
March 2016 NASDAQ: PULM Engineering the Future for Inhaled Therapeutics Exhibit 99.1

Disclaimers & Forward Looking Statements This presentation contains forward-looking statements. Forward-looking statements may be identified by the use of forward-looking terms such as “anticipates,” “assumes,” “believes,” “can,” “could,” “estimates,” “expects,” “forecasts,” “guides,” “intends,” “is confident that,” “may,” “plans,” “seeks,” “projects,” “targets,” and “would” or the negative of such terms or other variations on such terms or comparable terminology. These statements are not guarantees of future performance, are based on current expectations of future events and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements, including risks that we will not have sufficient working capital, that we will have delays in obtaining, or we will be unable to obtain, FDA or other regulatory approvals for our products, unable to establish collaborations, or that our products will not be commercially viable, among other risks. Additional information about the risk factors that may affect the realization of forward-looking statements is set forth in our filings with the Securities and Exchange Commission (SEC) including our quarterly report on Form 10-Q for the period ended September 30, 2015. Investors and security holders are urged to read these documents free of charge on the SEC’s website at http://www.sec.gov. Forward-looking statements contained in this presentation are made as of this date, and the Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise.
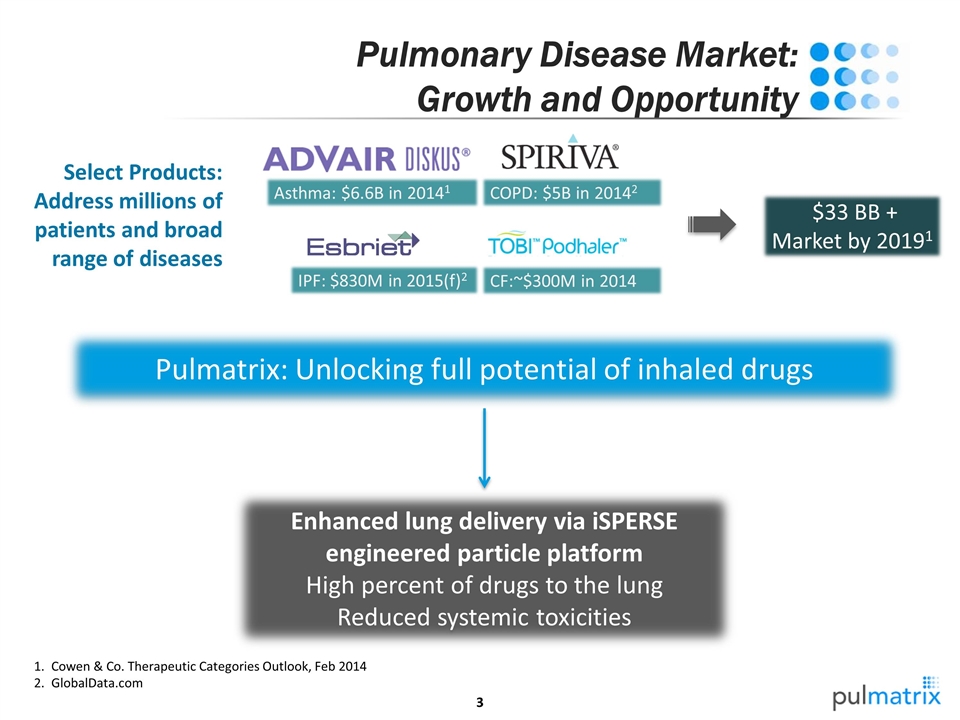
Enhanced lung delivery via iSPERSE engineered particle platform High percent of drugs to the lung Reduced systemic toxicities Pulmatrix: Unlocking full potential of inhaled drugs $33 BB + Market by 20191 Select Products: Address millions of patients and broad range of diseases Pulmonary Disease Market: Growth and Opportunity Asthma: $6.6B in 20141 COPD: $5B in 20142 IPF: $830M in 2015(f)2 CF:~$300M in 2014 Cowen & Co. Therapeutic Categories Outlook, Feb 2014 GlobalData.com
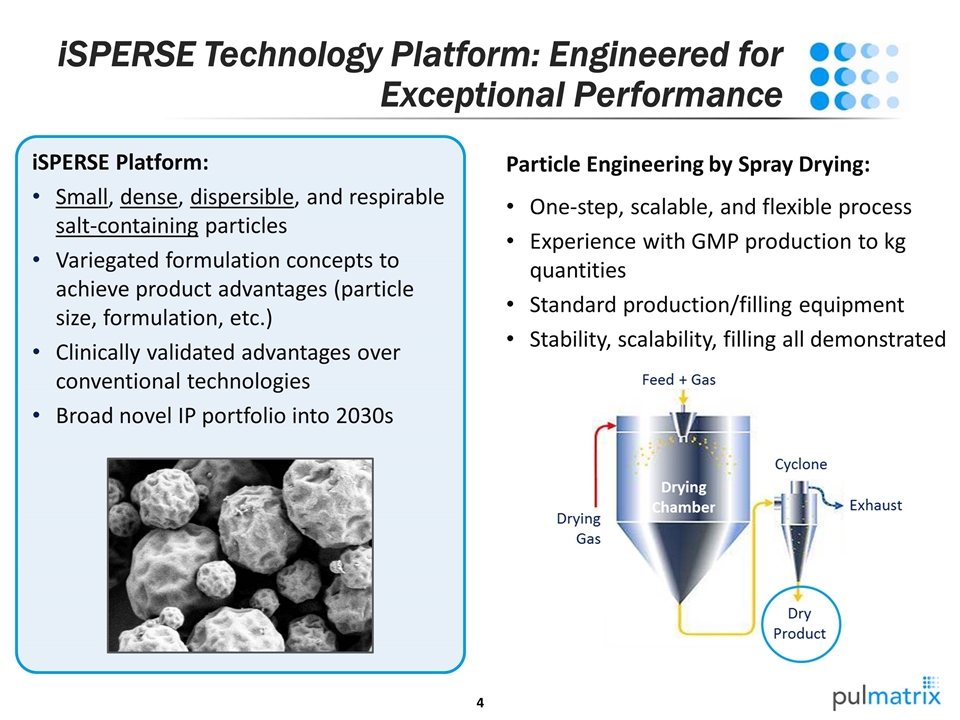
iSPERSE Platform: Small, dense, dispersible, and respirable salt-containing particles Variegated formulation concepts to achieve product advantages (particle size, formulation, etc.) Clinically validated advantages over conventional technologies Broad novel IP portfolio into 2030s Particle Engineering by Spray Drying: One-step, scalable, and flexible process Experience with GMP production to kg quantities Standard production/filling equipment Stability, scalability, filling all demonstrated iSPERSE Technology Platform: Engineered for Exceptional Performance
