
December 30, 2024 AXS-05 Alzheimer’s Disease�Agitation Phase 3 Clinical Program ACCORD-2, ADVANCE-2, and Long-term safety Phase 3 trial topline results

Forward Looking Statements & Safe Harbor Certain matters discussed in this press release are “forward-looking statements”. The Company may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the continued commercial success of the Company’s Sunosi® and Auvelity® products and the success of the Company’s efforts to obtain any additional indication(s) with respect to solriamfetol and/or AXS-05; the Company’s ability to maintain and expand payer coverage; the success, timing and cost of the Company’s ongoing clinical trials and anticipated clinical trials for the Company’s current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including the Company’s ability to fully fund the Company’s disclosed clinical trials, which assumes no material changes to the Company’s currently projected revenues or expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of the Company’s ongoing clinical trials, and/or data readouts, and the number or type of studies or nature of results necessary to support the filing of a new drug application (“NDA”) for any of the Company’s current product candidates, including statements regarding the ability of the ACCORD and ADVANCE clinical trials to support the filing of an NDA for Alzheimer’s disease agitation; the Company’s ability to fund additional clinical trials to continue the advancement of the Company’s product candidates; the timing of and the Company’s ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, the Company’s product candidates, including statements regarding the timing of any NDA submission; whether issues identified by FDA in the complete response letter may impact the potential approvability of the Company’s NDA for AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to the Company’s special protocol assessment for the MOMENTUM clinical trial; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s products and product candidates, if approved; the Company’s anticipated capital requirements, including the amount of capital required for the continued commercialization of Sunosi and Auvelity and for the Company’s commercial launch of its other product candidates, if approved, and the potential impact on the Company’s anticipated cash runway; the Company’s ability to convert sales to recognized revenue and maintain a favorable gross to net sales; unforeseen circumstances or other disruptions to normal business operations arising from or related to domestic political climate, geo-political conflicts or a global pandemic and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation contains statements regarding the Company’s observations based upon the reported clinical data. