.00 | | | | 18,000.00 | | | | 2,000.00 | |
| Leachtenauer, Paul C. | | | 141,601.81 | | | | 15,000.00 | | | | 126,601.81 | |
| Leaseke , Paul | | | 30,463.92 | | | | 15,000.00 | | | | 15,463.92 | |
| Logan, Particia | | | 10,000.00 | | | | 9,000.00 | | | | 1,000.00 | |
| Mamot, Keith | | | 20,000.00 | | | | 18,000.00 | | | | 2,000.00 | |
| Mamot, Michael | | | 10,000.00 | | | | 9,000.00 | | | | 1,000.00 | |
| Mansell, Donna J. | | | 13,661.20 | | | | 10,928.96 | | | | 2,732.24 | |
| McConnell, Rita | | | 13,661.20 | | | | 1,400.00 | | | | 12,261.20 | |
| Mills, Andy | | | 27,210.88 | | | | 6,802.72 | | | | 20,408.16 | |
| Moulds, Elizabeth | | | 136,612.02 | | | | 50,000.00 | | | | 86,612.02 | |
| Novick, Robert | | | 100,000.00 | | | | 10,000.00 | | | | 90,000.00 | |
| Patnaik, Goutam | | | 112,881.07 | | | | 22,577.00 | | | | 90,304.07 | |
| Porter , Peg Connor | | | 46,012.27 | | | | 4,600.00 | | | | 41,412.27 | |
| Poullos, Peter | | | 94,027.80 | | | | 18,000.00 | | | | 76,027.80 | |
| Power, Joanne | | | 19,125.68 | | | | 15,300.54 | | | | 3,825.14 | |
| Quinn, Brian R. | | | 16,802.72 | | | | 3,500.00 | | | | 13,302.72 | |
| Reiger, Mark A. | | | 13,661.20 | | | | 10,928.96 | | | | 2,732.24 | |
| Robinson, Jimmy D. | | | 22,269.94 | | | | 20,000.00 | | | | 2,269.94 | |
| Rudd, Gary | | | 20,464.48 | | | | 6,800.00 | | | | 13,664.48 | |
| Sadeghipour, Keyanoush | | | 38,201.08 | | | | 10,000.00 | | | | 28,201.08 | |
| Schilling, William C. | �� | | 16,802.72 | | | | 15,122.45 | | | | 1,680.27 | |
| Schlidt, Robert | | | 225,270.44 | | | | 100,000.00 | | | | 125,270.44 | |
| Six, Randall G. | | | 13,661.20 | | | | 10,928.96 | | | | 2,732.24 | |
| Sulser, Daniel F. | | | 1,827,626.37 | | | | 100,000.00 | | | | 1,727,626.37 | |
| Tarka, Christopher J. | | | 26,455.03 | | | | 21,164.02 | | | | 5,291.01 | |
| Tarka, Elizabeth Ann | | | 26,455.03 | | | | 21,164.02 | | | | 5,291.01 | |
| Tobin, Robert | | | 179,829.26 | | | | 149,208.43 | | | | 30,620.83 | |
| Tomlinson, David J. | | | 13,661.20 | | | | 10,928.96 | | | | 2,732.24 | |
| Toussaint, R. James | | | 25,641.03 | | | | 20,512.82 | | | | 5,128.21 | |
| Walker, Thomas J. | | | 17,006.80 | | | | 15,306.12 | | | | 1,700.68 | |
| Wells, Donald | | | 54,948.04 | | | | 15,200.00 | | | | 39,748.04 | |
| Wilkinson DPM, Michael P. | | | 40,000.00 | | | | 20,000.00 | | | | 20,000.00 | |
| Workman, Ernest | | | 93,926.02 | | | | 23,962.02 | | | | 69,964.00 | |
| Grand Total | | | 5,809,122.38 | | | | 1,904,761.90 | | | | 3,904,360.48 | |
The Company is offering 1,904,761.90 Shares for the accounts of 74 Selling Shareholders, as described in the table above. This represents approximately 4.21% of the outstanding Common Shares as of the date of this Offering Circular. The Shares on account of Selling Shareholders will not be sold from the beginning of the Offering but in accordance with the schedule described below:
- The first $5,000,000 of the net Proceeds (defined below) will be allocated 100% to the Company;
- The next $5,000,000 of the net Proceeds will be allocated 80% to the Company and 20% to the accounts of Selling Shareholders who purchased Shares for cash in one or more of the Company’s prior offerings; and,
- The remaining $30,000,000 of the net Proceeds will be allocated 70% to the Company and 30% to the accounts of all of the Selling Shareholders.
The term “net Proceeds” refers to the gross Proceeds received by the Company less any commissions and other Offering fees. (See discussion on this “Plan of Distribution” above).
Bonus Share Program
Certain Investors will be eligible to receive additional Shares of Common Stock (“Bonus Shares”) depending upon the amount invested by such Investors. The Company will not absorb the cost of the issuance of the Bonus Shares; to the extent any are issued, it will not reduce the Proceeds that the Company receives. The Company will issue the Bonus Shares from its authorized Shares. The issuance of these Bonus Shares will have a maximum potential dilutive effect of 55% consisting of (1) the Bonus Shares issued pursuant to investment amount for a maximum of 50% (3,809,523.81 Bonus Shares); and, (2) Bonus Shares issued pursuant to time of investment for a maximum of 5% (380,952.38 Bonus Shares) - meaning the Company is Offering a total of 11,809,523.81 Shares through this Offering.
Bonus Shares issued pursuant to investment amount
The following table describes the ratio of Bonus Shares due to an Investor based on the size of initial investment:
| A | B |
| Investment Amount | Ratio of Bonus Shares |
| $5,000 - $24,999.99 | | 3% |
| $25,000- $49,999.99 | | 5% |
| $50,000 - $99,999.99 | | 10% |
| $100,000 - $499,999.99 | | 15% |
| $500,000 - $999,999.99 | | 25% |
| $1,000,000 and above | | 50% |
For clarity, the number of Bonus Shares owed to an Investor as per the table above equals the number of Shares owed to the Investor upon purchase at the stated Investment Amount ranges in column A multiplied by the percentage in column B. For example, if an Investor purchases $5,250 worth of Shares, the Investor will be awarded 30 Bonus Shares, for a total of 1,030 Shares.
Bonus Shares issued pursuant to time of investment
In addition to the Bonus Shares issued pursuant to investment amount – if an Investor invests within the first six weeks following the start of the Offering, that Investor will be granted a bonus of 5% Bonus Shares. These Bonus Shares are in addition to any Bonus Shares issued pursuant to investment amount. For example, if an Investor invests $5,250 within the first six weeks of the Offering, that Investor will receive 1,080 Shares.
USE OF PROCEEDS
| | | | 25% | | | | 50% | | | | 75% | | | | 100% | |
| �� | | | | | | | | | | | | | | | | |
| 1. Marketing/Sales/Distribution | | $ | 2,500,000 | | | $ | 3,500,000 | | | $ | 6,000,000 | | | $ | 7,000,000 | |
| 2. Finance/Administrative | | $ | 300,000 | | | $ | 500,000 | | | $ | 1,000,000 | | | $ | 1,750,000 | |
| 3. Product Development | | $ | 200,000 | | | $ | 500,000 | | | $ | 1,500,000 | | | $ | 1,500,000 | |
| 4. Quality/Compliance | | $ | 100,000 | | | $ | 200,000 | | | $ | 500,000 | | | $ | 750,000 | |
| 5. Planning/Partnerships/Acquisitions | | $ | 100,000 | | | $ | 300,000 | | | $ | 2,000,000 | | | $ | 5,000,000 | |
| 6. Investor Acquisition | | $ | 1,000,000 | | | $ | 2,000,000 | | | $ | 3,000,000 | | | $ | 4,000,000 | |
| 7. Working Capital | | $ | 4,400,000 | | | $ | 8,000,000 | | | $ | 8,000,000 | | | $ | 8,000,000 | |
| 8. PPE Expansion | | $ | 400,000 | | | $ | 1,000,000 | | | $ | 1,000,000 | | | $ | 2,000,000 | |
| 9. Proceeds to Selling Shareholders | | $ | 1,000,000 | | | $ | 4,000,000 | | | $ | 7,000,000 | | | $ | 10,000,000 | |
| Total | | $ | 10,000,000 | | | $ | 20,000,000 | | | $ | 30,000,000 | | | $ | 40,000,000 | |
Upon successful Offering whereby the Company receives gross Proceeds equaling $40,000,000, the Company expects to use the Proceeds as shown in (a) the categories defined in the left-hand column of the table above and (b) in the amounts shown in the table’s right-most column. Deployment of partial Proceeds are shown in the middle columns labeled 25%, 50%, and 75% from left to right. Investment in each of the categories and amounts shown in the table above are anticipated to be as follows.
THE COMPANY RESERVES THE RIGHT TO CHANGE ITS USE OF THE PROCEEDS AT ANY TIME, AT THE SOLE DISCRETION OF THE COMPANY, ITS DIRECTOR(S), OR OFFICERS WITHOUT PRIOR NOTICE TO SHAREHOLDERS OR POTENTIAL INVESTORS.
1. Marketing/Sales/Distribution/Customer Service $7,000,000
The Company anticipates to devote up to $7,00,000 of the Proceeds in the branding, marketing, selling, distribution, and customer service/training of its products to hospitals, surgical centers, and individual orthopedic surgeons. This will include the expansion of the Company’s sales force, support employees, educational materials, marketing materials, and online and in-person advertising.
2. Finance/Administration $1,750,000
The Company will use up to $1,750,000 of the Proceeds for staffing and equipping its finance/accounting and administrative departments. Finance and accounting will require resources in treasury, control, intangible asset accounting, external audit, and accounting systems. Administration requires investment in human resources, public relations, risk management, legal, and information technology.
3. Product Development $1,500,000
A competitive strength of the Company is its intellectual property: patents and proprietary knowhow. The Company has numerous new products in various stages of development that require investment to bring them to market. For this purpose, the Company intends to increase its current level of product development spending up to $1,500,000 of the Proceeds.
4. Quality Assurance/Compliance $750,000
Quality assurance and regulatory compliance are major responsibilities that the Company owes to its customers, government regulators (e.g., FDA), and international standards setting organizations. The strong programs already operative in the company will need to additional resources to expand as the Company grows. The Company expects to spend $750,000 on these activities.
5. Planning/Partnerships/Acquisitions $5,000,000
A key source of growth for the Company in coming years will involve licensing, product development partnerships and possible acquisitions of various technologies. The Company anticipates spending as much as $5,000,000 on these activities. No such technologies have been identified as of the date of this Offering Circular.
6. Investor Acquisition $4,000,000
The Company’s cost of presenting itself to potential Investors nationally and internationally, and of properly administering their investments in the Company, is significant. The Company expects that up to $4,000,000 of the Proceeds will be required to compensate to investment intermediaries, transfer agents, market makers, attorneys, and other advisors involved is completing a successful offering.
