Exhibit 99.3
ALLERGAN TO ACQUIRE VITAE PHARMACEUTICALS
Strengthening Allergan’s Medical Dermatology Pipeline
ALLERGAN CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This presentation contains “forward-looking statements” relating to the acquisition of Vitae by Allergan. Such forward-looking statements include the ability of Vitae, Parent and Merger Sub to complete the transactions contemplated by the merger agreement, including the parties’ ability to satisfy the conditions to the consummation of the offer and the other conditions set forth in the merger agreement and the possibility of any termination of the merger agreement. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. Actual results may differ materially from current expectations because of risks associated with uncertainties as to the timing of the offer and the subsequent merger; uncertainties as to how many of Vitae’s stockholders will tender their shares in the offer; the risk that competing offers or acquisition proposals will be made; the possibility that various conditions to the consummation of the offer or the merger may not be satisfied or waived; the effects of disruption from the transactions contemplated by the merger agreement on Vitae’s business and the fact that the announcement and pendency of the transactions may make it more difficult to establish or maintain relationships with employees, suppliers and other business partners; the risk that stockholder litigation in connection with the offer or the merger may result in significant costs of defense, indemnification and liability; other uncertainties pertaining to the business of Vitae, including those set forth in the “Risk Factors” and “Management’s Discussion and
Analysis of Financial Condition and Results of Operations” sections of Vitae’s Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, which are on file with the Securities and Exchange
Commission and available on the Securities and Exchange Commission’s website at www.sec.gov. In addition to the risks described above and in Vitae’s other filings with the Securities and Exchange Commission, other unknown or unpredictable factors could also affect Vitae’s results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information contained in this presentation is provided only as of the date of this report, and Vitae undertakes no obligation to update any forward-looking statements either contained in or incorporated by reference into this report on account of new information, future events, or otherwise, except as required by law
2
NOTICE TO INVESTORS
The tender offer for the outstanding common stock of Vitae referred to in this communication has not yet commenced. The description contained in this communication is neither an offer to purchase nor a solicitation of an offer to sell any securities. The solicitation and the offer to buy shares of Vitae common stock will be made pursuant to an offer to purchase and related materials that Allergan intends to file with the Securities and Exchange Commission. At the time the offer is commenced, Allergan will file a tender offer statement on Schedule TO with the Securities and Exchange Commission, and thereafter Vitae will file a solicitation/recommendation statement on Schedule 14D-9 with respect to the offer. The tender offer statement (including an offer to purchase, a related letter of transmittal and other offer documents) and the solicitation/recommendation statement will contain important information that should be read carefully and considered before any decision is made with respect to the tender offer. Additionally, Vitae and Allergan will file other relevant materials in connection with the proposed acquisition of Vitae by Allergan pursuant to the terms of the merger agreement. These materials will be sent free of charge to all stockholders of Vitae when available. In addition, all of these materials (and all other materials filed by Vitae with the Securities and Exchange Commission) will be available at no charge from the Securities and Exchange Commission through its website at www.sec.gov. Free copies of the offer to purchase, the related letter of transmittal and certain other offering documents will be made available by Allergan and when available may be obtained by directing a request to Allergan’s Investor Relations Department at (862) 261-7488. Investors and security holders may also obtain free copies of the documents filed with the Securities and Exchange Commission by Vitae by contacting Vitae Investor Relations at (215) 461-2000.
INVESTORS AND SHAREHOLDERS OF VITAE ARE ADVISED TO READ THE SCHEDULE TO AND THE SCHEDULE 14D-9, AS EACH MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME, AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION WHEN THEY BECOME AVAILABLE BEFORE THEY MAKE ANY DECISION WITH RESPECT TO THE TENDER OFFER OR MERGER, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES THERETO.
