
A Phase I Study Investigating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamic Activity of the Hepcidin Antagonist PRS-080#022. Results from a Randomized, Placebo Controlled, Double-Blind Study Following Single Administration to Healthy Subjects December 07, 2015 57th Annual Meeting of the American Society of Hematology, Orlando, FL Exhibit 99.2
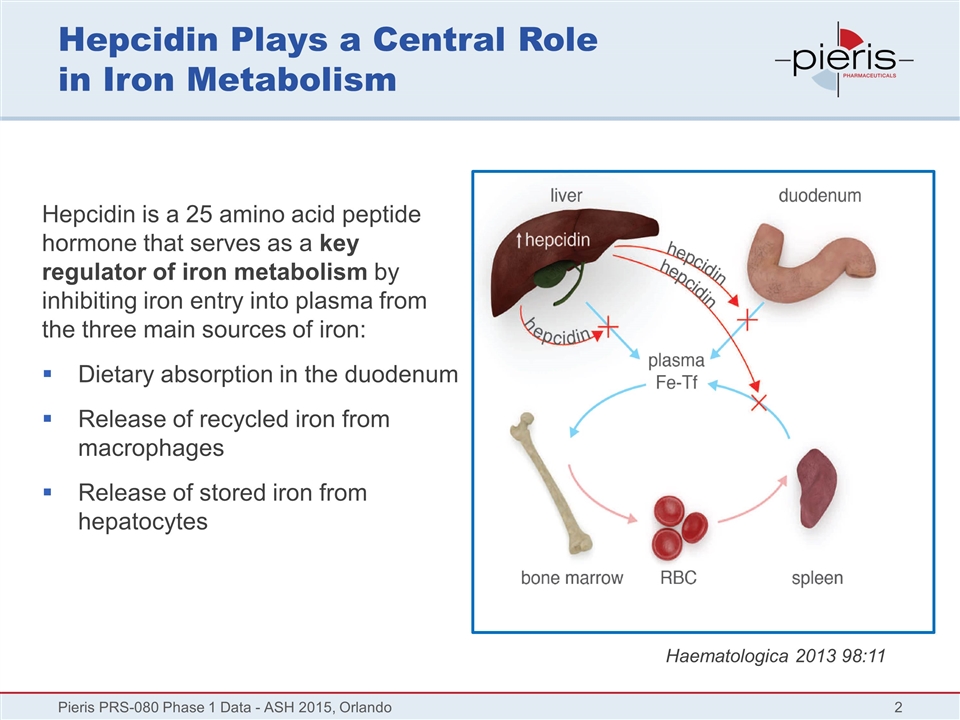
Hepcidin Plays a Central Role in Iron Metabolism Hepcidin is a 25 amino acid peptide hormone that serves as a key regulator of iron metabolism by inhibiting iron entry into plasma from the three main sources of iron: Dietary absorption in the duodenum Release of recycled iron from macrophages Release of stored iron from hepatocytes Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Haematologica 2013 98:11
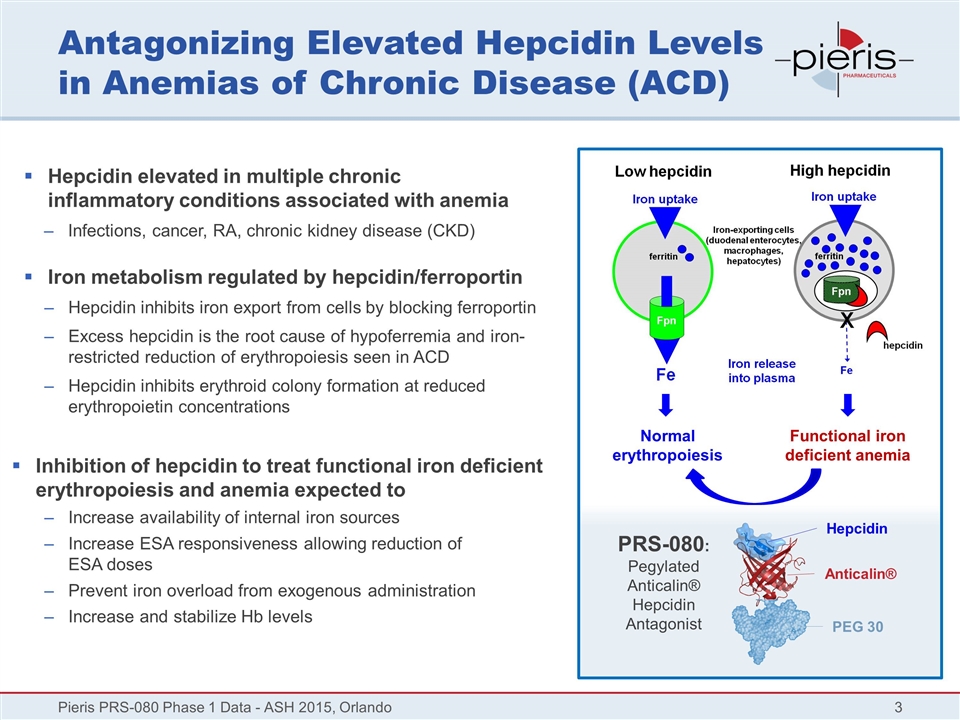
Antagonizing Elevated Hepcidin Levels in Anemias of Chronic Disease (ACD) Hepcidin elevated in multiple chronic inflammatory conditions associated with anemia Infections, cancer, RA, chronic kidney disease (CKD) Iron metabolism regulated by hepcidin/ferroportin Hepcidin inhibits iron export from cells by blocking ferroportin Excess hepcidin