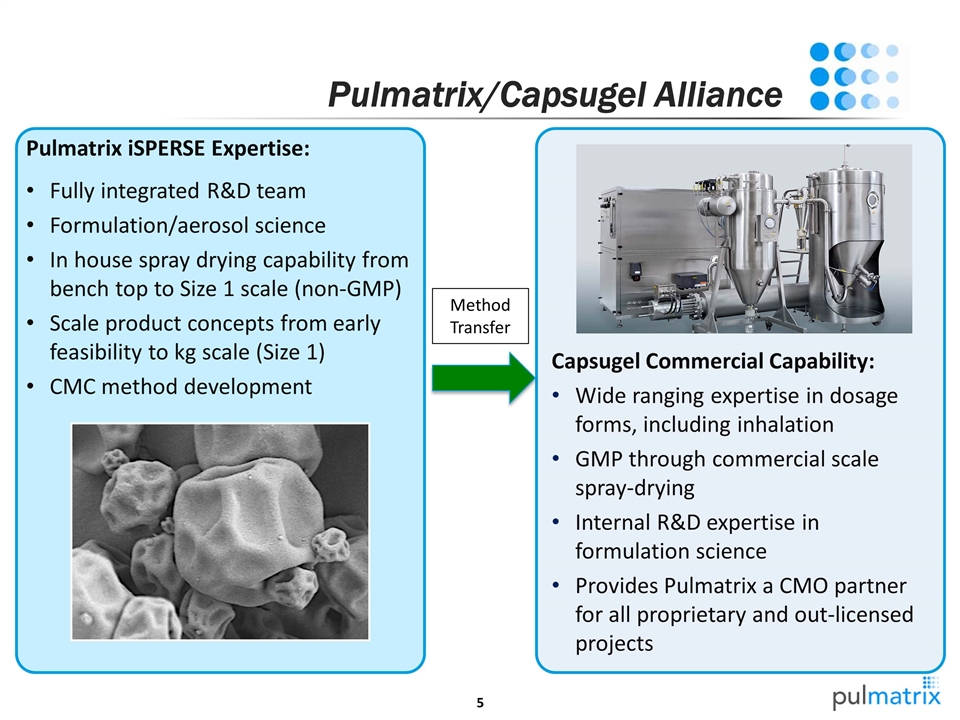
Pulmatrix/Capsugel Alliance Capsugel Commercial Capability: Wide ranging expertise in dosage forms, including inhalation GMP through commercial scale spray-drying Internal R&D expertise in formulation science Provides Pulmatrix a CMO partner for all proprietary and out-licensed projects Pulmatrix iSPERSE Expertise: Fully integrated R&D team Formulation/aerosol science In house spray drying capability from bench top to Size 1 scale (non-GMP) Scale product concepts from early feasibility to kg scale (Size 1) CMC method development Method Transfer
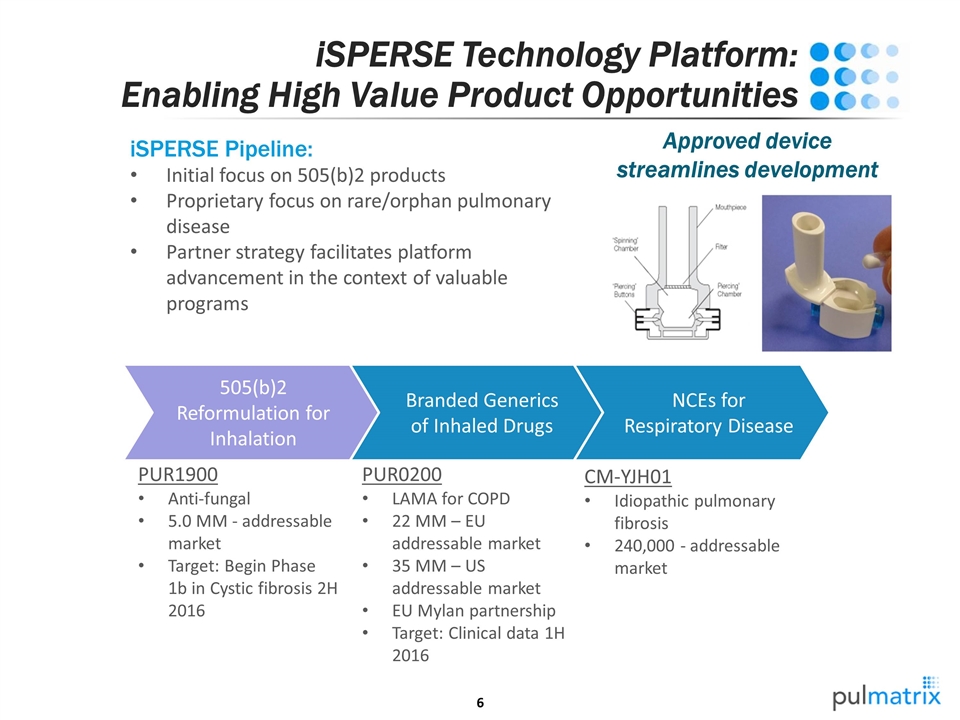
iSPERSE Technology Platform: Enabling High Value Product Opportunities iSPERSE Pipeline: Initial focus on 505(b)2 products Proprietary focus on rare/orphan pulmonary disease Partner strategy facilitates platform advancement in the context of valuable programs NCEs for Respiratory Disease Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation PUR0200 LAMA for COPD 22 MM – EU addressable market 35 MM – US addressable market EU Mylan partnership Target: Clinical data 1H 2016 PUR1900 Anti-fungal 5.0 MM - addressable market Target: Begin Phase 1b in Cystic fibrosis 2H 2016 CM-YJH01 Idiopathic pulmonary fibrosis 240,000 - addressable market Approved device streamlines development
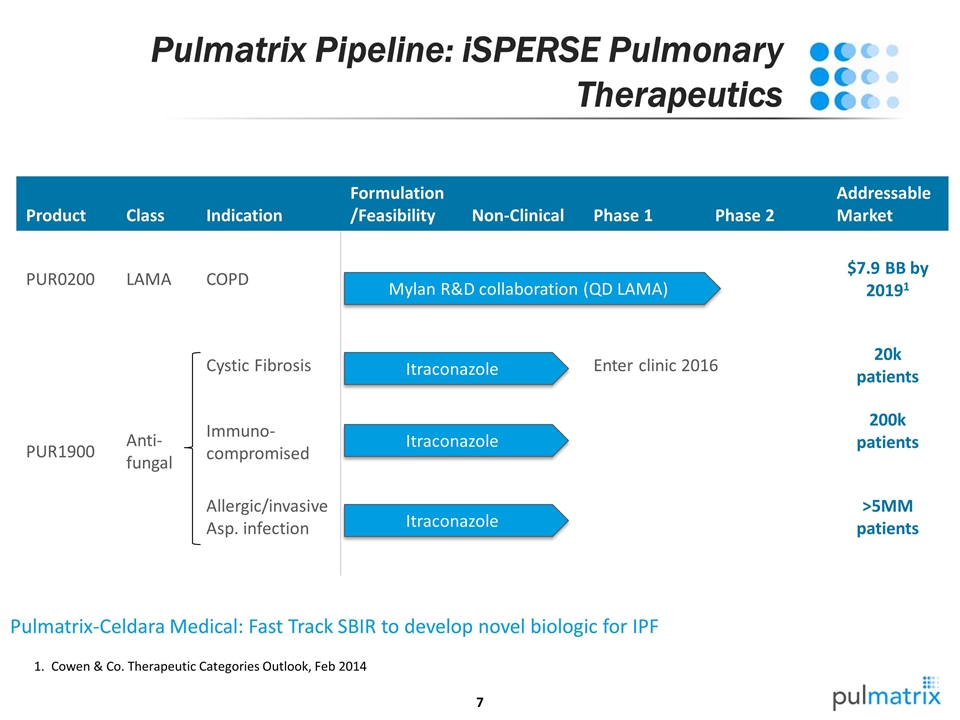
Pulmatrix Pipeline: iSPERSE Pulmonary Therapeutics Product Class Indication Formulation/Feasibility Non-Clinical Phase 1 Phase 2 Addressable Market PUR0200 LAMA COPD $7.9 BB by 20191 PUR1900 Anti-fungal Cystic Fibrosis Enter clinic 2016 20k patients Immuno- compromised 200k patients Allergic/invasive Asp. infection >5MM patients Pulmatrix-Celdara Medical: Fast Track SBIR to develop novel biologic for IPF Mylan R&D collaboration (QD LAMA) Itraconazole Cowen & Co. Therapeutic Categories Outlook, Feb 2014 Itraconazole Itraconazole
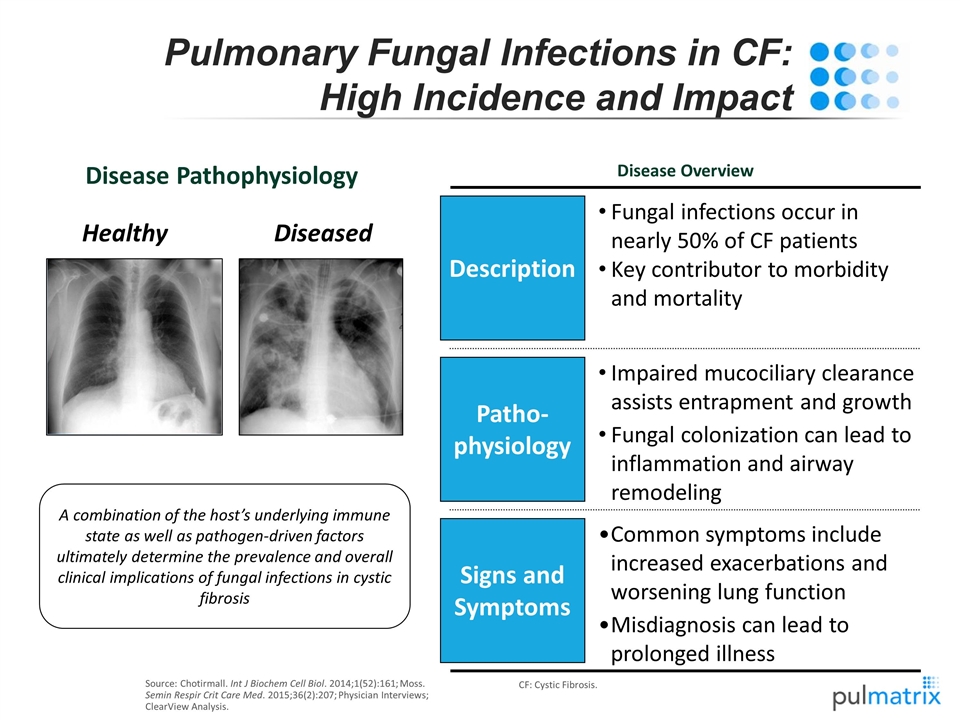
