Exhibit 99.1
| Theravance Biopharma, Inc. (NASDAQ: TBPH) Rick E Winningham Chief Executive Officer Bank of America Merrill Lynch 2015 Health Care Conference |
| Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, and the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products and risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-K filed with the Securities and Exchange Commission (SEC) on March 13, 2015 and other periodic reports filed with the SEC. |
| Theravance Biopharma Today (NASDAQ: TBPH) Theravance Biopharma was created to drive value from a unique and diverse set of assets An approved product A pipeline of high-value assets A productive research platform |
| The Theravance Biopharma Strategy Payors Partners Acute Care Leverage Our Insights to Make a Difference for Patients and Create Meaningful Value for Shareholders Insight and Innovation Patient Provider Payor |
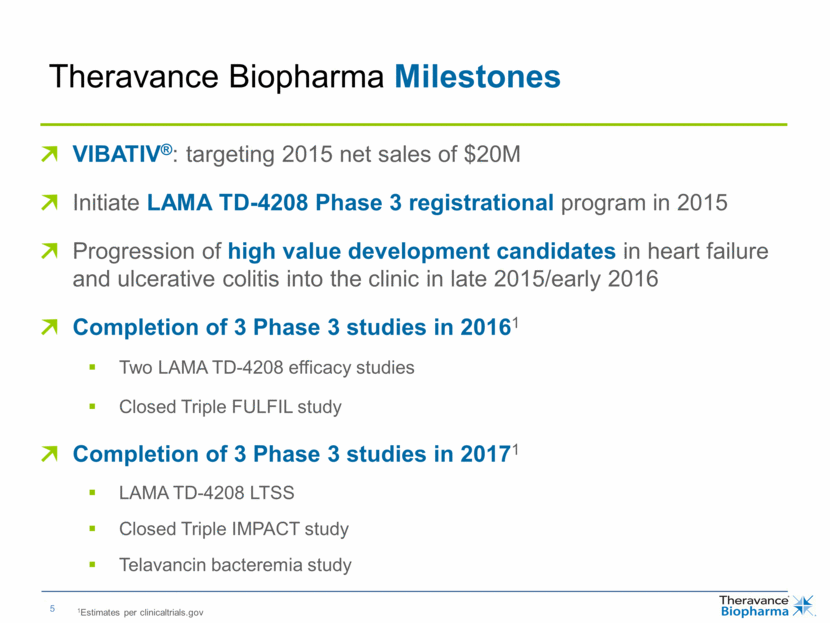
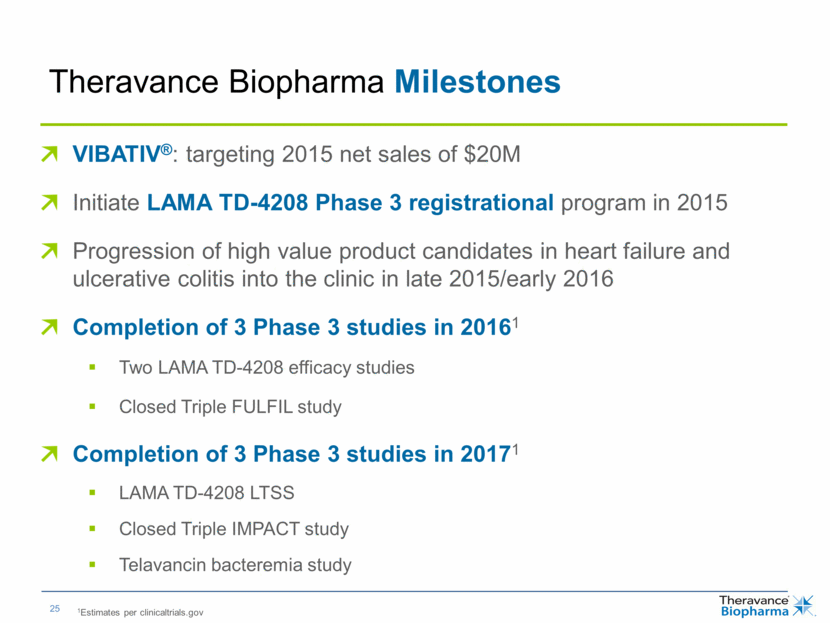
| Theravance Biopharma Milestones VIBATIV®: targeting 2015 net sales of $20M Initiate LAMA TD-4208 Phase 3 registrational program in 2015 Progression of high value development candidates in heart failure and ulcerative colitis into the clinic in late 2015/early 2016 Completion of 3 Phase 3 studies in 20161 Two LAMA TD-4208 efficacy studies Closed Triple FULFIL study Completion of 3 Phase 3 studies in 20171 LAMA TD-4208 LTSS Closed Triple IMPACT study Telavancin bacteremia study 1Estimates per clinicaltrials.gov |
| VIBATIV® (telavancin) |
| What is VIBATIV®? First FDA approved lipoglycopeptide exhibiting concentration-dependent bactericidal activity via a dual mechanism of action that inhibits cell wall synthesis and disrupts membrane barrier function Active against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) Intravenously administered; once-daily dosing VIBATIV® (telavancin) prescribing information Approved in the U.S. for treatment of the following infections in adult patients caused by designated susceptible bacteria: Complicated skin and skin structure infections (cSSSI) Hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible isolates of Staphylococcus aureus when alternative treatments are not suitable |
