Exhibit 99.2

VYN202 P1a SAD/MAD Data December 2024

Forward Looking Statements and Important Notes This presentation by VYNE Therapeutics Inc . (“VYNE”) includes forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 including, but not limited to, statements regarding VYNE’s development plans and timelines for VYN 201 and VYN 202 , VYNE’s InhiBET platform, planned trial designs, potential market opportunities, VYNE's cash runway and other statements regarding the future expectations, plans and prospects of VYNE . All statements in this presentation which are not historical facts are forward - looking statements . Any forward - looking statements are based on VYNE’s current knowledge and its present beliefs and expectations regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially and adversely from those set forth or implied by such forward - looking statements . These risks and uncertainties include, but are not limited to : VYNE’s ability to successfully develop its product candidates ; the timing of commencement of future preclinical studies and clinical trials ; VYNE’s ability to enroll patients and successfully progress, complete, and receive favorable results from, clinical trials of its product candidates ; VYNE’s ability to comply with various regulations applicable to its business ; VYNE’s ability to create intellectual property and the scope of protection it is able to establish and maintain for intellectual property rights covering its product candidates, including the projected terms of patent protection ; risks that any of VYNE’s patents may be held to be narrowed, invalid or unenforceable or one or more of VYNE’s patent applications may not be granted and potential competitors may also seek to design around VYNE’s granted patents or patent applications ; estimates of VYNE’s expenses and capital requirements, and its ability to obtain additional capital on acceptable terms or at all ; VYNE’s expectations regarding licensing, business transactions and strategic operations ; VYNE’s future financial performance and liquidity ; and volatility in VYNE’s stock price may result in rapid and substantial increases or decreases in the stock price that may or may not be related to VYNE’s operating performance or prospects . For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE’s