|
Exhibit 99.1 
|
WELCOME BIOMARIN ANALYST AND INVESTOR DAY 2014 Jean-Jacques Bienaime Chief Executive Officer Hank Fuchs Chief Medical Officer
ANALYST DAY 2014 Safe Harbor Statement This non-confidential presentation contains ‘forward-looking statements’ about the business prospects of BioMarin Pharmaceutical Inc., including potential future products in different areas of therapeutic research and development. Results may differ materially depending on the progress of BioMarin’s product programs, actions of regulatory authorities, availability of capital, future actions in the pharmaceutical market and developments by competitors, and those factors detailed in BioMarin’s filings with the Securities and Exchange Commission such as 10-Q, 10-K and 8-K reports. 2
|

|
ANALYST DAY 2014 Additional Information The offer by BioMarin to purchase ordinary shares of Prosensa Holding N.V. (“Prosensa”) contemplated by the Purchase Agreement, dated November 23, 2014, between BioMarin, Prosensa and BioMarin Falcons B.V. (the “Offer”) has not yet commenced. This communication is neither an offer to purchase nor a solicitation of an offer to sell any ordinary shares of Prosensa or any other securities. On the commencement date of the Offer, a tender offer statement on Schedule TO, including an offer to purchase, a letter of transmittal and related documents, will be filed with the United States Securities and Exchange Commission (the “SEC”). Thereafter, Prosensa will file a solicitation/recommendation statement on Schedule 14D-9 with the SEC. The offer to purchase ordinary shares of Prosensa will only be made pursuant to the offer to purchase, the letter of transmittal and related documents filed as a part of the Schedule TO. INVESTORS AND SECURITY HOLDERS OF PROSENSA ARE URGED TO READ BOTH THE SCHEDULE TO (AND THE INCLUDED OFFER TO PURCHASE) AND THE SOLICITATION/ RECOMMENDATION STATEMENT, AS THEY MAY BE AMENDED FROM TIME TO TIME, AND OTHER RELEVANT DOCUMENTS FILED WITH THE SEC WHEN THEY BECOME AVAILABLE BEFORE THEY MAKE ANY DECISION WITH RESPECT TO THE TENDER OFFER, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE TERMS OF THE OFFER, THE PROPOSED TRANSACTIONS AND THE PARTIES THERETO. The tender offer statement will be filed with the SEC by BioMarin and the solicitation/recommendation statement will be filed with the SEC by Prosensa. Investors and security holders may obtain a free copy of these statements (when available) and other documents filed with the SEC at the website maintained by the SEC at www.sec.gov or by directing such requests to the information agent for the tender offer that will be named in the tender offer statement on Schedule TO. 3
|
|
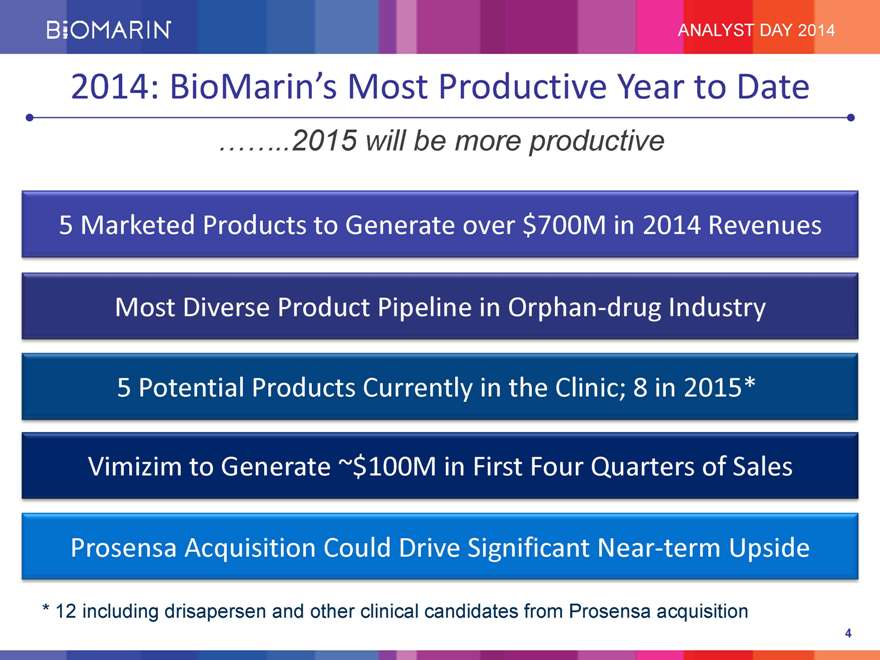
ANALYST DAY 2014 2014: BioMarin’s Most Productive Year to Date 2015 will be more productive 5 Marketed Products to Generate over $700M in 2014 Revenues Most Diverse Product Pipeline in Orphan-drug Industry 5 Potential Products Currently in the Clinic; 8 in 2015* Vimizim to Generate ~$100M in First Four Quarters of Sales Prosensa Acquisition Could Drive Significant Near-term Upside * 12 including drisapersen and other clinical candidates from Prosensa acquisition 4
|

|
ANALYST DAY 2014 Prosensa Acquisition Highlights Great strategic fit and value creator for BioMarin Drisapersen has a large body of clinical data that could support early regulatory approval Leverages BioMarin’s extensive experience getting orphan drugs approved DMD is one of the largest monogenetic disorders (approx. 75,000 WW) Leverages our global commercial infrastructure and leadership in orphan disease market Adds what could be our largest product to a well diversified product portfolio With early approvals, accretive to operating and GAAP earnings in 2017 A potentially transformative investment that is risk managed
|

|
ANALYST DAY 2014 Why Prosensa? First to file in the U.S. for the treatment of Duchenne muscular dystrophy Our belief that the totality of drisapersen data combined with the significant unmet medical need of this patient population provide a path to early approvals Largest data set in DMD comprising three placebo-controlled studies and two long-term open label studies, treating over 300 patients New Drug Application (NDA) with Breakthrough Therapy designation currently under rolling review based on existing data; complete NDA submission expected 1Q15; MAA in EMA expected 2015 In-line with FDA guidance, two confirmatory post-approval studies to support accelerated approval planned to begin 1H15 Issued U.S. patents through 2023; EU through 2021; potential for extensions Low dose that supports sub-cutaneous route of administration Anticipated low COGS and available manufacturing capacity to meet market demand
|
|
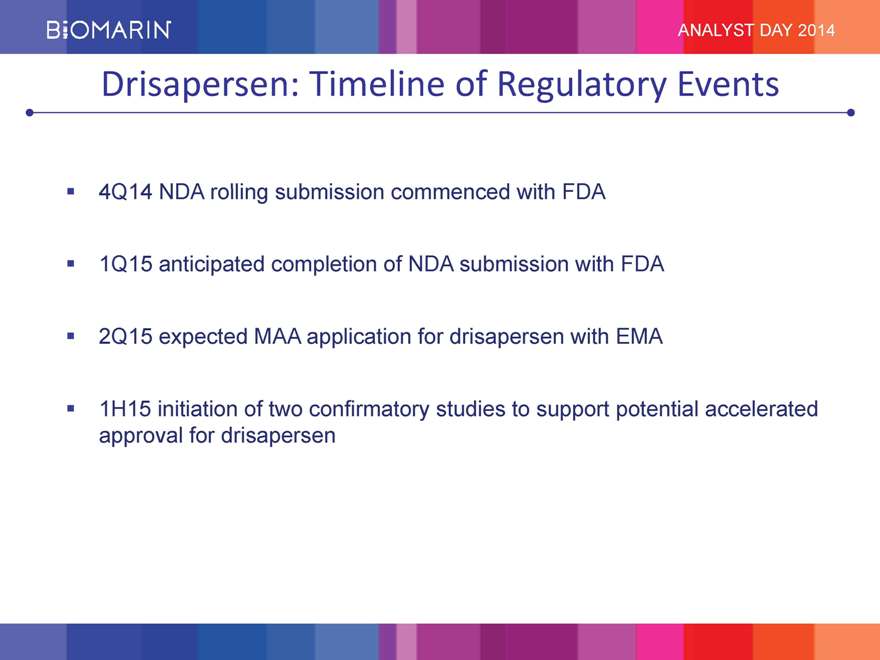
ANALYST DAY 2014 Drisapersen: Timeline of Regulatory Events 4Q14 NDA rolling submission commenced with FDA 1Q15 anticipated completion of NDA submission with FDA 2Q15 expected MAA application for drisapersen with EMA 1H15 initiation of two confirmatory studies to support potential accelerated approval for drisapersen
