Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future, "likely," "may," "should," "will" and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our business plans and objectives, timing and success of our clinical trials for LOXO-292, our ability to obtain regulatory approval or the timing of regulatory filings, the potential therapeutic benefits of LOXO-292, potential growth opportunities, the timing or success of commercialization, financing plans, competitive position and industry environment. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: those related to our future financial performance, our ability to raise additional funding when needed, our ability to develop and maintain partnerships, our ability to identify and develop new products in a timely manner, the outcome, cost and timing of our product development activities and clinical trials, market size and acceptance of our targeted small molecule therapeutics and diagnostics, our ability to maintain, protect and enhance our brand and intellectual property, our ability to continue to stay in compliance with applicable laws and regulations, our ability to scale our business and make key hires and such other factors as discussed under the section titled “Risk Factors” and elsewhere in our annual report on Form 10-K and quarterly reports on Form 10-Q that we filed with the Securities and Exchange Commission (“SEC”) as well as our other filings and the documents incorporated by reference therein, with the SEC. Any forward-looking statement made by us in this presentation and the accompanying oral presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Certain information in this slide deck on LOXO-292 is derived from the presentation made at the 2018 ASCO Annual Meeting by Dr. Drilon, an independent third-party investigator. 2

Agenda • Review of LOXO-292 data presented at ASCO • LOXO-292 next steps 3

LIBRETT0-001: A phase 1study of LOX0-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers Alexander Drilon,1Vivek Subbiah? Geoffrey R. Oxnard? Todd M. Bauer/1 Vamsidhar Velcheti,5 Nehal J. Lakhani,6 Benjamin Besse/ Keunchil Park,8 Jyoti D. Patel,9 Maria E. Cabanillas,2 Melissa L. Johnson/1 Karen L. Reckamp,10 Va lentina Boni,11 Herbert H. F. LoongP Martin Schlumberger/ Ben Solomon,B Scott Cruickshank,14 S. Michael Rothenberg, 14 Manisha H. Shah,15 and Lori J. Wirth16 lMemorial Sloan Kettering Cancer Center, New York, NY; 2MD Anderson Cancer Center, Houston, TX; Joana-Farber Cancer Institute, Boston, MA; 4Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN; 5Cieveland Clinic Taussig Cancer Institute,Cleveland, OH; 6START Midwest,Grand Rapids, Ml; 7/nstitut Gustave RoussVillejuif, France; 8Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea;5University