

FORWARD LOOKING STATEMENTS T h i s p r e s e n t a t i o n c o n t a i n s f o r w a r d - l o o k i n g s t a t e m e n t s w i t h i n t h e m e a n i n g of t h e U . S . P r i v a t e S e c u r i t i e s L i t i g a t i o n R e f o r m A c t of 1 9 9 5 . A l l s t a t e m e n t s c o n t a i n e d in t h i s p r e s e n t a t i o n t h a t do not r e l a t e to m a t t e r s of h i s t o r i c a l f a c t s h o u l d be c o n s i d e r e d f o r w a r d - l o o k i n g s t a t e m e n t s , i n c l u d i n g , b u t n o t l i m i t e d t o , s t a t e m e n t s r e g a r d i n g o u r e x p e c t a t i o n s a b o u t t h e t r i a l s , r e g u l a t o r y a p p r o v a l , m a n u f a c t u r i n g , d i s t r i b u t i o n a n d c o m m e r c i a l i z a t i o n of o u r c u r r e n t a n d f u t u r e p r o d u c t c a n d i d a t e s , and s t a t e m e n t s r e g a r d i n g o u r a n t i c i p a t e d r e v e n u e s , e x p e n s e s , m a r g i n s , p r o f i t s and u s e of c a s h . T h e s e f o r w a r d - l o o k i n g s t a t e m e n t s a r e b a s e d on o u r c u r r e n t e x p e c t a t i o n s . T h e s e s t a t e m e n t s a r e n o t p r o m i s e s or g u a r a n t e e s , b u t i n v o l v e k n o w n a n d u n k n o w n r i s k s , u n c e r t a i n t i e s and o t h e r i m p o r t a n t f a c t o r s t h a t m a y c a u s e o u r a c t u a l r e s u l t s to be m a t e r i a l l y d i f f e r e n t f r o m a n y f u t u r e r e s u l t s e x p r e s s e d or i m p l i e d by t h e f o r w a r d - l o o k i n g s t a t e m e n t s . T h e s e r i s k s i n c l u d e , b u t a r e not l i m i t e d t o , t h e f o l l o w i n g : o u r l i m i t e d o p e r a t i n g h i s t o r y and e x p e c t a t i o n s of l o s s e s f o r t h e f o r e s e e a b l e f u t u r e ; t h e a b s e n c e of s i g n i f i c a n t r e v e n u e f r o m o u r p r o d u c t c a n d i d a t e s f o r t h e f o r e s e e a b l e f u t u r e ; o u r p o t e n t i a l i n a b i l i t y to o b t a i n a n y n e c e s s a r y a d d i t i o n a l f i n a n c i n g ; o u r s u b s t a n t i a l d e p e n d e n c e on t h e s u c c e s s of o u r l e a d p r o d u c t c a n d i d a t e s , w h i c h m a y n o t be s u c c e s s f u l l y c o m m e r c i a l i z e d e v e n if t h e y a r e a p p r o v e d f o r m a r k e t i n g ; t h e e f f e c t of c o m p e t i t i o n ; our p o t e n t i a l i n a b i l i t y to o b t a i n r e g u l a t o r y a p p r o v a l f o r o u r e x i s t i n g or f u t u r e p r o d u c t c a n d i d a t e s ; our d e p e n d e n c e on t h i r d p a r t i e s to c o n d u c t s o m e of o u r d e v e l o p m e n t a c t i v i t i e s ; o u r d e p e n d e n c e u p o n t h i r d - p a r t y m a n u f a c t u r e r s f o r s u p p l i e s of o u r p r o d u c t c a n d i d a t e s ; u n c e r t a i n t i e s r e g a r d i n g t h e o u t c o m e s of t r i a l s r e g a r d i n g o u r p r o d u c t c a n d i d a t e s ; our p o t e n t i a l f a i l u r e to a t t r a c t and r e t a i n s e n i o r m a n a g e m e n t a n d k e y s c i e n t i f i c p e r s o n n e l ; u n c e r t a i n t y a b o u t o u r a b i l i t y to d e v e l o p a s a t i s f a c t o r y s a l e s o r g a n i z a t i o n ; o u r s i g n i f i c a n t c o s t s of o p e r a t i n g as a p u b l i c c o m p a n y ; our p o t e n t i a l i n a b i l i t y to o b t a i n p a t e n t p r o t e c t i o n a n d o t h e r i n t e l l e c t u a l p r o p e r t y p r o t e c t i o n f o r o u r p r o d u c t c a n d i d a t e s ; p o t e n t i a l c l a i m s by t h i r d p a r t i e s a l l e g i n g o u r i n f r i n g e m e n t of t h e i r p a t e n t s and o t h e r i n t e l l e c t u a l p r o p e r t y r i g h t s ; our p o t e n t i a l f a i l u r e to c o m p l y w i t h r e g u l a t o r y r e q u i r e m e n t s , w h i c h a r e s u b j e c t to c h a n g e on an o n g o i n g b a s i s ; t h e p o t e n t i a l v o l a t i l i t y of o u r s t o c k p r i c e ; and t h e s i g n i f i c a n t c o n t r o l o v e r our b u s i n e s s by our p r i n c i p a l s t o c k h o l d e r s and m a n a g e m e n t . F o r a f u r t h e r d e s c r i p t i o n of t h e s e r i s k s and o t h e r r i s k s t h a t we f a c e , p l e a s e s e e t h e r i s k f a c t o r s d e s c r i b e d in o u r f i l i n g s w i t h t h e U . S . S e c u r i t i e s a n d E x c h a n g e C o m m i s s i o n ( t h e S E C ) , i n c l u d i n g t h e r i s k f a c t o r s d i s c u s s e d u n d e r t h e c a p t i o n " R i s k F a c t o r s " in o u r A n n u a l R e p o r t on F o r m 10- K a n d a n y s u b s e q u e n t u p d a t e s t h a t m a y be c o n t a i n e d in o u r Q u a r t e r l y R e p o r t s on F o r m 10- Q f i l e d w i t h t h e SEC. As a r e s u l t of t h e r i s k s d e s c r i b e d a b o v e and in o u r f i l i n g s w i t h t h e S E C , a c t u a l r e s u l t s m a y d i f f e r m a t e r i a l l y f r o m t h o s e i n d i c a t e d by t h e f o r w a r d - l o o k i n g s t a t e m e n t s m a d e in t h i s p r e s e n t a t i o n . F o r w a r d - l o o k i n g s t a t e m e n t s c o n t a i n e d in t h i s p r e s e n t a t i o n s p e a k o n l y as of t h e d a t e of t h i s p r e s e n t a t i o n a n d we u n d e r t a k e no o b l i g a t i o n to u p d a t e or r e v i s e t h e s e s t a t e m e n t s , e x c e p t as m a y be r e q u i r e d by l a w . A u g u s t 31, 2018

