Exhibit 99.1

Enumeral Overview May 2016

Forward Looking Statements OTC QB: ENUM THIS PRESENTATION CONTAINS FORWARD - LOOKING STATEMENTS THAT ARE BASED ON THE COMPANY’S CURRENT EXPECTATIONS, ASSUMPTIONS, ESTIMATES AND PROJECTIONS ABOUT THE COMPANY AND THE PHARMACEUTICAL INDUSTRY . THE COMPANY MAKES NO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS . FORWARD - LOOKING STATEMENTS ARE INDICATED BY WORDS SUCH AS : MAY, WILL, SHOULD, PREDICT, CONTINUE, PLAN, EXPECT, ANTICIPATE, ESTIMATE, INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS . FORWARD - LOOKING STATEMENTS MAY INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS CONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS ; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS ; DURATION ; SIZE ; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS ; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY ; BUSINESS MIX ; REVENUES AND GROWTH IN OUR PARTNER BASE ; MARKET OPPORTUNITIES ; COMPETING TECHNOLOGIES, INDUSTRY CONDITIONS AND TRENDS ; AND REGULATORY DEVELOPMENTS . ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATED RESULTS DUE TO SUBSTANTIAL RISKS AND UNCERTAINTIES RELATED TO THE COMPANY AND THE BIOPHARMACEUTICAL INDUSTRY IN WHICH THE COMPANY OPERATES . 2
Enumeral Overview • Potential first - in - class non - competitive PD - 1 antagonist – Novel mechanism in a proven immuno - oncology pathway • Clinical significance: – Potential to treat failure and relapse due to differentiated mechanism: drives both arms of the immune system – Not all patients benefit from current therapies • Commercial advantage: – Large market exists for a more effective PD - 1 directed therapy – Antibody immunotherapies generating ~$5B in annualized revenues* – Many other I/O modalities face clinical or commercialization challenges • Value creation opportunity could be significant: – Possible ‘clear line of sight’ to registration if responses are seen in prior failure/relapse patients • Enumeral’s platform and human biopsy approach is differentiated – Ex vivo translational human biopsy approach informs development of next - generation I/O opportunities 3 *BMS, Merck, and Amgen SEC filings; as of 3/31/2016

Agenda 1. Optimizing Drugs Against PD - 1: Lessons Learned 2. ENUM 244C8 – A Differentiated Anti - PD - 1 Antibody That Drives Adaptive and Innate Immune Cell Activation 3. Opportunity 4. Pipeline, Platform, and Collaborators 5. Management and Directors 4
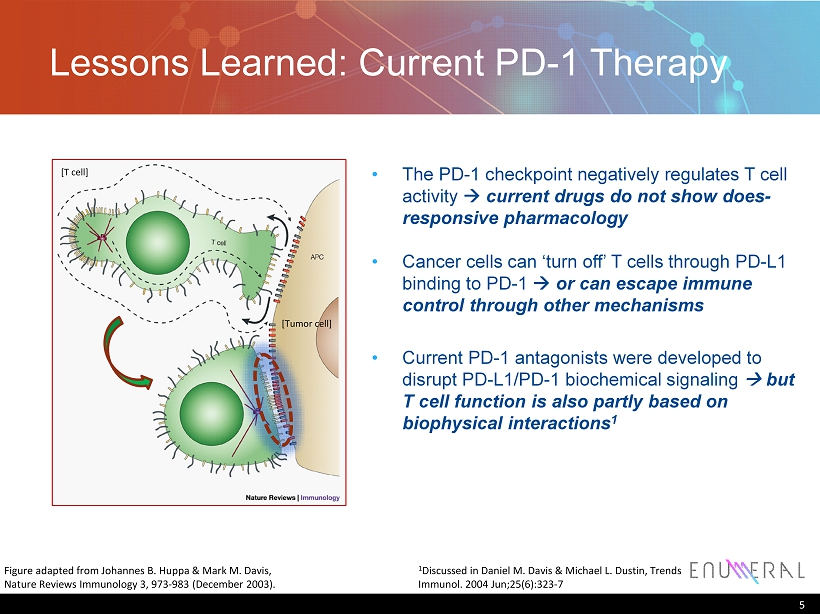
Lessons Learned: Current PD - 1 Therapy 5 Figure adapted from Johannes B. Huppa & Mark M. Davis, Nature Reviews Immunology 3, 973 - 983 (December 2003). • The PD - 1 checkpoint negatively regulates T cell activity à current drugs do not show does - responsive pharmacology • Cancer cells can ‘turn off’ T cells through PD - L1 binding to PD - 1 à or can escape immune control through other mechanisms • Current PD - 1 antagonists were developed to disrupt PD - L1/PD - 1 biochemical signaling à but T cell function is also partly based on biophysical interactions 1 [Tumor cell] [T cell] 1 Discussed in Daniel M. Davis & Michael L. Dustin, Trends Immunol. 2004 Jun;25(6):323 - 7
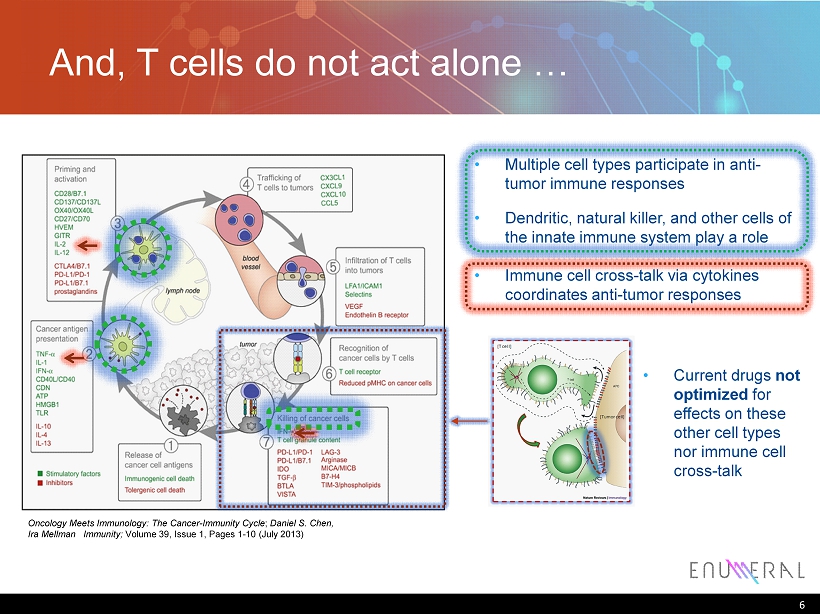
And, T cells do not act alone … 6 • Multiple cell types participate in anti - tumor immune responses Oncology Meets Immunology: The Cancer - Immunity Cycle ; Daniel S. Chen, Ira Mellman Immunity; Volume 39, Issue 1, Pages 1 - 10 (July 2013) • Dendritic, natural killer, and other cells of the innate immune system play a role responses • Immune cell cross - talk via cytokines coordinates anti - tumor responses • Current drugs not optimized for effects on these other cell types nor immune cell cross - talk

