Exhibit 99.1
Cowen and Company 36th Annual Health Care Conference March 2016 Theravance Biopharma, Inc. (NASDAQ: TBPH)

Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, and the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities, and the finalization of financial results for the three months and twelve months ended December 31, 2015 and the audit of those results by us and our independent auditors may result in changes from the expected results disclosed in this presentation. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on November 12, 2015, and other periodic reports filed with the SEC.
Theravance Biopharma Investment Highlights Productive Research Engine Pipeline of High Value Assets Robust Business Model Team Track Record of Success Strong Financial Position
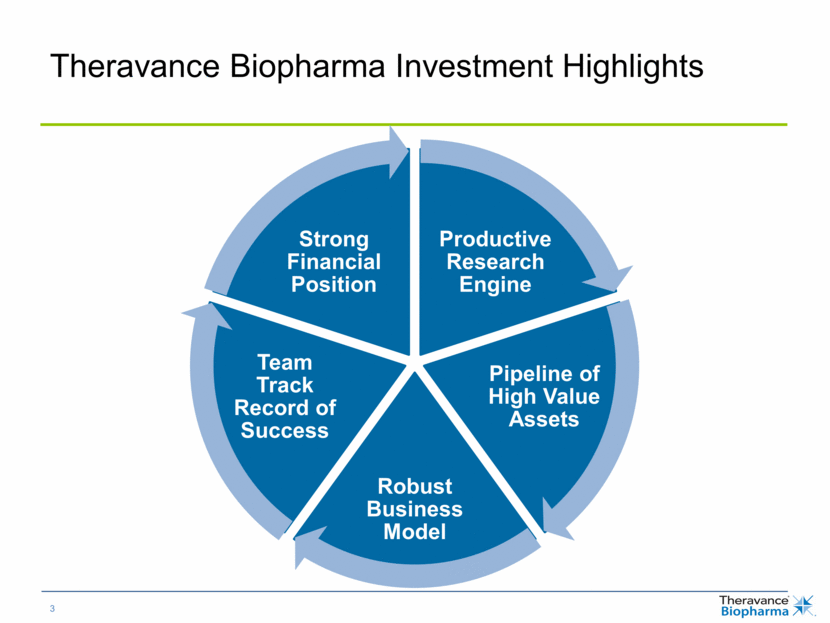
Program Phase 1 Phase 2 Phase 3 Filed Approved VIBATIV® (telavancin) cSSSI, HABP/VABP sNDA Concurrent Bacteremia & cSSSI sNDA Concurrent Bacteremia & HABP/VABP Phase 3 Registrational Study – Bacteremia Revefenacin (TD-4208) Phase 3 Efficacy Studies (2) – COPD Phase 3 Long-Term Safety Study – COPD TD-0714 (NEP Inhibitor) Phase 1 Study TD-1473 (JAK Inhibitor) Phase 1 Study 2016 Focus
Neprilysin Inhibitor (NEPi) Program Potential Best-in-Class Therapeutic for Cardiovascular and Renal Disease

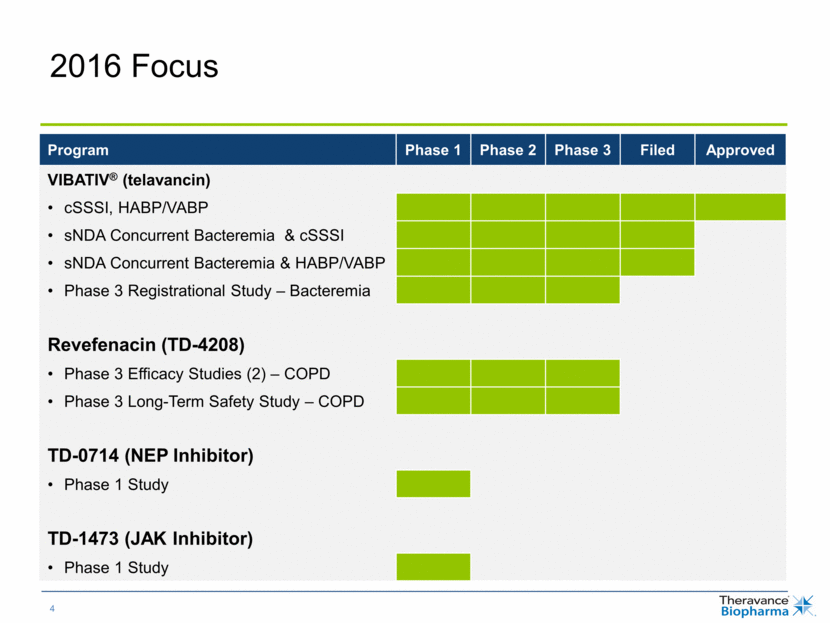
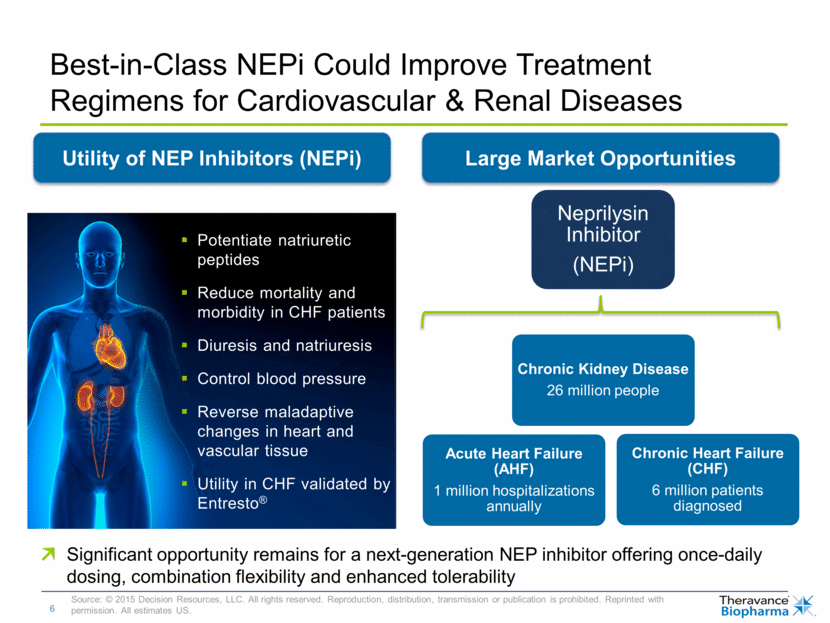
