Exhibit 99.1
1 1 Rencofilstat (CRV431): A Novel Drug Candidate for NASH, Fibrosis, and HCC October 2022 Creating a Therapeutic Ecosystem NASDAQ: HEPA

2 This presentation may contain forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Such forward - looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words . You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward - looking information . Such statements are only predictions, and our actual results may differ materially from those anticipated in these forward - looking statements . We believe that it is important to communicate future expectations to investors . However, there may be events in the future that we are not able to accurately predict or control . Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger - scale clinical trials, the risk that we will not obtain approval to market our products, risks associated with delays, increased costs and funding shortages caused by the COVID - 19 pandemic ; the risks associated with dependence upon key personnel and the need for additional financing . We do not assume any obligation to update forward - looking statements as circumstances change . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Hepion Pharmaceuticals or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . Forward - Looking Statements
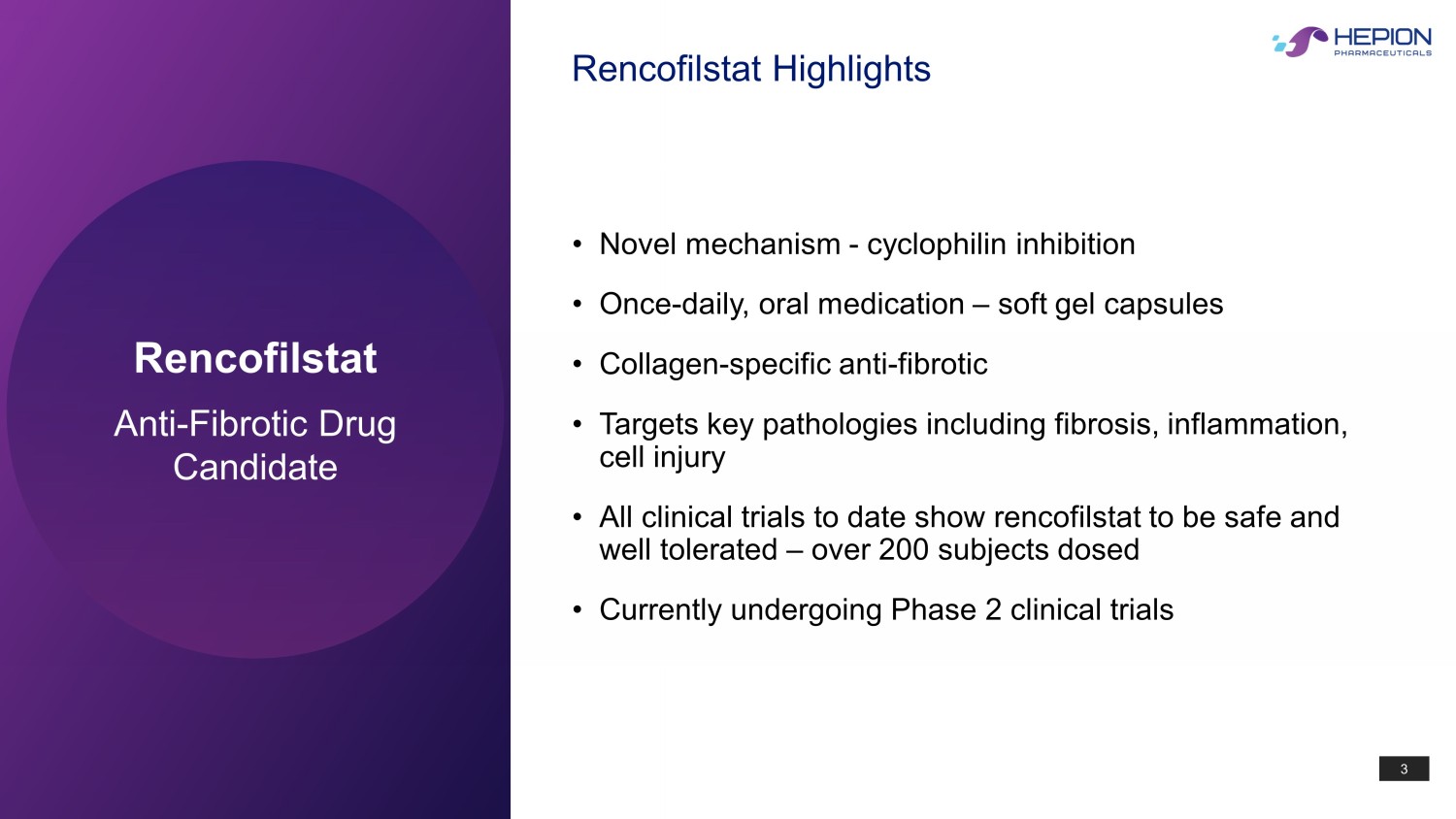
3 Rencofilstat Anti - Fibrotic Drug Candidate • Novel mechanism - cyclophilin inhibition • Once - daily, oral medication – soft gel capsules • Collagen - specific anti - fibrotic • Targets key pathologies including fibrosis, inflammation, cell injury • All clinical trials to date show rencofilstat to be safe and well tolerated – over 200 subjects dosed • Currently undergoing Phase 2 clinical trials Rencofilstat Highlights
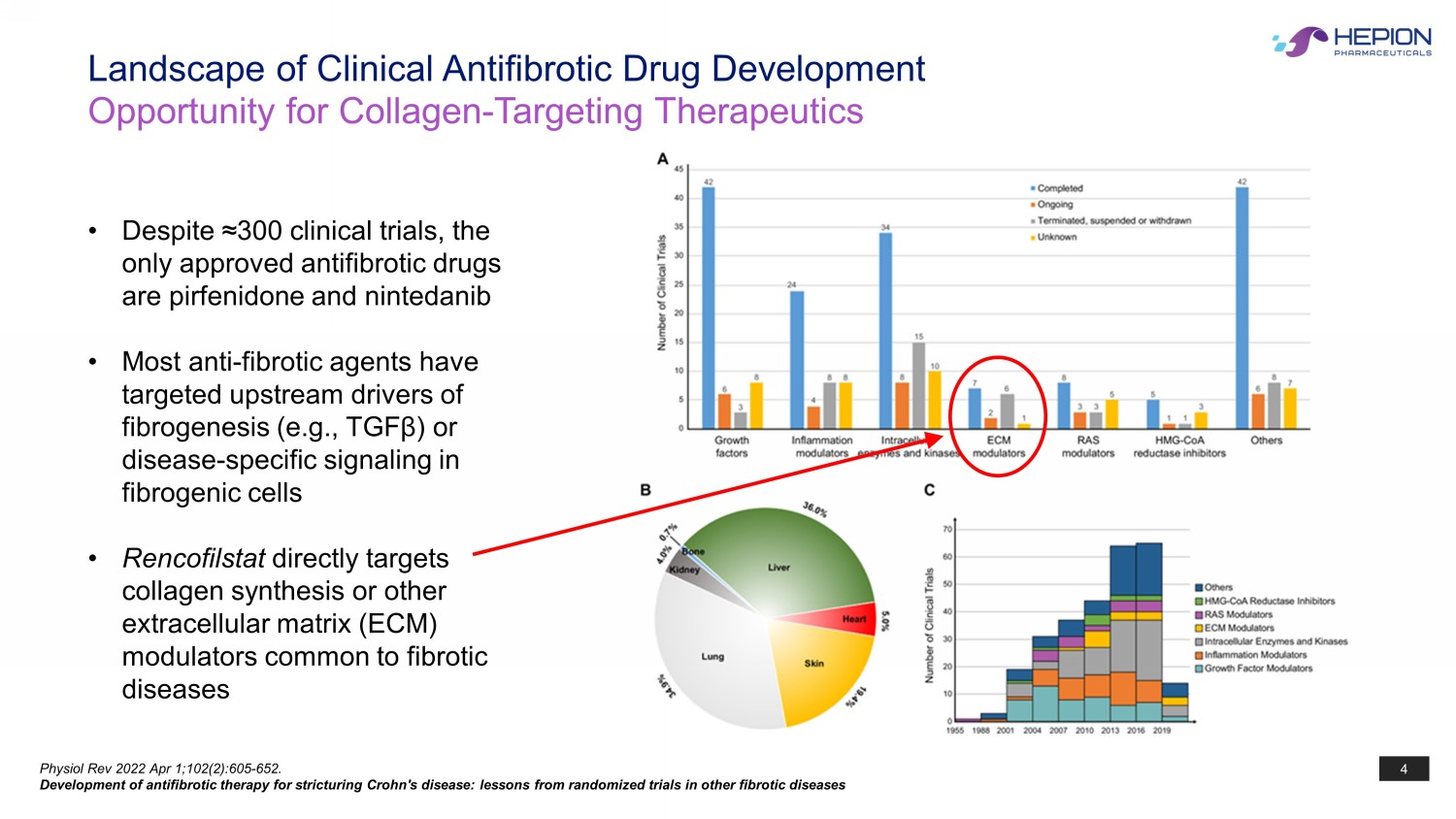
