
Mathew Cooper, PhD, MBA Chief Scientific Officer November 20, 2020 Preeclampsia R&D Day Exhibit 99.1

Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, which statements are subject to substantial risks and uncertainties and are based on estimates and assumptions. All statements, other than statements of historical facts included in this presentation, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, financing needs, plans or intentions relating to product candidates, estimates of market size, estimates of market growth, business trends, expected testing supply and demand, the anticipated timing, design and conduct of our planned clinical trials, the development of our product candidates, including the timing and likelihood of regulatory filings and approvals for our product candidates, our ability to commercialize our product candidates, if approved, the pricing and reimbursement of our product candidates, if approved, the potential to develop future product candidates, the potential benefits of strategic collaborations and our intent to enter into any strategic arrangements, the timing and likelihood of success, plans and objectives of management for future operations and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this presentation, including those described in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, and elsewhere in such filings and in other subsequent disclosure documents filed with the U.S. Securities and Exchange Commission (SEC). We cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. Forward-looking statements are not historical facts, and reflect our current views with respect to future events. Given the significant uncertainties, you should evaluate all forward-looking statements made in this presentation in the context of these risks and uncertainties and not place undue reliance on these forward-looking statements as predictions of future events. All forward-looking statements in this presentation apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this presentation. We disclaim any intent to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances, except as required by law. Industry and Market Data: We obtained the industry, market, and competitive position data used throughout this Presentation from our own internal estimates and research, as well as from industry and general publications, and research, surveys, and studies conducted by third parties. Internal estimates are derived from publicly available information released by industry analysts and third-party sources, our internal research and our industry experience, and are based on assumptions made by us based on such data and our knowledge of the industry and market, which we believe to be reasonable. In addition, while we believe the industry, market, and competitive position data included in this prospectus is reliable and based on reasonable assumptions, we have not independently verified any third-party information, and all such data involve risks and uncertainties and are subject to change based on various factors. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.

Preecludia™ - A high Potential Rule Out Test for Preeclampsia Established a fundamental understanding of preeclampsia and the great clinical unmet need that exists today in assessing patient risk in our channel: Ob/Gyn and MFM Showed the incremental healthcare cost burden of $9B+ associated with managing a preeclamptic pregnancy and its support for premium reimbursement Shared our compelling prospective, blinded clinical verification data from 400 enrolled subjects for our Preecludia™ LDT rule-out test achieving high sensitivity of 88% and 98.2% NPV* with a rule out window of up to 14 days Provided support for the clinical utility of a rule-out test and its place in the clinical management care path to help improve standard of care Demonstrated our ability to advance our program and our efforts to generate strong adoption rates within our women's health channel towards a $3B US market * NPV calculated using a 10% prevalence rate

Agenda Opening remarks and Introductions Closing Remarks Preecludia™: Clinical Evidence Development Program, Christina Settler, MS, CGC Preeclampsia: The Gravity of the Condition and the Unmet Need, Dr. Christopher Robinson, MD, MSCR, FACOG Preecludia™: The Development Journey from Bench to Bedside, Dr. Pankaj Oberoi, Ph.D Preeclampsia Cost of Illness, Dr. Mathew Cooper, PhD, MBA Introduction to Preeclampsia, Dr. Douglas Woelkers, MD The Patient Burden, Eleni Tsigas, CEO of Preeclampsia Foundation

Introduction to preeclampsia Douglas Woelkers, MD Professor Obstetrics, Gynecology, and Reproductive Science University of California, San Diego

What is preeclampsia? A disease of pregnancy Characterized by High blood pressure Multi-organ injury Fetal compromise Named for the syndrome that precedes eclampsia (seizures) Unique to humans Related to presence of placenta Resolves spontaneously after pregnancy
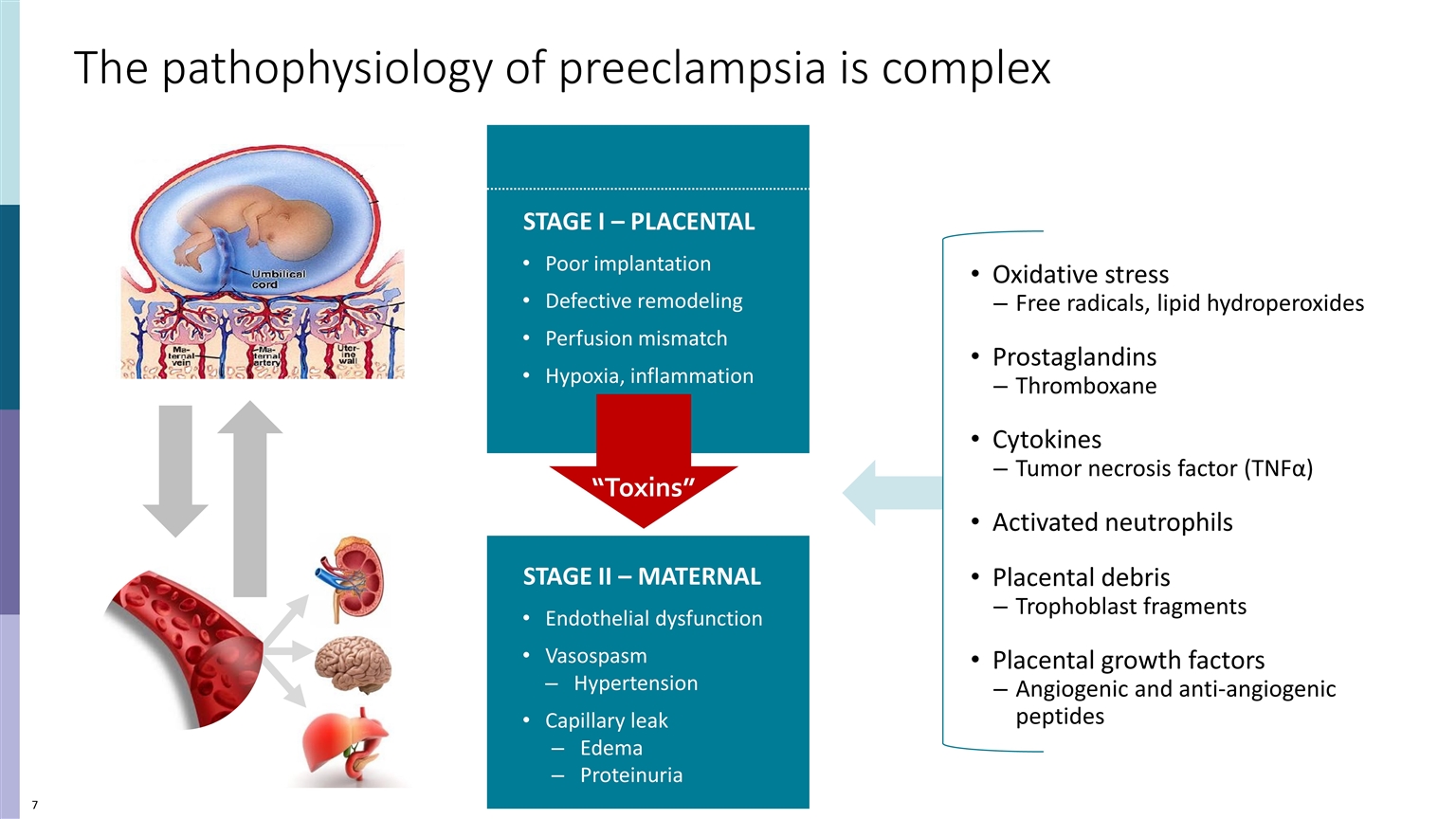
The pathophysiology of preeclampsia is complex STAGE II – MATERNAL Endothelial dysfunction Vasospasm Hypertension Capillary leak Edema Proteinuria STAGE I – PLACENTAL Poor implantation Defective remodeling Perfusion mismatch Hypoxia, inflammation Oxidative stress Free radicals, lipid hydroperoxides Prostaglandins Thromboxane Cytokines Tumor necrosis factor (TNFα) Activated neutrophils Placental debris Trophoblast fragments Placental growth factors Angiogenic and anti-angiogenic peptides “Toxins”
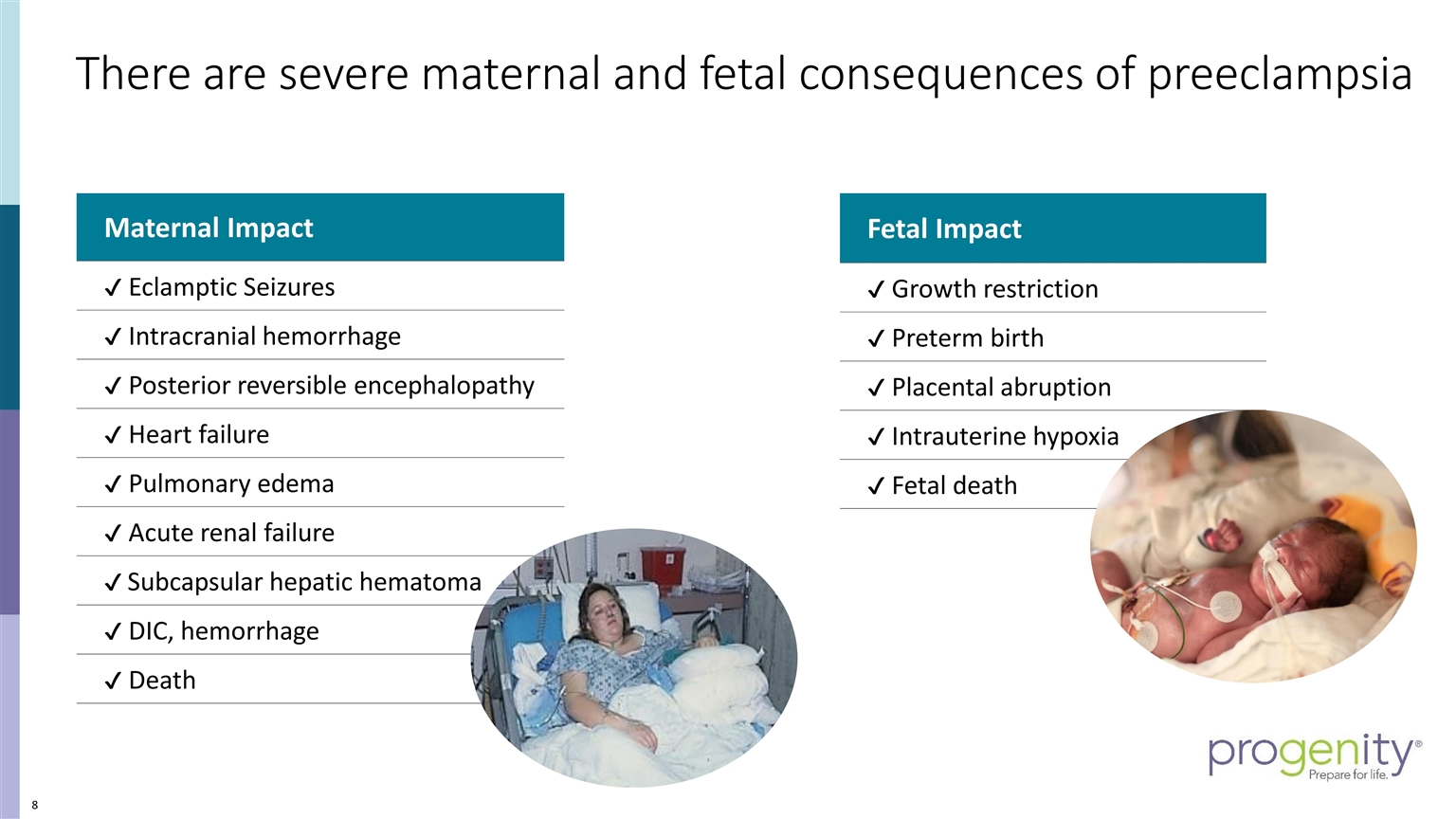
There are severe maternal and fetal consequences of preeclampsia Maternal Impact ✔ Eclamptic Seizures ✔ Intracranial hemorrhage ✔ Posterior reversible encephalopathy ✔ Heart failure ✔ Pulmonary edema ✔ Acute renal failure ✔ Subcapsular hepatic hematoma ✔ DIC, hemorrhage ✔ Death Fetal Impact ✔ Growth restriction ✔ Preterm birth ✔ Placental abruption ✔ Intrauterine hypoxia ✔ Fetal death