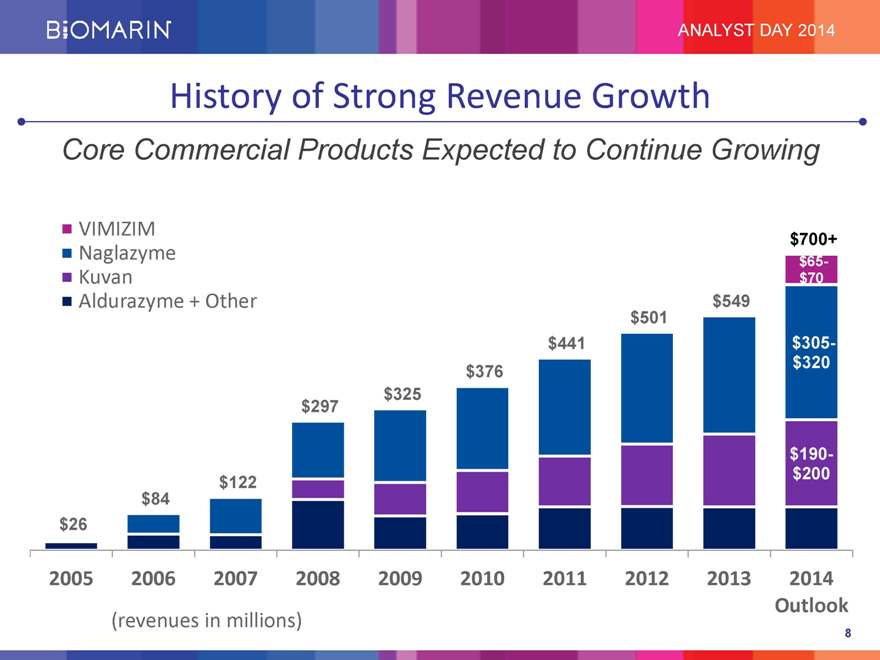
ANALYST DAY 2014 History of Strong Revenue Growth Core Commercial Products Expected to Continue Growing VIMIZIM Naglazyme Kuvan Aldurazyme + Other $26 2005 $84 2006 $122 2007 $297 2008 $325 2009 $376 2010 $441 2011 $501 2012 $549 2013 $700+ $65- $70 $305- $320 $190- $200 2014 (revenues in millions) Outlook 8
ANALYST DAY 2014 What’s New Today? Drisapersen Potential near-term registration opportunity in large market with unmet need Seamless fit within BioMarin’s clinical, regulatory and commercial expertise BMN 111 for Achondroplasia First 3 cohorts enrolled Leveraging the natural history study to inform our understanding of our patients and analytic approaches BMN 190 for CLN2, Late Infantile Form of Batten Disease Enrollment completion imminent Current study exhibiting excellent safety profile to date BMN 270 for Hemophilia A Launching Phase 1 study 1H15 on track two years after licensing Expanded pre-clinical proof of concept to include “real world” (clotting) assays
|
|
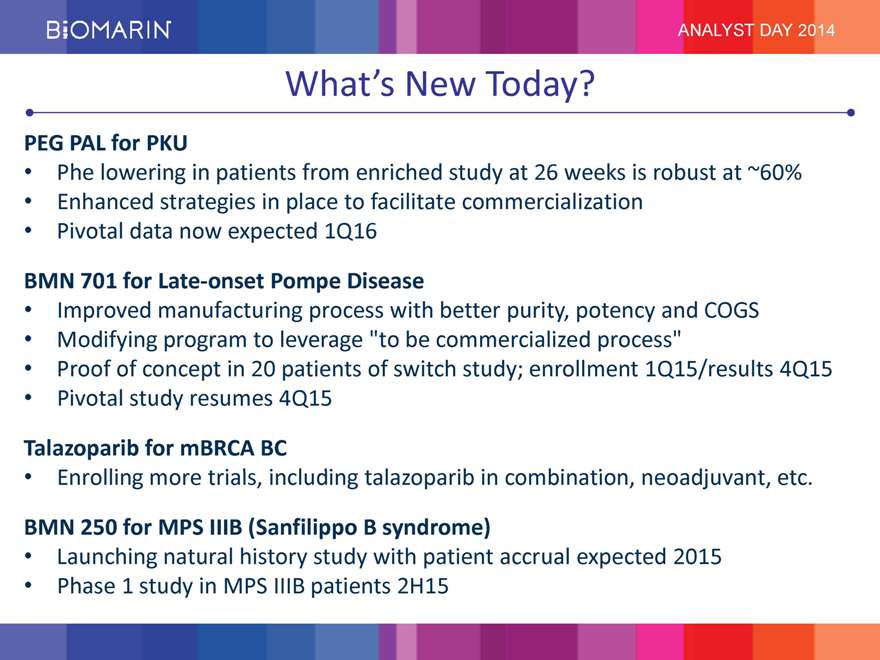
ANALYST DAY 2014 What’s New Today? PEG PAL for PKU Phe lowering in patients from enriched study at 26 weeks is robust at ~60% Enhanced strategies in place to facilitate commercialization Pivotal data now expected 1Q16 BMN 701 for Late-onset Pompe Disease Improved manufacturing process with better purity, potency and COGS Modifying program to leverage “to be commercialized process” Proof of concept in 20 patients of switch study; enrollment 1Q15/results 4Q15 Pivotal study resumes 4Q15 Talazoparib for mBRCA BC Enrolling more trials, including talazoparib in combination, neoadjuvant, etc. BMN 250 for MPS IIIB (Sanfilippo B syndrome) Launching natural history study with patient accrual expected 2015 Phase 1 study in MPS IIIB patients 2H15
|

|
ANALYST DAY 2014 Agenda and Welcome External Speakers Cary O. Harding, M.D., F.A.C.M.G. Professor of Molecular and Medical Genetics and Pediatrics at Oregon Health & Science University Medical Director of the OHSU Biochemical Genetics Laboratory Alfried Kohlschutter, M.D. Expert in NCL disorders (Neuronal Ceroid Lipofuscinoses, Batten disease) Specialty Clinic for Degenerative Brain Disorders in Children and Adolescents, associated with the Department of Pediatrics, University Medical Center in Hamburg, Germany Cathleen Raggio, M.D. Attending Orthopedic Surgeon, Co-Director, The Kathryn O. and Alan C. Greenberg Center for Skeletal Dysplasias at Hospital for Special Surgery, New York Craig McDonald, M.D. Professor and Chair, Department of Physical Medicine & Rehabilitation, Center for Healthcare Policy and Research U.C. Davis Children’s Hospital Expert in the clinical management and rehabilitation of neuromuscular diseases including muscular dystrophies and the development of novel outcome measures for clinical trials Glenn Pierce, M.D., Ph.D. Expert in the field of hemophilia with more than 20 years of experience Previously served as the Chief Medical Officer and Senior Vice President of Biogen Idec Hemophilia Therapeutic Area and senior vice president of Hematology, Cell and Gene Therapies at Biogen Idec Inc. 11
|

|
PKU PROGRAM OVERVIEW – FOCUS ON PEG PAL BIOMARIN ANALYST AND INVESTOR DAY 2014 Wolfgang Dummer, M.D., Ph.D., Vice President, Clinical Development BioMarin
Drisapersen: an overview of the clinical program in Duchenne muscular dystrophy
Craig McDonald, MD Professor and Chair Physical Medicine & Rehabilitation Professor of Pediatrics
Disclosures
Consulting work on Duchenne muscular dystrophy clinical trials for
– PTC Therapeutics
– Sarepta
– Prosensa
– Pfizer
– Eli Lilly
– Halo Therapeutics
– Bristol Myers Squib
– Novartis
– Italfarmaco
– Mitokyne
– Cardero Therapeutics
Site investigator for phase 3 trials for ataluren and eteplirsen Site investigator and study PI for DEMAND V study of drisapersen Site investigator for drisapersen extension trial
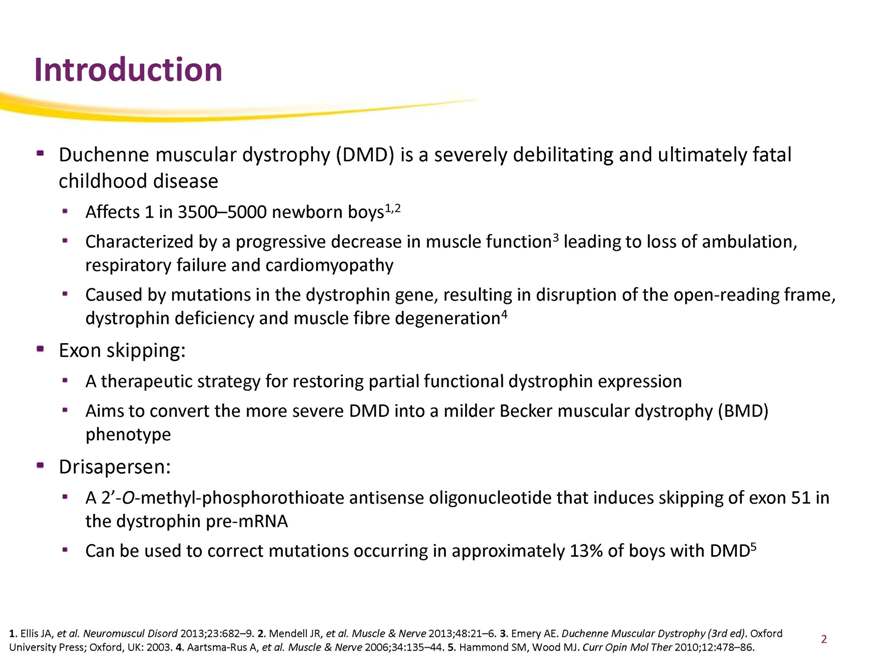
Introduction
Duchenne muscular dystrophy (DMD) is a severely debilitating and ultimately fatal childhood disease
Affects 1 in 3500–5000 newborn boys1,2
Characterized by a progressive decrease in muscle function3 leading to loss of ambulation, respiratory failure and cardiomyopathy Caused by mutations in the dystrophin gene, resulting in disruption of the open-reading frame, dystrophin deficiency and muscle fibre degeneration4
Exon skipping:
A therapeutic strategy for restoring partial functional dystrophin expression
Aims to convert the more severe DMD into a milder Becker muscular dystrophy (BMD) phenotype
Drisapersen:
A 2’-O-methyl-phosphorothioate antisense oligonucleotide that induces skipping of exon 51 in the dystrophin pre-mRNA
Can be used to correct mutations occurring in approximately 13% of boys with DMD5
1. Ellis JA, et al. Neuromuscul Disord 2013;23:682–9. 2. Mendell JR, et al. Muscle & Nerve 2013;48:21–6. 3. Emery AE. Duchenne Muscular Dystrophy (3rd ed). Oxford University Press; Oxford, UK: 2003. 4. Aartsma-Rus A, et al. Muscle & Nerve 2006;34:135–44. 5. Hammond SM, Wood MJ. Curr Opin Mol Ther 2010;12:478–86.