actual results to differ from those contained in the forward - looking statements, see the section titled “Risk Factors” in VYNE’s Annual Report on Form 10 - K for the year ended December 31 , 2023 , as well as discussions of potential risks, uncertainties, and other important factors in VYNE’s subsequent filings with the U . S . Securities and Exchange Commission . Although VYNE believes these forward - looking statements are reasonable, they speak only as of the date of this presentation and VYNE undertakes no obligation to update publicly such forward - looking statements to reflect subsequent events or circumstances, except as otherwise required by law . Given these risks and uncertainties, you should not rely upon forward - looking statements as predictions of future events . Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third - party sources and VYNE’s own internal estimates and research . While VYNE believes these third - party sources to be reliable as of the date of this presentation, VYNE has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while VYNE believes its own internal research is reliable, such research has not been verified by any independent source . You are cautioned not to give undue weight to any such information, projections and estimates . The trademarks included herein are the property of the owners thereof and are used for reference purposes only . This presentation concerns product candidates that are under clinical investigation . None of such product candidates have been approved for marketing by the FDA or the EMA, and such product candidates are currently limited to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated . 2
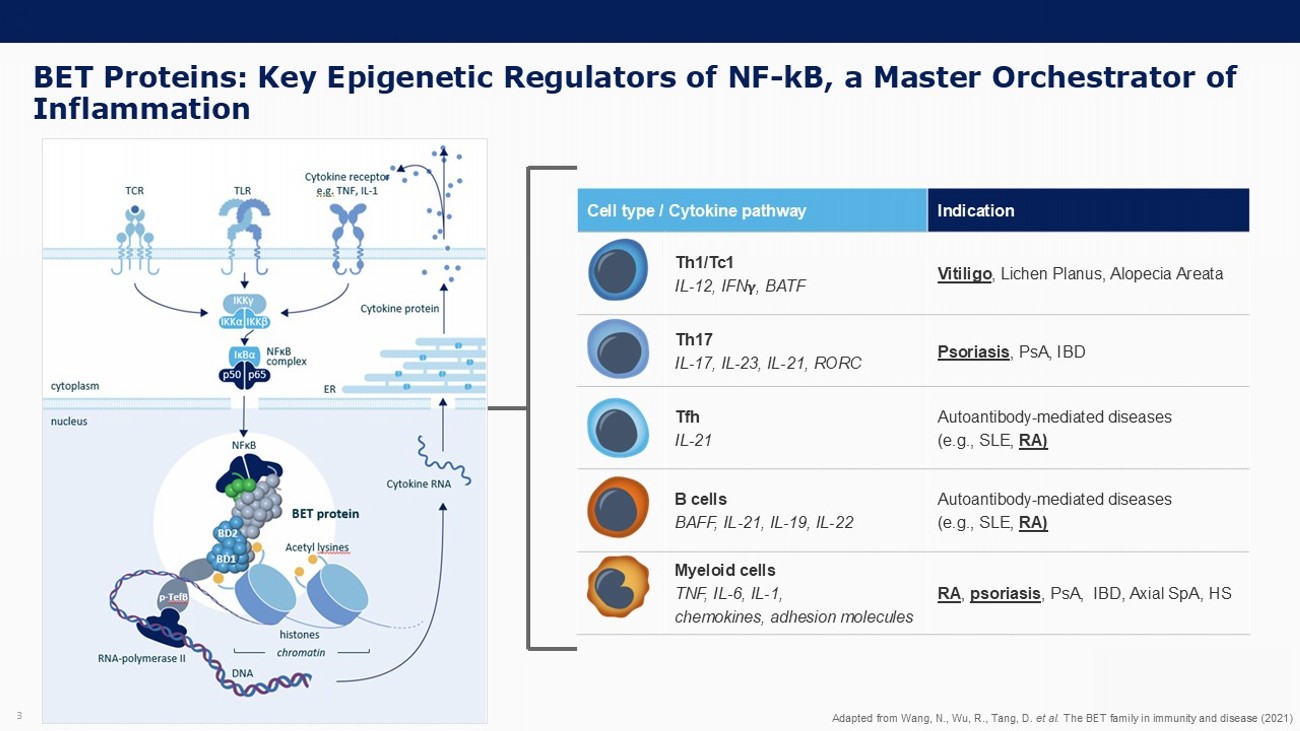
3 BET Proteins: Key Epigenetic Regulators of NF - kB, a Master Orchestrator of Inflammation Indication Cell type / Cytokine pathway Vitiligo , Lichen Planus, Alopecia Areata Th1/Tc1 IL - 12, IFN , BATF Psoriasis , PsA, IBD Th17 IL - 17, IL - 23, IL - 21, RORC Autoantibody - mediated diseases (e.g., SLE, RA) Tfh IL - 21 Autoantibody - mediated diseases (e.g., SLE, RA) B cells BAFF, IL - 21, IL - 19, IL - 22 RA , psoriasis , PsA, IBD, Axial SpA , HS Myeloid cells TNF, IL - 6, IL - 1, chemokines, adhesion molecules Adapted from Wang, N., Wu, R., Tang, D. et al. The BET family in immunity and disease (2021)
