Exhibit 99.1
1 1 Rencofilstat (CRV431): A liver - targeting drug candidate for NASH and HCC January 2022 | INVESTOR PRESENTATION smart drug smart technology smart development

2 This presentation may contain forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Such forward - looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words . You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward - looking information . Such statements are only predictions, and our actual results may differ materially from those anticipated in these forward - looking statements . We believe that it is important to communicate future expectations to investors . However, there may be events in the future that we are not able to accurately predict or control . Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger - scale clinical trials, the risk that we will not obtain approval to market our products, risks associated with delays, increased costs and funding shortages caused by the COVID - 19 pandemic ; the risks associated with dependence upon key personnel and the need for additional financing . We do not assume any obligation to update forward - looking statements as circumstances change . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Hepion Pharmaceuticals or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . Statements Forward - Looking
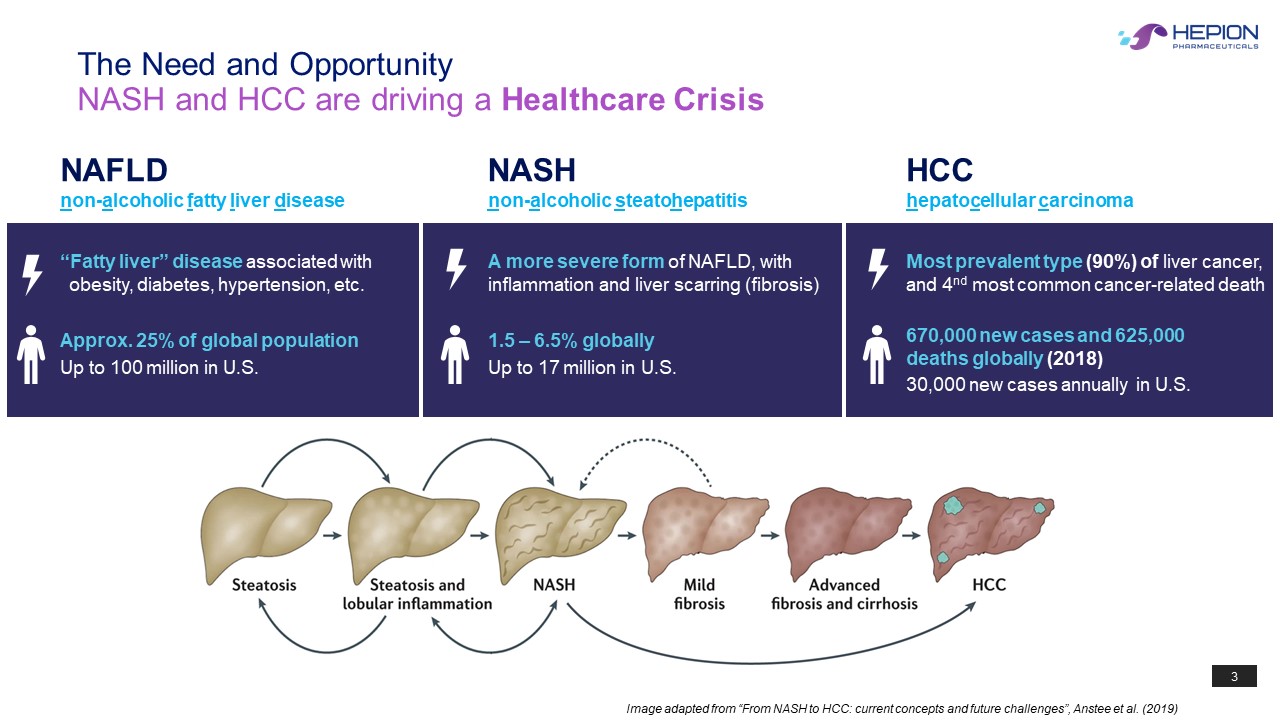
3 NASH and HCC are driving a Healthcare Crisis The Need and Opportunity Image adapted from “From NASH to HCC: current concepts and future challenges”, Anstee et al. (2019) NAFLD n on - a lcoholic f atty l iver d isease Approx. 25% of global population Up to 100 million in U.S. “Fatty liver” disease associated with obesity, diabetes, hypertension, etc. 1.5 – 6.5% globally Up to 17 million in U.S. A more severe form of NAFLD, with inflammation and liver scarring (fibrosis) NASH n on - a lcoholic s teato h ep atitis HCC h epato c ellular c arcinoma Most prevalent type (90%) of liver cancer, and 4 nd most common cancer - related death 670,000 new cases and 625,000 deaths globally (2018) 30,000 new cases annually in U.S.