4 Physiol Rev 2022 Apr 1;102(2):605 - 652. Development of antifibrotic therapy for stricturing Crohn's disease: lessons from randomized trials in other fibrotic diseases • Despite ≈300 clinical trials, the only approved antifibrotic drugs are pirfenidone and nintedanib • Most anti - fibrotic agents have targeted upstream drivers of fibrogenesis (e.g., TGF β ) or disease - specific signaling in fibrogenic cells • Rencofilstat directly targets collagen synthesis or other extracellular matrix (ECM) modulators common to fibrotic diseases Landscape of Clinical Antifibrotic Drug Development Opportunity for Collagen - Targeting Therapeutics
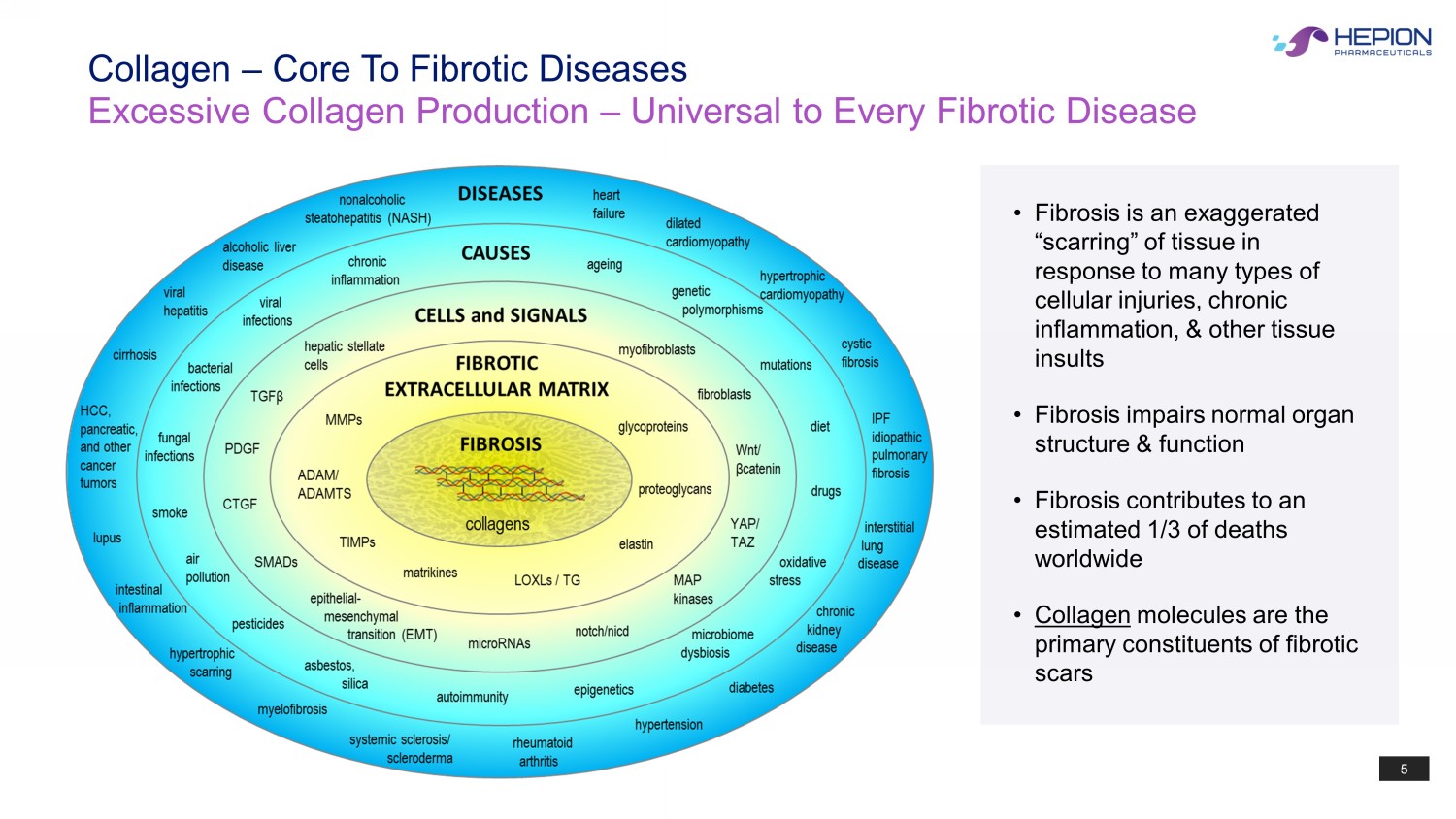
5 Collagen – Core To Fibrotic Diseases Excessive Collagen Production – Universal to Every Fibrotic Disease • Fibrosis is an exaggerated “scarring” of tissue in response to many types of cellular injuries, chronic inflammation, & other tissue insults • Fibrosis impairs normal organ structure & function • Fibrosis contributes to an estimated 1/3 of deaths worldwide • Collagen molecules are the primary constituents of fibrotic scars
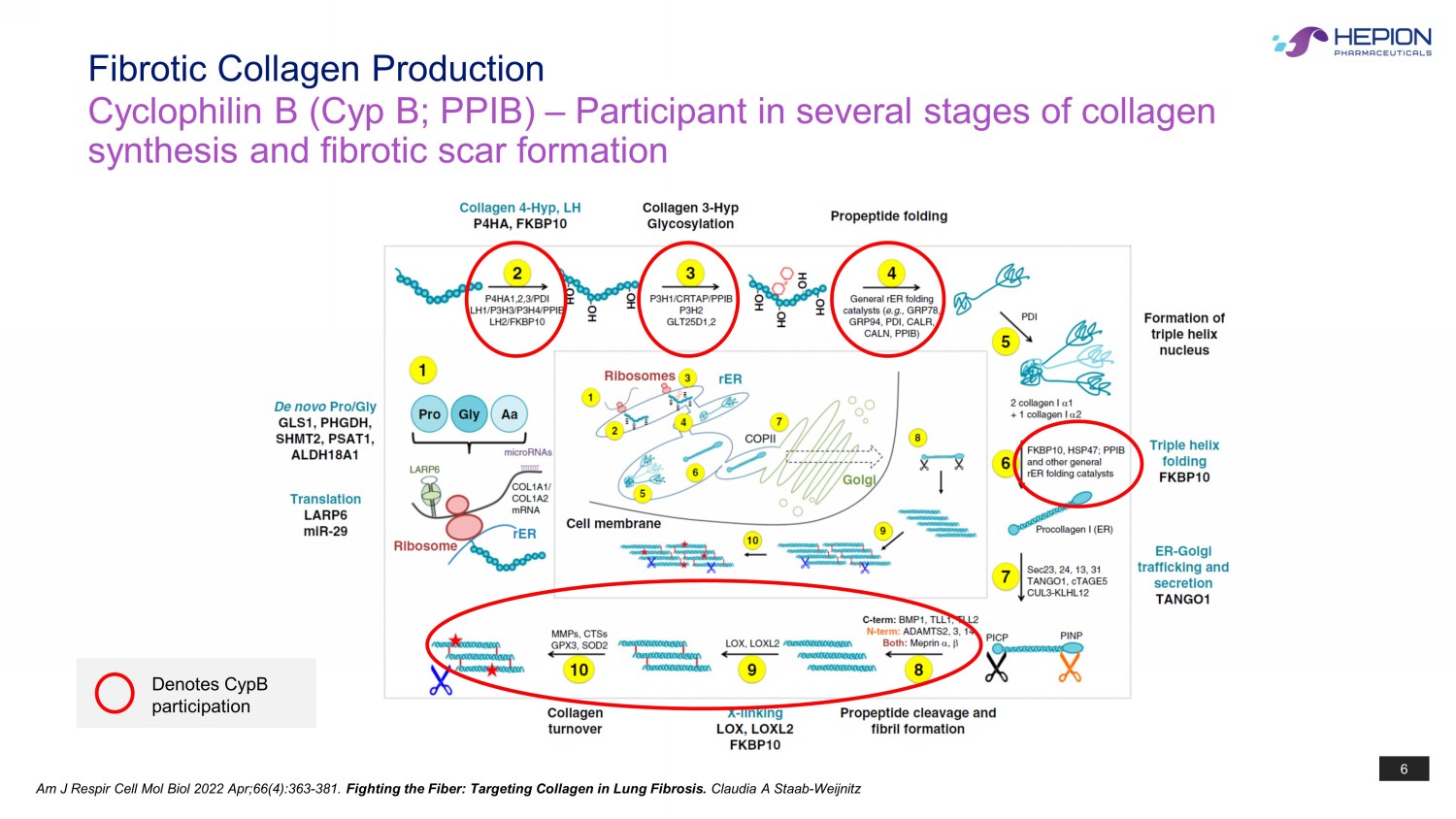
6 Am J Respir Cell Mol Biol 2022 Apr;66(4):363 - 381. Fighting the Fiber: Targeting Collagen in Lung Fibrosis. Claudia A Staab - Weijnitz Denotes CypB participation Fibrotic Collagen Production Cyclophilin B ( Cyp B; PPIB) – Participant in several stages of collagen synthesis and fibrotic scar formation
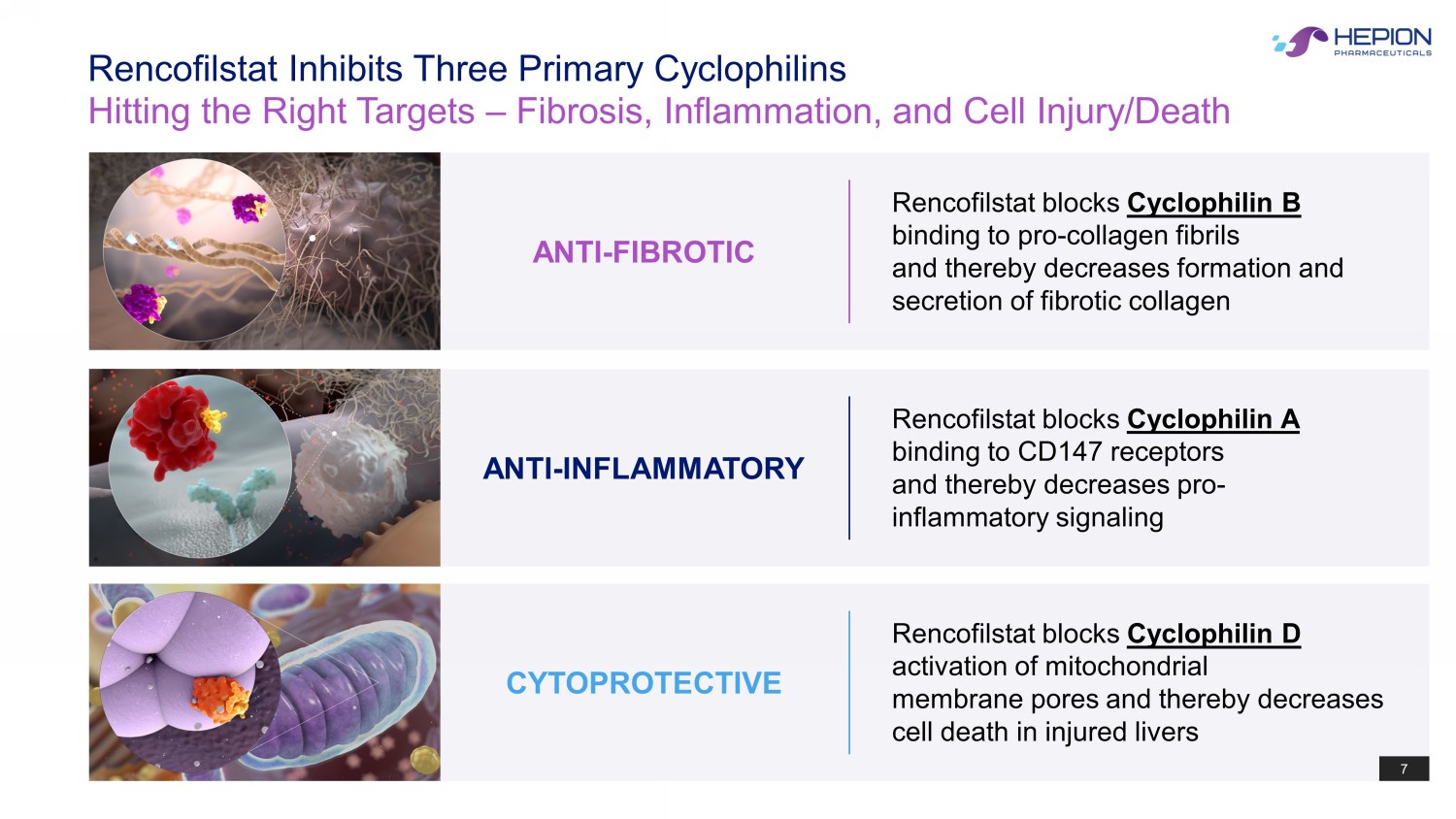
7 Rencofilstat Inhibits Three Primary Cyclophilins Hitting the Right Targets – Fibrosis, Inflammation, and Cell Injury/Death Rencofilstat blocks Cyclophilin D activation of mitochondrial membrane pores and thereby decreases cell death in injured livers CYTOPROTECTIVE 7 Rencofilstat blocks Cyclophilin A binding to CD147 receptors and thereby decreases pro - inflammatory signaling ANTI - INFLAMMATORY Rencofilstat blocks Cyclophilin B binding to pro - collagen fibrils and thereby decreases formation and secretion of fibrotic collagen ANTI - FIBROTIC