Best-in-Class NEPi Could Improve Treatment Regimens for Cardiovascular & Renal Diseases Source: © 2015 Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission. All estimates US. Utility of NEP Inhibitors (NEPi) Large Market Opportunities Potentiate natriuretic peptides Reduce mortality and morbidity in CHF patients Diuresis and natriuresis Control blood pressure Reverse maladaptive changes in heart and vascular tissue Utility in CHF validated by Entresto® Significant opportunity remains for a next-generation NEP inhibitor offering once-daily dosing, combination flexibility and enhanced tolerability Neprilysin Inhibitor (NEPi) Acute Heart Failure (AHF) 1 million hospitalizations annually Chronic Heart Failure (CHF) 6 million patients diagnosed Chronic Kidney Disease 26 million people
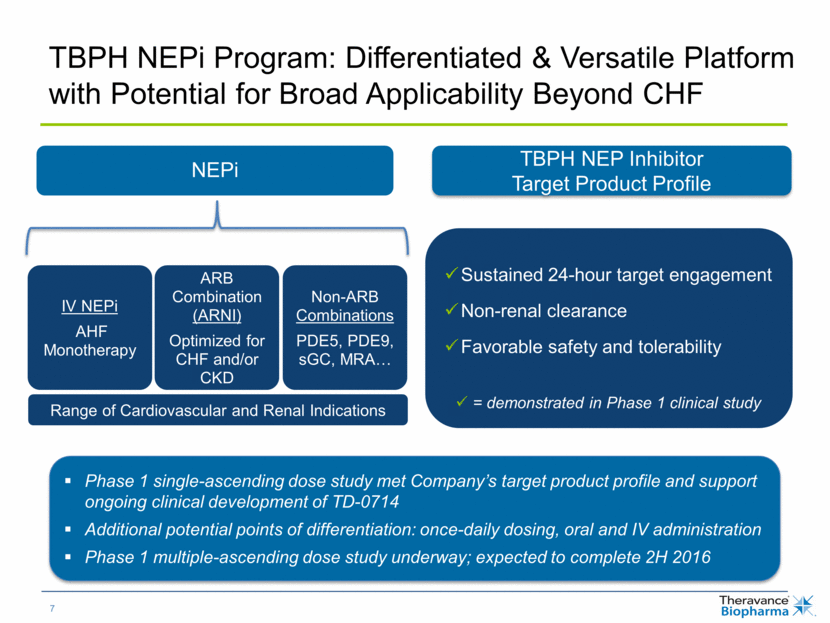
TBPH NEPi Program: Differentiated & Versatile Platform with Potential for Broad Applicability Beyond CHF Range of Cardiovascular and Renal Indications TBPH NEP Inhibitor Target Product Profile Phase 1 single-ascending dose study met Company’s target product profile and support ongoing clinical development of TD-0714 Additional potential points of differentiation: once-daily dosing, oral and IV administration Phase 1 multiple-ascending dose study underway; expected to complete 2H 2016 Sustained 24-hour target engagement Non-renal clearance Favorable safety and tolerability = demonstrated in Phase 1 clinical study NEPi IV NEPi AHF Monotherapy ARB Combination (ARNI) Optimized for CHF and/or CKD Non-ARB Combinations PDE5, PDE9, sGC, MRA