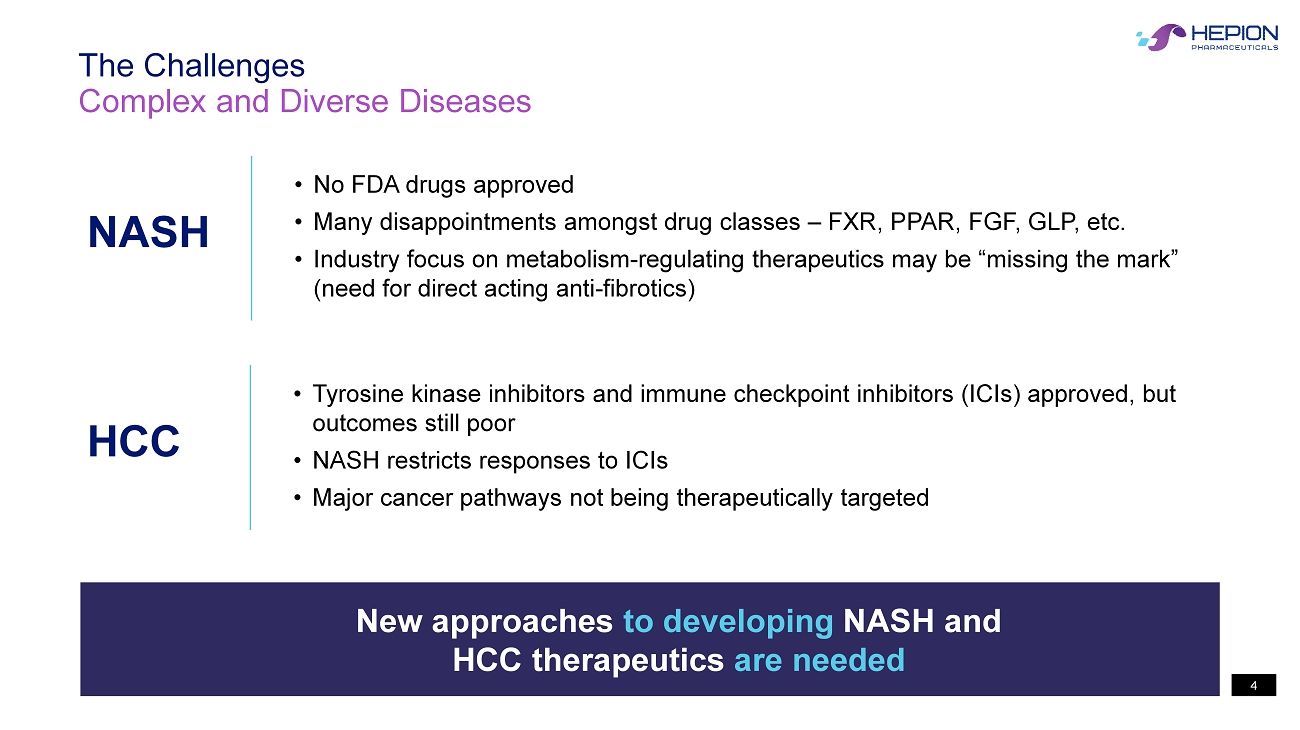
4 Complex and Diverse Diseases The Challenges New approaches to developing NASH and HCC therapeutics are needed NASH • No FDA drugs approved • Many disappointments amongst drug classes – FXR, PPAR, FGF, GLP, etc. • Industry focus on metabolism - regulating therapeutics may be “missing the mark” (need for direct acting anti - fibrotics ) HCC • Tyrosine kinase inhibitors and immune checkpoint inhibitors (ICIs) approved, but outcomes still poor • NASH restricts responses to ICIs • Major cancer pathways not being therapeutically targeted 4
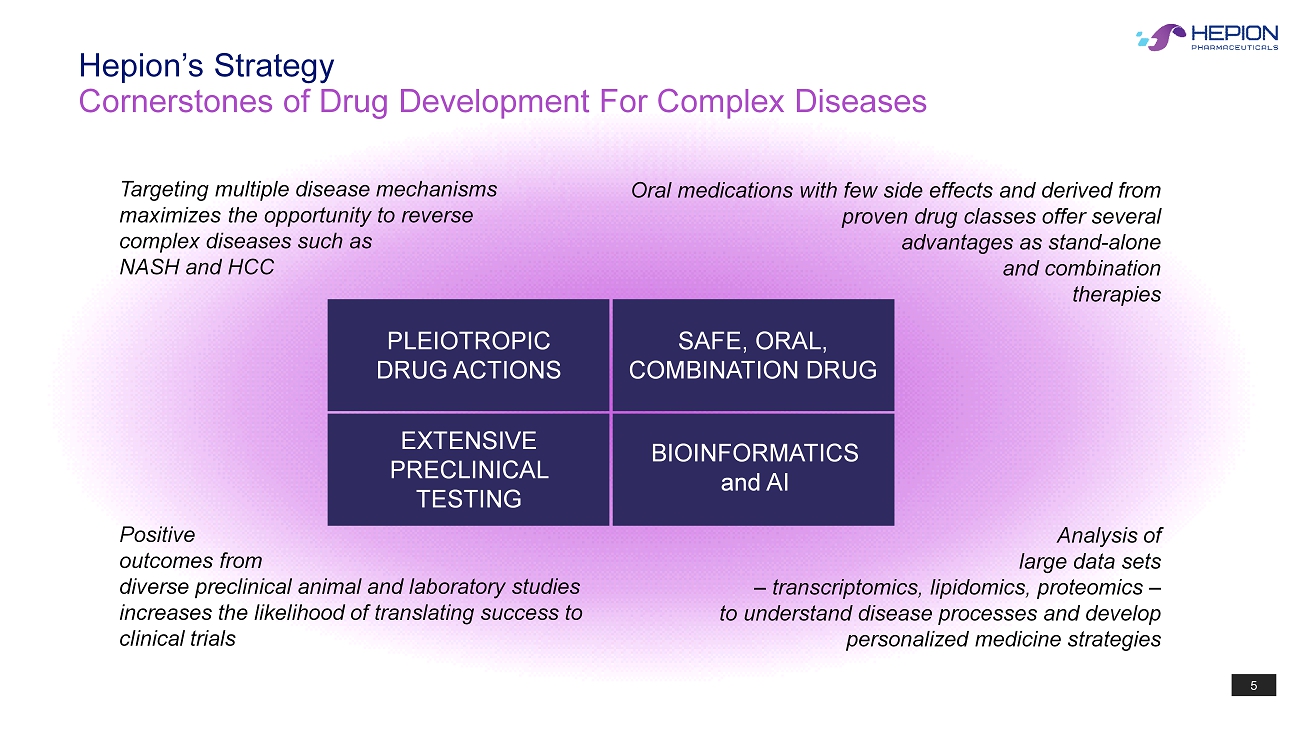
5 Cornerstones of Drug Development For Complex Diseases Hepion’s Strategy PLEIOTROPIC DRUG ACTIONS SAFE, ORAL, COMBINATION DRUG EXTENSIVE PRECLINICAL TESTING BIOINFORMATICS and AI Targeting multiple disease mechanisms maximizes the opportunity to reverse complex diseases such as NASH and HCC Oral medications with few side effects and derived from proven drug classes offer several advantages as stand - alone and combination therapies Positive outcomes from diverse preclinical animal and laboratory studies increases the likelihood of translating success to clinical trials Analysis of large data sets – transcriptomics, lipidomics , proteomics – to understand disease processes and develop personalized medicine strategies
6 Rencofilstat Drug Candidate for NASH and HCC • Cyclophilin inhibitor – new drug class • Once - daily, oral medication – soft gel capsules • No serious adverse effects • Liver targeting: [liver] > [blood] • Pleiotropic activities in preclinical models of NASH, liver fibrosis, and hepatocellular carcinoma (HCC) • Clinical Studies – 159 subjects dosed successfully • Phase 2 NASH and HCC – to be initiated, H2, 2022 Rencofilstat
