
AK006 Phase 1 CSU Study Results �& Business Update January 27, 2025 Exhibit 99.1

Disclaimer This presentation contains forward-looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding the expectations of Allakos Inc. (“Allakos,” the “Company,” “we” or “our”) regarding its financial position and guidance, including estimated costs of restructuring activities to closeout AK006 development, the timing of payment of such restructuring costs, and estimated ending 2024 and second quarter 2025 cash, cash equivalents and investments; restructuring activities and plans; and exploration of strategic alternatives, are forward-looking statements. Allakos has based these forward-looking statements on its estimates and assumptions and its current expectations and projections about future events. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this presentation speak only as of the date of this presentation and are subject to a number of risks, uncertainties, and assumptions, including, but not limited to: uncertainties related to Allakos’ ability to realize the contemplated benefits of its restructuring activities and related reduction in force; Allakos’ ability to accurately forecast financial results, including restructuring and other costs and expenses; availability of suitable third parties with which to conduct contemplated strategic alternative transactions; whether Allakos will be able to pursue strategic alternative transactions, or whether any transaction, if pursued, will be completed on attractive terms; whether Allakos’ cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; Allakos’ ability to maintain the listing of its common stock on Nasdaq; general economic and market conditions, both domestic and international; risks associated with volatility and uncertainty in the capital markets for biotechnology companies; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” included in our periodic filings that we have made and will make with the Securities and Exchange Commission (“SEC”). In addition, Allakos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Allakos’ management to predict all risks, nor can Allakos assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements that Allakos may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Allakos does not undertake any obligation to update or revise any forward-looking statements, to conform these statements to actual results or to make changes in Allakos’ expectations, except as required by law. Additional Information: The Company has filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about the Company. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated.
