Exhibit 99.1

THE POWER ofHUMAN™ May 2015

Forward Looking Statements OTC QB: ENUM THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT ARE BASED ON THE COMPANY’S CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS ABOUT THE COMPANY AND THE PHARMACEUTICAL INDUSTRY. THE COMPANY MAKES NO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS. FORWARD-LOOKING STATEMENTS ARE INDICATED BY WORDS SUCH AS: MAY, WILL, SHOULD, PREDICT, CONTINUE, PLAN, EXPECT, ANTICIPATE, ESTIMATE, INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS. FORWARD-LOOKING STATEMENTS MAY INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS CONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS; DURATION; SIZE; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY; BUSINESS MIX; REVENUES AND GROWTH IN OUR PARTNER BASE; MARKET OPPORTUNITIES; COMPETING TECHNOLOGIES, INDUSTRY CONDITIONS AND TRENDS; AND REGULATORY DEVELOPMENTS. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATED RESULTS DUE TO SUBSTANTIAL RISKS AND UNCERTAINTIES RELATED TO THE COMPANY AND THE BIOPHARMACEUTICAL INDUSTRY IN WHICH THE COMPANY OPERATES. 2

EnumeralInvestment Opportunity 3 A biopharma company developing potential ‘best- in-class’ antibodies against both proven and new targets using a proprietary immuno-oncology profiling technology.

EnumeralOverview 4 Initial goal: develop best-in-class PD-1 antibody • Opportunity: higher initial response rates and treatment of refractory cancers • First clinical trial targeted for 2016; results expected 2017 Ability to potentially develop multiple best-in-class antibodies through ‘Human immune system on a chip’ • MIT/Harvard-developed platform uniquely leverages patient- derived immune cells (TILs) • Antibodies selected based on modulation of human effector cell function • Additional antibodies targeting other checkpoints, costimulatory receptors, and TME targets • Novel target discovery and validation for combinations and next- gen immunotherapies Highly productive platform generates additional programs to drive growth

Enumeral’sDifferentiated Approach 5 Create broad antibody diversity Measure antibody function using our ‘Human immune system on a chip’ platform Select best-in-class candidates that modulate desired immune effector cells

EnumeralOrigins 6 July 2014December 2009 Completed reverse merger transaction; gross proceeds of $21.5M through private placement offering, began public trading on the OTC Markets (OTCQB:ENUM) Exclusive patent license with MIT Company incorporated First equity financing closed April 2011 June 2011 “Lights on” in Kendall Square lab, first scientist hired Additional financings: ~$11M total deployed on technology transfer, development, and program initiation Initial evaluation of first PD-1 antibody candidates

Experienced Leadership Team 7 John J. Rydzewski ExecutiveChairman, Co-Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co-Founder, Director CokeyNguyen, Ph.D. Vice President,Research&Development Isabel Chiu, Ph.D. Vice President, Translational& Clinical Sciences Kevin G. Sarney Vice President, Finance, &Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel

Accomplished Board of Directors 8 John J. Rydzewski ExecutiveChairman, Co-Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co-Founder, Director BarryBuckland, Ph.D. Co-Founder, Chairman, Scientific Advisory Board Allan Rothstein Director PaulJ. Sekhri Director Robert L. Van Nostrand Director Daniel Wolfe, Ph.D. Director

Immunotherapy Market Opportunity 9 *Citigroup **Bristol Myers Squibb Estimated sales of first- in-class products over next decade $35 billion per year (treating up to 60% of cancers*) Yervoy sales (launched in 2011) $1 billion in revenue (year ended June 20, 2014**) Strong interest in new approaches among Big Pharma, especially for antibody approaches Merck, Bristol Myers Squibb, AstraZeneca, Roche leading development; others actively investing in space