| VIBATIV®: Focus for 2015 Targeting 2015 net sales of approximately $20 million Increasing sales force to 50 reps in targeted U.S. territories Regional partners outside the U.S. contribute cash plus insight to drive commercial success Establishing VIBATIV in the market as a differentiated product In vitro potency as great or greater than any other approved Gram+ antibiotic Aiming for broadest set of indications among branded anti MRSA agents Generating additional efficacy data in patients Phase 3 registrational bacteremia study in ~250 patients Patient registry study (TOUR) in ~1,000 patients |
| Why Physicians Choose VIBATIV® In Vitro Activity Dual Mechanism of Action; Bactericidal against clinically important Gram+ organisms Active against S. aureus strains with reduced susceptibility to other agents VAN MIC > 1 µg/mL VISA, hVISA strains Daptomycin and linezolid-resistant No resistance detected in bacterial strains in Phase 2 and 3 cSSSI and HABP/VABP clinical programs; resistance rarely reported during marketed use. Clinically Relevant Penetration into important sites of infection, including the lung Drug levels remain above the MIC90 for MRSA over 24 hours Clinical efficacy shown in largest HABP/VABP studies to date in a broad population of patients with multiple co-morbidities Convenience of once daily dosing (with no required therapeutic dose monitoring) Safety profile characterized in large clinical studies in both cSSSI and HABP/VABP Louis D. Saravolatz, MD, MACP; St. John Hospital, Detroit, MI; Infectious Disease Specialist |
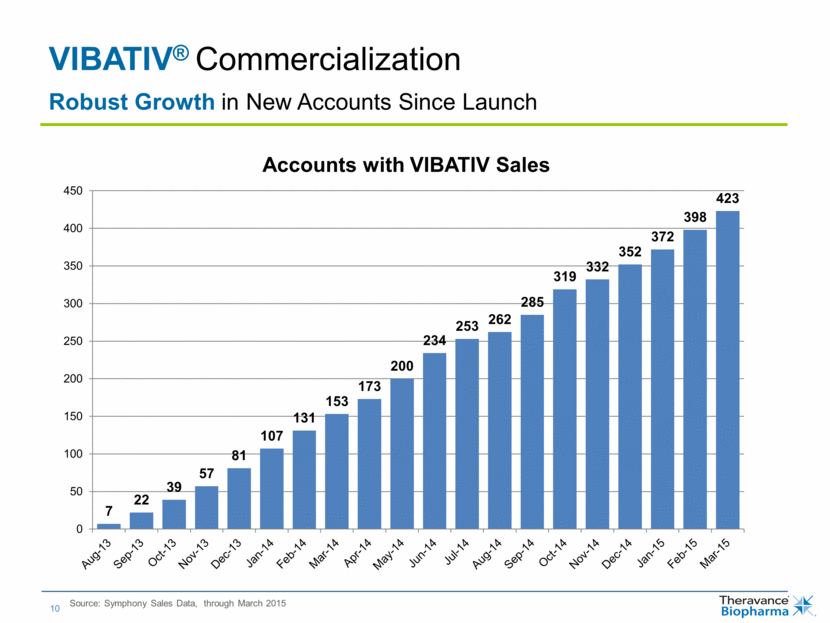
| VIBATIV® Commercialization Source: Symphony Sales Data, through March 2015 Robust Growth in New Accounts Since Launch 7 22 39 57 81 107 131 153 173 200 234 253 262 285 319 332 352 372 398 423 0 50 100 150 200 250 300 350 400 450 Accounts with VIBATIV Sales |
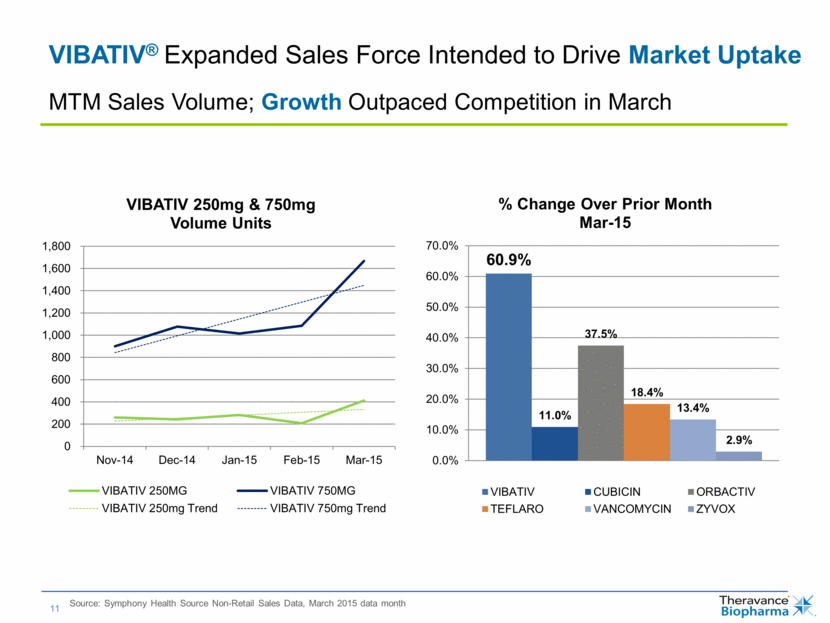
| VIBATIV® Expanded Sales Force Intended to Drive Market Uptake Source: Symphony Health Source Non-Retail Sales Data, March 2015 data month MTM Sales Volume; Growth Outpaced Competition in March 0 200 400 600 800 1,000 1,200 1,400 1,600 1,800 Nov-14 Dec-14 Jan-15 Feb-15 Mar-15 VIBATIV 250mg & 750mg Volume Units VIBATIV 250MG VIBATIV 750MG VIBATIV 250mg Trend VIBATIV 750mg Trend 60.9% 11.0% 37.5% 18.4% 13.4% 2.9% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% % Change Over Prior Month Mar - 15 VIBATIV CUBICIN ORBACTIV TEFLARO VANCOMYCIN ZYVOX |