Preeclampsia can affect any pregnancy 3.75 million total births US, 2019 3 to 8% of all births complicated by preeclampsia 112,500 to ~300,000 cases per year Occurs in 2nd half of pregnancy After 20 weeks Mostly diagnosed near term (37 to 42 weeks) But disproportionate effect on preterm births “ it is important to remember that most cases of preeclampsia occur in healthy nulliparous women with no obvious risk factors.” - ACOG Practice Bulletin 222, June 2020 Reliance on maternal symptoms may occasionally be problematic in clinical practice. Thus, an astute and circumspect diagnostic approach is required when other corroborating signs and symptoms are missing. ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020 Jun;135(6):e237-e260
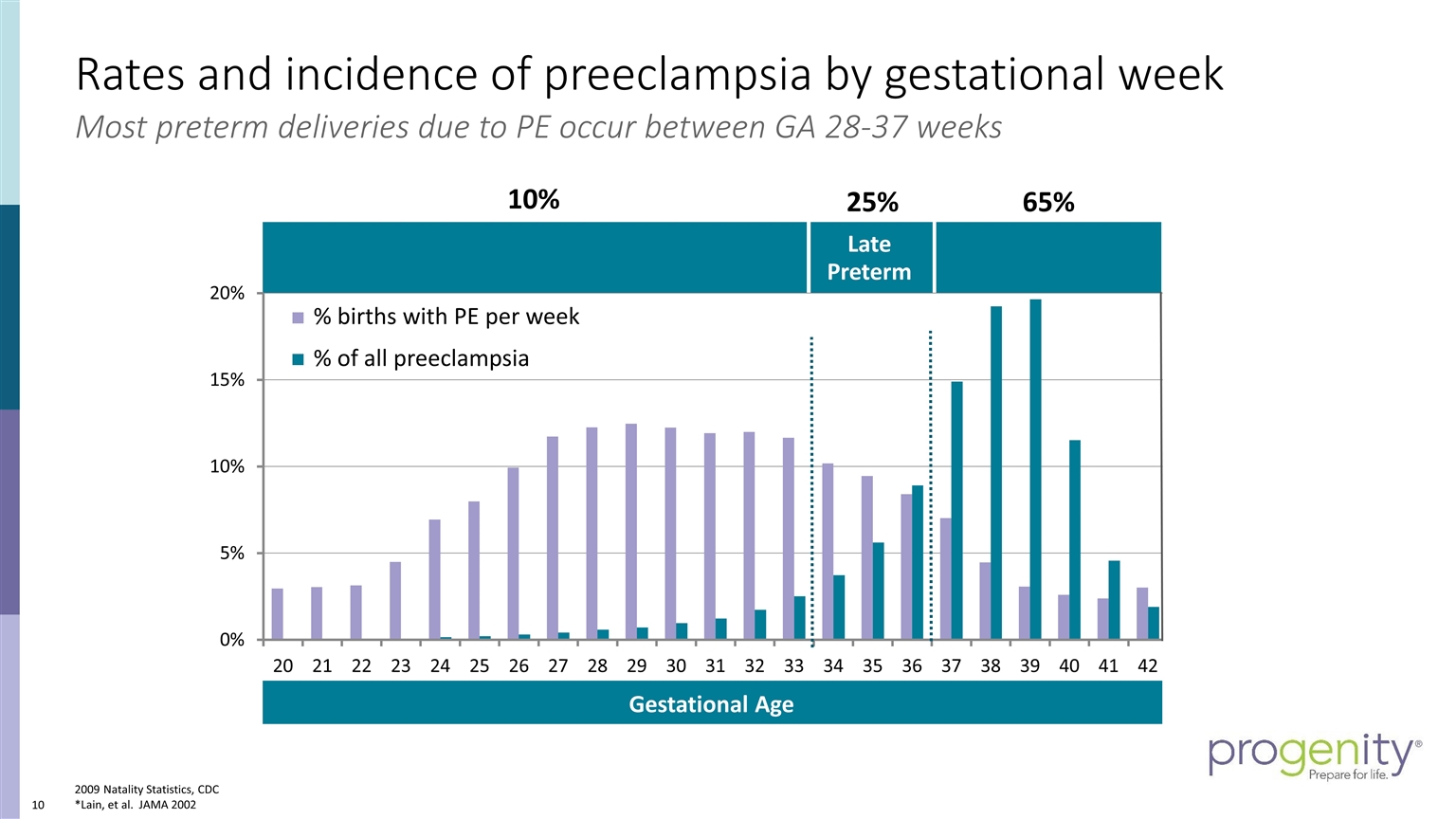
Rates and incidence of preeclampsia by gestational week 2009 Natality Statistics, CDC *Lain, et al. JAMA 2002 Late Preterm % of all preeclampsia % births with PE per week 65% 10% 25% Most preterm deliveries due to PE occur between GA 28-37 weeks Gestational Age
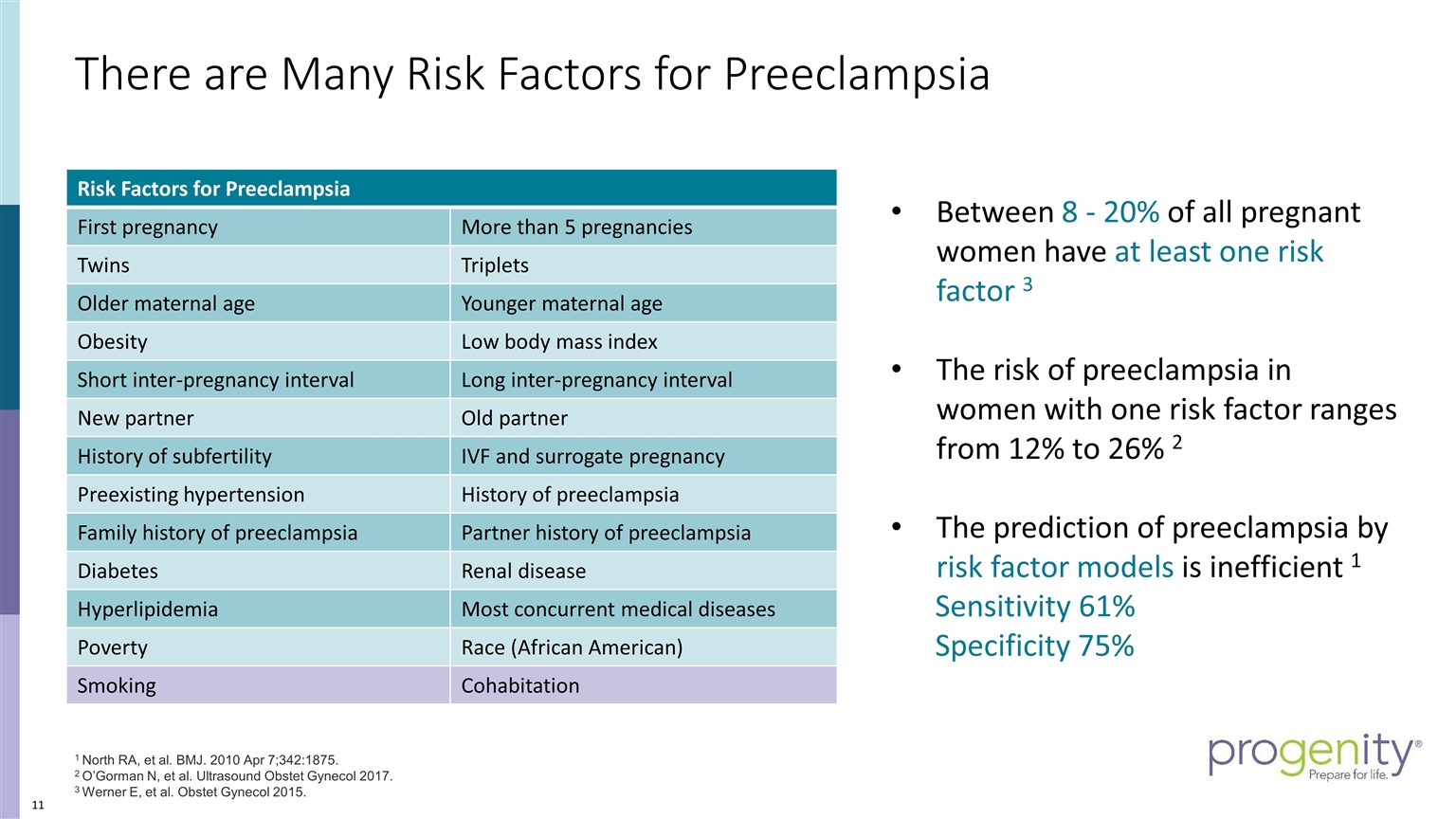
Risk Factors for Preeclampsia First pregnancy More than 5 pregnancies Twins Triplets Older maternal age Younger maternal age Obesity Low body mass index Short inter-pregnancy interval Long inter-pregnancy interval New partner Old partner History of subfertility IVF and surrogate pregnancy Preexisting hypertension History of preeclampsia Family history of preeclampsia Partner history of preeclampsia Diabetes Renal disease Hyperlipidemia Most concurrent medical diseases Poverty Race (African American) Smoking Cohabitation There are Many Risk Factors for Preeclampsia Between 8 - 20% of all pregnant women have at least one risk factor 3 The risk of preeclampsia in women with one risk factor ranges from 12% to 26% 2 The prediction of preeclampsia by risk factor models is inefficient 1 Sensitivity 61% Specificity 75% 1 North RA, et al. BMJ. 2010 Apr 7;342:1875. 2 O’Gorman N, et al. Ultrasound Obstet Gynecol 2017. 3 Werner E, et al. Obstet Gynecol 2015.
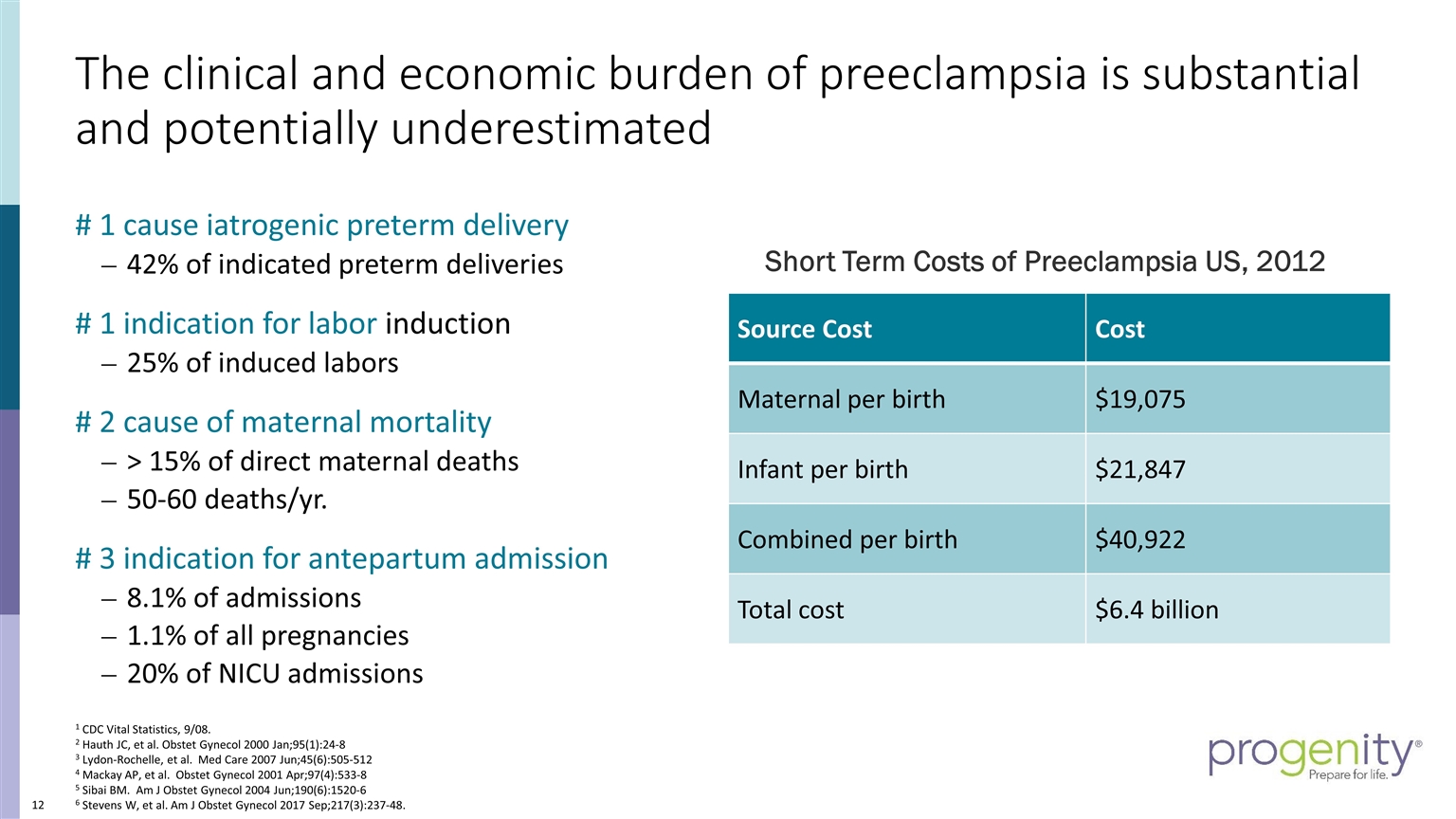
The clinical and economic burden of preeclampsia is substantial and potentially underestimated # 1 cause iatrogenic preterm delivery 42% of indicated preterm deliveries # 1 indication for labor induction 25% of induced labors # 2 cause of maternal mortality > 15% of direct maternal deaths 50-60 deaths/yr. # 3 indication for antepartum admission 8.1% of admissions 1.1% of all pregnancies 20% of NICU admissions 1 CDC Vital Statistics, 9/08. 2 Hauth JC, et al. Obstet Gynecol 2000 Jan;95(1):24-8 3 Lydon-Rochelle, et al. Med Care 2007 Jun;45(6):505-512 4 Mackay AP, et al. Obstet Gynecol 2001 Apr;97(4):533-8 5 Sibai BM. Am J Obstet Gynecol 2004 Jun;190(6):1520-6 6 Stevens W, et al. Am J Obstet Gynecol 2017 Sep;217(3):237-48. Source Cost Cost Maternal per birth $19,075 Infant per birth $21,847 Combined per birth $40,922 Total cost $6.4 billion Short Term Costs of Preeclampsia US, 2012
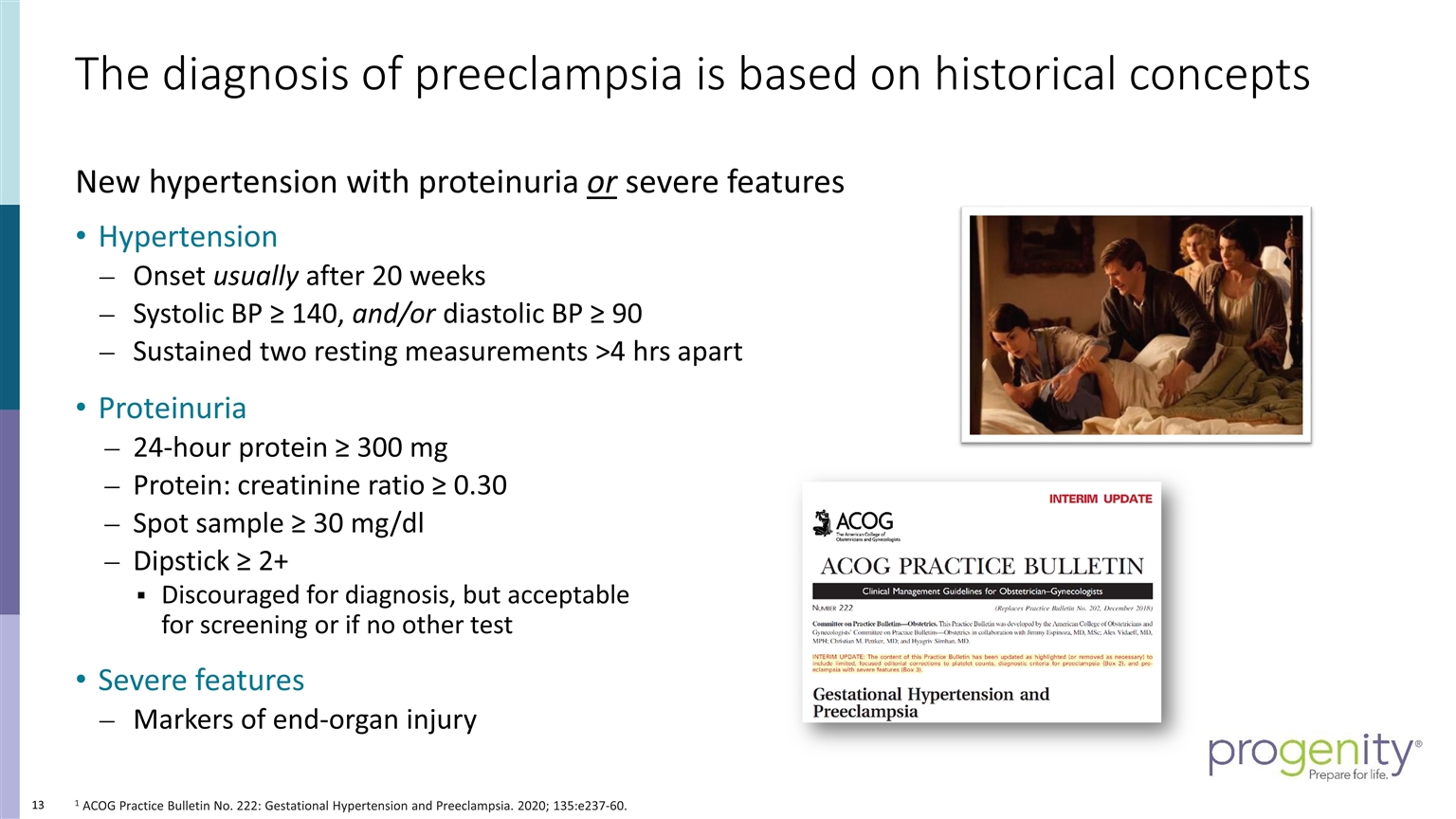
The diagnosis of preeclampsia is based on historical concepts 1 ACOG Practice Bulletin No. 222: Gestational Hypertension and Preeclampsia. 2020; 135:e237-60. New hypertension with proteinuria or severe features Hypertension Onset usually after 20 weeks Systolic BP ≥ 140, and/or diastolic BP ≥ 90 Sustained two resting measurements >4 hrs apart Proteinuria 24-hour protein ≥ 300 mg Protein: creatinine ratio ≥ 0.30 Spot sample ≥ 30 mg/dl Dipstick ≥ 2+ Discouraged for diagnosis, but acceptable for screening or if no other test Severe features Markers of end-organ injury
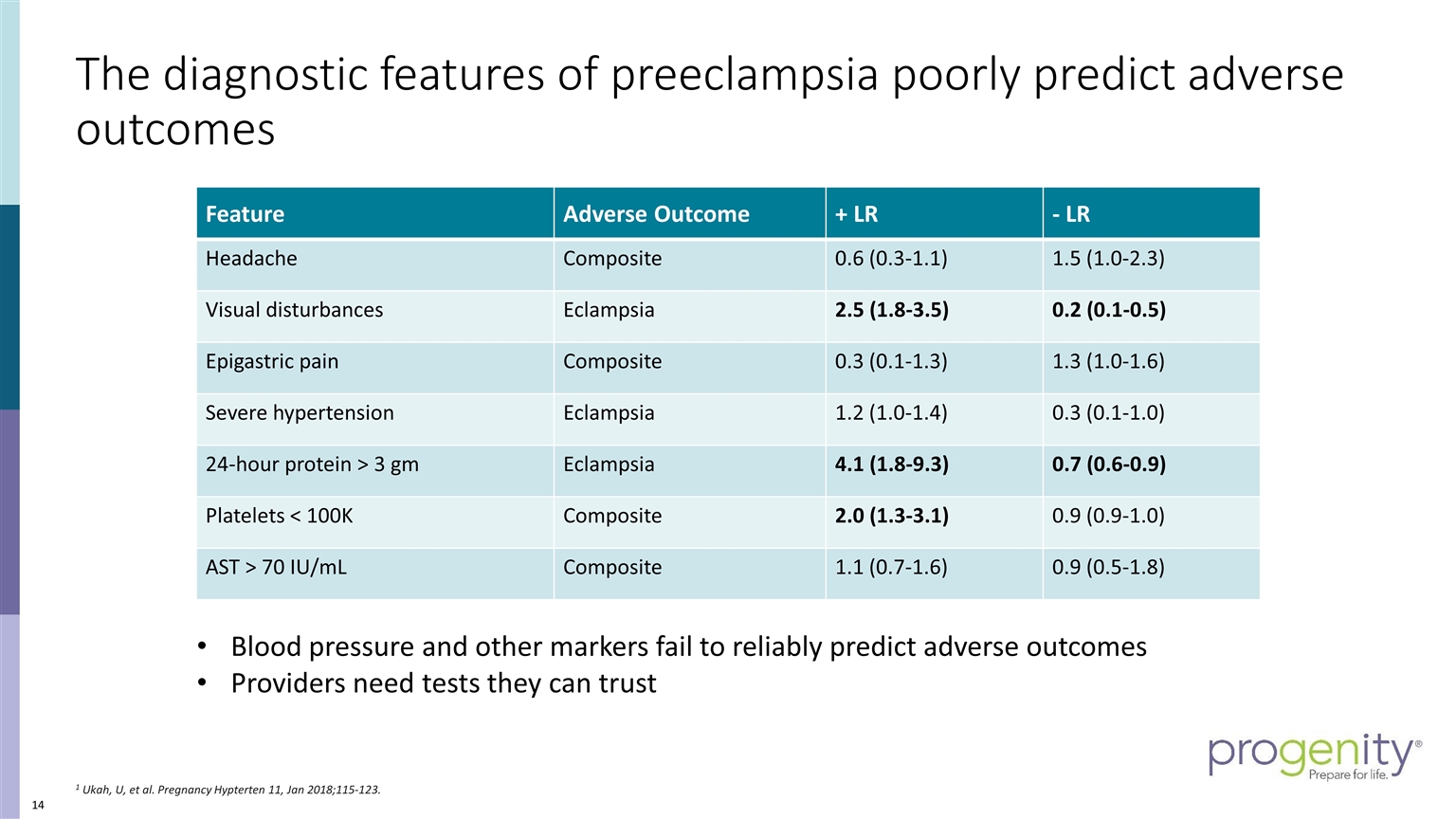
The diagnostic features of preeclampsia poorly predict adverse outcomes 1 Ukah, U, et al. Pregnancy Hypterten 11, Jan 2018;115-123. Blood pressure and other markers fail to reliably predict adverse outcomes Providers need tests they can trust Feature Adverse Outcome + LR - LR Headache Composite 0.6 (0.3-1.1) 1.5 (1.0-2.3) Visual disturbances Eclampsia 2.5 (1.8-3.5) 0.2 (0.1-0.5) Epigastric pain Composite 0.3 (0.1-1.3) 1.3 (1.0-1.6) Severe hypertension Eclampsia 1.2 (1.0-1.4) 0.3 (0.1-1.0) 24-hour protein > 3 gm Eclampsia 4.1 (1.8-9.3) 0.7 (0.6-0.9) Platelets < 100K Composite 2.0 (1.3-3.1) 0.9 (0.9-1.0) AST > 70 IU/mL Composite 1.1 (0.7-1.6) 0.9 (0.5-1.8)
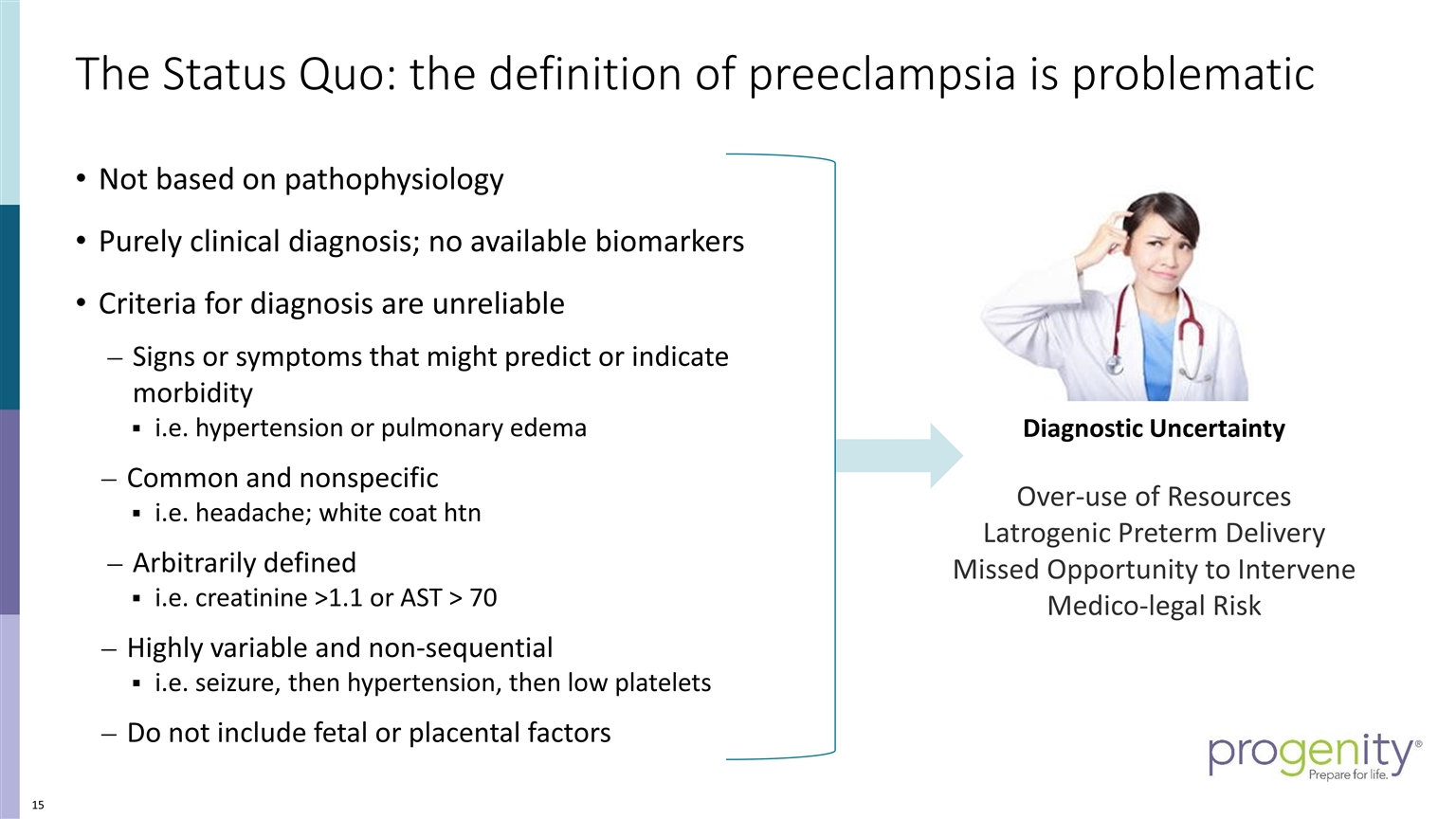
The Status Quo: the definition of preeclampsia is problematic Diagnostic Uncertainty Over-use of Resources Latrogenic Preterm Delivery Missed Opportunity to Intervene Medico-legal Risk Not based on pathophysiology Purely clinical diagnosis; no available biomarkers Criteria for diagnosis are unreliable Signs or symptoms that might predict or indicate morbidity i.e. hypertension or pulmonary edema Common and nonspecific i.e. headache; white coat htn Arbitrarily defined i.e. creatinine >1.1 or AST > 70 Highly variable and non-sequential i.e. seizure, then hypertension, then low platelets Do not include fetal or placental factors

At UCSD, 30% of pregnancies were evaluated for suspected preeclampsia 2402 deliveries at UCSD (2009) 725 (30%) had lab test(s) for preeclampsia 406 (56%) tested more than once 146 (20%) ultimately diagnosed with PE 75 (46%) severe PE 654 (90%) had a urine P/C ratio 336 (51%) had repeat assays 159 (22%) had a 24-hour urine 48 (30%) had repeat collection Add image of UCSD Hospital or OBGYN Team who conducted the study…. UCSD Medical Center La Jolla, CA
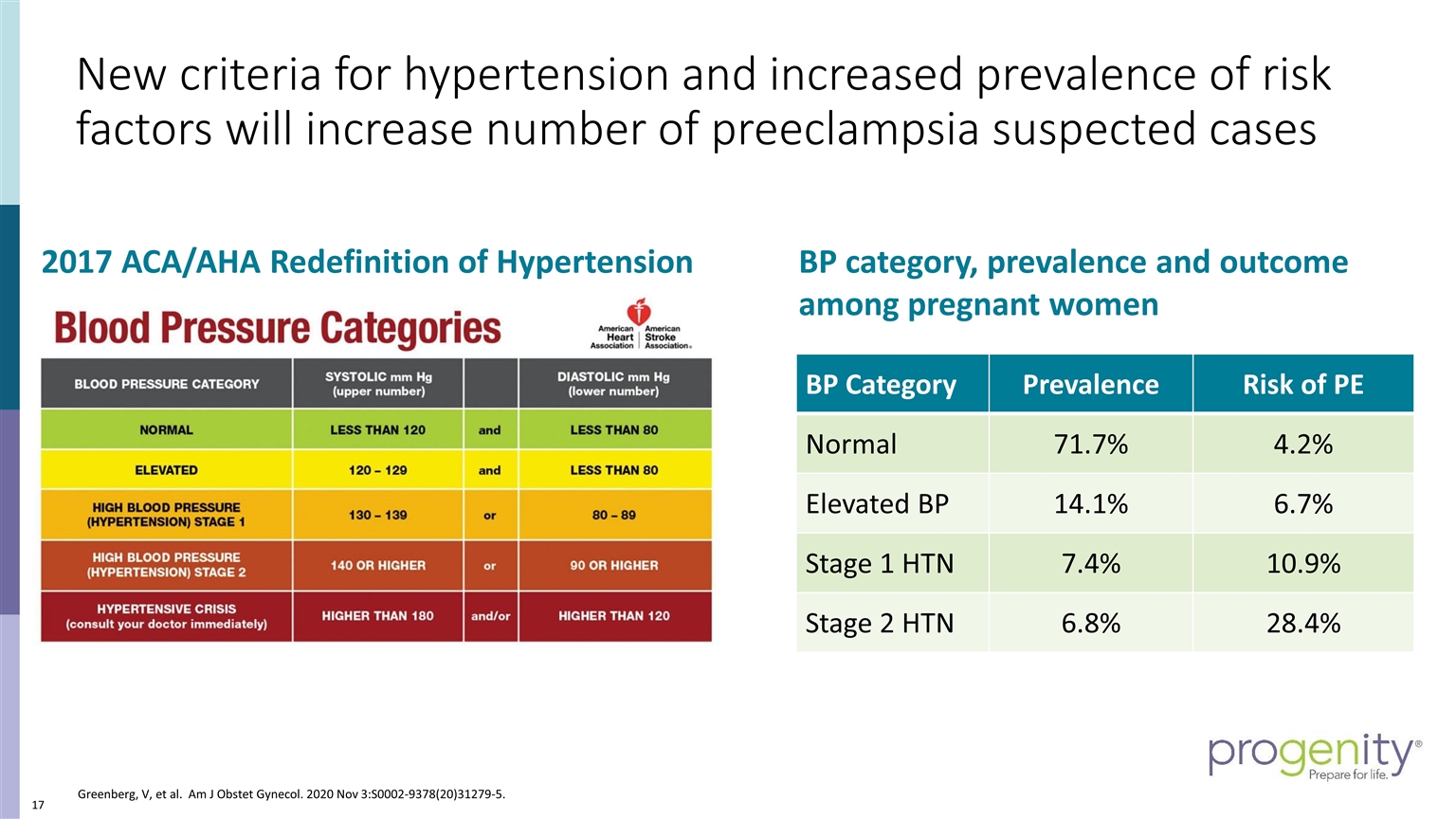
New criteria for hypertension and increased prevalence of risk factors will increase number of preeclampsia suspected cases 2017 ACA/AHA Redefinition of Hypertension BP Category Prevalence Risk of PE Normal 71.7% 4.2% Elevated BP 14.1% 6.7% Stage 1 HTN 7.4% 10.9% Stage 2 HTN 6.8% 28.4% BP category, prevalence and outcome among pregnant women Greenberg, V, et al. Am J Obstet Gynecol. 2020 Nov 3:S0002-9378(20)31279-5.
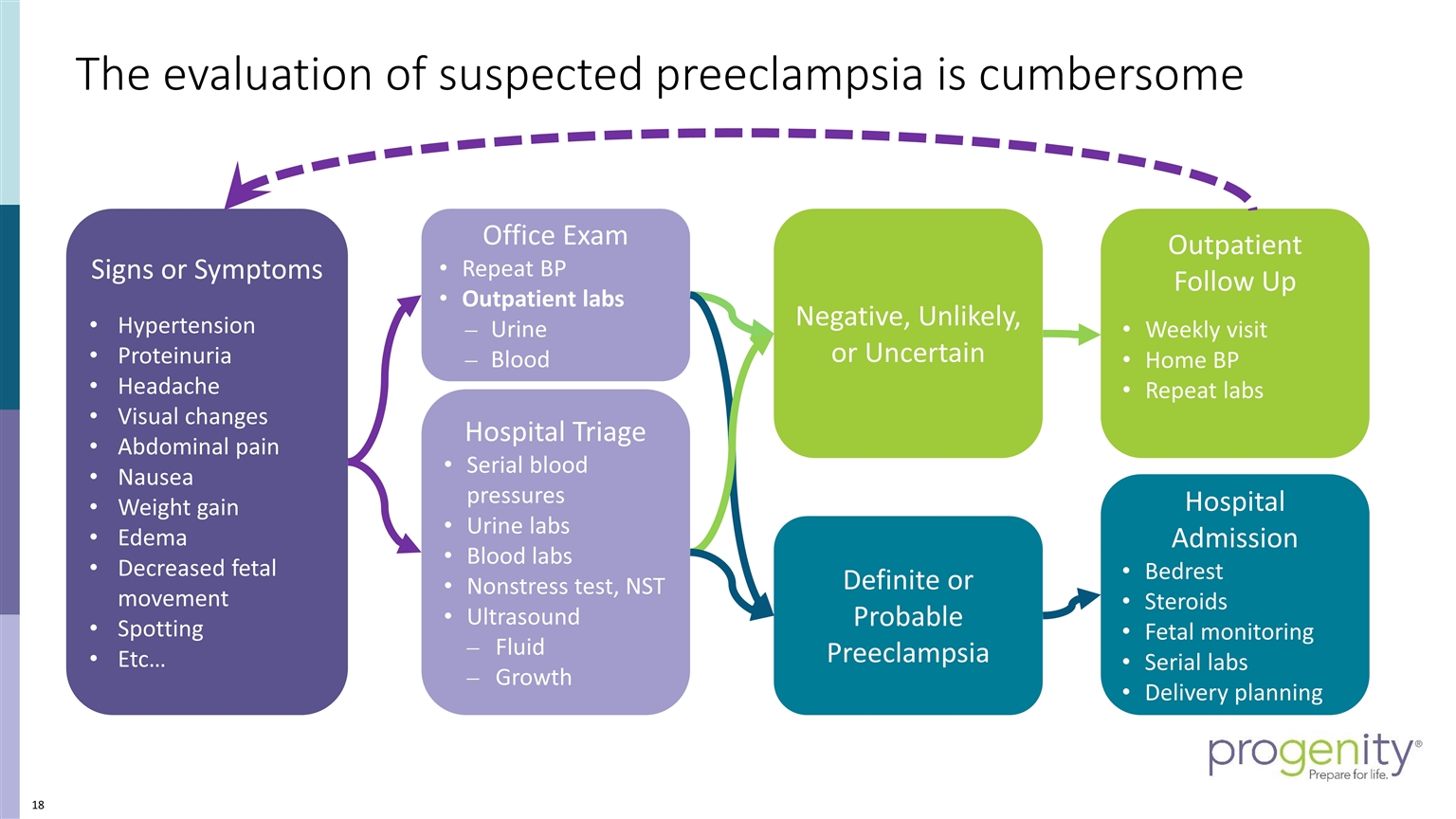
The evaluation of suspected preeclampsia is cumbersome Signs or Symptoms Hypertension Proteinuria Headache Visual changes Abdominal pain Nausea Weight gain Edema Decreased fetal movement Spotting Etc… Office Exam Repeat BP Outpatient labs Urine Blood Hospital Triage Serial blood pressures Urine labs Blood labs Nonstress test, NST Ultrasound Fluid Growth Definite or Probable Preeclampsia Negative, Unlikely, or Uncertain Hospital Admission Bedrest Steroids Fetal monitoring Serial labs Delivery planning Outpatient Follow Up Weekly visit Home BP Repeat labs