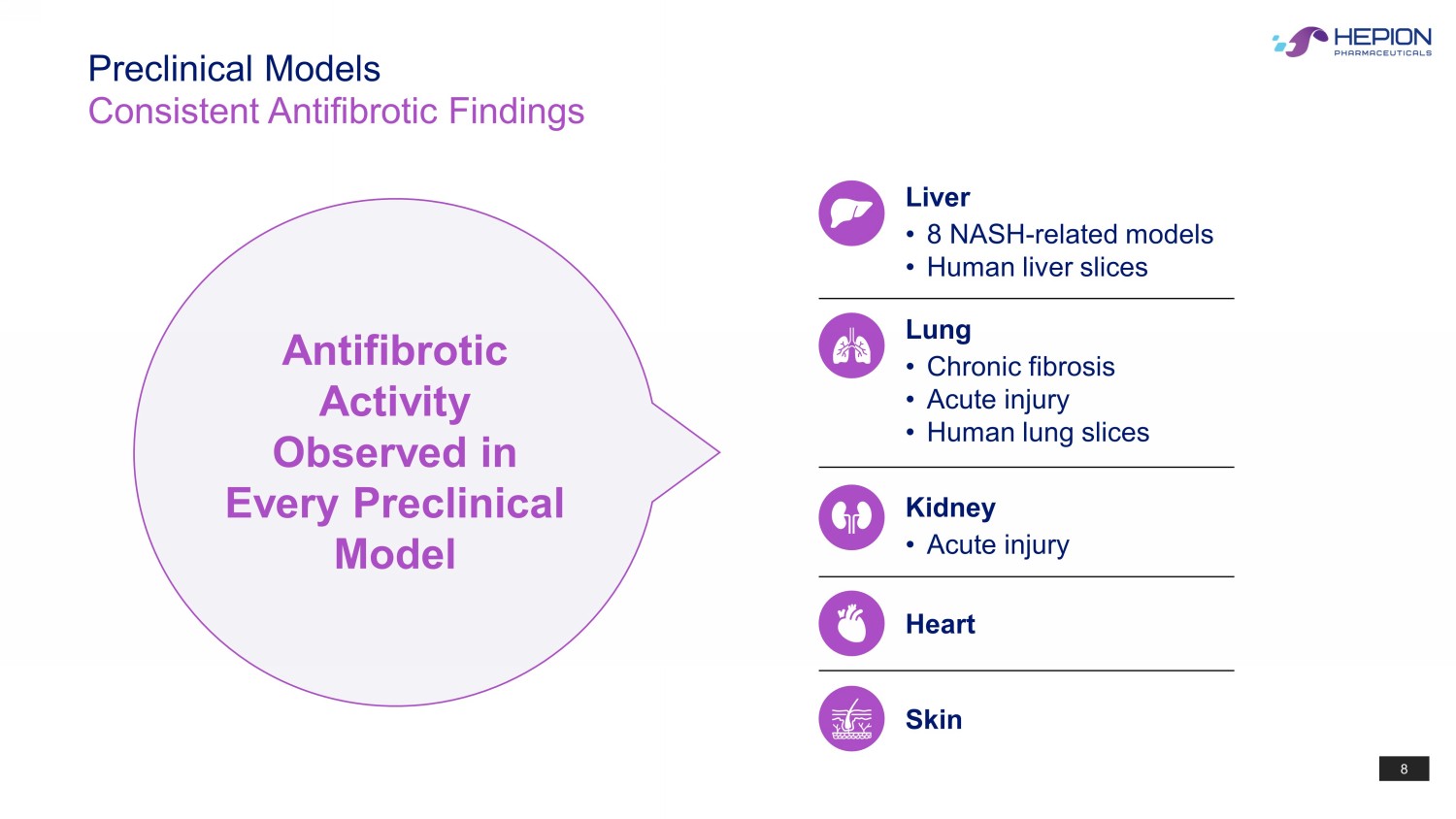
8 Preclinical Models Consistent Antifibrotic Findings Antifibrotic Activity Observed in Every Preclinical Model Liver • 8 NASH - related models • Human liver slices Lung • Chronic fibrosis • Acute injury • Human lung slices Kidney • Acute injury Heart Skin
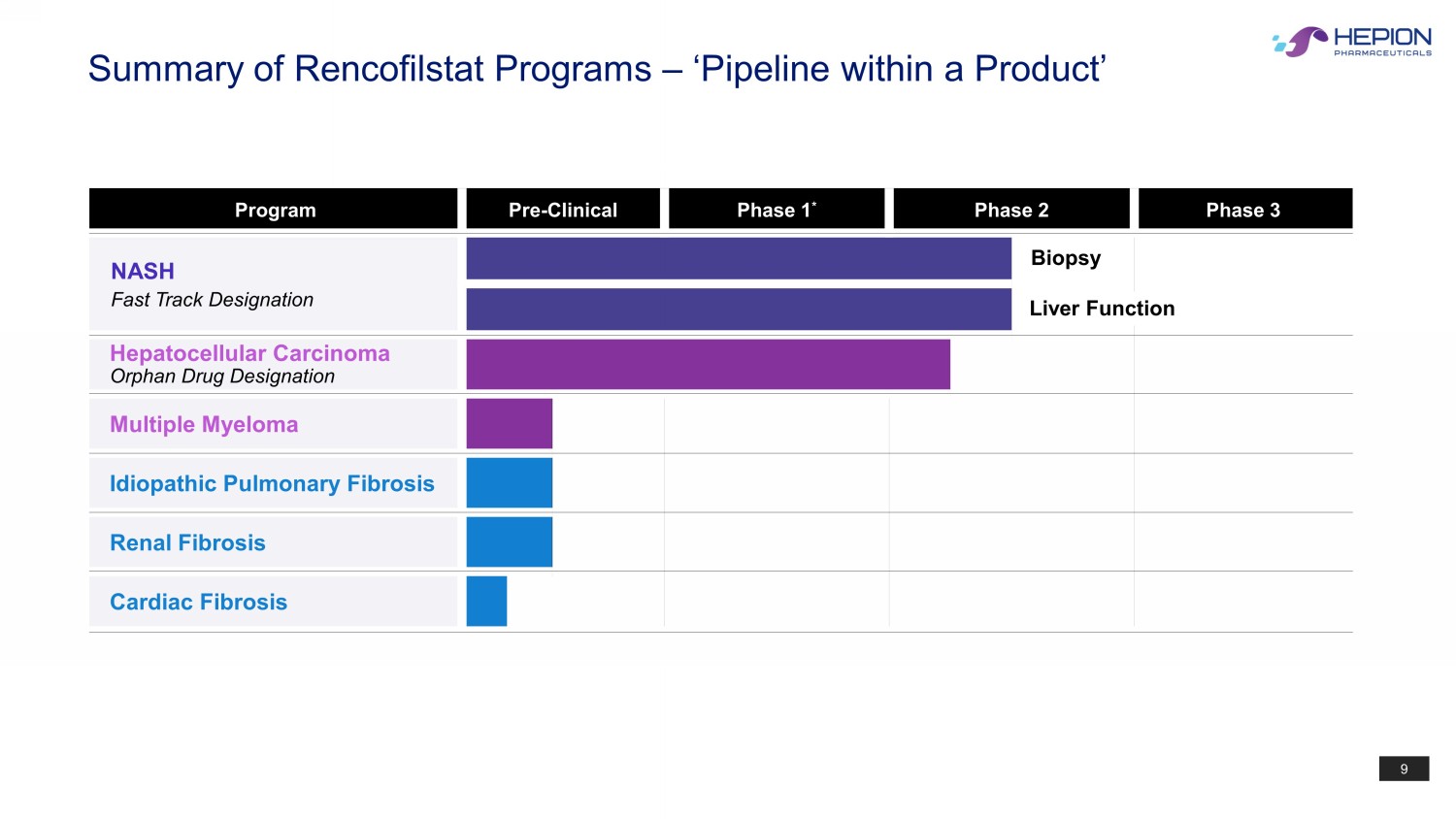
9 Program Pre - Clinical Phase 1 * Phase 2 Phase 3 NASH Fast Track Designation Hepatocellular Carcinoma Orphan Drug Designation Multiple Myeloma Idiopathic Pulmonary Fibrosis Renal Fibrosis Cardiac Fibrosis Biopsy Summary of Rencofilstat Programs – ‘Pipeline within a Product’ Liver Function

10 NASH Fibrotic Liver Disease
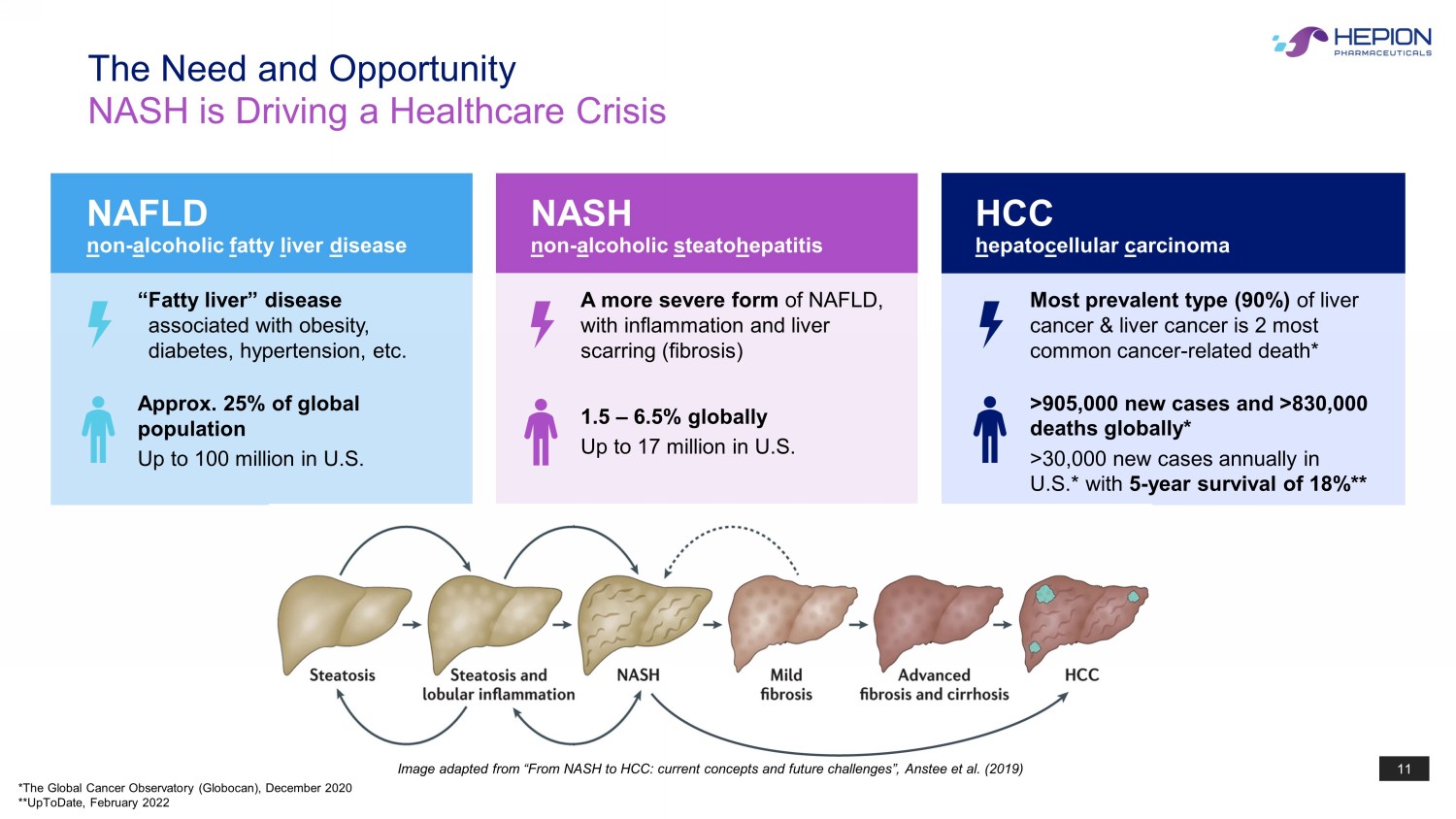
11 Image adapted from “From NASH to HCC: current concepts and future challenges”, Anstee et al. (2019) The Need and Opportunity NASH is Driving a Healthcare Crisis NAFLD n on - a lcoholic f atty l iver d isease Approx. 25% of global population Up to 100 million in U.S. “Fatty liver” disease associated with obesity, diabetes, hypertension, etc. 1.5 – 6.5% globally Up to 17 million in U.S. A more severe form of NAFLD, with inflammation and liver scarring (fibrosis) NASH n on - a lcoholic s teato h ep atitis HCC h epato c ellular c arcinoma Most prevalent type (90%) of liver cancer & liver cancer is 2 most common cancer - related death* >905,000 new cases and >830,000 deaths globally* >30,000 new cases annually in U.S.* with 5 - year survival of 18%** *The Global Cancer Observatory ( Globocan ), December 2020 **UpToDate, February 2022
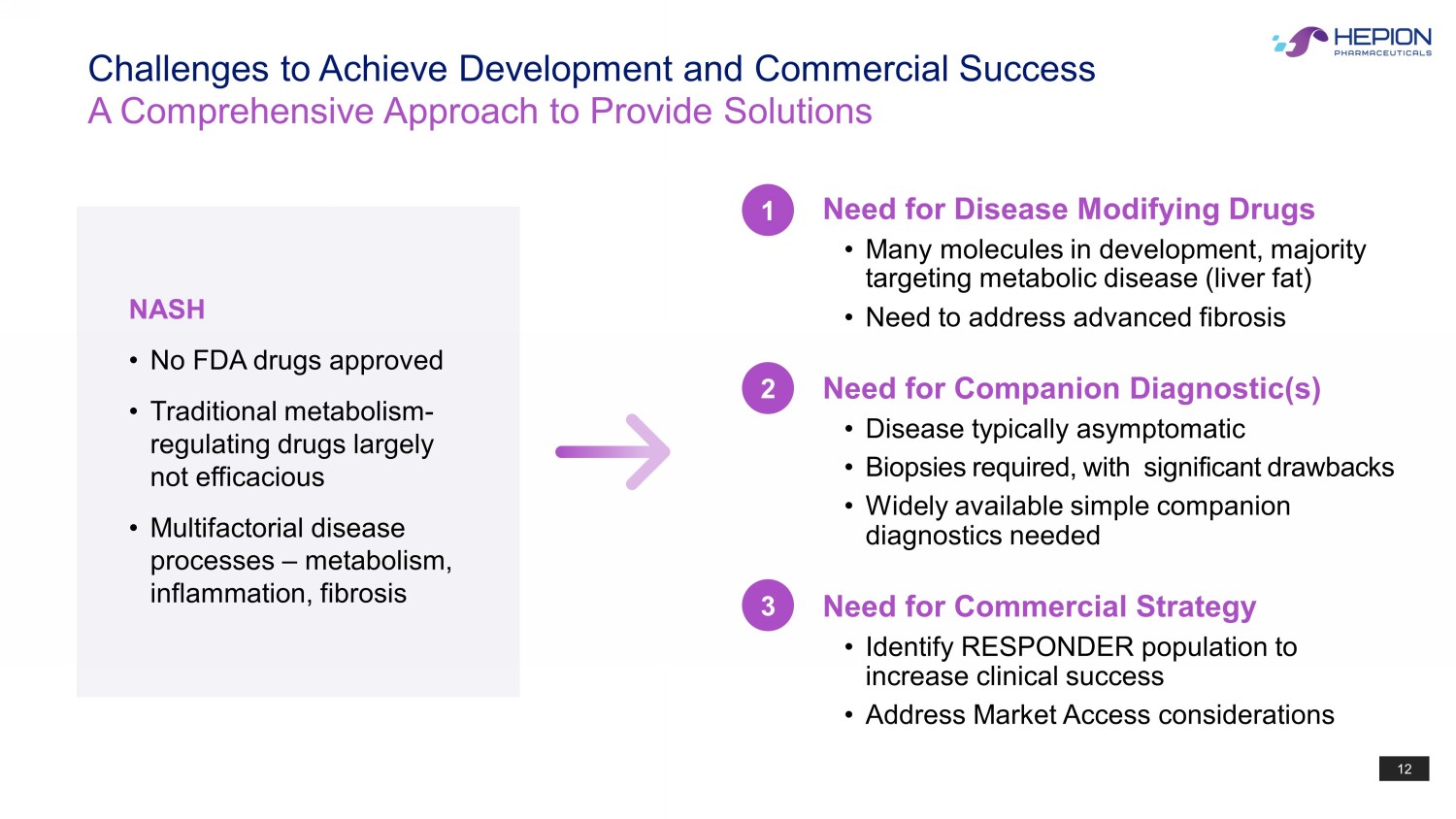
12 Challenges to Achieve Development and Commercial Success A Comprehensive Approach to Provide Solutions NASH • No FDA drugs approved • Traditional metabolism - regulating drugs largely not efficacious • Multifactorial disease processes – metabolism, inflammation, fibrosis Need for Disease Modifying Drugs • Many molecules in development, majority targeting metabolic disease (liver