
Investor Presentation July 2021 Exhibit 99.1

Statements contained in this presentation and oral statements made regarding the subject of this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied by such forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding our business plans and objectives, including future plans or expectations for our product candidates, clinical trials of our product candidates, and expectations regarding our uses and sufficiency of capital; and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” “could,” “continue” and similar expressions, although not all forward-looking statements contain these identifying words. Risks that contribute to the uncertain nature of the forward-looking statements include: uncertainties regarding the success, cost, and timing of the our product candidate development activities and clinical trials; uncertainties as to the potential impact to our clinical trials and operations of the COVID-19 pandemic; the risk that results from a clinical trial may not be predictive of the results of other future clinical trials; potential regulatory developments in the United States and foreign countries; uncertainties inherent in estimating future expenses, capital requirements and other financial results; risks with respect to our ability to fund our operations on a continuing basis; as well as the other risks and uncertainties set forth in our Quarterly Report on Form 10-Q for the period ended March 31, 2021, filed with the Securities and Exchange Commission and in subsequent filings with the Securities and Exchange Commission. All forward-looking statements contained in this presentation speak only as of the date of this presentation. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that these third-party sources and estimates are reliable but have not independently verified them. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors that could cause results to differ materially from those expressed in the estimates made by third parties and by us. Forward-Looking Statements Disclaimer
Executive Summary Differentiated MOA Only oral dual acting MOA in clinical development that works both peripherally and centrally to rebalance the kappa and mu receptors, both of which are important in mediating pruritus and cough Strong Pruritus Data Statistically significant pruritus reduction in uremic pruritus Ph2b/3 trial Prurigo nodularis Ph2a WI-NRS reduction vs. placebo Prurigo nodularis Ph2a open-label extension demonstrated disease improvement Near Term Data for Lead Indications Near term data in large attractive markets with broad expansion opportunities Prurigo nodularis Ph2b/3 and IPF Ph2a topline data in 1H 2022 Experienced Management Experienced management team with several successful drug approvals in relevant indications Strong IP 18 issued US and foreign patents with terms expiring from 2026 to 2032 and additional applications with terms, if issued, expiring from 2032 to 2041 Prurigo nodularis Ph2a open-label extension demonstrated disease improvement in patients on HADUVIO ≥6 months

Experienced Leadership with Strong Financial Backing Shareholders (as of April 19, 2021) Management Team Jennifer Good President & Chief Executive Officer Thomas Sciascia, M.D. Chief Medical Officer William Forbes, Pharm.D. Chief Development Officer Farrell Simon, Pharm.D. VP, Head of U.S. Marketing David Meeker, M.D. Rhythm Pharmaceuticals Board of Directors Ed Mathers New Enterprise Associates, Inc Insider held ≈60% Jennifer Good Trevi Therapeutics Anne VanLent AMV Advisors James Cassella, Ph.D. Concert Pharmaceuticals Michael Heffernan Collegium Pharmaceutical, Inc. Dominick Colangelo Vericel Corporation