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about the Company's industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. Axsome, Auvelity, Sunosi, and MoSEIC, are trademarks or registered trademarks of Axsome Therapeutics, Inc. or its affiliates. Except as with respect to Auvelity and Sunosi for their approved indications, the development products referenced herein have not been approved by the FDA.
Agenda Mark Jacobson, MA�Chief Operating Officer Herriot Tabuteau, MD�Founder and Chief Executive Officer Sue Giordano, PhD�Vice President, Medical Affairs Dr. Jeffrey Cummings, MD, ScD Herriot Tabuteau, MD Mark Jacobson, MA Sue Giordano, PhD Introduction Phase 3 trial results�ACCORD-2, ADVANCE-2, long-term safety trial Alzheimer’s disease agitation�Disease overview Q&A Dr. Jeffrey Cummings, MD, ScD�Vice Chair of Research, UNLV Department of Brain Health Clinical perspective

Develop and deliver transformative medicines �for the hundreds of millions of people impacted by central nervous system conditions
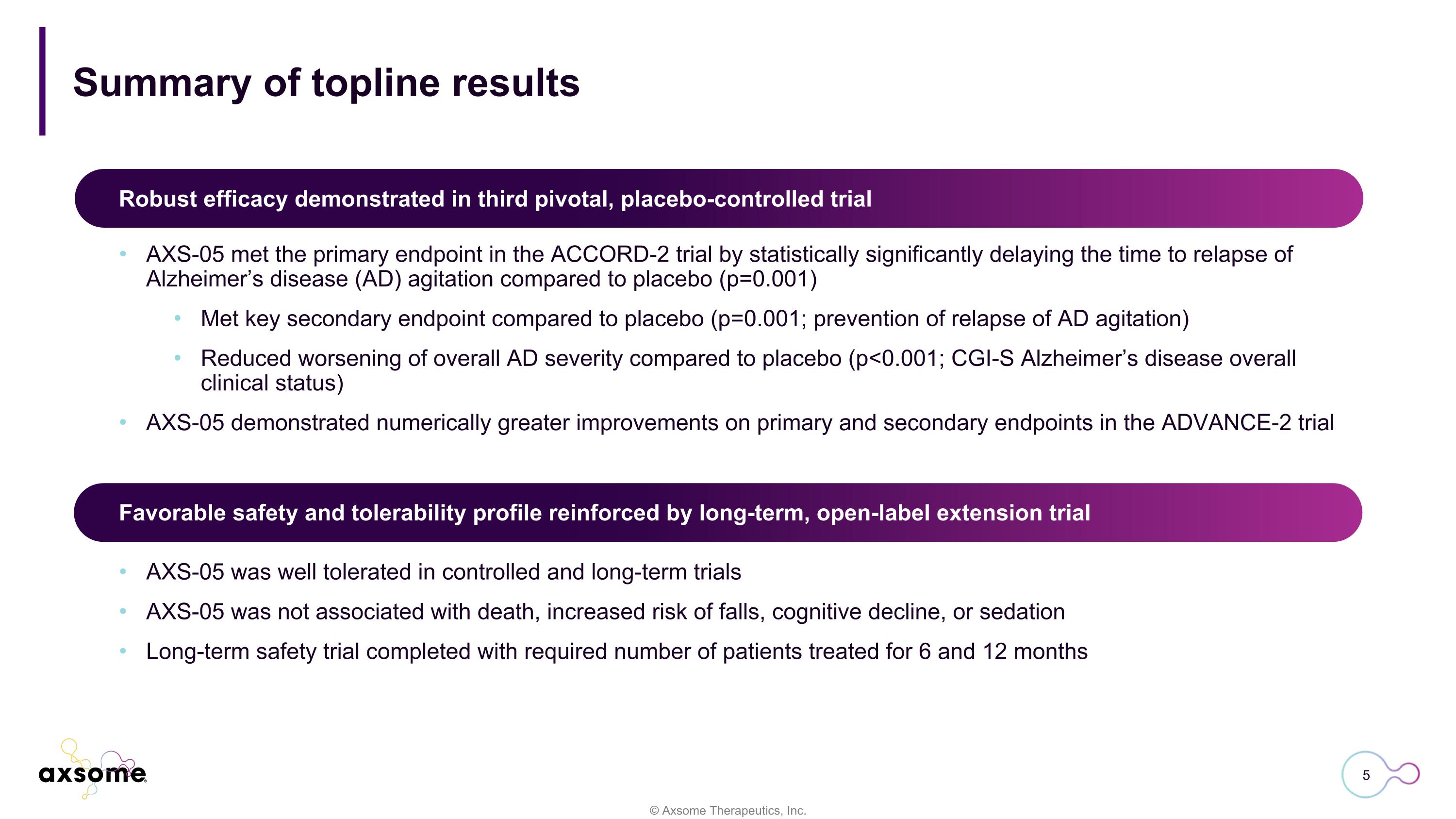
Summary of topline results AXS-05 met the primary endpoint in the ACCORD-2 trial by statistically significantly delaying the time to relapse of Alzheimer’s disease (AD) agitation compared to placebo (p=0.001) Met key secondary endpoint compared to placebo (p=0.001; prevention of relapse of AD agitation) Reduced worsening of overall AD severity compared to placebo (p<0.001; CGI-S Alzheimer’s disease overall clinical status) AXS-05 demonstrated numerically greater improvements on primary and secondary endpoints in the ADVANCE-2 trial Robust efficacy demonstrated in third pivotal, placebo-controlled trial Favorable safety and tolerability profile reinforced by long-term, open-label extension trial AXS-05 was well tolerated in controlled and long-term trials AXS-05 was not associated with death, increased risk of falls, cognitive decline, or sedation Long-term safety trial completed with required number of patients treated for 6 and 12 months
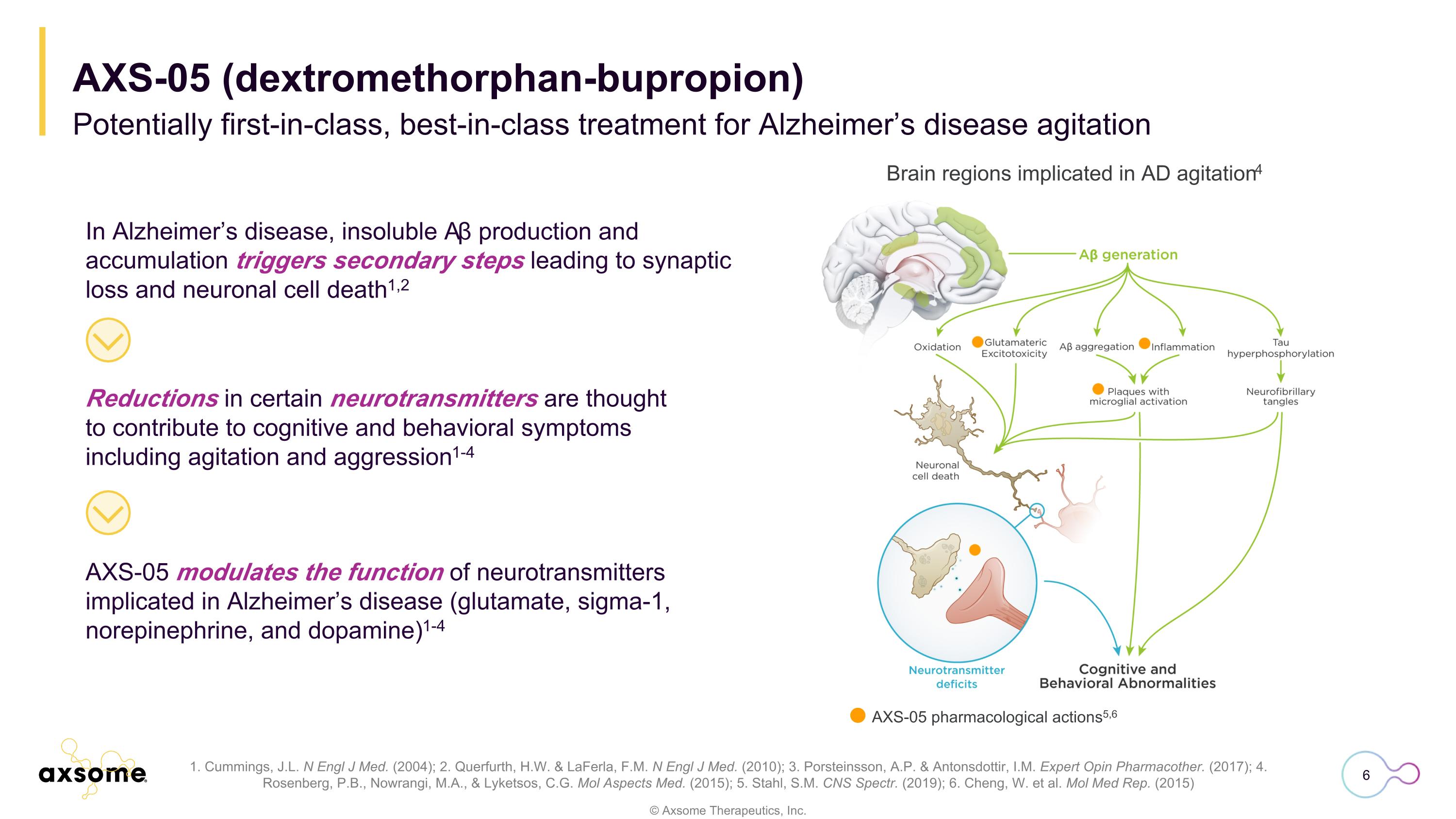
AXS-05 modulates the function of neurotransmitters implicated in Alzheimer’s disease (glutamate, sigma-1, norepinephrine, and dopamine)1-4 1. Cummings, J.L. N Engl J Med. (2004); 2. Querfurth, H.W. & LaFerla, F.M. N Engl J Med. (2010); 3. Porsteinsson, A.P. & Antonsdottir, I.M. Expert Opin Pharmacother. (2017); 4. Rosenberg, P.B., Nowrangi, M.A., & Lyketsos, C.G. Mol Aspects Med. (2015); 5. Stahl, S.M. CNS Spectr. (2019); 6. Cheng, W. et al. Mol Med Rep. (2015) In Alzheimer’s disease, insoluble Aβ production and accumulation