7. Addition to Working Capital $8,000,000
Anticipated growth in the Company’s revenue, production and marketing/selling operations, staffing, and possibly partnering/acquisition opportunities will require the Company to hold significant cash reserves. The Company expects to set aside up to $8,000,000 for cash reserves and working capital.
8. Property, Plant, and Equipment Expansion $2,000,000
As demand for the Company’s Products grows, its manufacturing facilities will also increase the size and complexity. The Company anticipates investing up to $2,000,000 of the Proceeds for plant expansion and new equipment.
9. Proceeds to Selling Shareholders $10,000,000
The Company anticipates up to $10,000,000 of the Proceeds to go to selling shareholders. See “Selling Shareholders” above.
DESCRIPTION OF THE BUSINESS
Corporate History
McGinley Orthopaedic Innovations, Inc. (the “Company”) is an orthopedic surgical device/implant innovator and manufacturer based in Casper, Wyoming. The Company was founded as McGinley Orthopaedic Innovations, LLC in 2012. The Company converted to a Wyoming Corporation on May 13, 2022 by filing Articles of Incorporation and Statement of Conversion with the Wyoming Secretary of State (See Exhibit 2A “Certificate of Incorporation and Other Documents”).
Summary of the Company
The Company was established in 2012 with the goal of increasing patient safety and physician confidence through technological advances in the orthopedic field. The Company has developed and is marketing patented, FDA-cleared IntelliSense Drill Technology, a set of orthopedic power tools and accessories, and the Lever Action Plate System bone fixation implant system. The Company aims to make its growing product lines the “standard of care” in orthopedic surgery worldwide. Its mission is three-fold: increase patient safety, improve patient care, and reduce surgical cost.
The patented and proprietary sensing, navigation, and robotic capabilities of the IntelliSense Drills were cleared for commercial sale by the FDA on February 20, 2015 through clearance by FDA of the Company’s 510(k). The IntelliSense Drills exhibit capabilities far beyond those available to surgeons in the legacy devices offered by any other power tool manufacturer. “Legacy” devices is a reference to the current status quo products of the orthopedic surgery industry.
The Lever Action Plate System was cleared for commercial sale by the FDA on June 25, 2020 through clearance by FDA of the Company’s 510(k). The Company believes that the LADS will revolutionize wrist surgery and dramatically improve patient outcomes.
The Company currently owns issued or pending patents and has already attracted substantial interest from leading orthopedic device makers in the U.S. The Company is engaged in partnering discussions with several firms related to technology development, licensing, commercialization, and distribution in various segments of the national and global orthopedic power tool and implant markets.
The Company has two wholly-owned subsidiaries: DS Manufacturing LLC and McGinley Engineered Solutions, LLC. DS Manufacturing LLC, has a DBA as McGinley Manufacturing, is a wholly-owned subsidiary of the Company, and operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, welding, machining, replacement part fabrication, CNC plasma cutting, powder coating, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scale pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries.
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of the Company and holds title to all of the patents assigned to the Company.
IntelliSense Drill Technology


IntelliSense Technology includes series of surgical drills that have proprietary sensing, navigation, and robotic capabilities far beyond those available to surgeons in the devices offered by any other surgical power tool manufacturer.
The Company’s first products utilizing IntelliSense Technology received FDA 510(k) clearance in February 2015 and has been used in over 1,000 surgical procedures at over 25 orthopedic surgical centers and teaching hospitals across the United States. The hand-held, electric, surgical drill is designed for routine use in many common orthopedic procedures that require the placement of hardware and bone drilling. Unique design features of IntelliSense Drills include (1) an ergonomic and distinctive drill housing; (2) an integrated and automated depth measurement component, a
chuck uniquely designed to engage the Company’s proprietary quick-release drill bits; (3) integrated lighting; (4) an electronic control unit that provides power, engages sensors, collects and analyzes data, and displays information in real time on a touchscreen visible to the entire surgical team. The IntelliSense Drill’s high-torque motor and a broad range depth sensor is suited to large/small bone drilling and fracture fixations. Furthermore, the IntelliSense Drill is significantly smaller, lighter, easier to use, and more accurate than other standard non-sensing orthopedic drills in service today.
IntelliSense Drill Technology has been reported by physicians to reduce the number of wasted screws, plates, and other consumables and may shorten the duration of a typical orthopedic surgery by 20 minutes or more.
Features of the IntelliSense Drill:
- The Drills include an integrated and automated depth measurement component that, together with a microprocessor, has several modes. The main feature of the IntelliSense Drill Technology is the depth measuring component that stops the Drill once the desired depth has been achieved. This significantly reduces the risk of drilling too deep into the bone and “plunging”, or drilling through the bone. The measuring device will stop the drilling automatically depending on which mode the user selects. These modes include (1) Bicortical (Drill stops upon breaching the second cortex); (2) Freehand (Drill operates as a standard orthopedic drill while including depth measurement); and (3) Set Depth (Drill stops at a predetermined depth), and Multicortex (Drill stops upon breaching a preset number of cortices).
- Orthopedic drill bits are notoriously difficult to sterilize. Minimizing infection risk depends on the consistent use of a new drill bit for each surgery. Preventing surgical site infection remains one of the key patient safety goal standards per the National Patient Safety Goals Joint Commission (NPSG.07.05.01). To assure the IntelliSense Drill bits are proprietary, the Company has designed a patented interlock sensitive to autoclave sterilization which prevents Drill bit reuse.
- LED lights mounted to the front of the Drill housing that illuminates the operative site. It can be activated throughout the procedure, only during active drilling, or turned off. Enhanced visibility is achieved without cumbersome, outside sources such as headlamps, ceiling-hung, and roll-around lights that cast shadows.
IntelliSense Drills Accessories

The accessories include a keyed chuck, keyless chuck, standard AO chuck, and a patented universal pin driver. While these chucks allow the IntelliSense Drill to be used with a wide range of generic products, the benefits of the IntelliSense Drill Technology - in particular the auto-stop and depth measurement features – will not function while using the generic products. Replacement of the Drill bits will drive a secondary and constant revenue stream for the Company.
The Equalizer®:

A companion tool to the IntelliSense Drill, the patented Equalizer, determines the size and length differences that are manufactured into plate and screw sets developed by different manufacturers. With some plate systems, the plates, screws, and depth gauges are manufactured for exclusivity within its own system. Using a depth gauge from one company will give accurate results for plates and screw sets made by that company, but may be completely unreliable for plates and screws made by a different company because of their proprietary offsets. These systems do not give an actual measurement, but only a screw size number; therefore, they are incompatible with the IntelliSense Drill, which is a true measurement device. The Equalizer was developed to eliminate the issue of manufactured plate offsets. The Equalizer can determine the offset of a plate needed for a surgical procedure, and that offset can be input to the IntelliSense Drill for compatibility with all plating systems.
The Revolver® Universal Drill Bit Guide System

The patented Revolver revolutionizes conventional drill bit guides by replacing multiple standard drill bit guides with one 10-piece kit. It features a rotating chamber that adjusts to multiple drill bit diameters from 1.0mm to 5.0mm. It has a range of attachments that extend the length of the guide up to 220mm for percutaneous applications. It has a non-slip tip providing stability on the bone and a comfortable, ergonomic handle. The surgeon’s hand placement is safely away from the drilling point. The Revolver is universal with most standard drill bits. Some of its benefits include cost savings by eliminating numerous conventional drill bit guides, ease of inventory management and sterilization, reducing surgical time in switching and locating various guides and requires little to no training.
IntelliSense Pins
The pin technology currently used to transfix bone for traction or fixation has remained unchanged for decades. The Company has developed a new pin design to be used with the IntelliSense Pin Pilot (a specialized drill bit), with the goal of reducing heat generation while maintaining proper fixation. IntelliSense Pins will be required when using the IntelliSense Pin Pilot.
Advantages of the IntelliSense Drill Technology
Speed of IntelliSense Drill Technology
Hospitals rigorously schedule operating rooms to maximize utilization of scarce resources. According to studies from Akron General Hospital and Northwestern Memorial Hospital, typical Level 1 Trauma Center Operating Room cost in the range of $20.93 to $97.00 per minute. According to a 2005 study of 100 U.S. hospitals, the average charge for the Operating Room was $62.00 per minute, not including extra resources specific to the procedure or provider fees and anesthesia. The time savings from the automatic measuring features of IntelliSense can add one procedure per day of operating room utilization.
An average trauma case uses 10 fixation screws. Surgeons spend one minute (or more) per screw to stop, measure and re-drill each hole (10 mins x $62/min = $620).
Competing Technology for Measuring Drilling Depth
Fluoroscopy is used to confirm screw measurements. For each image, the team must move the fluoroscope to the patient, position the machine arm over the incision, and move team members away from the radiation. Then they take the image, move the equipment back out of the way, and finally reposition the team and resume drilling. In a trauma setting, this takes approximately four to eight minutes per case; in a spine procedure, 25-40 minutes. Using the lower trauma figure, IntelliSense® will save OR time costs attributable only to fluoroscopy by approximately $496.
Less Screw Replacement
On average, when a screw is incorrectly sized, it takes five to ten minutes to remove and replace the screw and additional time to re-image the new screw, according to a surgeon at a hospital in New York. The Company estimates (statistically) the cost of screw replacement at $370 per typical trauma procedure.
Fewer Wasted Screws
Published literature indicates a screw error rate requiring removal and replacement of between 20% and 24.7% that is directly attributable to measurement error. Based on a minimum of six screws used per orthopedic surgery, the Company estimates 1 to 2 screw(s) requiring replacement per case. Fracture fixation screws cost approximately $35 to $150; uni-axial pedicle screws for certain spine surgeries cost up to $1,000 and multi-axial pedicle screws may cost up to $1,500. When surgeons recognize an incorrect screw length during surgery and replace it with one of proper length, hospitals often times pay for the error time, costing hundreds of dollars.
Mitigated Drill Plunging
When surgeons drill too far through a bone blood vessels, nerves, tendons, and organs such as the spinal cord can be damaged. Plunging is a common and under-reported problem. In an informal experiment at a major trade show in 2015, over 150 surgeons were asked to drill holes in saw bones and avoid plunging. The surgeons averaged a 6.67mm plunge with a standard deviation of 3.32mm. Many critical organs and structures lie within 7mm of a bone. This simple experiment shows that, despite frequent denials, surgeons often plunge even when they consciously try not to. In the worst cases, plunging too far can "wind up" nerves and vessels in an instant, causing serious injury or even death
Fewer Screw Injuries
Measurement errors may result in excessively long screws that protrude through bones or short screws causing an unstable plate long screws can rub or erode tissue causing pain or harm as seen in the testimonial IntelliSense Drill commercial. Especially dangerous for patients is a spinal pedicle screw that is too long as it can compress the outer wall of the aorta threatening to erode and puncture the vessel. It is potentially fatal
Automatic and Speedy Measuring
To avoid plunging and screw length errors, orthopedic surgeons interrupt the surgical procedure several times to measure each hole. Errors in screw length are common in orthopedic surgeries. Average trauma surgeries consume up to ten screws, so interrupting the surgical process to measure hole depth consumes significant amounts of very expensive operating room time
Lever Action Plate System® for Distal Radius Fractures
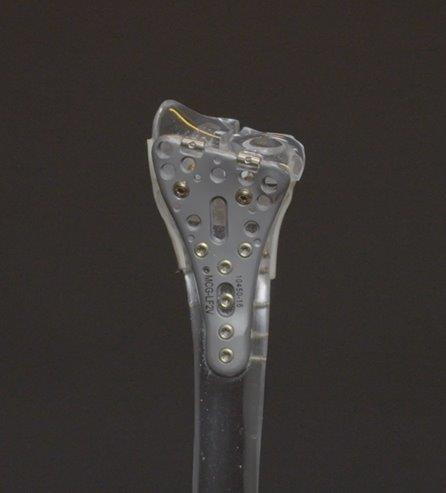
The Company’s second major product is the Lever Action Plate System for Distal Radius Fractures. The Lever Action Plate System received FDA 510(k) clearance in June 2020. The Company acquired exclusive rights to manufacture this technology along with associated issued patents.


