
Talis Biomedical Corporate Presentation May 2023 Decentralizing Women’s and Sexual Health Testing

Disclaimer This presentation may contain forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements because they contain words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would,” or the negative of these words or other similar terms or expressions. Talis Biomedical (“Talis Bio,” “we,” “our”) has based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and results of operations. Forward-looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: our plans to advance our pipeline, including our plans to develop Talis One assays in the women’s and sexual health markets; the size and potential of our opportunity in the women’s and sexual health markets; our ability to capitalize on any competitive advantages; the potential to realize the benefit of our restructuring plan in the fourth quarter of 2022; our ability to position Talis Bio to provide durable value to our shareholders; our future revenue growth and profit margins; and our ability to lower our cash burn, extend operations and our cash runway. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and other factors that could cause actual results and events to differ materially and adversely from those indicated by such forward-looking statements including, among others: risks and uncertainties associated with development and regulatory approval, the impact to our business from global economic conditions, including the ongoing COVID-19 pandemic, and inflationary pressures and any related impact on our ability to develop our pipeline products, our ability to achieve or sustain profitability, our ability to launch and gain market acceptance for our pipeline products and to accurately forecast and meet customer demand, our ability to compete successfully and our ability to enhance our product offerings, development and manufacturing, capacity constraints or delays in production of our products, product defects or failures. These and other risks and uncertainties are described more fully in the “Risk Factors” section and elsewhere in our filings with the Securities and Exchange Commission and available at www.sec.gov, including in our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. Any forward-looking statements that we make in this presentation speak only as of the date of this presentation, and Talis Bio assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise after the date of this presentation, except as required under applicable law. This presentation also contains estimates and information concerning our industry and business, including estimated market size and projected growth rates of the markets in which Talis Bio participates. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Talis Bio has not independently verified the statistical and other industry data generated by independent parties and contained in this presentation and, accordingly, we cannot guarantee their accuracy or completeness. In addition, projections, assumptions and estimates of our future performance and the future performance of the industries in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by Talis Bio.

Differentiated to Lead in Significant Untapped Market Advancing health equity and outcomes by delivering accurate infectious disease testing in the moment of need, at the point of care Talis One® System Established innovative, high-performing diagnostic platform at the point of care PROMISING PIPELINE OPPORTUNITY Refocused product roadmap in growing women’s and sexual health markets OPERATIONAL OPTIMIZATION Demonstrated scalable manufacturing capabilities with path to attractive margins WELL-POSITIONED FOR COMMERICIALIZATION Built strong commercial infrastructure
Women’s/Sexual Health Providers Eager to Test at Point-of-Care (POC) INHERENT CLINICAL ADVANTAGE Accurate, immediate treatment reduces empirical prescribing, office visits and patients lost to follow-up ECONOMIC BENEFIT Shifts reimbursement to those providing care COVID ACCELERATED POC PLATFORM ADOPTION Created demand and channel for broader infectious �disease testing at the point of care
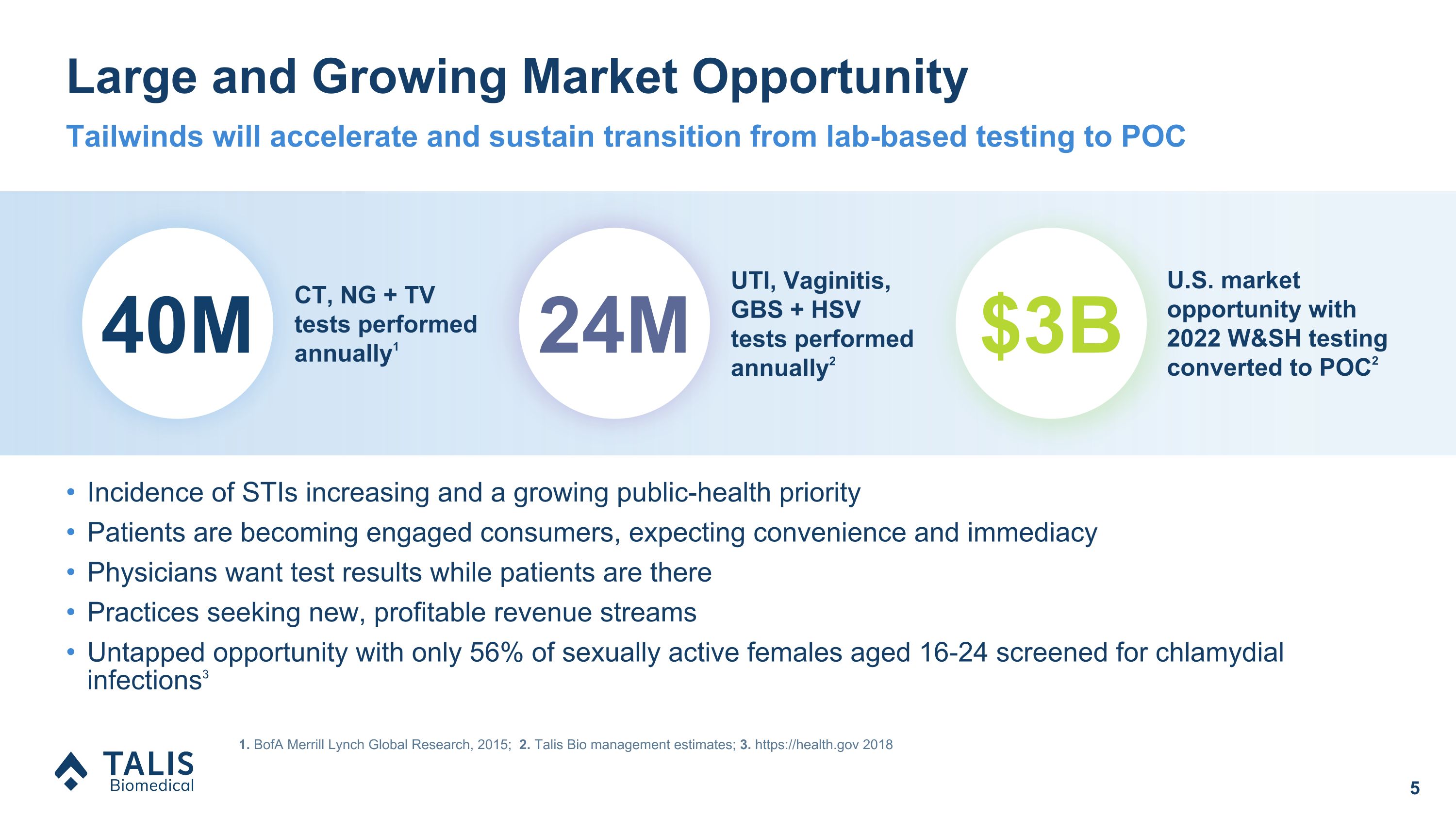
Large and Growing Market Opportunity 1. BofA Merrill Lynch Global Research, 2015; 2. Talis Bio management estimates; 3. https://health.gov 2018 Tailwinds will accelerate and sustain transition from lab-based testing to POC Incidence of STIs increasing and a growing public-health priority Patients are becoming engaged consumers, expecting convenience and immediacy Physicians want test results while patients are there Practices seeking new, profitable revenue streams Untapped opportunity with only 56% of sexually active females aged 16-24 screened for chlamydial infections3 40M CT, NG + TV tests performed annually1 24M UTI, Vaginitis, GBS + HSV �tests performed annually2 $3B U.S. market opportunity with 2022 W&SH testing converted to POC2
The Talis One System is Designed to Win in POC Market SENSITIVITY & SPECIFICITY Embedded Sample Prep, �Nucleic Acid Extraction RAPID TURNAROUND Results in <30 Minutes DESIGNED FOR UNTRAINED USERS CLIA Waiver for POC Market RELEVANT MENU Women’s & Sexual Health Menu + Respiratory LOW COST Automated Manufacturing Drives Down Cost