Only oral dual acting MOA in clinical development that works both peripherally and centrally to rebalance the kappa and mu receptors, both of which are important in mediating pruritus and cough Lead Indications Pruritus Associated with Prurigo Nodularis (PN) - PRISM 730k patients WW, 300k in U.S. with no current approved therapies Only oral compound in clinical development in $3B PN category1 Shown reductions in pruritus in moderate to severe itch patients (Ph2a) Chronic Cough in Idiopathic Pulmonary Fibrosis (IPF) - CANAL 1M+ patients WW, 130k in U.S. with no current approved therapies Most advanced oral compound in clinical development for cough in $4B IPF category2 Broad Pipeline Potential in Each Indication Potential for broad application in $20B pruritus and $10B chronic cough categories3,4 HADUVIOTM (nalbuphine ER) Overview Anticipated Milestones PRISM Ph2b/3 PN trial enrollment completion (2H 2021) and topline data (1H 2022) CANAL Ph2a IPF trial topline data (1H 2022) HADUVIO™ (nalbuphine ER) is an investigational drug 12031 est. Prurigo Nodularis Market Report May 2020 22028 est. Research Idiopathic Pulmonary Fibrosis Report Feb 2021 32026 est. Global Pruritus Therapeutics Market Prurigo 42027 est. The Insight Partners Jun 2020
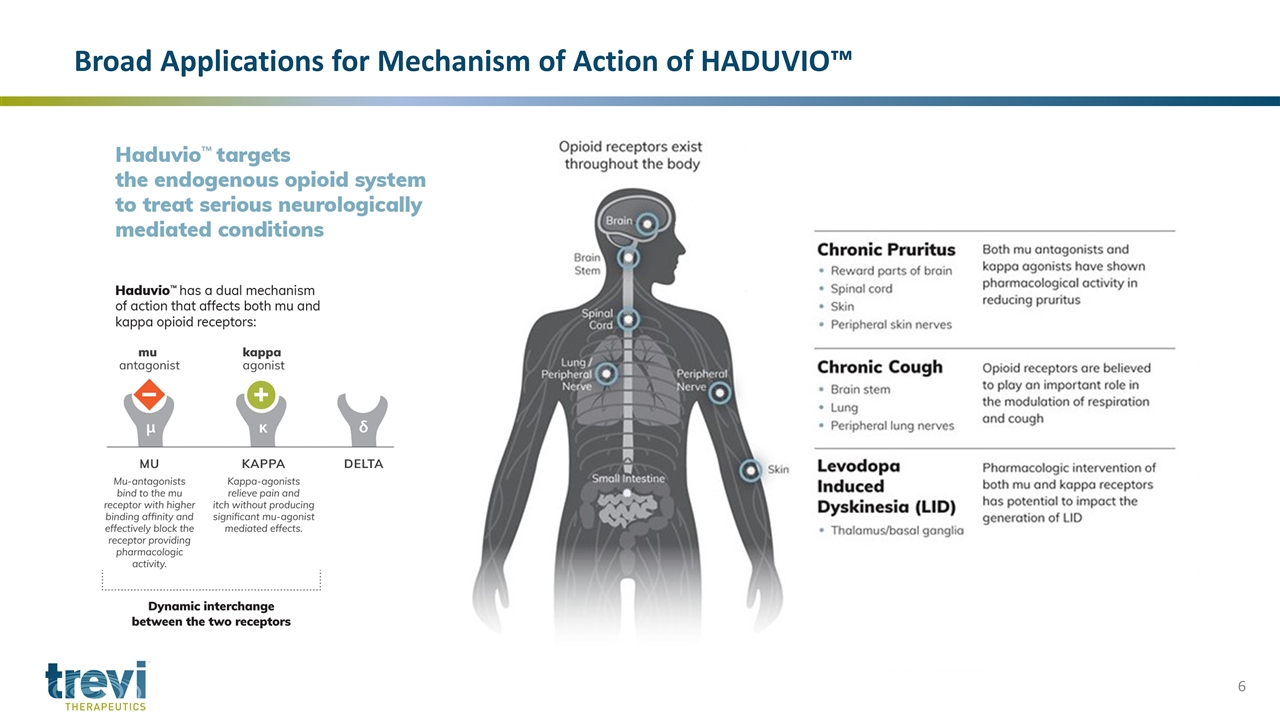
Broad Applications for Mechanism of Action of HADUVIO™
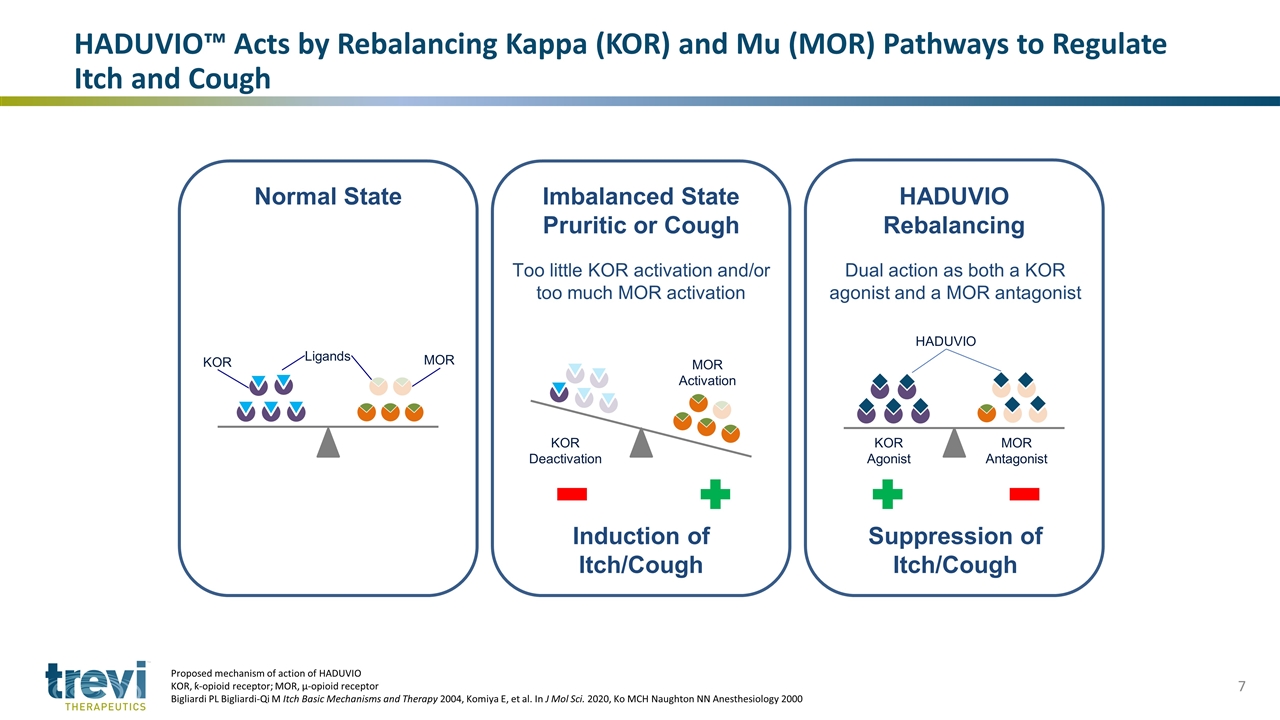
Normal State Imbalanced State Pruritic or Cough Induction of Itch/Cough Too little KOR activation and/or too much MOR activation HADUVIO Rebalancing Suppression of Itch/Cough Dual action as both a KOR agonist and a MOR antagonist MOR KOR Ligands MOR Activation KOR Deactivation MOR Antagonist KOR Agonist HADUVIO HADUVIO™ Acts by Rebalancing Kappa (KOR) and Mu (MOR) Pathways to Regulate Itch and Cough Proposed mechanism of action of HADUVIO KOR, ƙ-opioid receptor; MOR, µ-opioid receptor Bigliardi PL Bigliardi-Qi M Itch Basic Mechanisms and Therapy 2004, Komiya E, et al. In J Mol Sci. 2020, Ko MCH Naughton NN Anesthesiology 2000
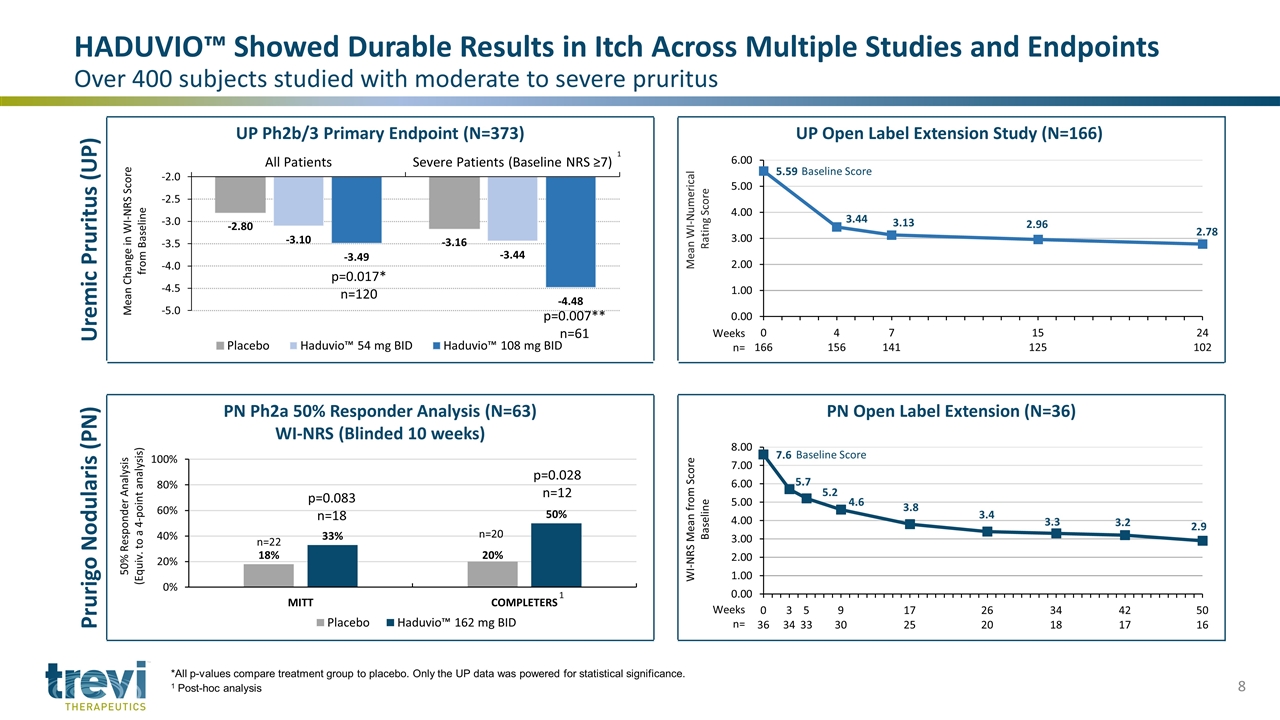
HADUVIO™ Showed Durable Results in Itch Across Multiple Studies and Endpoints Over 400 subjects studied with moderate to severe pruritus 1 p=0.017* n=120 p=0.007** n=61 Baseline Score Weeks n= Baseline Score Weeks n= n=20 p=0.028 n=12 p=0.083 n=18 n=22 *All p-values compare treatment group to placebo. Only the UP data was powered for statistical significance. 1 Post-hoc analysis 1 Uremic Pruritus (UP) UP Ph2b/3 Primary Endpoint (N=373) UP Open Label Extension Study (N=166) Prurigo Nodularis (PN) PN Ph2a 50% Responder Analysis (N=63) WI-NRS (Blinded 10 weeks) PN Open Label Extension (N=36)
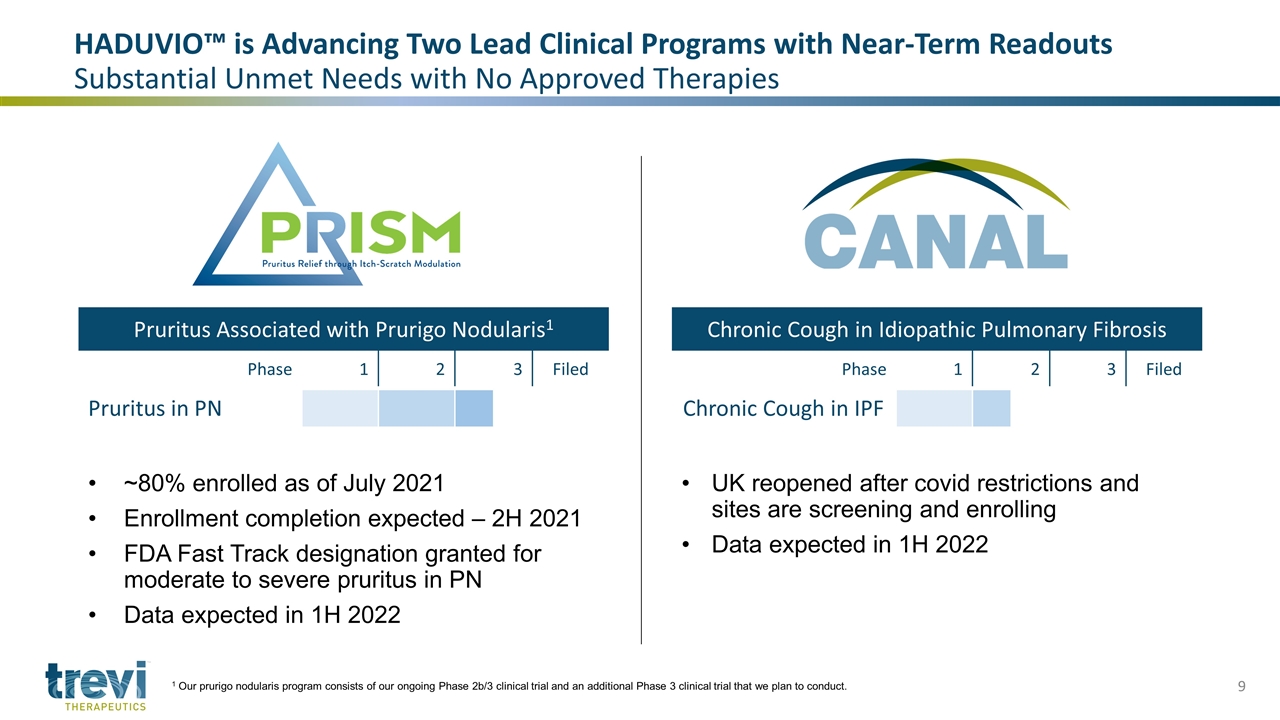
Phase 1 2 3 Filed Pruritus in PN Phase 1 2 3 Filed Chronic Cough in IPF UK reopened after covid restrictions and sites are screening and enrolling Data expected in 1H 2022 ~80% enrolled as of July 2021 Enrollment completion expected – 2H 2021 FDA Fast Track designation granted for moderate to severe pruritus in PN Data expected in 1H 2022 HADUVIO™ is Advancing Two Lead Clinical Programs with Near-Term Readouts Substantial Unmet Needs with No Approved Therapies Pruritus Associated with Prurigo Nodularis1 Chronic Cough in Idiopathic Pulmonary Fibrosis 1 Our prurigo nodularis program consists of our ongoing Phase 2b/3 clinical trial and an additional Phase 3 clinical trial that we plan to conduct.
