UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
_______________________________________________
FORM 10-Q
_______________________________________________
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
Commission file number 001-36353
_______________________________________________
Perrigo Company plc
(Exact name of registrant as specified in its charter)
_______________________________________________
| Ireland | Not Applicable | |||||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
The Sharp Building, Hogan Place, Dublin 2, Ireland D02 TY74
+353 1 7094000
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
| Ordinary shares, €0.001 par value | PRGO | New York Stock Exchange | ||||||
| 3.900% Notes due 2024 | PRGO24 | New York Stock Exchange | ||||||
| 4.375% Notes due 2026 | PRGO26 | New York Stock Exchange | ||||||
| 4.400% Notes due 2030 | PRGO30 | New York Stock Exchange | ||||||
| 5.300% Notes due 2043 | PRGO43 | New York Stock Exchange | ||||||
| 4.900% Notes due 2044 | PRGO44 | New York Stock Exchange | ||||||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | |||||||||||||||||||||||||
| Emerging growth company | ☐ | |||||||||||||||||||||||||||||||
| If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | ☐ | |||||||||||||||||||||||||||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
As of August 5, 2022, there were 134,618,001 ordinary shares outstanding.
PERRIGO COMPANY PLC
FORM 10-Q
INDEX
PAGE NUMBER | ||||||||
| PART I. FINANCIAL INFORMATION | ||||||||
| 1 | ||||||||
| 2 | ||||||||
| 3 | ||||||||
| 4 | ||||||||
| 5 | ||||||||
| 6 | ||||||||
| 7 | ||||||||
| 8 | ||||||||
| 9 | ||||||||
| 10 | ||||||||
| 11 | ||||||||
| 12 | ||||||||
| 13 | ||||||||
| 14 | ||||||||
| 15 | ||||||||
| 16 | ||||||||
| 17 | ||||||||
| PART II. OTHER INFORMATION | ||||||||
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this report are “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and are subject to the safe harbor created thereby. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our, or our industry’s actual results, levels of activity, performance or achievements to be materially different from those expressed or implied by any forward-looking statements. In particular, statements about our expectations, beliefs, plans, objectives, assumptions, future events or future performance contained in this report, including certain statements contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” "forecast," “predict,” “potential” or the negative of those terms or other comparable terminology.
The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control, including: the effect of the coronavirus (COVID-19) pandemic and its variants and associated supply chain impacts on the Company’s business; general economic, credit, and market conditions; the impact of the war in Ukraine and any escalation thereof, including the effects of economic and political sanctions imposed by the United States, United Kingdom, European Union, and other countries related thereto; the outbreak or escalation of conflict in other regions where we do business; future impairment charges; customer acceptance of new products; competition from other industry participants, some of whom have greater marketing resources or larger market shares in certain product categories than the Company does; pricing pressures from customers and consumers; resolution of uncertain tax positions, including the Company’s appeal of the draft and final Notices of Proposed Assessment (“NOPAs”) issued by the U.S. Internal Revenue Service and the impact that an adverse result in any such proceedings would have on operating results, cash flows, and liquidity; pending and potential third-party claims and litigation, including litigation relating to the Company’s restatement of previously-filed financial information and litigation relating to uncertain tax positions, including the NOPAs; potential impacts of ongoing or future government investigations and regulatory initiatives; uncertainty regarding the timing of, and the Company’s ability to obtain and maintain, certain regulatory approvals, including the sale of daily over-the-counter oral contraceptives; potential costs and reputational impact of product recalls or sales halts; the impact of tax reform legislation and/or changes in healthcare policy; the timing, amount and cost of any share repurchases; fluctuations in currency exchange rates and interest rates; the Company’s ability to achieve the benefits expected from the sale of its RX business and the risk that potential costs or liabilities incurred or retained in connection with that transaction may exceed the Company’s estimates or adversely affect the Company’s business or operations; the Company’s ability to achieve the benefits expected from the acquisition of Héra SAS ("HRA Pharma") and the risks that the Company’s synergy estimates are inaccurate or that the Company faces higher than anticipated integration or other costs in connection with the acquisition; risks associated with the integration of HRA Pharma, including the risk that growth rates are adversely affected by any delay in the integration of sales and distribution networks; the consummation and success of other announced and unannounced acquisitions or dispositions, and the Company’s ability to realize the desired benefits thereof; and the Company’s ability to execute and achieve the desired benefits of announced cost-reduction efforts and strategic and other initiatives, including the Company’s ability to achieve the expected benefits from its supply chain reinvention program. An adverse result with respect to the Company’s appeal of any material outstanding tax assessments or pending litigation, including securities or drug pricing matters, could ultimately require the use of corporate assets to pay such assessments, damages from third-party claims, and related interest and/or penalties, and any such use of corporate assets would limit the assets available for other corporate purposes. There can be no assurance that the FDA will approve the sale of daily oral contraceptives without a prescription in the United States. These and other important factors, including those discussed in our Form 10-K for the year ended December 31, 2021, this report under “Risk Factors” and in any subsequent filings with the United States Securities and Exchange Commission, may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. The forward-looking statements in this report are made only as of the date hereof, and unless otherwise required by applicable securities laws, we disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
This report contains trademarks, trade names and service marks that are the property of Perrigo Company plc, as well as, for informational purposes, trademarks, trade names, and service marks that are the property of other organizations. Solely for convenience, certain trademarks, trade names, and service marks referred to in this report appear without the ®, ™ and SM symbols, but those references are not intended to indicate that we or the applicable owner, as the case may be, will not assert, to the fullest extent under applicable law, our or their rights to such trademarks, trade names, and service marks.
1
Perrigo Company plc - Item 1
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (UNAUDITED)
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in millions, except per share amounts)
(unaudited)
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| Net sales | $ | 1,121.7 | $ | 981.1 | $ | 2,196.2 | $ | 1,991.1 | |||||||||||||||
| Cost of sales | 749.6 | 632.1 | 1,486.3 | 1,273.7 | |||||||||||||||||||
| Gross profit | 372.1 | 349.0 | 709.9 | 717.4 | |||||||||||||||||||
| Operating expenses | |||||||||||||||||||||||
| Distribution | 29.5 | 24.1 | 53.9 | 45.8 | |||||||||||||||||||
| Research and development | 31.5 | 33.0 | 60.8 | 64.1 | |||||||||||||||||||
| Selling | 150.8 | 139.8 | 286.4 | 275.2 | |||||||||||||||||||
| Administration | 157.8 | 110.4 | 280.1 | 237.6 | |||||||||||||||||||
| Impairment charges | — | 158.6 | — | 158.6 | |||||||||||||||||||
| Restructuring | 9.5 | 9.0 | 13.1 | 10.7 | |||||||||||||||||||
| Other operating expense (income), net | (0.1) | — | 0.8 | — | |||||||||||||||||||
| Total operating expenses | 379.0 | 474.9 | 695.1 | 792.0 | |||||||||||||||||||
| Operating income (loss) | (6.9) | (125.9) | 14.8 | (74.6) | |||||||||||||||||||
| Interest expense, net | 38.3 | 31.6 | 74.1 | 63.6 | |||||||||||||||||||
| Other (income) expense, net | 53.8 | (0.4) | 52.7 | 1.9 | |||||||||||||||||||
| Loss on extinguishment of debt | 9.3 | — | 9.3 | — | |||||||||||||||||||
| Income (loss) from continuing operations before income taxes | (108.3) | (157.1) | (121.3) | (140.1) | |||||||||||||||||||
| Income tax expense (benefit) | (43.4) | (45.2) | (55.1) | (31.0) | |||||||||||||||||||
| Income (loss) from continuing operations | (64.9) | (111.9) | (66.2) | (109.1) | |||||||||||||||||||
| Income (loss) from discontinued operations, net of tax | (0.2) | 54.2 | (1.4) | 89.5 | |||||||||||||||||||
| Net income (loss) | $ | (65.1) | $ | (57.7) | $ | (67.6) | $ | (19.6) | |||||||||||||||
| Earnings (loss) per share | |||||||||||||||||||||||
| Basic | |||||||||||||||||||||||
| Continuing operations | $ | (0.48) | $ | (0.84) | $ | (0.49) | $ | (0.82) | |||||||||||||||
| Discontinued operations | — | 0.41 | (0.01) | 0.67 | |||||||||||||||||||
| Basic earnings (loss) per share | $ | (0.48) | $ | (0.43) | $ | (0.50) | $ | (0.15) | |||||||||||||||
| Diluted | |||||||||||||||||||||||
| Continuing operations | $ | (0.48) | $ | (0.84) | $ | (0.49) | $ | (0.82) | |||||||||||||||
| Discontinued operations | — | 0.41 | (0.01) | 0.67 | |||||||||||||||||||
| Diluted earnings (loss) per share | $ | (0.48) | $ | (0.43) | $ | (0.50) | $ | (0.15) | |||||||||||||||
| Weighted-average shares outstanding | |||||||||||||||||||||||
| Basic | 134.6 | 133.6 | 134.3 | 133.4 | |||||||||||||||||||
| Diluted | 134.6 | 133.6 | 134.3 | 133.4 | |||||||||||||||||||
See accompanying Notes to the Condensed Consolidated Financial Statements.
2
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in millions)
(unaudited)
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| Net income (loss) | $ | (65.1) | $ | (57.7) | $ | (67.6) | $ | (19.6) | |||||||||||||||
| Other comprehensive income (loss): | |||||||||||||||||||||||
| Foreign currency translation adjustments | (182.5) | 32.5 | (207.0) | (79.1) | |||||||||||||||||||
| Change in fair value of derivative financial instruments, net of tax | 21.2 | (1.3) | 31.5 | (7.3) | |||||||||||||||||||
| Change in post-retirement and pension liability, net of tax | (0.5) | (0.8) | (6.8) | (1.5) | |||||||||||||||||||
| Other comprehensive income (loss), net of tax | (161.8) | 30.4 | (182.3) | (87.9) | |||||||||||||||||||
| Comprehensive income (loss) | $ | (226.9) | $ | (27.3) | $ | (249.9) | $ | (107.5) | |||||||||||||||
See accompanying Notes to the Condensed Consolidated Financial Statements.
3
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED BALANCE SHEETS
(in millions, except per share amounts)
(unaudited)
| July 2, 2022 | December 31, 2021 | ||||||||||
| Assets | |||||||||||
| Cash and cash equivalents | $ | 485.3 | $ | 1,864.9 | |||||||
| Accounts receivable, net of allowance for credit losses of $7.3 and $7.2, respectively | 750.3 | 652.9 | |||||||||
| Inventories | 1,079.6 | 1,020.2 | |||||||||
| Prepaid expenses and other current assets | 336.3 | 305.8 | |||||||||
| Current assets held for sale | — | 16.1 | |||||||||
| Total current assets | 2,651.5 | 3,859.9 | |||||||||
| Property, plant and equipment, net | 840.3 | 864.1 | |||||||||
| Operating lease assets | 207.1 | 166.9 | |||||||||
| Goodwill and indefinite-lived intangible assets | 3,582.5 | 3,004.7 | |||||||||
| Definite-lived intangible assets, net | 3,222.7 | 2,146.1 | |||||||||
| Deferred income taxes | 6.9 | 6.5 | |||||||||
| Other non-current assets | 408.6 | 377.5 | |||||||||
| Total non-current assets | 8,268.1 | 6,565.8 | |||||||||
| Total assets | $ | 10,919.6 | $ | 10,425.7 | |||||||
| Liabilities and Shareholders’ Equity | |||||||||||
| Accounts payable | $ | 490.4 | $ | 411.2 | |||||||
| Payroll and related taxes | 104.1 | 118.5 | |||||||||
| Accrued customer programs | 143.6 | 125.6 | |||||||||
| Other accrued liabilities | 240.7 | 279.4 | |||||||||
| Accrued income taxes | 5.0 | 16.5 | |||||||||
| Current indebtedness | 30.8 | 603.8 | |||||||||
| Current liabilities held for sale | — | 32.9 | |||||||||
| Total current liabilities | 1,014.6 | 1,587.9 | |||||||||
| Long-term debt, less current portion | 4,086.1 | 2,916.7 | |||||||||
| Deferred income taxes | 392.1 | 239.3 | |||||||||
| Other non-current liabilities | 577.0 | 530.1 | |||||||||
| Total non-current liabilities | 5,055.2 | 3,686.1 | |||||||||
| Total liabilities | 6,069.8 | 5,274.0 | |||||||||
| Contingencies - Refer to Note 15 | 0 | 0 | |||||||||
| Shareholders’ equity | |||||||||||
| Controlling interests: | |||||||||||
| Preferred shares, $0.0001 par value per share, 10 shares authorized | — | — | |||||||||
| Ordinary shares, €0.001 par value per share, 10,000 shares authorized | 6,991.1 | 7,043.2 | |||||||||
| Accumulated other comprehensive income | (146.8) | 35.5 | |||||||||
| Retained earnings (accumulated deficit) | (1,994.5) | (1,927.0) | |||||||||
| Total shareholders’ equity | 4,849.8 | 5,151.7 | |||||||||
| Total liabilities and shareholders' equity | $ | 10,919.6 | $ | 10,425.7 | |||||||
| Supplemental Disclosures of Balance Sheet Information | |||||||||||
| Preferred shares, issued and outstanding | — | — | |||||||||
| Ordinary shares, issued and outstanding | 134.6 | 133.8 | |||||||||
See accompanying Notes to the Condensed Consolidated Financial Statements.
4
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in millions, except per share amounts)
(unaudited)
| Ordinary Shares Issued | Accumulated Other Comprehensive Income | Retained Earnings (Accumulated Deficit) | Total | ||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||
| Balance at December 31, 2020 | 133.1 | $ | 7,118.2 | $ | 395.0 | $ | (1,858.1) | $ | 5,655.1 | ||||||||||||||||||||
| Net income | — | — | — | 38.1 | 38.1 | ||||||||||||||||||||||||
| Other comprehensive loss | — | — | (118.3) | — | (118.3) | ||||||||||||||||||||||||
| Restricted stock plan | 0.6 | — | — | — | — | ||||||||||||||||||||||||
| Compensation for stock options | — | 0.4 | — | — | 0.4 | ||||||||||||||||||||||||
| Compensation for restricted stock | — | 24.6 | — | — | 24.6 | ||||||||||||||||||||||||
| Cash dividends, $0.24 per share | — | (32.6) | — | — | (32.6) | ||||||||||||||||||||||||
| Shares withheld for payment of employees' withholding tax liability | (0.2) | (9.3) | — | — | (9.3) | ||||||||||||||||||||||||
| Balance at April 3, 2021 | 133.5 | $ | 7,101.3 | $ | 276.7 | $ | (1,820.0) | $ | 5,558.0 | ||||||||||||||||||||
| Net loss | — | — | — | (57.7) | (57.7) | ||||||||||||||||||||||||
| Other comprehensive income | — | — | 30.4 | — | 30.4 | ||||||||||||||||||||||||
| Restricted stock plan | 0.1 | — | — | — | — | ||||||||||||||||||||||||
| Compensation for stock options | — | 0.2 | — | — | 0.2 | ||||||||||||||||||||||||
| Compensation for restricted stock | — | 13.9 | — | — | 13.9 | ||||||||||||||||||||||||
| Cash dividends, $0.24 per share | — | (32.5) | — | — | (32.5) | ||||||||||||||||||||||||
| Shares withheld for payment of employees' withholding tax liability | — | (1.2) | — | — | (1.2) | ||||||||||||||||||||||||
| Balance at July 3, 2021 | 133.6 | $ | 7,081.7 | $ | 307.1 | $ | (1,877.7) | $ | 5,511.1 | ||||||||||||||||||||
| Ordinary Shares Issued | Accumulated Other Comprehensive Income | Retained Earnings (Accumulated Deficit) | Total | ||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||
| Balance at December 31, 2021 | 133.8 | $ | 7,043.2 | $ | 35.5 | $ | (1,927.0) | $ | 5,151.7 | ||||||||||||||||||||
| Net loss | — | — | — | (2.4) | (2.4) | ||||||||||||||||||||||||
| Other comprehensive loss | — | — | (20.5) | — | (20.5) | ||||||||||||||||||||||||
| Restricted stock plan | 1.2 | — | — | — | — | ||||||||||||||||||||||||
| Compensation for restricted stock | — | 26.3 | — | — | 26.3 | ||||||||||||||||||||||||
| Cash dividends, $0.26 per share | — | (34.2) | — | — | (34.2) | ||||||||||||||||||||||||
| Shares withheld for payment of employees' withholding tax liability | (0.4) | (16.4) | — | — | (16.4) | ||||||||||||||||||||||||
| Balance at April 2, 2022 | 134.6 | $ | 7,018.9 | $ | 15.0 | $ | (1,929.4) | $ | 5,104.5 | ||||||||||||||||||||
| Net loss | — | — | — | (65.1) | (65.1) | ||||||||||||||||||||||||
| Other comprehensive income | — | — | (161.8) | — | (161.8) | ||||||||||||||||||||||||
| Compensation for restricted stock | — | 9.0 | — | — | 9.0 | ||||||||||||||||||||||||
| Cash dividends, $0.26 per share | — | (35.4) | — | — | (35.4) | ||||||||||||||||||||||||
| Shares withheld for payment of employees' withholding tax liability | — | (1.4) | — | — | (1.4) | ||||||||||||||||||||||||
| Balance at July 2, 2022 | 134.6 | $ | 6,991.1 | $ | (146.8) | $ | (1,994.5) | $ | 4,849.8 | ||||||||||||||||||||
See accompanying Notes to the Condensed Consolidated Financial Statements.
5
Perrigo Company plc - Item 1
PERRIGO COMPANY PLC
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
(unaudited)
| Six Months Ended | |||||||||||
| July 2, 2022 | July 3, 2021 | ||||||||||
| Cash Flows From (For) Operating Activities | |||||||||||
| Net income (loss) | $ | (67.6) | $ | (19.6) | |||||||
| Adjustments to derive cash flows: | |||||||||||
| Depreciation and amortization | 153.0 | 165.3 | |||||||||
| Foreign currency remeasurement loss | 39.4 | — | |||||||||
| Share-based compensation | 37.3 | 39.1 | |||||||||
| Restructuring charges | 13.1 | 10.7 | |||||||||
| Deferred income taxes | 12.6 | (25.4) | |||||||||
| Loss on sale of business | 1.4 | — | |||||||||
| Impairment charges | — | 158.6 | |||||||||
| (Gain) on sale of assets | (5.8) | — | |||||||||
| Amortization of debt premium | (4.3) | (1.4) | |||||||||
| Other non-cash adjustments, net | (4.8) | 18.8 | |||||||||
| Subtotal | 174.3 | 346.1 | |||||||||
| Increase (decrease) in cash due to: | |||||||||||
| Accounts receivable | (56.4) | (108.2) | |||||||||
| Inventories | (50.5) | (106.0) | |||||||||
| Prepaid expenses | 16.8 | 1.8 | |||||||||
| Accounts payable | 64.2 | (22.5) | |||||||||
| Payroll and related taxes | (38.8) | (61.1) | |||||||||
| Accrued customer programs | 16.9 | 4.3 | |||||||||
| Accrued liabilities | 14.2 | (32.1) | |||||||||
| Accrued income taxes | (99.9) | (135.6) | |||||||||
| Other, net | 21.4 | 31.2 | |||||||||
| Subtotal | (112.1) | (428.2) | |||||||||
| Net cash from (for) operating activities | 62.2 | (82.1) | |||||||||
| Cash Flows From (For) Investing Activities | |||||||||||
| Proceeds from royalty rights | 2.0 | 1.9 | |||||||||
| Settlement of acquisition-related foreign currency derivatives | (37.1) | — | |||||||||
| Acquisitions of businesses, net of cash acquired | (1,901.4) | — | |||||||||
| Asset acquisitions | (10.0) | (70.6) | |||||||||
| Additions to property, plant and equipment | (48.7) | (68.4) | |||||||||
| Net proceeds from sale of businesses | 58.7 | — | |||||||||
| Proceeds from sale of assets | 24.8 | — | |||||||||
| Other investing, net | — | 1.3 | |||||||||
| Net cash from (for) investing activities | (1,911.7) | (135.8) | |||||||||
| Cash Flows From (For) Financing Activities | |||||||||||
| Issuances of long-term debt | 1,587.7 | — | |||||||||
| Payments on long-term debt | (958.9) | — | |||||||||
| Borrowings (repayments) of revolving credit agreements and other financing, net | — | (5.8) | |||||||||
| Payments for debt issuance costs | (19.5) | — | |||||||||
| Premiums on early debt retirement | (12.2) | — | |||||||||
| Cash dividends | (69.6) | (65.1) | |||||||||
| Other financing, net | (20.4) | (13.5) | |||||||||
| Net cash from (for) financing activities | 507.1 | (84.4) | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | (51.6) | (3.2) | |||||||||
| Net increase (decrease) in cash and cash equivalents | (1,394.0) | (305.5) | |||||||||
| Cash and cash equivalents of continuing operations, beginning of period | 1,864.9 | 631.5 | |||||||||
| Cash and cash equivalents held for sale, beginning of period | 14.4 | 10.0 | |||||||||
| Less cash and cash equivalents held for sale, end of period | — | (18.5) | |||||||||
| Cash and cash equivalents of continuing operations, end of period | $ | 485.3 | $ | 317.5 | |||||||
See accompanying Notes to the Condensed Consolidated Financial Statements.
6
Perrigo Company plc - Item 1
Note 1
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
General Information
The Company
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
Our vision is to make lives better by bringing Quality, Affordable Self-Care Products that consumers trust everywhere they are sold. We are a leading provider of over-the-counter ("OTC") health and wellness solutions that are designed to enhance individual well-being.
Basis of Presentation
The accompanying unaudited Condensed Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles ("GAAP") for interim financial information and with the instructions to Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. The unaudited Condensed Consolidated Financial Statements should be read in conjunction with the Consolidated Financial Statements and footnotes included in our Annual Report on Form 10-K for the year ended December 31, 2021. In the opinion of management, all adjustments (consisting of normal recurring accruals and other adjustments) considered necessary for a fair presentation of the unaudited Condensed Consolidated Financial Statements have been included and include our accounts and the accounts of all majority-owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Segment Reporting
Our reporting and operating segments are as follows:
•Consumer Self-Care Americas ("CSCA") comprises our consumer self-care business (OTC, infant formula, and oral care categories, and contract manufacturing) in the U.S. and Canada, and HRA Pharma self-care business (Women's Health and Skin-Care categories) in the U.S. and Canada since it was acquired on April 29, 2022. CSCA previously included our Mexico and Brazil-based OTC businesses ("Latin America businesses") until they were disposed on March 9, 2022.
•Consumer Self-Care International ("CSCI") comprises our consumer self-care business in Europe and Australia, which are primarily branded, our store brand business in the United Kingdom and parts of Europe and Asia, and HRA Pharma self-care business (Women's Health, Skin-Care and Rare-Disease categories) in Europe since it was acquired on April 29, 2022.
We previously had an RX segment, which was comprised of our prescription pharmaceuticals business in
the U.S. and other pharmaceuticals and diagnostic business in Israel, which businesses have been divested. Following the divestiture, there were no substantial assets or operations left in this segment. The RX segment was reported as Discontinued Operations in 2021, and is presented as such for all periods in this report (refer to Note 8).
Non-U.S. Operations
We translate our non-U.S. dollar-denominated operations’ assets and liabilities into U.S. dollars at current rates of exchange as of the balance sheet date and income and expense items at the average exchange rate for the
reporting period. Translation adjustments resulting from exchange rate fluctuations are recorded in the cumulative
translation account, a component of Accumulated other comprehensive income (loss) ("AOCI"). Gains or losses
from foreign currency transactions are included in Other (income) expense, net.
7
Perrigo Company plc - Item 1
Note 1
Allowance for Credit Losses
Expected credit losses on trade receivables and contract assets are measured collectively by geographic location. The estimate of expected credit losses considers historical credit loss information that is adjusted for current conditions and for reasonable and supportable forecasts. Historical credit loss experience provides the primary basis for estimation of expected credit losses. Adjustments to historical loss information may be made for significant changes in a geographic location’s economic conditions. Receivables that do not share risk characteristics are evaluated on an individual basis. These receivables are not included in the collective evaluation.
The allowance for credit losses is a valuation account that is deducted from the instruments’ cost basis to present the net amount expected to be collected. Trade receivables and contract assets are charged off against the allowance when the balance is no longer deemed collectible.
The following table presents the allowance for credit losses activity (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| Balance at beginning of period | $ | 7.1 | $ | 9.1 | $ | 7.2 | $ | 6.5 | |||||||||||||||
| Provision for credit losses, net | 2.2 | 0.4 | 2.0 | 3.7 | |||||||||||||||||||
| Receivables written-off | (1.7) | (0.6) | (1.9) | (0.9) | |||||||||||||||||||
| Transfer to held for sale | — | (1.4) | — | (1.4) | |||||||||||||||||||
| Currency translation adjustment | (0.3) | 0.2 | — | (0.2) | |||||||||||||||||||
| Balance at end of period | $ | 7.3 | $ | 7.7 | $ | 7.3 | $ | 7.7 | |||||||||||||||
NOTE 2 – REVENUE RECOGNITION
Revenue is recognized when or as a customer obtains control of promised products. The amount of revenue recognized reflects the consideration we expect to be entitled to receive in exchange for these products.
Disaggregation of Revenue
We generated net sales in the following geographic locations(1) (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| U.S. | $ | 717.6 | $ | 590.3 | $ | 1,400.6 | $ | 1,201.6 | |||||||||||||||
Europe(2) | 381.9 | 348.1 | 733.6 | 704.1 | |||||||||||||||||||
All other countries(3) | 22.2 | 42.7 | 62.0 | 85.4 | |||||||||||||||||||
| Total net sales | $ | 1,121.7 | $ | 981.1 | $ | 2,196.2 | $ | 1,991.1 | |||||||||||||||
(1) The net sales by geographic locations is derived from the location of the entity that sells to a third party.
(2) Includes Ireland net sales of $7.3 million and $13.9 million for the three and six months ended July 2, 2022, respectively, and $5.3 million and $9.8 million for the three and six months ended July 3, 2021, respectively.
(3) Includes net sales generated primarily in Mexico, Australia and Canada.
Product Category
As a result of the completed acquisition of HRA Pharma, the Company has updated its global reporting product categories beginning in the second quarter of 2022. These product category updates have been adjusted retrospectively to reflect the changes. The following updates have no impact on the Company's historical consolidated financial position, results of operations, or cash flows:
•The creation of a new "Women's Health" reporting category, comprised of the women's health portfolio of HRA Pharma, in addition to legacy Perrigo women's health products;
•The creation of a new "Skin Care" reporting category, comprised of all of the products in the legacy Perrigo "Skincare and Personal Hygiene" category except for legacy Perrigo women's health products; and
•The "Other" category in the CSCI segment now includes the HRA Pharma Rare Diseases business.
