Exhibit 99.1
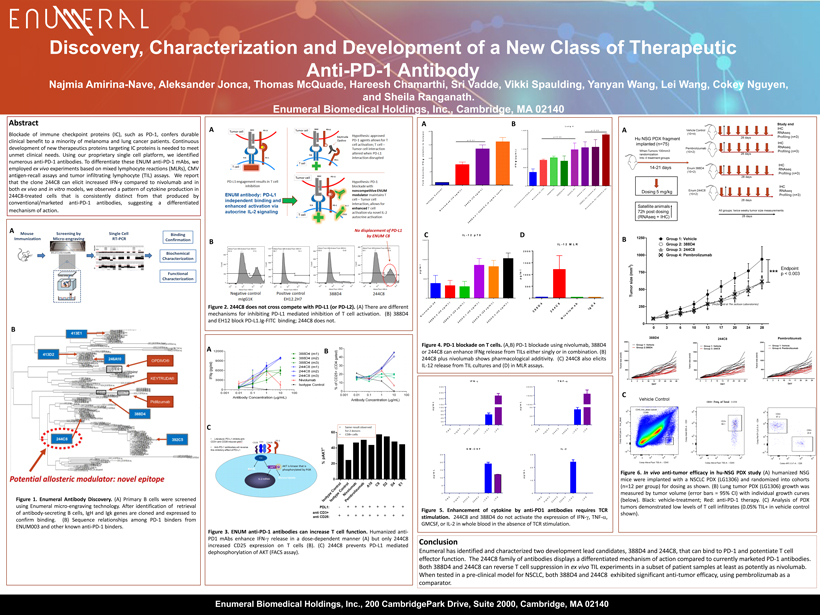
N i v o l u m a b - 2 0 3 8 8 D 4 - 2 ( 2 0 u g / m L ) 3 8 8 D 4 - 3 ( 2 0 u g / m L ) 2 4 4 C 8 - 1 ( 2 0 u g / m L ) 2 4 4 C 8 - 2 ( 2 0 u g / m L ) 2 4 4 C 8 - 3 ( 2 0 u g / m L ) 0 500 1000 1500 IL-12 p70 p g / m l Discovery, C haracterization and Development of a New C lass of T herapeutic A nti - PD - 1 A ntibody Abstract Blockade of immune checkpoint proteins (IC), such as PD - 1 , confers durable clinical benefit to a minority of melanoma and lung cancer patients . Continuous development of new therapeutics proteins targeting IC proteins is needed to meet unmet clinical needs . Using our proprietary single cell platform , we identified numerous anti - PD - 1 antibodies . To differentiate these ENUM anti - PD - 1 mAbs , we employed ex vivo experiments based on mixed lymphocyte reactions ( MLRs), CMV antigen - recall assays and tumor infiltrating lymphocyte ( TIL) assays . We report that the clone 244 C 8 can elicit increased IFN - y compared to nivolumab and in both ex vivo and in vitro models, we observed a pattern of cytokine production in 244 C 8 - treated cells that is consistently distinct from that produced by conventional/marketed anti - PD - 1 antibodies, suggesting a differentiated mechanism of action . Figure 1 . Enumeral Antibody Discovery . (A) Primary B cells were screened using Enumeral micro - engraving technology . After identification of retrieval of antibody - secreting B cells, IgH and Igk genes are cloned and expressed to confirm binding . (B) Sequence relationships among PD - 1 binders from ENUM 003 and other known anti - PD - 1 binders . Conclusion Enumeral has identified and characterized two development lead candidates, 388D4 and 244C8, that can bind to PD - 1 and potentiate T cell effector function. The 244C8 family of antibodies displays a differentiated mechanism of action compared to currently marketed PD - 1 antibodies. Both 388D4 and 244C8 can reverse T cell suppression in ex vivo TIL experiments in a subset of patient samples at least as potently as nivolumab . When tested in a pre - clinical model for NSCLC, both 388D4 and 244C8 exhibited significant anti - tumor efficacy, using pembrolizumab as a comparator. Figure 3 . ENUM anti - PD - 1 a ntibodies can increase T cell function . Humanized anti - PD 1 mAbs enhance IFN - g release in a dose - dependent manner (A ) but only 244 C 8 increased CD 25 expression on T cells (B) . (C) 244 C 8 prevents PD - L 1 mediated dephosphorylation of AKT (FACS assay) . A B A A B Najmia Amirina - Nave, Aleksander Jonca, Thomas McQuade, Hareesh Chamarthi, Sri Vadde, Vikki Spaulding, Yanyan Wang, Lei Wang, Cok ey Nguyen, and Sheila Ranganath. Enumeral Biomedical Holdings, Inc., Cambridge, MA 02140 Figure 6 . In vivo anti - tumor efficacy in hu - NSG PDX study (A) humanized NSG mice were implanted with a NSCLC PDX (LG 1306 ) and randomized into cohorts (n= 12 per group) for dosing as shown . (B) Lung tumor PDX (LG 1306 ) growth was measured by tumor volume (error bars = 95 % CI) with individual growth curves (below) . Black : vehicle - treatment ; Red : anti - PD - 1 therapy . (C) Analysis of PDX tumors demonstrated low levels of T cell infiltrates ( 0 . 05 % TIL+ in vehicle control shown) . Figure 2 . 244 C 8 does not cross compete with PD - L 1 (or PD - L 2 ) . (A) There are different mechanisms for inhibiting PD - L 1 mediated inhibition of T cell activation . (B) 388 D 4 and EH 12 block PD - L 1 . Ig - FITC binding ; 244 C 8 does not . Figure 4 . PD - 1 blockade on T cells . (A,B) PD - 1 blockade using nivolumab , 388 D 4 or 244 C 8 can enhance IFNg release from TILs either singly or in combination . (B) 244 C 8 plus nivolumab shows pharmacological additivity . (C) 244 C 8 also elicits IL - 12 release from TIL cultures and (D) in MLR assays . Figure 5 . Enhancement of cytokine by anti - PD 1 antibodies requires TCR stimulation . 244 C 8 and 388 D 4 do not activate the expression of IFN - g , TNF - a , GMCSF, or IL - 2 in whole blood in the absence of TCR stimulation . A B C B C A B I s o t y p e C o n t r o l N i v o l u m a b ( 1 0 u g / m L ) 2 4 4 C 8 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 + 2 4 4 C 8 - 2 ( 5 u g / m L e a c h ) 0 5 10 15 20 F o l d I n d u c t i o n I F N - ( r e l a t i v e t o I s o t y p e ) p<0.01 C (Performed at The Jackson Laboratories) Enumeral Biomedical Holdings, Inc., 200 CambridgePark Drive, Suite 2000, Cambridge, MA 02140 3 8 8 D 4 2 4 4 C 8 N i v o l u m a b I g G 4 0 500 1000 1500 2000 IL-12 MLR p g / m l a n t i - C D 3 + a n t i - C D 2 8 o n l y N i v o l u m a b ( 1 0 ) 3 8 8 D 4 ( 5 ) 3 8 8 D 4 ( 1 0 ) 2 4 4 C 8 ( 5 ) 2 4 4 C 8 ( 1 0 ) 3 8 8 D 4 ( 5 ) + 2 4 4 C 8 ( 5 ) N i v o l u m a b ( 5 ) + 2 4 4 C 8 - 2 ( 5 ) 0 500 1,000 1,500 Lung 4 I F N - ( p g / m L ) p<0.05 p<0.05 D