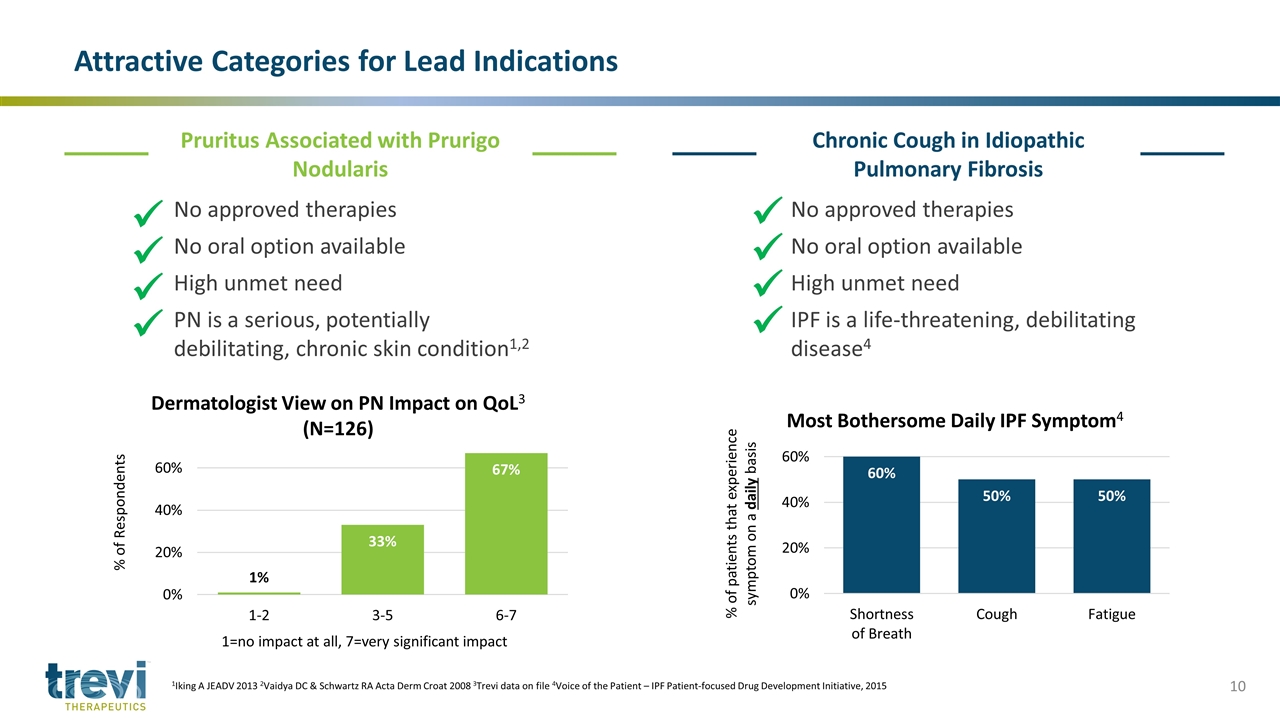
Attractive Categories for Lead Indications 1Iking A JEADV 2013 2Vaidya DC & Schwartz RA Acta Derm Croat 2008 3Trevi data on file 4Voice of the Patient – IPF Patient-focused Drug Development Initiative, 2015 Pruritus Associated with Prurigo Nodularis Chronic Cough in Idiopathic Pulmonary Fibrosis No approved therapies No oral option available High unmet need PN is a serious, potentially debilitating, chronic skin condition1,2 1=no impact at all, 7=very significant impact ü ü ü ü No approved therapies No oral option available High unmet need IPF is a life-threatening, debilitating disease4 ü ü ü ü
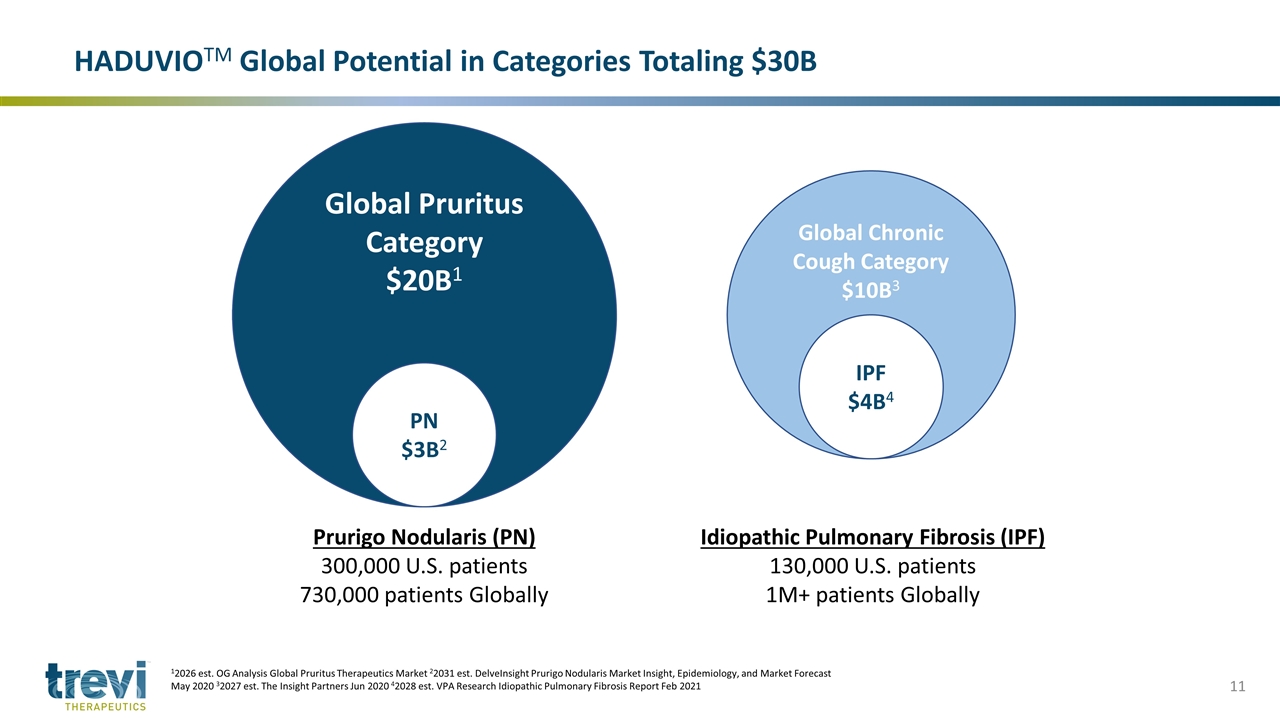
Global Pruritus Category $20B1 PN $3B2 Global Chronic Cough Category $10B3 IPF $4B4 Prurigo Nodularis (PN) 300,000 U.S. patients 730,000 patients Globally Idiopathic Pulmonary Fibrosis (IPF) 130,000 U.S. patients 1M+ patients Globally HADUVIOTM Global Potential in Categories Totaling $30B 12026 est. OG Analysis Global Pruritus Therapeutics Market 22031 est. DelveInsight Prurigo Nodularis Market Insight, Epidemiology, and Market Forecast May 2020 32027 est. The Insight Partners Jun 2020 42028 est. VPA Research Idiopathic Pulmonary Fibrosis Report Feb 2021

Chronic Pruritus in Prurigo Nodularis
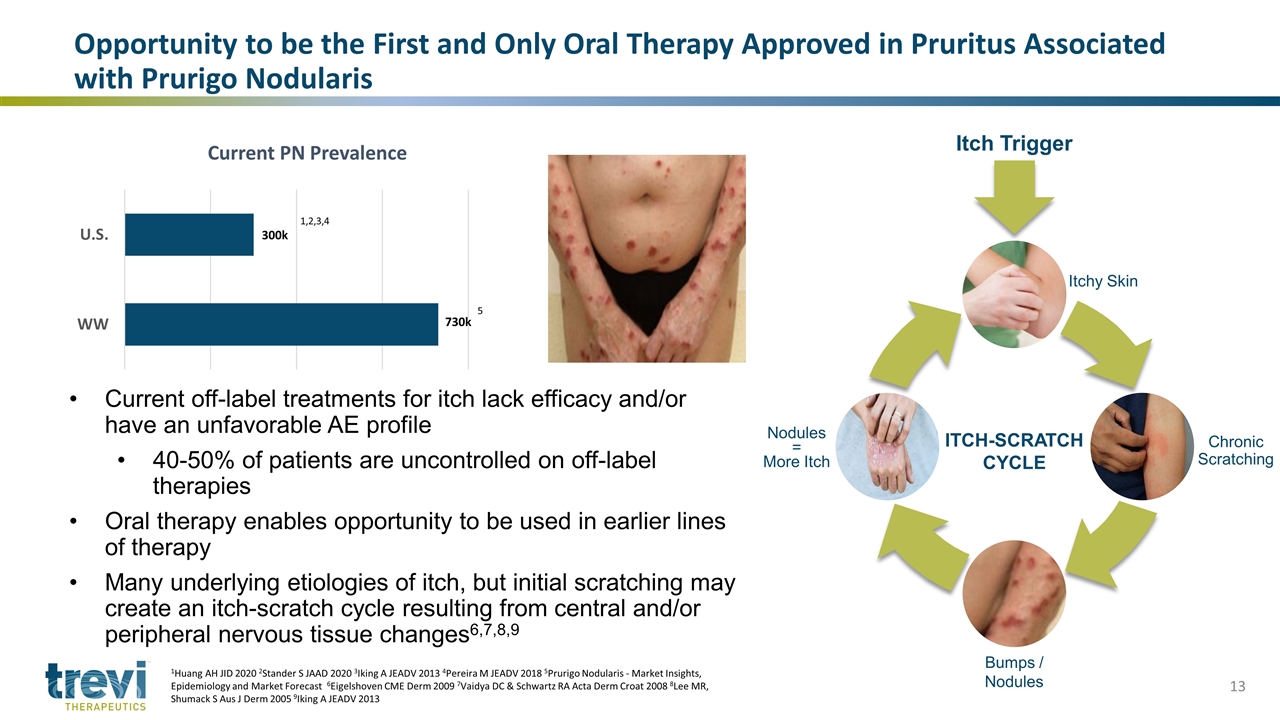
Opportunity to be the First and Only Oral Therapy Approved in Pruritus Associated with Prurigo Nodularis 1Huang AH JID 2020 2Stander S JAAD 2020 3Iking A JEADV 2013 4Pereira M JEADV 2018 5Prurigo Nodularis - Market Insights, Epidemiology and Market Forecast 6Eigelshoven CME Derm 2009 7Vaidya DC & Schwartz RA Acta Derm Croat 2008 8Lee MR, Shumack S Aus J Derm 2005 9Iking A JEADV 2013 Current off-label treatments for itch lack efficacy and/or have an unfavorable AE profile 40-50% of patients are uncontrolled on off-label therapies Oral therapy enables opportunity to be used in earlier lines of therapy Many underlying etiologies of itch, but initial scratching may create an itch-scratch cycle resulting from central and/or peripheral nervous tissue changes6,7,8,9 Current PN Prevalence 730k 1,2,3,4 5 Bumps / Nodules ITCH-SCRATCH CYCLE Itchy Skin Chronic Scratching Itch Trigger Nodules = More Itch