of Chicago, Chicago, IL; 1°City of Hope Comprehensive Cancer Center, Duarte, CA; 11START Madrid C/OCC Hospital Universitario Sanchinarro, Madrid, Spain; '1The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong; •Peter MacCallum Cancer Centre, East Melbourne, Australia; 14Loxo OncologStamford, CT; 15The Ohio State University Comprehensive Cancer Center, Columbus, OH; 16Massachusetts General Hospital Cancer Center, Boston, MA 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 4 ONCOLOGY
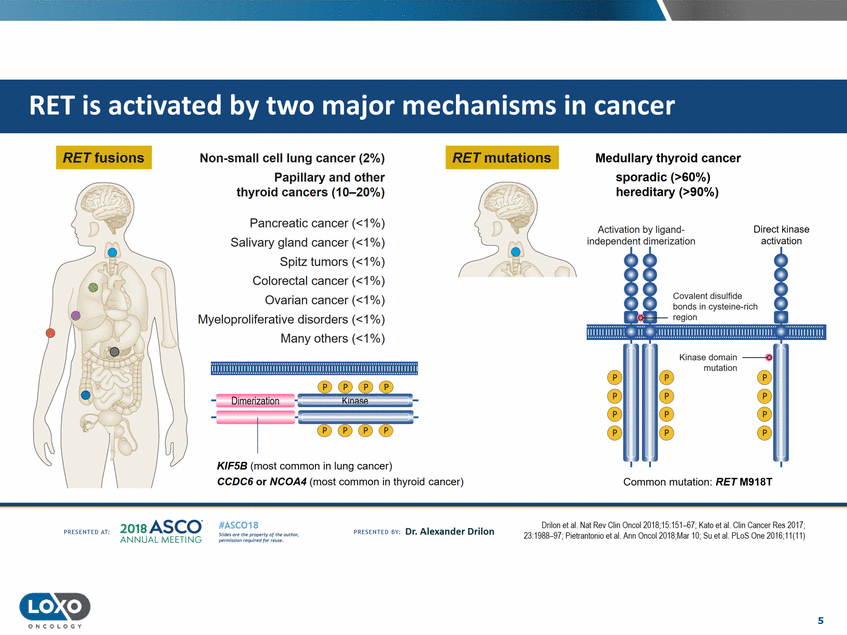
RET fusions RET mutations Non-small cell lung cancer (2%) Papillary and other thyroid cancers (10-20%) Medullary thyroid cancer sporadic (>60%) hereditary (>90%) Pancreatic cancer (<1%) Salivary gland cancer (<1%) Spitz tumors (<1%) Colorectal cancer (<1%) Ovarian cancer (<1%) Myeloproliferative disorders (<1%) Many others (<1%) Activation by ligand independent dimerization Direct kinase activation Covalent disulfide mutation ). ®®®® ----1----5 5 Dimerization··maPK.s 5t,_-® ®®® KIF5B (most common in lung cancer) CCDC6 or NCOA4 (most common in thyroid cancer) Common mutation: RET M918T 2o1a Asco· ANNUA L MEET NG Drilon et al. Nat Rev Chn Onea0l18;1o:1o1-6f ; Kato et al. Clin Cancer ResO tf ; 23:1988-97;Pietrantonio etaL Ann Oncol20 IB;Mar 10;Suet aL PLoS One 2016;11{"11) #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 5 O N COLOGY ................................................................................................ 1.!!.!!.1.!!.1.!.1.1).1. .!!!!.!.!1.!!.1.1).!.!1.!1.1!. 1.1.11.11 .1 11!111!1!.1 1 )1..1 11).1.1 ).1 1.1.!. 1.!.1 two major mechanisms in cancer by RET is activated
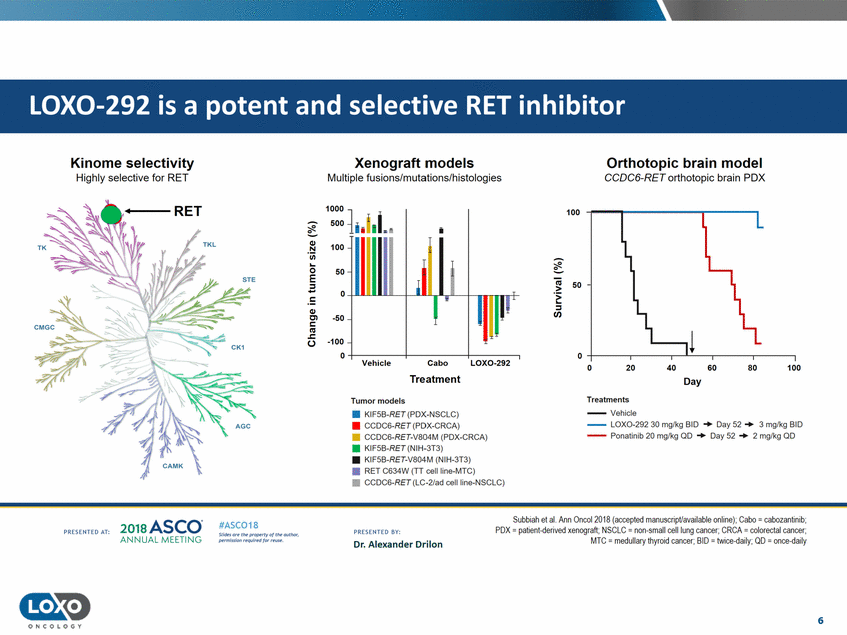
Kinome selectivity Highly selective for RET Xenograft models Multiple fusions/mutations/histologies Orthotopic brain model CCDC6-RET orthotopic brain PDX 100 50 - · ::s Ill ..... ,t ---.------.-----. 0 ,_-----.------.-Cabo Treatment LOX0-292 0 40 80 100 20 60 Day Treatments --Vehicle --LOX0-292 30 mg/kg BID + Day 52 + 3 mglkg BID --Ponatinib 20 mg/kg QD + Day 52 + 2 mglkg QD Tumor models • KIF5B-RET (PDX-NSCLC) • CCOC6-RET(PDX-CRCA) • CCDC6-RET-V804M (PDX-CRCA) • KIF5B-RET (NIH-3T3) • KIF!iB-RET-V804M (NIH-3T3) • RET C634W (TT cell line-MTC) • CCDC6-RET (LC-2/ad cellline-NSCLC) Sullbiah et al. Ann Onool 2018 (aooepted manuscriptlw,ailable online), Cabo = cabozarrtinib, POX = patienl-{!erived xenograft; NSCLC = non-small cell lung cancer; CRCA = colorectal cancer; MTC = medullary thyroid cancer;BID = IVJice.(Jaily; OD = or ee-<laily 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor• tN;tt11Ht'f'l t1f rMout :«. PR CSOHEO AT: PRESOHCO 6Y· Dr. Alexander Drilon Pt'.T.Iis!iol't r QH'f'N {(X . 6 ONCOLOGY LOX0-292 is a potent and selective RET inhibitor