VYN202: A Novel BD2 - Selective BET Inhibitor for Immune - Mediated Diseases 4 1. Based on readily available public information such as clintrials.gov, academic publications and corporate websites/present ati ons. • VYN202 is an innovative, oral BD2 - Selective BET inhibitor • VYN202 is believed to be the most potent and BD2 - selective BET Inhibitor in clinical development 1 which is designed to improve efficacy and tolerability • VYN202 has demonstrated a significant inhibitory effect on key disease - related inflammatory biomarkers that correspond with reduced disease severity observed across multiple diverse preclinical models of autoimmune disease • Phase 1 SAD and MAD studies in healthy volunteers complete: • VYN202 was generally well tolerated with no drug - related adverse events historically associated with earlier generation, less selective BET inhibitors • Favorable PK profile demonstrated for VYN202, supporting once - daily dosing regimen • VYN202 demonstrated robust pharmacodynamic activity including evidence of target engagement and significant inhibition of inflammatory biomarkers relevant to several immune - mediated disorders in ex vivo stimulation assays Compelling data support VYN202’s potential as a novel, once - daily oral treatment for a broad range of immune - mediated disorders

Maximizing On - target Potency vs. BD2 and Minimizing Affinity to BD1 May B e T he Key to Optimizing the Benefit/Risk Profile of BET Inhibitors for Autoimmune Diseases 5 Selectivity ** (BD1/BD2) Potency vs. BD2 * ( nM ) Compound ID ~10,000 1 VYN202 (VYNE) 1,460x (FRET) 2 NUV - 868 ( Nuvation ) 2 753x (FRET) 28 ABBV - 744 (AbbVie) 3 220x 79 GSK620 (GSK) 4 5x (FRET) 17 Pelabresib (NVS/MOR) 2 2.6x 13 ABBV - 075 3 1.5x 26 MK - 8628/OTX - 015 5 0.1x 41 BI - 894999 6 *Lower number denotes higher potency **Higher number denotes higher selectivity. Data based on nanoBRET assay unless otherwise indicated. 1. Based on readily available public information such as clintrials.gov, academic publications and corporate websites/present ati ons. 2. Nuvation corporate presentation (August 2024); 3. Faivre et al 2020; 4. Delmont et al 2020; 5. Wang et al 2017 6. Kraut et al 2018; Data on file VYN202 is believed to be the most potent and BD2 - selective BET Inhibitor in clinical development 1 which is designed to improve efficacy and tolerability BD1 BD2 EXT BD2 activity is associated with inflammatory gene induction that drives inflammatory responses in autoimmune disease BD1 regulates “housekeeping” gene activity