triggers secondary steps leading to synaptic loss and neuronal cell death1,2 Reductions in certain neurotransmitters are thought to contribute to cognitive and behavioral symptoms including agitation and aggression1-4 Brain regions implicated in AD agitation4 AXS-05 pharmacological actions5,6 Potentially first-in-class, best-in-class treatment for Alzheimer’s disease agitation AXS-05 (dextromethorphan-bupropion)
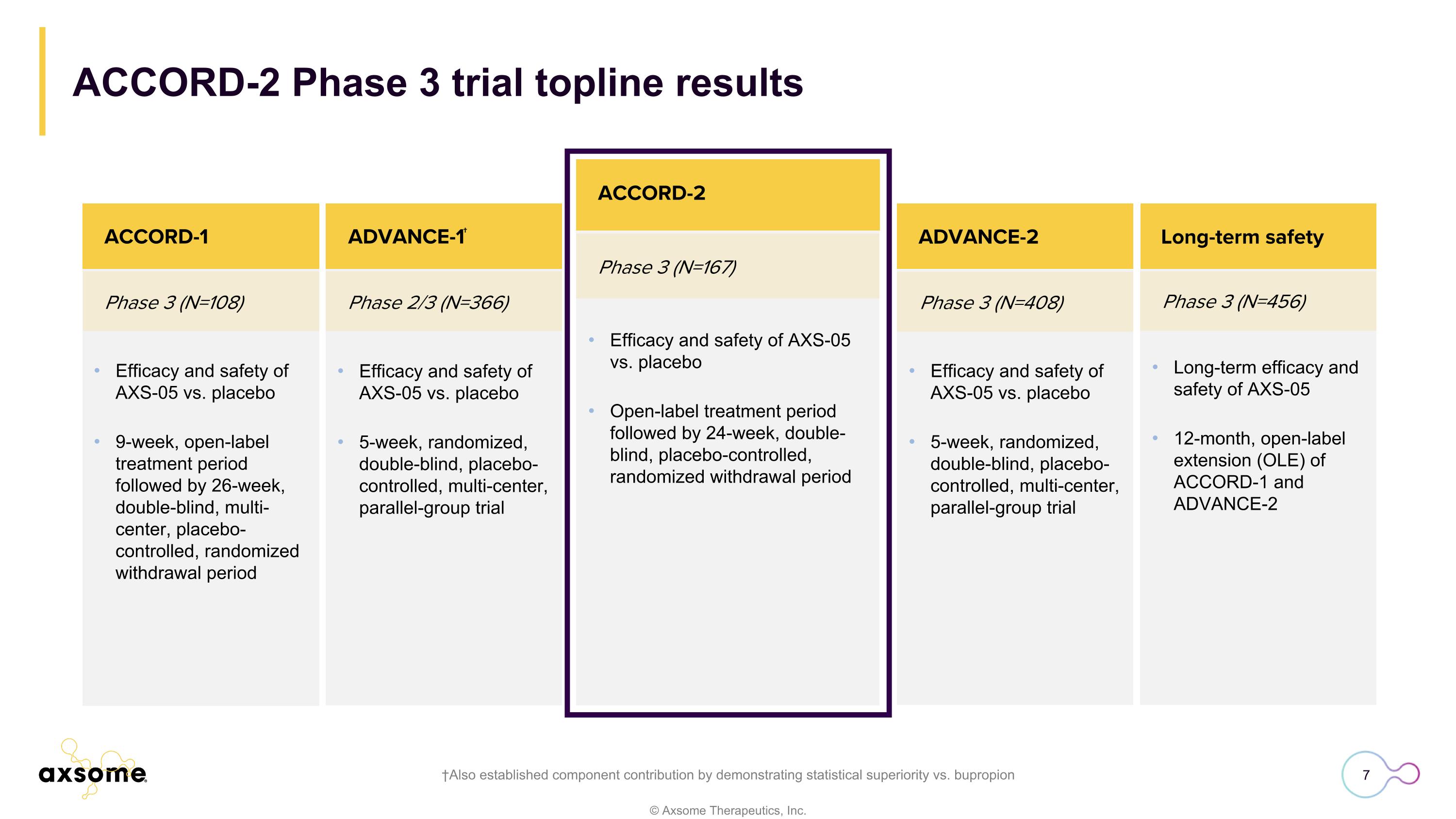
ACCORD-2 Phase 3 trial topline results †Also established component contribution by demonstrating statistical superiority vs. bupropion Phase 3 (N=167) ACCORD-2 Efficacy and safety of AXS-05 vs. placebo Open-label treatment period followed by 24-week, double-blind, placebo-controlled, randomized withdrawal period Efficacy and safety of AXS-05 vs. placebo 9-week, open-label treatment period followed by 26-week, double-blind, multi-center, placebo-controlled, randomized withdrawal period Phase 3 (N=108) Phase 2/3 (N=366) ACCORD-1 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial ADVANCE-1† Phase 3 (N=408) ADVANCE-2 Phase 3 (N=456) ACCORD-2 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial Long-term efficacy and safety of AXS-05 12-month, open-label extension (OLE) of ACCORD-1 and ADVANCE-2 Long-term safety
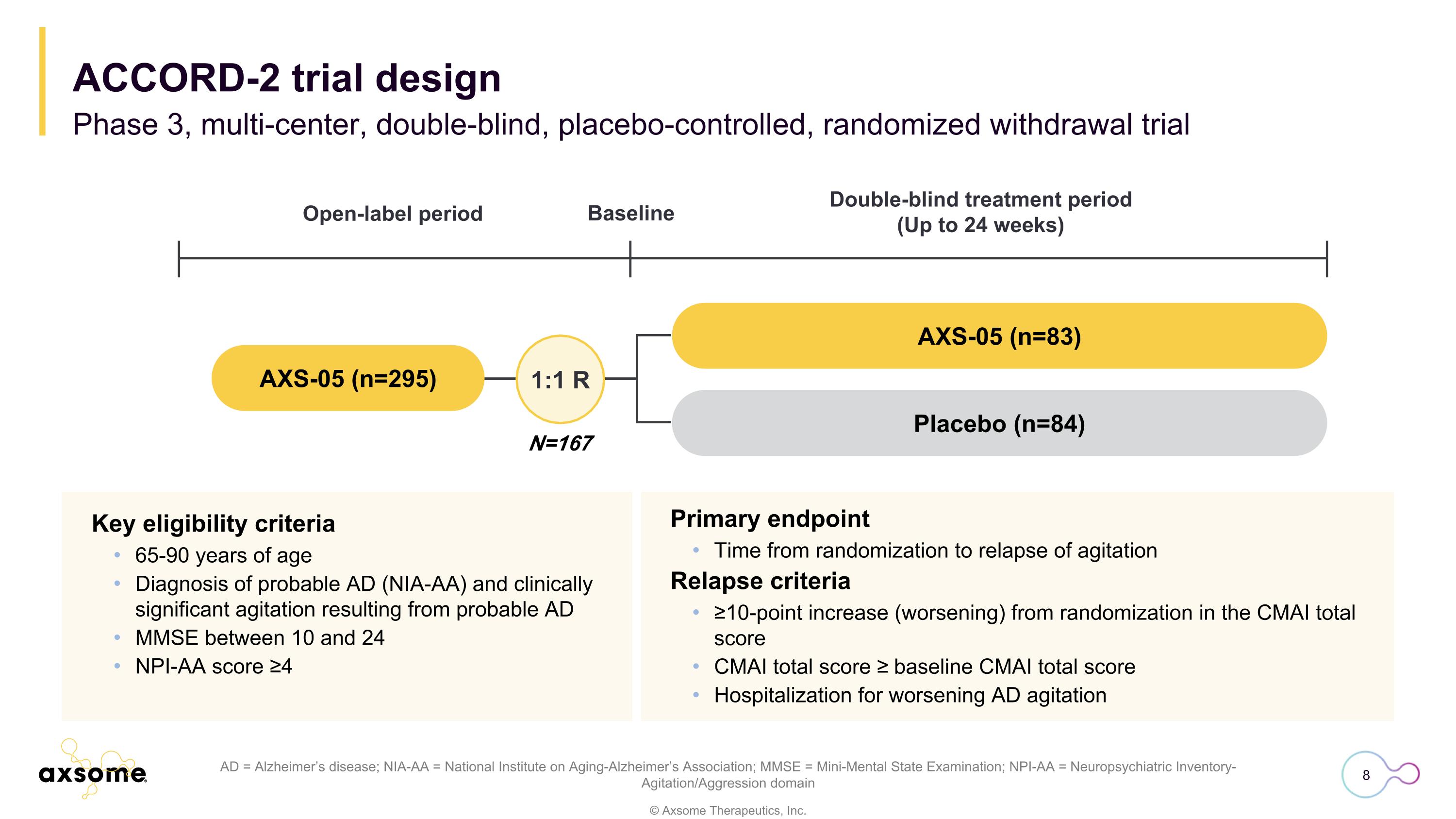
Primary endpoint Time from randomization to relapse of agitation Relapse criteria ≥10-point increase (worsening) from randomization in the CMAI total score CMAI total score ≥ baseline CMAI total score Hospitalization for worsening AD agitation AD = Alzheimer’s disease; NIA-AA = National Institute on Aging-Alzheimer’s Association; MMSE = Mini-Mental State Examination; NPI-AA = Neuropsychiatric Inventory-Agitation/Aggression domain Key eligibility criteria 65-90 years of age Diagnosis of probable AD (NIA-AA) and clinically significant agitation resulting from probable AD MMSE between 10 and 24 NPI-AA score ≥4 N=167 1:1 R AXS-05 (n=83) Placebo (n=84) Open-label period Double-blind treatment period (Up to 24 weeks) Baseline AXS-05 (n=295) ACCORD-2 trial design Phase 3, multi-center, double-blind, placebo-controlled, randomized withdrawal trial
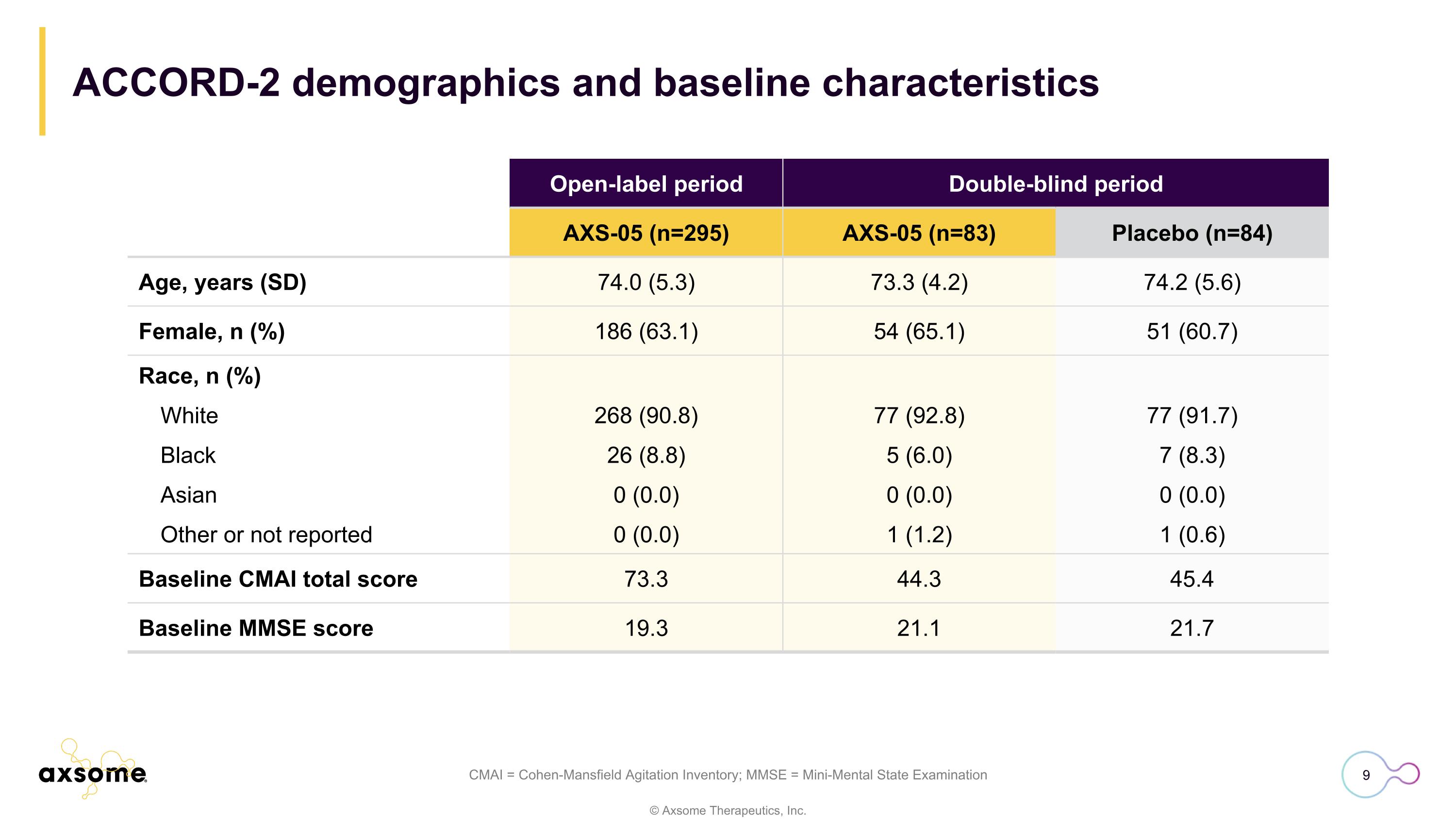
CMAI = Cohen-Mansfield Agitation Inventory; MMSE = Mini-Mental State Examination Open-label period Double-blind period AXS-05 (n=295) AXS-05 (n=83) Placebo (n=84) Age, years (SD) 74.0 (5.3) 73.3 (4.2) 74.2 (5.6) Female, n (%) 186 (63.1) 54 (65.1) 51 (60.7) Race, n (%) White 268 (90.8) 77 (92.8) 77 (91.7) Black 26 (8.8) 5 (6.0) 7 (8.3) Asian 0 (0.0) 0 (0.0) 0 (0.0) Other or not reported 0 (0.0) 1 (1.2) 1 (0.6) Baseline CMAI total score 73.3 44.3 45.4 Baseline MMSE score 19.3 21.1 21.7 ACCORD-2 demographics and baseline characteristics