KINDREDBIO AT-A-GLANCE One of the only veterinary biopharmaceutical companies in the world Innovative therapies leveraging validated human drugs in high growth, underserved market Commercial-stage firm with rich pipeline that offers attractive ROI potential Industry-leading biologics capabilities – the future of veterinary medicine 3
OUR STRATEGY: REPURPOSE HUMAN DRUGS FOR PETS Pursue molecules already Average of $5-8M to develop known to work each drug in 3-6 years Reduce technical risk Portfolio approach Shorten timelines Reduce financial risk 4

COMMERCIAL-STAGE With a rich pipeline, our goal is to launch an average of two drugs per year ATTRACTIVE ROI POTENTIAL Market sizes are 10 times smaller than human markets but the cost of development is 100 times lower 5

WE SPEND GENEROUSLY ON PETS $ 6 9 . 4 $ 1 . 5 $700 B I L L I O N B I L L I O N M I L L I O N We spend $69.4 We spend $1.5 We spend $700 billion a year on billion a year on million a year on pets knee surgeries for Valentine’s Day dogs presents for pets Sources: 2017-2018 APPA National Pet Owners Survey 6 Journal of the American Veterinary Medical Association, November 15, 2005, Vol. 227, No. 10, Pages 1604-1607, https://doi.org/10.2460/javma.2005.227.1604 Valentine’s Day Gift Spend: National Retail Foundation—http://consumerist.com/2015/01/28/americans-will-spend-703-million-on-valentines-day-gifts-for-pets