Agenda 1. Optimizing Drugs Against PD - 1: Lessons Learned 2. ENUM 244C8 – A Differentiated Anti - PD - 1 Antibody That Drives Adaptive and Innate Immune Cell Activation 3. Opportunity 4. Pipeline, Platform, and Collaborators 5. Management and Directors 7
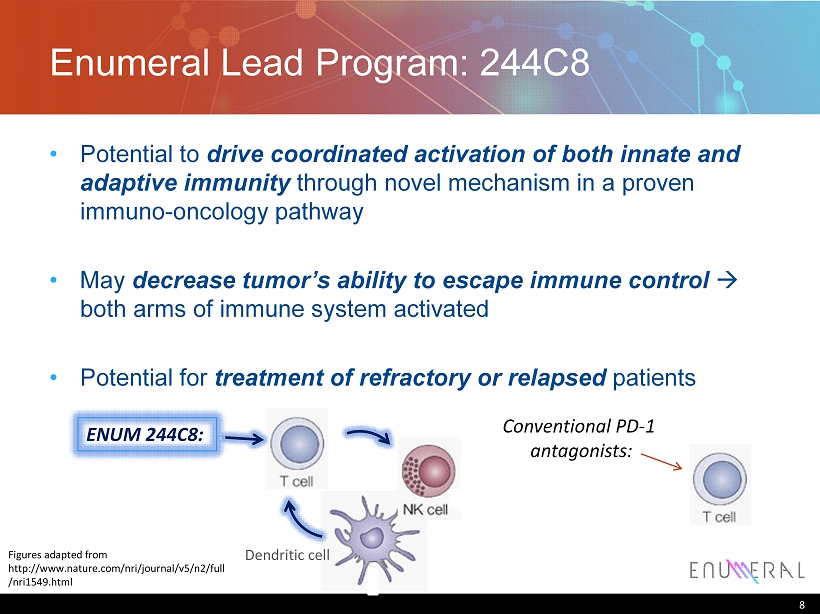
Enumeral Lead Program: 244C8 • Potential to drive coordinated activation of both innate and adaptive immunity through novel mechanism in a proven immuno - oncology pathway • May decrease tumor’s ability to escape immune control à both arms of immune system activated • Potential for treatment of refractory or relapsed patients 8 Figures adapted from http://www.nature.com/nri/journal/v5/n2/full /nri1549.html Conventional PD - 1 antagonists: Dendritic cell ENUM 244C8:
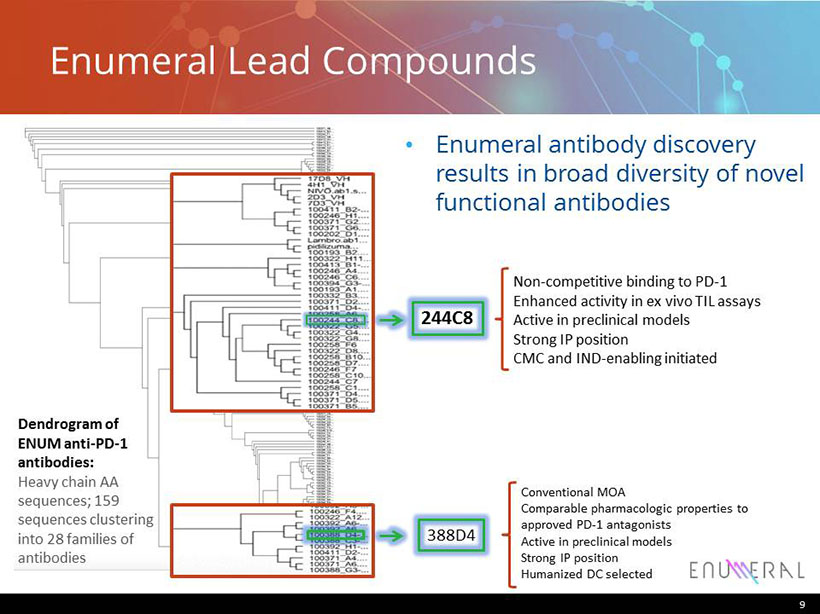
Enumeral Lead Compounds • Enumeral antibody discovery results in broad diversity of novel functional antibodies 9 Dendrogram of ENUM anti - PD - 1 antibodies: Heavy chain AA sequences; 159 sequences clustering into 28 families of antibodies Conventional MOA Comparable pharmacologic properties to approved PD - 1 antagonists Active in preclinical models Strong IP position Humanized DC selected Non - competitive binding to PD - 1 Enhanced activity in ex vivo TIL assays Active in preclinical models Strong IP position CMC and IND - enabling initiated 244C8 388D4
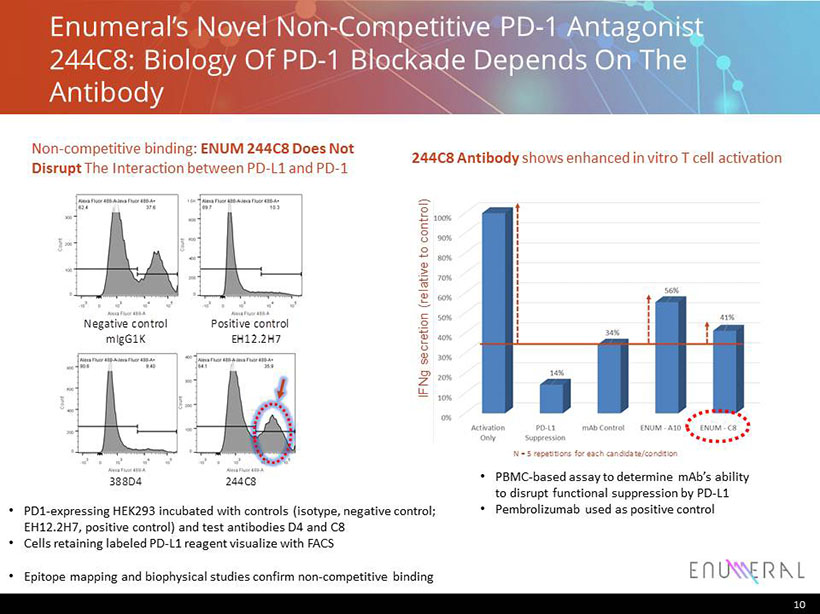
Enumeral’s Novel Non - Competitive PD - 1 Antagonist 244C8: Biology Of PD - 1 Blockade Depends On The Antibody 10 244C8 Antibody shows enhanced in vitro T cell activation IFNg secretion (relative to control) N = 5 repetitions for each candidate/condition • PBMC - based assay to determine mAb’s ability to disrupt functional suppression by PD - L1 • Pembrolizumab used as positive control • PD1 - expressing HEK293 incubated with controls (isotype, negative control; EH12.2H7, positive control) and test antibodies D4 and C8 • Cells retaining labeled PD - L1 reagent