The Lever Action Plate System® is a novel solution for distal radius fractures, a wide range of wrist fractures that affect the joint. This innovative plate system features proprietary beams that align volar tilt, an angle of the bones in the wrist. The beams are inserted into the bone fragment and with the turn of a screw, the beams and the fragment elevated into the surgeon’s desired placement. An improvement on the current, generic plates available on the market, the system has optional variable angle screws of varying sizes with patented locking technology. The contour of the plate is designed to adhere comfortably to the watershed line, a theoretical line on the radius in relation to tendons and nerves.
Since FDA clearance, Company engineers have partnered with physicians to perfect the design and bring the device to market. The plates are currently manufactured by Affiliate McGinley Manufacturing. The Company reserves the right to use third party manufacturers for the manufacture of any of the Products, including the Drills and Lever Action Plate Systems.
Clinical Collaborations for the Company’s Products
The Company supports and collaborates on independent and joint research projects with several academic and medical institutions across the country. Such collaborations enable the Company to get its products into the hands of leading medical researchers and refine the IntelliSense Technology. The institutions the Company collaborate with include:
- University of Wyoming;
- New York University
- Shriners Hospital for Children - Philadelphia
- Temple University
- University of Pennsylvania Medical Center - Hamot Hospital
- University of Southern California
Intellectual Property
The Company’s intellectual property assets consist of patents, FDA 510(k) clearances, proprietary knowledge in orthopedics and sports medicine, trade secrets, mechanical and electrical engineering know-how, radiology know-how, registered trademarks, and copyrights.
Patents Summary
The Company currently owns a total of 71 patents issued by United States Patent Office (“USPTO”) and foreign jurisdictions. Approximately 20 more patents are in process of preparation and filing with various jurisdictions.
The Company continues to invest significantly in (i) research & development directed at solving longstanding problems in orthopedic surgery, and in (ii) protecting its discoveries and innovations via the patent process.
FDA 510(k) Clearances
The Company has applied for and received two 510(k) clearances from the FDA. These 510(k) clearances were for the IntelliSense Drills and accessories and (ii) the Lever Action Plate System bone fixation implant system.
Patent/Product Categories
The Company’s issued and pending US patents, and their foreign registrations, can be grouped into six product categories or families. These categories, and the number of the Company’s US and foreign registrations that are core technologies for each, are as follows:
· IntelliSense Drill and Related Systems/Improvements/Features 39
· Orthopedic Implants 9
· IntelliSense Pin Pilot, Driver and Accessories 10
· IntelliSense© Bone Saw and Related Systems/Improvements/Features 8
· High Speed Burr 3
· Hand Held Surgical Device Navigation System 2
Total Issued Patents: 71
Affiliates
DS Manufacturing LLC d/b/a McGinley Manufacturing
In April of 2015, the Company acquired DS Manufacturing LLC, its primary supplier of machined parts and conducts business as, by filing a “doing business as”, McGinley Manufacturing LLC (“McGinley Manufacturing”). McGinley Manufacturing has ten (10) employees and revenue exceeding $1.0 million. McGinley Manufacturing operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, welding, machining, replacement part fabrication, CNC plasma cutting, powder coating, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scale pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries.
McGinley Engineered Solutions LLC
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of the Company, and holds title to all of the patents used by the Company. This is the sole purpose of McGinley Engineered Solutions LLC.
TriOpportunity Investment Group LLC
TriOpportunity Investment Group LLC is owned by Dr. Joseph McGinley and his wife, Diane McGinley. TriOpportunity Investment Group LLC is controlled by Dr. Joseph McGinley. TriOpportunity Investment Group LLC owns the building from which the Company leases its offices.
Markets for Company Products
IntelliSense Drill Technology Market
The Company’s market opportunity exists in the over five million orthopedic surgeries performed annually in the United States alone, a number that grows each year. Each of these surgeries is time consuming, costly, and potentially risky for patients and surgical teams. In 2019, orthopedic surgeries consumed over six million drill bits worldwide (approximately four million in the U.S.), at a value exceeding $650 million. Sale of consumables associated with orthopedic surgeries (drill bits, pins, saw blades, high speed burrs), estimated to be 10.1 million in 2021, are expected to grow in parallel with drill sales to 14.5 million by 2027. The Company estimates U.S. sales of power drills – relatively low in volume but high in margin – to be approximately 24,000 units in 2020. The bulk of current annual sales volume is driven by replacement or updating of old equipment, and by restocking of consumables such as drill bits, pins, saw blades, burrs, and batteries. The Company’s global unit sales volume should increase as the IntelliSense Products are adopted by hospitals as the necessary standard of care in orthopedic surgery and the obsolete devices currently in use are replaced. The Company’s IntelliSense Products address a market in the U.S. alone exceeding $1 billion annually.
Customer Profile
The Company will market the IntelliSense Drills to orthopedic surgeons, surgical centers, and hospitals throughout the United States and globally. The main value proposition derives from the increased safety of using the IntelliSense Drill Technology versus the current ‘dumb’ drills which are the current status quo.
Studies show that waste of implants (primarily plates and fixation screws) occurs in 12% to 30% of orthopedic surgical cases at a cost exceeding $162 million annually (U.S.). Globally, preventable orthopedic surgical errors cost in excess of $1.5 billion per year. This cost falls on patients, health care providers, and health insurers. The sensors, software, and real-time monitoring and measurement capabilities of IntelliSense Drills may dramatically reduce orthopedic surgical errors, waste, and their associated economic costs.
The IntelliSense Drill Technology assists surgeons and patients in numerous ways. It may possibly make trauma and spine surgeries safer and less costly by (i) reducing the risk of surgeons drilling through bones (i.e. “plunging”) into patient’s arteries, veins, nerves, and vital organs; (ii) giving surgeons precise depth measurement and accurate screw sizing in real time; (iii) reducing or eliminating the need for x-rays during surgery, and (iv) precisely recording data for each bone
penetration drilled by the surgeon. Independent studies show that use of IntelliSense Drill Technology can reduce the cost of orthopedic surgery by approximately $1,850 per procedure.
In contrast to IntelliSense Drills, conventional orthopedic power tools and current “standard of care” surgical procedures are obsolete. Conventional devices lack the sensors, software, real-time data recording, and artificial intelligence embedded in Company devices. Existing “standard of care” orthopedic surgical practices force surgeons to rely on “feel” to avoid plunging into the patient’s blood vessels, nerves, tendons and other structures and organs.
Lever Action Plate System Market
The LAPS is a major advance in the repair of complex wrist fractures. This innovative plate system features proprietary beams that align volar tilt, an angle of the bones in the wrist. In the global plates and screws market, plates account for 83%, or $4.3 billion in 2020 and are expected to reach $5.6 billion by 2027.
Wrist fractures are the most common upper extremity fracture experienced in the U.S. population. The market size of volar distal radius plates is estimated to by 1.1 million units with a CAGR of 5.8% over the next five years. Volar distal radius plates make up 92% of the market, while dorsal plates are only 8%.
This innovative technology addresses a real and prevalent need in orthopedics. There are around 67 upper extremity fractures per 10,000 people annually in the U.S.; 25% of these fractures occur in the distal radius and ulna. In addition to the ability to restore volar tilt, the Lever Action Plate System has optional variable angle screws with patented locking technology. This hybrid type of plate and screw system gives surgeons flexibility to decide which product type that best suits the needs of the patient.
The global market for fracture fixation products, including plate systems, is estimated to reach $12.1 billion by 2025. This estimate is driven by an increasing incidence of fractures in the elderly population and a rapid adoption of internal fixation devices for small bones. The fracture fixation market is made up of both external and internal fixation devices, which include frames, pins, plates, intramedullary nails, cables, staples, and wires.
The leading competitors for the fracture fixation market are Johnson & Johnson, Stryker, Zimmer Biomet, and Smith & Nephew. Implanted medical devices are one of the most profitable businesses in the domestic healthcare industry.
Distal radius fractures account for about 20% of all fractures treated in emergency departments and 8% to 15% of all bony injuries in adults. A complication rate of 15% has been reported with traditional volar plating and post-op complication rates can be as high as 80%.
Distribution of the Company’s Products
The Company’s marketing and sales efforts are focused on (i) building the Company’s sales “funnel”; (ii) empowering patients to advocate directly for IntelliSense Technology and LAPS use;
(iii) rolling out a nationwide team of experienced medical device sales representatives; and, (iv) adding distributors to support the nationwide sales team.
The Company’s management and nationwide sales and distribution team have established a large number of evaluation-for-purchase trials at leading orthopedic hospitals and medical schools across the country. The key selling points emphasize clinical, economic, and patient outcome benefits to the hospital, surgeons, and staff.
To date, the Company has focused its sales efforts on teaching institutions and hospitals with the most reputable surgeons. Leading orthopedic surgeons are demonstrating IntelliSense and LAPS Products to residents, fellows, and interns, thus building future customers. Several of these surgeons have become champions of the Products and have aided in word-of-mouth efforts.
The Company will use the Proceeds to focus its sales and distribution team on national group purchase organizations (“GPOs”) and ambulatory surgery centers, the most rapidly growing segment among providers of orthopedic surgery.
The Company uses a traditional regional sales and distribution strategy to affect sales of its Products. The Company has four regional representatives on its internal sales team. The Company will continue to recruit qualified individuals to the sales team in coming quarters. As sales opportunities in discrete geographic area increase, additional distributors will be brought on board.
The Company has priced the its Products competitively with industry standards. In addition to outright sale of the IntelliSense Drills, the Company offers hospitals convenient lease-to-own and per-use rental arrangements that eliminate the need for capital purchases that must endure the time-consuming consideration approval of the hospitals’ capital acquisition committees prior to purchase.