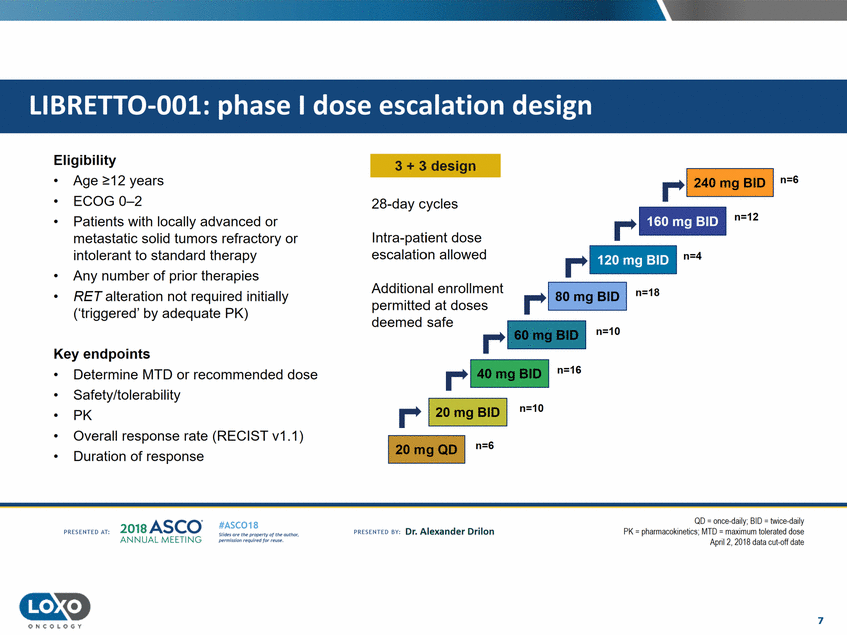
Eligibility 3 + 3 design r-1 240 mg BID I n=S • • • Age 2::12 years ECOG 0-2 Patients with locally advanced or metastatic solid tumors refractory or intolerant to standard therapy Any number of prior therapies RET alteration not required initially ('triggered' by adequate PK) 28-day cycles r n=12 Intra-patient dose escalation allowed r n=4 • • Additional enrollment permitted at doses deemed safe n=18 n=10 Key endpoints n=16 • • • • • Determine MTD or recommended dose Safety/tolerability PK Overall response rate (RECIST v1.1) Duration of response r-1 20 mg BID I n=10 1 20mg QD I n=G QD - once daily; BID - twice daily PK = pll<llmacokineti<.:s.MTD = maximumllle1utelJ dose April 2, 2018 data cut-off date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 7 ONCOLOGY 120 mg BID 160 mg BID LIBRETT0-001: phase I dose escalation design
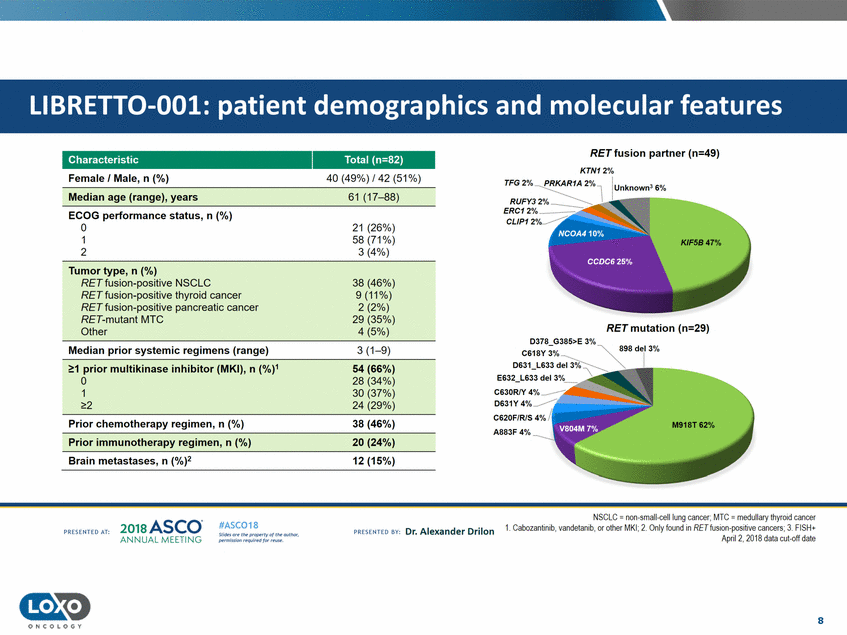
features RET fusion partner (n=49) KTN12% Characteristic Female I Male, n (%) Total (n=82) 40 (49%) /42 (51%) Median age (range),years 61 (17-88) ECOG performance status, n (%) 0 1 2 CLIP1 21 (26%) 58 (71%) 3 (4%) Tumor type,n ( %) RETfusion-positive NSCLC RET fusion-positive thyroid cancer RET fusion-positive pancreatic cancer RET-mutant MTC Other 38 (46%) 9(11%) 2 (2%) 29 (35%) 4 (5%) RET mutation (n=29) Median prior systemic regimens (range) 3 (1-9) 1 prior multikinase in1hibitor (MKI),n (5%4)(66%) 0 1 28 (34%) 30 (37%) 24 (29%) C630RIY 2 Prior chemotherapy regimen, n (%) 38 (46%) Prior immunotherapy regimen,n (%) 20 (24%) Brain metastases,n (%)2 12 (15%) NSCLC = non-small-celllung cancer;MTC =medullary thyroid cancer 1. Cabozantinib,vandetanib, or oij1er MKI; 2. Only foundin RET fusion-positive cancers;3. FISH+ Apnl 2, 2018 dala cul-off dale 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 8 ONCOLOGY LIBRETT0-001: patient demographics and molecular
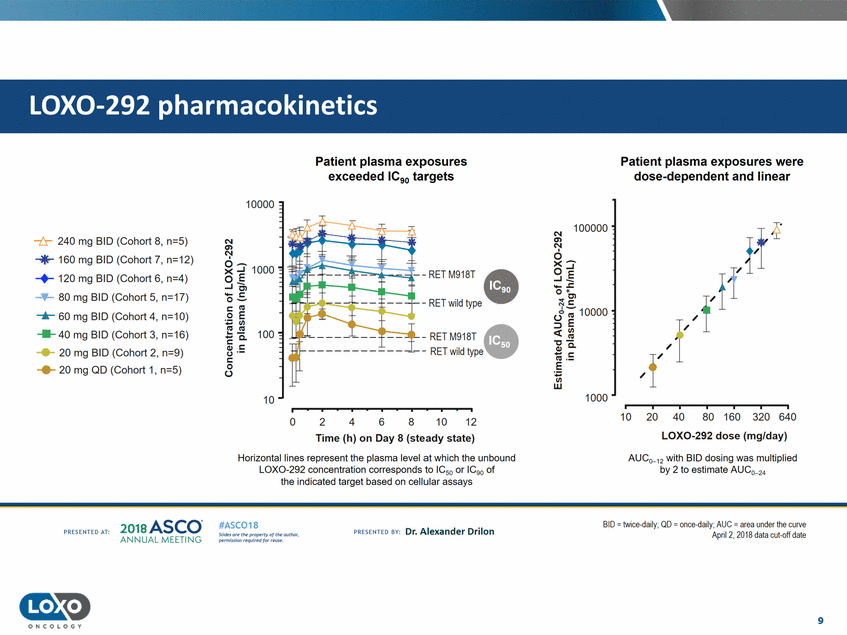
pharmacokinetics Patient plasma exposures exceeded IC90 targets Patient plasma exposures were dose-dependent and linear 10000 N 100000 -t:r-240 mg BID (Cohort 8, n=5) en Ns-'"'*" 160 mg BID (Cohort 7, n=12) -+-120 mg BID (Cohort 6, n=4) -+ 80 mg BID (Cohort 5, n=17) ....... 60 mg BID (Cohort 4, n=10) .....40 mg BID (Cohort 3, n=16) ...... 20 mg BID (Cohort 2, n=9) ..... 20 mg QD (Cohort 1, n=5) o... ...J.E ..,_.s:; 0 .. ... Cl J-t:; 10000 :::> E c:( - RET M918T - RET wild type -c QJ ' .... t : "'·-E ; Cl) w 1000 10 10 20 40 80 160 320 640 2 4 10 12 0 6 8 LOX0-292 dose (mg/day) Time (h) on Day 8 (steady state) Horizontal lines represent the plasma level at which the unbound LOX0-292 concentration corresponds to IC50 or IC90 of the indicated target based on cellular assays AUC0_12 with BID dosing was multiplied by 2 to estimate AUC0_2• 201a ASCO. ANNUA L MEET NG BID - twice daily; QD - once daily;AUC - area under the curve Apri1 2, 2018 data cul-ofl dale #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 9 ONCOLOGY LOX0-292