Agenda Robert Alexander, CEO Introduction Chin Lee, CMO AK006 Phase 1 CSU Study Results Baird Radford, CFO Restructuring and Financial Implications Q&A

Phase 1 Study of AK006 in �Chronic Spontaneous Urticaria
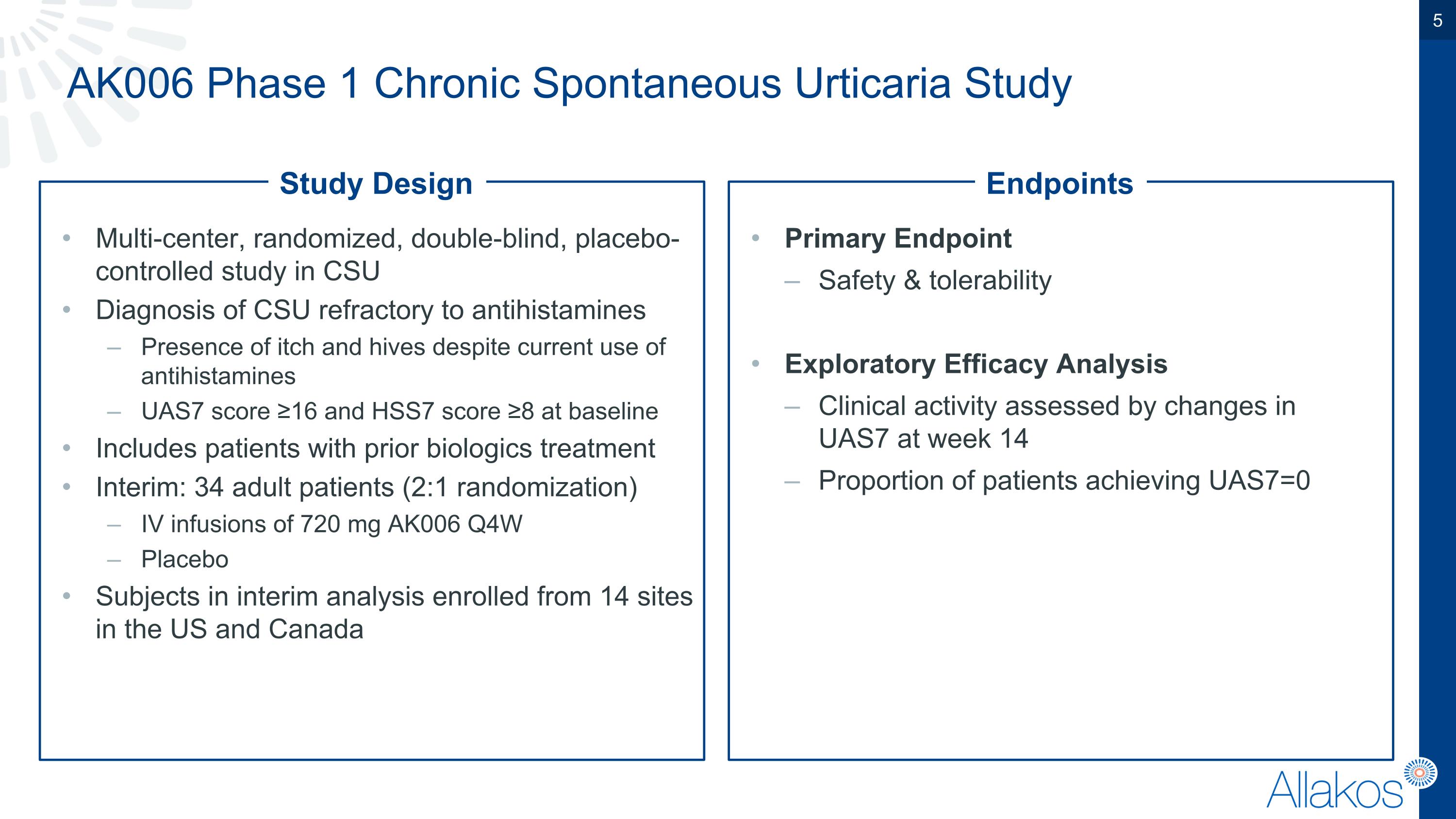
AK006 Phase 1 Chronic Spontaneous Urticaria Study Multi-center, randomized, double-blind, placebo-controlled study in CSU Diagnosis of CSU refractory to antihistamines Presence of itch and hives despite current use of antihistamines UAS7 score ≥16 and HSS7 score ≥8 at baseline Includes patients with prior biologics treatment Interim: 34 adult patients (2:1 randomization) IV infusions of 720 mg AK006 Q4W Placebo Subjects in interim analysis enrolled from 14 sites in the US and Canada Primary Endpoint Safety & tolerability Exploratory Efficacy Analysis Clinical activity assessed by changes in UAS7 at week 14 Proportion of patients achieving UAS7=0 Study Design Endpoints
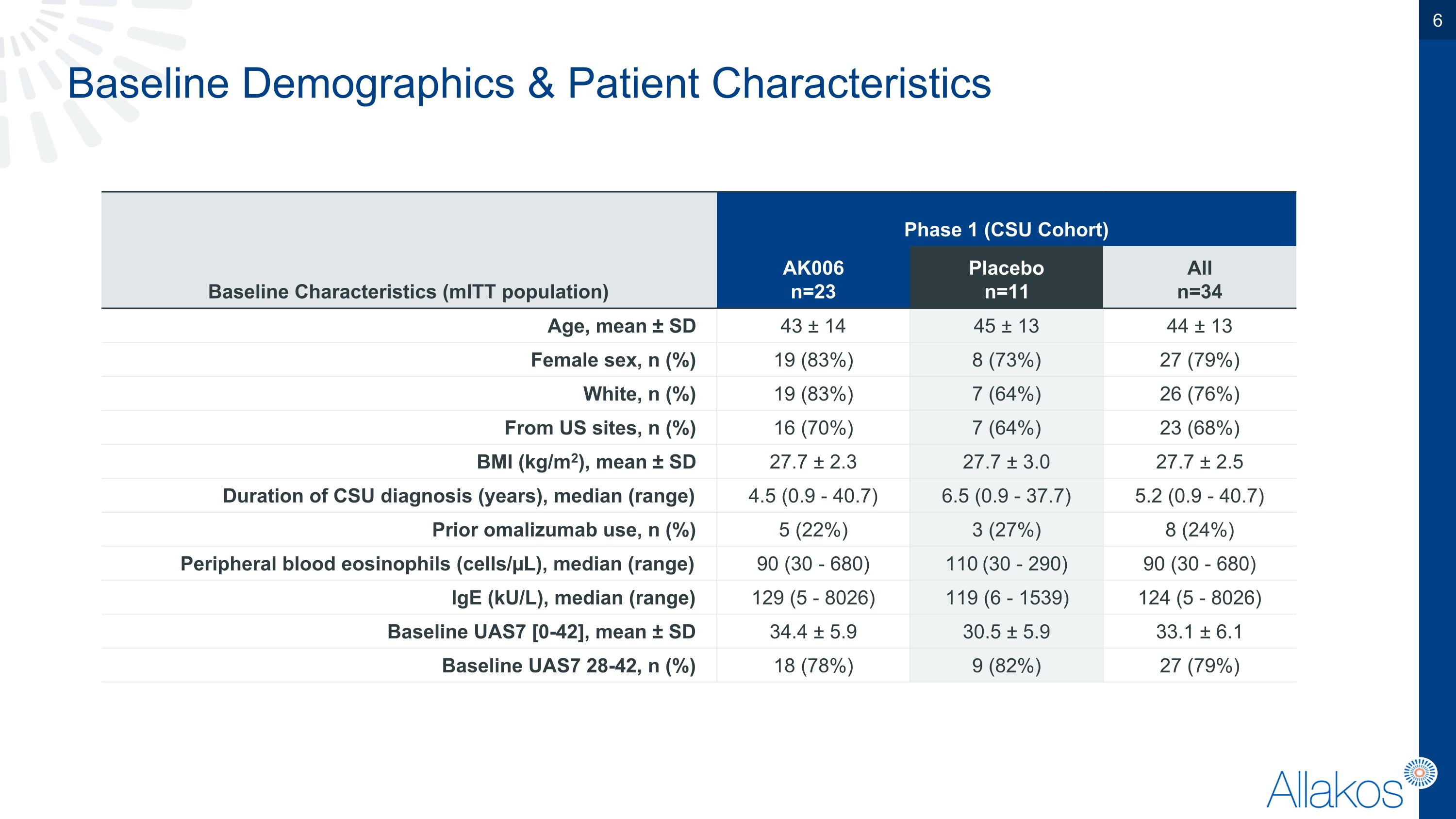
Baseline Demographics & Patient Characteristics Baseline Characteristics (mITT population) Phase 1 (CSU Cohort) Patient Characteristics AK006 n=23 Placebo n=11 All n=34 Age, mean ± SD 43 ± 14 45 ± 