2
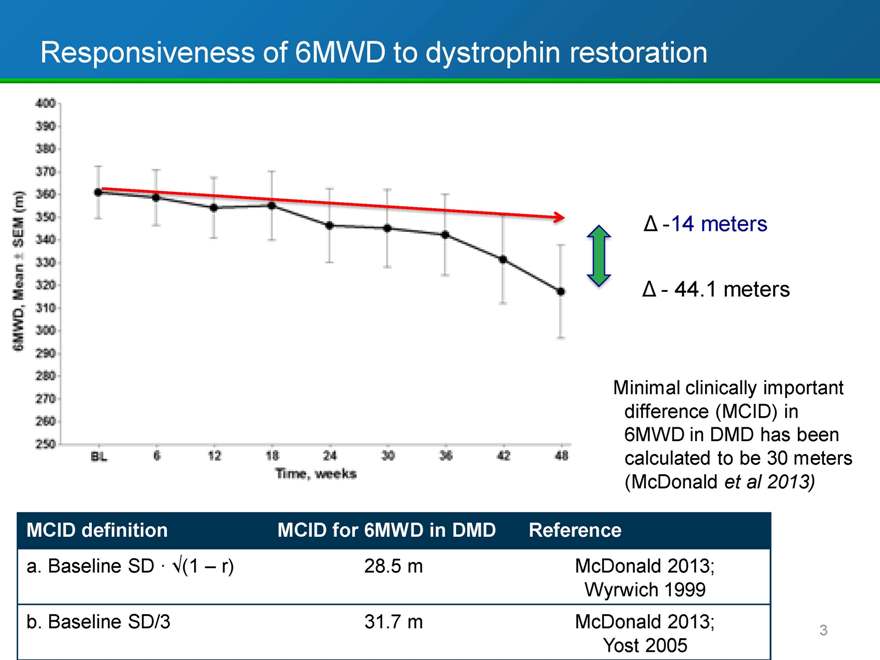
Responsiveness of 6MWD to dystrophin restoration
-14 meters
- 44.1 meters
Minimal clinically important difference (MCID) in 6MWD in DMD has been calculated to be 30 meters (McDonald et al 2013)
MCID definition MCID for 6MWD in DMD Reference
a. Baseline SD · (1 r) 28.5 m McDonald 2013;
Wyrwich 1999
b. Baseline SD/3 31.7 m McDonald 2013;
Yost 2005
3
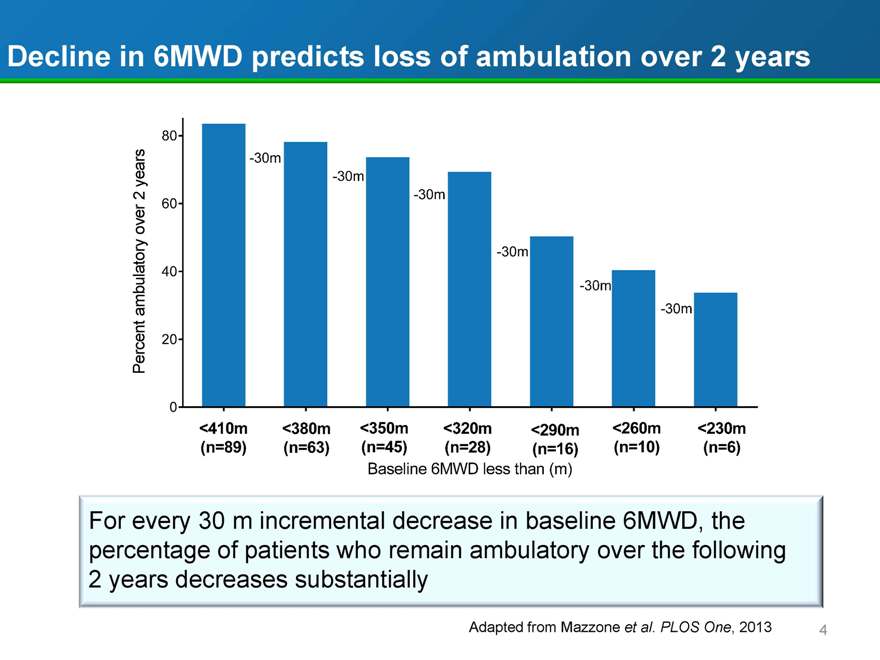
Decline in 6MWD predicts loss of ambulation over 2 years
Percent ambulatory over 2 years
80 60 40 20 0
-30m -30m
-30m
-30m
-30m
-30m
<410m <380m <350m <320m <290m <260m <230m
(n=89)
(n=63) (n=45) (n=28) (n=16) (n=10) (n=6)
Baseline 6MWD less than (m)
For every 30 m incremental decrease in baseline 6MWD, the percentage of patients who remain ambulatory over the following 2 years decreases substantially
Adapted from Mazzone et al. PLOS One, 2013
4
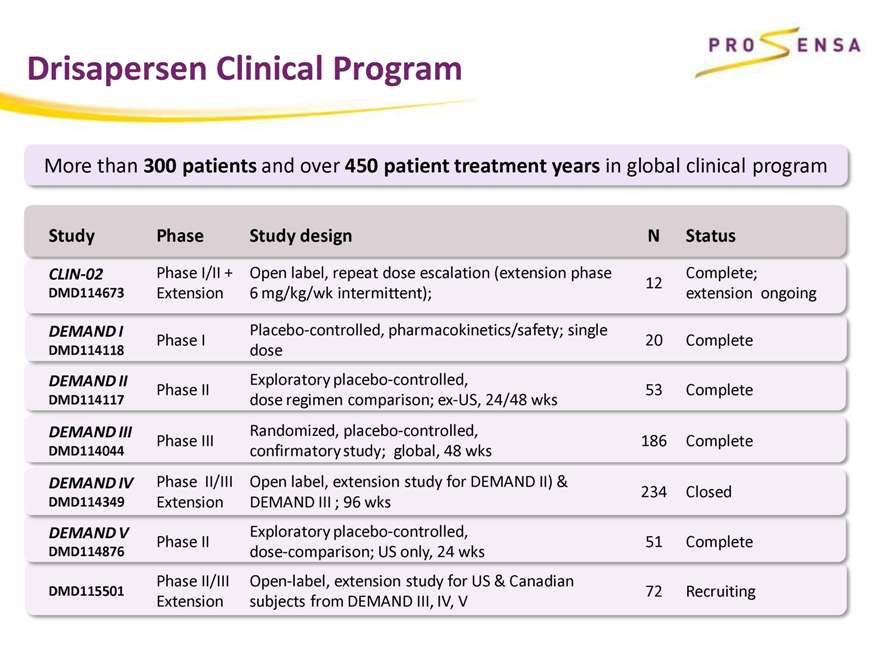
Drisapersen Clinical Program
More than 300 patients and over 450 patient treatment years in global clinical program
Study Phase Study design N Status
CLIN-02 Phase I/II + Open label, repeat dose escalation (extension phase Complete;
12
DMD114673 Extension 6 mg/kg/wk intermittent); extension ongoing
DEMAND I Placebo-controlled, pharmacokinetics/safety; single
Phase I 20 Complete
DMD114118 dose
DEMAND II Exploratory placebo-controlled,
Phase II 53 Complete
DMD114117 dose regimen comparison; ex-US, 24/48 wks
DEMAND III Randomized, placebo-controlled,
Phase III 186 Complete
DMD114044 confirmatory study; global, 48 wks
DEMAND IV Phase II/III Open label, extension study for DEMAND II) & 234 Closed
DMD114349 Extension DEMAND III ; 96 wks
DEMAND V Exploratory placebo-controlled,
Phase II 51 Complete
DMD114876 dose-comparison; US only, 24 wks
Phase II/III Open-label, extension study for US & Canadian
DMD115501 72 Recruiting
Extension subjects from DEMAND III, IV, V
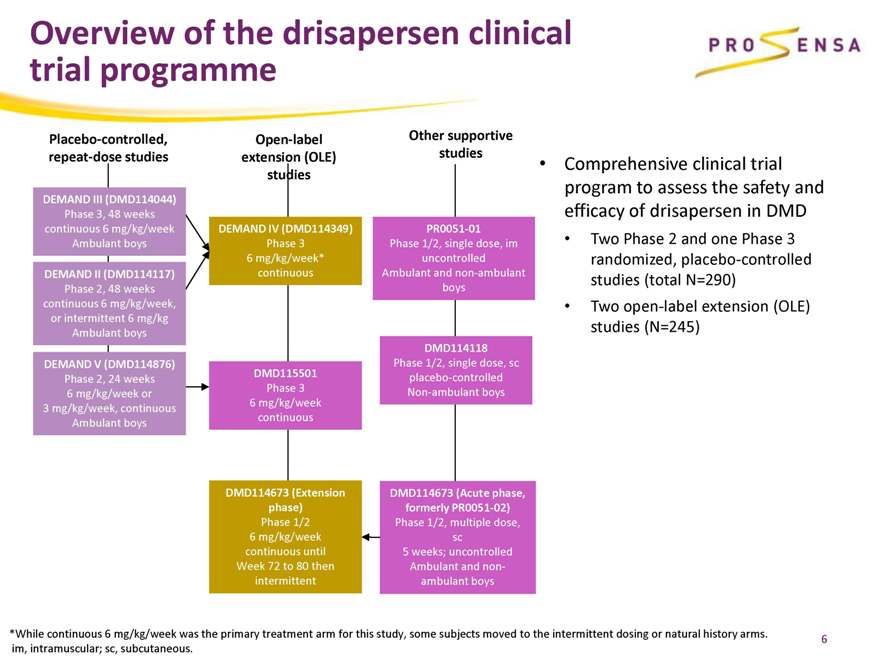
Overview of the drisapersen clinical trial programme
Placebo-controlled, repeat-dose studies
Open-label extension (OLE) studies
Other supportive studies
DEMAND III (DMD114044)
Phase 3, 48 weeks continuous 6 mg/kg/week Ambulant boys
DEMAND II (DMD114117)
Phase 2, 48 weeks continuous 6 mg/kg/week, or intermittent 6 mg/kg Ambulant boys
DEMAND V (DMD114876)
Phase 2, 24 weeks 6 mg/kg/week or 3 mg/kg/week, continuous Ambulant boys
DEMAND IV (DMD114349)
Phase 3 6 mg/kg/week* continuous
DMD115501
Phase 3 6 mg/kg/week continuous
DMD114673 (Extension phase)
Phase 1/2 6 mg/kg/week continuous until Week 72 to 80 then intermittent
PR0051-01
Phase 1/2, single dose, im uncontrolled Ambulant and non-ambulant boys
DMD114118
Phase 1/2, single dose, sc placebo-controlled Non-ambulant boys
DMD114673 (Acute phase, formerly PR0051-02)
Phase 1/2, multiple dose, sc 5 weeks; uncontrolled Ambulant and non-ambulant boys
Comprehensive clinical trial program to assess the safety and efficacy of drisapersen in DMD
Two Phase 2 and one Phase 3 randomized, placebo-controlled studies (total N=290)
Two open-label extension (OLE) studies (N=245)
*While continuous 6 mg/kg/week was the primary treatment arm for this study, some subjects moved to the intermittent dosing or natural history arms. im, intramuscular; sc, subcutaneous.
6
The totality of the clinical program efficacy data suggest:
benefit with drisapersen versus placebo in boys with less severe disease clinically meaningful benefit after longer duration of drisapersen treatment in boys who are, on average, more severely affected
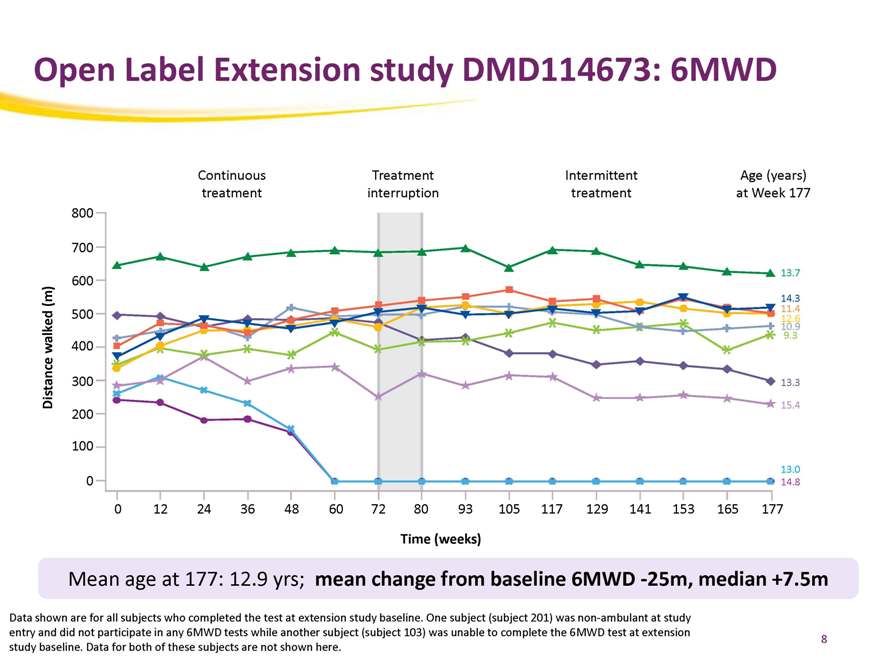
Open Label Extension study DMD114673: 6MWD
Continuous Treatment Intermittent Age (years) treatment interruption treatment at Week 177
800
700
13.7
600
(m) 14.3 500 11.4 12 10. .9 6
9.3
walked 400
300 13.3
Distance 15.4
200
100
13.0
0 14.8
0 12 24 36 48 60 72 80 93 105 117 129 141 153 165 177
Time (weeks)
Mean age at 177: 12.9 yrs; mean change from baseline 6MWD -25m, median +7.5m
Data shown are for all subjects who completed the test at extension study baseline. One subject (subject 201) was non-ambulant at study entry and did not participate in any 6MWD tests while another subject (subject 103) was unable to complete the 6MWD test at extension
study baseline. Data for both of these subjects are not shown here.