BECAUSE PETS ARE FAMILY 67 37 71 P E R C E N T P E R C E N T P E R C E N T 67% of pet 37% of pet 71% of pets sleep parents would parents would in bed with their give up their give up their pet parents vacation to pay cellphone to pay for pet emergency for pet emergency Sources: 7 American Institute of Certified Public Accountants-https://www.aicpa.org/content/aicpa/press/pressreleases/2017/survey-explores-financial-sacrifices-americans- make-for-pets.html 2017-2018 APPA National Pet Owners Survey

VETERINARY MARKET IS GROWING RAPIDLY 68 42 P E R C E N T P E R C E N T The veterinary care Animal health stocks market grew 68% have risen 42% in the from 2007 to 2017 past 12 months Sources: 2017-2018 APPA National Pet Owners Survey & 2007-2008 APPA National Pet Owners Survey 8 https://www.motifinvesting.com/motifs/pet-passion
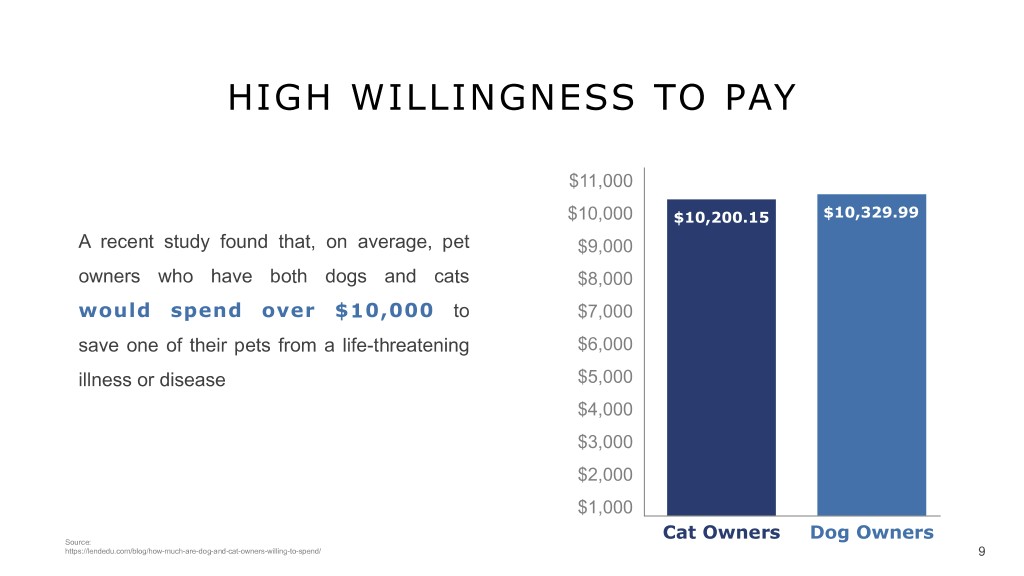
HIGH WILLINGNESS TO PAY $11,000 $10,000 $10,200.15 $10,329.99 A recent study found that, on average, pet $9,000 owners who have both dogs and cats $8,000 would spend over $10,000 to $7,000 save one of their pets from a life-threatening $6,000 v illness or disease $5,000 $4,000 $3,000 $2,000 $1,000 Cat Owners Dog Owners Source: https://lendedu.com/blog/how-much-are-dog-and-cat-owners-willing-to-spend/ 9

LOW GENERIC PENETRATION Generic Dispensing Rates There are very few generic companies, and no automatic substitution at the pharmacy 81% Human Many products reach peak sales several years after patent expiration 7% Companion There is no biosimilar pathway Animal Note: Companion Animal dispensing rate within the veterinary clinic. Source: IMS Health, Putney, BofA Merrill Lynch Global Research 10

M I R A T A Z ®️ (mirtazapine transdermal ointment) Important Safety Information: Mirataz® (mirtazapine transdermal ointment) is for topical use in cats only under veterinary supervision. Do not use in cats with a known hypersensitivity to mirtazapine or any of the excipients or in cats treated with monoamine oxidase inhibitors (MAOIs). Not for human use. Keep out of reach of children. Wear gloves to apply and wash hands after. Avoid contact with treated cat for 2 hours following application. The most common adverse reactions include application site reactions, behavioral abnormalities (vocalization and hyperactivity) and vomiting. For complete safety information, see the product insert at the end of the presentation.