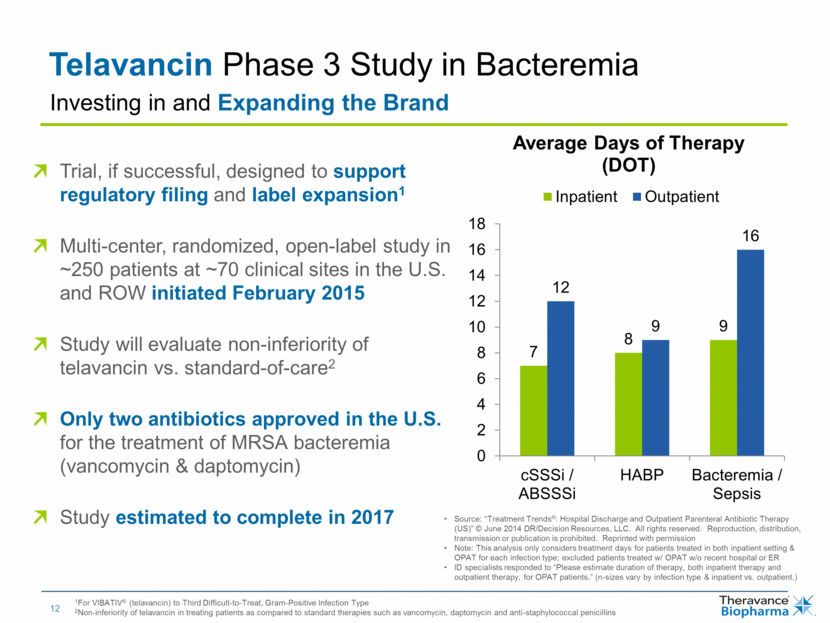
| Telavancin Phase 3 Study in Bacteremia Trial, if successful, designed to support regulatory filing and label expansion1 Multi-center, randomized, open-label study in ~250 patients at ~70 clinical sites in the U.S. and ROW initiated February 2015 Study will evaluate non-inferiority of telavancin vs. standard-of-care2 Only two antibiotics approved in the U.S. for the treatment of MRSA bacteremia (vancomycin & daptomycin) Study estimated to complete in 2017 Investing in and Expanding the Brand 1For VIBATIV® (telavancin) to Third Difficult-to-Treat, Gram-Positive Infection Type 2Non-inferiority of telavancin in treating patients as compared to standard therapies such as vancomycin, daptomycin and anti-staphylococcal penicillins Source: “Treatment Trends®: Hospital Discharge and Outpatient Parenteral Antibiotic Therapy (US)” © June 2014 DR/Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission Note: This analysis only considers treatment days for patients treated in both inpatient setting & OPAT for each infection type; excluded patients treated w/ OPAT w/o recent hospital or ER ID specialists responded to “Please estimate duration of therapy, both inpatient therapy and outpatient therapy, for OPAT patients.” (n-sizes vary by infection type & inpatient vs. outpatient.) 7 8 9 12 9 16 0 2 4 6 8 10 12 14 16 18 cSSSi / ABSSSi HABP Bacteremia / Sepsis Average Days of Therapy (DOT) Inpatient Outpatient |
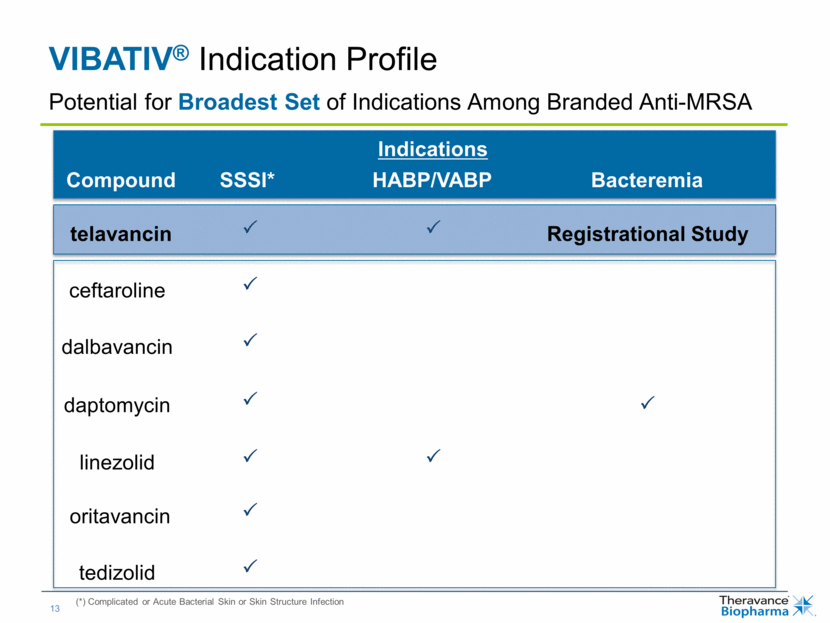
| VIBATIV® Indication Profile (*) Complicated or Acute Bacterial Skin or Skin Structure Infection Potential for Broadest Set of Indications Among Branded Anti-MRSA telavancin ceftaroline dalbavancin daptomycin linezolid oritavancin tedizolid Compound Indications SSSI* HABP/VABP Bacteremia Registrational Study |
| TD-4208 |
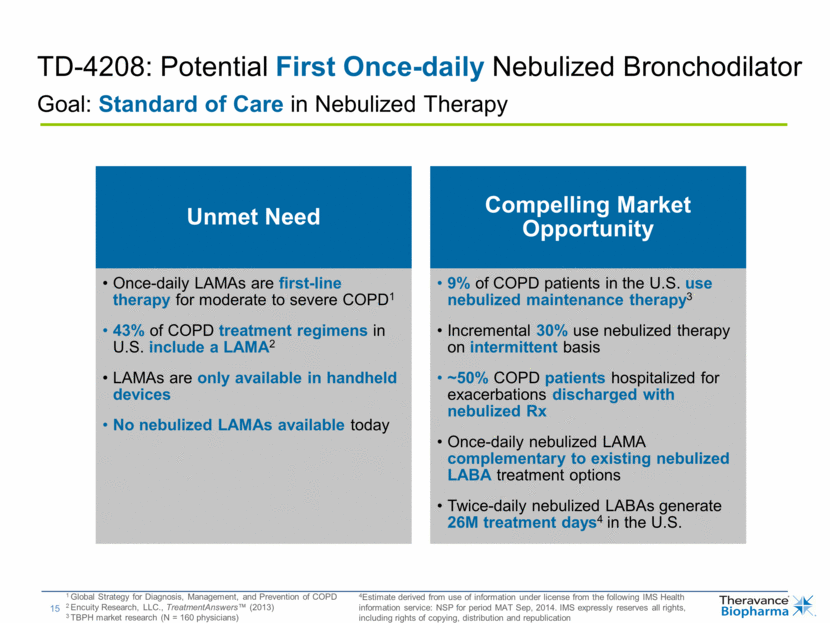
| TD-4208: Potential First Once-daily Nebulized Bronchodilator Goal: Standard of Care in Nebulized Therapy 1 Global Strategy for Diagnosis, Management, and Prevention of COPD 2 Encuity Research, LLC., TreatmentAnswers™ (2013) 3 TBPH market research (N = 160 physicians) 4Estimate derived from use of information under license from the following IMS Health information service: NSP for period MAT Sep, 2014. IMS expressly