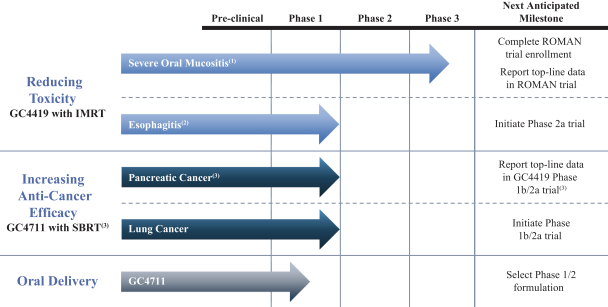
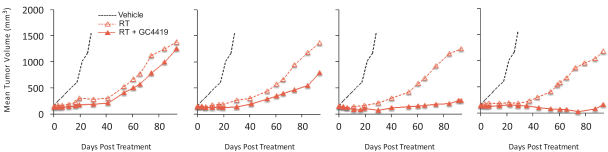
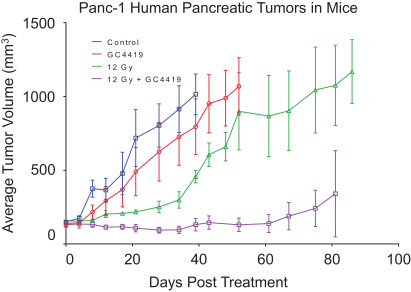
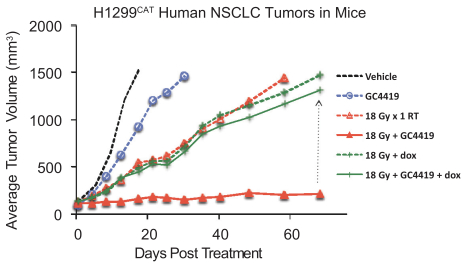
We also have pending patent families in the United States that cover certain combinations of our product candidates with several oncology products that may provide protection for the use of our product candidates in connection with those oncology products, which, if granted, are projected to expire between 2037 and 2039.
Trademarks and Trade Secrets
As of June 30, 2019, our trademark portfolio contained two U.S. trademark registrations, for GALERA and GALERA THERAPEUTICS.
Furthermore, we rely upon trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality and invention assignment agreements with our commercial partners, collaborators, employees, and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with an employee or a third party. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees, and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Royalty Agreement with Clarus
In November 2018, we entered into an Amended and Restated Purchase and Sale Agreement, or the Royalty Agreement, by and among us, Clarus IV Galera Royalty AIV, L.P., Clarus IV-A, L.P., Clarus IV-B, L.P., Clarus IV-C, L.P. and Clarus IV-D, L.P., or, collectively, Clarus. Pursuant to the Royalty Agreement, Clarus agreed to pay us, in the aggregate, up to $80 million, or the Royalty Purchase Price, in four tranches of $20 million each upon the achievement of specified clinical milestones in our ROMAN Trial. We agreed to apply the proceeds from such payments primarily to support clinical development and regulatory activities for GC4419, GC4711 and any pharmaceutical product comprising or containing GC4419 or GC4711, or, collectively, the Products, as well as to satisfy working capital obligations and for general corporate expenses. We achieved the first milestone under the Royalty Agreement and received the first tranche of the Royalty Purchase Price in November 2018 and received the second tranche of the Royalty Purchase Price in April 2019 in connection with the achievement of the second milestone under the Royalty Agreement in March 2019.
In connection with the payment of each tranche of the Royalty Purchase Price, we have agreed to sell, convey, transfer and assign to Clarus all of our right, title and interest in a mid-single digit percentage of (i) the gross amount from the worldwide sale of the Products less certain items, including refunds, allowances, credits for recalls, customary discounts for purchase chargebacks, taxes in connection with transportation and/or delivery of Products, income-based taxes, customary distributor fees and commissions and customary distribution and transportation charges, and (ii) all recoveries, consideration, compensation, payments, collections, settlements and other amounts (including damages, awards, interest and penalties) of any kind or nature actually received by us or our affiliates, licensees and sublicensees in substitution or compensation for, or otherwise in lieu of, any net sales of the Products arising out of or resulting from any proceeding brought, or assertion made, by us against any third party relating to or arising out of any infringement, misuse or misappropriation by such third party of our intellectual property rights in the Products, less all out-of-pocket costs and expenses incurred by us or our affiliates, licensees and sublicensees in connection with such enforcement action, or, collectively, the Product Payments, during the Royalty Period. The Royalty Period means, on a Product-by-Product and country-by-country basis, the period of time commencing on the commercial launch of such Product in such country and ending on the latest to occur of (i) the 12th anniversary of such commercial launch, (ii) the expiration of all valid claims of our patents covering such Product in such country, and (iii) the expiration of regulatory data protection or market exclusivity or similar regulatory
123