is the root cause of hypoferremia and iron-restricted reduction of erythropoiesis seen in ACD Hepcidin inhibits erythroid colony formation at reduced erythropoietin concentrations Inhibition of hepcidin to treat functional iron deficient erythropoiesis and anemia expected to Increase availability of internal iron sources Increase ESA responsiveness allowing reduction of ESA doses Prevent iron overload from exogenous administration Increase and stabilize Hb levels Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Normal erythropoiesis Functional iron deficient anemia Hepcidin Anticalin® PEG 30 PRS-080: Pegylated Anticalin® Hepcidin Antagonist
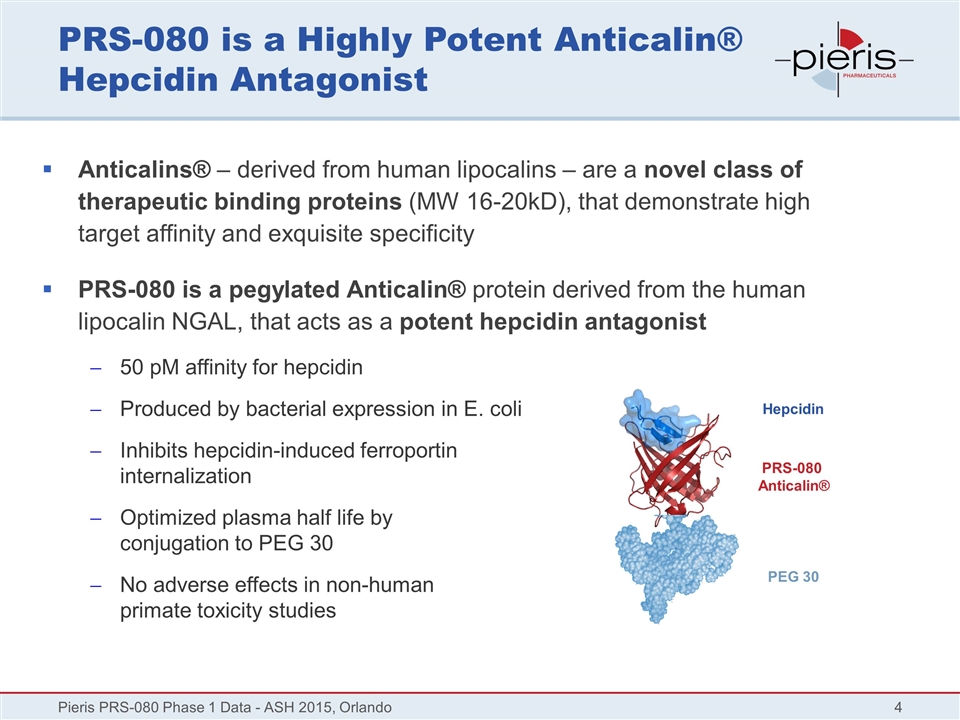
PRS-080 is a Highly Potent Anticalin® Hepcidin Antagonist Anticalins® – derived from human lipocalins – are a novel class of therapeutic binding proteins (MW 16-20kD), that demonstrate high target affinity and exquisite specificity PRS-080 is a pegylated Anticalin® protein derived from the human lipocalin NGAL, that acts as a potent hepcidin antagonist 50 pM affinity for hepcidin Produced by bacterial expression in E. coli Inhibits hepcidin-induced ferroportin internalization Optimized plasma half life by conjugation to PEG 30 No adverse effects in non-human primate toxicity studies Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Hepcidin PRS-080 Anticalin® PEG 30