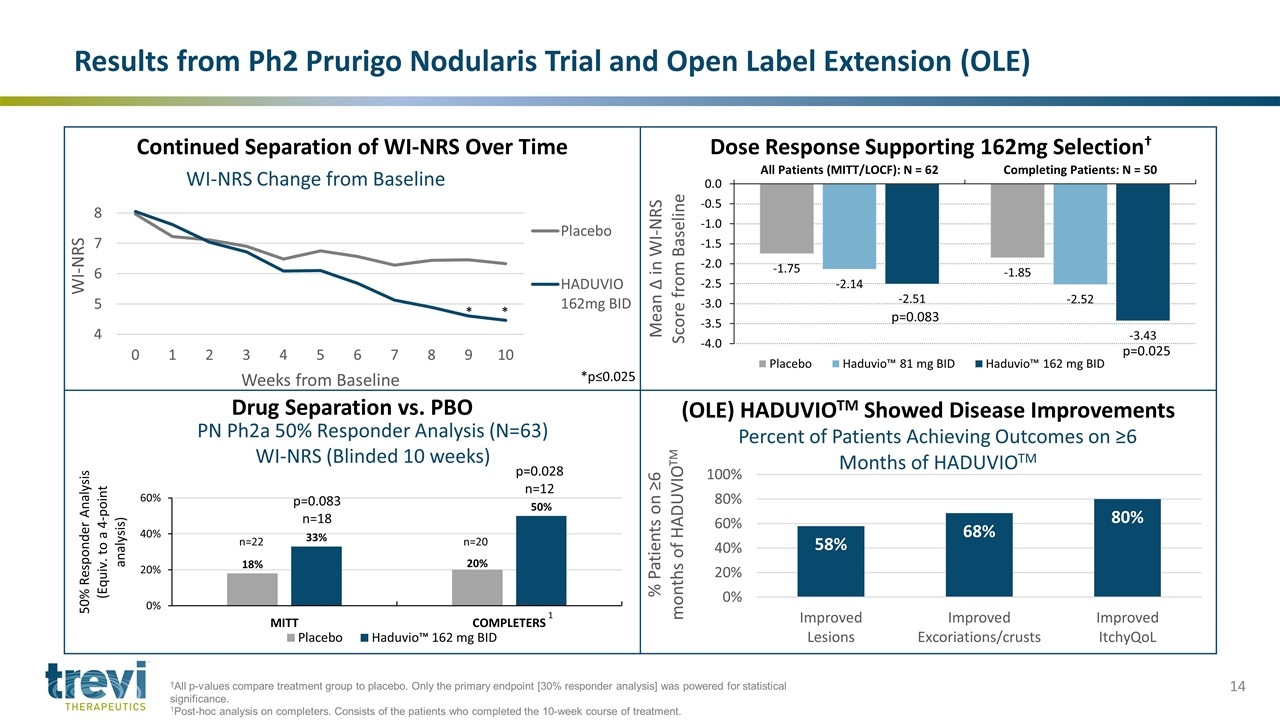
Results from Ph2 Prurigo Nodularis Trial and Open Label Extension (OLE) Continued Separation of WI-NRS Over Time Dose Response Supporting 162mg Selection† Drug Separation vs. PBO (OLE) HADUVIOTM Showed Disease Improvements PN Ph2a 50% Responder Analysis (N=63) WI-NRS (Blinded 10 weeks) n=20 p=0.028 n=12 p=0.083 n=18 n=22 * * *p≤0.025 †All p-values compare treatment group to placebo. Only the primary endpoint [30% responder analysis] was powered for statistical significance. 1Post-hoc analysis on completers. Consists of the patients who completed the 10-week course of treatment. p=0.083 p=0.025 1
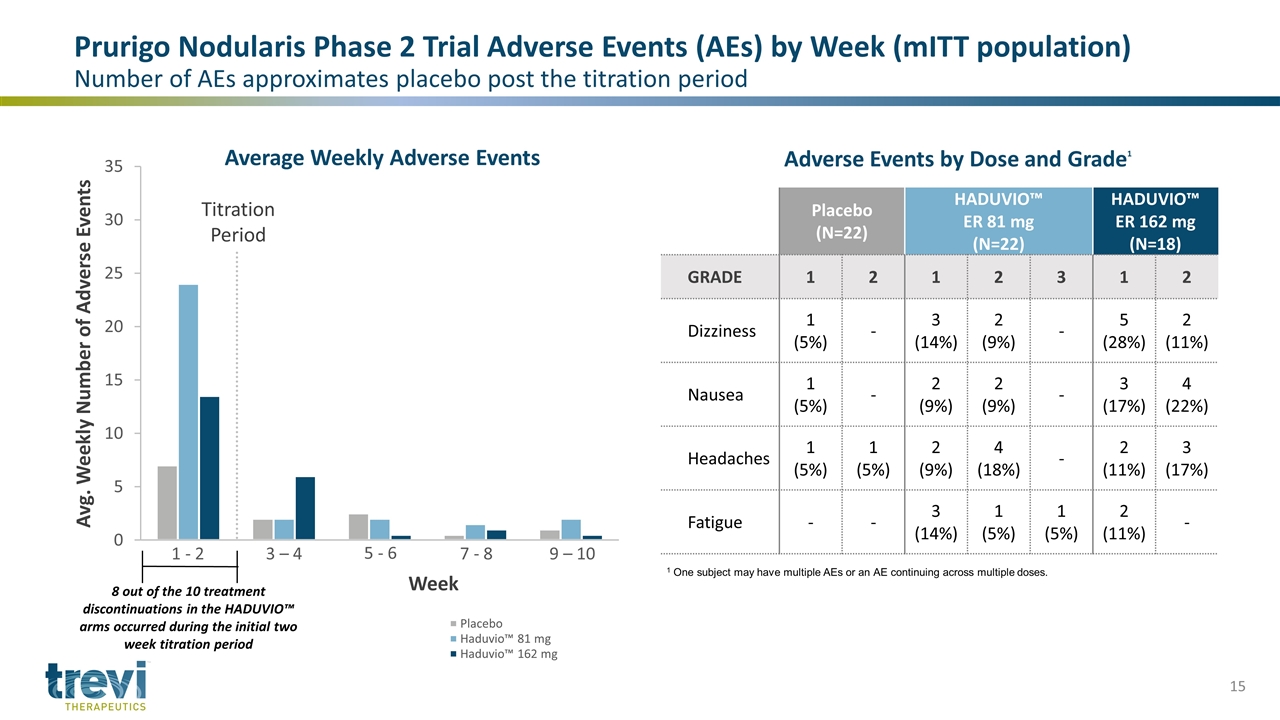
Avg. Weekly Number of Adverse Events Titration Period Week 1 - 2 3 – 4 7 - 8 9 – 10 8 out of the 10 treatment discontinuations in the HADUVIO™ arms occurred during the initial two week titration period Placebo (N=22) HADUVIO™ ER 81 mg (N=22) HADUVIO™ ER 162 mg (N=18) GRADE 1 2 1 2 3 1 2 Dizziness 1 (5%) - 3 (14%) 2 (9%) - 5 (28%) 2 (11%) Nausea 1 (5%) - 2 (9%) 2 (9%) - 3 (17%) 4 (22%) Headaches 1 (5%) 1 (5%) 2 (9%) 4 (18%) - 2 (11%) 3 (17%) Fatigue - - 3 (14%) 1 (5%) 1 (5%) 2 (11%) - Prurigo Nodularis Phase 2 Trial Adverse Events (AEs) by Week (mITT population) Number of AEs approximates placebo post the titration period 1 One subject may have multiple AEs or an AE continuing across multiple doses. Adverse Events by Dose and Grade1 5 - 6
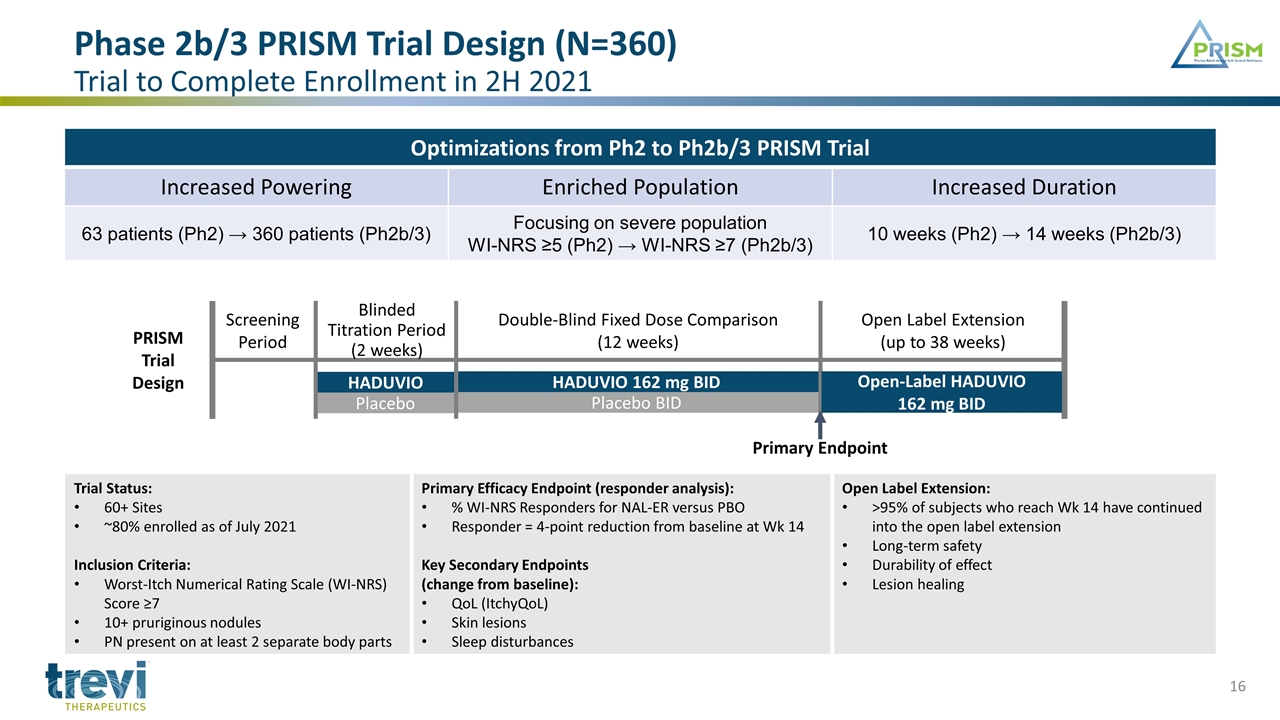
Phase 2b/3 PRISM Trial Design (N=360) Trial to Complete Enrollment in 2H 2021 Optimizations from Ph2 to Ph2b/3 PRISM Trial Increased Powering Enriched Population Increased Duration 63 patients (Ph2) → 360 patients (Ph2b/3) Focusing on severe population WI-NRS ≥5 (Ph2) → WI-NRS ≥7 (Ph2b/3) 10 weeks (Ph2) → 14 weeks (Ph2b/3) HADUVIO