
Galera Therapeutics (GRTX) Driving Better Outcomes for Breast Cancer Patients January 2025 Exhibit 99.2

Forward-Looking Statements ©2025 Galera Therapeutics, Inc. Certain information contained in this presentation and statements made orally during this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Galera’s own internal estimates and research. “While Galera believes these third-party sources to be reliable as of the date of this presentation, it has not been independently verified, and Galera makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources.” While Galera believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, the safety, efficacy, regulatory and clinical progress and timing thereof, and therapeutic potential of current and prospective product candidates, plans and timing for the commencement of, and the release of data from, clinical trials, planned clinical trials and preclinical activities, potential product approvals and related commercial opportunity, current and prospective collaborations, and timing and likelihood of success, plans and objectives of management for future operations, are forward-looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The information in this presentation, including without limitation the forward-looking statements contained herein, represent our views as of the date of this presentation. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. The forward-looking statements in this presentation involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the drug development process and the regulatory approval process, our reliance on third parties over which we may not always have full control, and other important risks and uncertainties that are described in Galera’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with the U.S. Securities Exchange Commission (SEC) and Galera’s other filings with the SEC. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties.
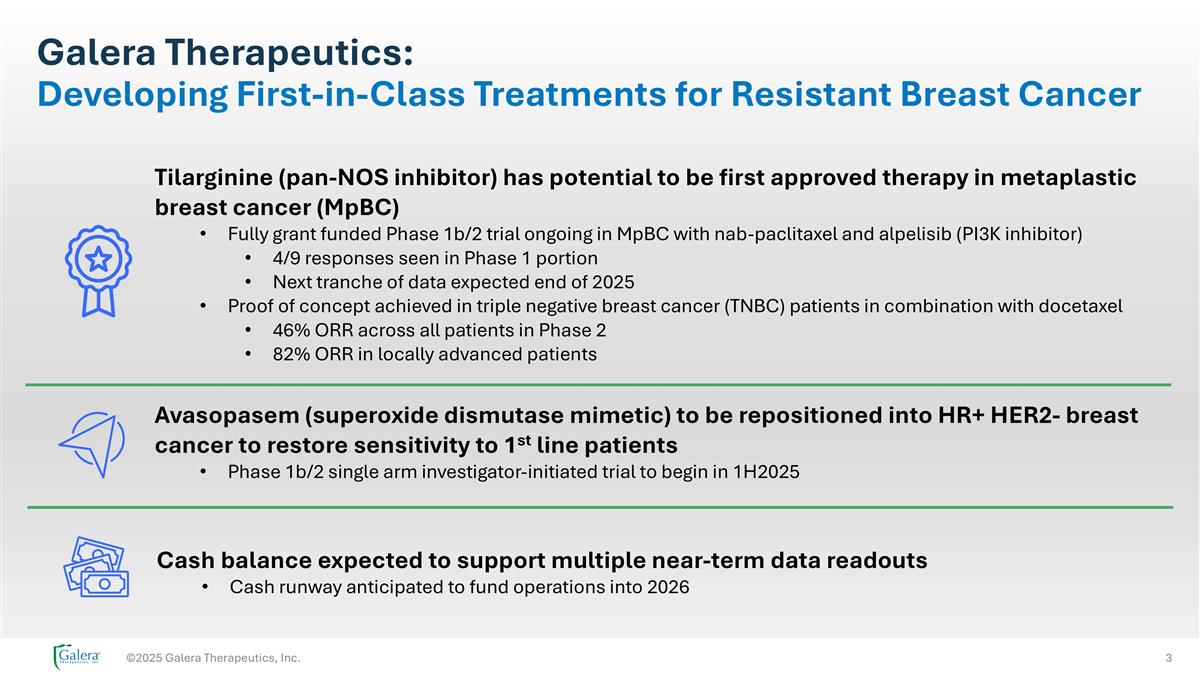
©2025 Galera Therapeutics, Inc. Tilarginine (pan-NOS inhibitor) has potential to be first approved therapy in metaplastic breast cancer (MpBC) Fully grant funded Phase 1b/2 trial ongoing in MpBC with nab-paclitaxel and alpelisib (PI3K inhibitor) 4/9 responses seen in Phase 1 portion Next tranche of data expected end of 2025 Proof of concept achieved in triple negative breast cancer (TNBC) patients in combination with docetaxel 46% ORR across all patients in Phase 2 82% ORR in locally advanced patients Avasopasem (superoxide dismutase mimetic) to be repositioned into HR+ HER2- breast cancer to restore sensitivity to 1st line patients Phase 1b/2 single arm investigator-initiated trial to begin in 1H2025 Cash balance expected to support multiple near-term data readouts Cash runway anticipated to fund operations into 2026 Galera Therapeutics: Developing First-in-Class Treatments for Resistant Breast Cancer
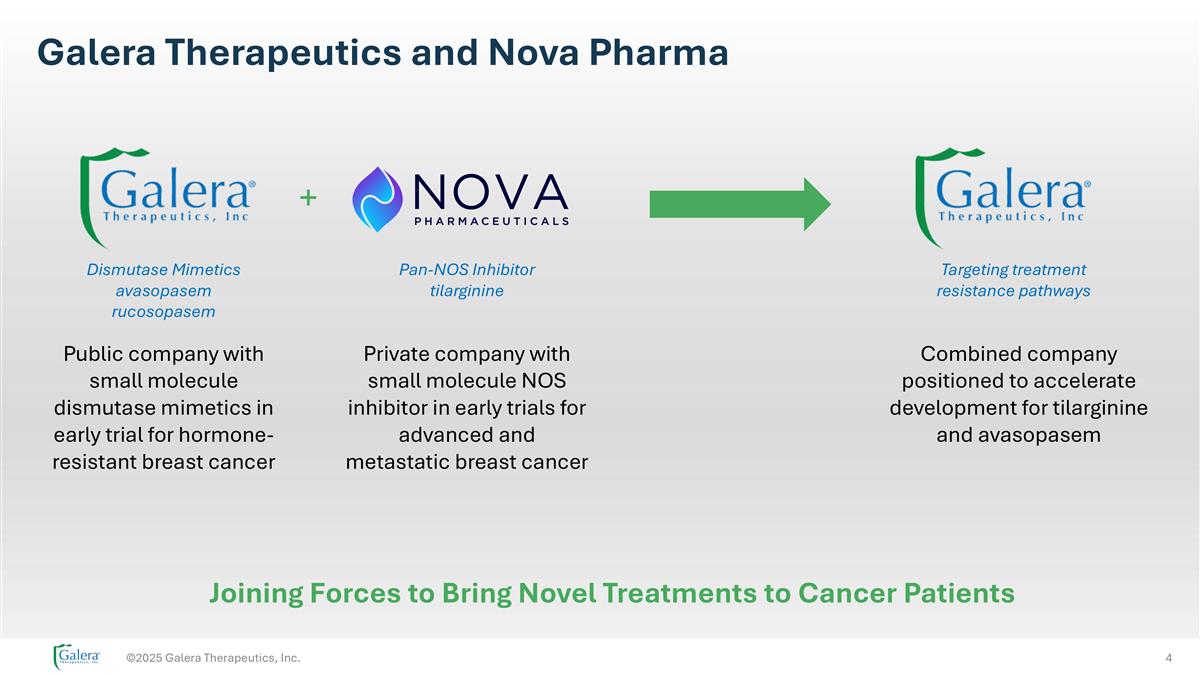
Galera Therapeutics and Nova Pharma ©2025 Galera Therapeutics, Inc. + Public company with small molecule dismutase mimetics in early trial for hormone-resistant breast cancer Private company with small molecule NOS inhibitor in early trials for advanced and metastatic breast cancer Combined company positioned to accelerate development for tilarginine and avasopasem Dismutase Mimetics avasopasem rucosopasem Pan-NOS Inhibitor tilarginine Targeting treatment resistance pathways Joining Forces to Bring Novel Treatments to Cancer Patients