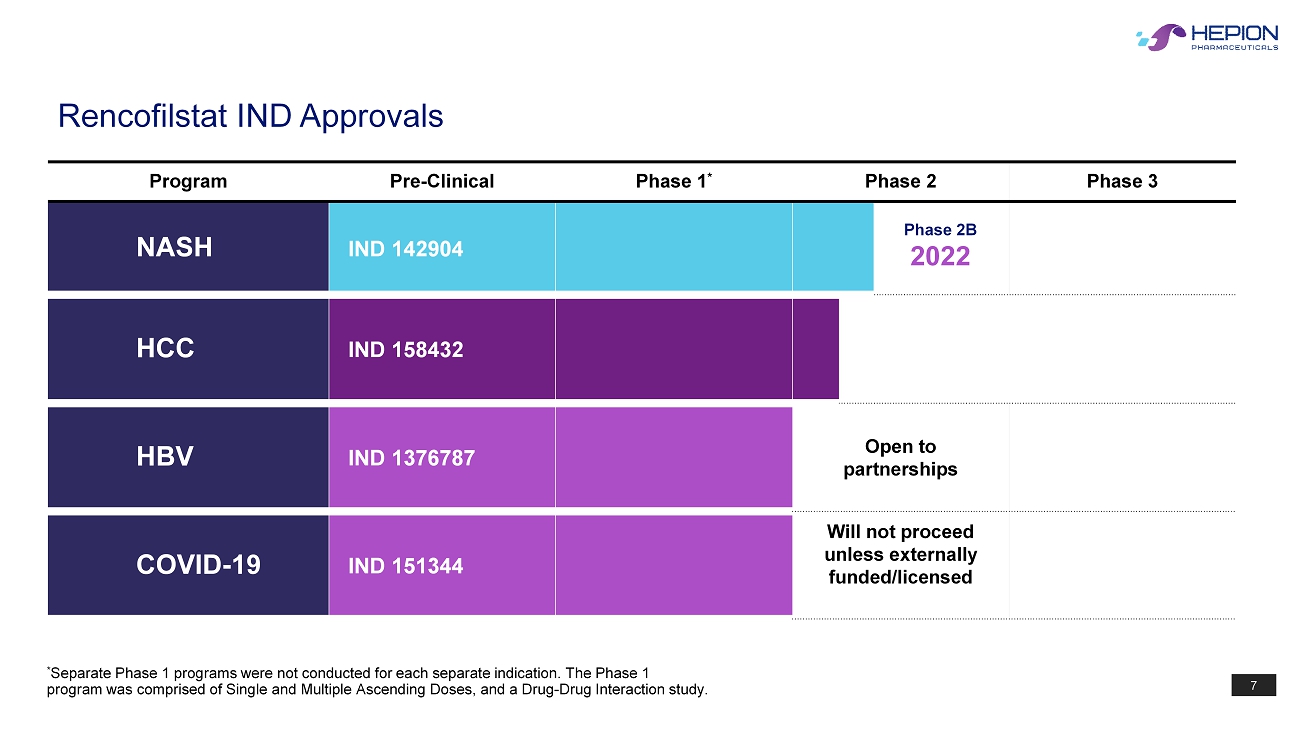
7 Program Pre - Clinical Phase 1 * Phase 2 Phase 3 NASH IND 142904 HCC IND 158432 HBV IND 1376787 Open to partnerships COVID - 19 IND 151344 Will not proceed unless externally funded/licensed Rencofilstat IND Approvals * Separate Phase 1 programs were not conducted for each separate indication. The Phase 1 program was comprised of Single and Multiple Ascending Doses, and a Drug - Drug Interaction study. Phase 2B 2022
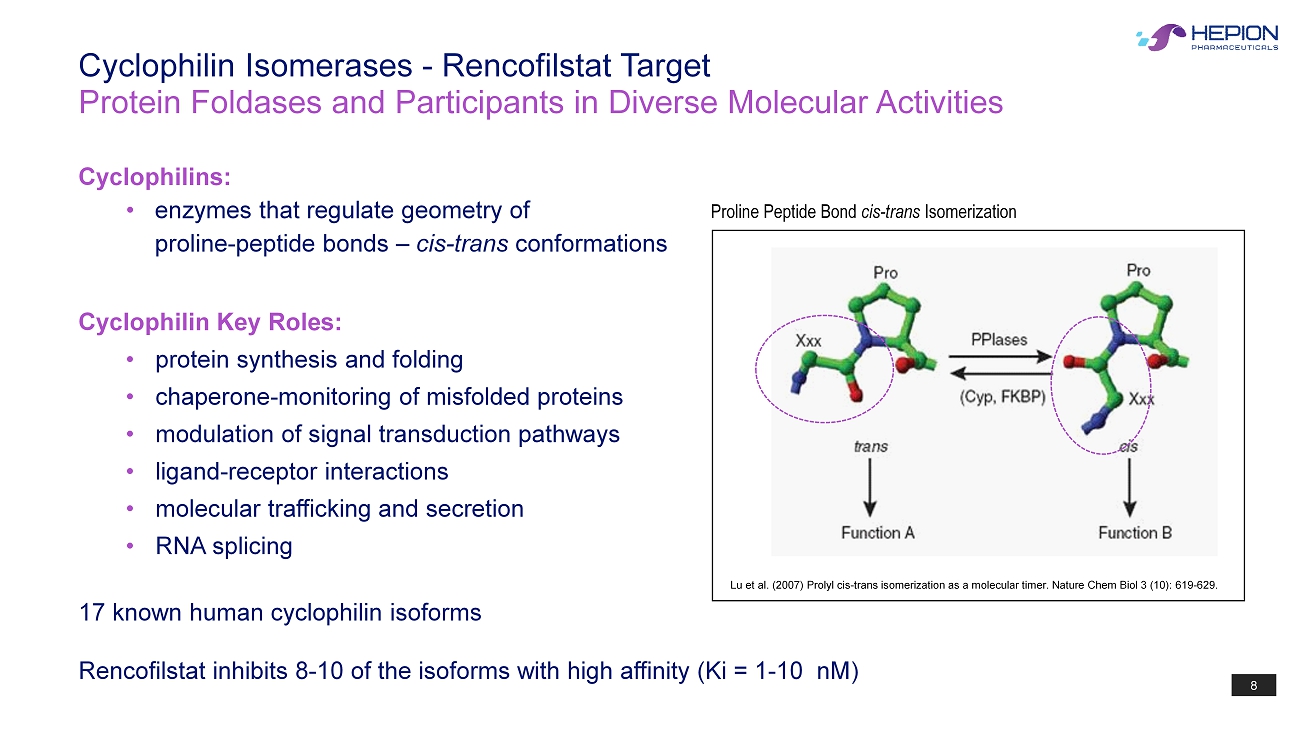
8 Protein Foldases and Participants in Diverse Molecular Activities Cyclophilin Isomerases - Rencofilstat Target Cyclophilins: • enzymes that regulate geometry of proline - peptide bonds – cis - trans conformations Cyclophilin Key Roles: • protein synthesis and folding • chaperone - monitor ing of misfolded proteins • modulation of signal transduction pathways • ligand - receptor interactions • molecular trafficking and secretion • RNA splicing 17 known human cyclophilin isoforms Rencofilstat inhibits 8 - 10 of the isoforms with high affinity (Ki = 1 - 10 nM ) Lu et al. (2007) Prolyl cis - trans isomerization as a molecular timer. Nature Chem Biol 3 (10): 619 - 629. Proline Peptide Bond cis - trans Isomerization
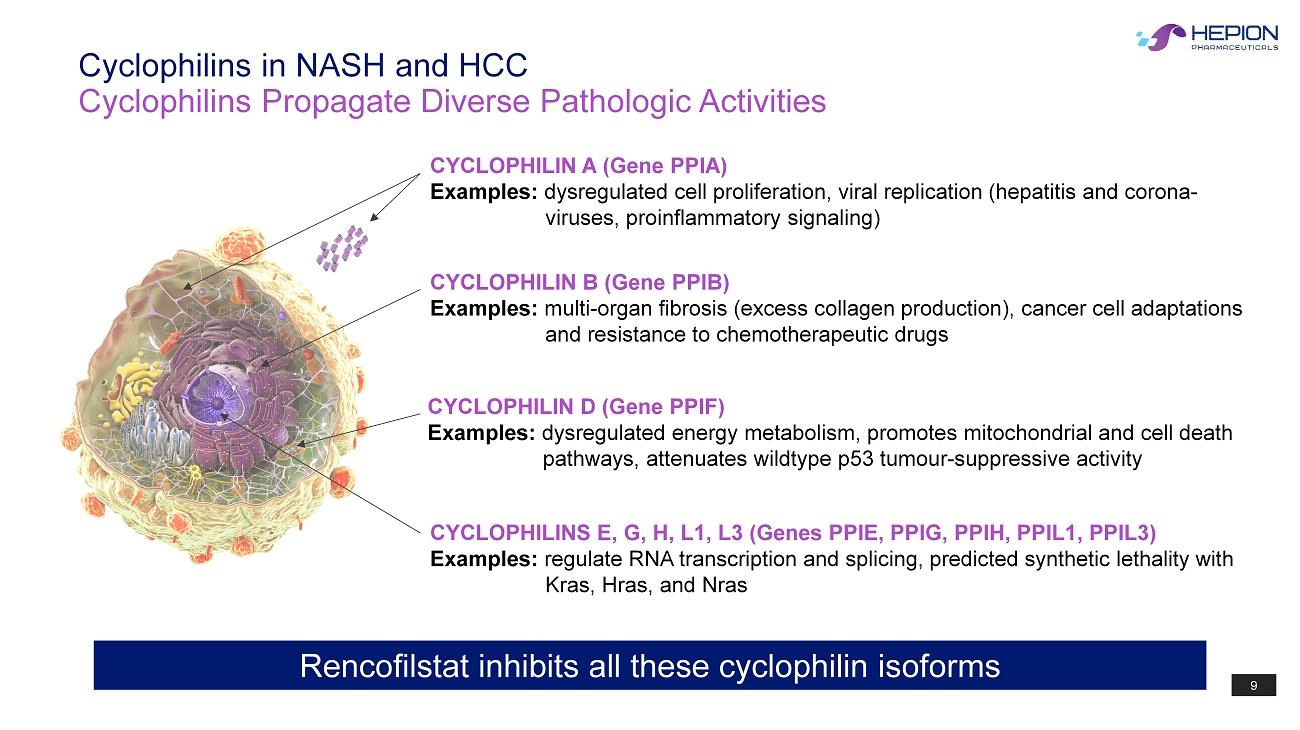
9 Cyclophilins Propagate Diverse Pathologic Activities Cyclophilins in NASH and HCC CYCLOPHILIN A (Gene PPIA) Examples: dysregulated cell proliferation, viral replication (hepatitis and corona - viruses, proinflammatory signaling) CYCLOPHILIN B (Gene PPIB) Examples: multi - organ fibrosis (excess collagen production), cancer cell adaptations and resistance to chemotherapeutic drugs CYCLOPHILIN D (Gene PPIF) Examples: dysregulated energy metabolism, promotes mitochondrial and cell death