Mirataz® (mirtazapine transdermal ointment) now available The first and only FDA-approved transdermal medication for the management of weight loss in cats Mirataz is classified pharmacologically as a weight-gain drug 12

FELINE WEIGHT LOSS CAN BE FATAL Unintended weight loss is a leading cause of feline veterinary visits Caused by underlying conditions, such as chronic kidney disease, cancer and diabetes 9 million cats are diagnosed with unintended weight loss each year 3 million cats are currently treated for unintended weight loss each year Sources: 2012 U.S. Pet Ownership & Demographics Sourcebook American Veterinary Medical Association (n=50,000 U.S. Households) 2016 U.S. Veterinarian Mirtazapine Research, Wise Insights May 2016 (n=89 U.S. small animal Veterinarians). Data on file at Kindred Biosciences. 13 2017 Mirataz Pricing Research, Kynetec, September 2017 (n=204 U.S. small animal veterinarians). Data on file at Kindred Biosciences.
COMPLIANCE IS KEY 74% of veterinarians say ease of administration is a primary factor in selecting a medication for feline weight loss Mirataz® (mirtazapine transdermal ointment) is applied topically to the cat’s inner ear Provides attractive application route versus oral dosing Source: 14 2016 U.S. Veterinarian Mirtazapine Phase 3 Research, Ipsos, September 2016 (n=201 U.S. small animal Veterinarians). Data on file at Kindred Biosciences.

15

OPPORTUNITY 71% of veterinarians use mirtazapine and the majority say they would switch to the transdermal gel formulation 59% 34% 7% 59% of 34% of Only 7% of veterinarians would veterinarians would veterinarians would replace most or all replace some of the not replace any mirtazapine mirtazapine mirtazapine Source: 16 2016 U.S. Veterinarian Mirtazapine Phase 3 Research, Ipsos, September 2016 (n=201 U.S. small animal Veterinarians). Data on file at Kindred Biosciences.

C O M M E R C I A L I Z A T I O N

COMPANION ANIMAL COMMERCIALIZATION Commercialize with 20-25 person direct sales force, in conjunction with distributors EQUINE COMMERCIALIZATION Commercialize with 3-5 person direct sales force, in conjunction with distributors 18

WORLD-CLASS COMMERCIAL TEAM 15+ YEARS >50 TOP EXPERIENCE L A U N C H E S C O M P A N I E S Sales force has Team members The team comes average have launched from top experience of over 50 products veterinary 15.7 years companies Source: 19 Data on file at Kindred Biosciences. 19

D E E P P I P E L I N E
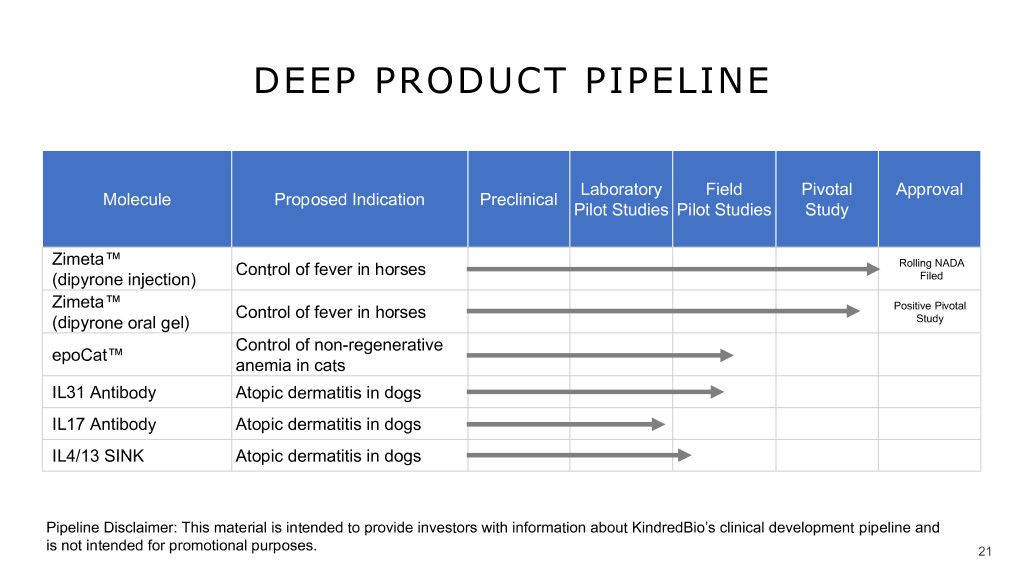
DEEP PRODUCT PIPELINE Laboratory Field Pivotal Approval Molecule Proposed Indication Preclinical Pilot Studies Pilot Studies Study Zimeta™ Control of fever in horses Rolling NADA (dipyrone injection) Filed Zimeta™ Control of fever in horses Positive Pivotal (dipyrone oral gel) Study Control of non-regenerative epoCat™ anemia in cats IL31 Antibody Atopic dermatitis in dogs IL17 Antibody Atopic dermatitis in dogs IL4/13 SINK Atopic dermatitis in dogs Pipeline Disclaimer: This material is intended to provide investors with information about KindredBio’s clinical development pipeline and is not intended for promotional purposes. 21