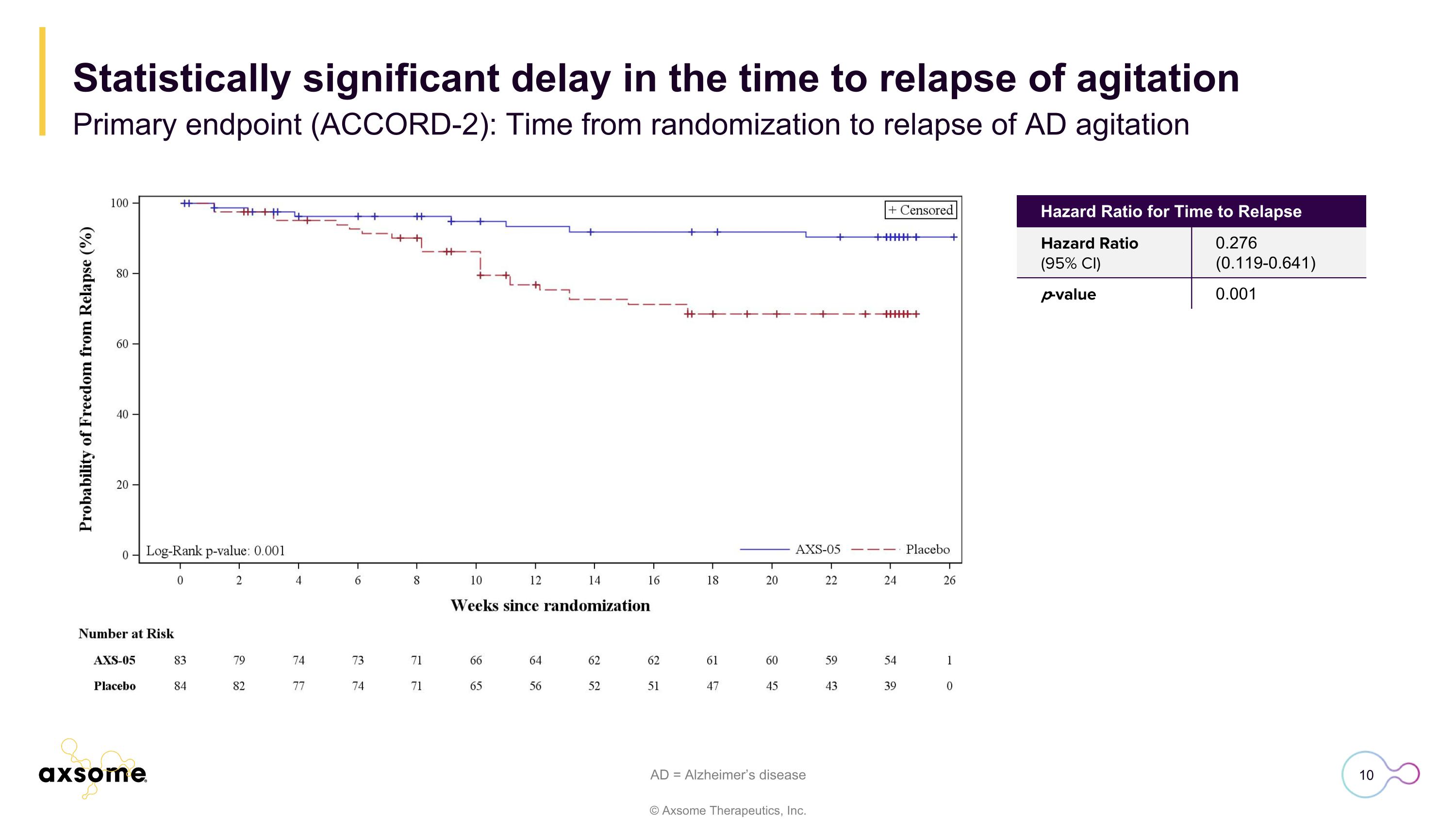
AD = Alzheimer’s disease Hazard Ratio for Time to Relapse Hazard Ratio (95% CI) 0.276 (0.119-0.641) p-value 0.001 Statistically significant delay in the time to relapse of agitation Primary endpoint (ACCORD-2): Time from randomization to relapse of AD agitation
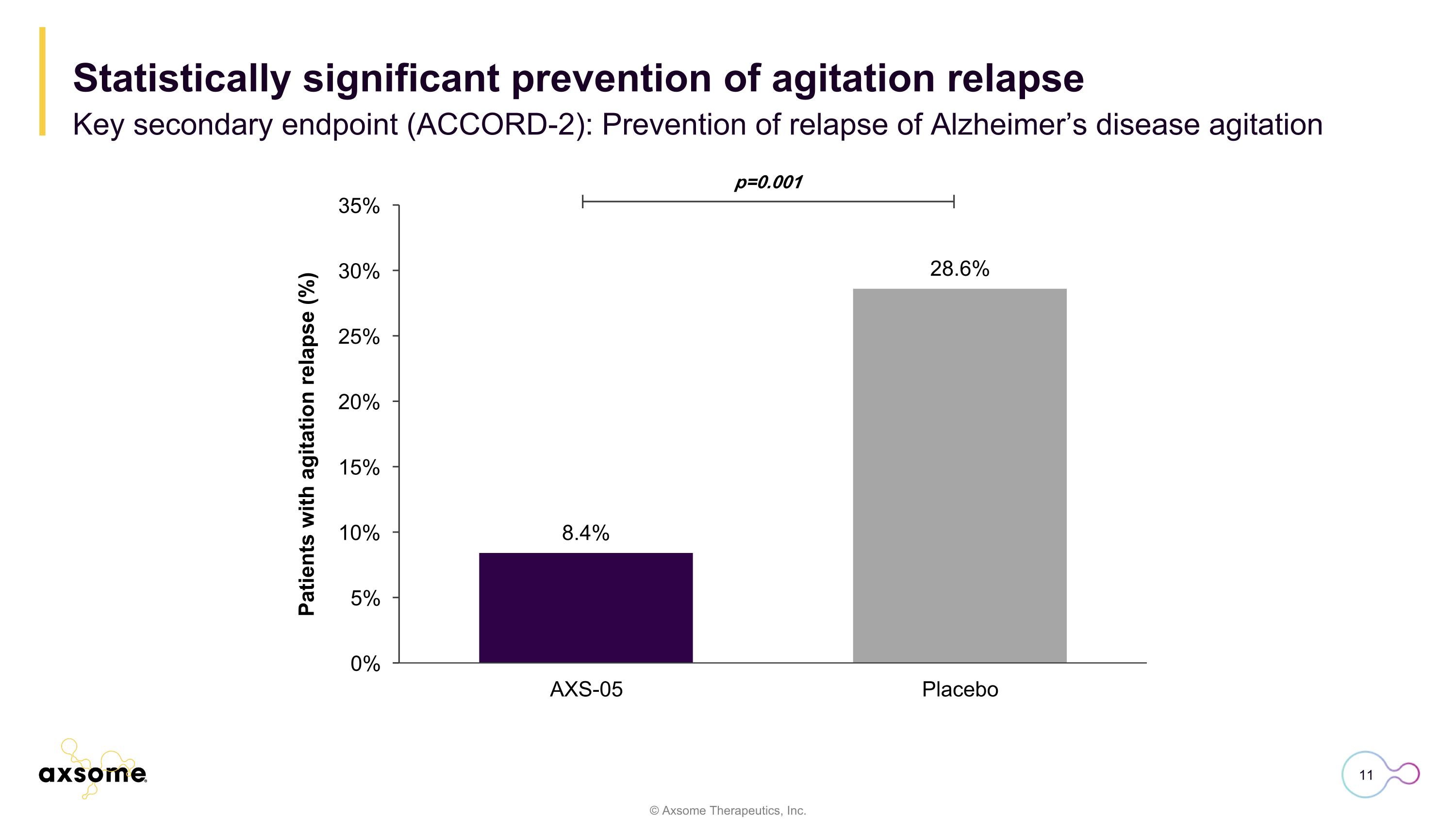
p=0.001 Statistically significant prevention of agitation relapse Key secondary endpoint (ACCORD-2): Prevention of relapse of Alzheimer’s disease agitation
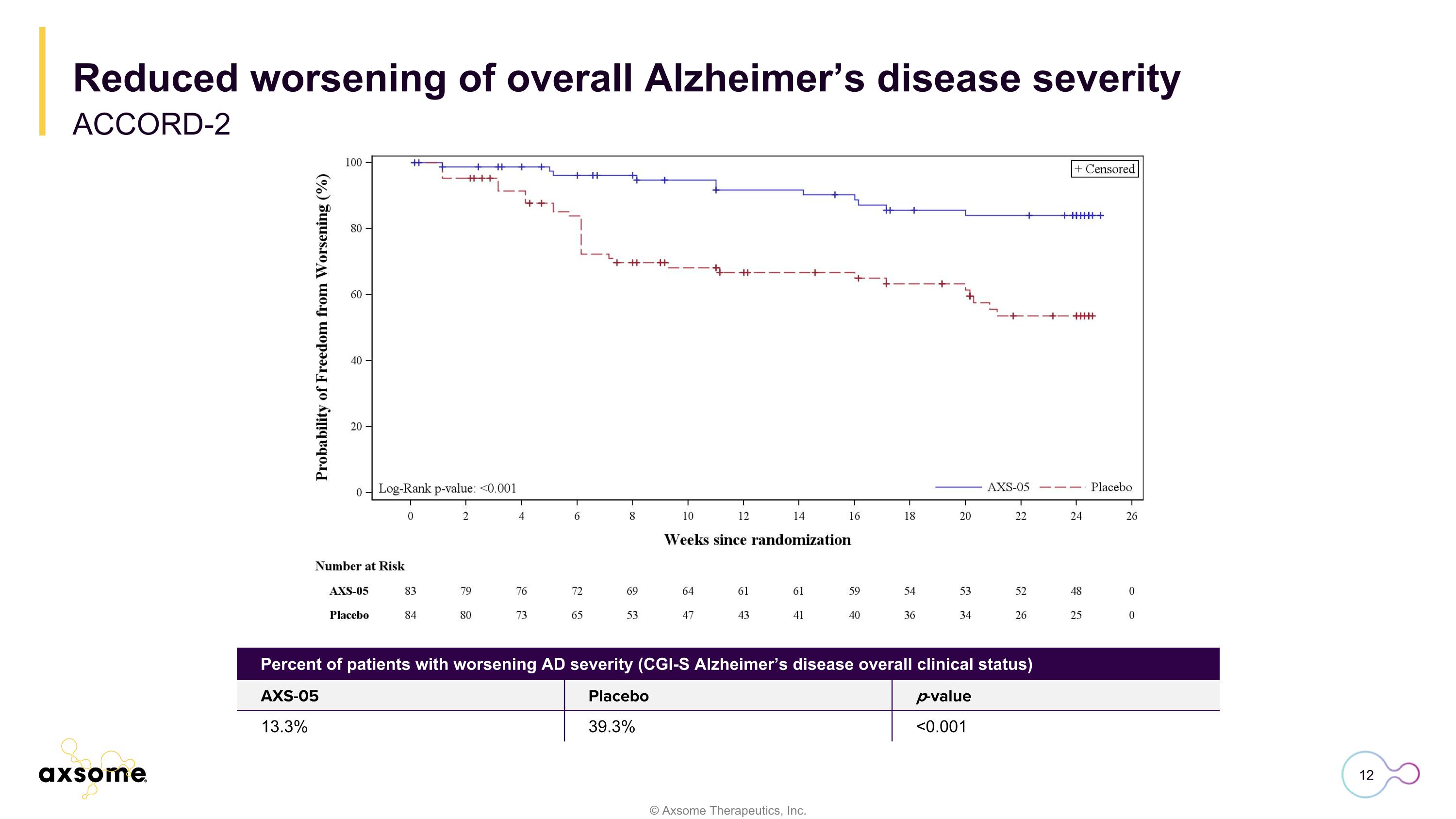
Reduced worsening of overall Alzheimer’s disease severity ACCORD-2 Percent of patients with worsening AD severity (CGI-S Alzheimer’s disease overall clinical status) AXS-05 Placebo p-value 13.3% 39.3% <0.001
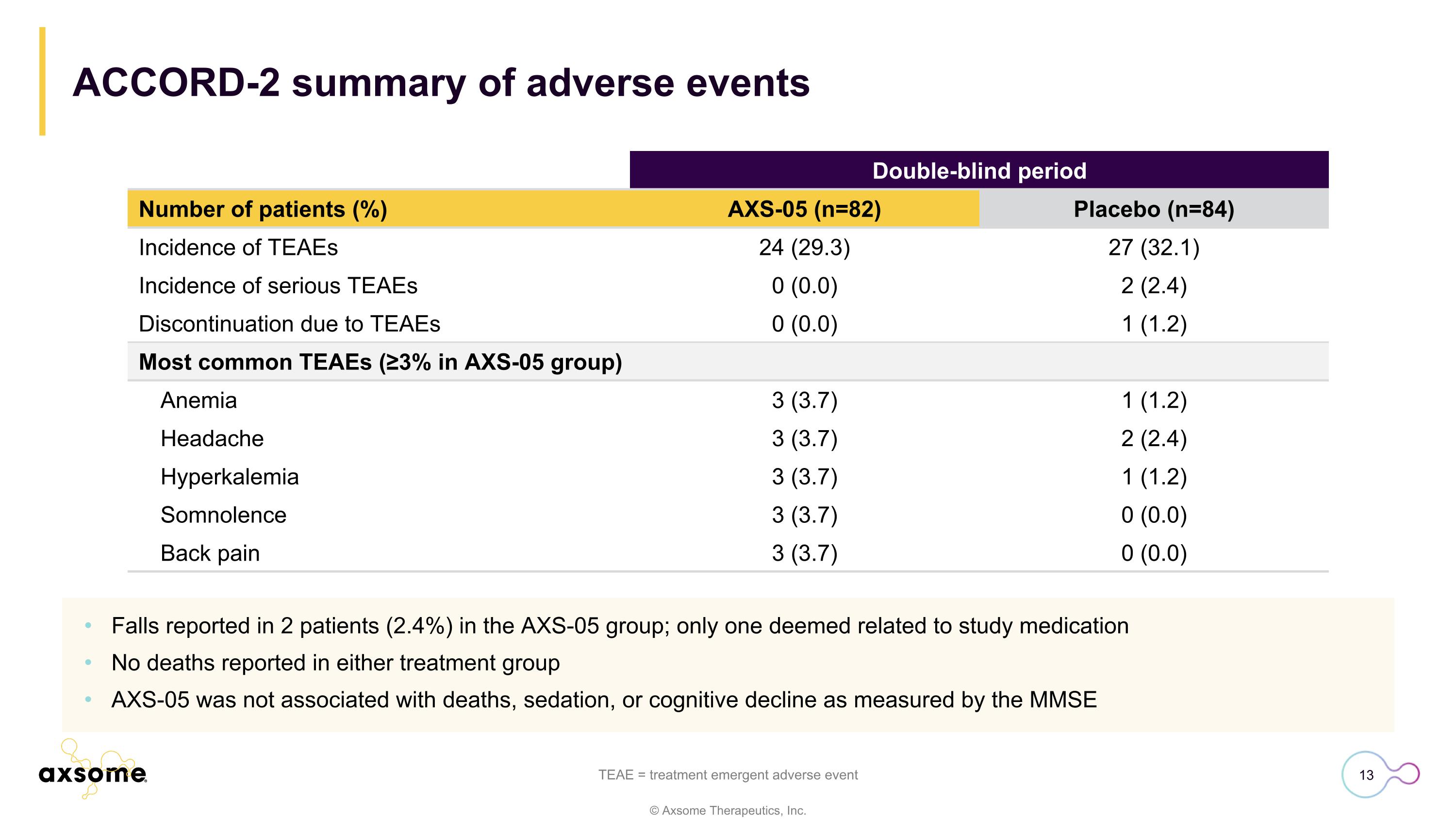
ACCORD-2 summary of adverse events Falls reported in 2 patients (2.4%) in the AXS-05 group; only one deemed related to study medication No deaths reported in either treatment group AXS-05 was not associated with deaths, sedation, or cognitive decline as measured by the MMSE TEAE = treatment emergent adverse event Double-blind period Number of patients (%) AXS-05 (n=82) Placebo (n=84) Incidence of TEAEs 24 (29.3) 27 (32.1) Incidence of serious TEAEs 0 (0.0) 2 (2.4) Discontinuation due to TEAEs 0 (0.0) 1 (1.2) Most common TEAEs (≥3% in AXS-05 group) Anemia 3 (3.7) 1 (1.2) Headache 3 (3.7) 2 (2.4) Hyperkalemia 3 (3.7) 1 (1.2) Somnolence 3 (3.7) 0 (0.0) Back pain 3 (3.7) 0 (0.0)
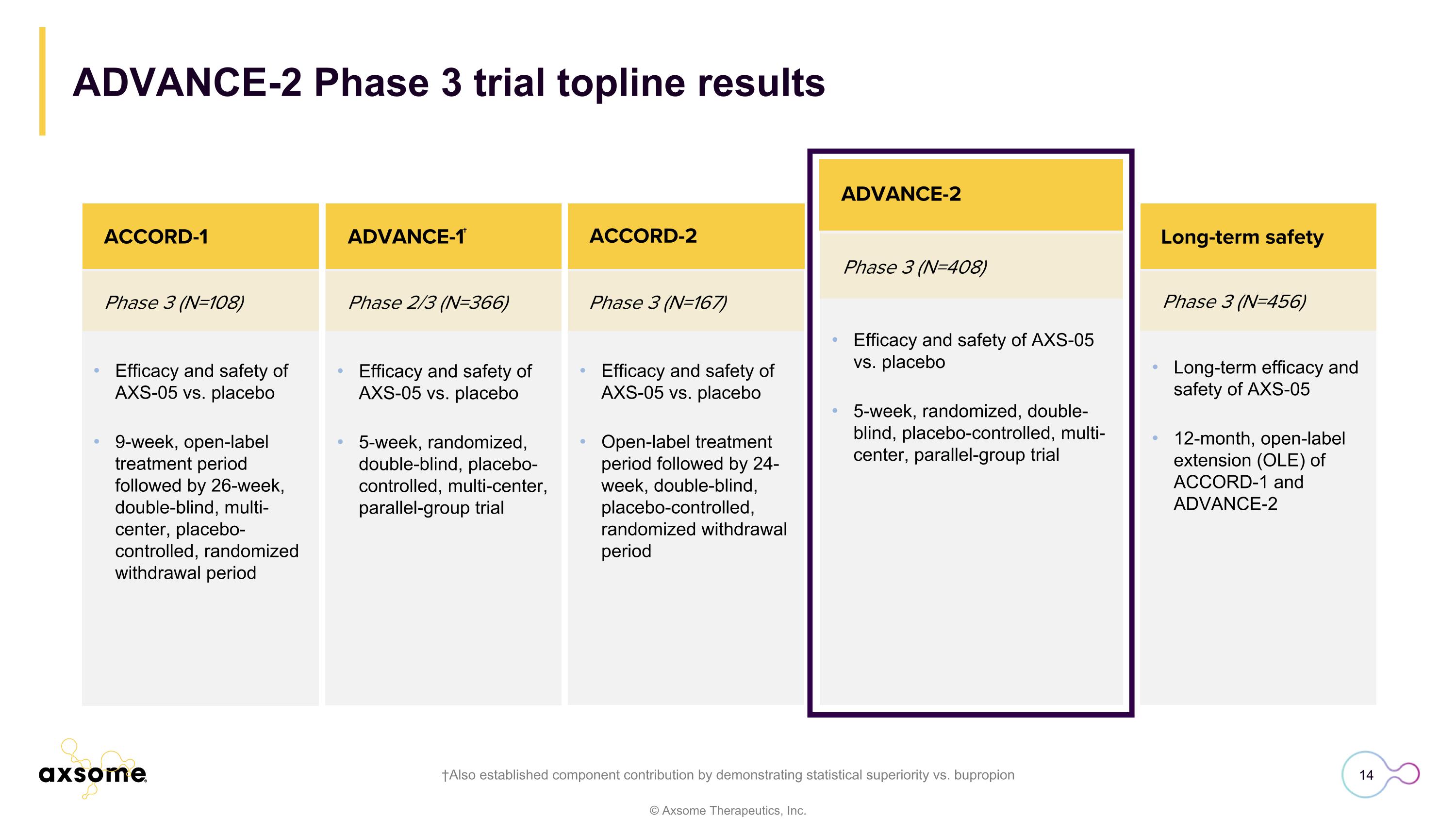
Phase 3 (N=456) ACCORD-2 Long-term efficacy and safety of AXS-05 12-month, open-label extension (OLE) of ACCORD-1 and ADVANCE-2 Long-term safety Efficacy and safety of AXS-05 vs. placebo 9-week, open-label treatment period followed by 26-week, double-blind, multi-center, placebo-controlled, randomized withdrawal period Phase 3 (N=108) Phase 2/3 (N=366) ACCORD-1 ACCORD-2 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial Efficacy and safety of AXS-05 vs. placebo Open-label treatment period followed by 24-week, double-blind, placebo-controlled, randomized withdrawal period ADVANCE-1† Phase 3 (N=408) ADVANCE-2 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial ADVANCE-2 Phase 3 trial topline results †Also established component contribution by demonstrating statistical superiority vs. bupropion Phase 3 (N=167)
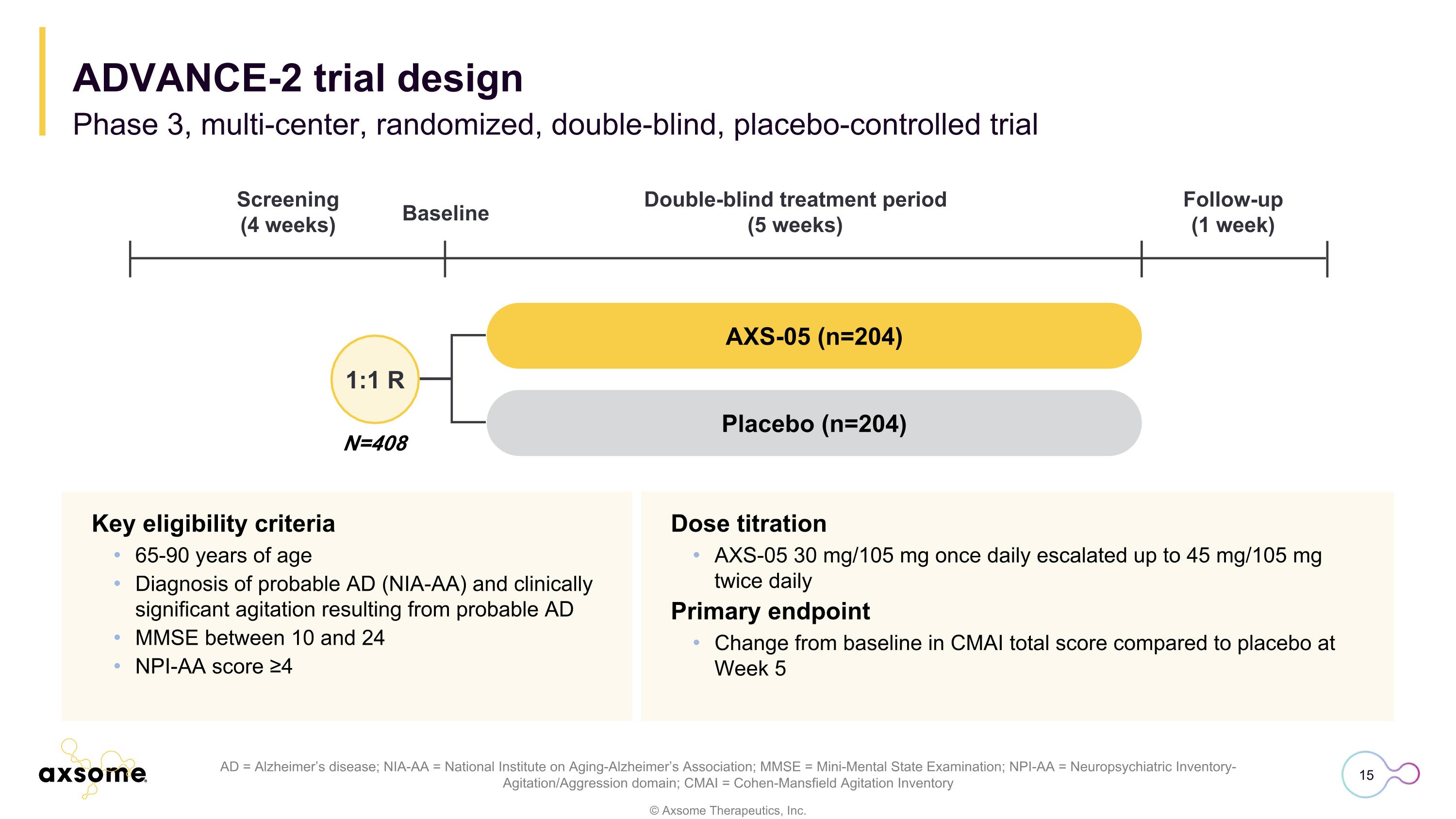
