FORM 1-K PART II
Annual Report
For
McGinley Orthopaedic Innovations, Inc.
A Wyoming Corporation
May 01, 2023
THIS ANNUAL REPORT MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE ANNUAL REPORT, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
TABLE OF CONTENTS
BUSINESS
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
DIRECTORS AND OFFICERS
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
FINANCIAL STATEMENTS
Item 1.
BUSINESS
Corporate History
McGinley Orthopaedic Innovations, Inc. (the “Company”) is an orthopedic surgical device/implant innovator and manufacturer based in Casper, Wyoming. The Company was founded as McGinley Orthopaedic Innovations, LLC in 2012. The Company converted to a Wyoming Corporation on May 13, 2022 by filing Articles of Incorporation and Statement of Conversion with the Wyoming Secretary of State (See Exhibit 2A “Certificate of Incorporation and Other Documents”).
Summary of the Company
The Company was established in 2012 with the goal of increasing patient safety and physician confidence through technological advances in the orthopedic field. The Company has developed and is marketing patented, FDA-cleared IntelliSense Drill Technology®, a set of orthopedic power tools and accessories, and the Lever Action Plate System® bone fixation implant system. The Company aims to make its growing product lines the “standard of care” in orthopedic surgery worldwide. Its mission is three-fold: increase patient safety, improve patient care, and reduce surgical cost.
The patented and proprietary sensing, navigation, and robotic capabilities of the IntelliSense Drills were cleared for commercial sale by the FDA on February 20, 2015 through clearance by FDA of the Company’s 510(k). The IntelliSense Drills exhibit capabilities far beyond those available to surgeons in the legacy devices offered by any other power tool manufacturer. “Legacy” devices is a reference to the current status quo products of the orthopedic surgery industry.
The Lever Action Plate System® (“LAPS”) was cleared for commercial sale by the FDA on June 25, 2020 through clearance by FDA of the Company’s 510(k). The Company believes that the LAPS will revolutionize wrist surgery and dramatically improve patient outcomes.
The Company’s third major product is the Freedom Nail: Intramedullary Hindfoot Nail. The Company is applying for FDA 510(k) clearance in Q2 2023, is estimating FDA clearance in Q3 2023, and planning for commercial launch in Q4 2023. The Company acquired exclusive rights to manufacture this technology along with associated issued patents.
An additional product launching in 2023 is the IntelliSense Cannulated Drill Bit. This product gives the surgeon the ability to drill a hole over a guide wire to help insure proper trajectory. These drill bits are often used in lower extremity procedures and will provide expanded use of the IntelliSense Drill Technology®. The Company anticipates that this product will be commercially available in Q3 2023.
The IntelliSense Drill Technology® and accessories will be referred to as the “IntelliSense Drill(s)” or “IntelliSense products”. The Lever Action Plate System® line of products will be referred to as “LAPS” or “LAPS products”. Both “IntelliSense Drill Technology" and “Lever Action Plate System” are registered trademarks owned by the Company. Collectively the IntelliSense products and LAPS products will be referred to collectively as the (the “Products”).
The Company currently owns 129 issued or pending patents and has already attracted substantial interest from leading orthopedic device makers in the U.S. The Company is engaged in partnering discussions with several firms related to technology development, licensing, commercialization, and distribution in various segments of the national and global orthopedic power tool and implant markets.
The Company has two wholly-owned subsidiaries: DS Manufacturing LLC and McGinley Engineered Solutions, LLC. DS Manufacturing LLC, has a DBA as McGinley Manufacturing, is a wholly-owned subsidiary of the Company, and operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, machining, replacement part fabrication, CNC plasma cutting, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scale pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries.
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of the Company and holds title to all of the patents assigned to the Company.
IntelliSense Drill Technology®

IntelliSense Technology includes a series of surgical drills that have proprietary sensing, navigation, and robotic capabilities far beyond those available to surgeons in the devices offered by any other surgical power tool manufacturer.
The Company’s first products using IntelliSense Technology received FDA 510(k) clearance in February 2015 and have been used in over 1,000 surgical procedures at over 25 orthopedic surgical centers and teaching hospitals across the United States. The hand-held, electric, surgical drill is designed for routine use in many common orthopedic procedures that require the placement of hardware and bone drilling. Unique design features of IntelliSense Drills include (1) an ergonomic and distinctive drill housing; (2) an integrated and automated depth measurement component, (3) a chuck uniquely designed to engage the Company’s proprietary quick-release drill bits; (4) proprietary integrated lighting; (5) an electronic control unit that provides power, engages sensors, collects and analyzes data, and displays information in real time on a touchscreen visible to the entire surgical team and (6) an auto-stop safety feature that can be set to stop the forward progress of the bit in multiple modes. The IntelliSense Drill’s high-torque motor and a broad range depth sensor is suited to large/small bone drilling and fracture fixations. Furthermore, the IntelliSense Drill is significantly smaller, lighter, easier to use, and more accurate than other standard non-sensing orthopedic drills in service today.
IntelliSense Drill Technology® has been reported by physicians to reduce the number of wasted screws, plates, and other consumables and may shorten the duration of a typical orthopedic surgery by 20 minutes or more.
Features of the IntelliSense Drill:
· The IntelliSense Drill includes an integrated and automated depth measurement component that, together with a microprocessor, has several modes. The main feature of the IntelliSense Drill Technology® is the depth measuring component that stops the drill once the desired depth has been achieved. This significantly reduces the risk of drilling too deep into the bone and “plunging,” or drilling through the bone. The measuring device will stop the drilling automatically depending on which mode the user selects. These modes include (1) Bicortical (Drill stops upon breaching the second cortex); (2) Freehand (Drill operates as a standard orthopedic drill while including depth measurement); (3) Set Depth (Drill stops at a predetermined depth), and (4) Multicortex (Drill stops upon breaching a preset number of cortices).
· Orthopedic drill bits are notoriously difficult to sterilize. Minimizing infection risk depends on the consistent use of a new drill bit for each surgery. Preventing surgical site infection remains one of the key patient safety goal standards per the National Patient Safety Goals Joint Commission (NPSG.07.05.01). To assure the IntelliSense Drill bits are proprietary, the Company has designed a patented interlock sensitive to autoclave sterilization which prevents drill bit reuse. Replacement of the Drill bits will drive a secondary and constant revenue stream for the Company.
· LED lights mounted to the front of the drill housing that illuminates the operative site are patented and unique to the IntelliSense Drill. In-Sight Surgical Lighting® can be activated throughout the procedure, only during active drilling, or turned off. Enhanced visibility is achieved without cumbersome, outside sources such as headlamps, ceiling-hung, and roll-around lights that cast shadows.
IntelliSense Drill Accessories
Universal Chuck Set for the IntelliSense Drill

The accessories include a keyed chuck, keyless chuck, standard AO chuck, and a patented universal pin driver. While these chucks allow the IntelliSense Drill to be used with a wide range of generic products, the benefits of the IntelliSense Drill Technology® - in particular the auto-stop and depth measurement features – will not function while using the generic products.
The Equalizer: Offset Reference Guide®

A companion tool to the IntelliSense Drill, the patented Equalizer: Offset Reference Guide®, determines the size and length differences that are manufactured into plate and screw sets developed by different manufacturers. With some plate systems, the plates, screws, and depth gauges are manufactured for exclusivity within its own system. Using a depth gauge from one company will give accurate results for plates and screw sets made by that company but may be completely unreliable for plates and screws made by a different company because of their proprietary offsets. These systems do not give an actual measurement, but only a screw size number; therefore, they are incompatible with the IntelliSense Drill, which is a true measurement device. The Equalizer was developed to eliminate the issue of manufactured plate offsets. The Equalizer can determine the offset of a plate needed for a surgical procedure, and that offset can be inputted in the IntelliSense Drill for compatibility with all plating systems.
The Revolver® Universal Drill Bit Guide System

The patented Revolver revolutionizes conventional drill bit guides by replacing multiple standard drill bit guides with one 10-piece kit. It features a rotating chamber that adjusts to multiple drill bit diameters from 1.0mm to 5.0mm. It has a range of attachments that extend the length of the guide up to 220mm for percutaneous applications. It has a non-slip tip providing stability on the bone and a comfortable, ergonomic handle. The surgeon’s hand placement is safely away from the drilling point. The Revolver is universal with most standard drill bits. Some of its benefits include cost savings by eliminating numerous conventional drill bit guides, ease of inventory management and sterilization, reducing surgical time in switching and locating various guides and requires little to no training.
Advantages of the IntelliSense Drill Technology®
Speed of IntelliSense Drill Technology®
Hospitals rigorously schedule operating rooms to maximize utilization of scarce resources. According to studies from Akron General Hospital and Northwestern Memorial Hospital, typical Level 1 Trauma Center Operating Room cost is in the range of $20.93 to $97.00 per minute. According to a 2005 study of 100 U.S. hospitals, the average charge for the Operating Room was $62.00 per minute, not including extra resources specific to the procedure or provider fees and anesthesia. The time savings from the automatic measuring features of IntelliSense can add one procedure per day of operating room utilization.
Imaging to Confirm Measurement
Fluoroscopy is used to confirm screw measurements. For each image, the team must move the fluoroscope to the patient, position the machine arm over the incision, and move team members away from the radiation. Then they take the image, move the equipment back out of the way, and finally reposition the team and resume drilling. In a trauma setting, this takes approximately four to eight minutes per case; in a spine procedure, 25-40 minutes. Using the lower trauma figure, IntelliSense® will save OR time costs attributable only to fluoroscopy by approximately $496.
Less Screw Replacement
On average, when a screw is incorrectly sized, it takes five to ten minutes to remove and replace the screw and additional time to re-image the new screw, according to a surgeon at a hospital in New York. The Company estimates (statistically) the cost of screw replacement at $370 per typical trauma procedure.
Fewer Wasted Screws
Published literature indicates a screw error rate requiring removal and replacement of between 20% and 24.7% that is directly attributable to measurement error. Based on a minimum of six screws used per orthopedic surgery, the Company estimates 1 to 2 screw(s) requiring replacement per case. Fracture fixation screws cost approximately $35 to $150; uni-axial pedicle screws for certain spine surgeries cost up to $1,000 and multi-axial pedicle screws may cost up to $1,500. When surgeons recognize an incorrect screw length during surgery and replace it with one of proper length, hospitals often times pay for the error time, costing hundreds of dollars.