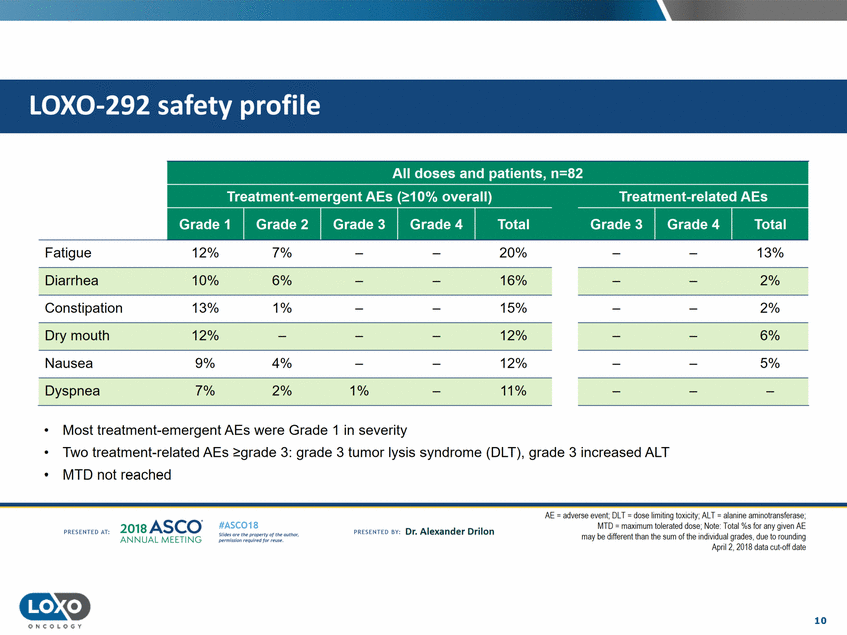
profile 12% 7% 20% 13% Fatigue Diarrhea 10% 13% 6% 1% 16% 15% 2% 2% Constipation Dry mouth 12% 9% 12% 12% 6% 4% 2% 5% Nausea Dyspnea 7% 1% 11% • • • Most treatment-emergent AEs were Grade 1 in severity Two treatment-related AEsgrade 3: grade 3 tumor lysis syndrome (DLT), grade 3 increased ALT MTD not reached AE = adverse event; DLT = dose limiting toxicity;AlT = alanine aminotransferase; MTD - maxtmum tolerated dose;Note· Total%s for any given AE may be different than the sum of the indtvidual grades,due to rounding Apnl 2,2018 data cut-off date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 10 ONCOLOGY LOX0-292 safety
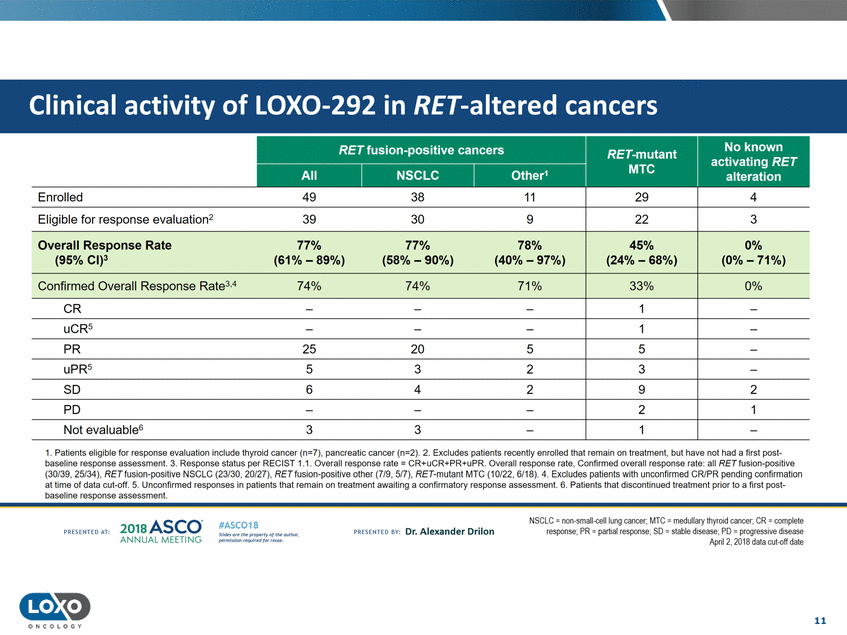
RET fusion-positive cancers 1.Patients eligible for response evaluation include thyroid cancer (n=7), pancreatic cancer (n=2).2.Excludes patients recently enrolled that remain on treatment, but have not had a first post baseline response assessment. 3. Response status per RECIST 1.1. Overall response rate = CR+uCR+PR+uPR. Overall response rate,Confirmed overall response rate: all RET fusion-positive (30f39, 25/34), RETfusion-positive NSCLC (23/30, 20/27),RETfusion-positive other (7f9, 5f7), RET-mutant MTC (10/22, 6/18).4.Excludes patients with unconfirmed CR/PR pending confirmation at time of data cut-off.5.Unconfirmed responses in patients that remain on treatment awaiting a confirmatory response assessment. 6. Pa tients that discontinued treatment prior to a first post baseline response assessment. 201a ASCO. ANNUAL MEET NG NSCLC = non-small-celllung cancer; MTC =medullary thyrcid cancer:CR = ccmplete response;PR = partialresponse;SD = stable disease: PD = progressive disease Apnl 2, 2018 data cut-oft date #ASC018 Uf.:•s or• tN ;tt11Ht'f'l t1f rM out :«. PR Escrnco sv· Dr. Alexander Drilon PRCSCIHEO AT: Pt'.T.Iis!iol't r QH' f'N {(X . 11 O N COLOGY Enrolled 49 38 11 Eligible for response evaluation2 39 30 9 Overall Response Rate 77% 77% 78% (95% Cl)3 (61% - 89%) (58% -90%)(40% -97%) Confirmed Overall Response Rate3.474% 74% 71% 29 22 45% (24% - 68%) 33% 4 3 0% (0% -71%) 0% CR PR 2520 5 5 uPR5 53 2 3 so 6 4 2 9 2 PO 2 1 Not evaluable63 3 Clinical activity of LOX0-292 in RET-altered cancers
RET fusion-positive cancers 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 12 ONCOLOGY

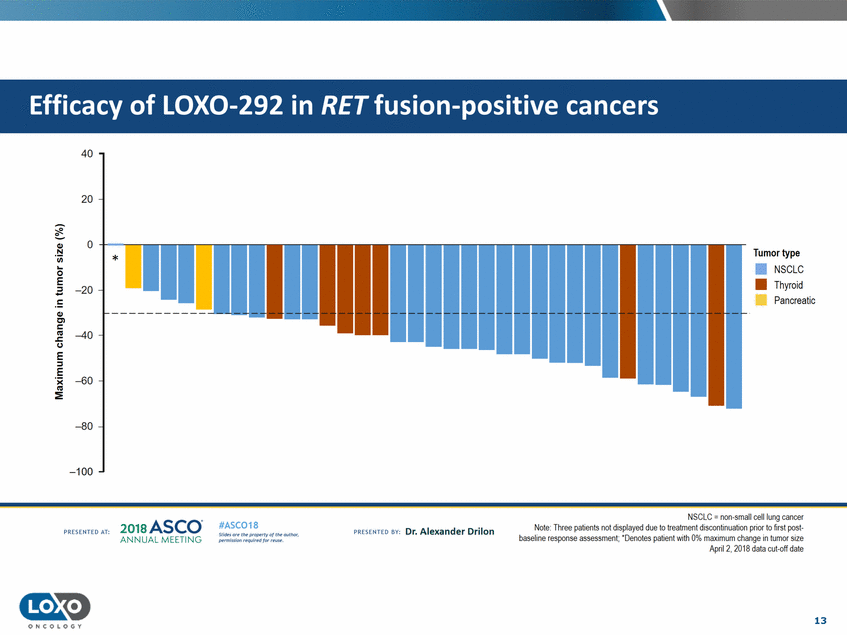