visualize with FACS • Epitope mapping and biophysical studies confirm non - competitive binding Positive control EH12.2H7 Negative control mIgG1K 388D4 244C8 Positive control EH12.2H7 Negative control mIgG1K 388D4 244C8 Non - competitive binding: ENUM 244C8 Does Not Disrupt The Interaction between PD - L1 and PD - 1 Positive control EH12.2H7 Negative control mIgG1K 388D4 244C8 Positive control EH12.2H7 Negative control mIgG1K 388D4 244C8
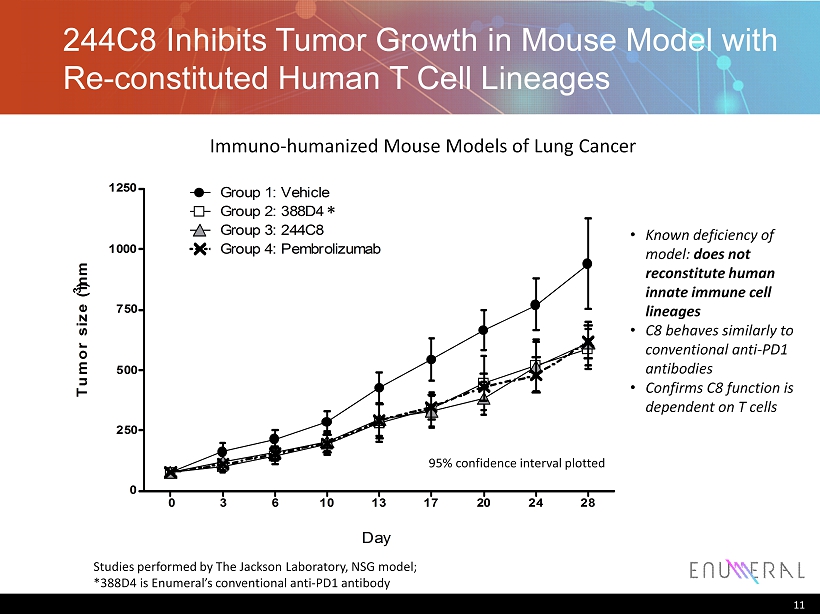
244C8 Inhibits Tumor Growth in Mouse Model with Re - constituted Human T Cell Lineages 11 Tumor size (mm 3 ) 0 3 6 10 13 17 20 24 28 0 250 500 750 1000 1250 Group 1: Vehicle Group 2: 388D4 Group 3: 244C8 Group 4: Pembrolizumab Day 95% confidence interval plotted Studies performed by The Jackson Laboratory, NSG model; *388D4 is Enumeral’s conventional anti - PD1 antibody Immuno - humanized Mouse Models of Lung Cancer * • Known deficiency of model: does not reconstitute human innate immune cell lineages • C8 behaves similarly to conventional anti - PD1 antibodies • Confirms C8 function is dependent on T cells
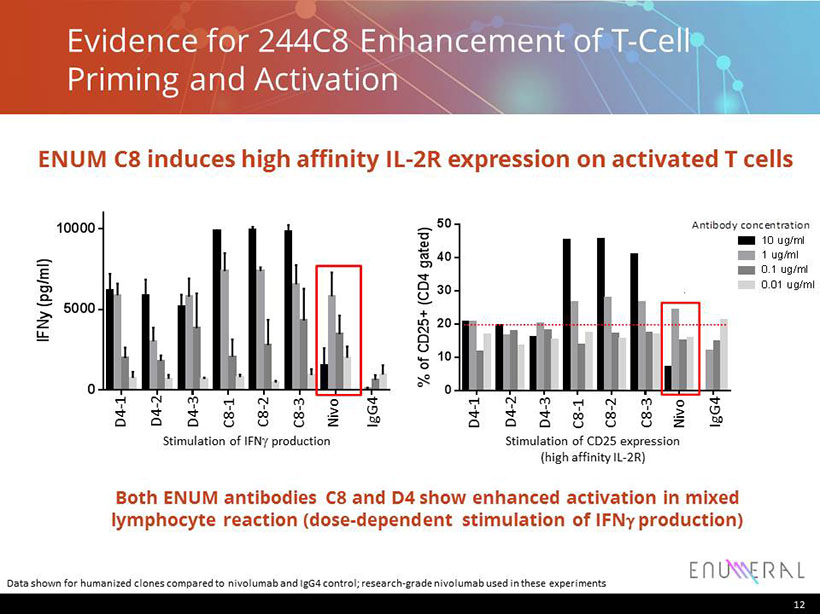
D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 5000 10000 MLR Donor 337+23 (IFNy) I F N y ( p g / m l ) 10 ug/ml 1 ug/ml 0.1 ug/ml 0.01 ug/ml Antibody concentration Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of IFN g production Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of CD25 expression (high affinity IL - 2R) Nivo IgG4 D4 - 1 D4 - 2 D4 - 3 C8 - 1 C8 - 2 C8 - 3 Nivo IgG4 D4 - 1 D4 - 2 D4 - 3 C8 - 1 C8 - 2 C8 - 3 Evidence for 244C8 Enhancement of T - Cell Priming and Activation 12 ENUM C8 induces high affinity IL - 2R expression on activated T cells Data shown for humanized clones compared to nivolumab and IgG4 control; research - grade nivolumab used in these experiments Both ENUM antibodies C8 and D4 show enhanced activation in mixed lymphocyte reaction (dose - dependent stimulation of IFN g production)

