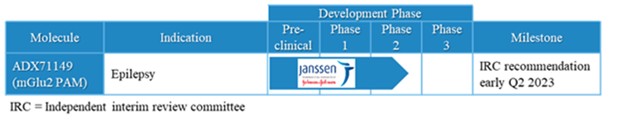
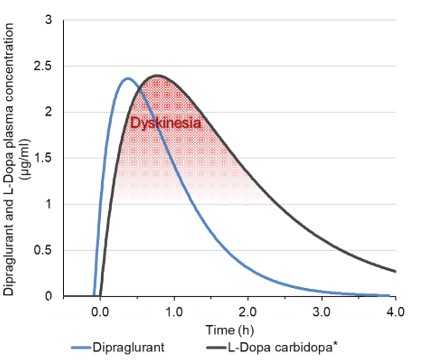
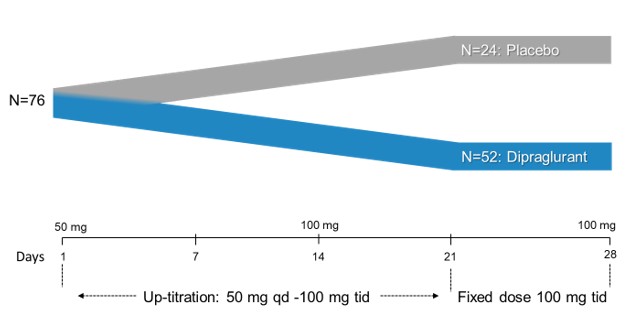
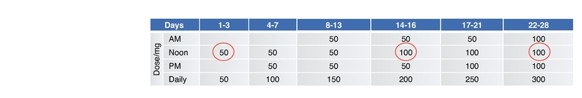
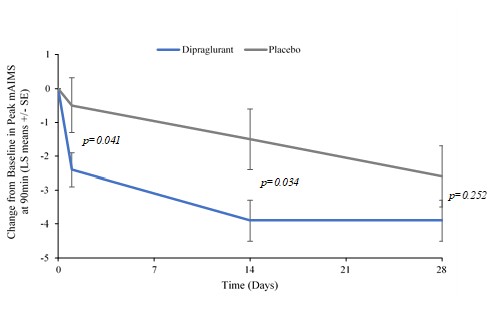
Janssen has sole responsibility, including funding liability, for development of selected compounds under the agreement through preclinical and clinical trials, as well as registration procedures and commercialization, if any, in the United States, Japan, the United Kingdom, Germany, France, Spain and Italy. Janssen has the right to design development programs for selected compounds under the agreement. Through our participation in a joint development committee, we review, in an advisory capacity, any development programs designed by Janssen. However, Janssen has authority over all aspects of the development of selected compounds and may develop or commercialize third-party compounds with a different mechanism of action for identical use.
Under the terms of the Janssen agreement, we received an upfront fee of € 4.6 million and research funding of € 6.4 million during the research period, which ran from 2005 to 2007. In addition, we are eligible for payments on successful achievement of pre-specified clinical and regulatory milestones. We are also eligible for low double digit royalties on net sales of applicable products on a country-by country-basis and on a product by product basis, for a period commencing on the date of first sale and ending upon the latest of the expiration of twelve years from the date of first sale of a product in a given country or the last to expire of our patents containing a claim covering composition of compound comprised in a product sold by Janssen, its affiliates or sublicensees in such country. We received a € 1.5 million milestone payment in relation to the entry of ADX71149 into Phase 1 in July 2009 and a € 2.6 million milestone payment in relation to the entry of ADX71149 into Phase 2 in June 2011. We are eligible for a further €109 million in success-based development and regulatory milestones and low double-digit royalties on net sales.
In the event that no compounds are discovered or identified during the research period, the agreement shall terminate.
Intellectual Property
Patents and Proprietary Rights
As at December 31, 2022, we owned 12 U.S. and 179 foreign patents and a number of pending patent applications that cover various aspects of our allosteric modulator technologies and discovery platform, including several classes of compounds which are potentially useful as modulators of mGlu5, mGlu2, mGlu4, mGlu7 and GABAB. More specifically, our patents and patent applications cover compounds, pharmaceutical compositions, polymorphs and uses of compounds for medical treatment.
Our patent strategy is to file patent applications on innovations and improvements to cover a majority of the major pharmaceutical markets in the world. We typically file priority applications at the United Kingdom Patent Office to establish a priority date for the generic subject matter and examples which are available at the filing date of each invention. Subsequently, we file international applications under the Patent Cooperation Treaty or PCT, with extra examples to support the scope of the claims (International Phase). After the International Phase, we file patent applications in selected countries representing potential major markets for our drug candidates (National/Regional Phase).
Generally, patents have a term of twenty years from the filing date, assuming all maintenance fees are paid. In some instances, patent terms can be increased or decreased, depending on the laws and regulations of the country or jurisdiction that issued the patent. Wherever appropriate and legally possible, we aim at obtaining patent protection for novel molecules, composition of matter and uses for drugs and inventions originating from our research and development efforts, as well as new manufacturing and other processes and formulations. In each case, we carefully balance the value of patent protection against the advantage of keeping the know-how regarding the invention confidential. We aim to position the claims of our applications to exploit gaps in prior art.
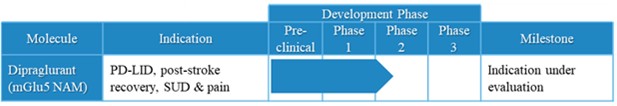
We have two patent families covering dipraglurant as a composition of matter and its polymorphs which are useful as mGlu5 NAMs: 111 patents have been granted to us, including 4 in the United States and 97 in other international jurisdictions (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Great Britain, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Monaco, Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland/Liechtenstein, Turkey, Armenia, Australia, Brazil, Canada, China, Hong Kong, Indonesia, Israel, Japan, South Korea, Mexico, New Zealand, Philippines, Russia, Singapore, South Africa and Ukraine). We also have 4 patent applications pending.
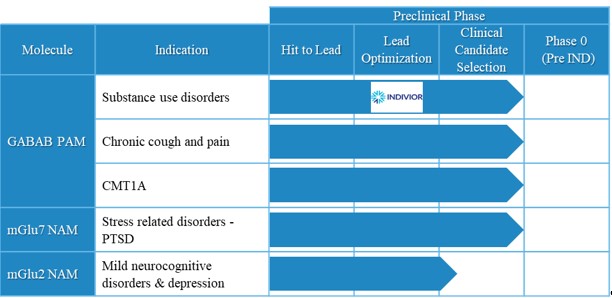
We have one patent family covering compounds which are useful as GABAB PAMs, of which 24 patents have been granted, including 2 in the United States and 22 in other international jurisdictions (Austria, Belgium, Denmark, Finland, France, Germany, Great Britain, Ireland, Italy, Netherlands, Norway, Spain, Sweden, Switzerland/Liechtenstein, Australia, Canada, China, India, Israel, Japan, and New Zealand).