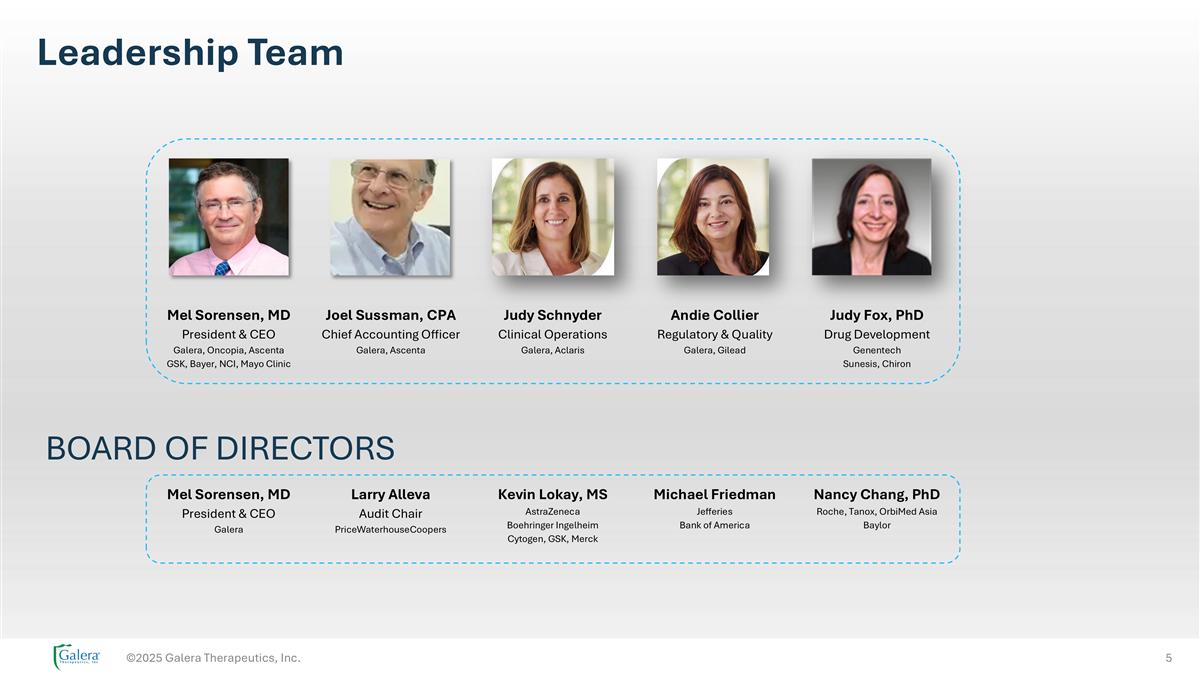
©2025 Galera Therapeutics, Inc. Mel Sorensen, MD President & CEO Galera, Oncopia, Ascenta GSK, Bayer, NCI, Mayo Clinic Judy Schnyder Clinical Operations Galera, Aclaris Andie Collier Regulatory & Quality Galera, Gilead Judy Fox, PhD Drug Development Genentech Sunesis, Chiron Joel Sussman, CPA Chief Accounting Officer Galera, Ascenta BOARD OF DIRECTORS Mel Sorensen, MD President & CEO Galera Kevin Lokay, MS AstraZeneca Boehringer Ingelheim Cytogen, GSK, Merck Michael Friedman Jefferies Bank of America Nancy Chang, PhD Roche, Tanox, OrbiMed Asia Baylor Larry Alleva Audit Chair PriceWaterhouseCoopers Leadership Team
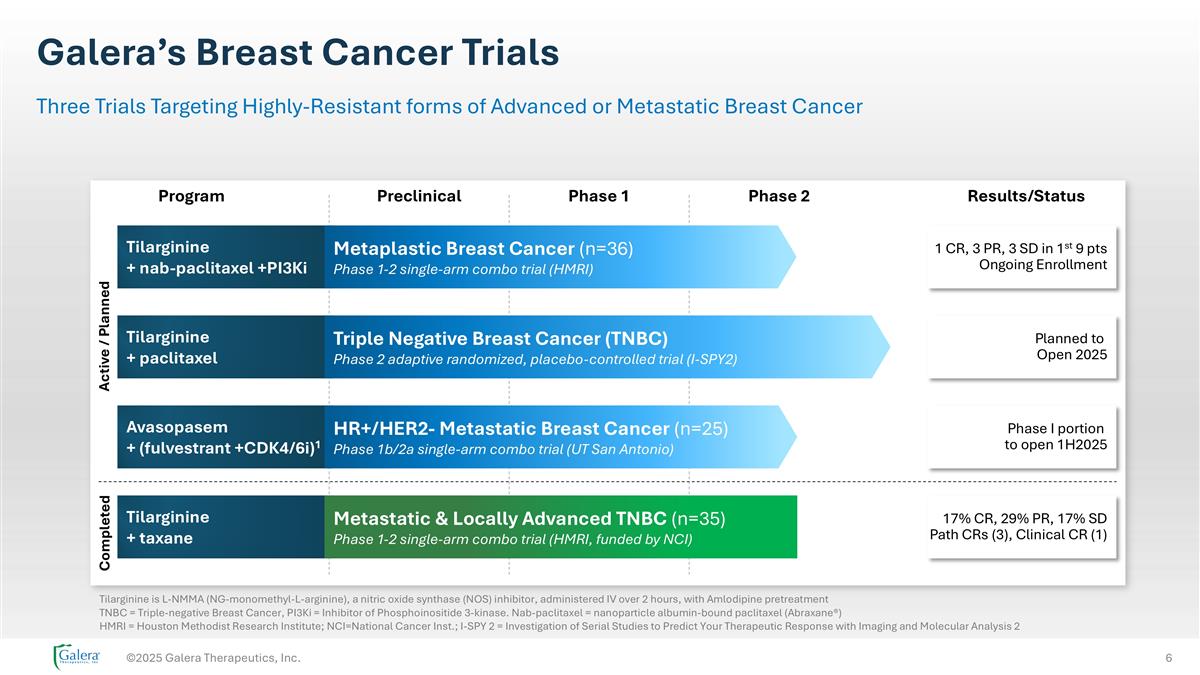
Galera’s Breast Cancer Trials ©2025 Galera Therapeutics, Inc. Three Trials Targeting Highly-Resistant forms of Advanced or Metastatic Breast Cancer Tilarginine is L-NMMA (NG-monomethyl-L-arginine), a nitric oxide synthase (NOS) inhibitor, administered IV over 2 hours, with Amlodipine pretreatment TNBC = Triple-negative Breast Cancer, PI3Ki = Inhibitor of Phosphoinositide 3-kinase. Nab-paclitaxel = nanoparticle albumin-bound paclitaxel (Abraxane®) HMRI = Houston Methodist Research Institute; NCI=National Cancer Inst.; I-SPY 2 = Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2 Program Preclinical Phase 1 Phase 2 Results/Status Active / Planned Avasopasem + (fulvestrant +CDK4/6i)1 HR+/HER2- Metastatic Breast Cancer (n=25) Phase 1b/2a single-arm combo trial (UT San Antonio) Tilarginine + nab-paclitaxel +PI3Ki Metaplastic Breast Cancer (n=36) Phase 1-2 single-arm combo trial (HMRI) Tilarginine + paclitaxel Triple Negative Breast Cancer (TNBC) Phase 2 adaptive randomized, placebo-controlled trial (I-SPY2) 1 CR, 3 PR, 3 SD in 1st 9 pts Ongoing Enrollment Planned to Open 2025 Phase I portion to open 1H2025 Tilarginine + taxane Metastatic & Locally Advanced TNBC (n=35) Phase 1-2 single-arm combo trial (HMRI, funded by NCI) 17% CR, 29% PR, 17% SD Path CRs (3), Clinical CR (1) Completed

Tilarginine in Triple Negative Breast Cancer

Promising Early Results With Tilarginine in TNBC ©2025 Galera Therapeutics, Inc. Long-term research with nitric oxide synthase inhibition in cancer has demonstrated potential to translate into the clinic Jenny Chang, MD Breast Oncologist Houston Methodist A research team led by Jenny Chang, M.D., a breast medical oncologist and director of the Houston Methodist Dr. Mary and Ron Neal Cancer Center, found variants in a gene called RPL39 that work through a pathway called nitric oxide. Through the combination of chemotherapy and a nitric oxide synthase inhibitor, tilarginine or L-NMMA, developed at Houston Methodist, researchers were able to regress tumor growth of triple negative breast cancer and prevent the cancer from spreading. Historically, patients with cancers resistant to chemotherapy have about a 10-15% chance of responding when used with older drugs that target the immune system. The response rate using the Houston Methodist-discovered drug Tilarginine has been approximately 45% in initial trials in TNBC and Metaplastic breast cancer.
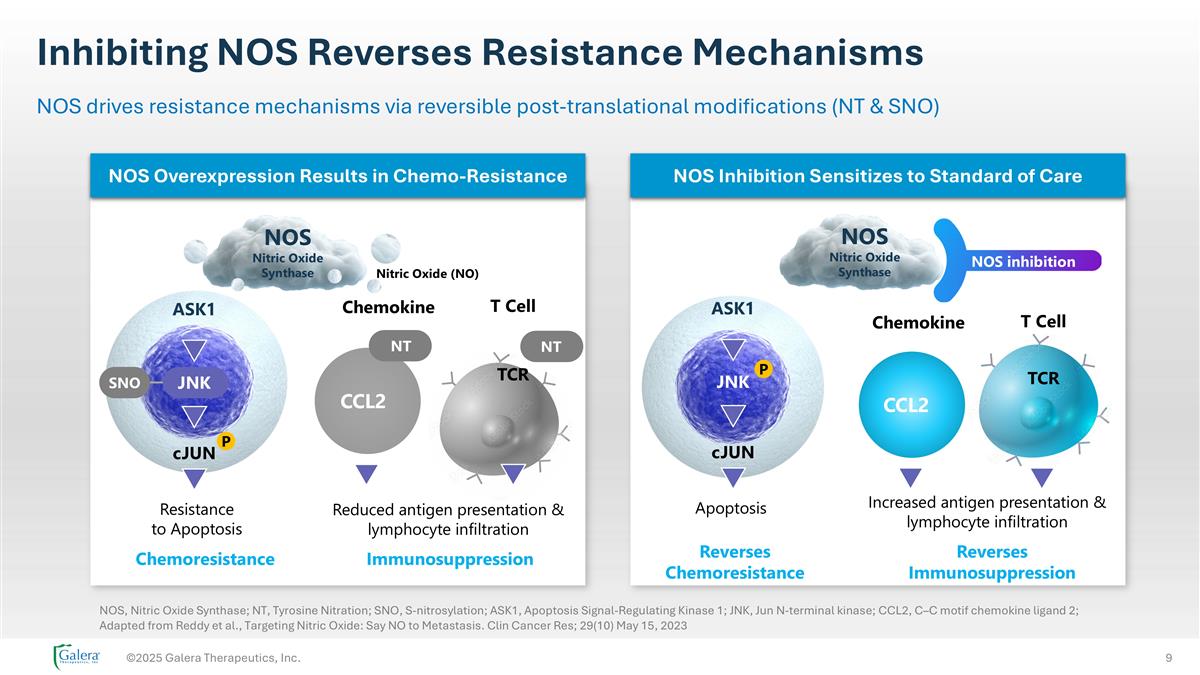