Patient Case 27 y.o. 1st pregnancy, generally healthy Early blood pressure 127/82, overweight, family history diabetes 34 weeks blood pressure 144/85, repeat 136/82 Urine 1+ protein Reports headaches, occasional visual floaters, swelling of legs and feet, post meal abdominal pain Sent to hospital. First 2 BPs >140/90, but mostly normal after rest Urine P/C ratio 0.34 (positive); urinalysis contaminated – possible infection Normal Cr, Platelets High LFT’s (ALT 65, AST 54). Concern for liver disease Admitted to observation and studies Fetal US, Abdominal US (fatty liver), Serial labs (only LFT’s high) 24-hour protein 218 gm (normal), Urine culture is positive (treated for UTI) Discharged home after 36 hours.
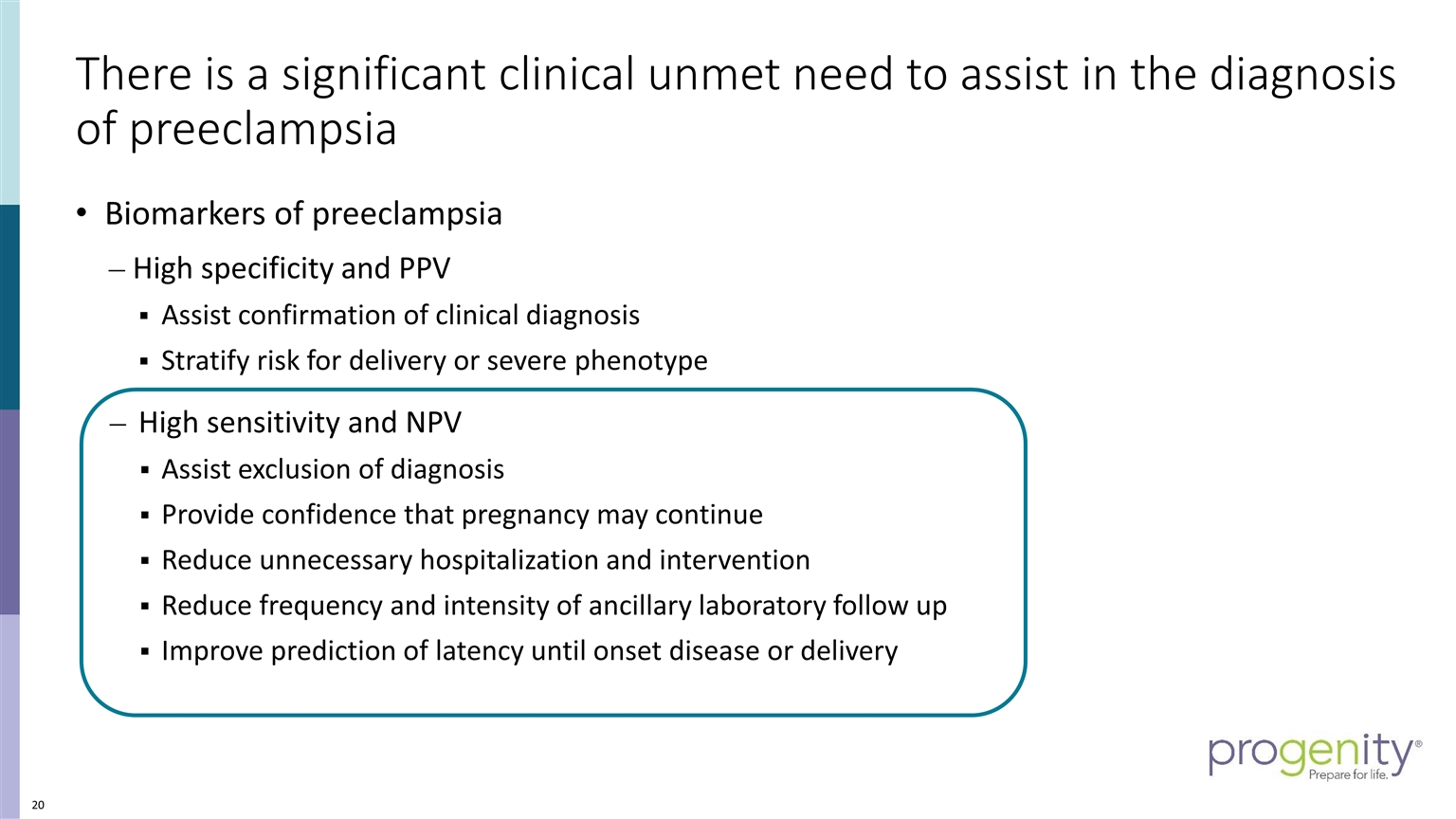
There is a significant clinical unmet need to assist in the diagnosis of preeclampsia Biomarkers of preeclampsia High specificity and PPV Assist confirmation of clinical diagnosis Stratify risk for delivery or severe phenotype High sensitivity and NPV Assist exclusion of diagnosis Provide confidence that pregnancy may continue Reduce unnecessary hospitalization and intervention Reduce frequency and intensity of ancillary laboratory follow up Improve prediction of latency until onset disease or delivery

Summary Preeclampsia presents in many ways and is difficult to diagnose Overlooking mild signs may lead to missed diagnosis and adverse outcomes Overreacting to mild signs may lead to unnecessary intervention Providers (MDs and midwives) need accurate, reliable diagnostics to modernize their approach to evaluation While high specificity is helpful for rare cases, high sensitivity is more useful in everyday situations to simplify triage of healthy women Similar to cardiac troponins, a preeclampsia test that rules out severe or impending disease would be a breakthrough advancement in women’s healthcare

Preeclampsia cost of illness study Maternal & infant costs Mathew Cooper, PhD, MBA Chief Scientific Officer
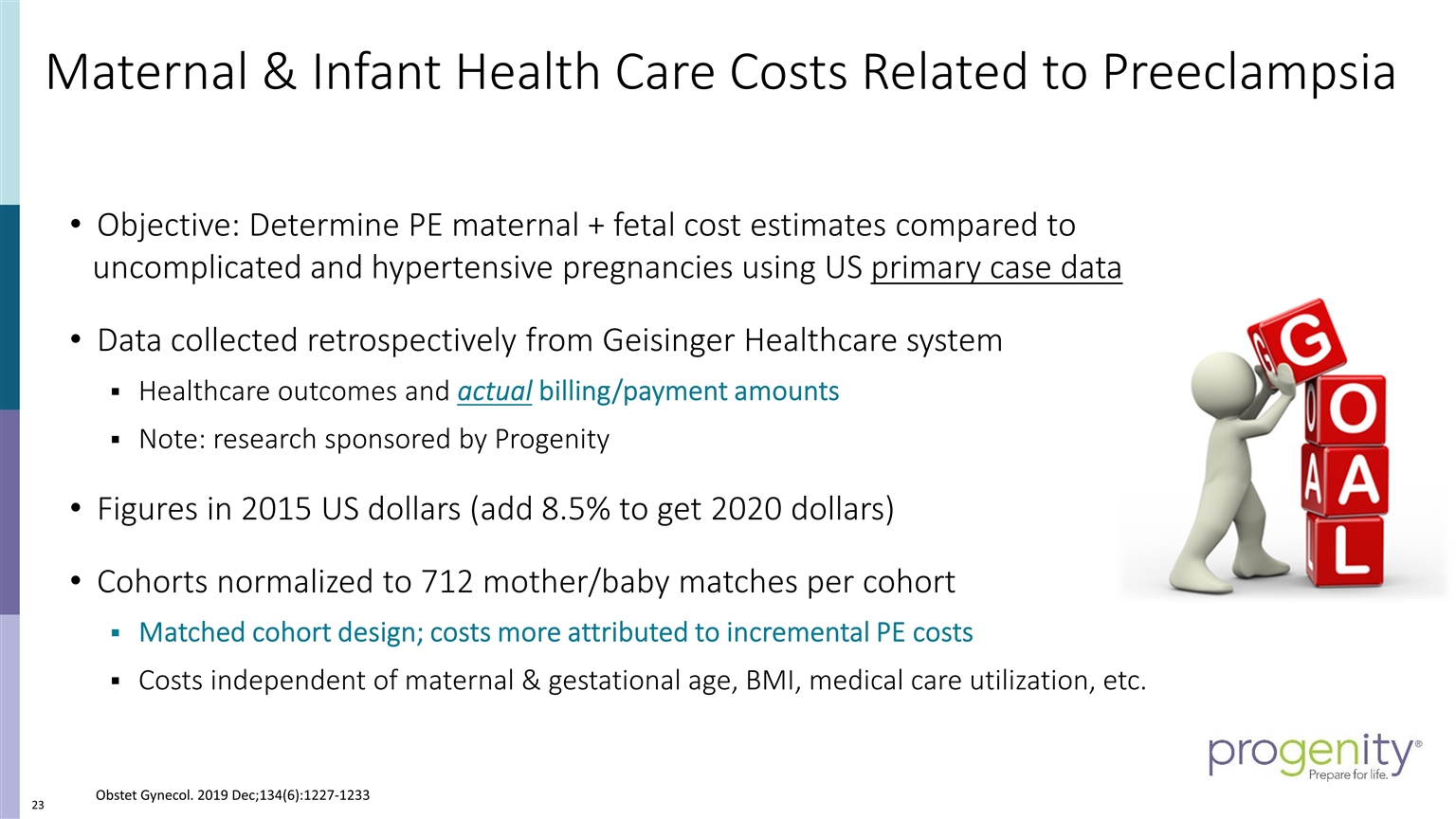
Maternal & Infant Health Care Costs Related to Preeclampsia Objective: Determine PE maternal + fetal cost estimates compared to uncomplicated and hypertensive pregnancies using US primary case data Data collected retrospectively from Geisinger Healthcare system Healthcare outcomes and actual billing/payment amounts Note: research sponsored by Progenity Figures in 2015 US dollars (add 8.5% to get 2020 dollars) Cohorts normalized to 712 mother/baby matches per cohort Matched cohort design; costs more attributed to incremental PE costs Costs independent of maternal & gestational age, BMI, medical care utilization, etc. Obstet Gynecol. 2019 Dec;134(6):1227-1233

Methods Overview US, maternal + fetal costs, payer perspective, from 2010-2015 Compared PE to hypertensive and uncomplicated cohorts Equivalence using maternal age, parity, BMI, comorbidities Mothers of singleton pregnancies only Costs GA 20 weeks to 6 weeks post delivery maternal Birth to 12 months for infant Study used EMR data to link mother/infant pairs Captures healthcare outcomes and actual (not imputed) costs No self-reported data
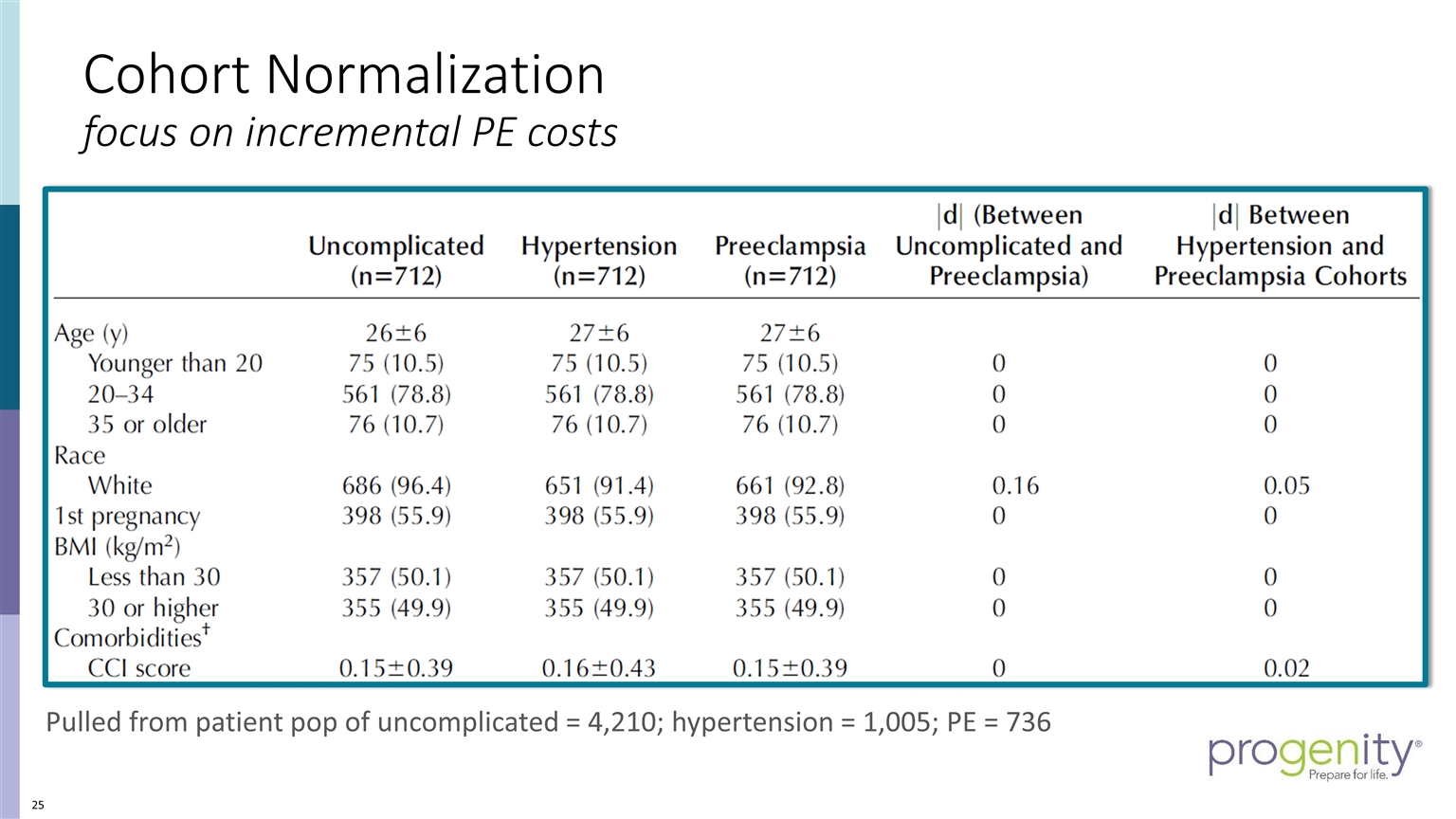
Cohort Normalization focus on incremental PE costs Pulled from patient pop of uncomplicated = 4,210; hypertension = 1,005; PE = 736
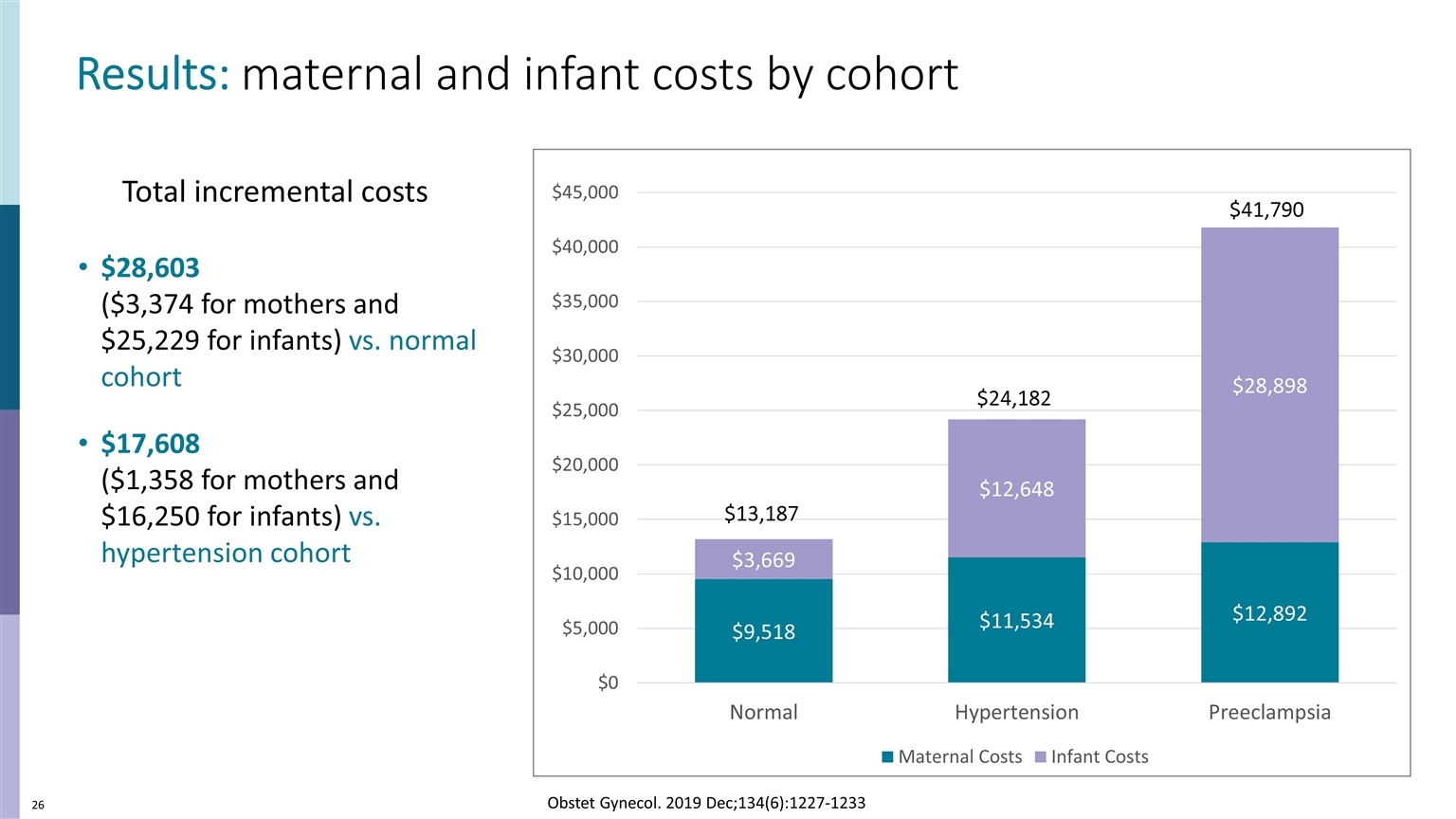
$41,790 $24,182 $13,187 Results: maternal and infant costs by cohort $28,603 ($3,374 for mothers and $25,229 for infants) vs. normal cohort $17,608 ($1,358 for mothers and $16,250 for infants) vs. hypertension cohort Obstet Gynecol. 2019 Dec;134(6):1227-1233 Total incremental costs
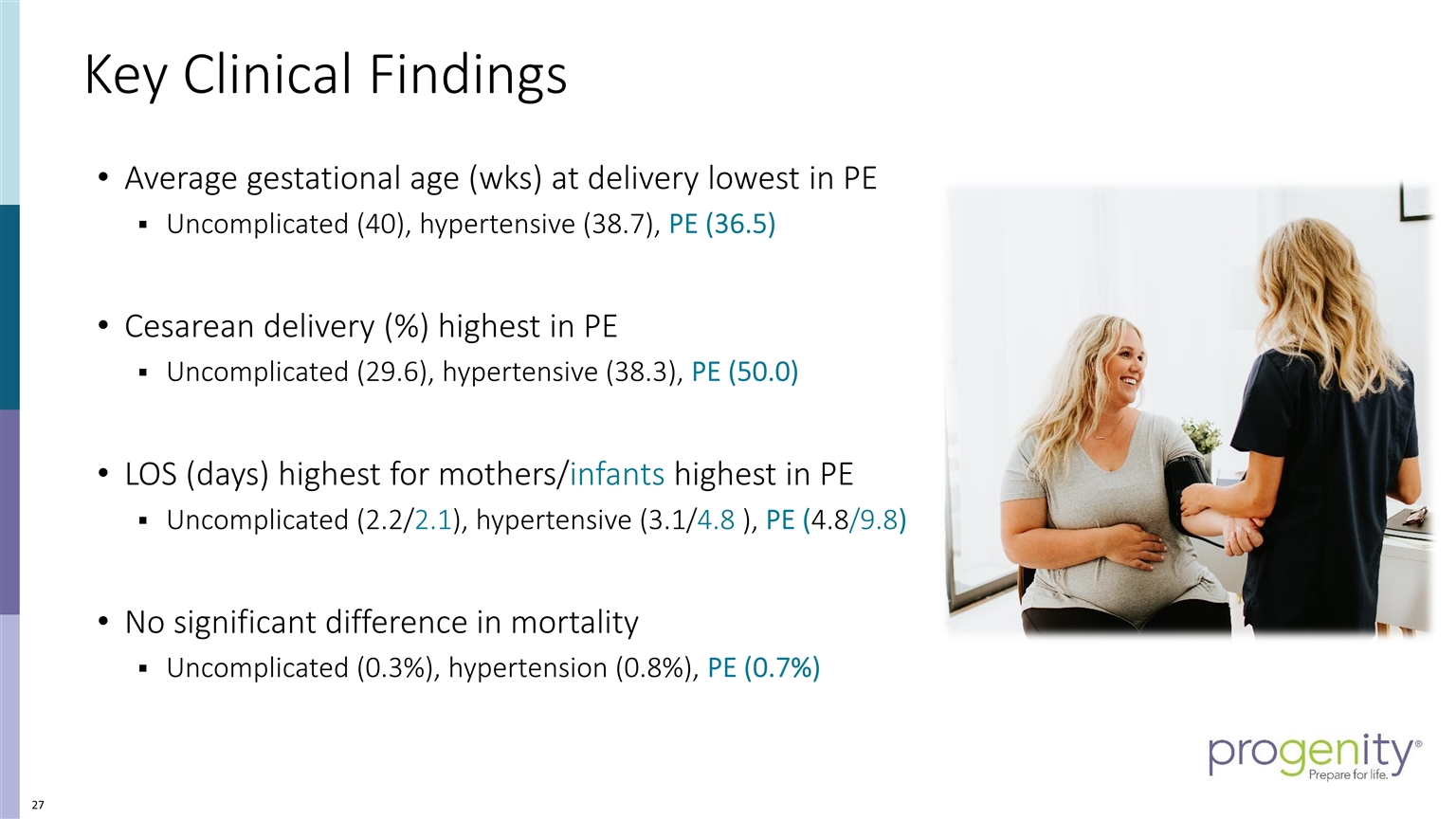
Key Clinical Findings Average gestational age (wks) at delivery lowest in PE Uncomplicated (40), hypertensive (38.7), PE (36.5) Cesarean delivery (%) highest in PE Uncomplicated (29.6), hypertensive (38.3), PE (50.0) LOS (days) highest for mothers/infants highest in PE Uncomplicated (2.2/2.1), hypertensive (3.1/4.8 ), PE (4.8/9.8) No significant difference in mortality Uncomplicated (0.3%), hypertension (0.8%), PE (0.7%)
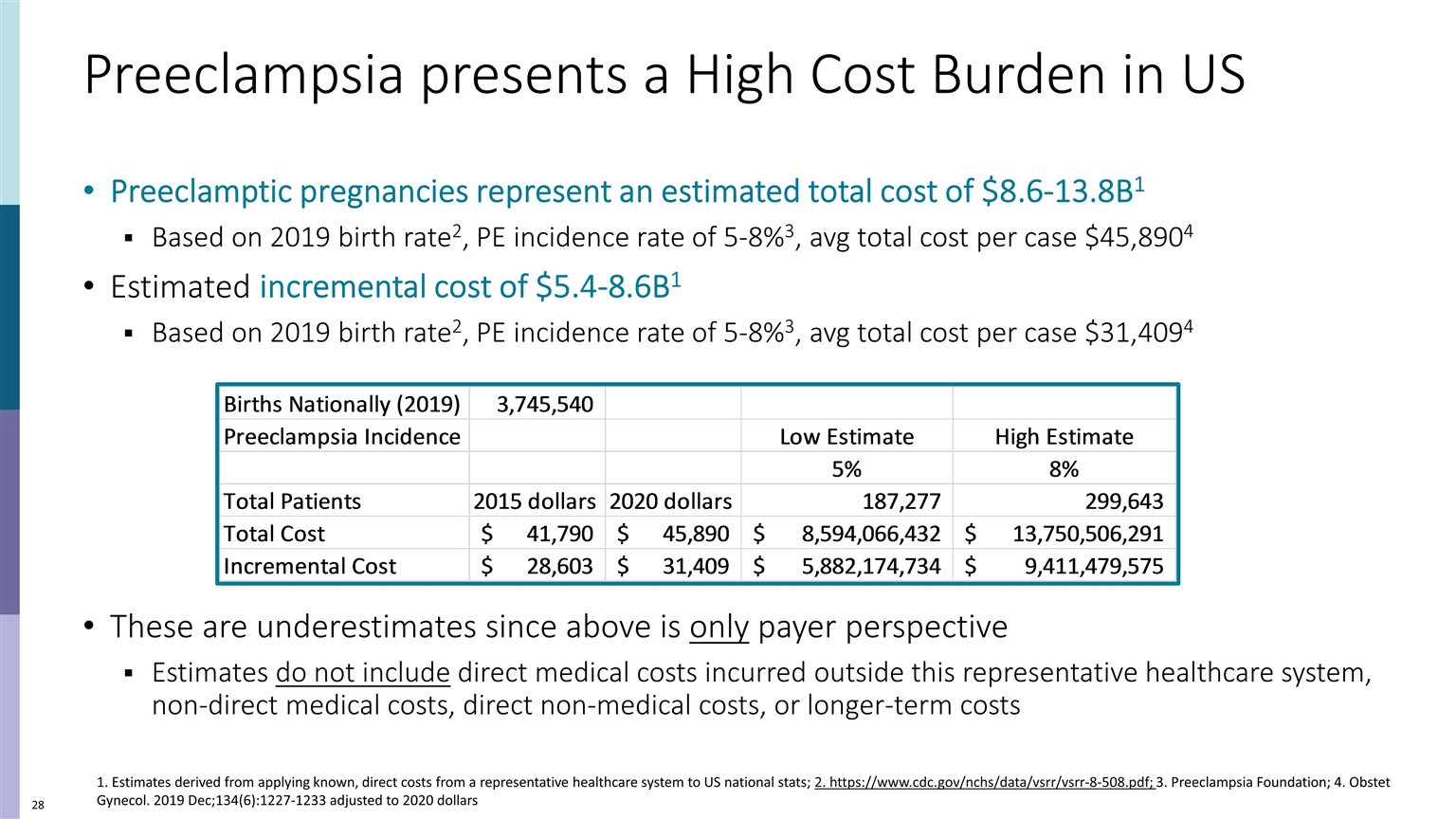
Preeclampsia presents a High Cost Burden in US Preeclamptic pregnancies represent an estimated total cost of $8.6-13.8B1 Based on 2019 birth rate2, PE incidence rate of 5-8%3, avg total cost per case $45,8904 Estimated incremental cost of $5.4-8.6B1 Based on 2019 birth rate2, PE incidence rate of 5-8%3, avg total cost per case $31,4094 These are underestimates since above is only payer perspective Estimates do not include direct medical costs incurred outside this representative healthcare system, non-direct medical costs, direct non-medical costs, or longer-term costs 1. Estimates derived from applying known, direct costs from a representative healthcare system to US national stats; 2. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf; 3. Preeclampsia Foundation; 4. Obstet Gynecol. 2019 Dec;134(6):1227-1233 adjusted to 2020 dollars