Placebo HADUVIO 162 mg BID Placebo BID Screening Period Blinded Titration Period (2 weeks) Double-Blind Fixed Dose Comparison (12 weeks) Open Label Extension (up to 38 weeks) Open-Label HADUVIO 162 mg BID Trial Status: 60+ Sites ~80% enrolled as of July 2021 Inclusion Criteria: Worst-Itch Numerical Rating Scale (WI-NRS) Score ≥7 10+ pruriginous nodules PN present on at least 2 separate body parts Primary Efficacy Endpoint (responder analysis): % WI-NRS Responders for NAL-ER versus PBO Responder = 4-point reduction from baseline at Wk 14 Key Secondary Endpoints (change from baseline): QoL (ItchyQoL) Skin lesions Sleep disturbances Open Label Extension: >95% of subjects who reach Wk 14 have continued into the open label extension Long-term safety Durability of effect Lesion healing Primary Endpoint PRISM Trial Design
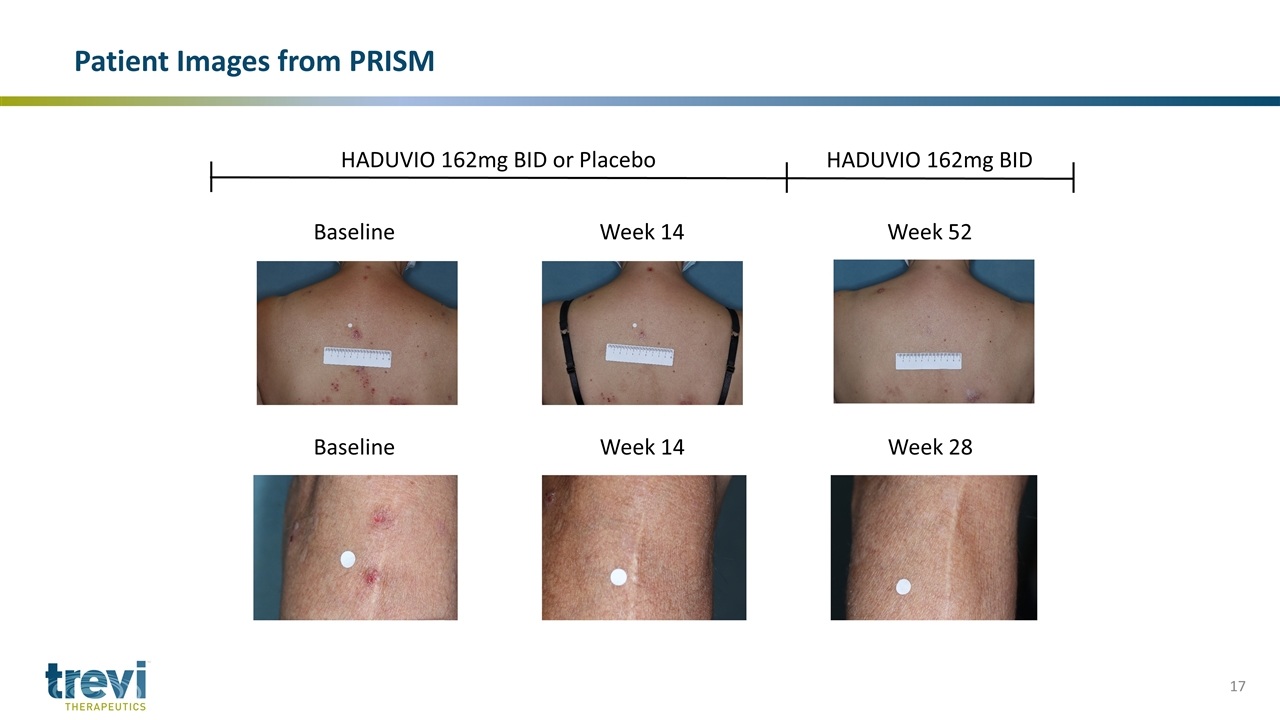
Patient Images from PRISM Baseline Week 14 Week 52 Baseline Week 14 Week 28 HADUVIO 162mg BID or Placebo HADUVIO 162mg BID
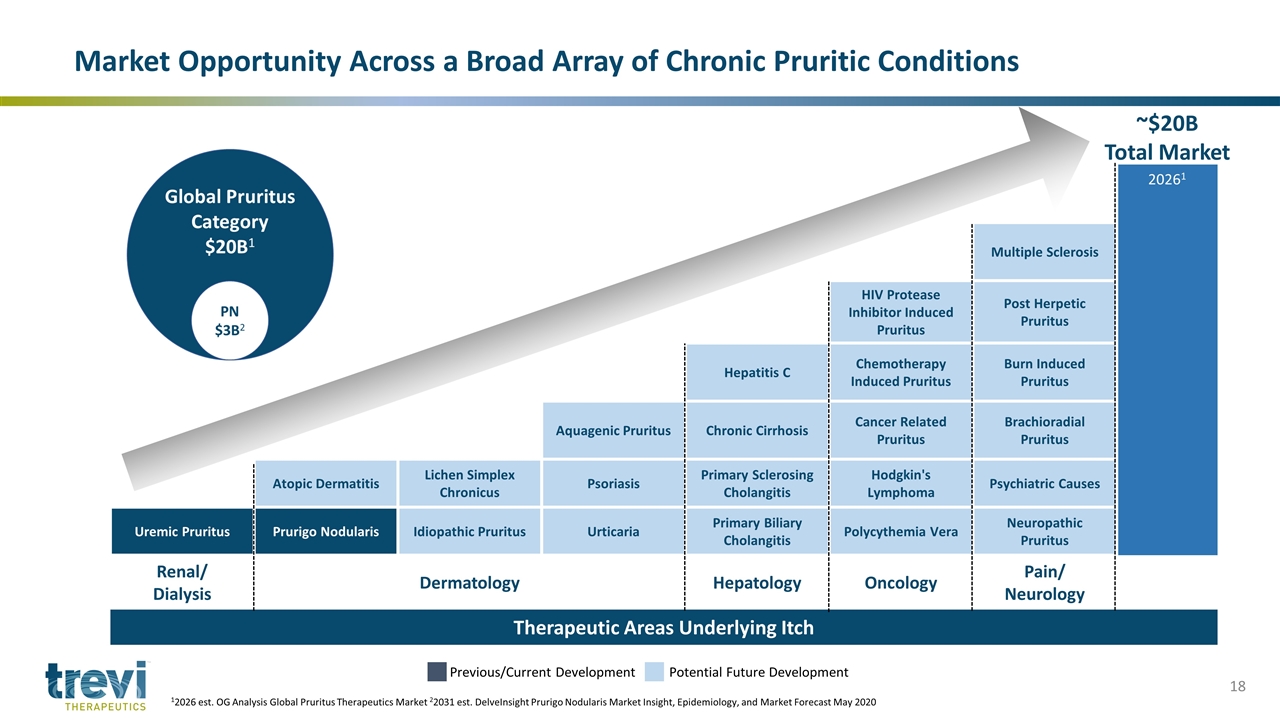
20261 Multiple Sclerosis HIV Protease Inhibitor Induced Pruritus Post Herpetic Pruritus Hepatitis C Chemotherapy Induced Pruritus Burn Induced Pruritus Aquagenic Pruritus Chronic Cirrhosis Cancer Related Pruritus Brachioradial Pruritus Atopic Dermatitis Lichen Simplex Chronicus Psoriasis Primary Sclerosing Cholangitis Hodgkin's Lymphoma Psychiatric Causes Uremic Pruritus Prurigo Nodularis Idiopathic Pruritus Urticaria Primary Biliary Cholangitis Polycythemia Vera Neuropathic Pruritus Renal/ Dialysis Dermatology Hepatology Oncology Pain/ Neurology Therapeutic Areas Underlying Itch Previous/Current Development Potential Future Development Market Opportunity Across a Broad Array of Chronic Pruritic Conditions ~$20B Total Market 12026 est. OG Analysis Global Pruritus Therapeutics Market 22031 est. DelveInsight Prurigo Nodularis Market Insight, Epidemiology, and Market Forecast May 2020
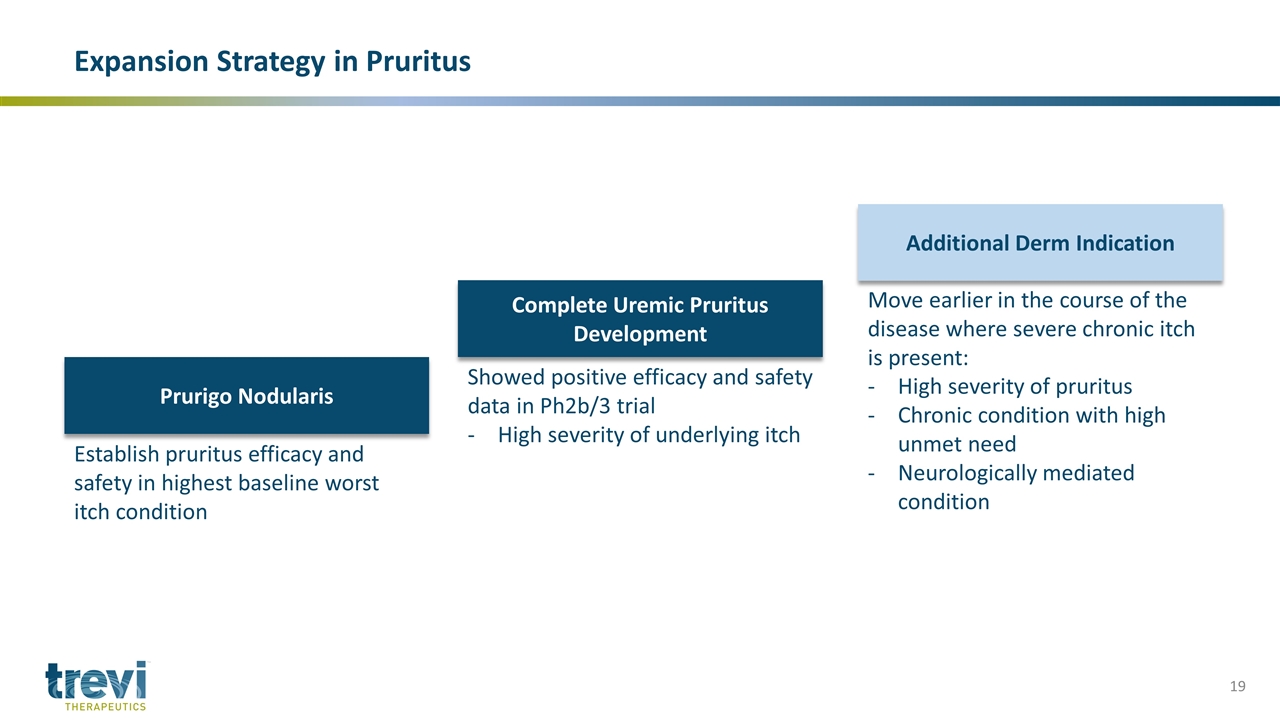