Inhibiting NOS Reverses Resistance Mechanisms ©2025 Galera Therapeutics, Inc. NOS drives resistance mechanisms via reversible post-translational modifications (NT & SNO) Chemoresistance cJUN P Resistance to Apoptosis Nitric Oxide (NO) ASK1 NOS Nitric Oxide Synthase Immunosuppression Chemokine T Cell TCR CCL2 NT NT JNK SNO Reduced antigen presentation & lymphocyte infiltration Increased antigen presentation & lymphocyte infiltration Reverses Immunosuppression Reverses Chemoresistance Apoptosis NOS Nitric Oxide Synthase JNK cJUN ASK1 P Chemokine T Cell TCR CCL2 NOS inhibition NOS Overexpression Results in Chemo-Resistance NOS Inhibition Sensitizes to Standard of Care NOS, Nitric Oxide Synthase; NT, Tyrosine Nitration; SNO, S-nitrosylation; ASK1, Apoptosis Signal-Regulating Kinase 1; JNK, Jun N-terminal kinase; CCL2, C–C motif chemokine ligand 2; Adapted from Reddy et al., Targeting Nitric Oxide: Say NO to Metastasis. Clin Cancer Res; 29(10) May 15, 2023
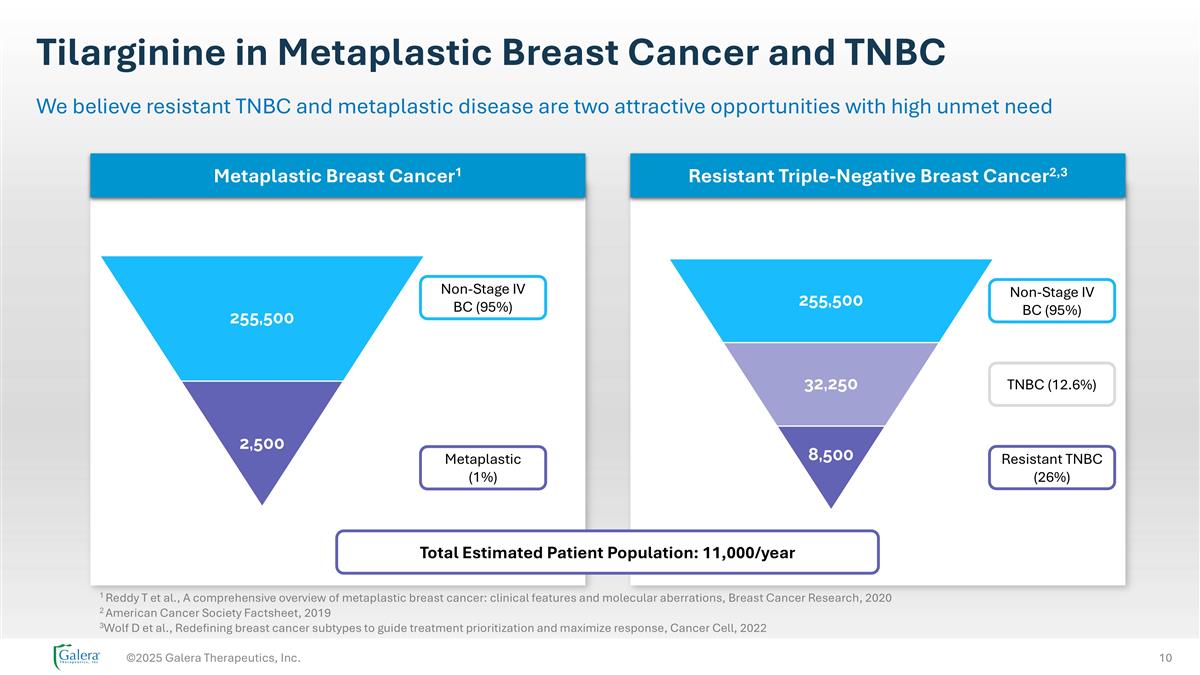
Tilarginine in Metaplastic Breast Cancer and TNBC ©2025 Galera Therapeutics, Inc. We believe resistant TNBC and metaplastic disease are two attractive opportunities with high unmet need Non-Stage IV BC (95%) Metaplastic (1%) Non-Stage IV BC (95%) TNBC (12.6%) Resistant TNBC (26%) Total Estimated Patient Population: 11,000/year Metaplastic Breast Cancer1 Resistant Triple-Negative Breast Cancer2,3 1 Reddy T et al., A comprehensive overview of metaplastic breast cancer: clinical features and molecular aberrations, Breast Cancer Research, 2020 2 American Cancer Society Factsheet, 2019 3Wolf D et al., Redefining breast cancer subtypes to guide treatment prioritization and maximize response, Cancer Cell, 2022
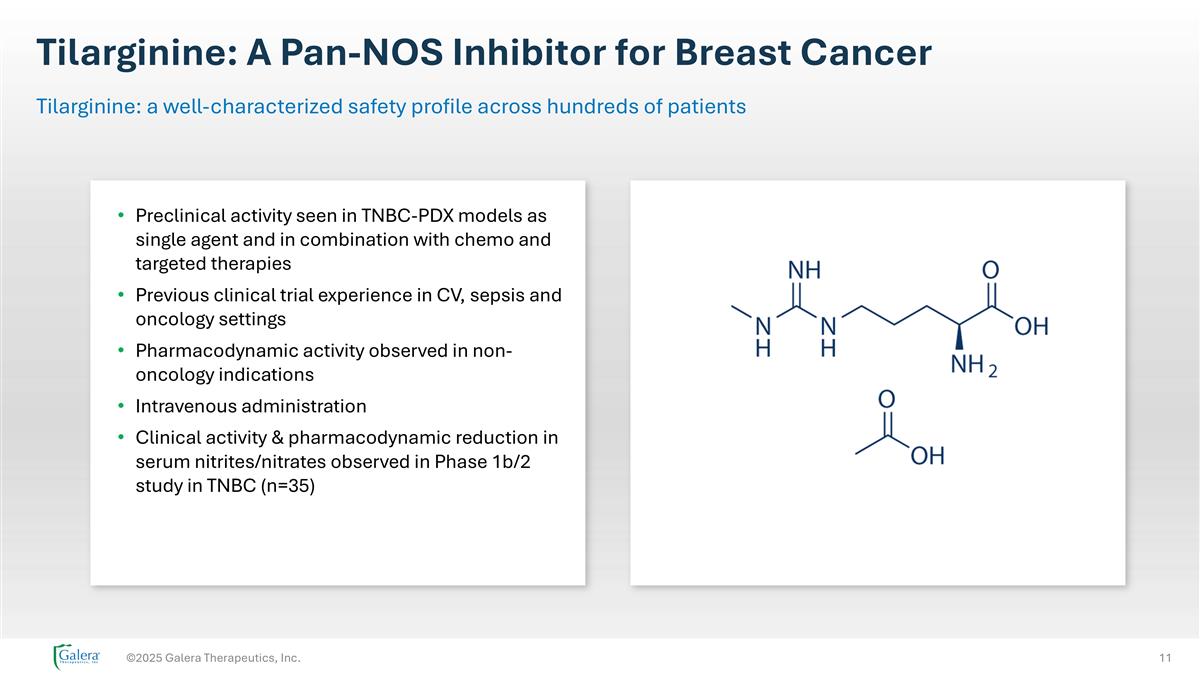
Tilarginine: A Pan-NOS Inhibitor for Breast Cancer ©2025 Galera Therapeutics, Inc. Tilarginine: a well-characterized safety profile across hundreds of patients Preclinical activity seen in TNBC-PDX models as single agent and in combination with chemo and targeted therapies Previous clinical trial experience in CV, sepsis and oncology settings Pharmacodynamic activity observed in non-oncology indications Intravenous administration Clinical activity & pharmacodynamic reduction in serum nitrites/nitrates observed in Phase 1b/2 study in TNBC (n=35)
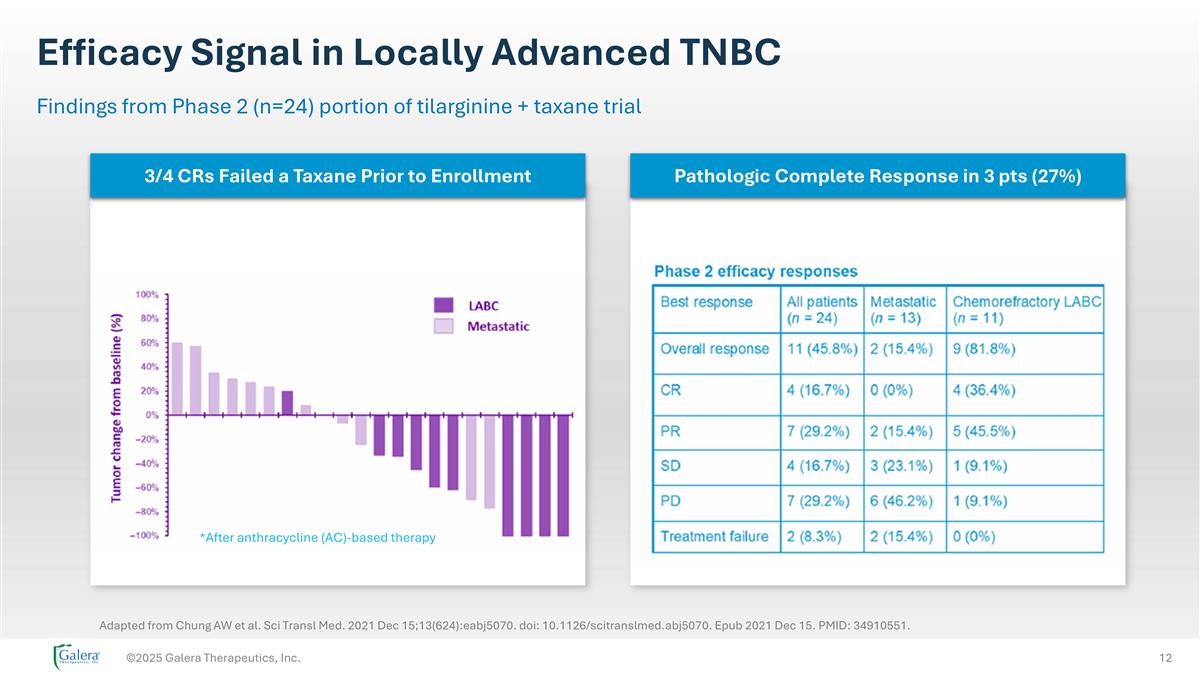
Efficacy Signal in Locally Advanced TNBC ©2025 Galera Therapeutics, Inc. Findings from Phase 2 (n=24) portion of tilarginine + taxane trial *After anthracycline (AC)-based therapy Adapted from Chung AW et al. Sci Transl Med. 2021 Dec 15;13(624):eabj5070. doi: 10.1126/scitranslmed.abj5070. Epub 2021 Dec 15. PMID: 34910551. 3/4 CRs Failed a Taxane Prior to Enrollment Pathologic Complete Response in 3 pts (27%)
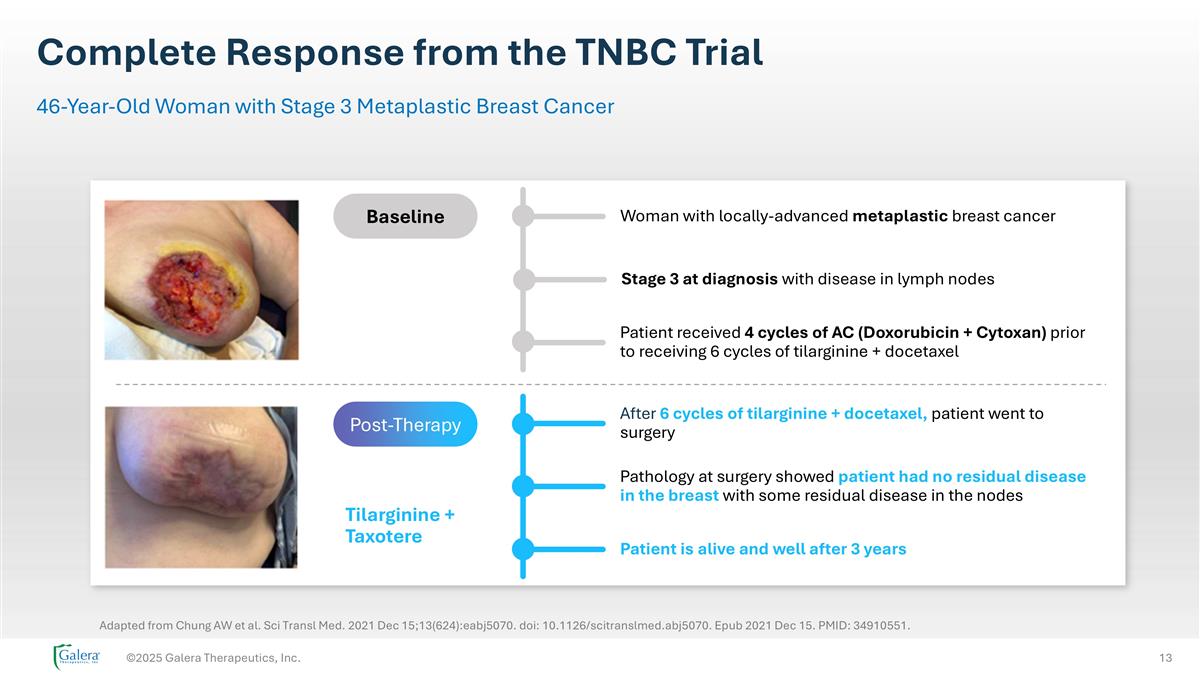
Complete Response from the TNBC Trial ©2025 Galera Therapeutics, Inc. 46-Year-Old Woman with Stage 3 Metaplastic Breast Cancer Patient is alive and well after 3 years Woman with locally-advanced metaplastic breast cancer Stage 3 at diagnosis with disease in lymph nodes Patient received 4 cycles of AC (Doxorubicin + Cytoxan) prior to receiving 6 cycles of tilarginine + docetaxel After 6 cycles of tilarginine + docetaxel, patient went to surgery Pathology at surgery showed patient had no residual disease in the breast with some residual disease in the nodes Post-Therapy Tilarginine + Taxotere Baseline Post-Therapy Adapted from Chung AW et al. Sci Transl Med. 2021 Dec 15;13(624):eabj5070. doi: 10.1126/scitranslmed.abj5070. Epub 2021 Dec 15. PMID: 34910551.