8
Perrigo Company plc - Item 1
Note 2
The following is a summary of our net sales by category (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
CSCA(1) | |||||||||||||||||||||||
| Upper Respiratory | $ | 145.9 | $ | 105.3 | $ | 298.7 | $ | 223.9 | |||||||||||||||
| Nutrition | 125.1 | 95.8 | 252.3 | 188.0 | |||||||||||||||||||
| Digestive Health | 125.1 | 115.0 | 243.7 | 233.4 | |||||||||||||||||||
| Pain and Sleep-Aids | 102.6 | 89.4 | 205.5 | 184.5 | |||||||||||||||||||
| Oral Care | 76.6 | 75.7 | 147.0 | 150.7 | |||||||||||||||||||
| Healthy Lifestyle | 67.3 | 64.7 | 134.9 | 140.9 | |||||||||||||||||||
| Skin Care | 48.6 | 47.1 | 89.5 | 92.7 | |||||||||||||||||||
| Women's Health | 12.0 | 8.1 | 20.2 | 18.3 | |||||||||||||||||||
| Vitamins, Minerals, and Supplements ("VMS") | 8.2 | 8.4 | 15.9 | 16.2 | |||||||||||||||||||
Other CSCA(2) | 16.6 | 12.8 | 30.3 | 14.2 | |||||||||||||||||||
| Total CSCA | 728.0 | 622.3 | 1,438.0 | 1,262.8 | |||||||||||||||||||
| CSCI | |||||||||||||||||||||||
| Skin Care | 120.3 | 98.7 | 209.7 | 190.1 | |||||||||||||||||||
| Upper Respiratory | 59.8 | 42.4 | 121.4 | 85.1 | |||||||||||||||||||
| Pain and Sleep-Aids | 48.8 | 48.6 | 101.8 | 97.7 | |||||||||||||||||||
| VMS | 47.5 | 53.9 | 100.2 | 116.6 | |||||||||||||||||||
| Healthy Lifestyle | 38.5 | 46.7 | 80.4 | 97.4 | |||||||||||||||||||
| Women's Health | 24.4 | 14.4 | 37.4 | 28.7 | |||||||||||||||||||
| Oral Care | 20.6 | 22.5 | 44.9 | 47.5 | |||||||||||||||||||
| Digestive Health | 4.8 | 4.9 | 9.2 | 9.8 | |||||||||||||||||||
Other CSCI(3) | 29.0 | 26.7 | 53.2 | 55.4 | |||||||||||||||||||
| Total CSCI | 393.7 | 358.8 | 758.2 | 728.3 | |||||||||||||||||||
| Total net sales | $ | 1,121.7 | $ | 981.1 | $ | 2,196.2 | $ | 1,991.1 | |||||||||||||||
(1) Includes net sales from our OTC contract manufacturing business.
(2) Consists primarily of product sales and royalty income related to supply and distribution agreements, diagnostic products and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales.
(3) Consists primarily of liquid licensed products, our distribution business, rare disease products, and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales.
While the majority of revenue is recognized at a point in time, certain of our product revenue is recognized over time. Customer contracts recognized over time exist predominately in contract manufacturing arrangements, which occur in both the CSCA and CSCI segments. Contract manufacturing revenue was $88.2 million and $168.4 million for the three and six months ended July 2, 2022, respectively, and $69.8 million and $132.9 million for the three and six months ended July 3, 2021, respectively.
We also recognize a portion of the store brand OTC product revenues in the CSCA segment over time; however, the timing difference between over time and point in time revenue recognition for store brand contracts is not significant due to the short time period between the customization of the product and shipment or delivery.
Contract Balances
The following table provides information about contract assets from contracts with customers (in millions):
| Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||
| Short-term contract assets | Prepaid expenses and other current assets | $ | 26.8 | $ | 40.2 | ||||||||||||
9
Perrigo Company plc - Item 1
Note 3
NOTE 3 – ACQUISITIONS AND DIVESTITURES
Acquisitions During the Six Months Ended July 2, 2022
HRA Pharma
On April 29, 2022, we completed the previously announced acquisition of 100% of the outstanding equity interest in HRA Pharma for total consideration of €1.8 billion, or approximately $1.9 billion. We funded the transaction with cash on hand and borrowings under our New Senior Secured Credit Facilities (as defined in Note 11).
HRA Pharma is a self-care based company with consumer brands such as Compeed®, ellaOne® and Mederma®, as well as a trusted rare disease portfolio. The acquisition completed our transformation to a consumer self-care company. HRA Pharma’s operations are reported in both our CSCA and CSCI segments.
The acquisition of HRA Pharma was accounted for as a business combination and has been reported in our Consolidated Statements of Operations as of the acquisition date. From April 29, 2022 through July 2, 2022, HRA Pharma generated net sales of $57.8 million and a net loss of $13.1 million, inclusive of $6.5 million of cost of goods sold related to the acquisition step up to fair value on inventories sold and $16.4 million of amortization related to intangible assets recognized on acquisition.
During three and six months ended July 2, 2022, we incurred $36.3 million and $43.3 million of transaction costs related to the acquisition (legal, banking and other professional fees), respectively. The amounts were recorded in Administration expense and were not allocated to an operating segment.
We are in the process of gathering significant relevant information needed to complete the valuation for the assets acquired and liabilities assumed. As a result, the initial accounting for the acquisition is incomplete. The provisional acquisition amounts recognized for assets acquired and liabilities assumed will be finalized as soon as possible but no later than one year from the acquisition date. The final determination may result in asset and liability fair values and tax bases that differ from the preliminary estimates and require changes to the preliminary amounts recognized.
10
Perrigo Company plc - Item 1
Note 3
The following table summarizes the consideration paid for HRA Pharma and the provisional amounts of the assets acquired and liabilities assumed (in millions):
| HRA Pharma | |||||
| Purchase Price | $ | 1,945.6 | |||
| Assets Acquired | |||||
| Cash and cash equivalents | $ | 44.1 | |||
| Accounts receivable | 78.1 | ||||
| Inventories | 38.5 | ||||
| Prepaid expenses and other current assets | 16.8 | ||||
| Property, plant and equipment | 4.6 | ||||
| Operating lease assets | 9.7 | ||||
| Goodwill | 616.2 | ||||
| Definite-lived intangible assets | |||||
| Trademarks and trade names | 1,072.3 | ||||
| Developed product technology | 170.2 | ||||
| Distribution networks | 82.3 | ||||
| Indefinite lived intangibles | |||||
| In-process research and development | 63.3 | ||||
| Total intangible assets | 1,388.1 | ||||
| Deferred income taxes | 11.2 | ||||
| Other non-current assets | 0.8 | ||||
| Total assets | 2,208.1 | ||||
| Liabilities assumed | |||||
| Accounts payable | 44.5 | ||||
| Payroll and related taxes | 16.1 | ||||
| Accrued customer programs | 9.0 | ||||
| Other accrued liabilities | 13.9 | ||||
| Deferred income taxes | 168.8 | ||||
| Other non-current liabilities | 10.1 | ||||
| Total liabilities | 262.4 | ||||
| Non-Controlling Interest | 0.1 | ||||
| Net Assets Acquired | $ | 1,945.6 | |||
11
Perrigo Company plc - Item 1
Note 3
Goodwill of $616.2 million arising from the acquisition consists largely of the anticipated growth from new product sales, sales to new customers, HRA Pharma's assembled workforce, and the synergies expected from combining the operations of Perrigo and HRA Pharma. Goodwill of $106.1 million and $510.1 million was allocated to our CSCA and CSCI segments, respectively. We are currently evaluating the tax deductibility of the provisional goodwill; however, we do not expect deductibility for income tax purposes. The definite-lived intangible assets acquired consist of trademarks and trade names, developed product technologies, and distribution networks. Trademarks and trade names were assigned useful lives that range from 20 to 26 years. Developed product technologies were assigned 8 to 21-year useful lives. Distribution networks were assigned useful lives ranging from 2 to 23-years reflecting the intent to integrate certain external distributors and sales forces within the CSCI segment. Trademarks and trade names, developed product technology, and in-process research and development ("IPR&D") were valued using the multi-period excess earnings method. Significant judgment was applied in estimating the fair value of the intangible assets acquired, which involved the use of significant estimates and assumptions with respect to the timing and amounts of cash flow projections, including revenue growth rates, projected profit margins, and discount rates.
Pro Forma Impact of Business Combinations
The following table presents unaudited pro forma information as if the HRA Pharma acquisition had occurred on January 1, 2021 and had been combined with the results reported in our Consolidated Statements of Operations for all periods presented (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| (Unaudited) | July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||||
| Net sales | $ | 1,151.3 | $ | 1,069.2 | $ | 2,304.7 | $ | 2,146.2 | |||||||||||||||
Income (loss) from continuing operations(1) | $ | (39.5) | $ | (123.6) | $ | (42.6) | $ | (213.8) | |||||||||||||||
(1) Includes $66.3 million of nonrecurring acquisition-related charges for the six months ended July 3, 2021.
The unaudited pro forma information is presented for information purposes only and is not indicative of the results that would have been achieved if the acquisition had taken place at such time. The unaudited pro forma information presented above includes adjustments primarily for amortization charges for acquired intangible assets, depreciation of property, plant and equipment that have been revalued, certain acquisition-related charges, and related tax effects.
Divestitures During the Six Months Ended July 2, 2022
On March 9, 2022, we completed the sale of our Latin America businesses to Advent International for total consideration of $23.9 million, consisting of $5.4 million in cash, installment receivables due 12 and 18 months from completion totaling $11.3 million based on the Mexican peso exchange rate at the time of sale, and contingent consideration of $7.2 million based on the Brazilian real exchange rate at the time of sale. The sale resulted in a pre-tax loss of $1.4 million, net of professional fees, recorded in Other operating expense, net on the Condensed Statements of Operations.
On March 24, 2022, we completed the sale of ScarAway®, a leading U.S. OTC scar management brand, to Alliance Pharmaceuticals Ltd. for cash consideration of $20.7 million. The sale resulted in a pre-tax gain of $3.6 million recorded in our CSCA segment in Other operating expense, net on the Condensed Statements of Operations.
Divestitures During the Year Ended December 31, 2021
RX business
12
Perrigo Company plc - Item 1
Note 4
NOTE 4 – GOODWILL AND INTANGIBLE ASSETS
Goodwill
Changes in the carrying amount of goodwill, by reportable segment, were as follows (in millions):
| December 31, 2021 | Business acquisitions | Currency translation adjustments | July 2, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||
CSCA(1) | $ | 1,902.4 | $ | 106.1 | $ | (1.6) | $ | 2,006.9 | ||||||||||||||||||||||||||||||||||||||||||
CSCI(2) | 1,097.0 | 510.1 | (99.1) | 1,508.0 | ||||||||||||||||||||||||||||||||||||||||||||||
| Total goodwill | $ | 2,999.4 | $ | 616.2 | $ | (100.7) | $ | 3,514.9 | ||||||||||||||||||||||||||||||||||||||||||
(1) We had no accumulated goodwill impairments as of July 2, 2022 and $6.1 million as of December 31, 2021.
(2) We had accumulated goodwill impairments of $878.4 million as of July 2, 2022 and as of December 31, 2021.
Intangible Assets
Intangible assets and related accumulated amortization consisted of the following (in millions):
| July 2, 2022 | December 31, 2021 | ||||||||||||||||||||||||||||||||||
| Gross | Accumulated Amortization | Gross | Accumulated Amortization | ||||||||||||||||||||||||||||||||
| Indefinite-lived intangibles: | |||||||||||||||||||||||||||||||||||
| Trademarks, trade names, and brands | $ | 3.2 | $ | — | $ | 3.5 | $ | — | |||||||||||||||||||||||||||
| In-process research and development | 64.4 | — | 1.8 | — | |||||||||||||||||||||||||||||||
| Total indefinite-lived intangibles | $ | 67.6 | $ | — | $ | 5.3 | $ | — | |||||||||||||||||||||||||||
| Definite-lived intangibles: | |||||||||||||||||||||||||||||||||||
| Distribution and license agreements and supply agreements | $ | 80.1 | $ | 55.8 | $ | 73.2 | $ | 56.9 | |||||||||||||||||||||||||||
| Developed product technology, formulations, and product rights | 464.7 | 198.0 | 300.2 | 191.4 | |||||||||||||||||||||||||||||||
| Customer relationships and distribution networks | 1,788.8 | 883.4 | 1,820.7 | 887.8 | |||||||||||||||||||||||||||||||
| Trademarks, trade names, and brands | 2,433.5 | 407.2 | 1,482.3 | 394.2 | |||||||||||||||||||||||||||||||
| Non-compete agreements | 2.1 | 2.1 | 2.1 | 2.1 | |||||||||||||||||||||||||||||||
| Total definite-lived intangibles | $ | 4,769.2 | $ | 1,546.5 | $ | 3,678.5 | $ | 1,532.4 | |||||||||||||||||||||||||||
| Total intangible assets | $ | 4,836.8 | $ | 1,546.5 | $ | 3,683.8 | $ | 1,532.4 | |||||||||||||||||||||||||||
We recorded amortization expense of $62.4 million and $110.9 million for the three and six months ended July 2, 2022, respectively, and $53.4 million and $106.6 million for the three and six months ended July 3, 2021, respectively.
On March 17, 2022, we announced that we received final approval from the U.S. Food and Drug Administration for the over-the-counter use of Nasonex® 24HR Allergy (mometasone furoate monohydrate 50mcg). The approval triggered a $10.0 million milestone payment to the licensor, which was made in the second quarter of 2022 and capitalized as a definite-lived intangible asset.
13
Perrigo Company plc - Item 1
Note 5
NOTE 5 – INVENTORIES
Major components of inventory were as follows (in millions):
| July 2, 2022 | December 31, 2021 | ||||||||||
| Finished goods | $ | 582.1 | $ | 549.2 | |||||||
| Work in process | 265.5 | 251.9 | |||||||||
| Raw materials | 232.0 | 219.1 | |||||||||
| Total inventories | $ | 1,079.6 | $ | 1,020.2 | |||||||
NOTE 6 – FAIR VALUE MEASUREMENTS
The table below summarizes the valuation of our financial instruments carried at fair value by the applicable pricing categories (in millions):
| July 2, 2022 | December 31, 2021 | |||||||||||||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||||||||||||||||
| Measured at fair value on a recurring basis: | ||||||||||||||||||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||||||||||||||
| Investment securities | $ | — | $ | — | $ | — | $ | 0.4 | $ | — | $ | — | ||||||||||||||||||||||||||
| Foreign currency forward contracts | — | 4.9 | — | — | 5.7 | — | ||||||||||||||||||||||||||||||||
| Cross-currency swap | — | 38.7 | — | — | — | — | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | — | 4.8 | — | — | — | — | ||||||||||||||||||||||||||||||||
| Foreign currency option contracts | — | — | — | — | 5.0 | — | ||||||||||||||||||||||||||||||||
| Total assets | $ | — | $ | 48.4 | $ | — | $ | 0.4 | $ | 10.7 | $ | — | ||||||||||||||||||||||||||
| Liabilities: | ||||||||||||||||||||||||||||||||||||||
| Cross-currency swap | $ | — | $ | — | $ | — | $ | — | $ | 13.8 | $ | — | ||||||||||||||||||||||||||
| Foreign currency forward contracts | — | 3.1 | — | — | 2.4 | — | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | — | 11.0 | — | — | — | — | ||||||||||||||||||||||||||||||||
| Total liabilities | $ | — | $ | 14.1 | $ | — | $ | — | $ | 16.2 | $ | — | ||||||||||||||||||||||||||
| Measured at fair value on a non-recurring basis: | ||||||||||||||||||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||||||||||||||
Goodwill(1) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 71.7 | ||||||||||||||||||||||||||
| Total assets | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 71.7 | ||||||||||||||||||||||||||
| Liabilities | ||||||||||||||||||||||||||||||||||||||
Liabilities held for sale, net(2) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 16.8 | ||||||||||||||||||||||||||
| Total liabilities | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 16.8 | ||||||||||||||||||||||||||
(1) During the year ended December 31, 2021, goodwill with a carrying value of $81.7 million was written down to a fair value of $71.7 million
(2) We measured the net assets held for sale for impairment purposes and recorded a total impairment of $162.2 million, resulting in a net liability held for sale balance as of December 31, 2021.
There were no transfers within Level 3 fair value measurements during the three and six months ended July 2, 2022 or the year ended December 31, 2021.
Non-recurring Fair Value Measurements
The non-recurring fair values represent only those assets whose carrying values were adjusted to fair value during the prior reporting period.
14
Perrigo Company plc - Item 1
Note 6
Fixed Rate Long-term Debt
Our fixed rate long-term debt consisted of the following (in millions):
| July 2, 2022 | December 31, 2021 | ||||||||||||||||||||||
| Level 1 | Level 2 | Level 1 | Level 2 | ||||||||||||||||||||
| Public Bonds | |||||||||||||||||||||||
| Carrying Value (excluding discount) | $ | 2,544.4 | $ | — | $ | 2,760.0 | $ | — | |||||||||||||||
| Fair value | $ | 2,271.1 | $ | — | $ | 2,847.2 | $ | — | |||||||||||||||
| Private placement note | |||||||||||||||||||||||
| Carrying value (excluding premium) | $ | ��� | $ | — | $ | — | $ | 153.5 | |||||||||||||||
| Fair value | $ | — | $ | — | $ | — | $ | 162.6 | |||||||||||||||
The fair values of our public bonds for all periods were based on quoted market prices. The fair values of our private placement note for all periods were based on interest rates offered for borrowings of a similar nature and remaining maturities.
The carrying amounts of our other financial instruments, consisting of cash and cash equivalents, accounts receivable, accounts payable, short-term debt, revolving credit agreements, promissory notes related to our equity method investment in Kazmira, and variable rate long-term debt, approximate their fair value.
NOTE 7 – INVESTMENTS
The following table summarizes the measurement category, balance sheet location, and balances of our equity securities (in millions):
| Measurement Category | Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||||
| Fair value method | Prepaid expenses and other current assets | $ | 0.3 | $ | 0.4 | |||||||||||||||
Fair value method(1) | Other non-current assets | $ | 1.7 | $ | 1.8 | |||||||||||||||
| Equity method | Other non-current assets | $ | 65.1 | $ | 66.4 | |||||||||||||||
(1) Measured at fair value using the Net Asset Value practical expedient.
The following table summarizes the expense (income) recognized in earnings of our equity securities (in millions):
| Three Months Ended | Six Months Ended | |||||||||||||||||||||||||||||||
| Measurement Category | Income Statement Location | July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||||||||
| Fair value method | Other (income) expense, net | $ | — | $ | 0.9 | $ | 0.2 | $ | 0.9 | |||||||||||||||||||||||
| Equity method | Other (income) expense, net | $ | 0.6 | $ | — | $ | 1.3 | $ | 0.7 | |||||||||||||||||||||||
NOTE 8 – DISCONTINUED OPERATIONS
Our discontinued operations consist of our generic prescription pharmaceuticals business in the U.S. and our pharmaceuticals and diagnostic businesses in Israel (collectively, the “RX business”).
On March 1, 2021, we announced a definitive agreement to sell our RX business to Altaris Capital Partners, LLC (“Altaris”). On July 6, 2021, we completed the sale of the RX business for aggregate consideration of $1.55 billion. The consideration included a $53.3 million reimbursement related to an Abbreviated New Drug Application (“ANDA") for a generic topical lotion which Altaris delivered in cash to Perrigo pursuant to the terms of the definitive agreement during the three months ended April 2, 2022. The sale resulted in a 2021 pre-tax gain, net
15
Perrigo Company plc - Item 1
Note 8
of professional fees, of $47.5 million recorded in Other (income) expense, net on the Statement of Operations for discontinued operations. The gain included a $159.3 million increase from the write-off of foreign currency translation adjustment from Accumulated other comprehensive income. The transaction gain was subject to final settlements under the Agreement, which were finalized in the first quarter of 2022 with no change to the gain reported for the year ended December 31, 2021.
As of March 1, 2021, we determined that the RX business met the criteria to be classified as a discontinued operation and, as a result, its historical financial results have been reflected in our consolidated financial statements as a discontinued operation and its assets and liabilities have been classified as held for sale. We ceased recording depreciation and amortization on the RX business assets from March 1, 2021. We have not allocated any general corporate overhead to the discontinued operation.
Under the terms of a transition services agreement ("TSA"), we will provide transition services for up to 24 months after the close of the transaction. We also entered into reciprocal supply agreements pursuant to which Perrigo will supply certain products to the RX business and the RX business will supply certain products to Perrigo. The supply agreements have a term of four years, extendable up to seven years by the party who is the purchaser of the products under such agreement. We also extended distribution rights to the RX business for certain OTC products owned and manufactured by Perrigo that may be fulfilled through pharmacy channels, in return for a share of the net profits.
The following table summarizes the results of the TSA and supply agreements:
| Three Months Ended | Six Months Ended | |||||||||||||
| Financial Statement Location | July 2, 2022 | July 2, 2022 | ||||||||||||
| TSA income recognized | Administration expense | $ | 3.4 | $ | 6.8 | |||||||||
| TSA income collected | Administration expense | $ | 2.3 | $ | 5.7 | |||||||||
| Product & Royalty sales recognized | Net sales | $ | 38.0 | $ | 70.8 | |||||||||
| Product & Royalty sales collected | Net sales | $ | 37.9 | $ | 68.6 | |||||||||
| Purchases | Inventory | $ | 6.4 | $ | 15.1 | |||||||||
| Inventory payments | Inventory | $ | 3.7 | $ | 18.0 | |||||||||
Additionally, under the TSA, we net settle any receipts received or payments made on behalf of the RX business’ customers or vendors. As of July 2, 2022, we recorded a receivable in the amount of $15.1 million in Prepaid expenses and other current assets for the net reimbursement due to Perrigo.
In the transaction, Perrigo retained certain pre-closing liabilities arising out of antitrust (refer to Note 15 - Contingencies under the header "Price-Fixing Lawsuits") and opioid matters and the Company’s Albuterol recall, subject to, in each case, Altaris' obligation to indemnify the Company for 50 percent of these liabilities up to an aggregate cap on Altaris' obligation of $50.0 million. We did not incur changes in liabilities or request payments from Altaris related to the indemnity of these liabilities during the three and six months ended July 2, 2022.
16
Perrigo Company plc - Item 1
Note 8
For the three and six months ended July 2, 2022, we incurred $0.2 million and $1.4 million of net loss, respectively, from discontinued operations, which primarily related to legal fees for retained liabilities and related tax benefit. Prior year income from discontinued operations, net of tax was as follows (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||||||||
| July 3, 2021 | July 3, 2021 | ||||||||||||||||||||||||||||
| Net sales | $ | 204.5 | $ | 404.5 | |||||||||||||||||||||||||
| Cost of sales | 119.9 | 258.2 | |||||||||||||||||||||||||||
| Gross profit | 84.6 | 146.3 | |||||||||||||||||||||||||||
| Operating expenses | |||||||||||||||||||||||||||||
| Distribution | 2.8 | 6.1 | |||||||||||||||||||||||||||
| Research and development | 17.3 | 30.6 | |||||||||||||||||||||||||||
| Selling | 8.8 | 16.2 | |||||||||||||||||||||||||||
| Administration | 12.4 | 30.6 | |||||||||||||||||||||||||||
| Other operating expense (income) | 0.5 | (0.4) | |||||||||||||||||||||||||||
| Total operating expenses | 41.8 | 83.1 | |||||||||||||||||||||||||||
| Operating income | 42.8 | 63.2 | |||||||||||||||||||||||||||
| Interest expense, net | 0.2 | 0.8 | |||||||||||||||||||||||||||
| Other (income) expense, net | (0.2) | (1.7) | |||||||||||||||||||||||||||
| Income from discontinued operations before tax | 42.8 | 64.1 | |||||||||||||||||||||||||||
| Income tax benefit | (11.4) | (25.4) | |||||||||||||||||||||||||||
| Income from discontinued operations, net of tax | $ | 54.2 | $ | 89.5 | |||||||||||||||||||||||||
During the three months and six months ended July 3, 2021, we incurred $2.4 million and $11.7 million, respectively, of separation costs related to the sale of the RX business, which are recorded in administration expenses. We incurred no such costs in 2022.
Select cash flow information related to discontinued operations was as follows (in millions):
| Six Months Ended | |||||||||||||||||
| July 3, 2021 | |||||||||||||||||
| Cash flows from discontinued operations operating activities: | |||||||||||||||||
| Depreciation and amortization | $ | 15.3 | |||||||||||||||
| Cash flows from discontinued operations investing activities: | |||||||||||||||||
| Asset acquisitions | $ | (69.7) | |||||||||||||||
| Additions to property, plant and equipment | (6.1) | ||||||||||||||||
Asset acquisitions related to discontinued operations consisted of 2 ANDAs purchased under a contractual arrangement. On December 31, 2020, we purchased an ANDA for a generic topical gel for $16.4 million, which was subsequently paid during the three months ended April 3, 2021 and on March 8, 2021, we purchased an ANDA for a generic topical lotion for $53.3 million. These ANDAs were acquired by Altaris as part of the RX business sale.
17
Perrigo Company plc - Item 1
Note 9
NOTE 9 – DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
Foreign Currency Option Contracts
In September 2021, to economically hedge the foreign currency exposure associated with the planned payment of the euro-denominated purchase price for HRA Pharma, we entered into 2 non-designated currency option contracts with a total notional amount of $1.1 billion that were scheduled to mature in September 2022. In April 2022, due to market conditions, we unwound the 2 options and entered into 2 new undesignated options to economically hedge the purchase price for HRA Pharma for a total notional amount of $2.0 billion. All premiums associated with the HRA Pharma related currency options were settled in April 2022 for $37.1 million, and we recorded a loss within Other (income) expense of $12.7 million and $16.2 million for the three and six months ended July 2, 2022, respectively.
Interest Rate Swaps
Interest rate swap agreements are contracts to exchange fixed or floating rates over the life of the agreement without the exchange of the underlying notional amounts. The notional amounts of the interest rate swap agreements are used to measure interest to be paid or received and do not represent the amount of exposure to credit loss. The differential paid or received on the interest rate swap agreements is recognized as an adjustment to interest expense.
In April 2022, to economically hedge the interest rate risk of the New Senior Secured Credit Facilities (as defined in Note 11), we entered into 5 variable-to-fixed interest rate swap agreements. NaN of the interest rate swaps were designated as cash flow hedges to fix the interest rate on a substantial portion of the 2022 Term Loan B Facility (as defined in Note 11). The interest rate swaps cover an interest period ranging from June 1, 2022, through April 1, 2029, on notional balances that decline from $1.0 billion to $812.5 million over the term. The other 2 interest rate swaps were designated as cash flow hedges to fix the interest rate on a substantial portion of the 2022 Term Loan A Facility (as defined in Note 11). The interest rate swaps cover an interest period ranging from June 1, 2022, through April 1, 2027, on notional balances that decline from $487.5 million to $387.5 million over the term.
As a designated cash flow hedge, gains and losses will be deferred in Accumulated Other Comprehensive Income (“AOCI”) and recognized within Interest expense, net when interest is paid on the New Senior Secured Credit Facilities.
Cross-currency Swaps
In April 2022, we entered into 3 fixed-for-fixed cross currency interest rate swaps designated as net investment hedge to hedge the EUR currency exposure of our investment in European operations. Over the term, we receive EUR interest payments and make USD interest payments followed by an exchange of notional currencies at the expiration of the contract. The following are the terms and notional amounts outstanding:
•$300 million notional amount outstanding from April 20, 2022 through December 15, 2024;
•$700 million notional amount outstanding from April 29, 2022 through March 15, 2026; and
•$500 million notional amount outstanding from April 22, 2022 through June 15, 2030.
As a designated net investment hedge, gains and losses related to the EUR spot exchange rate will be deferred within the Cumulative Translation Adjustment, a component of AOCI, and recognized in the Statement of Operations when the hedged EUR net investment is substantially liquidated. Gains and losses on excluded components (e.g. interest differentials) will be recorded in Interest expense, net on a systematic and rational basis.
In August 2019, we entered into a cross-currency swap designated as a net investment hedge to hedge the EUR currency exposure of our net investment in European operations. This agreement is a contract to exchange floating-rate EUR payments for floating-rate USD payments through August 15, 2022. We terminated this cross-currency swap on January 28, 2022.
18
Perrigo Company plc - Item 1
Note 9
Foreign Currency Forwards
Foreign currency forward contracts were as follows (in millions):
| Notional Amount | ||||||||||||||
| July 2, 2022 | December 31, 2021 | |||||||||||||
| British Pound (GBP) | $ | 232.2 | $ | 135.8 | ||||||||||
| Swedish Krona (SEK) | 61.2 | 47.8 | ||||||||||||
| Danish Krone (DKK) | 49.6 | 37.5 | ||||||||||||
| European Euro (EUR) | 39.1 | 232.6 | ||||||||||||
| Chinese Yuan (CNH) | 37.7 | 37.7 | ||||||||||||
| Canadian Dollar (CAD) | 29.2 | 29.0 | ||||||||||||
| Polish Zloty (PLZ) | 15.3 | 21.0 | ||||||||||||
| Mexican Peso (MXN) | 12.8 | 1.0 | ||||||||||||
| United States Dollar (USD) | 9.3 | 22.9 | ||||||||||||
| Norwegian Krone (NOK) | 7.4 | 11.0 | ||||||||||||
| Brazilian Real (BRL) | 6.8 | — | ||||||||||||
| Australian Dollar (AUD) | 4.0 | 1.6 | ||||||||||||
| Turkish Lira (TRY) | 3.0 | 3.1 | ||||||||||||
| Romanian New Leu (RON) | 2.2 | 1.6 | ||||||||||||
| Switzerland Franc (CHF) | 1.2 | 1.9 | ||||||||||||
| Other | 7.0 | 3.6 | ||||||||||||
| Total | $ | 518.0 | $ | 588.1 | ||||||||||
The maximum term of our forward currency exchange contracts is 60 months.