pathways, attenuates wildtype p53 tumour - suppressive activity CYCLOPHILINS E, G, H, L1, L3 (Genes PPIE, PPIG, PPIH, PPIL1, PPIL3) Examples: regulate RNA transcription and splicing, predicted synthetic lethality with Kras , Hras , and Nras Rencofilstat inhibits all these cyclophilin isoforms
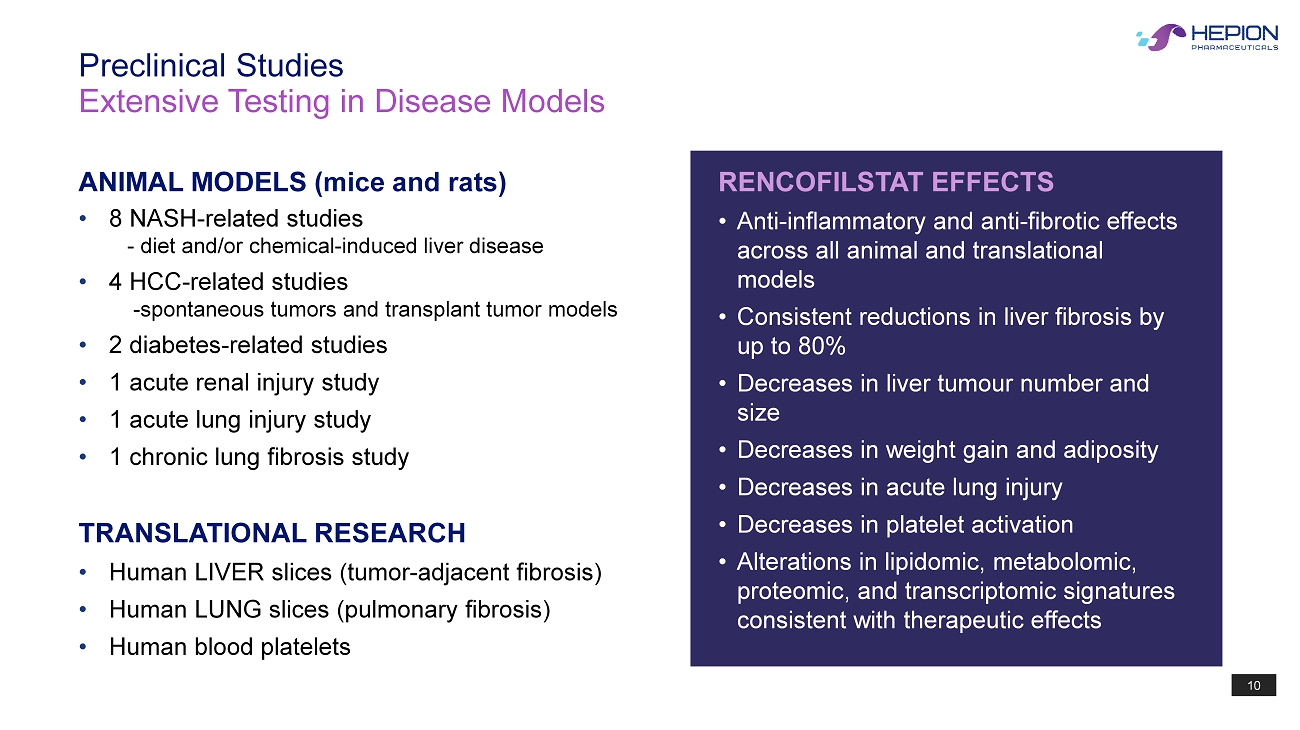
10 Extensive Testing in Disease Models Preclinical Studies ANIMAL MODELS (mice and rats) TRANSLATIONAL RESEARCH • 8 NASH - related studies - diet and/or chemical - induced liver disease • 4 HCC - related studies - spontaneous tumors and transplant tumor models • 2 diabetes - related studies • 1 acute renal injury study • 1 acute lung injury study • 1 chronic lung fibrosis study • Human LIVER slices (tumor - adjacent fibrosis) • Human LUNG slices (pulmonary fibrosis) • Human blood platelets RENCOFILSTAT EFFECTS • Anti - inflammatory and anti - fibrotic effects across all animal and translational models • Consistent reductions in liver fibrosis by up to 80% • Decreases in liver tumour number and size • Decreases in weight gain and adiposity • Decreases in acute lung injury • Decreases in platelet activation • Alterations in lipidomic, metabolomic, proteomic, and transcriptomic signatures consistent with therapeutic effects

11 Overview of PHASE 2A ‘AMBITION’ NASH TRIAL
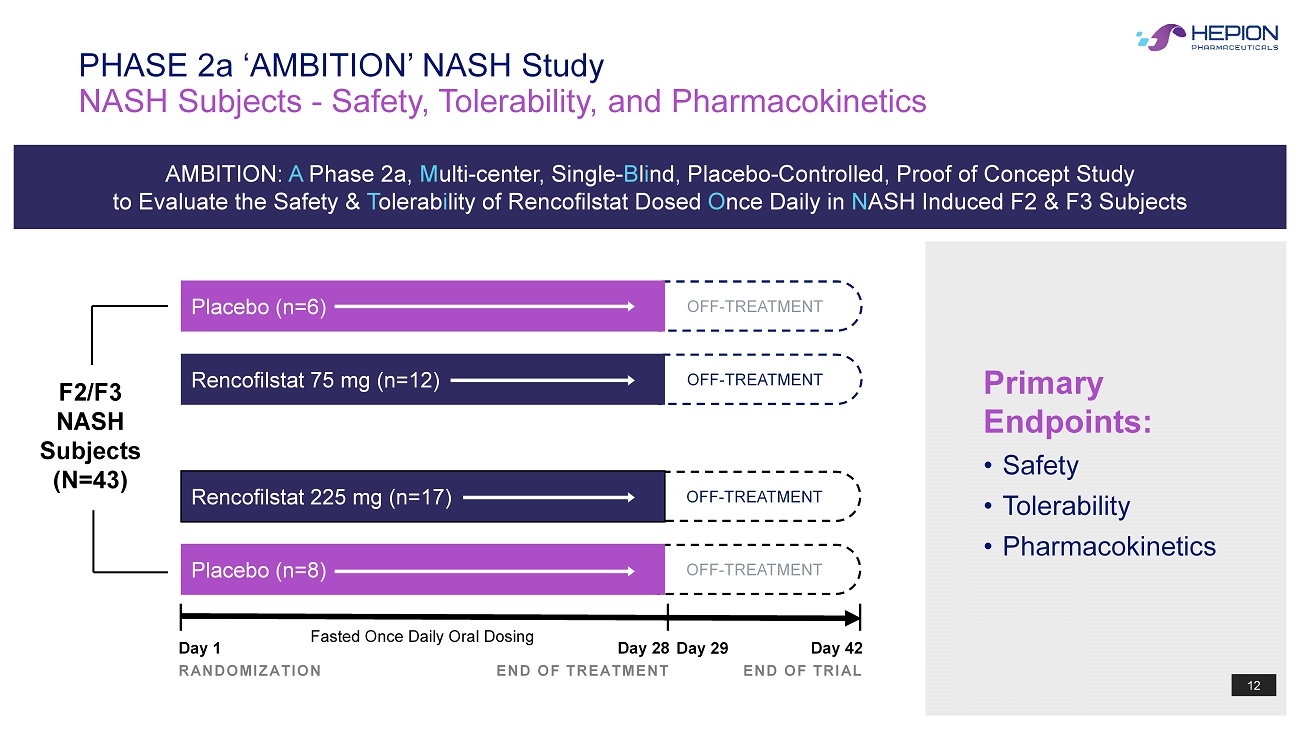
12 O FF - TREATMENT O FF - TREATMENT O FF - TREATMENT O FF - TREATMENT NASH Subjects - Safety, Tolerability, and Pharmacokinetics PHASE 2a ‘AMBITION’ NASH Study Primary Endpoints: • Safety • Tolerability • Pharmacokinetics Day 1 RANDOMIZATION Day 28 END OF TREATMENT Day 42 END OF TRIAL Rencofilstat 225 mg (n=17) Placebo (n=8) F2/F3 NASH Subjects (N=43) Fasted Once Daily Oral Dosing Day 29 Placebo (n=6) Rencofilstat 75 mg (n=12) AMBITION: A Phase 2a, M ulti - center, Single - B l i nd, Placebo - Controlled, Proof of Concept Study to Evaluate the Safety & T olerab i lity of Rencofilstat Dosed O nce Daily in N ASH Induced F2 & F3 Subjects 12