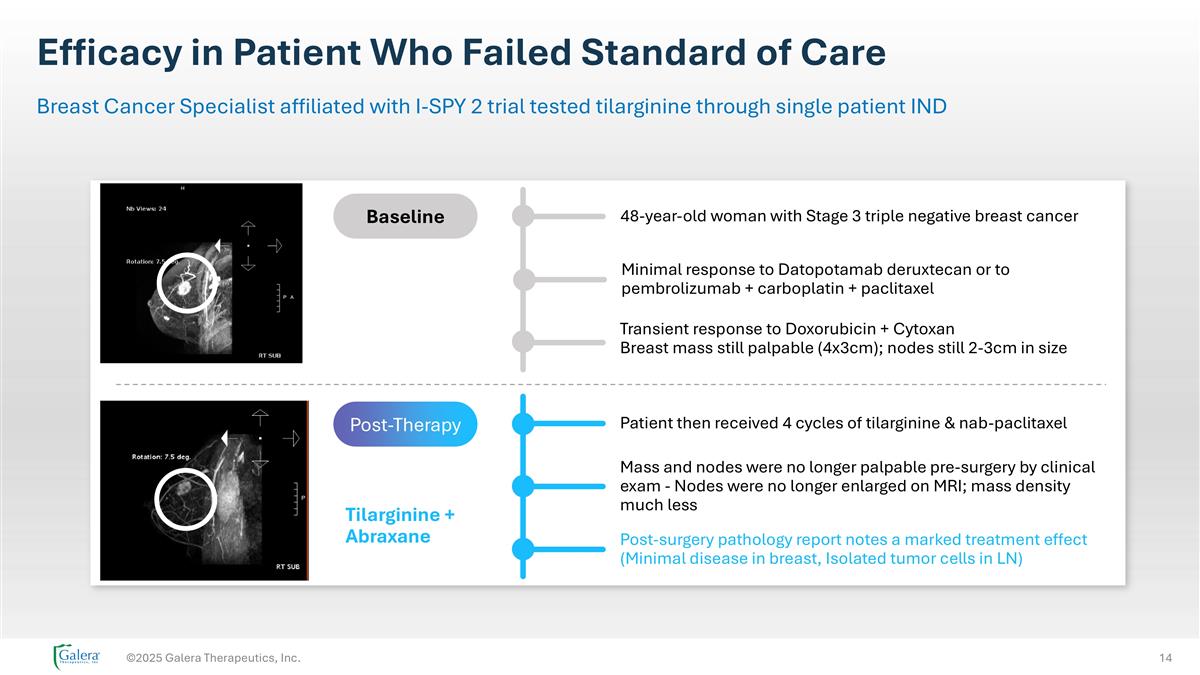
Efficacy in Patient Who Failed Standard of Care ©2025 Galera Therapeutics, Inc. Breast Cancer Specialist affiliated with I-SPY 2 trial tested tilarginine through single patient IND Tilarginine + Abraxane Baseline Post-Therapy Post-surgery pathology report notes a marked treatment effect (Minimal disease in breast, Isolated tumor cells in LN) 48-year-old woman with Stage 3 triple negative breast cancer Minimal response to Datopotamab deruxtecan or to pembrolizumab + carboplatin + paclitaxel Transient response to Doxorubicin + Cytoxan Breast mass still palpable (4x3cm); nodes still 2-3cm in size Patient then received 4 cycles of tilarginine & nab-paclitaxel Mass and nodes were no longer palpable pre-surgery by clinical exam - Nodes were no longer enlarged on MRI; mass density much less
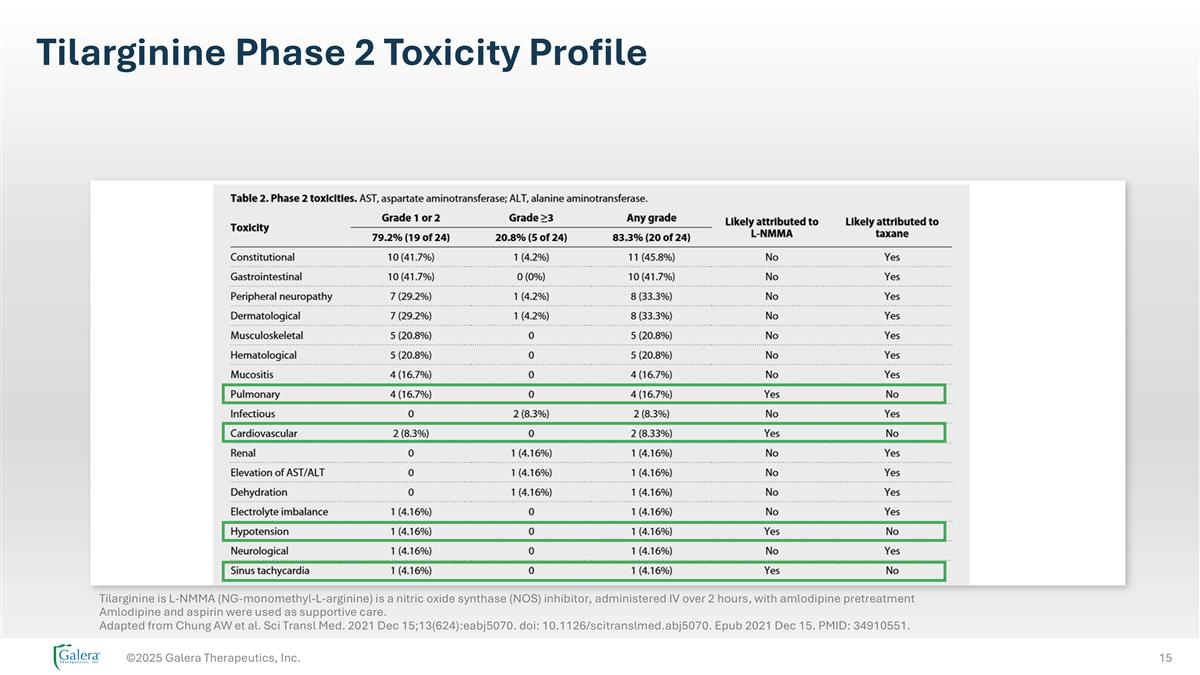
Tilarginine Phase 2 Toxicity Profile ©2025 Galera Therapeutics, Inc. Tilarginine is L-NMMA (NG-monomethyl-L-arginine) is a nitric oxide synthase (NOS) inhibitor, administered IV over 2 hours, with amlodipine pretreatment Amlodipine and aspirin were used as supportive care. Adapted from Chung AW et al. Sci Transl Med. 2021 Dec 15;13(624):eabj5070. doi: 10.1126/scitranslmed.abj5070. Epub 2021 Dec 15. PMID: 34910551.
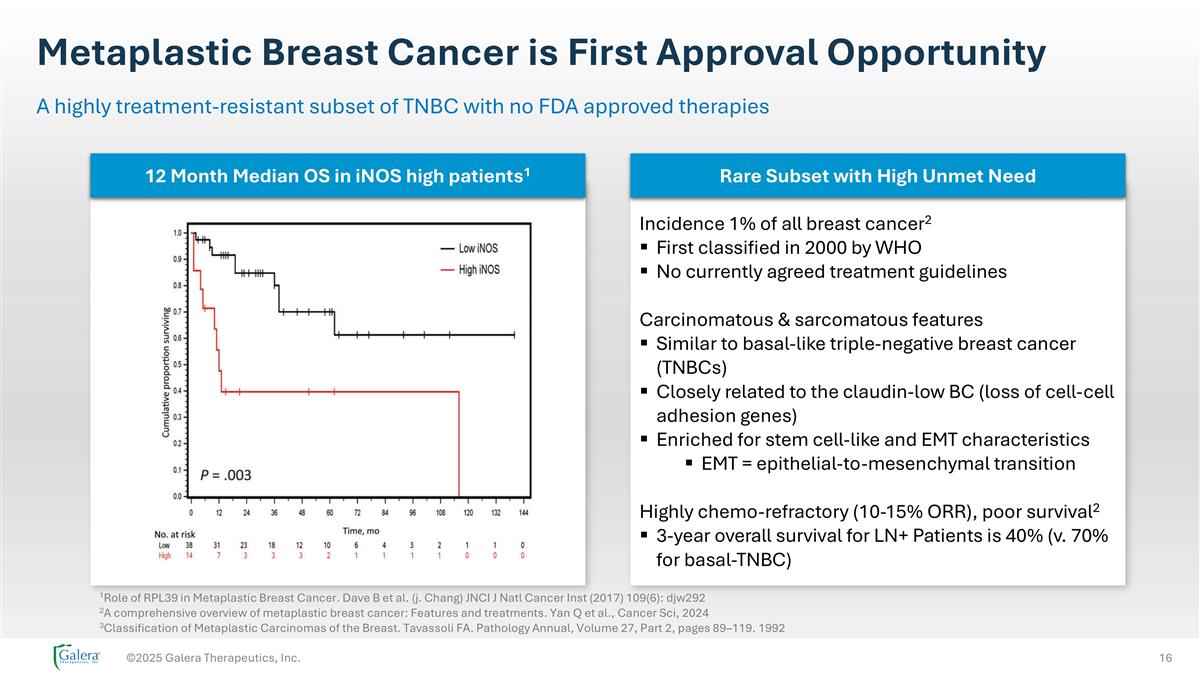
Metaplastic Breast Cancer is First Approval Opportunity ©2025 Galera Therapeutics, Inc. A highly treatment-resistant subset of TNBC with no FDA approved therapies 12 Month Median OS in iNOS high patients1 Rare Subset with High Unmet Need Incidence 1% of all breast cancer2 First classified in 2000 by WHO No currently agreed treatment guidelines Carcinomatous & sarcomatous features Similar to basal-like triple-negative breast cancer (TNBCs) Closely related to the claudin-low BC (loss of cell-cell adhesion genes) Enriched for stem cell-like and EMT characteristics EMT = epithelial-to-mesenchymal transition Highly chemo-refractory (10-15% ORR), poor survival2 3-year overall survival for LN+ Patients is 40% (v. 70% for basal-TNBC) 1Role of RPL39 in Metaplastic Breast Cancer. Dave B et al. (j. Chang) JNCI J Natl Cancer Inst (2017) 109(6): djw292 2A comprehensive overview of metaplastic breast cancer: Features and treatments. Yan Q et al., Cancer Sci, 2024 3Classification of Metaplastic Carcinomas of the Breast. Tavassoli FA. Pathology Annual, Volume 27, Part 2, pages 89–119. 1992
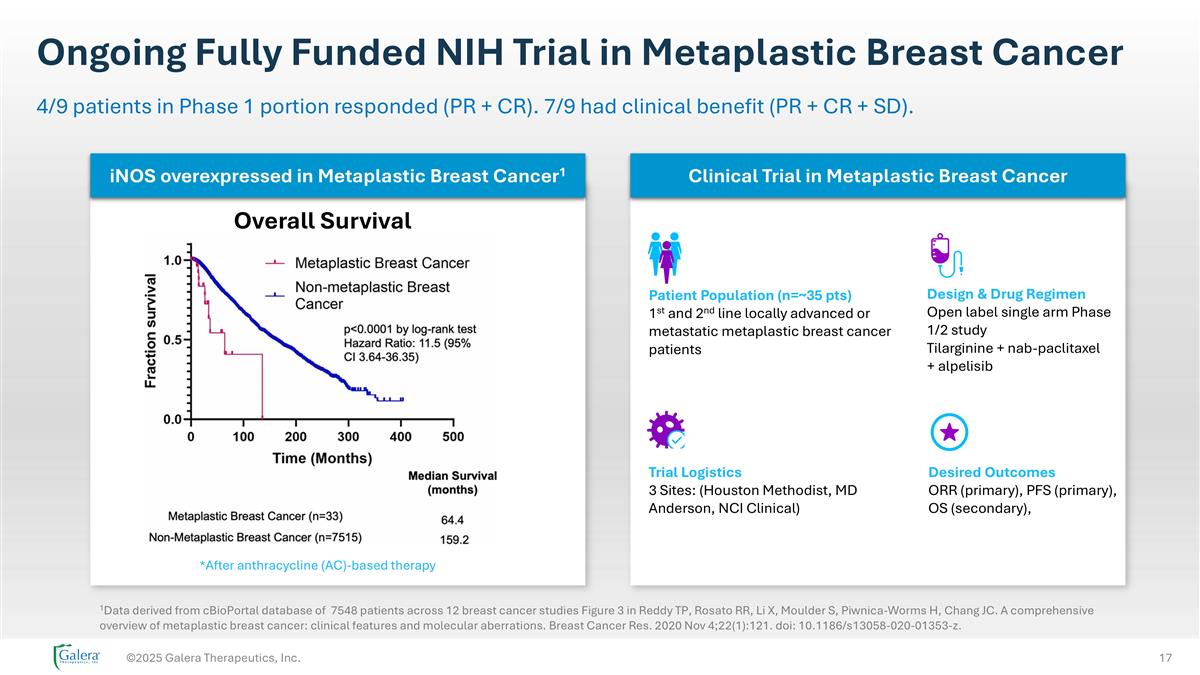