cancers 40 20 0 Q,) Tumor type N 'i.i.i. * • NSCLC •Thyroid 0 -::J E -20 Pancreatic !: Q,) C'l c liS .J::. C.) -40 E ::J -E :E -60 -80 -100 NSCLC = non-small cell lung cancer Note· Three patients not displayed dtJe to treatment discontni uation prior to first pest baseline response assessment. 'Denotes patient with 0% maximum change in tumor size Apn12, 2018 data cut-oft date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't rQH'f'N {(X. PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 13 ONCOLOGY Efficacy of LOX0-292 in RET fusion-positive
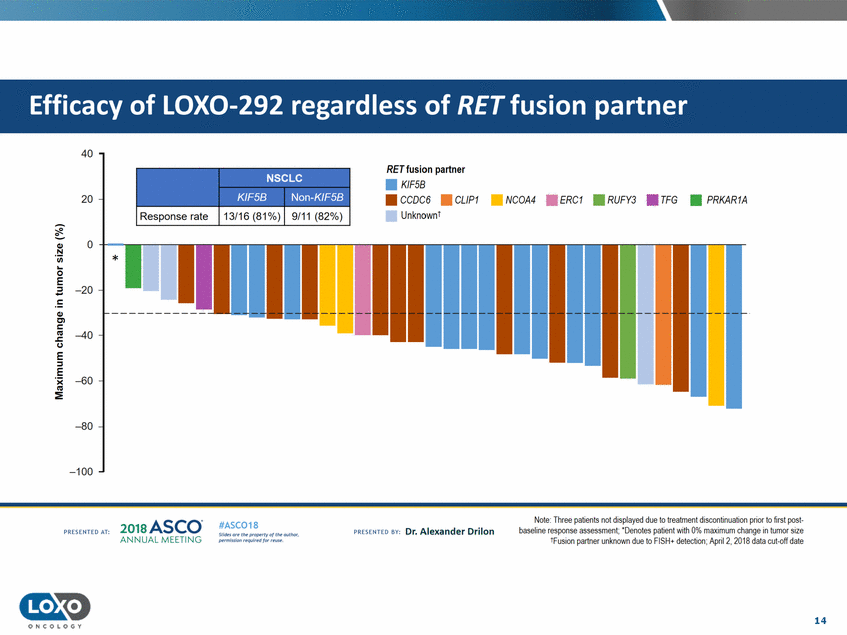
partner 40 RETfusion partner • KIF5B 20 • CCOC6 • UnknownI CL/P1 • NCOA4 • ERC1 • RUFY3 • TFG •PRKAR1A Response rate 0 Q,) N 'i.i.i. 0 E -20 Q,) C'l c C.) -40 E ::;, E · :E -60 -80 -100 Note: Ihree pat1ents not d1splayed due to treatment d1scont1nuat1on pnor to llrst post· baseline response assessment; ' Denotes patient Vlith 0% maximum change in tumor size 1FIJSIOn partner unknown due to FISH+ detection; Apri12, 201A data cut-off date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 14 ONCOLOGY --Non-Kir!:>B KU:SB NSCLC Efficacy of LOX0-292 regardless of RET fusion
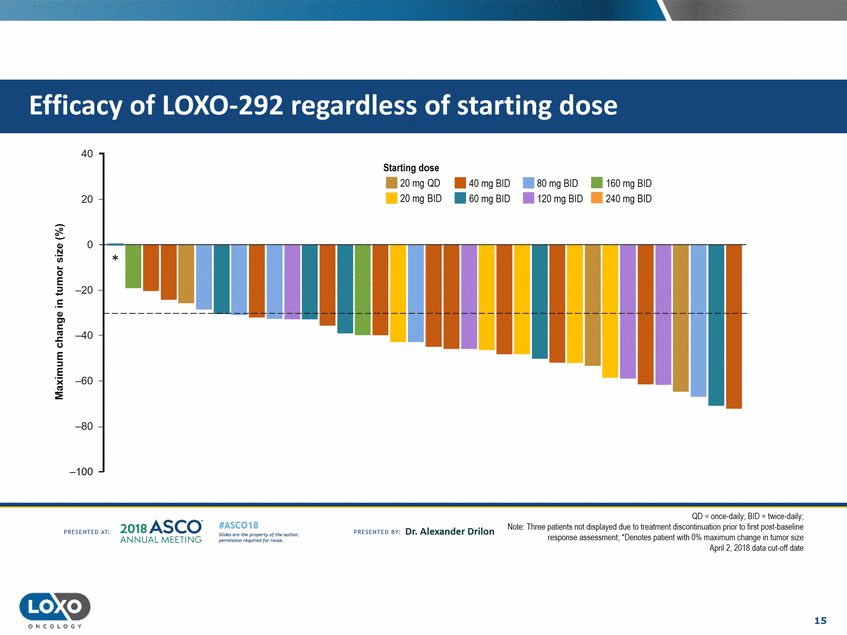
dose 40 Starting dose . . 20mgQD 20mg BID • 40 mg BID • • 80 mg BID 120 mg BID • 160 mg BID • 240 mg BID 20 • 60 mg BID 0 -+-..---Q,) N 'i.i.i. * 0 E -20 Q,) C'l c C.) -40 E ::;, E - -60 :E -80 -100 QD = once-daily;BID = twice-daily; Note: Three patients not displayed due to treatment discontinuabon prior to rst post-baseline response assessment; ' Denotes patient Vlith 0% maximum change in tumor stze April?, ?01A rlata cut-off rlatf! 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor• tN;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH' f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 15 ONCOLOGY Efficacy of LOX0-292 regardless of starting
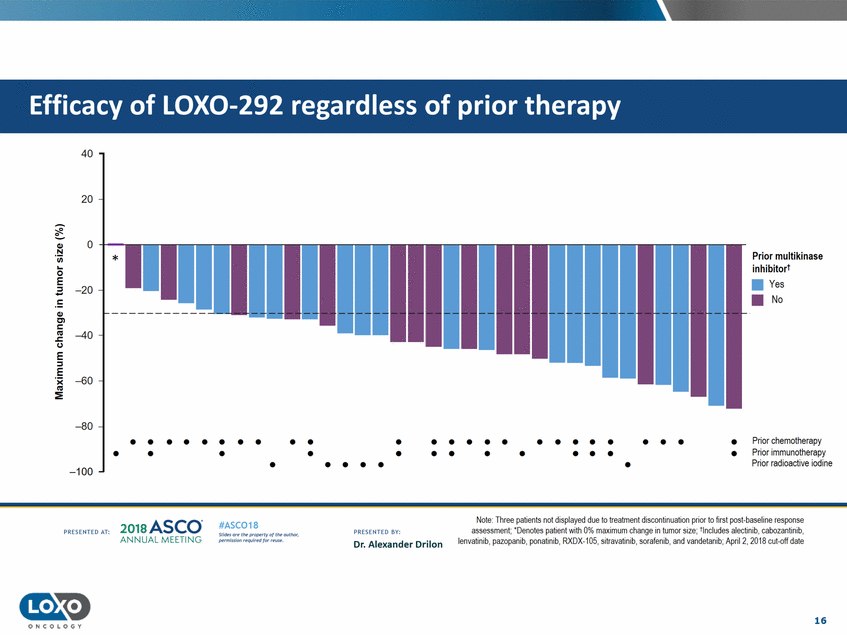
therapy 40 20 0 Q,) N Prior multikinase 'i.i.i. inhibitort •Yes 0 -::J E • -20 No !: Q,) C'l c liS .J::. C.) -40 E ::J -E :E -60 -80 • • • • • • • • • • • • • • • • • • • • • • • • • Prior chemotherapy Prior immunotherapy Prior radioactive iodine • • • • • • • • • • • • • • • • • • • -100 Note:Ihree pat1ents not d1splayed due to treatment d1soonbnuabon pnor to hrst post-baseline response assessment;·Denotes patient with0% maximum change in tumor size;tlncludes alectinib, cabozantinib, lenvatinib. pazopanib,ponatinib,RXDX-105,sitravat nib,sorafenib. and vandetanib;April 2, 2018 cut-off date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't rQH'f'N {(X. PRCSOHEO AT: PRESOHCO 6Y· Dr. Alexander Drilon 16 O N C O LOGY Efficacy of LOX0-292 regardless of prior
RET-mutant medullary thyroid cancer PRCSOHEO AT: 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon 17 ONCOLOGY
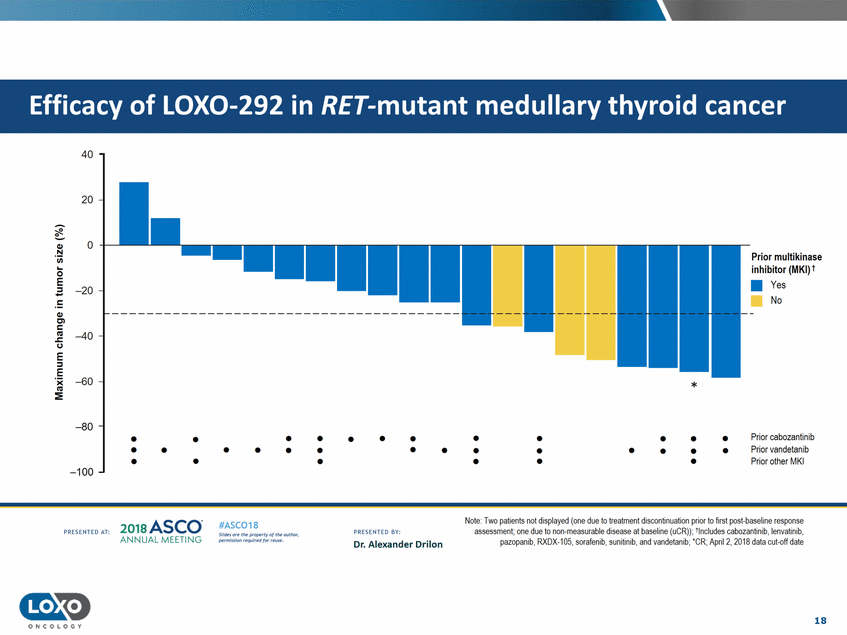