PRS-080 Has Been Investigated in Phase I in Healthy Subjects Single dose escalating study in healthy male subjects, n=48 Randomized, double blinded, placebo controlled study 6 dose cohorts 0.08, 0.4, 1.2, 4.0, 8.0, 16.0 mg/kg (based on API without PEG) I.V. infusion over 2 hours 6 subjects receiving PRS-080, 2 subjects receiving placebo per cohort Endpoints Safety and maximal tolerated dose Pharmacokinetics Pharmacodynamics (serum iron, transferrin saturation) Hepcidin plasma concentrations Immunogenicity Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando

PRS-080 Was Well Tolerated in Healthy Subjects No serious Adverse Events (AE) 39 treatment emergent AEs (TEAE) in 22 subjects 30 mild TEAEs 9 moderate TEAEs Headache was most common TEAE (10 subjects) Otherwise, no association of AEs to specific organs No apparent correlation between dose and number of TEAEs No hypersensitivity, no infusion reactions Vital signs and ECG without changes No cytokine release upon PRS-080 administration IFN-g, IL-1b, IL-6 and TNF-a Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando
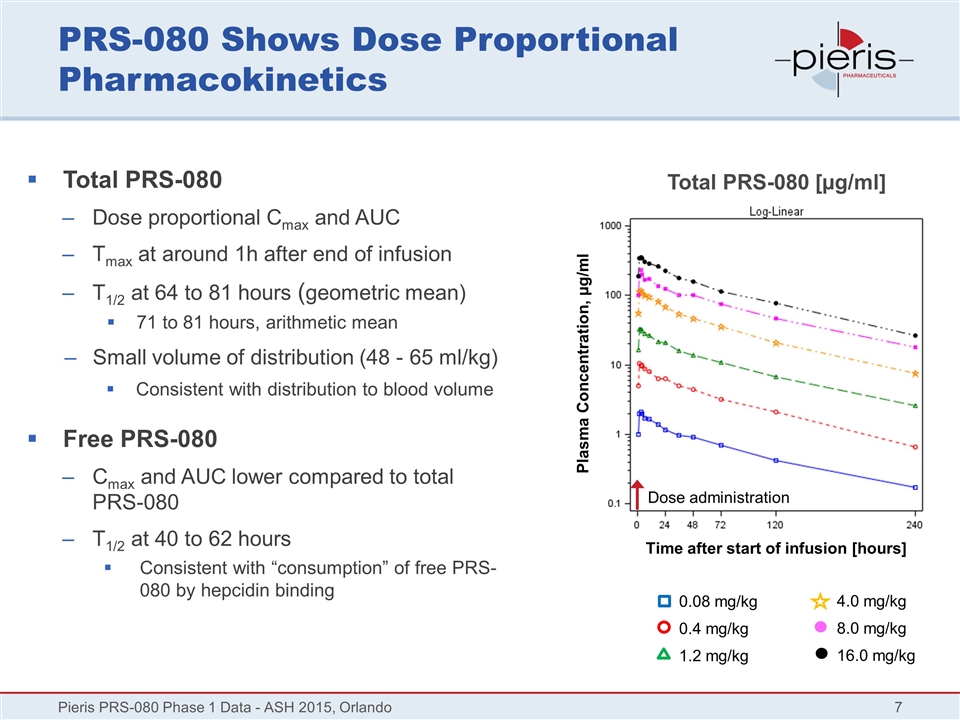
PRS-080 Shows Dose Proportional Pharmacokinetics Total PRS-080 Dose proportional Cmax and AUC Tmax at around 1h after end of infusion T1/2 at 64 to 81 hours (geometric mean) 71 to 81 hours, arithmetic mean Small volume of distribution (48 - 65 ml/kg) Consistent with distribution to blood volume Free PRS-080 Cmax and AUC lower compared to total PRS-080 T1/2 at 40 to 62 hours Consistent with “consumption” of free PRS-080 by hepcidin binding Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Plasma Concentration, µg/ml Total PRS-080 [µg/ml] Time after start of infusion [hours] 0.08 mg/kg 0.4 mg/kg 1.2 mg/kg 4.0 mg/kg 8.0 mg/kg 16.0 mg/kg Dose administration
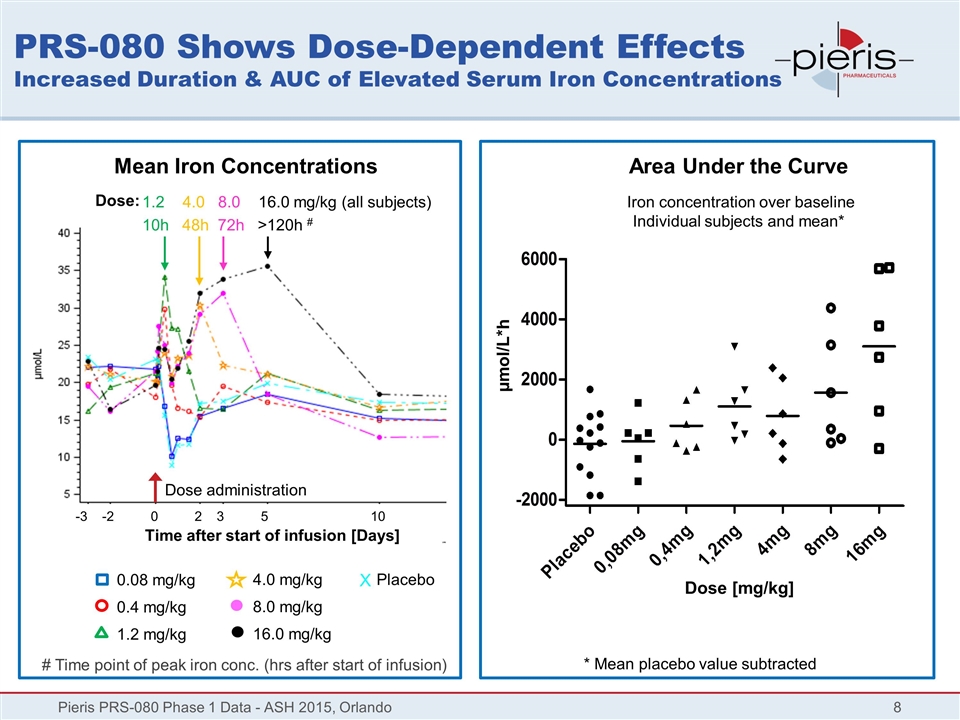
PRS-080 Shows Dose-Dependent Effects Increased Duration & AUC of Elevated Serum Iron Concentrations Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Placebo Mean Iron Concentrations Dose administration X Area Under the Curve Dose [mg/kg] * Mean placebo value subtracted µmol/L*h 1.2 4.0 8.0 16.0 mg/kg (all subjects) 10h 48h 72h >120h # # Time point of peak iron conc. (hrs