Move earlier in the course of the disease where severe chronic itch is present: High severity of pruritus Chronic condition with high unmet need Neurologically mediated condition Expansion Strategy in Pruritus Prurigo Nodularis Complete Uremic Pruritus Development Additional Derm Indication Establish pruritus efficacy and safety in highest baseline worst itch condition Showed positive efficacy and safety data in Ph2b/3 trial High severity of underlying itch
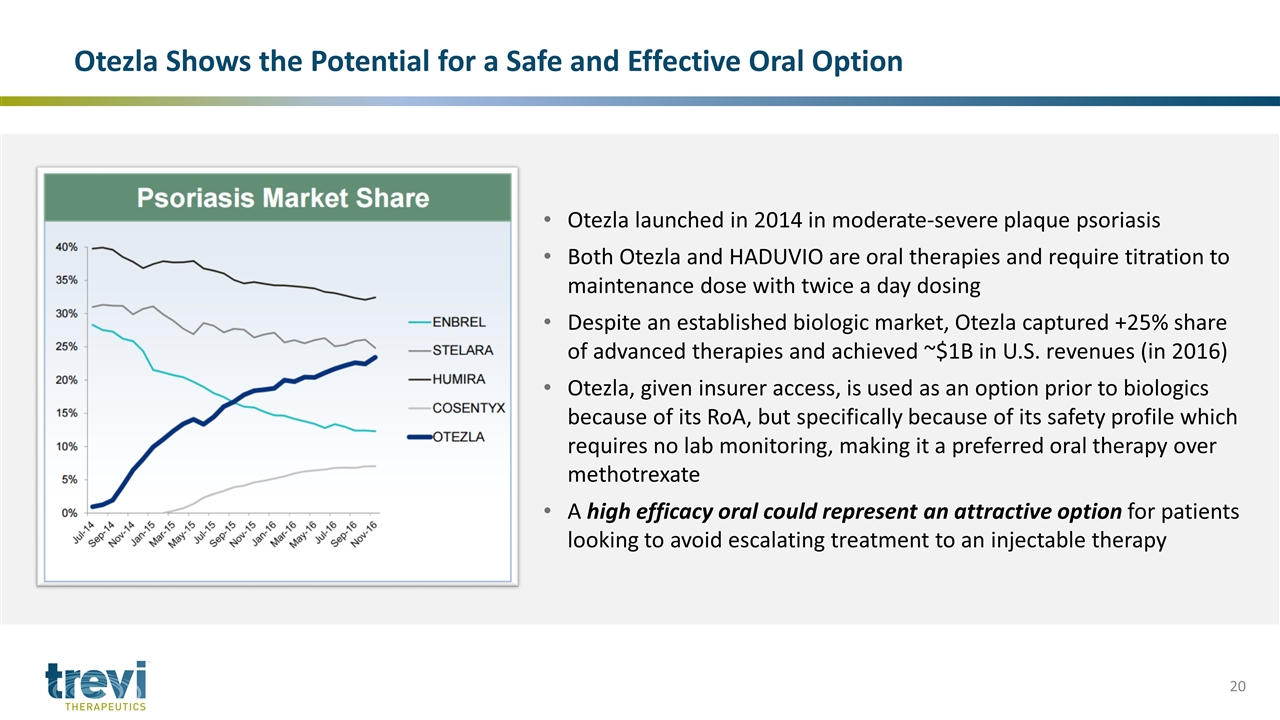
Otezla Shows the Potential for a Safe and Effective Oral Option Otezla launched in 2014 in moderate-severe plaque psoriasis Both Otezla and HADUVIO are oral therapies and require titration to maintenance dose with twice a day dosing Despite an established biologic market, Otezla captured +25% share of advanced therapies and achieved ~$1B in U.S. revenues (in 2016) Otezla, given insurer access, is used as an option prior to biologics because of its RoA, but specifically because of its safety profile which requires no lab monitoring, making it a preferred oral therapy over methotrexate A high efficacy oral could represent an attractive option for patients looking to avoid escalating treatment to an injectable therapy

Chronic Cough in Idiopathic Pulmonary Fibrosis
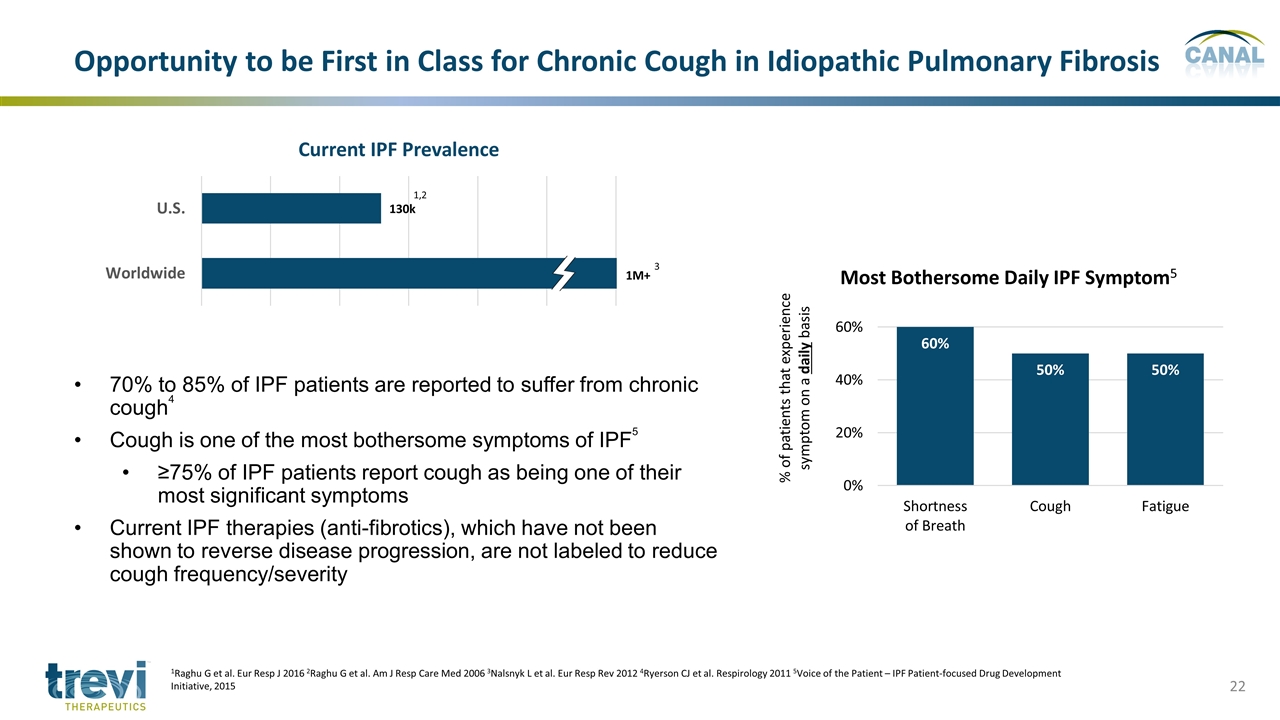
70% to 85% of IPF patients are reported to suffer from chronic cough4 Cough is one of the most bothersome symptoms of IPF5 ≥75% of IPF patients report cough as being one of their most significant symptoms Current IPF therapies (anti-fibrotics), which have not been shown to reverse disease progression, are not labeled to reduce cough frequency/severity Current IPF Prevalence 1M+ 1,2 3 Opportunity to be First in Class for Chronic Cough in Idiopathic Pulmonary Fibrosis 1Raghu G et al. Eur Resp J 2016 2Raghu G et al. Am J Resp Care Med 2006 3Nalsnyk L et al. Eur Resp Rev 2012 4Ryerson CJ et al. Respirology 2011 5Voice of the Patient – IPF Patient-focused Drug Development Initiative, 2015
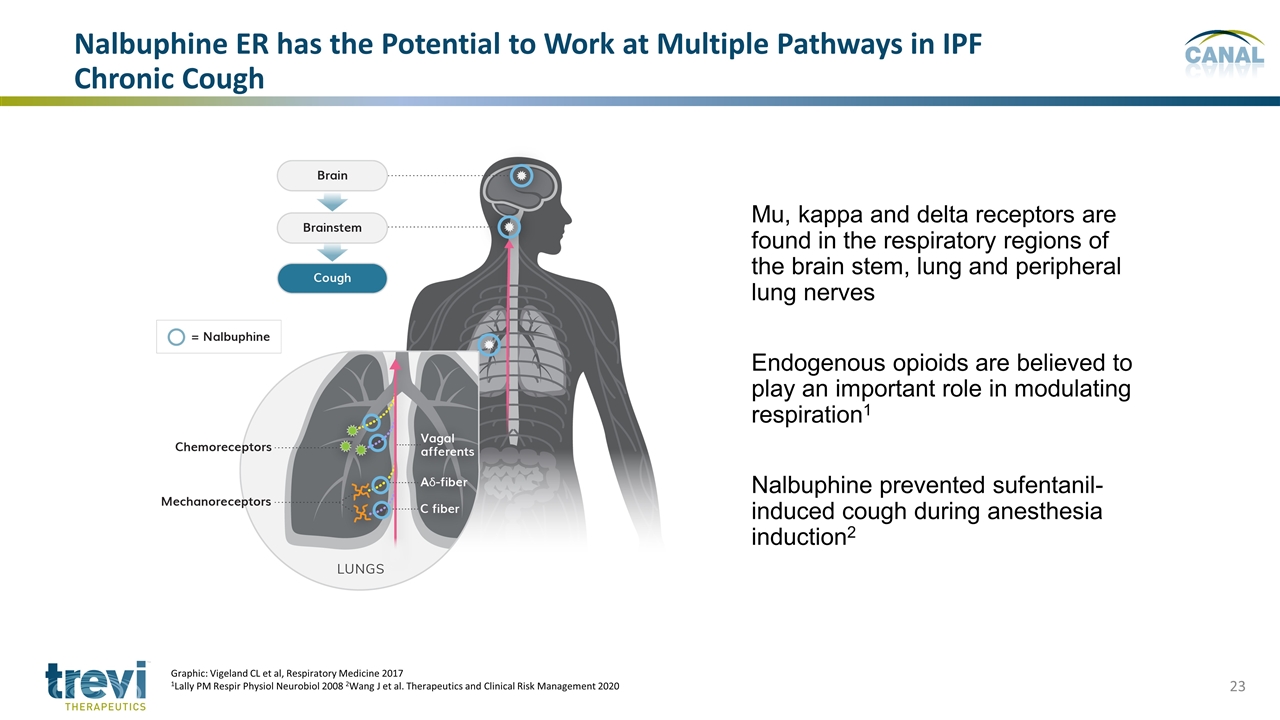