fat) • Need to address advanced fibrosis Need for Companion Diagnostic(s) • Disease typically asymptomatic • Biopsies required, with significant drawbacks • Widely available simple companion diagnostics needed Need for Commercial Strategy • Identify RESPONDER population to increase clinical success • Address Market Access considerations 1 2 3

13 Overview of Phase 1 Studies (completed)
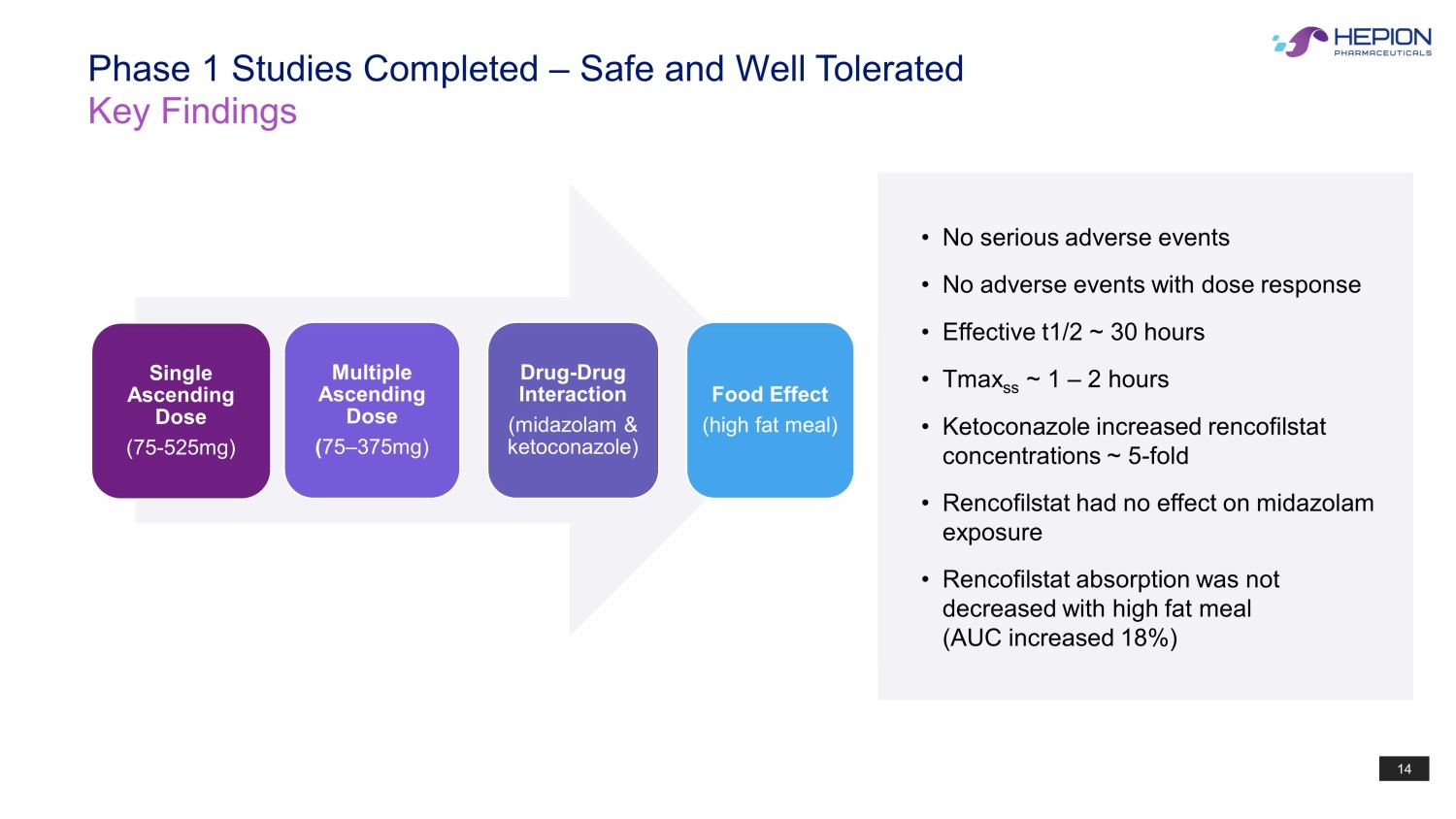
14 Phase 1 Studies Completed – Safe and Well Tolerated Key Findings • No serious adverse events • No adverse events with dose response • Effective t1/2 ~ 30 hours • Tmax ss ~ 1 – 2 hours • Ketoconazole increased rencofilstat concentrations ~ 5 - fold • Rencofilstat had no effect on midazolam exposure • Rencofilstat absorption was not decreased with high fat meal (AUC increased 18%) Single Ascending Dose (75 - 525mg) Multiple Ascending Dose ( 75 – 375mg) Drug - Drug Interaction (midazolam & ketoconazole) Food Effect (high fat meal)

15 Overview of Phase 2a ‘AMBITION’ NASH Trial (completed)
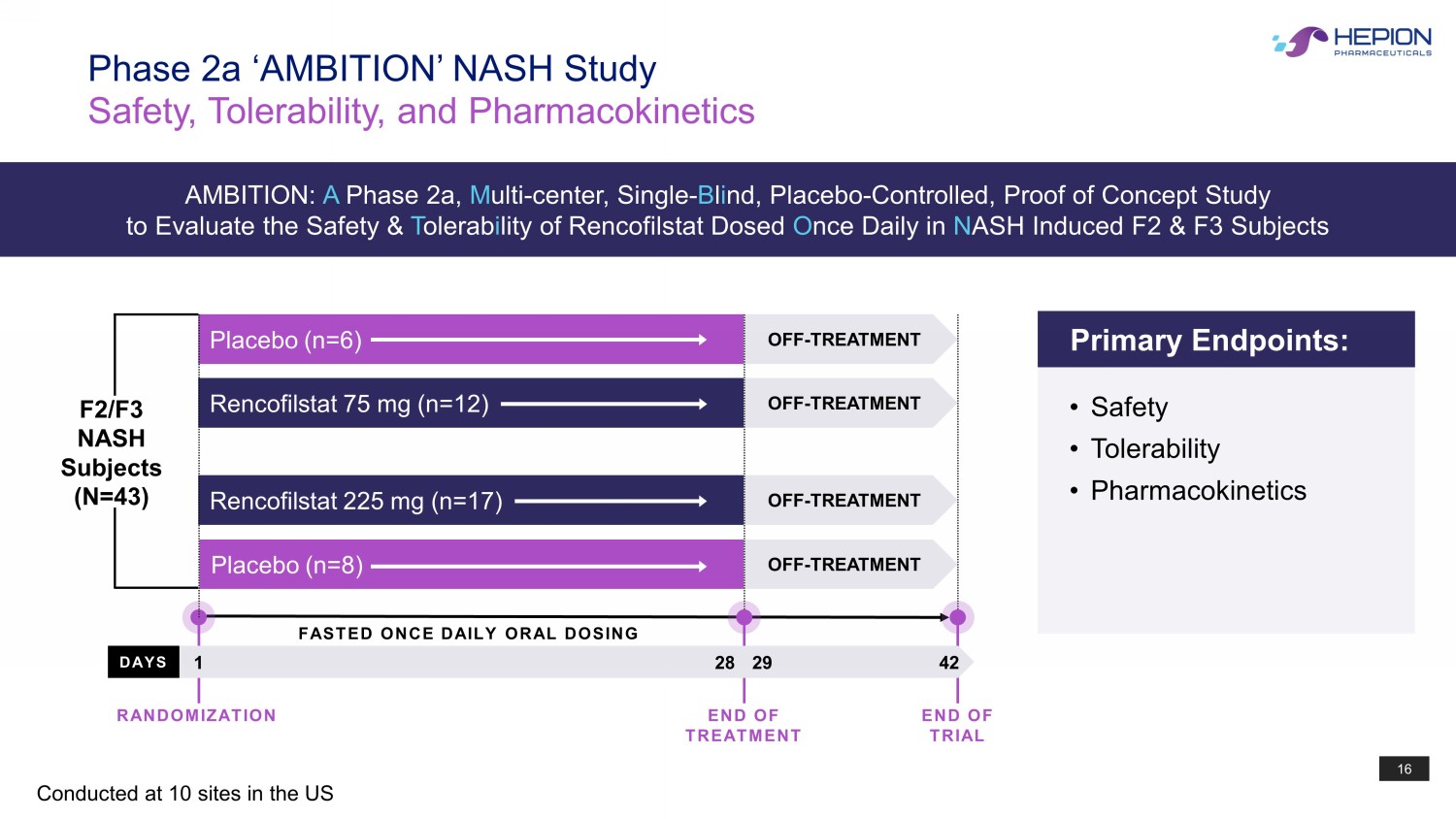
16 F2/F3 NASH Subjects (N=43) Rencofilstat 225 mg (n=17) Placebo (n=8) Placebo (n=6) Rencofilstat 75 mg (n=12) OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT DAYS FASTED ONCE DAILY ORAL DOSING Phase 2a ‘AMBITION’ NASH Study Safety, Tolerability, and Pharmacokinetics Conducted at 10 sites in the US 1 28 42 Primary Endpoints: • Safety • Tolerability • Pharmacokinetics RANDOMIZATION END OF TREATMENT END OF TRIAL 29 AMBITION: A Phase 2a, M ulti - center, Single - B l i nd, Placebo - Controlled, Proof of Concept Study to Evaluate the Safety & T olerab i lity of Rencofilstat Dosed O nce Daily in N ASH Induced F2 & F3 Subjects