DEEP PRODUCT PIPELINE OTHER PRODUCTS IN DEVELOPMENT Laboratory Field Pivotal Approval Molecule Proposed Indication Preclinical Pilot Studies Pilot Studies Study KIND-014 Equine gastric ulcers Metabolic syndrome KIND-015 in horses Anti-TNF Antibody Sick newborn foals Inflammatory bowel disease Anti-TNF Antibody in dogs Other products in development (partial list) include KIND-bodies, IgE antibody, CD20 antibody, VEGF antibody, and Checkpoint Inhibitors Pipeline Disclaimer: This material is intended to provide investors with information about KindredBio’s clinical development pipeline and is not intended for promotional purposes. 22

Z I M E T A ™️ (dipyrone injection)

ZIMETA™️ OPPORTUNITY IV and Oral drug for the control of pyrexia (fever) in horses 8-9 million horses in the US 690,000 horses treated for fever annually Sources: The Economic Impact of the Horse Industry on the United States, 2005, American Horse Council Foundation (n=18,648 U.S. horse owners/industry suppliers). 24 Zimeta Pricing Research, Ipsos Ag & Animal Health, May 2016 (n=160 U.S. equine Veterinarians). Data on file at Kindred Biosciences.
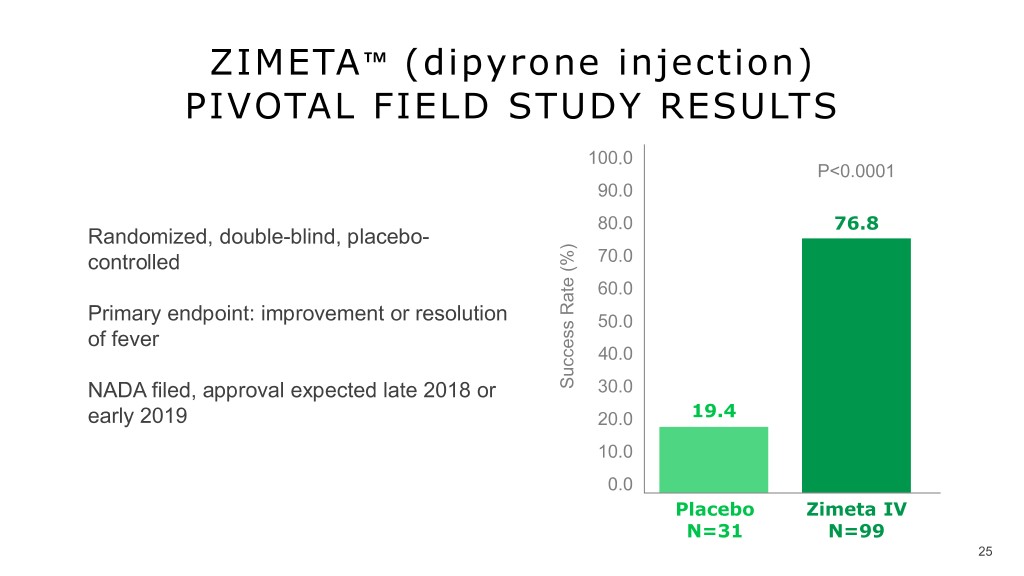
ZIMETA™️ (dipyrone injection) PIVOTAL FIELD STUDY RESULTS 100.0 P<0.0001 90.0 80.0 76.8 Randomized, double-blind, placebo- controlled 70.0 60.0 Primary endpoint: improvement or resolution 50.0 v of fever 40.0 NADA filed, approval expected late 2018 or Success Rate (%) 30.0 early 2019 20.0 19.4 10.0 0.0 Placebo Zimeta IV N=31 N=99 25