Mu, kappa and delta receptors are found in the respiratory regions of the brain stem, lung and peripheral lung nerves Endogenous opioids are believed to play an important role in modulating respiration1 Nalbuphine prevented sufentanil-induced cough during anesthesia induction2 Nalbuphine ER has the Potential to Work at Multiple Pathways in IPF Chronic Cough Graphic: Vigeland CL et al, Respiratory Medicine 2017 1Lally PM Respir Physiol Neurobiol 2008 2Wang J et al. Therapeutics and Clinical Risk Management 2020
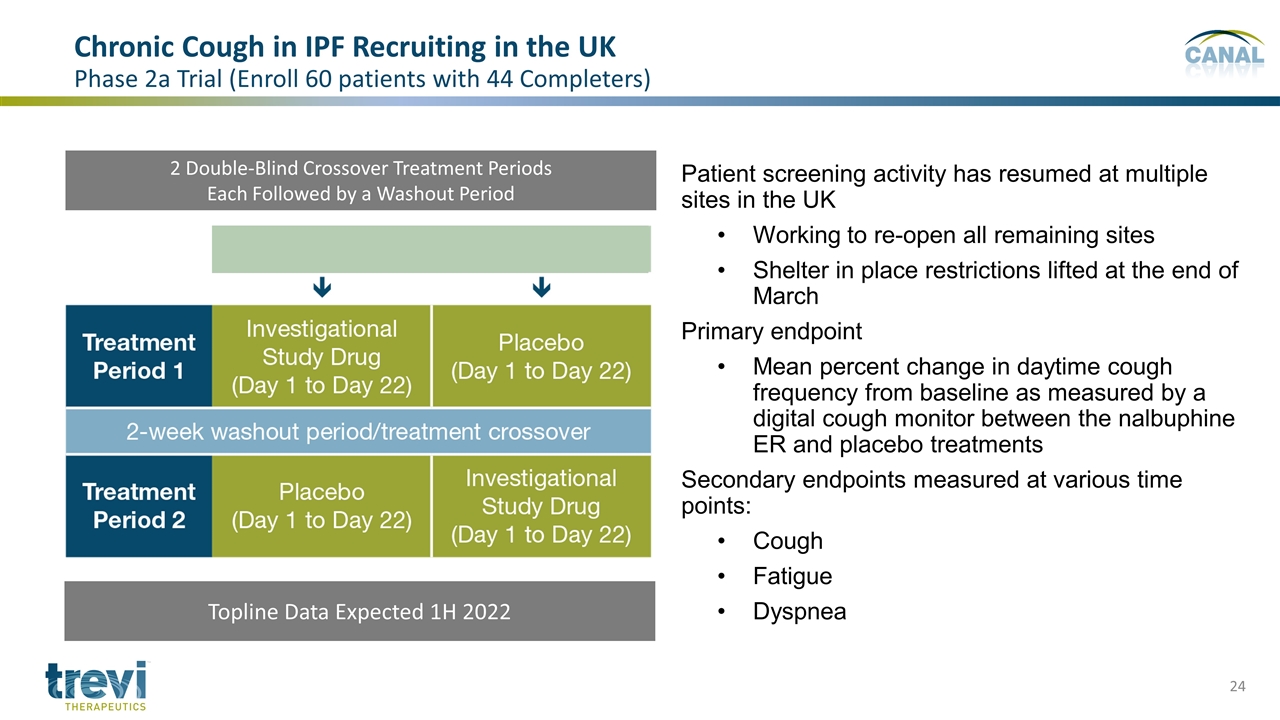
2 Double-Blind Crossover Treatment Periods Each Followed by a Washout Period Patient screening activity has resumed at multiple sites in the UK Working to re-open all remaining sites Shelter in place restrictions lifted at the end of March Primary endpoint Mean percent change in daytime cough frequency from baseline as measured by a digital cough monitor between the nalbuphine ER and placebo treatments Secondary endpoints measured at various time points: Cough Fatigue Dyspnea Chronic Cough in IPF Recruiting in the UK Phase 2a Trial (Enroll 60 patients with 44 Completers) Topline Data Expected 1H 2022
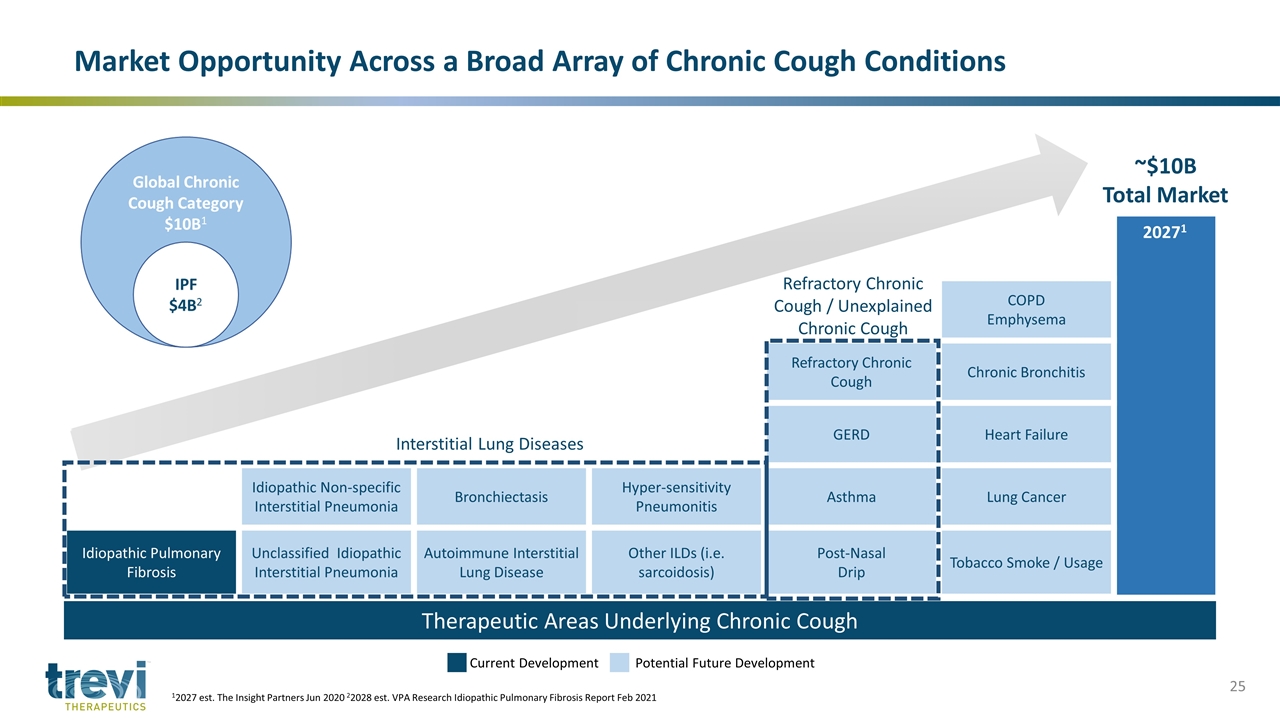
20271 COPD Emphysema Refractory Chronic Cough Chronic Bronchitis GERD Heart Failure Idiopathic Non-specific Interstitial Pneumonia Bronchiectasis Hyper-sensitivity Pneumonitis Asthma Lung Cancer Idiopathic Pulmonary Fibrosis Unclassified Idiopathic Interstitial Pneumonia Autoimmune Interstitial Lung Disease Other ILDs (i.e. sarcoidosis) Post-Nasal Drip Tobacco Smoke / Usage Therapeutic Areas Underlying Chronic Cough Interstitial Lung Diseases Refractory Chronic Cough / Unexplained Chronic Cough Current Development Potential Future Development ~$10B Total Market Market Opportunity Across a Broad Array of Chronic Cough Conditions 12027 est. The Insight Partners Jun 2020 22028 est. VPA Research Idiopathic Pulmonary Fibrosis Report Feb 2021
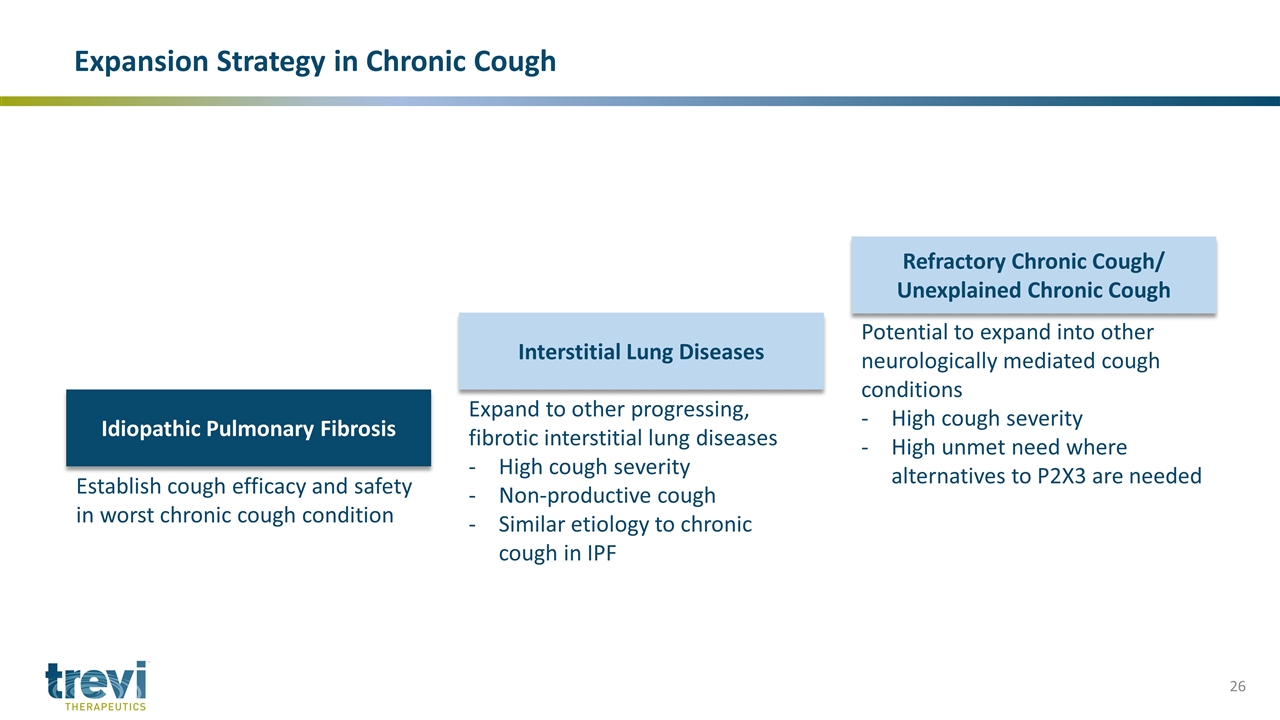