Announced Products
The Company is developing new products based on its issued and pending patents that will, when introduced in the market over the coming five years, greatly increase the Company’s market opportunity and growth rate. These new products (and the product families of which they are a part) include the following:
· IntelliSense Drill for Spine (Drill)
• IntelliSense Drill with Navigation (Navigation)
• IntelliSense MicroDrill with Navigation (Drill)
· Lever Action Plates® for Tibial Plateau Fracture (Implants)
• Independent Navigation for Orthopedic Devices (Navigation)
• IntelliSense Bone Saw (Saw)
· Ankle Fixation Plate System (Implants)
The Company has announced the above products to the general public. As of the Date of this Offering Circular, none of these announced products are ready for commercial distribution.
Several of these following products either do not require 510(k) clearance or they will be covered under the Company’s current 510(k) as predicate devices.
IntelliSense Cannulated Drill Bit: Cannulated drill bits provide the surgeon with the ability to drill a hole over a guide wire to help insure proper trajectory. These drill bits are often used in lower extremity procedures and will provide expanded use of the IntelliSense Drill Technology. The Company anticipates that this product will be commercially available in Q4-2022.
IntelliSense Pin Pilot: The Pin Pilot revolutionizes pin/wire drivers and employs technological advances for pin and K-wire placement. With IntelliSense Drill Technology, this device can auto-stop after breaching a predetermined number of cortices, subchondral or endosteal, while simultaneously providing depth measurement and cortical edge detection.
The Company anticipates this product will be covered by the Company’s existing 510(k) for the IntelliSense Drill. The Company anticipates that this product will be commercially available in Q4-2022.
IntelliSense Spine (Specialized Products and Software): IntelliSense Spine embodies patented and proprietary algorithms in software controlling the IntelliSense Drill and adapts the latter to surgery or the spine. The IntelliSense Drill Technology will include four activities, three spine locations, and six modes of operation, including endosteal and subchondral modes. The Company believes that no 510(k) is needed for this product and anticipates commercial sales to begin in 2023.
IntelliSense Drill and MicroDrill with Navigation: IntelliSense Drill with Navigation will provide the surgeon real-time point-of-contact navigation. This navigation system may also be an accessory to conventional drills. The MicroDrill with Navigation is a miniaturized device employing all features of the IntelliSense Drill plus revolutionary navigation software, for use in specialized orthopedic surgeries requiring the utmost delicacy and precision (e.g., fingers and spine surgeries).
The Company anticipates this product may be covered by the Company’s existing 510(k) for the IntelliSense drill. In the event the current 510(k) does not cover this product as a predicate device, the Company will submit a new 510(k) application prior to commercial sales. The Company anticipates that this product will be commercially available in 2024.
IntelliSense Bone Saw: The IntelliSense Bone Saw employs Company patented and proprietary technology in an orthopedic saw device. This product addresses the pressing need for greatly improved precision in joint replacement surgeries.
The Company anticipates this product may be covered by the Company’s existing 510(k) for the IntelliSense drill. In the event the current 510(k) does not cover this product as a predicate device, the Company will submit a new 510(k) clearance application prior to commercial sales. The Company anticipates that this product will be commercially available in 2024.
Ankle Fusion Implants: Ankle fusion is a surgical procedure used to treat ankle pain due to severe degenerative arthritis. This surgery involves removing the inflamed cartilage and surgically joining
two or more ankle bones together. The Company is working with a partner surgeon in the early stage of product development for a unique product that can be used for these procedures.
The Company does not believe any 510(k) will cover this product as a predicate device. The Company will submit a new 510(k) clearance application prior to commercial sales. The Company anticipates that this product will be commercially available in 2024.
Special Characteristics of the Company’s Operations and Competing Products/Procedures
The Company is subject to regulation of its Products before, during, and after clearance by the FDA Products for commercial distribution of the Products
Facilities Regulation
Regulation of the Company as a Device Manufacturer
The Company may outsource some of manufacturing of the Company’s Products, in part, to third party manufacturers.
Quality System Regulation and Good Manufacturing: Medical Devices
After clearance of the Products by the FDA through the 510(k) process, the Company will be subject to FDA Quality Systems Regulation (“QS Regulation”) and Good Manufacturing Practices” (“GMPs”). Manufacturers must establish and follow quality systems to help ensure that their medical device products consistently meet applicable requirements and specifications known as current good manufacturing practices. Continued Compliance with these QS Regulations and GMPs is an ongoing duty of the Company. On February 23, 2022, the FDA proposed a regulation which would incorporate the international standard specific for medical device quality management systems set by the International Organization for Standardization (“ISO”), ISO 13485:2016 Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. The Company intends to use contract manufacturers and McGinley Manufacturing for manufacturing of its Products. All of these contract manufacturers will be required by the Company to be ISO 13485:2016, and thus compliant with FDA GMP.
The Company does not anticipate that this proposed regulation will affect the operations of the Company, since the Company intends on complying with ISO 13485:2016 prior to the proposed rule change in development of its facilities.
The Company has also directed McGinley Manufacturing to maintain certification with AS 9100-Aerospace Management Standard.
Employees
The Company, not including its subsidiaries, has nine (9) full time employees and one (1) part-time employee.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF THE FINANCIAL CONDITION OF THE ISSUER
The following is a discussion of the financial condition of the Company. On May 13, 2022 the Company executed a conversion from McGinley Orthopaedic Innovations, LLC, a Wyoming limited liability company, to McGinley Orthopaedic Innovations, Inc., a Wyoming corporation. This conversion occurred after the end of FY2021 on December 31, 2021. Therefore, all figures and disclosures in this section will be those of McGinley Orthopaedic Innovations, LLC. For this reason, references to the “Company” will include McGinley Orthopaedic Innovations, LLC and McGinley Orthopaedic Innovations, Inc. only throughout this section.
Components of Results of Operations
The following discussion of the Company’s financial conditions and results of operations should be read in conjunction with the financial statements and the related notes included elsewhere in this Offering Statement. The following discussion contains forward-looking statements that reflect the Company’s plans, estimates and beliefs. The Company’s actual results could differ significantly from those discussed in the forward-looking statements. Unless otherwise indicated, the latest results discussed below are as of December 31, 2021.
Revenue/Net Loss from Operating Activities
The Company is, and has been, solely focused on development and commercialization of its Products and development of new related products. As of the date of this Offering Circular, the Company has never been profitable.
In 2021 the Company’s revenues were $1,352,296, an increase of $335,000 (33%) over revenues of $1,017,289 in 2020. The reason for this increase was due to medical sales and mining sales increasing after the economic effects caused by COVID-19 recovered, and the launch of the LAPS product line.
In FY2021 the Company had a net loss of $2,857,806. In FY2020 the Company’s net loss was $2,199,694. Net loss increased in 2021 by $658,000 (33%) over 2020. The reason for this increase was due to operating expenses increasing by $837,000 (26%) and other income being reduced by $49,000 (11 %).
Loss from Operations increased to $3,273,899 in 2021 from $2,664,432 in 2020, an increase of $613,000 (23%). The reason for this increase was due to operating expenses increasing by $837,000.
Operating Expenses
The $613,000 increase in Operating Loss in 2021 over 2020 was driven primarily by an increase of $837,000 in Selling, General and Administrative expenses (“SG&A”) in 2021 over 2020. The Company classifies its SG&A expenses as wages and benefits, general office, research and
development, professional fees, rent, insurance, advertising, other expenses, dues and subscriptions, depreciation and amortization, taxes and licenses, computer and internet, and postage and shipping. Over 80% of SG&A in 2021 was accounted for by wages and benefits, general office, Research & Development, professional (legal and accounting) fees, and travel and entertainment. The reason for this increase in SG&A expenses was due to an increase in the in-house sales team, rent and insurance for a new location and 2020 spending being generally lower due to Covid-19.
Wages and Benefits
In FY2021, the Company spent $2,338,656 on wages, benefits, contractor and other compensation expenses. In FY2020, the Company spent $1,890,629 on wages, benefits, contractor and other compensation. The increase of $448,000 (24%) in 2021 over 2020 was driven primarily by enlargement of the sales force. The Company expects that significantly increased spending in all personnel categories – particularly in marketing and selling expense – will follow a successful Offering by the Company, as discussed in Use of Proceeds discussed elsewhere in this document.
General Office Expenses
In FY2021, the Company spent $494,047 on general office expenses. In FY2020, the Company spent $398,757 on general office expenses. The increase of $95,000 (24%) in 2021 over 2020 was driven primarily by (1) a $15,000 increase in office supplies related to the Company’s new office location; (2) a $41,000 increase in tradeshow spending due to COVID-19 restrictions being lifted; and, (3) $42,000 used in recruiting a Vice President of Sales.
Research and Development Expense
The Company’s research and development efforts are focused on the continued development of new products related to the Company’s IntelliSense Technology and LAPS Products. Research and development expenses consist primarily of materials, equipment, and manufacturing costs to design, prototype, develop, and test the Company’s new products, and to acquire the parts, tools and equipment needed to do so.
In FY2021, the Company spent $305,331 on research and development expenses. In FY2020, the Company spent $353,460 on research and development expenses. The decrease of $48,000 (14%) from 2020 to 2021 was due primarily to the need to reallocate resources to the Company’s enlarged sales force.
The Company anticipates that annual increases in R&D expenses will resume upon a successful Offering by the Company, as discussed in Use of Proceeds discussed elsewhere in this Offering Circular.
Professional fees
In FY2021, the Company spent $249,910 on professional fees. In FY2020, the Company spent $184,685 on professional fees. The increase of $65,225 (35%) in 2021 over 2020 was driven
primarily by an increase in spending for the contract CFO, patent attorney, and engineering/quality assurance programs. The Company anticipates that annual increases in professional fees will continue upon a successful Offering by the Company, as discussed in Use of Proceeds discussed elsewhere in this document
Travel and Entertainment
In FY2021, the Company spent $148,282 on travel and entertainment. In FY2020, the Company spent $63,423 on travel and entertainment. The increase of $84,850 (234%) in 2021 over 2020 was driven primarily by primarily by an increase in the internal sales force and Covid 19 restrictions being lifted. The Company anticipates that increases in travel and entertainment professional fees will continue upon a successful Offering by the Company, as discussed in Use of Proceeds discussed elsewhere in this document.
Membership Units/Accrued Membership Units
Between August 15, 2019 and March 31, 2022, the Company issued 573,633.16 Membership Units and accrued 353,846.15 Accrued Membership Units for future issuance to members of the Company’s Board of Advisors, pursuant to Board of Advisor agreements executed by each Board of Advisors member. These Units are discussed in the Company’s financial statements in Note I to the balance sheet.
Liquidity and Capital Resources
As of December 31, 2021, the Company had cash of $1,624,273, working capital of $3,067,379, and total assets of $4,983,251. On December 31, 2020, the Company had cash of $2,026,934, working capital of $3,368,779, and total assets of $5,121,205. From December 31, 2020 to December 31, 2021, cash decreased by $402,661 (20 %), working capital decreased by $301,400 ( 9 %), and total assets decreased by $ 137,954 (3 %).