Items to Note One healthcare system, not a national estimate Geography, race known confounders Payer perspective limits cost burden to direct medical care costs Not a comprehensive societal perspective, which would include additional cost components (direct nonmedical and indirect costs) and a longer time horizon for maternal/infant adverse outcomes Medical cost estimates do not capture medical care services provided outside the single integrated health care system

Acknowledgements and additional information Geisinger Study Team Susan R. Snyder, PhD; Principal Investigator Jing Hao, PhD, MD; Co-Investigator Jove Graham, PhD, Co-Investigator Michael Paglia, MD, PhD; Co-Investigator Dina Hassen, MPP; Economic Research Analyst Qiang Hao, MS, PhD Candidate; Intern Jason Brown, MS; Senior Data Analyst Victoria Schlieder, MS; Project Manager Sponsor: Progenity, Inc. Presentations Posters: International Society of Pharmacoeconomics and Outcome Research (ISPOR), 5/2018; AcademyHealth, 6/2018; Society for Maternal and Fetal Medicine, 2/2019 Manuscript: Obstetrics & Gynecology (Green Journal), December 2019 Preeclampsia reference source NIH Genetics Home Reference: https://ghr.nlm.nih.gov/condition/ preeclampsia#genes

November 20, 2020 Preecludia™ | The development journey from bench to bedside. Pankaj Oberoi, PhD Vice President Research & Development, Progenity
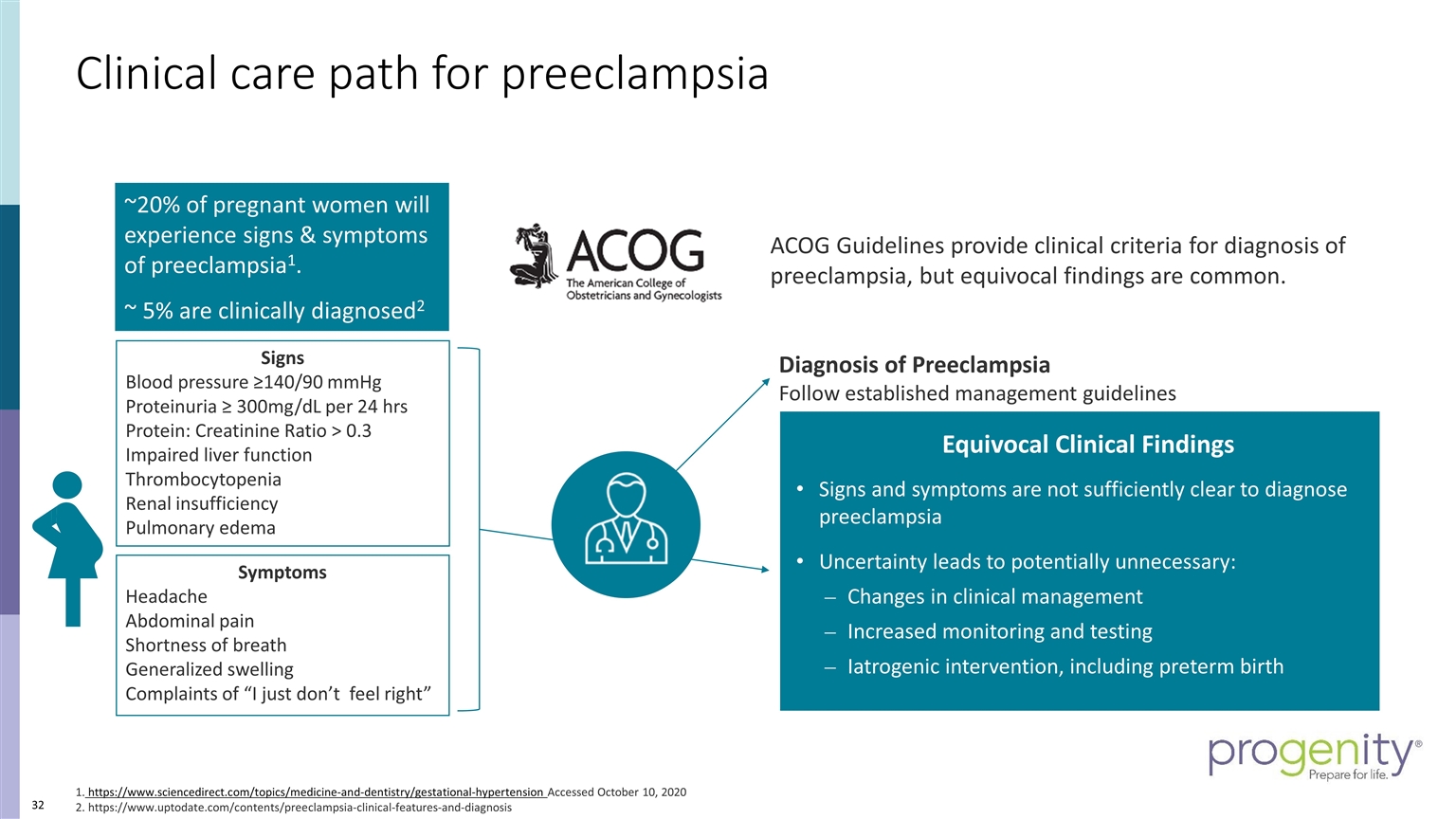
ACOG Guidelines provide clinical criteria for diagnosis of preeclampsia, but equivocal findings are common. Symptoms Headache Abdominal pain Shortness of breath Generalized swelling Complaints of “I just don’t feel right” Signs Blood pressure ≥140/90 mmHg Proteinuria ≥ 300mg/dL per 24 hrs Protein: Creatinine Ratio > 0.3 Impaired liver function Thrombocytopenia Renal insufficiency Pulmonary edema Diagnosis of Preeclampsia Follow established management guidelines Equivocal Clinical Findings Signs and symptoms are not sufficiently clear to diagnose preeclampsia Uncertainty leads to potentially unnecessary: Changes in clinical management Increased monitoring and testing Iatrogenic intervention, including preterm birth 1. https://www.sciencedirect.com/topics/medicine-and-dentistry/gestational-hypertension Accessed October 10, 2020 2. https://www.uptodate.com/contents/preeclampsia-clinical-features-and-diagnosis ~20% of pregnant women will experience signs & symptoms of preeclampsia1. ~ 5% are clinically diagnosed2 Clinical care path for preeclampsia
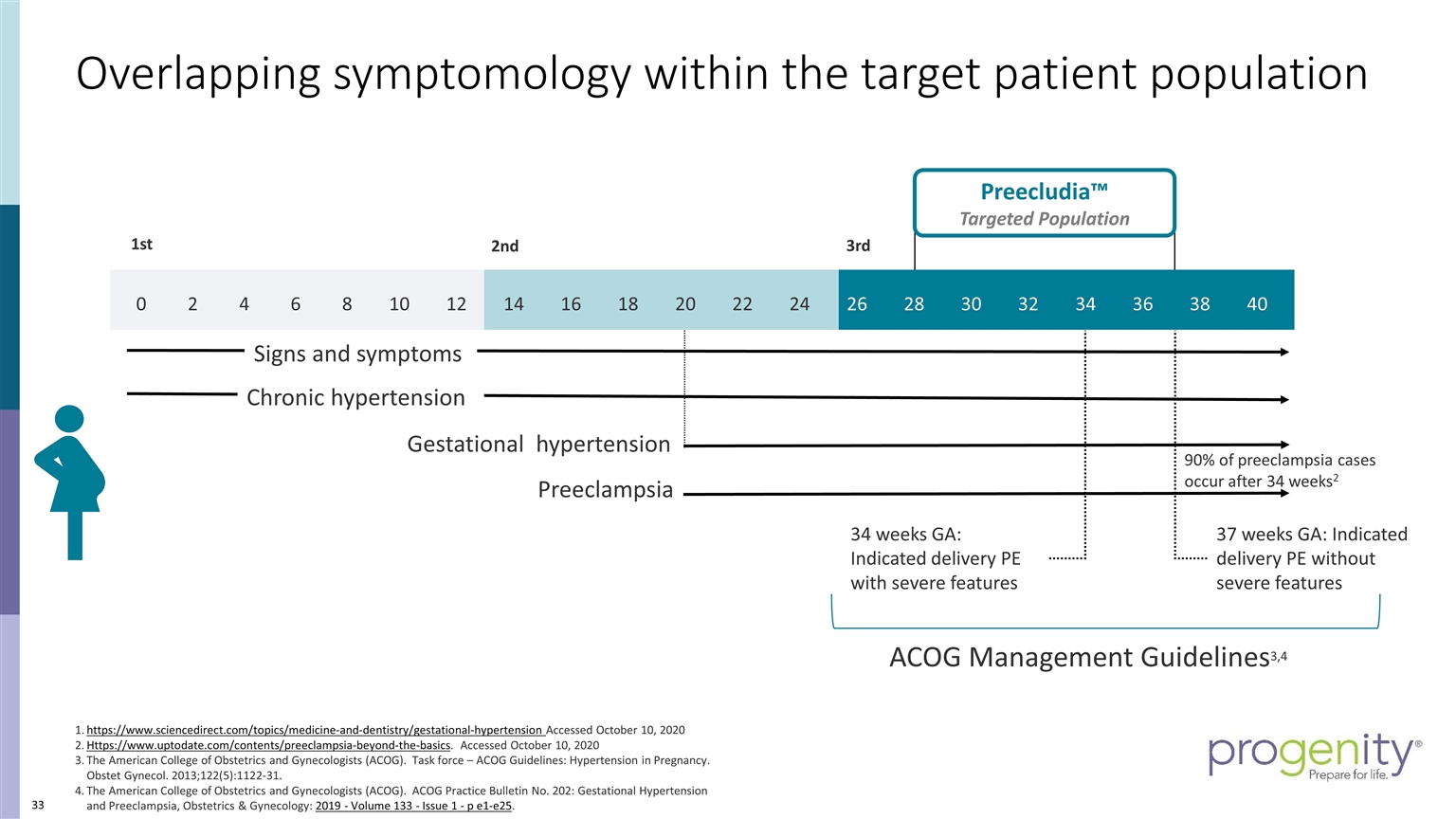
3rd 2nd 1st Preecludia™ Targeted Population 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 37 weeks GA: Indicated delivery PE without severe features 34 weeks GA: Indicated delivery PE with severe features https://www.sciencedirect.com/topics/medicine-and-dentistry/gestational-hypertension Accessed October 10, 2020 Https://www.uptodate.com/contents/preeclampsia-beyond-the-basics. Accessed October 10, 2020 The American College of Obstetrics and Gynecologists (ACOG). Task force – ACOG Guidelines: Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-31. The American College of Obstetrics and Gynecologists (ACOG). ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia, Obstetrics & Gynecology: 2019 - Volume 133 - Issue 1 - p e1-e25. 90% of preeclampsia cases occur after 34 weeks2 ACOG Management Guidelines3,4 Preeclampsia Chronic hypertension Signs and symptoms Gestational hypertension Overlapping symptomology within the target patient population
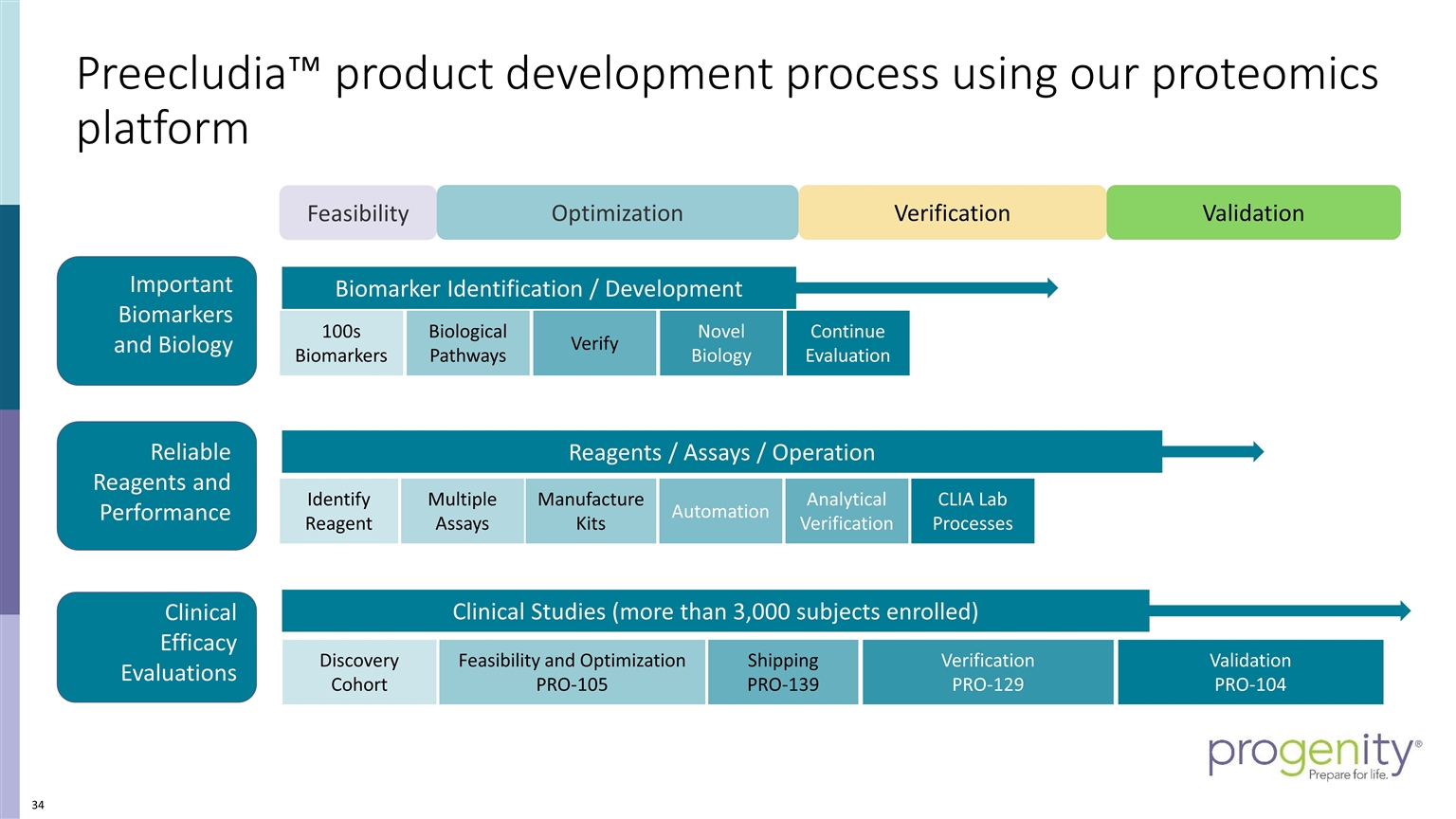
Feasibility Optimization Reagents / Assays / Operation Analytical Verification CLIA Lab Processes Manufacture Kits Multiple Assays Automation Identify Reagent Clinical Studies (more than 3,000 subjects enrolled) Discovery Cohort Feasibility and Optimization PRO-105 Verification PRO-129 Validation PRO-104 Shipping PRO-139 Biomarker Identification / Development 100s Biomarkers Biological Pathways Verify Novel Biology Continue Evaluation Verification Validation Important Biomarkers and Biology Reliable Reagents and Performance Clinical Efficacy Evaluations Preecludia™ product development process using our proteomics platform
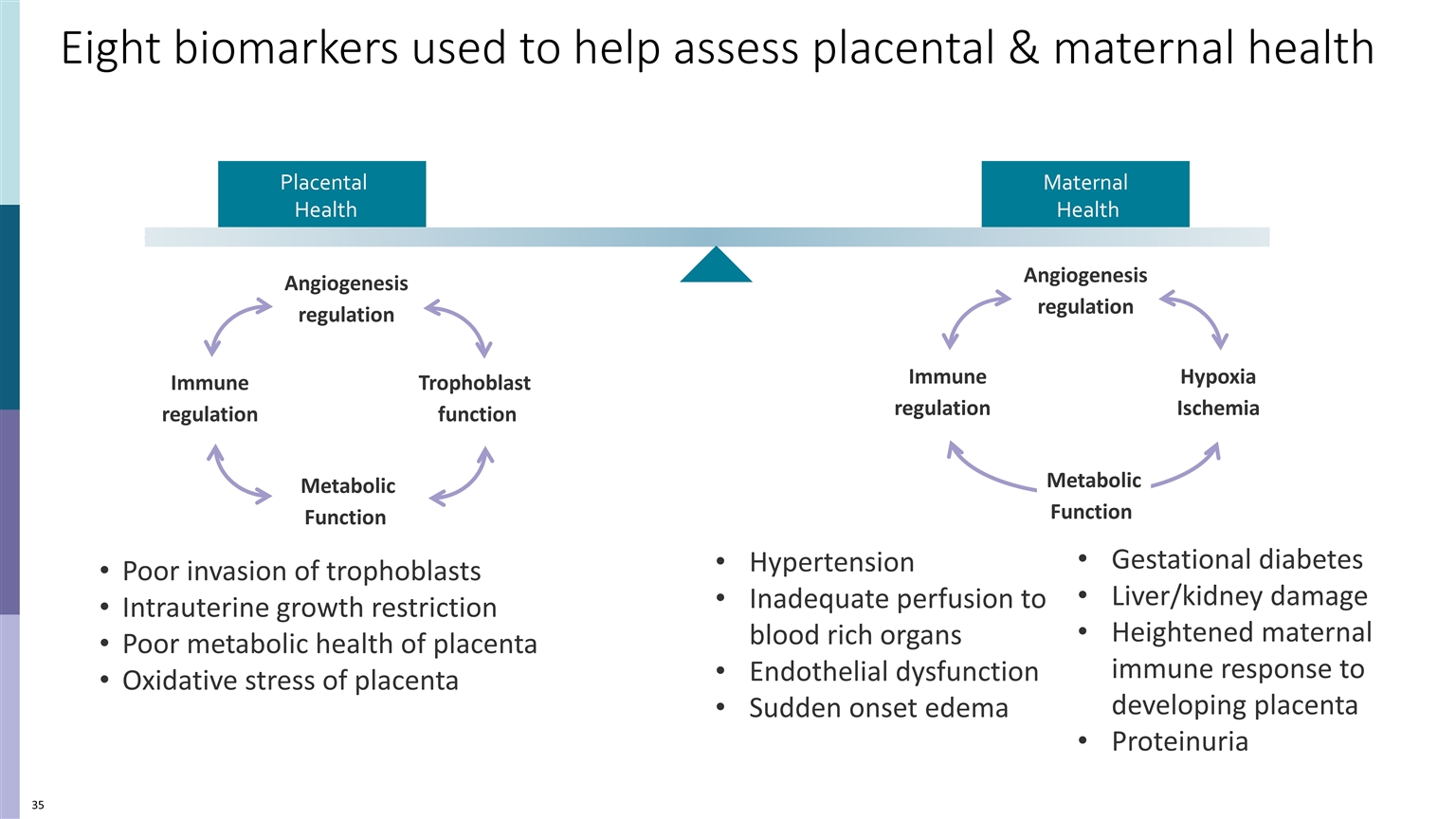
Eight biomarkers used to help assess placental & maternal health Poor invasion of trophoblasts Intrauterine growth restriction Poor metabolic health of placenta Oxidative stress of placenta Gestational diabetes Liver/kidney damage Heightened maternal immune response to developing placenta Proteinuria Hypertension Inadequate perfusion to blood rich organs Endothelial dysfunction Sudden onset edema Maternal Health Placental Health Trophoblast function Angiogenesis regulation Metabolic Function Immune regulation Immune regulation Angiogenesis regulation Hypoxia Ischemia Metabolic Function
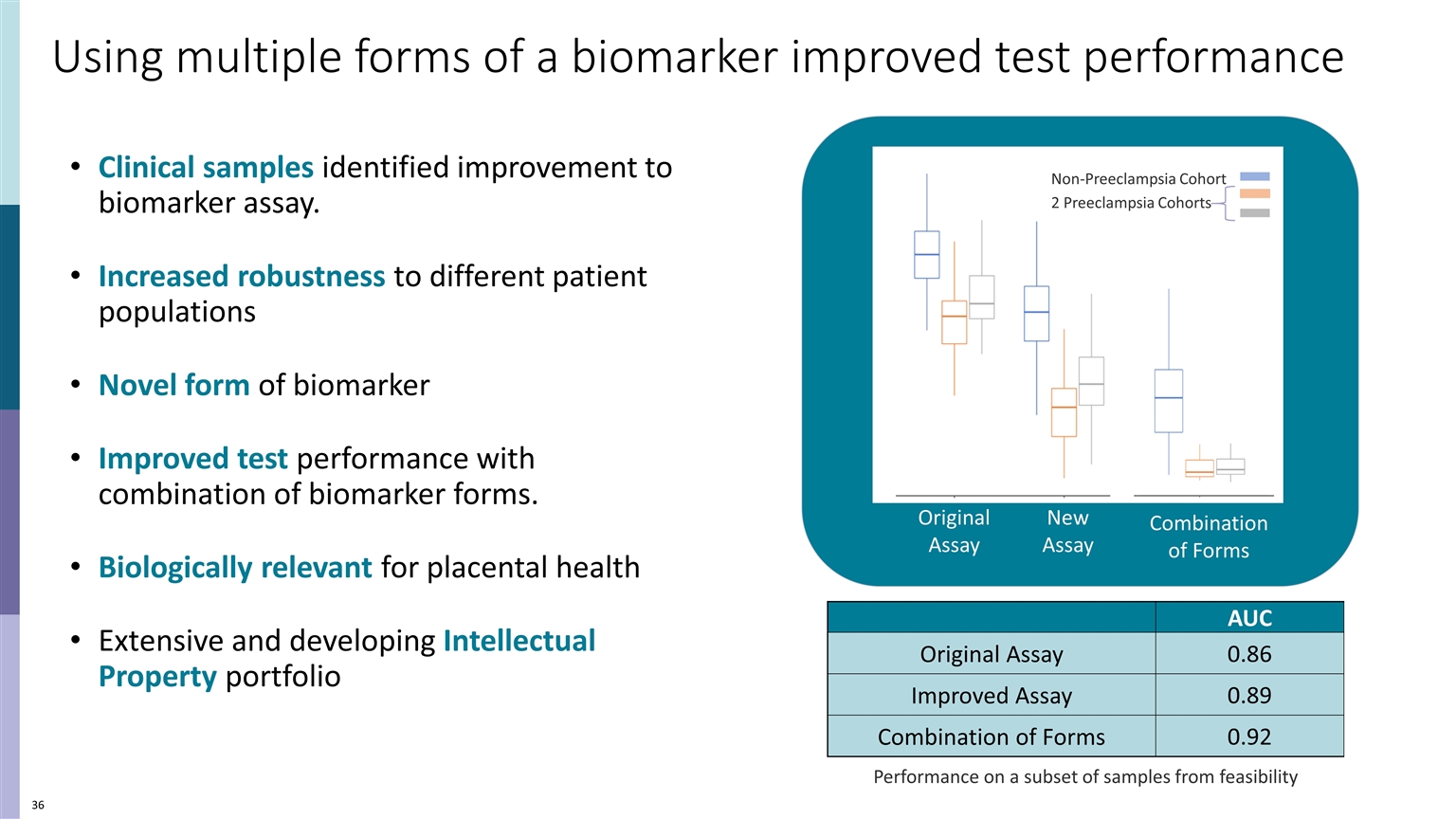