Talis One System Technology Overview
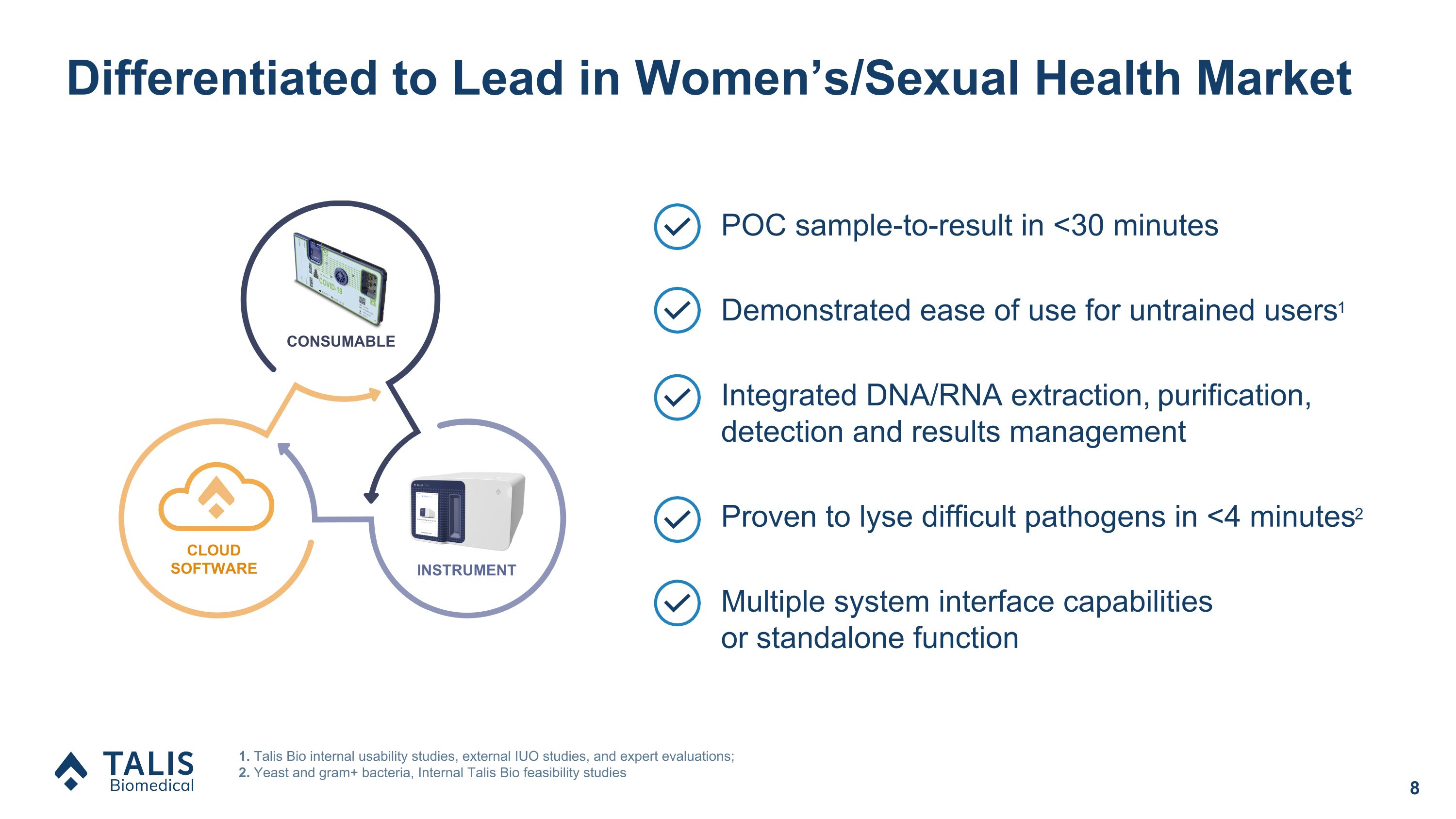
Differentiated to Lead in Women’s/Sexual Health Market POC sample-to-result in <30 minutes Demonstrated ease of use for untrained users1 Integrated DNA/RNA extraction, purification, detection and results management Proven to lyse difficult pathogens in <4 minutes2 Multiple system interface capabilities �or standalone function 1. Talis Bio internal usability studies, external IUO studies, and expert evaluations; 2. Yeast and gram+ bacteria, Internal Talis Bio feasibility studies CLOUD SOFTWARE INSTRUMENT CONSUMABLE
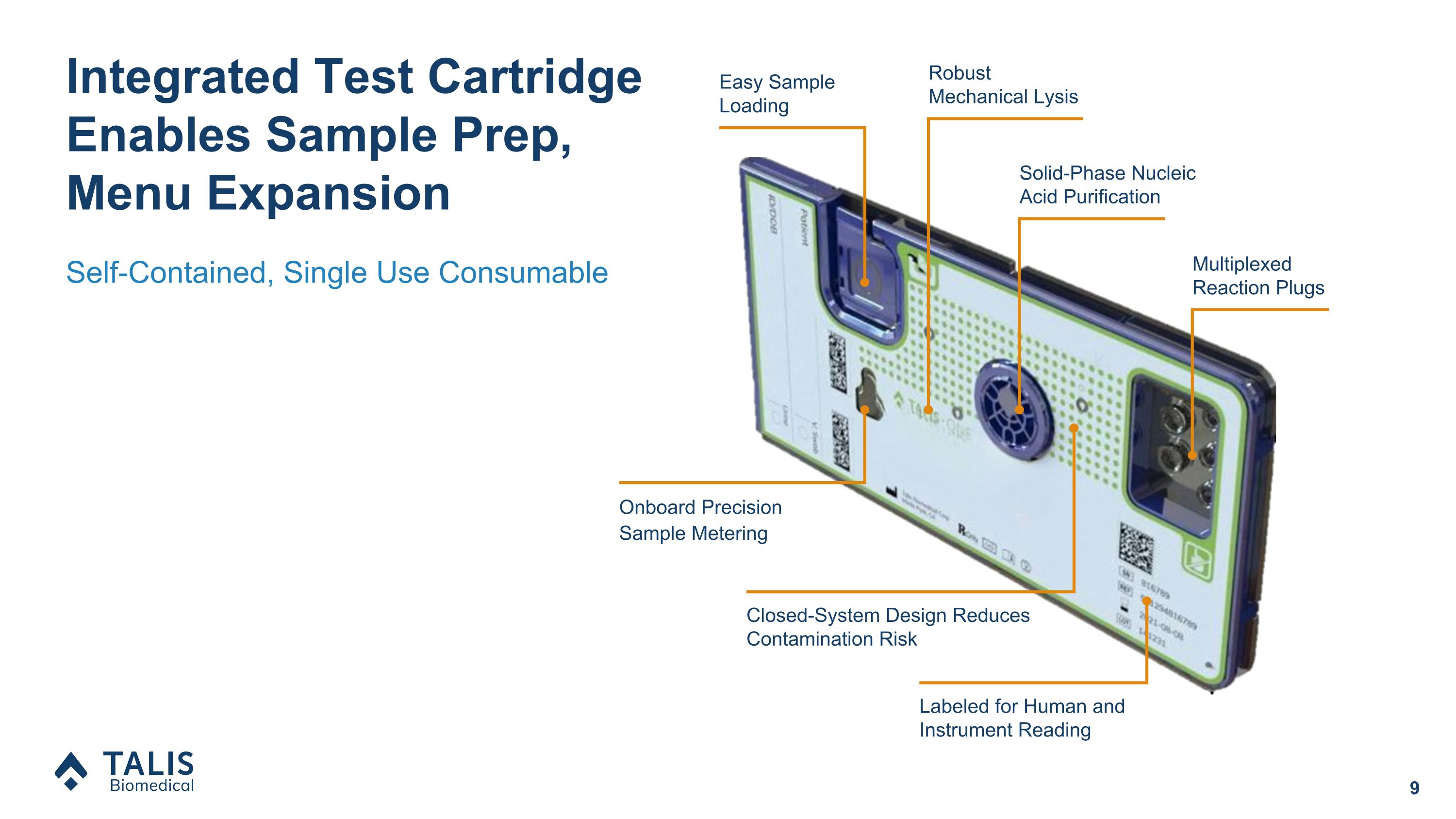
Onboard Precision Sample Metering Labeled for Human and Instrument Reading Multiplexed Reaction Plugs Robust �Mechanical Lysis Easy Sample Loading Solid-Phase Nucleic Acid Purification Closed-System Design Reduces Contamination Risk Integrated Test Cartridge Enables Sample Prep, Menu Expansion Self-Contained, Single Use Consumable

Designed for Untrained User Touchscreen Interface Cloud Connectivity Flexible Multiplexing Instrument Designed for Ease-of-Use
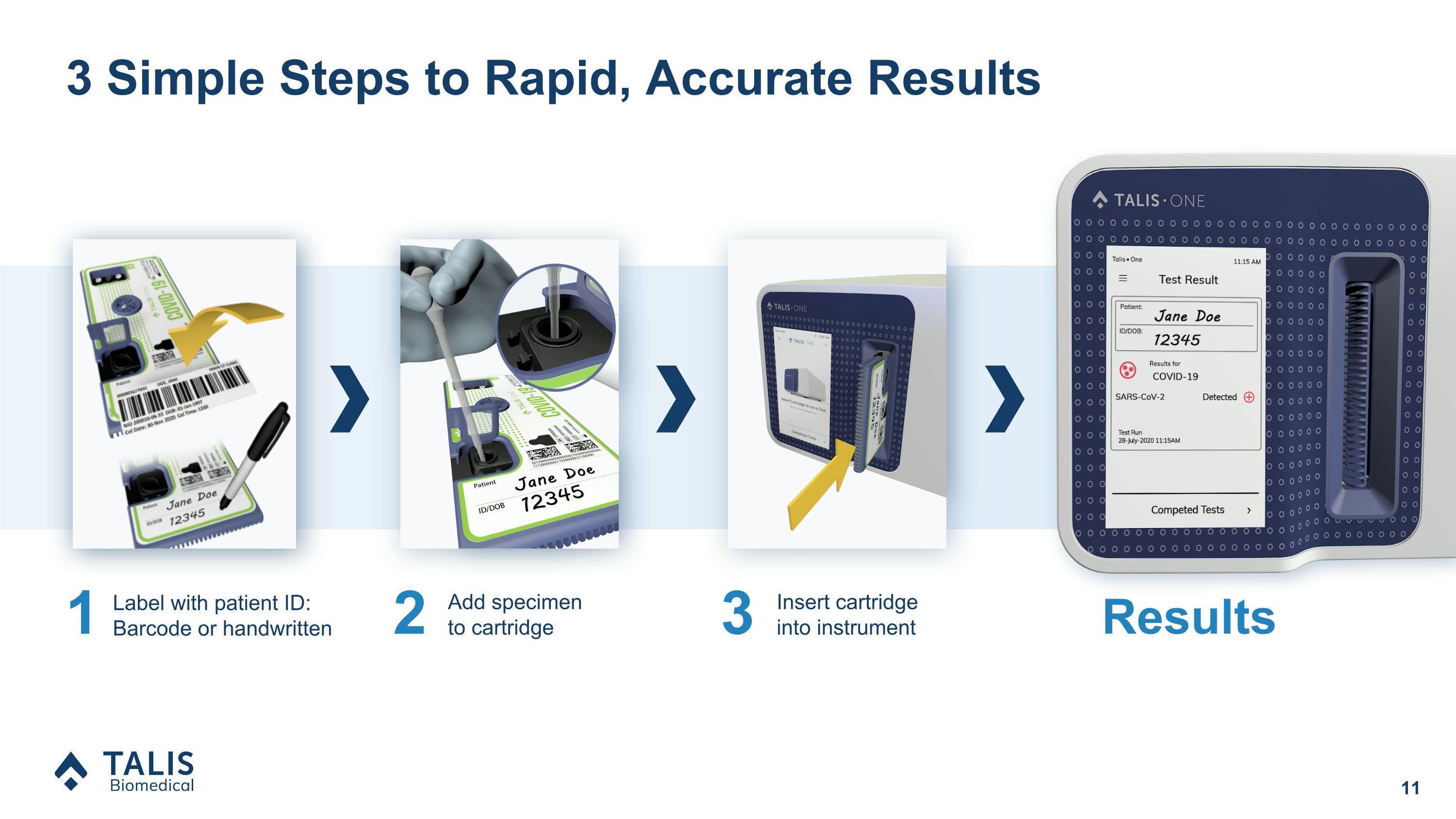