Ongoing Fully Funded NIH Trial in Metaplastic Breast Cancer ©2025 Galera Therapeutics, Inc. 4/9 patients in Phase 1 portion responded (PR + CR). 7/9 had clinical benefit (PR + CR + SD). *After anthracycline (AC)-based therapy 1Data derived from cBioPortal database of 7548 patients across 12 breast cancer studies Figure 3 in Reddy TP, Rosato RR, Li X, Moulder S, Piwnica-Worms H, Chang JC. A comprehensive overview of metaplastic breast cancer: clinical features and molecular aberrations. Breast Cancer Res. 2020 Nov 4;22(1):121. doi: 10.1186/s13058-020-01353-z. Patient Population (n=~35 pts) 1st and 2nd line locally advanced or metastatic metaplastic breast cancer patients Design & Drug Regimen Open label single arm Phase 1/2 study Tilarginine + nab-paclitaxel + alpelisib Trial Logistics 3 Sites: (Houston Methodist, MD Anderson, NCI Clinical) Desired Outcomes ORR (primary), PFS (primary), OS (secondary), iNOS overexpressed in Metaplastic Breast Cancer1 Clinical Trial in Metaplastic Breast Cancer Overall Survival
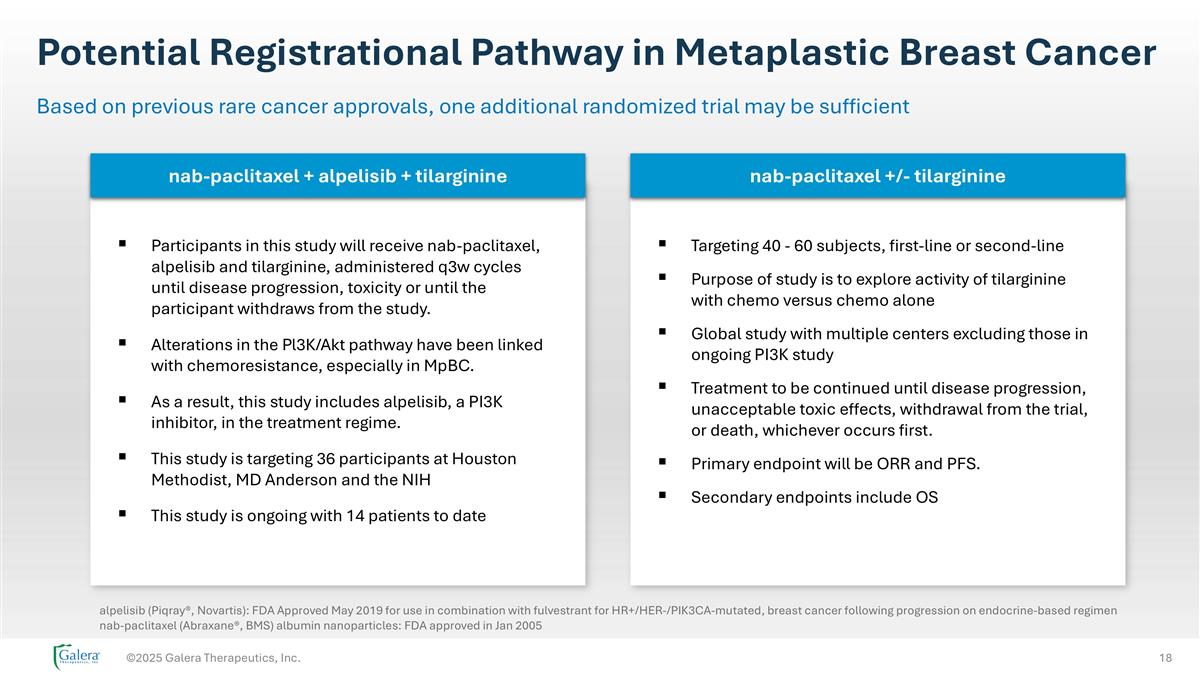
Potential Registrational Pathway in Metaplastic Breast Cancer ©2025 Galera Therapeutics, Inc. Based on previous rare cancer approvals, one additional randomized trial may be sufficient Participants in this study will receive nab-paclitaxel, alpelisib and tilarginine, administered q3w cycles until disease progression, toxicity or until the participant withdraws from the study. Alterations in the Pl3K/Akt pathway have been linked with chemoresistance, especially in MpBC. As a result, this study includes alpelisib, a PI3K inhibitor, in the treatment regime. This study is targeting 36 participants at Houston Methodist, MD Anderson and the NIH This study is ongoing with 14 patients to date Targeting 40 - 60 subjects, first-line or second-line Purpose of study is to explore activity of tilarginine with chemo versus chemo alone Global study with multiple centers excluding those in ongoing PI3K study Treatment to be continued until disease progression, unacceptable toxic effects, withdrawal from the trial, or death, whichever occurs first. Primary endpoint will be ORR and PFS. Secondary endpoints include OS alpelisib (Piqray®, Novartis): FDA Approved May 2019 for use in combination with fulvestrant for HR+/HER-/PIK3CA-mutated, breast cancer following progression on endocrine-based regimen nab-paclitaxel (Abraxane®, BMS) albumin nanoparticles: FDA approved in Jan 2005 nab-paclitaxel + alpelisib + tilarginine nab-paclitaxel +/- tilarginine
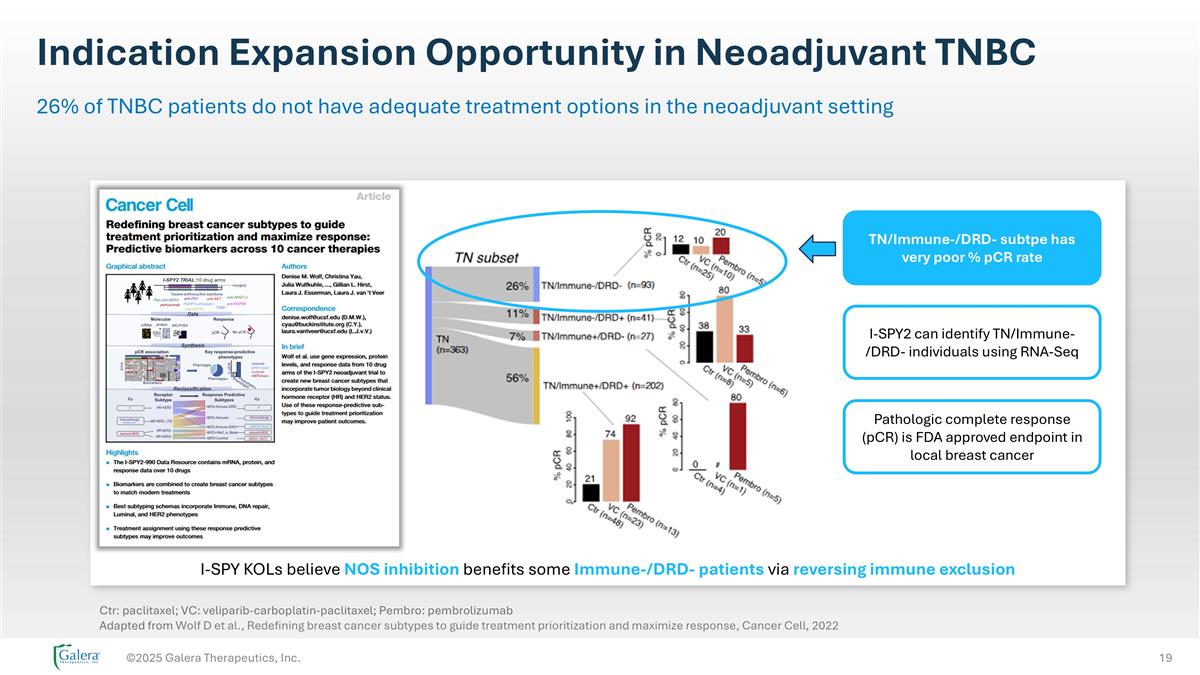
Indication Expansion Opportunity in Neoadjuvant TNBC ©2025 Galera Therapeutics, Inc. 26% of TNBC patients do not have adequate treatment options in the neoadjuvant setting Ctr: paclitaxel; VC: veliparib-carboplatin-paclitaxel; Pembro: pembrolizumab Adapted from Wolf D et al., Redefining breast cancer subtypes to guide treatment prioritization and maximize response, Cancer Cell, 2022 TN/Immune-/DRD- subtpe has very poor % pCR rate I-SPY2 can identify TN/Immune-/DRD- individuals using RNA-Seq Pathologic complete response (pCR) is FDA approved endpoint in local breast cancer I-SPY KOLs believe NOS inhibition benefits some Immune-/DRD- patients via reversing immune exclusion