The Company has a $1,000,000 line of credit with a bank which matures February 8, 2024. Interest is subject to change on a monthly basis, calculated at the prime plus a variable rate (3.50% as of December 31, 2021). There was no outstanding balance on the line of credit as of December 31, 2021 and 2020.
The Company has additional capital requirements during the current fiscal year 2022 to be used in expanding its marketing and sales team and program, adding additional skills to its management team, and undertaking other initiatives as summarized in “Use of Proceeds” above. The Company is seeking additional capital via the sale of its securities in this Offering. The Company’s Offering is for a maximum of 7,619,047.62 Shares (not including Bonus Shares) at price of $5.25 per Share, with potential aggregate gross Proceeds of $40,000,000.
There can be no assurance that the Company will be successful in acquiring additional funding at levels sufficient to fund its future capital requirements. If the Company is unable to raise additional capital in sufficient amounts or on terms acceptable to it, the Company may have to significantly reduce, delay, scale back or discontinue the development of its technologies and patents or discontinue operations completely.
DESCRIPTION OF PROPERTY
The Company owns real property through its wholly-owned subsidiary DS Manufacturing d/b/a McGinley Manufacturing. The property is an 8400 sq.ft. manufacturing plant located at 585 E Birch St Glenrock, WY 82637. The plant is a highly-specialized and highly-technical manufacturing facility capable of manufacturing all of the Company’s Products. In some occurrences, the Company must use third-party contract manufacturer for processes such as heat treating, anodizing, and heat treatment. The facility is and will continue to be ISO 13485:2016 and AS 9100 certified.
DIRECTORS, OFFICERS AND SIGNIFICANT EMPLOYEES
Directors
| Name | | Position | | Age | | Term of Office | | Approximate Hours per week |
| Dr. Joseph McGinley | | | Director | | | | 48 | | | | June 2012 - Present | | | Full Time |
Officers
| Name | | Position | | Age | | Term of Office | | Approximate Hours per week |
| Dr. Joseph McGinley | | CEO/President/Treasurer/Secretary | | | 48 | | | | June 2012 - Present | | | Full Time |
Significant Employees
| Name | | Position | | Age | | Term of Office | | Approximate Hours per week |
| Dr. Richard C. McGinity | | | Director of Finance | | | | 78 | | | | July 2016 - Present | | | Full Time |
| Diane McGinley | | | Director of Operations | | | | 46 | | | | June 2012 - Present | | | Full Time |
| Ben Warren | | Vice President- McGinley Manufacturing | | | 55 | | | | June 2015-Present | | | Full Time |
| Adam Johnson | | Director of Engineering | | | 46 | | | | June 2012- Present | | | Full Time |
| Michael Hays | | Manager of Finances | | | 58 | | | | June 2015 | | | Full Time |
| Travis Sides | | Operations Manager | | | 48 | | | | August 2015-Present | | | Full Time |
Directors/Officers
Dr. Joseph McGinley: Founder, Chairman of the Board of Directors, Chief Executive Officer, President, Treasurer, Secretary
Full Time
Duties of Dr. Joseph McGinley as CEO/President, Treasurer and Secretary
The duties of the CEO and President are set by the Bylaws.
The President is the Chief Executive Officer and President of the Company, subject to the control of the Board of Directors, has responsibility for the conduct and management of the business and fiscal affairs of the Company and the general supervision of its property, business interests, and agents. The President, or a person designated by the President, must preside at all meetings of the Shareholders and Directors, unless otherwise ordered by the Board of Directors. The President may sign, with the Secretary or any other proper officer of the Company authorized by the Board of Directors, certificates for Shares of the Company and deeds, mortgages, bonds, contracts, or other instruments that the Board of Directors has authorized to be executed, except in cases where the signing and execution thereof is expressly delegated by the Board of Directors or the Bylaws to some other Officer or agent of the Company, or is required by law to be otherwise signed or executed.
The Treasurer is responsible for the administration of the financial affairs of the Company and has charge and custody of, and is responsible for, all funds and securities of the Company. The Treasurer also gives receipts for moneys due and payable to the Company from any source whatsoever, and deposits all such money in the name of the Company in such banks, trust companies, or other depositories as selected by the board of directors, and, if required by the Board of Directors, will give a bond for the faithful discharge of the Treasurer’s duties in such sums and with such security or surety as the Board of Directors may require.
The Secretary keeps or causes to be kept a book of minutes of all meetings of Directors and Shareholders, a stock transfer book, or a duplicate stock transfer book, showing the names of the Shareholders and their addresses, the number and classes of Shares held by each, the number and date of certificates issued for such Shares, if any, and the number and date of cancellation of certificates surrendered for cancellation, if any. The Secretary also gives or causes to be given notice of the meetings of the Shareholders and Board of Directors as is required by the Bylaws, and, when requested or required, certifies any records of the Company.
Significant Employees
Dr. Richard McGinity: Director of Finance
Dr. Richard McGinity joined the Company as Director of Finance in July 2016, and is a member of its Board of Advisors. Dr. McGinity holds degrees from Princeton University (AB), Harvard University (MBA, DBA), and is President Emeritus of the University of Wyoming. Prior to joining the University of Wyoming faculty in 2007 as Daniels Chair of Business Ethics, Dr. McGinity was a general partner in a Boston-based venture capital fund and subsequently launched his own investment banking firm serving technology firms and their founders. He has extensive public and private transaction structuring and execution experience, has served on numerous boards of publicly-traded and private companies in the technology, energy, and banking industries, and is an “audit committee financial expert” as defined in Sarbanes-Oxley Section 407. He deployed three times to Vietnam as a Navy pilot, earning two Air Medals.
Duties of Dr. Richard McGinity as Director of Finance
The duties of the Director of Finances of the Company include oversight of the three corporate functions of (i) accounting and control, (ii) economic strategy and forecasting, and (iii) investor and financial community relations. The latter includes compliance with financial accounting standards, all financial disclosure and regulatory reporting requirements, and state and federal tax regulations. The Director of Finance serves at the direction of the CEO and President Dr. Joseph McGinley.
Diane McGinley M.S.: Director of Operations
Diane McGinley joined the Company in 2012 upon the Company’s inception. She is a graduate of Ohio University, with an M.S. in Education from Gwynedd Mercy College. Her early career was as an educator, curriculum director, and instructional facilitator. She has experience in corporate finance and governance as a board member of Hilltop Bank in Casper, WY, in which she serves as a member of seven committees: Assets and Liabilities, Trust, Trust Audit, Board Credit, Compensation, Marketing and Building.
Duties of Diane McGinley, MS
Diane McGinley oversees human resources, general operations and marketing and those departments’ personnel.
Ben Warren: Vice President of Manufacturing, McGinley Manufacturing
Ben Warren has more than 22 years of experience in the machining industry. He established DS Manufacturing, Inc. in 2009 to serve the hard rock mining industry with after-market parts. In 2015, he partnered with the Company to form McGinley Manufacturing, a full-service machining and manufacturing facility for the medical, oil and gas, and mining industries. Ben now oversees all manufacturing operations for the Company at its plant in Glenrock, WY.
Duties of Ben Warren as Vice President of Manufacturing, McGinley Manufacturing
The duties of the Vice President of McGinley Manufacturing are to oversee production, operations, and personnel at the facility. He is to ensure that quality systems certification requirements are being met and followed. He is in charge of ensuring quality of products, meeting OSHA requirements for safety and is the direct supervisor to the manufacturing operations manager.
Adam Johnson: Director of Engineering
Adam Johnson has over 22 years of experience in custom engineering design and manufacturing. His experience includes positions in production facility development, machine design, composite lumber manufacturing, business development, and continuous improvement training. He holds a Bachelor of Science degree in Mechanical Engineering from Colorado State University.
Duties of Adam Johnson as Director of Engineering
The duties of the Director of Engineering include overseeing the engineering department. He is responsible for creating engineer work flows, document submissions to the FDA, ensuring adherence to the quality system and managing personnel. He also oversees product specialists and their work in the field with end user customers.
Michael Hays: Manager of Accounting and Finance
Michael Hays joined the Company in 2015 and has over 32 years of experience in corporate accounting, with over 19 years in the manufacturing industries and more than five years in healthcare accounting. He holds a degree in finance from the University of Wyoming.
Duties of Michael Hays as Manager of Accounting and Finance
The duties of the Manager of Accounting and Finance include responsibility for the Company’s accounting, treasury, and bank relationship activities.
Travis Sides: Manufacturing Operations Manager
Tavis Sides joined the Company in 2020 and has over 17 years of sales, safety, and quality experience. He enjoys challenging work and has worked on many projects that improved work conditions for his team members. He graduated with an Associates degree in Psychology from Casper College in 2000.
Duties of Travis Sides as Manufacturing Operations Manager
The duties of Manufacturing Operation Manager include creating a production schedule, monitoring and adjusting manufacturing work flows, customer relationships including recruiting new customers and overseeing machinists and other facility personnel. This position has responsibility roles in the quality system certifications.
Family Relationship Disclosure
Dr. Joseph McGinley and Diane McGinley are husband and wife.
COMPENSATION OF DIRECTORS AND OFFICERS
Directors
| Name | | Capacity of Compensation | | Cash Compensation | | Other Compensation | | Total Compensation |
| Dr. Joseph McGinley | | | Director | | | $ | 20,000 | | | $ | 162,500.03* | | | $ | 182,500.03 | |
Officers
| Name | | Capacity of Compensation | | Cash Compensation | | Other Compensation | | Total Compensation |
| Dr. Joseph McGinley | | CEO, President, Treasurer, Secretary | | $ | 25,000 | | | $ | 0 | | | $ | 25,000 | |
Dr. Joseph McGinley’s compensation in his capacities as an Officer and as a Director are segregated and are Dr. Joseph McGinley’s compensation in his capacities as an Officer and as a Director are segregated and are reflected in the above tables.
*The Director accrued the following Shares that are accrued but not issued to the Director within the past calendar year pursuant to the table below:
| NAME | | DATE ACCRUED | | NUMBER OF SHARES ISSUED/ACCRUED | | VALUE OF SHARES ISSUED/ACCRUED |
| Joseph McGinley | | 3/31/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 6/17/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 9/24/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 12/31/2021 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 2/17/2022 | | | 6,410.26 | | | $ | 1.95 | |
| Joseph McGinley | | 5/15/2022 | | | 51,282.05 | | | $ | 1.95 | |
| Total Shares Issued/Accrued | | | | | 83,333.35 | | | $ | 162,500.03 | |
All of the Shares above were accrued at the price of $1.95 per Share. When the above persons desire to have the Shares issued, they will be required to pay $1.95 to receive the Shares.
Aggregate Compensation to Directors
$207,500.03
There are no anticipated increases to compensation for Directors and Officers planned by the Company at this time.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
| Title of Class | | Name and Address of Beneficial Owner | | Amount of Shares Owned | | Amount and nature of beneficial ownership acquirable | | Percent of Class |
| Common Stock | | Dr. Joseph McGinley | | | 30,267,901.81 | | | | 83,333.35* | | | | 67.05% | |
(1) Dr. Joseph McGinley 234 E. 1st St. Suite 242 Casper, WY 82601. None of the Shares beneficially owned or acquirable by Dr. Joseph McGinley are being offered for sale in this Offering.