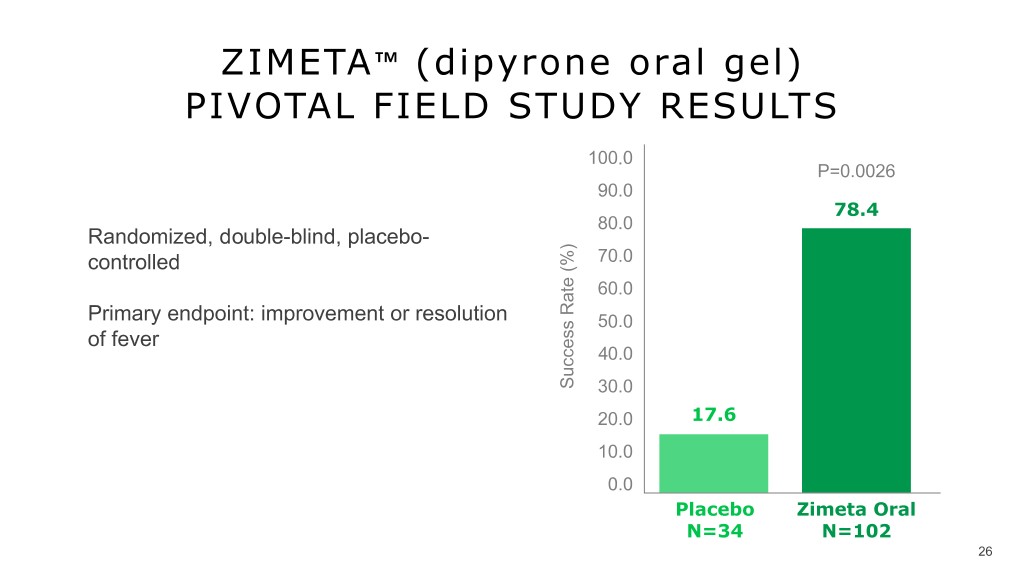
ZIMETA™️ (dipyrone oral gel) PIVOTAL FIELD STUDY RESULTS 100.0 P=0.0026 90.0 78.4 80.0 Randomized, double-blind, placebo- controlled 70.0 60.0 Primary endpoint: improvement or resolution 50.0 v of fever 40.0 Success Rate (%) 30.0 20.0 17.6 10.0 0.0 Placebo Zimeta Oral N=34 N=102 26

ZIMETA™️ (dipyrone injection) OPPORTUNITY 95% 83% 95% believe that 83% would use Zimeta is a good fit Zimeta in the for their practice first year Source: 27 Zimeta Pricing Research, Ipsos Ag & Animal Health, May 2016 (n=160 U.S. equine Veterinarians) . Data on file at Kindred Biosciences. 27

B I O L O G I C S

BIOLOGICS: THE FUTURE OF VETERINARY MEDICINE Veterinary medicine to follow human market, where top drugs are biologics Industry leading biologic programs & state-of-the-art manufacturing plant Highly experienced biologics team responsible for leading human drugs End-to-end capabilities & new technologies (KIND-bodies) 29

epoCat™ FELINE ERYTHROPOIETIN Recombinant long-acting feline erythropoietin for non-regenerative anemia in cats Half of elderly cats develop kidney disease, which can cause anemia Initial laboratory study has been completed and the results were positive, as evidenced by an increase in new red blood cells Pilot field efficacy study is underway 30