Avasopasem in HR+ HER2- Breast Cancer
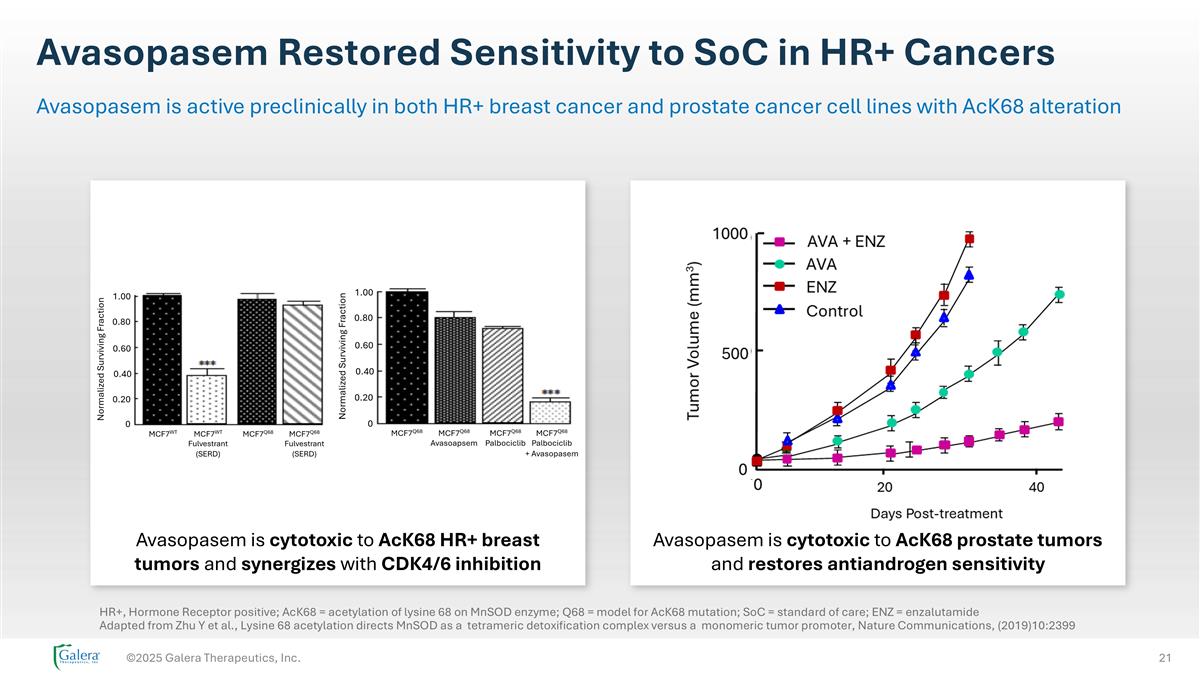
Avasopasem Restored Sensitivity to SoC in HR+ Cancers ©2025 Galera Therapeutics, Inc. Avasopasem is active preclinically in both HR+ breast cancer and prostate cancer cell lines with AcK68 alteration Avasopasem is cytotoxic to AcK68 HR+ breast tumors and synergizes with CDK4/6 inhibition Avasopasem is cytotoxic to AcK68 prostate tumors and restores antiandrogen sensitivity HR+, Hormone Receptor positive; AcK68 = acetylation of lysine 68 on MnSOD enzyme; Q68 = model for AcK68 mutation; SoC = standard of care; ENZ = enzalutamide Adapted from Zhu Y et al., Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter, Nature Communications, (2019)10:2399
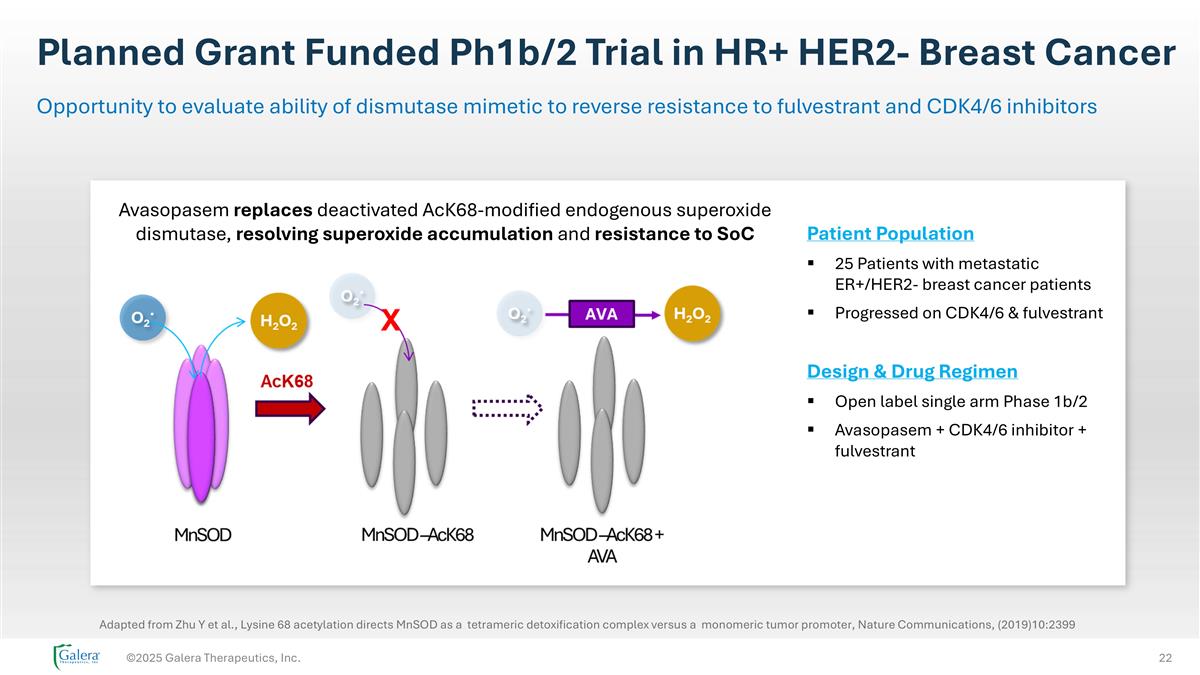
Planned Grant Funded Ph1b/2 Trial in HR+ HER2- Breast Cancer ©2025 Galera Therapeutics, Inc. Opportunity to evaluate ability of dismutase mimetic to reverse resistance to fulvestrant and CDK4/6 inhibitors Avasopasem replaces deactivated AcK68-modified endogenous superoxide dismutase, resolving superoxide accumulation and resistance to SoC Patient Population 25 Patients with metastatic ER+/HER2- breast cancer patients Progressed on CDK4/6 & fulvestrant Design & Drug Regimen Open label single arm Phase 1b/2 Avasopasem + CDK4/6 inhibitor + fulvestrant Adapted from Zhu Y et al., Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter, Nature Communications, (2019)10:2399
©2025 Galera Therapeutics, Inc. Tilarginine potential to be first approved therapy in metaplastic breast cancer (MpBC) Fully grant funded Phase 1b/2 trial ongoing in MpBC with nab-paclitaxel and alpelisib (PI3K inhibitor) 4/9 responses seen in Phase 1 portion Next tranche of data expected end of 2025 Proof of concept achieved in TNBC patients in combination with docetaxel 46% ORR across all patients in Phase 2 82% ORR in locally advanced patients Avasopasem to be repositioned into HR+ HER2- breast cancer to restore sensitivity to 1st line patients Phase 1b/2 single arm investigator-initiated trial to begin in 1H2025 Cash balance supports multiple near-term data readouts Cash runway anticipated to fund operations into 2026 Executive Summary and Key Milestones
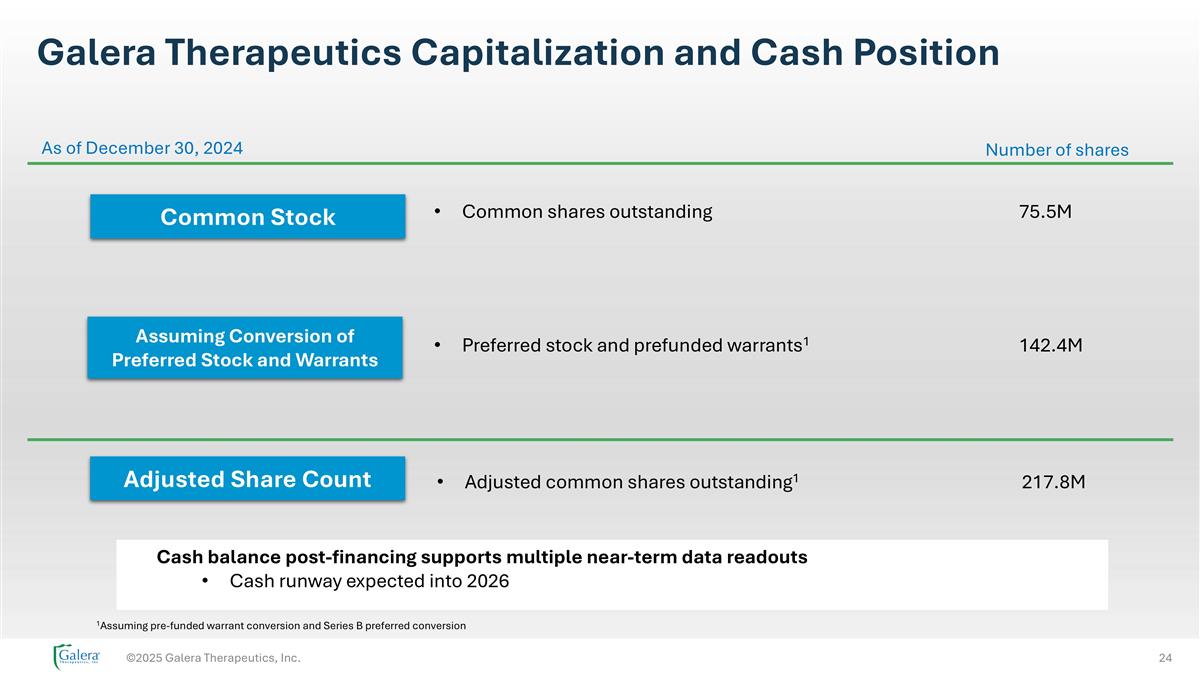
©2025 Galera Therapeutics, Inc. Cash balance post-financing supports multiple near-term data readouts Cash runway expected into 2026 Galera Therapeutics Capitalization and Cash Position Common Stock As of December 30, 2024 Assuming Conversion of Preferred Stock and Warrants Adjusted Share Count Common shares outstanding75.5M Number of shares Preferred stock and prefunded warrants1142.4M Adjusted common shares outstanding1217.8M 1Assuming pre-funded warrant conversion and Series B preferred conversion