Effects of Derivatives on the Financial Statements
The below tables indicate the effects of all derivative instruments on the Condensed Consolidated Financial Statements. All amounts exclude income tax effects.
The balance sheet location and gross fair value of our derivative instruments were as follows (in millions):
| Asset Derivatives | |||||||||||||||||
| Fair Value | |||||||||||||||||
| Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||
| Designated derivatives: | |||||||||||||||||
| Foreign currency forward contracts | Prepaid expenses and other current assets | $ | 2.9 | $ | 3.5 | ||||||||||||
| Interest rate swap agreements | Prepaid expenses and other current assets | 4.8 | — | ||||||||||||||
| Foreign currency forward contracts | Other non-current assets | 0.6 | 1.3 | ||||||||||||||
| Cross-currency swap | Other non-current assets | 38.7 | — | ||||||||||||||
| Total designated derivatives | $ | 47.0 | $ | 4.8 | |||||||||||||
| Non-designated derivatives: | |||||||||||||||||
| Foreign currency forward contracts | Prepaid expenses and other current assets | $ | 1.4 | $ | 0.9 | ||||||||||||
| Foreign currency options | Prepaid expenses and other current assets | — | 5.0 | ||||||||||||||
| Total non-designated derivatives | $ | 1.4 | $ | 5.9 | |||||||||||||
19
Perrigo Company plc - Item 1
Note 9
| Liability Derivatives | |||||||||||||||||
| Fair Value | |||||||||||||||||
| Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||
| Designated derivatives: | |||||||||||||||||
| Foreign currency forward contracts | Other accrued liabilities | $ | 0.4 | $ | 1.2 | ||||||||||||
| Cross-currency swap | Other accrued liabilities | — | 13.8 | ||||||||||||||
| Interest rate swap agreements | Other non-current liabilities | 11.0 | — | ||||||||||||||
| Total designated derivatives | $ | 11.4 | $ | 15.0 | |||||||||||||
| Non-designated derivatives: | |||||||||||||||||
| Foreign currency forward contracts | Other accrued liabilities | $ | 2.7 | $ | 1.2 | ||||||||||||
The following tables summarize the effect of derivative instruments designated as hedging instruments in AOCI (in millions):
| Three Months Ended | ||||||||||||||||||||||||||||||||
| July 2, 2022 | ||||||||||||||||||||||||||||||||
| Instrument | Amount of Gain/(Loss) Recorded in OCI | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing | |||||||||||||||||||||||||||
| Cash flow hedges: | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | $ | (6.2) | Interest expense, net | $ | (1.6) | Interest expense, net | $ | — | ||||||||||||||||||||||||
| Foreign currency forward contracts | 2.3 | Net sales | 0.3 | Net sales | (0.1) | |||||||||||||||||||||||||||
| Cost of sales | (0.9) | Cost of sales | (0.1) | |||||||||||||||||||||||||||||
| Other (income) expense, net | (0.8) | |||||||||||||||||||||||||||||||
| $ | (3.9) | $ | (2.2) | $ | (1.0) | |||||||||||||||||||||||||||
| Net investment hedges: | ||||||||||||||||||||||||||||||||
| Cross-currency swap | $ | 38.7 | Interest expense, net | $ | 3.6 | |||||||||||||||||||||||||||
20
Perrigo Company plc - Item 1
Note 9
| Six Months Ended | ||||||||||||||||||||||||||||||||
| July 2, 2022 | ||||||||||||||||||||||||||||||||
| Instrument | Amount of Gain/(Loss) Recorded in OCI(1) | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing | |||||||||||||||||||||||||||
| Cash flow hedges: | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | (6.2) | Interest expense, net | (2.0) | Interest expense, net | — | |||||||||||||||||||||||||||
| Foreign currency forward contracts | (4.9) | Net sales | 0.5 | Net sales | (0.2) | |||||||||||||||||||||||||||
| Cost of sales | (1.3) | Cost of sales | — | |||||||||||||||||||||||||||||
| Other (income) expense, net | (0.7) | |||||||||||||||||||||||||||||||
| $ | (11.1) | $ | (2.8) | $ | (0.9) | |||||||||||||||||||||||||||
| Net investment hedges: | ||||||||||||||||||||||||||||||||
| Cross-currency swap | $ | 34.1 | Interest expense, net | $ | 4.2 | |||||||||||||||||||||||||||
(1) Net gain of $6.4 million is expected to be reclassified out of AOCI into earnings during the next 12 months.
| Three Months Ended | ||||||||||||||||||||||||||||||||
| July 3, 2021 | ||||||||||||||||||||||||||||||||
| Instrument | Amount of Gain/(Loss) Recorded in OCI | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing | |||||||||||||||||||||||||||
| Cash flow hedges: | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | $ | — | Interest expense, net | $ | (0.4) | Interest expense, net | $ | — | ||||||||||||||||||||||||
| Foreign currency forward contracts | 1.1 | Net sales | (1.0) | Net sales | — | |||||||||||||||||||||||||||
| Cost of sales | 0.9 | Cost of sales | 0.4 | |||||||||||||||||||||||||||||
| Other (income) expense, net | 0.4 | |||||||||||||||||||||||||||||||
| $ | 1.1 | $ | (0.5) | $ | 0.8 | |||||||||||||||||||||||||||
| Net investment hedges: | ||||||||||||||||||||||||||||||||
| Cross-currency swap | $ | (1.5) | Interest expense, net | $ | (1.1) | |||||||||||||||||||||||||||
21
Perrigo Company plc - Item 1
Note 9
| Six Months Ended | ||||||||||||||||||||||||||||||||
| July 3, 2021 | ||||||||||||||||||||||||||||||||
| Instrument | Amount of Gain/(Loss) Recorded in OCI | Classification of Gain/(Loss) Reclassified from AOCI into Earnings | Amount of Gain/(Loss) Reclassified from AOCI into Earnings | Classification of Gain/(Loss) Recognized into Earnings Related to Amounts Excluded from Effectiveness Testing | Amount of Gain/(Loss) Recognized in Earnings on Derivatives Related to Amounts Excluded from Effectiveness Testing | |||||||||||||||||||||||||||
| Cash flow hedges: | ||||||||||||||||||||||||||||||||
| Interest rate swap agreements | — | Interest expense, net | (0.9) | Interest expense, net | — | |||||||||||||||||||||||||||
| Foreign currency forward contracts | (1.2) | Net sales | (1.9) | Net sales | — | |||||||||||||||||||||||||||
| Cost of sales | 2.8 | Cost of sales | 0.5 | |||||||||||||||||||||||||||||
| Other Income/Expense | $ | 0.4 | ||||||||||||||||||||||||||||||
| $ | (1.2) | $ | — | $ | 0.9 | |||||||||||||||||||||||||||
| Net investment hedges: | ||||||||||||||||||||||||||||||||
| Cross-currency swap | $ | (2.0) | Interest expense, net | $ | (2.2) | |||||||||||||||||||||||||||
The amounts of (income)/expense recognized in earnings related to our non-designated derivatives on the Condensed Consolidated Statements of Operations were as follows (in millions):
| Three Months Ended | Six Months Ended | |||||||||||||||||||||||||||||||
| Non-Designated Derivatives | Income Statement Location | July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||||||||
| Foreign currency forward contracts | Other (income) expense, net | $ | 1.6 | $ | (3.1) | $ | 2.0 | $ | (5.9) | |||||||||||||||||||||||
| Interest expense, net | 0.8 | 0.4 | (0.4) | 0.9 | ||||||||||||||||||||||||||||
| $ | 2.4 | $ | (2.7) | $ | 1.6 | $ | (5.0) | |||||||||||||||||||||||||
| Foreign currency options | Other (income) expense, net | $ | 12.7 | $ | — | $ | 16.2 | $ | — | |||||||||||||||||||||||
The classification and amount of gain/(loss) recognized in earnings on fair value and hedging relationships were as follows (in millions):
22
Perrigo Company plc - Item 1
Note 9
| Three Months Ended | ||||||||||||||||||||||||||
| July 2, 2022 | ||||||||||||||||||||||||||
| Net Sales | Cost of Sales | Interest Expense, net | Other (Income) Expense, net | |||||||||||||||||||||||
| Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | $ | 1,121.7 | $ | 749.6 | $ | 38.3 | $ | 53.8 | ||||||||||||||||||
| The effects of cash flow hedging: | ||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships | ||||||||||||||||||||||||||
| Foreign currency forward contracts | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | 0.3 | $ | (0.9) | $ | — | $ | — | ||||||||||||||||||
| Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | $ | (0.1) | $ | (0.1) | $ | — | $ | (0.8) | ||||||||||||||||||
| Interest rate swap agreements | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | — | $ | — | $ | (1.6) | $ | — | ||||||||||||||||||
| Six Months Ended | ||||||||||||||||||||||||||
| July 2, 2022 | ||||||||||||||||||||||||||
| Net Sales | Cost of Sales | Interest Expense, net | Other (Income) Expense, net | |||||||||||||||||||||||
| Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | $ | 2,196.2 | $ | 1,486.3 | $ | 74.1 | $ | 52.7 | ||||||||||||||||||
| The effects of cash flow hedging: | ||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships | ||||||||||||||||||||||||||
| Foreign currency forward contracts | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | 0.5 | $ | (1.3) | $ | — | $ | — | ||||||||||||||||||
| Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | $ | (0.2) | $ | — | $ | — | $ | (0.7) | ||||||||||||||||||
| Treasury locks | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
| Interest rate swap agreements | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | — | $ | — | $ | (2.0) | $ | — | ||||||||||||||||||
23
Perrigo Company plc - Item 1
Note 9
| Three Months Ended | ||||||||||||||||||||||||||
| July 3, 2021 | ||||||||||||||||||||||||||
| Net Sales | Cost of Sales | Interest Expense, net | Other (Income) Expense, net | |||||||||||||||||||||||
| Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | $ | 981.1 | $ | 632.1 | $ | 31.6 | $ | (0.4) | ||||||||||||||||||
| The effects of cash flow hedging: | ||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships | ||||||||||||||||||||||||||
| Foreign currency forward contracts | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | (1.0) | $ | 0.9 | $ | — | $ | — | ||||||||||||||||||
| Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | $ | — | $ | 0.4 | $ | — | $ | 0.4 | ||||||||||||||||||
| Interest rate swap agreements | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | — | $ | — | $ | (0.4) | $ | — | ||||||||||||||||||
| Six Months Ended | ||||||||||||||||||||||||||
| July 3, 2021 | ||||||||||||||||||||||||||
| Net Sales | Cost of Sales | Interest Expense, net | Other (Income) Expense, net | |||||||||||||||||||||||
| Total amounts of income and expense line items presented on the Condensed Consolidated Statements of Operations in which the effects of fair value or cash flow hedges are recorded | $ | 1,991.1 | $ | 1,273.7 | $ | 63.6 | $ | 1.9 | ||||||||||||||||||
| The effects of cash flow hedging: | ||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships | ||||||||||||||||||||||||||
| Foreign currency forward contracts | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | (1.9) | $ | 2.8 | $ | — | $ | — | ||||||||||||||||||
| Amount excluded from effectiveness testing recognized using a systematic and rational amortization approach | $ | — | $ | 0.5 | $ | — | $ | 0.4 | ||||||||||||||||||
| Interest rate swap agreements | ||||||||||||||||||||||||||
| Amount of gain or (loss) reclassified from AOCI into earnings | $ | — | $ | — | $ | (0.9) | $ | — | ||||||||||||||||||
24
Perrigo Company plc - Item 1
Note 10
NOTE 10 – LEASES
The balance sheet locations of our lease assets and liabilities were as follows (in millions):
| Assets | Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||||
| Operating | Operating lease assets | $ | 207.1 | $ | 166.9 | |||||||||||||||
| Finance | Other non-current assets | 24.4 | 27.9 | |||||||||||||||||
| Total | $ | 231.5 | $ | 194.8 | ||||||||||||||||
| Liabilities | Balance Sheet Location | July 2, 2022 | December 31, 2021 | |||||||||||||||||
| Current | ||||||||||||||||||||
| Operating | Other accrued liabilities | $ | 27.2 | $ | 26.0 | |||||||||||||||
| Finance | Current indebtedness | 4.2 | 4.9 | |||||||||||||||||
| Non-Current | ||||||||||||||||||||
| Operating | Other non-current liabilities | 178.6 | 147.3 | |||||||||||||||||
| Finance | Long-term debt, less current portion | 18.6 | 20.9 | |||||||||||||||||
| Total | $ | 228.6 | $ | 199.1 | ||||||||||||||||
The below tables show our lease assets and liabilities by reporting segment (in millions):
| Assets | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Operating | Financing | |||||||||||||||||||||||||||||||||||||||||||||||||
| July 2, 2022 | December 31, 2021 | July 2, 2022 | December 31, 2021 | |||||||||||||||||||||||||||||||||||||||||||||||
| CSCA | $ | 102.8 | $ | 98.2 | $ | 14.5 | $ | 15.3 | ||||||||||||||||||||||||||||||||||||||||||
| CSCI | 33.5 | 30.7 | 6.9 | 7.9 | ||||||||||||||||||||||||||||||||||||||||||||||
| Unallocated | 70.8 | 38.0 | 3.0 | 4.7 | ||||||||||||||||||||||||||||||||||||||||||||||
| Total | $ | 207.1 | $ | 166.9 | $ | 24.4 | $ | 27.9 | ||||||||||||||||||||||||||||||||||||||||||
| Liabilities | ||||||||||||||||||||||||||
| Operating | Financing | |||||||||||||||||||||||||
| July 2, 2022 | December 31, 2021 | July 2, 2022 | December 31, 2021 | |||||||||||||||||||||||
| CSCA | $ | 103.9 | $ | 99.7 | $ | 15.5 | $ | 16.0 | ||||||||||||||||||
| CSCI | 34.9 | 31.8 | 4.3 | 5.0 | ||||||||||||||||||||||
| Unallocated | 67.0 | 41.8 | 3.0 | 4.8 | ||||||||||||||||||||||
| Total | $ | 205.8 | $ | 173.3 | $ | 22.8 | $ | 25.8 | ||||||||||||||||||
Lease expense was as follows (in millions):
| Three Months Ended | Six Months Ended | |||||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||||
Operating leases(1) | $ | 10.8 | $ | 9.8 | $ | 20.6 | $ | 19.7 | ||||||||||||||||||
| Finance leases | ||||||||||||||||||||||||||
| Amortization | $ | 1.4 | $ | 1.5 | $ | 2.9 | $ | 3.0 | ||||||||||||||||||
| Interest | 0.2 | 0.2 | 0.4 | 0.4 | ||||||||||||||||||||||
| Total finance leases | $ | 1.6 | $ | 1.7 | $ | 3.3 | $ | 3.4 | ||||||||||||||||||
(1) Includes short-term leases and variable lease costs, which are immaterial.
25
Perrigo Company plc - Item 1
Note 10
The annual future maturities of our leases as of July 2, 2022 are as follows (in millions):
| Operating Leases | Finance Leases | Total | ||||||||||||||||||
| 2022 | $ | 16.9 | $ | 2.6 | $ | 19.5 | ||||||||||||||
| 2023 | 30.3 | 3.8 | 34.1 | |||||||||||||||||
| 2024 | 26.4 | 2.3 | 28.7 | |||||||||||||||||
| 2025 | 23.0 | 2.2 | 25.2 | |||||||||||||||||
| 2026 | 18.6 | 2.0 | 20.6 | |||||||||||||||||
| After 2026 | 123.4 | 13.6 | 137.0 | |||||||||||||||||
| Total lease payments | 238.6 | 26.5 | 265.1 | |||||||||||||||||
| Less: Interest | 32.8 | 3.7 | 36.5 | |||||||||||||||||
| Present value of lease liabilities | $ | 205.8 | $ | 22.8 | $ | 228.6 | ||||||||||||||
Our weighted average lease terms and discount rates are as follows:
| July 2, 2022 | July 3, 2021 | |||||||||||||
| Weighted-average remaining lease term (in years) | ||||||||||||||
| Operating leases | 11.45 | 11.59 | ||||||||||||
| Finance leases | 9.37 | 9.24 | ||||||||||||
| Weighted-average discount rate | ||||||||||||||
| Operating leases | 2.53 | % | 2.71 | % | ||||||||||
| Finance leases | 2.86 | % | 2.76 | % | ||||||||||
Our lease cash flow classifications are as follows (in millions):
| Six Months Ended | ||||||||||||||
| July 2, 2022 | July 3, 2021 | |||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities | ||||||||||||||
| Operating cash flows for operating leases | $ | 16.6 | $ | 18.2 | ||||||||||
| Operating cash flows for finance leases | $ | 0.3 | $ | 0.4 | ||||||||||
| Financing cash flows for finance leases | $ | 2.6 | $ | 2.6 | ||||||||||
| Leased assets obtained in exchange for new finance lease liabilities | $ | — | $ | 4.4 | ||||||||||
| Leased assets obtained in exchange for new operating lease liabilities | $ | 42.8 | $ | 34.9 | ||||||||||
26
Perrigo Company plc - Item 1
Note 11
NOTE 11 – INDEBTEDNESS
On April 20, 2022, we and our wholly owned subsidiary, Perrigo Investments, LLC, entered into new senior secured credit facilities that consist of (i) a $1.0 billion five-year revolving credit facility (the “2022 Revolver”), (ii) a $500 million five-year Term Loan A facility (the “2022 Term Loan A Facility”), and (iii) a $1.1 billion seven-year Term Loan B facility (the “2022 Term Loan B Facility” and, together with the 2022 Revolver and 2022 Term Loan A Facility, the “New Senior Secured Credit Facilities”), pursuant to a new Term Loan and Revolving Credit Agreement. The New Senior Secured Credit Facilities are fully guaranteed, along with any hedging or cash management obligations entered into with a lender, by us and certain of our direct and indirect wholly-owned subsidiaries organized in the United States, Ireland, Belgium and England and Wales (subject to certain exceptions) (the “Guarantor Subsidiaries”). The Guarantor Subsidiaries and Perrigo Investments, LLC provide full and unconditional guarantees, jointly and severally, on a senior unsecured basis, of the 5.300% Notes due 2043 issued by the Company, and the Guarantor Subsidiaries, Perrigo Investments, LLC and the Company provide full and unconditional guarantees, jointly and severally, on a senior unsecured basis, of the 3.900% Notes due 2024, the 4.375% Notes due 2026, the 4.400% Notes due 2030 and the 4.900% Notes due 2044 issued by Perrigo Finance Unlimited Company.
Total borrowings are summarized as follows (in millions):
| July 2, 2022 | December 31, 2021 | ||||||||||||||||||||||
| Term loan | |||||||||||||||||||||||
2019 Term loan due August 15, 2022(1) | $ | — | $ | 600.0 | |||||||||||||||||||
| 2022 Term loan A due April 1, 2027 | 500.0 | — | |||||||||||||||||||||
| 2022 Term loan B due April 1, 2029 | 1,100.0 | — | |||||||||||||||||||||
| Total term loans | 1,600.0 | 600.0 | |||||||||||||||||||||
| Notes and Bonds | |||||||||||||||||||||||
| Coupon | Due | ||||||||||||||||||||||
| 5.105% | July 28, 2023(1,2) | $ | — | $ | 153.5 | ||||||||||||||||||
| 4.000% | November 15, 2023(1) | — | 215.6 | ||||||||||||||||||||
| 3.900% | December 15, 2024 | 700.0 | 700.0 | ||||||||||||||||||||
| 4.375% | March 15, 2026 | 700.0 | 700.0 | ||||||||||||||||||||
| 4.400% | June 15, 2030(3) | 750.0 | 750.0 | ||||||||||||||||||||
| 5.300% | November 15, 2043 | 90.5 | 90.5 | ||||||||||||||||||||
| 4.900% | December 15, 2044 | 303.9 | 303.9 | ||||||||||||||||||||
| Total notes and bonds | 2,544.4 | 2,913.5 | |||||||||||||||||||||
| Other financing | 22.8 | 25.8 | |||||||||||||||||||||
| Unamortized premium (discount), net | (17.3) | (4.8) | |||||||||||||||||||||
| Deferred financing fees | (33.0) | (14.0) | |||||||||||||||||||||
| Total borrowings outstanding | 4,116.9 | 3,520.5 | |||||||||||||||||||||
| Current indebtedness | (30.8) | (603.8) | |||||||||||||||||||||
| Total long-term debt less current portion | $ | 4,086.1 | $ | 2,916.7 | |||||||||||||||||||
(1) Redeemed in connection with the New Senior Secured Credit Facilities entered into during the second quarter of 2022.
(2) Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
(3) The coupon rate noted above is that as of July 2, 2022, following a step up in rate from 3.150% to 3.900%, effective December 16, 2021. Due to a credit ratings downgrade by S&P and Moody's in the first quarter of 2022, the interest rate has stepped up from 3.900% to 4.400% starting after June 15, 2022.
Revolving Credit Agreements
On April 20, 2022 we terminated the revolving credit agreement maturing in March 2023 (the "2018 Revolver") and entered into the 2022 Revolver. There were no borrowings outstanding under the 2022 Revolver or 2018 Revolver as of July 2, 2022 or December 31, 2021, respectively.
27
Perrigo Company plc - Item 1
Note 11
Term Loan and Notes
On April 20, 2022, we repaid the prior $600.0 million term loan, maturing on August 15, 2022 (the "2019 Term Loan") with the proceeds of the New Senior Secured Credit Facilities. The remaining $500.0 million of proceeds were used to redeem the 4.00% Senior Notes due 2023 on May 19, 2022 and the 5.1045% Guaranteed Senior Notes due 2023 on May 19, 2022. In relation to the New Senior Secured Credit Facilities, we deferred $30.8 million of financing fees, which will be amortized to interest expense over the term of the facilities. We recorded $9.3 million in Loss on extinguishment of debt on the Condensed Statement of Operations, consisting of the write-off of certain new and previously deferred financing fees and make whole payments on the redeemed 4.00% Senior Notes due 2023. The proceeds from the New Senior Secured Credit Facilities were also used, in part, along with cash on hand to fund the acquisition of HRA Pharma (Refer to Note 3).
We had $1.1 billion and $500.0 million outstanding under our 2022 Term Loan B Facility and 2022 Term Loan A Facility as of July 2, 2022, respectively. There were no borrowings outstanding under our 2019 Term Loan as of July 2, 2022. We had $600.0 million outstanding under our 2019 Term Loan as of December 31, 2021.
We are in compliance with all the covenants under our debt agreements.
Other Financing
We have overdraft facilities available that we use to support our cash management operations. We report any balances outstanding in the above table under "Other financing". There were no borrowings outstanding under the facilities as of July 2, 2022 or December 31, 2021.
In June 2020, we incurred debt of $34.3 million related to our equity method investment in Kazmira pursuant to 2 promissory notes. In December 2020, we repaid the $3.7 million balance due on the November 2020 portion of the promissory notes. In June 2021, we repaid the $5.8 million balance due on the May 2021 portion of the promissory notes and in December 2021, we repaid the $24.8 million balance due on the November 2021 portion, settling the debt in full.
We have financing leases that are reported in the above table under "Other financing" (refer to Note 10).
NOTE 12 – EARNINGS PER SHARE AND SHAREHOLDERS' EQUITY
Earnings per Share
A reconciliation of the numerators and denominators used in the basic and diluted earnings per share ("EPS") calculation is as follows (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| Numerator: | |||||||||||||||||||||||
| Income (loss) from continuing operations | $ | (64.9) | $ | (111.9) | $ | (66.2) | $ | (109.1) | |||||||||||||||
| Income (loss) from discontinued operations, net of tax | (0.2) | 54.2 | (1.4) | 89.5 | |||||||||||||||||||
| Net income (loss) | $ | (65.1) | $ | (57.7) | $ | (67.6) | $ | (19.6) | |||||||||||||||
| Denominator: | |||||||||||||||||||||||
| Weighted average shares outstanding for basic EPS | 134.6 | 133.6 | 134.3 | 133.4 | |||||||||||||||||||
| Dilutive effect of share-based awards* | — | — | — | — | |||||||||||||||||||
| Weighted average shares outstanding for diluted EPS | 134.6 | 133.6 | 134.3 | 133.4 | |||||||||||||||||||
| Anti-dilutive share-based awards excluded from computation of diluted EPS* | — | — | — | — | |||||||||||||||||||
| * In the period of a net loss, diluted shares equal basic shares. | |||||||||||||||||||||||
28
Perrigo Company plc - Item 1
Note 13
Shareholders' Equity
Share Repurchases
In October 2018, our Board of Directors authorized up to $1.0 billion of share repurchases with no expiration date, subject to the Board of Directors’ approval of the pricing parameters and amount that may be repurchased under each specific share repurchase program (the "2018 Authorization"). We did not repurchase any shares during the three and six months ended July 2, 2022 or July 3, 2021.
NOTE 13 – ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
Changes in our AOCI balances, net of tax were as follows (in millions):
| Fair Value of Derivative Financial Instruments, net of tax | Foreign Currency Translation Adjustments (1) | Post-Retirement and Pension Liability Adjustments, net of tax(1) | Total AOCI | ||||||||||||||||||||||||||
| Balance at December 31, 2021 | $ | (22.0) | $ | 67.4 | $ | (9.9) | $ | 35.5 | |||||||||||||||||||||
| OCI before reclassifications | 28.7 | (163.4) | (8.3) | (143.0) | |||||||||||||||||||||||||
| Amounts reclassified from AOCI | 2.8 | (43.6) | 1.5 | (39.3) | |||||||||||||||||||||||||
| Other comprehensive income (loss) | $ | 31.5 | $ | (207.0) | $ | (6.8) | $ | (182.3) | |||||||||||||||||||||
| Balance at July 2, 2022 | $ | 9.5 | $ | (139.6) | $ | (16.7) | $ | (146.8) | |||||||||||||||||||||
(1) Amounts reclassified from AOCI relate to the divestiture of the Latin America businesses
NOTE 14 – INCOME TAXES
The effective tax rates were as follows:
| Three Months Ended | Six Months Ended | |||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||
| 40.1 | % | 28.8 | % | 45.4 | % | 22.1 | % | |||||||||||||
The effective tax rate on the pre-tax loss for the three and six months ended July 2, 2022, increased compared to the effective tax rate on the pre-tax loss for the three and six months ended July 3, 2021, primarily due to the tax benefit of the loss on sale of our Latin America businesses and costs related to the acquisition of HRA Pharma that are not deductible for tax purposes. The increase in the effective tax rate was offset, in part, by the income tax expense associated with legal entity restructuring recognized for the six months ended July 2, 2022, and the income tax expense on intra-entity transfers of intellectual property recognized in the prior year.
Internal Revenue Service Audits of Perrigo Company, a U.S. Subsidiary
Perrigo Company, our U.S. subsidiary ("Perrigo U.S."), is engaged in a series of tax disputes in the U.S. relating primarily to transfer pricing adjustments including income in connection with the purchase, distribution, and sale of store-brand OTC pharmaceutical products in the United States, including the generic heartburn medication omeprazole. On August 27, 2014, we received a statutory notice of deficiency from the IRS relating to our fiscal tax years ended June 27, 2009, and June 26, 2010 (the “2009 tax year” and “2010 tax year”, respectively). On April 20, 2017, we received a statutory notice of deficiency from the IRS for the years ended June 25, 2011 and June 30, 2012 (the “2011 tax year” and “2012 tax year”, respectively). Specifically, both statutory notices proposed adjustments related to the offshore reporting of profits on sales of omeprazole in the United States resulting from the assignment of an omeprazole distribution contract to an Israeli affiliate. In addition to the transfer pricing adjustments, which applied to all 4 tax years, the statutory notice of deficiency for the 2011 and 2012 tax years included adjustments requiring the capitalization and amortization of certain legal expenses that were deducted when paid or incurred in defending against certain patent infringement lawsuits related to ANDAs filed with a Paragraph IV Certification.