8
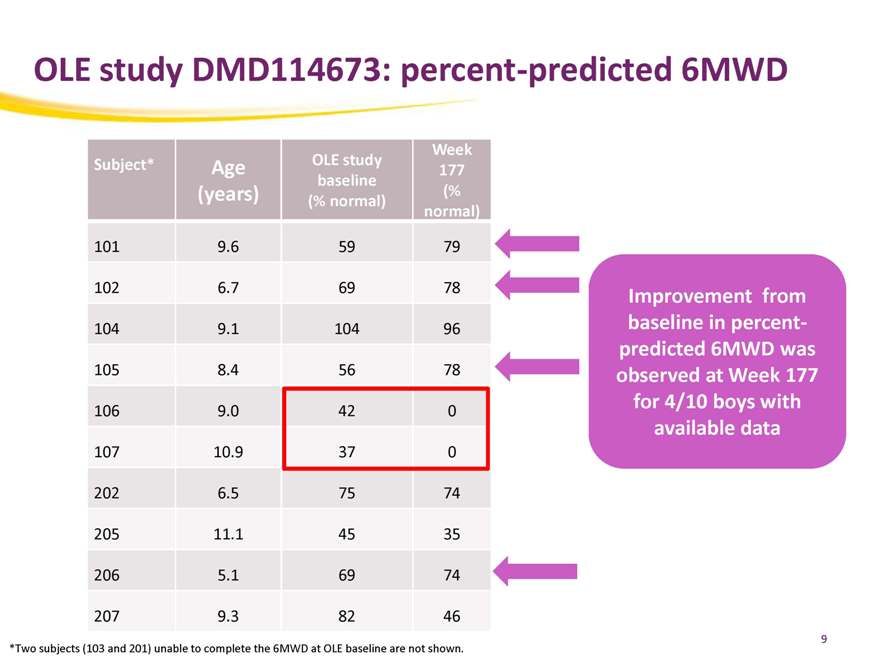
OLE study DMD114673: percent-predicted 6MWD
Week
Subject* Age OLE study 177
baseline
(years) (% normal) (%
normal)
101 9.6 59 79
102 6.7 69 78
104 9.1 104 96
105 8.4 56 78
106 9.0 42 0
107 10.9 37 0
202 6.5 75 74
205 11.1 45 35
206 5.1 69 74
207 9.3 82 46
Improvement from baseline in percent-predicted 6MWD was observed at Week 177 for 4/10 boys with available data
*Two subjects (103 and 201) unable to complete the 6MWD at OLE baseline are not shown.
9
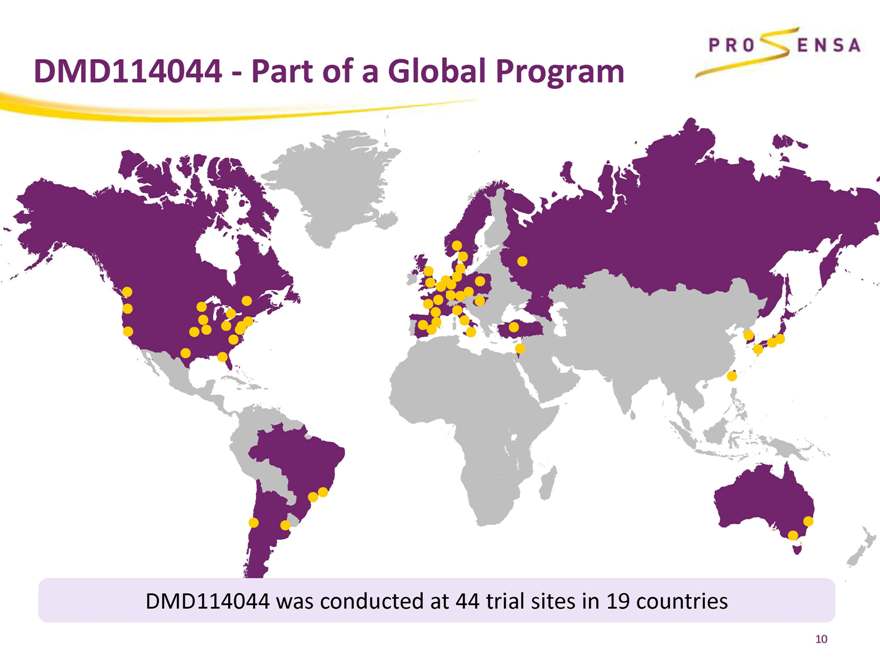
DMD114044 - Part of a Global Program
DMD114044 was conducted at 44 trial sites in 19 countries
10
Mean change from baseline in 6MWD in the Phase 2 and 3 studies
DMD114117
80 70
60
(m 50 40 baseline 30 from 20)
10 0 change –10 n mea –20
–30
–40
Adjusted –50 Drisapersen 6mg/kg intermittent (n=17)
–60 Drisapersen 6 mg/kg/week (n=18)
–70 Placebo (n=18)
–80
Week 1 Day 1 Week 13 Week 25 Week 37 Week 49
DMD114876
Treatment phase Post-treatment phase 60 50
(m) 40 30 baseline 20 from 10 0 change –10 n –20 mea –30
–40
Adjusted –50 Drisapersen 3 mg/kg/week (n=17)
–60 Drisapersen 6 mg/kg/week (n=18)
–70 Placebo (combined; n=16)
–80
Randomization Week 12 Week 24 Week 36 Week 48
Week 1 Day 1 Week 13 Week 25 Week 37 Week 49
DMD114044
60 50
(m) 40 e 30 baselin 20
10 from 0
–10 change –20
–30 mean –40
–50
Adjusted –60
–70 Drisapersen 6 mg/kg/week (n=125)
–80 Placebo (n=61)
–90
Randomization Week 12 Week 24 Week 38 Week 48
Difference in change from baseline in 6MWD for drisapersen 6 mg/kg/week versus placebo
DMD114117
Week 25: 35 m; p=0.014 Week 49: 36 m; p=0.051
DMD114876
Week 24: 27 m; p=0.069
DMD114044
Week48: 10 m; p=0.415
11
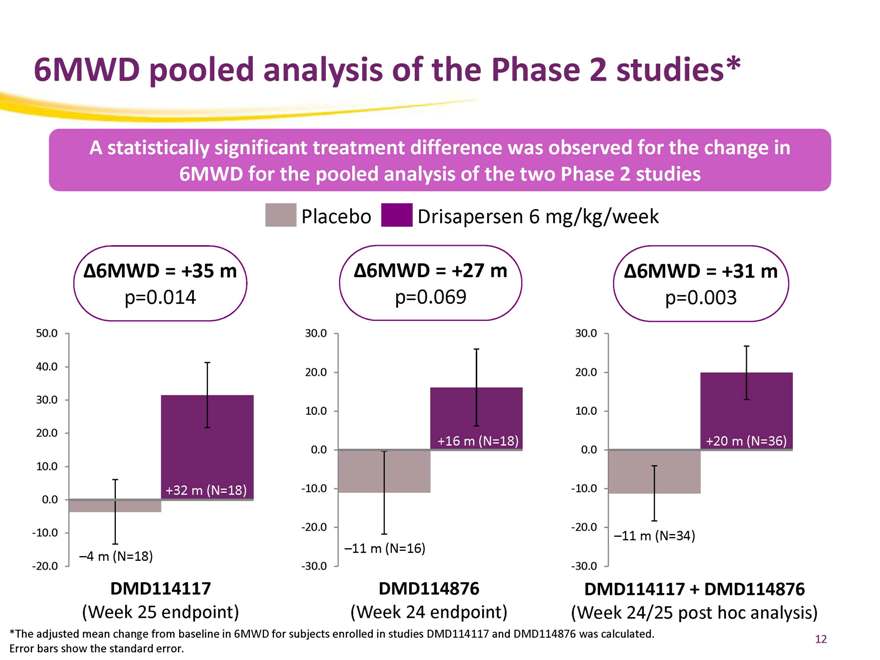
6MWD pooled analysis of the Phase 2 studies*
A statistically significant treatment difference was observed for the change in 6MWD for the pooled analysis of the two Phase 2 studies
Placebo Drisapersen 6 mg/kg/week
Ä6MWD = +35 m Ä6MWD = +27 m Ä6MWD = +31 m p=0.014 p=0.069 p=0.003
50.0 30.0 30.0
40.0 20.0 20.0
30.0
10.0 10.0
20.0
+16 m (N=18) +20 m (N=36)
0.0 0.0
10.0
+32 m (N=18) -10.0 -10.0
0.0
-20.0 -20.0
-10.0 –11 m (N=34)
–4 m (N=18) –11 m (N=16)
-20.0 -30.0 -30.0
DMD114117 DMD114876 DMD114117 + DMD114876
(Week 25 endpoint) (Week 24 endpoint) (Week 24/25 post hoc analysis)
*The adjusted mean change from baseline in 6MWD for subjects enrolled in studies DMD114117 and DMD114876 was calculated. Error bars show the standard error.
12
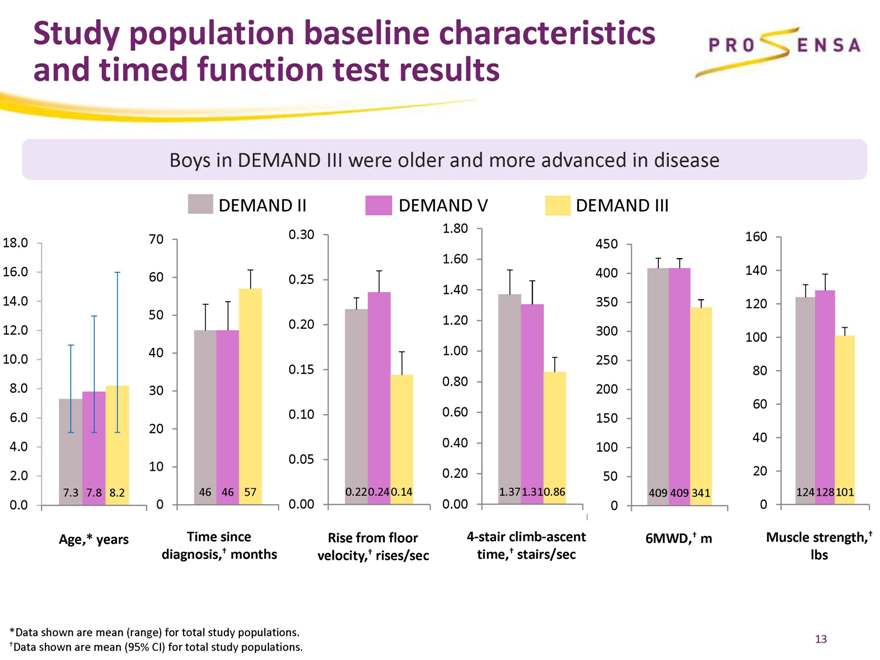
Study population baseline characteristics and timed function test results
Boys in DEMAND III were older and more advanced in disease
DEMAND II DEMAND V DEMAND III
18.0 70 0.30 450 160
1.60
16.0 60 0.25 400 140
1.40
14.0 350 120
50
0.20 1.20
12.0 300 100
40 1.00
10.0 250
0.15 80
0.80
8.0 30 200
60
6.0 0.10 0.60 150
20
0.40 40
4.0 100
10 0.05
2.0 0.20 50 20
7.3 7.8 8.2 46 46 57 0.22 0.24 0.14 1.371.310.86 409 409 341 124 128 101
0.0 0 0.00 0.00 0 0
Age,* years Time since Rise from floor 4-stair climb-ascent 6MWD,† m Muscle strength,† diagnosis,† months velocity,† rises/sec time,† stairs/sec lbs
*Data shown are mean (range) for total study populations. †Data shown are mean (95% CI) for total study populations.