10 Enumeral’sPipeline 2015 2016 2017 Target Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 PD-1 Tim3 Lag-3 OX40 VISTA Undisclosed Screening Screening Screening Screening PreclinicalEfficacy Preclinical Efficacy Preclinical Efficacy Preclinical Efficacy Preclinical Efficacy IND EnablingStudies & CMC IND Enabling Studies & CMC IND Enabling Studies & CMC IND Enabling Studies & CMC IND Enabling Studies & CMC IND Filing IND Enabling Studies Phase I • Advance pipeline via platform insights: evaluating PD-1 and Tim3 on human TILs from patients for enrichment of patients for accelerated clinical development and to drive combination strategy • Leverage patient-centric platform insights to develop combination strategy to drive co-development partnerships in oncology

Breaking the “Immuno-Oncology Code” Proprietary ‘humanimmune system on a chip’ selects best-in-class antibodies and optimal target combinations 11 • Developing pipeline of antibody product candidates modulating Tumor- Infiltrating Lymphocyte (TIL) function • Next-generation checkpoint modulators derived from proprietary target-specific antibody libraries • Opportunity to rationally define combinatorial immunotherapy within oncology, and new uses beyond cancer: inflammation and autoimmunity

Validation of Technology Platform* 12 *Publications from laboratory of scientific founder, J. Christopher Love, Ph.D.

Recognition of Enumeral’sApproach • Merck: collaboration with leading immuno-oncology pharmaceutical company – Focused on using Enumeral'splatform to interrogate the tumor microenvironment in colorectal cancer tissues to identify functional cellular responses to therapies being developed by Merck – R&D funding and undisclosed milestone payments – Merck has exclusive rights to data related to its proprietary compounds • NCI Phase 2 Contract: $1M over two years – Automation of human tissue immuno-oncology profiling – Opens door to broader pipeline and potentially accelerated development – Collaboration with leading scientists in the field: – JeddWolchok’sgroup at MSKCC, pioneers in the development of approved cancer immunotherapies – Doug Kwon’sgroup at MGH/RagonInstitute, leaders in colorectal mucosal immunology 13

Enumeral’sApproach to T Cell-selective Antibody Discovery: PICTURE TM* 14 *Patent pending PICTURE: Precision Immune Cell Targeting Using Real-time Enumeration Effector T cells Hypothetical output of platform*: Antibodies selective for T cells

Functional Diversity: Potential for Best-in-Class Pharmacologic and MOA Differentiation 15 Screen 1-12 Heavy chain AA sequences from PD1 N= 349 sequences; 28 families of antibodies OPDIVO® KEYTRUDA® Pidilizumab • Enumeral proprietary antibody libraries - unprecedented functional diversity • PD-1: ~10 months - generation of 28 families • Potential for strong IP position around validated targets • Ability to select potential ‘best-in- class’ candidates

Platform Enables Identification of Antibodies That Selectively Modulate T Cells 16 Biopsy from colorectal cancer patient; No sorting or enrichment • Preclinical animal models poorly developed • Enumeral‘human immune system on a chip’ enables us to measure the effect our antibody candidates have on immune cells from patients • Enables selection of antibodies based on comparative ability to modulate T cells

Enumeral’s Broadly Enabling Platform 17 84,672 microwells • High-density bio-chip and one-day process • Measures key parameters of function of individual immune cells from patient samples • Uniquely enumerates and rapidly identifies rare immune cellscritical to responses • Validates targets and efficiently generates new antibodies Determine cell function through cell secretions Determine cell type and biological function through cytometry Recover cells of interest and gene sequences important to disease

ENUM Antibodies Demonstrate pMAffinity mAb EC 50 246A10 0.020 244C8 0.005 388D4 0.003 413Ec1 0.013 413D2 0.005 392C5 0.015 Keytruda 0.066 18 Several ENUM mAbs show superior EC 50 vs Keytruda (*Data Provided From Internal Enumeral Tests) 0 0.5 1 1.5 2 2.5 3 3.5 a b s ( 4 0 5 n m ) mAbconcentration (M) 388D4 413cE1 413D2 246A10 244C8 392C5 Preclinical Benchmarking*

