Exhibit 99.2
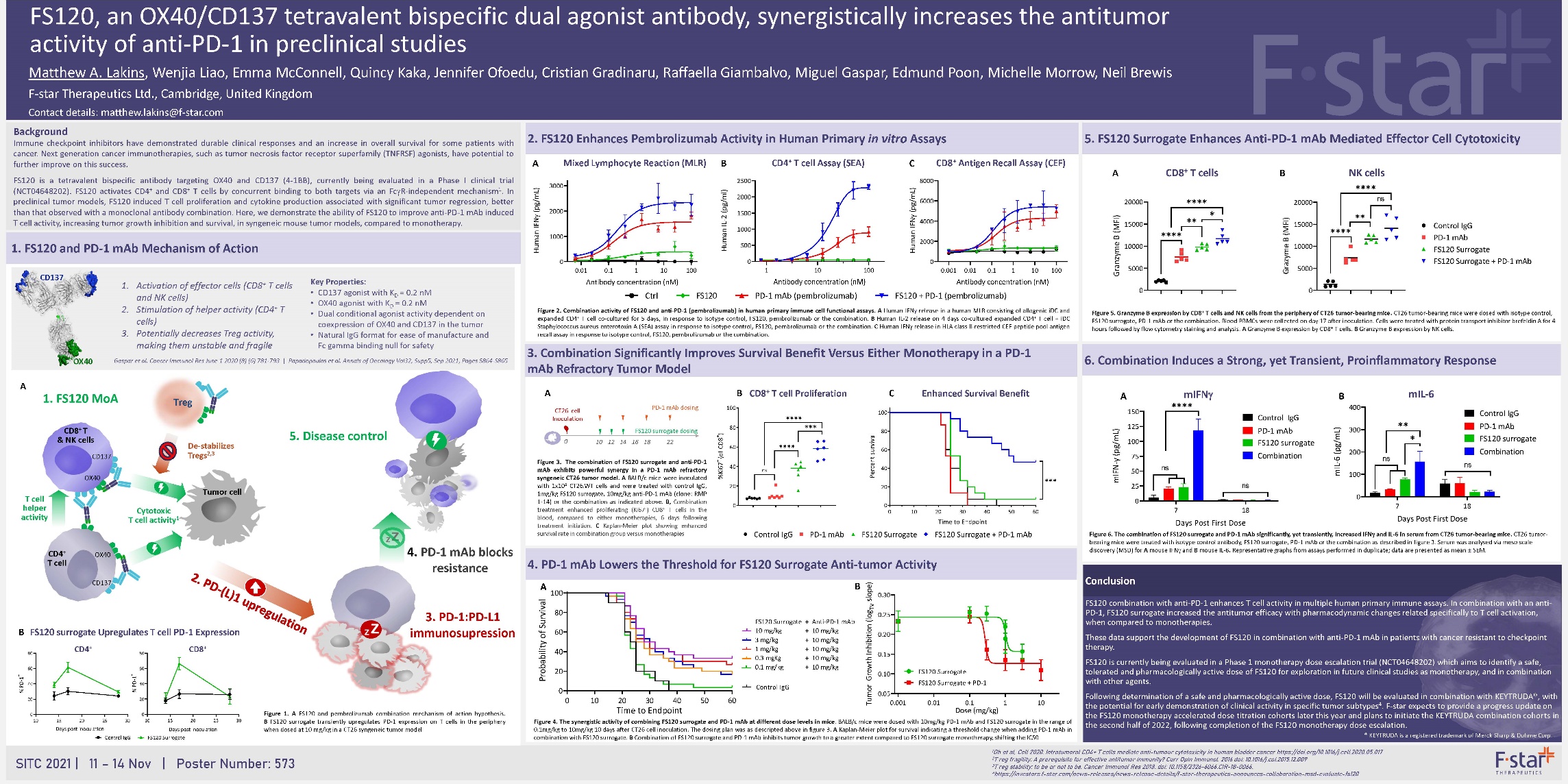
FS120, an OX40/CD137 tetravalent bispecific dual agonist antibody, synergistically increases the antitumor activity of anti-PD-1 in preclinical studies 1. FS120 MoA 5. Disease control T cell helper activity 4. PD-1 mAb blocks resistance 3. PD-1:PD-L1 immunosupression FS120 surrogate Upregulates T cell PD-1 Expression Mixed Lymphocyte Reaction (MLR) CD4+ T cell Assay (SEA) CD8+ Antigen Recall Assay (CEF) Antibody concentration (nM) Human IFNg (pg/mL) 0.001 0.01 0.1 1 10 100 0 2000 4000 6000 8000 Antibody concentration (nM) FS120 surrogate Upregulates T cell PD-1 Expression CD4+ CD8+ Figure 1. A FS120 and pembrolizumab combination mechanism of action hypothesis. B FS120 surrogate transiently upregulates PD-1 expression on T cells in the periphery when dosed at 10 mg/kg in a CT26 syngeneic tumor model Key Properties: • CD137 agonist with KD = 0.2 nM • OX40 agonist with KD = 0.2 nM • Dual conditional agonist activity dependent on coexpression of OX40 and CD137 in the tumor • Natural IgG format for ease of manufacture and Fc gamma binding null for safety 1. Activation of effector cells (CD8+ T cells and NK cells) 2. Stimulation of helper activity (CD4+ T cells) 3. Potentially decreases Treg activity, making them unstable and fragile Gaspar et al. Cancer Immunol Res June 1 2020 (8) (6) 781-793 | Papadopoulos et al. Annals of Oncology Vol32, Supp5, Sep 2021, Pages S864-S865 Figure 2. Combination activity of FS120 and anti-PD-1 (pembrolizumab) in human primary immune cell functional assays. A Human IFNγ release in a human MLR consisting of allogenic iDC and expanded CD4+ T cell co-cultured for 5 days, in response to isotype control, FS120, pembrolizumab or the combination. B Human IL-2 release on 4 days co-cultured expanded CD4+ T cell + iDC Staphylococcus aureus enterotoxin A (SEA) assay in response to isotype control, FS120, pembrolizumab or the combination. C Human IFNg release in HLA class II-restricted CEF peptide pool antigen recall assay in response to isotype control, FS120, pembrolizumab or the combination. Ctrl FS120 PD-1 mAb (pembrolizumab) 1 (pembrolizumab) 3. Combination Significantly Improves Survival Benefit Versus Either Monotherapy in a PD-1 mAb Refractory Tumor Model Figure 3. The combination of FS120 surrogate and anti-PD-1 mAb exhibits powerful synergy in a PD-1 mAb refractory syngeneic CT26 tumor model. A BALB/c mice were inoculated with 1x105 CT26.WT cells and were treated with control IgG, 1mg/kg FS120 surrogate, 10mg/kg anti-PD-1 mAb (clone: RMP 1-14) or the combination as indicated above. B, Combination treatment enhanced proliferating (Ki67+) CD8+ T cells in the blood, compared to either monotherapies, 6 days following treatment initiation. C Kaplan-Meier plot showing enhanced survival rate in combination group versus monotherapies 4. PD-1 mAb Lowers the Threshold for FS120 Surrogate Anti-tumor Activity Figure 4. The synergistic activity of combining FS120 surrogate and PD-1 mAb at different dose levels in mice. BALB/c mice were dosed with 10mg/kg PD-1 mAb and FS120 surrogate in the range of 0.1mg/kg to 10mg/kg 10 days after CT26 cell inoculation. The dosing plan was as descripted above in figure 3. A Kaplan-Meier plot for survival indicating a threshold change when adding PD-1 mAb in combination with