Clinical samples identified improvement to biomarker assay. Increased robustness to different patient populations Novel form of biomarker Improved test performance with combination of biomarker forms. Biologically relevant for placental health Extensive and developing Intellectual Property portfolio Using multiple forms of a biomarker improved test performance AUC Original Assay 0.86 Improved Assay 0.89 Combination of Forms 0.92 Performance on a subset of samples from feasibility Original Assay New Assay Combination of Forms Non-Preeclampsia Cohort 2 Preeclampsia Cohorts
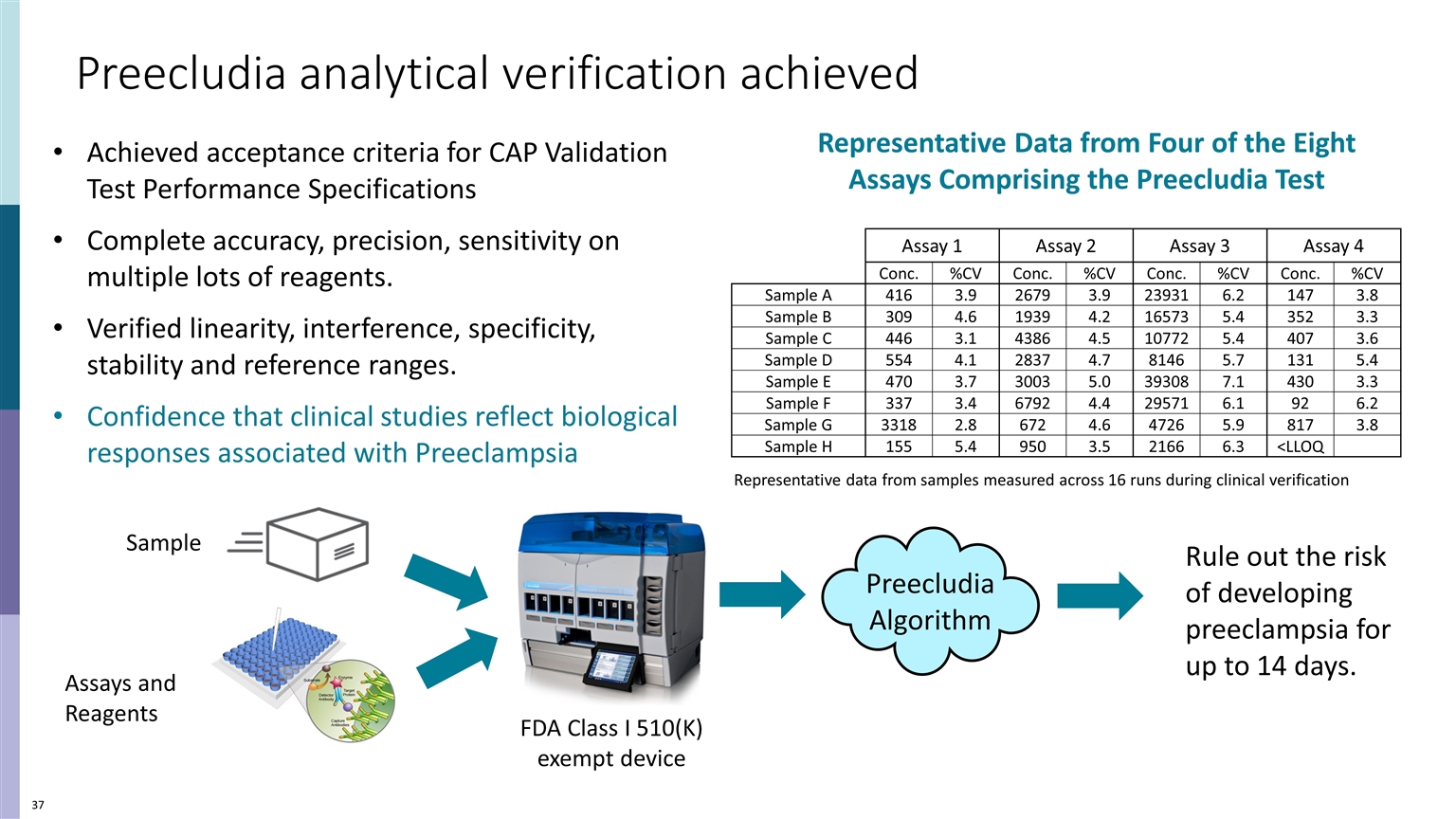
UNMET NEED CLINICAL DILEMMA Preecludia analytical verification achieved Achieved acceptance criteria for CAP Validation Test Performance Specifications Complete accuracy, precision, sensitivity on multiple lots of reagents. Verified linearity, interference, specificity, stability and reference ranges. Confidence that clinical studies reflect biological responses associated with Preeclampsia Assay 1 Assay 2 Assay 3 Assay 4 Conc. %CV Conc. %CV Conc. %CV Conc. %CV Sample A 416 3.9 2679 3.9 23931 6.2 147 3.8 Sample B 309 4.6 1939 4.2 16573 5.4 352 3.3 Sample C 446 3.1 4386 4.5 10772 5.4 407 3.6 Sample D 554 4.1 2837 4.7 8146 5.7 131 5.4 Sample E 470 3.7 3003 5.0 39308 7.1 430 3.3 Sample F 337 3.4 6792 4.4 29571 6.1 92 6.2 Sample G 3318 2.8 672 4.6 4726 5.9 817 3.8 Sample H 155 5.4 950 3.5 2166 6.3 <LLOQ Representative data from samples measured across 16 runs during clinical verification FDA Class I 510(K) exempt device Assays and Reagents Preecludia Algorithm Rule out the risk of developing preeclampsia for up to 14 days. Representative Data from Four of the Eight Assays Comprising the Preecludia Test Sample
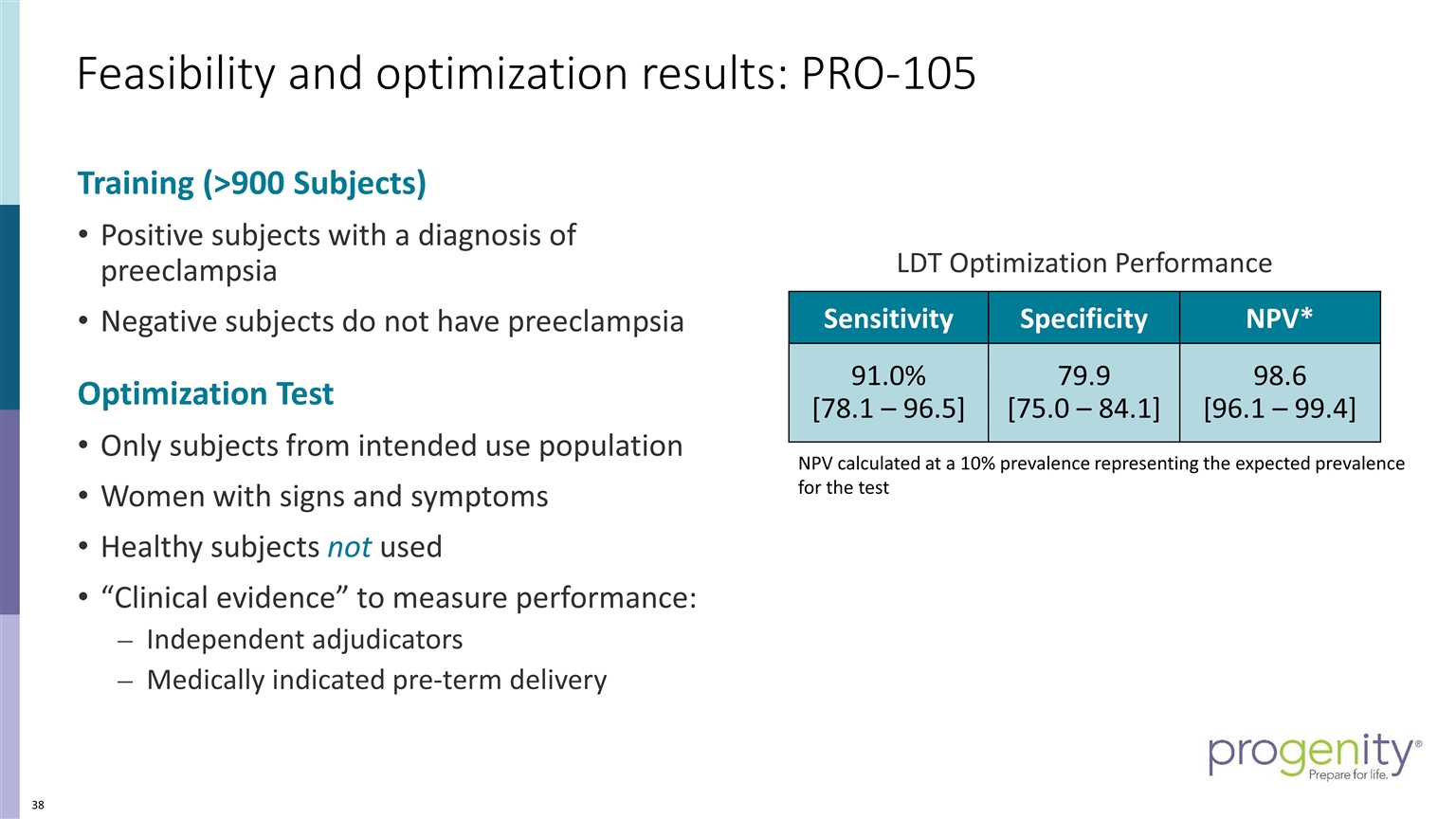
Training (>900 Subjects) Positive subjects with a diagnosis of preeclampsia Negative subjects do not have preeclampsia Optimization Test Only subjects from intended use population Women with signs and symptoms Healthy subjects not used “Clinical evidence” to measure performance: Independent adjudicators Medically indicated pre-term delivery Sensitivity Specificity NPV* 91.0% [78.1 – 96.5] 79.9 [75.0 – 84.1] 98.6 [96.1 – 99.4] NPV calculated at a 10% prevalence representing the expected prevalence for the test LDT Optimization Performance Feasibility and optimization results: PRO-105
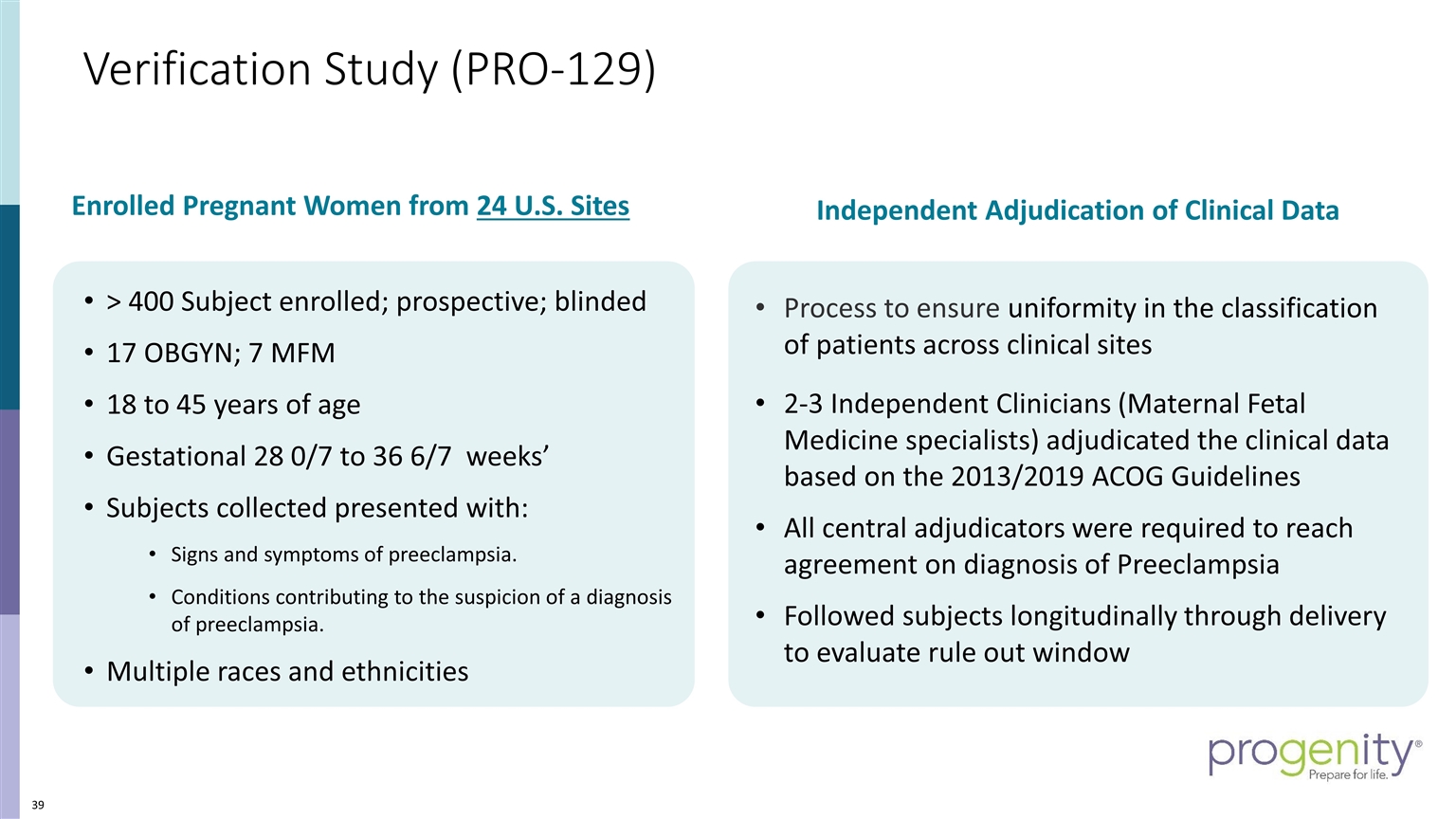
Verification Study (PRO-129) Process to ensure uniformity in the classification of patients across clinical sites 2-3 Independent Clinicians (Maternal Fetal Medicine specialists) adjudicated the clinical data based on the 2013/2019 ACOG Guidelines All central adjudicators were required to reach agreement on diagnosis of Preeclampsia Followed subjects longitudinally through delivery to evaluate rule out window > 400 Subject enrolled; prospective; blinded 17 OBGYN; 7 MFM 18 to 45 years of age Gestational 28 0/7 to 36 6/7 weeks’ Subjects collected presented with: Signs and symptoms of preeclampsia. Conditions contributing to the suspicion of a diagnosis of preeclampsia. Multiple races and ethnicities Independent Adjudication of Clinical Data Enrolled Pregnant Women from 24 U.S. Sites
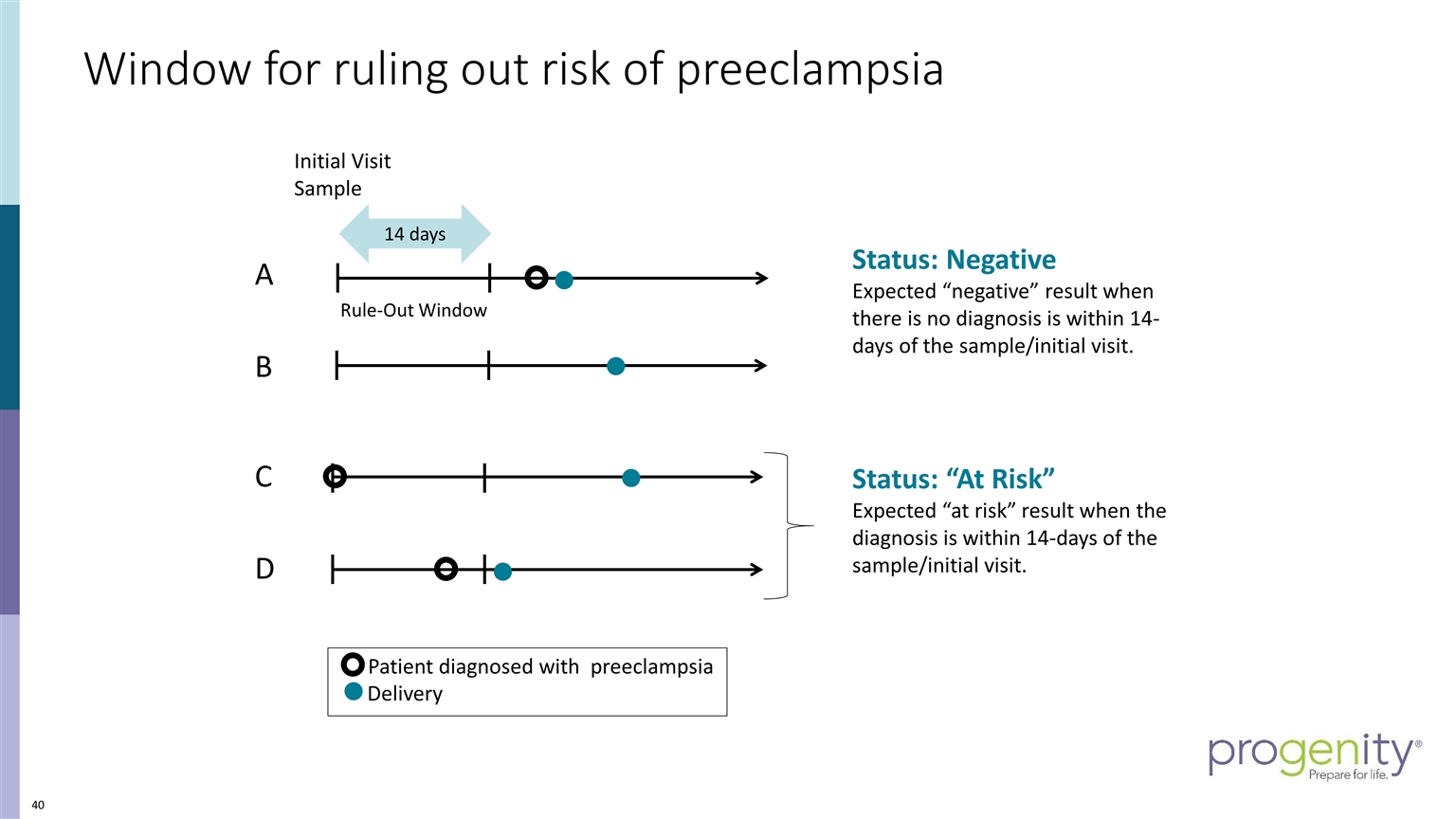
Initial Visit Sample A B Patient diagnosed with preeclampsia Delivery C 14 days Rule-Out Window Window for ruling out risk of preeclampsia Status: “At Risk” Expected “at risk” result when the diagnosis is within 14-days of the sample/initial visit. Status: Negative Expected “negative” result when there is no diagnosis is within 14-days of the sample/initial visit. D
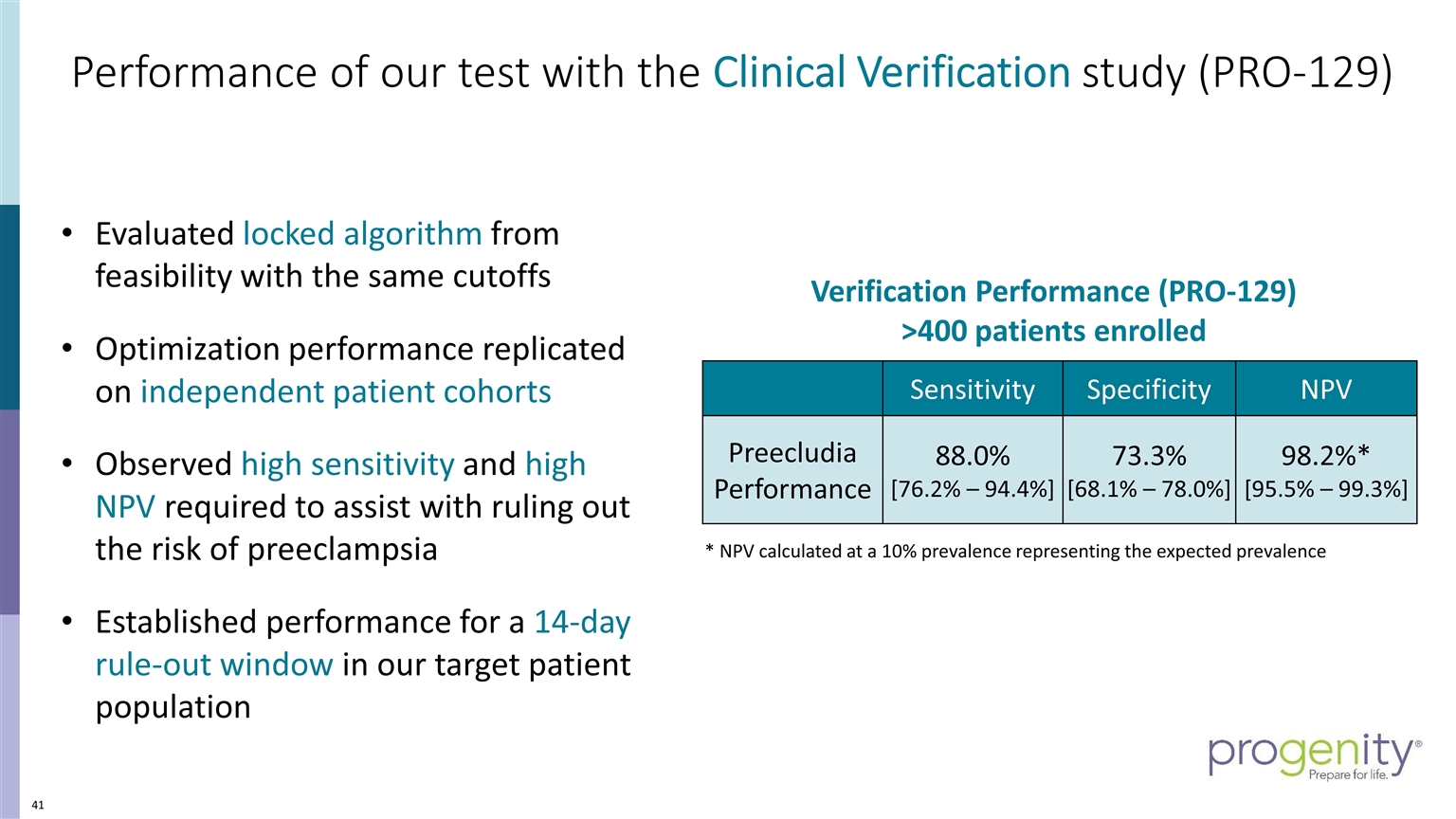
Performance of our test with the Clinical Verification study (PRO-129) 4 Evaluated locked algorithm from feasibility with the same cutoffs Optimization performance replicated on independent patient cohorts Observed high sensitivity and high NPV required to assist with ruling out the risk of preeclampsia Established performance for a 14-day rule-out window in our target patient population Sensitivity Specificity NPV Preecludia Performance 88.0% [76.2% – 94.4%] 73.3% [68.1% – 78.0%] 98.2%* [95.5% – 99.3%] * NPV calculated at a 10% prevalence representing the expected prevalence Verification Performance (PRO-129) >400 patients enrolled
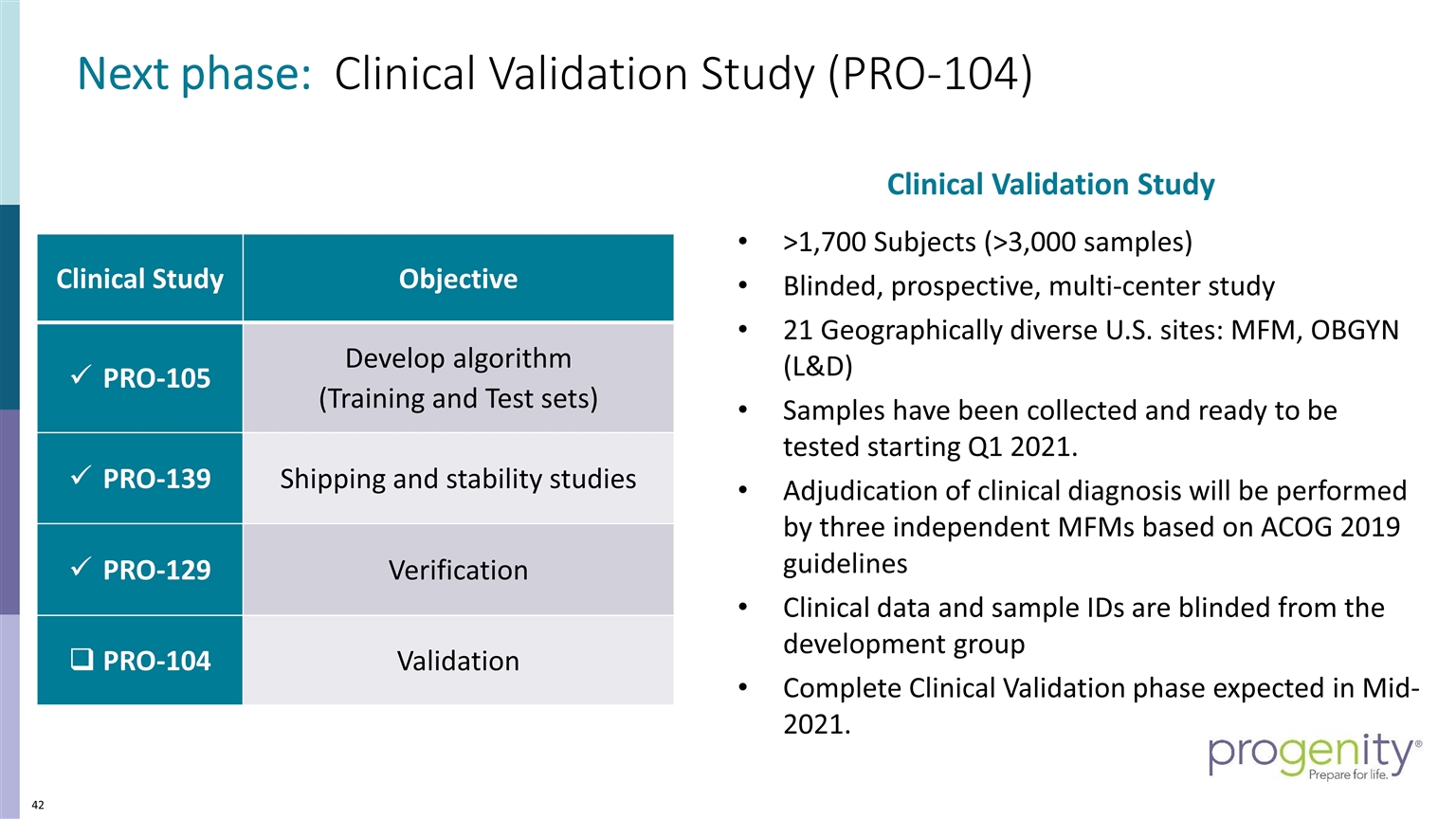
Next phase: Clinical Validation Study (PRO-104) >1,700 Subjects (>3,000 samples) Blinded, prospective, multi-center study 21 Geographically diverse U.S. sites: MFM, OBGYN (L&D) Samples have been collected and ready to be tested starting Q1 2021. Adjudication of clinical diagnosis will be performed by three independent MFMs based on ACOG 2019 guidelines Clinical data and sample IDs are blinded from the development group Complete Clinical Validation phase expected in Mid-2021. Clinical Study Objective PRO-105 Develop algorithm (Training and Test sets) PRO-139 Shipping and stability studies PRO-129 Verification PRO-104 Validation Clinical Validation Study