* Dr. Joseph McGinley is the beneficial owner of these Shares pursuant to accrual of those Shares (See “Dilution” above)
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
Related Party Operating Lease
TriOpportunity Investment Group LLC is owned by Dr. Joseph McGinley and his wife, Diane McGinley. TriOpportunity Investment Group LLC is controlled by Dr. Joseph McGinley. TriOpportunity Investment Group LLC owns the building from which the Company leases its offices. The Company is a tenant in this building located at 234 E. 1st St. Suite 242 Casper, WY 82601. The Company has payables due to TriOpportunity Investment Group LLC
The Company has an operating lease for office space from TriOpportunity Investment Group LLC which began in January 2021. The lease is for seven years and expires in December 2027. Rent expense is recognized on a straight-line basis over the term of the lease. The lease is also subject to a three percent minimum annual increase. In connection with the related party lease, approximately $102,000 was paid to the lessor for specific leasehold improvements requested by the Company.
Future minimum rental payments under non-cancellable operating leases (with initial or remaining lease terms in excess of one year) for each of the five years subsequent to December 31, 2021 are:
2022 - $113,221
2023 - $113,280
2024 - $113,341
2025 - $113,403
Thereafter - $113,467
Total $ 566,712
Total combined rent expense associated with the operating leases amounted to approximately $114,000 and $0 for the years ended December 31, 2021 and 2020, respectively.
DESCRIPTION OF THE SECURITIES
The Company has one class of authorized stock, Common Stock. The following is a description of some of the rights associated with the Common Stock.
Dividends
The Board may authorize, and the Company may make, distributions (including dividends on its outstanding shares) in the manner and on the terms and conditions provided by law and the Articles of Incorporation.
Before payment of any dividend, there may be set aside out of any funds of the Company available for dividends such sum or sums as the Board, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the Company, or for such other purpose as the Board shall think conducive to the interests of the Company.
Voting
Shareholders are only permitted to vote on the following matters:
- Removal of a Director
- Election of Directors
- Termination and dissolution of the Company
- Amendment of the Articles of Incorporation
- Any other matter requiring a vote of the Shareholders in the Articles of Incorporation or the Wyoming Business Corporation Act
Each Share is entitled to one vote.
Preemptive rights
There are no preemptive rights for Shareholders of the Company. This includes no right to purchase any classes of stock, option, warrants, or any other security convertible into Company stock.
Redemption Provisions
The Articles of Incorporation and Bylaws do not provide for redemption rights.
Sinking Fund Provisions
The Articles of Incorporation and Bylaws do not contain sinking fund provisions.
Liability to Further Calls
Shareholders are not required to participate in future capital calls.
Restrictions on Alienability
There are no restrictions of alienability for the Shares.
Drag Along Rights
In the event 65% of the shareholders and the Board approve and agree to a sale, merger, or reclassification of the Company where an excess of 50% of the Company’s voting share or capital is transferred, the Shareholders will have a drag-along right to participate in the sale.
Part F/S
Consolidated Financial Statements and Report of
Independent Certified Public Accountants
McGinley Orthopaedic Innovations, LLC
December 31, 2021 and 2020
REPORT OF INDEPENDENT CERTIFIED PUBLIC ACCOUNTANTS
To the Members of
McGinley Orthopaedic Innovations, LLC:
Opinion
We have audited the accompanying consolidated financial statements of McGinley Orthopaedic Innovations, LLC (the “Company”), which comprise of the consolidated balance sheets as of December 31, 2021 and 2020, and the related consolidated statements of operations, changes in members’ equity, and cash flows for the years then ended, and the related notes to the consolidated financial statements.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Basis of Opinion
We conducted our audits in accordance with auditing standards generally accepted in the United States of America (“U.S. GAAS”). Our responsibilities under those standards are further described in the Auditor’s Responsibilities for the Audit of the Consolidated Financial Statements section of our report. We are required to be independent of the Company and to meet our other ethical responsibilities in accordance with the relevant ethical requirements relating to our audits. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note C to the consolidated financial statements, the Company had net losses of approximately $2,878,000 and $2,205,000 for the years ended December 31, 2021 and 2020, respectively, as well as negative cash flow from operations of approximately $2,055,000 and $1,485,000, respectively. Management’s evaluation of the events and conditions and management’s plans regarding those matters are also described in Note C. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to that matter.
Responsibilities of Management for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of the consolidated financial statements in accordance with U.S. GAAP, and for the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the consolidated financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are available to be issued.
Auditor’s Responsibilities for the Audit of the Consolidated Financial Statements
Our objectives are to obtain reasonable assurance about whether the consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with U.S. GAAS will always detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate, they would influence the judgement made by a reasonable user based on the consolidated financial statements.
In performing an audit accordance with U.S. GAAS, we:
Exercise professional judgement and maintain professional skepticism throughout the audit.
Identity and assets the risks of material misstatement of the consolidated financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements.
Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such opinion is expressed.
Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the consolidated financial statements.
Conclude whether, in our judgement, there are conditions or events, considered in the aggregate that raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time.
We are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit, significant audit findings, and certain internal control related matters that we identified during the audit.
s//: Assurance Dimensions
Jacksonville, Florida
July 13, 2022
McGinley Orthopaedic Innovations, LLC
Consolidated Balance Sheets
As of December 31, 2021 and 2020
| ASSETS | 2021 | | 2020 |
| Current assets: | | | | |
| Cash | | $ | 1,624,273 | | | $ | 2,026,934 | |
| Accounts receivable, net | | | 137,360 | | | | 63,311 | |
| Due from related parties | | | 72,394 | | | | 6,658 | |
| Inventories | | | 1,355,011 | | | | 1,373,094 | |
| Prepaid expenses | | | 101,414 | | | | 74,672 | |
| Total current assets | | | 3,290,452 | | | | 3,544,669 | |
| | | | | | | | | |
| Property and equipment, net | | | 1,190,846 | | | | 1,073,317 | |
| Intangible assets, net | | | 501,953 | | | | 503,219 | |
| Total assets | | $ | 4,983,251 | | | $ | 5,121,205 | |
| LIABILITIES AND MEMBERS’ EQUITY | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 129,292 | | | $ | 57,861 | |
| Accrued liabilities | | | 93,781 | | | | 53,742 | |
| Notes payable – related parties | | | — | | | | 64,287 | |
| Total liabilities | | | 223,073 | | | | 175,890 | |
| | | | | | | | | |
| Members’ equity: | | | | | | | | |
| Paid-in capital | | | 22,867,040 | | | | 21,082,704 | |
| Member units to be issued | | | 888,333 | | | | — | |
| Accumulated deficit | | | (18,995,195 | ) | | | (16,137,389 | ) |
| Total members’ equity | | | 4,760,178 | | | | 4,945,315 | |
| Total liabilities and members’ equity | | $ | 4,983,251 | | | $ | 5,121,205 | |
The accompanying notes are an integral part of these consolidated financial statements.
McGinley Orthopaedic Innovations, LLC
Consolidated Statements of Operations
For the Years Ended December 31, 2021 and 2020
| | | 2021 | | 2020 |
| Revenues | | $ | 1,352,296 | | | $ | 1,017,289 | |
| Cost of goods sold | | | 555,276 | | | | 448,127 | |
| Gross margin | | | 797,020 | | | | 569,162 | |
| | | | | | | | | |
| Selling, general and administrative expenses: | | | | | | | | |
| Wages and benefits | | | 2,338,656 | | | | 1,890,629 | |
| General office | | | 494,047 | | | | 398,757 | |
| Research and development | | | 305,331 | | | | 353,460 | |
| Professional fees | | | 249,910 | | | | 184,685 | |
| Travel & entertainment | | | 148,282 | | | | 63,423 | |
| Rent | | | 115,416 | | | | 26,189 | |
| Insurance | | | 107,643 | | | | 102,857 | |
| Advertising | | | 73,610 | | | | 33,421 | |
| Other expenses | | | 65,240 | | | | 34,343 | |
| Dues & subscriptions | | | 55,233 | | | | 19,538 | |
| Depreciation and amortization | | | 50,966 | | | | 58,513 | |
| Taxes & licenses | | | 24,967 | | | | 33,831 | |
| Computer & interest | | | 24,659 | | | | 24,301 | |
| Postage & shipping | | | 16,959 | | | | 9,647 | |
| Total selling, general and administrative expenses | | | 4,070,919 | | | | 3,233,594 | |
| | | | | | | | | |
| Loss from operations | | | (3,273,899 | ) | | | (2,664,432 | ) |
| | | | | | | | | |
| Other income: | | | | | | | | |
| Gain on extinguishment of debt | | | 316,500 | | | | 316,500 | |
| Interest income | | | 3,630 | | | | 8,179 | |
| Miscellaneous income | | | 95,963 | | | | 140,059 | |
| Total other income | | | 416,093 | | | | 464,738 | |
| Net loss before income taxes | | | (2,857,806 | ) | | | (2,199,694 | ) |
| Provision for income taxes | | | — | | | | — | |
| Net loss | | $ | (2,857,806 | ) | | $ | (2,199,694 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
McGinley Orthopaedic Innovations, LLC
Consolidated Statements of Operations
For the Years Ended December 31, 2021 and 2020
| | | Membership Units | | Membership Units | | Member Units to be issued | | Accumulated deficit | | Total |
| | | Units | | Amount | | | | | | |
| Balance at December 31, 2019 | | | 42,398,572 | | | $ | 19,057,422 | | | $ | — | | | $ | (13,937,695 | ) | | $ | 5,119,727 | |
| Issuance of membership units, net of issuance costs | | | 646,688 | | | | 1,205,278 | | | | — | | | | — | | | | 1,205,278 | |
| Issuance of membership units for loan repayment | | | 39,440 | | | | 64,287 | | | | — | | | | — | | | | 64,287 | |
| Stock-based compensation expense | | | 390,986 | | | | 755,717 | | | | — | | | | — | | | | 755,717 | |
| Net loss | | | — | | | | — | | | | — | | | | (2,199,694 | ) | | | (2,199,694 | ) |
| Balance at December 31, 2020 | | | 43,475,686 | | | | 21,082,704 | | | | — | | | | (16,137,389 | ) | | | 4,945,315 | |
| Issuance of membership units, net of issuance costs | | | 903,846 | | | | 1,720,049 | | | | — | | | | — | | | | 1,720,049 | |
| Issuance of membership units for loan repayment | | | 39,440 | | | | 64,287 | | | | — | | | | — | | | | 64,287 | |
| Stock-based compensation expense | | | — | | | | — | | | | 888,333 | | | | — | | | | 888,333 | |
| Net loss | | | — | | | | — | | | | — | | | | (2,857,806 | ) | | | (2,857,806 | ) |
| Balance at December 31, 2021 | | | 44,418,972 | | | $ | 22,867,040 | | | $ | 888,333 | | | $ | (18,995,195 | ) | | $ | 4,760,178 | |
The accompanying notes are an integral part of these consolidated financial statements.