Financial & Investor Information ©2025 Galera Therapeutics, Inc. OTC: GRTX In December 2024, Galera entered into a private placement financing Net proceeds of $3M combined with Galera’s net cash of $5.7M is expected to provide cash runway into 2026 On an as-converted basis after accounting for the acquisition of Nova Pharmaceuticals, Inc. and the financing, the total shares of Galera common stock outstanding would be 217.8M The following data is as of September 30, 2024 Cash and cash equivalents - $8.455M Outstanding shares of common stock – 54.4M

Appendix

Other Completed Trials ©2025 Galera Therapeutics, Inc. Two Completed Trials Tilarginine is L-NMMA (NG-monomethyl-L-arginine), a nitric oxide synthase (NOS) inhibitor, administered IV over 2 hours, with Amlodipine pretreatment SBRT is Stereotactic Body Radiotherapy, where high doses are administered over a 1-2 week period HMRI = Houston Methodist Research Institute Program Preclinical Phase 1 Phase 2 Results/Status Tilarginine + pembrolizumab Solid Tumors (n=12) Ph 1b single-arm combo trial (HMRI) Combination Well tolerated Avasopasem + SBRT x 5 days Locally-Advanced Pancreatic Cancer (n=42) Pilot randomized Phase 1-2 combo trial Improved response rate, tumor control, PFS and OS
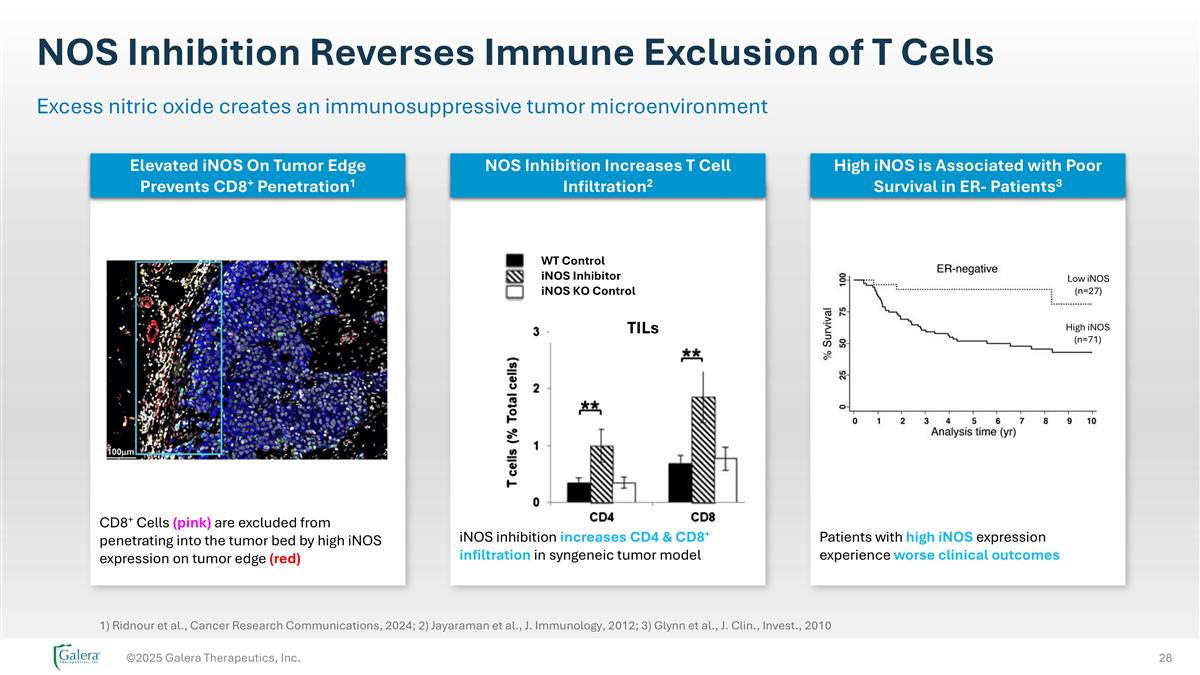
NOS Inhibition Reverses Immune Exclusion of T Cells ©2025 Galera Therapeutics, Inc. Excess nitric oxide creates an immunosuppressive tumor microenvironment CD8+ Cells (pink) are excluded from penetrating into the tumor bed by high iNOS expression on tumor edge (red) iNOS inhibition increases CD4 & CD8+ infiltration in syngeneic tumor model Patients with high iNOS expression experience worse clinical outcomes TILs WT Control iNOS Inhibitor iNOS KO Control Low iNOS (n=27) High iNOS (n=71) 1) Ridnour et al., Cancer Research Communications, 2024; 2) Jayaraman et al., J. Immunology, 2012; 3) Glynn et al., J. Clin., Invest., 2010 NOS Inhibition Increases T Cell Infiltration2 High iNOS is Associated with Poor Survival in ER- Patients3 Elevated iNOS On Tumor Edge Prevents CD8+ Penetration1
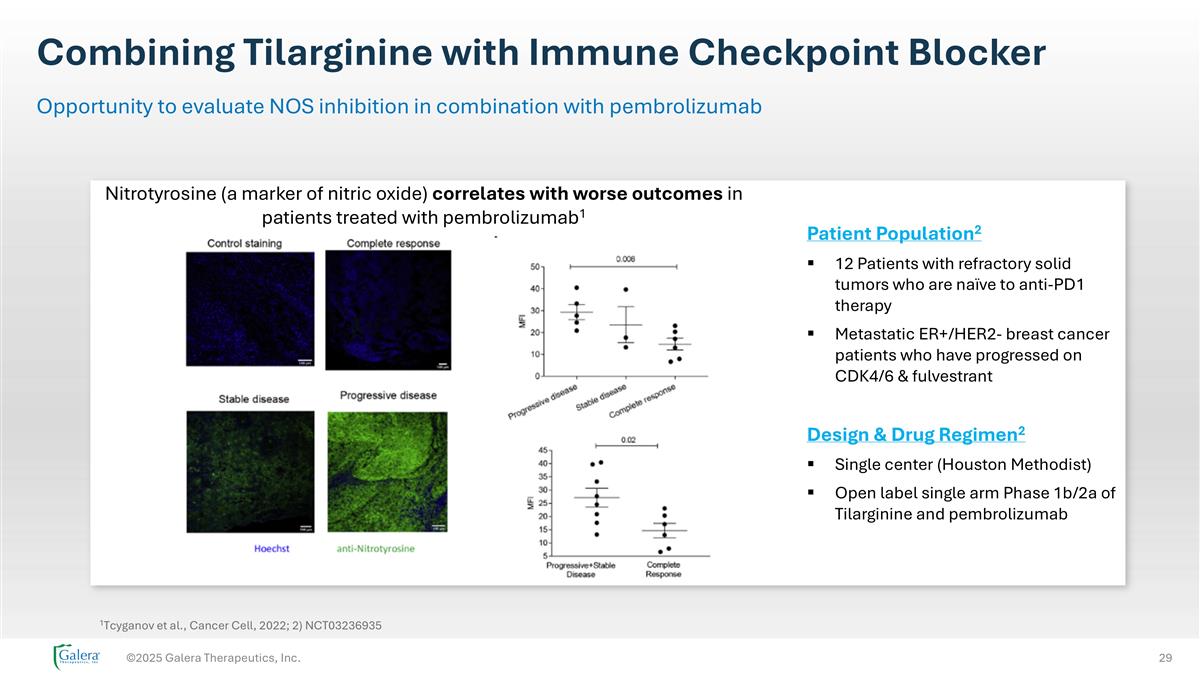
Combining Tilarginine with Immune Checkpoint Blocker ©2025 Galera Therapeutics, Inc. Opportunity to evaluate NOS inhibition in combination with pembrolizumab 1Tcyganov et al., Cancer Cell, 2022; 2) NCT03236935 Patient Population2 12 Patients with refractory solid tumors who are naïve to anti-PD1 therapy Metastatic ER+/HER2- breast cancer patients who have progressed on CDK4/6 & fulvestrant Design & Drug Regimen2 Single center (Houston Methodist) Open label single arm Phase 1b/2a of Tilarginine and pembrolizumab Nitrotyrosine (a marker of nitric oxide) correlates with worse outcomes in patients treated with pembrolizumab1

Avasopasem in Locally Advanced Pancreatic Cancer