17 Rencofilstat is safe and well - tolerated Efficacy signals were observed in only 28 days including: • Reduction in ALT (marker of inflammation & fibrosis) • Reduction in Pro - C3 (marker of fibrosis) • Downregulation of collagen genes • Upregulation of genes associated with liver recovery and favorable lipid dynamics Early evidence of a concentration - effect relationship was observed with both ALT and Pro - C3 Rencofilstat concentrations are not significantly altered by NASH Rencofilstat concentrations expected to be effective in NASH endpoints (ALT and Pro - C3) were achieved All Primary Endpoints Met Phase 2a ‘AMBITION’ NASH Study

18 Hepion’s Proprietary Artificial Intelligence
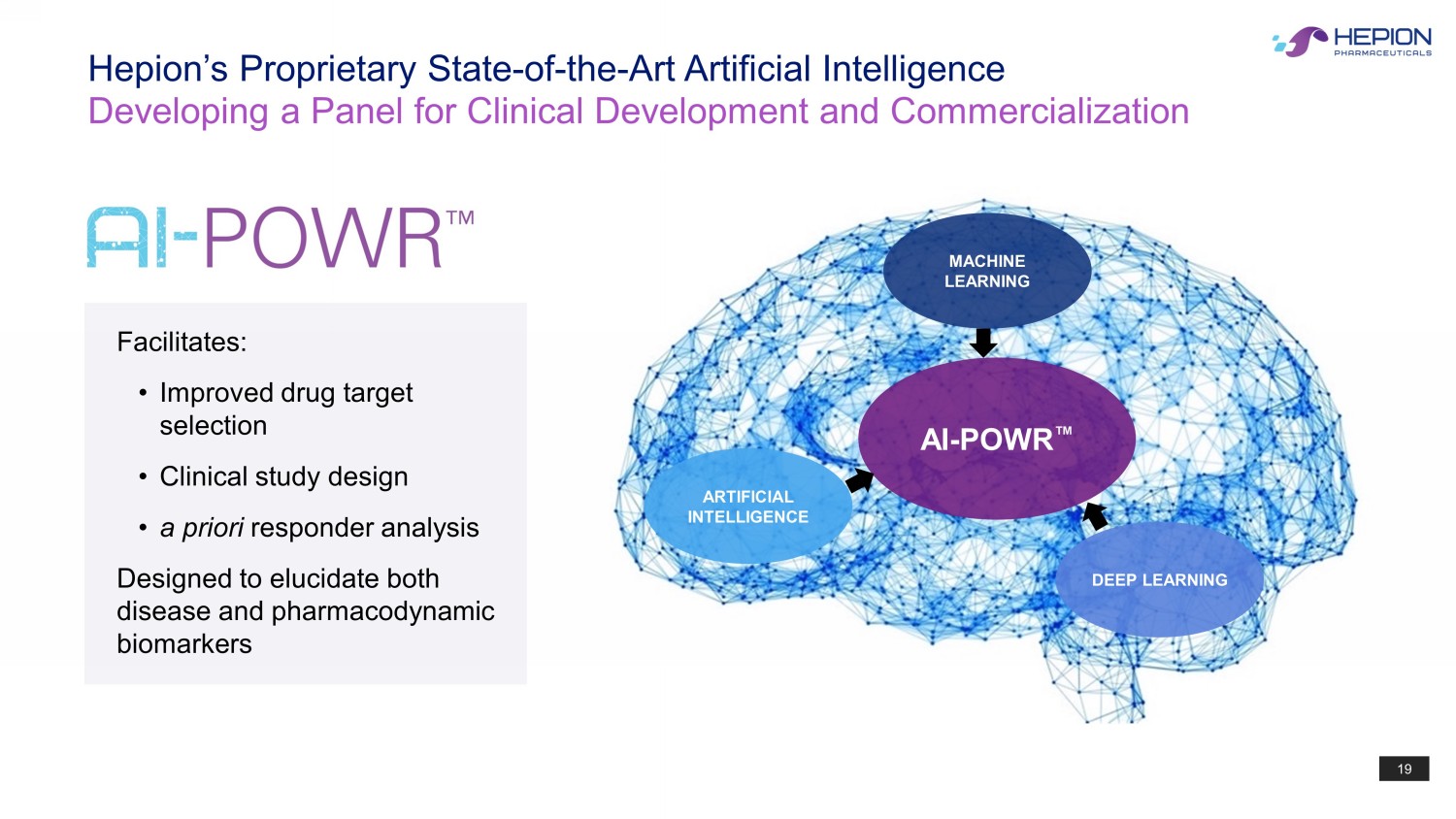
19 Hepion’s Proprietary State - of - the - Art Artificial Intelligence Developing a Panel for Clinical Development and Commercialization AI - POWR Œ MACHINE LEARNING DEEP LEARNING ARTIFICIAL INTELLIGENCE F acilitates: • I mproved drug target selection • Clinical study design • a priori responder analysis Designed to elucidate both disease and pharmacodynamic biomarkers
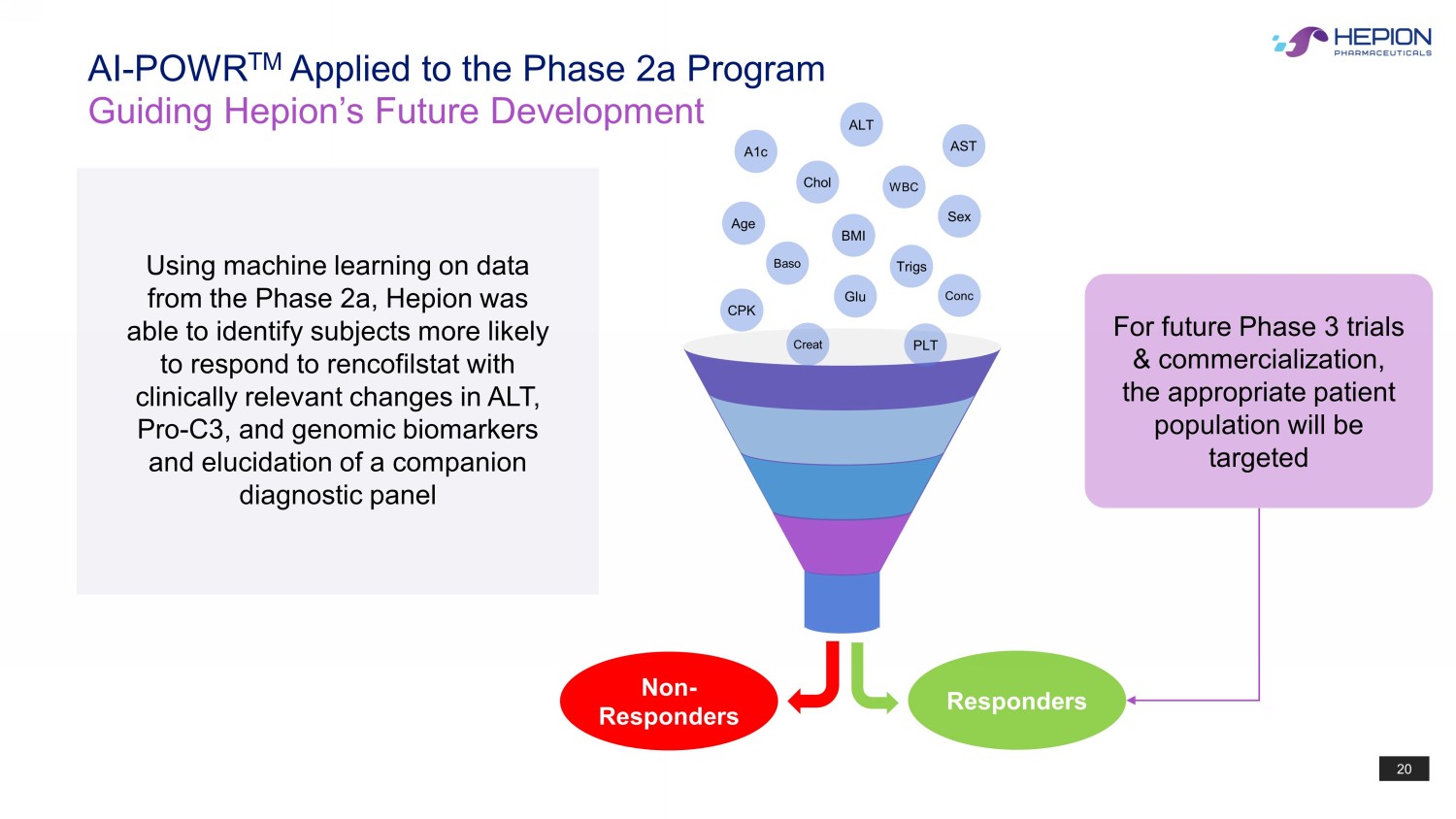
20 AI - POWR TM Applied to the Phase 2a Program Guiding Hepion’s Future Development CPK Baso Glu Conc Trigs Age BMI Sex Creat PLT WBC Chol AST ALT A1c Responders Non - Responders Using machine learning on data from the Phase 2a, Hepion was able to identify subjects more likely to respond to rencofilstat with clinically relevant changes in ALT, Pro - C3, and genomic biomarkers and elucidation of a companion diagnostic panel For future Phase 3 trials & commercialization, the appropriate patient population will be targeted

21 A1B G SDC 2 MM P23 B FBN 2 EMID1 LOXL1 SERP INB8 ORM1 ICAM1 VCAN CLEC3B PXDN HTRA1 F13A1 COCH COL9A2 COL4A3 EMILIN2 MMRN1 LAMB1 COL6AE LAMA5 ADAM9 LTBP1 TIMP2 COL 26A1 HRNR COLQ HSPG2 C1QB CLU ADAM 19 BCAM MXRA7 PLSCR1 PCSK6 BCHE N1D1 F5 FN1 FBN1 HGF PKLR NID2 COL3A1 LAMB2 LAMC1 IL2RA EGFL7 PLSCR4 USH2A MMP8 CLUL1 TMEM256 - PLSCR3 PKM ACHE Fibrosis - Associated Gene Network Observed in Phase 2a Blood Samples Supports Rencofilstat Antifibrotic Efficacy in 28 - Day Study collagen - containing extracellular matrix collagen binding collagen type IV trimer collagen type IX trimer collagen fibril organization collagen catabolic process Rencofilstat impacted gene expression related to biosynthesis, remodeling, and degradation of collagens.