Source: Chotirmall. Int J Biochem Cell Biol. 2014;1(52):161; Moss. Semin Respir Crit Care Med. 2015;36(2):207; Physician Interviews; ClearView Analysis. CF: Cystic Fibrosis. Pulmonary Fungal Infections in CF: High Incidence and Impact A combination of the host’s underlying immune state as well as pathogen-driven factors ultimately determine the prevalence and overall clinical implications of fungal infections in cystic fibrosis Disease Pathophysiology Disease Overview Healthy Diseased Description Patho-physiology Signs and Symptoms Impaired mucociliary clearance assists entrapment and growth Fungal colonization can lead to inflammation and airway remodeling Common symptoms include increased exacerbations and worsening lung function Misdiagnosis can lead to prolonged illness Fungal infections occur in nearly 50% of CF patients Key contributor to morbidity and mortality
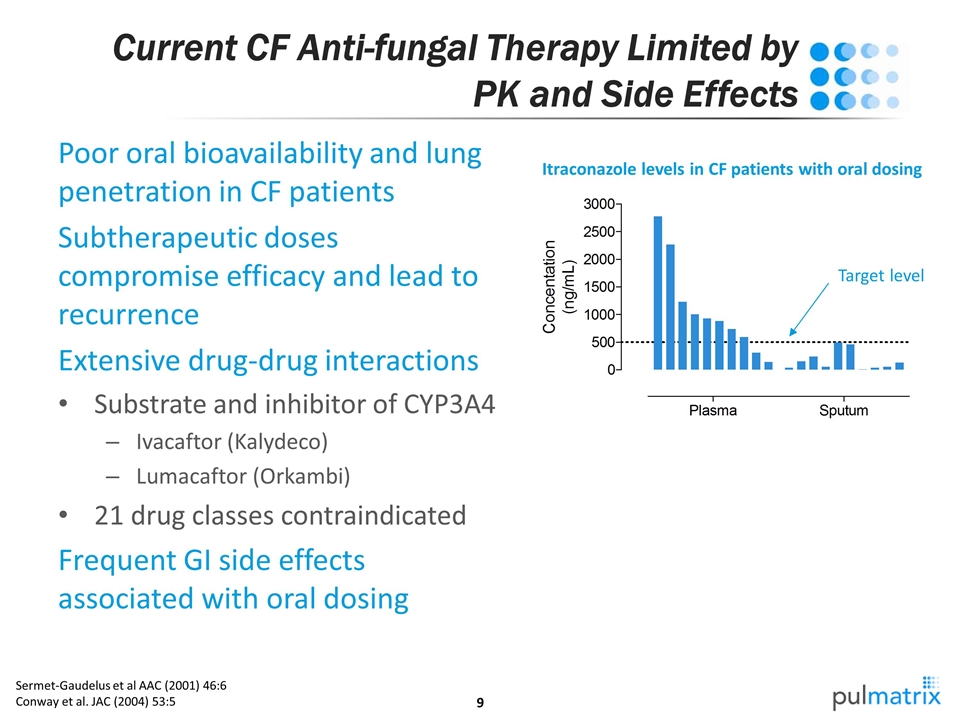
Current CF Anti-fungal Therapy Limited by PK and Side Effects Poor oral bioavailability and lung penetration in CF patients Subtherapeutic doses compromise efficacy and lead to recurrence Extensive drug-drug interactions Substrate and inhibitor of CYP3A4 Ivacaftor (Kalydeco) Lumacaftor (Orkambi) 21 drug classes contraindicated Frequent GI side effects associated with oral dosing Sermet-Gaudelus et al AAC (2001) 46:6 Conway et al. JAC (2004) 53:5 Itraconazole levels in CF patients with oral dosing Target level
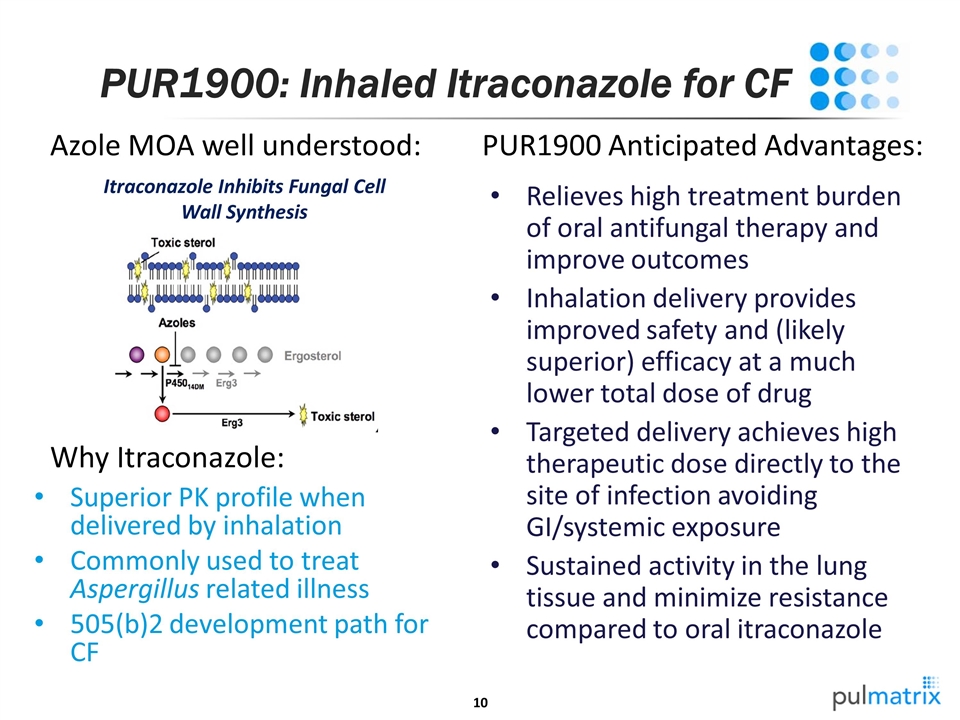
Relieves high treatment burden of oral antifungal therapy and improve outcomes Inhalation delivery provides improved safety and (likely superior) efficacy at a much lower total dose of drug Targeted delivery achieves high therapeutic dose directly to the site of infection avoiding GI/systemic exposure Sustained activity in the lung tissue and minimize resistance compared to oral itraconazole PUR1900: Inhaled Itraconazole for CF Itraconazole Inhibits Fungal Cell Wall Synthesis Superior PK profile when delivered by inhalation Commonly used to treat Aspergillus related illness 505(b)2 development path for CF Azole MOA well understood: Why Itraconazole: PUR1900 Anticipated Advantages:
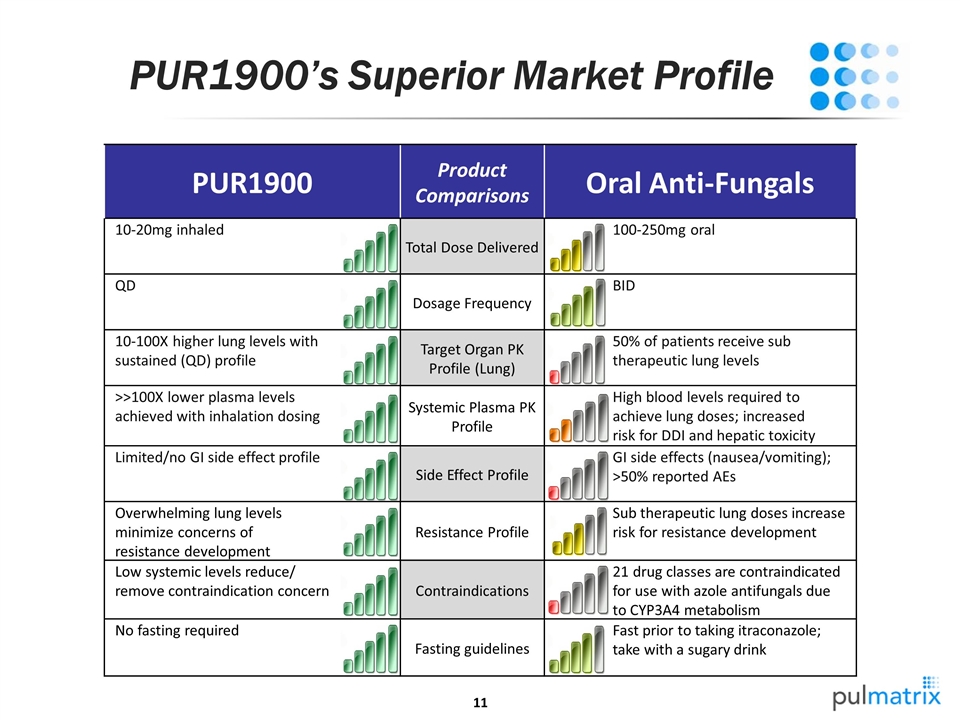
PUR1900’s Superior Market Profile PUR1900 Product Comparisons Oral Anti-Fungals 10-20mg inhaled Total Dose Delivered 100-250mg oral QD Dosage Frequency BID 10-100X higher lung levels with sustained (QD) profile Target Organ PK Profile (Lung) 50% of patients receive sub therapeutic lung levels >>100X lower plasma levels achieved with inhalation dosing Systemic Plasma PK Profile High blood levels required to achieve lung doses; increased risk for DDI and