KEY FOCUS AREA ATOPIC DERMATITIS Canine atopic dermatitis, an allergic skin disease, is a >$500M a year market and growing KindredBio has an industry-leading portfolio of atopic dermatitis candidates 31
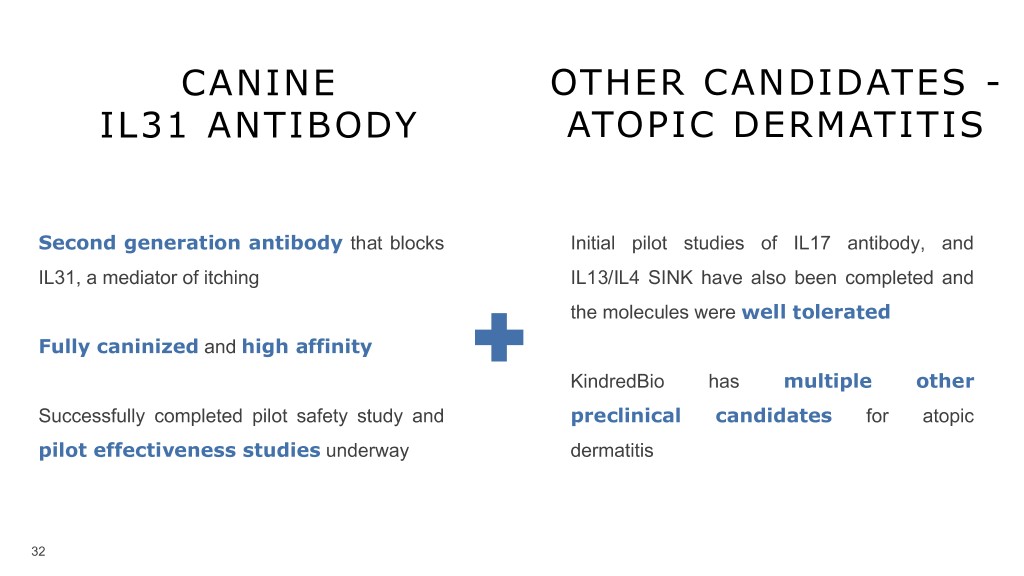
CANINE OTHER CANDIDATES - IL31 ANTIBODY ATOPIC DERMATITIS Second generation antibody that blocks Initial pilot studies of IL17 antibody, and IL31, a mediator of itching IL13/IL4 SINK have also been completed and the molecules were well tolerated Fully caninized and high affinity KindredBio has multiple other Successfully completed pilot safety study and preclinical candidates for atopic pilot effectiveness studies underway dermatitis 32