Mitigated Drill Plunging
When surgeons drill too far through a bone, blood vessels, nerves, tendons, and organs such as the spinal cord can be damaged. Plunging is a common and under-reported problem. In an informal experiment at a major trade show in 2015, over 150 surgeons were asked to drill holes in saw bones and avoid plunging. The surgeons averaged a 6.67mm plunge with a standard deviation of 3.32mm. Many critical organs and structures lie within 7mm of a bone. This simple experiment shows that, despite frequent denials, surgeons often plunge even when they consciously try not to. In the worst cases, plunging too far can "wind up" nerves and vessels in an instant, causing serious injury or even death.
Fewer Screw Injuries
Measurement errors may result in excessively long screws that protrude through bones or short screws causing an unstable plate. Long screws can rub or erode tissue causing pain or harm as seen in the testimonial IntelliSense Drill commercial. Especially dangerous for patients is a spinal pedicle screw that is too long as it can compress the outer wall of the aorta threatening to erode and puncture the vessel. It is potentially fatal.
Automatic and Speedy Measuring
To avoid plunging and screw length errors, orthopedic surgeons interrupt the surgical procedure several times to measure each hole. Errors in screw length are common in orthopedic surgeries. Average trauma surgeries consume up to ten screws, so interrupting the surgical process to measure hole depth consumes significant amounts of very expensive operating room time.
An average trauma case uses 10 fixation screws. Surgeons spend one minute (or more) per screw to stop, measure and re-drill each hole (10 mins x $62/min = $620).
Lever Action Plate System® for Distal Radius Fractures

The Company’s second major product is the Lever Action Plate System® for Distal Radius Fractures. The Lever Action Plate System® received FDA 510(k) clearance in June 2020. The Company acquired exclusive rights to manufacture this technology along with associated issued patents.

The Lever Action Plate System® is a novel solution for distal radius fractures, a wide range of wrist fractures that affect the joint. This innovative plate system features proprietary beams that align volar tilt, an angle of the bones in the wrist. The beams are inserted into the bone fragment and with the turn of a screw, the beams and the fragment elevated into the surgeon’s desired placement. An improvement on the current, generic plates available on the market, the system has optional variable angle screws of varying sizes with patented locking technology. The contour of the plate is designed to adhere comfortably to the watershed line, a theoretical line on the radius in relation to tendons and nerves.

Since FDA clearance, Company engineers have partnered with physicians to perfect the design and bring the device to market. The plates are currently manufactured by Affiliate McGinley Manufacturing. The Company reserves the right to use third party manufacturers for the manufacture of any of the Products, including the IntelliSense Drills and Lever Action Plate Systems.
Ankle Fusion Nail System for End Stage Ankle Osteoarthritis (Launching in 2023)

The Company’s third major product is the Freedom Nail: Intramedullary Hindfoot Nail. The Company is applying for FDA 510(k) clearance in Q2 2023, is estimating FDA clearance in Q3 2023, and planning for commercial launch in Q4 2023. The Company acquired exclusive rights to manufacture this technology along with associated issued patents.
Ankle fusions are the most commonly done surgery for end stage ankle osteoarthritis, and with previously existing technology are complex procedures requiring the surgeon to make tradeoffs among competing needs for effective infection prevention, precise preparation of the fusion site, and placement of stable rigid fixation. Current techniques of fixation include percutaneous cannulated screws and plates, and fusion of both of the joints in an ankle. The Company’s Freedom Nail can be a much simpler, safer, and more effective procedure because it (i) provides a rigid, stable fixation beyond that of a plate, (ii) requires fusion of only one of the two joints in an ankle, instead of both, and (iii) and is implanted through small incisions away from the fusion site, thus decreasing the risk of wound complications.
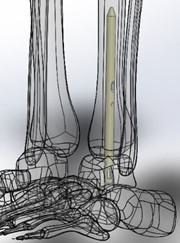
The most common current ankle fusion surgeries require fusing both of the two joints in an ankle. The Company’s ankle fusion nail enables the surgeon to fuse only one of the two joints in an ankle, rather than both as in the current standard of care. The Freedom Nail’s various screw locations make it easier to insert the nail, even without a skilled surgical assistant. The patient can remain in a supine position for both the arthroscopic preparation of the tibiotalar joint and for implantation of the nail device. There is no need to reposition the patient during the procedure. What this means for the patient: the entire system is safer. What this means for the surgeon: the entire system is more reliable and easier to use. What this means for the hospital and the payor: the procedure takes less time and costs less to perform.
Clinical Collaborations for the Company’s Products
The Company supports and collaborates on independent and joint research projects with several academic and medical institutions across the country. Such collaborations enable the Company to get its products into the hands of leading medical researchers and refine IntelliSense Technology. The institutions with which the Company collaborates include:
· University of Wyoming (IntelliSense: artificial intelligence/augmented reality)
· New York University (IntelliSense)
· Shriners Hospital for Children – Philadelphia (IntelliSense)
· Temple University (IntelliSense)
· University of Pennsylvania Medical Center - Hamot Hospital (IntelliSense)
· University of Southern California (IntelliSense)
Intellectual Property
The Company’s intellectual property assets consist of patents, FDA 510(k) clearances, proprietary knowledge in orthopedics and sports medicine, trade secrets, mechanical and electrical engineering know-how, radiology know-how, artificial intelligence, registered trademarks, and copyrights. The Company continues to invest in building and protecting its portfolio of patents and proprietary know-how, spending approximately 10% and 9.24% of operating cost for this purpose in 2021 and 2022 respectively. It was issued 4 new patents and filed applications for 7 new patents in 2022.
Patents Summary
The Company has been issued 114 patents by the United States Patent and Trademark Office (“USPTO”) and foreign jurisdictions, and has 15 pending patent applications. Approximately 20 more patents are in process of preparation and filing with various jurisdictions.
FDA 510(k) Clearances
The Company has applied for and received two 510(k) clearances from the FDA. These 510(k) clearances were for the IntelliSense Drill Technology® and accessories and the Lever Action Plate System® bone fixation implant system. For its new Ankle Fusion Implant System, the Company expects to apply for a third 510(k) clearance from the FDA during Q2 2023.
Patent/Product Categories
The Company’s issued and pending US patents, and their foreign registrations, can be grouped into six product categories or families. These categories, and the number of the Company’s US and foreign registrations that are core technologies for each, are as follows:
| IntelliSense Drill and Related Systems/Improvements/Features | 69 |
| Orthopedic Implants | 21 |
| InteliiSense Pin Pilot, Driver and Accessories | 13 |
| Bone Saw and Related Systems/Improvements/Features | 16 |
| High Speed Burr | 3 |
| Hand Held Surgical Device Navigation System | 7 |
| Total Issued/Pending Patents | 129 |
Affiliates
DS Manufacturing LLC d/b/a McGinley Manufacturing
In April of 2015, the Company acquired DS Manufacturing LLC, its primary supplier of machined parts. In connection with that acquisition, DS Manufacturing LLC filed a “doing business as” and now conducts operations as McGinley Manufacturing. McGinley Manufacturing has nine (9) employees and revenue exceeding $1.0 million. McGinley Manufacturing operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, welding, machining, replacement part fabrication, CNC plasma cutting, powder coating, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scale pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries. McGinley Manufacturing holds the quality certifications ISO 13485:2016 (Medical devices - Quality Management Systems) and AS9100D/ISO 9001:2015 (Quality Management Systems – Requirements for Aviation, Space, and Defense Organizations).
McGinley Engineered Solutions LLC
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of the Company, and holds title to all of the patents used by the Company. This is the sole purpose of McGinley Engineered Solutions LLC.
TriOpportunity Investment Group LLC
TriOpportunity Investment Group LLC is owned by Dr. Joseph McGinley and his wife, Diane McGinley. TriOpportunity Investment Group LLC is controlled by Dr. Joseph McGinley. TriOpportunity Investment Group LLC owns the building from which the Company leases its offices.
McGinley Education Innovations LLC
McGinley Education Innovations LLC is wholly-owned by Dr. Joseph McGinley. McGinley Education Innovations LLC provides education and training for physicians. Dr. Joseph McGinley is a practicing physician, and McGinley Education Innovations LLC is the vehicle by which Dr. Joseph McGinley’s procedures are taught to other physicians. These procedures are unrelated to the Company’s Products.
Markets for Company Products
IntelliSense Drill Technology® Market
The Company’s market opportunity exists in the over seven million orthopedic surgeries performed annually in the United States alone, a number that grows each year. Each of these surgeries is time consuming, costly, and potentially risky for patients and surgical teams. In 2019, orthopedic surgeries consumed over six million drill bits worldwide (approximately four million in the U.S.), at a value exceeding $650 million. Sale of consumables associated with orthopedic surgeries (drill bits, pins, saw blades, high speed burrs), estimated to be 10.1 million in 2021, are expected to grow in parallel with drill sales to 14.5 million by 2027. The Company estimates U.S. sales of power drills – relatively low in volume but high in margin – to be approximately 24,000 units in 2020. The bulk of current annual sales volume is driven by replacement or updating of old equipment, and by restocking of consumables such as drill bits, pins, saw blades, burrs, and batteries. The Company’s global unit sales volume should increase as the IntelliSense Products are adopted by hospitals as the necessary standard of care in orthopedic surgery and the obsolete devices currently in use are replaced. The Company’s IntelliSense Products address a market in the U.S. alone exceeding $2.7 billion annually.
Customer Profile
The Company is marketing the IntelliSense Drill to orthopedic surgeons, surgical centers, and hospitals throughout the United States and globally. The main value proposition derives from the increased safety of using the IntelliSense Drill Technology® versus the current ‘dumb’ drills which are the current status quo.
Studies show that waste of implants (primarily plates and fixation screws) occurs in 12% to 30% of orthopedic surgical cases at a cost exceeding $162 million annually (U.S.). Globally, preventable orthopedic surgical errors cost in excess of $1.5 billion per year. This cost falls on patients, health care providers, and health insurers. The sensors, software, and real-time monitoring and measurement capabilities of the IntelliSense Drill may dramatically reduce orthopedic surgical errors, waste, and their associated economic costs.
The IntelliSense Drill Technology® assists surgeons and patients in numerous ways. It may make trauma and spine surgeries safer and less costly by (i) reducing the risk of surgeons drilling through bones (i.e., “plunging”) into patient’s arteries, veins, nerves, and vital organs; (ii) giving surgeons precise depth measurement and accurate screw sizing in real time; (iii) reducing or eliminating the need for x-rays during surgery, and (iv) precisely recording data for each bone penetration drilled by the surgeon. Independent studies show that use of IntelliSense Drill Technology® can reduce the cost of orthopedic surgery by approximately $1,850 per procedure.