hepatic toxicity Limited/no GI side effect profile Side Effect Profile GI side effects (nausea/vomiting); >50% reported AEs Overwhelming lung levels minimize concerns of resistance development Resistance Profile Sub therapeutic lung doses increase risk for resistance development Low systemic levels reduce/ remove contraindication concern Contraindications 21 drug classes are contraindicated for use with azole antifungals due to CYP3A4 metabolism No fasting required Fasting guidelines Fast prior to taking itraconazole; take with a sugary drink
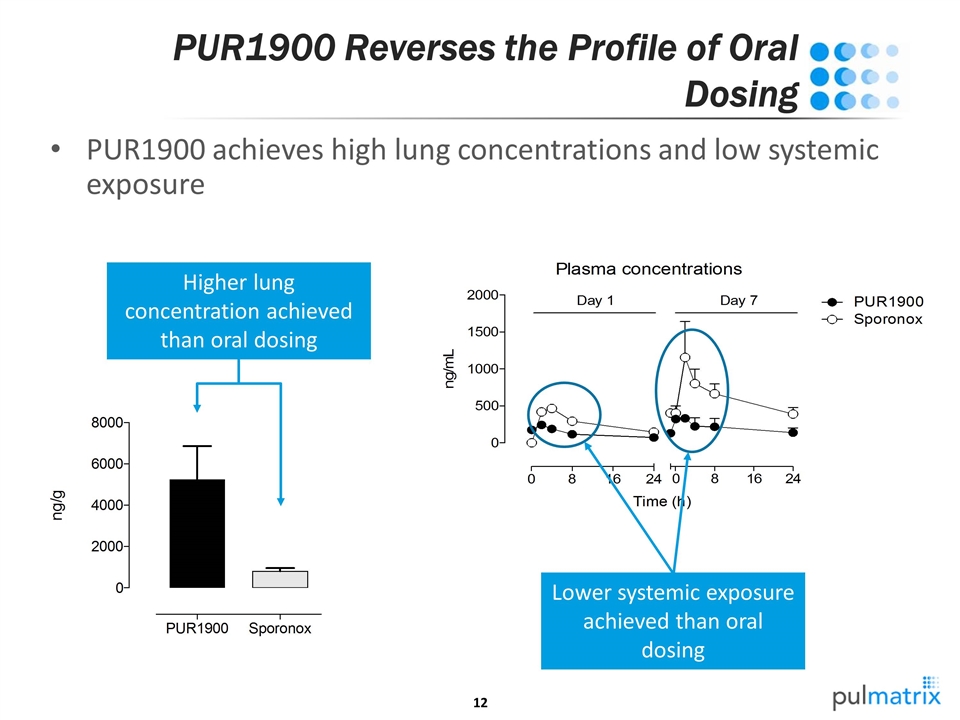
PUR1900 Reverses the Profile of Oral Dosing PUR1900 achieves high lung concentrations and low systemic exposure Higher lung concentration achieved than oral dosing Lower systemic exposure achieved than oral dosing
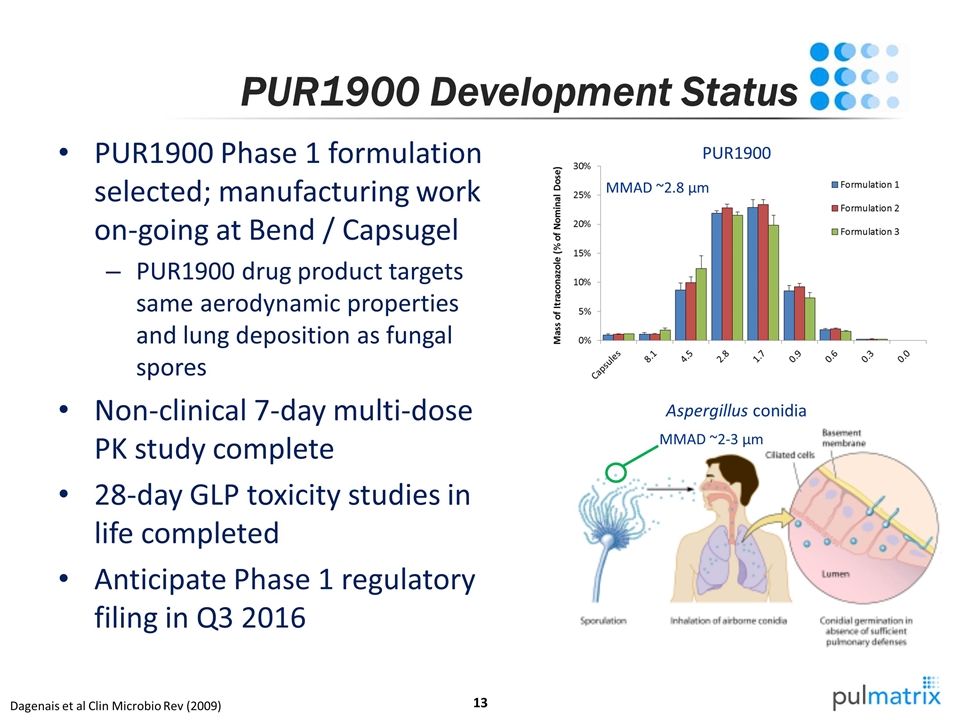
PUR1900 Development Status PUR1900 Phase 1 formulation selected; manufacturing work on-going at Bend / Capsugel PUR1900 drug product targets same aerodynamic properties and lung deposition as fungal spores Non-clinical 7-day multi-dose PK study complete 28-day GLP toxicity studies in life completed Anticipate Phase 1 regulatory filing in Q3 2016 Dagenais et al Clin Microbio Rev (2009) MMAD ~2.8 µm MMAD ~2-3 µm PUR1900 Aspergillus conidia
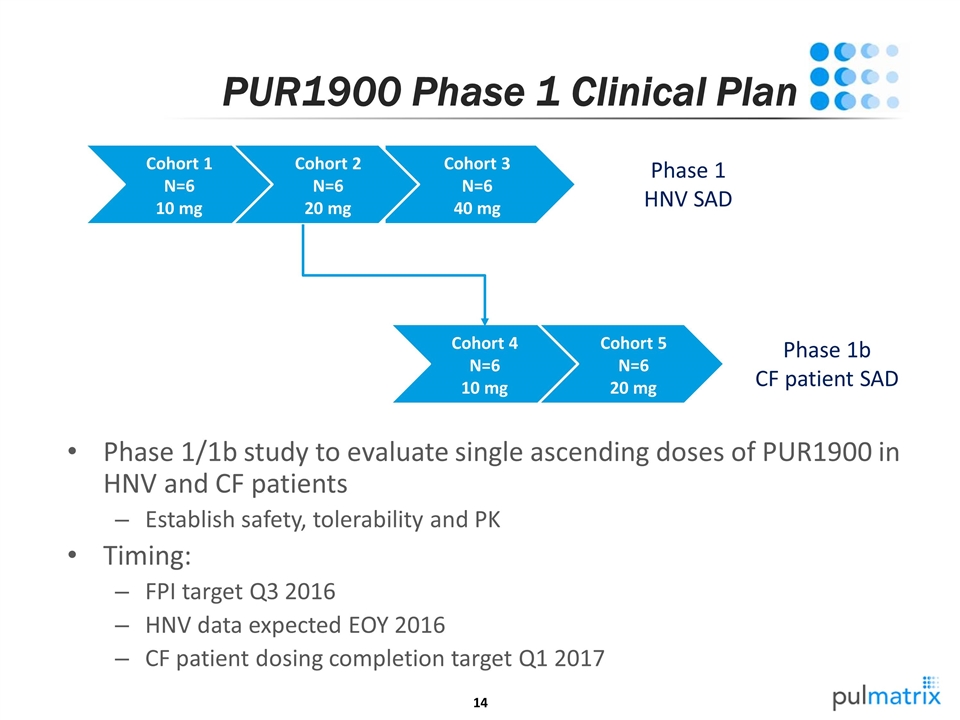
PUR1900 Phase 1 Clinical Plan Phase 1/1b study to evaluate single ascending doses of PUR1900 in HNV and CF patients Establish safety, tolerability and PK Timing: FPI target Q3 2016 HNV data expected EOY 2016 CF patient dosing completion target Q1 2017 Cohort 1 N=6 10 mg Cohort 2 N=6 20 mg Cohort 3 N=6 40 mg Phase 1 HNV SAD Cohort 4 N=6 10 mg Cohort 5 N=6 20 mg Phase 1b CF patient SAD
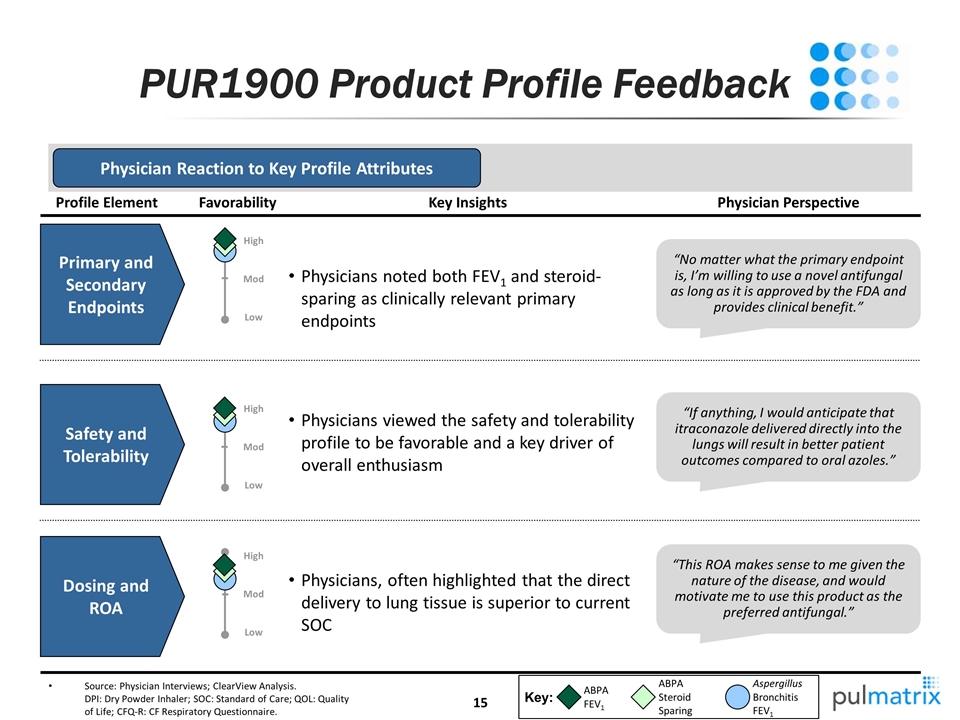
PUR1900 Product Profile Feedback Source: Physician Interviews; ClearView Analysis. DPI: Dry Powder Inhaler; SOC: Standard of Care; QOL: Quality of Life; CFQ-R: CF Respiratory Questionnaire. Profile Element Safety and Tolerability Dosing and ROA “If anything, I would anticipate that itraconazole delivered directly into the lungs will result in better patient outcomes compared to oral azoles.” “This ROA makes sense to me given the nature of the disease, and would motivate me to use this product as the preferred antifungal.” Physician Perspective Physicians viewed the safety and tolerability profile to be favorable and a key driver of overall enthusiasm Physicians, often