13 Phase 2a Study Summary • Rencofilstat, safe and well - tolerated, while showing efficacy signals in only 28 days dosing, including: • Reduction in ALT (marker of inflammation) • Reduction in Pro - C3 (marker of fibrosis) • Blood exposures in subjects with NASH similar to healthy subjects • Data to support AI - POWR training and a priori prediction of rencofilstat responders • Data was utilized to support the addition of a 150 mg dose cohort for the Phase 2b protocol and to adjust inclusion criteria (e.g., Pro - C3 > 14 ng/mL)
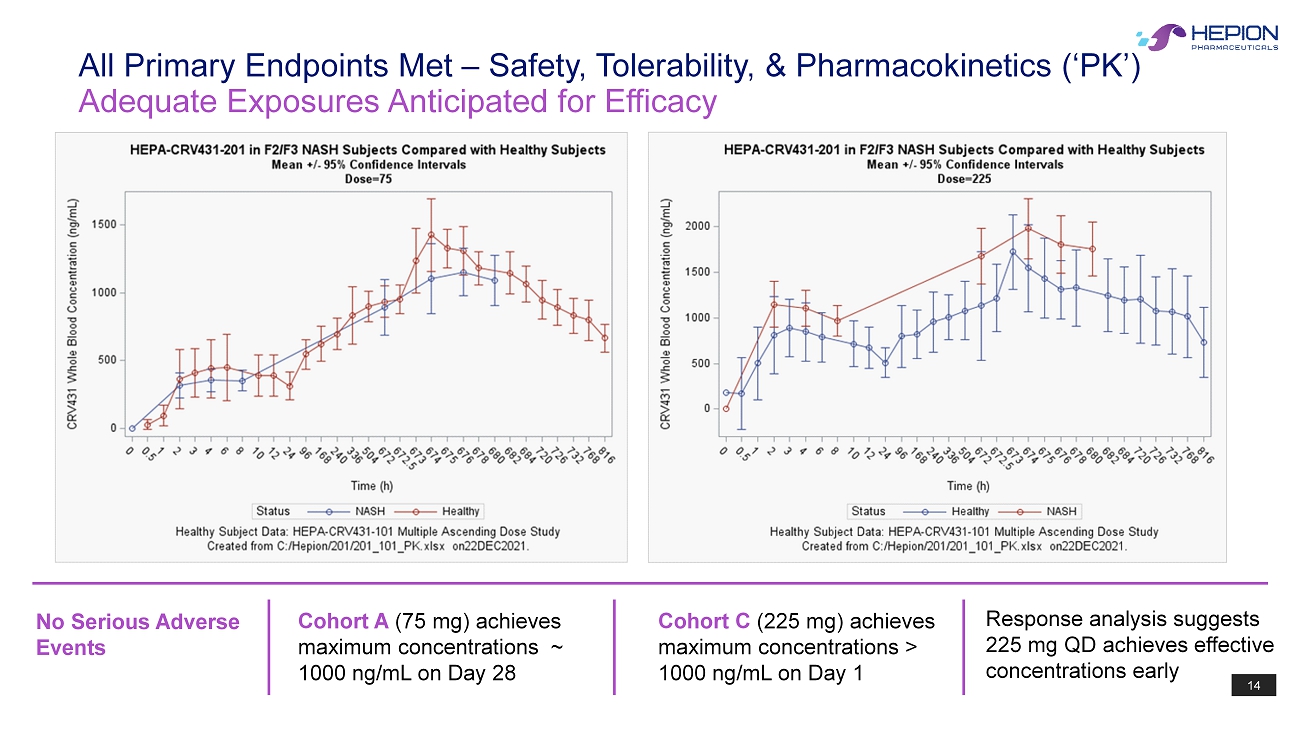
14 No Serious Adverse Events Adequate Exposures Anticipated for Efficacy All Primary Endpoints Met – Safety, Tolerability, & Pharmacokinetics (‘PK’) Cohort C (225 mg) achieves maximum concentrations > 1000 ng/mL on Day 1 Cohort A (75 mg) achieves maximum concentrations ~ 1000 ng/mL on Day 28 Response analysis suggests 225 mg QD achieves effective concentrations early
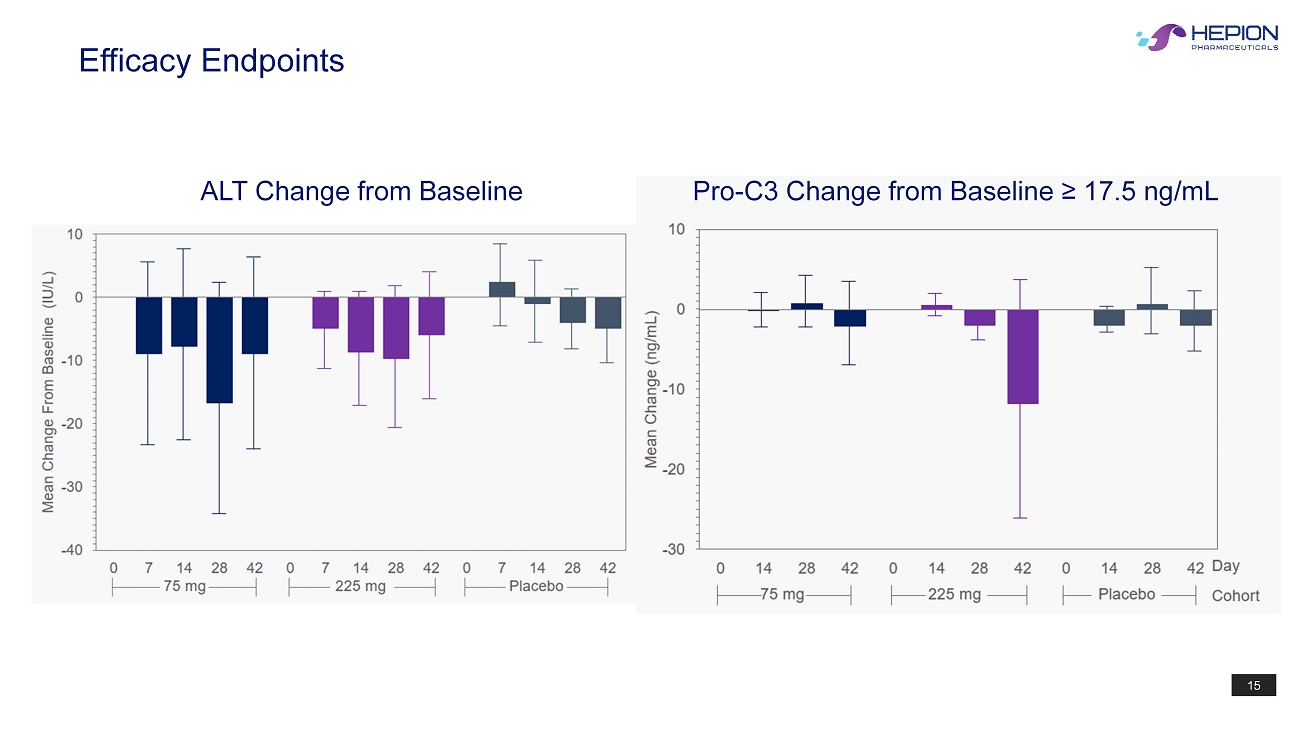
15 Efficacy Endpoints ALT Change from Baseline Pro - C3 Change from Baseline ≥ 17.5 ng/mL
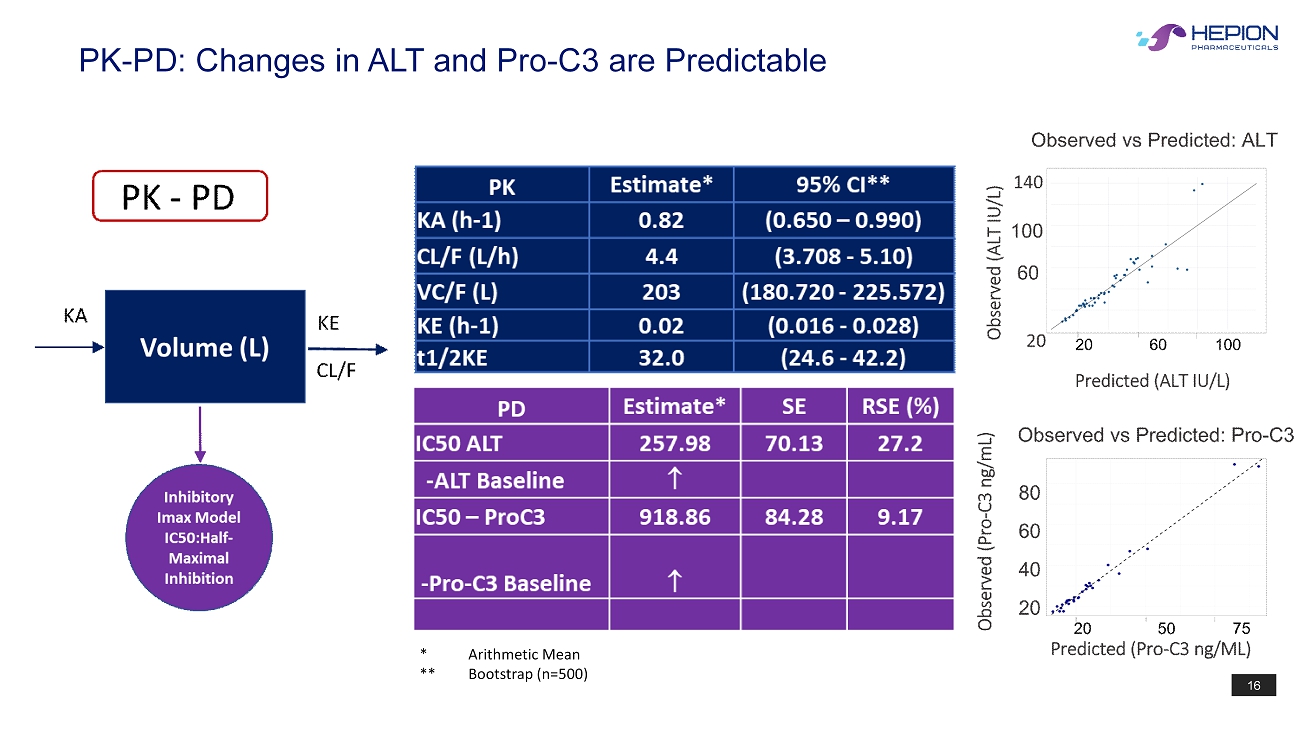
16 PK - PD: Changes in ALT and Pro - C3 are Predictable * Arithmetic Mean ** Bootstrap (n=500)
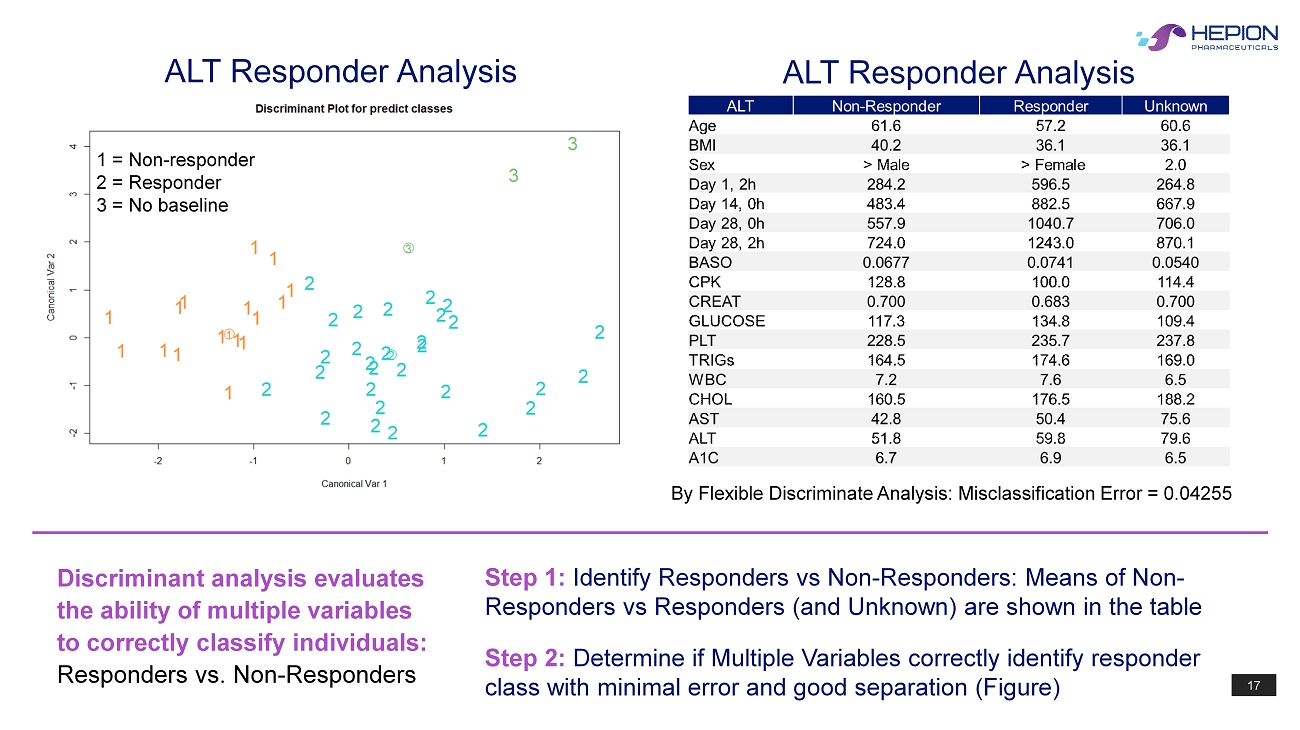
17 1 = Non - responder 2 = Responder 3 = No baseline By Flexible Discriminate Analysis: Misclassification Error = 0.04255 ALT Responder Analysis ALT Responder Analysis Discriminant analysis evaluates the ability of multiple variables to correctly classify individuals: Responders vs. Non - Responders Step 1: Identify Responders vs Non - Responders: Means of Non - Responders vs Responders (and Unknown) are shown in the table Step 2: Determine if Multiple Variables correctly identify responder class with minimal error and good separation (Figure)