C O R P O R A T E I N F O
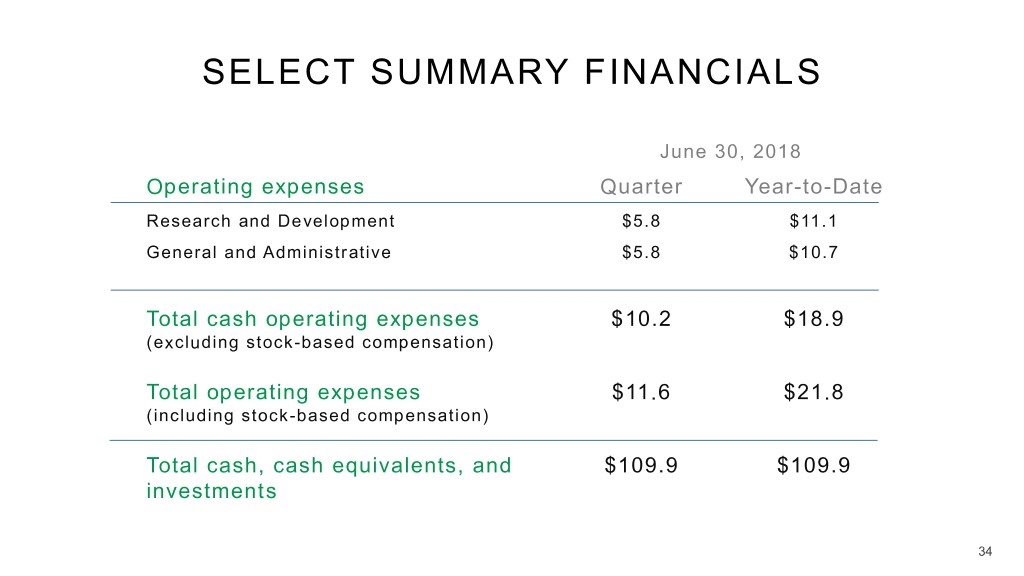
SELECT SUMMARY FINANCIALS June 30, 2018 Operating expenses Quarter Year-to-Date Research and Development $5.8 $11.1 General and Administrative $5.8 $10.7 Total cash operating expenses $10.2 $18.9 (excluding stock-based compensation) Total operating expenses $11.6 $21.8 (including stock-based compensation) Total cash, cash equivalents, and $109.9 $109.9 investments 34
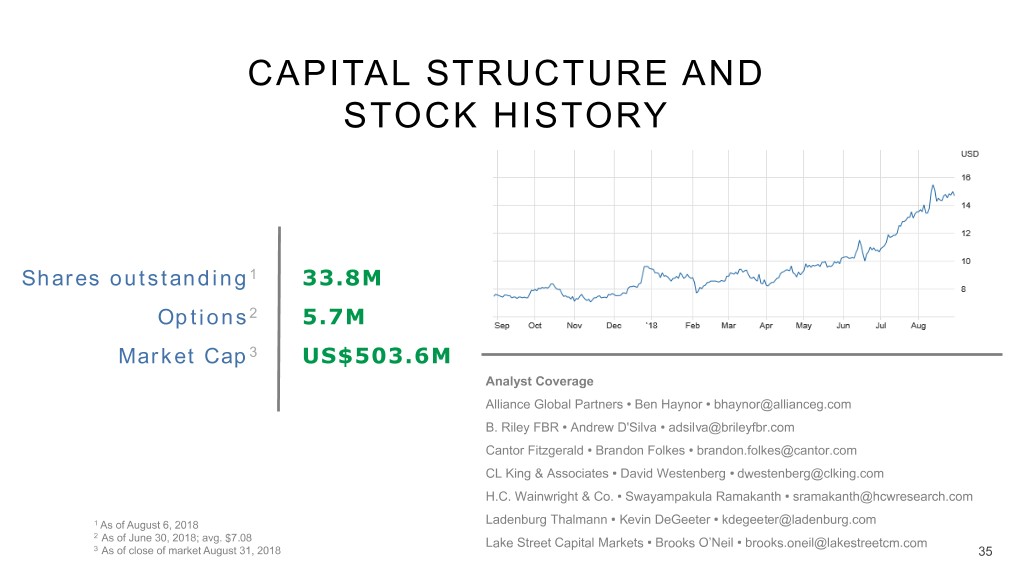
CAPITAL STRUCTURE AND STOCK HISTORY Shares outstanding 1 33.8M Options2 5.7M Market Cap3 US$503.6M Analyst Coverage Alliance Global Partners • Ben Haynor • bhaynor@allianceg.com B. Riley FBR • Andrew D'Silva • adsilva@brileyfbr.com Cantor Fitzgerald • Brandon Folkes • brandon.folkes@cantor.com CL King & Associates • David Westenberg • dwestenberg@clking.com H.C. Wainwright & Co. • Swayampakula Ramakanth • sramakanth@hcwresearch.com 1 As of August 6, 2018 Ladenburg Thalmann • Kevin DeGeeter • kdegeeter@ladenburg.com 2 As of June 30, 2018; avg. $7.08 Lake Street Capital Markets • Brooks O’Neil • brooks.oneil@lakestreetcm.com 3 As of close of market August 31, 2018 35

NEWS FLOW IL31 – Results from pilot effectiveness epoCat™ – Pilot studies efficacy results Late 2018 / Early 2019 2019 Late 2019 2018 Zimeta™ IL4/IL13 – Pilot (dipyrone injection) efficacy results 36

S U M M A R Y

SUMMARY KindredBio develops innovative therapies for companion animals by leveraging validated human drugs. The markets are ten times smaller than human markets, but the cost of development is a hundred times lower. KindredBio plans to launch an average of two drugs per year. 38

Prescribing Information

Prescribing Information








