In contrast to the IntelliSense Drill, conventional orthopedic power tools and current “standard of care” surgical procedures are obsolete. Conventional devices lack sensors, software, real-time data recording, and artificial intelligence embedded in the Company’s devices. Existing “standard of care” orthopedic surgical practices force surgeons to rely on “feel” to avoid plunging into the patient’s blood vessels, nerves, tendons and other structures and organs.
Lever Action Plate System® (“LAPS”) Market
The LAPS is a major advance in the repair of complex wrist fractures. This innovative plate system features proprietary beams that align volar tilt, an angle of the bones in the wrist. In the global plates and screws market, plates account for 83%, or $4.3 billion in 2020 and are expected to reach $5.6 billion by 2027.
Wrist fractures are the most common upper extremity fracture experienced in the U.S. population. The market size of volar distal radius plates is estimated to be 1.1 million units with a CAGR of 5.8% over the next five years. Volar distal radius plates make up 92% of the market, while dorsal plates are only 8%.
This innovative technology addresses a real and prevalent need in orthopedics. There are around 67 upper extremity fractures per 10,000 people annually in the U.S.; 25% of these fractures occur in the distal radius and ulna. In addition to the ability to restore volar tilt, the Lever Action Plate System has optional variable angle screws with patented locking technology. This hybrid type of plate and screw system gives surgeons flexibility to decide which product type that best suits the needs of the patient.
The global market for fracture fixation products, including plate systems, is estimated to reach $12.1 billion by 2025. This estimate is driven by an increasing incidence of fractures in the elderly population and a rapid adoption of internal fixation devices for small bones. The fracture fixation market is made up of both external and internal fixation devices, which include frames, pins, plates, intramedullary nails, cables, staples, and wires.
The leading competitors for the fracture fixation market are Johnson & Johnson, Stryker, Zimmer Biomet, and Smith & Nephew. Implanted medical devices are one of the most profitable businesses in the domestic healthcare industry.
Distal radius fractures account for about 20% of all fractures treated in emergency departments and 8% to 15% of all bony injuries in adults. A complication rate of 15% has been reported with traditional volar plating and post-op complication rates can be as high as 80%.
Freedom Nail: Intramedullary Hindfoot Nail Market
The Company’s Freedom Nail: Intramedullary Hindfoot Nail (trademark pending) is a major advance in ankle fusion. This innovation fusion offers two kinds of nails. Starting the traditional Tibiotalarcalcaneal (TTC) nail but adding on a second option, the first of its kind, a Tibiotalar (TT) nail. The TT nail offers the opportunity to fuse the Tibiotalar joint space and leaves the talocalcaneal joint free to move. Additionally, our intramedullary nail system offers the physician anterior and lateral screw insertion. These options allow for greater freedom for the surgeon and an option for those patients whose joint damage may not be as severe but could benefit from a partial fusion of the ankle joint. This safer, less time-consuming method is a first of its kind.
The global market for ankle fusion devices is estimated to be $1.4 billion with an expected compounded growth of 7.3%. Business Wire analysis says within the next seven years, 2023 to 2029 the estimated value will be $2.3 billion.
The leading competitors for ankle devices are Stryker, Arthrex, and DePuy Synthes.
Distribution of the Company’s Products
The Company’s marketing and sales efforts are focused on (i) building the Company’s sales “funnel”; (ii) empowering patients to advocate directly for surgeon use of IntelliSense, LAPS, and Freedom Nail; (iii) rolling out a nationwide team of experienced medical device sales representatives; and, (iv) adding distributors to support the nationwide sales team.
The Company’s management and nationwide sales and distribution team have established a large number of evaluation-for-purchase trials at leading orthopedic hospitals and medical schools across the country. The key selling points emphasize clinical, economic, and patient safety/outcome benefits to the hospital, surgeons, and staff.
To date, the Company has focused its sales efforts on teaching institutions and hospitals with the most reputable surgeons. Leading orthopedic surgeons are demonstrating IntelliSense and LAPS Products to residents, fellows, and interns, thus building future customers. Several of these surgeons have become champions of the Products and have aided in word-of-mouth efforts.
The Company will use the Proceeds to focus its sales and distribution team on national group purchase organizations (“GPOs”) and ambulatory surgery centers, the most rapidly growing segment among providers of orthopedic surgery.
The Company uses a traditional regional sales and distribution strategy to affect sales of its Products. The Company has four regional representatives on its internal sales team. The Company will continue to recruit qualified individuals to the sales team in coming quarters. As sales opportunities in discrete geographic areas increase, additional distributors will be brought on board.
In early 2023, the Company’s IntelliSense product suite received an Innovative Technology contract from Vizient, Inc., the nation’s largest member-driven healthcare performance improvement company. The contract was awarded based on the recommendation of IntelliSense Drill by hospital experts who serve on one of Vizient’s member-led councils, and it signifies to Vizient members unique qualities that potentially bring improvement to the healthcare industry. This contract will allow the Company the ability to offer negotiated pricing for the IntelliSense Drill to Vizient’s vast membership of healthcare providers, which includes more than 60% of the nation’s acute care providers and 25% of the non-acute market. (Vizient contract MS7259)
The Company has priced its Products competitively with industry standards. In addition to outright sale of the IntelliSense Drill, the Company offers hospitals convenient lease-to-own and per-use rental arrangements that eliminate the need for capital purchases that must endure the time-consuming consideration approval of the hospitals’ capital acquisition committees prior to purchase.
Announced Products
The Company is developing new products based on its 129 issued and pending patents that will, when introduced in the market over the coming five years, greatly increase the Company’s market opportunity and growth rate. These new products (and the product families of which they are a part) include the following:
- FreedomNail: Intramedullary Hindfoot Nail (Implants)
- IntelliSense Cannulated Drill Bit (Drill)
- IntelliSense Drill for Spine (Drill)
- IntelliSense Drill with Navigation (Navigation)
- IntelliSense MicroDrill with Navigation (Drill)
- Lever Action Plates for Tibial Plateau Fracture (Implants)
- Independent Navigation for Orthopedic Devices (Navigation)
The Company has announced the above products to the general public. As of the Date of this Annual Report, none of these announced products are ready for commercial distribution. Several of these following products either do not require 510(k) clearance or they will be covered under the Company’s current 510(k) as predicate devices.
IntelliSense Drill Version 2
The next generation of the IntelliSense Drill is expected to be released in Q3 2023. This new generation will provide an additional 30mm of depth measurement. Version 2 will also simplify the sterilization procedure so as to improve throughput in sterile processing equipment and departments.
IntelliSense Cannulated Drill Bit
Cannulated drill bits provide the surgeon with the ability to drill a hole over a guide wire to help insure proper trajectory. These drill bits are often used in lower extremity procedures and will provide expanded use of the IntelliSense Drill Technology®. The Company anticipates that this product will be commercially available in Q3 2023.
IntelliSense Pin Pilot
The Pin Pilot revolutionizes pin/wire drivers and employs technological advances for pin and K-wire placement. With IntelliSense Drill Technology®, this device can auto-stop after breaching a predetermined number of cortices, subchondral or endosteal, while simultaneously providing depth measurement and cortical edge detection.
The Company anticipates this product will be covered by the Company’s existing 510(k) for the IntelliSense Drill. The Company anticipates that this product will be commercially available in Q4 2023.
IntelliSense Spine (Specialized Products and Software)
IntelliSense Spine embodies patented and proprietary algorithms in software controlling the IntelliSense Drill and adapts the latter to surgery or the spine. The IntelliSense Drill Technology® will include four activities, three spine locations, and six modes of operation, including endosteal and subchondral modes. The Company believes that no 510(k) is needed for this product and anticipates commercial sales to begin in 2024.
IntelliSense Drill and MicroDrill with Navigation
IntelliSense Drill with Navigation will provide the surgeon with real-time point-of-contact navigation. This navigation system may also be an accessory to conventional drills. The MicroDrill with Navigation is a miniaturized device employing all features of the IntelliSense Drill plus revolutionary navigation software, for use in specialized orthopedic surgeries requiring the utmost delicacy and precision (e.g., fingers and spine surgeries).
The Company anticipates this product may be covered by the Company’s existing 510(k) for the IntelliSense drill. In the event the current 510(k) does not cover this product as a predicate device, the Company will submit a new 510(k) application prior to commercial sales. The Company anticipates that this product will be commercially available in 2024.
Special Characteristics of the Company’s Operations and Competing Products/Procedures
The Company is subject to regulation of its Products before, during, and after clearance by the FDA for commercial distribution of the Products.
Facilities Regulation
Regulation of the Company as a Device Manufacturer
The Company may outsource some of the manufacturing of the Company’s Products, in part, to third party manufacturers. The Company will contract only with manufacturers who meet the regulatory requirements of a medical device manufacturer.
Quality System Regulation and Good Manufacturing: Medical Devices
After clearance of the Products by the FDA through the 510(k) process, the Company will be subject to FDA Quality Systems Regulation (“QSR”) and Good Manufacturing Practices” (“GMPs”). Manufacturers must establish and follow quality systems to help ensure that their medical device products consistently meet applicable requirements and specifications known as current good manufacturing practices. Continued Compliance with the QSRs and GMPs is an ongoing duty of the Company. On February 23, 2022, the FDA proposed a regulation which would incorporate the international standard specific for medical device quality management systems set by the International Organization for Standardization (“ISO”), ISO 13485:2016 Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. The Company intends to use contract manufacturers and McGinley Manufacturing for manufacturing of its Products. All of these contract manufacturers will be required by the Company to be ISO 13485:2016, and thus compliant with FDA GMPs.
The Company does not anticipate that this proposed regulation will affect the operations of the Company, since the Company already is certified ISO 13485:2016.
The Company will maintain certification with AS9100-Aerospace Management Standard ensuring the possibility of growth in the aerospace and defense industries at McGinley Manufacturing outside the current medical device and mining capabilities.
Employees
The Company, not including its subsidiaries, has nine (9) full-time employees and four (4) part-time employees.
Item 2.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS.
The following is a discussion of the financial condition of the Company. On May 13, 2022 the Company executed a conversion from McGinley Orthopaedic Innovations, LLC, a Wyoming limited liability company, to McGinley Orthopaedic Innovations, Inc., a Wyoming corporation. All existing membership units at that time were converted to common stock on a 1 to 1 basis. All figures and disclosures in this section will be those of McGinley Orthopaedic Innovations, Inc. and references to the “Company” will only include McGinley Orthopaedic Innovations, Inc. throughout this section.