McGinley Orthopaedic Innovations, LLC
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2021 and 2020
| | | 2021 | | 2020 |
| Cash flows from operating activities: | | | | |
| Net loss | | $ | (2,857,806 | ) | | $ | (2,199,694 | ) |
| Adjustments to reconcile net loss to net cash used by operating activities: | | | | | | | | |
| Depreciation and amortization | | | 202,141 | | | | 199,714 | |
| Stock-based compensation expense | | | 888,333 | | | | 755,717 | |
| Gain on extinguishment of debt | | | (316,500 | ) | | | (316,500 | ) |
| Changes in assets and liabilities: | | | | | | | | |
| Accounts receivable, net | | | (74,049 | ) | | | 77,110 | |
| Inventories | | | 18,083 | | | | 47,936 | |
| Prepaid expenses | | | (26,741 | ) | | | 45,096 | |
| Accounts payable and accrued liabilities | | | 111,470 | | | | (94,590 | ) |
| Net cash used by operating activities | | | (2,055,069 | ) | | | (1,485,211 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | (281,489 | ) | | | (35,126 | ) |
| Investment in intangible assets | | | (36,915 | ) | | | (44,732 | ) |
| Net advances to related parties | | | (65,736 | ) | | | (2,997 | ) |
| Net cash used by investing activities | | | (384,140 | ) | | | (82,855 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from PPP loans | | | 316,500 | | | | 316,500 | |
| Proceeds from membership units issued | | | 1,762,501 | | | | 1,217,239 | |
| Capital raise costs | | | (42,452 | ) | | | (11,961 | ) |
| Net cash provided by financing activities | | | 2,036,549 | | | | 1,521,778 | |
| | | | | | | | | |
| Net change in cash | | | (402,660 | ) | | | (46,288 | ) |
| Cash at beginning of year | | | 2,026,933 | | | | 2,073,222 | |
| Cash at end of year | | $ | 1,624,273 | | | $ | 2,026,934 | |
| | | | | | | | | |
| Supplemental and non-cash disclosures | | | | | | | | |
| Cash paid for income taxes | | $ | — | | | $ | — | |
| Cash paid for interest | | $ | — | | | $ | — | |
| Issuance of member units for payment on note payable | | $ | 64,287 | | | $ | 64,287 | |
The accompanying notes are an integral part of these consolidated financial statements.
McGinley Orthopaedic Innovations, LLC
Notes to the Consolidated Financial Statements
December 31, 2021 and 2020
Notes A – Nature of Business
McGinley Orthopaedic Innovations, LLC (“MOI”) is a Wyoming limited liability corporation. MOI was established in 2012 with the goal of increasing patient safety and physician confidence through technological advances in the orthopedic field. MOI has developed and is marketing patented, FDA-approved Intellisense Drill Technology®, an orthopedic power tool, and the Lever Action Plate System® bone fixation implant system.
DS Manufacturing LLC, known commonly as McGinley Manufacturing, is a wholly-owned subsidiary of MOI, and operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, welding, machining, replacement part fabrication, CNC plasma cutting, powder coating, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scal pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries.
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of MOI, and holds title to all of the patents assigned to MOI.
Note B – Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements and related notes include MOI and its wholly owned subsidiaries described in Note A. Hereinafter, they are collectively referred to as the Company. All significant inter-company accounts and transactions have been eliminated in consolidation.
Basis of Accounting
The Company prepares its consolidated financial statements using the accrual methods of accounting in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Such estimates include inventory reserves for obsolescence, allowance for uncollectible receivables, depreciable lives of property and equipment and costs of patents applied for. Actual results could differ from those estimates.
Recent Accounting Pronouncements
The Company periodically reviews new accounting standards that issued as Accounting Standards Updates (“ASU”) by the Financial Accounting Standards Board (“FASB”). The Company carefully considers all new pronouncements that alter previous U.S. GAAP, and has identified the following new accounting standards that it believes merits further discussion. Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption.
The Company adopted Accounting Standards Codification (“ASC”) 606.Revenue from Contracts with Customers as of January 1, 2020 using the modified retrospective method for all contracts with customers. The Adoption did not result in an impact on operating retained earnings. No significant judgements were made in the application of the guidance in ASC 606.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This guidance amends the existing accounting standards for lease accounting, including requiring lessees to recognize most leases on their balance sheet. Under ASU 2020-05, ASU 2016-02 is effective for years beginning on or after December 15, 2021. Management is evaluating the impact of this ASU on the Company’s financial reporting.
Cash
The Company deposits cash with financial institutions which management believes are of high credit quality. The Company does not believe it is exposed to significant risk. At December 31, 2021 and 2020, there were no cash equivalents.
Accounts Receivable
Accounts receivable are stated at the amount management expects to collect for outstanding balances. Management provides for probable uncollectible accounts through an allowance for doubtful accounts. The allowance is estimated based on historical performance and management’s assessment of the current status of individual accounts. Management estimated an allowance of approximately $5,000 and $7,000 at December 31, 2021 and 2020, respectively, which is based on an analysis of individual trade accounts, historical experience with customers and general economic conditions.
Inventory
Inventories are valued on the lower of cost or market. Inventory is valued at the lower of first-in, first-out cost or net realizable value, which is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The Company has established the policy of capitalizing manufacturing materials and supplies, labor, overhead, and expenses allocable to finished goods and assemblies for the inventory valuation. The Company also carries expendable supplies inventory. This includes expendable items that are consumed in the manufacturing process.
As of December 31, 2021 and 2020, management has recorded a reserve of approximately $1,327,000 for both years. Inventories consisted of the following at December 31:
| | | 2021 | | 2020 |
| Materials and supplies | | $ | 201,953 | | | $ | 163,695 | |
| Work in process and finished goods | | | 2,479,665 | | | | 2,536,006 | |
| Inventory reserve | | | (1,326,607 | ) | | | (1,326,607 | ) |
| Total inventories | | $ | 1,355,011 | | | $ | 1,373,094 | |
Property and Equipment
Property and equipment are recorded at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. Maintenance and repairs are charged to expense as incurred. Betterments and renewals are capitalized. When property and equipment are sold or otherwise disposed of, the asset account and related accumulated depreciation account are relieved, and any gain or loss is included in operations. The straight-line method of depreciation is used over the following estimated useful lives:
| | | Years |
| Machinery | | 5-15 |
| Leasehold improvements | | 5-15 |
| Computers and software | | | 5 | |
| Furniture and equipment | | | 5-15 | |
| Buildings | | | 39 | |
| Vehicles | | | 5 | |
| Land | | | 10 | |
Additions and improvements are capitalized, while replacements, maintenance, and repairs, which do not improve or extend the life of the respective assets, are expensed as incurred. Gains and losses from the disposition of assets are recorded in the year of disposition.
Intangible Assets
Intangible assets consist of patents and patents pending. Patents and patents pending are being amortized on the straight-line method over twenty years, but not exceeding the initial expiration date of the patent.
Impairment of Long-Lived Assets
Long-lived assets such as property and equipment, and intangible assets subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If this review reveals an indicator of impairment, as determined based on estimated undiscounted cash flows, the carrying amounts of the related long-lived assets are adjusted to fair value. Management has determined that there has been no impairment in the carrying value of its long-lived assets as of December 31, 2021 and 2020.
Revenue Recognition
All revenues from exchange transactions are recorded in accordance with ASC 606, which is recognized when: (i) a contract with a customer has been identified, (ii) the performance obligation(s) in the contract have been identified, (iii) the transaction price has been determined, (iv) the transaction price has been allocated to each performance obligation in the contract, and (v) the Company has satisfied the applicable performance obligation at a point in time or over time.
The Company’s revenues are primarily generated from the sale of products, and represent a single performance obligation and are earned at a point in time when the goods have been shipped. Payment for the remaining customer’s is due between 15-30 days. The Company’s revenues also contain drill system rentals to customers and are recognized on a per use and/or monthly basis. All revenue is earned in the United States.
The Company’s revenue by type are summarized as follows for the year ended December 31:
| | | 2021 | | 2020 |
| Product sales | | $ | 1,219,511 | | | $ | 1,004,696 | |
| Drill system rentals | | | 132,785 | | | | 12,593 | |
| Total revenues | | $ | 1,352,296 | | | $ | 1,017,289 | |
Research and Development
Research and development costs that do not meet the criteria for capitalization are expensed as incurred. Research and development expenses include compensation, employee benefits, and incentive-unit-based compensation for employees, as well as fees paid to outside consultants. Nonrefundable advanced payments, for goods and services that will be used in future research development activities are expensed when the activity is performed or when the goods have been received, rather than when payment is made, in accordance with ASC 730, Research and Development.
Stock-based Compensation
For stock-based compensation awards, the Company measures compensation costs for these awards to employees and non-employees based upon the fair value of the award on the date of grant.
Income Taxes
Effective January 1, 2016, the Company elected to be taxed as a C corporation. The Company files a consolidated income tax return with its subsidiaries in the U.S. Federal jurisdiction. Generally, the Company’s tax returns remain open for three years for Federal income tax examination. At December 31, 2021, the Company believes there are no significant uncertain tax positions or liabilities, or interest and penalties associated with uncertain tax positions.
The Company’s income tax expenses consists of the following components: current and deferred. Current income tax expense reflects taxes to be paid or refunded for the current period by applying the provisions of the enacted tax law to the taxable income or excess of deductions over revenues. The Company determines deferred income taxes using the liability (or balance sheet) method. Under this method, the net deferred tax asset or liability is based on the tax effects of the differences between the book and tax bases of assets and liabilities, and enacted changes in tax rates and laws are recognized in the period in which they occur.
Deferred income tax expenses results from changes in deferred tax assets and liabilities between periods. Deferred tax assets are recognized if it is more likely than not, based on the technical merits, that the tax position will be realized or sustained upon examination. The term more likely than not means a likelihood of more than 50 percent; the terms examined and upon examination also include resolution of the related appeals or litigation processes, if any. A tax position that meets the more-likely-than-not recognition threshold is initially and subsequently measured as the largest amount of tax benefit that has a greater than 50 percent likelihood of being realized upon settlement with a taxing authority that has full knowledge of all relevant information. The determination of whether or not a tax position has met the more-likely-than-not recognition threshold considers the facts, circumstances, and information available at the reporting date and is subject to management’s judgement. Deferred tax assets are reduced by a valuation allowance if, based on the weight of evidence available, it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Note C – Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. For the years ended December 31, 2021 and 2020, the Company incurred net losses of approximately $2,878,000 and $2,205,000, respectively, and had negative cash flows from operations in the amount of approximately $2,055,000 and $1,485,000, respectively. As of December 31, 2021 and 2020, the Company had an accumulated deficit of approximately $19,021,000 and $16,143,000, respectively. These matters raise substantial doubt about the Company’s ability to continue as a going concern.