3
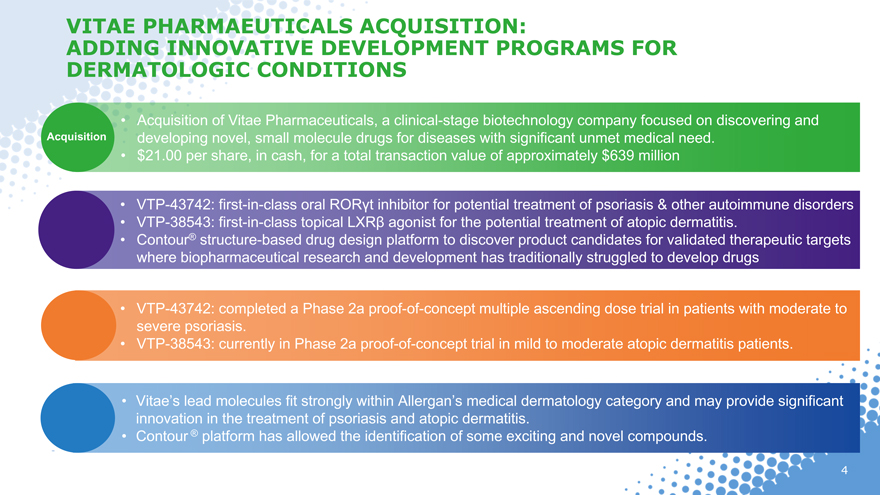
VITAE PHARMAEUTICALS ACQUISITION:
ADDING INNOVATIVE DEVELOPMENT PROGRAMS FOR DERMATOLOGIC CONDITIONS
Acquisition
Acquisition of Vitae Pharmaceuticals, a clinical-stage biotechnology company focused on discovering and developing novel, small molecule drugs for diseases with significant unmet medical need.
$21.00 per share, in cash, for a total transaction value of approximately $639 million
VTP-43742: first-in-class oral RORt inhibitor for potential treatment of psoriasis & other autoimmune disorders
VTP-38543: first-in-class topical LXR agonist for the potential treatment of atopic dermatitis.
Contour® structure-based drug design platform to discover product candidates for validated therapeutic targets where biopharmaceutical research and development has traditionally struggled to develop drugs
VTP-43742: completed a Phase 2a proof-of-concept multiple ascending dose trial in patients with moderate to severe psoriasis.
VTP-38543: currently in Phase 2a proof-of-concept trial in mild to moderate atopic dermatitis patients.
Vitae’s lead molecules fit strongly within Allergan’s medical dermatology category and may provide significant innovation in the treatment of psoriasis and atopic dermatitis.
Contour ® platform has allowed the identification of some exciting and novel compounds.
4
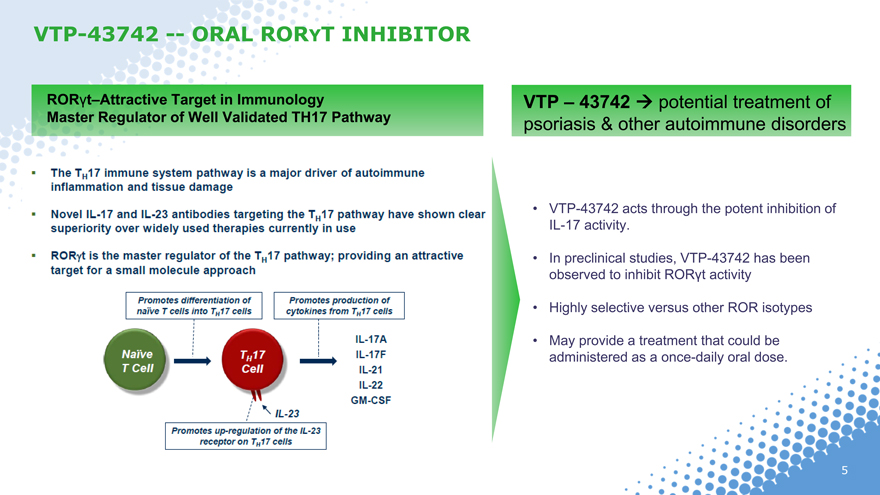
VTP-43742 — ORAL RORYT INHIBITOR
RORt–Attractive Target in Immunology Master Regulator of Well Validated TH17 Pathway
The TH17 immune system pathway is a major driver of autoimmune inflammation and tissue damage
Novel IL-17 and IL-23 antibodies targeting the TH17 pathway have shown clear superiority over widely used therapies currently in use
RORt is the master regulator of the TH17 pathway; providing an attractive target for a small molecule approach
Promotes differentiation of naïve T cells into TH17 cells
Naïve T cell
TH17 cell
IL-23
Promotes up-regulation of the IL-23 receptor on TH17 cells
Promotes production of cytokines from TH17 cells
IL-17A IL17F IL-21 IL-22 GM-CSF
VTP – 43742 potential treatment of psoriasis & other autoimmune disorders
VTP-43742 acts through the potent inhibition of IL-17 activity.
In preclinical studies, VTP-43742 has been observed to inhibit RORt activity
Highly selective versus other ROR isotypes
May provide a treatment that could be administered as a once-daily oral dose.