Components of Results of Operations
The following discussion of the Company’s financial conditions and results of operations should be read in conjunction with the financial statements and the related notes included elsewhere in this Annual Report. The following discussion contains forward-looking statements that reflect the Company’s plans, estimates and beliefs. The Company’s actual results could differ significantly from those discussed in the forward-looking statements. Unless otherwise indicated, the latest results discussed below are as of December 31, 2022.
Revenue/Net Loss from Operating Activities
The Company is, and has been, solely focused on development and commercialization of its Products and development of new related products. As of the date of this Annual Report, the Company has never been profitable.
In 2022 the Company’s revenues were $1,458,547 an increase of $106,251 (8%) over revenues of $1,352,296 in 2021. The reason for this increase was due to mining sales increasing by 34% over 2021.
In FY2022 the Company had a net loss of $1,927,410. In FY2021 the Company’s net loss was $2,857,806. Net loss decreased in 2022 by $930,396 (33%) over 2021. The reason for this decrease was due to operating expenses decreasing by $1,162,995 (29%) which was offset by other income being reduced by $334,772 (80%).
Loss from Operations decreased to $2,008,731 in 2022 from $3,273,899 in 2021, a decrease of $1,265,168 (39%). The reason for this decrease was due to Gross Margin increasing by $102,173 (9%) and operating expenses decreasing by $1,162,995 (29%).
Operating Expenses
The $1,265,168 decrease in Operating Loss in 2022 over 2021 was driven primarily by a decrease of $1,162,995 in Selling, General and Administrative expenses (“SG&A”) in 2022 over 2021. The Company classifies its SG&A expenses as wages and benefits, general office, research and development, professional fees, rent, insurance, advertising, other expenses, dues and subscriptions, depreciation and amortization, taxes and licenses, computer and internet, and postage and shipping. Over 79% of SG&A in 2022 was accounted for by wages and benefits, general office, Research & Development, professional (legal and accounting) fees, and travel and entertainment. The reason for this decrease in SG&A expenses was due to a reduction in salary expense for the sales team and the forfeiture of Accrued Membership Units (AMU) by two members of the Board of Advisors.
Wages and Benefits
In FY2022, the Company spent $1,146,594 on wages, benefits, contractor and other compensation expenses. In FY2021, the Company spent $2,338,656 on wages, benefits, contractor and other compensation. The decrease of $1,1192,062 (51%) in 2022 over 2021 was driven primarily by a transition of the sales force from traditional, regional salespeople to an in-house Product Specialist model. The Product Specialist are more technically oriented and have lower salaries in general than the previous sales force. Also contributing were resignations of two members of the Board of Advisors and consequent savings in Stock Compensation expense of $338,333.
General Office Expenses
In FY2022, the Company spent $381,176 on general office expenses. In FY2021, the Company spent $494,047 on general office expenses. The decrease of $112,871 (23%) in 2022 over 2021 was driven primarily by 2021 general office expenses being unusually high.
Research and Development Expense
The Company’s research and development efforts are focused on the continued development of new products related to the Company’s IntelliSense Technology. LAPS, and Freedom Nail products. Research and development expenses consist primarily of materials, equipment, and manufacturing costs to design, prototype, develop, and test the Company’s new products, and to acquire the parts, tools and equipment needed to do so.
In FY2022, the Company spent $155,325 on research and development expenses. In FY2021, the Company spent $305,331 on research and development expenses. The decrease of $150,000 (49%) from 2022 to 2021 was due primarily to lower development costs for the Lever Action Plate System which was successfully launched in 2021.
Professional fees
In FY2022, the Company spent $482,983 on professional fees. In FY2021, the Company spent $249,910 on professional fees. The increase of $233,073 (93%) in 2022 over 2021 was driven primarily by an increase in spending for the contract CFO, patent attorney, and engineering/quality assurance programs. The Company anticipates that annual increases in professional fees will continue upon a successful Offering by the Company, as discussed in “Use of Proceeds” in the Company’s SEC Form 1-A Offering Statement of August 11, 2022 (incorporated by reference ).
Travel and Entertainment
In FY2022, the Company spent $151,974 on travel and entertainment. In FY2021, the Company spent $148,282 on travel and entertainment. The increase of $3,692 (2%) in 2022 over 2021 was driven primarily by the smaller Product Specialist force traveling from Casper, Wyoming which is generally more expensive than the travel for the previous regional sales force was.
Accrued Common Stock
In FY2022, the Company accrued 203,913.31 and reversed 173,504.26 shares previously accrued for a net accrual of 30,409.05 shares for future issuance to members of the Company’s Board of Advisors, pursuant to Board of Advisor agreements executed by each Board of Advisors member. These shares are discussed in the Company’s financial statements in Note I to the balance sheet.
Liquidity and Capital Resources
As of December 31, 2022, the Company had cash of $1,721,610, working capital of $2,845,204 and total assets of $5,453,173. On December 31, 2021, the Company had cash of $1,624,273 working capital of $3,067,379 and total assets of $4,983,251. From December 31, 2021 to December 31, 2022, cash increased by $97,337 (6 %), working capital decreased by $222,175 (7 %), and total assets increased by $469,922 (9 %).
The Company has a $1,000,000 line of credit with a bank which matures February 8, 2024. Interest is subject to change on a monthly basis, calculated at the prime plus a variable rate (5.0% as of December 31, 2022). There was no outstanding balance on the line of credit as of December 31, 2022 and 2021.
The Company has additional capital requirements during the current fiscal year 2023 to be used in expanding its marketing and sales team and program, adding additional skills to its management team, and undertaking other initiatives as summarized in “Use of Proceeds” in Company’s SEC Form 1-A Offering Statement of August 11, 2022 (incorporated by reference).
There can be no assurance that the Company will be successful in acquiring additional funding at levels sufficient to fund its future capital requirements. If the Company is unable to raise additional capital in sufficient amounts or on terms acceptable to it, the Company may have to significantly reduce, delay, scale back or discontinue the development of its technologies and patents or discontinue operations completely.
DESCRIPTION OF PROPERTY
The Company owns real property through its wholly-owned subsidiary DS Manufacturing d/b/a McGinley Manufacturing. The property is an 8400 sq.ft. manufacturing plant located at 585 E Birch St Glenrock, WY 82637. The plant is a highly-specialized and highly-technical manufacturing facility capable of manufacturing all of the Company’s Products . In some occurrences, the Company must use a third-party contract manufacturer for processes such as heat treating and anodizing. The facility is and will continue to be ISO 13485:2016 and AS 9100 certified.
Item 3.
DIRECTORS, OFFICERS AND SIGNIFICANT EMPLOYEES
Directors
| Name | | Position | | Age | | Term of Office | | Approximate Hours per Week |
| Dr. Joseph McGinley | | | Director | | | | 49 | | | | June 2012 - Present | | | Full Time |
Officers
| Name | | Position | | Age | | Term of Office | | Approximate Hours per Week |
| Dr. Joseph McGinley | | | CEO/President/Treasurer/Secretary | | | | 49 | | | | June 2012 - Present | | | Full Time |
Significant Employees
| Name | | Position | | Age | | Term of Office | | Approximate Hours per Week |
| Dr. Richard C. McGinity | | Director of Finance | | | 79 | | | | July 2016 - Present | | | Full Time |
| Dian McGinley | | Director of Operations and Administration | | | 47 | | | | June 2012 - Present | | | Full Time |
| Ben Warren | | Vice President - McGinley Manufacturing | | | 56 | | | | June 2015 - Present | | | Full Time |
| Adam Johnson | | Vice President of Engineering | | | 47 | | | | June 2012 - Present | | | Full Time |
| Michael Hays | | Manager of Finances | | | 59 | | | | June 2015 - Present | | | Full Time |
| Travis Sides | | Operations Manager | | | 49 | | | | August 2020 - Present | | | Full Time |
Directors/Officers/Significant Employees (continued)
Dr. Joseph McGinley: Founder, Chairman of the Board of Directors, Chief Executive Officer, President
Full Time.
Duties of Dr. Joseph McGinley as CEO/ President, Treasurer, and Secretary
The duties of the CEO and President are set by the Bylaws.
The President is the Chief Executive Officer and President of the Company, subject to the control of the Board of Directors, has responsibility for the conduct and management of the business and fiscal affairs of the Company and the general supervision of its property, business interests, and agents. The President, or a person designated by the President, must preside at all meetings of the Shareholders and directors, unless otherwise ordered by the board of directors. The President may sign, with the Secretary or any other proper officer of the Corporation authorized by the Board of Directors, certificates for Shares of the Company and deeds, mortgages, bonds, contracts, or other instruments that the Board of Directors has authorized to be executed, except in cases where the signing and execution thereof is expressly delegated by the Board of Directors or the Bylaws to some other Officer or agent of the Company, or is required by law to be otherwise signed or executed.
Significant Employees
Dr. Richard McGinity: Director of Finance
Dr. Richard McGinity joined the Company as Director of Finance in July 2016, and is a member of its Board of Advisors. Dr. McGinity holds degrees from Princeton University (AB), Harvard University (MBA, DBA), and is President Emeritus of the University of Wyoming. Prior to joining the University of Wyoming faculty in 2007 as Daniels Chair of Business Ethics, Dr. McGinity was a general partner in a Boston-based venture capital fund and subsequently launched his own investment banking firm serving technology firms and their founders. He has extensive public and private transaction structuring and execution experience, has served on numerous boards of publicly-traded and private companies in the technology, energy, and banking industries, and is an “audit committee financial expert” as defined in Sarbanes-Oxley Section 407. He deployed three times to Vietnam as a Navy pilot, earning two Air Medals.
Duties of Dr. Richard McGinity as Director of Finance
The duties of the Director of Finance of the Company include oversight of the four corporate functions of (i) accounting and control, (ii) treasury, (iii) economic strategy and forecasting, and (iv) investor and financial community relations. The latter includes compliance with financial accounting standards, all financial disclosure and regulatory reporting requirements, and state and federal tax regulations.
Diane McGinley M.S: Director of Operations and Administration
Diane McGinley joined the company in 2012 upon the company’s inception. She is a graduate of Ohio University, with an M.S. in Education from Gwynedd Mercy College. Her early career was as an educator, curriculum director, and instructional facilitator. She has experience in corporate finance and governance as a board member of Hilltop Bank in Casper, WY, in which she serves as a member of seven committees: Assets and Liabilities, Trust, Trust Audit, Board Credit, Compensation, Marketing and Building.
Duties of Diane McGinley, MS
Diane McGinley oversees human resources, general operations and marketing and those department’s personnel.
Ben Warren: Vice President of Manufacturing, McGinley Manufacturing
Ben Warren has more than 22 years of experience in the machining industry. He established DS Manufacturing, Inc. in 2009 to serve the hard rock mining industry with after-market parts. In 2015, he partnered with the Company to form McGinley Manufacturing, a full-service machining and manufacturing facility for the medical, oil and gas, and mining industries. Ben now oversees all manufacturing operations for the Company at its plant in Glenrock, WY.