22 Phase 2b ‘ASCEND - NASH’ Trial (in progress)
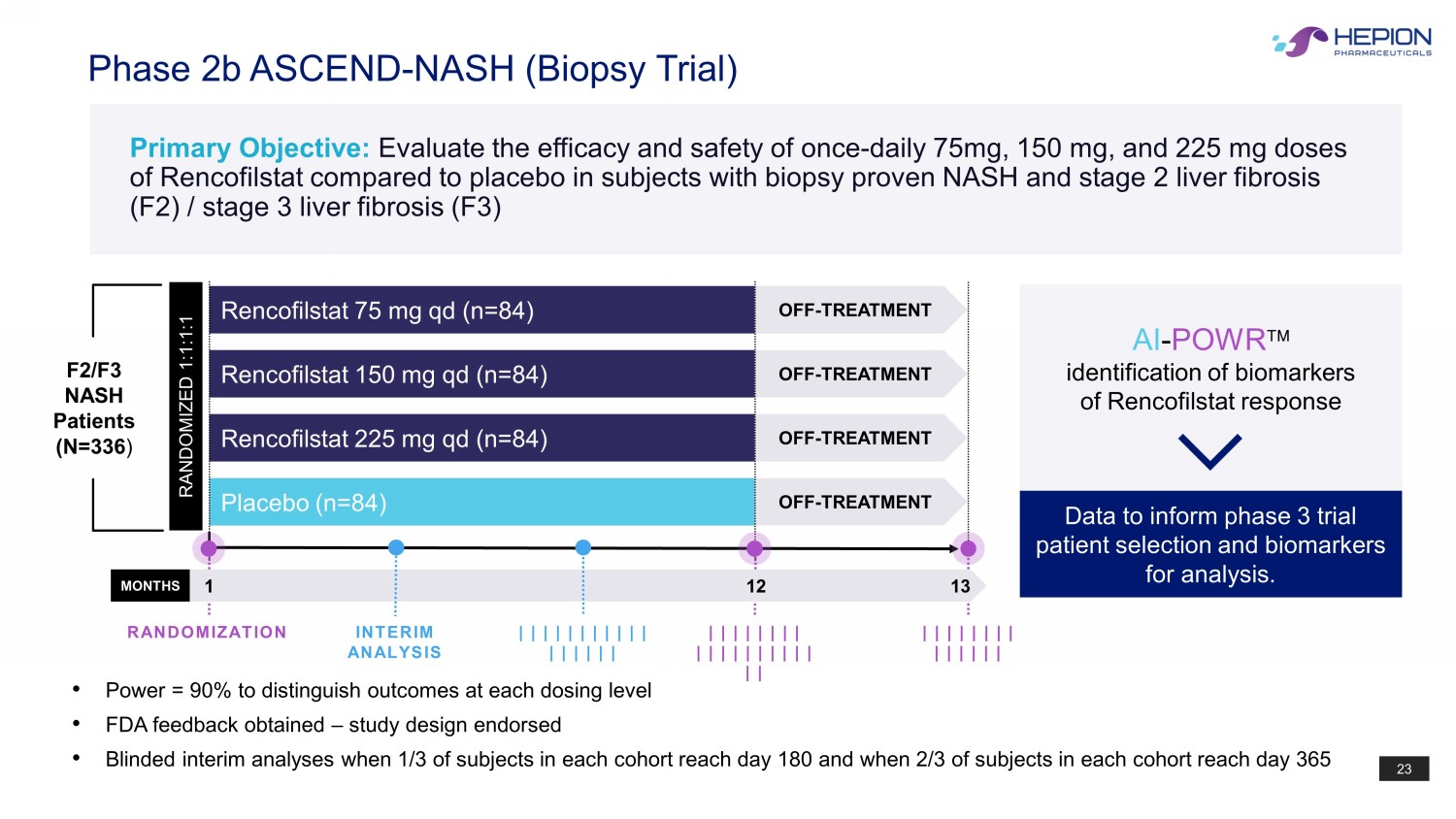
23 Phase 2b ASCEND - NASH (Biopsy Trial) Rencofilstat 225 mg qd (n=84) Placebo (n=84) Rencofilstat 75 mg qd (n=84) Rencofilstat 150 mg qd (n=84) RANDOMIZED 1:1:1:1 OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT MONTHS 1 12 13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | INTERIM ANALYSIS | | | | | | | | | | | | | | | | | RANDOMIZATION Data to inform phase 3 trial patient selection and biomarkers for analysis. AI - POWR identification of biomarkers of Rencofilstat response F2/F3 NASH Patients (N=336 ) • Power = 90% to distinguish outcomes at each dosing level • FDA feedback obtained – study design endorsed • Blinded interim analyses when 1/3 of subjects in each cohort reach day 180 and when 2/3 of subjects in each cohort reach day 365 Primary Objective: Evaluate the efficacy and safety of once - daily 75mg, 150 mg, and 225 mg doses of Rencofilstat compared to placebo in subjects with biopsy proven NASH and stage 2 liver fibrosis (F2) / stage 3 liver fibrosis (F3)
24 Phase 2b ASCEND - NASH Primary Efficacy Endpoint: Superiority of rencofilstat compared to placebo on liver histology at month 12 relative to the screening biopsy, by assessing the proportion of subjects with improvement in fibrosis by at least 1 stage (NASH CRN system) OR NASH resolution without worsening of fibrosis Secondary Efficacy Endpoints: Superiority of rencofilstat compared to placebo on histology at month 12 relative to screening by assessing the proportion of subjects with improvement in fibrosis by: • At least 1 stage regardless of effect on NASH • At least 2 stages regardless of effect on NASH • At least 2 stages AND no worsening of NASH.