3 Simple Steps to Rapid, Accurate Results Results 1 Label with patient ID: Barcode or handwritten 2 Add specimen to cartridge 3 Insert cartridge into instrument

>$100M investment in automated manufacturing to �deliver 1M tests/month at full scale High speed assembly lines consistently �produce cartridges Ability to build instruments efficiently Talis One System performing well in hands of study users Established Ability to Manufacture at Scale Investment in automation provides advantages of quality, throughput and cost
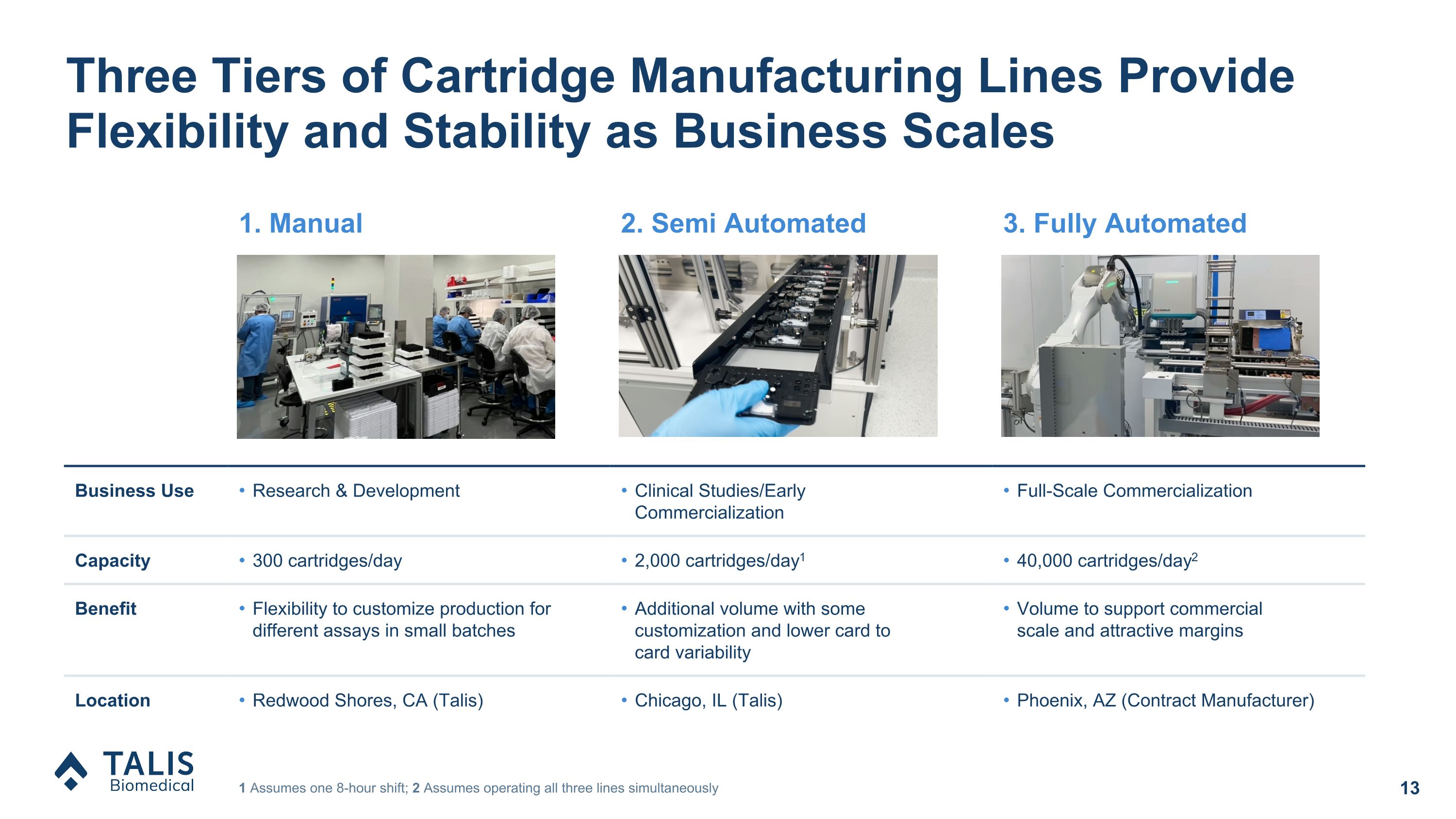
Three Tiers of Cartridge Manufacturing Lines Provide Flexibility and Stability as Business Scales 1 Assumes one 8-hour shift; 2 Assumes operating all three lines simultaneously 1. Manual 2. Semi Automated 3. Fully Automated Business Use Research & Development Clinical Studies/Early Commercialization Full-Scale Commercialization Capacity 300 cartridges/day 2,000 cartridges/day1 40,000 cartridges/day2 Benefit Flexibility to customize production for different assays in small batches Additional volume with some customization and lower card to �card variability Volume to support commercial �scale and attractive margins Location Redwood Shores, CA (Talis) Chicago, IL (Talis) Phoenix, AZ (Contract Manufacturer)