cancer 40 20 0 Ql .!::! Prior multikinase inhibitor (MKI) t • Yes No C..l.l 0 =E -20 c Ql Cl c u E ::I -40 E 'i( C'G :::!E -60 * -80 • • • • • • • • • • • • • Prior cabozantinib Prior vandetanib Prior other MKI • • • • • • • • • • • • • • • • • • • -100 Note· Two pa!ients not displayed (one due to treatment discontinuation pnor to first post-baseline response assessment;one due to non-measurable disease at baseline (uCR}i; -lnclucles cabozanlinib, lenvatinib, pazopamb,f{)(UX-105,sorafen1b, sumhmb,and vandetamb;·cl<; Apnl2,2018 data cut-oft date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PRCSOHEO AT: PRESOHCO 6Y· Dr. Alexander Drilon 18 O N COLOGY Efficacy of LOX0-292 in RET-mutant medullary thyroid
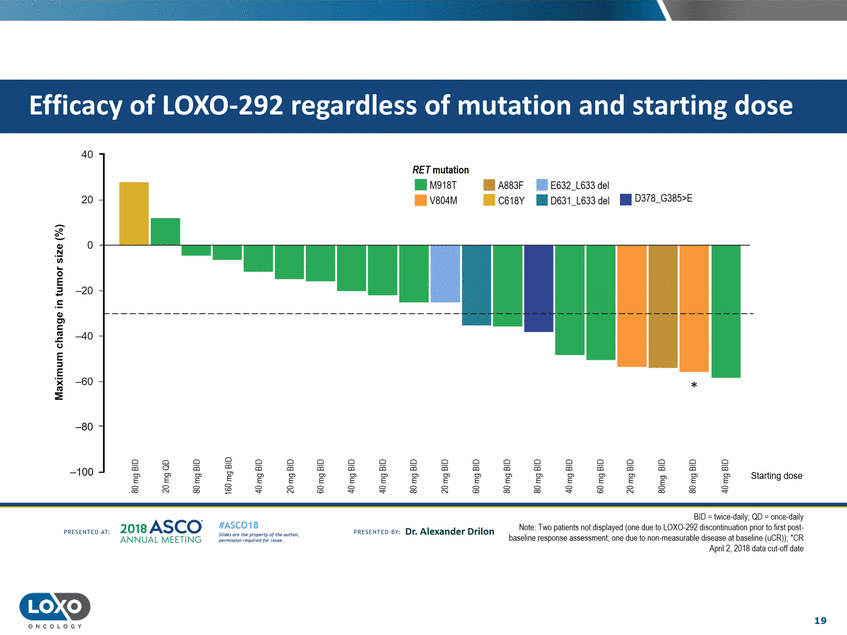
dose 40 RET mutation . . M918T V804M • A883F • C618Y • E632_L633 del 20 • 0631_L633 del • D378_G385>E 0 +-__L_ --' Ql .!::! C..l.l 0 =E -20 Ql Cl s::: u E ::I -40 E 'i( C'G :::!E -60 * -80 Cl a5 Cl 0 0 "E'"' Cl Cl Cl iii E' Cl Cl iii E' Cl Cl Cl iii E' Cl Cl Cl Cl Cl Cl Cl iii Cl iii Cl iii a:; a:; a:; E' a:; e a:; a:; a:; a:; iii iii iii iii -100 e' e' e e e "E" e' e' e' Starting dose E' E' E' 0: gE g g g g g g 0 g g 0 <'< BID = twice-daily; QD = once-daily Note TVlo patients not displayed (one due to LOX0-292 discontinuation prior to first post baseline response assessment;one due to non measurable disease at baseline (uCR)); ·cR April2, 2018 data t:ul-Q[[dalr,! 201a ASCO. ANNUA L MEET NG #ASC018 Uf.:•s or• tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH' f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 19 ONCOLOGY Efficacy of LOX0-292 regardless of mutation and starting
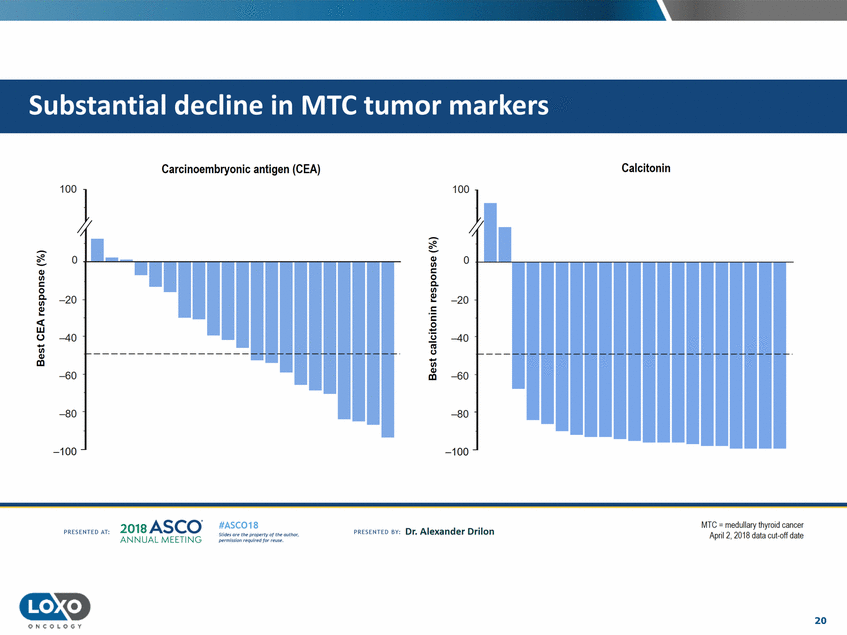
Calcitonin Carcinoembryonic antigen (CEA) 'n I ..-. ..-. 0 0 Q/ 1/1 c: Q/ 0 Q. 1/1 c: 0 Q. Q../. 1/1 -20 -20 Q../. 1/1 c: - ..... ..... c: <w 0 0-'i3 -40 -40 iii -c.J ------------------1/1 Q/ cc 1/1 Q/ -60 -60 Ill -80 -80 '--..._._ .... ...... -100 -100 201a ASCO. ANNUA L MEET NG MIc.; = medullary thyror d cancer April2, 2018 data cut-off date #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 20 ONCOLOGY r-1""""1 1-----r- - ·-r- - · - - ·-r- - ·-... - ·-r- - ·-r- ..... . .......... ...... ............... uuu ·-i ....... · ...... - ..... ·-E- - --,...._ . ........... ............. ..... ................... tumor markers Substantial decline in MTC
therapy V..I. Ql (,) c (,) • NSCLC • Thyroid • Pancreatic • MTC 0 First response • Treatment after progression ·.w 'iii 0a. c. 0 'iii 44/49 (90%) of patients with RET fusion-positive cancers remain on therapy .:.:.:.! 1-...... Still on treatment () :-E 1--c :::! .E.:. 27/29 (93%) of patients with RET-mutant medullary thyroid cancer remain on therapy aLU: 0 2 3 4 5 6 7 8 9 10 11 Time on treatment (months) SCLC - non small cell lung cancer; MTC = mduii<Jr y UryroillCatl(;er April2. 2018 data cut-<>ff date 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•s or• tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH' f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 21 ONCOLOGY Duration of LOX0-292