Duties of Ben Warren as Vice President of Manufacturing, McGinley Manufacturing
The duties of the Vice President of McGinley Manufacturing are to oversee production, operations, and personnel at the facility. He is to ensure that quality systems certification requirements are being met and followed. He is in charge of ensuring quality of products, meeting OSHA requirements for safety and is the direct supervisor to the manufacturing operations manager.
Adam Johnson: Vice President of Engineering
Adam Johnson has over 22 years of experience in custom engineering design and manufacturing. His experience includes positions in production facility development, machine design, composite lumber manufacturing, business development, and continuous improvement training. He holds a Bachelor of Science degree in Mechanical Engineering from Colorado State University.
Duties of Adam Johnson as Vice President of Engineering
The duties of the Director of Engineering include overseeing the engineering department. He is responsible for creating engineer work flows, document submissions to the FDA, ensuring adherence to the quality system and managing personnel. He also oversees product specialists and their work in the field with end user customers.
Michael Hays: Manager of Accounting and Finance 2015
Michael Hays joined the Company in 2015 and has over 32 years of experience in corporate accounting, with over 19 years in the manufacturing industries and more than five years in healthcare accounting. He holds a degree in finance from the University of Wyoming.
Duties of Michael Hays as Manager of Accounting and Finance
The duties of the Manager of Accounting and Finance include responsibility for the Company’s accounting, treasury, budgeting, forecasting, Capitalization Table and bank relationship activities.
Michael Anderson: Product Specialist
Michael Anderson joined the Company in 2022 and has over 18 years of experience in Sonography, Ultrasound Applications and Ultrasound Product Management. He holds a degree in Diagnostic Medical Sonography from Mercy College.
Duties of Michael Anderson as Product Specialist
Works with teams to develop pro-active competitive strategies and sales campaigns. Educate the team with product sales tactics and strategy. Act as a team role model to promote corporate code of business. Establish relationships with key accounts and customers. Ensure corporate initiatives are communicated clearly and on a timely basis. Manage an assigned territory and independent agencies to maximize sales revenues and meet corporate objectives. Helps to facilitate and manage hospital account contracts and pricing. Management of travel and sales expense.
Travis Sides: Manufacturing Operations Manager
Travis Sides joined the Company in 2020 and has over 17 years of sales, safety, and quality experience. He enjoys challenging work and has worked on many projects that improved work conditions for his team members. He graduated with an Associates degree in Psychology from Casper College in 2000.
Duties of Travis Sides as Manufacturing Operations Manager
The duties of Manufacturing Operation Manager include creating a production schedule, monitoring and adjusting manufacturing work flows, customer relationships including recruiting new customers and overseeing machinists and other facility personnel. This position has responsibility roles in the quality system certifications.
Family Relationship Disclosure
Dr. Joseph McGinley and Diane McGinley are husband and wife.
COMPENSATION OF DIRECTORS AND OFFICERS
Directors
| Name | | Capacity of Compensation | | Cash Compensation | | Other Compensation | | Total Compensation |
| Dr. Joseph McGinley | | | Director | | | $ | 10,000 | | | $ | 65,000 | | | $ | 75,000 | |
Officers
| Name | | Capacity of Compensation | | Cash Compensation | | Other Compensation | | Total Compensation |
| Dr. Joseph McGinley | | CEO, President, Treasurer, Secretary | | $ | 25,000 | | | $ | 0 | | | $ | 25,000 | |
Dr. Joseph McGinley’s compensation in his capacities as an Officer and as a Director are segregated and are reflected in the above tables. Dr. McGinley also had stock compensation of $65,000 in 2022 for participating on the Board of Advisors.
Aggregate Compensation to Directors
$100,000
There are no anticipated increases to compensation for Directors and Officers planned by the Company at this time.
Item 4.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
| Title of Class | | Name and Address of Beneficial Owner | | Amount of Shares Owned | | Amount and nature of beneficial ownership acquirable | | Percent of Class |
| Common Stock | | Dr. Joseph McGinley | | | 30,267,901.81 | | | | 52,527.49 | * | | | 64.4 | % |
(1) Dr. Joseph McGinley 234 E. 1st St. Suite 242 Casper, WY 82601. None of the Shares beneficially owned or acquirable by Dr. Joseph McGinley are being offered for sale in this Offering.
* Dr. Joseph McGinley is the beneficial owner of these Shares pursuant to accrual of those Shares (See the “Dilution” section of the Preliminary Offering Circular).
Item 5.
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
Related Party Receivables
The Company has receivables due from McGinley Education Innovations LLC (MEI) and McGinley Medical PC (MC) of approximately $2,657 and $18,308 respectively as of December 31, 2022. Both of the entities above are wholly-owned by Dr. Joseph McGinley. The transactions in question were regarding payments made by the Company to third parties for employee benefits, shared expenses, miscellaneous expenses, etc.
Related Party Operating Lease
TriOpportunity Investment Group LLC is owned by Dr. Joseph McGinley and his wife, Diane McGinley. TriOpportunity Investment Group LLC is controlled by Dr. Joseph McGinley. TriOpportunity Investment Group LLC owns the building from which the Company leases its offices. The Company is a tenant in this building located at 234 E. 1st St. Suite 242 Casper, WY 82601.
The Company has an operating lease for office space from TriOpportunity Investment Group LLC which began in January 2021. The lease is for seven years and expires in December 2027. Rent expense is recognized on a straight-line basis over the term of the lease. The lease is also subject to a three percent minimum annual increase. In connection with the related party lease, approximately $102,000 was paid to the lessor for specific leasehold improvements requested by the Company. The ROU asset for the year-ended December 31, 2022 is summarized below:
| Operating lease ROU asset | | $ | 722,073 |
| Less accumulated reduction | | | (116,172 |
| Balance of ROU asset | | $ | 605,901 |
Operating lease liability related to the ROU asset is summarized below:
| Operating lease liability | $ | 7,22,073 |
| Reduction of lease liability | | (107,072 |
| Total | $ | 615,001 |
Future minimum rental payments under non-cancellable operating leases (with initial or remaining lease terms in excess of one year) for each of the five years subsequent to December 31, 2022 are:
| | 2023 | | | $ | 120,056 | |
| | 2024 | | | $ | 123,658 | |
| | 2025 | | | $ | 127,368 | |
| | 2026 | | | $ | 131,189 | |
| | Thereafter | | | $ | 135,124 | |
| | Total | | | $ | 637,395 | |
Total combined rent expense associated with the operating leases amounted to approximately $127,000 and $114,000 for the years ended December 31, 2022 and 2021, respectively.
Item 7.
FINANCIAL STATEMENTS
Consolidated Financial Statements and Report of
Independent Certified Public Accountants
McGinley Orthopaedic Innovations, Inc.
Devember 31, 2022 and 2021
To the Stockholders of
McGinley Orthopaedic Innovations, Inc.:
Opinion
We have audited the accompanying consolidated financial statements of McGinley Orthopaedic Innovations, Inc. (the “Company”), which comprise of the consolidated balance sheets as of December 31, 2022 and 2021, and the related consolidated statements of operations, changes in equity, and cash flows for the years then ended, and the related notes to the consolidated financial statements.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Basis of Opinion
We conducted our audits in accordance with auditing standards generally accepted in the United States of America(“U.S. GAAS”). Our responsibilities under those standards are further described in the Auditor’s Responsibilities for the Audit of the Consolidated Financial Statements section of our report. We are required to be independent of the Company and to meet our other ethical responsibilities in accordance with the relevant ethical requirements relating to our audits. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note C to the consolidated financial statements, the Company had net losses of approximately $1,927,000 and $2,858,000 for the years ended December 31, 2022 and 2021, respectively, as well as negative cash flow from operations of approximately $1,425,000 and $2,055,000, respectively. Management’s evaluation of the events and conditions and management’s plans regarding those matters are also described in Note C. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to that matter.
Responsibilities of Management for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of the consolidated financial statements in accordance with U.S. GAAP, and for the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the consolidated financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are available to be issued.
Auditor’s Responsibilities for the Audit of the Consolidated Financial Statements
Our objectives are to obtain reasonable assurance about whether the consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with U.S. GAAS will always detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate, they would influence the judgment made by a reasonable user based on the consolidated financial statements.
In performing an audit in accordance with U.S. GAAS, we:
· Exercise professional judgment and maintain professional skepticism throughout the audit.
· Identify and assess the risks of material misstatement of the consolidated financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements.
· Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such opinion is expressed.
· Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the consolidated financial statements.
· Conclude whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time.
We are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit, significant audit findings, and certain internal control related matters that we identified during the audit.