13
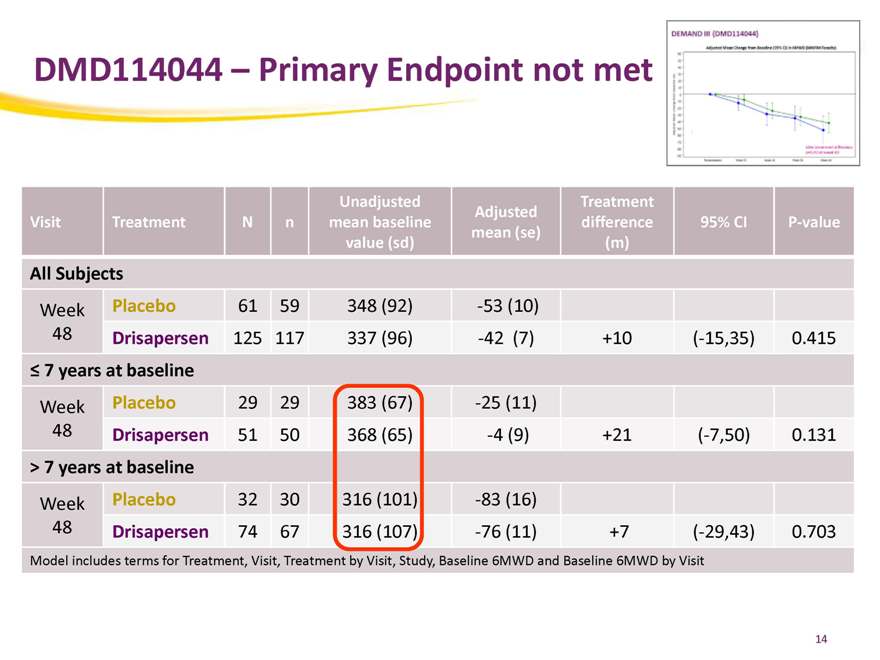
DMD114044 – Primary Endpoint not met
Unadjusted Treatment
Adjusted
Visit Treatment N n mean baseline difference 95% CI P-value
mean (se)
value (sd) (m)
All Subjects
Week Placebo 61 59 348 (92) -53 (10)
48 Drisapersen 125 117 337 (96) -42 (7) +10 (-15,35) 0.415
7 years at baseline
Week Placebo 29 29 383 (67) -25 (11)
48 Drisapersen 51 50 368 (65) -4 (9) +21 (-7,50) 0.131
> 7 years at baseline
Week Placebo 32 30 316 (101) -83 (16)
48 Drisapersen 74 67 316 (107) -76 (11) +7 (-29,43) 0.703
Model includes terms for Treatment, Visit, Treatment by Visit, Study, Baseline 6MWD and Baseline 6MWD by Visit
14
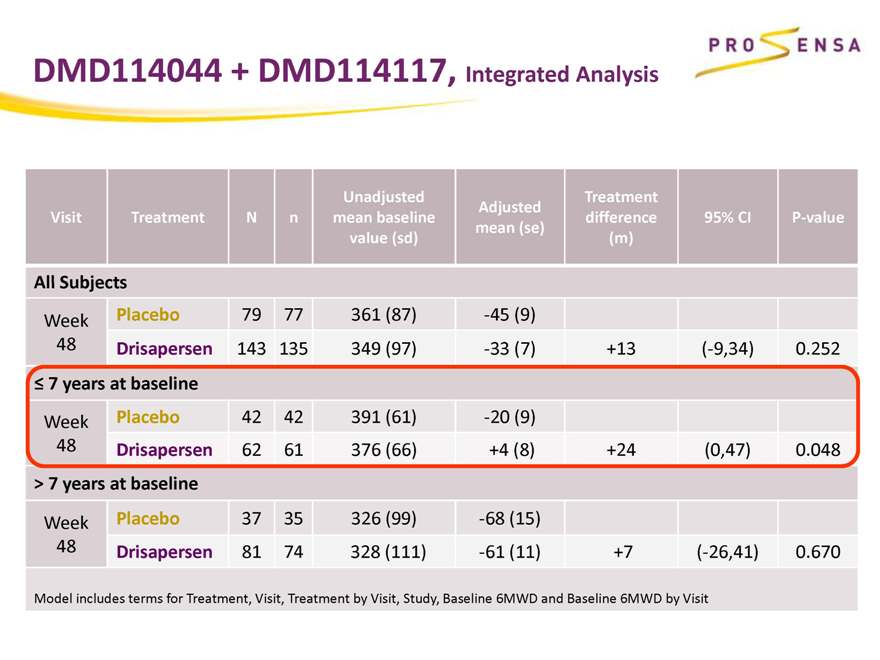
DMD114044 + DMD114117, Integrated Analysis
Unadjusted Treatment
Adjusted
Visit Treatment N n mean baseline difference 95% CI P-value
mean (se)
value (sd) (m)
All Subjects
Week Placebo 79 77 361 (87) -45 (9)
48 Drisapersen 143 135 349 (97) -33 (7) +13 (-9,34) 0.252
7 years at baseline
Week Placebo 42 42 391 (61) -20 (9)
48 Drisapersen 62 61 376 (66) +4 (8) +24 (0,47) 0.048
> 7 years at baseline
Week Placebo 37 35 326 (99) -68 (15)
48 Drisapersen 81 74 328 (111) -61 (11) +7 (-26,41) 0.670
Model includes terms for Treatment, Visit, Treatment by Visit, Study, Baseline 6MWD and Baseline 6MWD by Visit
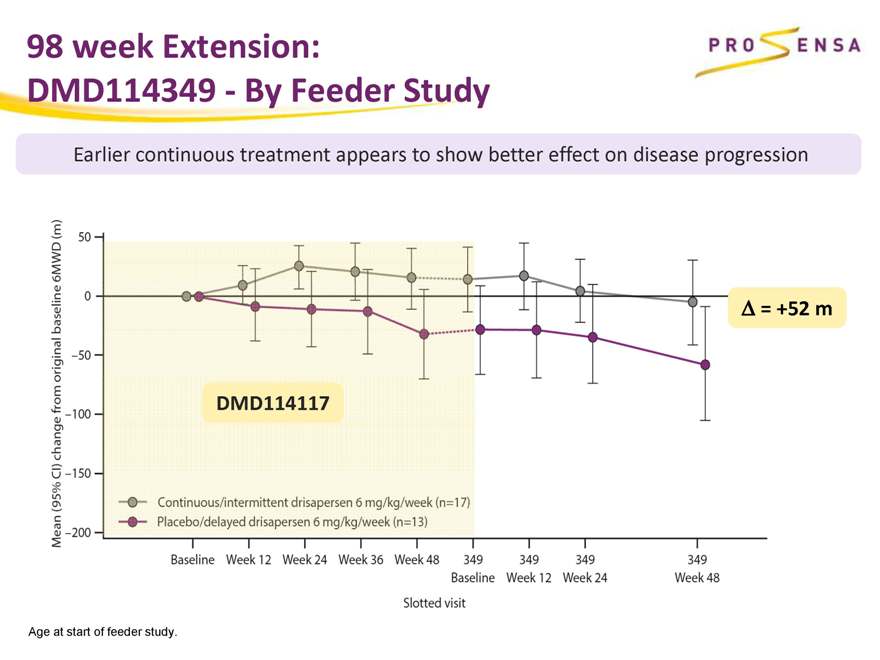
98 week Extension:
DMD114349—By Feeder Study
Earlier continuous treatment appears to show better effect on disease progression
Ä = +52 m DMD114117
Age at start of feeder study.