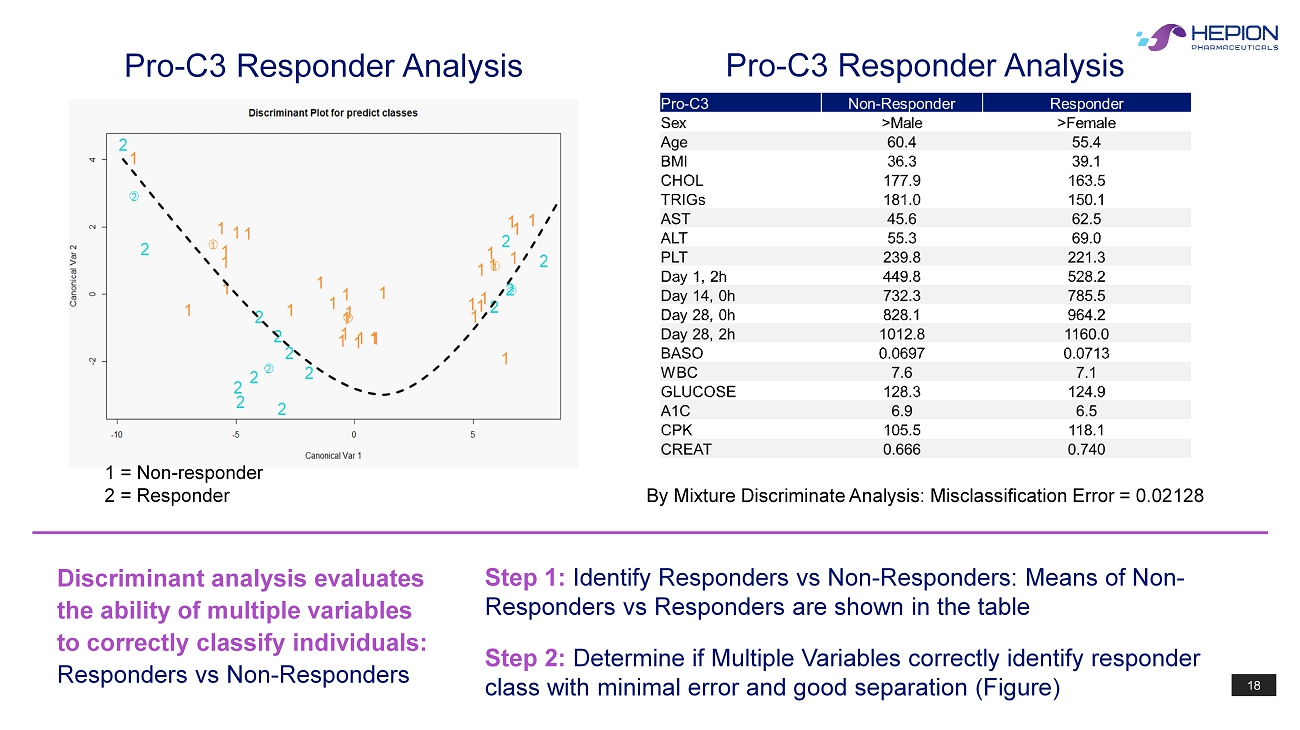
18 Pro - C3 Responder Analysis 1 = Non - responder 2 = Responder Pro - C3 Responder Analysis By Mixture Discriminate Analysis: Misclassification Error = 0.02128 Discriminant analysis evaluates the ability of multiple variables to correctly classify individuals: Responders vs Non - Responders Step 1: Identify Responders vs Non - Responders: Means of Non - Responders vs Responders are shown in the table Step 2: Determine if Multiple Variables correctly identify responder class with minimal error and good separation (Figure)
19 AI - POWR Œ NEURAL NETWORK MACHINE LEARNING BIG DATA DEEP LEARNING MULTI - OMICS NASH Rencofilstat: Multiple Beneficial Properties and State - of - the - Art Artificial Intelligence Hepion’s Artificial Intelligence Understand disease mechanisms Identify biomarkers Track disease progression and regression Predict drug responders Precision medicine
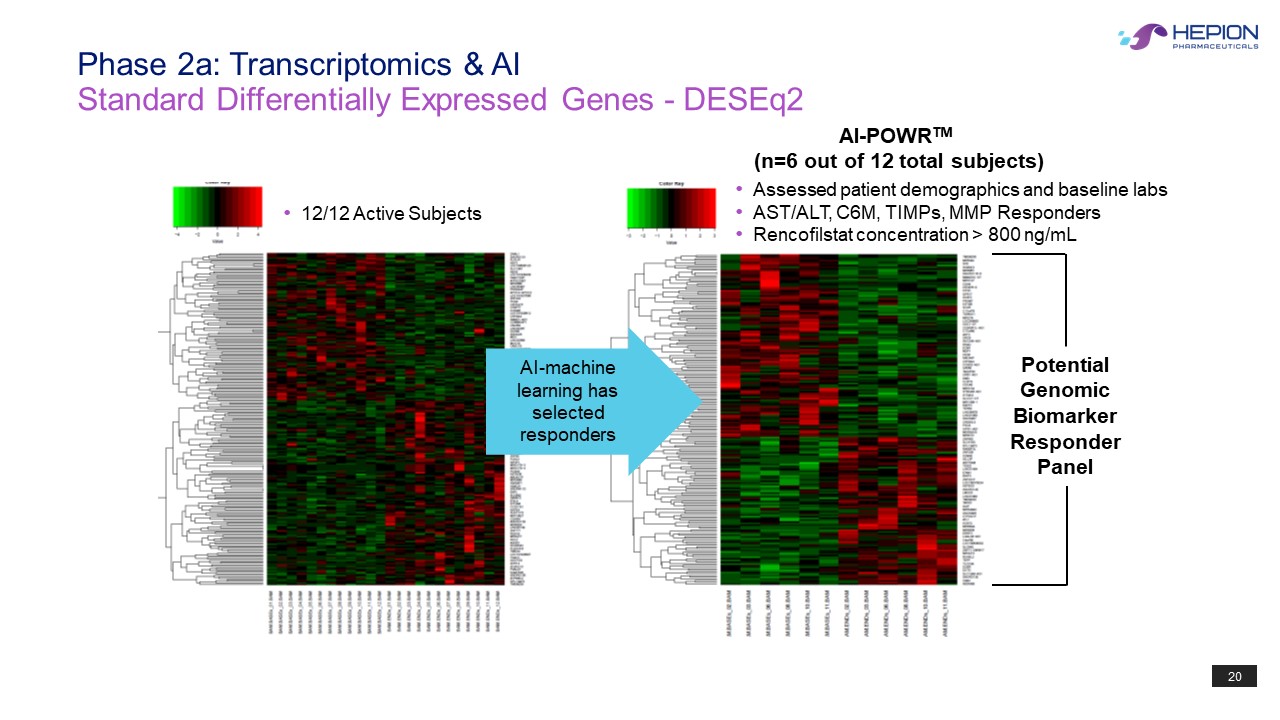
20 AI - POWR TM (n=6 out of 12 total subjects) • Assessed patient demographics and baseline labs • AST/ALT, C6M, TIMPs, MMP Responders • R encofilstat concentration > 800 ng / mL Potential Genomic Biomarke r Responder Panel • 12/12 Active Subjects AI - machine learning has selected responders Standard Differentially Expressed Genes - DESEq2 Phase 2a: Transcriptomics & AI 20
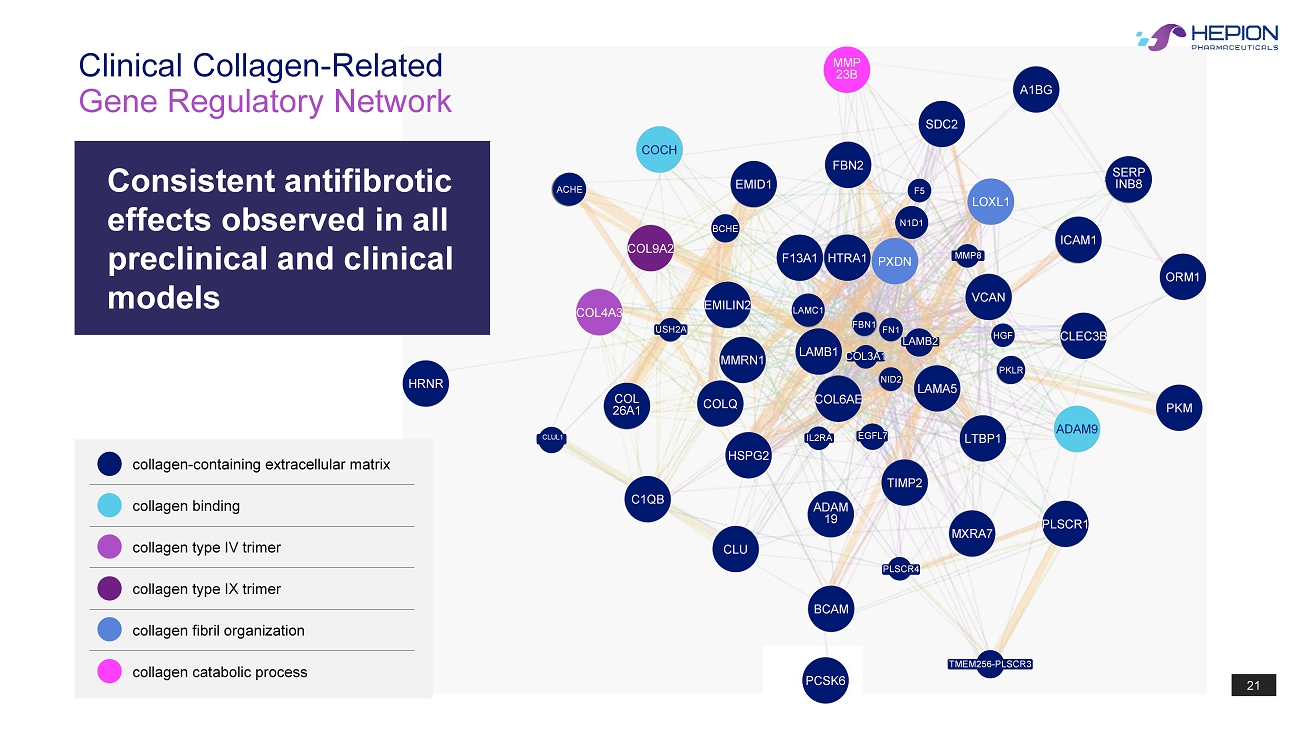
21 A1BG SDC2 MMP 23B FBN2 EMID1 LOXL1 SERP INB8 ORM1 ICAM1 VCAN CLEC3B PXDN HTRA1 F13A1 COCH COL9A2 COL4A3 EMILIN2 MMRN1 LAMB1 COL6AE LAMA5 ADAM9 LTBP1 TIMP2 COL 26A1 HRNR COLQ HSPG2 C1QB CLU ADAM 19 BCAM MXRA7 PLSCR1 PCSK6 BCHE N1D1 F5 FN1 FBN1 HGF PKLR NID2 COL3A1 LAMB2 LAMC1 IL2RA EGFL7 PLSCR4 USH2A MMP8 CLUL1 TMEM256 - PLSCR3 PKM ACHE Gene Regulatory Network Clinical Collagen - Related collagen - containing extracellular matrix collagen binding collagen type IV trimer collagen type IX trimer collagen fibril organization collagen catabolic process Consistent antifibrotic effects observed in all preclinical and clinical models