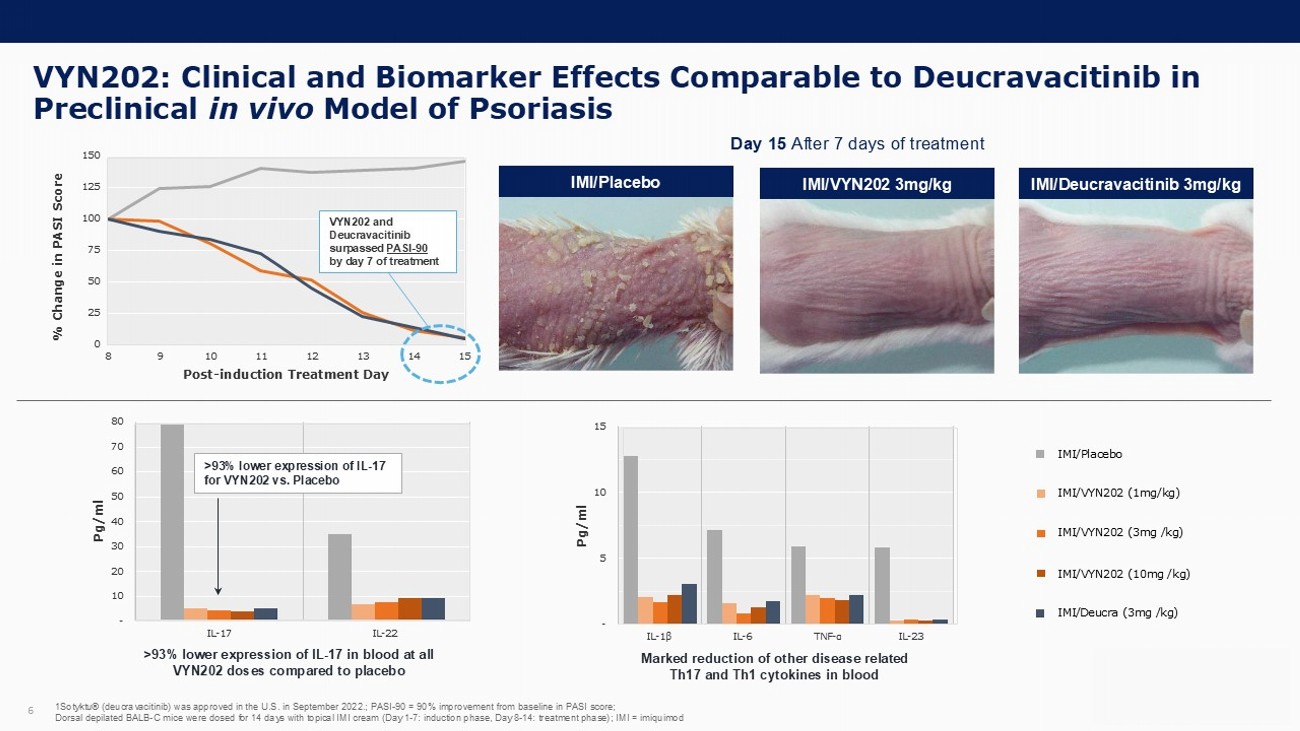
- 10 20 30 40 50 60 70 80 IL-17 IL-22 VYN202: Clinical and Biomarker Effects Comparable to Deucravacitinib in Preclinical in vivo Model of Psoriasis 6 Day 15 After 7 days of treatment IMI/VYN202 3mg/kg IMI/Placebo IMI/Deucravacitinib 3mg/kg - 5 10 15 IL - 1 β IL-6 TNF - α IL-23 >93% lower expression of IL - 17 in blood at all VYN202 doses compared to placebo Marked reduction of other disease related Th17 and Th1 cytokines in blood Pg/ml >93% lower expression of IL - 17 for VYN202 vs. Placebo Pg/ml 0 25 50 75 100 125 150 8 9 10 11 12 13 14 15 Post - induction Treatment Day % Change in PASI Score VYN202 and Deucravacitinib surpassed PASI - 90 by day 7 of treatment 1Sotyktu® ( deucravacitinib ) was approved in the U.S. in September 2022.; PASI - 90 = 90% improvement from baseline in PASI score; Dorsal depilated BALB - C mice were dosed for 14 days with topical IMI cream (Day 1 - 7: induction phase, Day 8 - 14: treatment phase) ; IMI = imiquimod IMI/Placebo IMI/VYN202 (1mg/kg) IMI/VYN202 (3mg /kg) IMI/VYN202 (10mg /kg) IMI/ Deucra (3mg /kg)
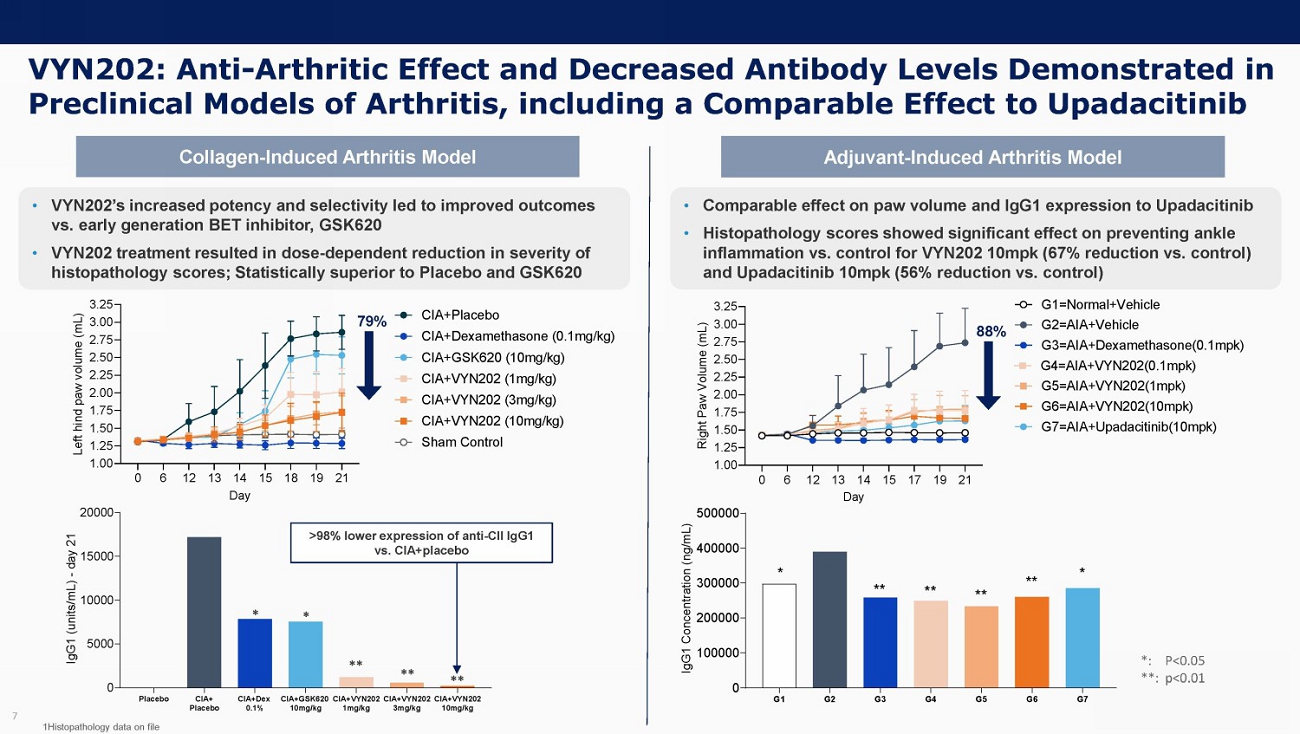
VYN202: Anti - Arthritic Effect and Decreased Antibody Levels Demonstrated in Preclinical Models of Arthritis, including a Comparable Effect to Upadacitinib 7 79% >98% lower expression of anti - CII IgG1 vs. CIA+placebo Collagen - Induced Arthritis Model Adjuvant - Induced Arthritis Model *: P<0.05 **: p<0.01 ** ** ** * * • VYN202’s increased potency and selectivity led to improved outcomes vs. early generation BET inhibitor, GSK620 • VYN202 treatment resulted in dose - dependent reduction in severity of histopathology scores; Statistically superior to Placebo and GSK620 • Comparable effect on paw volume and IgG1 expression to Upadacitinib • Histopathology scores showed significant effect on preventing ankle inflammation vs. control for VYN202 10mpk (67% reduction vs. control) and Upadacitinib 10mpk (56% reduction vs. control) 88 % 1Histopathology data on file