Delivering Clinical and Economic Value to Physicians Minimal capital costs and lower practice overhead with better informed diagnosis and treatment Pricing below established CPT codes Redirects reimbursement from central lab �to providers
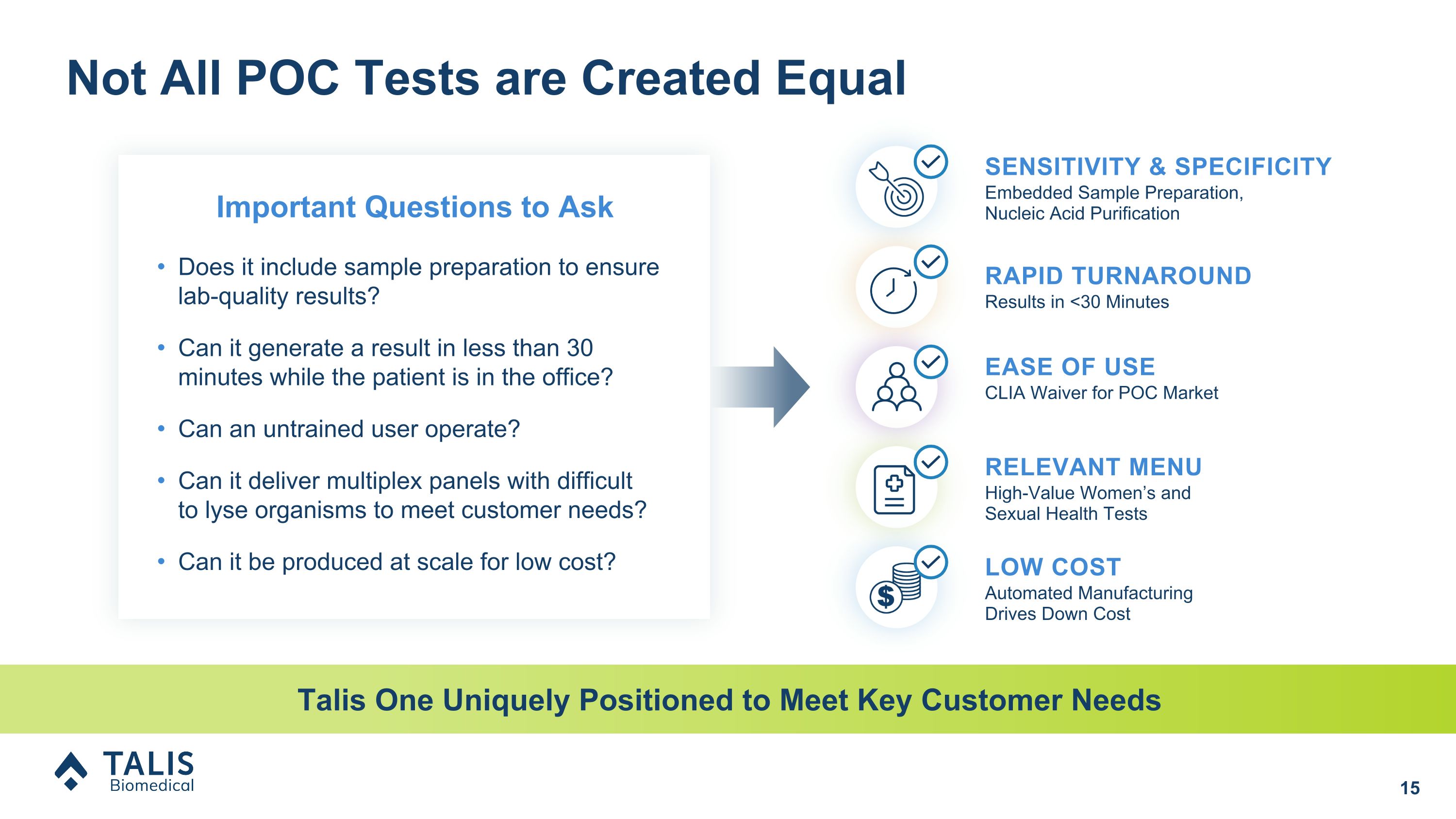
Not All POC Tests are Created Equal Does it include sample preparation to ensure lab-quality results? Can it generate a result in less than 30 minutes while the patient is in the office? Can an untrained user operate? Can it deliver multiplex panels with difficult �to lyse organisms to meet customer needs? Can it be produced at scale for low cost? Talis One Uniquely Positioned to Meet Key Customer Needs RAPID TURNAROUND Results in <30 Minutes SENSITIVITY & SPECIFICITY Embedded Sample Preparation, �Nucleic Acid Purification CLIA Waiver for POC Market EASE OF USE LOW COST Automated Manufacturing Drives Down Cost RELEVANT MENU High-Value Women’s and Sexual Health Tests Important Questions to Ask
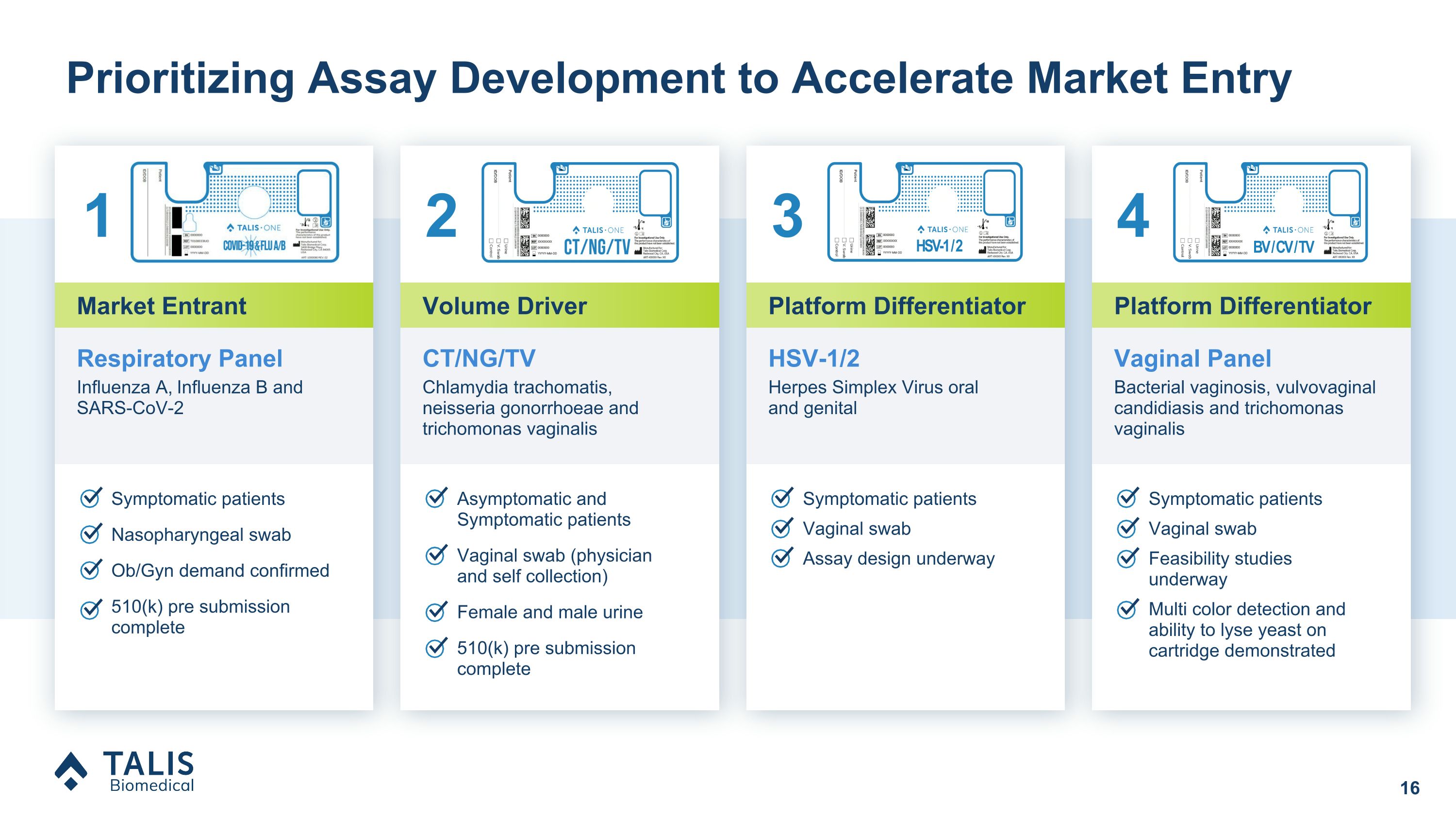
Prioritizing Assay Development to Accelerate Market Entry Symptomatic patients Nasopharyngeal swab Ob/Gyn demand confirmed 510(k) pre submission complete Asymptomatic and Symptomatic patients Vaginal swab (physician and self collection) Female and