s//: Assurance Dimensions
Jacksonville, Florida
May 1, 2023
| McGinley Orthopaedic Innovations, Inc. | | | | |
Consolidated Balance Sheets As of December 31, 2022 and 2021 | | | | |
| | | 2022 | | 2021 |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 1,721,610 | | | $ | 1,624,273 | |
| Accounts receivable, net | | | 168,229 | | | | 137,360 | |
| Due from related parties | | | 21,026 | | | | 72,394 | |
| Inventories, net | | | 1,237,909 | | | | 1,355,011 | |
| Prepaid expenses | | | 103,159 | | | | 101,414 | |
| Total current assets | | | 3,251,933 | | | | 3,290,452 | |
| Property and equipment, net | | | 1,072,983 | | | | 1,190,846 | |
| Operating lease right of use asset | | | 605,901 | | | | — | |
| Intangible assets, net | | | 522,356 | | | | 501,953 | |
| Total assets | | $ | 5,453,173 | | | $ | 4,983,251 | |
LIABILITIES AND EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 244,895 | | | $ | 129,292 | |
| Accrued liabilities | | | 49,696 | | | | 93,781 | |
| Current portion of operating lease liabilities | | | 112,138 | | | | — | |
| Total liabilities | | | 406,729 | | | | 223,073 | |
| Operating lease liabilities, net of current portion | | | 502,863 | | | | — | |
| Total liabilities | | | 502,863 | | | | — | |
Equity:
| Common stock, $0.001 par value, 100,000,000 shares authorized; 46,997,724 and 0 shares issued and outstanding for 2022 and 2021, respectively | | | 46,998 | | | | — | |
| Additional paid-in capital | | | 24,421,261 | | | | 22,867,040 | |
| Common stock to be issued | | | 997,927 | | | | 888,333 | |
| Accumulated deficit | | | (20,922,605 | ) | | | (18,995,195 | ) |
| Total equity | | | 4,543,581 | | | | 4,760,178 | |
| Total liabilities and equity | | $ | 5,453,173 | | | $ | 4,983,251 | |
| McGinley Orthopaedic Innovations, Inc. | | | | |
| Consolidated Statements of Operations | | | | |
| For the Years Ended December 31, 2022 and 2021 | | | | |
| | | 2022 | | 2021 |
Revenues | | $ | 1,458,547 | | | $ | 1,352,296 | |
| Cost of goods sold | | | 559,354 | | | | 555,276 | |
| Gross margin | | | 899,193 | | | | 797,020 | |
Selling, general and administrative expenses: | | | | | | | | |
| Wages and benefits | | | 1,146,594 | | | | 2,338,656 | |
| General office | | | 381,176 | | | | 494,047 | |
| Research and development | | | 155,325 | | | | 305,331 | |
| Professional fees | | | 482,983 | | | | 249,910 | |
| Travel & entertainment | | | 151,974 | | | | 148,282 | |
| Rent | | | 126,722 | | | | 115,416 | |
| Insurance | | | 140,587 | | | | 107,643 | |
| Advertising | | | 87,811 | | | | 73,610 | |
| Other expenses | | | 59,738 | | | | 65,240 | |
| Dues & subscriptions | | | 35,063 | | | | 55,233 | |
| Depreciation and amortization | | | 65,347 | | | | 50,966 | |
| Taxes & licenses | | | 31,644 | | | | 24,967 | |
| Computer & internet | | | 26,108 | | | | 24,659 | |
| Postage & shipping | | | 16,852 | | | | 16,959 | |
| Total selling, general and administrative expenses | | | 2,907,924 | | | | 4,070,919 | |
| Loss from operations | | | (2,008,731 | ) | | | (3,273,899 | ) |
| Other income: | | | | | | | | |
| Gain on extinguishment of debt | | | — | | | | 316,500 | |
| Interest income | | | 5,090 | | | | 3,630 | |
| Miscellaneous income | | | 76,231 | | | | 95,963 | |
| Total other income | | | 81,321 | | | | 416,093 | |
Net loss before income taxes Provision for income taxes | | | (1,927,410 | ) | | | (2,857,806 | ) |
| Net loss | | $ | (1,927,410 | ) | | $ | (2,857,806 | ) |
 McGinley Orthopaedic Innovations, Inc.
McGinley Orthopaedic Innovations, Inc.
Consolidated Statements of Changes in Members' Equity
For the Years Ended December 31, 2022 and 2021
| | | Membership Units | | Common Stock | | Additional Paid-in | | Common Stock | | Accumulated | | |
| | | Units | | Amount | | Units | | Amount | | Capital | | to be issued | | deficit | | Total |
| Balance at December 31, 2020 | | | 43,475,686.0 | | | $ | 21,082,704 | | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | (16,137,389 | ) | | $ | 4,945,315 | |
| Issuance of membership units, net of issuance costs | | | 903,846 | | | | 1,720,049 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,720,049 | |
| Issuance of membership units for loan repayment | | | 39,440 | | | | 64,287 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 64,287 | |
| Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | 888,333 | | | | — | | | | 888,333 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (2,857,806 | ) | | | (2,857,806 | ) |
| Balance at December 31, 2021 | | | 44,418,972 | | | | 22,867,040 | | | | — | | | | — | | | | — | | | | 888,333 | | | | (18,995,195 | ) | | | 4,760,178 | |
| Conversion of membership units to common stock | | | (44,418,972 | ) | | | (22,867,040 | ) | | | 44,418,972 | | | | 44,419 | | | | 22,822,621 | | | | — | | | | — | | | | — | |
| Issuance of common stock, net of issuance costs | | | — | | | | — | | | | 2,543,752 | | | | 2,544 | | | | 1,530,425 | | | | 11 | | | | — | | | | 1,532,980 | |
| Stock-based compensation expense | | | — | | | | — | | | | 35,000 | | | | 35 | | | | 68,215 | | | | 109,583 | | | | — | | | | 177,833 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,927,410 | ) | | | (1,927,410 | ) |
| Balance at December 31, 2022 | | | — | | | $ | — | | | | 46,997,724 | | | $ | 46,998 | | | $ | 24,421,261 | | | $ | 997,927 | | | $ | (20,922,605 | ) | | $ | 4,543,581 | |
McGinley Orthopaedic Innovations, Inc. Consolidated Statements of Cash Flows For the Years Ended December 31, 2022 and 2021 | | |
| | | 2022 | | 2021 |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (1,927,410 | ) | | $ | (2,857,806 | ) |
| Adjustments to reconcile net loss to net cash used | | | | | | | | |
| by operating activities: | | | | | | | | |
| Depreciation and amortization | | | 227,456 | | | | 202,141 | |
| Stock-based compensation expense | | | 177,719 | | | | 888,333 | |
| Gain on extinguishment of debt | | | — | | | | (316,500 | ) |
| Changes in assets and liabilities: | | | | | | | | |
| Accounts receivable, net | | | (30,869 | ) | | | (74,049 | ) |
| Inventories, net | | | 117,102 | | | | 18,083 | |
| Prepaid expenses | | | (1,745 | ) | | | (26,741 | ) |
| Accounts payable and accrued liabilities | | | 71,518 | | | | 111,470 | |
| Operating lease right of use asset | | | 9,100 | | | | — | |
| Net cash used by operating activities | | | (1,357,129 | ) | | | (2,055,069 | ) |
Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | (64,802 | ) | | | (281,489 | ) |
| Investment in intangible assets | | | (65,194 | ) | | | (36,915 | ) |
| Net payments from (advances to) related parties | | | 51,368 | | | | (65,736 | ) |
| Net cash used by investing activities | | | (78,628 | ) | | | (384,140 | ) |
Cash flows from financing activities: | | | | | | | | |
| Proceeds from PPP loans | | | — | | | | 316,500 | |
| Proceeds from stocks issued | | | 2,363,618 | | | | 1,762,501 | |
| Capital raise costs | | | (830,524 | ) | | | (42,452 | ) |
| Net cash provided by financing activities | | | 1,533,094 | | | | 2,036,549 | |
| Net change in cash | | | 97,337 | | | | (402,660 | ) |
| Cash at beginning of year | | | 1,624,273 | | | | 2,026,933 | |
| Cash at end of year | | $ | 1,721,610 | | | $ | 1,624,273 | |
Supplemental and non-cash disclosures | | | | | | | | |
| Cash paid for income taxes | | $ | — | | | $ | — | |
| Cash paid for interest | | $ | — | | | $ | — | |
| Issuance of member units for payment on note payable | | $ | — | | | $ | 64,287 | |
Operating lease right of use asset and liability recorded on adoption of ASC 842 | | $ | 722,073 | | | $ | — | |
Note A – Nature of Business
McGinley Orthopaedic Innovations, Inc. (“MOI”) is a Wyoming corporation. MOI was established in 2012 with the goal of increasing patient safety and physician confidence through technological advances in the orthopedic field. MOI has developed and is marketing patented, FDA-approved Intellisense Drill Technology®, an orthopedic power tool, and the Lever Action Plate System® bone fixation implant system.
On May 13, 2022, the Company filed to be a Wyoming corporation. The new name of the Company is McGinley Orthopaedic Innovations, Inc. The Company’s membership units converted to common stock, which have a par value of $0.001. The Board also established that the Company is authorized to issue 100,000,000 shares of common stock.
DS Manufacturing LLC, known commonly as McGinley Manufacturing, is a wholly-owned subsidiary of MOI, and operates a custom machine and fabrication shop with a fully integrated engineering team. Capabilities include custom engineering and design, high precision turning, milling, welding, machining, replacement part fabrication, CNC plasma cutting, powder coating, as well as 5-axis capability. McGinley Manufacturing also manufactures parts of varying sizes, including small scale pieces for precision hand tools to medium-sized pieces for hard rock mining projects for the mining and oil services industries.
McGinley Engineered Solutions, LLC is a wholly-owned subsidiary of MOI, and holds title to all of the patents assigned to MOI.
Note B – Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements and related notes include MOI and its wholly owned subsidiaries described in Note A. Hereinafter, they are collectively referred to as the Company. All significant inter- company accounts and transactions have been eliminated in consolidation.
Basis of Accounting
The Company prepares its consolidated financial statements using the accrual method of accounting in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Such estimates include inventory reserves for obsolescence, allowance for uncollectible receivables, depreciable lives of property and equipment and costs of patents applied for. Actual results could differ from those estimates.
Recently Adopted Standards
Effective January 1, 2022, the Company adopted the new lease accounting guidance in Accounting Standards Update No. 2016-02, Leases (Topic 842). The Company has elected the package of practical expedients permitted in Accounting Standards Codification (ASC) 842. Accordingly, the Company accounted for its existing operating lease as an operating lease under the new guidance, without reassessing (a) whether the contract contains a lease under ASC 842, (b) whether classification of the operating lease would be different in accordance with ASC 842, or (c) whether the unamortized initial direct costs before transition adjustments (as of December 31, 2021) would have met the definition of initial direct costs in ASC 842 at lease commencement. As a result of the adoption of the new lease accounting guidance, the Company recognized on January 1, 2022 (a) a lease liability of approximately $722,000, which represents the present value of the remaining lease payments of approximately $754,000, discounted using the risk-free rate of 1.43%, and (b) a right-of-use asset approximately of $722,000.
Note B – Summary of Significant Accounting Policies (continued)
Cash
The Company deposits cash with financial institutions which management believes are of high credit quality. The Company does not believe it is exposed to significant risk. At December 31, 2022 and 2021, there were no cash equivalents.
Accounts Receivable
Accounts receivable are stated at the amount management expects to collect from outstanding balances. Management provides for probable uncollectible accounts through an allowance for doubtful accounts. The allowance is estimated based on historical performance and management's assessment of the current status of individual accounts. Management estimated an allowance of approximately $4,000 and $5,000 at December 31, 2022 and 2021, respectively, which is based on an analysis of individual trade accounts, historical experience with customers and general economic conditions.
Inventories
Inventories are valued on the lower of cost or market. Inventory is valued at the lower of first-in, first-out cost or net realizable value, which is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The Company has established the policy of capitalizing manufacturing materials and supplies, labor, overhead, and expenses allocable to finished goods and assemblies for the inventory valuation. The Company also carries expendable supplies inventory. This includes expendable items that are consumed in the manufacturing process.