PD-L1 Suppression Assay 19 • PBMC-based assay to determine mAb’s ability to disrupt functional suppression by PD-L1 • ENUM candidates compared with: – Activation only – PD-L1 (suppression) – Positive control • ENUM A10 and C8 shown to reverse PD- L1 suppression more effectively than control I F N g s e c r e t i o n ( r e l a t i v e t o c o n t r o l ) N = 5 repetitions for each candidate/condition
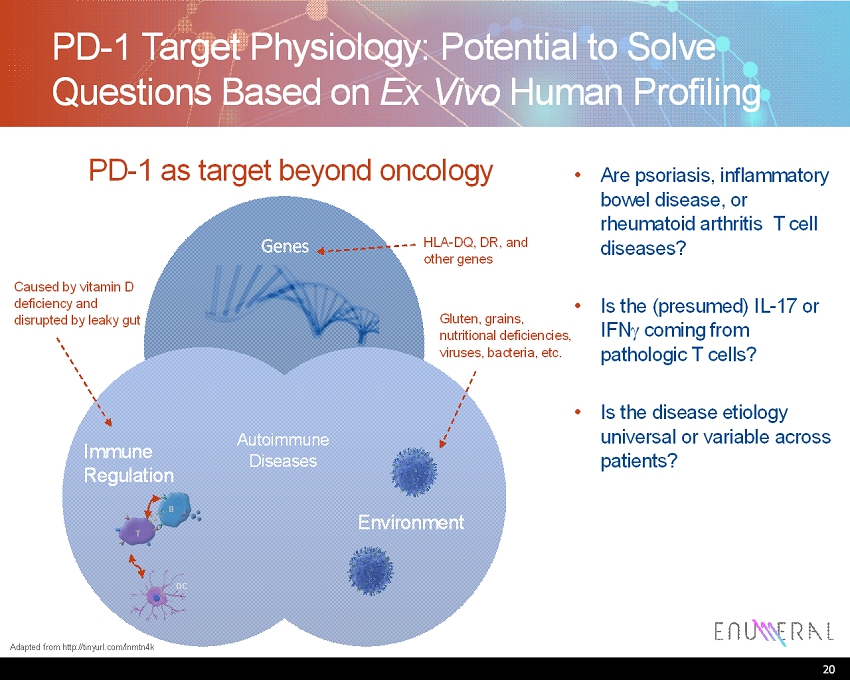
PD-1 Target Physiology: Potential to Solve Questions Based on Ex Vivo Human Profiling • Are psoriasis, inflammatory bowel disease, or rheumatoid arthritis T cell diseases? • Is the (presumed) IL-17 or IFN coming from pathologic T cells? • Is the disease etiology universal or variable across patients? 20 PD-1 as target beyond oncology Genes Immune Regulation Autoimmune Diseases Environment Caused by vitamin D deficiency and disrupted by leaky gut HLA-DQ, DR, and other genes Gluten, grains, nutritional deficiencies, viruses, bacteria, etc. B T DC Adapted from http://tinyurl.com/lnmtn4k

Value Creation 21 Value 2012 2013 2014 2015 2016 2017 Enumeralbelieves it is well-positioned to achieve significant value-generating milestones xNCI Phase 1 contract x$21.5M financing and public listing xNCI Phase 2 contract xMerck Collaboration xMSKCC and MGH collaborations Initiate IND-enabling studies in PD-1 program (2015) Initiate Phase 1 in PD- 1 program (2016) Phase 1 data from PD-1 program (2017) Denotes near-term corporate objectives Validation of PD-1 Lead Candidates (2015)

Financials & Capitalization Common Shares Outstanding 51,607,665 Warrants Outstanding* 24,803,409 Options Outstanding** 2,730,963 Burn Rate, Q1 ’15 ($2.5M) Total Debt $0.0M Cash, Cash Equivalents and Marketable Securities** $11.0M 22 OTC QB: ENUM Price: $0.65 Market Cap (5/15/2015) $34.58M *21,549,510 are exercisable at $2.00 **As of March 31, 2015 Enumeral: uniquely positioned to discover and develop novel ‘best-in- class’ immunotherapies

THE POWER ofHUMAN™ Arthur H. Tinkelenberg, Ph.D. President and CEO arthur@enumeral.com 617-500-2647






