reserves all rights, including rights of copying, distribution and republication Unmet Need Once-daily LAMAs are first-line therapy for moderate to severe COPD1 43% of COPD treatment regimens in U.S. include a LAMA2 LAMAs are only available in handheld devices No nebulized LAMAs available today Compelling Market Opportunity 9% of COPD patients in the U.S. use nebulized maintenance therapy3 Incremental 30% use nebulized therapy on intermittent basis ~50% COPD patients hospitalized for exacerbations discharged with nebulized Rx Once-daily nebulized LAMA complementary to existing nebulized LABA treatment options Twice-daily nebulized LABAs generate 26M treatment days4 in the U.S. |
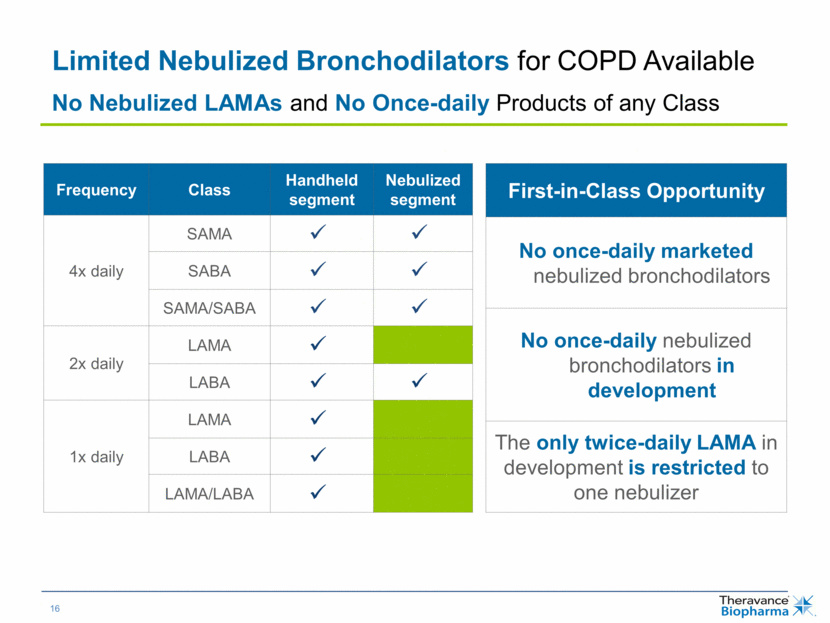
| Limited Nebulized Bronchodilators for COPD Available No Nebulized LAMAs and No Once-daily Products of any Class Frequency Class Handheld segment Nebulized segment 4x daily SAMA SABA SAMA/SABA 2x daily LAMA LABA 1x daily LAMA LABA LAMA/LABA First-in-Class Opportunity No once-daily marketed nebulized bronchodilators No once-daily nebulized bronchodilators in development The only twice-daily LAMA in development is restricted to one nebulizer |
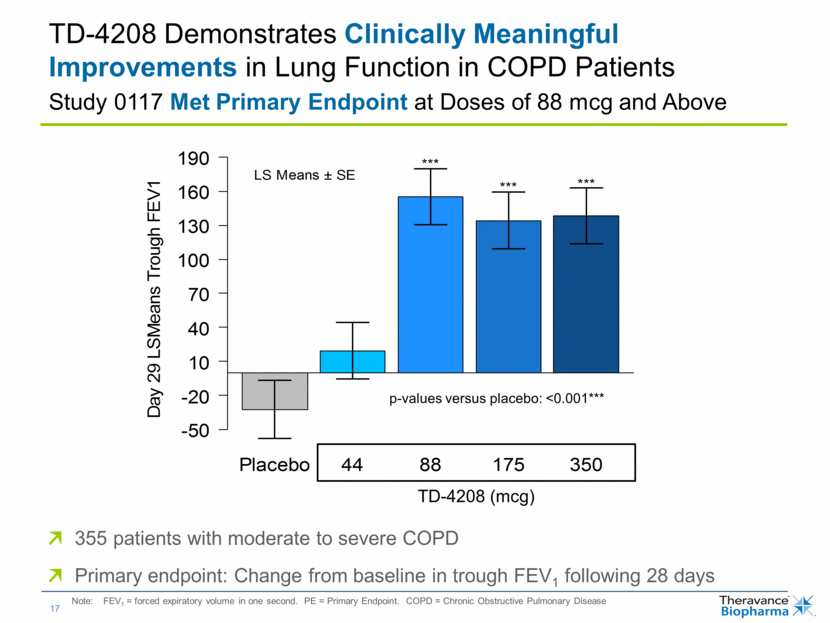
| TD-4208 Demonstrates Clinically Meaningful Improvements in Lung Function in COPD Patients Study 0117 Met Primary Endpoint at Doses of 88 mcg and Above 355 patients with moderate to severe COPD Primary endpoint: Change from baseline in trough FEV1 following 28 days Note: FEV1 = forced expiratory volume in one second. PE = Primary Endpoint. COPD = Chronic Obstructive Pulmonary Disease TD-4208 (mcg) p-values versus placebo: <0.001*** |
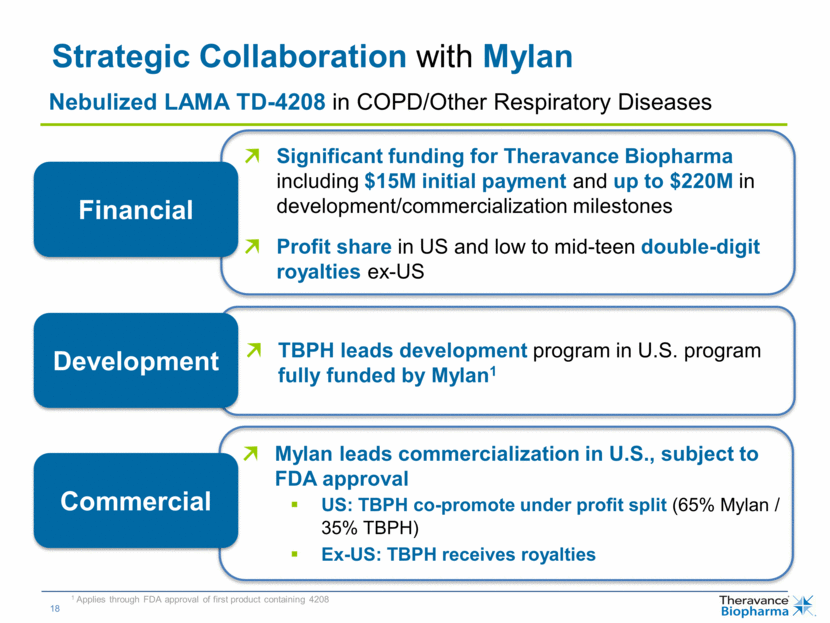
| Strategic Collaboration with Mylan Significant funding for Theravance Biopharma including $15M initial payment and up to $220M in development/commercialization milestones Profit share in US and low to mid-teen double-digit royalties ex-US TBPH leads development program in U.S. program fully funded by Mylan1 Mylan leads commercialization in U.S., subject to FDA approval US: TBPH co-promote under profit split (65% Mylan / 35% TBPH) Ex-US: TBPH receives royalties Financial Development Commercial 1 Applies through FDA approval of first product containing 4208 Nebulized LAMA TD-4208 in COPD/Other Respiratory Diseases |