22 PHASE 2B ‘ASCEND - NASH’ TRIAL
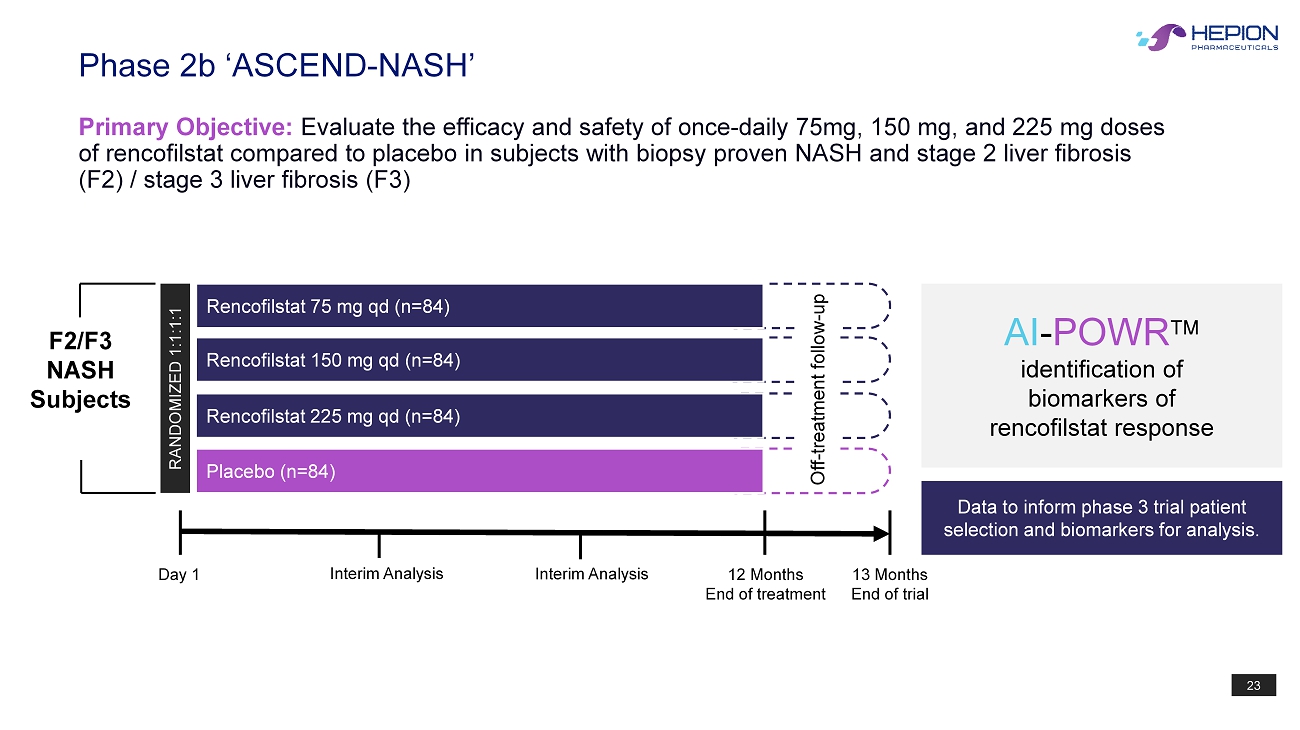
23 Phase 2b ‘ASCEND - NASH’ Primary Objective: Evaluate the efficacy and safety of once - daily 75mg, 150 mg, and 225 mg doses of rencofilstat compared to placebo in subjects with biopsy proven NASH and stage 2 liver fibrosis (F2) / stage 3 liver fibrosis (F3) RANDOMIZED 1:1:1:1 Day 1 12 Months End of treatment Rencofilstat 225 mg qd (n=84) Placebo (n=84) Rencofilstat 150 mg qd (n=84) 13 Months End of trial Data to inform phase 3 trial patient selection and biomarkers for analysis. AI - POWR identification of biomarkers of rencofilstat response Off - treatment follow - up Rencofilstat 75 mg qd (n=84) Interim Analysis Interim Analysis F2/F3 NASH Subjects

24 PHASE 2A HCC TRIAL
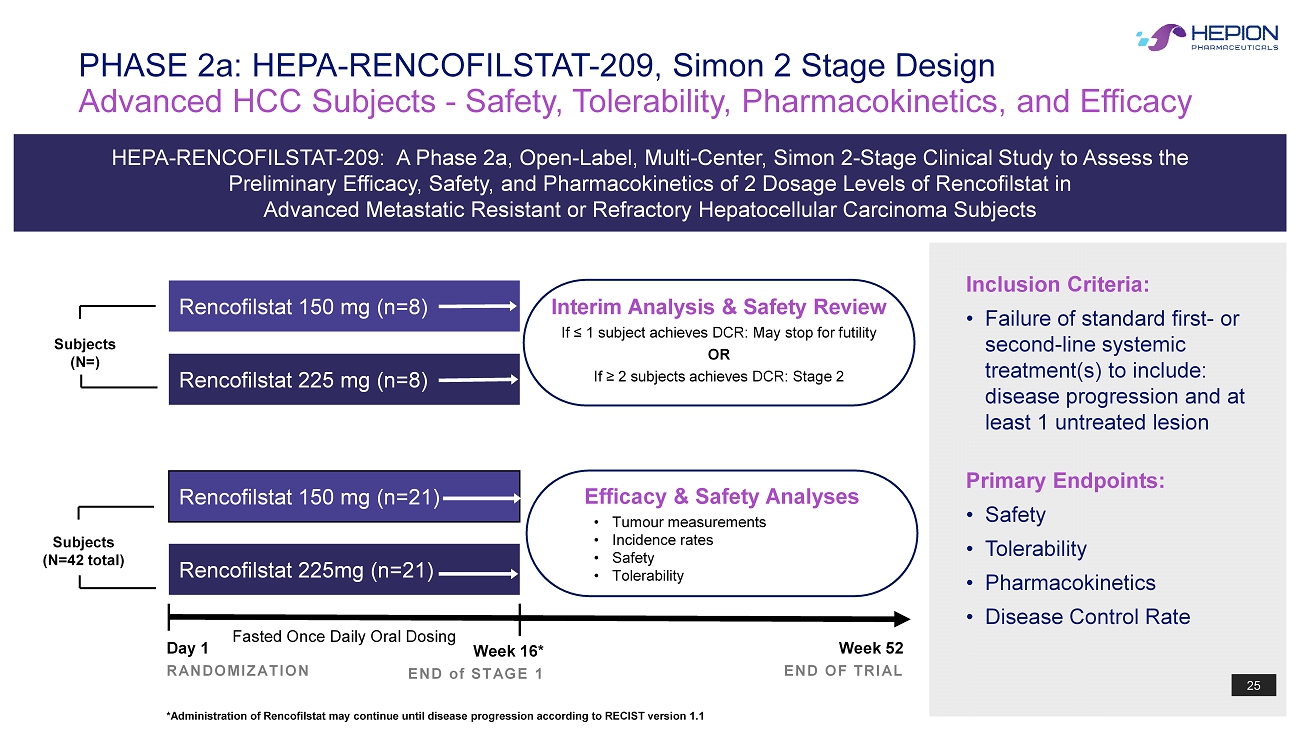
25 Interim Analysis & Safety Review If ≤ 1 subject achieves DCR: May stop for futility OR If ≥ 2 subjects achieves DCR: Stage 2 Advanced HCC Subjects - Safety, Tolerability, Pharmacokinetics, and Efficacy PHASE 2a: HEPA - RENCOFILSTAT - 209, Simon 2 Stage Design Day 1 RANDOMIZATION Week 16* END of STAGE 1 Week 52 END OF TRIAL Rencofilstat 150 mg (n= 21 ) Rencofilstat 225mg (n=21) Fasted Once Daily Oral Dosing Rencofilstat 150 mg (n=8) Rencofilstat 225 mg (n= 8 ) HEPA - RENCOFILSTAT - 209 : A Phase 2a, Open - Label, Multi - Center, Simon 2 - Stage Clinical Study to Assess the Preliminary Efficacy, Safety, and Pharmacokinetics of 2 Dosage Levels of Rencofilstat in Advanced Metastatic Resistant or Refractory Hepatocellular Carcinoma Subjects 25 Subjects (N=) Subjects (N=42 total) *Administration of Rencofilstat may continue until disease progression according to RECIST version 1.1 Efficacy & Safety Analyses • Tumour