29
Perrigo Company plc - Item 1
Note 14
We do not agree with the audit adjustments proposed by the IRS in either of the notices of deficiency. We paid the assessed amounts of tax, interest, and penalties set forth in the statutory notices and timely filed claims for refund on June 11, 2015 for the 2009 and 2010 tax years, and on June 7, 2017, for the 2011 and 2012 tax years. On August 15, 2017, following disallowance of such refund claims, we timely filed a complaint in the United States District Court for the Western District of Michigan seeking refunds of tax, interest, and penalties of $27.5 million for the 2009 tax year, $41.8 million for the 2010 tax year, $40.1 million for the 2011 tax year, and $24.7 million for the 2012 tax year, for a total of $134.1 million, plus statutory overpayment interest thereon from the dates of payment. The amounts sought in the complaint for the 2009 and 2010 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended March 28, 2015, and the amounts sought in the complaint for the 2011 and 2012 tax years were recorded as deferred charges in Other non-current assets on our balance sheet during the three months ended July 1, 2017.
A bench trial was held during the period May 25, 2021 to June 7, 2021 for the refund case in the United States District Court for the Western District of Michigan. The total amount of cumulative deferred charge that we are seeking to receive in this litigation is approximately $111.6 million, which reflects the impact of conceding that Perrigo U.S. should have received a 5.24% royalty on all omeprazole sales. That concession was previously paid and is the subject of the above refund claims. The issues outlined in the statutory notices of deficiency described above are continuing in nature, and the IRS will likely carry forward the adjustments set forth therein as long as the drug is sold, in the case of the omeprazole issue, and for all post-2012 Paragraph IV filings that trigger patent infringement suits, in the case of the ANDA issue. On April 30, 2021, we filed a Notice of New Authority in our refund case in the Western District of Michigan alerting the court to a United States Tax Court decision in Mylan v. Comm'r that ruled in favor of the taxpayer on nearly identical ANDA issues as we have before the court. Post-trial briefings were completed on September 24, 2021 and the case is now fully submitted for the court's decision. On January 28, 2022, the IRS filed a Notice of Appeal with the United States Court of Appeals of the Third Circuit to appeal the United States Tax Court's decision in Mylan v. Comm'r.
On January 13, 2021, the IRS issued a 30-day letter and Revenue Agent's Report ("RAR") with respect to its audit of our fiscal tax years ended June 29, 2013, June 28, 2014, and June 27, 2015. The 30-day letter proposed, among other modifications, transfer pricing adjustments in connection with the distribution of omeprazole in the aggregate amount of $141.6 million and ANDA-related adjustments in the aggregate amount of $21.9 million. The 30-day letter also set forth adjustments described in the next two paragraphs. We timely filed a protest to the 30-day letter for those additional adjustments but noting that due to the pending refund litigation described above, IRS Appeals would not consider the merits of the omeprazole or ANDA matters. We believe that we should prevail on the merits on both carryforward issues and have reserved for taxes and interest payable on the 5.24% deemed royalty on omeprazole through the tax year ended December 31, 2018. Beginning with the tax year ended December 31, 2019, we began reporting income commensurate with the 5.24% deemed royalty. We have not reserved for the ANDA-related issue described above. While we believe we should prevail on the merits of this case, the outcome remains uncertain. If our litigation position on the omeprazole issue is not sustained, the outcome for the 2009–2012 tax years could range from a reduction in the refund amount to denial of any refund. In addition, we expect that the outcome of the refund litigation could effectively bind future tax years. In that event, an adverse ruling on the omeprazole issue could have a material impact on subsequent periods, with additional tax liability in the range of $24.0 million to $112.0 million, not including interest and any applicable penalties.
The 30-day letter for the 2013-2015 tax years also proposed to reduce Perrigo U.S.'s deductible interest expense for the 2014 tax year and the 2015 tax year on $7.5 billion in certain intercompany debts owed by it to Perrigo Company plc. The debts were incurred in connection with the Elan merger transaction in 2013. On May 7, 2020, the IRS issued a NOPA capping the interest rate on the debts for U.S. federal tax purposes at 130.0% of the Applicable Federal Rate ("AFR") (a blended rate reduction of 4.0% per annum) on the stated ground that the loans were not negotiated on an arms-length basis. The NOPA proposes a reduction in gross interest expense of approximately $414.7 million for tax years 2014 and 2015. On January 13, 2021, we received a RAR, together with the 30-day letter, requiring our filing of a written protest to request IRS Appeals consideration. The protest was timely filed with the IRS on February 26, 2021. On January 20, 2022, the IRS responded to our protest with its rebuttal in which it revised its position on this interest rate issue by reasserting that implicit parental support considerations are necessary to determine the arm's length interest rates and proposing revised interest rates that are higher than the interest rates proposed under its 130.0% of AFR assertion. The blended interest rate proposed by the IRS rebuttal is 4.36%, an increase from the blended interest rate in the RAR of 2.57% but lower than the stated blended interest rate of the loans of 6.8%.We will pursue all available administrative and judicial remedies necessary to defend the deductibility of the interest expense on this indebtedness. If the IRS were to prevail in its
30
Perrigo Company plc - Item 1
Note 14
revised proposed adjustment, we estimate an increase in tax expense of approximately $72.9 million, excluding interest and penalties, for fiscal years ended June 28, 2014 through June 27, 2015. In addition, we expect the IRS to seek similar adjustments for the fiscal years ended December 31, 2015 through December 31, 2018 with potential section 163(j) carryover impacts beyond December 2018. If those further adjustments were sustained, based on preliminary calculations and subject to further analysis, our current best estimate is that the additional tax expense would not exceed $58.5 million, excluding interest and penalties. No further adjustments beyond this period are expected. We strongly disagree with the IRS position and we will pursue all available administrative and judicial remedies necessary. At this stage, an IRS Appeals meeting has not yet been scheduled to discuss this issue, and we are unable to estimate additional liability, if any, associated with this matter.
In addition, the 30-day letter for the 2013-2015 tax years expanded on a NOPA issued on December 11, 2019 and proposed to disallow reductions to gross sales income on the sale of prescription products to wholesalers for accrued wholesale customer pipeline chargebacks where the prescription products were not re-sold by such wholesalers to covered retailers by the end of the tax year. The NOPA asserts that the reduction of gross sales income of such chargebacks is an impermissible method of accounting and proposed a change in accounting method that would defer the reduction in gross sales income until the year the prescription products were re-sold to covered retailers. The NOPA proposes an increase in sales revenue of approximately $99.5 million for the 2013-2015 tax years. We filed a protest on February 26, 2021 to request IRS Appeals consideration. On January 20, 2022, the IRS responded to our protest with its rebuttal and reiterated the NOPA's position that the accrued chargebacks are not currently deductible in the tax year accrued because all events have not occurred to establish the fact of the liability in the year deducted. The first meeting to discuss this issue with IRS Appeals has been scheduled for October 12 and 13, 2022. We strongly disagree with the IRS’s proposed adjustment and will pursue all available administrative and judicial remedies necessary.
On December 2, 2021, the IRS commenced an audit of our federal income tax returns for the tax years ended December 31, 2015, through December 31, 2019.
Internal Revenue Service Audit of Athena Neurosciences, Inc., a U.S. Subsidiary
On April 26, 2019, we received a revised NOPA from the IRS regarding transfer pricing positions related to the IRS audit of Athena Neurosciences, LLC ("Athena") for the years ended December 31, 2011, December 31, 2012, and December 31, 2013. The NOPA carries forward the IRS's theory from its 2017 draft NOPA that when Elan took over the future funding of Athena's in-process research and development after acquiring Athena in 1996, Elan should have paid a substantially higher royalty rate for the right to exploit Athena’s intellectual property in various developmental products, including the Multiple Sclerosis drug Tysabri, rather than rates based on transfer pricing documentation prepared by Elan's external tax advisors. The NOPA proposes a payment of $843.0 million, which represents additional tax based on imputing royalty income to Athena using a 24.7% royalty rate derived by the IRS and a 40.0% accuracy-related penalty. This amount excludes consideration of offsetting tax attributes and any potential interest that may be imposed. We strongly disagree with the IRS position. On December 22, 2016, we also received a NOPA for these years denying the deductibility of settlement costs related to illegal marketing of Zonegran in the United States raised in a Qui Tam action under the U.S. False Claims Act. We strongly disagree with the IRS' position on this issue as well. Because we believe that any concession on these issues in Appeals would be contrary to our evaluation of the issues and to avoid double taxation of the same income in the United States and Ireland, we pursued our remedies under the Mutual Agreement Procedure of the U.S.-Ireland Income Tax Treaty to alleviate double taxation. On April 21 and 23, 2020, we filed requests for Competent Authority Assistance with the IRS and Irish Revenue on the Tysabri royalty issue, and those applications were accepted. On October 20, 2020, we amended our requests for Competent Authority Assistance to include the Zonegran issue and these supplemental requests were also accepted. On May 6, 2021, we had our opening conference with the IRS. A follow-up conference was held with the IRS on December 13, 2021 and we discussed our submission, which continues to be reviewed by the IRS. Our opening conference with Irish Revenue was held on July 23, 2021 and we discussed our submission, which continues to be reviewed by Irish Revenue. The U.S. and Irish Competent Authorities will seek to achieve a resolution that avoids double taxation on both the Tysabri royalty and Zonegran issues.
No payment of the additional amounts is required until these two matters are resolved with finality under the treaty, or any additional administrative or judicial process if treaty negotiations are unsuccessful.
31
Perrigo Company plc - Item 1
Note 14
Irish Revenue Audit of Fiscal Years Ended December 31, 2012 and December 31, 2013
On November 29, 2018, Irish Revenue issued a Notice of Amended Assessment (“NoA”) for the tax year ended December 31, 2013, related to the tax treatment of the 2013 sale of the Tysabri® intellectual property and related assets to Biogen Idec by Elan Pharma. On September 29, 2021, Elan reached an agreement with Irish Revenue providing for full and final settlement of the NoA on the following terms: (i) on a 'without prejudice basis' and, for purposes of the settlement, an alternative basis of taxation was applied, (ii) Irish Revenue to take no further action in relation to the NoA or any Tysabri related income or transactions, (iii) no interest or penalties applied, (iv) a total tax of €297.0 million charged as full and final settlement of all liabilities arising from the sale of the Tysabri patents for the fiscal years 2013 to 2021, and (v) after Irish Revenue credited taxes already paid and certain unused research and development ("R&D") credits against the €297.0 million charged settlement amount, the total cash payment of €266.1 million, $307.5 million as of the date of payment, was made on October 5, 2021. We recorded the payment as a component of income tax expense on the Consolidated Statements of Operations in the third quarter of 2021.
Israel Tax Authority Audit of Fiscal Year Ended June 27, 2015 and Calendar Years Ended December 31, 2015 through December 31, 2019
On December 29, 2020, we received a Stage A assessment from the Israel Tax Authority ("ITA") for the tax years ended December 31, 2015 through December 31, 2017 relating to attribution of intangible income to Israel, income qualifying for a lower preferential rate of tax, exemption from capital gains tax, and deduction of certain settlement payments. Through negotiations with the ITA, we resolved the audit by agreeing to add tax years ended December 31, 2018 and December 31, 2019 to the audit. Further, the agreement with the ITA required us to pay $19.0 million, after offset of refunds of $17.2 million, for the 5 taxable years. In addition, we paid $12.5 million to resolve a tax liability indemnity for the tax year ended December 31, 2017 relating to Perrigo API Ltd, which we disposed of in December 2017.
As a result of the settlement with the ITA, we reduced our liability recorded for uncertain tax positions by $38.3 million including interest.
Although we believe that our tax estimates are reasonable and that we prepare our tax filings in accordance with all applicable tax laws, the final determination with respect to any tax audit and any related litigation could be materially different from our estimates or from our historical income tax provisions and accruals. The results of an audit or litigation could have a material effect on operating results and/or cash flows in the periods for which that determination is made. In addition, future period earnings may be adversely impacted by litigation costs, settlements, penalties, and/or interest assessments.
Based on the final resolution of tax examinations, judicial or administrative proceedings, changes in facts or law, expirations of statute of limitations in specific jurisdictions or other resolutions of, or changes in, tax positions - one or more of which may occur within the next twelve months - it is reasonably possible that unrecognized tax benefits for certain tax positions taken on previously filed tax returns may change materially from those recorded as of July 2, 2022. However, we are not able to estimate a reasonably possible range of how these events may impact our unrecognized tax benefits in the next twelve months.
NOTE 15 – CONTINGENCIES
In view of the inherent difficulties of predicting the outcome of various types of legal proceedings, we cannot determine the ultimate resolution of the matters described below. We establish reserves for litigation and regulatory matters when losses associated with the claims become probable and the amounts can be reasonably estimated. The actual costs of resolving legal matters may be substantially higher or lower than the amounts reserved for those matters. For matters where the likelihood or extent of a loss is not probable or cannot be reasonably estimated as of July 2, 2022, we have not recorded a loss reserve. If certain of these matters are determined against us, there could be a material adverse effect on our financial condition, results of operations, or cash flows. We currently believe we have valid defenses to the claims in these lawsuits and intend to defend these lawsuits vigorously regardless of whether or not we have a loss reserve. Other than what is disclosed below, we do not expect the outcome of the litigation matters to which we are currently subject to, individually or in the aggregate, have a material adverse effect on our financial condition, results of operations, or cash flows.
32
Perrigo Company plc - Item 1
Note 15
Price-Fixing Lawsuits
Perrigo is a defendant in several cases in the generic pricing multidistrict litigation MDL No. 2724 (United States District Court for Eastern District of Pennsylvania). This multidistrict litigation, which has many cases that do not include Perrigo, includes class action and opt-out cases for federal and state antitrust claims, as well as complaints filed by certain states alleging violations of state antitrust laws.
On July 14, 2020, the court issued an order designating the following cases to proceed on a more expedited basis (as a bellwether) than the other cases in MDL No. 2724: (a) the May 2019 state case alleging an overarching conspiracy involving more than 120 products (which does not name Perrigo a defendant) and (b) class actions alleging “single drug” conspiracies involving Clomipramine, Pravastatin, and Clobetasol. Perrigo is a defendant in the Clobetasol cases but not the others. On February 9, 2021, the Court entered an order provisionally deciding to remove the May 2019 state case and the pravastatin class cases from the bellwether proceedings. On May 7, 2021, the Court ruled that the clobetasol end payer and direct purchaser class cases will remain part of the bellwether. The Court also ruled that the June 10, 2020 state complaint against Perrigo and approximately 35 other manufacturers will move forward as a bellwether case. The bellwether cases are proceeding in discovery, which must be completed by January 17, 2023 under the schedule set by the Court. No trial dates have been set for any of the bellwether cases, or any of the other cases in the MDL.
Class Action Complaints
(a) Single Drug Conspiracy Class Actions
We have been named as a co-defendant with certain other generic pharmaceutical manufacturers in a number of class actions alleging single-product conspiracies to fix or raise the prices of certain drugs and/or allocate customers for those products starting, in some instances, as early as June 2013. The class actions were filed on behalf of putative classes of (a) direct purchasers, (b) end payors, and (c) indirect resellers. The products in question are Clobetasol gel, Desonide, and Econazole. The court denied motions to dismiss each of the complaints alleging “single drug” conspiracies involving Perrigo, and the cases are proceeding in discovery. As noted above, the Clobetasol cases have been designated to proceed on a more expedited schedule than the other cases. That schedule culminates with summary judgment motions due to be filed no later than November 16, 2023. No trial dates have been set for the Clobetasol cases, and no schedules have been set for the other “single drug” conspiracy cases.
(b) “Overarching Conspiracy” Class Actions
The same 3 putative classes, including (a) direct purchasers, (b) end payors, and (c) indirect resellers, have filed 2 sets of class action complaints alleging that Perrigo and other manufacturers (and some individuals) entered into an “overarching conspiracy” that involved allocating customers, rigging bids and raising, maintaining, and fixing prices for various products. Each class brings claims for violations of Sections 1 and 3 of the Sherman Antitrust Act as well as several state antitrust and consumer protection statutes.
Filed in June 2018, and later amended in December 2018 (with respect to direct purchasers) and April 2019 (with respect to end payors and indirect resellers), the first set of “overarching conspiracy” class actions include allegations against Perrigo and approximately 27 other manufacturers involving 135 drugs with allegations dating back to March 2011. The allegations against Perrigo concern only 2 formulations (cream and ointment) of 1 of the products at issue, Nystatin. The court denied motions to dismiss the first set of “overarching conspiracy” class actions, and they are proceeding in discovery. NaN of these cases are included in the group of cases on a more expedited schedule pursuant to the court’s May 17, 2021 order.
In December 2019, the end payor plaintiffs filed a second "overarching conspiracy” class action against Perrigo, dozens of other manufacturers of generic prescription pharmaceuticals, and certain individuals dating back to July 2009, which complaint was amended on January 7, 2021 to add additional claims. The amended end payor complaint alleges that Perrigo conspired in connection with its sale of the following drugs: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fenofibrate, Fluocinonide, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Mometasone Furoate, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
33
Perrigo Company plc - Item 1
Note 15
In December, 2019, the indirect reseller class plaintiffs also filed an “overarching conspiracy” class action against Perrigo, dozens of other manufacturers of generic prescription pharmaceuticals, distributors of generic pharmaceuticals, and certain individuals, dating back to January 2010. In May 2020, the plaintiffs amended their complaint and alleged that Perrigo conspired in connection with its sales of Betamethasone Dipropionate lotion, Imiquimod cream, Desonide cream and ointment, and Hydrocortisone Valerate cream. On July 1, 2022, the indirect reseller plaintiffs filed a further amended complaint that removed claims relating to Imiquimod cream but added allegations about Olopatadine Nasal Spray. The indirect reseller plaintiffs filed their second “overarching conspiracy” complaint on December 15, 2020. The complaint alleges conspiracies relating to the sale of various products that are not at issue in the earlier-filed overarching conspiracy class actions, the majority of which Perrigo neither makes nor sells, dating back to January 2010. The December 2020 indirect reseller complaint alleges that Perrigo conspired in connection with its sales of Adapalene, Ammonium Lactate, Bromocriptine Mesylate, Calcipotriene, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Methazolamide, Mometasone Furoate, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
The direct purchaser plaintiffs filed their second round overarching conspiracy complaint in February 2020 with claims dating back to July 2009. On October 21, 2020, the direct purchaser plaintiffs amended their complaint. The amended direct purchaser complaint alleges that Perrigo conspired in connection with its sale of the following drugs: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Ciclopirox, Clindamycin Phosphate, Fenofibrate, Fluocinonide, Halobetasol Propionate, Hydrocortisone Valerate, Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
Perrigo has not yet responded to the second set of overarching conspiracy complaints and responses are currently stayed.
Opt-Out Complaints
On January 22, 2018, Perrigo was named a co-defendant along with 35 other manufacturers in a complaint filed by 3 supermarket chains alleging that defendants conspired to fix prices of 31 generic prescription pharmaceutical products starting in 2013. On December 21, 2018, an amended complaint was filed that adds additional products and allegations against a total of 39 manufacturers for 33 products. The only allegations specific to Perrigo relate to Clobetasol, Desonide, Econazole, and Nystatin. Perrigo moved to dismiss this complaint on February 21, 2019, and also signed onto a joint motion to dismiss the complaint filed by all of the Defendants named in the complaint on March 1, 2019. The joint motion was denied on August 15, 2019. The case is proceeding in discovery. Perrigo’s individual motion to dismiss is still pending. On February 3, 2020, the plaintiffs requested leave to file a second amended complaint, which it has withdrawn and refiled several times since, with the latest requested amendment filed in August 2020. The proposed amended complaint adds dozens of additional products and allegations to the original complaint. Perrigo is discussed in connection with allegations concerning an additional drug, Fenofibrate. Defendants opposed the motion for leave to file a second amended complaint and the court has yet to rule on the issue.
On August 3, 2018, a large managed care organization filed a complaint alleging price-fixing and customer allocation concerning 17 different products among 27 manufacturers including Perrigo. The only allegations specific to Perrigo in the initial complaint concerned Clobetasol. Perrigo moved to dismiss this complaint on February 21, 2019 and filed an additional, joint, motion to dismiss on March 1, 2019. Plaintiff filed a second amended complaint on April 1, 2019 that added additional products and allegations. The amended allegations that concern Perrigo include: Clobetasol, Desonide, Econazole, and Nystatin. The joint motion to dismiss was denied on August 15, 2019, but Perrigo’s individual motion to dismiss is still pending. The case is proceeding in discovery.
The same organization amended a different complaint that it had filed in October 2019, which did not name Perrigo, on December 15, 2020, adding Perrigo as a defendant and asserting new allegations of alleged antitrust violations involving Perrigo and dozens of other generic pharmaceutical manufacturers. The allegations relating to Perrigo concern: Adapalene, Betamethasone Dipropionate, Bromocriptine Mesylate, Ciclopirox, Clindamycin Phosphate, Fenofibrate, Fluocinonide, Halobetasol Propionate, Hydrocortisone Valerate, Imiquimod, Permethrin, Prochlorperazine Maleate, and Triamcinolone Acetonide.
34
Perrigo Company plc - Item 1
Note 15
The same organization filed a third complaint on December 15, 2020, naming Perrigo and dozens of other manufacturers alleging antitrust violations concerning generic pharmaceutical drugs. The allegations relating to Perrigo concern: Ammonium Lactate, Calcipotriene Betamethasone Dipropionate, Erythromycin, Fluticasone Propionate, Hydrocortisone Acetate, Methazolamide, Promethazine HCL, and Tacrolimus.
On January 16, 2019, a health insurance carrier filed a complaint in the U.S. District Court for the District of Minnesota alleging a conspiracy to fix prices of 30 products among 30 defendants. The only allegations specific to Perrigo concerned Clobetasol, Desonide, Econazole, and Nystatin. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations that concern Perrigo relate to Fluocinonide.
The same health insurance carrier filed a new complaint on December 15, 2020, naming Perrigo and dozens of other manufacturers alleging antitrust violations concerning generic pharmaceutical drugs. The allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On July 18, 2019, 87 health plans filed a Praecipe to Issue Writ of Summons in Pennsylvania state court to commence an action against 53 generic pharmaceutical manufacturers and 17 individuals, alleging antitrust violations concerning generic pharmaceutical drugs. While Perrigo was named as a defendant, no complaint has been filed and the precise allegations and products at issue have not been identified. Proceedings in the case, including the filing of a complaint, have been stayed at the request of the plaintiffs.
On December 11, 2019, a health care service company filed a complaint against Perrigo and 38 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other multi-district litigation ("MDL") complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin cream/ointment. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fenofibrate, Fluocinonide, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On December 16, 2019, a Medicare Advantage claims recovery company filed a complaint against Perrigo and 39 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, and Econazole. The complaint was originally filed in the District of Connecticut but has been consolidated into the MDL. Perrigo has not yet had the opportunity to respond to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Desoximetasone, Erythromycin, Fenofibrate, Fluocinonide, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On December 23, 2019, several counties in New York filed an amended complaint against Perrigo and 28 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin. The complaint was originally filed in New York State court but was removed to federal court and has been consolidated into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Betamethasone Dipropionate, Bromocriptine Mesylate,
35
Perrigo Company plc - Item 1
Note 15
Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Mometasone Furoate, Nystatin, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide. On June 30, 2021, the counties filed a proposed revised second amended complaint. Perrigo has not yet responded to the complaint, and responses are currently stayed.
On December 27, 2019, a healthcare management organization filed a complaint against Perrigo and 25 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin. The complaint was filed originally in the Northern District of California but has been consolidated into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fenofibrate, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On March 1, 2020, Harris County of Texas filed a complaint against Perrigo and 29 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The products at issue that plaintiffs claim Perrigo manufacturers or sells include: Adapalene, Betamethasone Dipropionate, Ciclopirox, Clindamycin, Clobetasol, Desonide, Econazole, Ethinyl Estradiol/Levonorgestrel, Fenofibrate, Fluocinolone, Fluocinonide, Gentamicin, Glimepiride, Griseofulvin, Halobetasol Propionate, Hydrocortisone Valerate, Ketoconazole, Mupirocin, Nystatin, Olopatadine, Permethrin, Prednisone, Promethazine, Scopolamine, and Triamcinolone Acetonide. The complaint was originally filed in the Southern District of Texas but has been transferred to the MDL. Harris County amended its complaint in May 2020. Perrigo has not yet responded to the complaint, and responses are currently stayed.
In May 2020, 7 health plans filed a writ of summons in the Pennsylvania Court of Common Pleas in Philadelphia concerning an as-yet unfiled complaint against Perrigo, 3 dozen other manufacturers, and 17 individuals, concerning alleged antitrust violations in connection with the pricing and sale of generic prescription pharmaceutical products. No complaint has yet been filed, so the precise allegations and products at issue are not yet clear. Proceedings in the case have been stayed.
On June 9, 2020, a health insurance carrier filed a complaint against Perrigo and 25 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin. The complaint was filed in the Eastern District of Pennsylvania and has been transferred into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fluocinonide, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On July 9, 2020, a drugstore chain filed a complaint against Perrigo and 39 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin. Perrigo is also listed in connection with Fenofibrate. The complaint was filed in the Eastern District of Pennsylvania and will be transferred into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed. On December 15, 2020, the complaint was amended to add additional defendants and claims. The new allegations relating to Perrigo concern: Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fenofibrate, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod,
36
Perrigo Company plc - Item 1
Note 15
Methazolamide, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide.
On August 27, 2020, Suffolk County of New York filed a complaint against Perrigo and 35 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin cream and ointment. The other products at issue that plaintiffs claim Perrigo manufacturers or sells include: Adapalene gel, Albuterol, Benazepril HCTZ, Clotrimazole, Diclofenac Sodium, Fenofibrate, Fluocinonide, Glimepiride, Ketoconazole, Meprobamate, Imiquimod, Triamcinolone Acetonide, Erythromycin/Ethyl Solution, Betamethasone Valerate, Ciclopirox Olamine, Terconazole, Hydrocortisone Valerate, Fluticasone Propionate, Desoximetasone, Clindamycin Phosphate, Halobetasol Propionate, Hydrocortisone Acetate, Promethazine HCL, Mometasone Furoate, and Amiloride HCTZ. The complaint was filed in the Eastern District of New York and has been transferred into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed.
On September 4, 2020, a drug wholesaler and distributor filed a complaint against Perrigo and 39 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin, Clobetasol, Desonide, Econazole, Erythromycin, Fenofibrate, Fluticasone, Halobetasol, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Mometasone furoate, Nystatin, Prochlorperazine, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide. The complaint was filed in the Eastern District of Pennsylvania and has been transferred into the MDL. Perrigo has not yet responded to the complaint, and responses are currently stayed.
On December 11, 2020, a drugstore chain filed a complaint against Perrigo and 45 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on Adapalene, Ammonium Lactate, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Clobetasol, Desonide, Econazole, Erythromycin, Fenofibrate, Fluticasone Propionate, Halobetasol, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Nystatin, Permethrin, Prochlorperazine, Promethazine HCL, Tacrolimus, and Triamcinolone. The complaint was filed in the Eastern District of Pennsylvania and has been transferred into the MDL.
On December 14, 2020, a supermarket chain filed a complaint against Perrigo and 45 other manufacturers (as well as certain individuals) alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on Betamethasone Dipropionate, Bromocriptine Mesylate, Ciclopirox, Clindamycin Phosphate, Clobetasol, Desonide, Econazole, Fenofibrate, Halobetasol, Hydrocortisone Valerate, Nystatin, Permethrin, and Triamcinolone Acetonide. The complaint was filed in the Eastern District of Pennsylvania and has been transferred into the MDL.