male urine 510(k) pre submission complete CT/NG/TV Chlamydia trachomatis, �neisseria gonorrhoeae and trichomonas vaginalis Vaginal Panel Bacterial vaginosis, vulvovaginal candidiasis and trichomonas vaginalis BV / CV / TV Volume Driver Platform Differentiator Respiratory Panel Influenza A, Influenza B and SARS-CoV-2 Market Entrant HSV-1/2 Herpes Simplex Virus oral �and genital HSV-1 / 2 Platform Differentiator Symptomatic patients Vaginal swab Assay design underway Symptomatic patients Vaginal swab Feasibility studies underway Multi color detection and ability to lyse yeast on cartridge demonstrated 1 2 3 4
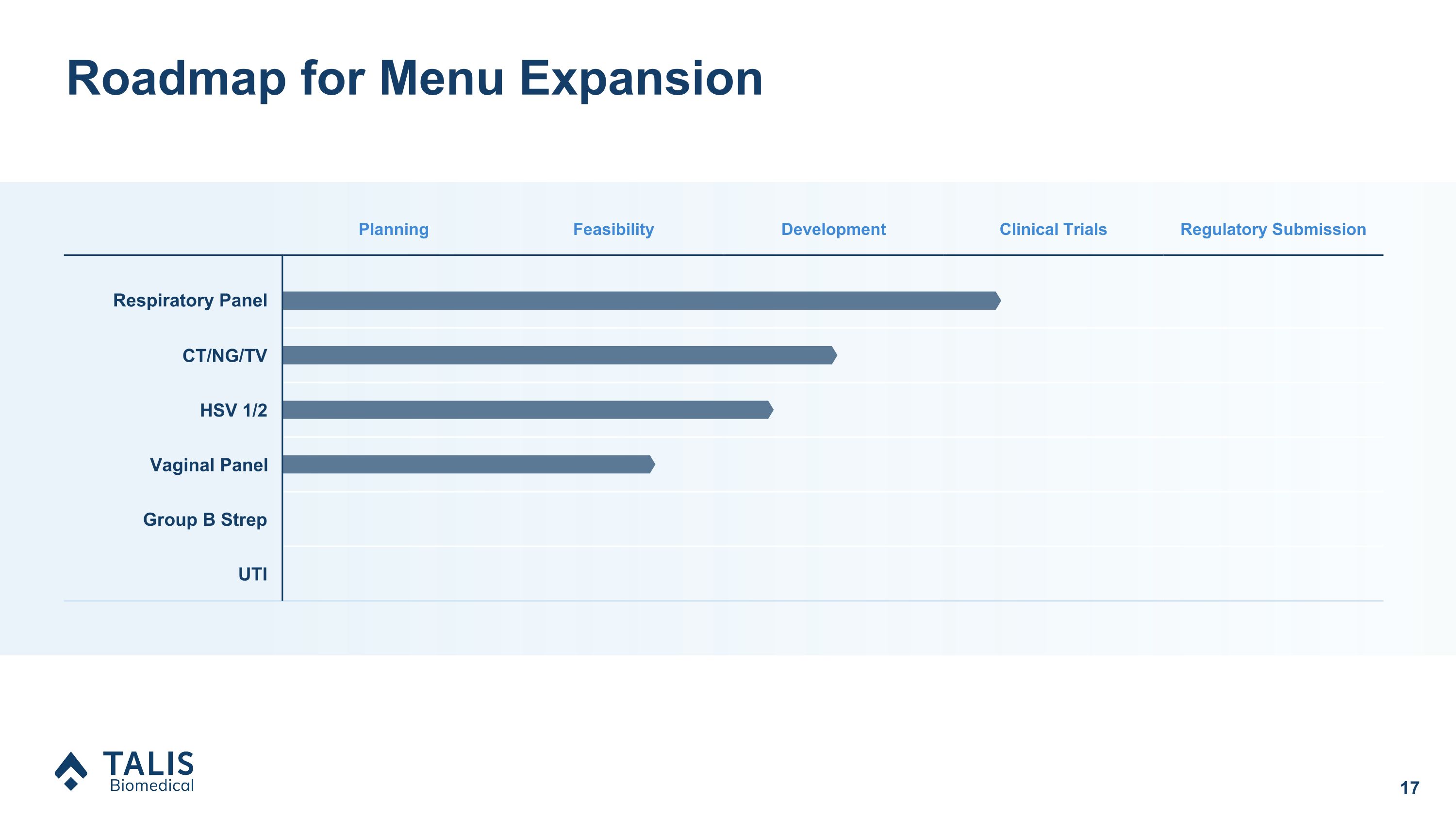
Roadmap for Menu Expansion Planning Feasibility Development Clinical Trials Regulatory Submission Respiratory Panel CT/NG/TV HSV 1/2 Vaginal Panel Group B Strep UTI

Talis Biomedical Wins Over Time Nascent and growing $3B U.S. market opportunity addressing women’s and sexual health testing Talis One System designed to deliver accuracy, speed, and ease of use High-value efficient product roadmap and clear path to attractive margins Capitalized to execute strategy into 2025 with cash ending 1Q23 of $113M Large unmet need with shifting diagnostic testing from centralized labs to the point of care

Thank you.


