5
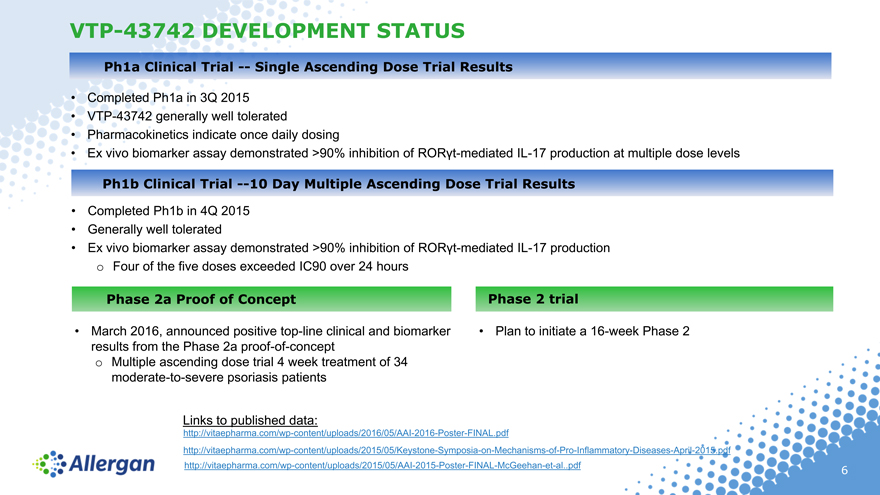
VTP-43742 DEVELOPMENT STATUS
Ph1a Clinical Trial — Single Ascending Dose Trial Results
Completed Ph1a in 3Q 2015
VTP-43742 generally well tolerated
Pharmacokinetics indicate once daily dosing
Ex vivo biomarker assay demonstrated >90% inhibition of RORt-mediated IL-17 production at multiple dose levels
Ph1b Clinical Trial —10 Day Multiple Ascending Dose Trial Results
Completed Ph1b in 4Q 2015
Generally well tolerated
Ex vivo biomarker assay demonstrated >90% inhibition of RORt-mediated IL-17 production
o Four of the five doses exceeded IC90 over 24 hours
Phase 2a Proof of Concept
March 2016, announced positive top-line clinical and biomarker results from the Phase 2a proof-of-concept
Multiple ascending dose trial 4 week treatment of 34 moderate-to-severe psoriasis patients
Phase 2 trial
• Plan to initiate a 16-week Phase 2
Links to published data:
http://vitaepharma.com/wp-content/uploads/2016/05/AAI-2016-Poster-FINAL.pdf http://vitaepharma.com/wp-content/uploads/2015/05/Keystone-Symposia-on-Mechanisms-of-Pro-Inflammatory-Diseases-April-2015.pdf http://vitaepharma.com/wp-content/uploads/2015/05/AAI-2015-Poster-FINAL-McGeehan-et-al pdf
6
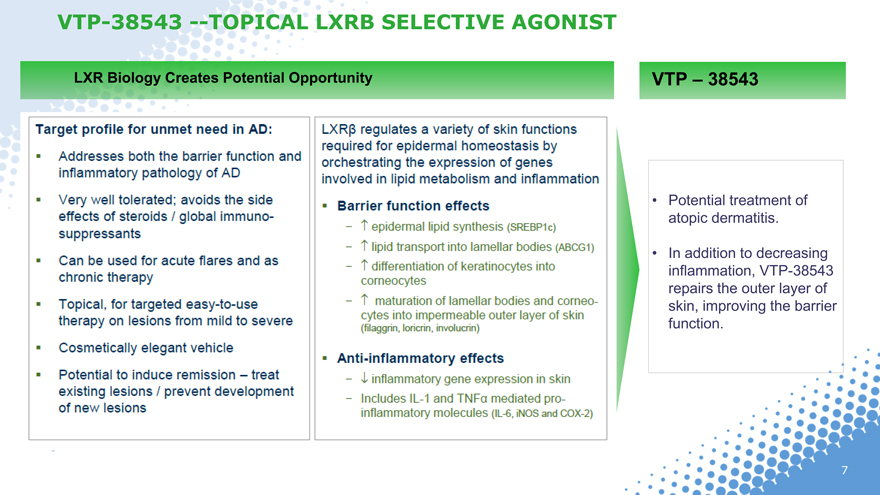
VTP-38543 —TOPICAL LXR SELECTIVE AGONIST
LXR Biology Creates Potential Opportunity
Target profile for unmet need in AD:
Addresses both the barrier function and inflammatory pathology of AD
Very well tolerated; avoids the side effects of steroids / global immune-suppressants
Can be used for acute flares and as chronic therapy
Topical, for targeted easy-to-use therapy on lesions from mild to severe
Cosmetically elegant vehicle
Potential to induce remission – treat existing lesions / prevent development of new lesions
LXR regulates a variety of skin functions required for epidermal homeostatis by orchestrating the expression of genes involved in lipid metabolism and inflammation
Barrier function effects
epidermal lipid synthesis (SREBP1c)
lipid transport into lamellar bodies (ABCG1)
differentiation of keratinocytes into corneocytes
maturation of lamellar bodies an comeocytes into impermeable outer layer of skin (filaggrin, loricrin, involucrin)
Anti-inflammtory effects
Inflammatory gene expression in skin
Includes IL-1 and TNF mediated pro-inflammatory molecules (IL-6, iNOS and COX-2)
VTP – 38543
Potential treatment of atopic dermatitis.