On December 15, 2020, a drugstore chain filed a complaint against Perrigo and 45 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The complaint lists 63 drugs that the chain purchased from Perrigo, but the product conspiracies allegedly involving Perrigo focus on Adapalene, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Desonide, Econazole, Erythromycin, Fluocinonide, Fluticasone Propionate, Halobetasol, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Nystatin, Prochlorperazine, Promethazine HCL, Tacrolimus, and Triamcinolone. The complaint was filed in the Eastern District of Pennsylvania and has been transferred into the MDL.
On December 15, 2020, several counties in New York filed a complaint against Perrigo and 45 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens products, most of which Perrigo neither makes nor sells. The allegations that concern Perrigo include: Adapalene, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Erythromycin, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate,
37
Perrigo Company plc - Item 1
Note 15
Hydrocortisone Valerate, Imiquimod, Methazolamide, Mometasone Furoate, Nystatin, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide. The complaint was originally filed in New York State court but has been removed to federal court and consolidated into the MDL. The counties filed an amended complaint on June 30, 2021.
On August 30, 2021, the county of Westchester, NY filed a complaint in New York State court against Perrigo and 45 other pharmaceutical companies alleging an overarching conspiracy to fix, raise or stabilize prices of dozens products, most of which Perrigo neither makes nor sells. The allegations that concern Perrigo include: Adapalene, Betamethasone Dipropionate, Bromocriptine Mesylate, Calcipotriene Betamethasone Dipropionate, Ciclopirox, Clindamycin Phosphate, Clobetasol, Desonide, Econazole, Erythromycin, Fluticasone Propionate, Halobetasol Propionate, Hydrocortisone Acetate, Hydrocortisone Valerate, Imiquimod, Methazolamide, Mometasone Furoate, Nystatin, Permethrin, Prochlorperazine Maleate, Promethazine HCL, Tacrolimus, and Triamcinolone Acetonide. The case has been removed to federal court and consolidated into the MDL.
On October 8, 2021, approximately 20 health plans filed a Praecipe to Issue Writ of Summons in Pennsylvania state court to commence an action against 46 generic pharmaceutical manufacturers and 24 individuals, alleging antitrust violations concerning generic pharmaceutical drugs. While Perrigo was named as a defendant, no complaint has been filed and the precise allegations and products at issue have not been identified. On January 3, 2022, the plaintiffs filed a second Praecipe. Proceedings in the case, including the filing of a complaint, have not yet occurred. The case remains in deferred status.
State Attorney General Complaint
On June 10, 2020, the Connecticut Attorney General’s office filed a lawsuit on behalf of Connecticut and 50 other states and territories against Perrigo, 35 other generic pharmaceutical manufacturers, and certain individuals (including 2 former Perrigo employees), alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of 80 products. The allegations against Perrigo focus on the following drugs: Adapalene cream, Ammonium Lactate cream and lotion, Betamethasone dipropionate lotion, Bromocriptine tablets, Calcipotriene Betamethasone Dipropionate ointment, Ciclopirox cream, shampoo and solution, Clindamycin solution, Desonide cream and ointment, Econazole cream, Erythromycin base alcohol solution, Fluocinonide cream, Fluticasone lotion, Halobetasol cream and ointment, Hydrocortisone Acetate suppositories, Hydrocortisone Valerate cream, Imiquimod cream, Methazolamide tablets, Nystatin ointment, Prochlorperazine suppositories, Promethazine HCL suppositories, Tacrolimus ointment, and Triamcinolone cream and ointment. The Complaint was filed in the District of Connecticut, but has been transferred into the MDL. On May 7, 2021, the Court ruled that this case will move forward as a bellwether case. On September 9, 2021, the States filed an amended complaint, although the substantive allegations against Perrigo did not change. Perrigo moved to dismiss the Complaint on November 12, 2021. That motion has been fully briefed and is pending. This case is included among the “bellwether cases” designated to move on a more expedited schedule than the other cases in the MDL, and, as such, it will be subject to the January 17, 2023 discovery deadline and November 16, 2023 summary judgment deadline if the Complaint survives the pending motions to dismiss. Like the other cases in the MDL, no trial date has been set for this case.
Canadian Class Action Complaint
In June 2020, an end payor filed a class action in Ontario, Canada against Perrigo and 29 other manufacturers alleging an overarching conspiracy to allocate customers and/or fix, raise or stabilize prices of dozens of products, most of which Perrigo neither makes nor sells. The product conspiracies allegedly involving Perrigo focus on the same products as those involved in other MDL complaints naming Perrigo: Clobetasol, Desonide, Econazole, and Nystatin. In December 2020, Plaintiffs amended their complaint to add additional claims based on the State AG complaint of June 2020.
At this stage, we cannot reasonably estimate the outcome of the liability if any, associated with the claims listed above.
Securities Litigation
In the United States (cases related to events in 2015-2017)
On May 18, 2016, a shareholder filed a securities case against us and our former CEO, Joseph Papa, in the U.S. District Court for the District of New Jersey (Roofers’ Pension Fund v. Papa, et al.). The plaintiff purported to
38
Perrigo Company plc - Item 1
Note 15
represent a class of shareholders for the period from April 21, 2015 through May 11, 2016, inclusive. The original complaint alleged violations of Securities Exchange Act sections 10(b) (and Rule 10b5) and 14(e) against both defendants and 20(a) control person liability against Mr. Papa. In general, the allegations concerned the actions taken by us and the former executive to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015. The plaintiff also alleged that the defendants provided inadequate disclosure concerning alleged integration problems related to the Omega acquisition in the period from April 21, 2015 through May 11, 2016. On July 19, 2016, a different shareholder filed a securities class action against us and our former CEO, Joseph Papa, also in the District of New Jersey (Wilson v. Papa, et al.). The plaintiff purported to represent a class of persons who sold put options on our shares between April 21, 2015 and May 11, 2016. In general, the allegations and the claims were the same as those made in the original complaint filed in the Roofers' Pension Fund case described above. On December 8, 2016, the court consolidated the Roofers' Pension Fund case and the Wilson case under the Roofers' Pension Fund case number. In February 2017, the court selected the lead plaintiffs for the consolidated case and the lead counsel to the putative class. In March 2017, the court entered a scheduling order.
On June 21, 2017, the court-appointed lead plaintiffs filed an amended complaint that superseded the original complaints in the Roofers’ Pension Fund case and the Wilson case. In the amended complaint, the lead plaintiffs seek to represent 3 classes of shareholders: (i) shareholders who purchased shares during the period from April 21, 2015 through May 3, 2017 on the U.S. exchanges; (ii) shareholders who purchased shares during the same period on the Tel Aviv stock exchange; and (iii) shareholders who owned shares on November 12, 2015 and held such stock through at least 8:00 a.m. on November 13, 2015 (the final day of the Mylan tender offer) regardless of whether the shareholders tendered their shares. The amended complaint names as defendants us and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The amended complaint alleges violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals. In general, the allegations concern the actions taken by us and the former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure throughout the entire class period related to purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company and at Omega, alleges price fixing activities with respect to 6 generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The amended complaint does not include an estimate of damages. During 2017, the defendants filed motions to dismiss, which the plaintiffs opposed. On July 27, 2018, the court issued an opinion and order granting the defendants’ motions to dismiss in part and denying the motions to dismiss in part. The court dismissed without prejudice defendants Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, Donal O’Connor, and Marc Coucke. The court also dismissed without prejudice claims arising from the Tysabri® accounting issue described above and claims alleging incorrect disclosure of organic growth described above. The defendants who were not dismissed are Perrigo Company plc, Joe Papa, and Judy Brown. The claims (described above) that were not dismissed relate to the integration issues regarding the Omega acquisition, the defense against the Mylan tender offer, and the alleged price fixing activities with respect to 6 generic prescription pharmaceuticals. The defendants who remain in the case (the Company, Mr. Papa, and Ms. Brown) have filed answers denying liability, and the discovery stage of litigation began in late 2018. Discovery in the class action ended on January 31, 2021. In early April 2021, the defendants filed various post-discovery motions, including summary judgment motions; the briefing of which was completed in early July 2021. The motions are now before the court. The court held oral argument on April 7, 2022. We intend to defend the lawsuit vigorously.
On November 14, 2019, the court granted the lead plaintiffs’ motion and certified 3 classes for the case: (i) all those who purchased shares between April 21, 2015 through May 2, 2017 inclusive on a U.S. exchange and were damaged thereby; (ii) all those who purchased shares between April 21, 2015 through May 2, 2017 inclusive on the Tel Aviv stock exchange and were damaged thereby; and (iii) all those who owned shares as of November 12, 2015 and held such stock through at least 8:00 a.m. on November 13, 2015 (whether or not a person tendered shares in response to the Mylan tender offer) (the "tender offer class"). Defendants filed a petition for leave to appeal in the Third Circuit challenging the certification of the tender offer class. On April 30, 2020, the Third Circuit denied leave to appeal. The District Court has approved the issuance of a notice of the pendency of the class action, and the notice has been sent to shareholders who are eligible to participate in the classes.
In early July 2021, the Court assigned the securities class action case (Roofer’s case) to a new judge within the U.S. District Court for the District of New Jersey. Unless otherwise noted, each of the lawsuits discussed in the following sections is pending in the U.S. District Court for the District of New Jersey and remains with the originally assigned judge. The allegations in the complaints relate to events during certain portions of the 2015 through 2017
39
Perrigo Company plc - Item 1
Note 15
calendar years, including the period of the Mylan tender offer. All but one of these lawsuits allege violations of federal securities laws, but none are class actions. NaN lawsuit (Highfields) alleges only state law claims. Discovery in all these cases, except Starboard Value and Highfields, ended in November, 2021. The cases pending in federal court in New Jersey listed below have been suspended since January 2022, pending the ruling on the summary judgment motions in the class action case (Roofers case). We intend to defend all these lawsuits vigorously.
Carmignac, First Manhattan and Similar Cases. The following 7 cases were filed by the same law firm and generally make the same factual assertions but, at times, differ as to which securities laws violations they allege:
| Case | Date Filed | ||||
| Carmignac Gestion, S.A. v. Perrigo Company plc, et al. | 11/1/2017 | ||||
| First Manhattan Co. v. Perrigo Company plc, et al. | 2/16/2018; amended 4/20/2018 | ||||
| Nationwide Mutual Funds, et al. v. Perrigo Company plc, et al. | 10/29/2018 | ||||
| Schwab Capital Trust, et al. v. Perrigo Company plc, et al. | 1/31/2019 | ||||
| Aberdeen Canada Funds -- Global Equity Fund, et al. v. Perrigo Company plc, et al. | 2/22/2019 | ||||
| Principal Funds, Inc., et al. v. Perrigo Company plc, et al. | 3/5/2020 | ||||
| Kuwait Investment Authority, et al. v. Perrigo Company plc, et al. | 3/31/2020 | ||||
The original complaints in the Carmignac case and the First Manhattan case named Perrigo, Mr. Papa, Ms. Brown, and Mr. Coucke as defendants. Mr. Coucke was dismissed as a defendant after the plaintiffs agreed to apply the July 2018 ruling in the Roofers' Pension Fund case to these two cases. The complaints in each of the other cases name only Perrigo, Mr. Papa, and Ms. Brown as defendants.
Each complaint asserts claims under Sections 10(b) (and Rule 10b-5 thereunder) and all cases except Aberdeen assert claims under Section 14(e) of the Securities Exchange Act against all defendants, as well as control person liability under Section 20(a) of the Securities Exchange Act against the individual defendants. The control person claims against the individual defendants are limited to the period from April 2015 through April 2016 in the Carmignac case. The complaints in the Carmignac and First Manhattan cases also assert claims under Section 18 of the Exchange Act.
Each complaint alleges inadequate disclosures concerning the valuation and integration of Omega, the financial guidance we provided, our reporting about the generic prescription pharmaceutical business and its prospects, and the activities surrounding the efforts to defeat the Mylan tender offer during 2015, and, in each of the cases other than Carmignac, alleged price fixing activities with respect to 6 generic prescription pharmaceuticals. The First Manhattan complaint also alleges improper accounting for the Tysabri® asset. With the exception of Carmignac, each of these cases relates to events during the period from April 2015 through May 2017. Many of the allegations in these cases overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case, though the Nationwide Mutual, Schwab Capital, Aberdeen, Principal Funds and Kuwait complaints do not include the factual allegations that the court dismissed in the July 2018 ruling in the Roofers' Pension Fund case.
After the court issued its July 2018 opinion in the Roofers’ Pension Fund case, the parties in Carmignac and First Manhattan conferred and agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in their cases. The later filed cases adopted a similar posture. The defendants in the Carmignac and other cases listed above filed motions to dismiss addressing the additional allegations in such cases. On July 31, 2019, the court granted such motions to dismiss in part and denied them in part. That ruling applies to each of the above cases. The defendants have filed answers in each case denying liability. Discovery in these cases has ended.
40
Perrigo Company plc - Item 1
Note 15
Mason Capital, Pentwater and Similar Cases. The following 8 cases were filed by the same law firm and generally make the same factual allegations:
| Case | Date Filed | ||||
| Mason Capital L.P., et al. v. Perrigo Company plc, et al. | 1/26/2018 | ||||
| Pentwater Equity Opportunities Master Fund Ltd., et al. v. Perrigo Company plc, et al. | 1/26/2018 | ||||
| WCM Alternatives: Event-Drive Fund, et al. v. Perrigo Co., plc, et al. | 11/15/2018 | ||||
| Hudson Bay Master Fund Ltd., et al. v. Perrigo Co., plc, et al. | 11/15/2018 | ||||
| Discovery Global Citizens Master Fund, Ltd., et al. v. Perrigo Co. plc, et al. | 12/18/2019 | ||||
| York Capital Management, L.P., et al. v. Perrigo Co. plc, et al. | 12/20/2019 | ||||
| Burlington Loan Management DAC v. Perrigo Co. plc, et al. | 2/12/2020 | ||||
| Universities Superannuation Scheme Limited v. Perrigo Co. plc, et al. | 3/2/2020 | ||||
The complaints in the Mason Capital case and the Pentwater case originally named Perrigo and 11 current or former directors and officers of Perrigo as defendants. In the July 2018 Roofers’ Pension Fund ruling, the court dismissed without prejudice each of the defendants other than Perrigo, Mr. Papa and Ms. Brown from that case; these plaintiffs later agreed that this ruling would apply to their cases as well. The complaints in each of the other cases in the above table name only Perrigo, Mr. Papa, and Ms. Brown as defendants.
Each complaint asserts claims under Section 14(e) of the Securities Exchange Act against all defendants, as well as control person liability under Section 20(a) of the Securities Exchange Act against the individual defendants. The complaints in the WCM case and the Universities Superannuation Scheme case also assert claims under Section 10(b) of the Exchange Act and Rule 10b-5 thereunder.
Each complaint alleges inadequate disclosure during the tender offer period in 2015 and at various times concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to 6 generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset. The WCM complaint also makes these allegations for the period through May 2017 and the Universities Superannuation Scheme complaint also concerns certain times during 2016. Many of the factual allegations in these cases overlap with the allegations of the June 2017 amended complaint in the Roofers’ Pension Fund case, and the Mason Capital and Pentwater cases include factual allegations similar to those in the Carmignac case described above.
After the court issued its July 2018 opinion in the Roofers’ Pension Fund case, the parties in each of the above cases conferred and agreed that the ruling in the Roofers’ Pension Fund case would apply equally to the common allegations in their cases. The defendants in each of these cases have filed answers denying liability, and the discovery phase in each of these cases has ended.
Harel Insurance and TIAA-CREF Cases. The following 2 cases were filed by the same law firm and generally make the same factual allegations relating to the period from February 2014 through May 2017 (in the Harel case) and from August 2014 through May 2017 (in the TIAA-CREF case):
| Case | Date Filed | ||||
| Harel Insurance Company, Ltd., et al. v. Perrigo Company plc, et al. | 2/13/2018 | ||||
| TIAA-CREF Investment Management, LLC., et al. v. Perrigo Company plc, et al. | 4/20/2018 | ||||
The complaints in the Harel and TIAA-CREF cases originally named Perrigo and 13 current or former directors and officers of Perrigo as defendants (adding 2 more individual defendants not sued in the other cases described in this section). In the July 2018 Roofers’ Pension Fund ruling, the court dismissed without prejudice 8 of the 11 defendants other than Perrigo, Mr. Papa and Ms. Brown from that case. These plaintiffs later agreed that that ruling would apply to these cases as well and also dismissed their claims against the two additional individuals that only these plaintiffs had named as defendants.
Each complaint asserts claims under Sections 10(b) and 14(e) of the Securities Exchange Act and Rule 10b-5 thereunder against all defendants, as well as control person liability under Section 20(a) of the Securities
41
Perrigo Company plc - Item 1
Note 15
Exchange Act against the individual defendants. The complaint in the Harel case also asserts claims based on Israeli securities laws.
Each of the complaints alleges inadequate disclosure around the tender offer events in 2015 and at various times during the relevant periods concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to 6 generic prescription pharmaceuticals, and alleged improper accounting for the Tysabri® asset from February 2014 until the withdrawal of past financial statements in April 2017.
After the court issued its July 2018 opinion in the Roofers’ Pension Fund case, the parties in the Harel and TIAA-CREF cases conferred and agreed that such ruling would apply equally to the common allegations in their cases. The defendants in each of these cases have filed answers denying liability, and the discovery phase in each of these cases has ended.
Other Cases Related to Events in 2015-2017. Certain allegations in the following 3 cases also overlap with the allegations of the June 2017 amended complaint in the Roofers' Pension Fund case and with allegations in 1 or more of the other individual cases described in the sections above:
| Case | Date Filed | ||||
| Sculptor Master Fund (f/k/a OZ Master Fund, Ltd.), et al. v. Perrigo Company plc, et al. | 2/6/2019 | ||||
| Highfields Capital I LP, et al. v. Perrigo Company plc, et al. | 6/4/2020 | ||||
| BlackRock Global Allocation Fund, Inc., et al. v. Perrigo Co. plc, et al. | 4/21/2020 | ||||
| Starboard Value and Opportunity C LP, et al. v. Perrigo Company plc, et al. | 2/25/2021 | ||||
Each of the above complaints names Perrigo, Mr. Papa, and Ms. Brown as defendants.
The Sculptor Master Fund (formerly OZ) complaint asserts claims under Sections 10(b) and 14(e) of the Securities Exchange Act and Rule 10b-5 thereunder against all defendants, as well as control person liability under Section 20(a) of the Securities Exchange Act against the individual defendants. The parties have agreed that the court's rulings in July 2018 in the Roofers' Pension Fund case and in July 2019 in the Carmignac and related cases will apply to this case as well. The defendants have filed answers denying liability. The plaintiffs participated in the discovery proceedings in the Roofers' Pension Fund case and the various individual cases described above. The discovery phase in this case has ended.
The BlackRock Global complaint also asserts claims under Securities Exchange Act section 10(b) (and Rule 10b-5) and section 14(e) against all defendants and section 20(a) control person claims against the individual defendants largely based on the same events during the period from April 2015 through May 2017. Plaintiffs contend that the defendants provided inadequate disclosure during the tender offer period in 2015 and point to disclosures at various times during the period concerning valuation and integration of Omega, the financial guidance provided by us during that period, alleged price fixing activities with respect to 6 generic prescription pharmaceuticals, alleged lower performance in the generic prescription drug business during 2015 and alleged improper accounting for the Tysabri® asset. The defendants have filed answers denying liability. The plaintiffs participated in the discovery proceedings in the Roofers' Pension Fund case and the various individual cases described above. The discovery phase in this case has ended.
The Starboard Value and Opportunity C LP complaint also asserts claims under Securities Exchange Act section 10(b) (and Rule 10b-5) against all defendants and section 20(a) control person claims against the individual defendants based on events related to alleged price fixing activities with respect to generic prescription drugs during periods that overlap to some extent with the period alleged in the various other cases described above. Plaintiffs contend that the defendants provided inadequate disclosure during 2016 about generic prescription drug business and those alleged matters. The lawsuit was filed on February 25, 2021; but by agreement the case was administratively terminated by the court in June 2021 pending a decision on the same defendants’ motions currently pending before the court in the Roofers' Pension Fund case described above.
The Highfields federal case complaint asserted claims under Sections 14(e) and 18 of the Securities Exchange Act against all defendants, as well as control person liability under Section 20(a) of the Securities Exchange Act against the individual defendants. As originally filed in the U.S. District Court for the District of
42
Perrigo Company plc - Item 1
Note 15
Massachusetts, the Highfields complaint also alleged claims under the Massachusetts Unfair Business Methods Law (chapter 93A) and Massachusetts common law claims of tortious interference with prospective economic advantage, common law fraud, negligent misrepresentation, and unjust enrichment. The factual allegations generally were similar to the factual allegations in the Amended Complaint in the Roofers' Pension Fund case described above, except that the Highfields plaintiffs did not include allegations about alleged collusive pricing of generic prescription drugs. In March 2020, the District of Massachusetts court granted defendants’ motion and transferred the case to the U.S. District Court for the District of New Jersey so that the activities in the case could proceed in tandem with the other cases in the District of New Jersey described above. After the transfer, in June 2020, the Highfields plaintiffs voluntarily dismissed their federal lawsuit.
The same Highfields plaintiffs the same day then filed a new lawsuit in Massachusetts State Court asserting the same factual allegations as in their federal lawsuit and alleging only Massachusetts state law claims under the Massachusetts Unfair Business Methods Law (chapter 93A) and Massachusetts common law claims of tortious interference with prospective economic advantage, common law fraud, negligent misrepresentation, and unjust enrichment. Defendants’ motion to dismiss was fully briefed as of late November 2020, argument occurred in early May 2021. In December 2021, the Massachusetts State Court granted Defendants’ motion to dismiss in part and denied it in part. Defendants’ filed their answers in January 2022 denying liability. The discovery phase in this case has begun (including discovery related to some factual allegations that were not part of the discovery in the actions in New Jersey federal court). The Court held a discovery conference and approved fact discovery deadlines into May, 2023 and later deadlines to complete expert discovery.
In Israel (cases related to events in 2015-2017)
Because our shares were traded on the Tel Aviv stock exchange under a dual trading arrangement until February 23, 2022, we are potentially subject to securities litigation in Israel. NaN cases were filed; 1 was voluntarily dismissed in each of 2017 and 2018 and 1 was stayed in 2018. We are consulting with Israeli counsel about our response to these allegations and we intend to defend this case vigorously.
On June 28, 2017, a plaintiff filed a complaint in Tel Aviv District Court styled Israel Elec. Corp. Employees’ Educ. Fund v. Perrigo Company plc, et al. The lead plaintiff seeks to represent a class of shareholders who purchased Perrigo stock on the Tel Aviv stock exchange during the period from April 24, 2015 through May 3, 2017 and also a claim for those that owned shares on the final day of the Mylan tender offer (November 13, 2015). The amended complaint names as defendants the Company, Ernst & Young LLP (the Company’s auditor), and 11 current or former directors and officers of Perrigo (Mses. Judy Brown, Laurie Brlas, Jacqualyn Fouse, Ellen Hoffing, and Messrs. Joe Papa, Marc Coucke, Gary Cohen, Michael Jandernoa, Gerald Kunkle, Herman Morris, and Donal O’Connor). The complaint alleges violations under U.S. securities laws of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against all defendants and 20(a) control person liability against the 11 individuals or, in the alternative, under Israeli securities laws. In general, the allegations concern the actions taken by us and our former executives to defend against the unsolicited takeover bid by Mylan in the period from April 21, 2015 through November 13, 2015 and the allegedly inadequate disclosure concerning purported integration problems related to the Omega acquisition, alleges incorrect reporting of organic growth at the Company, alleges price fixing activities with respect to 6 generic prescription pharmaceuticals, and alleges improper accounting for the Tysabri® royalty stream. The plaintiff indicates an initial, preliminary class damages estimate of 2.7 billion NIS (approximately $760.0 million at 1 NIS = 0.28 cents). After the other 2 cases filed in Israel were voluntarily dismissed, the plaintiff in this case agreed to stay this case pending the outcome of the Roofers’ Pension Fund case in the U.S. (described above). The Israeli court approved the stay, and this case is now stayed. We intend to defend the lawsuit vigorously.
In Israel (case related to Irish Tax events)
On December 31, 2018, a shareholder filed an action against the Company, our CEO Murray Kessler, and our former CFO Ronald Winowiecki in Tel Aviv District Court (Baton v. Perrigo Company plc, et. al.). The case is a securities class action brought in Israel making similar factual allegations for the same period as those asserted in the In re Perrigo Company plc Sec. Litig case in New York federal court in which the settlement received final approval in February 2022. This case alleges that persons who purchased securities through the Tel Aviv stock exchange and suffered damages can assert claims under Israeli securities law that will follow the liability principles of Sections 10(b) and 20(a) of the U.S. Securities Exchange Act. The plaintiff does not provide an estimate of class damages. In 2019, the court granted 2 requests by Perrigo to stay the proceedings pending the resolution of proceedings in the United States. Perrigo filed a further request for a stay in February 2020, and the court granted the stay indefinitely. The plaintiff filed a motion to lift the stay then later agreed that the case should remain stayed
43
Perrigo Company plc - Item 1
Note 15
through February 2021. The stay continued in place during 2021. After the settlement of the U.S. case (In re Perrigo Company plc Sec. Litig.), Perrigo’s counsel informed the Israeli Court of the final approval of the settlement of the U.S. case. The plaintiff filed papers in late April 2022 requesting that the Court allow the parties some additional months to explore options, and the Court required the parties to provide an update no later than June 30, 2022. In June 2022 the Company and the plaintiff reported to the court that they are attempting mediation, and the Court ordered the parties to provide a further update by September 22, 2022. The case has been transferred to a newly-appointed judge as the original judge has moved to a higher court. We intend to defend the lawsuit vigorously.
Other Matters
Talcum Powder
The Company has been named, together with other manufacturers, in product liability lawsuits in state courts in California, Florida, Mississippi, Missouri, New Jersey, Louisiana, Ohio, Oregon and Illinois alleging that the use of body powder products containing talcum powder causes mesothelioma and lung cancer due to the presence of asbestos. All but one of these cases involve legacy talcum powder products that have not been manufactured by the Company since 1999. One of the pending actions involves a current prescription product that contains talc as an excipient. As of July 2, 2022, the Company is currently named in 69 individual lawsuits seeking compensatory and punitive damages and has accepted a tender for a portion of the defense costs and liability from a retailer for 1 additional matter. The Company has several defenses and intends to aggressively defend these lawsuits. Trials for these lawsuits are currently scheduled throughout 2022, 2023 and 2024, with the earliest trial date in August 2022.
Ranitidine
After regulatory bodies announced worldwide that ranitidine may potentially contain N-nitrosodimethylamine ("NDMA"), a known environmental contaminant, the Company promptly began testing its externally-sourced ranitidine API and ranitidine-based products. On October 8, 2019, the Company halted shipments of the product based upon preliminary results and on October 23, 2019, the Company made the decision to conduct a voluntary retail market withdrawal.
In February 2020, the resulting actions involving Zantac® and other ranitidine products were transferred for coordinated pretrial proceedings to a Multi-District Litigation (In re Zantac®/Ranitidine Products Liability Litigation MDL No. 2924) in the U.S. District Court for the Southern District of Florida. After the Company successfully moved to dismiss the first set of Master Complaints in the MDL, it now includes 3: 1) an Amended Master Personal Injury Complaint; 2) a Consolidated Amended Consumer Economic Loss Class Action Complaint; and 3) a Consolidated Medical Monitoring Class Action Complaint. All 3 name the Company. Plaintiffs appealed 1 of the original Master Complaints, the Third-Party Payor Complaint, and 2 individual plaintiffs appealed their individual personal injury claims on limited grounds. The Company is not named in the appeals.
On June 30, 2021, the Court dismissed all claims against the retail and distributor defendants with prejudice, thereby reducing the Company’s potential for exposure and liability related to possible indemnification. On July 8, 2021, the Court dismissed all claims against the Company with prejudice. Appeals of these dismissal orders to the U.S. Court of Appeals for the 11th Circuit have been filed, as well several state level claims related to the theories advanced in the MDL litigation. The Company will continue to vigorously defend each of these lawsuits.