25 Phase 2 ‘ALTITUDE - NASH’ Trial (in progress)
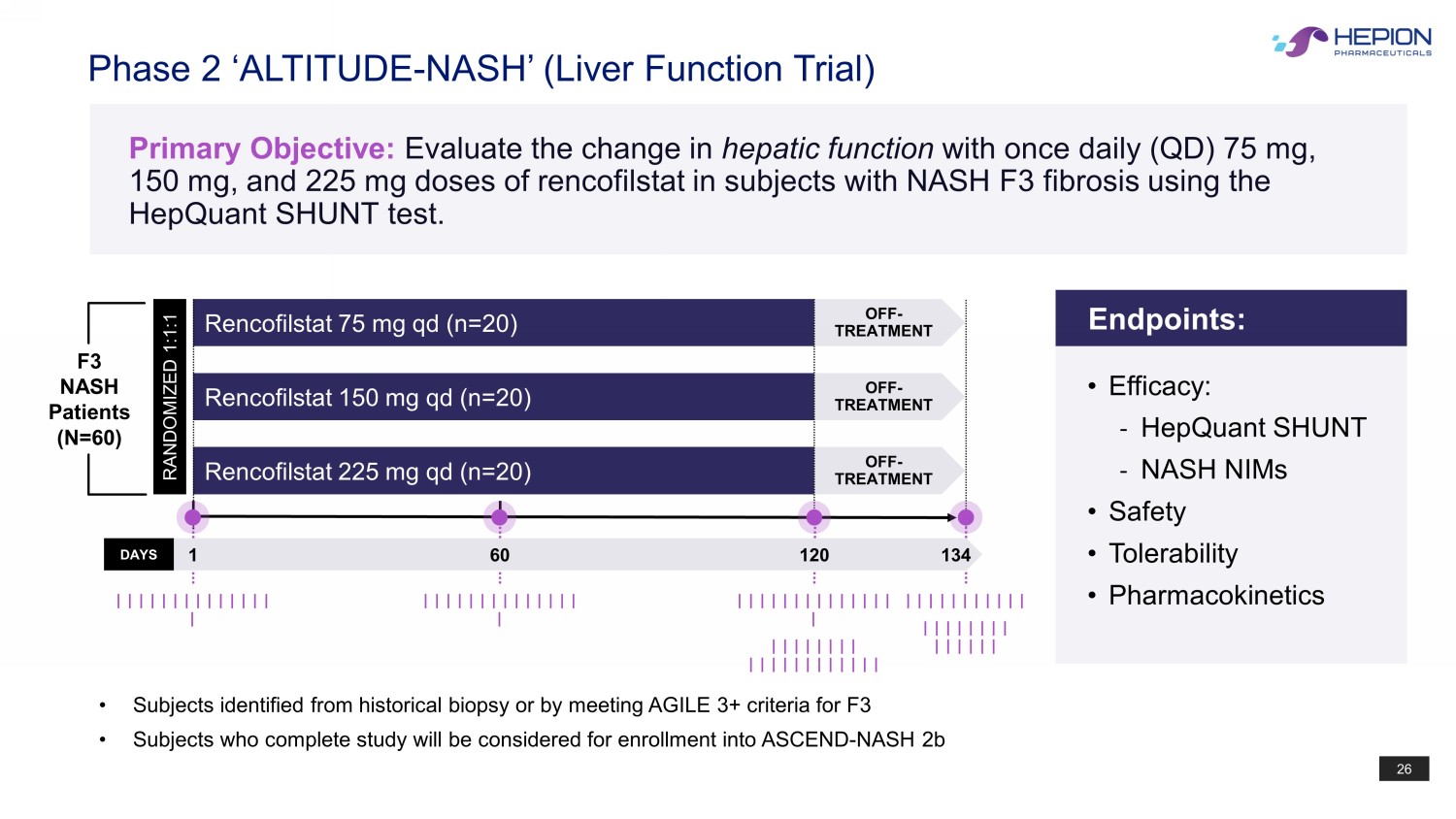
26 • Subjects identified from historical biopsy or by meeting AGILE 3+ criteria for F3 • Subjects who complete study will be considered for enrollment into ASCEND - NASH 2b Phase 2 ‘ALTITUDE - NASH’ (Liver Function Trial) Primary Objective: Evaluate the change in hepatic function with once daily (QD) 75 mg, 150 mg, and 225 mg doses of rencofilstat in subjects with NASH F3 fibrosis using the HepQuant SHUNT test. Rencofilstat 225 mg qd (n=20) Rencofilstat 75 mg qd (n=20) Rencofilstat 150 mg qd (n=20) RANDOMIZED 1:1:1 OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT DAYS 1 120 134 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | F3 NASH Patients (N=60) 60 | | | | | | | | | | | | | | | Endpoints: • Efficacy: - HepQuant SHUNT - NASH NIMs • Safety • Tolerability • Pharmacokinetics
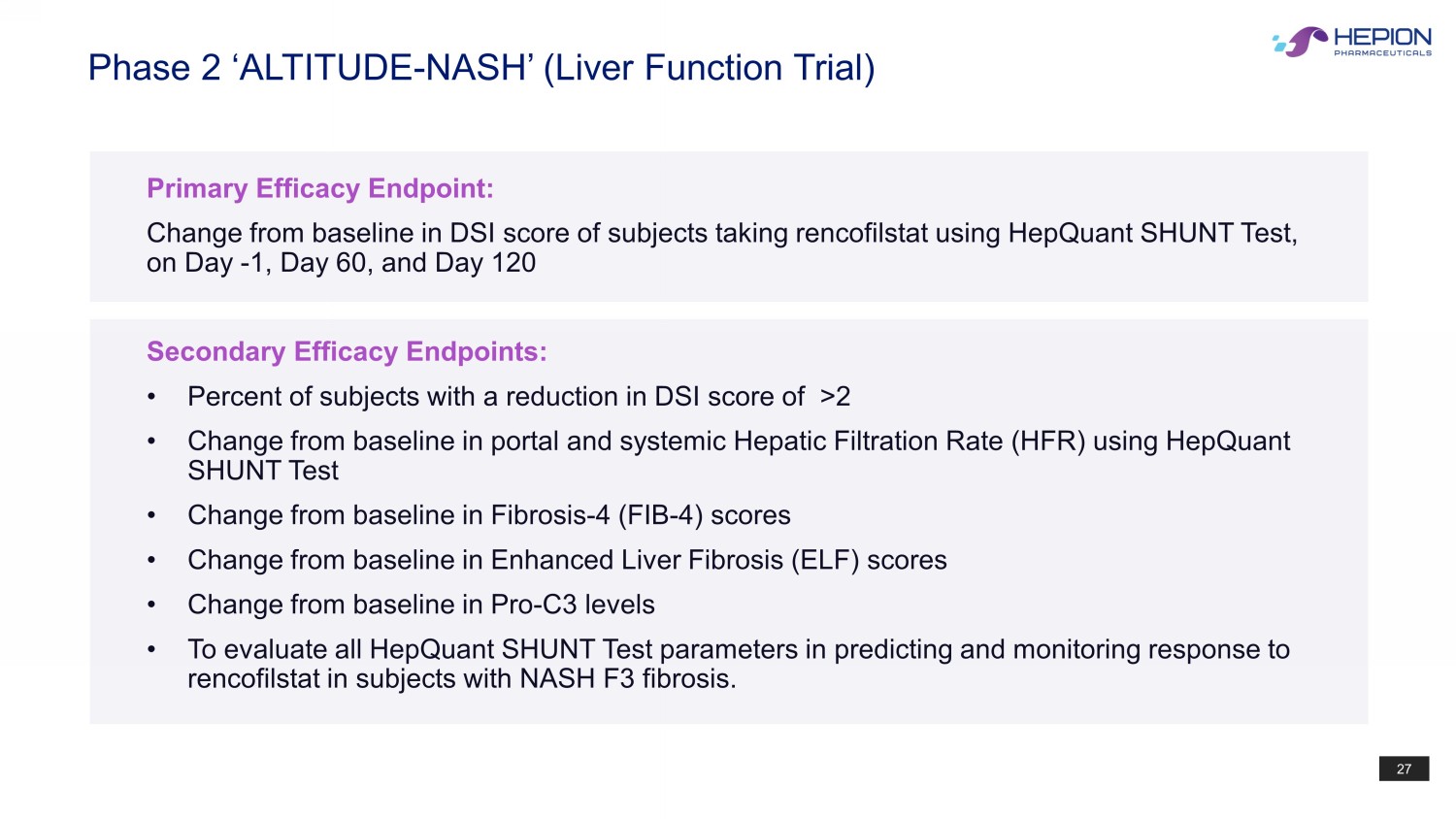
27 Primary Efficacy Endpoint: Change from baseline in DSI score of subjects taking rencofilstat using HepQuant SHUNT Test, on Day - 1, Day 60, and Day 120 Secondary Efficacy Endpoints: • Percent of subjects with a reduction in DSI score of >2 • Change from baseline in portal and systemic Hepatic Filtration Rate (HFR) using HepQuant SHUNT Test • Change from baseline in Fibrosis - 4 (FIB - 4) scores • Change from baseline in Enhanced Liver Fibrosis (ELF) scores • Change from baseline in Pro - C3 levels • To evaluate all HepQuant SHUNT Test parameters in predicting and monitoring response to rencofilstat in subjects with NASH F3 fibrosis. Phase 2 ‘ALTITUDE - NASH’ (Liver Function Trial)

28 Phase 2a HCC Trial (upcoming)
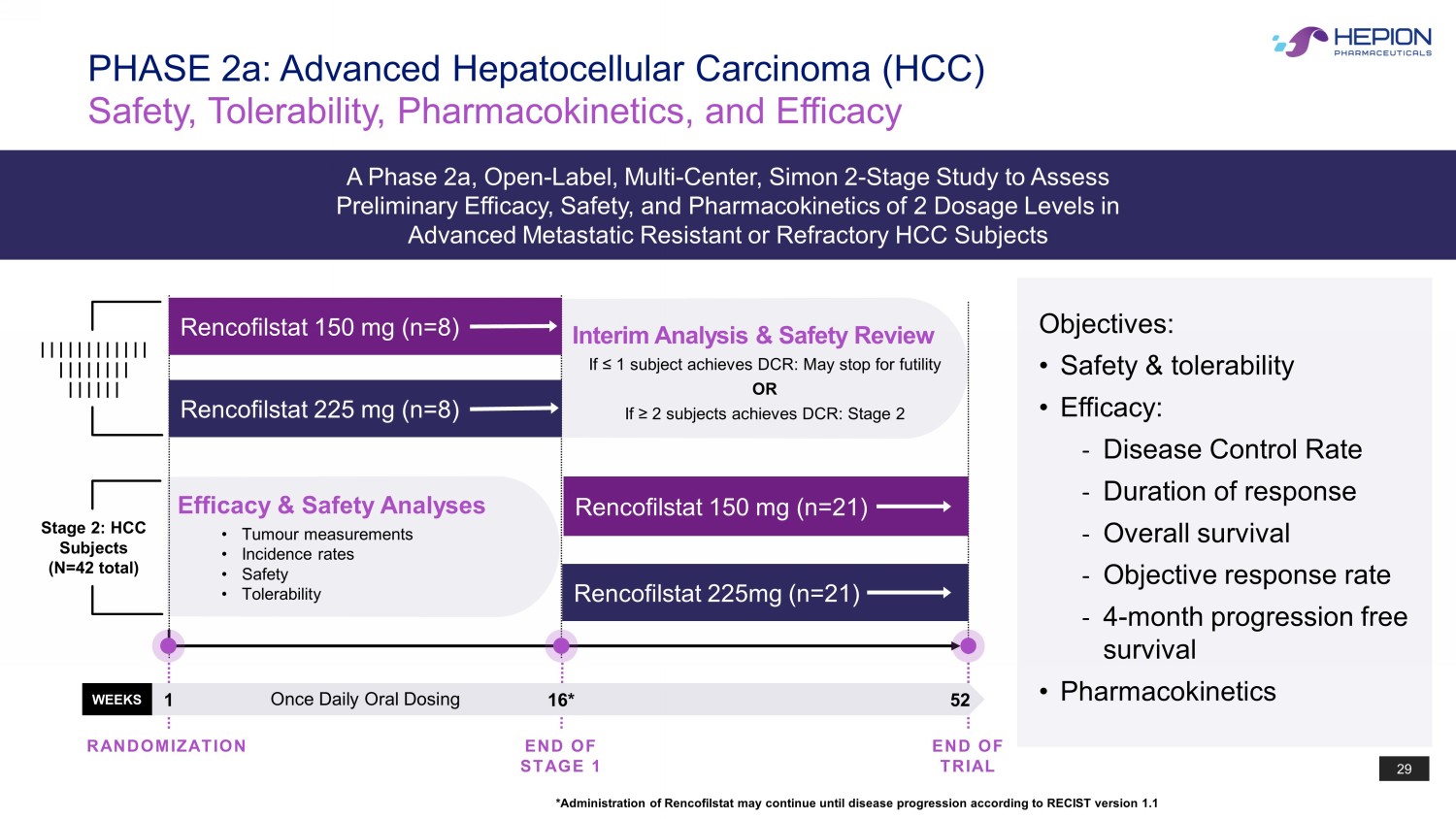
29 Objectives: • Safety & tolerability • Efficacy: - Disease Control Rate - Duration of response - Overall survival - Objective response rate - 4 - month progression free survival • Pharmacokinetics PHASE 2a: Advanced Hepatocellular Carcinoma (HCC) Safety, Tolerability, Pharmacokinetics, and Efficacy A Phase 2a, Open - Label, Multi - Center, Simon 2 - Stage Study to Assess Preliminary Efficacy, Safety, and Pharmacokinetics of 2 Dosage Levels in Advanced Metastatic Resistant or Refractory HCC Subjects *Administration of Rencofilstat may continue until disease progression according to RECIST version 1.1 WEEKS 1 16* 52 END OF STAGE 1 END OF TRIAL RANDOMIZATION Once Daily Oral Dosing Efficacy & Safety Analyses • Tumour measurements • Incidence rates • Safety • Tolerability Rencofilstat 150 mg (n= 21 ) Rencofilstat 225mg (n=21) Stage 2: HCC Subjects (N=42 total) Interim Analysis & Safety Review If ≤ 1 subject achieves DCR: May stop for futility OR If ≥ 2 subjects achieves DCR: Stage 2 Rencofilstat 150 mg (n=8) Rencofilstat 225 mg (n= 8 ) | | | | | | | | | | | | | | | | | | | | | | | | | |