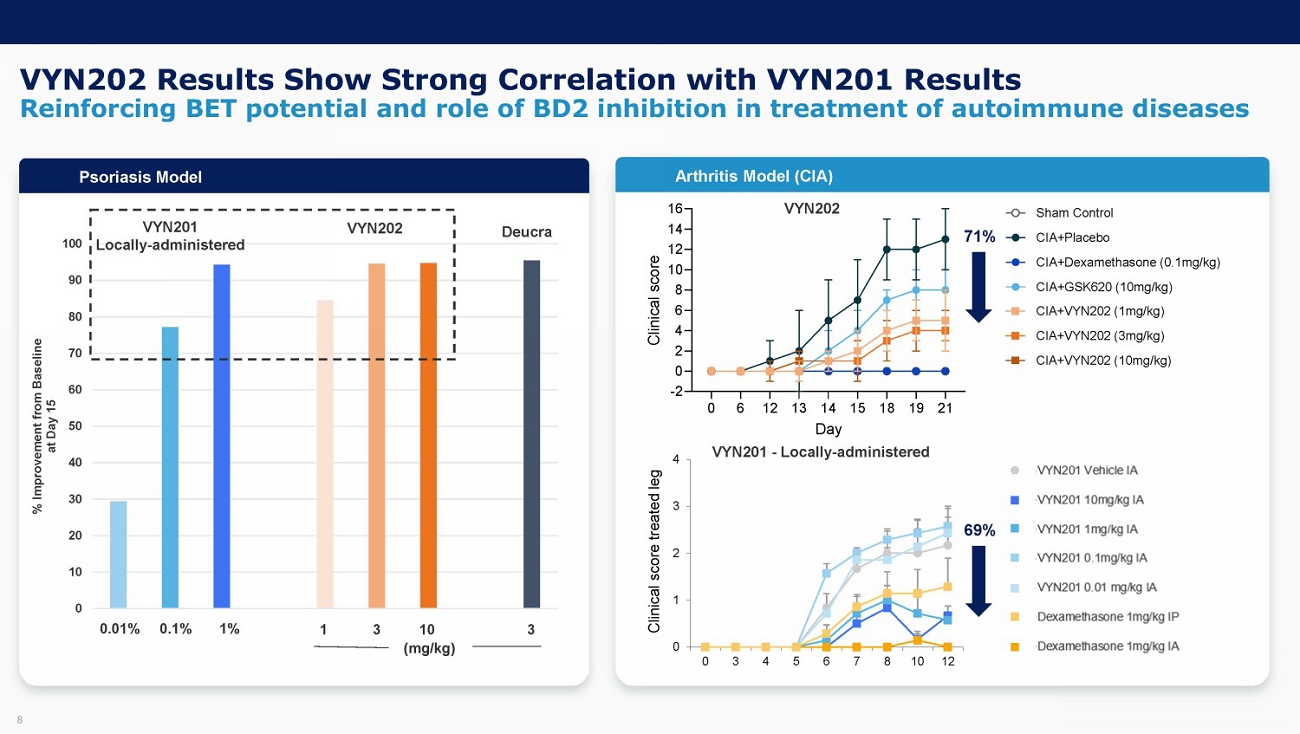
8 VYN202 Results Show Strong Correlation with VYN201 Results Reinforcing BET potential and role of BD2 inhibition in treatment of autoimmune diseases 0 10 20 30 40 50 60 70 80 90 100 % Improvement from Baseline at Day 15 Psoriasis Model VYN201 Locally - administered 0.01% 0.1% 1% 1 (mg/kg) 3 10 3 VYN202 Deucra 71% 0 1 2 3 4 0 3 4 5 6 7 8 10 12 Clinical score treated leg 69% VYN201 - Locally - administered VYN202 Psoriasis Model Arthritis Model (CIA)

VYN202: Phase 1a SAD/MAD Data
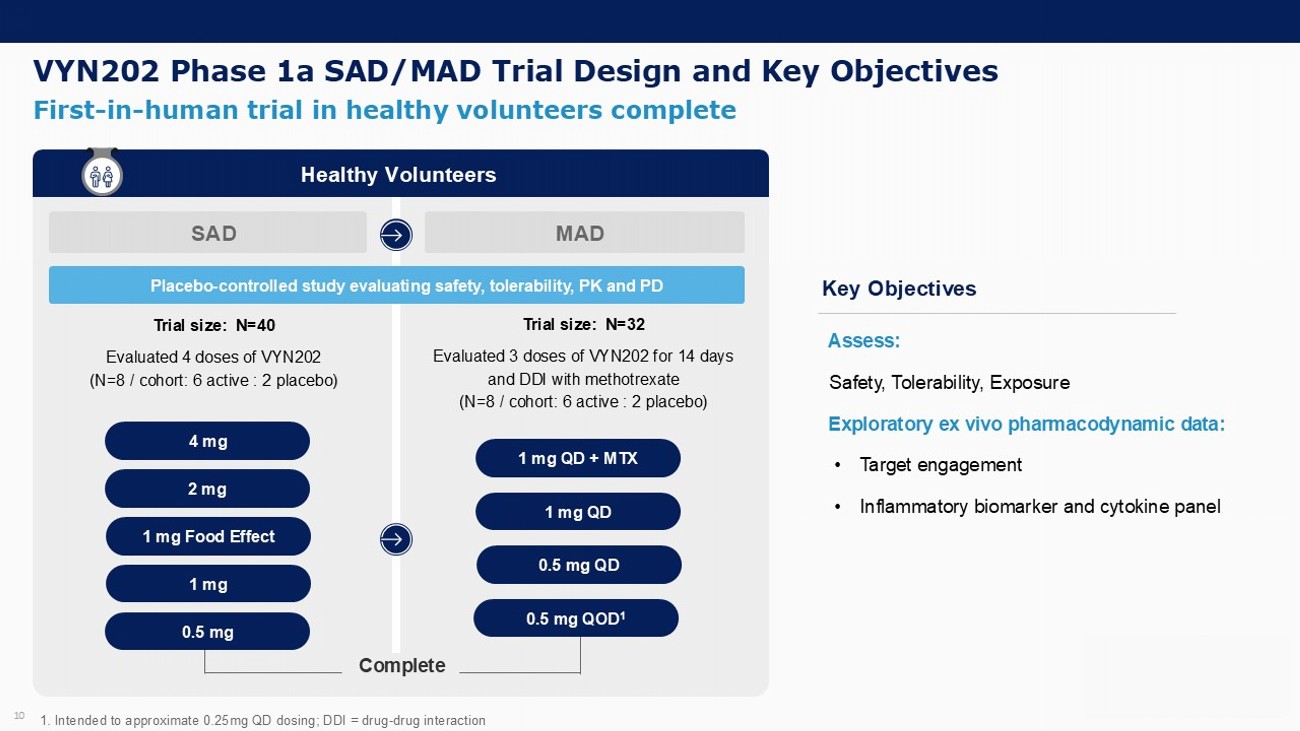
VYN202 Phase 1a SAD/MAD Trial Design and Key Objectives 10 Placebo - controlled study evaluating safety, tolerability, PK and PD First - in - human trial in healthy volunteers complete 1. Intended to approximate 0.25mg QD dosing; DDI = drug - drug interaction Assess: Safety, Tolerability, Exposure Exploratory ex vivo pharmacodynamic data: • Target engagement • Inflammatory biomarker and cytokine panel Key Objectives Healthy Volunteers SAD MAD Trial size: N=40 Evaluated 4 doses of VYN202 (N=8 / cohort: 6 active : 2 placebo) 4 mg 2 mg 1 mg Complete Trial size: N=32 Evaluated 3 doses of VYN202 for 14 days and DDI with methotrexate (N=8 / cohort: 6 active : 2 placebo) 1 mg QD + MTX 1 mg QD 0.5 mg QD 1 mg Food Effect 0.5 mg QOD 1 0.5 mg
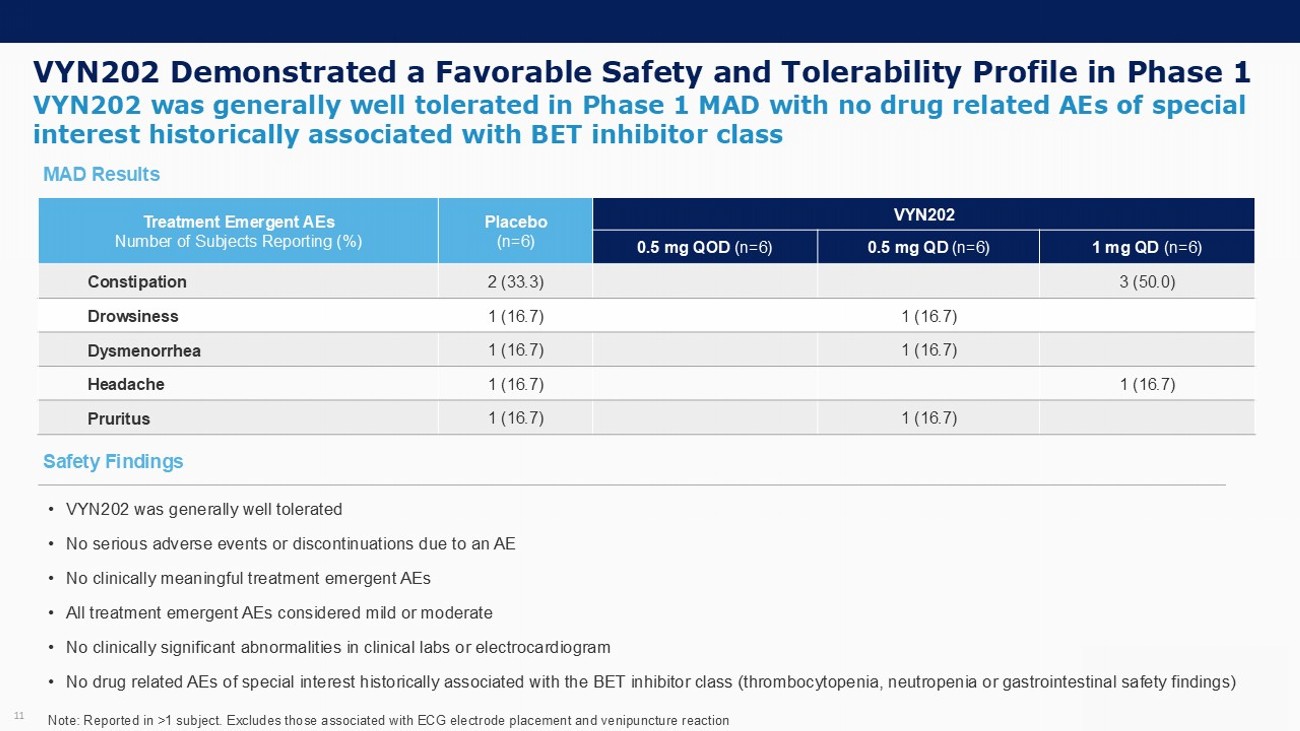
VYN202 Demonstrated a Favorable Safety and Tolerability Profile in Phase 1 11 VYN202 was generally well tolerated in Phase 1 MAD with no drug related AEs of special interest historically associated with BET inhibitor class VYN202 Placebo (n=6) Treatment Emergent AEs Number of Subjects Reporting (%) 1 mg QD (n=6) 0.5 mg QD (n=6) 0.5 mg QOD (n=6) 3 (50.0) 2 (33.3) Constipation 1 (16.7) 1 (16.7) Drowsiness 1 (16.7) 1 (16.7) Dysmenorrhea 1 (16.7) 1 (16.7) Headache 1 (16.7) 1 (16.7) Pruritus Note: Reported in >1 subject. Excludes those associated with ECG electrode placement and venipuncture reaction • VYN202 was generally well tolerated • No serious adverse events or discontinuations due to an AE • No clinically meaningful treatment emergent AEs • All treatment emergent AEs considered mild or moderate • No clinically significant abnormalities in clinical labs or electrocardiogram • No drug related AEs of special interest historically associated with the BET inhibitor class (thrombocytopenia, neutropenia o r g astrointestinal safety findings) Safety Findings MAD Results