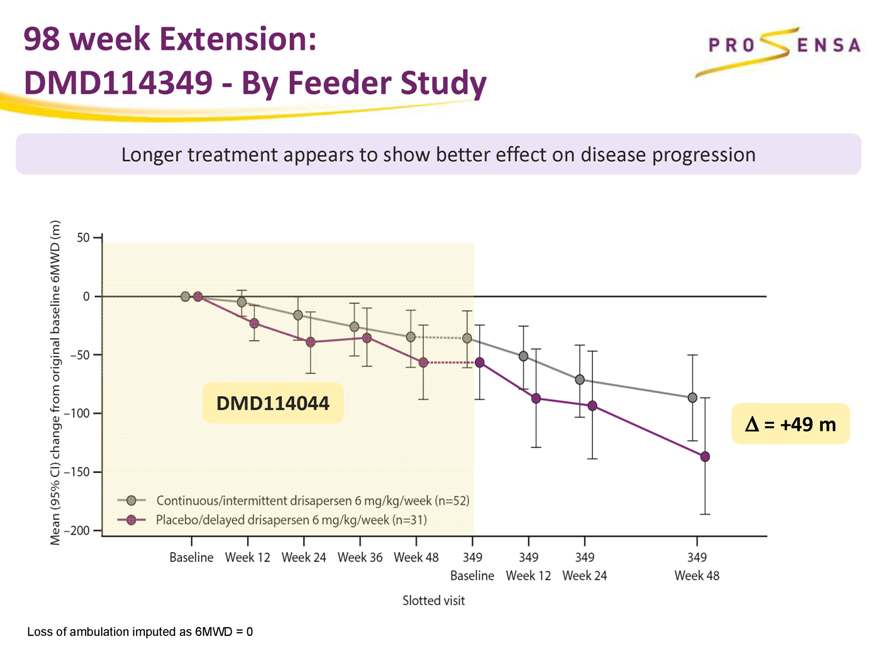
98 week Extension:
DMD114349—By Feeder Study
Longer treatment appears to show better effect on disease progression
DMD114044
Ä = +49 m
Loss of ambulation imputed as 6MWD = 0
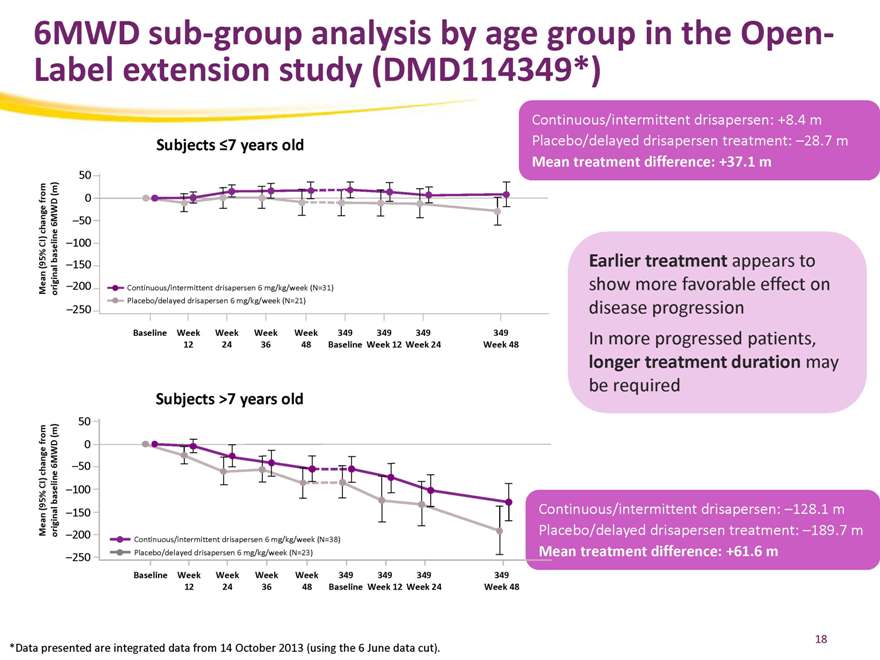
6MWD sub-group analysis by age group in the Open-Label extension study (DMD114349*)
Subjects 7 years old
50
m (m ) fro 0 6MWD –50 change CI) baseline –100 (95% –150 an ginal
Me ori –200 Continuous/intermittent drisapersen 6 mg/kg/week (N=31) Placebo/delayed drisapersen 6 mg/kg/week (N=21)
–250
Baseline Week Week Week Week 349 349 349 349
12 24 36 48 Baseline Week 12 Week 24 Week 48
Continuous/intermittent drisapersen: +8.4 m Placebo/delayed drisapersen treatment: –28.7 m
Mean treatment difference: +37.1 m
Earlier treatment appears to show more favorable effect on disease progression In more progressed patients, longer treatment duration may be required
Subjects >7 years old
50
from(m) 6MWD 0 hange –50 c baseline CI)
–100
(95% Mean original –150
–200
Continuous/intermittent drisapersen 6 mg/kg/week (N=38) Placebo/delayed drisapersen 6 mg/kg/week (N=23)
–250
Baseline Week Week Week Week 349 349 349 349
12 24 36 48 Baseline Week 12 Week 24 Week 48
Continuous/intermittent drisapersen: –128.1 m Placebo/delayed drisapersen treatment: –189.7 m
Mean treatment difference: +61.6 m
*Data presented are integrated data from 14 October 2013 (using the 6 June data cut).
18
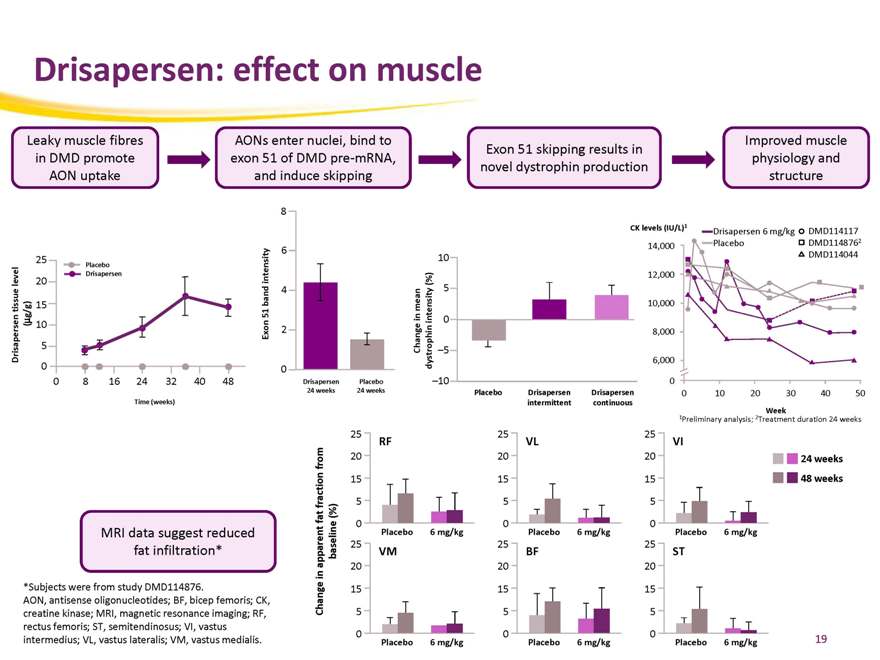
Drisapersen: effect on muscle
Leaky muscle fibres in DMD promote AON uptake
AONs enter nuclei, bind to exon 51 of DMD pre-mRNA, and induce skipping
Exon 51 skipping results in novel dystrophin production
Improved muscle physiology and structure
25
Placebo evel 20 Drisapersen l tissue 15 g/g)
µ ( 10
Drisapersen 5 0
0 8 16 24 32 40 48
Time (weeks)
intensity 6 band 4 51 Exon 2 0
Drisapersen Placebo 24 weeks 24 weeks
Change in mean – dystrophin intensity (%)
10 5 – 0 5 10
Placebo intermittent Drisapersen continuous Drisapersen
CK levels (IU/L)1
Drisapersen 6 mg/kg DMD114117 Placebo DMD1148762 14,000 DMD114044
12,000 10,000 8,000
6,000
0
0 10 20 304050
Week
1Preliminary analysis; 2Treatment duration 24 weeks
25
RF from 20 15 fraction 5 (%) fat 0
Placebo 6 mg/kg
25 apparent baseline VM
20 in
15
Change 5
0 Placebo 6 mg/kg
25
VL
20 15 5
0 Placebo 6 mg/kg
25
BF
20 15 5
0 Placebo 6 mg/kg
25
VI
20 15 5
0 Placebo 6 mg/kg
25
ST
20 15 5
0 Placebo 6 mg/kg
24 weeks
48 weeks
MRI data suggest reduced fat infiltration*
*Subjects were from study DMD114876.
AON, antisense oligonucleotides; BF, bicep femoris; CK, creatine kinase; MRI, magnetic resonance imaging; RF, rectus femoris; ST, semitendinosus; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
19
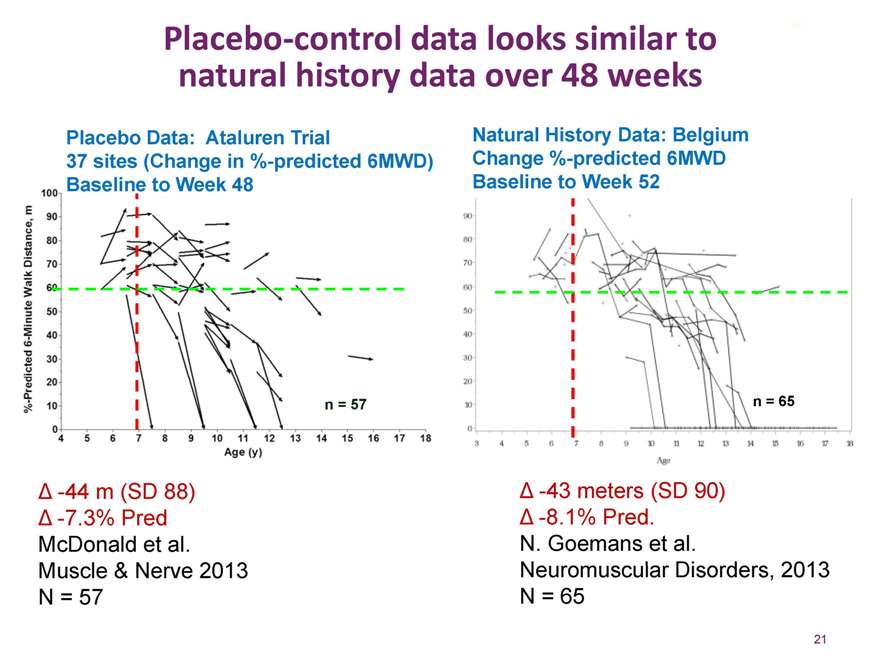
Prosensa and now Biomarin are leveraging what they have learned from natural history studies to design confirmatory studies of drisapersen in DMD
Placebo-control data looks similar to natural history data over 48 weeks
Placebo Data: Ataluren Trial
37 sites (Change in %-predicted 6MWD) Baseline to Week 48
Natural History Data: Belgium Change %-predicted 6MWD Baseline to Week 52
n = 57
n = 65
-44 m (SD 88)
-7.3% Pred McDonald et al. Muscle & Nerve 2013 N = 57
-43 meters (SD 90)
-8.1% Pred.