As of July 2, 2022, the Company has been named in three hundred and thirteen (313) personal injury lawsuits, most of which are in the MDL tied to various federal courts alleging that plaintiffs developed various types of cancers or are placed at higher risk of developing cancer as a result of ingesting products containing ranitidine. The Company has also been named in a handful of similar lawsuits in the state courts of California, Illinois, New York and Pennsylvania. The Company is named in these lawsuits with manufacturers of the national brand Zantac® and other manufacturers of ranitidine products, as well as distributors, repackagers, and/or retailers. Plaintiffs seek compensatory and punitive damages, and in some instances seek applicable remedies under state consumer protection laws.
The Company has also been named in a Complaint brought by the New Mexico Attorney General based on the following theories: violation of a New Mexico public nuisance statute, NMSA 30-8-1 to -14; common law nuisance; and negligence and gross negligence. The Company is named in this lawsuit with manufacturers of the national brand Zantac® and other manufacturers of ranitidine products and/or retailers. Brand name manufactures named in the lawsuit also face claims under the state’s Unfair Practices & False Advertising acts. Likewise, the
44
Perrigo Company plc - Item 1
Note 15
Company has also been named in a Complaint brought by the Mayor and City Council of Baltimore, along with manufacturers of the national brand Zantac® and other manufacturers of ranitidine products and/or retailers. This action brings claims under the Maryland Consumer Protection Act against the brand name defendants only, as well as public nuisance and negligence for the remaining defendants. The Company was originally able to consolidate the New Mexico and Baltimore Actions to the MDL, however both actions were recently remanded to state court. The Company filed motions to dismiss in both actions. The New Mexico District Court denied the Company’s Motion to Dismiss and litigation continues. The Maryland Circuit Court has not issued a ruling on the Company’s Motion. The Company will continue to vigorously defend each of these lawsuits. On January 28, 2022 the Baltimore Circuit Court dismissed all of Plaintiffs’ claims in full against Perrigo. Plaintiffs have not sought certification to appeal the Circuit Court’s ruling.
Some of the Company’s retailer customers are seeking indemnity from the Company for a portion of their defense costs and liability relating to these cases.
Contingencies Accruals
As a result of the matters discussed in this Note, the Company has established a loss accrual for litigation contingencies where we believe a loss to be probable and for which an amount of loss can be reasonably estimated. However, we cannot determine a reasonable estimate of the maximum possible loss or range of loss for these matters given that they are at various stages of the litigation process and each case is subject to inherent uncertainties of litigation. At July 2, 2022, the loss accrual for litigation contingencies reflected on the balance sheet in Other accrued liabilities was approximately $67.8 million. The Company also recorded an insurance recovery receivable reflected on the balance sheet in Prepaid expenses and other current assets of approximately $43.5 million related to these litigation contingencies because it believes such amount is recoverable based on communications with its insurers to date; however, the Company may erode this insurance receivable as it incurs defense costs associated with defending the matters. The Company’s management believes these accruals for contingencies are reasonable and sufficient based upon information currently available to management; however, there can be no assurance that final costs related to these contingencies will not exceed current estimates or that all of the final costs related to these contingencies will be covered by insurance. (See "Insurance Coverage Litigation," below.) In addition, we have other litigation matters pending for which we have not recorded any accruals because our potential liability for those matters is not probable or cannot be reasonably estimated based on currently available information. For those matters where we have not recorded an accrual but a loss is reasonably possible, we cannot determine a reasonable estimate of the maximum possible loss or range of loss for these matters given that they are at various stages of the litigation process and each case is subject to the inherent uncertainties of litigation.
Insurance Coverage Litigation
In May 2021, insurers on multiple policies of D&O insurance filed an action in the High Court in Dublin against the Company and multiple current and former directors and officers of the Company seeking declaratory judgments on certain coverage issues. Those coverage issues include claims that policies for periods beginning in December 2015 and December 2016, respectively, do not have to provide coverage for the securities actions described above pending in the District of New Jersey or in Massachusetts state court concerning the events of 2015-2017. The policy for the period beginning December 2014 is currently providing coverage for those matters, and the litigation would not affect that existing coverage. However, if the plaintiffs are successful, the total amount of insurance coverage available to defend such lawsuits and to satisfy any judgment or settlement costs thereunder would be limited to 1 policy period. The insurers’ lawsuit also challenges coverage for Krueger derivatively on behalf of nominal defendant Perrigo Company plc v. Alford et al., a prior derivative action filed in the District of New Jersey that was dismissed in August 2020, and for the counterclaims brought in the Omega arbitration proceedings. Perrigo responded on November 1, 2021; Perrigo’s response includes its position that the policies for the periods beginning December 2015 and December 2016 provide coverage for the underlying litigation matters and seeks a ruling to that effect. The discovery stage of the case has begun. We intend to defend the lawsuit vigorously.
45
Perrigo Company plc - Item 1
Note 17
NOTE 16 – RESTRUCTURING CHARGES
We periodically take action to reduce redundant expenses and improve operating efficiencies. Restructuring activity includes severance, lease exit costs, and related consulting fees. The following reflects our restructuring activity (in millions):
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | ||||||||||||||||||||
| Beginning balance | $ | 8.2 | $ | 6.2 | $ | 6.9 | $ | 9.1 | |||||||||||||||
| Additional charges | 9.5 | 9.0 | 13.0 | 10.7 | |||||||||||||||||||
| Payments | (3.9) | (5.1) | (6.0) | (9.5) | |||||||||||||||||||
| Non-cash adjustments | (0.3) | 0.1 | (0.4) | (0.1) | |||||||||||||||||||
| Ending balance | $ | 13.5 | $ | 10.2 | $ | 13.5 | $ | 10.2 | |||||||||||||||
The charges incurred during the three and six months ended July 2, 2022 and July 3, 2021 were primarily associated with actions taken on supply chain restructuring in 2022 and actions to streamline the organization in 2021.
There were no other material restructuring programs for the three and six months ended July 2, 2022 and July 3, 2021. All charges are recorded in Restructuring expense on the Condensed Consolidated Statements of Operations. The remaining $13.5 million liability for employee severance benefits and consulting fees is expected to be paid within the next year.
46
Perrigo Company plc - Item 2
Note 18
NOTE 17 – SEGMENT INFORMATION
Our segment reporting structure is consistent with the way our management makes operating decisions,
The tables below show select financial measures by reporting segment (in millions):
| Total Assets | ||||||||||||||
| July 2, 2022 | December 31, 2021 | |||||||||||||
| CSCA | $ | 5,088.4 | $ | 5,983.8 | ||||||||||
| CSCI | 5,831.2 | 4,425.8 | ||||||||||||
| Held for sale | — | 16.1 | ||||||||||||
| Total | $ | 10,919.6 | $ | 10,425.7 | ||||||||||
| Three Months Ended | |||||||||||||||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | ||||||||||||||||||||||||||||||||||
| Net Sales | Operating Income (Loss) | Intangible Asset Amortization | Net Sales | Operating Income (Loss) | Intangible Asset Amortization | ||||||||||||||||||||||||||||||
| CSCA | $ | 728.0 | $ | 86.3 | $ | 13.6 | $ | 622.3 | $ | (72.0) | $ | 12.9 | |||||||||||||||||||||||
| CSCI | 393.7 | 1.5 | 48.8 | 358.8 | 1.3 | 40.5 | |||||||||||||||||||||||||||||
| Unallocated | — | (94.7) | — | — | (55.2) | — | |||||||||||||||||||||||||||||
| Continuing Operations Total | $ | 1,121.7 | $ | (6.9) | $ | 62.4 | $ | 981.1 | $ | (125.9) | $ | 53.4 | |||||||||||||||||||||||
| Six Months Ended | |||||||||||||||||||||||||||||||||||
| July 2, 2022 | July 3, 2021 | ||||||||||||||||||||||||||||||||||
| Net Sales | Operating Income (Loss) | Intangible Asset Amortization | Net Sales | Operating Income (Loss) | Intangible Asset Amortization | ||||||||||||||||||||||||||||||
| CSCA | $ | 1,438.0 | $ | 164.8 | $ | 26.0 | $ | 1,262.8 | $ | 23.6 | $ | 25.8 | |||||||||||||||||||||||
| CSCI | 758.2 | 17.7 | 84.9 | 728.3 | 18.8 | 80.8 | |||||||||||||||||||||||||||||
| Unallocated | — | (167.7) | — | — | (117.0) | — | |||||||||||||||||||||||||||||
| Continuing Operations Total | $ | 2,196.2 | $ | 14.8 | $ | 110.9 | $ | 1,991.1 | $ | (74.6) | $ | 106.6 | |||||||||||||||||||||||
47
Perrigo Company plc - Item 2
Executive Overview
ITEM 2. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the financial statements included in this Form 10-Q, and our Form 10-K for the year ended December 31, 2021 (the “2021 Form 10-K”). These historical financial statements may not be indicative of our future performance. This discussion contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks referred to under “Risk Factors” in Item 1A of our 2021 Form 10-K and Part II. Item 1A of this Form 10-Q.
EXECUTIVE OVERVIEW
Perrigo Company plc was incorporated under the laws of Ireland on June 28, 2013 and became the successor registrant of Perrigo Company, a Michigan corporation, on December 18, 2013 in connection with the acquisition of Elan Corporation, plc ("Elan"). Unless the context requires otherwise, the terms "Perrigo," the "Company," "we," "our," "us," and similar pronouns used herein refer to Perrigo Company plc, its subsidiaries, and all predecessors of Perrigo Company plc and its subsidiaries.
Our vision is to make lives better by bringing quality, affordable Self-Care Products that consumers trust everywhere they are sold. We are a leading provider of over-the-counter ("OTC") health and wellness solutions that are designed to enhance individual well-being.
Our core competencies are geared to fully take advantage of the massive global trend towards self-care. We define self-care as not just treating disease or helping individuals feel better after taking a product, but also maintaining and enhancing their overall health and wellness. Consistent with our vision, in 2019 Perrigo’s management and board of directors launched a three-year strategy to transform the Company into a consumer self-care leader. We advanced our transformation to a consumer self-care company in 2021 by reconfiguring the portfolio through the divestiture of our RX business, announcement of the acquisition of HRA Pharma that closed in the second quarter of 2022, and removal of significant uncertainty through settlement of a tax exposure. In addition, we continue to invest in growth initiatives to drive future consistent and sustainable results in line with consumer-packaged goods peers.
Our reporting and operating segments reflect the way our management makes operating decisions, allocates resources and manages the growth and profitability of the Company. Our segments are as follows:
•Consumer Self-Care Americas ("CSCA") comprises our consumer self-care business in the U.S. and Canada, including HRA Pharma since it was acquired on April 29, 2022. CSCA previously included our Latin America businesses until they were disposed on March 9, 2022.
•Consumer Self-Care International ("CSCI") comprises our consumer self-care business in Europe and Australia, including HRA Pharma since it was acquired on April 29, 2022, which are primarily branded, and our store brand business in the United Kingdom and parts of Europe and Asia.
Highlights and Recent Developments
•On April 20, 2022, pursuant to a new credit agreement, we entered into new senior secured credit facilities consisting of (i) a $1.0 billion five-year revolving credit facility (the “2022 Revolver”) and (ii) a $500 million five-year Term Loan A facility (the "2022 Term Loan A Facility") and a $1.1 billion seven-year Term Loan B facility (the “2022 Term Loan B Facility" and, together with the 2022 Term Loan A Facility and the 2022 Revolver, the “New Senior Secured Credit Facilities”) through our indirect wholly-owned subsidiary, Perrigo Investments, LLC. We used a portion of the proceeds from the New Senior Secured Credit Facilities, together with cash on hand, to finance the acquisition of HRA Pharma and to repay our outstanding term loan facility. We also redeemed our 4.00% Senior Notes due 2023 and Perrigo Holding N.V.’s 5.1045% Guaranteed Senior Notes due 2023 on May 20, 2022, using the proceeds from the New Senior Secured Credit Facilities.
48
Perrigo Company plc - Item 2
Executive Overview
•On April 29, 2022, we completed the previously announced acquisition of HRA Pharma for €1.8 billion, or approximately $1.9 billion based on exchange rates at the time of closing on an enterprise value basis and using a lockbox mechanism set forth in the Purchase Agreement. HRA Pharma is one of the fastest growing OTC companies globally, with three category-leading self-care brands in blister care (Compeed®), women’s health (ellaOne®) and scar care (Mederma®), and brings expertise in prescription-to-OTC switches. This acquisition is expected to strengthen our presence in Europe, improve our financial profile and margins, and completed our transformation to a consumer self-care company. Operating results are reported within both our CSCA and CSCI segments.
•As described in more detail in Item 1. Note 9, in April, 2022, we entered into several financing hedge activities associated with the acquisition of HRA Pharma and New Senior Secured Credit Facilities to economically hedge the purchase price for HRA Pharma, fix the interest rate on a substantial portion of the 2022 Term Loan A Facility and 2022 Term Loan B Facility, and to reduce the Euro exposure of our net investment in European operations.
•On May 26, 2022, we received final approval from the U.S. Food and Drug Administration ("FDA") for Omeprazole Magnesium Delayed-Release Mini Capsules, 20 mg over-the-counter ("OTC"). This launch represents a first-to-market mini capsule form of omeprazole, which was developed to treat frequent heartburn. We expect to launch Omeprazole Minis later this year.
•On July 11, 2022, HRA Pharma, a Perrigo company, announced that it submitted its application for an Rx-to-OTC switch for Opill®, a progestin-only daily birth control pill (also referred to as a mini pill or non-estrogen pill). If approved, this would be the first daily birth control pill available OTC without a prescription in the U.S.
•Several leadership changes were announced during the three months ended July 2, 2022:
•On June 1, 2022, Alison Ives was promoted to Executive Vice President and Chief Scientific Officer, responsible for global oversight and coordination of Perrigo’s R&D, Regulatory and Innovation functions.
•On May 11, 2022, Eduardo Bezerra was named Executive Vice President and Chief Financial Officer, effective May 16, 2022, succeeding Ray Silcock, who retired on July 15, 2022.
•On May 11, 2022, Kyle Hanson was named Executive Vice President, General Counsel and Corporate Secretary, effective June 1, 2022, succeeding Todd Kingma, who announced his intent to retire effective August 31, 2022.
•As a result of the completed acquisition of HRA Pharma, the Company has updated its global reporting product categories beginning in the second quarter of 2022. These product category updates have been adjusted retrospectively to reflect the changes. The following updates have no impact on the Company's historical consolidated financial position, results of operations, or cash flows:
•The creation of a new "Women's Health" reporting category, comprised of the women's health portfolio of HRA Pharma, including ellaOne® and Hana®, in addition to legacy Perrigo women's health products, including feminine hygiene and pregnancy products;
•The creation of a new "Skin Care" reporting category, comprised of Compeed®, Mederma®, and all of the products in the legacy Perrigo "Skincare and Personal Hygiene" category except for legacy Perrigo women's health products; and
•The "Other" category now includes the HRA Pharma Rare Diseases business.
49
Perrigo Company plc - Item 2
Executive Overview
•The Company has initiated restructuring actions within our supply chain and operations to generate and identify possible strategic opportunities that will make Perrigo’s supply chains even more competitive to support our business objectives, as well as our current and future customer and consumer expectations. $7.6 million of expense associated with these actions was recognized in the second quarter of 2022.
War in Ukraine
The Russian invasion of Ukraine and resulting economic and political sanctions imposed by the United States, United Kingdom, European Union, and other countries on Russia, Belarus, and occupied regions in Ukraine have negatively impacted our results from operations in the region. We currently have 97 employees working out of our Ukraine subsidiary. We do not have a subsidiary or employees in Russia. We have no manufacturing facilities in either Russia or Ukraine and we sold products into Russia entirely through distributors. For the year ended December 31, 2021, these countries included approximately $27 million of net sales, $15 million of gross profit, and $8 million of operating income. In March 2022, we halted all sales to distributors in Russia and sales in Ukraine were severely depressed. While there is a possibility that sales levels may rebound in Ukraine, they are not likely to fully materialize in the short-term.
More broadly, there could be additional impacts to our net sales, earnings and cash flows, including, among other potential impacts, lower economic growth or recessions in certain neighboring countries, or globally, due to increases in global commodity costs and inflationary impacts and the limited availability of energy, and other supply chain items we procure. Additionally, our suppliers, distributors and retailers may be impacted by the war and related sanctions, and any such challenges could in turn impact our operations or negatively impact the sales, cost, or availability of our products. We will continue to monitor any impact that the war in Ukraine and existing and proposed sanctions may have on our business operations, as well as the impact that such events may have on economic and political conditions in the region and on our industry generally. If the conflict spreads or materially escalates, or economic conditions deteriorate, the impact on our business and results of operations could be material.
Impact of COVID-19 Pandemic and Economic Conditions
As the COVID-19 global pandemic and the emergence of new variants continues to evolve, the spread of the disease and actions to slow it have impacted, and continue to impact, our business and the global self-care markets in which we compete. This evolution may contribute to economic recessions or a slowdown of economic growth in certain countries or globally, which may result in increased or reduced demand for our products. COVID-19 has and could lead to future volatility in consumer preferences and access to our products (due to government actions or key material, transportation and labor shortages impacting our ability to produce and ship products), or impact consumers’ movements and access to our products.
Currently, most of the markets in which we operate have relaxed COVID-19 related restrictions and have returned to in-person activities, leading to higher incidences of factors that may impact sales of our products, including cough, cold and flu-like illnesses, sun burn care, and head lice as children return to school. For instance, we experienced higher demand in the first half of 2022 for our cough/cold sales relative to the first half of 2021 when there was very low incidence of cough, cold and flu-like illness, which we attributed to COVID-19 social distancing and mask requirements.
We continue to closely monitor and adjust our COVID-19 safety protocols for employees in response to the changing incidence rate, rules and guidelines in each jurisdiction in which we operate. While most of our facilities have maintained production at high levels despite the challenges posed by the impact of the COVID-19 pandemic, our global operations have been negatively impacted by the worldwide supply chain challenges. This has resulted in increased costs of materials, labor, logistics and distribution networks. We have taken steps to substantially mitigate these and other inflationary cost pressures, including pricing actions, productivity improvements and reducing discretionary costs.
While the current trend of increased consumer takeaway and retailer restocking suggests that the volatility in consumer behavior is stabilizing, the emergence and spread of new disease variants or additional outbreaks in markets in which we operate could result in new or reimposed restrictions or cause these trends to change, slow or reverse. Moving forward, it remains uncertain if the consumer and customer behavior surrounding COVID-19 that had impacted net sales will continue to normalize, or change, and how the increase in operating costs and supply chain disruptions will evolve going forward. Any change in these trends will likely depend on the duration and severity of the COVID-19 pandemic, including the emergence of new strains of the virus that are more contagious or
50
Perrigo Company plc - Item 2
Executive Overview
harmful, each individual country's evolving response to the pandemic, as well as the availability, acceptance and efficacy of the COVID-19 vaccines and therapeutics. Moreover, disruptions in global supply chains, labor shortages, inflation and rising interest rates may further exacerbate challenging economic conditions as a result of the pandemic or otherwise. The magnitude of any such adverse impact cannot currently be determined due to a number of uncertainties surrounding COVID-19.
Internal Revenue Service Audits of Perrigo Company, a U.S. Subsidiary
As described in more detail in Item 1. Note 14, Perrigo Company, our U.S. subsidiary ("Perrigo U.S."), is engaged in a series of tax disputes in the United States relating primarily to transfer pricing adjustments including income in connection with the purchase, distribution, and sale of store-brand OTC pharmaceutical products in the United States, including the heartburn medication omeprazole. The trial of the refund case relating to the dispute of the amount of taxable income on omeprazole sales was held during the period May 25, 2021 to June 7, 2021 in the United States District Court for the Western District of Michigan. Post-trial briefings were completed on September 24, 2021 and the case is now fully submitted for the court's decision.
On May 7, 2020, we received a final Notice of Proposed Adjustment ("NOPA") from the IRS regarding the deductibility of interest related to the IRS audit of Perrigo U.S. for the years ended June 28, 2014 and June 27, 2015. The NOPA capped the interest rate on certain intercompany debt for U.S. federal tax purposes at 130.0% of the Applicable Federal Rate (a blended rate reduction of approximately 4.0% per annum) on the stated ground that the loans were not negotiated on an arms-length basis. The IRS followed-up the NOPA with the issuance of a Revenue Agent's Report ("RAR"), together with a 30-day letter on January 13, 2021, restating its adjustments in the NOPA. On May 3, 2021, the IRS notified us that it would no longer pursue the 130.0% of AFR position as indicated in the NOPA due to a change in IRS policy. On January 20, 2022, the IRS responded to our protest to request IRS Appeals consideration, which we filed on February 26, 2021, with its rebuttal, and revised its position on this interest rate issue by reasserting that implicit parental support considerations are necessary to determine the arm's length interest rate and proposing revised interest rates that are higher than the interest rates proposed under its 130.0% of AFR assertion. The blended interest rate proposed by the IRS rebuttal is 4.36%, an increase from the blended interest rate in the NOPA of 2.57%, but lower than the stated blended interest rate of the loans of 6.8%. An IRS Appeals meeting has not yet been scheduled to discuss this issue. We will pursue all available administrative and judicial remedies necessary to defend the deductibility of the interest expense on this indebtedness.
In addition, the 30-day letter and Revenue Agent's Report for the 2013-2015 tax years expanded on a NOPA issued on December 11, 2019 and proposed to disallow reductions to gross sales income on the sale of prescription products to wholesalers for accrued wholesale customer pipeline chargebacks where the prescription products were not re-sold by such wholesalers to covered retailers by the end of the tax year. The NOPA asserted that the reduction of gross sales income of such chargebacks is an impermissible method of accounting and proposed a change in accounting method that would defer the reduction in gross sales income until the year the prescription products were re-sold to covered retailers. The NOPA proposed an increase in sales revenue of approximately $99.5 million for the 2013-2015 tax years. We filed a protest on February 26, 2021 to request IRS Appeals consideration. On January 20, 2022, the IRS responded to our protest with its rebuttal and reiterated the NOPA's position that the accrued chargebacks are not currently deductible in the tax year accrued because all events have not occurred to establish the fact of the liability in the year deducted. The first meeting to discuss this issue with IRS Appeals has been scheduled for October 12 and 13, 2022. We strongly disagree with the IRS’s proposed adjustment and will pursue all available administrative and judicial remedies necessary.
On December 2, 2021, the IRS commenced an audit of our federal income tax returns for the tax years ended December 31, 2015, through December 31, 2019.
Internal Revenue Service Audit of Athena Neurosciences, LLC, a U.S. Subsidiary
As described in more detail in Item 1. Note 14, on April 26, 2019, we received a revised NOPA from the IRS regarding transfer pricing positions related to the IRS audit of Athena for the years ended December 31, 2011, December 31, 2012 and December 31, 2013. The dispute involves the royalties payable to Athena and reportable by Athena as U.S. royalty income for its contribution of its early-stage intellectual property in several in-process products, including the Multiple Sclerosis drug Tysabri for further development by non-U.S. entities. To avoid double taxation of Tysabri income in the U.S. and Ireland, Athena made requests for Competent Authority Assistance with the IRS and Irish Revenue on April 21 and 23, 2020 pursuant to the Mutual Agreement Procedure in the U.S.-
51
Perrigo Company plc - Item 2
Executive Overview
Ireland Income Tax Treaty, which were accepted by the IRS and Irish Revenue. Supplemental requests for Competent Authority assistance to resolve a dispute with the IRS over the deductibility for U.S. tax purposes of a litigation settlement payment made in 2011 for the drug Zonegran were also accepted. An opening conference with the IRS was held on May 6, 2021 with a follow-up conference held on December 3, 2021. An opening conference with Irish Revenue was held on July 23, 2021. Athena has subsequently responded to multiple requests for information from both Competent Authorities. The respective Competent Authorities will attempt to reach a resolution that avoids double taxation on both issues.
RESULTS OF OPERATIONS
Currency Translation
Currency translation effects described below represent estimates of the net differences between translation of foreign currency transactions into U.S. dollars for the three and six months ended July 2, 2022 at the average exchange rates for the reporting period and average exchange rates for the three and six months ended July 3, 2021.
CONSOLIDATED
Consolidated Financial Results
Three Month Comparison
| Three Months Ended | |||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||
| Net sales | $ | 1,121.7 | $ | 981.1 | |||||||||||||||||||
| Gross profit | $ | 372.1 | $ | 349.0 | |||||||||||||||||||
| Gross profit % | 33.2 | % | 35.6 | % | |||||||||||||||||||
| Operating income (loss) | $ | (6.9) | $ | (125.9) | |||||||||||||||||||
| Operating income (loss) % | (0.6) | % | (12.8) | % | |||||||||||||||||||
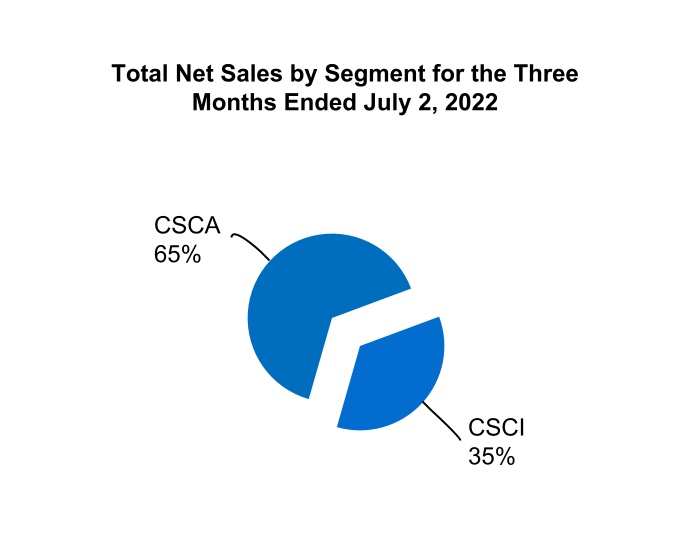
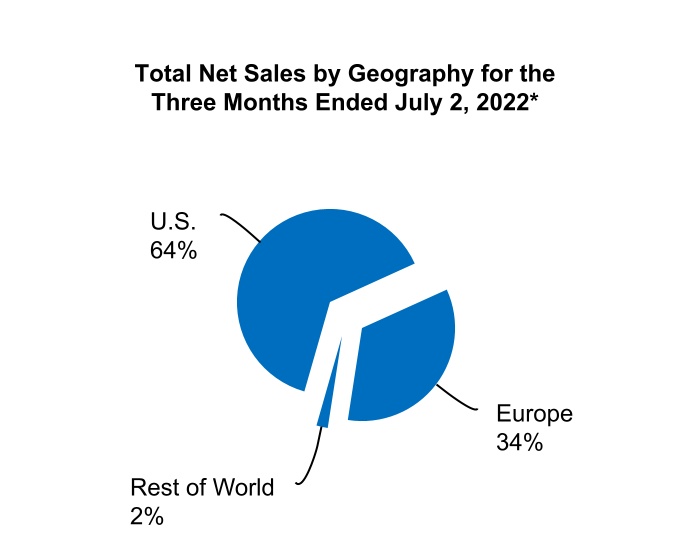
* Total net sales by geography is derived from the location of the entity that sells to a third party.
52
Perrigo Company plc - Item 2
Consolidated
Three Months Ended July 2, 2022 vs. Three Months Ended July 3, 2021
Net sales increased $140.6 million, or 14.3%, primarily due to:
•$163.5 million increase, or 17.2% due primarily to a $71.5 million increase in cough/cold sales resulting from higher incidences of cough/cold and flu-like illnesses globally, an increase of $29.3 million in U.S. Nutrition business stemming from store brand infant formula share gains due partly to a competitor recall, an increase of $38.0 million from the addition of contract manufacturing sales to the divested RX business, and positive pricing; and
•$57.8 million increase from the addition of HRA Pharma; partially offset by
•$29.6 million decrease from divestitures of Latin America businesses and the ScarAway® brand asset; and
•$51.1 million decrease from unfavorable foreign currency translation.