| TD-4208: Phase 3 Registrational Program Plan to initiate Phase 3 program in second half of 2015 Phase 3 program Two replicate 3-month efficacy studies expected to read-out in 2016 Single 12-month safety study expected to read-out in 2017 ~2,200 patients across three studies Studies will test two doses: 88 mcg and 175 mcg administered once-daily Fully-funded by Mylan and Executed by Theravance Biopharma |
| NEPI Program |
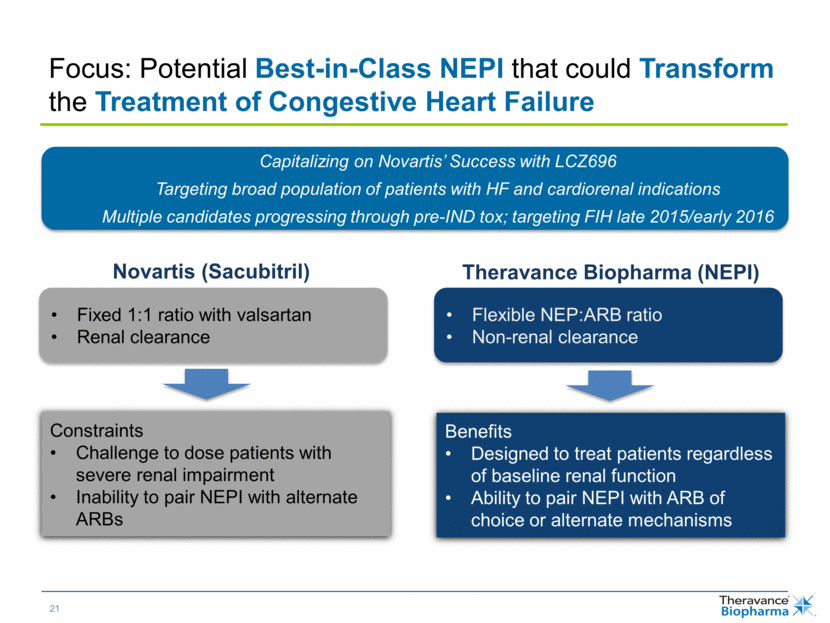
| Focus: Potential Best-in-Class NEPI that could Transform the Treatment of Congestive Heart Failure Fixed 1:1 ratio with valsartan Renal clearance Novartis (Sacubitril) Flexible NEP:ARB ratio Non-renal clearance Theravance Biopharma (NEPI) Constraints Challenge to dose patients with severe renal impairment Inability to pair NEPI with alternate ARBs Benefits Designed to treat patients regardless of baseline renal function Ability to pair NEPI with ARB of choice or alternate mechanisms Capitalizing on Novartis’ Success with LCZ696 Targeting broad population of patients with HF and cardiorenal indications Multiple candidates progressing through pre-IND tox; targeting FIH late 2015/early 2016 |
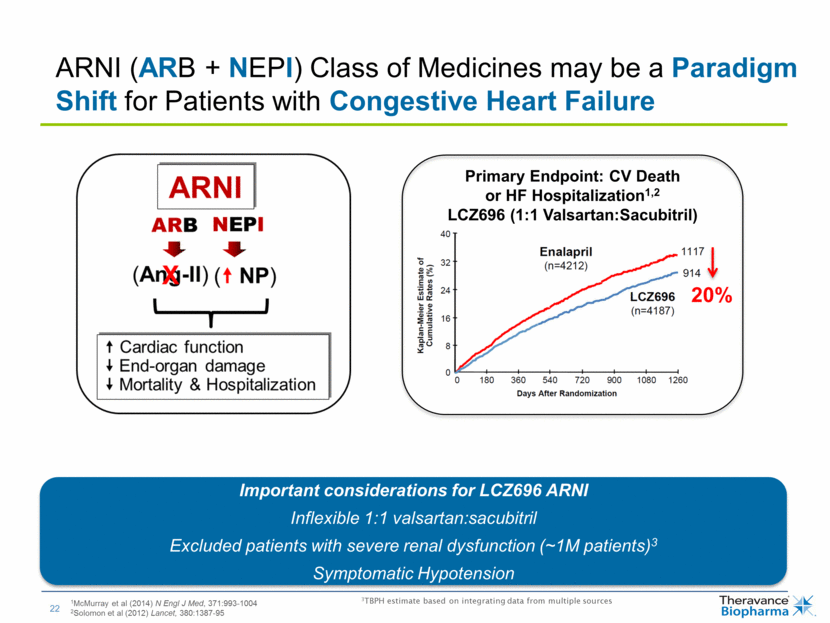
| Primary Endpoint: CV Death or HF Hospitalization1,2 LCZ696 (1:1 Valsartan:Sacubitril) 20% 1McMurray et al (2014) N Engl J Med, 371:993-1004 2Solomon et al (2012) Lancet, 380:1387-95 3TBPH estimate based on integrating data from multiple sources ARNI (ARB + NEPI) Class of Medicines may be a Paradigm Shift for Patients with Congestive Heart Failure Important considerations for LCZ696 ARNI Inflexible 1:1 valsartan:sacubitril Excluded patients with severe renal dysfunction (~1M patients)3 Symptomatic Hypotension |
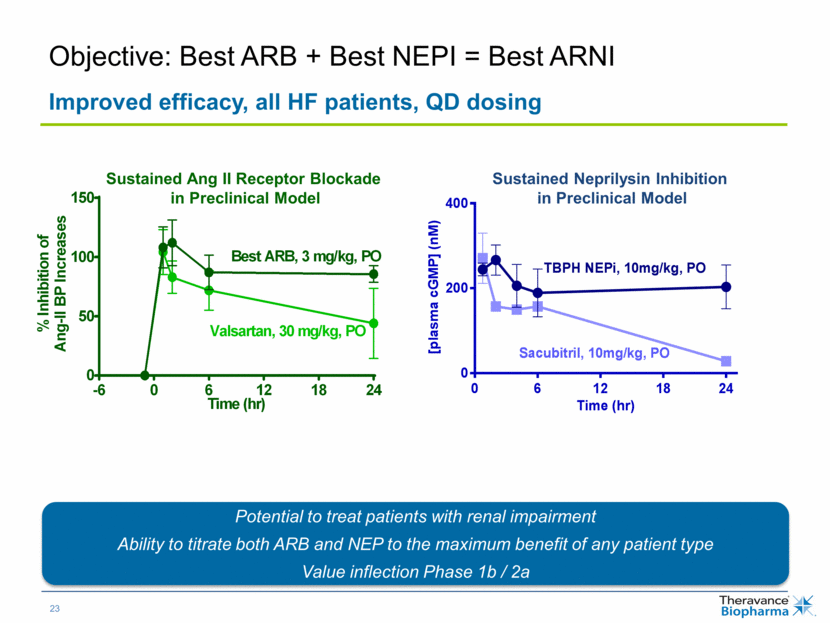
| Sustained Ang II Receptor Blockade in Preclinical Model Potential to treat patients with renal impairment Ability to titrate both ARB and NEP to the maximum benefit of any patient type Value inflection Phase 1b / 2a Sustained Neprilysin Inhibition in Preclinical Model Objective: Best ARB + Best NEPI = Best ARNI Improved efficacy, all HF patients, QD dosing Time (hr) % I n h i b i t i o n o f A n g - I I B P I n c r e a s e s -6 0 6 12 18 24 0 50 100 150 Valsartan, 30 mg/kg, PO Best ARB, 3 mg/kg, PO Time (hr) [ p l a s m a c G M P ] ( n M ) 0 6 12 18 24 0 200 400 Sacubitril, 10mg/kg, PO TBPH NEPi, 10mg/kg, PO |