Dose titration AXS-05 30 mg/105 mg once daily escalated up to 45 mg/105 mg twice daily Primary endpoint Change from baseline in CMAI total score compared to placebo at Week 5 AD = Alzheimer’s disease; NIA-AA = National Institute on Aging-Alzheimer’s Association; MMSE = Mini-Mental State Examination; NPI-AA = Neuropsychiatric Inventory-Agitation/Aggression domain; CMAI = Cohen-Mansfield Agitation Inventory Key eligibility criteria 65-90 years of age Diagnosis of probable AD (NIA-AA) and clinically significant agitation resulting from probable AD MMSE between 10 and 24 NPI-AA score ≥4 N=408 1:1 R AXS-05 (n=204) Placebo (n=204) Screening (4 weeks) Double-blind treatment period (5 weeks) Follow-up (1 week) Baseline Phase 3, multi-center, randomized, double-blind, placebo-controlled trial ADVANCE-2 trial design
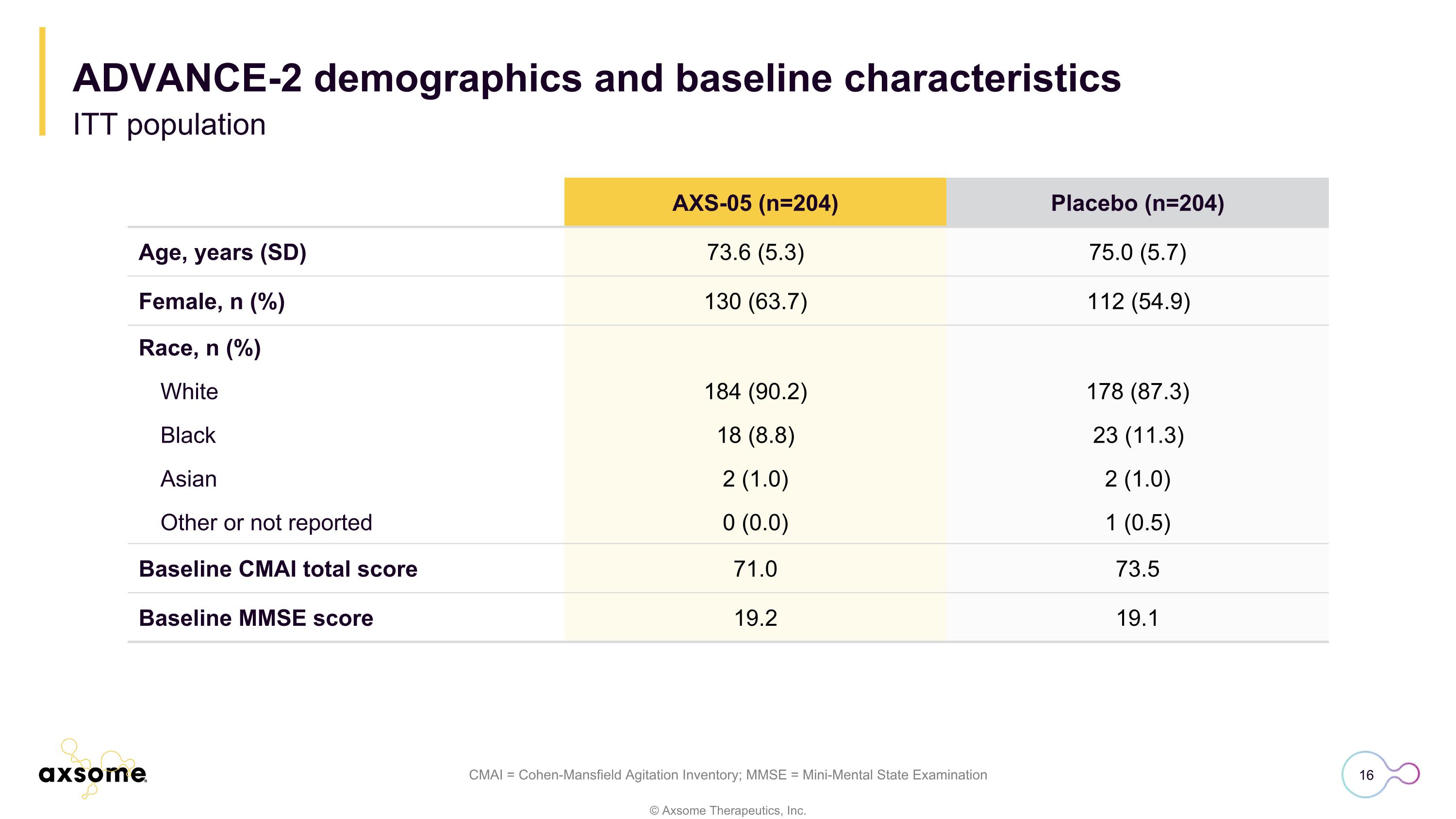
ADVANCE-2 demographics and baseline characteristics ITT population CMAI = Cohen-Mansfield Agitation Inventory; MMSE = Mini-Mental State Examination AXS-05 (n=204) Placebo (n=204) Age, years (SD) 73.6 (5.3) 75.0 (5.7) Female, n (%) 130 (63.7) 112 (54.9) Race, n (%) White 184 (90.2) 178 (87.3) Black 18 (8.8) 23 (11.3) Asian 2 (1.0) 2 (1.0) Other or not reported 0 (0.0) 1 (0.5) Baseline CMAI total score 71.0 73.5 Baseline MMSE score 19.2 19.1
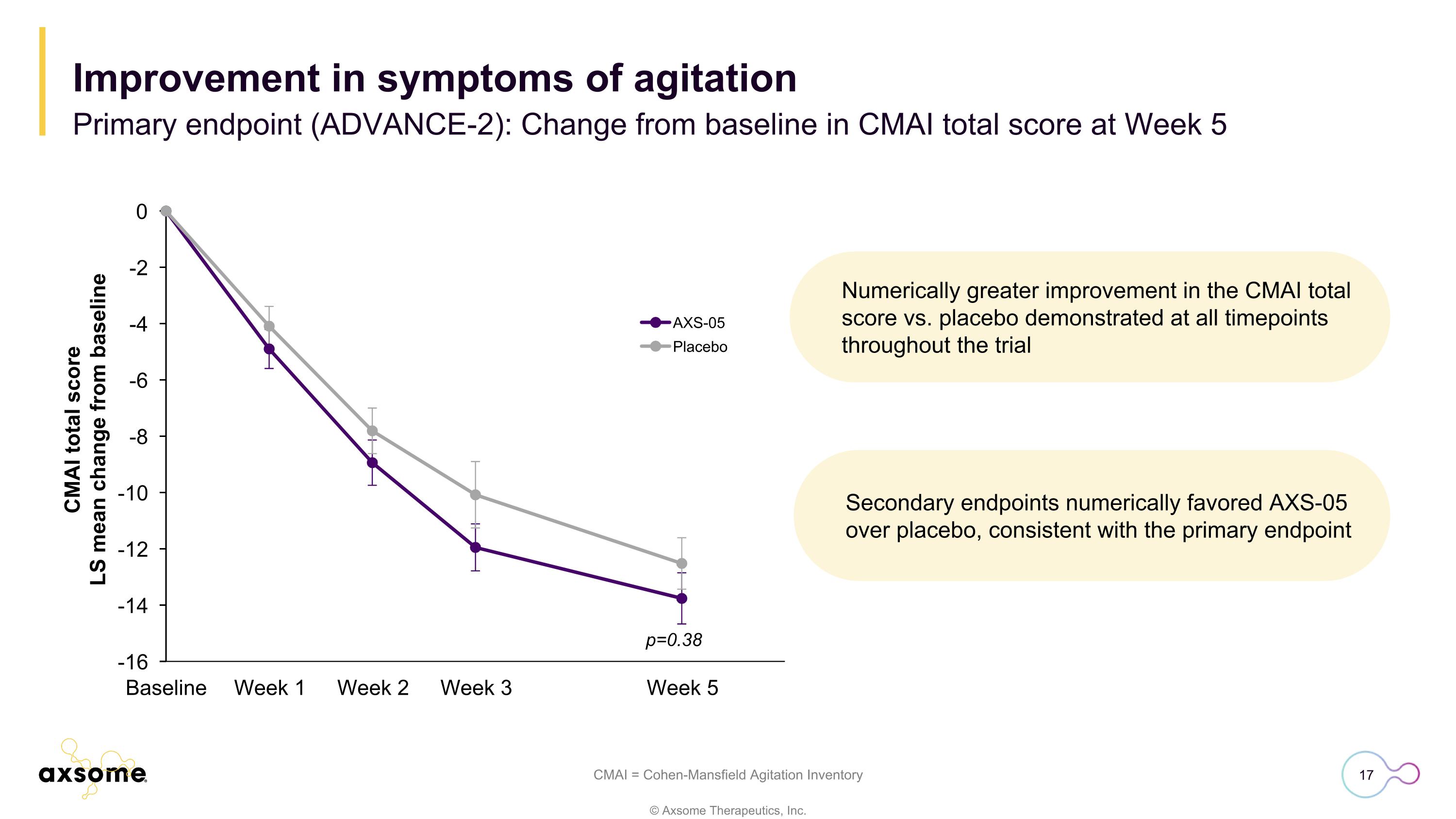
Numerically greater improvement in the CMAI total score vs. placebo demonstrated at all timepoints throughout the trial CMAI = Cohen-Mansfield Agitation Inventory p=0.38 Improvement in symptoms of agitation Primary endpoint (ADVANCE-2): Change from baseline in CMAI total score at Week 5 Secondary endpoints numerically favored AXS-05 over placebo, consistent with the primary endpoint
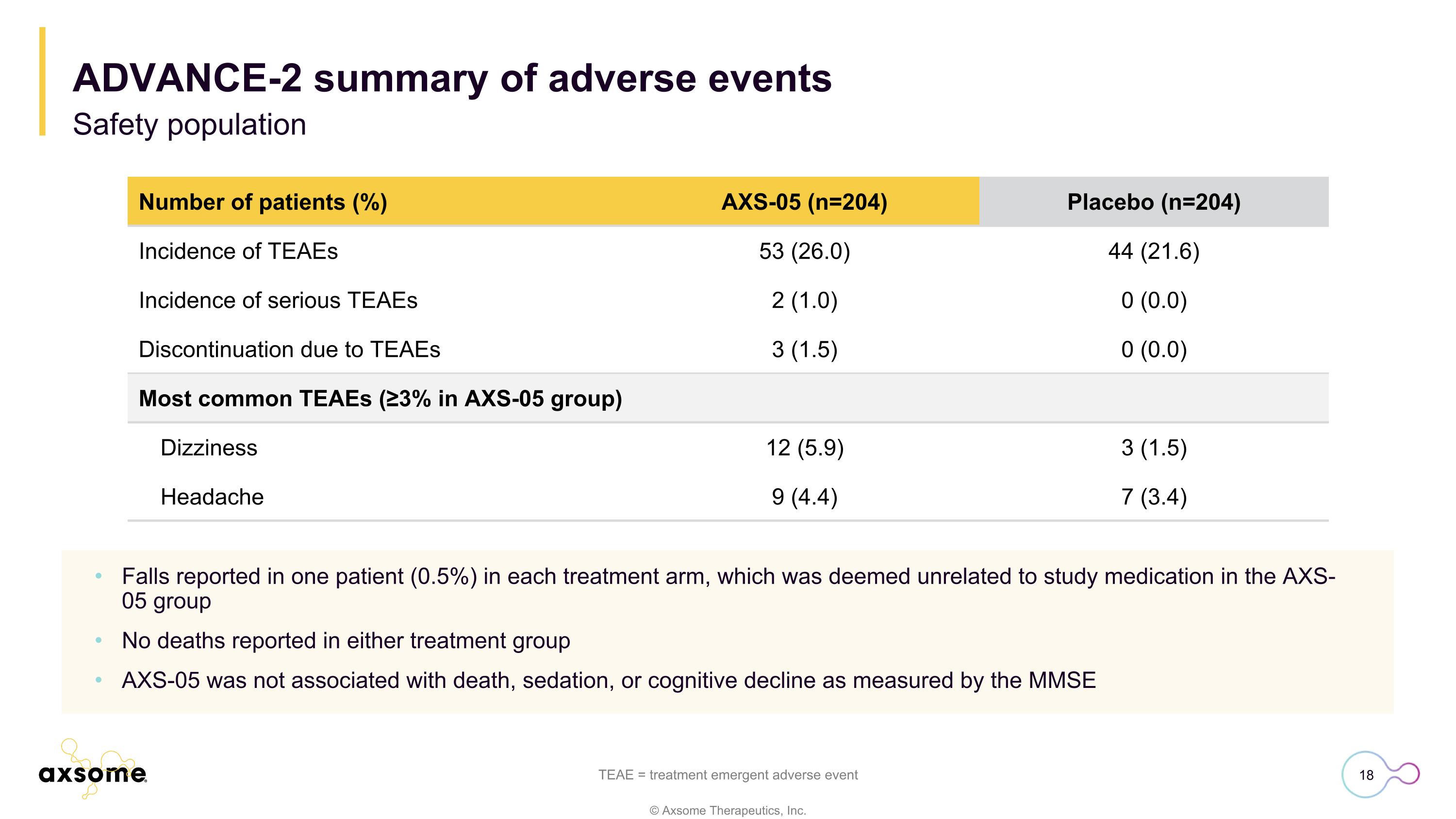
Number of patients (%) AXS-05 (n=204) Placebo (n=204) Incidence of TEAEs 53 (26.0) 44 (21.6) Incidence of serious TEAEs 2 (1.0) 0 (0.0) Discontinuation due to TEAEs 3 (1.5) 0 (0.0) Most common TEAEs (≥3% in AXS-05 group) Dizziness 12 (5.9) 3 (1.5) Headache 9 (4.4) 7 (3.4) Falls reported in one patient (0.5%) in each treatment arm, which was deemed unrelated to study medication in the AXS-05 group No deaths reported in either treatment group AXS-05 was not associated with death, sedation, or cognitive decline as measured by the MMSE TEAE = treatment emergent adverse event ADVANCE-2 summary of adverse events Safety population
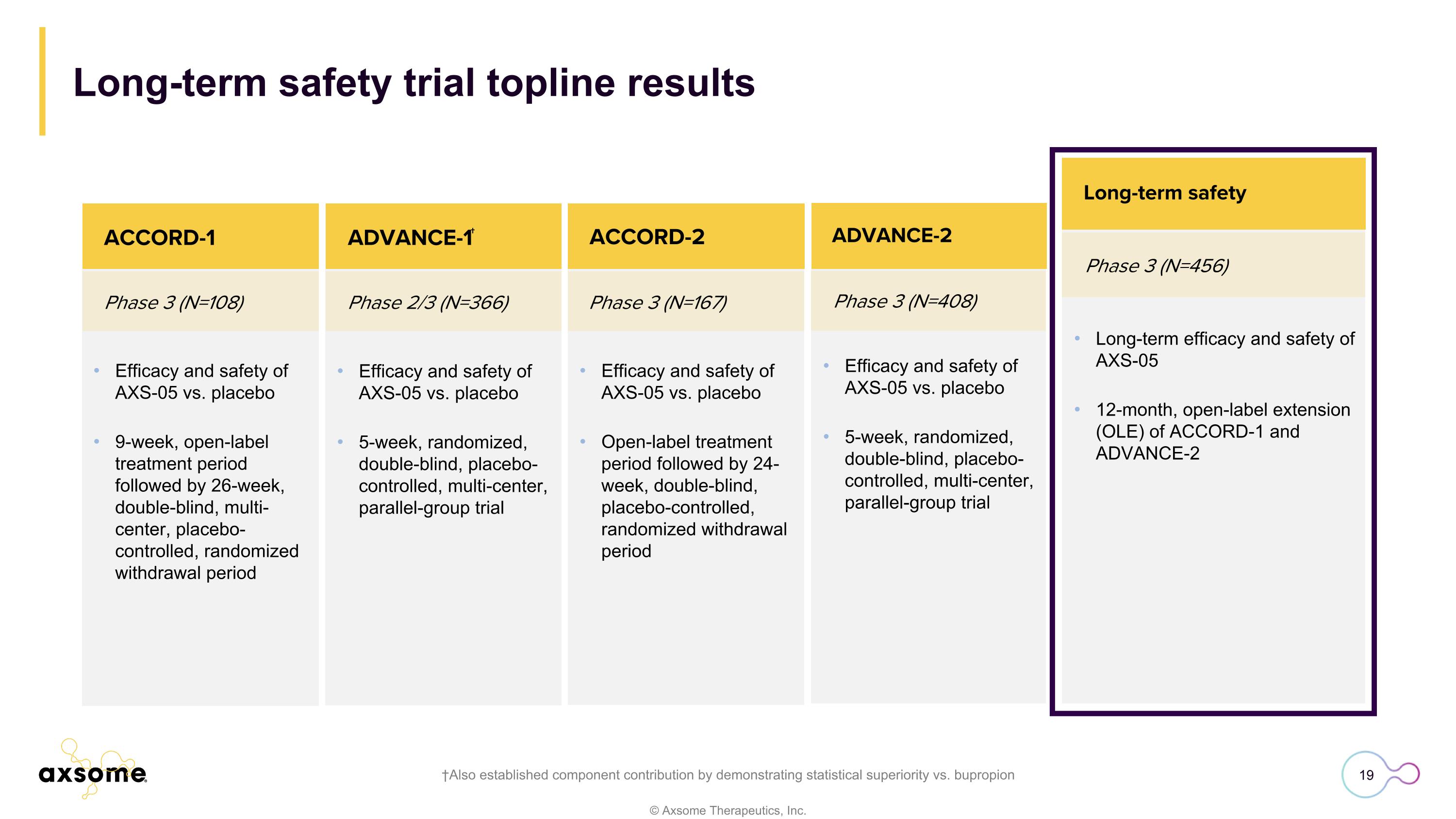
Phase 3 (N=408) ACCORD-2 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial ADVANCE-2 Complete Efficacy and safety of AXS-05 vs. placebo 9-week, open-label treatment period followed by 26-week, double-blind, multi-center, placebo-controlled, randomized withdrawal period Phase 3 (N=108) Phase 2/3 (N=366) Phase 3 (N=167) ACCORD-1 ACCORD-2 Efficacy and safety of AXS-05 vs. placebo 5-week, randomized, double-blind, placebo-controlled, multi-center, parallel-group trial Efficacy and safety of AXS-05 vs. placebo Open-label treatment period followed by 24-week, double-blind, placebo-controlled, randomized withdrawal period ADVANCE-1† Long-term safety trial topline results †Also established component contribution by demonstrating statistical superiority vs. bupropion Phase 3 (N=456) Long-term safety Long-term efficacy and safety of AXS-05 12-month, open-label extension (OLE) of ACCORD-1 and ADVANCE-2