30 Summary
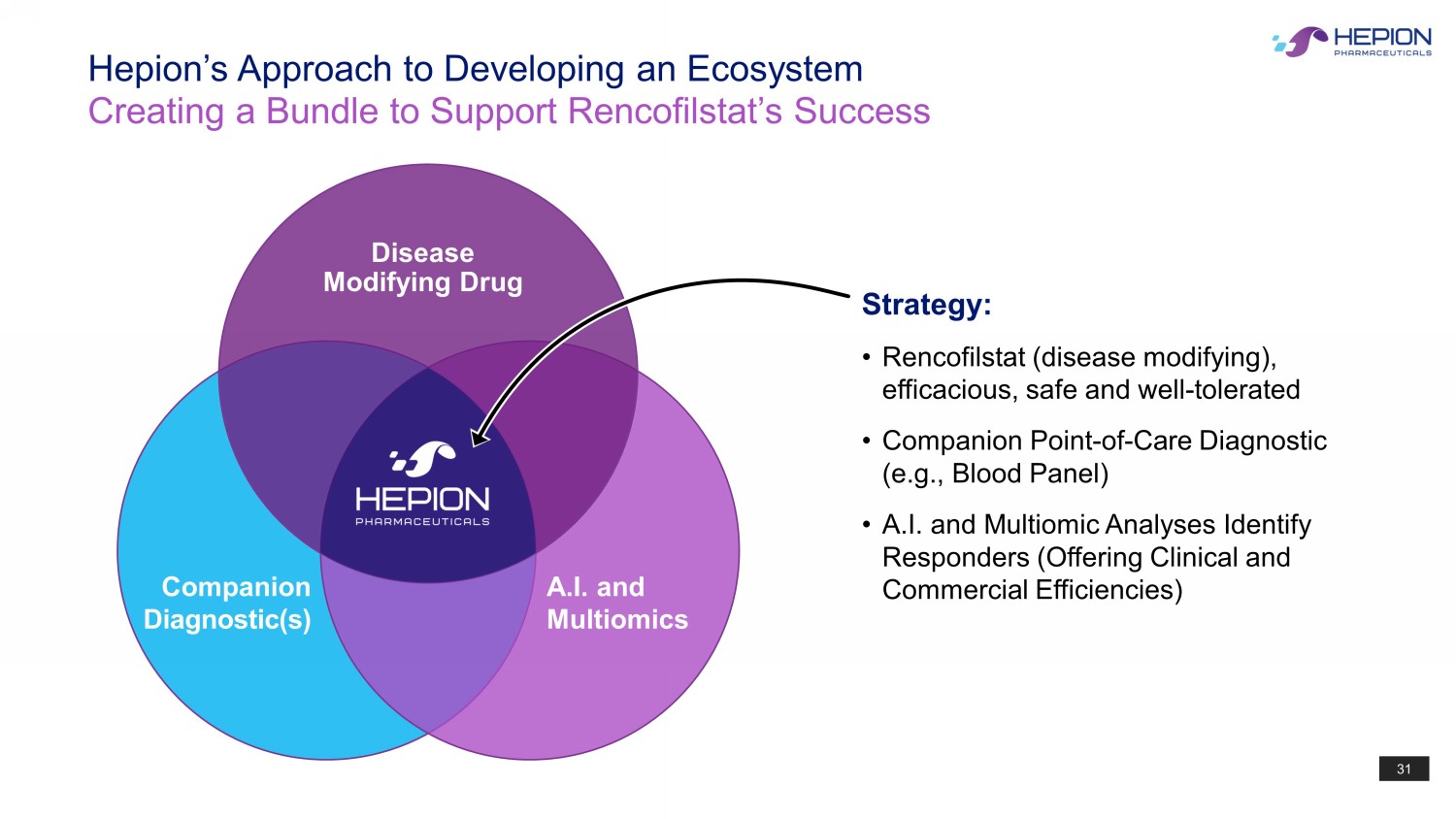
31 Hepion’s Approach to Developing an Ecosystem Creating a Bundle to Support Rencofilstat’s Success Strategy: • Rencofilstat (disease modifying), efficacious, safe and well - tolerated • Companion Point - of - Care Diagnostic (e.g., Blood Panel) • A.I. and Multiomic Analyses Identify Responders (Offering Clinical and Commercial Efficiencies) Disease Modifying Drug A.I. and Multiomics Companion Diagnostic(s)
32 $71.7 M Cash as of 6/30/22 Financials Two Value Drivers Tackling Fibrotic Diseases • Rencofilstat , once - daily oral, targeting key drivers of pathology, tested in over 200 subjects • Two Phase 2 NASH trials underway • Upcoming Phase 2 for HCC • Developing companion A.I. for clinical development and commercialization strategy • Core scientific team discovered and developed voclosporin (currently marketed) • Robust IP 76.2 M Common Shares Outstanding 32

33 Launa Aspeslet , PhD COO Formerly COO of Isotechnika ( Aurinia ) from 1996 - 2013. Was CEO of an oncology CRO from 2013 until joining HEPA in 2022. Core R&D team has collectively > 120 yrs of experience with cyclophilin inhibition drug development, most notably voclosporin ( Lupkynis ®) for lupus nephritis while at Isotechnika ( Aurinia , NASDAQ:AUPH) Experienced Team Robert Foster, PharmD, PhD CEO Founded Isotechnika ( Aurinia ) in 1993, and served mostly as CEO & Chairman, until 2014. Joined HEPA in 2016 as CSO and became CEO in 2018. John Cavan, MBA CFO Formerly of Pine Hill, Stemline , Aegerion, AlgoRx , Alpharma , Sony, American Express and International Specialty Products, joined HEPA in 2016. Todd Hobbs, MD CMO Formerly Chief Medical Officer of Novo Nordisk, joined HEPA in 2021. Daren Ure , PhD CSO Joined Isotechnika ( Aurinia ) in 2003 and joined HEPA in 2016. Daniel Trepanier, PhD SVP, Drug Development Joined Isotechnika ( Aurinia ) in 1997 and joined HEPA in 2016. Patrick Mayo, PhD SVP, Clinical Pharmacology and Analytics Joined Isotechnika ( Aurinia ) in 2002 and joined HEPA in 2019.
CONTACT US Hepion Pharmaceuticals Inc. 399 Thornall Street, First Floor Edison, New Jersey, USA, 08837 Email: info@hepionpharma.com Phone: 732 - 902 - 4000 www.hepionpharma.com

