As of December 31, 2022 and 2021, management has recorded a reserve of approximately $518,000 and $1,327,000, respectively. Inventories consisted of the following at December 31:
| | | 2022 | | 2021 |
| Materials and supplies | | $ | 208,909 | | | $ | 201,953 | |
| Work in process and finished goods | | | 1,547,270 | | | | 2,479,665 | |
| Inventory reserve | | | (518,270 | ) | | | (1,326,607 | ) |
| Total inventories, net | | $ | 1,237,909 | | | $ | 1,355,011 | |
Property and Equipment
Property and equipment are recorded at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. Maintenance and repairs are charged to expense as incurred. Betterments and renewals are capitalized. When property and equipment are sold or otherwise disposed of, the asset account and related accumulated depreciation account are relieved, and any gain or loss is included in operations. The straight-line method of depreciation is used over the following estimated useful lives:
| | | Years |
| Machinery | | 5-15 |
| Leasehold improvements | | | 5-15 | |
| Computers and software | | | 5 | |
| Furniture and equipment | | | 5-15 | |
| Buildings | | | 39 | |
| Vehicles | | | 5 | |
| Land | | | 10 | |
Additions and improvements are capitalized, while replacements, maintenance, and repairs, which do not improve or extend the life of the respective assets, are expensed as incurred. Gains and losses from the disposition of assets are recorded in the year of disposition.
Note B – Summary of Significant Accounting Policies (continued)
Intangible Assets
Intangible assets consist of patents and patents pending. Patents and patents pending are being amortized on the straight-line method over twenty years, but not exceeding the initial expiration date of the patent.
Impairment of Long-Lived Assets
Long-lived assets such as property and equipment, and intangible assets subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If this review reveals an indicator of impairment, as determined based on estimated undiscounted cash flows, the carrying amounts of the related long-lived assets are adjusted to fair value. Management has determined that there has been no impairment in the carrying value of its long-lived assets as of December 31, 2022 and 2021.
Revenue Recognition
All revenues from exchange transactions are recorded in accordance with ASC 606, which is recognized when: (i) a contract with a customer has been identified, (ii) the performance obligation(s) in the contract have been identified, (iii) the transaction price has been determined, (iv) the transaction price has been allocated to each performance obligation in the contract, and (v) the Company has satisfied the applicable performance obligation at a point in time or over time.
The Company’s revenues are primarily generated from the sale of products, and represent a single performance obligation and are earned at a point in time when the goods have been shipped. Payment for the remaining customer’s is due between 15-30 days. The Company’s revenues also contain drill system rentals to customers and are recognized on a per use and/or monthly basis. All revenue is earned in the United States.
The Company’s revenue by type are summarized as follows for the year ended December 31:
| | | 2022 | | 2021 |
| Product sales | | $ | 1,418,962 | | | $ | 1,219,511 | |
| Drill system rentals | | | 39,585 | | | | 132,785 | |
| Total revenues | | $ | 1,458,547 | | | $ | 1,352,296 | |
Research and Development
Research and development costs that do not meet the criteria for capitalization are expensed as incurred. Research and development expenses include compensation, employee benefits, and incentive-unit-based compensation for employees, as well as fees paid to outside consultants. Nonrefundable advanced payments, for goods and services that will be used in future research and development activities are expensed when the activity is performed or when the goods have been received, rather than when payment is made, in accordance with ASC 730, Research and Development.
Stock-based Compensation
For stock-based compensation awards, the Company measures compensation costs for these awards to employees and non-employees based upon the fair value of the award on the date of grant.
Income Taxes
Effective January 1, 2016, the Company elected to be taxed as a C corporation. On May 13, 2022, the Company filed to be a Wyoming corporation. The Company files a consolidated income tax return with its subsidiaries in the U.S. Federal jurisdiction. Generally, the Company’s tax returns remain open for three years for Federal income tax examination. At December 31, 2022 and 2021, the Company believes there are no significant uncertain tax positions or liabilities, or interest and penalties associated with uncertain tax positions.
Note B – Summary of Significant Accounting Policies (continued)
The Company’s income tax expense consists of the following components: current and deferred. Current income tax expense reflects taxes to be paid or refunded for the current period by applying the provisions of the enacted tax law to the taxable income or excess of deductions over revenues. The Company determines deferred income taxes using the liability (or balance sheet) method. Under this method, the net deferred tax asset or liability is based on the tax effects of the differences between the book and tax bases of assets and liabilities, and enacted changes in tax rates and laws are recognized in the period in which they occur.
Deferred income tax expense results from changes in deferred tax assets and liabilities between periods. Deferred tax assets are recognized if it is more likely than not, based on the technical merits, that the tax position will be realized or sustained upon examination. The term more likely than not means a likelihood of more than 50 percent; the terms examined and upon examination also include resolution of the related appeals or litigation processes, if any. A tax position that meets the more-likely-than-not recognition threshold is initially and subsequently measured as the largest amount of tax benefit that has a greater than 50 percent likelihood of being realized upon settlement with a taxing authority that has full knowledge of all relevant information. The determination of whether or not a tax position has met the more-likely-than-not recognition threshold considers the facts, circumstances, and information available at the reporting date and is subject to management’s judgment. Deferred tax assets are reduced by a valuation allowance if, based on the weight of evidence available, it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Note C – Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. For the years ended December 31, 2022 and 2021, the Company incurred net losses of approximately $1,927,000 and $2,858,000, respectively, and had negative cash flows from operations in the amount of approximately $1,425,000 and $2,055,000, respectively. As of December 31, 2022 and 2021, the Company had an accumulated deficit of approximately $20,923,000 and $18,995,000, respectively. These matters raise substantial doubt about the Company's ability to continue as a going concern.
The ability of the Company to continue as a going concern is dependent upon increasing sales and obtaining additional capital and financing. Management intends to attempt to raise funds by way of private offerings. While the Company believes in the viability of its strategy to increase sales and in its ability to raise additional funds, there can be no assurances to that effect. The financial statements do not include adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Note D – Property and Equipment
Property and equipment consisted of the following as of December 31:
| | | 2022 | | 2021 |
| Machinery | | $ | 1,576,585 | | | $ | 1,555,335 | |
| Leasehold improvements | | | 172,822 | | | | 152,752 | |
| Computers and software | | | 99,094 | | | | 75,612 | |
| Furniture and equipment | | | 46,341 | | | | 46,341 | |
| Buildings | | | 27,482 | | | | 27,482 | |
| Vehicles | | | 20,244 | | | | 20,244 | |
| Land | | | 10,806 | | | | 10,806 | |
| | | | 1,953,374 | | | | 1,888,572 | |
| Less: accumulated depreciation | | | (880,391 | ) | | | (697,726 | ) |
| Property and equipment, net | | $ | 1,072,983 | | | $ | 1,190,846 | |
Note D – Property and Equipment (continued)
For the years ended December 31, 2022 and 2021, the depreciation expense was approximately $183,000 and $164,000 respectively. The expense is split between cost of goods sold and selling, general and administrative expenses. Depreciation expense included in costs of goods sold was approximately $162,000 and $151,000, respectively.
Note E – Intangible Assets
Intangible assets consisted of the following as of December 31:
| | | | | | | Useful Life |
| | | 2022 | | 2021 | | (years) |
| Intellectual property | | $ | 867,774 | | | $ | 802,580 | | | | 20 | |
| Less: accumulated amortization | | | (345,418 | ) | | | (300,627 | ) | | | | |
| Intangible assets, net | | $ | 522,356 | | | $ | 501,953 | | | | | |
Amortization expense charged to operations for the years ended December 31, 2022 and 2021 was approximately
$45,000 and $38,000, respectively. The following is a schedule of the estimated amortization expense for intangible assets over the remaining useful life:
| Years ending December 31, |
| | 2023 | | | $ | 37,922 | |
| | 2024 | | | | 37,922 | |
| | 2025 | | | | 37,922 | |
| | 2026 | | | | 37,922 | |
| | 2027 | | | | 37,529 | |
| | Thereafter | | | | 333,139 | |
| | | | | $ | 522,356 | |
Note F – Note Payable – Related Parties
On April 17, 2015 the Company entered into agreement to purchase DS Manufacturing. The purchase agreement called for the Company to purchase DS Manufacturing for a total of $450,010, which was to be paid in membership units of the Company over six years. The balance of the note payable was $0 as of December 31, 2022 and 2021. For the years ended December 31, 2022 and 2021, the Company issued 0 and 39,440 membership units, respectively. These payments represent non-cash financing payments. As of December 31, 2021, the note payable was paid in full.
Note G – Notes Payable – Paycheck Protection Program Loans
In March 2020, Congress established the Paycheck Protection Program (“PPP”) to provide relief to small businesses during the coronavirus pandemic (“COVID-19”) as part of the Coronavirus Aid, Relief, and Economic Security Act. The legislation authorized Treasury to use the Small Business Association’s 7(a) small business lending program to fund forgivable loans that qualifying businesses could spend to cover payroll, mortgage interest, rent, and utilities during the “Covered Period” defined as the 24-week period starting on the date the PPP loan proceeds are received. Upon meeting certain criteria as specified in the PPP program, the loan is eligible for partial or total forgiveness. The Company received two PPP2 loans of $316,500 in January 2021. These loans were fully forgiven in the respective year they were received, and are reflected as a gain on extinguishment of debt on the consolidated statements of operations.
Note H – Line of Credit
The Company has a $1,000,000 line of credit with a bank which matures February 8, 2024. Interest is subject to change on a monthly basis, calculated at the prime plus a variable rate (5.00% as of December 31, 2022). There was no outstanding balance on the line of credit as of December 31, 2022 and 2021.
Note I – Equity
Conversion from Membership Units
With the change in the Company’s corporate structure from a limited liability company to a corporation, the membership units converted to common stock on a 1:1 basis, which have a par value of $0.001. The membership units of 44,418,972 as of December 31, 2021 were converted to common stock in 2022. As of December 31, 2022, the Company had 46,997,724 common stock issued and outstanding. All stockholders have equal voting rights and allocation of profits and losses.
For the years ended December 31, 2022 and 2021, the Company issued 2,543,751 common stock and 903,846 membership units for cash of approximately $1,472,000 and $1,720,000, respectively, net of issuance costs.
Common Stock Issued
During the year ended December 31, 2022, the Company had two separate capital raises.
During 2022, the Company completed an internal capital raise issuing 838,207 shares for proceeds of $1,634,503. In September 2022, the Company opened a Regulation A+ capital raise campaign for shares of stock to be sold at
$5.25 per share. The Company subscribed and issued 1,705,545 shares of stock. Of these shares, 1,597,300 were issued to pay for capital raise costs. The Company had gross proceeds $569,888 from the issuances, and incurred capital raise costs of $889,265 in payments to third parties for the year ended December 31, 2022. As of December 31, 2022, the Company had 11 shares of common stock to be issued associated with this capital raise.