In addition to decreasing inflammation, VTP-38543 repairs the outer layer of skin, improving the barrier function.
7
VTP-38543 DEVELOPMENT STATUS
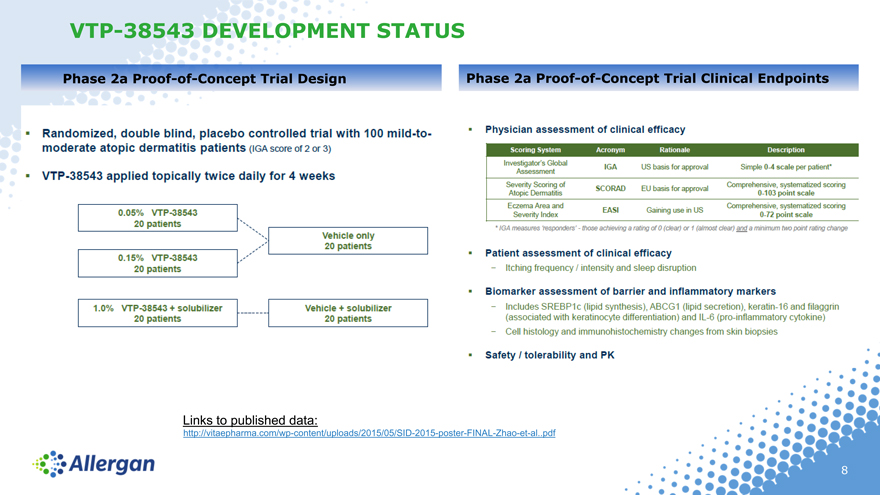
Phase 2a Proof-of-Concept Trial Design
Randomized, double blind, placebo controlled trial with 100 mild-to-moderate adopic dermatitis patients (IGA score of 2 or 3)
VTP-38543 applied topically twice daily for 4 weeks
0.05% VTP-38543 20 patients
0.15% VTP-38543 20 patients
1.0% VTP-38543 + solubilizer 20 patients
Vehicle only 20 patients
Vehicle + solubilizer 20 patients
Phase 2a Proof-of-Concept Trial Clinical Endpoints
Physician assessment of clinical efficacy
Scoring System Acronym Rationale Description
Investigator’s Global Assessment IGA US basis for approval Simple 0-4 scale per patient’
Severity Scoring of Atopic Dematitis SCORAD EU basis for approval Comprehensive, systematized scoring 0-103 point scale
Eczema Area and Severity Index EASI Gaining use in US Comprehensive, systematized scoring 0-72 point scale
*IGA measures ‘responders’ – those achieving a rating of 0 (clear) or 1 (almost clear) and a minimum two point rating change
Patient assessment of clinical efficacy
Itching frequency / intensity and sleep disruption
Biomarker assessment of barrier and inflammatory markers
Includes SREBP1c (lipid synthesis), ABCG1 (lipid secretion), keratin-16 and filaggrin (associated with keratinocyte differentiation) and IL-6 (pro-inflammatory cytokine)
Cell histology and immunohistochemistry changes from skin biopsies
Safety / tolerability and PK
Links to published data:
http://vitaepharma.com/wp-content/uploads/2015/05/SID-2015-poster-FINAL-Zhao-et-al pdf
8
PSORIASIS AND ATOPIC DERMATITIS ARE AREAS OF MEDICINE WHERE INNOVATION IS NEEDED FOR PATIENTS
PSORIASIS
• Affects ~7.5 million people in the U.S.
• Chronic autoimmune disorder affecting the skin.
• Causes cells to rapidly multiply and build up on the skin’s surface, resulting in red scaly patches; often itchy and painful.
Atopic Dermatitis
• Skin condition affecting ~17.5 infants, adolescents and adults in the U.S.
• Characterized by intense itching caused by both inflammation and a breakdown of the skin’s barrier function.
Additional information about Vitae, VTP-43742 and VTP-38543, as well as the unmet medical need in the treatment of psoriasis and atopic dermatitis, is available on Vitae’s website at http://vitaepharma.com/pipeline/
9