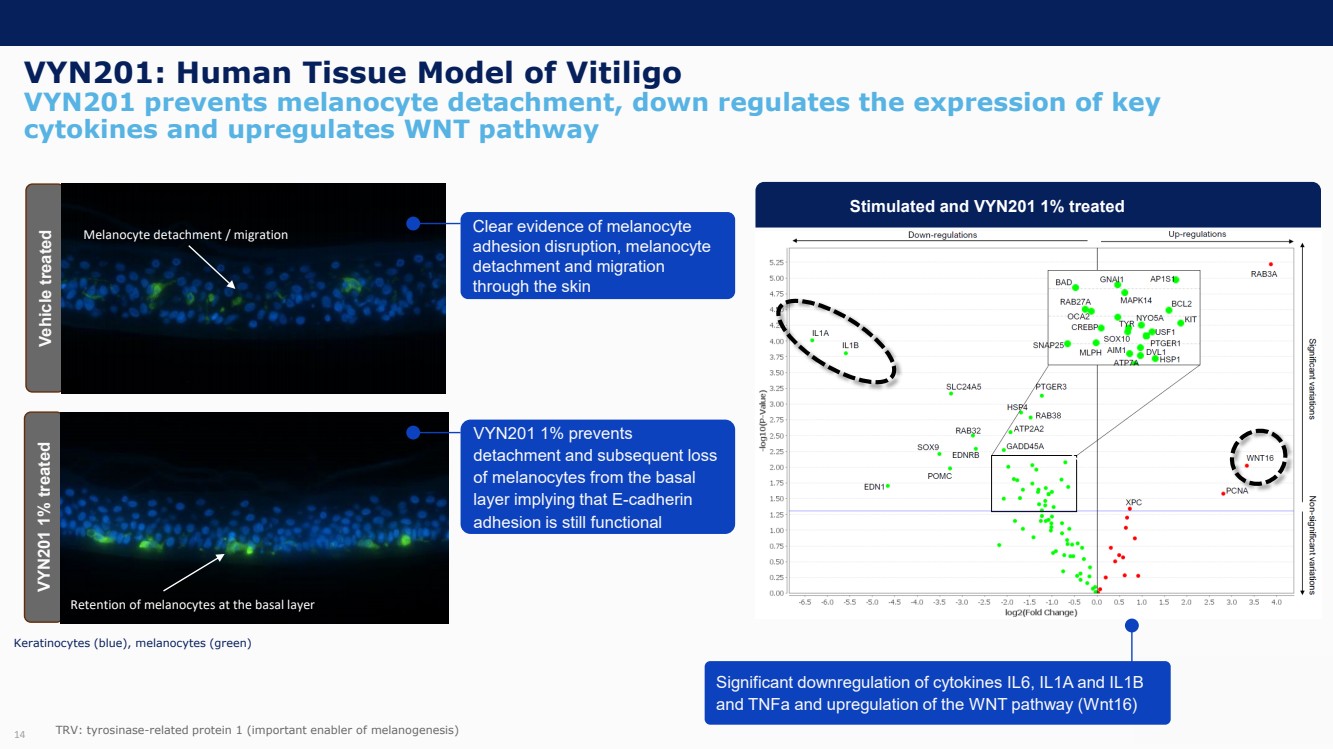
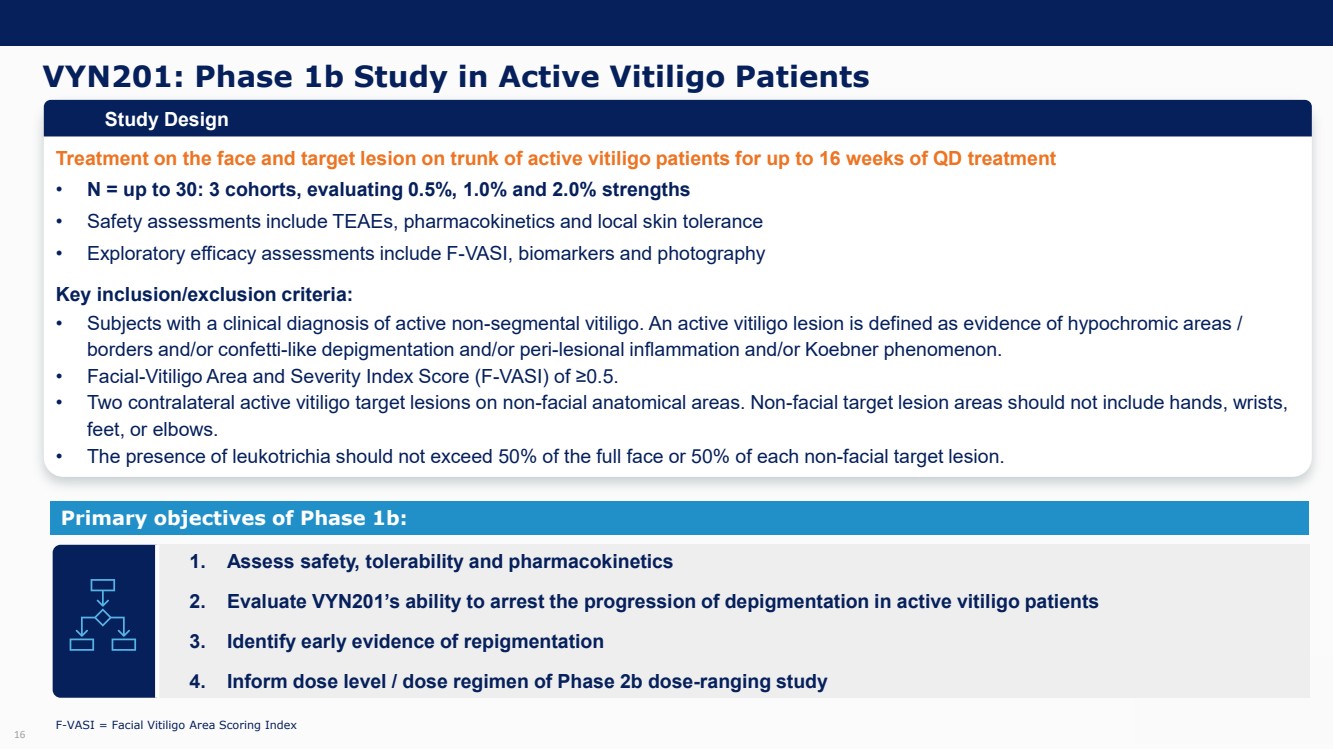
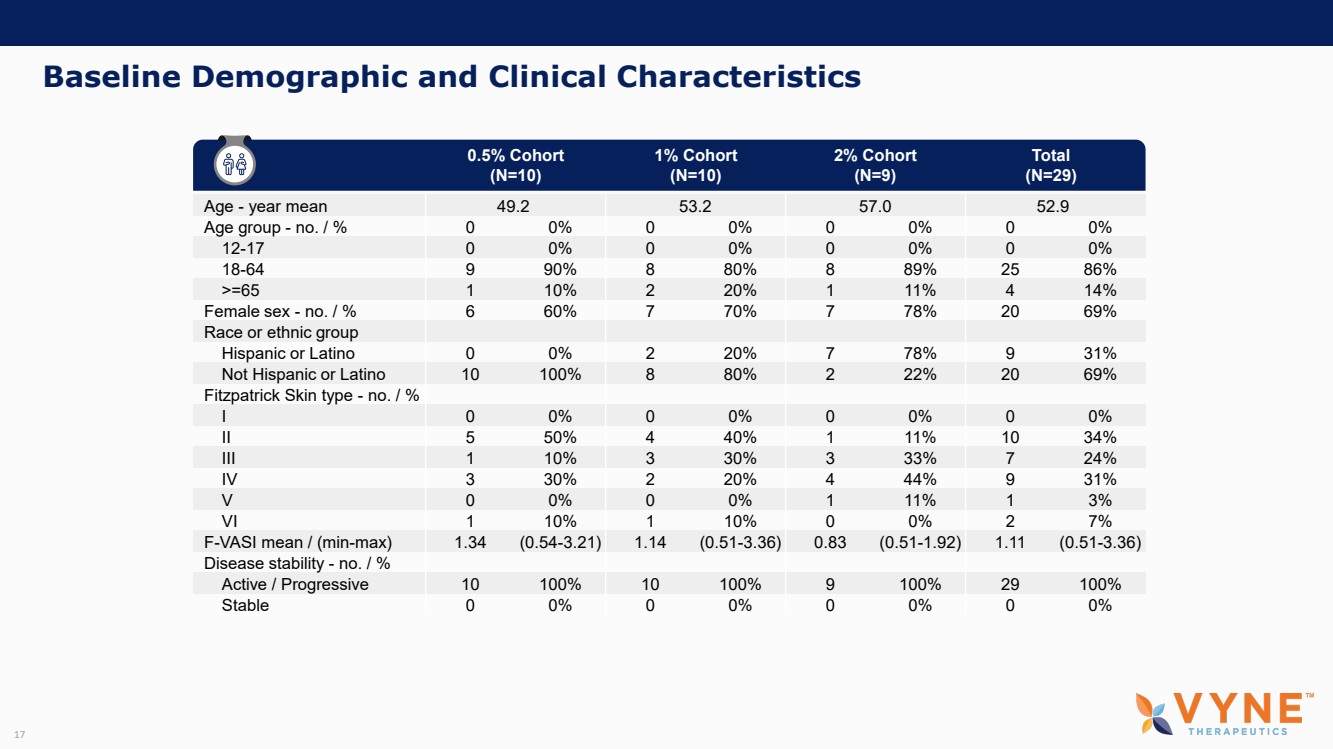
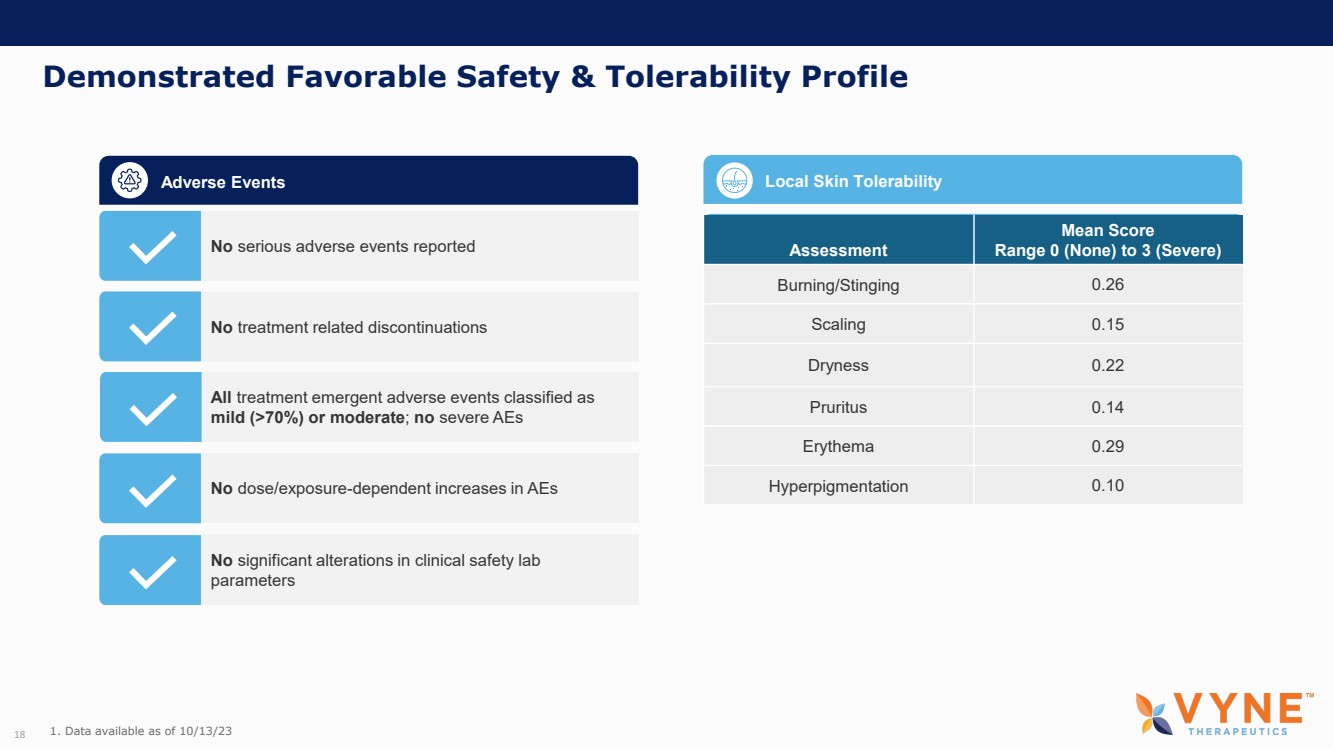
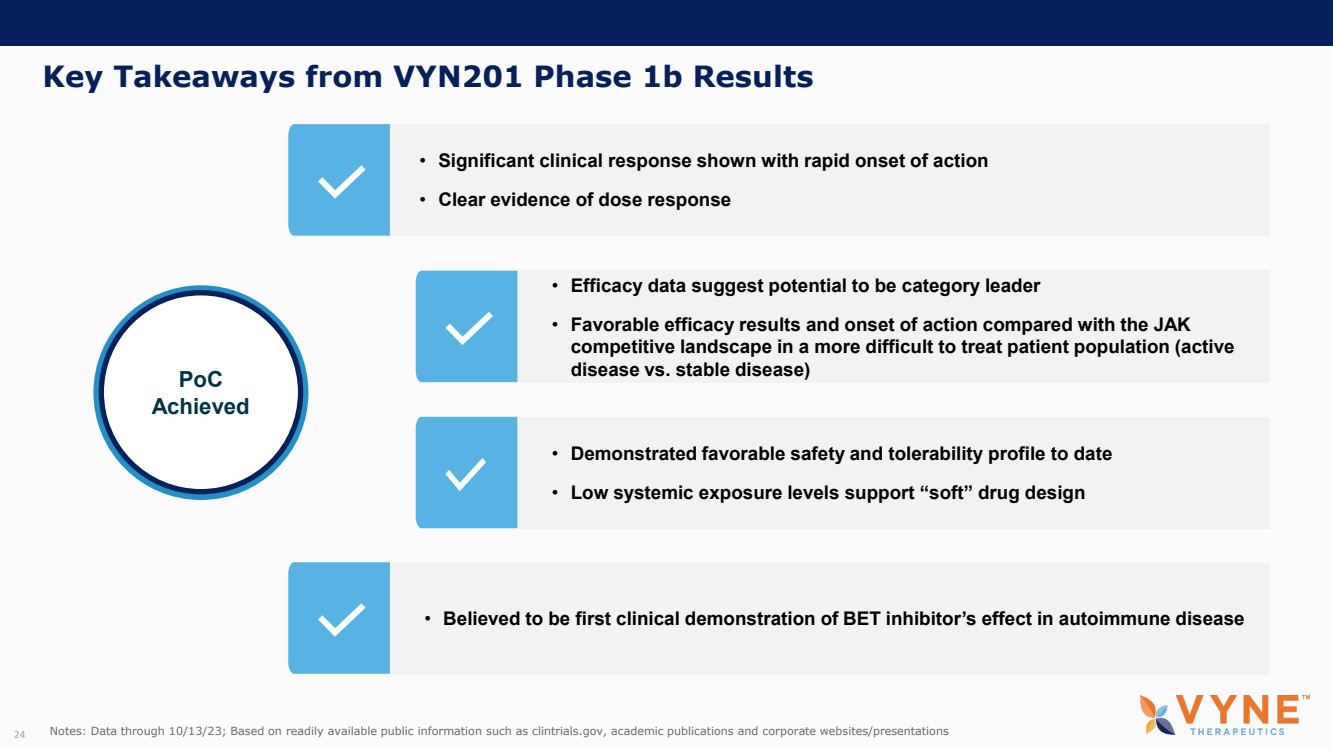
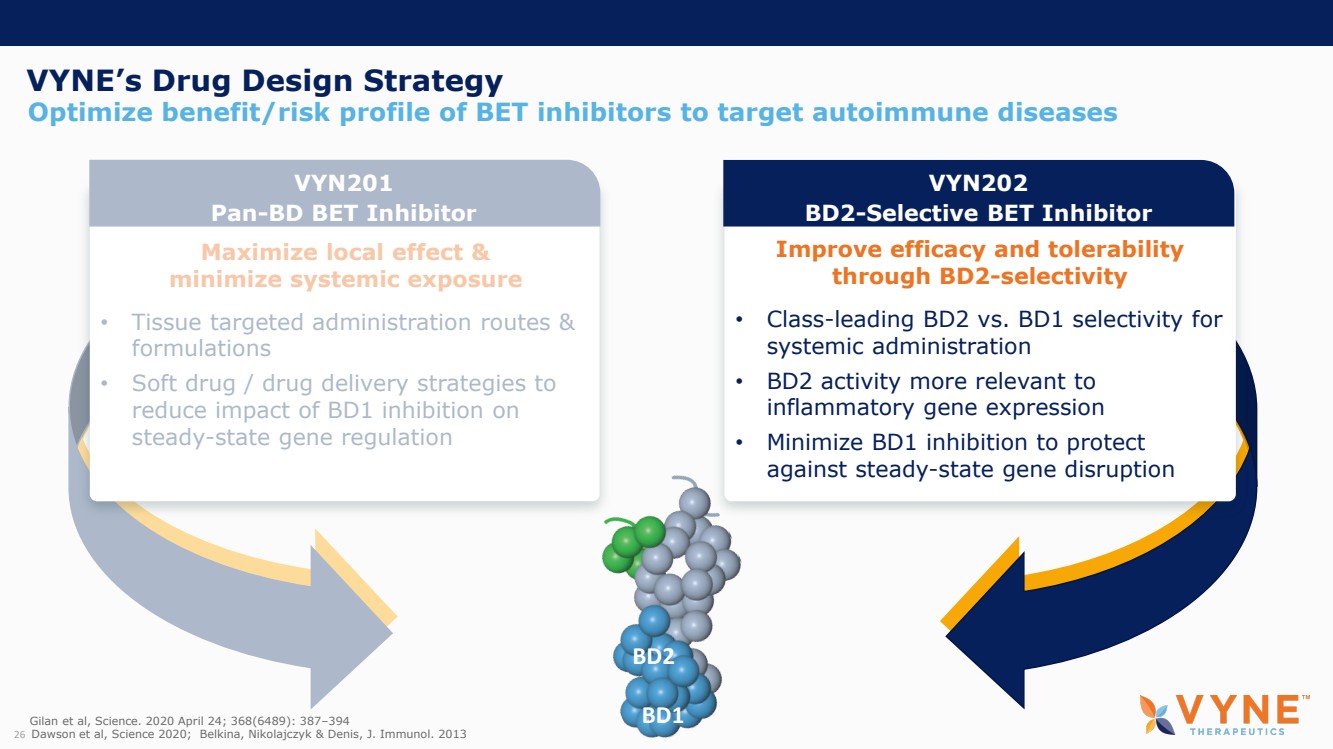
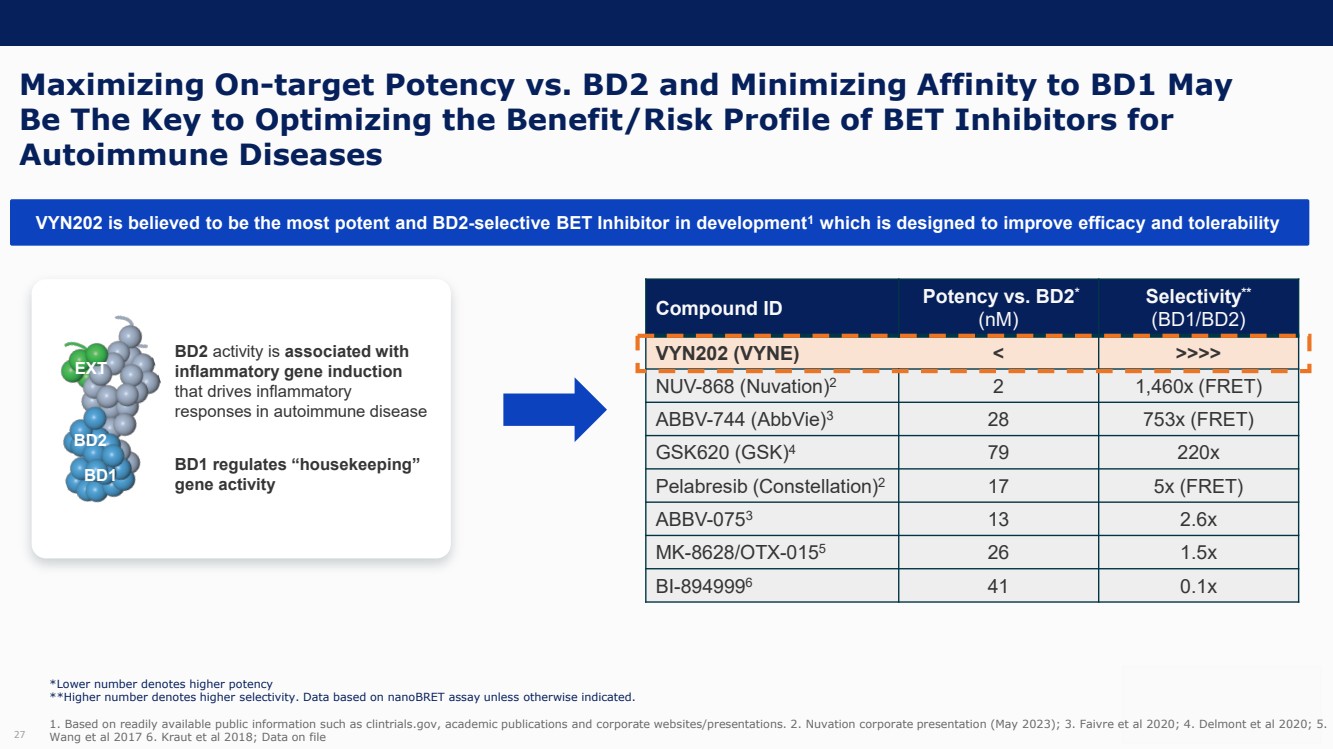
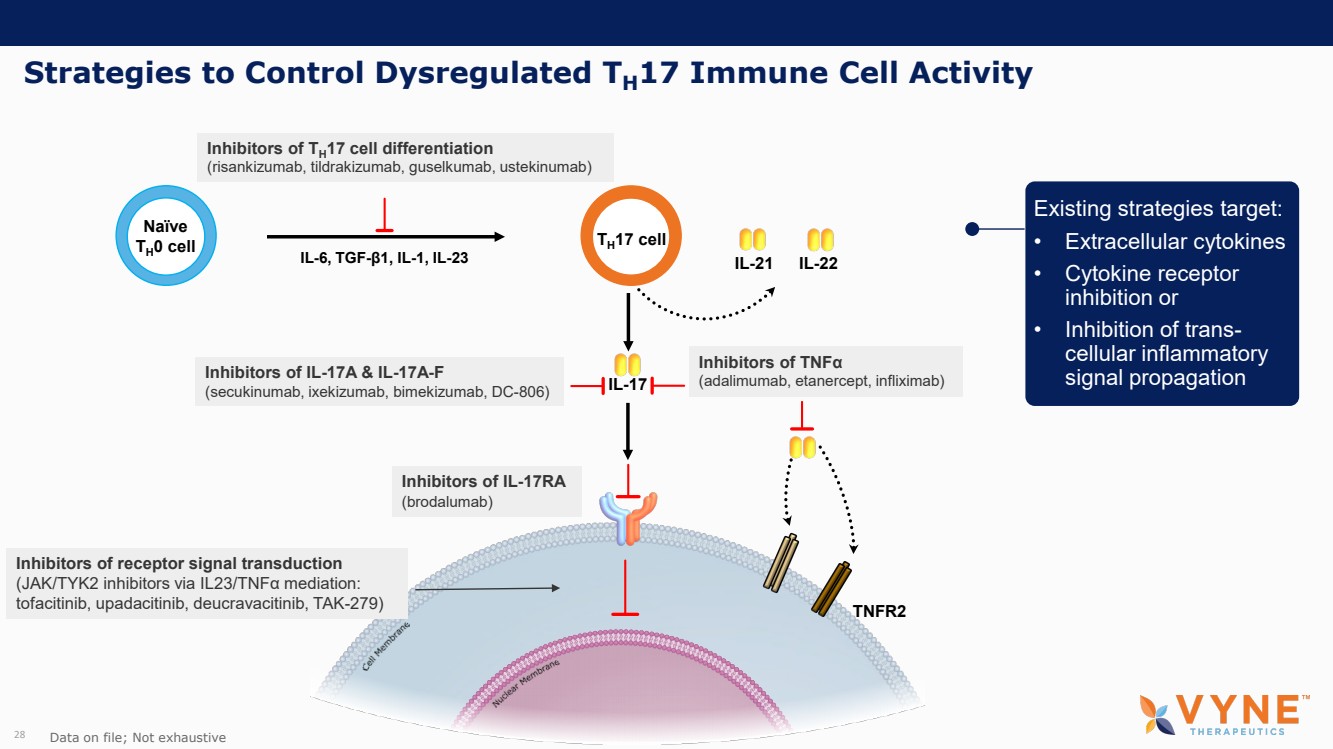
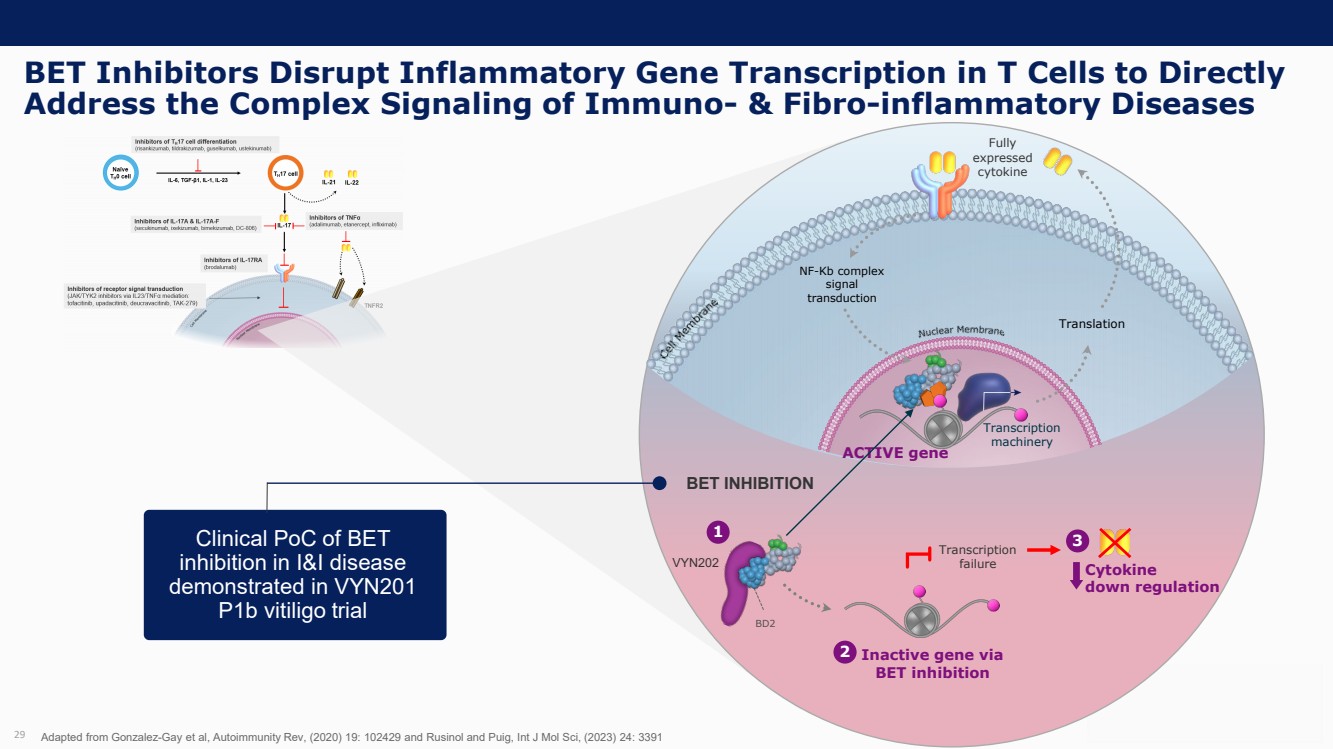
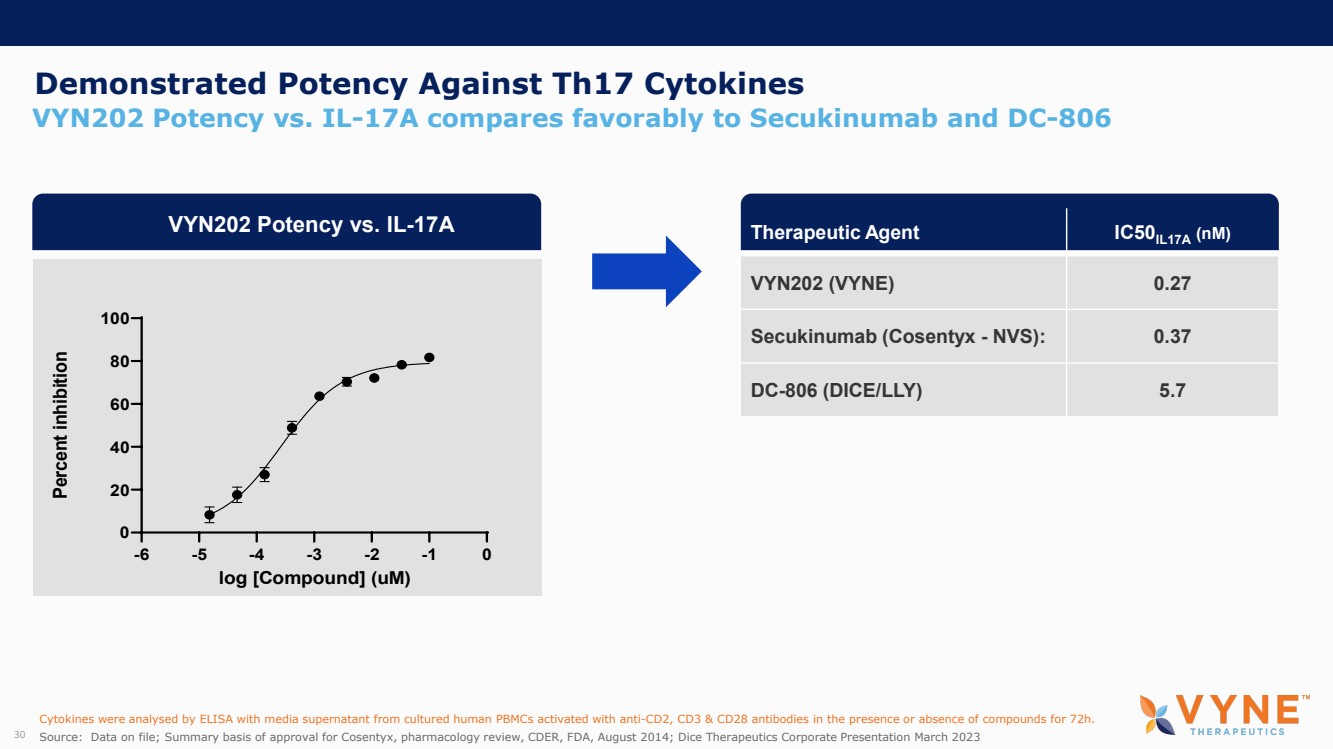
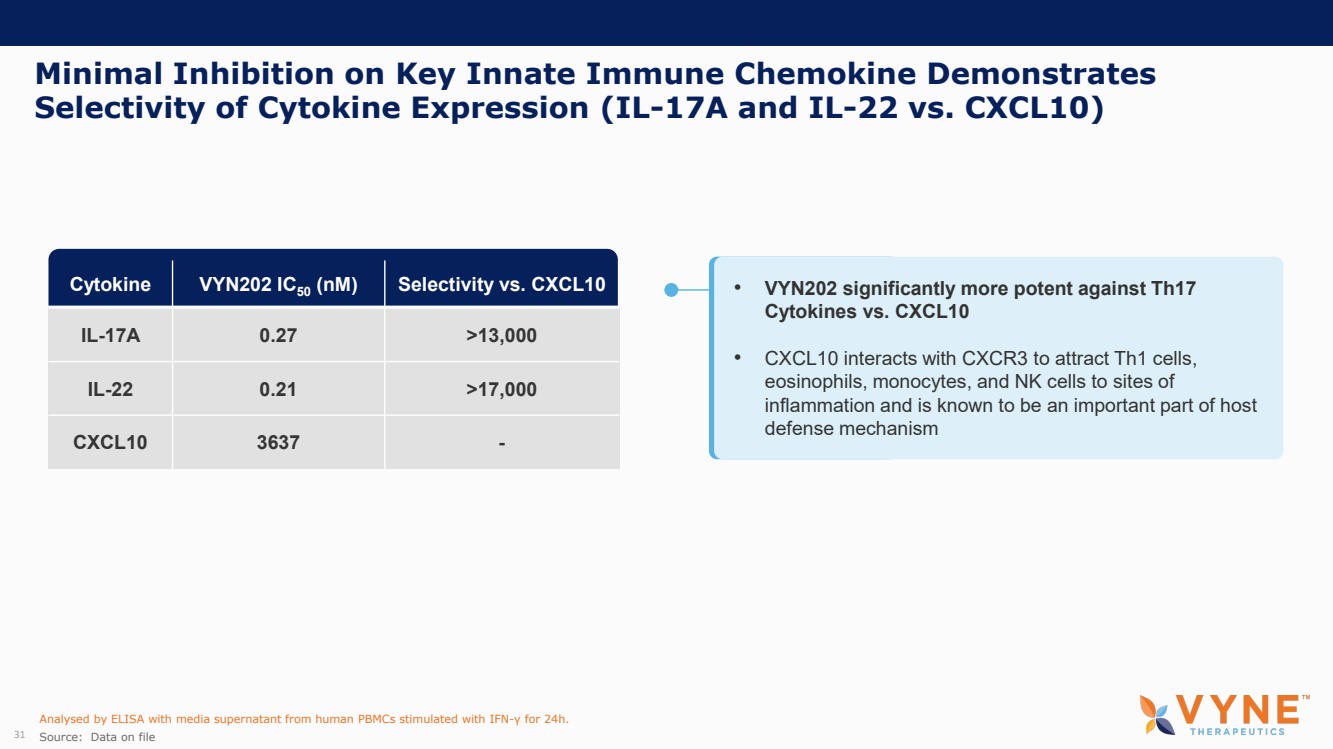
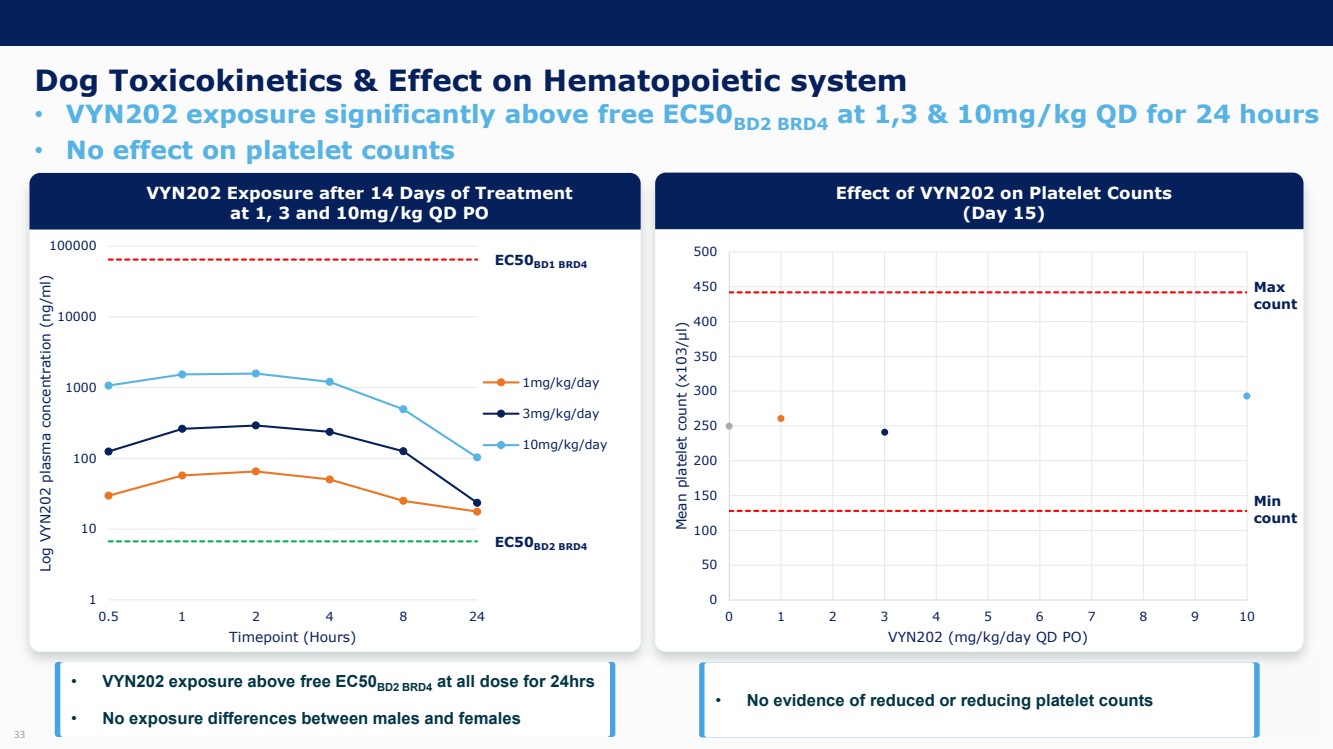
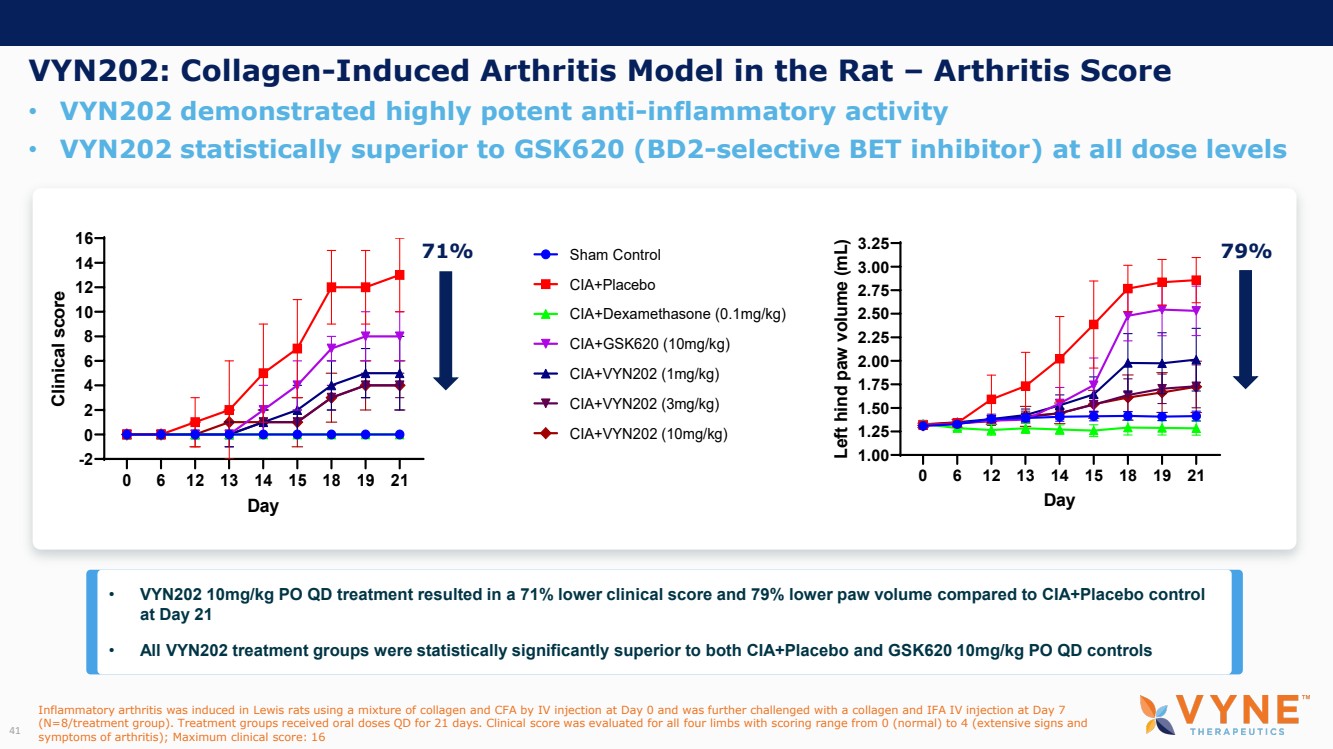
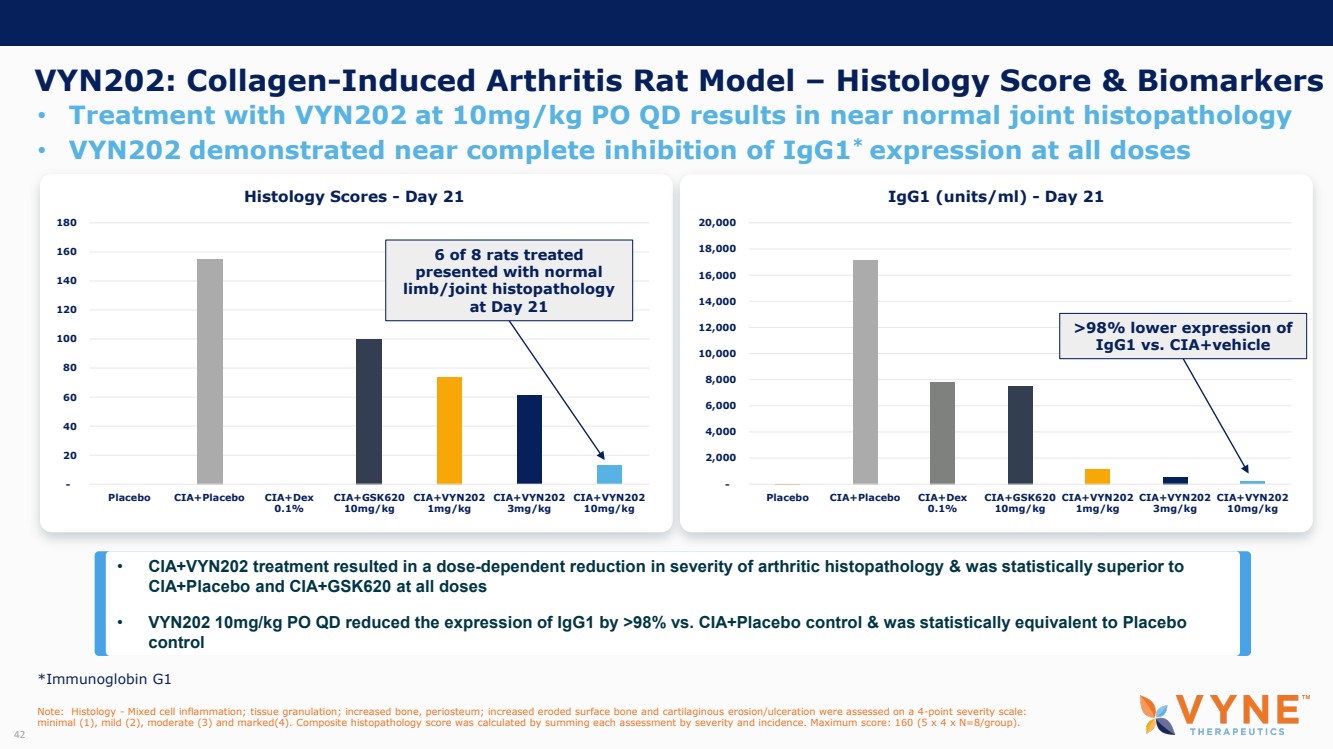
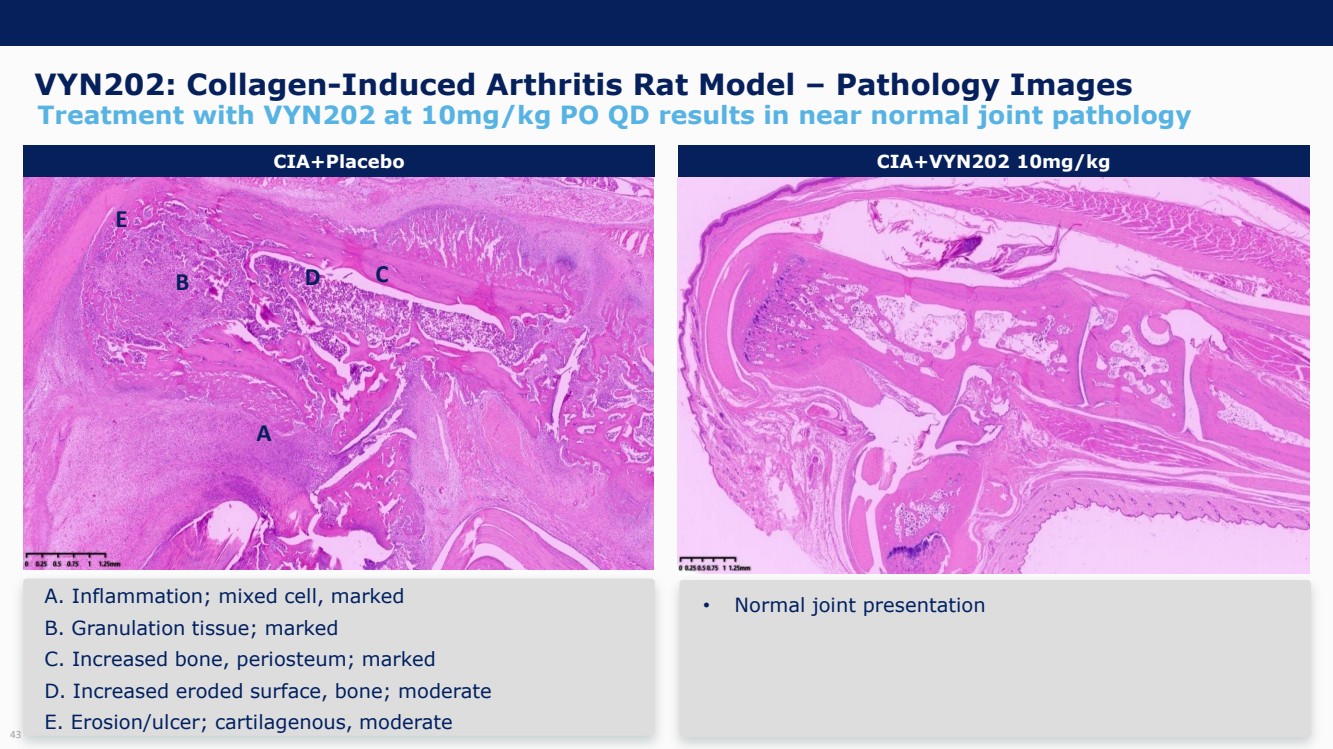