N. Goemans et al.
Neuromuscular Disorders, 2013 N = 65
21
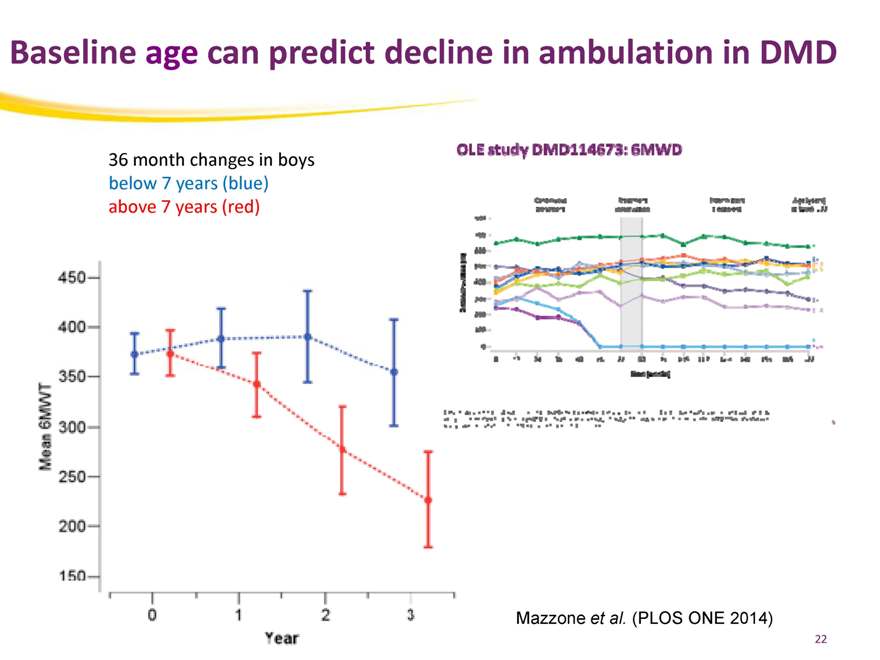
Baseline age can predict decline in ambulation in DMD
36 month changes in boys below 7 years (blue) above 7 years (red)
Mazzone et al. (PLOS ONE 2014)
22
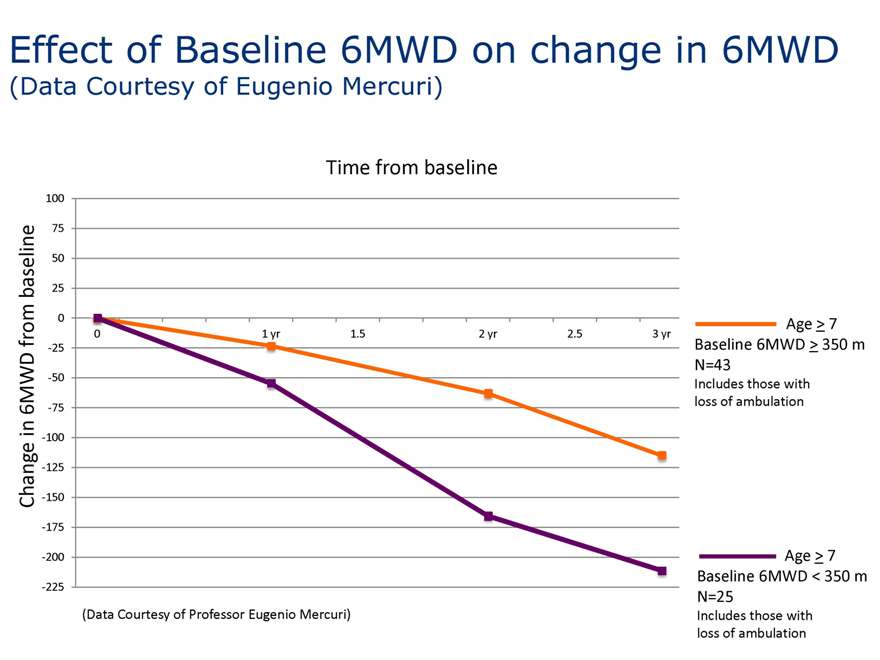
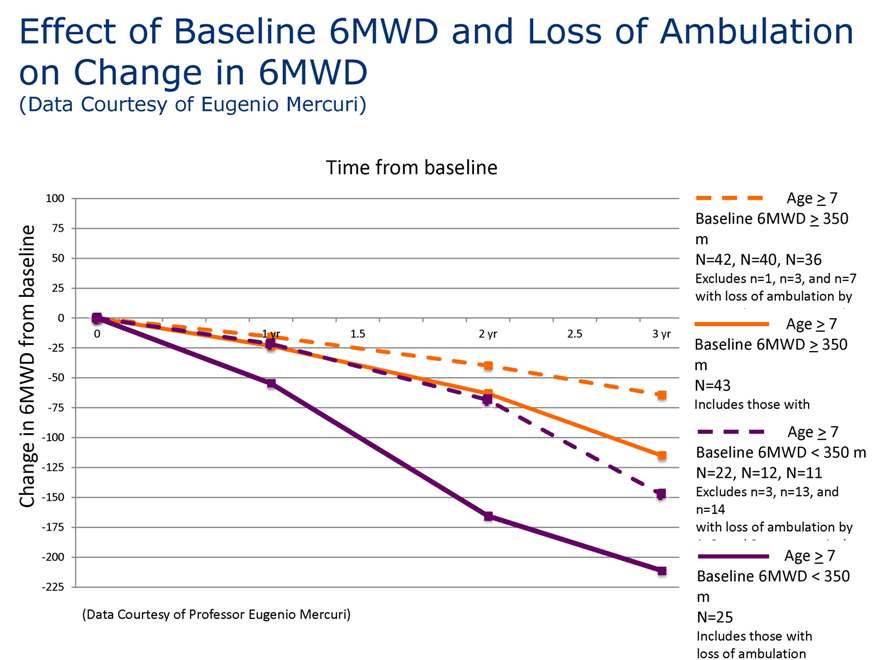
Effect of Baseline 6MWD on change in 6MWD
(Data Courtesy of Eugenio Mercuri)
Time from baseline
100
75
aseline 50
b 25 0 from 0 1 yr 1.5 2 yr 2.5 3 yr -25 WD -50
6M -75
in
-100 nge -125 Cha -150 -175
-200
-225
Age > 7 Baseline 6MWD > 350 m N=43
Includes those with loss of ambulation
Age > 7 Baseline 6MWD < 350 m N=25
Includes those with loss of ambulation
(Data Courtesy of Professor Eugenio Mercuri)
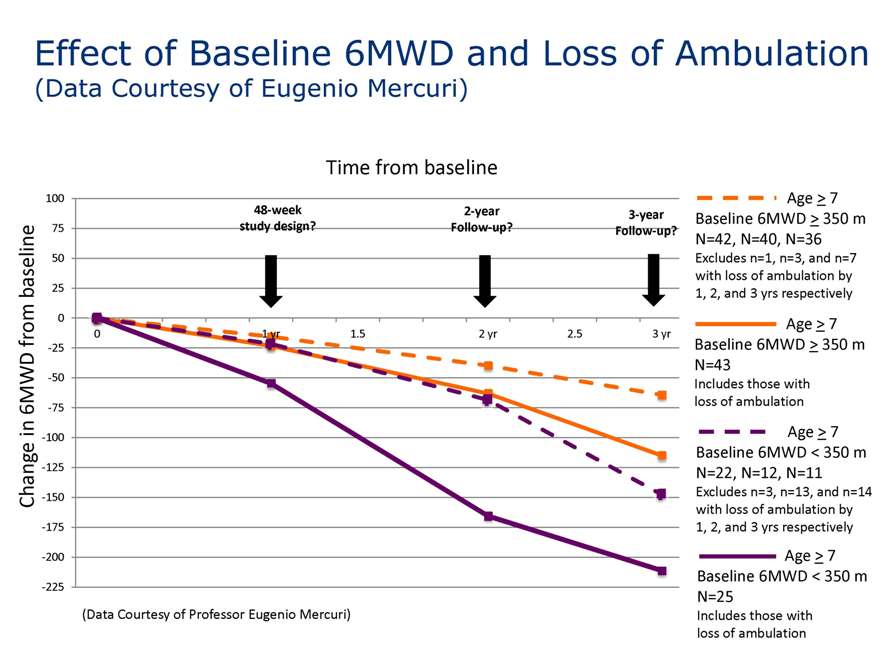
Effect of Baseline 6MWD and Loss of Ambulation on Change in 6MWD
(Data Courtesy of Eugenio Mercuri)
Time from baseline
100
75
aseline 50
b 25 0 from 0 1 yr 1.5 2 yr 2.5 3 yr -25 WD -50
6M -75
in
-100 nge -125 Cha -150 -175
-200
-225
(Data Courtesy of Professor Eugenio Mercuri)
Age > 7 Baseline 6MWD > 350 m N=42, N=40, N=36
Excludes n=1, n=3, and n=7 with loss of ambulation by
Age > 7 Baseline 6MWD > 350 m N=43
Includes those with
Age > 7 Baseline 6MWD < 350 m N=22, N=12, N=11
Excludes n=3, n=13, and n=14 with loss of ambulation by
Age > 7 Baseline 6MWD < 350 m N=25
Includes those with loss of ambulation
Effect of Baseline 6MWD and Loss of Ambulation
(Data Courtesy of Eugenio Mercuri)
Time from baseline
100
48-week 2-year 3-year 75 study design? Follow-up? Follow-up?
aseline 50
b 25 0 from 0 1 yr 1.5 2 yr 2.5 3 yr -25 WD -50
6M -75
in
-100 nge -125 Cha -150 -175
-200
-225
Age > 7 Baseline 6MWD > 350 m N=42, N=40, N=36
Excludes n=1, n=3, and n=7 with loss of ambulation by
1, 2, and 3 yrs respectively
Age > 7 Baseline 6MWD > 350 m N=43
Includes those with loss of ambulation
Age > 7 Baseline 6MWD < 350 m N=22, N=12, N=11
Excludes n=3, n=13, and n=14 with loss of ambulation by
1, 2, and 3 yrs respectively
Age > 7 Baseline 6MWD < 350 m N=25
Includes those with loss of ambulation
(Data Courtesy of Professor Eugenio Mercuri)
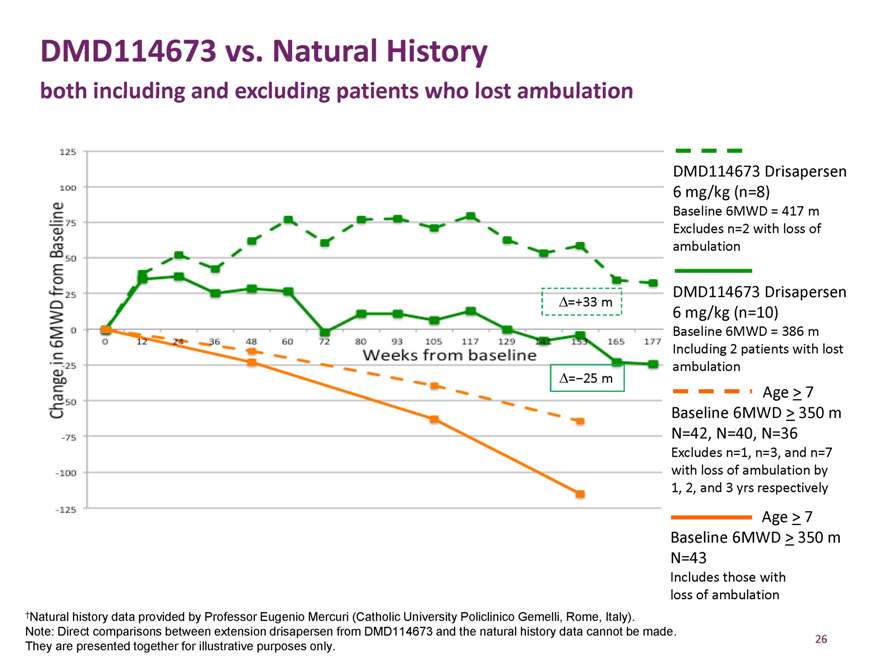
DMD114673 vs. Natural History
both including and excluding patients who lost ambulation
Ä=+33 m
Ä=-25 m
DMD114673 Drisapersen 6 mg/kg (n=8)
Baseline 6MWD = 417 m Excludes n=2 with loss of ambulation
, ,
DMD114673 Drisapersen 6 mg/kg (n=10)
Baseline 6MWD = 386 m Including 2 patients with lost ambulation
Age > 7 Baseline 6MWD > 350 m N=42, N=40, N=36
Excludes n=1, n=3, and n=7 with loss of ambulation by
1, 2, and 3 yrs respectively
Age > 7 Baseline 6MWD > 350 m N=43
Includes those with loss of ambulation
†Natural history data provided by Professor Eugenio Mercuri (Catholic University Policlinico Gemelli, Rome, Italy). Note: Direct comparisons between extension drisapersen from DMD114673 and the natural history data cannot be made. They are presented together for illustrative purposes only.