after start of infusion) Dose: 0.08 mg/kg 0.4 mg/kg 1.2 mg/kg 4.0 mg/kg 8.0 mg/kg 16.0 mg/kg Iron concentration over baseline Individual subjects and mean* Time after start of infusion [Days] -3 -2 0 2 3 5 10
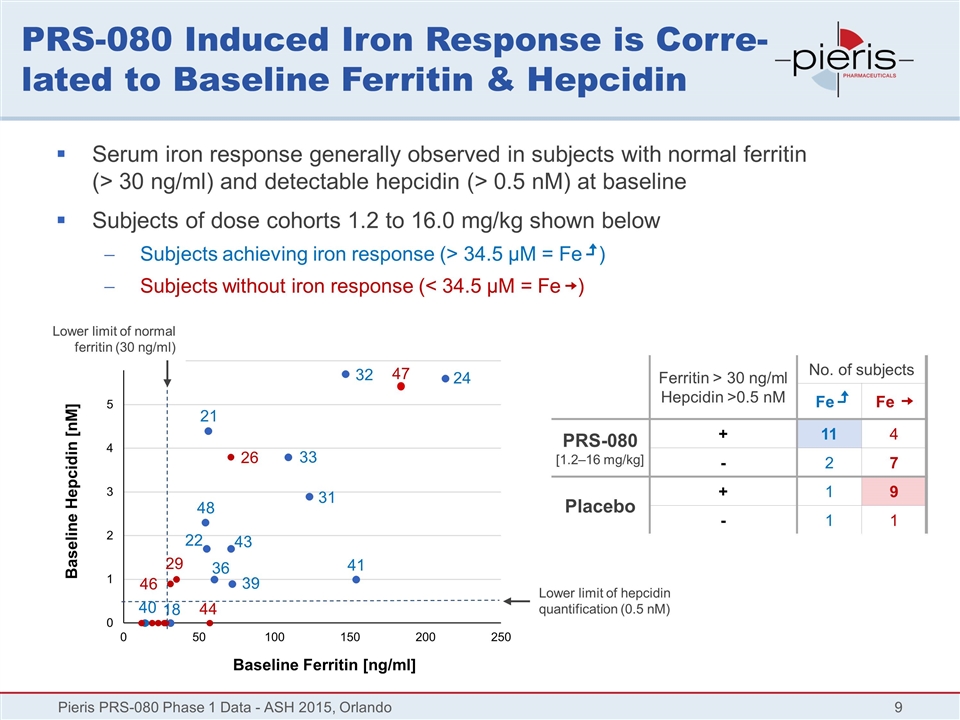
PRS-080 Induced Iron Response is Corre-lated to Baseline Ferritin & Hepcidin Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Baseline Ferritin [ng/ml] Baseline Hepcidin [nM] Serum iron response generally observed in subjects with normal ferritin (> 30 ng/ml) and detectable hepcidin (> 0.5 nM) at baseline Subjects of dose cohorts 1.2 to 16.0 mg/kg shown below Subjects achieving iron response (> 34.5 µM = Fe ) Subjects without iron response (< 34.5 µM = Fe ) 32 24 41 33 31 26 43 22 48 21 36 18 39 46 29 44 Lower limit of hepcidin quantification (0.5 nM) Ferritin > 30 ng/ml Hepcidin >0.5 nM No. of subjects Fe Fe PRS-080 [1.2–16 mg/kg] + 11 4 - 2 7 Placebo + 1 9 - 1 1 40 Lower limit of normal ferritin (30 ng/ml) 47
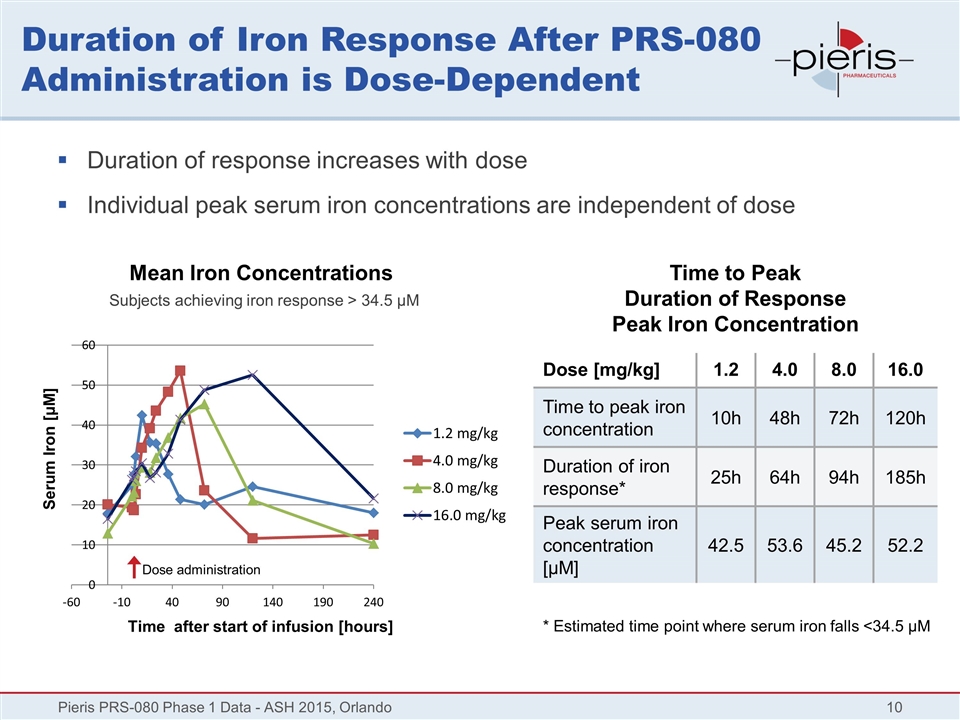