| Theravance Biopharma Value Creation |
| Theravance Biopharma Milestones VIBATIV®: targeting 2015 net sales of $20M Initiate LAMA TD-4208 Phase 3 registrational program in 2015 Progression of high value product candidates in heart failure and ulcerative colitis into the clinic in late 2015/early 2016 Completion of 3 Phase 3 studies in 20161 Two LAMA TD-4208 efficacy studies Closed Triple FULFIL study Completion of 3 Phase 3 studies in 20171 LAMA TD-4208 LTSS Closed Triple IMPACT study Telavancin bacteremia study 1Estimates per clinicaltrials.gov |
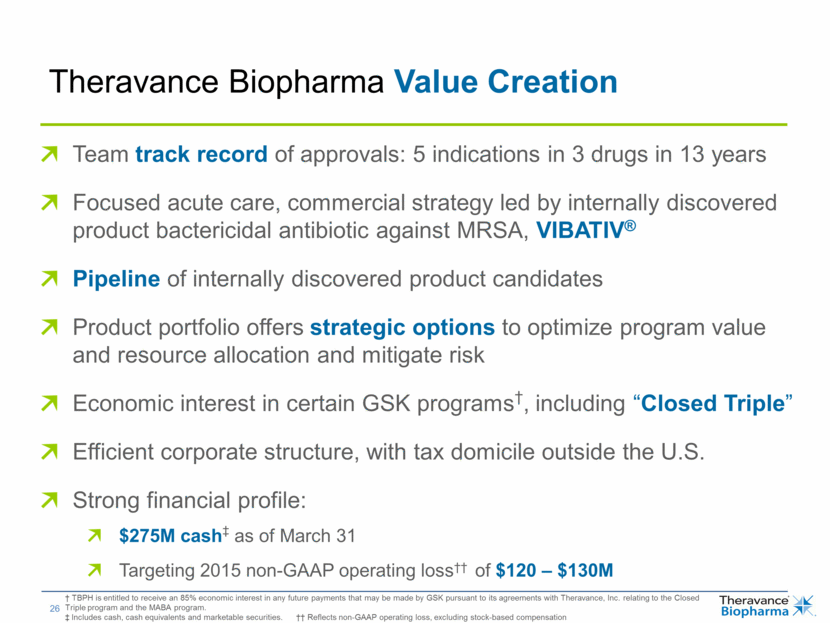
| Theravance Biopharma Value Creation † TBPH is entitled to receive an 85% economic interest in any future payments that may be made by GSK pursuant to its agreements with Theravance, Inc. relating to the Closed Triple program and the MABA program. ‡ Includes cash, cash equivalents and marketable securities. †† Reflects non-GAAP operating loss, excluding stock-based compensation Team track record of approvals: 5 indications in 3 drugs in 13 years Focused acute care, commercial strategy led by internally discovered product bactericidal antibiotic against MRSA, VIBATIV® Pipeline of internally discovered product candidates Product portfolio offers strategic options to optimize program value and resource allocation and mitigate risk Economic interest in certain GSK programs†, including “Closed Triple” Efficient corporate structure, with tax domicile outside the U.S. Strong financial profile: $275M cash‡ as of March 31 Targeting 2015 non-GAAP operating loss†† of $120 – $130M |
| Thank you |
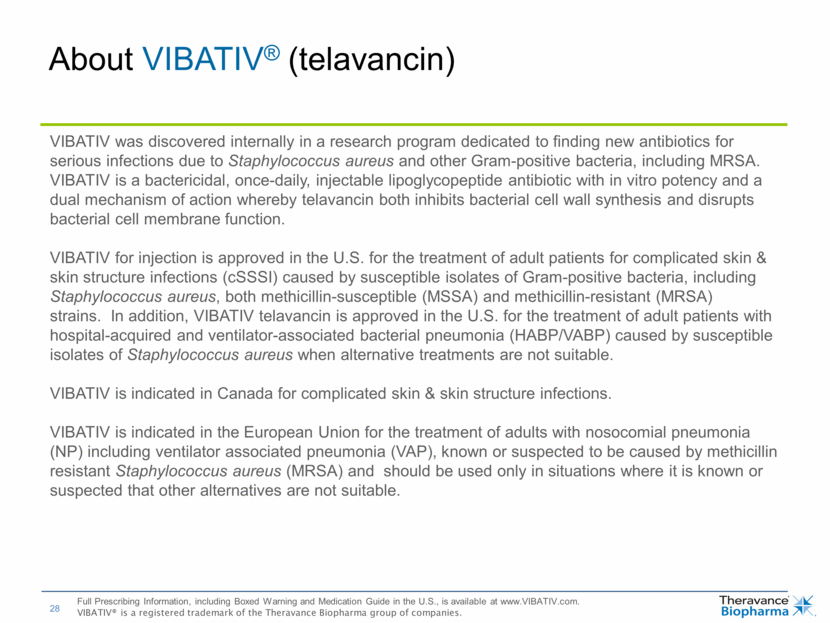
| About VIBATIV® (telavancin) VIBATIV was discovered internally in a research program dedicated to finding new antibiotics for serious infections due to Staphylococcus aureus and other Gram-positive bacteria, including MRSA. VIBATIV is a bactericidal, once-daily, injectable lipoglycopeptide antibiotic with in vitro potency and a dual mechanism of action whereby telavancin both inhibits bacterial cell wall synthesis and disrupts bacterial cell membrane function. VIBATIV for injection is approved in the U.S. for the treatment of adult patients for complicated skin & skin structure infections (cSSSI) caused by susceptible isolates