November 20, 2020 Preeclampsia: The gravity of the condition and the unmet need in assessment Christopher Robinson, MD, MSCR, FACOG Partner, Charleston Maternal Fetal Medicine

Preeclampsia – the great obstetrical syndrome Proteinuria – Lever/Simpson 1843 “Father of Preeclampsia Investigation” Recognized the “spectrum of preeclampsia” Leon C. Chesley, Ph.D. 1908-2000
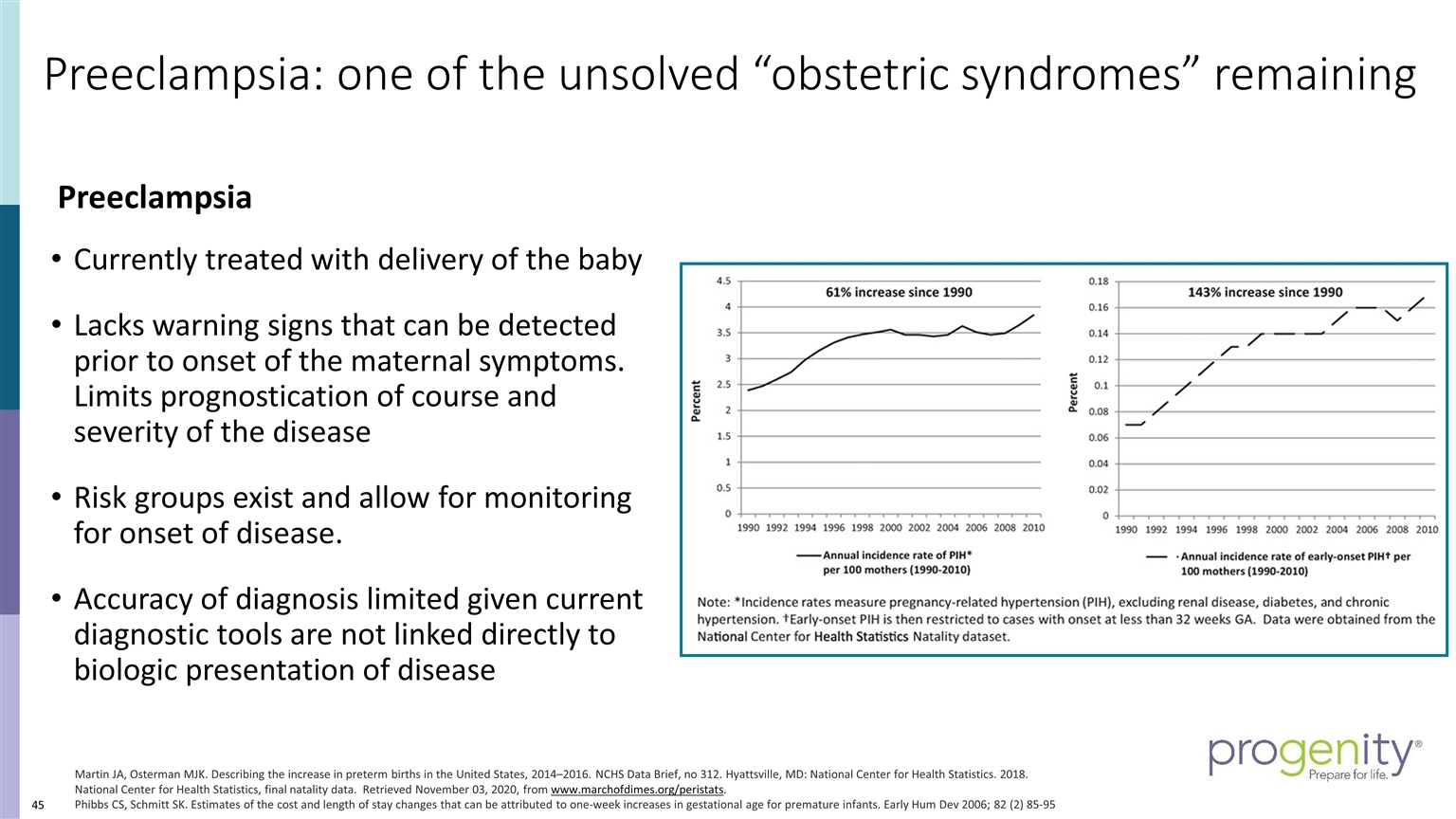
Preeclampsia: one of the unsolved “obstetric syndromes” remaining Preeclampsia Currently treated with delivery of the baby Lacks warning signs that can be detected prior to onset of the maternal symptoms. Limits prognostication of course and severity of the disease Risk groups exist and allow for monitoring for onset of disease. Accuracy of diagnosis limited given current diagnostic tools are not linked directly to biologic presentation of disease Martin JA, Osterman MJK. Describing the increase in preterm births in the United States, 2014–2016. NCHS Data Brief, no 312. Hyattsville, MD: National Center for Health Statistics. 2018. National Center for Health Statistics, final natality data. Retrieved November 03, 2020, from www.marchofdimes.org/peristats. Phibbs CS, Schmitt SK. Estimates of the cost and length of stay changes that can be attributed to one-week increases in gestational age for premature infants. Early Hum Dev 2006; 82 (2) 85-95
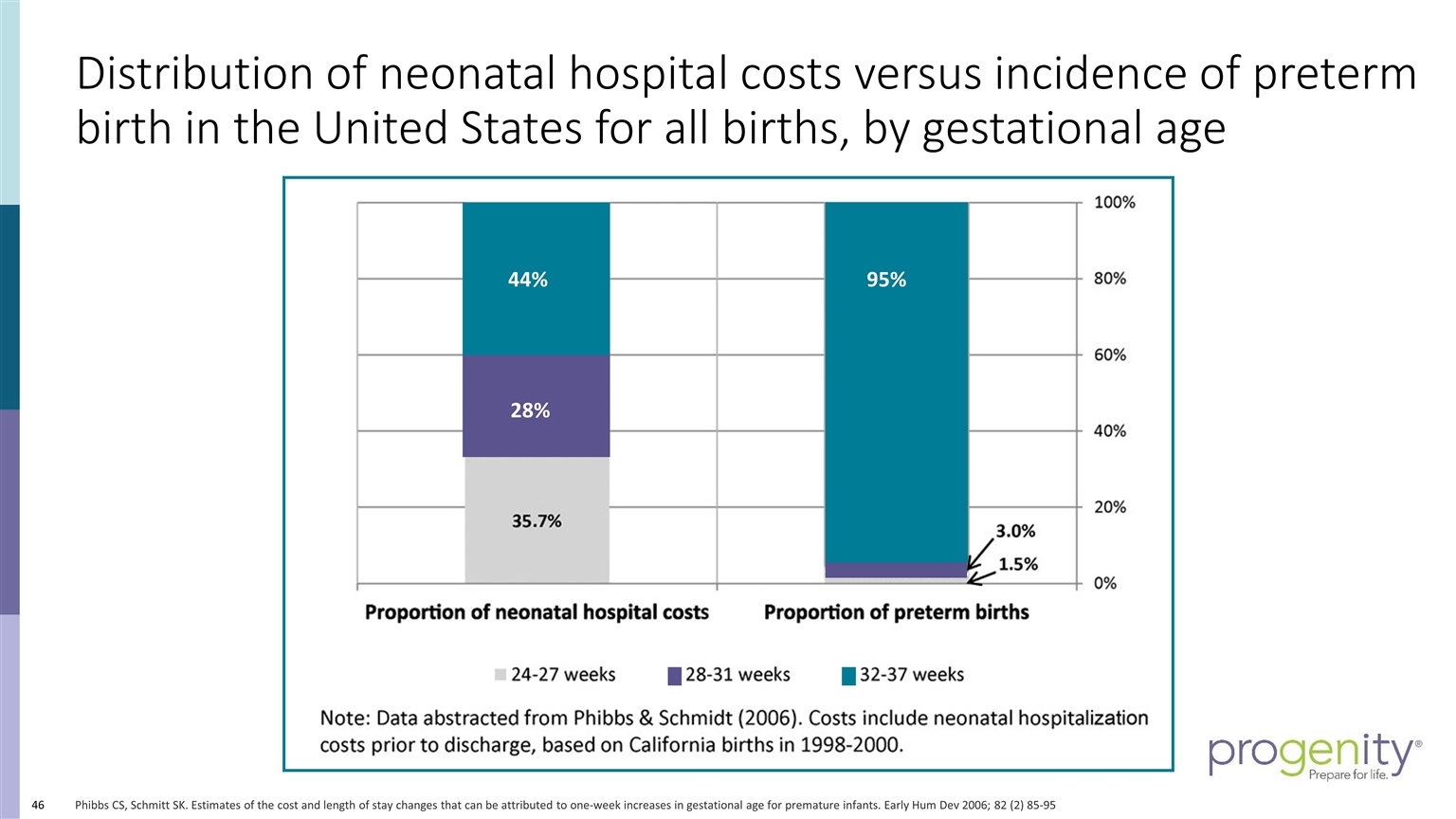
Distribution of neonatal hospital costs versus incidence of preterm birth in the United States for all births, by gestational age Phibbs CS, Schmitt SK. Estimates of the cost and length of stay changes that can be attributed to one-week increases in gestational age for premature infants. Early Hum Dev 2006; 82 (2) 85-95 44% 95% 28%
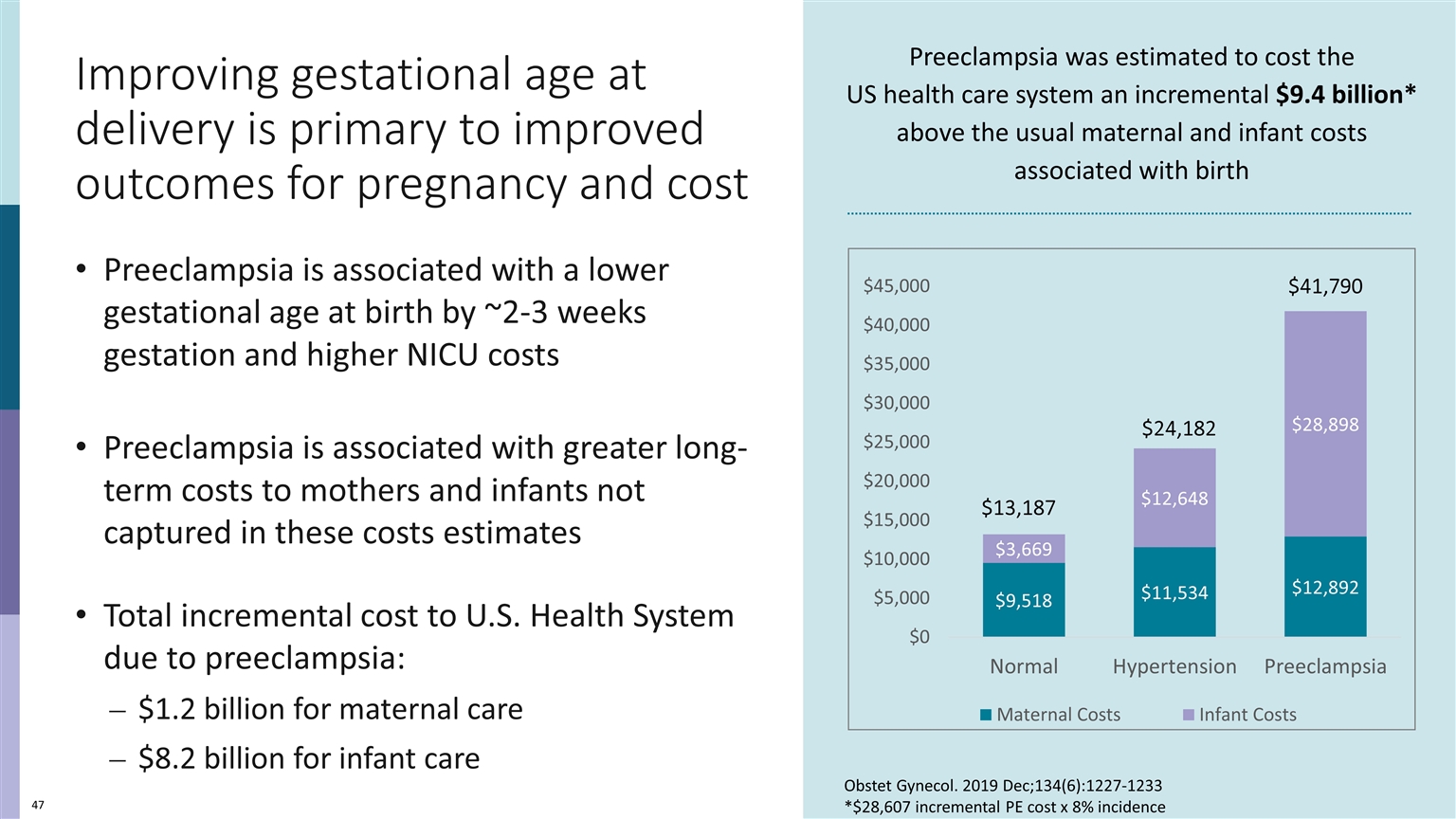
Improving gestational age at delivery is primary to improved outcomes for pregnancy and cost Preeclampsia is associated with a lower gestational age at birth by ~2-3 weeks gestation and higher NICU costs Preeclampsia is associated with greater long-term costs to mothers and infants not captured in these costs estimates Total incremental cost to U.S. Health System due to preeclampsia: $1.2 billion for maternal care $8.2 billion for infant care Preeclampsia was estimated to cost the US health care system an incremental $9.4 billion* above the usual maternal and infant costs associated with birth $41,790 $24,182 $13,187 Obstet Gynecol. 2019 Dec;134(6):1227-1233 *$28,607 incremental PE cost x 8% incidence
Despite the increasing rates of preeclampsia There have been no advances in diagnostic assessment There have been no improvements in triage of patients with suspected preeclampsia
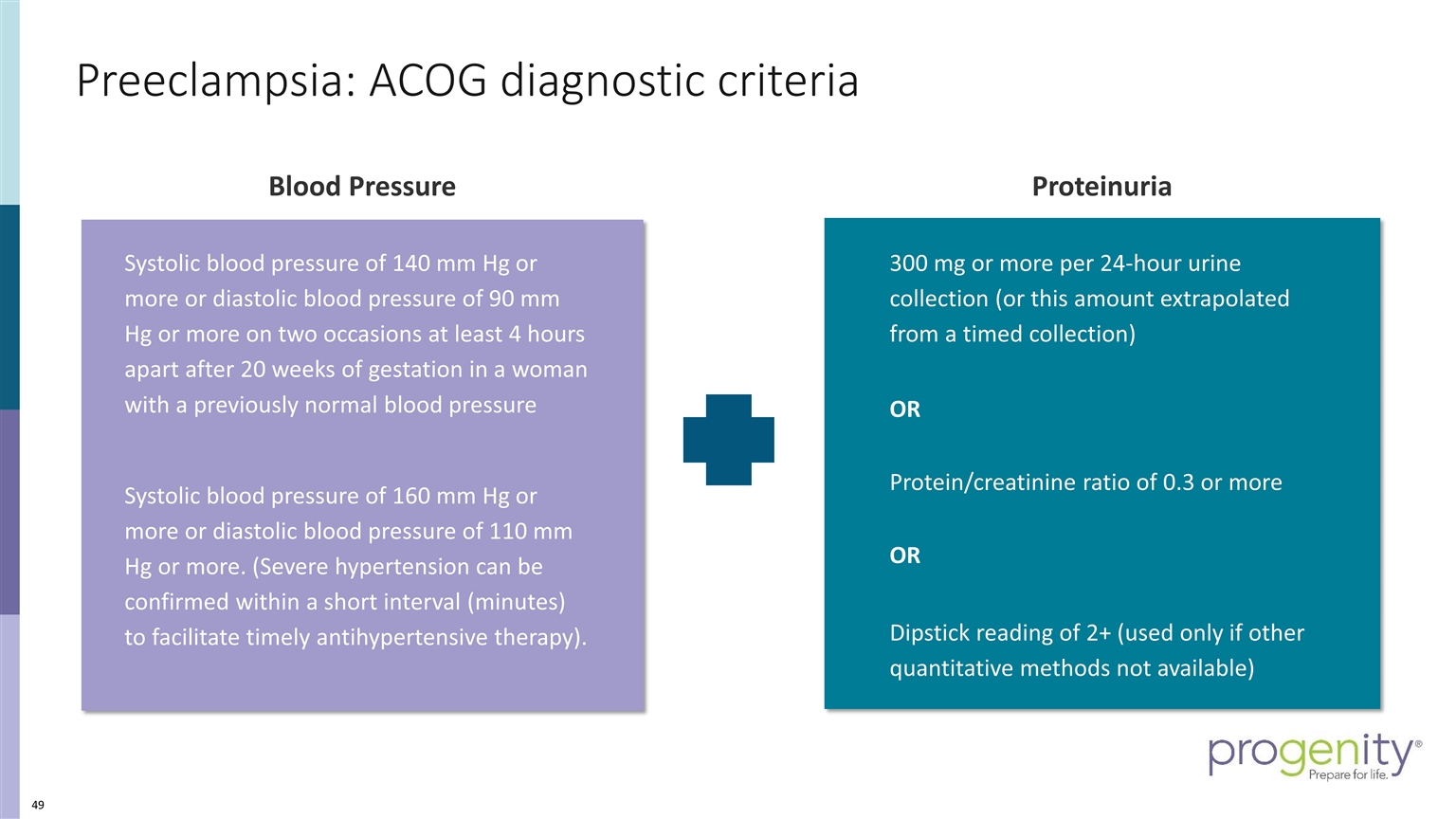
Preeclampsia: ACOG diagnostic criteria Blood Pressure 300 mg or more per 24-hour urine collection (or this amount extrapolated from a timed collection) OR Protein/creatinine ratio of 0.3 or more OR Dipstick reading of 2+ (used only if other quantitative methods not available) Proteinuria Systolic blood pressure of 140 mm Hg or more or diastolic blood pressure of 90 mm Hg or more on two occasions at least 4 hours apart after 20 weeks of gestation in a woman with a previously normal blood pressure Systolic blood pressure of 160 mm Hg or more or diastolic blood pressure of 110 mm Hg or more. (Severe hypertension can be confirmed within a short interval (minutes) to facilitate timely antihypertensive therapy).
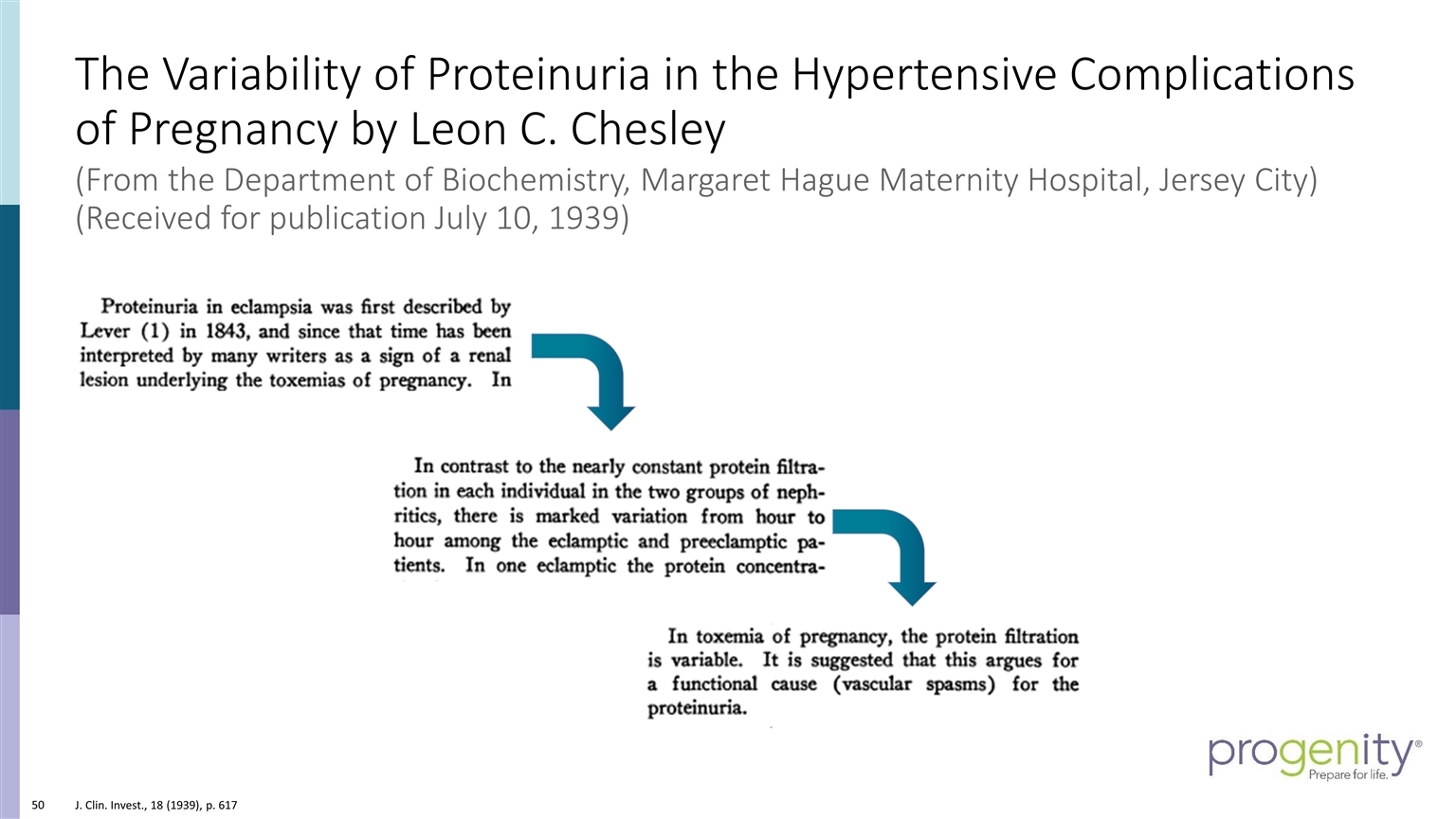