244C8 Elicits Cytokine Signature Consistent with Activation of Innate Immunity 13 Ex vivo cytokine production in NSCLC tumor biopsy consistent with focal activation of both innate & adaptive anti - tumor immunity B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 10000 20000 30000 40000 Lung IL-6 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 20 40 60 80 100 Lung IL-12 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL-18 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 Lung TNF-? p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 2500 Lung GMCSF p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL1-? p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 10000 20000 30000 40000 Lung IL-6 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 20 40 60 80 100 Lung IL-12 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL-18 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 Lung TNF-? p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 2500 Lung GMCSF p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL1-? p g / m l Assays performed using lung biopsy obtained from patients undergoing staging surgeries. Trends observed in three independent experiments.
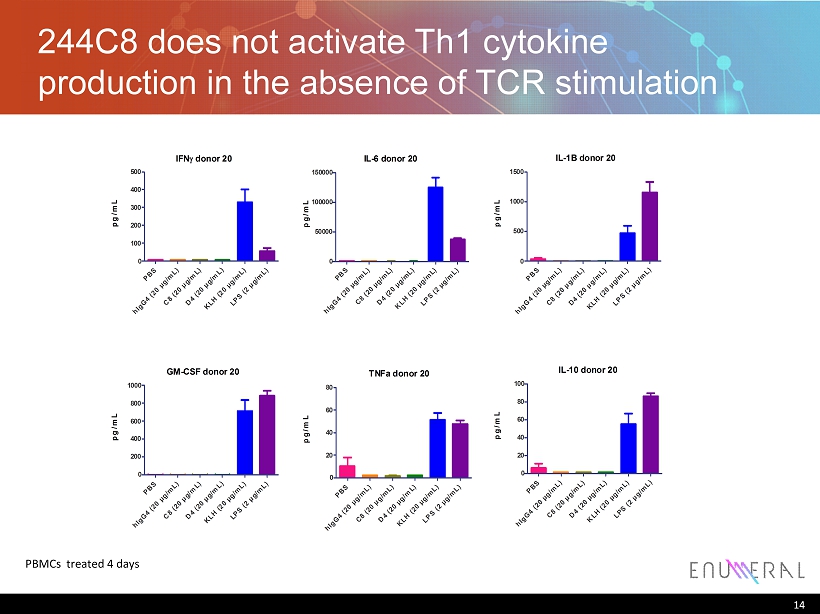
244C8 does not activate Th1 cytokine production in the absence of TCR stimulation IFN? donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 100 200 300 400 500 IL-6 donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 50000 100000 150000 IL-1B donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 500 1000 1500 GM-CSF donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 200 400 600 800 1000 TNFa donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 20 40 60 80 IL-10 donor 20 pg/mL PBS hIgG4 (20 µg/mL)C8 (20 µg/mL)D4 (20 µg/mL)KLH (20 µg/mL)LPS (2 µg/mL) 0 20 40 60 80 100 14 PBMCs treated 4 days
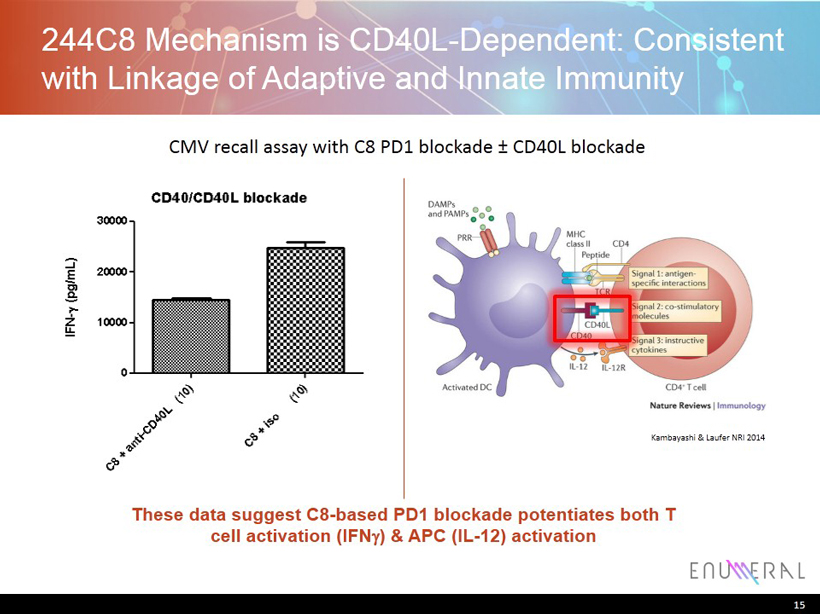
244C8 Mechanism is CD40L - Dependent: Consistent with Linkage of Adaptive and Innate Immunity 15 Kambayashi & Laufer NRI 2014 CMV recall assay with C8 PD1 blockade ± CD40L blockade These data suggest C8 - based PD1 blockade potentiates both T cell activation ( IFN g ) & APC (IL - 12) activation