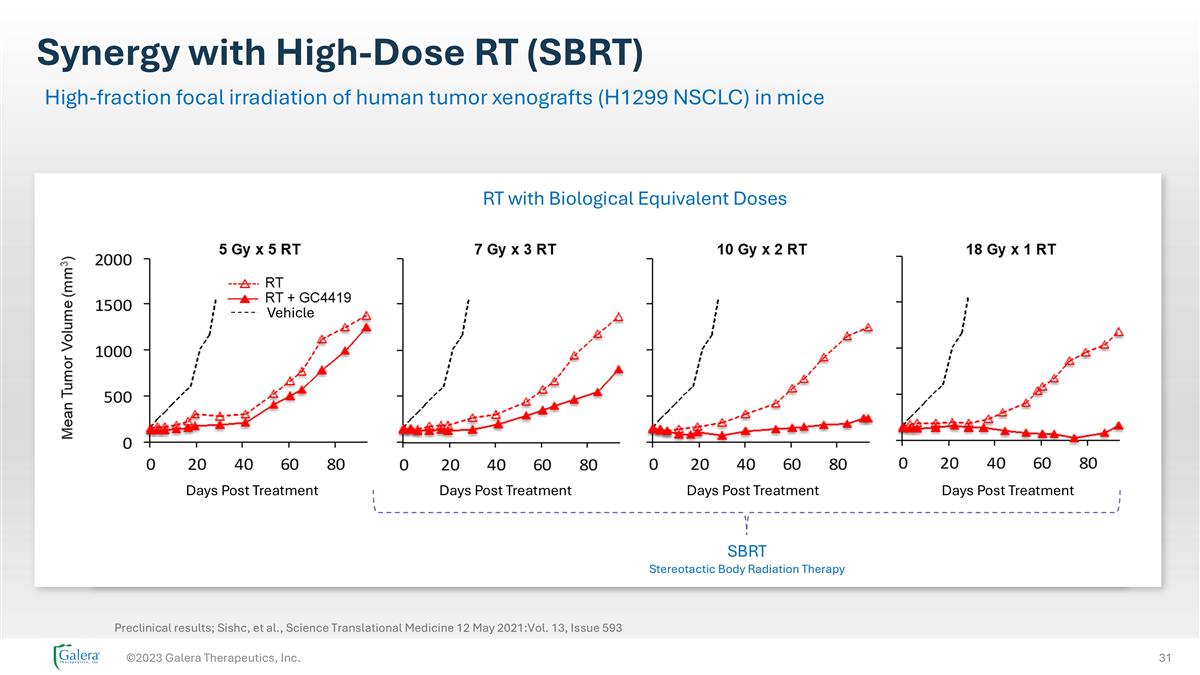
Synergy with High-Dose RT (SBRT) ©2023 Galera Therapeutics, Inc. High-fraction focal irradiation of human tumor xenografts (H1299 NSCLC) in mice Vehicle Days Post Treatment Days Post Treatment Days Post Treatment Days Post Treatment SBRT Stereotactic Body Radiation Therapy RT with Biological Equivalent Doses Preclinical results; Sishc, et al., Science Translational Medicine 12 May 2021:Vol. 13, Issue 593
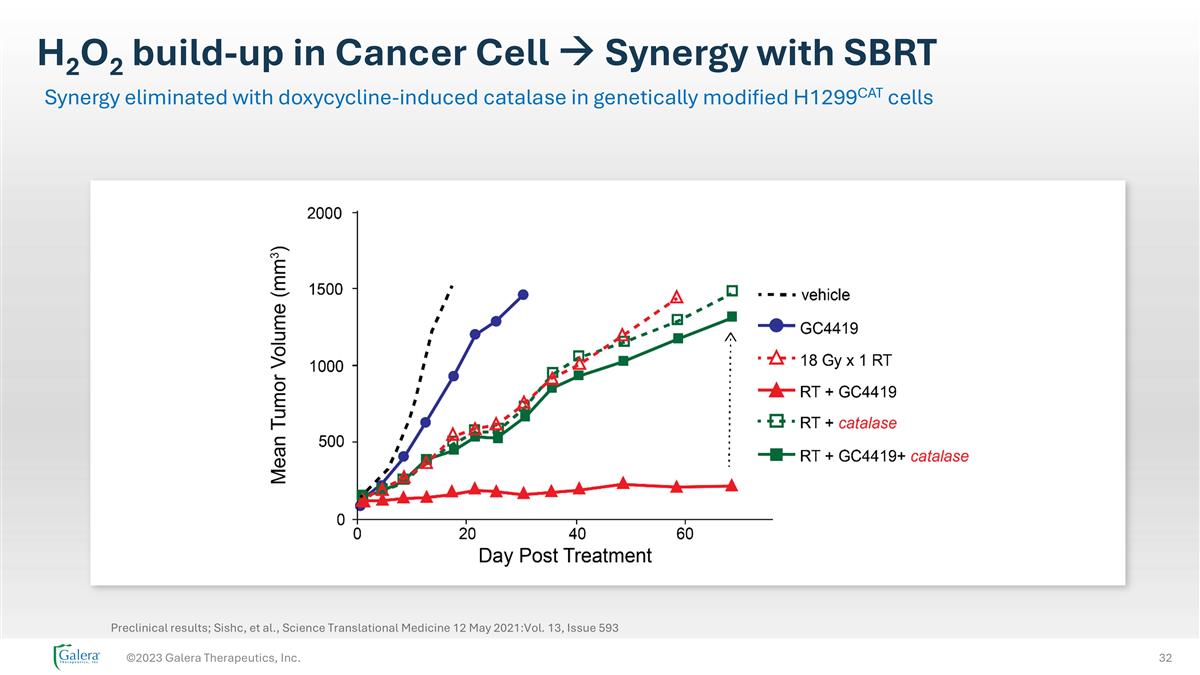
H2O2 build-up in Cancer Cell à Synergy with SBRT ©2023 Galera Therapeutics, Inc. Synergy eliminated with doxycycline-induced catalase in genetically modified H1299CAT cells Preclinical results; Sishc, et al., Science Translational Medicine 12 May 2021:Vol. 13, Issue 593
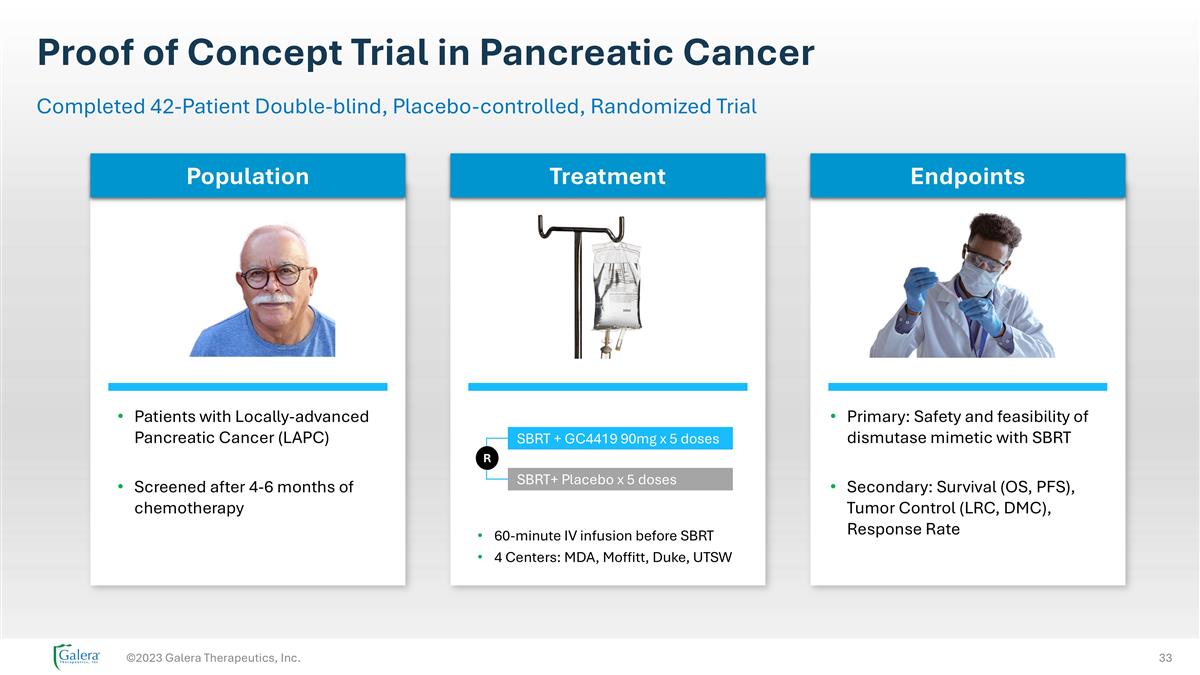
Proof of Concept Trial in Pancreatic Cancer ©2023 Galera Therapeutics, Inc. Completed 42-Patient Double-blind, Placebo-controlled, Randomized Trial 60-minute IV infusion before SBRT 4 Centers: MDA, Moffitt, Duke, UTSW Patients with Locally-advanced Pancreatic Cancer (LAPC) Screened after 4-6 months of chemotherapy Primary: Safety and feasibility of dismutase mimetic with SBRT Secondary: Survival (OS, PFS), Tumor Control (LRC, DMC), Response Rate SBRT + GC4419 90mg x 5 doses SBRT+ Placebo x 5 doses R Treatment Endpoints Population
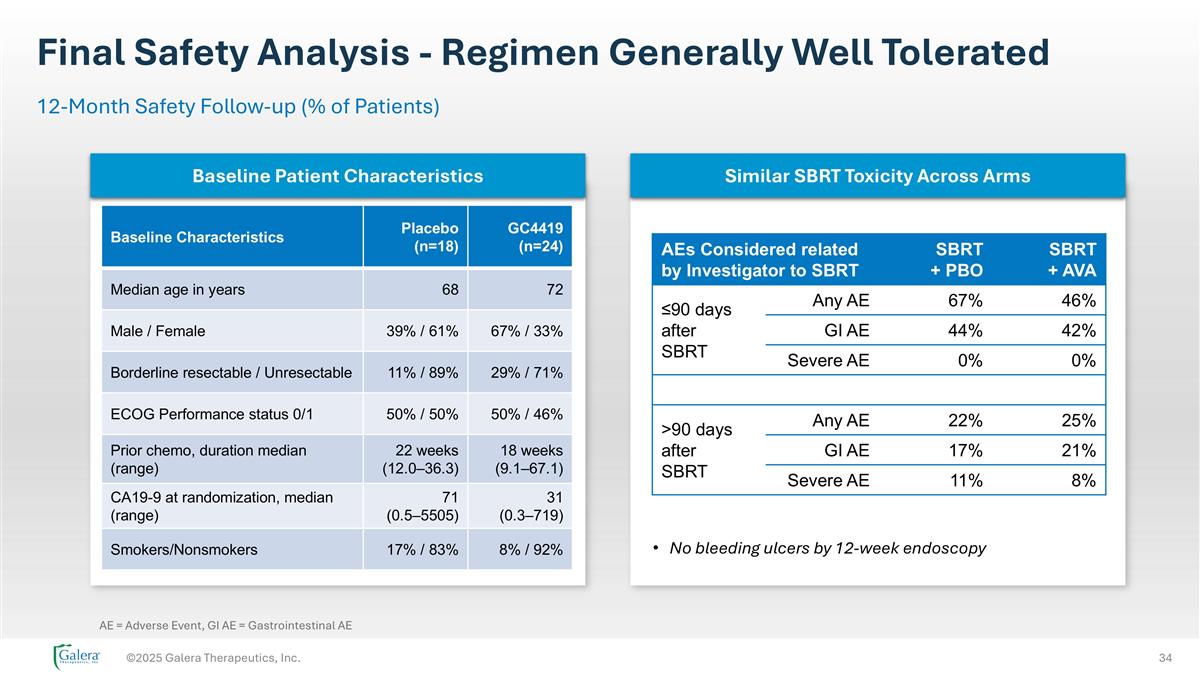
Final Safety Analysis - Regimen Generally Well Tolerated ©2025 Galera Therapeutics, Inc. 12-Month Safety Follow-up (% of Patients) AEs Considered related by Investigator to SBRT SBRT + PBO SBRT + AVA ≤90 days after SBRT Any AE 67% 46% GI AE 44% 42% Severe AE 0% 0% >90 days after SBRT Any AE 22% 25% GI AE 17% 21% Severe AE 11% 8% No bleeding ulcers by 12-week endoscopy Similar SBRT Toxicity Across Arms Baseline Patient Characteristics Baseline Characteristics Placebo (n=18) GC4419 (n=24) Median age in years 68 72 Male / Female 39% / 61% 67% / 33% Borderline resectable / Unresectable 11% / 89% 29% / 71% ECOG Performance status 0/1 50% / 50% 50% / 46% Prior chemo, duration median (range) 22 weeks (12.0–36.3) 18 weeks (9.1–67.1) CA19-9 at randomization, median (range) 71 (0.5–5505) 31 (0.3–719) Smokers/Nonsmokers 17% / 83% 8% / 92% AE = Adverse Event, GI AE = Gastrointestinal AE
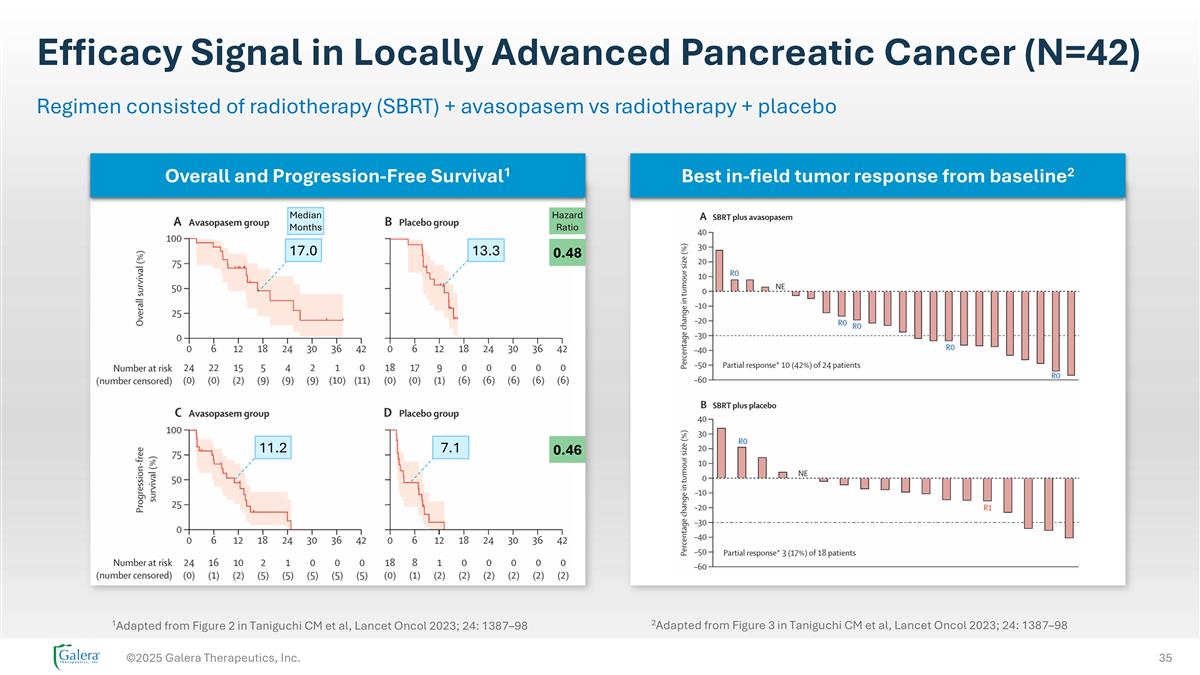
Efficacy Signal in Locally Advanced Pancreatic Cancer (N=42) ©2025 Galera Therapeutics, Inc. Regimen consisted of radiotherapy (SBRT) + avasopasem vs radiotherapy + placebo 1Adapted from Figure 2 in Taniguchi CM et al, Lancet Oncol 2023; 24: 1387–98 Overall and Progression-Free Survival1 Best in-field tumor response from baseline2 11.2 7.1 17.0 13.3 Median Months Hazard Ratio 0.48 0.46 2Adapted from Figure 3 in Taniguchi CM et al, Lancet Oncol 2023; 24: 1387–98


