$119.0 million lower Operating loss, or 94.5% improvement, due primarily to:
•$23.1 million increase in gross profit due primarily to the $27.8 million increase from the addition of HRA Pharma and higher gross profit flow-through resulting from higher sales volumes, partially offset by unfavorable foreign currency translation of $25.1 million, cost of goods sold inflation and increased freight expenses (which were largely offset by pricing benefits); and divestitures of Latin America businesses and ScarAway®. Gross profit as a percentage of net sales decreased 240 basis points compared to the prior year due to the addition of third party sales to the divested RX business, which have a lower margin profile, cost of goods sold inflation and increased freight expenses, and unfavorable foreign currency translation, partially offset by the same factors that drove gross profit.
•$95.9 million decrease in operating expenses favorably impacted operating income due primarily to:
◦The absence of $158.6 million of prior year impairment charges on goodwill and assets related to the divested Latin America businesses, partially offset by
◦an increase in consulting and other expenses related to the acquisition of HRA Pharma, a divestiture gain recognized in the prior year, and expenses associated with supply chain restructuring, and
◦higher employee expenses, higher distribution costs due primarily to increased third party logistics and warehouse costs, the inclusion of HRA Pharma expenses, partially offset by cost savings related to restructuring initiatives and favorable foreign currency translation.
Six Month Comparison
| Six Months Ended | |||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||
| Net sales | $ | 2,196.2 | $ | 1,991.1 | |||||||||||||||||||
| Gross profit | $ | 709.9 | $ | 717.4 | |||||||||||||||||||
| Gross profit % | 32.3 | % | 36.0 | % | |||||||||||||||||||
| Operating income (loss) | $ | 14.8 | $ | (74.6) | |||||||||||||||||||
| Operating income (loss) % | 0.7 | % | (3.7) | % | |||||||||||||||||||
53
Perrigo Company plc - Item 2
Consolidated
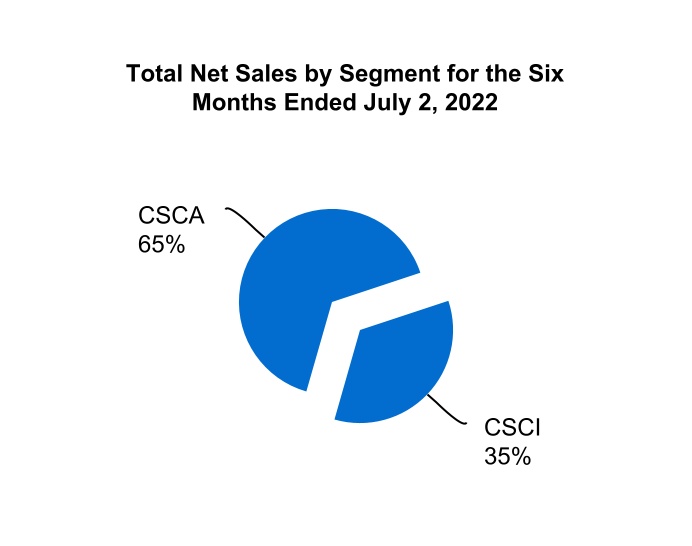
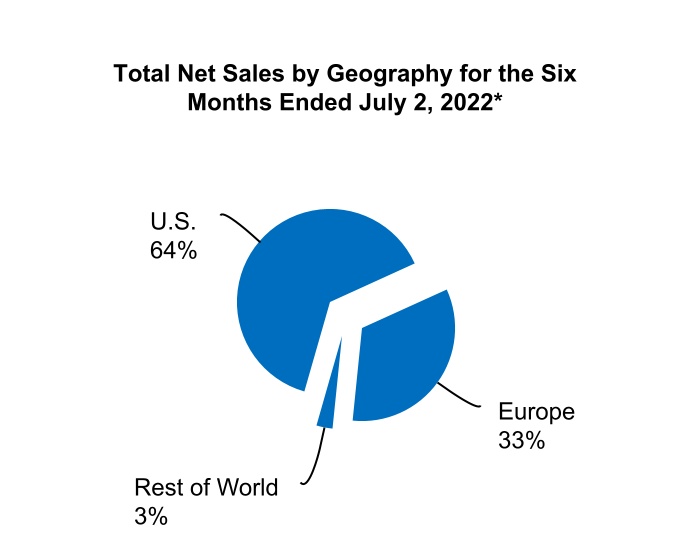
* Total net sales by geography is derived from the location of the entity that sells to a third party.
Six Months Ended July 2, 2022 vs. Six Months Ended July 3, 2021
Net sales increased $205.1 million, or 10.3% due primarily to:
•$261.7 million increase, or 13.3% due primarily to a $120.6 million increase in cough/cold sales resulting from higher incidences of cough/cold and flu-like illnesses globally, an increase of $64.3 million in U.S. Nutrition business stemming from store brand infant formula share gains due partly to a competitor recall, an increase of $70.8 million from the addition of contract manufacturing sales to the divested RX business, and positive pricing, partially offset by net sales declines in certain other product categories; and
•$57.8 million increase from the addition of HRA Pharma; partially offset by
•$29.6 million decrease from divestitures of Latin America businesses and the ScarAway® brand asset; and
•$84.8 million decrease from unfavorable foreign currency translation.
Operating income increased $89.4 million, or 119.8%, due primarily to:
•$7.5 million decrease in gross profit due primarily to $42.3 million of unfavorable foreign currency translation, cost of goods sold inflation and increased freight expenses (which were partially offset by pricing benefits), lower operating efficiencies, an increase in customer service penalties, and divestitures of Latin America businesses and ScarAway®, partially offset by higher gross profit flow-through resulting from higher sales volumes, and $27.8 million from the addition of HRA Pharma. Gross profit as a percentage of net sales decreased 370 basis points compared to the prior year due to the same factors that drove gross profit, and from the addition of third party sales to the divested RX business.
•$96.9 million decrease in operating expenses due primarily to:
◦The absence of $158.6 million of prior year impairment charges on goodwill and assets related to the divested Latin America businesses, partially offset by
◦an increase in consulting and other expenses related to the acquisition of HRA Pharma, a divestiture gain recognized in the prior year, a litigation settlement, and expenses associated with supply chain restructuring, and
◦higher employee expenses, higher distribution costs due primarily to increased third party logistics and warehouse costs, higher planned advertising and promotion expenses to support brand growth, the inclusion of HRA Pharma expenses, partially offset by cost savings related to restructuring initiatives, favorable foreign currency translation and a gain on the sale of ScarAway®.
54
Perrigo Company plc - Item 2
CSCA
CONSUMER SELF-CARE AMERICAS
Recent Trends and Developments
•Currently, most of the markets in which we operate have relaxed COVID-19 related restrictions and have returned to in-person activities, leading to normalizing incidence levels of factors that may impact sales of our products, including cough, cold and flu-like illnesses. For instance, we experienced higher demand in the first half of 2022 for our Upper Respiratory and Pain and Sleep-Aids categories relative to the first half of 2021 when there was very low incidence of cough, cold and flu-like illness, which we attributed to COVID-19 social distancing and mask requirements. We expect consumer purchasing patterns to continue to normalize over the long-term but such patterns may be less predictable and may be impacted by the duration and severity of COVID-19 in the short-term. Refer to "Impact of COVID-19 Pandemic" above.
•Gross margin has been negatively impacted by significant inflation and disruption costs in our supply chain, and more recently less availability in our labor markets and wage rate inflation. Starting in the second half of 2021, supply chain disruptions, including a lack of truck drivers in the U.S. and record delays at global shipping ports, led to higher unfulfilled customer orders and higher input costs compared to the prior year. We took a series of actions to improve the situation, including reconfiguring our distribution system for short-term shipments, outsourcing highly complex product lines to a third party logistic provider, adding regional carriers for challenged shipping lanes, hiring additional distribution center personnel, and increasing the purchase cycle as it relates to the manufacturing process. Subsequent ongoing supply chain disruption resulting from the COVID-19 pandemic and Russian war in Ukraine has driven prices higher for many of the cost of goods sold items and commodities we procure. Additionally, limited labor market availability has become more challenging with higher vacancies and increased attrition, driving the need for higher compensation, such as wage rate increases and other retention benefits. As such, we implemented additional pricing and procurement actions, which offset the negative gross profit dollar impact in the second quarter of 2022. Continued benefits from our actions are anticipated to substantially offset inflationary pressures in the second half of 2022, however the duration and extent of inflation pressure, including impacts from the Russian war in Ukraine, labor market availability and wage rates, as well as the acceptance of pricing actions in the markets we operate, is uncertain.
Segment Financial Results
Three Month Comparison
| Three Months Ended | |||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||
| Net sales | $ | 728.0 | $ | 622.3 | |||||||||||||||||||
| Gross profit | $ | 192.3 | $ | 187.3 | |||||||||||||||||||
| Gross profit % | 26.4 | % | 30.1 | % | |||||||||||||||||||
| Operating income (loss) | $ | 86.3 | $ | (72.0) | |||||||||||||||||||
| Operating income (loss) % | 11.9 | % | (11.6) | % | |||||||||||||||||||
55
Perrigo Company plc - Item 2
CSCA
Three Months Ended July 2, 2022 vs. Three Months Ended July 3, 2021
Net sales increased $105.7 million, or 17.0%, due primarily to:
•a strong rebound in categories that were negatively impacted by the reduced COVID-19 pandemic-related consumer demand in the prior year (including cough, cold and flu related products), pricing actions, share gains and benefits from a competitor recall in the Nutrition segment, the addition of HRA Pharma, the addition of contract manufacturing sales to the divested RX business, and new products sales, partially offset by divestitures of Latin America businesses and the ScarAway® brand asset.
| Sales | Three Months Ended | ||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 2, 2021 | $ Change | % Change | |||||||||||||||||||
| Upper Respiratory | $ | 145.9 | $ | 105.3 | $ | 40.6 | 38.6 | % | |||||||||||||||
| Nutrition | 125.1 | 95.8 | 29.3 | 30.6 | % | ||||||||||||||||||
| Digestive Health | 125.1 | 115.0 | 10.1 | 8.8 | % | ||||||||||||||||||
| Pain and Sleep-Aids | 102.6 | 89.4 | 13.2 | 14.8 | % | ||||||||||||||||||
| Oral Care | 76.6 | 75.7 | 0.9 | 1.2 | % | ||||||||||||||||||
| Healthy Lifestyle | 67.3 | 64.7 | 2.6 | 4.0 | % | ||||||||||||||||||
| Skin Care | 48.6 | 47.1 | 1.5 | 3.2 | % | ||||||||||||||||||
| Women's Health | 12.0 | 8.1 | 3.9 | 48.1 | % | ||||||||||||||||||
| Vitamins, Minerals, and Supplements ("VMS") | 8.2 | 8.4 | (0.2) | (2.4) | % | ||||||||||||||||||
| Other CSCA | 16.6 | 12.8 | 3.8 | 29.7 | % | ||||||||||||||||||
| Total CSCA | $ | 728.0 | $ | 622.3 | $ | 105.7 | 17.0% | ||||||||||||||||
Global product category reporting was updated in the second quarter of 2022 and results were adjusted retrospectively to reflect the changes. Refer to Item 1. Note 2.
Sales drivers in each category are provided below:
•Upper Respiratory: Net sales of $145.9 million increased 38.6% due primarily to higher incidences of cough/cold and flu-like illnesses that led to strong demand, online and in-store, for cough/cold-related products. Sales of allergy products were particularly strong, despite lower levels of incidence compared to the year ago period, due primarily to increased promotions at a particular customer;
•Nutrition: Net sales of $125.1 million increased 30.6% due primarily to strong growth in U.S. store brand and contract manufacturing for national brands infant formula stemming from share gains and a competitor recall, new product launches within infant formula and continued growth in the oral electrolytes business;
•Digestive Health: Net sales of $125.1 million increased 8.8% due primarily to growth in store brand proton pump inhibitor products, including the store brand versions of Esomeprazole and Omeprazole, driven by share gains, in addition to growth in store brand versions of Famotidine;
•Pain and Sleep-aids: Net sales of $102.6 million increased 14.8% due primarily to strong demand, online and in-store, for analgesics products stemming from higher incidences of cough/cold and flu-like illnesses, including COVID-19;
•Oral Care: Net sales of $76.6 million increased 1.2% due primarily to increased sales of store brand products, particularly non-power toothbrushes, mostly offset by a decline in branded offerings stemming from delayed receipt of products manufactured outside the U.S., leading to unfulfilled customer orders;
•Healthy Lifestyle: Net sales of $67.3 million increased 4.0% due primarily to increased distribution of store brand smoking cessation products;
•Skin Care: Net sales of $48.6 million increased 3.2% due primarily to the addition of HRA Pharma brands, including Mederma® and Compeed®, partially offset by the divested ScarAway® brand asset;
•Women's Health: Net sales of $12.0 million increased 48.1% due primarily to the addition of HRA Pharma brands, including ellaOne®; and
56
Perrigo Company plc - Item 2
CSCA
•VMS and Other: Net sales of $24.8 million increased 17.0% due primarily to contract manufacturing sales to the divested RX business in the Other category.
Operating income increase of $158.3 million, or 219.9%, due primarily to:
•$5.0 million increase in gross profit due to gross profit flow-through resulting from higher sales volumes and pricing benefits, plus the addition of HRA Pharma; partially offset by cost of goods sold inflation and increased freight expenses, lower operating efficiencies, and divestitures of Latin America businesses and ScarAway®. Gross profit as a percentage of net sales decreased 370 basis points compared to the prior year due to the addition of third party sales to the divested RX business, partially offset by the same factors that drove gross profit. Despite continued challenges in our supply chain, gross profit as a percentage of sales improved sequentially by 210 basis points over the first quarter of 2022 from benefits of pricing actions and a decrease in customer service penalties.
•$153.3 million decrease in operating expenses due primarily to the absence of $158.6 million of prior year impairment charges on goodwill and assets related to the divested Latin America businesses, partially offset by higher distribution costs due primarily to increased third party logistics and warehouse costs and a litigation settlement.
Six Month Comparison
| Six Months Ended | |||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||
| Net sales | $ | 1,438.0 | $ | 1,262.8 | |||||||
| Gross profit | $ | 364.8 | $ | 381.8 | |||||||
| Gross profit % | 25.4 | % | 30.2 | % | |||||||
| Operating income | $ | 164.8 | $ | 23.6 | |||||||
| Operating income % | 11.5 | % | 1.9 | % | |||||||
Six Months Ended July 2, 2022 vs. Six Months Ended July 3, 2021
Net sales increased $175.2 million, or 13.9%, due primarily to:
•a strong rebound in categories that were negatively impacted by the reduced COVID-19 pandemic-related consumer demand in the prior year (including cough, cold and flu related products), pricing actions, share gains and a competitor recall in the Nutrition segment, the recognition of contract manufacturing sales to the now-divested RX business, the addition of HRA Pharma, and new products sales.
57
Perrigo Company plc - Item 2
CSCA
| Sales | Six Months Ended | ||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | $ Change | % Change | |||||||||||||||||||
| Upper Respiratory | $ | 298.7 | $ | 223.9 | $ | 74.8 | 33.4 | % | |||||||||||||||
| Nutrition | 252.3 | 188.0 | 64.3 | 34.2 | % | ||||||||||||||||||
| Digestive Health | 243.7 | 233.4 | 10.3 | 4.4 | % | ||||||||||||||||||
| Pain and Sleep-Aids | 205.5 | 184.5 | 21.0 | 11.4 | % | ||||||||||||||||||
| Oral Care | 147.0 | 150.7 | (3.7) | (2.5) | % | ||||||||||||||||||
| Healthy Lifestyle | 134.9 | 140.9 | (6.0) | (4.3) | % | ||||||||||||||||||
| Skin Care | 89.5 | 92.7 | (3.2) | (3.5) | % | ||||||||||||||||||
| Women's Health | 20.2 | 18.3 | 1.9 | 10.4 | % | ||||||||||||||||||
| VMS | 15.9 | 16.2 | (0.3) | (1.9) | % | ||||||||||||||||||
| Other CSCA | 30.3 | 14.2 | 16.1 | 113.4 | % | ||||||||||||||||||
| Total CSCA | $ | 1,438.0 | $ | 1,262.8 | $ | 175.2 | 13.9% | ||||||||||||||||
Global product category reporting was updated in the second quarter of 2022 and results were adjusted retrospectively to reflect the changes. Refer to Item 1. Note 2.
Sales in each category were driven primarily by:
•Upper Respiratory: Net sales of $298.7 million increased 33.4% due primarily to higher incidences of cough/cold and flu-like illnesses that led to strong demand, online and in-store, for cough/cold products, and demand for allergy products;
•Nutrition: Net sales of $252.3 million increased 34.2% due primarily to strong growth in U.S. store brand infant formula stemming from share gains and a competitor recall, new product launches within infant formula and continued growth in the oral electrolytes business;
•Digestive Health: Net sales of $243.7 million increased 4.4% due primarily to growth in store brand proton pump inhibitor products and e-commerce;
•Pain and Sleep-Aids: Net sales of $205.5 million increased 11.4% due primarily to strong demand for analgesics products stemming from higher incidences of cough/cold and flu-like illnesses, including COVID-19;
•Oral Care: Net sales of $147.0 million decreased 2.5% due primarily to delayed receipt of products manufactured outside the U.S., leading to unfulfilled customer orders;
•Healthy Lifestyle: Net sales of $134.9 million decreased 4.3% due primarily to the discontinuation of diabetes products;
•Skin Care: Net sales of $89.5 million decreased 3.5% due primarily to the divested ScarAway® brand asset, partially offset by the addition of HRA Pharma, including Mederma® and Compeed®;
•Women's Health: Net sales of $20.2 million increased 10.4% due primarily to the addition of HRA Pharma, including ella®; and
•VMS and Other: Net sales of $46.2 million increased 52.0% due primarily to contract manufacturing sales to the divested RX business in the Other category.
58
Perrigo Company plc - Item 2
CSCA
Operating income increased $141.2 million, or 598.3%, due primarily to:
•$17.0 million decrease in gross profit due primarily to cost of goods sold inflation and increased freight expenses, lower operating efficiencies, an increase in customer service penalties, and divestitures of Latin America businesses and ScarAway®, which were partially offset by higher gross profit flow-through resulting from higher sales volumes, pricing benefits, and the addition of HRA Pharma. Gross profit as a percentage of net sales decreased 480 basis points compared to the prior year due to the addition of third party sales to the divested RX business and the same factors that drove gross profit.
•$158.2 million decrease in operating expenses due primarily to the absence of $158.6 million of prior year impairment charges on goodwill and assets related to the divested Latin America businesses. Additionally, higher distribution costs due primarily to increased third party logistics and warehouse costs and costs from a litigation settlement were largely offset by a gain on the sale of ScarAway®, and lower R&D expenses.
CONSUMER SELF-CARE INTERNATIONAL
Recent Trends and Developments
•Currently, most of the markets in which we operate have relaxed COVID-19 related restrictions and have returned to in-person activities, leading to higher incidences of factors that may impact sales of our products, including cough, cold and flu-like illnesses. For instance, we experienced higher demand in the first half of 2022 for our Upper Respiratory and Pain and Sleep-Aids categories relative to the first half of 2021 when there was very low incidence of cough, cold and flu-like illness, which we attributed to COVID-19 social distancing and mask requirements. We expect consumer purchasing patterns to continue to normalize over the long-term but such patterns may be less predictable and may be impacted by the duration and severity of COVID-19 in the short-term. Refer to "Impact of COVID-19 Pandemic" above.
•The Russia invasion of Ukraine and resulting economic and political sanctions imposed by the United States, United Kingdom, European Union, and other countries on Russia, Belarus, and occupied regions in Ukraine has negatively impacted our results of operations in the region. For the year ended December 31, 2021, these countries included approximately $27 million of net sales, $15 million of gross profit, and $8 million of operating income. In March 2022, we halted all sales to distributors in Russia and sales in Ukraine have been severely depressed. While there is a possibility that sales levels may rebound in Ukraine, they are not likely to fully materialize in the short-term. Refer to "War in Ukraine" above.
•Supply chain inflation and labor market availability and wage rates are driving higher input costs compared to the prior year. As such, we implemented a series of actions to improve the situation, including pricing and procurement actions, which offset the negative gross profit dollar impact in the second quarter of 2022; however, the duration and extent of inflation pressure, including impacts from the Russian war in Ukraine, labor market availability and wage rates, as well as the acceptance of pricing actions in the markets we operate, is uncertain.
Segment Financial Results
Three Month Comparison
| Three Months Ended | |||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||||||||||||||
| Net sales | $ | 393.7 | $ | 358.8 | |||||||||||||||||||
| Gross profit | $ | 179.8 | $ | 161.7 | |||||||||||||||||||
| Gross profit % | 45.7 | % | 45.1 | % | |||||||||||||||||||
| Operating income | $ | 1.5 | $ | 1.3 | |||||||||||||||||||
| Operating income % | 0.4 | % | 0.4 | % | |||||||||||||||||||
59
Perrigo Company plc - Item 2
CSCI
Three Months Ended July 2, 2022 vs. Three Months Ended July 3, 2021
Net sales increased $34.9 million, or 9.7%, due primarily to:
•$86.0 million, or 24.0%, increase driven primarily by the addition of $48.1 million from HRA Pharma, a strong rebound in categories that were negatively impacted by the reduced COVID-19 pandemic-related consumer demand in the prior year, pricing actions and new products sales; partially offset by
•$51.1 million decrease from unfavorable foreign currency translation.
| Sales | Three Months Ended | ||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | $ Change | % Change | |||||||||||||||||||
| Skin Care | $ | 120.3 | $ | 98.7 | $ | 21.6 | 21.9 | % | |||||||||||||||
| Upper Respiratory | 59.8 | 42.4 | 17.4 | 41.0 | % | ||||||||||||||||||
| Pain and Sleep-Aids | 48.8 | 48.6 | 0.2 | 0.4 | % | ||||||||||||||||||
| VMS | 47.5 | 53.9 | (6.4) | (11.9) | % | ||||||||||||||||||
| Healthy Lifestyle | 38.5 | 46.7 | (8.2) | (17.6) | % | ||||||||||||||||||
| Oral Care | 20.6 | 22.5 | (1.9) | (8.4) | % | ||||||||||||||||||
| Women's Health | 24.4 | 14.4 | 10.0 | 69.4 | % | ||||||||||||||||||
| Digestive Health | 4.8 | 4.9 | (0.1) | (2.0) | % | ||||||||||||||||||
| Other CSCI | 29.0 | 26.7 | 2.3 | 8.6 | % | ||||||||||||||||||
| Total CSCI | $ | 393.7 | $ | 358.8 | $ | 34.9 | 9.7 | % | |||||||||||||||
Global product category reporting was updated in the second quarter of 2022 and results were adjusted retrospectively to reflect the changes. Refer to Item 1. Note 2.
Sales in each category were driven primarily by:
•Skin Care: Net sales of $120.3 million increased 21.9%, inclusive of a 17.3% negative effect of currency translation, driven primarily by the addition of HRA Pharma brands, including Compeed®, strong performance in the Sebamed and ACO skincare lines, and increased net sales of anti-parasite offerings, due to the easing of COVID-19-related restrictions. These benefits were partially offset by lower sales in Ukraine and Russia;
•Upper Respiratory: Net sales of $59.8 million increased 41.0%, inclusive of a 18.0% negative effect of currency translation, as the higher incidences of cough/cold and flu-like illnesses led to strong demand for cough/cold products, including the Bronchonolo, Bronchostop and Coldrex brands and U.K. store brands. In addition, a relatively stronger hayfever season drove strong performance in allergy related products, particularly the Beconase brand in the U.K;
•Pain & Sleep-Aids: Net sales of $48.8 million increased 0.4%, inclusive of a 12.4% negative effect of currency translation, due primarily to higher demand for Solpadeine, a paracetamol-based analgesics product, as well as an increase in U.K. store brand consumption within the category. These increases were driven primarily by strong demand for analgesics products stemming from higher incidences of cough/cold and flu-like illnesses, including COVID-19;
•VMS: Net sales of $47.5 million decreased 11.9%, inclusive of a 11.5% negative effect of currency translation, due primarily to lower overall category consumption and lower sales of Probify in certain geographies, partially offset by the restocking of the Abtei brand in Germany following the third quarter 2021 recall of certain batches;
•Healthy Lifestyle: Net sales of $38.5 million decreased 17.6%, inclusive of a 9.9% negative effect of currency translation, due primarily to lower net sales in the XLS Medical weight management franchise stemming from lower category consumption, partially offset by strong performance of NiQuitin smoking cessation product sales due to customer restocking following supply constraints earlier in the year;
•Women's Health: Net sales of $24.4 million increased 69.4%, inclusive of a 11.9% negative effect of currency translation, due primarily to the addition of HRA Pharma brands, including ellaOne® and NorLevo®; and
60
Perrigo Company plc - Item 2
CSCI
•Digestive Health, Oral Care and Other: Net sales of $54.4 million increased 0.6%, inclusive of a 14.4% negative effect of currency translation, due primarily to the addition of the HRA Pharma's Rare Diseases portfolio in the Other category.
Operating income increased $0.2 million, or 15.4%, due primarily to:
•$18.1 million increase in gross profit due primarily to higher gross profit flow-through resulting from higher sales volumes, pricing actions, and the addition of HRA Pharma, partially offset by unfavorable foreign currency translation, higher freight and input costs, and negative impact from the Russian war in Ukraine. Gross profit as a percentage of net sales increased 60 basis points due primarily to the same factors that drove gross profit.
•$17.9 million increase in operating expenses due primarily to higher employee expenses and the addition of HRA Pharma, partially offset by favorable foreign currency translation.
Six Month Comparison
| Six Months Ended | |||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | |||||||||
| Net sales | $ | 758.2 | $ | 728.3 | |||||||
| Gross profit | $ | 345.1 | $ | 335.6 | |||||||
| Gross profit % | 45.5 | % | 45.9 | % | |||||||
| Operating income | $ | 17.7 | $ | 18.8 | |||||||
| Operating income % | 2.3 | % | 2.6 | % | |||||||
Six Months Ended July 2, 2022 vs. Six Months Ended July 3, 2021
Net sales increased $29.9 million, or 4.1%, due to:
•$114.7 million, or 15.7%, net increase driven primarily by the addition of $48.1 million from HRA Pharma, a strong rebound in categories that were negatively impacted by the reduced COVID-19 pandemic-related consumer demand in the prior year, pricing actions and new products sales; partially offset by
•$84.8 million decrease from unfavorable foreign currency translation.
61
Perrigo Company plc - Item 2
CSCI
| Sales | Six Months Ended | ||||||||||||||||||||||
| (in millions, except percentages) | July 2, 2022 | July 3, 2021 | $ Change | % Change | |||||||||||||||||||
| Skin Care | $ | 209.7 | $ | 190.1 | $ | 19.6 | 10.3 | % | |||||||||||||||
| Upper Respiratory | 121.4 | 85.1 | 36.3 | 42.7 | % | ||||||||||||||||||
| VMS | 100.2 | 116.6 | (16.4) | (14.1) | % | ||||||||||||||||||
| Pain and Sleep-Aids | 101.8 | 97.7 | 4.1 | 4.2 | % | ||||||||||||||||||
| Healthy Lifestyle | 80.4 | 97.4 | (17.0) | (17.5) | % | ||||||||||||||||||
| Oral Care | 44.9 | 47.5 | (2.6) | (5.5) | % | ||||||||||||||||||
| Digestive Health | 9.2 | 9.8 | (0.6) | (6.1) | % | ||||||||||||||||||
| Women's Health | 37.4 | 28.7 | 8.7 | 30.3 | % | ||||||||||||||||||
| Other CSCI | 53.2 | 55.4 | (2.2) | (4.0) | % | ||||||||||||||||||
| Total CSCI | $ | 758.2 | $ | 728.3 | $ | 29.9 | 4.1 | % | |||||||||||||||
Global product category reporting was updated in the second quarter of 2022 and results were adjusted retrospectively to reflect the changes. Refer to Item 1. Note 2.