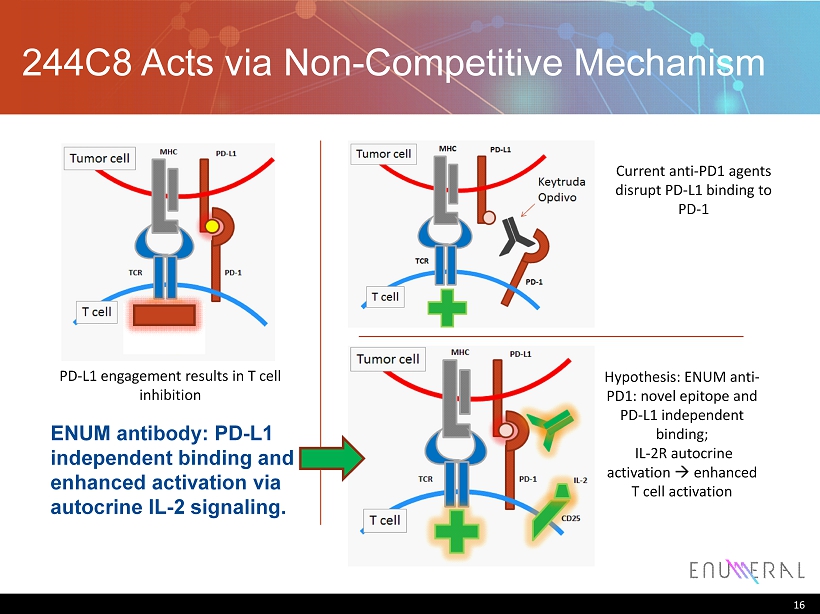
244C8 Acts via Non - Competitive Mechanism 16 PD - L1 engagement results in T cell inhibition Current anti - PD1 agents disrupt PD - L1 binding to PD - 1 Hypothesis: ENUM anti - PD1: novel epitope and PD - L1 independent binding; IL - 2R autocrine activation à enhanced T cell activation ENUM antibody: PD - L1 independent binding and enhanced activation via autocrine IL - 2 signaling.
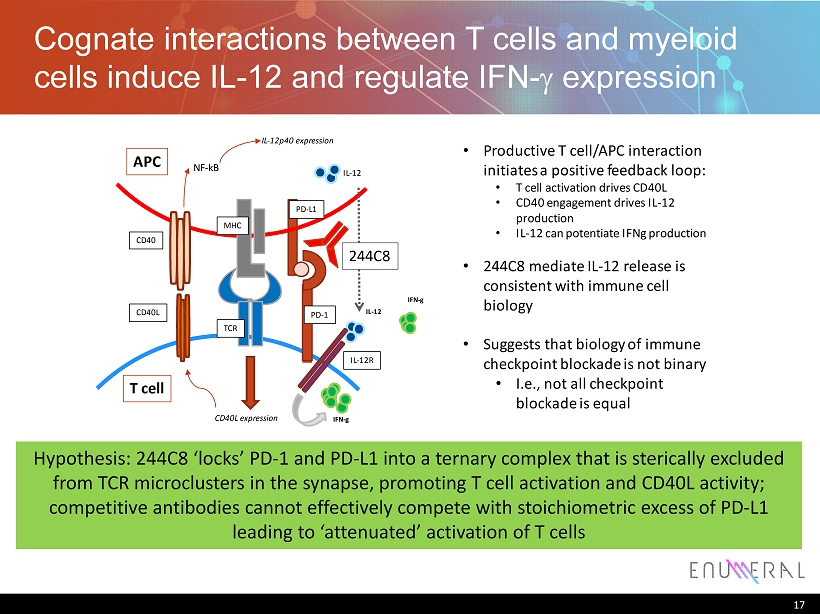
Cognate interactions between T cells and myeloid cells induce IL - 12 and regulate IFN - g expression 17 Hypothesis: 244C8 ‘locks’ PD - 1 and PD - L1 into a ternary complex that is sterically excluded from TCR microclusters in the synapse, promoting T cell activation and CD40L activity; competitive antibodies cannot effectively compete with stoichiometric excess of PD - L1 leading to ‘attenuated’ activation of T cells IL - 12p40 expression CD40L expression CD40L CD40 NF - kB IL - 12 IL - 12 IFN - g IL - 12R APC T cell PD - 1 244C8 TCR MHC IFN - g PD - L1 • Productive T cell/APC interaction initiates a positive feedback loop: • T cell activation drives CD40L • CD40 engagement drives IL - 12 production • IL - 12 can potentiate IFNg production • 244C8 mediate IL - 12 release is consistent with immune cell biology • Suggests that biology of immune checkpoint blockade is not binary • I.e., not all checkpoint blockade is equal
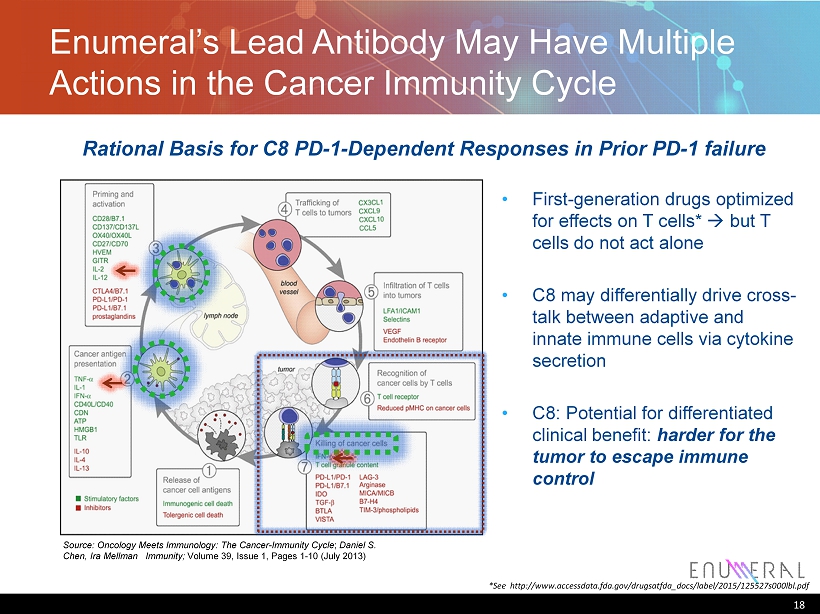
Enumeral’s Lead Antibody May Have Multiple Actions in the Cancer Immunity Cycle 18 Source: Oncology Meets Immunology: The Cancer - Immunity Cycle ; Daniel S. Chen, Ira Mellman Immunity; Volume 39, Issue 1, Pages 1 - 10 (July 2013) • First - generation drugs optimized for effects on T cells* à but T cells do not act alone • C8 may differentially drive cross - talk between adaptive and innate immune cells via cytokine secretion • C8: Potential for differentiated clinical benefit: harder for the tumor to escape immune control Rational Basis for C8 PD - 1 - Dependent Responses in Prior PD - 1 failure *See http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125527s000lbl.pdf