Idiopathic Pulmonary Fibrosis Interstitial Lung Diseases Refractory Chronic Cough/ Unexplained Chronic Cough Establish cough efficacy and safety in worst chronic cough condition Expand to other progressing, fibrotic interstitial lung diseases High cough severity Non-productive cough Similar etiology to chronic cough in IPF Potential to expand into other neurologically mediated cough conditions High cough severity High unmet need where alternatives to P2X3 are needed Expansion Strategy in Chronic Cough
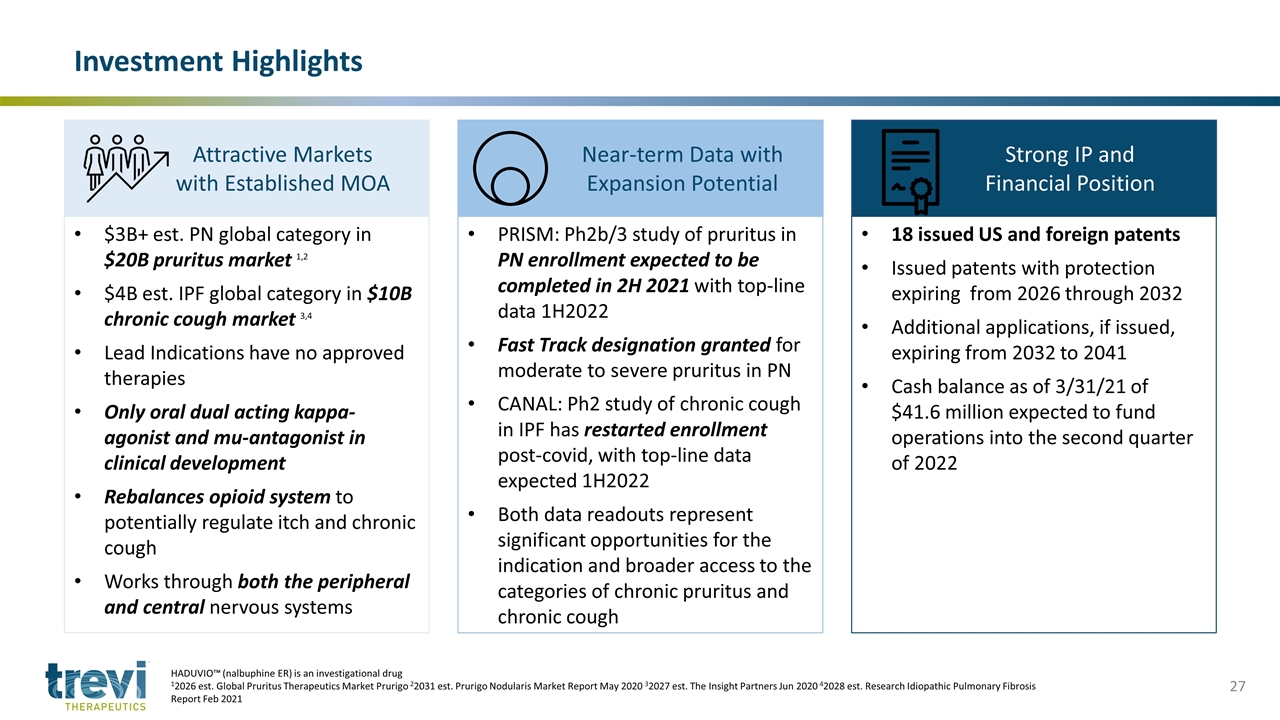
Investment Highlights HADUVIO™ (nalbuphine ER) is an investigational drug 12026 est. Global Pruritus Therapeutics Market Prurigo 22031 est. Prurigo Nodularis Market Report May 2020 32027 est. The Insight Partners Jun 2020 42028 est. Research Idiopathic Pulmonary Fibrosis Report Feb 2021 Attractive Markets with Established MOA Near-term Data with Expansion Potential Strong IP and Financial Position $3B+ est. PN global category in $20B pruritus market 1,2 $4B est. IPF global category in $10B chronic cough market 3,4 Lead Indications have no approved therapies Only oral dual acting kappa-agonist and mu-antagonist in clinical development Rebalances opioid system to potentially regulate itch and chronic cough Works through both the peripheral and central nervous systems PRISM: Ph2b/3 study of pruritus in PN enrollment expected to be completed in 2H 2021 with top-line data 1H2022 Fast Track designation granted for moderate to severe pruritus in PN CANAL: Ph2 study of chronic cough in IPF has restarted enrollment post-covid, with top-line data expected 1H2022 Both data readouts represent significant opportunities for the indication and broader access to the categories of chronic pruritus and chronic cough 18 issued US and foreign patents Issued patents with protection expiring from 2026 through 2032 Additional applications, if issued, expiring from 2032 to 2041 Cash balance as of 3/31/21 of $41.6 million expected to fund operations into the second quarter of 2022

Thank You



