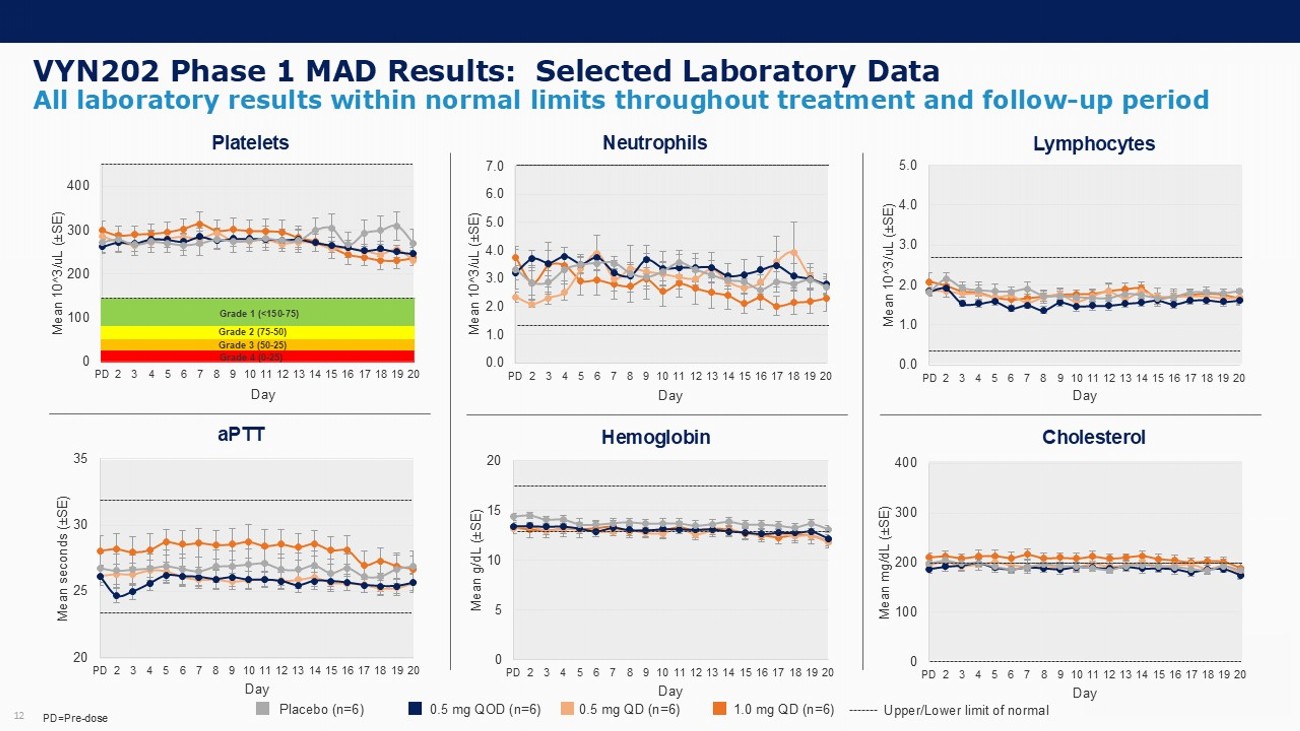
VYN202 Phase 1 MAD Results: Selected Laboratory Data 12 All laboratory results within normal limits throughout treatment and follow - up period 0 100 200 300 400 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean 10^3/ uL ( “ SE) Day 20 25 30 35 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean seconds ( “ SE) Day 0.0 1.0 2.0 3.0 4.0 5.0 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean 10^3/ uL ( “ SE) Day 0 5 10 15 20 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean g/dL ( “ SE) Day Platelets Neutrophils Lymphocytes Cholesterol Hemoglobin aPTT Placebo (n=6) 0.5 mg QOD (n=6) 0.5 mg QD (n=6) 1.0 mg QD (n=6) Upper/Lower limit of normal PD=Pre - dose Grade 1 (<150 - 75) Grade 2 (75 - 50) 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean 10^3/ uL ( “ SE) Day Grade 4 (0 - 25) Grade 3 (50 - 25) 0 100 200 300 400 PD 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Mean mg/dL ( “ SE) Day
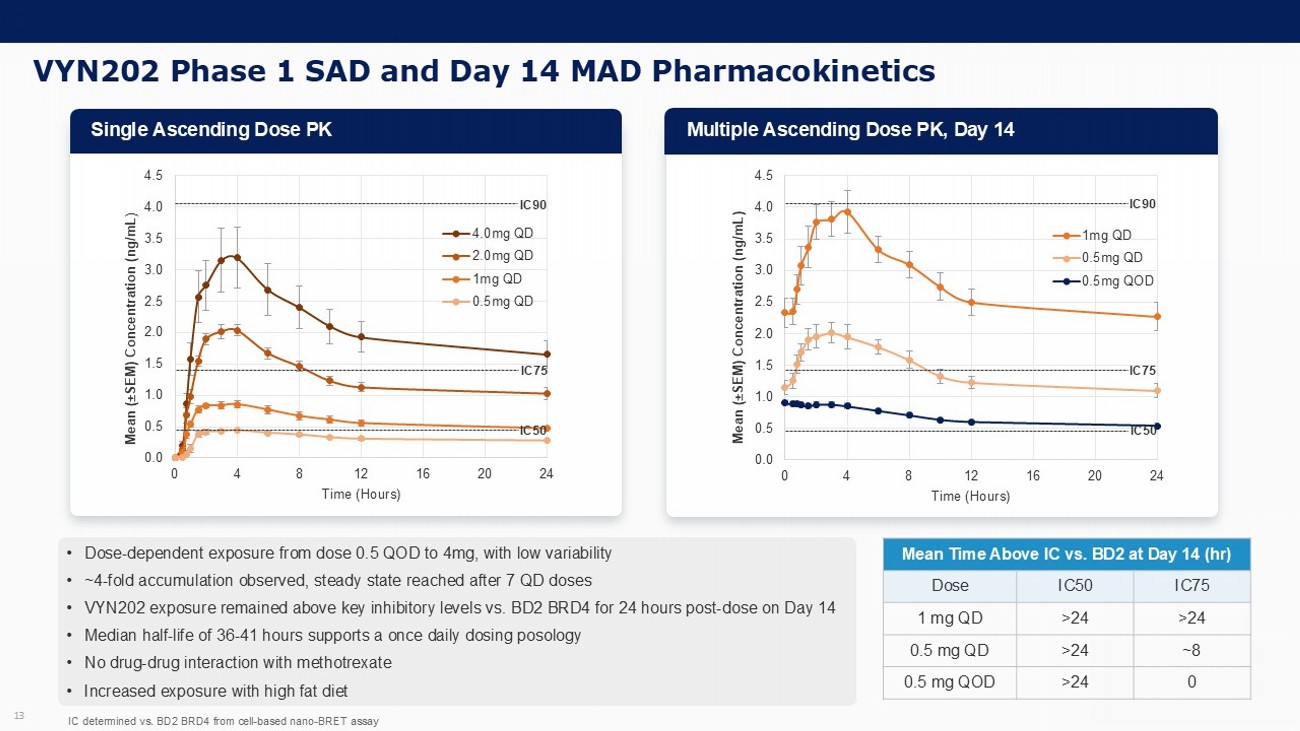
VYN202 Phase 1 SAD and Day 14 MAD Pharmacokinetics 13 • Dose - dependent exposure from dose 0.5 QOD to 4mg, with low variability • ~ 4 - fold accumulation observed, steady state reached after 7 QD doses • VYN202 exposure remained above key inhibitory levels vs. BD2 BRD4 for 24 hours post - dose on Day 14 • Median half - life of 36 - 41 hours supports a once daily dosing posology • No drug - drug interaction with methotrexate • Increased exposure with high fat diet 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 0 4 8 12 16 20 24 Mean ( “ SEM) Concentration (ng/mL) Time (Hours) 1mg QD 0.5mg QD 0.5mg QOD 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 0 4 8 12 16 20 24 Mean ( “ SEM) Concentration (ng/mL ) Time (Hours) 4.0mg QD 2.0mg QD 1mg QD 0.5mg QD IC50 IC90 Mean Time Above IC vs. BD2 at Day 14 ( hr ) IC75 IC50 Dose >24 >24 1 mg QD ~8 >24 0.5 mg QD 0 >24 0.5 mg QOD IC determined vs. BD2 BRD4 from cell - based nano - BRET assay IC50 IC90 IC75 IC75 Single Ascending Dose PK Multiple Ascending Dose PK, Day 14
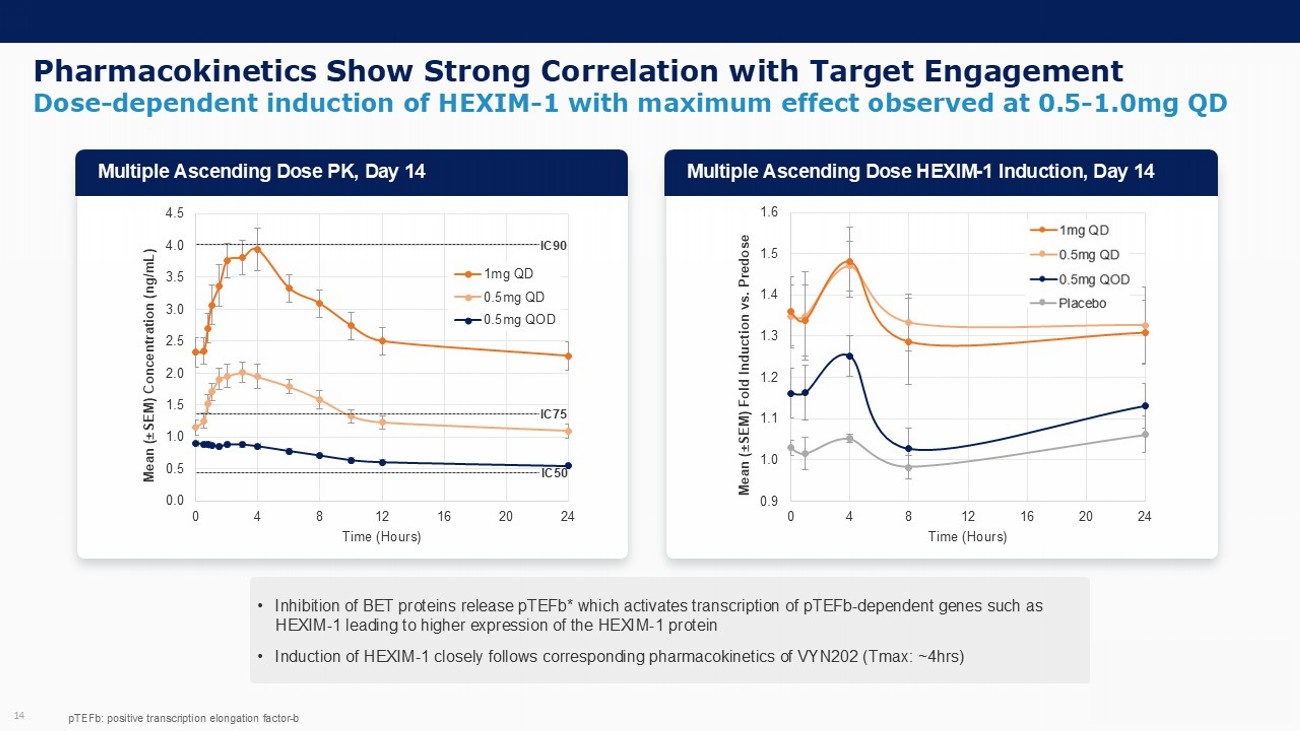