TD-1473 Oral GI-Targeted Pan-JAK Inhibitor for Ulcerative Colitis and Other Inflammatory Intestinal Diseases
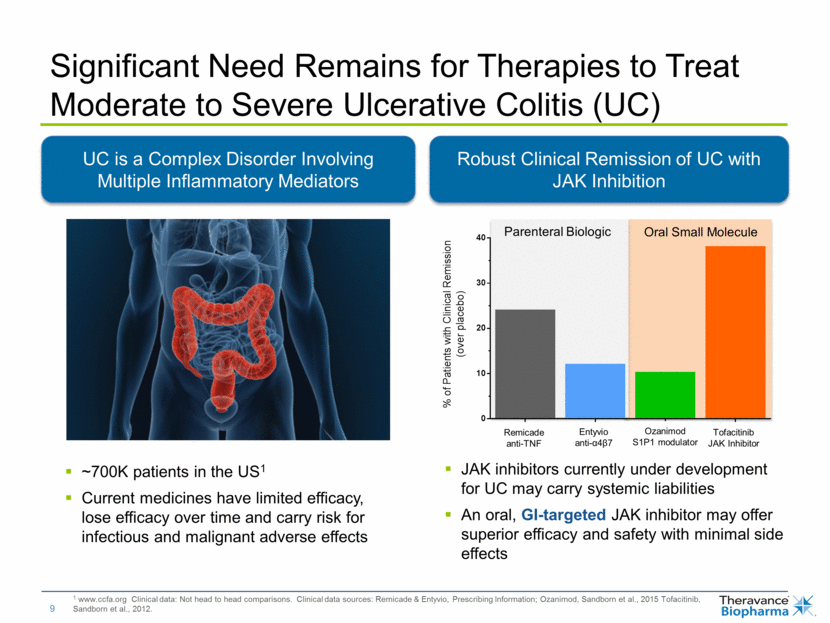
1 www.ccfa.org Clinical data: Not head to head comparisons. Clinical data sources: Remicade & Entyvio, Prescribing Information; Ozanimod, Sandborn et al., 2015 Tofacitinib, Sandborn et al., 2012. Significant Need Remains for Therapies to Treat Moderate to Severe Ulcerative Colitis (UC) UC is a Complex Disorder Involving Multiple Inflammatory Mediators ~700K patients in the US1 Current medicines have limited efficacy, lose efficacy over time and carry risk for infectious and malignant adverse effects Robust Clinical Remission of UC with JAK Inhibition Parenteral Biologic Oral Small Molecule Remicade anti-TNF Entyvio anti-α4β7 Ozanimod S1P1 modulator Tofacitinib JAK Inhibitor JAK inhibitors currently under development for UC may carry systemic liabilities An oral, GI-targeted JAK inhibitor may offer superior efficacy and safety with minimal side effects
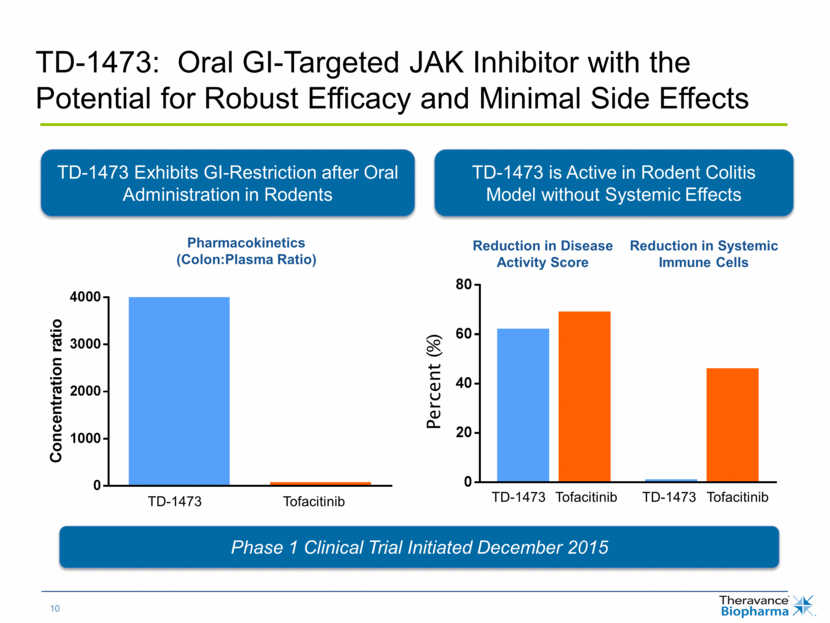