of Gram-positive bacteria, including Staphylococcus aureus, both methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) strains. In addition, VIBATIV telavancin is approved in the U.S. for the treatment of adult patients with hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible isolates of Staphylococcus aureus when alternative treatments are not suitable. VIBATIV is indicated in Canada for complicated skin & skin structure infections. VIBATIV is indicated in the European Union for the treatment of adults with nosocomial pneumonia (NP) including ventilator associated pneumonia (VAP), known or suspected to be caused by methicillin resistant Staphylococcus aureus (MRSA) and should be used only in situations where it is known or suspected that other alternatives are not suitable. Full Prescribing Information, including Boxed Warning and Medication Guide in the U.S., is available at www.VIBATIV.com. VIBATIV® is a registered trademark of the Theravance Biopharma group of companies. |
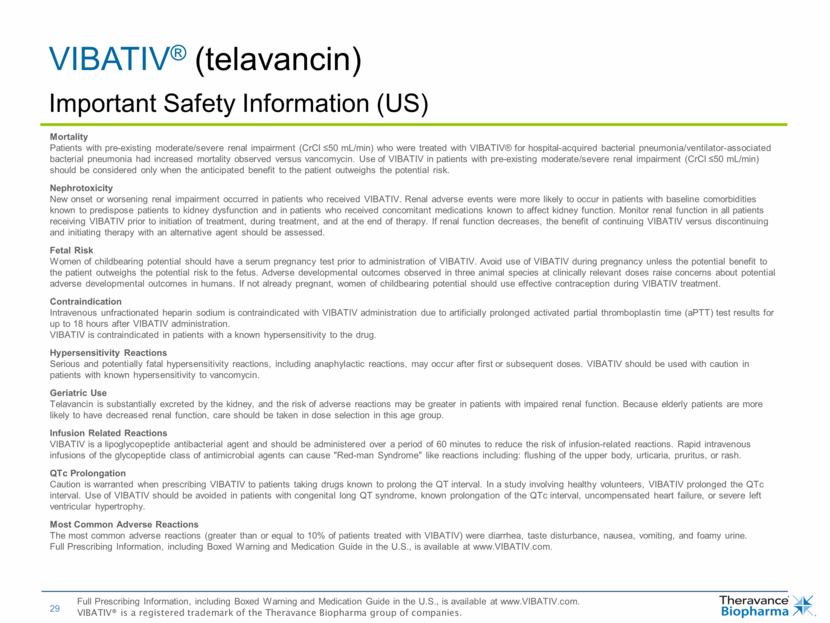
| VIBATIV® (telavancin) Important Safety Information (US) Mortality Patients with pre-existing moderate/severe renal impairment (CrCl <50 mL/min) who were treated with VIBATIV® for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia had increased mortality observed versus vancomycin. Use of VIBATIV in patients with pre-existing moderate/severe renal impairment (CrCl <50 mL/min) should be considered only when the anticipated benefit to the patient outweighs the potential risk. Nephrotoxicity New onset or worsening renal impairment occurred in patients who received VIBATIV. Renal adverse events were more likely to occur in patients with baseline comorbidities known to predispose patients to kidney dysfunction and in patients who received concomitant medications known to affect kidney function. Monitor renal function in all patients receiving VIBATIV prior to initiation of treatment, during treatment, and at the end of therapy. If renal function decreases, the benefit of continuing VIBATIV versus discontinuing and initiating therapy with an alternative agent should be assessed. Fetal Risk Women of childbearing potential should have a serum pregnancy test prior to administration of VIBATIV. Avoid use of VIBATIV during pregnancy unless the potential benefit to