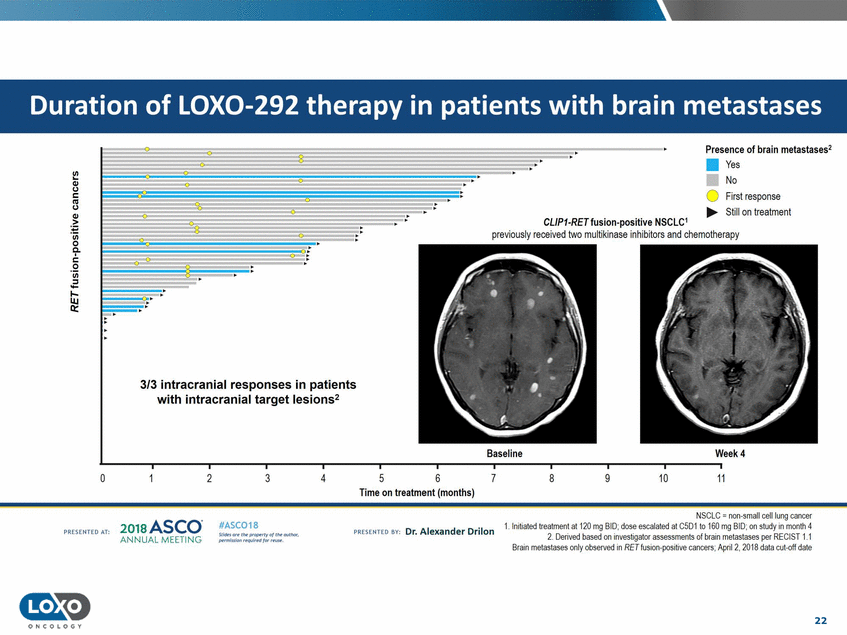
metastases .· . Presence of brain metastases2 • Yes No 0 First response ..... Still on treatment .· . .. CL/P1-RETfusion-positive NSCLC1 previously received two multikinase inhibitors and chemotherapy .: patients lesions 2 Baseline Week4 0 2 3 5 6 7 8 9 10 11 4 Time on treatment (months) NSCLC = non.small cell lung canoer 1.1nitiated tre< tment at 120 mg BID;dose escalated at C5D1 to 160 mg BID;on study in month 4 2.Denved based on 1nvesbgator assessments of bram metastases per KI::CISI1.1 Grain metastases only observed in RET fusion-positive canoers; Aprli 2. 2018 data cut-of date 201s ASCO. ANNUAL MEET NG IASC018 Uf141tN';.r(1!fWI't!f 111 rM outr.or, Pt'tm ioN'4U 1 frxr r.. . vn Esonco sv· Dr. Al exander Drilon PR[S[IH(O AT: 22 ONCO L OG Y 0 . .. 3/3 intracranial responses in with intracranial target Duration of LOX0-292 therapy in patients with brain
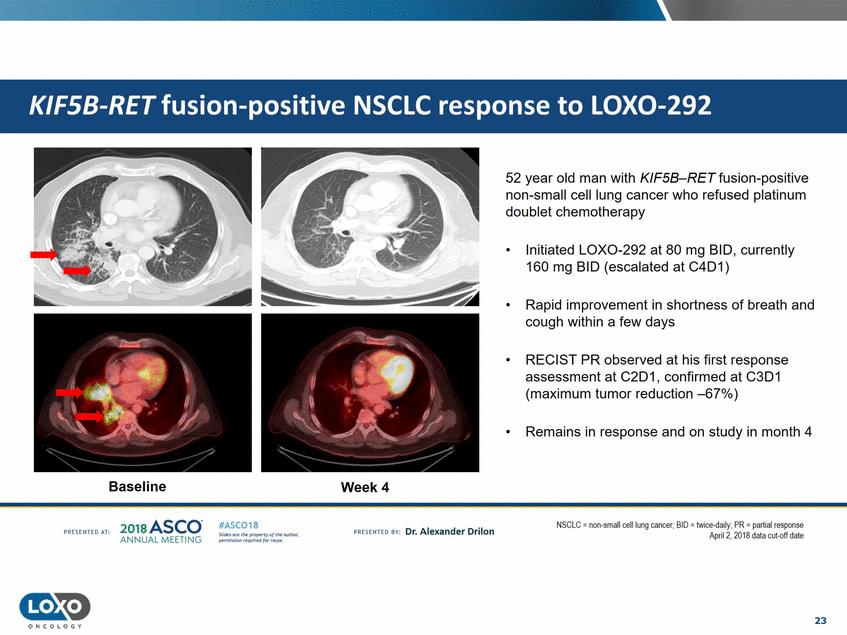
52 year old man with KIF58-RET fusion-positive non-small cell lung cancer who refused platinum doublet chemotherapy • Initiated LOX0-292 at 80 mg BID, currently 160 mg BID (escalated at C4D1) • Rapid improvement in shortness of breath and cough within a few days • RECIST PR observed at his first response assessment at C2D1, confirmed at C3D1 (maximum tumor reduction -67%) • Remains in response and on study in month 4 Baseline Week4 201s ASCO. ANNUAL MEET NG N:iGLC = non-smallcell lung cancer; 81U = t.w ee-da1ly;I'K = part1al response April2. 2018 data cut-cff date IASC018 Uf141tN';.r(1!fWI't!f 111 rM outr.or, Pt'tm ioN'4U 1 frxr r.. . vn Esonco sv· Dr. Al exander Drilon PR[S(IH(O AT: 23 O N COLOGY KIFSB-RET fusion-positive NSCLC response to LOX0-292
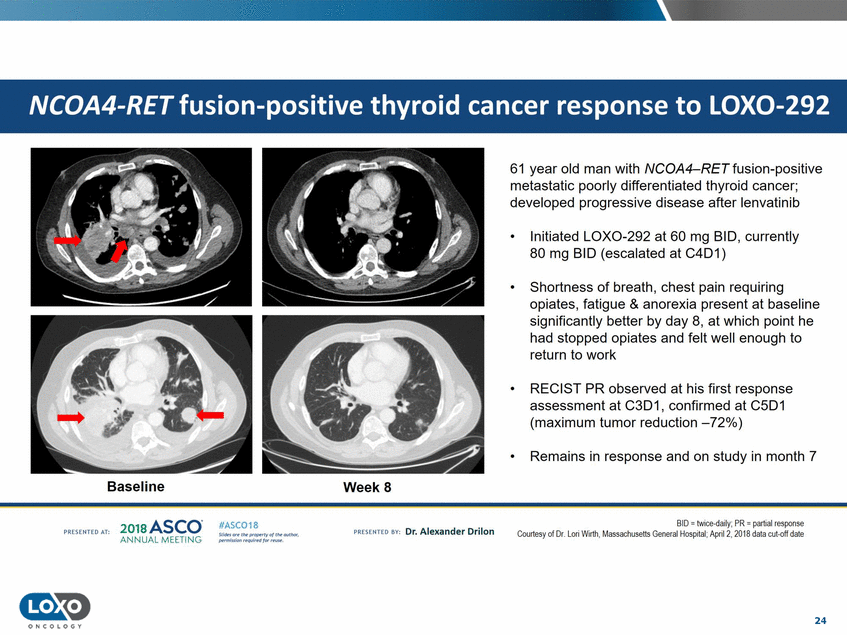
61 year old man with NCOA4-RET fusion-positive metastatic poorly differentiated thyroid cancer; developed progressive disease after lenvatinib Initiated LOX0-292 at 60 mg BID, currently 80 mg BID (escalated at C4D1) • • Shortness of breath, chest pain requiring opiates, fatigue & anorexia present at baseline significantly better by day 8, at which point he had stopped opiates and felt well enough to return to work • RECIST PR observed at his first response assessment at C3D1, confirmed at C5D1 (maximum tumor reduction -72%) • Remains in response and on study in month 7 Baseline WeekS 201s ASCO. ANNUAL MEET NG BDI = tw1oe-daily;PR = part1alresponse Courtesy of Dr. Lori Wirth, Massachusetts General llospital. April 2,2018 data cut-off date IASC018 Uf141tN';.r(1!fWI't!f 111 rM outr.or, Pt'tm ioN'4U 1 frxr r.. . vn Esonco sv· Dr. Al exander Drilon PR[S(IH(O AT: 24 O N COLOGY NCOA4-RET fusion-positive thyroid cancer response to LOX0-292
