Exhibit 99.2
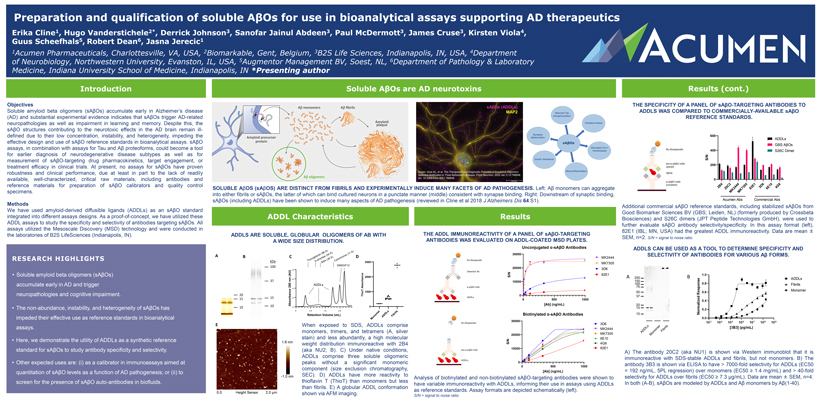
Preparation and qualification of soluble AßOs for use in bioanalytical assays supporting AD therapeutics Erika Cline1, Hugo Vanderstichele2*, Derrick Johnson3, Sanofar Jainul Abdeen3, Paul McDermott3, James Cruse3, Kirsten Viola4, Guus Scheefhals5, Robert Dean6, Jasna Jerecic1 1Acumen Pharmaceuticals, Charlottesville, VA, USA, 2Biomarkable, Gent, Belgium, 3B2S Life Sciences, Indianapolis, IN, USA, 4Department of Neurobiology, Northwestern University, Evanston, IL, USA, 5Augmentor Management BV, Soest, NL, 6Department of Pathology & Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN *Presenting author Introduction Objectives Soluble amyloid beta oligomers (sAßOs) accumulate early in Alzheimer’s disease (AD) and substantial experimental evidence indicates that sAßOs trigger AD-related neuropathologies as well as impairment in learning and memory. Despite this, the sAßO structures contributing to the neurotoxic effects in the AD brain remain ill-defined due to their low concentration, instability, and heterogeneity, impeding the effective design and use of sAßO reference standards in bioanalytical assays. sAßO assays, in combination with assays for Tau and Aß proteoforms, could become a tool for earlier diagnosis of neurodegenerative disease subtypes as well as for measurement of sAßO-targeting drug pharmacokinetics, target engagement, or treatment efficacy in clinical trials. At present, no assays for sAßOs have proven robustness and clinical performance, due at least in part to the lack of readily available, well-characterized, critical raw materials, including antibodies and reference materials for preparation of sAßO calibrators and quality control specimens. Methods We have used amyloid-derived diffusible ligands (ADDLs) as an sAßO standard integrated into different assays designs. As a proof-of-concept, we have utilized these ADDL assays to study the specificity and selectivity of antibodies targeting sAßOs. All assays utilized the Mesoscale Discovery (MSD) technology and were conducted in the laboratories of B2S LifeSciences (Indianapolis, IN). RESEARCH HIGHLIGHTS • Soluble amyloid beta oligomers (sAßOs) accumulate early in AD and trigger neuropathologies and cognitive impairment. • The non-abundance, instability, and heterogeneity of sAßOs has impeded their effective use as reference standards in bioanalytical assays. • Here, we demonstrate the utility of ADDLs as a synthetic reference standard for sAßOs to study antibody specificity and selectivity. • Other expected uses are: (i) as a calibrator in immunoassays aimed at quantitation of sAßO levels as a function of AD pathogenesis; or (ii) to screen for the presence of sAßO auto-antibodies in biofluids. Soluble AßOs are AD neurotoxins sAßOs (ADDLs) MAP2 Image: Viola KL, et al. The Therapeutic and Diagnostic Potential of Amyloid ß Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front Neurosci. 