highlighted that the direct delivery to lung tissue is superior to current SOC Key Insights Favorability Physician Reaction to Key Profile Attributes Key: ABPA FEV1 ABPA Steroid Sparing Aspergillus Bronchitis FEV1 Primary and Secondary Endpoints “No matter what the primary endpoint is, I’m willing to use a novel antifungal as long as it is approved by the FDA and provides clinical benefit.” Physicians noted both FEV1 and steroid-sparing as clinically relevant primary endpoints Low High Mod Low High Mod High Low Mod
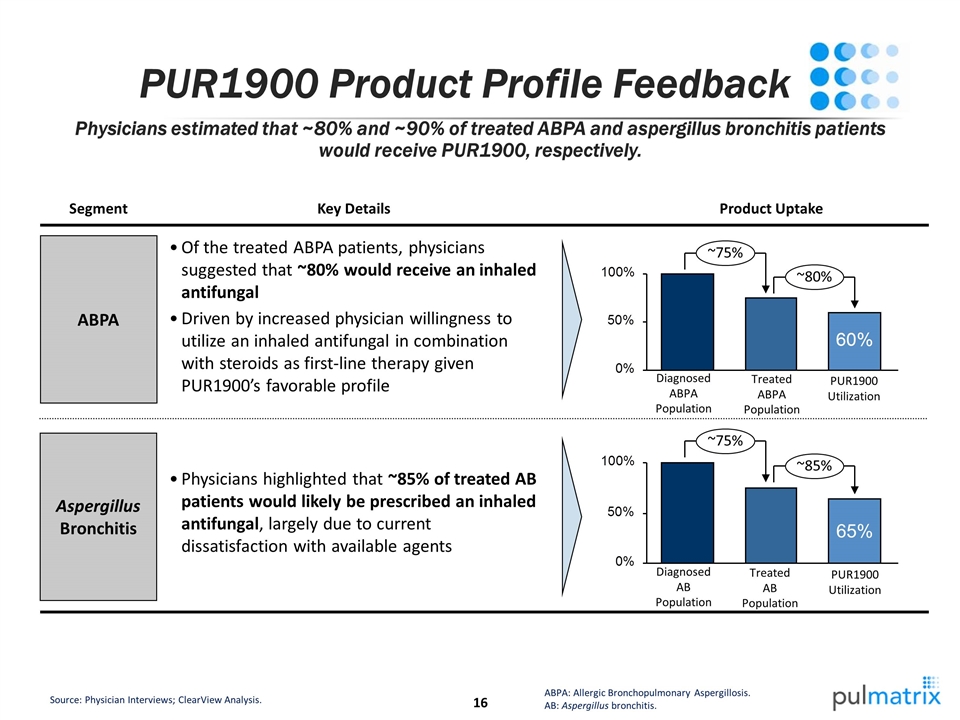
PUR1900 Product Profile Feedback Physicians highlighted that ~85% of treated AB patients would likely be prescribed an inhaled antifungal, largely due to current dissatisfaction with available agents Source: Physician Interviews; ClearView Analysis. ABPA: Allergic Bronchopulmonary Aspergillosis. AB: Aspergillus bronchitis. Physicians estimated that ~80% and ~90% of treated ABPA and aspergillus bronchitis patients would receive PUR1900, respectively. ~75% ~80% Of the treated ABPA patients, physicians suggested that ~80% would receive an inhaled antifungal Driven by increased physician willingness to utilize an inhaled antifungal in combination with steroids as first-line therapy given PUR1900’s favorable profile Key Details ABPA Aspergillus Bronchitis Segment Product Uptake Treated ABPA Population Diagnosed ABPA Population PUR1900 Utilization ~75% ~85% 65% Treated AB Population Diagnosed AB Population PUR1900 Utilization
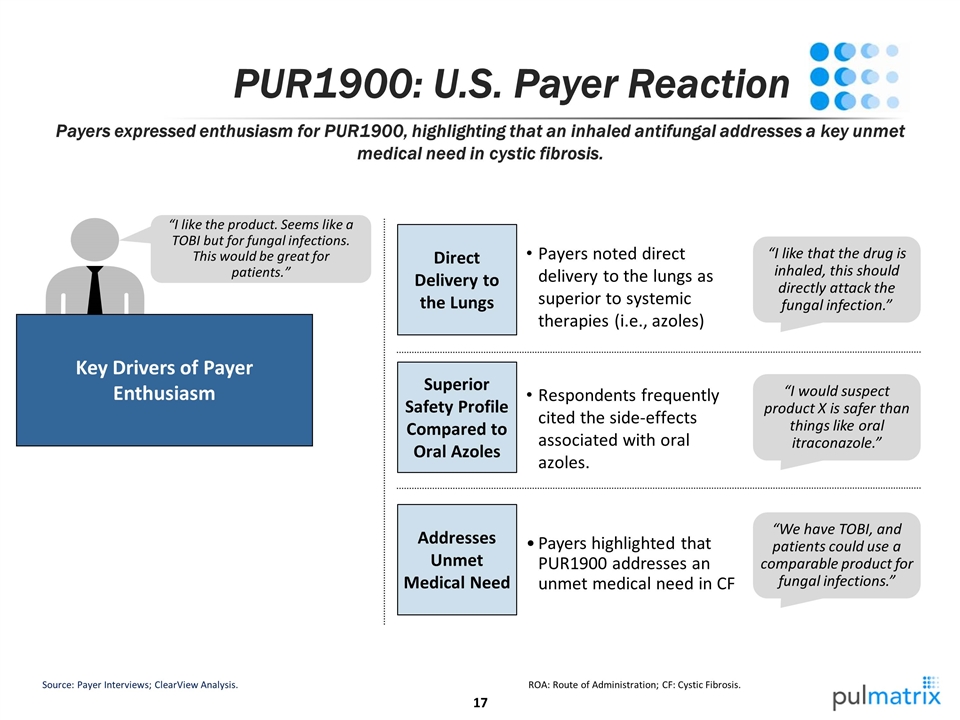
PUR1900: U.S. Payer Reaction Payers expressed enthusiasm for PUR1900, highlighting that an inhaled antifungal addresses a key unmet medical need in cystic fibrosis. Source: Payer Interviews; ClearView Analysis. ROA: Route of Administration; CF: Cystic Fibrosis. “I like the product. Seems like a TOBI but for fungal infections. This would be great for patients.” Key Drivers of Payer Enthusiasm Direct Delivery to the Lungs “I like that the drug is inhaled, this should directly attack the fungal infection.” Payers noted direct delivery to the lungs as superior to systemic therapies (i.e., azoles) “I would suspect product X is safer than things like oral itraconazole.” Superior Safety Profile Compared to Oral Azoles Respondents frequently cited the side-effects associated with oral azoles. “We have TOBI, and patients could use a comparable product for fungal infections.” Addresses Unmet Medical Need Payers highlighted that PUR1900 addresses an unmet medical need in CF