TD-1473: Oral GI-Targeted JAK Inhibitor with the Potential for Robust Efficacy and Minimal Side Effects Phase 1 Clinical Trial Initiated December 2015 TD-1473 Exhibits GI-Restriction after Oral Administration in Rodents TD-1473 is Active in Rodent Colitis Model without Systemic Effects Pharmacokinetics (Colon:Plasma Ratio) Reduction in Disease Activity Score Reduction in Systemic Immune Cells TD-1473 Tofacitinib TD-1473 Tofacitinib TD-1473 Tofacitinib Percent (%) C o n c e n t r a t i o n r a t i o 0 1000 2000 3000 4000 TD-1473 Tofacitinib % I n h i b i t i o n ( r e l a t i v e t o v e h i c l e c o n t r o l ) TD-1473 Tofacitinib TD-1473 Tofacitinib 0 20 40 60 80
Theravance Biopharma Opportunities for Value Creation

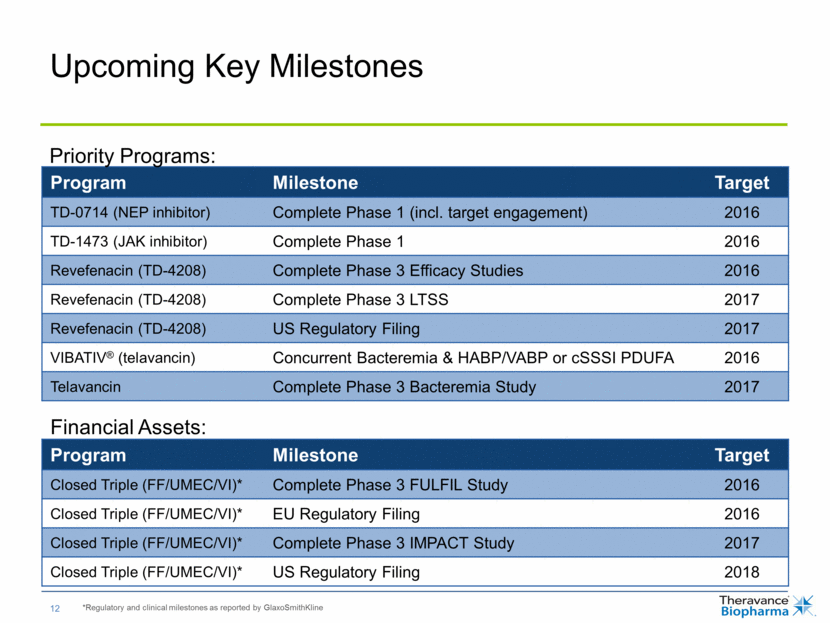
Upcoming Key Milestones Program Milestone Target TD-0714 (NEP inhibitor) Complete Phase 1 (incl. target engagement) 2016 TD-1473 (JAK inhibitor) Complete Phase 1 2016 Revefenacin (TD-4208) Complete Phase 3 Efficacy Studies 2016 Revefenacin (TD-4208) Complete Phase 3 LTSS 2017 Revefenacin (TD-4208) US Regulatory Filing 2017 VIBATIV® (telavancin) Concurrent Bacteremia & HABP/VABP or cSSSI PDUFA 2016 Telavancin Complete Phase 3 Bacteremia Study 2017 *Regulatory and clinical milestones as reported by GlaxoSmithKline Program Milestone Target Closed Triple (FF/UMEC/VI)* Complete Phase 3 FULFIL Study 2016 Closed Triple (FF/UMEC/VI)* EU Regulatory Filing 2016 Closed Triple (FF/UMEC/VI)* Complete Phase 3 IMPACT Study 2017 Closed Triple (FF/UMEC/VI)* US Regulatory Filing 2018 Financial Assets: Priority Programs:
Thank You