Agenda 1. Optimizing Drugs Against PD - 1: Lessons Learned 2. ENUM 244C8 – A Differentiated Anti - PD - 1 Antibody That Drives Adaptive and Innate Immune Cell Activation 3. Opportunity 4. Pipeline, Platform, and Collaborators 5. Management and Directors 19
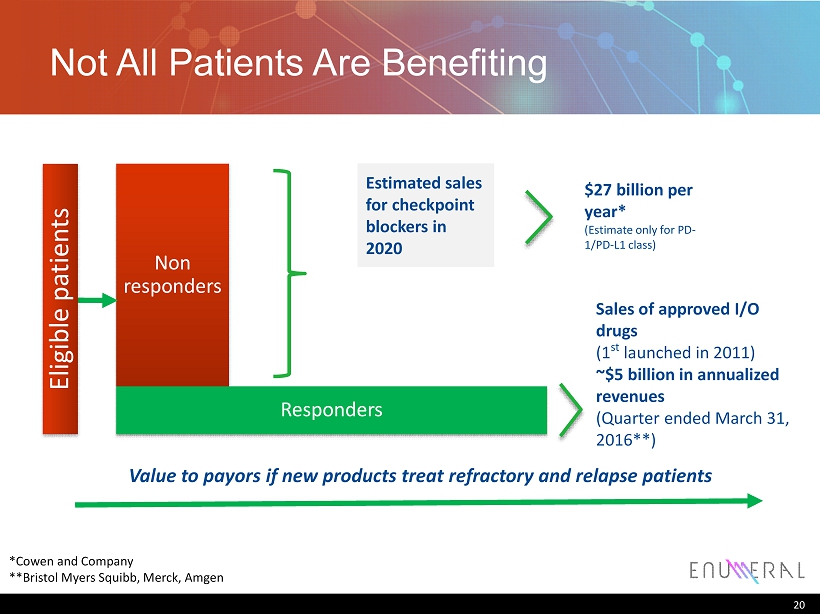
Not All Patients Are Benefiting 20 Non responders Responders Value to payors if new products treat refractory and relapse patients Estimated sales for checkpoint blockers in 2020 $27 billion per year* (Estimate only for PD - 1/PD - L1 class ) *Cowen and Company **Bristol Myers Squibb, Merck, Amgen Sales of approved I/O drugs (1 st launched in 2011) ~$5 billion in annualized revenues (Quarter ended March 31, 2016**) Eligible patients