Common Stock to be Issued and Stock-based Compensation Expense
Board of Advisors Accrued Shares
The Company entered into separate agreements with each of the Board of Advisors to award a total of 1,366,667 membership units (which were converted to shares of common stock as noted above). In exchange for their investment in the Company and their consulting services, the Company agreed to award them membership units on a quarterly basis over three years through 2023. For the years ended December 31, 2022 and 2021, the Company recognized stock-based compensation expense related to these agreements of approximately $178,000 and $888,000, respectively. The awards were based on the value of the share price at the date of the award. These costs are included in wages and benefits on the consolidated statements of operations. At December 31, 2022, there were 60,952 shares left to award and approximately $320,000 of unrecognized stock-based compensation expense.
A summary of the status of the Company's outstanding Board of Advisors accrued shares as of December 31, 2022 and 2021, and changes during the two years is as follows:
| | | Shares |
| Outstanding at December 31, 2020 | | - |
| | Granted | | | | 455,556 | |
| | Exercised | | | | — | |
| | Forfeited | | | | — | |
| | Outstanding at December 31, 2021 | | | | 455,556 | |
| | Granted | | | | 203,913 | |
| | Exercised | | | | — | |
| | Forfeited | | | | (173,504 | ) |
| | Outstanding at December 31, 2022 | | | | 485,965 | |
Stock-based Compensation Expense – Other Services
The Company issued 35,000 shares for other services during 2022, and recorded this as stock-based compensation in the amount of approximately $68,000.
Note J – Income Taxes
No provision or benefit for federal or state income taxes has been reflected for the year ended December 31, 2022 and 2021 since the Company reported losses and has established a valuation allowance against the total net deferred tax asset.
Significant components of the Company’s net deferred tax assets are as follows as of December 31:
| | | 2022 | | 2021 |
| Net operating loss carryforward | | $ | 2,703,000 | | $ | 2,127,185 |
| Property and equipment | | | (248000 | | | (84,562 |
| Inventory reserve | | | 279,000 | | | 278,587 |
| Intangible assets | | | (87,000 | | | 10,424 |
| Stock-based compensation | | | 37,000 | | | 186,550 |
| Prepaid expenses | | | 7,000 | | | — |
| Accrued expenses | | | 2,000 | | | — |
| Capitalized R&D | | | 142,000 | | | — |
| Valuation allowance | | | (2,835,000) | | | (2,518,184) |
| Net deferred tax asset | | $ | — | | $ | — |
At December 31, 2022 and 2021, the Company had federal net operating loss carryforwards of approximately $14,423,000 and $10,129,000, respectively. The federal net operating losses beginning in 2018 may be carried forward indefinitely but are limited to 80% of the taxable income in any one tax period. The deferred tax asset has been adjusted to account for the limitation. The pre-2018 net operating losses are approximately $2,378,000 and can be carried back two years and forward 20 years, and will begin expiring in 2036. The Company’s federal and state blended tax rate was 21% for the years ended December 31, 2022 and 2021, respectively.
The entire balance of the deferred tax asset has been offset by a valuation allowance since there may be limitations on the amount of net operating loss carryforwards which can be used in future years, and utilization of net operating loss carryforwards cannot be sufficiently assured at December 31, 2022 and 2021.
Note K - Related Party Transactions
Related Party Receivables
The Company has receivables due from various related parties of approximately $21,000 and $72,000 as of December 31, 2022 and 2021, respectively.
Related Party Payables
The Company has payables due to various related parties of approximately $0 and $6,000 as of December 31, 2022 and 2021, respectively. These are included in accounts payable on the accompanying consolidated balance sheets.
Related Party Operating Lease
The Company has an operating lease for office space from a related party which began in January 2021. The lease is for seven years and expires in December 2027. Rent expense is recognized on a straight-line basis over the term of the lease. The lease is also subject to a three percent minimum annual increase. In connection with the related party lease, approximately $102,000 was paid to the lessor for specific leasehold improvements requested by the Company.
The ROU asset for the year-ended December 31, 2022 is summarized below:
| Operating lease ROU asset | | $ | 722,073 | |
| Less accumulated reduction | | | (116,172 | ) |
| Balance of ROU asset | | $ | 605,901 | |
Note K - Related Party Transactions (continued)
Operating lease liability related to the ROU asset is summarized below:
| Operating lease liability | | $ | 722,073 | |
| Reduction of lease liability | | | (107,072 | ) |
| Total | | $ | 615,001 | |
As of December 31, 2022, the minimum lease payments under this related party lease are as follows:
| | 2023 | | | $ | 120,056 | |
| | 2024 | | | | 123,658 | |
| | 2025 | | | | 127,368 | |
| | 2026 | | | | 131,189 | |
| | 2027 | | | | 135,124 | |
| | Thereafter | | | | — | |
| | Total lease payments | | | | 637,395 | |
| | Less: interest | | | | 22,394 | |
| | Present value of lease payments | | | | 615,001 | |
| | Less: current portion of lease liabilities | | | | 120,256 | |
| | Operating lease liabilities, net of current portion | | | $ | 494,745 | |
Total lease and rent expense amounted to approximately $127,000 and $114,000 for the years ended December 31, 2022 and 2021, respectively.
Weighted average remaining lease terms as of December 31, 2022 is 0.9 years and the weighted average discount rates for December 31, 2022 is 0.26%.
Note L – Subsequent Events
Subsequent events have been evaluated through May 01, 2023, which is the date the consolidated financial statements were available to be issued.
Governance Letter
May 1, 2023
To the Stockholders of McGinley Orthopaedic Innovations, Inc.
We have audited the consolidated financial statements of McGinley Orthopaedic Innovations, Inc. (the
“Company”) for the years ended December 31, 2022 and 2021 (hereinafter, referred to as the “financial statements”), and have issued our report thereon dated May 1, 2023. Professional standards require that we provide you with information about our responsibilities under generally accepted auditing standards, as well as certain information related to the planned scope and timing of our audit. We have communicated such information in our engagement letter to you dated October 17, 2022, and in an email dated February 13, 2023. Professional standards also require that we communicate to you the following information related to our audit.
Significant Audit Findings
Qualitative Aspects of Accounting Practices
Management is responsible for the selection and use of appropriate accounting policies. The significant accounting policies used by the Company are described in Note B to the combined financial statements. Effective January 1, 2022, the Company adopted the new lease accounting guidance in Accounting Standards Update No. 2016-02, Leases (Topic 842). The Company has elected the package of practical expedients permitted in Accounting Standards Codification (ASC) 842. Accordingly, the Company accounted for its existing operating lease as an operating lease under the new guidance, without reassessing (a) whether the contract contains a lease under ASC 842, (b) whether classification of the operating lease would be different in accordance with ASC 842, or (c) whether the unamortized initial direct costs before transition adjustments (as of December 31, 2021) would have met the definition of initial direct costs in ASC 842 at lease commencement. As a result of the adoption of the new lease accounting guidance, the Company recognized on January 1, 2022 (a) a lease liability of approximately $722,000, which represents the present value of the remaining lease payments of approximately $754,000, discounted using the risk-free rate of 1.43%, and (b) a right-of-use asset approximately of $722,000.
We noted no transactions entered into by the Company during the year for which there is a lack of authoritative guidance or consensus. All significant transactions have been recognized in the financial statements in the proper period.
Accounting estimates are an integral part of the financial statements prepared by management and are based on management’s knowledge and experience about past and current events and assumptions about future events. Certain accounting estimates are particularly sensitive because of their significance to the financial statements and because of the possibility that future events affecting them may differ significantly from those expected. There were no particularly sensitive estimates affecting the financial statements noted.
Certain financial statement disclosures are particularly sensitive because of their significance to financial statement users. The most sensitive disclosures affecting the financial statements were:
Notes B, C, I, J and K
The financial statement disclosures are neutral, consistent, and clear.
Corrected and Uncorrected Misstatements
Professional standards require us to accumulate all misstatements identified during the audit, other than those that are clearly trivial, and communicate them to the appropriate level of management. You have corrected all such misstatements.
Disagreements with Management
For purposes of this letter, a disagreement with management is a financial accounting, reporting, or auditing matter, whether or not resolved to our satisfaction, that could be significant to the financial statements or the auditor’s report. We are pleased to report that no such disagreements arose during the course of our audit.
Management Representations
We have requested certain representations from management that are included in the management representation letter dated May 1, 2023.
Management Consultations with Other Independent Accountants
In some cases, management may decide to consult with other accountants about auditing and accounting matters, similar to obtaining a “second opinion” on certain situations. If a consultation involves application of an accounting principle to the Company’s financial statements or a determination of the type of auditor’s opinion that may be expressed on those statements, our professional standards require the consulting accountant to check with us to determine that the consultant has all the relevant facts. To our knowledge, there were no such consultations with other accountants.
Other Audit Findings or Issues
We generally discuss a variety of matters, including the application of accounting principles and auditing standards, with management each year prior to retention as the Company’s auditors. However, these discussions occurred in the normal course of our professional relationship and our responses were not a condition to our retention.
This information is intended solely for the use of the owners and management of the Company, and is not intended to be and should not be used by anyone other than these specified parties.
Very truly yours,
s//: Assurance Dimensions
Item 8.
EXHIBITS
The documents listed in the Exhibit Index of this report are incorporated by reference or are filed with this report, in each case as indicated below.
CERTIFICATE OF INCORPORATION AND OTHER DOCUMENTS
BYLAWS
SUBSCRIPTION AGREEMENT
ESCROW AGREEMENT
CONSENT
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Casper, Wyoming, on May 01, 2023.
McGinley Orthopaedic, Inc.
By:
s/Dr. Joseph McGinley
Director, CEO, President, Treasurer, Secretary of McGinley Orthopaedic Innovations, Inc.
(Date): May 01, 2023
Location Signed: Casper, WY
This Annual Report has been signed by the following Officers in the capacities and on the dates indicated.
s/Dr. Joseph McGinley
CEO, President, Treasurer, Secretary of McGinley Orthopaedic Innovations, Inc.
(Date): May 01, 2023
Location Signed: Casper, WY
This Annual Report has been signed by the following Directors in the capacities and on the dates indicated.
s/Dr. Joseph McGinley
Director of McGinley Orthopaedic Innovations, Inc.
(Date): May 01, 2023
Location Signed: Casper, WY









 McGinley Orthopaedic Innovations, Inc.
McGinley Orthopaedic Innovations, Inc.