J. Clin. Invest., 18 (1939), p. 617 The Variability of Proteinuria in the Hypertensive Complications of Pregnancy by Leon C. Chesley (From the Department of Biochemistry, Margaret Hague Maternity Hospital, Jersey City) (Received for publication July 10, 1939)
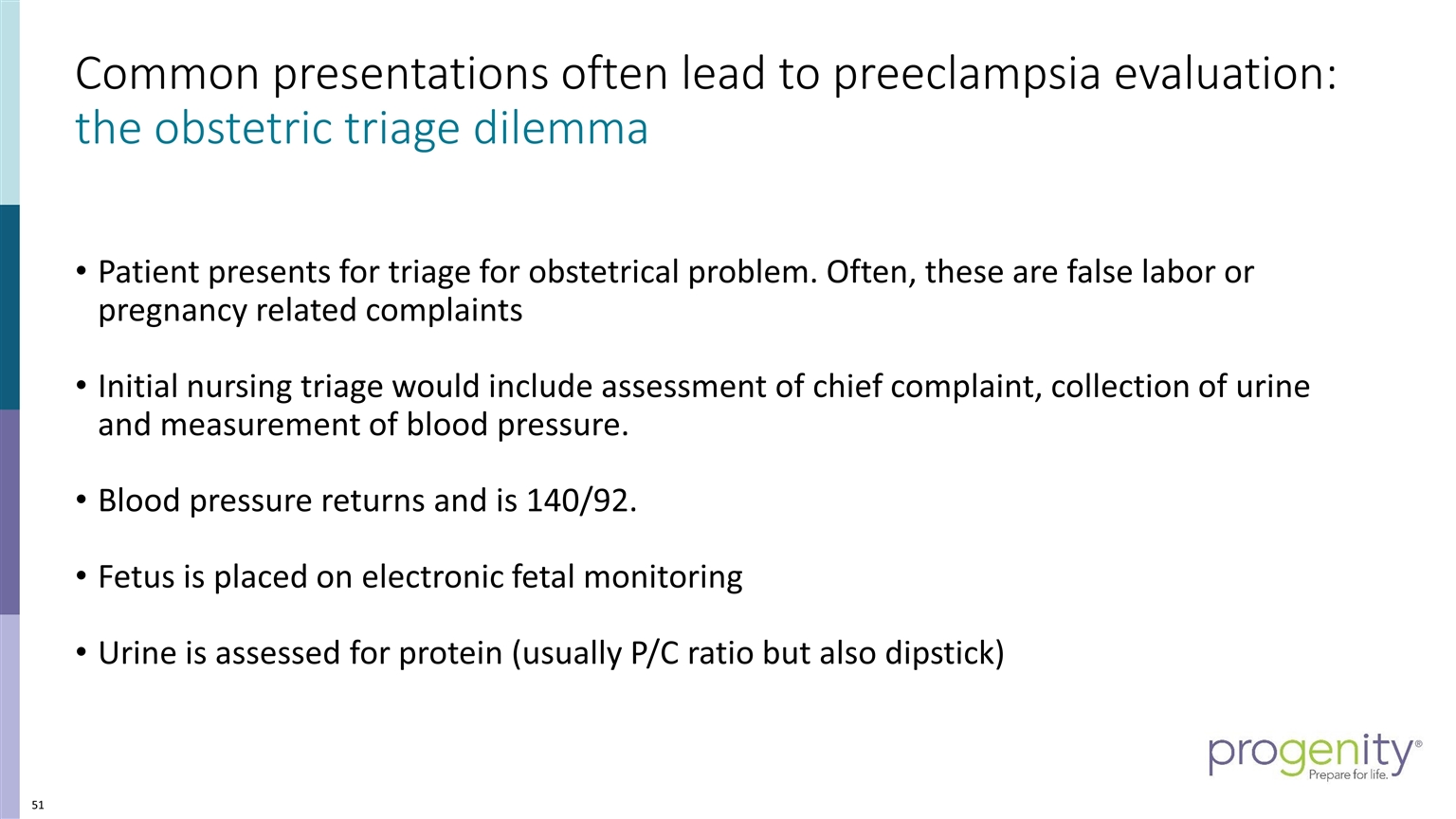
Common presentations often lead to preeclampsia evaluation: the obstetric triage dilemma Patient presents for triage for obstetrical problem. Often, these are false labor or pregnancy related complaints Initial nursing triage would include assessment of chief complaint, collection of urine and measurement of blood pressure. Blood pressure returns and is 140/92. Fetus is placed on electronic fetal monitoring Urine is assessed for protein (usually P/C ratio but also dipstick)
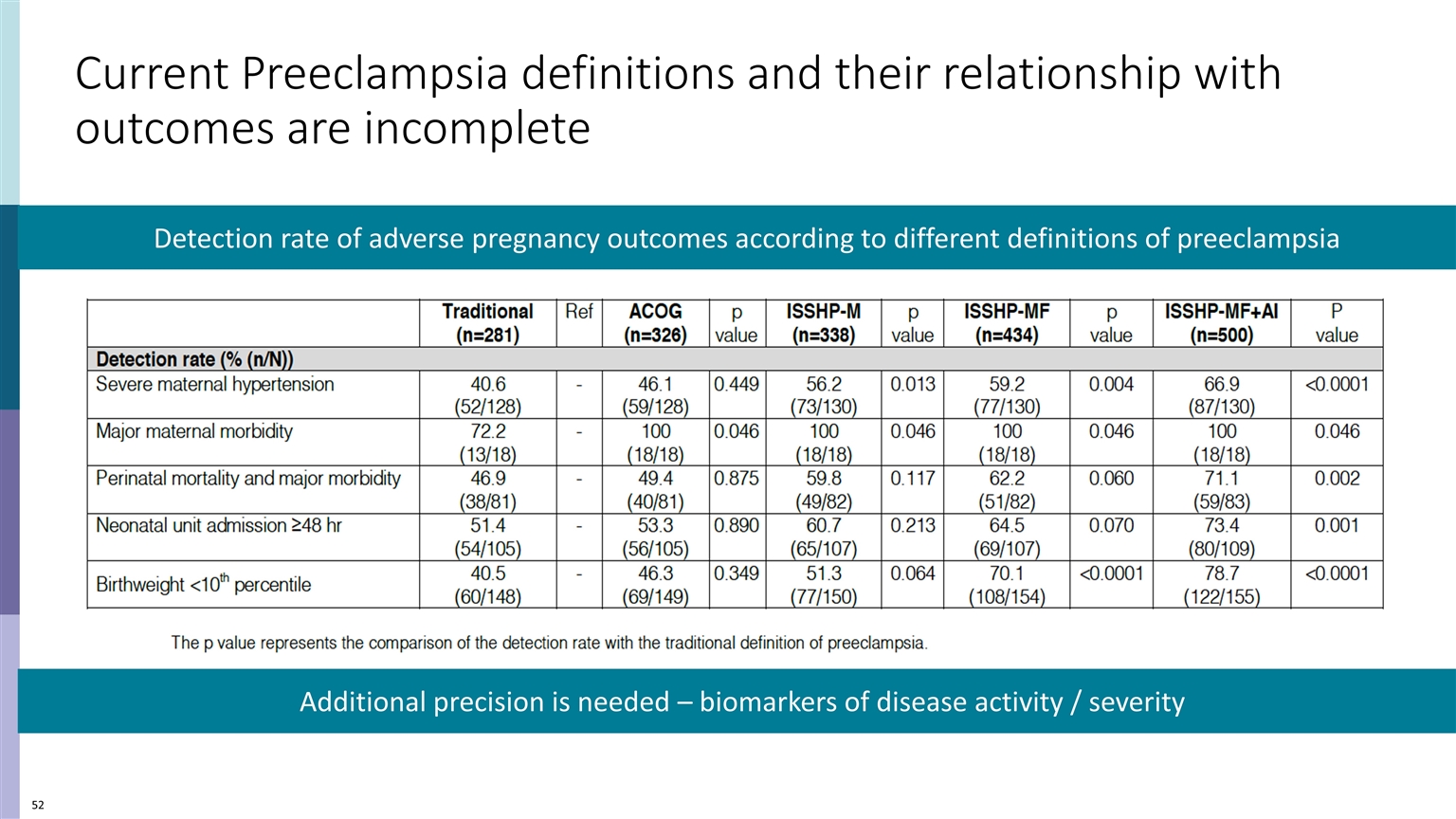
Current Preeclampsia definitions and their relationship with outcomes are incomplete Detection rate of adverse pregnancy outcomes according to different definitions of preeclampsia Additional precision is needed – biomarkers of disease activity / severity
Preeclampsia: more than just hypertension . . .
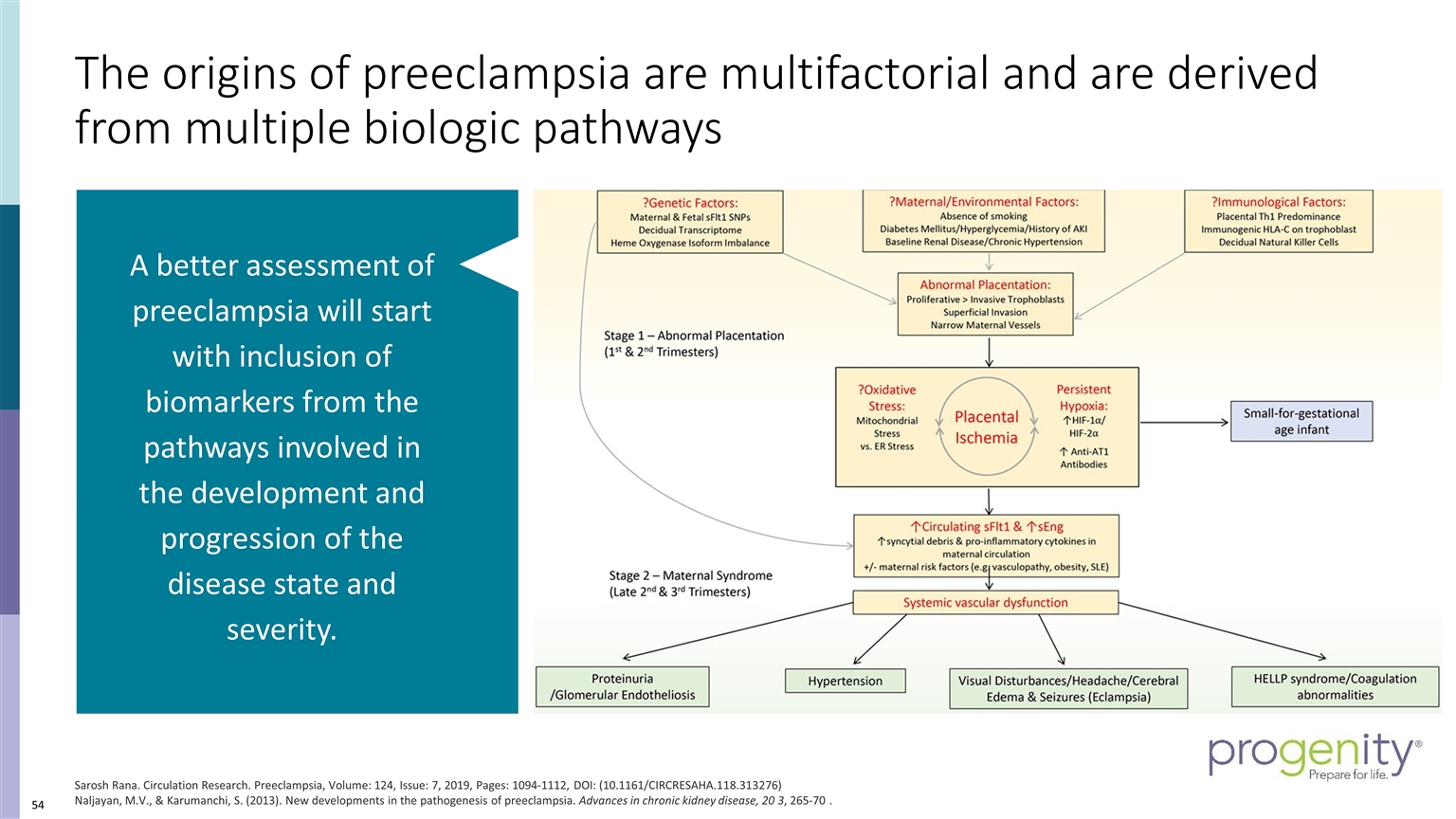
The origins of preeclampsia are multifactorial and are derived from multiple biologic pathways Sarosh Rana. Circulation Research. Preeclampsia, Volume: 124, Issue: 7, 2019, Pages: 1094-1112, DOI: (10.1161/CIRCRESAHA.118.313276) Naljayan, M.V., & Karumanchi, S. (2013). New developments in the pathogenesis of preeclampsia. Advances in chronic kidney disease, 20 3, 265-70 . A better assessment of preeclampsia will start with inclusion of biomarkers from the pathways involved in the development and progression of the disease state and severity.
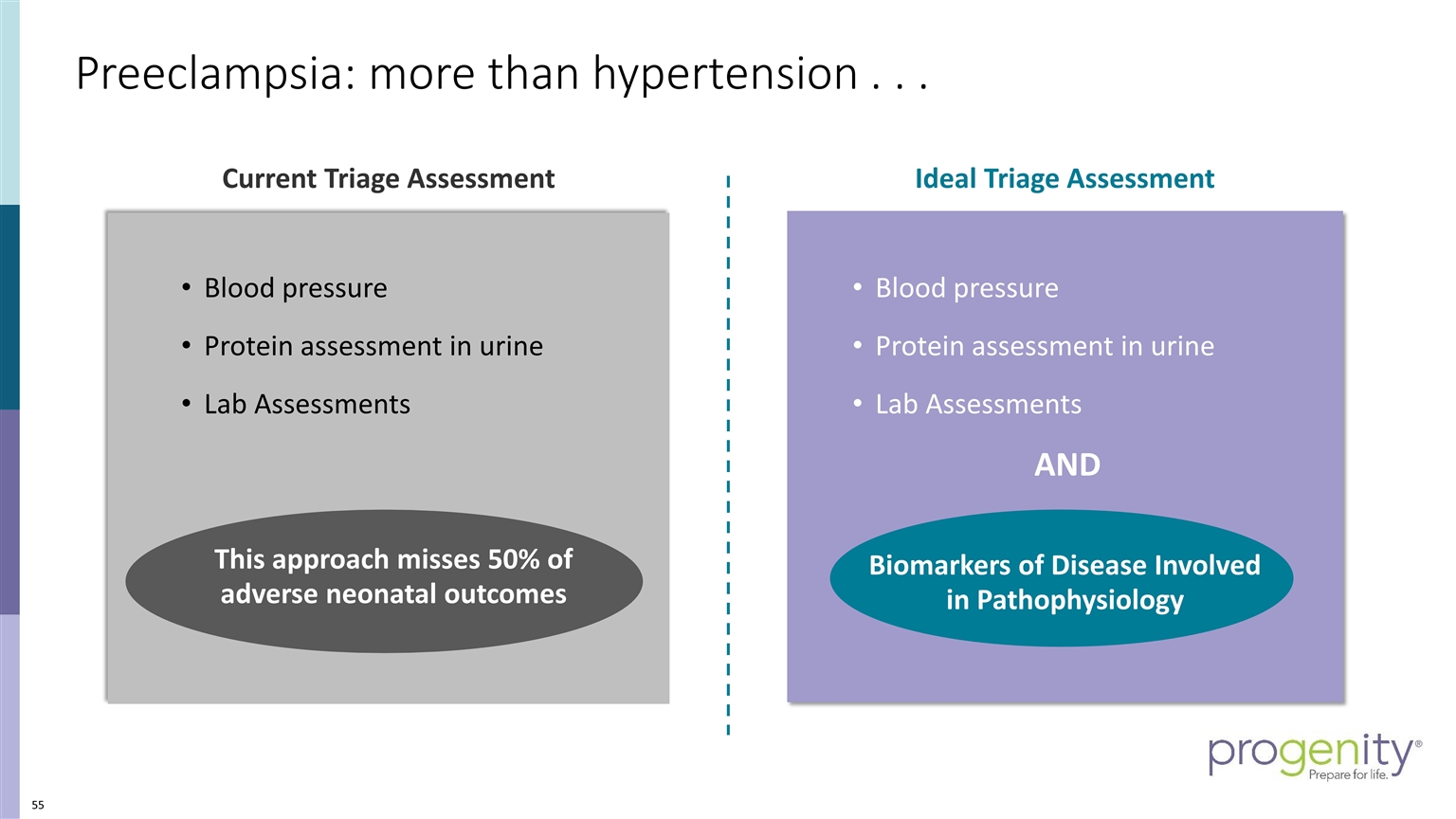
Current Triage Assessment Blood pressure Protein assessment in urine Lab Assessments AND Biomarkers of Disease Involved in Pathophysiology Ideal Triage Assessment Blood pressure Protein assessment in urine Lab Assessments This approach misses 50% of adverse neonatal outcomes Preeclampsia: more than hypertension . . .
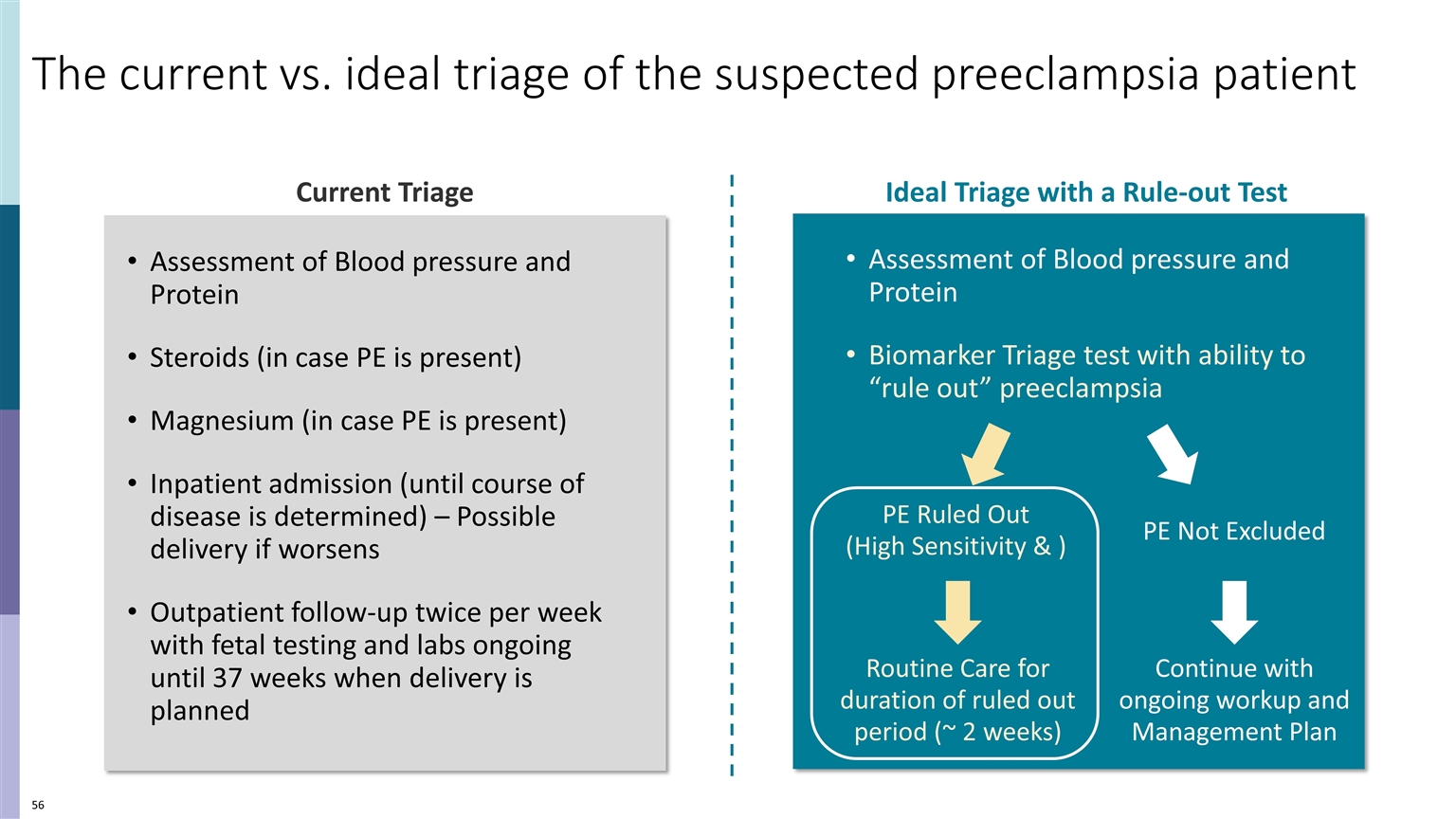
The current vs. ideal triage of the suspected preeclampsia patient Current Triage Assessment of Blood pressure and Protein Steroids (in case PE is present) Magnesium (in case PE is present) Inpatient admission (until course of disease is determined) – Possible delivery if worsens Outpatient follow-up twice per week with fetal testing and labs ongoing until 37 weeks when delivery is planned Assessment of Blood pressure and Protein Biomarker Triage test with ability to “rule out” preeclampsia Ideal Triage with a Rule-out Test PE Ruled Out (High Sensitivity & ) PE Not Excluded Routine Care for duration of ruled out period (~ 2 weeks) Continue with ongoing workup and Management Plan
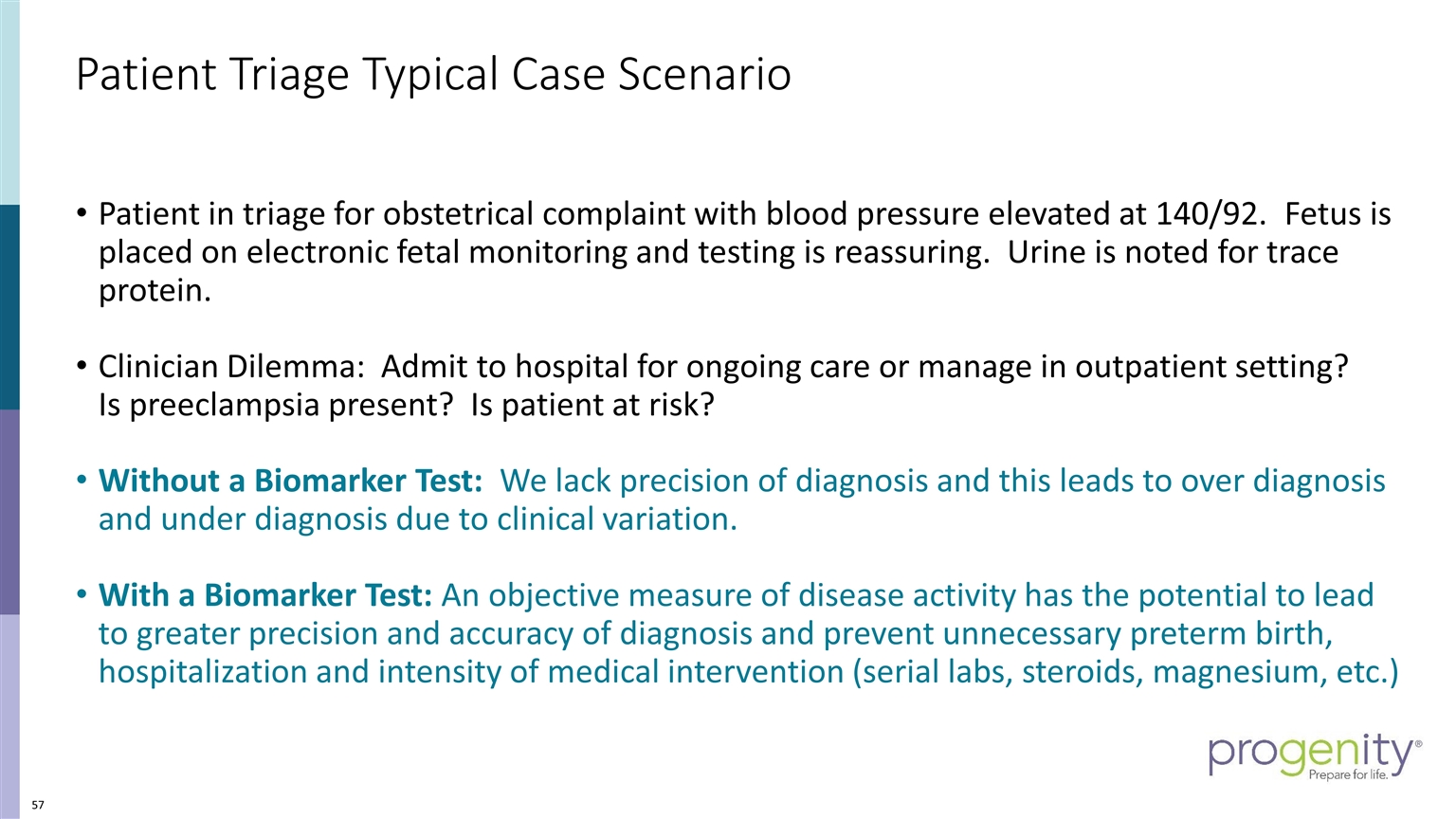
Patient Triage Typical Case Scenario Patient in triage for obstetrical complaint with blood pressure elevated at 140/92. Fetus is placed on electronic fetal monitoring and testing is reassuring. Urine is noted for trace protein. Clinician Dilemma: Admit to hospital for ongoing care or manage in outpatient setting? Is preeclampsia present? Is patient at risk? Without a Biomarker Test: We lack precision of diagnosis and this leads to over diagnosis and under diagnosis due to clinical variation. With a Biomarker Test: An objective measure of disease activity has the potential to lead to greater precision and accuracy of diagnosis and prevent unnecessary preterm birth, hospitalization and intensity of medical intervention (serial labs, steroids, magnesium, etc.)