FS120 surrogate. B Combination of FS120 surrogate and PD-1 mAb inhibits tumor growth to a greater extent compared to FS120 surrogate monotherapy, shifting the IC50 5. FS120 Surrogate Enhances Anti-PD-1 mAb Mediated Effector Cell Cytotoxicity Figure 5. Granzyme B expression by CD8+ T cells and NK cells from the periphery of CT26 tumor-bearing mice. CT26 tumor-bearing mice were dosed with isotype control, FS120 surrogate, PD-1 mAb or the combination. Blood PBMCs were collected on day 17 after inoculation. Cells were treated with protein transport inhibitor brefeldin A for 4 hours followed by flow cytometry staining and analysis. A Granzyme B expression by CD8+ T cells. B Granzyme B expression by NK cells. 6. Combination Induces a Strong, yet Transient, Proinflammatory Response Figure 6. The combination of FS120 surrogate and PD-1 mAb significantly, yet transiently, increased IFNg and IL-6 in serum from CT26 tumor-bearing mice. CT26 tumorbearing mice were treated with isotype control antibody, FS120 surrogate, PD-1 mAb or the combination as described in figure 3. Serum was analysed via meso scale discovery (MSD) for A mouse IFNg and B mouse IL-6. Representative graphs from assays performed in duplicate; data are presented as mean ± SEM. Conclusion FS120 combination with anti-PD-1 enhances T cell activity in multiple human primary immune assays. In combination with an anti- PD-1, FS120 surrogate increased the antitumor efficacy with pharmacodynamic changes related specifically to T cell activation, when compared to monotherapies. These data support the development of FS120 in combination with anti-PD-1 mAb in patients with cancer resistant to checkpoint therapy. FS120 is currently being evaluated in a Phase 1 monotherapy dose escalation trial (NCT04648202) which aims to identify a safe, tolerated and pharmacologically active dose of FS120 for exploration in future clinical studies as monotherapy, and in combination with other agents. Following determination of a safe and pharmacologically active dose, FS120 will be evaluated in combination with KEYTRUDA®, with the potential for early demonstration of clinical activity in specific tumor subtypes4. F-star expects to provide a progress update on the FS120 monotherapy accelerated dose titration cohorts later this year and plans to initiate the KEYTRUDA combination cohorts in the second half of 2022, following completion of the FS120 monotherapy dose escalation. ® KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp. SITC 2021 | 11 – 14 Nov | Poster Number: 573 1Oh et al, Cell 2020. Intratumoral CD4+ T cells mediate anti-tumour cytotoxicity in human bladder cancer https://doi.org/10.1016/j.cell.2020.05.017 2T reg fragility: A prerequisite for effective antitumor immunity? Curr Opin Immunol. 2016 doi: 10.1016/j.coi.2015.12.009 3T reg stability: to be or not to be. Cancer Immunol Res 2018. doi: 10.1158/2326-6066.CIR-18-0066. 4https://investors.f-star.com/news-releases/news-release-details/f-star-therapeutics-announces-collaboration-msd-evaluate-fs120 CD8+ T cell Proliferation Enhanced Survival Benefit mIFNg Control IgG PD-1 mAb FS120 surrogate ✱✱✱✱ ns Combination Control IgG PD-1 mAb FS120 surrogate ✱✱ ns Combination Days Post First Dose Days Post First Dose Control IgG PD-1 mAb FS120 Surrogate FS120 Surrogate + PD-1 mAb CD8+ T cells