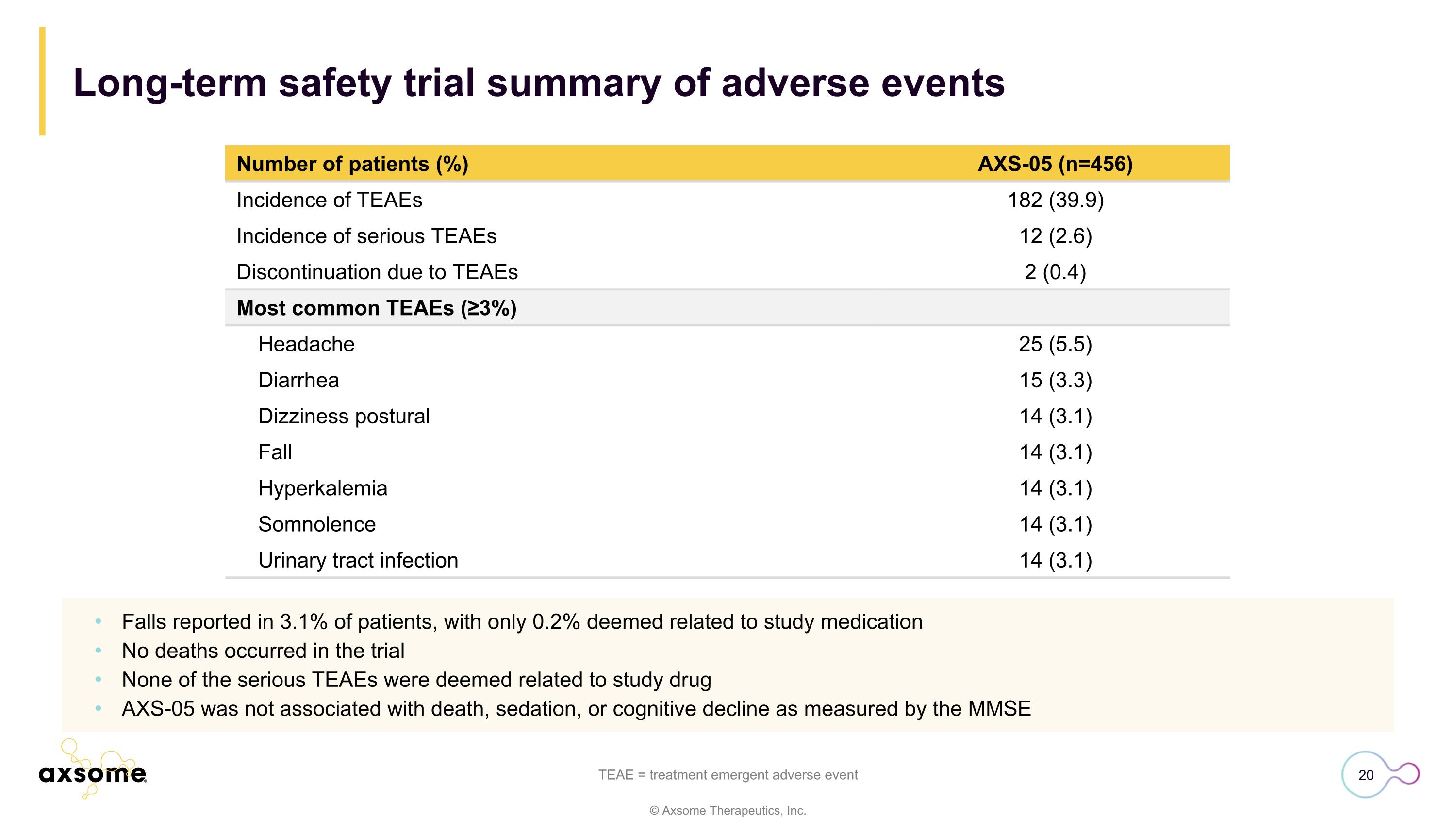
Long-term safety trial summary of adverse events TEAE = treatment emergent adverse event Number of patients (%) AXS-05 (n=456) Incidence of TEAEs 182 (39.9) Incidence of serious TEAEs 12 (2.6) Discontinuation due to TEAEs 2 (0.4) Most common TEAEs (≥3%) Headache 25 (5.5) Diarrhea 15 (3.3) Dizziness postural 14 (3.1) Fall 14 (3.1) Hyperkalemia 14 (3.1) Somnolence 14 (3.1) Urinary tract infection 14 (3.1) Falls reported in 3.1% of patients, with only 0.2% deemed related to study medication No deaths occurred in the trial None of the serious TEAEs were deemed related to study drug AXS-05 was not associated with death, sedation, or cognitive decline as measured by the MMSE
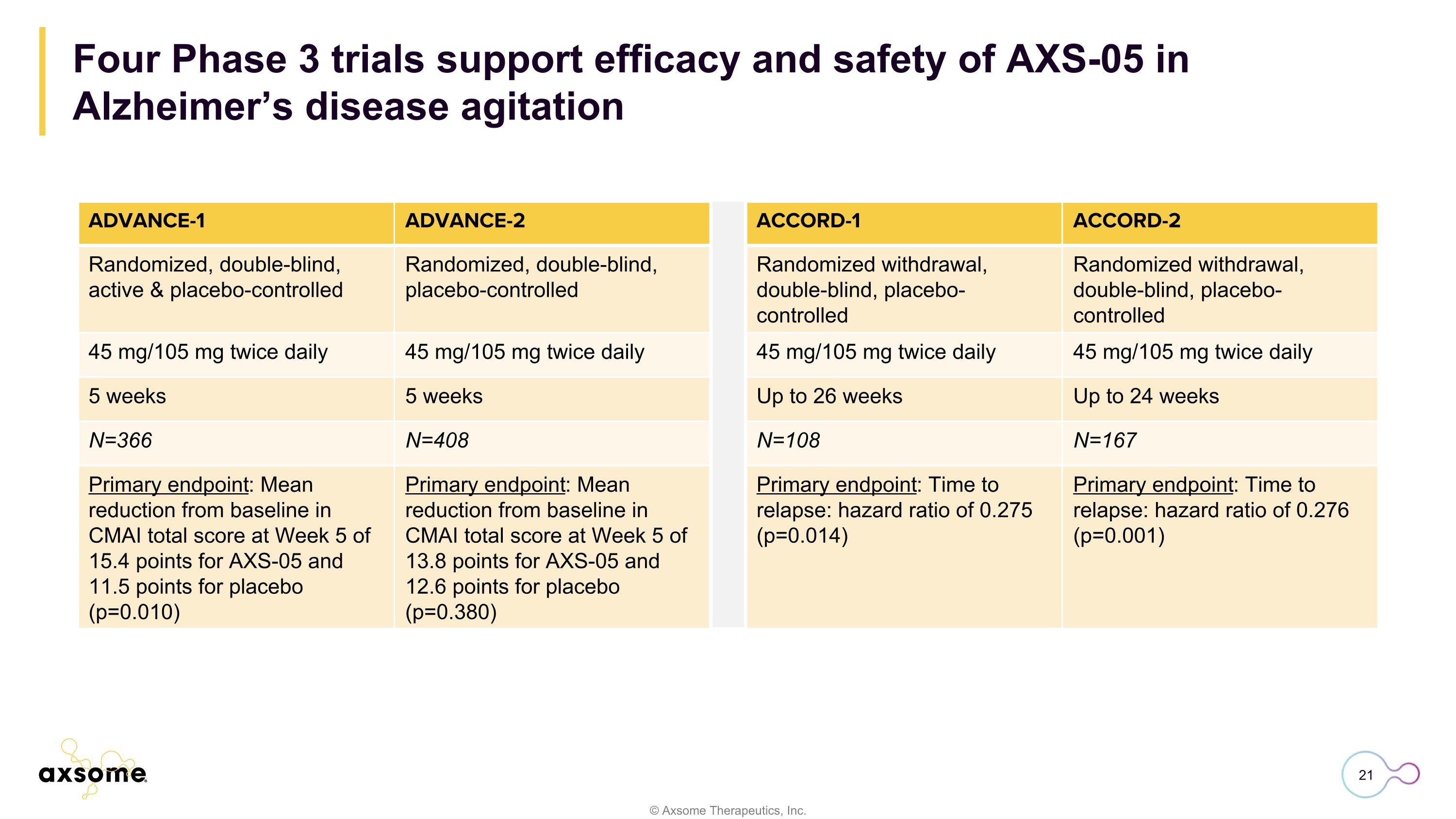
Four Phase 3 trials support efficacy and safety of AXS-05 in Alzheimer’s disease agitation ADVANCE-1 ADVANCE-2 ACCORD-1 ACCORD-2 Randomized, double-blind, active & placebo-controlled Randomized, double-blind, placebo-controlled Randomized withdrawal, double-blind, placebo-controlled Randomized withdrawal, double-blind, placebo-controlled 45 mg/105 mg twice daily 45 mg/105 mg twice daily 45 mg/105 mg twice daily 45 mg/105 mg twice daily 5 weeks 5 weeks Up to 26 weeks Up to 24 weeks N=366 N=408 N=108 N=167 Primary endpoint: Mean reduction from baseline in CMAI total score at Week 5 of 15.4 points for AXS-05 and 11.5 points for placebo (p=0.010) Primary endpoint: Mean reduction from baseline in CMAI total score at Week 5 of 13.8 points for AXS-05 and 12.6 points for placebo (p=0.380) Primary endpoint: Time to relapse: hazard ratio of 0.275 (p=0.014) Primary endpoint: Time to relapse: hazard ratio of 0.276 (p=0.001)

Sue Giordano, PhD Vice President of Medical Affairs Alzheimer’s disease agitation
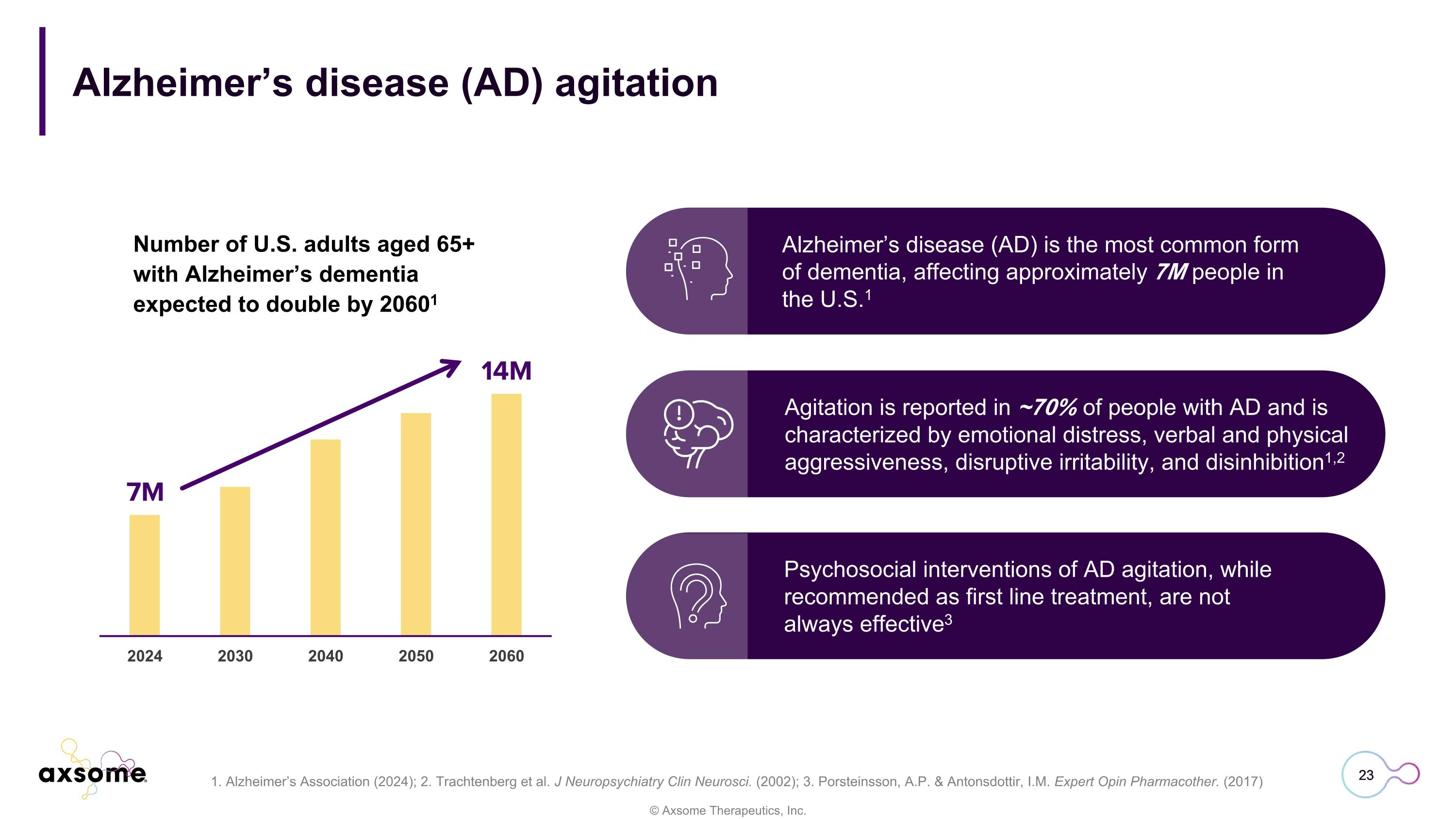
Alzheimer’s disease (AD) agitation Number of U.S. adults aged 65+ with Alzheimer’s dementia expected to double by 20601 23 Alzheimer’s disease (AD) is the most common form of dementia, affecting approximately 7M people in the U.S.1 Psychosocial interventions of AD agitation, while recommended as first line treatment, are not always effective3 Agitation is reported in ~70% of people with AD and is characterized by emotional distress, verbal and physical aggressiveness, disruptive irritability, and disinhibition1,2 1. Alzheimer’s Association (2024); 2. Trachtenberg et al. J Neuropsychiatry Clin Neurosci. (2002); 3. Porsteinsson, A.P. & Antonsdottir, I.M. Expert Opin Pharmacother. (2017)