What do clinicians need in the management and triage of patients with symptoms suspected of preeclampsia? A test that is based in the pathophysiology of preeclampsia Rapid ability to rule out disease and thereby prevent unnecessary interventions, hospitalizations and cost. Ability to notify patient of risk or lack of risk in pregnancy during triage Ability to prevent unnecessary delivery of pregnancy when preeclampsia does not exist. Increased confidence in performance of testing for preeclampsia.

The incidence of preeclampsia is increasing There is no effective cure for preeclampsia – delivery always remains the treatment of choice Current diagnostic testing for preeclampsia lacks a direct relationship to the biologic pathways involved in disease leading to missed opportunities in diagnostic accuracy Improved diagnostic assessment will lead to improved outcomes through targeted decisions for delivery in pregnancy at risk. Summary: Improving outcomes requires accuracy in assessment of preeclampsia risk

Preecludia™ Clinical Evidence Development Christina Settler, MS, CGC Vice President of Medical Affairs, Progenity
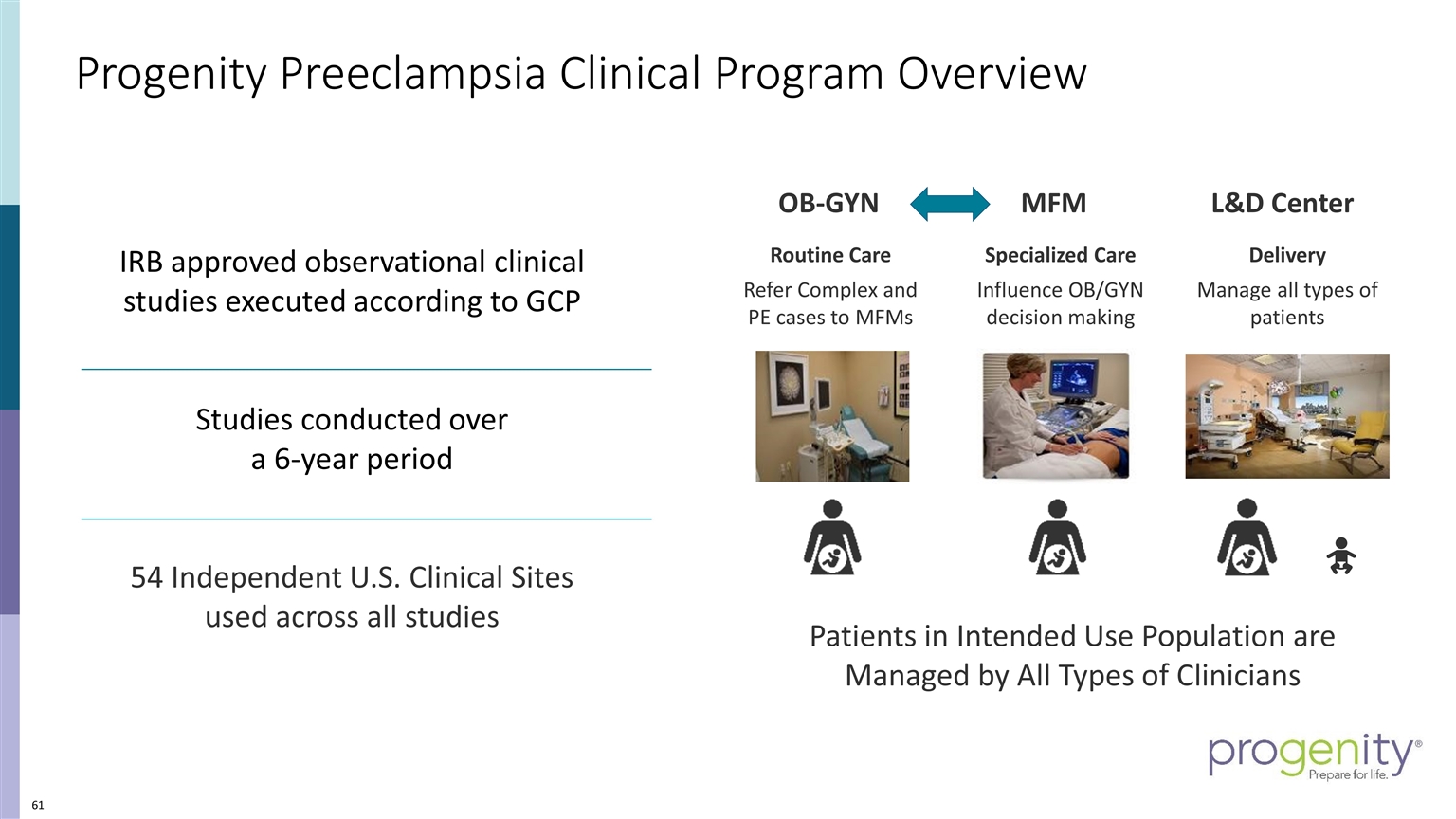
Progenity Preeclampsia Clinical Program Overview Patients in Intended Use Population are Managed by All Types of Clinicians OB-GYN MFM L&D Center Specialized Care Influence OB/GYN decision making Routine Care Refer Complex and PE cases to MFMs Delivery Manage all types of patients IRB approved observational clinical studies executed according to GCP Studies conducted over a 6-year period 54 Independent U.S. Clinical Sites used across all studies
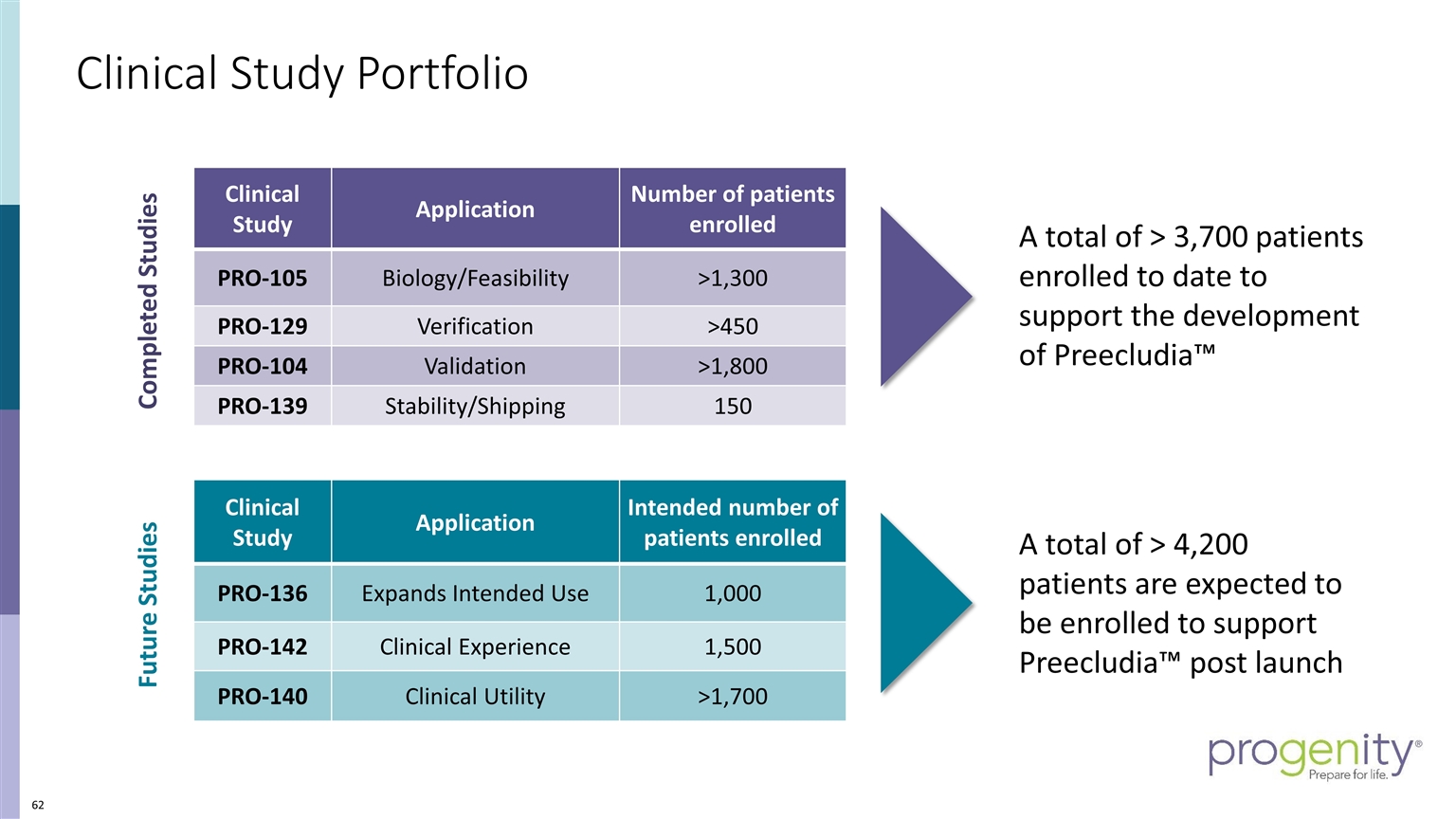
Clinical Study Portfolio Completed Studies Future Studies A total of > 3,700 patients enrolled to date to support the development of Preecludia™ Clinical Study Application Number of patients enrolled PRO-105 Biology/Feasibility >1,300 PRO-129 Verification >450 PRO-104 Validation >1,800 PRO-139 Stability/Shipping 150 Clinical Study Application Intended number of patients enrolled PRO-136 Expands Intended Use 1,000 PRO-142 Clinical Experience 1,500 PRO-140 Clinical Utility >1,700 A total of > 4,200 patients are expected to be enrolled to support Preecludia™ post launch

Planned publications & Future PE clinical program Summary Planned publications Clinical verification data Clinical validation data Meta analysis combining verification and validation data Preeclampsia disease biology and pathophysiology PRO-136: Observational Clinical Study; acquire clinical samples to enable expanding target patient population; study in the Start-Up Phase Q4 2020 PRO-142: Observational Clinical Study; assess initial user experience of the Progenity PE Test; enrollment targeted to begin Q3 2021 PRO-140: Randomized Controlled Clinical Study; to assess the Clinical Utility of the Progenity PE Test; enrollment targeted to begin Q4 2021 into 2022

Summary Progenity has a strong, well-established Clinical Operations Department including: Clinical Research coverage of the entire US Data-Management and biostatistics Progenity Biorepository in Ann Arbor We have a robust and diverse network of Clinical Researchers / Key Opinion Leaders necessary for the design, execution, and interpretation of our Clinical Studies Progenity has conducted multiple studies, enrolling over 7,500 participants to support the development of preecludia that will help improve the current standard of care.

Q&A

Closing Remarks Harry Stylli, PhD CEO, Chairman, and Co-founder, Progenity

Preecludia™ - A high Potential Rule Out Test for Preeclampsia Established a fundamental understanding of preeclampsia and the great clinical unmet need that exists today in assessing patient risk in our channel: Ob/Gyn and MFM Showed the incremental healthcare cost burden of $9B+ associated with managing a preeclamptic pregnancy and its support for premium reimbursement Shared our compelling prospective, blinded clinical verification data from 400 enrolled subjects for our Preecludia™ LDT rule-out test achieving high sensitivity of 88% and 98.2% NPV* with a rule out window of up to 14 days Provided support for the clinical utility of a rule-out test and its place in the clinical management care path to help improve standard of care Demonstrated our ability to advance our program and our efforts to generate strong adoption rates within our women's health channel towards a $3B US market * NPV calculated using a 10% prevalence rate

THANK YOU



































