Pharmacokinetics Show Strong Correlation with Target Engagement 14 • Inhibition of BET proteins release pTEFb * which activates transcription of pTEFb - dependent genes such as HEXIM - 1 leading to higher expression of the HEXIM - 1 protein • Induction of HEXIM - 1 closely follows corresponding pharmacokinetics of VYN202 ( Tmax : ~4hrs) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 0 4 8 12 16 20 24 Mean ( “ SEM) Concentration (ng/mL) Time (Hours) 1mg QD 0.5mg QD 0.5mg QOD IC50 IC90 pTEFb : positive transcription elongation factor - b IC75 Multiple Ascending Dose PK, Day 14 Multiple Ascending Dose HEXIM - 1 Induction, Day 14 0.9 1.0 1.1 1.2 1.3 1.4 1.5 1.6 0 4 8 12 16 20 24 Mean ( “ SEM) Fold Induction vs. Predose Time (Hours) 0.5mg QOD 0.5mg QD 1.0mg QD Placebo Dose - dependent induction of HEXIM - 1 with maximum effect observed at 0.5 - 1.0mg QD
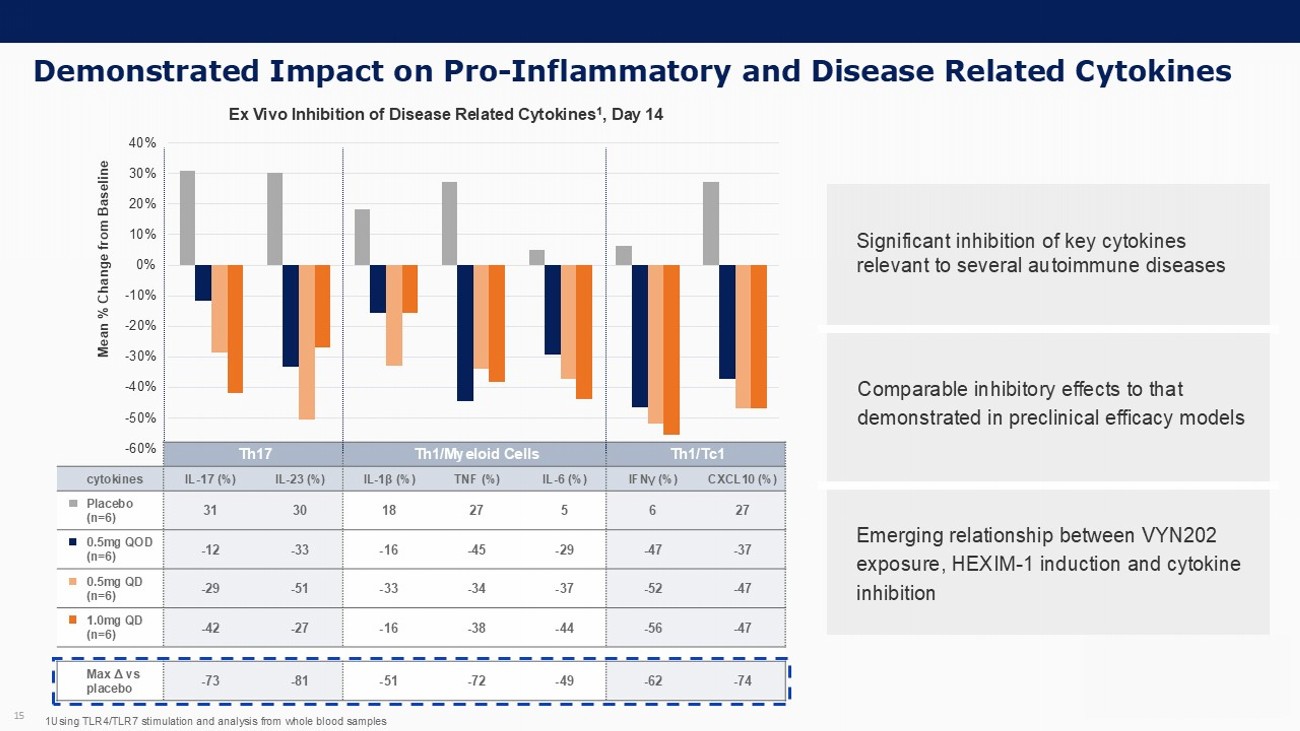
Demonstrated Impact on Pro - Inflammatory and Disease Related Cytokines 15 -60% -50% -40% -30% -20% -10% 0% 10% 20% 30% 40% Mean % Change from Baseline Ex Vivo Inhibition of Disease Related Cytokines 1 , Day 14 1U sing TLR4/TLR7 stimulation and analysis from whole blood samples Th1/Tc1 Th1/Myeloid Cells Th17 CXCL10 (%) IFN γ (%) IL - 6 (%) TNF (%) IL - 1 β (%) IL - 23 (%) IL - 17 (%) cytokines 27 6 5 27 18 30 31 Placebo (n=6) - 37 - 47 - 29 - 45 - 16 - 33 - 12 0.5mg QOD (n=6) - 47 - 52 - 37 - 34 - 33 - 51 - 29 0.5mg QD (n=6) - 47 - 56 - 44 - 38 - 16 - 27 - 42 1.0mg QD (n=6) - 74 - 62 - 49 - 72 - 51 - 81 - 73 Max Δ vs placebo Significant inhibition of key cytokines relevant to several autoimmune diseases Comparable inhibitory effects to that demonstrated in preclinical efficacy models Emerging relationship between VYN202 exposure, HEXIM - 1 induction and cytokine inhibition
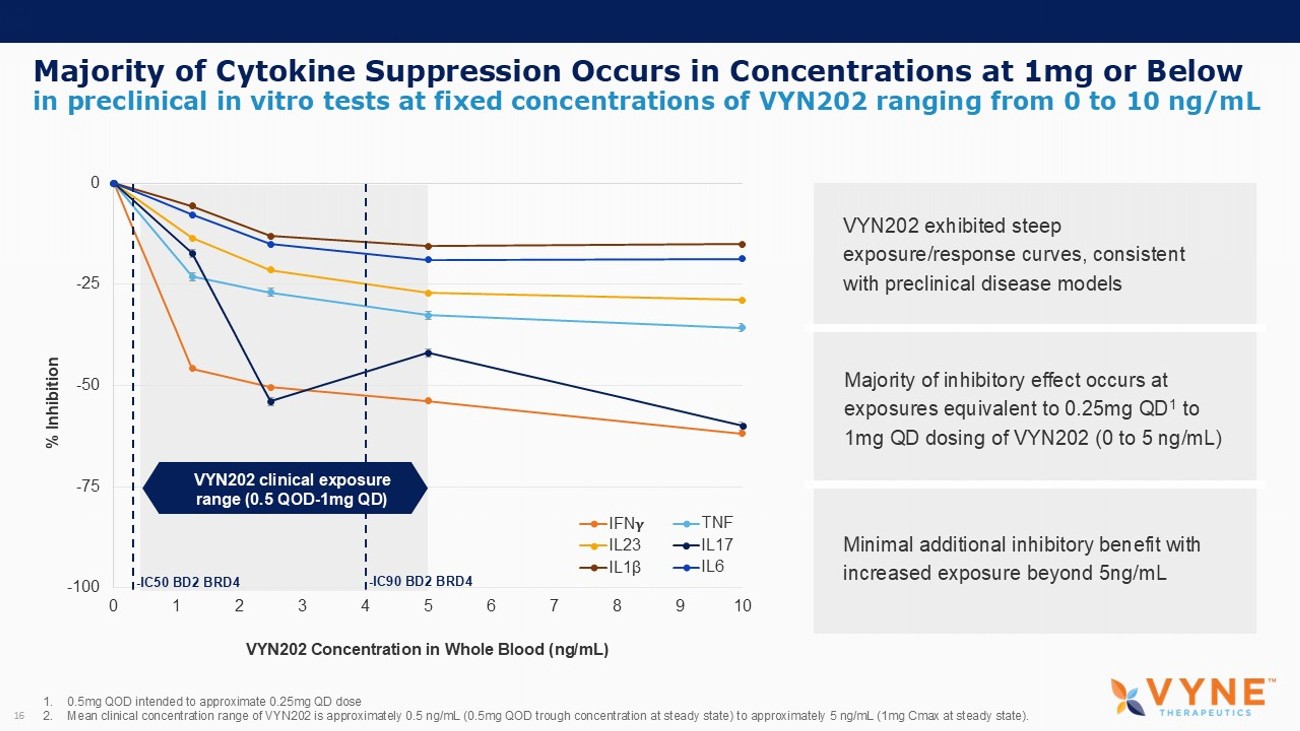
Majority of Cytokine Suppression Occurs in Concentrations at 1mg or Below 16 in preclinical in vitro tests at fixed concentrations of VYN202 ranging from 0 to 10 ng/mL 1. 0.5mg QOD intended to approximate 0.25mg QD dose 2. Mean clinical concentration range of VYN202 is approximately 0.5 ng/mL (0.5mg QOD trough concentration at steady state) to approximately 5 ng/mL (1mg Cmax at steady state). -100 -75 -50 -25 0 0 1 2 3 4 5 6 7 8 9 10 % Inhibition IFN TNF IL23 IL17 IL1 β IL6 - IC50 BD2 BRD4 - IC90 BD2 BRD4 VYN202 clinical exposure range (0.5 QOD - 1mg QD) VYN202 Concentration in Whole Blood (ng/mL) VYN202 exhibited steep exposure/response curves, consistent with preclinical disease models Majority of inhibitory effect occurs at exposures equivalent to 0.25mg QD 1 to 1mg QD dosing of VYN202 (0 to 5 ng/mL) Minimal additional inhibitory benefit with increased exposure beyond 5ng/mL