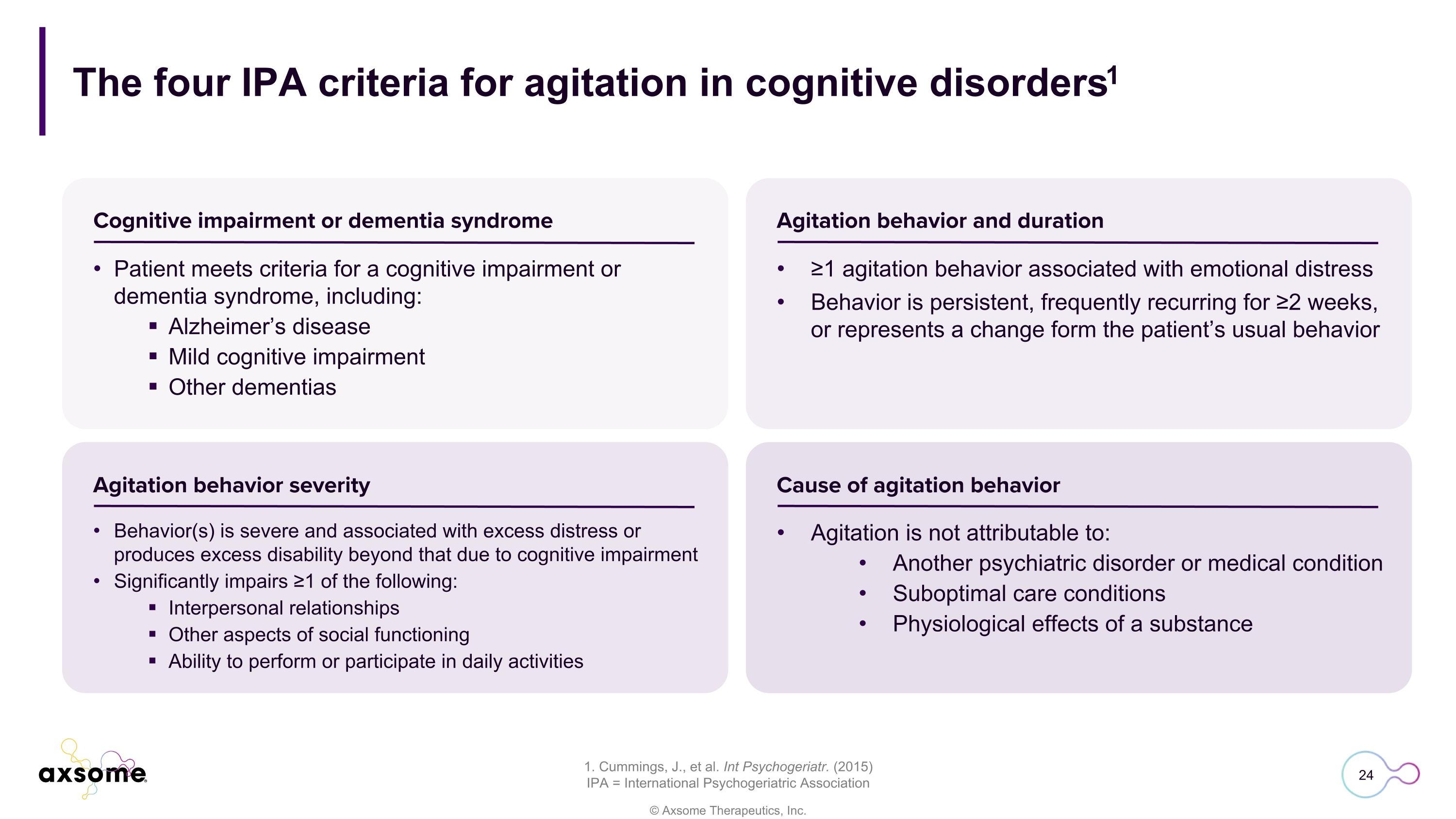
The four IPA criteria for agitation in cognitive disorders1 1. Cummings, J., et al. Int Psychogeriatr. (2015) IPA = International Psychogeriatric Association Patient meets criteria for a cognitive impairment or dementia syndrome, including: Alzheimer’s disease Mild cognitive impairment Other dementias ≥1 agitation behavior associated with emotional distress Behavior is persistent, frequently recurring for ≥2 weeks, or represents a change form the patient’s usual behavior Behavior(s) is severe and associated with excess distress or produces excess disability beyond that due to cognitive impairment Significantly impairs ≥1 of the following: Interpersonal relationships Other aspects of social functioning Ability to perform or participate in daily activities Agitation is not attributable to: Another psychiatric disorder or medical condition Suboptimal care conditions Physiological effects of a substance Cognitive impairment or dementia syndrome Agitation behavior and duration Agitation behavior severity Cause of agitation behavior
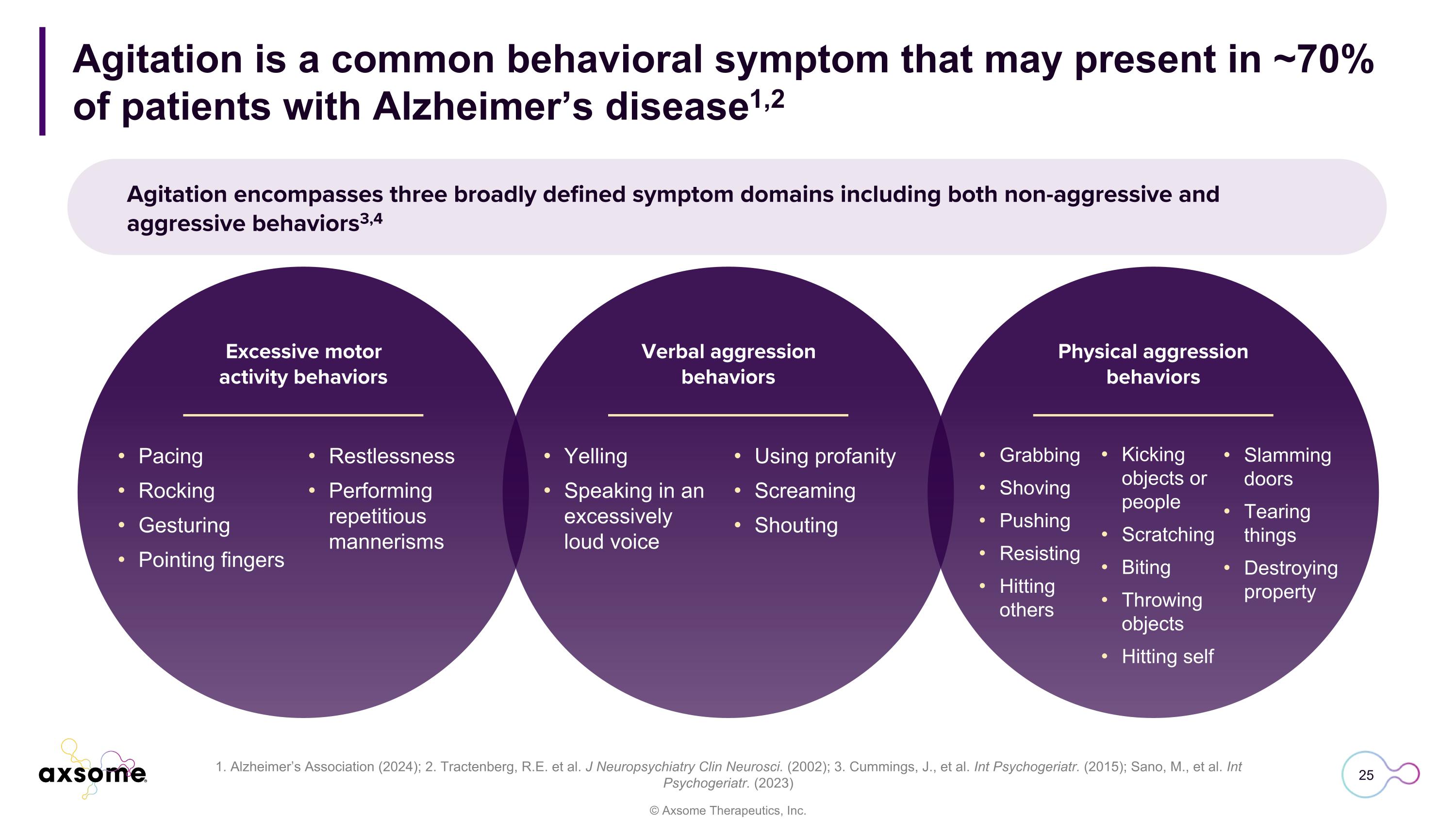
Agitation is a common behavioral symptom that may present in ~70% of patients with Alzheimer’s disease1,2 1. Alzheimer’s Association (2024); 2. Tractenberg, R.E. et al. J Neuropsychiatry Clin Neurosci. (2002); 3. Cummings, J., et al. Int Psychogeriatr. (2015); Sano, M., et al. Int Psychogeriatr. (2023) Agitation encompasses three broadly defined symptom domains including both non-aggressive and aggressive behaviors3,4 Pacing Rocking Gesturing Pointing fingers Excessive motor activity behaviors Verbal aggression behaviors Physical aggression behaviors Restlessness Performing repetitious mannerisms Yelling Speaking in an excessively loud voice Using profanity Screaming Shouting Grabbing Shoving Pushing Resisting Hitting others Kicking objects or people Scratching Biting Throwing objects Hitting self Slamming doors Tearing things Destroying property
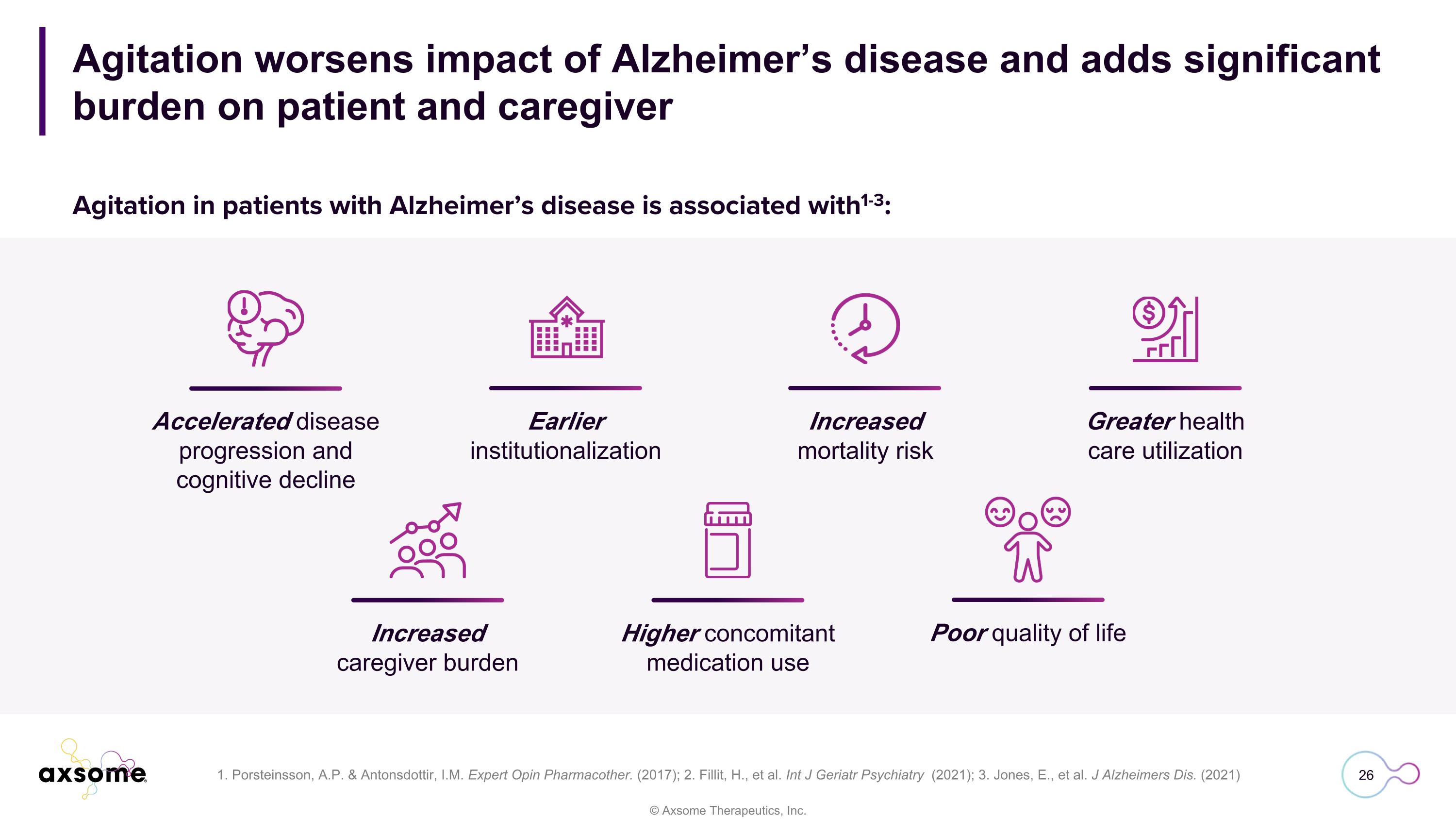
Agitation worsens impact of Alzheimer’s disease and adds significant burden on patient and caregiver 1. Porsteinsson, A.P. & Antonsdottir, I.M. Expert Opin Pharmacother. (2017); 2. Fillit, H., et al. Int J Geriatr Psychiatry (2021); 3. Jones, E., et al. J Alzheimers Dis. (2021) Accelerated disease progression and cognitive decline Earlier institutionalization Increased mortality risk Greater health care utilization Increased caregiver burden Higher concomitant medication use Poor quality of life Agitation in patients with Alzheimer’s disease is associated with1-3:

Dr. Jeffrey Cummings, MD, ScD Vice Chair of Research, UNLV Department of Brain Health Clinical perspective
Unmet need in the treatment of Agitation associated with Alzheimer’s disease Agitation affects the majority of patients with Alzheimer’s disease and is one of the most troubling and consequential aspects of Alzheimer’s disease for patients and caregivers. Current pharmacologic treatments are primarily off-label medications: Typical and atypical antipsychotics, benzodiazepines, antiepileptics, antidepressants Limitations of off-label medications: Sedation, extrapyramidal side effects, falls, worsening of cognition, cardiovascular and cerebrovascular events Modest efficacy Only 1 FDA-approved agent, an atypical antipsychotic There is an urgent unmet need for new effective pharmacological treatments with favorable safety and tolerability
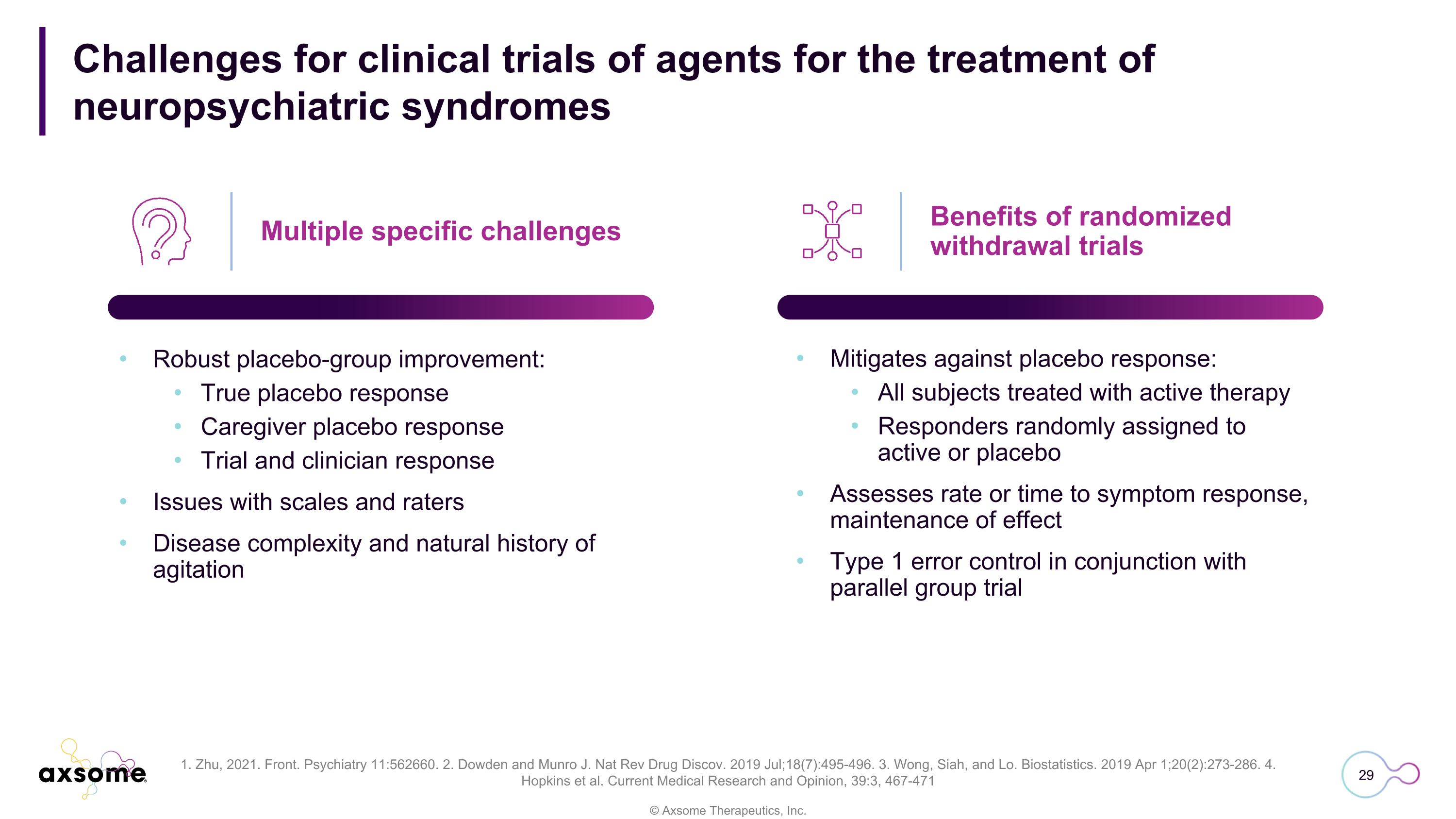
Challenges for clinical trials of agents for the treatment of�neuropsychiatric syndromes Multiple specific challenges Robust placebo-group improvement: True placebo response Caregiver placebo response Trial and clinician response Issues with scales and raters Disease complexity and natural history of agitation Mitigates against placebo response: All subjects treated with active therapy Responders randomly assigned to active or placebo Assesses rate or time to symptom response, maintenance of effect Type 1 error control in conjunction with parallel group trial Benefits of randomized withdrawal trials 1. Zhu, 2021. Front. Psychiatry 11:562660. 2. Dowden and Munro J. Nat Rev Drug Discov. 2019 Jul;18(7):495-496. 3. Wong, Siah, and Lo. Biostatistics. 2019 Apr 1;20(2):273-286. 4. Hopkins et al. Current Medical Research and Opinion, 39:3, 467-471
Perspective on AXS-05 in Alzheimer’s disease agitation Safety of AXS-05 in Alzheimer’s disease agitation Well tolerated across controlled and long-term studies ADVANCE-2 provides supportive controlled safety data No association with death, increased risk of falls, sedation, or cognitive decline observed Four controlled Phase 3 clinical trials evaluated AXS-05 in Alzheimer’s disease agitation Two distinct trial paradigms (parallel group and randomized withdrawal) is a strength Program evaluated both induction and maintenance effects of therapy Comprehensive Phase 3 clinical program Strong statistically significant and clinically meaningful efficacy demonstrated in ADVANCE-1, ACCORD-1, and ACCORD-2 Global improvement in Alzheimer’s disease severity observed Efficacy of AXS-05 in Alzheimer’s disease agitation

© Axsome Therapeutics, Inc. Q&A






