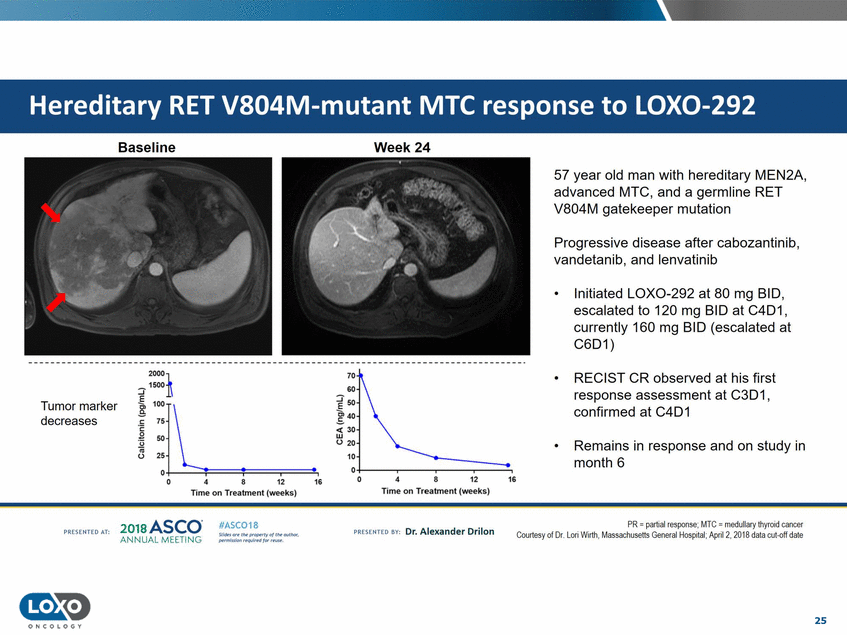
LOX0-292 Baseline Week 24 57 year old man with hereditary MEN2A, advanced MTC, and a germline RET V804M gatekeeper mutation Progressive disease after cabozantinib, vandetanib, and lenvatinib • Initiated LOX0-292 at 80 mg BID, escalated to 120 mg BID at C4D1, currently 160 mg BID (escalated at C6D1) 2000]. ::::;-15001\ 70 • RECIST CR observed at his first response assessment at C3D1, confirmed at C4D1 GO ?50 Tumor marker 40 !c: "75' \_ .s decreases 30 w (.) 20 10 50 25 0 • Remains in response and on study in month 6 0 0+--------- ----. • 0 4 8 12 16 12 D 4 8 16 Time on Treatment (weeks) Time on Treatment (weeks) 201s ASCO. ANNUAL MEET NG IASC018 Uf141tN';.r(1!fWI't!f 111 rM outr.or, Pt'tm ioN'4U 1 frxr r.. . PR - partial response;MTC - me<lullary thyro·d cancer Courtesy of Dr . L01i Wuth.Ma S<Jchuse ls Generul Hospr till,April2, 2018 dala cut-off d<rle vn Esonco sv· Dr. Al exander Drilon PA [S[IH[O AT: 25 O N COLOGY Hereditary RET V804M-mutant MTC response to
• LOX0-292 demonstrates robust anti-tumor activity across RET-altered cancers - - - 77% overall response rate in RETfusion-positive cancers, with intracranial activity 45% overall response rate in RET-mutant medullary thyroid cancer 71/78 (91%) of RET-altered patients remain on therapy, including all responding patients • Activity independent of RET fusion partner, RET mutation, or prior therapy - Heavily pretreated phase 1 population (66% of patients with prior multikinase inhibitor exposure) - Responses observed with RET V804M gatekeeper (causes resistance to multikinase inhibitors) • Safety and tolerability profile consistent with highly selective drug design - No evidence of off-target liabilities - Maximum tolerated dose not reached • LIBRETT0-001 expansion cohorts are open and enrolling, for patients with RET fusion positive solid tumors, medullary thyroid cancer, and other cancers with RET activation 2o1a Asco· ANNUA L MEET NG #ASC018 Uf.:•sor•tN ;tt11Ht'f'l t1f rM out :«. Pt'.T.Iis!iol't r QH'f'N {(X . PREscrnco sv· Dr. Alexander Drilon PRCSOHEO AT: 26 ONCOLOGY Conclusions

LOXO-292 Next Steps LIBRETTO-001 • – – Expansion cohorts currently enrolling at 160 mg BID Continuing to explore higher doses in parallel • Regulatory – Will engage with global regulators to determine paths and timelines approvals Future updates will be based on written meeting minutes to – Diagnostics • – – Enrolling based on local testing Working with Illumina to clinically validate TST170 as companion diagnostic • Planning to present updated clinical data in 2H 2018 27
Q&A 28