VYN202 Phase 1a MAD Data Summary 17 • Demonstrated favorable safety and tolerability profile • No drug - related adverse events historically associated with earlier generation, less selective BET inhibitors, including thrombocytopenia, neutropenia or gastrointestinal toxicity findings • No serious adverse events (AEs), discontinuations due to an AE or clinically meaningful treatment emergent adverse events (TEAEs) • All TEAEs were considered mild or moderate in severity • No drug - related adverse events associated with laboratory results • There were no AEs of any severity grade relating to thrombocytopenia, which is a known dose - limiting toxicity associated with earlier generations of BET inhibitors Safety Pharmacokinetics • Favorable PK profile • Data supports once - daily dosing regimen • VYN202 demonstrated dose dependent exposure that reached steady - state after 7 once - daily doses • VYN202 blood levels were within key inhibitory thresholds of IC50 to IC90 against BD2 BRD4 for at least 24 hours at all doses • No drug - drug interaction observed when VYN202 was co - administered with methotrexate, a treatment commonly used in the management of chronic immuno - inflammatory conditions Pharmacodynamics • Robust pharmacodynamic activity on target engagement and inflammatory biomarkers in ex vivo assays • VYN202 induced a dose - dependent increase in the target engagement biomarker HEXIM - 1 with a maximal effect observed at 0.5mg to 1.0 mg QD • VYN202 inhibited the production of multiple inflammatory biomarkers related to Th17, Th1/myeloid and Th1/Tc dysregulated activity, consistent with preclinical models of VYN202 • VYN202 exhibited steep exposure/response curves, consistent with preclinical disease models, with majority of inhibitory effect occurring at exposures equivalent to 0.25mg QD to 1mg QD dosing of VYN202 (0 to 5 ng/mL) Compelling data support VYN202’s potential as a novel, once - daily oral treatment for a broad range of immune - mediated disorders
