PUR1900 for CF: Significant Commercial Opportunity First product designed for topical delivery to the lung of an infected CF patient Anticipate a much better superior safety, tolerability, and efficacy profile to current standard of care Worldwide sales potential estimated at >$250MM annual peak sales Long-term value potential in CF and beyond: IP protection into the 2030’s Significant life cycle management opportunities
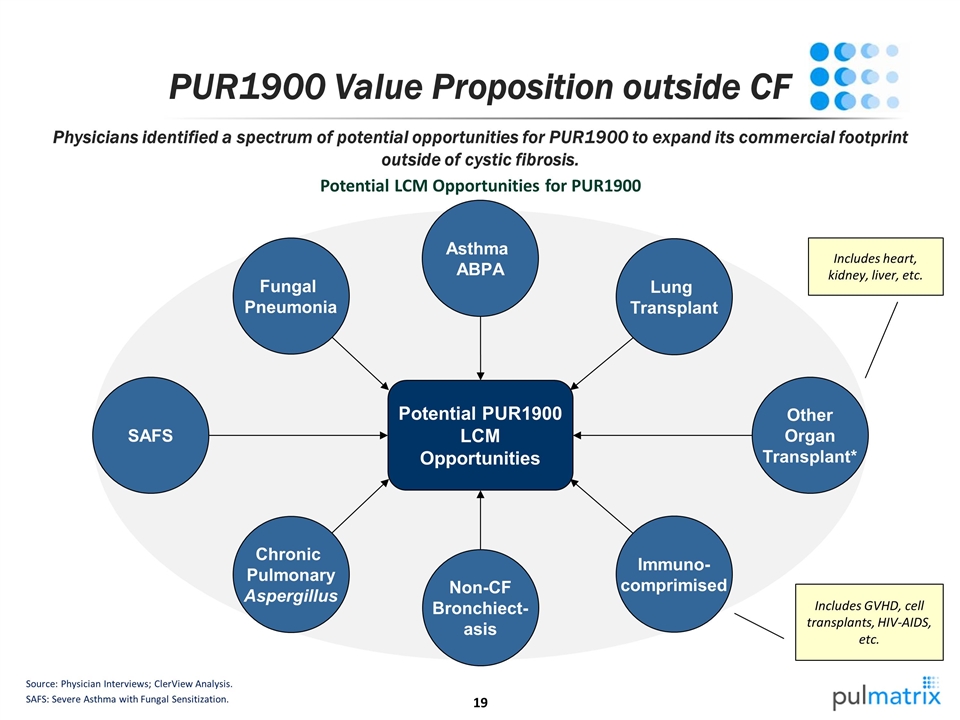
PUR1900 Value Proposition outside CF Source: Physician Interviews; ClerView Analysis. SAFS: Severe Asthma with Fungal Sensitization. Potential PUR1900 LCM Opportunities Potential LCM Opportunities for PUR1900 SAFS Fungal Pneumonia Other Organ Transplant* Asthma ABPA Lung Transplant Immuno- comprimised Chronic Pulmonary Aspergillus Non-CF Bronchiect- asis Includes heart, kidney, liver, etc. Includes GVHD, cell transplants, HIV-AIDS, etc. Physicians identified a spectrum of potential opportunities for PUR1900 to expand its commercial footprint outside of cystic fibrosis.
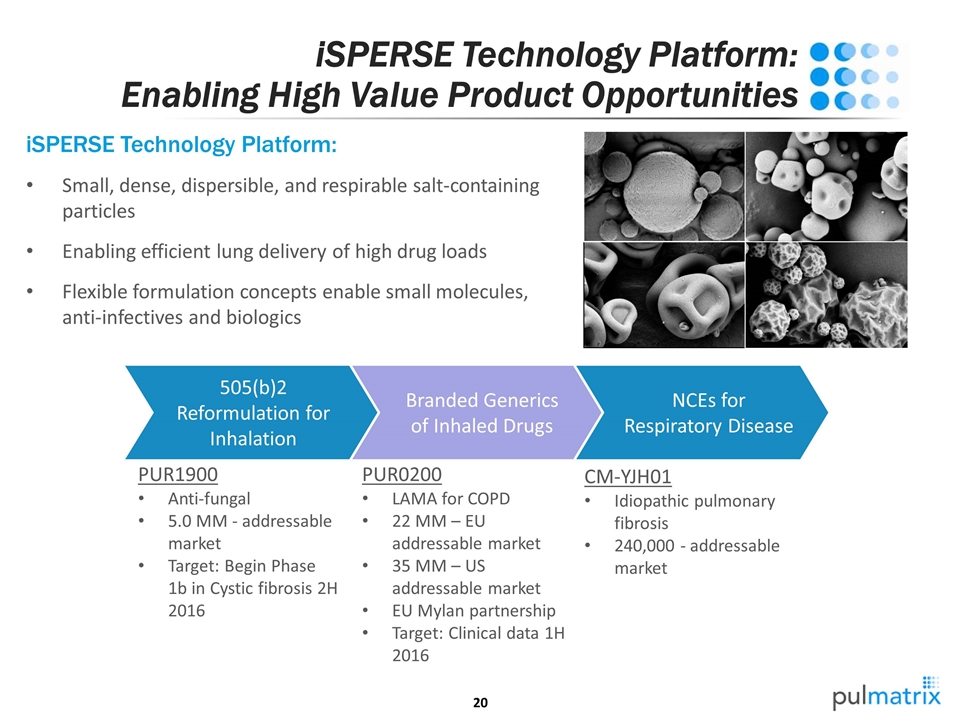
iSPERSE Technology Platform: Enabling High Value Product Opportunities iSPERSE Technology Platform: Small, dense, dispersible, and respirable salt-containing particles Enabling efficient lung delivery of high drug loads Flexible formulation concepts enable small molecules, anti-infectives and biologics NCEs for Respiratory Disease Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation PUR0200 LAMA for COPD 22 MM – EU addressable market 35 MM – US addressable market EU Mylan partnership Target: Clinical data 1H 2016 PUR1900 Anti-fungal 5.0 MM - addressable market Target: Begin Phase 1b in Cystic fibrosis 2H 2016 CM-YJH01 Idiopathic pulmonary fibrosis 240,000 - addressable market
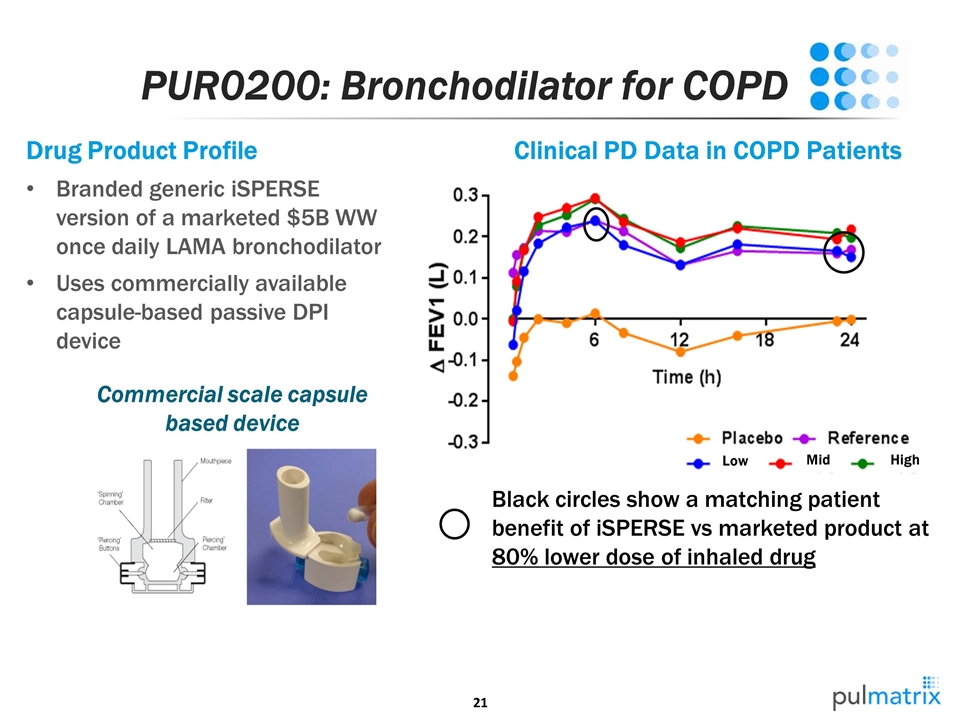
PUR0200: Bronchodilator for COPD Low Mid High Drug Product Profile Branded generic iSPERSE version of a marketed $5B WW once daily LAMA bronchodilator Uses commercially available capsule-based passive DPI device Commercial scale capsule based device Clinical PD Data in COPD Patients Black circles show a matching patient benefit of iSPERSE vs marketed product at 80% lower dose of inhaled drug
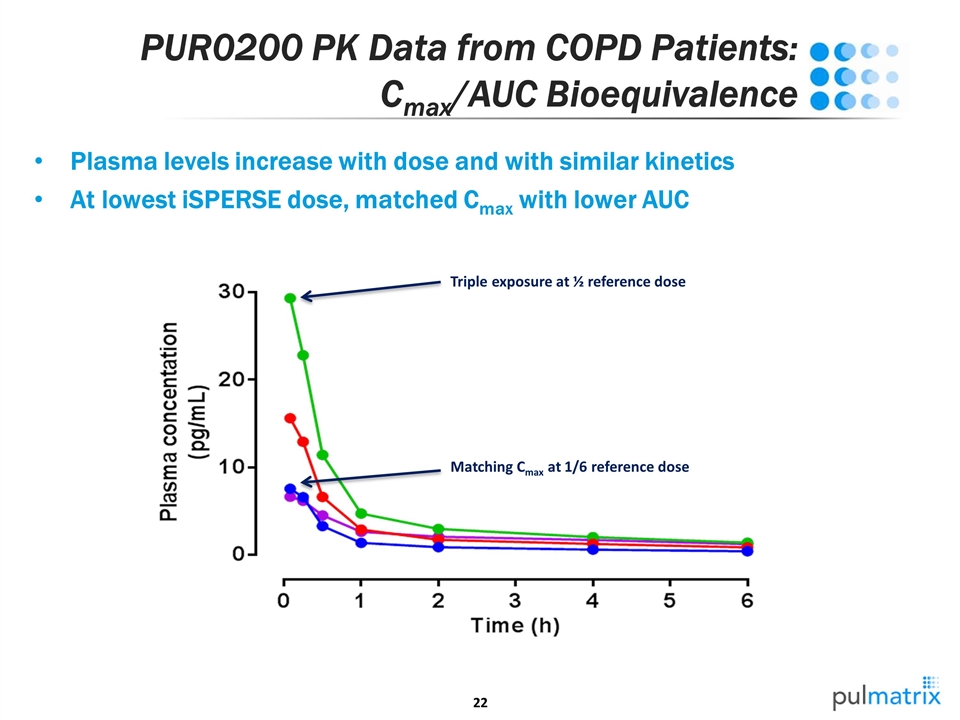
