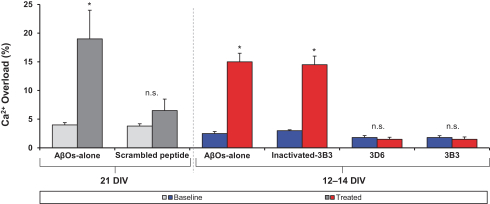
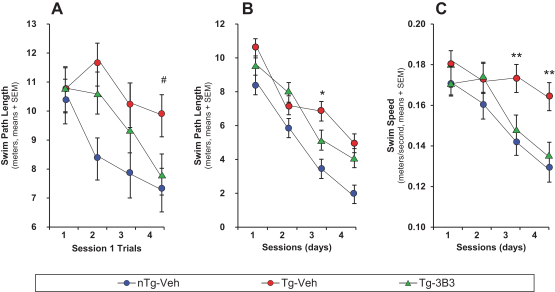
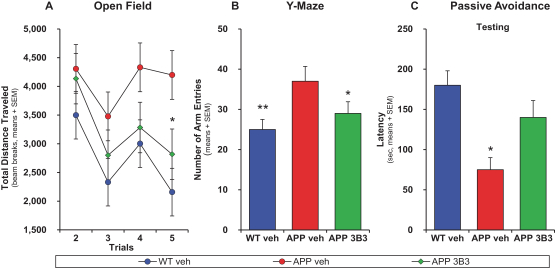
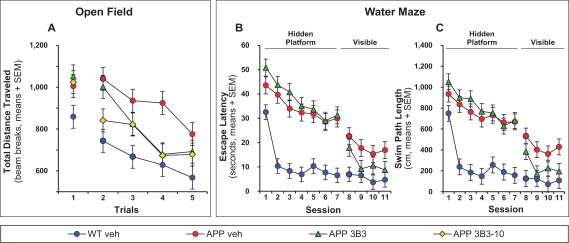
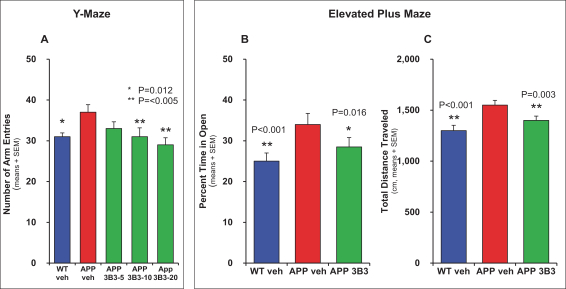
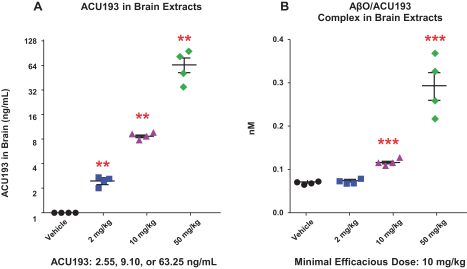
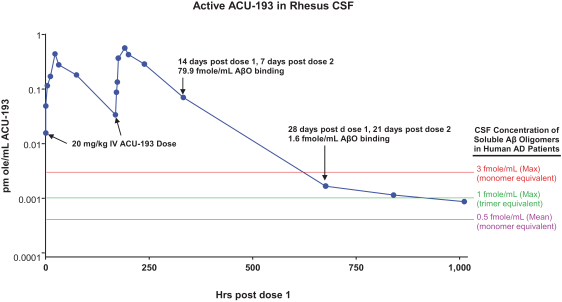
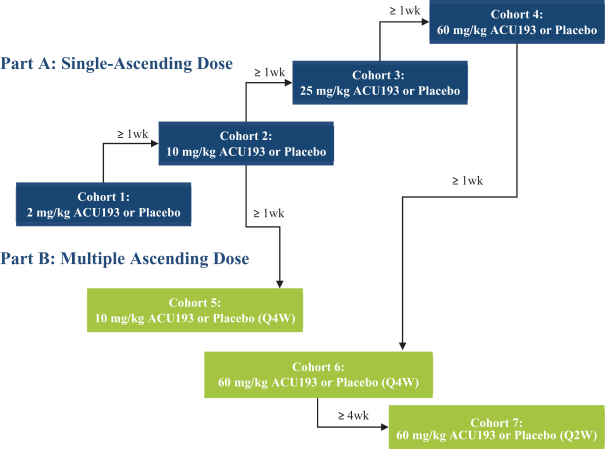
Manufacturing
We do not currently own or operate facilities for product manufacturing, storage and distribution, or testing. We contract with third parties for the manufacture of ACU193. Because we rely on contract manufacturers, we employ personnel with extensive technical, manufacturing, analytical and quality experience. Our staff has strong project management discipline to oversee contract manufacturing and testing activities, and to compile manufacturing and quality information for our regulatory submissions.