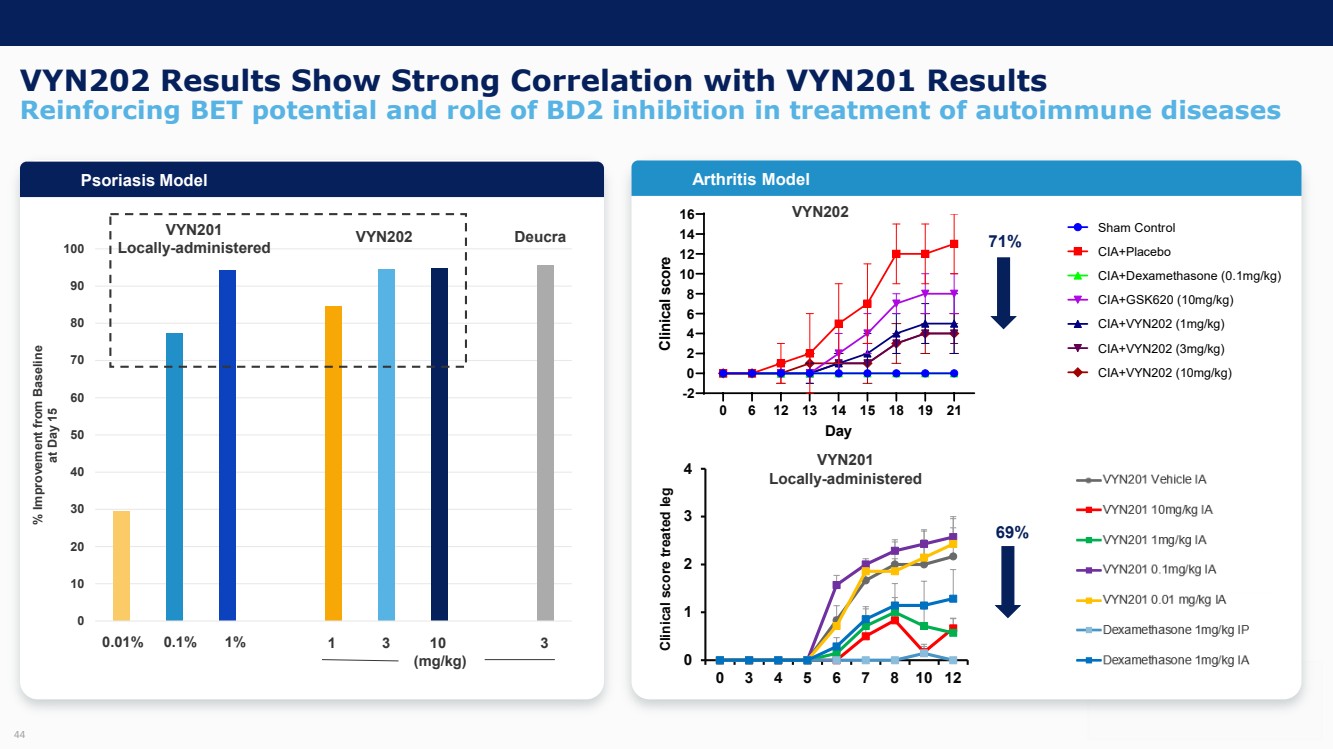
| Forward Looking Statements and Important Notes This presentation by VYNE Therapeutics Inc ..(" includes forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 including, but not limited to, statements regarding VYNE's plans, regulatory filings and development timelines for VYN 201 and VYN 202 VYNE's InhiBET platform VYNE's cash runway through the end of 2025 and other statements regarding the future expectations, plans and prospects of VYNE All statements in this presentation which are not historical facts are forward looking statements Any forward looking statements are based on VYNE's current