2022 Jan 3;15:768646. doi: 10.3389/fnins.2021.768646. SOLUBLE AßOS (sAßOS) ARE DISTINCT FROM FIBRILS AND EXPERIMENTALLY INDUCE MANY FACETS OF AD PATHOGENESIS. Left: Aß monomers can aggregate into either fibrils or sAßOs, the latter of which can bind cultured neurons in a punctate manner (middle) consistent with synapse binding. Right: Downstream of synaptic binding, sAßOs (including ADDLs) have been shown to induce many aspects of AD pathogenesis (reviewed in Cline et al 2018 J Alzheimers Dis 64:S1). ADDL Characteristics ADDLS ARE SOLUBLE, GLOBULAR OLIGOMERS OF AB WITH A WIDE SIZE DISTRIBUTION. Å Å) (86 Å) 17 lin 54 e ( A e ( B C globu las Å) hrom D ro y 6 oc hy -am (3 yt kDa T ß C 3000 BSA 100 DMSO/F12 8 (AU) 2000 37 nm 6 280 ADDLs Absorbance 4 1000 20 ThioT 15 15 Absorbance 2 10 10 0 0 5 10 15 20 r s s e il Retention Volume (mL) m br ono ADDL Fi M E When exposed to SDS, ADDLs comprise monomers, trimers, and tetramers (A, silver stain) and less abundantly, a high molecular weight distribution immunoreactive with 2B4 (aka NU2; B). C) Under native conditions, ADDLs comprise three soluble oligomeric peaks without a significant monomeric component (size exclusion chromatography, SEC). D) ADDLs have more reactivity to thioflavin T (ThioT) than monomers but less than fibrils. E) A globular ADDL conformation shown via AFM imaging. Results THE ADDL IMMUNOREACTIVITY OF A PANEL OF sAßO-TARGETING ANTIBODIES WAS EVALUATED ON ADDL-COATED MSD PLATES. Unconjugated α-sAßO Antibodies 30000 MK2444 MK7305 20000 3D6 S/N 82E1 10000 0 0 500 1000 [Ab] (ng/mL) Biotinylated α-sAßO Antibodies 30000 3D6 MK2444 20000 MK7305 6E10 S/N 4G8 10000 82E1 0 0 500 1000 [Ab] (ng/mL) Analysis of biotinylated and non-biotinylated sAßO-targeting antibodies were shown to have variable immunoreactivity with ADDLs, informing their use in assays using ADDLs as reference standards. Assay formats are depicted schematically (left). S/N = signal to noise ratio. Results (cont.) THE SPECIFICITY OF A PANEL OF sAßO-TARGETING ANTIBODIES TO ADDLS WAS COMPARED TO COMMERCIALLY-AVAILABLE sAßO REFERENCE STANDARDS. 600 ADDLs GBS AßOs 400 S26C Dimer S/N 200 0 B4 2 44 05 1 D6 10 G8 2 24 73 3 E 4 20C 82E 6 K K M M Acumen Abs Commercial Abs Additional commercial sAßO reference standards, including stabilized sAßOs from Good Biomarker Sciences BV (GBS; Leiden, NL) (formerly produced by Crossbeta Biosciences) and S26C dimers (JPT Peptide Technologies GmbH), were used to further evaluate sAßO antibody selectivity/specificity. In this assay format (left), 82E1 (IBL; MN, USA) had the greatest ADDL immunoreactivity. Data are mean ± SEM, n=2. S/N = signal to noise ratio. ADDLS CAN BE USED AS A TOOL TO DETERMINE SPECIFICITY AND SELECTIVITY OF ANTIBODIES FOR VARIOUS Aß FORMS. A B A) The antibody 20C2 (aka NU1) is shown via Western immunoblot that it is immunoreactive with SDS-stable ADDLs and fibrils, but not monomers. B) The antibody 3B3 is shown via ELISA to have > 7000-fold selectivity for ADDLs (EC50 = 192 ng/mL, 5PL regression) over monomers (EC50 ≥ 1.4 mg/mL) and > 40-fold selectivity for ADDLs over fibrils (EC50 ≥ 7.3 µg/mL). Data are mean ± SEM, n=4. In both (A-B), sAßOs are modeled by ADDLs and Aß monomers by Aß(1-40).