Manufacturing is subject to extensive regulation that imposes various procedural and documentation requirements and that governs record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, and more. Our systems and our contractors are required to be in compliance with these regulations, and compliance is assessed regularly through monitoring of performance and a formal audit program.
Our current supply chains for ACU193 involve several manufacturers that specialize in specific operations of the manufacturing process, including raw materials manufacturing, drug substance manufacturing and drug product manufacturing. We currently operate under work order programs for ACU193 with master services agreements in place that include specific supply timelines, volume and quality specifications. We believe our current manufacturers have the scale, the systems, and the experience to supply our currently planned clinical trials.
Competition
We face competition from several different institutions, including pharmaceutical and biotechnology companies, research institutions, governmental organizations and universities developing novel therapies for AD. We believe that the key factors affecting the clinical and commercial success of ACU193 will include safety profile, efficacy, cost, method of administration, level of marketing activity, insurance reimbursement and intellectual property protection.
If approved, ACU193 will compete with therapies currently approved for the treatment of AD, which have primarily been developed to treat the symptoms of AD rather than the underlying cause of the disease, such as memantine and cholinesterase inhibitors. ACU193 may also compete with one or more potentially disease-modifying therapeutics that target Ab or amyloid plaques, the most advanced of which is Biogen Inc.’s aducanumab, which the FDA approved in June 2021 under the accelerated approval pathway, which allows for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint. Regulatory approval of aducanumab is pending in Europe and Japan. Other companies known to be developing therapies with Ab/amyloid plaque-related targets include Alzheon, Inc., Alzinova AB, Chugai Pharmaceutical Co. Ltd., Cognition Therapeutics, Inc., Eisai Co., Ltd., Eli Lilly and Company, Grifols, S.A., KalGene Pharmaceuticals, Inc., Neurimmune AG, Novartis AG, ProMIS Neurosciences, Inc., Prothena Biosciences, Inc., Roche Holding AG (including Genentech, its wholly owned subsidiary) and Wren Therapeutics, Inc. Additionally, ACU193, if approved, may also compete with other potential therapies intended to address underlying causes of AD that are being developed by several companies, including AbbVie Inc., AC Immune SA, Alector, Inc., Anavex Life Sciences Corp., Annovis Bio, Inc., Athira Pharma, Inc., Biohaven Pharmaceuticals, Inc., Cassava Sciences, Inc., Cortexyme, Inc., Denali Therapeutics, Inc., Johnson & Johnson (including Janssen, its wholly-owned subsidiary) and Takeda Pharmaceutical Co. Ltd.
Collaboration Agreement with Merck
In December 2003, we entered into an exclusive license and research and development collaboration agreement with Merck to research, discover and develop certain technology related to amyloid beta-derived diffusible ligands, or ADDL, which agreement was amended and restated in October 2006. The agreement generally provided that, during the course of the collaboration, Merck would be responsible for the preclinical and clinical development and commercialization of any products covered by the agreement and, in return, we were eligible to receive potential nonclinical, clinical and regulatory milestone payments and royalties on future
124