knowledge and its present beliefs and expectations regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially and adversely from those set forth or implied by such forward looking statements These risks and uncertainties include, but are not limited to VYNE's ability to successfully develop its product candidates the timing of commencement of future preclinical studies and clinical trials VYNE's ability to enroll patients and successfully progress, complete, and receive favorable results in, clinical trials for its product candidates VYNE's ability to comply with various regulations applicable to its business VYNE's ability to create intellectual property and the scope of protection it is able to establish and maintain for intellectual property rights covering its product candidates, including the projected terms of patent protection risks that any of VYNE's patents may be held to be narrowed, invalid or unenforceable or one or more of VYNE's patent applications may not be granted and potential competitors may also seek to design around VYNE's granted patents or patent applications estimates of VYNE's expenses, capital requirements, its needs for additional financing and its ability to obtain additional capital on acceptable terms or at all VYNE's expectations regarding licensing, business transactions and strategic operations VYNE's future financial performance and liquidity and volatility in VYNE's stock price may result in rapid and substantial increases or decreases in the stock price that may or may not be related to VYNE's operating performance or prospects For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE's actual results to differ from those contained in the forward looking statements, see the section titled "Risk Factors" in VYNE's Annual Report on Form 10 K for the year ended December 31 2022 and Quarterly Report on Form 10 Q for the period ended June 30 2023 as well as discussions of potential risks, uncertainties, and other important factors in VYNE's subsequent filings with the U S Securities and Exchange Commission Although VYNE believes these forward looking statements are reasonable, they speak only as of the date of this presentation and VYNE undertakes no obligation to update publicly such forward looking statements to reflect subsequent events or circumstances, except as otherwise required by law Given these risks and uncertainties, you should not rely upon forward looking statements as predictions of future events This presentation includes preliminary clinical data from VYNE's Phase 1 b clinical trial evaluating topical VYN 201 in patients with nonsegmental vitiligo These preliminary data remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data included herein As a result, data should be viewed with caution until the final data are available in November 2023 Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and VYNE's own internal estimates and research While VYNE believes these third party sources to be reliable as of the date of this presentation, VYNE has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third party sources In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions Finally, while VYNE believes its own internal research is reliable, such research has not been verified by any independent source You are cautioned not to give undue weight to any such information, projections and estimates The trademarks included herein are the property of the owners thereof and are used for reference purposes only This presentation concerns product candidates that are under clinical investigation None of such product candidates have been approved for marketing by the FDA or the EMA, and such product candidates are currently limited to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. 2 |