the patient outweighs the potential risk to the fetus. Adverse developmental outcomes observed in three animal species at clinically relevant doses raise concerns about potential adverse developmental outcomes in humans. If not already pregnant, women of childbearing potential should use effective contraception during VIBATIV treatment. Contraindication Intravenous unfractionated heparin sodium is contraindicated with VIBATIV administration due to artificially prolonged activated partial thromboplastin time (aPTT) test results for up to 18 hours after VIBATIV administration. VIBATIV is contraindicated in patients with a known hypersensitivity to the drug. Hypersensitivity Reactions Serious and potentially fatal hypersensitivity reactions, including anaphylactic reactions, may occur after first or subsequent doses. VIBATIV should be used with caution in patients with known hypersensitivity to vancomycin. Geriatric Use Telavancin is substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection in this age group. Infusion Related Reactions VIBATIV is a lipoglycopeptide antibacterial agent and should be administered over a period of 60 minutes to reduce the risk of infusion-related reactions. Rapid intravenous infusions of the glycopeptide class of antimicrobial agents can cause "Red-man Syndrome" like reactions including: flushing of the upper body, urticaria, pruritus, or rash. QTc Prolongation Caution is warranted when prescribing VIBATIV to patients taking drugs known to prolong the QT interval. In a study involving healthy volunteers, VIBATIV prolonged the QTc interval. Use of VIBATIV should be avoided in patients with congenital long QT syndrome, known prolongation of the QTc interval, uncompensated heart failure, or severe left ventricular hypertrophy. Most Common Adverse Reactions The most common adverse reactions (greater than or equal to 10% of patients treated with VIBATIV) were diarrhea, taste disturbance, nausea, vomiting, and foamy urine. Full Prescribing Information, including Boxed Warning and Medication Guide in the U.S., is available at www.VIBATIV.com. Full Prescribing Information, including Boxed Warning and Medication Guide in the U.S., is available at www.VIBATIV.com. VIBATIV® is a registered trademark of the Theravance Biopharma group of companies. |
| Q&A Session |