The ability of the Company to continue as a going concern is dependent upon increasing sales and obtaining additional capital and financing. Managements intends to attempt to raise funds by way of private offerings. While the Company believes in the viability of its strategy to increase sales and in its ability to raise additional funds, there can be no assurances to that effect. The financial statements do not include adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Note D – Property and Equipment
Property and equipment consisted of the following as of December 31:
| | | 2021 | | 2020 |
| Machinery | | $ | 1,555,335 | | | $ | 1,448,200 | |
| Leasehold improvements | | | 152,752 | | | | 39,202 | |
| Computers and software | | | 75,612 | | | | 68,603 | |
| Furniture and equipment | | | 46,341 | | | | 30,834 | |
| Buildings | | | 27,482 | | | | — | |
| Vehicles | | | 20,244 | | | | 20,244 | |
| Land | | | 10,806 | | | | — | |
| | | | 1,888,572 | | | | 1,607,083 | |
| Less: accumulated depreciation | | | (697,726 | ) | | | (533,766 | ) |
| Property and equipment, net | | $ | 1,190,846 | | | $ | 1,073,317 | |
For the years ended December 31, 2021 and 2020, the depreciation expense was approximately $164,000 and $154,000 respectively. The expense is split between cost of goods sold and selling, general and administrative expenses. Depreciation expense included in costs of goods sold was approximately $151,000 and $141,000, respectively.
Note E – Intangible Assets
Intangible assets consisted of the following as of December 31:
| | | 2021 | | 2020 | | Useful Life (years) |
| Intellectual property | | $ | 802,580 | | | $ | 765,667 | | | | 20 | |
| Less: accumulated amortization | | | (300,627 | ) | | | (262,448 | ) | | | | |
| Intangible assets, net | | $ | 501,953 | | | $ | 503,219 | | | | | |
Amortization expense charged to operations for the years ended December 31, 2021 and 2020 was approximately $38,000 and $46,000, respectively. The following is a schedule of the estimated amortization expense for intangible assets over the remaining useful life:
| Years ending December 31, | | |
| | 2022 | | | | 35,908 | |
| | 2023 | | | | 35,908 | |
| | 2024 | | | | 35,908 | |
| | 2025 | | | | 35,908 | |
| | 2026 | | | | 35,515 | |
| | Thereafter | | | | 322,806 | |
| | | | | $ | 501,953 | |
Note F – Note Payable – Related Parties
On April 17, 2015 the Company entered into agreement to purchase DS Manufacturing. The purchase agreement called for the Company to purchase DS Manufacturing for a total of $450,010, which was to be paid in membership units of the Company over six years. The balance of the note payable as of December 31, 2021 and 2020, was zero and $64,287 each year. These payments represent non-cash financing payments. As of December 31, 2021, the note payable was paid in full.
Note G – Notes Payable – Paycheck Protection Program Loans
In March 2020, Congress established the Paycheck Protection Program (“PPP”) to provide relief to small businesses during the coronavirus pandemic (“COVID-19”) as part of the Coronavirus Aid, Relief, and Economic Security Act. The legislation authorized Treasury to use the Small Business Association’s 7(a) small business lending program to fund forgivable loans that qualifying businesses could spend to cover payroll, mortgage interest, rent, and utilities during the “Covered Period” defined as the 24-week period starting on the date the PPP loan proceeds are received. Upon meeting certain criteria as specified in the PPP program, the loan is eligible for partial or total forgiveness. The Company received two PPP loans totaling $316,500 in April 2020, and two PPP2 loans of $316,500 in January 2021. These loans were fully forgiven in the respective year they were received, and are reflected as a gain on extinguishment of debt on the consolidated statements of operations.
Note H – Line of Credit
The Company has a $1,000,000 line of credit with a bank which matures February 8, 2024. Interest is subject to change on a monthly basis, calculated at the prime plus a variable rate (3.50% as of December 31, 2021). There was no outstanding balance on the line of credit as of December 31, 2021 and 2020.
McGinley Orthopaedic Innovations, LLC
Notes to the Consolidated Financial Statements
December 31, 2021 and 2020
Note I – Member’s Equity
The Company has the ability to issue an unlimited number of membership units. As of December 31, 2021 and 2020, the Company had 44,418,972 and 43,475,686 membership units issued and outstanding. All membership units have equal voting rights and allocations of profits and losses.
For the years ended December 31, 2021 and 2020, the Company issued 903,846 and 646,688 membership units for cash of approximately $1,720,000 and $1,205,000, respectively, net of issuance costs.
Accrued Member Units
For the years ended December 31, 2021 and 2020, the Company awarded the nine Board of Advisor members 455,556 and 390,986 shares of member units, respectively, valued at $1.95 per member unit, which was based on the most recent issuance price. This compensation for management services was reported as stock-based expense in the amount of $888,333 and $755,717, respectively. These costs are included in wages and benefits on the consolidated statements of operations. As of December 31, 2021, the 455,556 member units earned by the nine Board of Advisor members had not been issued and are included in member units to be issued on the consolidated balance sheets.
As of December 31, 2021, the Company was committed to issuing the following member units to the Board of Advisors related to their agreements. The Company estimated the value of the member units based on the then current issuance price. These have not been accrued as of December 31, 2021 as the Board of Advisor must continue to participate as an advisor to earn the awards.
| Year-ended | | Awarded units | | Estimated value of the units |
| | 2022 | | | | 146,368 | | | $ | 285,417 | |
Note J – Income Taxes
No provision or benefit for federal or state income taxes has been reflected for the year ended December 31, 2021 and 2020 since the Company reported losses and has established a valuation allowance against the total net deferred tax asset.
Significant components of the Company’s net deferred tax assets are as follows as of December 31:
| | | 2021 | | 2020 |
| Net operating loss carryforward | | $ | 2,127,185 | | | $ | 1,810,683 | |
| Property and equipment | | | (84,562 | ) | | | (86,646 | ) |
| Inventory reserve | | | 278,587 | | | | 278,587 | |
| Intangible assets | | | 10,424 | | | | 12,399 | |
| Stock-based compensation | | | 186,550 | | | | — | |
| Valuation allowance | | | (2,518,184 | ) | | | (2,015,023 | ) |
| Net deferred tax asset | | $ | — | | | $ | — | |
At December 31, 2021 and 2020, the Company had federal net operating loss carryforwards of approximately $10,129,000 and $8,622,000, respectively. The federal net operating losses beginning in 2018 may be carried forward indefinitely but are limited to 80% of the taxable income in any one tax period. The deferred tax assets has been adjusted to account for the limitation. The pre-2018 net operating losses are approximately $2,378,000 and can be carried back two years and forward 20 years, and will begin expiring in 2036. The Company’s federal and state blended tax rate was 21% for the years ended December 31, 2021 and 2020, respectively.
The entire balance of the deferred tax asset has been offset by a valuation allowance since there may be limitations on the amount of net operating loss carryforwards which can be used in future years, and utilization of net operating loss carryforwards cannot be sufficiently assured at December 31, 2021 and 2020.
Note K – Related Party Transactions
Related Party Receivables
The Company has receivables due from various related parties of approximately $72,000 and $7,000 as of December 31, 2021 and 2020, respectively.
Related Party Payables
The Company has payables due to various related parties of approximately $6,000 and $9,000 as of December 31, 2021 and 2020, respectively. These are included in accounts payable on the accompanying consolidated balance sheets.
Related Party Operating Lease
The Company has an operating lease for office space from a related party which began in January 2021. The lease is for seven years and expires in December 2027. Rent expense is recognized on a straight-line basis over the term of the lease. The lease is also subject to a three percent minimum annual increase. In connection with the related party lease, approximately $102,000 was paid to the lessor for specific leasehold improvements requested by the Company.
Future minimum rental payments under non-cancellable operating leases (with initial or remaining lease terms in excess of one year) for each of the five years subsequent to December 31, 2021 are:
| | 2022 | | | $ | 113,221 | |
| | 2023 | | | | 113,280 | |
| | 2024 | | | | 113,341 | |
| | 2025 | | | | 113,403 | |
| | Thereafter | | | | 113,467 | |
| | Total | | | $ | 566,712 | |
Total combined rent expense associated with the operating leases amounted to approximately $114,000 and $0 for the years ended December 31, 2021 and 2020, respectively.
Note L – Concentrations and Commitments
Operating Leases
The Company leases office space under operating leases agreements that expire at various dates through July 2021. Rent expense was recognized on a straight-line basis over the term of the lease. Total combined rent expense associated with the operating leases amounted to approximately $64,000 and $20,000 for the years ended December 31, 2021 and 2020, respectively. Upon termination of these lease agreements, the Company leased office space with a related party as discussed at Note J.
COVID-19
Due to the COVID-19 outbreak in 2020, the Company was not closed at any point. Additionally, there were no staff furloughed or terminated due to COVID-19. Overall, the Company was not significantly affected by COVID-19.
Due to the level of risk this virus may have on the global economy, it is at least reasonably possible that it could have an impact on the operations of the Company in the near term that could materially impact the Company’s consolidated financial statements, however, management does not believe there will be any future impact. In addition, with vacancies and therapeutics coming to market, these will also help to mitigate any potential future losses.
Note M – Subsequent Events
On May 13, 2022, the Company filed to be a Wyoming corporation. The new name of the Company is McGinley Orthopaedic Innovations, Inc. The Company’s membership units converted to common stock, which have a par value of $0.001. The Board also established that the Company is authorized to issue 100,000,000 shares of common stock.
Between the balance sheet date for the year ending December 31, 2021 and July 13, 2022, the date the consolidated financial statements were available to be issued, the Copany issued 722,821 member units raising additional capital of approximately $1,410,000.
Subsequent events have been evaluated through July 13, 2022, which is the date the consolidated financial statements were available to be issued.
Exhibit Index
Exhibit 2A: Certificate of Incorporation and Other Documents
Exhibit 2B: Bylaws
Exhibit 4. Subscription Agreement
Exhibit 8. Escrow Agreement
Exhibit 11. Written Expert Consent Letter of Assurance Dimensions
Exhibit 12. Legal Opinion of Trae O’Neil High
Exhibit 13: Testing the Waters Materials
Signature Page
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Casper, WY, on August 11, 2022.
(Exact name of the Issuer as specified in its Charter)
McGinley Orthopaedic Innovations, Inc.
234 E. 1st St. Suite 242
Casper, WY 82601
(307) 315-6403
By:
s/Dr. Joseph McGinley
Director, CEO, President, Treasurer, Secretary of McGinley Orthopaedic Innovations, Inc.
(Date): August 11, 2022
Location Signed: Casper, WY
This Offering Statement has been signed by the following Officers in the capacities and on the dates indicated.
s/Dr. Joseph McGinley
CEO, President, Treasurer, Secretary of McGinley Orthopaedic Innovations, Inc.
(Date): August 11, 2022
Location Signed: Casper, WY
This Offering Statement has been signed by the following Directors in the capacities and on the dates indicated.
s/Dr. Joseph McGinley
Director of McGinley Orthopaedic Innovations, Inc.
(Date): August 11, 2022
Location Signed: Casper, WY