26
Safety:
Drisapersen was generally well tolerated
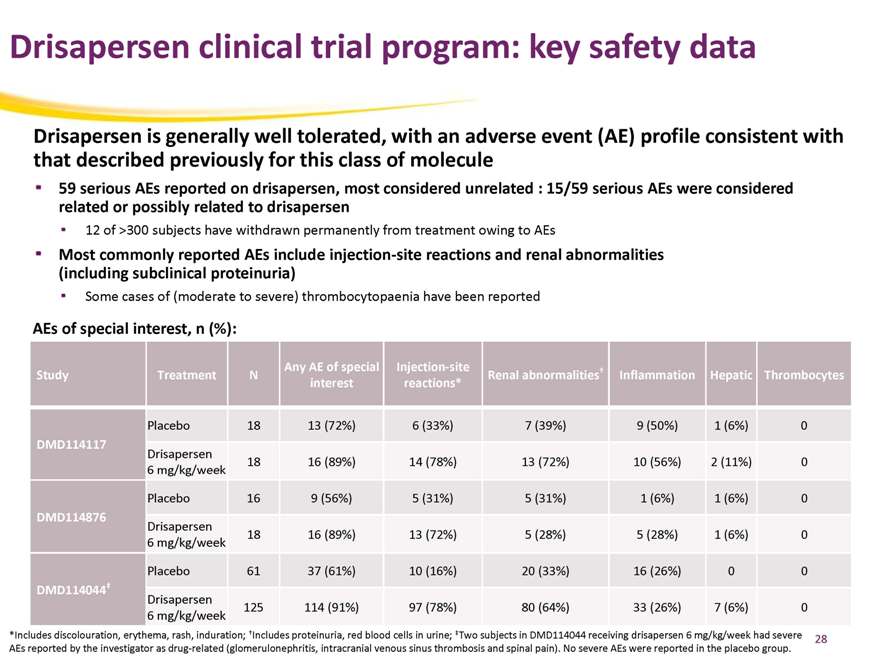
Drisapersen clinical trial program: key safety data
Drisapersen is generally well tolerated, with an adverse event (AE) profile consistent with that described previously for this class of molecule
59 serious AEs reported on drisapersen, most considered unrelated : 15/59 serious AEs were considered related or possibly related to drisapersen
12 of >300 subjects have withdrawn permanently from treatment owing to AEs
Most commonly reported AEs include injection-site reactions and renal abnormalities (including subclinical proteinuria)
Some cases of (moderate to severe) thrombocytopaenia have been reported
AEs of special interest, n (%):
Any AE of special Injection-site †
Study Treatment N Renal abnormalities Inflammation Hepatic Thrombocytes interest reactions*
Placebo 18 13 (72%) 6 (33%) 7 (39%) 9 (50%) 1 (6%) 0
DMD114117
Drisapersen
18 16 (89%) 14 (78%) 13 (72%) 10 (56%) 2 (11%) 0 6 mg/kg/week
Placebo 16 9 (56%) 5 (31%) 5 (31%) 1 (6%) 1 (6%) 0
DMD114876
Drisapersen
18 16 (89%) 13 (72%) 5 (28%) 5 (28%) 1 (6%) 0 6 mg/kg/week
Placebo 61 37 (61%) 10 (16%) 20 (33%) 16 (26%) 0 0
DMD114044‡
Drisapersen
125 114 (91%) 97 (78%) 80 (64%) 33 (26%) 7 (6%) 0 6 mg/kg/week
*Includes discolouration, erythema, rash, induration; †Includes proteinuria, red blood cells in urine; ‡Two subjects in DMD114044 receiving drisapersen 6 mg/kg/week had severe AEs reported by the investigator as drug-related (glomerulonephritis, intracranial venous sinus thrombosis and spinal pain). No severe AEs were reported in the placebo group.
28
Conclusions
Comprehensive clinical trial program (>300 subjects) has been conducted to assess safety and efficacy of drisapersen in DMD, comprising both placebo-controlled and OLE studies The clinical programme efficacy data suggest: benefit with drisapersen versus placebo in boys with less severe disease clinically meaningful benefit after longer duration of drisapersen treatment in boys who are, on average, more severely affected
Totality of the data warrant a filing for Accelerated Approval with the FDA and Conditional approval with the EMA
29
Conclusions
Prosensa and now Biomarin are leveraging what they have learned from natural history studies to design confirmatory studies of drisapersen in DMD characterized by
a) choice of the correct dose based on phase 2 studies; b) longer trial duration based on our experience with the multiple extension studies; and c) an emerging natural history comparison cohort
Drisapersen was generally well tolerated
AEs include sub-clinical proteinuria, local injection-site reactions and thrombocytopenia Addressed by phamacovigilence and emerging dosing regimes (Intermittent dosing after loading; IV route of administration) Overall safety profile is consistent with that described previously for this class of molecule
30
Glenn Pierce, M.D.
Former Chief Medical Officer, Senior Vice President of Biogen Idec Hemophilia Therapeutic Area
BIOMARIN ANALYST AND INVESTOR DAY 2014
BioMarin ANALYST DAY 2014 What’s New Today? Drisapersen • Potential near-term registration opportunity in large market with unmet need • Seamless fit within BioMarin’s clinical, regulatory and commercial expertise BMN 111 for Achondroplasia • First 3 cohorts enrolled • Leveraging the natural history study to inform our understanding of our patients and analytic approaches BMN 190 for CLN2, Late Infantile Form of Batten Disease • Enrollment completion imminent • Current study exhibiting excellent safety profile to date BMN 270 for Hemophilia A • Launching Phase 1 study 1H15 on track two years after licensing • Expanded pre-clinical proof of concept to include “real world” (clotting) assays
|
|
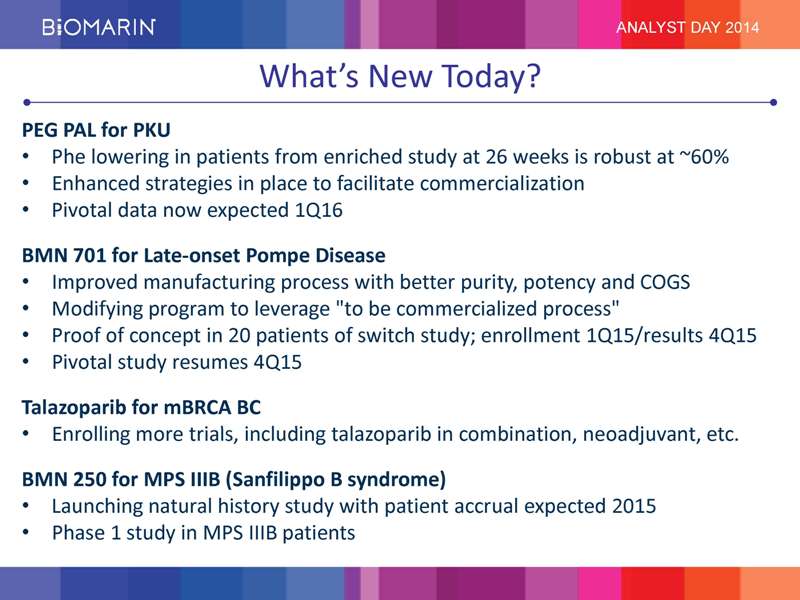
What’s New Today? PEG PAL for PKU • Phe lowering in patients from enriched study at 26 weeks is robust at ~60% • Enhanced strategies in place to facilitate commercialization • Pivotal data now expected 1Q16 BMN 701 for Late-onset Pompe Disease • Improved manufacturing process with better purity, potency and COGS • Modifying program to leverage “to be commercialized process” • Proof of concept in 20 patients of switch study; enrollment 1Q15/results 4Q15 • Pivotal study resumes 4Q15 Talazoparib for mBRCA BC • Enrolling more trials, including talazoparib in combination, neoadjuvant, etc. BMN 250 for MPS IIIB (Sanfilippo B syndrome) • Launching natural history study with patient accrual expected 2015 • Phase 1 study in MPS IIIB patients
|

|
Financial Outlook • Detailed financial guidance to be provided in February 2015 with year-end release • Consistent with past practice • Will allow for Prosensa budget exercise • 2015 Revenues: Generally comfortable with consensus estimates • 2015 Operating Expenses: Consensus R&D and SG&A expenses without Prosensa approximately 5% to 10% too low • GAAP Operating expenses include substantial non cash expenses • 2014 non GAAP loss guidance: $50 million to $65 million • 2015 non GAAP loss to be <$100 million • Prosensa impact. Details to be developed • Preliminary estimate $70 million to $100 million of additional operating expenses, primarily R&D • Estimate additional $60 million to $70 million of GAAP expense associated with CVR accretion
|
|
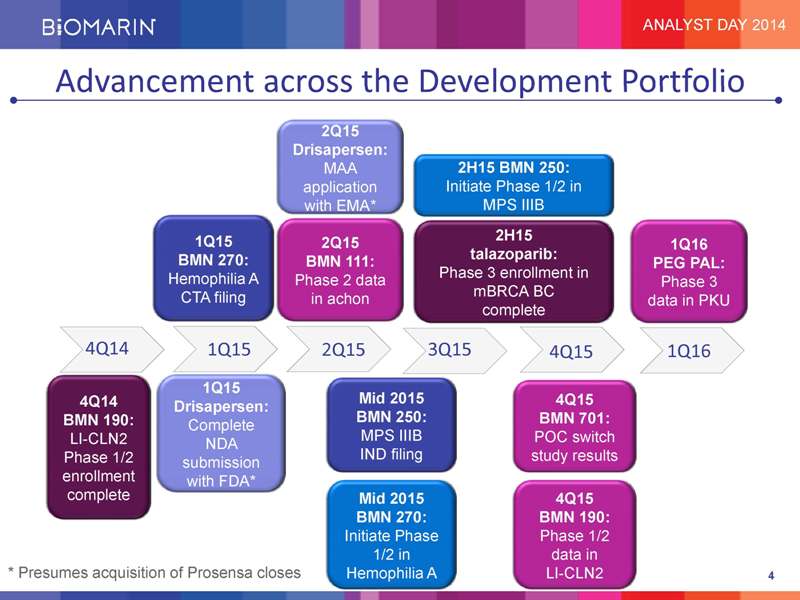
Advancement across the Development Portfolio 2Q15 Drisapersen: MAA 2H15 BMN 250: application Initiate Phase 1/2 in with EMA* MPS IIIB 1Q15 2H15 2Q15 1Q16 BMN 270: talazoparib: BMN 111: PEG PAL: Hemophilia A Phase 3 enrollment in Phase 2 data Phase 3 CTA filing mBRCA BC in achon data in PKU complete 4Q14 1Q15 2Q15 3Q15 4Q15 1Q16 1Q15 4Q14 Mid 2015 4Q15 Drisapersen: BMN 190: BMN 250: BMN 701: Complete LI-CLN2 MPS IIIB POC switch NDA Phase 1/2 IND filing study results enrollment submission with FDA* complete Mid 2015 4Q15 BMN 270: BMN 190: Initiate Phase Phase 1/2 1/2 in data in * Presumes acquisition of Prosensa closes Hemophilia A LI-CLN2 4
Q&A Jean-Jacques Bienaimé Chief Executive Officer Hank Fuchs Chief Medical Officer