13 44 ± 13 Female sex, n (%) 19 (83%) 8 (73%) 27 (79%) White, n (%) 19 (83%) 7 (64%) 26 (76%) From US sites, n (%) 16 (70%) 7 (64%) 23 (68%) BMI (kg/m2), mean ± SD 27.7 ± 2.3 27.7 ± 3.0 27.7 ± 2.5 Duration of CSU diagnosis (years), median (range) 4.5 (0.9 - 40.7) 6.5 (0.9 - 37.7) 5.2 (0.9 - 40.7) Prior omalizumab use, n (%) 5 (22%) 3 (27%) 8 (24%) Peripheral blood eosinophils (cells/µL), median (range) 90 (30 - 680) 110 (30 - 290) 90 (30 - 680) IgE (kU/L), median (range) 129 (5 - 8026) 119 (6 - 1539) 124 (5 - 8026) Baseline UAS7 [0-42], mean ± SD 34.4 ± 5.9 30.5 ± 5.9 33.1 ± 6.1 Baseline UAS7 28-42, n (%) 18 (78%) 9 (82%) 27 (79%)
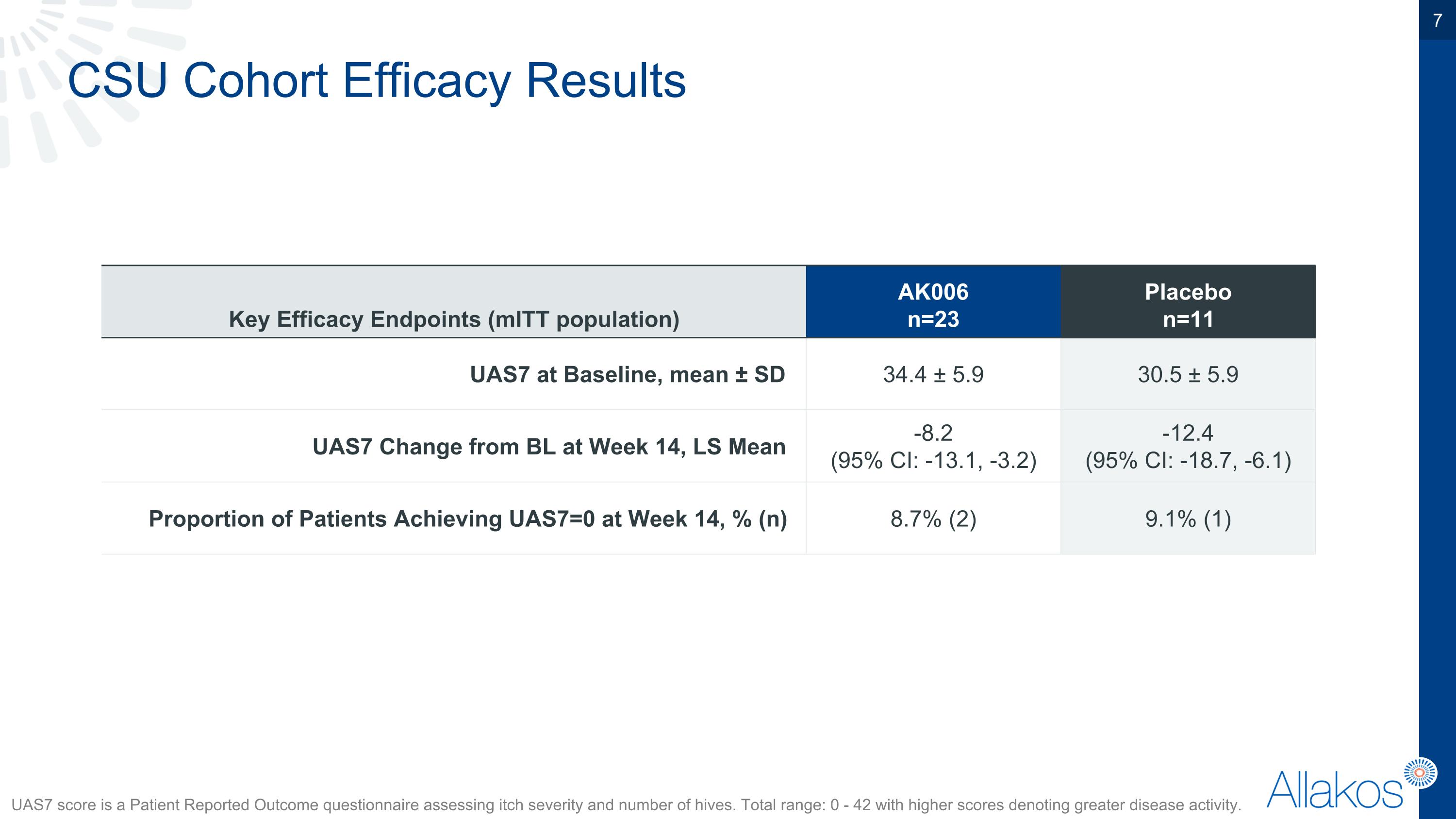
CSU Cohort Efficacy Results UAS7 score is a Patient Reported Outcome questionnaire assessing itch severity and number of hives. Total range: 0 - 42 with higher scores denoting greater disease activity. Key Efficacy Endpoints (mITT population) AK006 n=23 Placebo n=11 UAS7 at Baseline, mean ± SD 34.4 ± 5.9 30.5 ± 5.9 UAS7 Change from BL at Week 14, LS Mean -8.2 (95% CI: -13.1, -3.2) -12.4 (95% CI: -18.7, -6.1) Proportion of Patients Achieving UAS7=0 at Week 14, % (n) 8.7% (2) 9.1% (1)
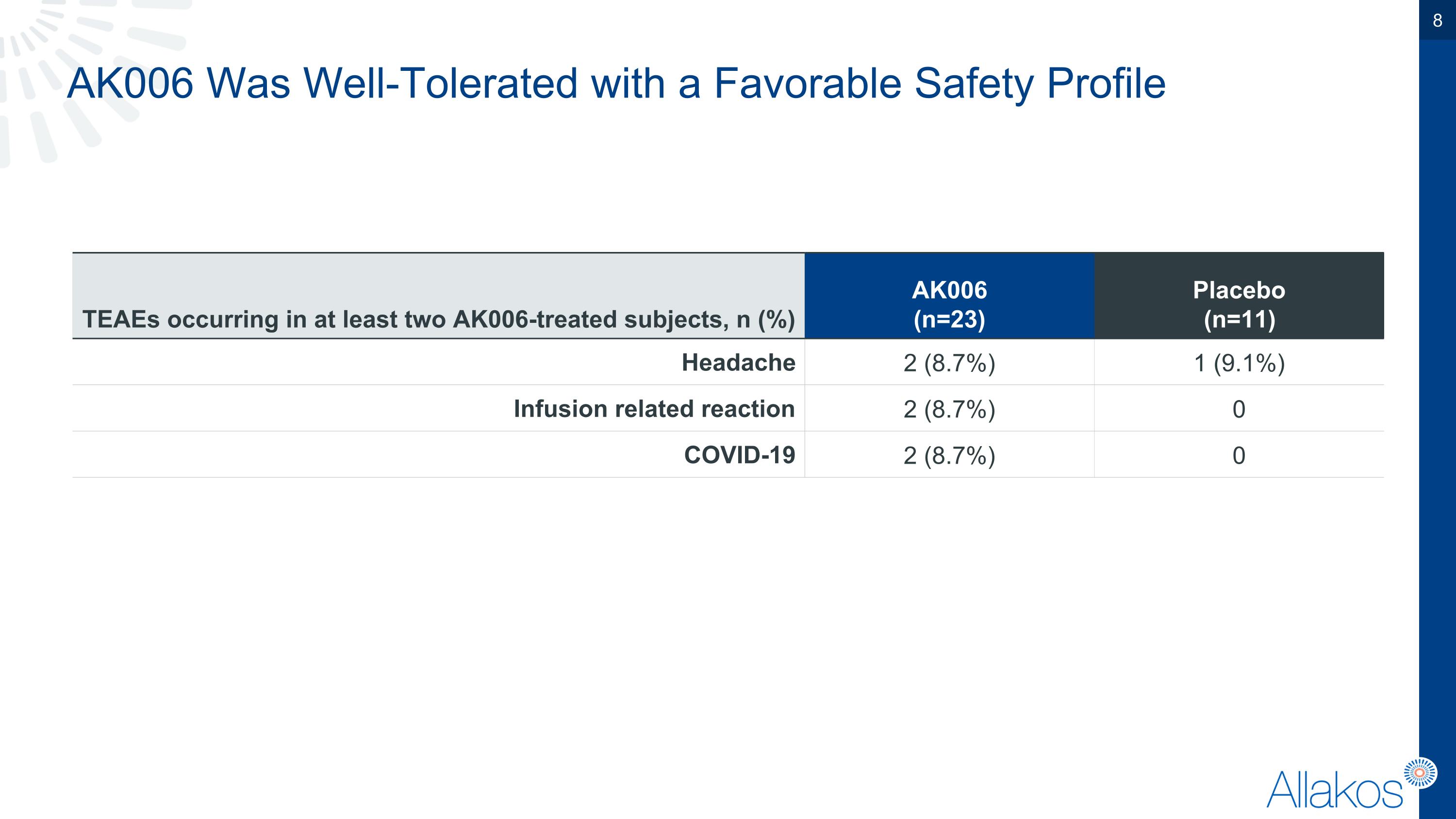
AK006 Was Well-Tolerated with a Favorable Safety Profile TEAEs occurring in at least two AK006-treated subjects, n (%) AK006 (n=23) Placebo (n=11) Headache 2 (8.7%) 1 (9.1%) Infusion related reaction 2 (8.7%) 0 COVID-19 2 (8.7%) 0

Business Update

Restructuring Activities and Planned Actions The Company plans to discontinue AK006-related activities across clinical, manufacturing, research and administrative functions and reduce its workforce �by approximately 75%. The Company plans to retain approximately 15 employees to explore strategic alternatives, maintain compliance with regulatory and financial reporting requirements, and wind-down the phase 1 clinical study.

Cash Guidance The Company ended the fourth quarter of 2024 with approximately $81 million in cash, cash equivalents, and investments (unaudited). The Company estimates that cash used in restructuring activities to closeout AK006 development, including severance and contractual payments to vendors will be approximately $34 million to $38 million. The Company also estimates that a significant majority of these restructuring costs will be paid over the first and second quarters of 2025. The Company estimates it will have cash, cash equivalents and investments in a range of approximately $35 million to $40 million at June 30, 2025.

Q&A Confidential

Thank You