PUR0200 PK Data from COPD Patients: Cmax/AUC Bioequivalence Plasma levels increase with dose and with similar kinetics At lowest iSPERSE dose, matched Cmax with lower AUC Matching Cmax at 1/6 reference dose Triple exposure at ½ reference dose
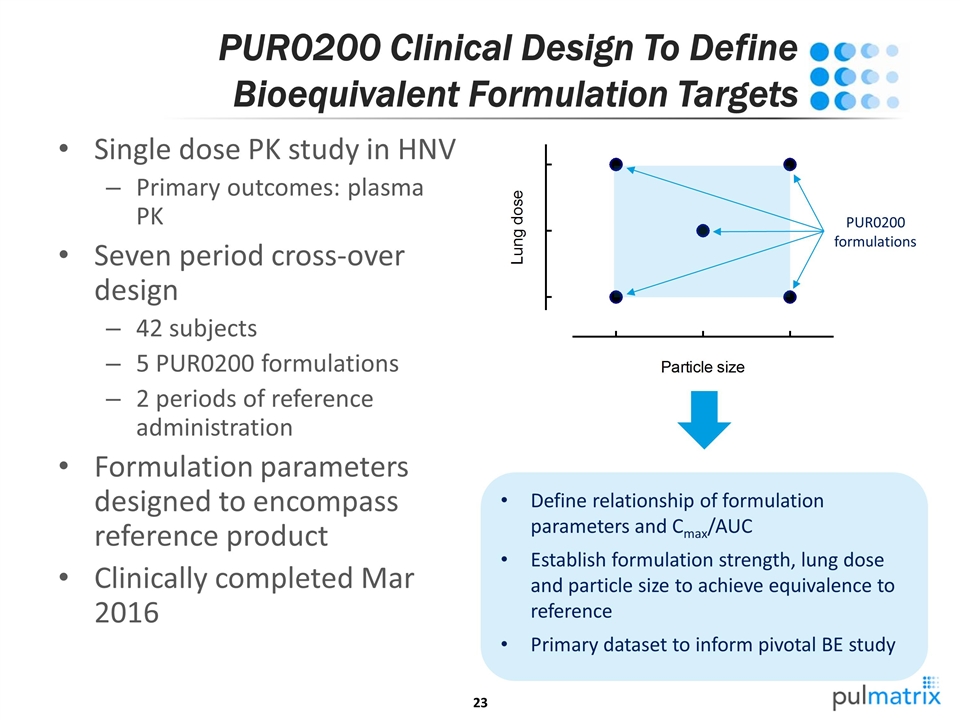
PUR0200 Clinical Design To Define Bioequivalent Formulation Targets Single dose PK study in HNV Primary outcomes: plasma PK Seven period cross-over design 42 subjects 5 PUR0200 formulations 2 periods of reference administration Formulation parameters designed to encompass reference product Clinically completed Mar 2016 PUR0200 formulations Define relationship of formulation parameters and Cmax/AUC Establish formulation strength, lung dose and particle size to achieve equivalence to reference Primary dataset to inform pivotal BE study
PUR0200 Fast Follower: Significant Near-Term Market Potential Drug Class Muscarinic antagonist (bronchodilator) Dosing profile Once daily, inhaled Regulatory Path EU PK Bioequivalence Development Status Clinical trial Q1 2016 Addressable Market Size (2019)* 22MM in the EU Data from current PK Bioequivalence study triggers Mylan negotiating option to EU rights US development & commercialization rights are not covered by the Mylan agreement; ongoing conversations with potential partners for US rights US estimated prevalent cases of COPD (2019)* = 35MM * GlobalData epidemiological forecast (estimated prevalent cases of COPD)
NCEs for Respiratory Disease Profile: Novel compounds to address high unmet need in COPD, CF and IPF iSPERSE enables: High drug loading and targeted lung delivery to maximize efficacy Evaluating multiple existing, development stage pharma compounds Pulmatrix: Building Future Value Reformulation for Inhalation Profile: Lung delivery for fast onset PK to overcome GI limited drug absorption and PK iSPERSE enables: High efficiency lung delivery with low GI exposure Assessing non-respiratory disease indications requiring fast onset action A Wide Range of Opportunities NCEs for Respiratory Disease Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation PUR0200 LAMA for COPD 22 MM – EU addressable market 35 MM – US addressable market EU Mylan partnership Target: Clinical data 1H 2016 PUR1900 Anti-fungal 5.0 MM - addressable market Target: Begin Phase 1b in Cystic fibrosis 2H 2016 CM-YJH01 Idiopathic pulmonary fibrosis 240,000 - addressable market