Limitations of 1 st Generation PD - 1 Inhibitors PD - 1 blockade through disrupting the PD - L1 interaction may not be the whole story • In the approved indications, response rates are 20% - 30% • Ability to predict responders based on PD - 1 or PDL - 1 expression is disappointing • Ability to predict responders based on the amount of tumor T cell infiltration is incomplete Drug development of I/O therapeutics limited by • Mouse models that do not predict human immuno - oncology • Complex target biology • Drugs not optimized for effects on non - T cell immune cells • Drugs do not show dose responsiveness 21

Commercial Opportunity Lead by Antibodies 22 Figure and text adapted from: http://www.nature.com/nrd/journal/vaop/ncurrent/full/nrd.2015.35.html *As of January 2015, reported revenues were ~$20M for Provenge ; no revenues reported by Valeant which acquired Provenge T cell therapies; ~$0 revenue*; Efficacy limited, Toxicity 1st generation bispecifics ; ~<$20M revenue since launch; Stability, Toxicity 1 st generation viruses; Approved Q4 ‘15; Moderate responses; Systemic effects Checkpoint antibodies ~$5B annualized revenues; Durable benefit in subset of patients

Agenda 1. Optimizing Drugs Against PD - 1: Lessons Learned 2. ENUM 244C8 – A Differentiated Anti - PD - 1 Antibody That Drives Adaptive and Innate Immune Cell Activation 3. Opportunity 4. Pipeline, Platform, and Collaborators 5. Management and Directors 23
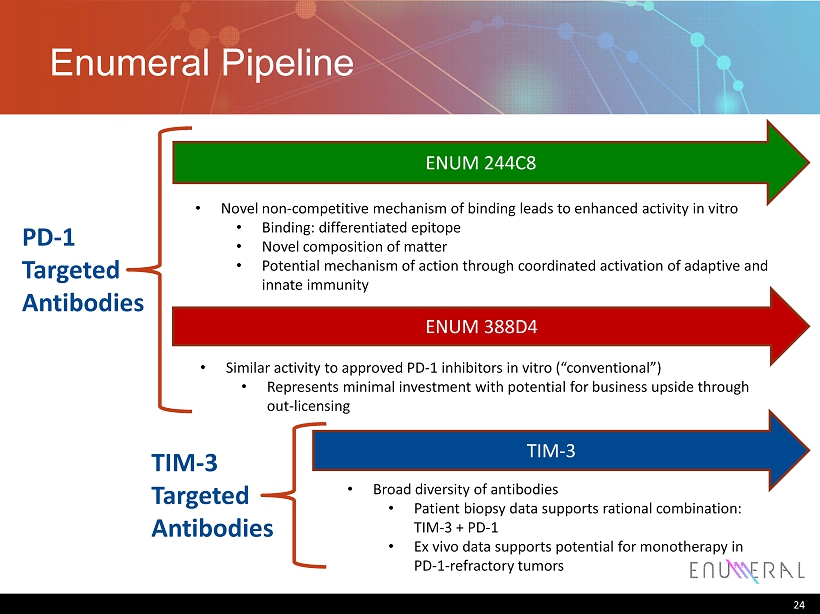
Enumeral Pipeline 24 ENUM 244C8 • Novel non - competitive mechanism of binding leads to enhanced activity in vitro • Binding: differentiated epitope • Novel composition of matter • Potential mechanism of action through coordinated activation of adaptive and innate immunity TIM - 3 • Broad diversity of antibodies • Patient biopsy data supports rational combination: TIM - 3 + PD - 1 • Ex vivo data supports potential for monotherapy in PD - 1 - refractory tumors PD - 1 Targeted Antibodies TIM - 3 Targeted Antibodies ENUM 388D4 • Similar activity to approved PD - 1 inhibitors in vitro (“conventional”) • Represents minimal investment with potential for business upside through out - licensing
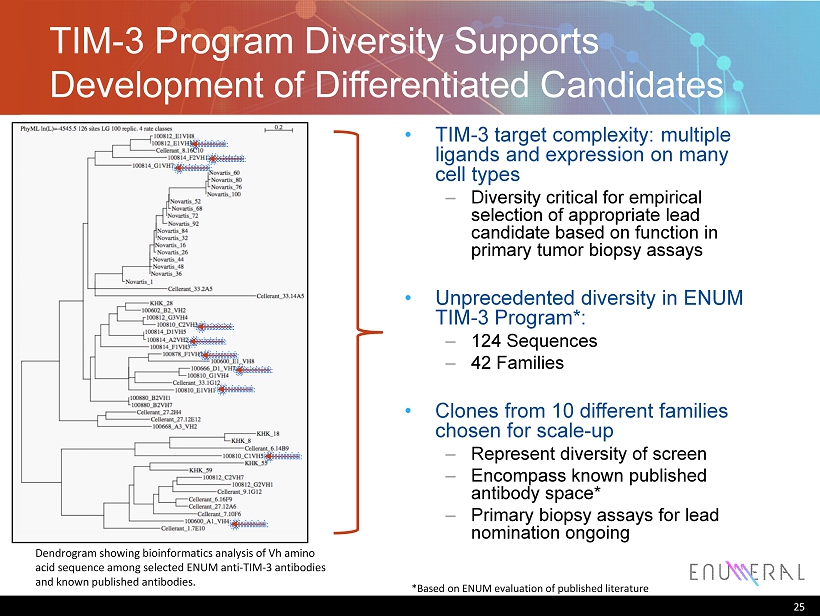
TIM - 3 Program Diversity Supports Development of Differentiated Candidates 25 *Based on ENUM evaluation of published literature Dendrogram showing bioinformatics analysis of Vh amino acid sequence among selected ENUM anti - TIM - 3 antibodies and known published antibodies. • TIM - 3 target complexity: multiple ligands and expression on many cell types – Diversity critical for empirical selection of appropriate lead candidate based on function in primary tumor biopsy assays • Unprecedented diversity in ENUM TIM - 3 Program*: – 124 Sequences – 42 Families • Clones from 10 different families chosen for scale - up – Represent diversity of screen – Encompass known published antibody space* – Primary biopsy assays for lead nomination ongoing

ENUM Anti - TIM3 Antibody Activates PD - 1 Blockade - Refractory NSCLC TILs Ex Vivo 26 NSCLC 22 TILs do not respond to PD - 1 blockade ex vivo h I g G 4 ( 1 0 u g / m L ) E N U M C 8 ( 1 0 u g / m L ) 0 5000 10000 15000 I F N - ? ( p g / m L ) Isotype control m I g G 1 k ( 1 u g / m L ) T I M - 3 # 5 ( 1 u g / m L ) 2 4 4 c 8 ( 1 0 u g / m L ) + T i m 3 # 5 ( 1 u g / m L ) 0 5000 10000 15000 I F N - ? ( p g / m L )