Duration of Iron Response After PRS-080 Administration is Dose-Dependent Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Mean Iron Concentrations Subjects achieving iron response > 34.5 µM Dose [mg/kg] 1.2 4.0 8.0 16.0 Time to peak iron concentration 10h 48h 72h 120h Duration of iron response* 25h 64h 94h 185h Peak serum iron concentration [µM] 42.5 53.6 45.2 52.2 Time after start of infusion [hours] Serum Iron [µM] Time to Peak Duration of Response Peak Iron Concentration Duration of response increases with dose Individual peak serum iron concentrations are independent of dose Dose administration * Estimated time point where serum iron falls <34.5 µM
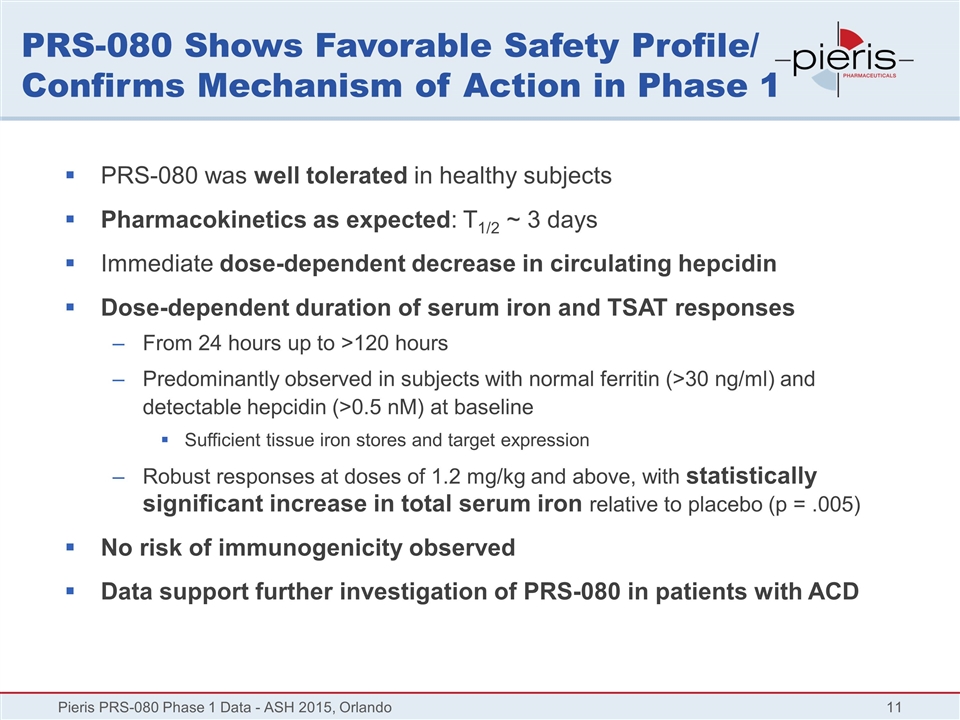
PRS-080 Shows Favorable Safety Profile/ Confirms Mechanism of Action in Phase 1 PRS-080 was well tolerated in healthy subjects Pharmacokinetics as expected: T1/2 ~ 3 days Immediate dose-dependent decrease in circulating hepcidin Dose-dependent duration of serum iron and TSAT responses From 24 hours up to >120 hours Predominantly observed in subjects with normal ferritin (>30 ng/ml) and detectable hepcidin (>0.5 nM) at baseline Sufficient tissue iron stores and target expression Robust responses at doses of 1.2 mg/kg and above, with statistically significant increase in total serum iron relative to placebo (p = .005) No risk of immunogenicity observed Data support further investigation of PRS-080 in patients with ACD Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando
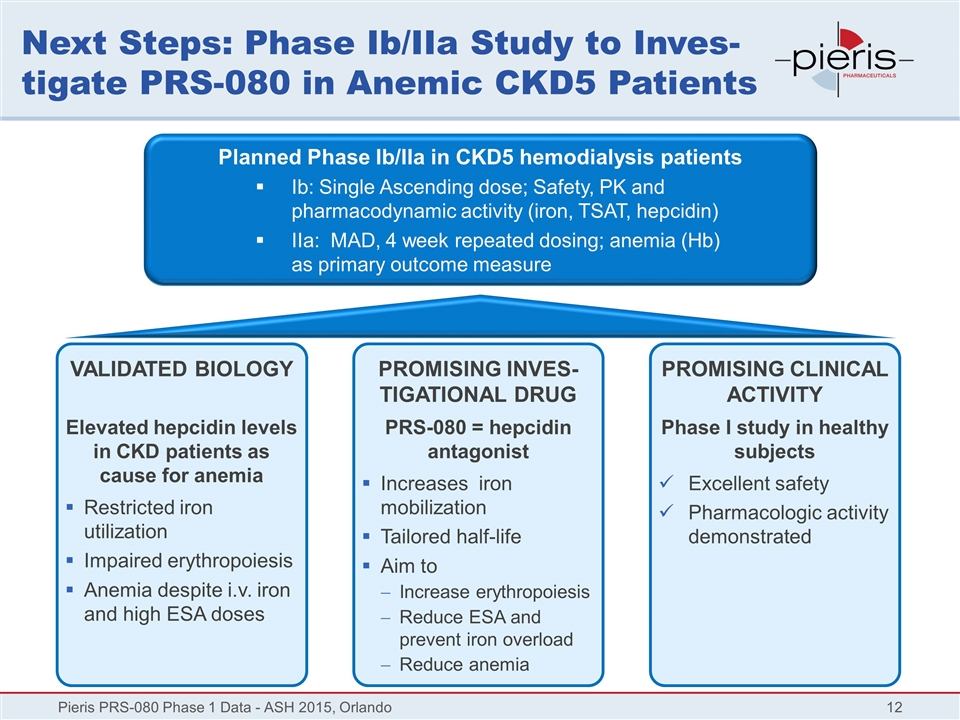
Next Steps: Phase Ib/IIa Study to Inves-tigate PRS-080 in Anemic CKD5 Patients Pieris PRS-080 Phase 1 Data - ASH 2015, Orlando Planned Phase Ib/IIa in CKD5 hemodialysis patients Ib: Single Ascending dose; Safety, PK and pharmacodynamic activity (iron, TSAT, hepcidin) IIa: MAD, 4 week repeated dosing; anemia (Hb) as primary outcome measure VALIDATED BIOLOGY Elevated hepcidin levels in CKD patients as cause for anemia Restricted iron utilization Impaired erythropoiesis Anemia despite i.v. iron and high ESA doses PROMISING INVES-TIGATIONAL DRUG PRS-080 = hepcidin antagonist Increases iron mobilization Tailored half-life Aim to Increase erythropoiesis Reduce ESA and prevent iron overload Reduce anemia PROMISING CLINICAL ACTIVITY Phase I study in healthy subjects Excellent safety Pharmacologic activity demonstrated

Pieris Pharmaceuticals GmbH Munich Site Lise-Meitner-Strasse 30 85354 Freising Germany Pieris Pharmaceuticals, Inc. Corporate Headquarters 255 State Street, 9th floor Boston, MA 02109 USA www.pieris.com Thank you