measurements • Incidence rates • Safety • Tolerability Inclusion Criteria: • Failure of standard first - or second - line systemic treatment(s) to include: disease progression and at least 1 untreated lesion Primary Endpoints: • Safety • Tolerability • Pharmacokinetics • Disease Control Rate
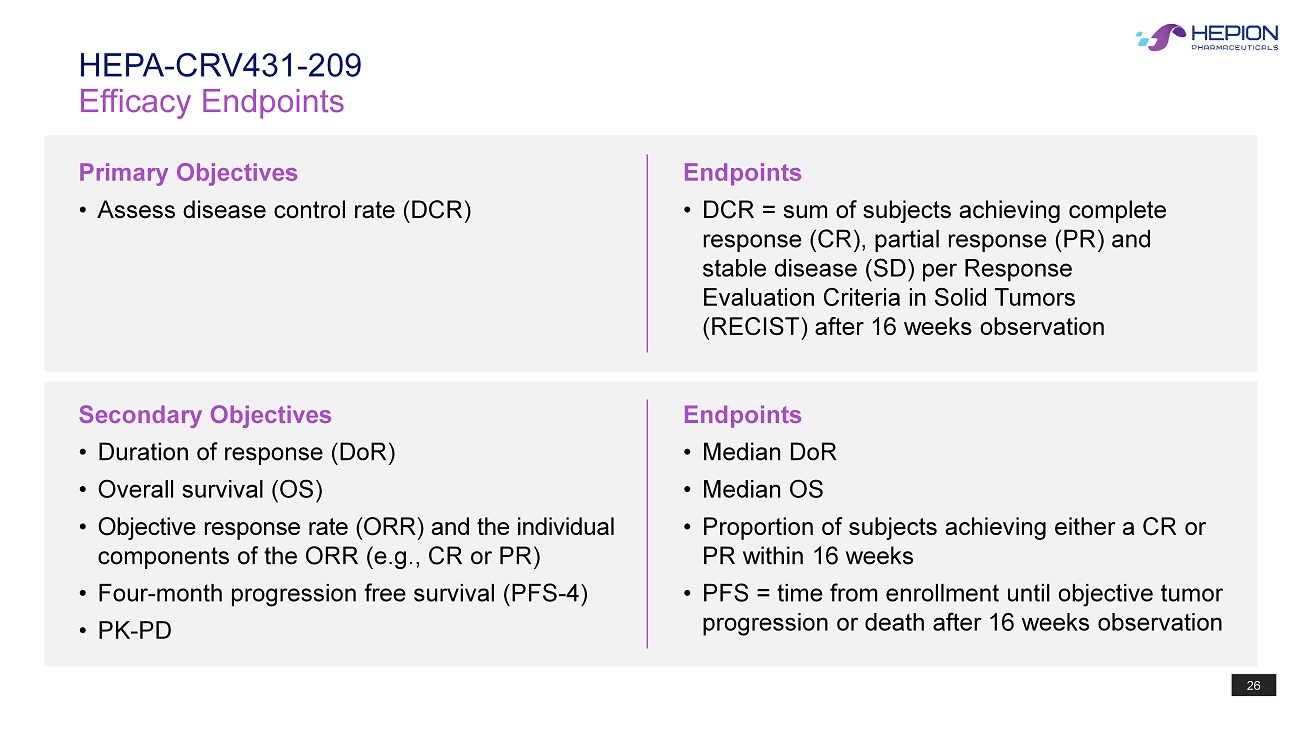
26 Efficacy Endpoints HEPA - CRV431 - 209 Primary Objectives • Assess disease control rate (DCR) Endpoints • DCR = sum of subjects achieving complete response (CR), partial response (PR) and stable disease (SD) per Response Evaluation Criteria in Solid Tumors (RECIST) after 16 weeks observation Endpoints • Median DoR • Median OS • Proportion of subjects achieving either a CR or PR within 16 weeks • PFS = time from enrollment until objective tumor progression or death after 16 weeks observation Secondary Objectives • Duration of response ( DoR ) • Overall survival (OS) • Objective response rate (ORR) and the individual components of the ORR (e.g., CR or PR) • Four - month progression free survival (PFS - 4) • PK - PD

27 $98.7 M Cash as of 9/30/21 Financials Two Value Drivers A Potential Therapy for NASH and HCC AI - Driven, Bioinformatic Platform Summary • Rencofilstat , once - daily oral, multi - modal • Phase 2a NASH trial completed with success • Phase 2b activities initiated for NASH • Phase 2a activities initiated for HCC • Hepion’s Proprietary Artificial Intelligence Platform (AI - POWR Ρ ) • Core scientific team with >100 years collective cyclophilin expertise • Core scientific team discovered and developed voclosporin (currently marketed) • Robust IP 76.2 M Common Shares Outstanding 27
CONTACT US Robert T. Foster, PharmD, Ph.D. Chief Executive Officer Hepion Pharmaceuticals Inc. 399 Thornall Street, First Floor Edison, New Jersey, USA, 08837 Email: rfoster@hepionpharma.com www.hepionpharma.com



