Other Enumeral Pipeline Programs BMS Immutep 27 GSK • ENUM LAG - 3 Antibodies To - Date: Broad Diversity – 102 Sequences – 40 Families – Encompass known published antibody space* • Other Screening Programs: – TIGIT – VISTA – OX40 – Others not disclosed Dendrogram showing bioinformatics analysis of Vh amino acid sequence among ENUM anti - LAG - 3 antibodies and known published antibodies. *Based on ENUM evaluation of published literature
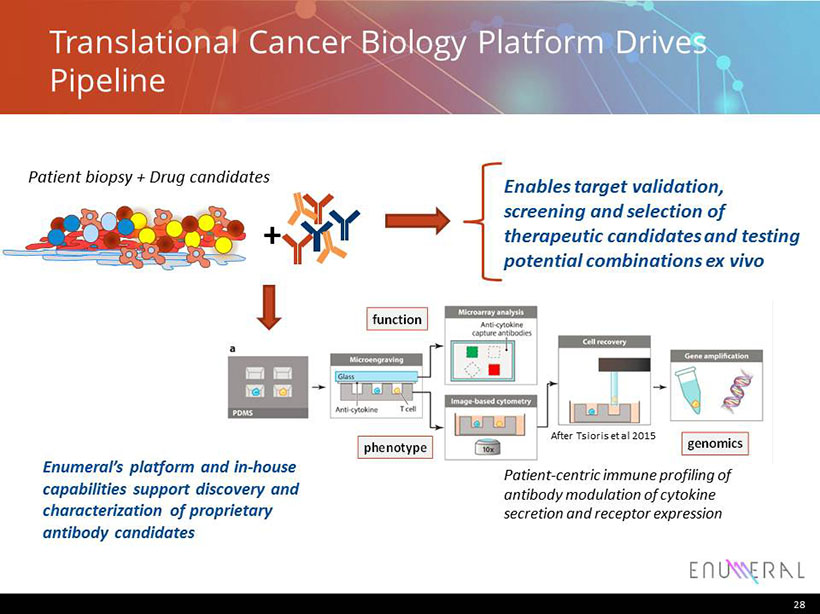
Translational Cancer Biology Platform Drives Pipeline 28 Patient biopsy + Drug candidates Enables target validation, screening and selection of therapeutic candidates and testing potential combinations ex vivo + Patient - centric immune profiling of antibody modulation of cytokine secretion and receptor expression function phenotype genomics function phenotype genomics function phenotype genomics After Tsioris et al 2015 Enumeral’s platform and in - house capabilities support discovery and characterization of proprietary antibody candidates
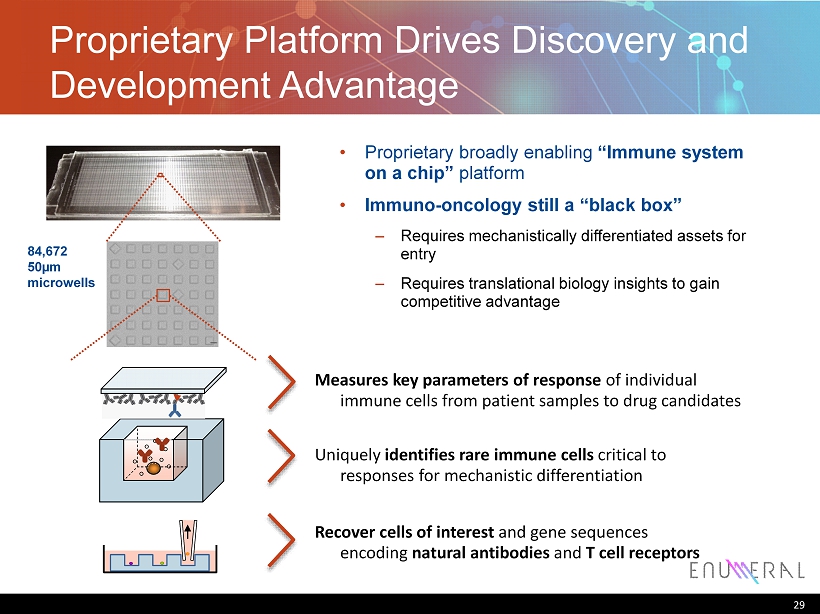
Proprietary Platform Drives Discovery and Development Advantage 29 84,672 50µm microwells • Proprietary broadly enabling “Immune system on a chip” platform • Immuno - oncology still a “black box” – Requires mechanistically differentiated assets for entry – Requires translational biology insights to gain competitive advantage Measures key parameters of response of individual immune cells from patient samples to drug candidates Uniquely identifies rare immune cells critical to responses for mechanistic differentiation Recover cells of interest and gene sequences encoding natural antibodies and T cell receptors
Strong Intellectual Property Position • Exclusive worldwide license with MIT/Harvard for platform technology – 8 issued patents in US and 41 issued in international jurisdictions – 17 pending patent applications (US & International) • Patents covering compositions of matter and methods – Applications pending or in preparation covering compositions of matter, methods of making, and methods of treating disease, for Enumeral discovered antibodies – Application pending for methods for cellular response profiling • Freedom to operate – Ability to navigate crowded patent landscape – Extensive searches and detailed analyses conducted on ongoing basis – Formal opinions of outside counsel obtained, where appropriate 30

Collaborators Provide External Recognition of Differentiated Approach • MERCK : collaboration with a leading immuno - oncology pharmaceutical company – Focused on using Enumeral's platform to interrogate the tumor microenvironment in colorectal cancer tissues to identify functional cellular responses to therapies being developed by Merck – R&D funding and undisclosed milestone payments – Merck has exclusive rights to data related to its proprietary compounds – ENUM recently achieved first milestone in the collaboration • NCI: awarded Phase 2 contract for ~$1 million over two years – Automation of human tissue immuno - oncology profiling – Opens door to broader pipeline and potentially accelerated development – Collaboration with leading scientists: 31 – Jedd Wolchok’s group at MSKCC − genetic basis for response to checkpoint inhibitors and novel immunotherapeutics – Doug Kwon’s group at MGH/ Ragon Institute − pioneering techniques for single cell immune cell analysis in biopsy

Strategic Collaboration with MD Anderson Cancer Center • Goal to Discover and Develop Novel Antibodies Against Specified Immunotherapy Targets – Utilizes Enumeral’s antibody discovery and patient - centric immune profiling platform – Leverages MD Anderson’s preclinical and development expertise and infrastructure – Collaboration with Oncology Research for Biologics and Immunotherapy Translation ( ORBIT ), a translational research platform of MD Anderson’s Moon Shots Program – Enumeral and MD Anderson will jointly fund research and development activities, and will share net income from product sales or any payments associated with third party partnering – Targets have not been disclosed • Impact on Potential Future Collaborations – Goal is for ENUM and MDACC to jointly out - license following clinical proof of concept, where a partner could step in to continue development 32

Agenda 1. Optimizing Drugs Against PD - 1: Lessons Learned 2. ENUM 244C8 – A Differentiated Anti - PD - 1 Antibody That Drives Adaptive and Innate Immune Cell Activation 3. Opportunity 4. Pipeline, Platform, and Collaborators 5. Management and Directors 33

Experienced Leadership Team 34 John J. Rydzewski Executive Chairman, Co - Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co - Founder, Director Cokey Nguyen, Ph.D. Vice President, Research & Development Isabel Chiu, Ph.D. Vice President, Translational & Clinical Sciences Kevin G. Sarney Vice President, Finance, & Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel Gary L. Creason , Ph.D. Vice President, Intellectual Property John J. Rydzewski Executive Chairman, Co - Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co - Founder, Director Cokey Nguyen, Ph.D. Vice President, Research & Development Isabel Chiu, Ph.D. Vice President, Translational & Clinical Sciences Kevin G. Sarney Vice President, Finance, & Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel Gary L. Creason , Ph.D. Vice President, Intellectual Property John J. Rydzewski Executive Chairman, Co - Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co - Founder, Director Cokey Nguyen, Ph.D. Vice President, Research & Development Isabel Chiu, Ph.D. Vice President, Translational & Clinical Sciences Kevin G. Sarney Vice President, Finance, & Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel Gary L. Creason , Ph.D. Vice President, Intellectual Property

Non - Management Directors 35 Barry Buckland, Ph.D. Co - Founder; Chairman, Scientific Advisory Board Robert J. Easton Allan Rothstein Paul J. Sekhri Robert L. Van Nostrand

THE POWER of HUMAN™



