iSPERSE for IPF Need for improvements on approved drugs Nintedanib and Pirfenidone Despite efficacy, significant unmet need remains GI side effects and oral bioavailability limit tolerability, use and efficacy Critical need for inhaled options to overcome side effects and enable drugs against new targets Product opportunities for Pulmatrix in 505(b)2 and NCE PUR1500: repurposing approved small molecule MOA for inhalation Celdara collaboration on novel biologic with extensive preclinical data
Pulmatrix Team: A History of Biotech Success Terry McGuire BOD Investor (AIR, Cubist, Ironwood) Robert Clarke, PhD CEO (12+ years) (AIR, Alkermes) Steve Gillis, PhD BOD Investor (Immunex, Bluebird) Kurt Graves BOD Independent (Vertex) Michael Higgins BOD-Audit Chair (Genzyme) Scott Rocklage, PhD BOD Investor (Cubist, Pearl) Board of Directors Management David Hava, PhD CSO (9+ years) (Harvard) Bill Duke, MBA CFO (Genzyme, Valeritas) Mark Iwicki Chairman (Civitas, Sepracor)
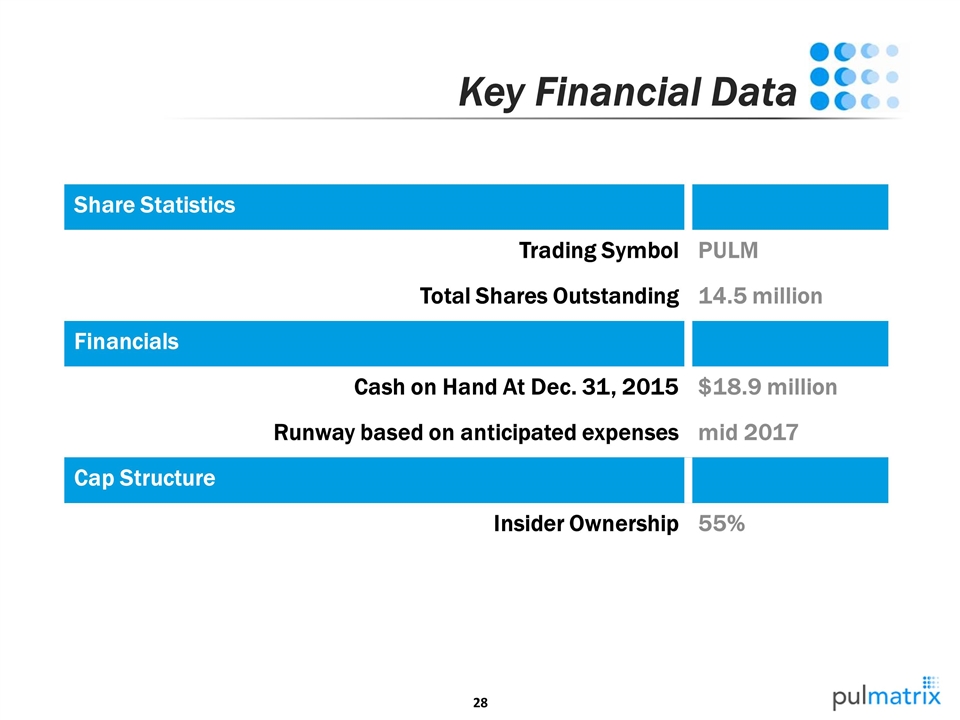
Share Statistics Trading Symbol PULM Total Shares Outstanding 14.5 million Financials Cash on Hand At Dec. 31, 2015 $18.9 million Runway based on anticipated expenses mid 2017 Cap Structure Insider Ownership 55% Key Financial Data

Anticipated Near Term Milestones PUR0200 Jan 2016 Initiated pilot BE PK study in EU Q2 2016 Clinical data from pilot BE PK study Q4 2016 Initiate development towards pivotal BE study with partner PUR1900 Jan 2016 Initiated GLP non-clinical safety program supporting Phase 1 Q2 2016 Completion of GLP non-clinical safety studies Q3 2016 Submission of CTA for Phase 1/1b clinical study 2H 2016 Initiate single ascending dose Phase 1/1b study in HNV/CF Q1 2017 Report data from Phase 1/1b clinical program 2H 2017 Initiate Phase 2 clinical study in US
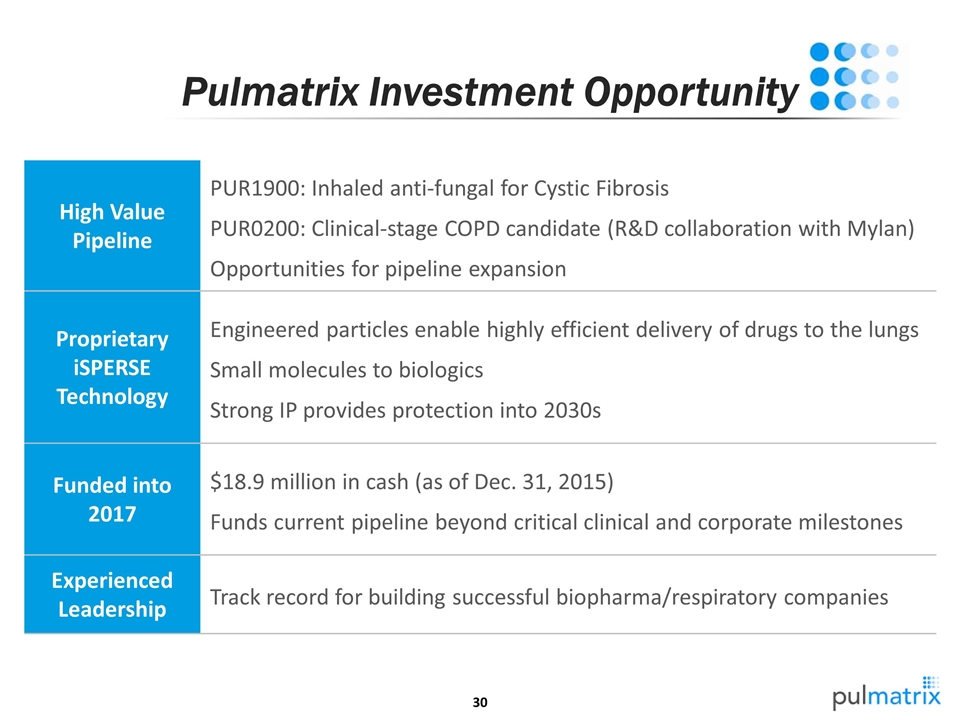
Pulmatrix Investment Opportunity High Value Pipeline PUR1900: Inhaled anti-fungal for Cystic Fibrosis PUR0200: Clinical-stage COPD candidate (R&D collaboration with Mylan) Opportunities for pipeline expansion Proprietary iSPERSE Technology Engineered particles enable highly efficient delivery of drugs to the lungs Small molecules to biologics Strong IP provides protection into 2030s Funded into 2017 $18.9 million in cash (as of Dec. 31, 2015) Funds current pipeline beyond critical clinical and corporate milestones Experienced Leadership Track record for building successful biopharma/respiratory companies

March 2016 NASDAQ: PULM Engineering the Future for Inhaled Therapeutics






