Sales in each category were driven primarily by:
•Skin Care: Net sales of $209.7 million increased 10.3%, inclusive of a 15.6% negative effect of currency translation, driven primarily by the addition of HRA Pharma sales, strong performance in the Sebamed and ACO skincare lines, and increased net sales of anti-parasite products;
•Upper Respiratory: Net sales of $121.4 million increased 42.7%, inclusive of a 13.7% negative effect of currency translation, as the higher incidences of cough/cold and flu-like illnesses led to strong demand for cough/cold products in addition to strong performance in allergy related products;
•VMS: Net sales of $100.2 million decreased 14.1%, inclusive of a 8.4% negative effect of currency translation, due primarily to lower category consumption and lower sales of Probify in certain markets;
•Pain & Sleep-Aids: Net sales of $101.8 million increased 4.2%, inclusive of a 8.7% negative effect of currency translation, due primarily to higher demand for Solpadeine and an increase in U.K. store brand sales within the category;
•Healthy Lifestyle: Net sales of $80.4 million decreased 17.5%, inclusive of a 7.4% negative effect of currency translation, due primarily to lower net sales in the XLS Medical weight management franchise stemming from lower category consumption;
•Women's Health: Net sales of $37.4 million increased 30.3%, inclusive of a 10.5% negative effect of currency translation, due primarily to the addition of HRA Pharma sales; and
•Digestive Health, Oral Care and Other: Net sales of $107.3 million decreased 4.8%, inclusive of a 13.1% negative effect of currency translation, due primarily to the addition of HRA Pharma's Rare Diseases portfolio in the Other category, partially offset by lower sales of distribution brands.
Operating income decreased $1.1 million, or 5.9%, due primarily to:
•$9.5 million increase in gross profit due primarily to higher gross profit flow-through resulting from higher sales volumes, pricing actions, and the addition of HRA Pharma, partially offset by unfavorable foreign currency translation, higher freight and input costs, and negative impact from the Russian war in Ukraine. Gross profit as a percentage of net sales decreased 40 basis points due primarily to less favorable product mix and lower operational productivity due to inflationary pressures; and
•$10.6 million increase in operating expenses due primarily to the addition of HRA Pharma, higher employee expenses, higher advertising and promotion investments to support brand growth, and a divestiture gain recognized in the prior year, partially offset by favorable foreign currency translation.
62
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
Unallocated Expenses
Unallocated expenses are comprised of certain corporate services not allocated to our reporting segments and are recorded in Operating income on the Condensed Consolidated Statements of Operations. Unallocated expenses were as follows (in millions):
| Three Months Ended | Six Months Ended | |||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||
| $ | 94.7 | $ | 55.2 | $ | 167.7 | $ | 117.0 | |||||||||||||
The increase of $39.5 million in unallocated expenses during the three months ended July 2, 2022 compared to the prior year period was due primarily to an increase in consulting and other fees related to the acquisition of HRA Pharma, higher employee expenses, and expenses associated with supply chain restructuring. This was partially offset by cost savings related to restructuring initiatives.
The increase of $50.7 million in unallocated expenses during the six months ended July 2, 2022 compared to the prior year period was due primarily to an increase in consulting and related acquisition fees, share-based compensation, a loss on disposal of a fixed asset, and expenses associated with supply chain restructuring. This was partially offset by lower legal expenses and cost savings related to restructuring initiatives.
Interest expense, net, and Other (income) expense, net
| Three Months Ended | Six Months Ended | ||||||||||||||||||||||
| (in millions) | July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||||
| Interest expense, net | $ | 38.3 | $ | 31.6 | $ | 74.1 | $ | 63.6 | |||||||||||||||
| Other (income) expense, net | $ | 53.8 | $ | (0.4) | $ | 52.7 | $ | 1.9 | |||||||||||||||
| Loss on extinguishment of debt | $ | 9.3 | $ | — | $ | 9.3 | $ | — | |||||||||||||||
Interest Expense, Net
The $6.7 million increase during the three months ended July 2, 2022, compared to the prior year period was due primarily to an increase in interest expense associated with the interest rates on our New Senior Secured Credit Facilities and associated interest rate swaps.
The $10.5 million increase for the six months ended July 2, 2022, compared to the prior year period was due primarily to an increase in interest expense associated with our New Senior Secured Credit Facilities and associated interest rate swaps, an increase in interest expense from a step up in interest rate on our 3.150% Senior Notes due 2030 from 3.150% to 3.900% during the fourth quarter of 2021, as well as our cross currency swaps and interest rate swap termination expenses.
Other (Income) Expense, Net
The $54.2 million increase in expense during the three months ended July 2, 2022 compared to the prior year period and the $50.8 million increase in expense during the six months ended July 2, 2022 compared to the prior year period was due primarily to unfavorable changes in revaluation of foreign currency expense associated with the acquisition of HRA Pharma and termination expense of the forward currency options related to the acquisition of HRA Pharma.
Loss on extinguishment of debt
The $9.3 million loss on extinguishment of debt expense during the three months ended July 2, 2022 consisted of the write-off of certain new and previously deferred financing fees and make whole payments on the redeemed 4.00% Senior Notes due 2023 (refer to Item 1. Note 11).
63
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
Income Taxes (Consolidated)
The effective tax rates were as follows:
| Three Months Ended | Six Months Ended | |||||||||||||||||||
| July 2, 2022 | July 3, 2021 | July 2, 2022 | July 3, 2021 | |||||||||||||||||
| 40.1 | % | 28.8 | % | 45.4 | % | 22.1 | % | |||||||||||||
The effective tax rate on the pre-tax loss for the three and six months ended July 2, 2022, increased compared to the effective tax rate on the pre-tax loss for the three and six months ended July 3, 2021, primarily due to the tax benefit of the loss on sale of our Latin America businesses and the non-deductibility of certain costs related to the acquisition of HRA Pharma for tax purposes. The increase in the effective tax rate was offset, in part, by income tax expense associated with internal legal entity restructuring recognized for the six months ended July 2, 2022, and the net income tax expense on intra-entity transfers of intellectual property recognized for the three and six months ended July 3, 2021.
FINANCIAL CONDITION, LIQUIDITY, AND CAPITAL RESOURCES
We finance our operations with internally generated funds, supplemented by credit arrangements with third parties and capital market financing. We routinely monitor current and expected operational requirements and financial market conditions to evaluate other available financing sources including term and revolving bank credit and securities offerings. In determining our future capital requirements, we regularly consider, among other factors, known trends and uncertainties, such as rising inflation rates, as well as the NOPAs from the IRS, the current COVID-19 pandemic, and other contingencies. We note that no payment of the additional amounts proposed by the IRS in the NOPAs is currently required, and no such payment is expected to be required, unless and until a settlement or other final determination of the matter is reached that is adverse to us (refer to Item 1. Note 14 for additional information on the NOPAs).
Based on the foregoing, management believes that our operations and borrowing resources are sufficient to provide for our short-term and long-term capital requirements, as described below. However, an adverse result with respect to our appeal of any material outstanding tax assessments or litigation, including securities or drug pricing matters and product liability cases, damages resulting from third-party claims, and related interest and/or penalties, could ultimately require the use of corporate assets to pay such assessments and any such use of corporate assets would limit the assets available for other corporate purposes. As such, we continue to evaluate the impact of the above factors on liquidity and may determine that modifications to our capital structure are appropriate if market conditions deteriorate, favorable capital market opportunities become available, or any change in conditions relating to the NOPAs, the COVID-19 pandemic or other contingencies have a material impact on our capital requirements.
Cash and Cash Equivalents
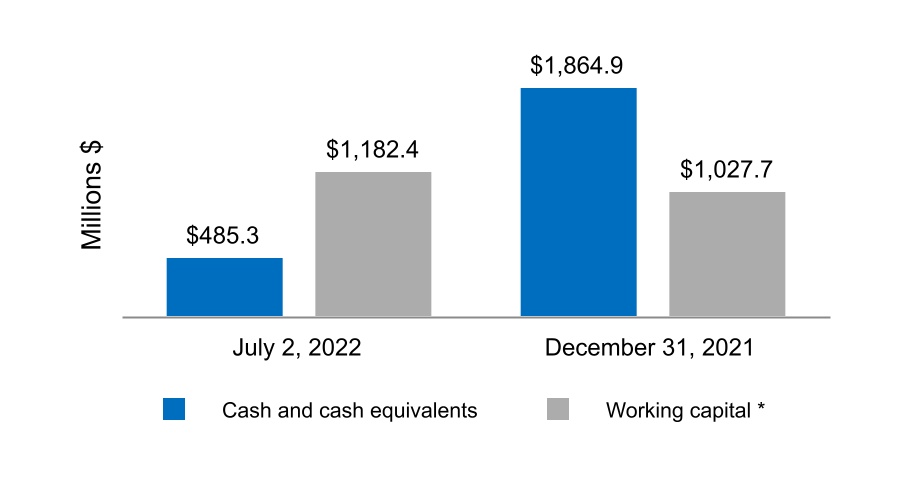
* Working capital represents current assets less current liabilities, excluding cash and cash equivalents, assets and liabilities held for sale, and excluding current indebtedness.
64
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Cash, cash equivalents, cash flows from operations, and borrowings available under our credit facilities are expected to be sufficient to finance our liquidity and capital expenditures in both the short and long term. Although our lenders have made commitments to make funds available to us in a timely fashion under our revolving credit agreements and overdraft facilities, if economic conditions worsen, including due to the COVID-19 pandemic, or new information becomes publicly available impacting the institutions’ credit rating or capital ratios, these lenders may be unable or unwilling to lend money pursuant to our existing credit facilities. Should our outlook on liquidity requirements change substantially from current projections, we may seek additional sources of liquidity in the future.
Cash Generated by (Used in) Operating Activities
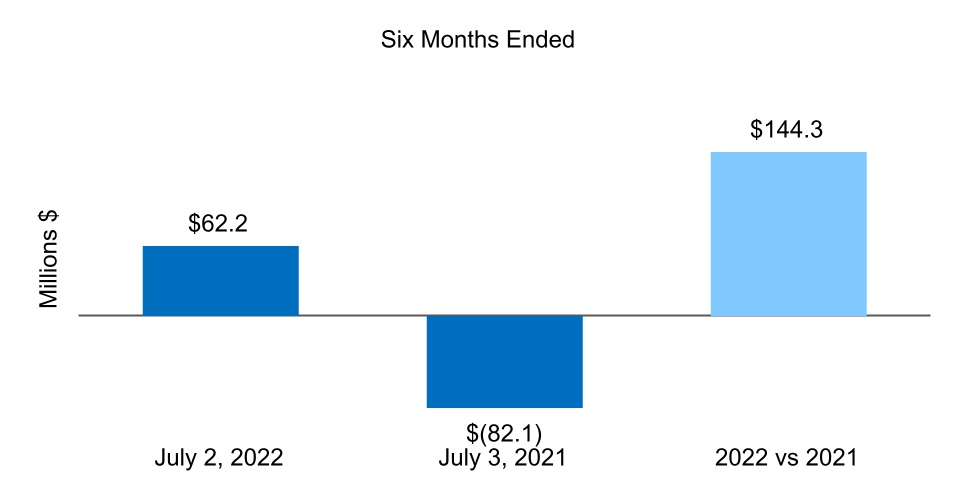
The $144.3 million increase in operating cash inflow was due primarily to:
•$86.7 million increase in cash flow from the change in accounts payable, due primarily to the timing of payments and mix of payment terms for the current year and in our discontinued operations in the prior year period;
•$55.5 million increase in cash flow from the change in inventory, due primarily to a higher build-up of inventory levels to support customer demand in the prior year period;
•$51.8 million increase in cash flow from the change in accounts receivable, due primarily to the timing of sales and receipt of payments for the current year and in our discontinued operations business in the prior year period;
•$46.3 million increase in cash flow from the change in accrued liabilities, due primarily to the change in accrued royalties and profit sharing, litigation and our discontinued operations;
•$35.7 million increase in cash flow from the change in accrued income taxes, due primarily to intra-entity IP transfers and settlement of Israeli tax audit in the prior year, partially offset by a reduction in the amount of interest and research and development costs deductible in the U.S. in 2022; and
•$22.3 million increase in cash flow from the change in accrued payroll and related taxes, due primarily to the decrease in annual management and employee bonus payments compared to the prior year period and the timing of payroll; partially offset by
•$171.8 million decrease in cash flow from the change in net earnings after adjustments for items including deferred income taxes, restructuring charges, share-based compensation, amortization of debt premium, loss on sale of businesses, gain on sale of brands, foreign currency remeasurement losses and depreciation and amortization.
65
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Cash Generated by (Used in) Investing Activities
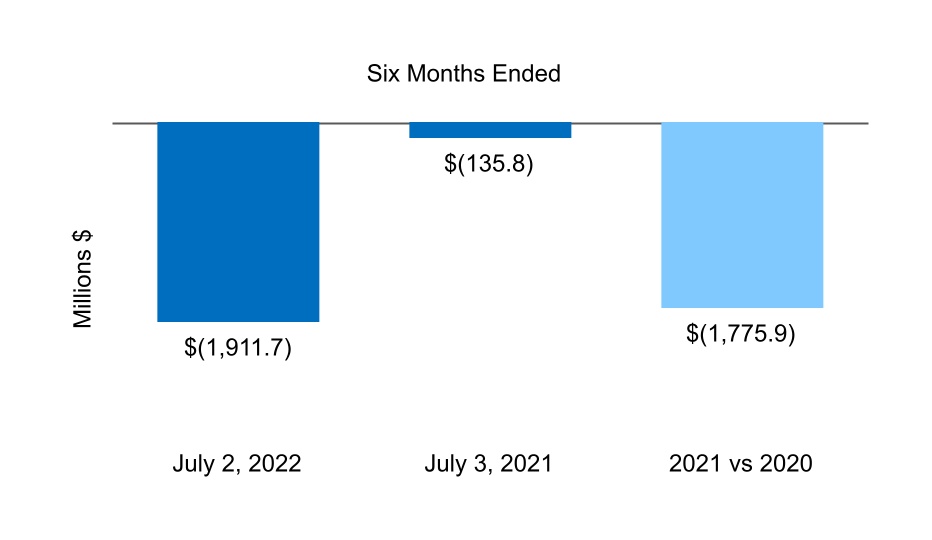
The $1.8 billion decrease in cash from investing cash flow was due primarily to:
•$1.9 billion decrease in cash due to the acquisition of HRA Pharma;
•$37.1 million decrease in cash due to the cash settlement of non-designated foreign currency derivatives we used to hedge the euro-denominated HRA Pharma purchase price; partially offset by
•$60.6 million increase in cash due primarily to the absence of cash paid in prior year for an ANDA for a generic topical gel and an ANDA for a generic topical lotion for $16.4 million and $53.3 million, respectively; partially offset by a $10.0 million milestone payment to the licensor for Nasonex® 24HR Allergy (refer to Item 1. Note 3);
•$58.7 million increase in cash due to the proceeds from the sale of our Latin America businesses and proceeds from an ANDA for a generic topical lotion related to our RX business sale (refer to Item 1. Note 8);
•$24.8 million increase in cash due primarily to the proceeds from the sale of ScarAway® (refer to Item 1. Note 3).
•$19.7 million increase in cash due to the change in capital spending, due primarily to reduced spending as a result of divestitures, and decreased spend related to our Nutrition plant expansion.
Cash Generated by (Used in) Financing Activities
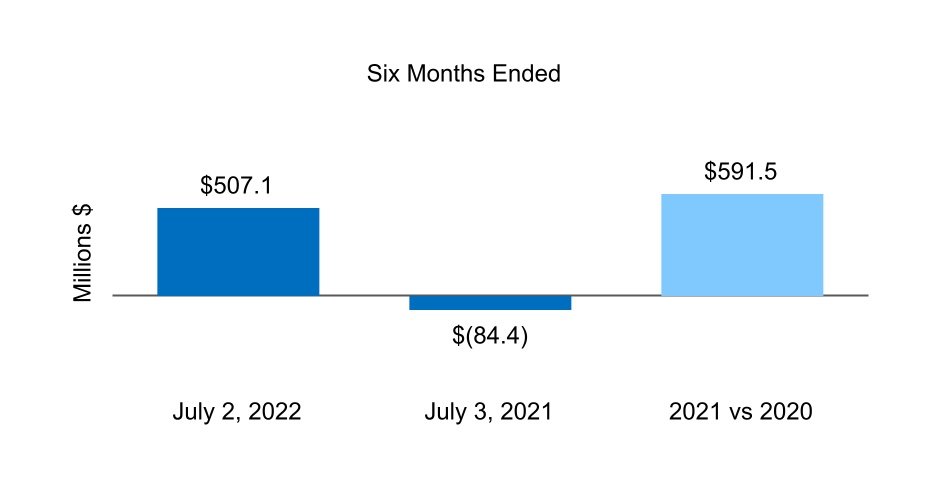
The $591.5 million increase in financing cash flow was due primarily to:
•$1.6 billion increase in cash due to the issuance of our New Senior Secured Credit Facilities; partially offset by
66
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
•$958.9 million decrease in cash due to the payment of our 2019 Term Loan, 4.00% Senior Notes due 2023, and 5.1045% Guaranteed Senior Notes due 2023;
•$19.5 million decrease in cash due to payments for debt issuance costs;
•$12.2 million decrease in cash due to a make-whole premium paid in connection with the redemption of our 4.00% Senior Notes due 2023;
•$6.9 million decrease in cash due to the amount of cash used to settle taxes on behalf of employees; and
•$4.5 million decrease in cash due to an increase in dividend payments.
Dividends
The declaration and payment of dividends, if any, is subject to the discretion of our Board of Directors and will depend on our earnings, financial condition, availability of distributable reserves, capital and surplus requirements, and other factors our Board of Directors may consider relevant.
Share Repurchases
In October 2018, our Board of Directors authorized up to $1.0 billion of share repurchases with no expiration date, subject to the Board of Directors’ approval of the pricing parameters and amount that may be repurchased under each specific share repurchase program. We did not repurchase any shares during the three and six months ended July 2, 2022 or July 3, 2021.
Borrowings and Capital Resources
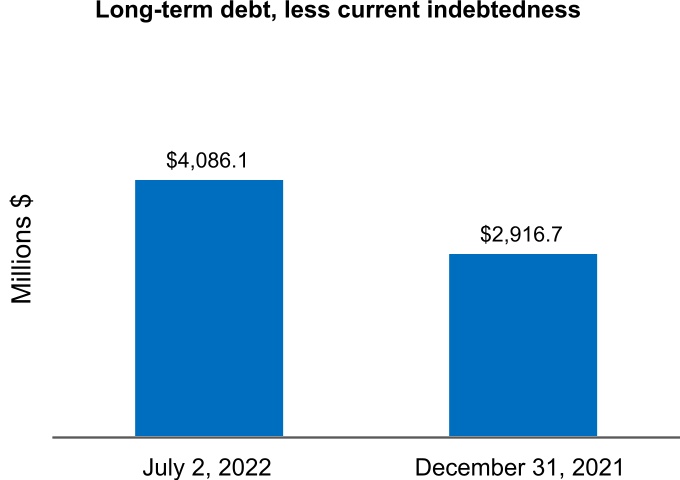
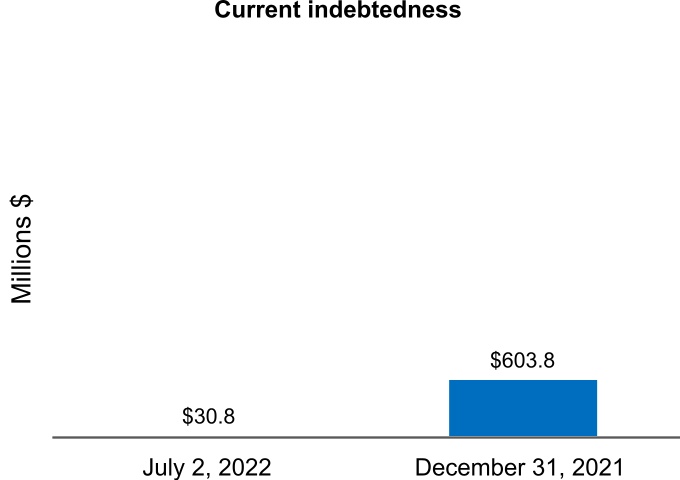
On April 20, 2022, we and our indirect wholly-owned subsidiary, Perrigo Investments, LLC (the "Borrower"), entered into new senior secured credit facilities that consist of (i) a $1.0 billion 2022 Revolver, (ii) a $500 million 2022 Term Loan A Facility, and (iii) a $1.1 billion 2022 Term Loan B Facility, pursuant to a new Term Loan and Revolving Credit Agreement (the "Credit Agreement"). The New Senior Secured Credit Facilities are guaranteed, along with any hedging or cash management obligations entered into with a lender, by us and certain of our wholly-owned subsidiaries organized in the U.S., Ireland, Belgium and the England and Wales (subject to certain exceptions) (the “Guarantor Subsidiaries” and together with the Company, the “Guarantors”). We refer to the Borrower and the Guarantors collectively as the “Loan Parties”.
Revolving Credit Agreements
On April 20, 2022 we terminated the 2018 Revolver and entered into the 2022 Revolver. There were no borrowings outstanding under the 2022 Revolver or 2018 Revolver as of July 2, 2022 or December 31, 2021, respectively.
67
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Term Loans and Notes
As of July 2, 2022, we had $1.1 billion and $500.0 million outstanding under our 2022 Term Loan B Facility and 2022 Term Loan A Facility, respectively. There were no borrowings outstanding under our 2019 Term Loan as of July 2, 2022. We had $600.0 million outstanding under our 2019 Term Loan as of December 31, 2021.
On April 20, 2022, we repaid the prior $600.0 million 2019 Term Loan with the proceeds of the New Senior Secured Credit Facilities. The remaining $500.0 million of proceeds were used to (i) redeem the 4.00% Senior Notes due 2023 on May 19, 2022 and the 5.1045% Guaranteed Senior Notes due 2023, on May 19, 2022 (collectively, the “Redeemed Notes”), (ii) to fund a portion of the cash consideration payable in connection with the acquisition of HRA Pharma (Refer to Item 1. Note 3), and (iii) to pay related fees and expenses. Upon the repayment in full of loans under the 2019 Term Loan and termination of the 2018 Revolver, such facilities were terminated and all guarantees thereunder were released. Upon the redemption of the Redeemed Notes, the guarantees related thereto were released.
The Loan Parties’ obligations under the Credit Agreement are secured, subject to customary permitted liens and other exceptions, by a security interest in all tangible and intangible assets of the Loan Parties, except for certain excluded assets. We may make voluntary prepayments at any time without payment of a premium or penalty, subject to certain exceptions, and are required to make certain mandatory prepayments of outstanding indebtedness under the Credit Agreement in certain circumstances. Principal repayments of the 2022 Term Loan B Facility, which are due quarterly, begin in September 2022 and are equal to 1.0% per annum of the original principal amount of the 2022 Term Loan B Facility incurred with any remaining balance payable on the maturity date. Principal repayments of the 2022 Term Loan A Facility, which are due quarterly, begin in September 2022 and are equal to (i) for the first year anniversary of the Closing Date (as such term is defined in such Credit Agreement), 2.5% per annum of the original principal amount of the 2022 Term Loan A Facility incurred and (ii) after the first year anniversary of the Closing Date (as such term is defined in such Credit Agreement), 5.0% per annum of the original principal amount of the 2022 Term Loan A Facility incurred, with any remaining balance payable on the maturity date. The Credit Agreement contains customary representations and warranties and customary affirmative and negative covenants applicable to the Borrower and its restricted subsidiaries, including, among other things, restrictions on indebtedness, liens, investments, mergers, dispositions, prepayment of junior indebtedness and dividends and other distributions. The Credit Agreement contains financial covenants that require the Borrower and its restricted subsidiaries to (a) not exceed a maximum first lien secured net leverage ratio of 3.00 to 1.00 at the end of each fiscal quarter and (b) not fall below a minimum interest coverage ratio of 3.00 to 1.00 at the end of each fiscal quarter, provided that such covenants apply only to the 2022 Revolver. The Credit Agreement also contains customary events of default relating to, among other things, failure to make payments, breach of covenants and breach of representations. If we consummate certain qualifying acquisitions during the term of the loan, the maximum first lien secured net leverage ratio covenant would increase to 3.25 to 1.00 for such quarter and the three following fiscal quarters thereafter.
We are in compliance with all the covenants under our debt agreements.
Overdraft Facilities
We have overdraft facilities available that we use to support our cash management operations. There were no borrowings outstanding under the facilities as of July 2, 2022 or December 31, 2021.
Leases
We had $228.6 million and $199.1 million of lease liabilities and $231.5 million and $194.8 million of lease assets as of July 2, 2022 and December 31, 2021, respectively.
68
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
Credit Ratings
Due to a credit ratings downgrade by S&P Global Ratings and Moody’s Investor Services in the first quarter of 2022, the interest of the 3.150% Senior Notes due 2030 stepped up from 3.900% to 4.400% on payments made after June 15, 2022. Credit rating agencies review their ratings periodically and, therefore, the credit rating assigned to us by each agency may be subject to revision at any time. Accordingly, we are not able to predict whether current credit ratings will remain as disclosed above. Factors that can affect our credit ratings include changes in operating performance, the economic environment, our financial position, and changes in business strategy. If changes in our credit ratings were to occur, they could impact, among other things, future borrowing costs, access to capital markets, and vendor financing terms. A credit rating is not a recommendation to buy, sell or hold securities.
Guarantor Financial Information
The Guarantor Subsidiaries and the Borrower provide full and unconditional guarantees, jointly and
severally, on a senior unsecured basis, of the 5.300% Notes due 2043 issued by the Company, and the Loan Parties provide full and unconditional guarantees, jointly and severally, on a senior unsecured basis, of the 3.900% Notes due 2024, the 4.375% Notes due 2026, the 4.400% Notes due 2030 and the 4.900% Notes due 2044 issued by Perrigo Finance Unlimited Company.
The guarantees of the Guarantor Subsidiaries, the Company and the Borrower are subject to release in limited circumstances only upon the occurrence of certain customary conditions. The guarantees of the Guarantor Subsidiaries, the Company and the Borrower rank senior in right of payment to any future subordinated indebtedness of the Company, equal in right of payment with all of the Company’s existing and future senior indebtedness and effectively subordinated to any of the Company’s existing and future secured indebtedness, to the extent of the value of the collateral securing such indebtedness.
Basis of Presentation
The following tables include summarized financial information of the obligor groups of debt issued by Perrigo Finance Unlimited Company and Perrigo Company plc. The summarized financial information of each obligor group is presented on a combined basis with balances and transactions within the obligor group eliminated. Investments in and the equity in earnings of non-guarantor subsidiaries, which would otherwise be consolidated in accordance with U.S. GAAP, are excluded from the below summarized financial information pursuant to SEC Regulation S-X Rule 13-01.
The summarized balance sheet information for the consolidated obligor group of debt issued by Perrigo Finance Unlimited Company and Perrigo Company plc is presented in the table below:
| July 2, 2022 | December 31, 2021 | ||||||||||
| Current Assets | $ | 2,033.7 | $ | 3,921.7 | |||||||
| Non-current Assets | $ | 4,884.8 | $ | 5,016.5 | |||||||
| Current liabilities | $ | 695.8 | $ | 1,307.4 | |||||||
| Non-current liabilities | $ | 11,018.9 | $ | 9,672.1 | |||||||
| Due to non-guarantors | $ | 6,330.2 | $ | 6,195.5 | |||||||
69
Perrigo Company plc - Item 2
Financial Condition, Liquidity and Capital Resources
The summarized results of operations information for the consolidated obligor group of debt issued by Perrigo Finance Unlimited Company and Perrigo Company plc is presented in the table below:
| Six Months Ended | Year Ended | ||||||||||
| July 2, 2022 | December 31, 2021 | ||||||||||
| Total Revenues | $ | 1,631.4 | $ | 3,046.2 | |||||||
| Gross Profit | $ | 731.9 | $ | 1,435.9 | |||||||
| Operating Income (loss) | $ | (60.1) | $ | 342.2 | |||||||
| Net Income (loss) | $ | (153.1) | $ | 985.1 | |||||||
| Revenue from non-guarantors | $ | 141.4 | $ | 241.7 | |||||||
| Operating Expenses to non-guarantors | $ | 0.6 | $ | 2.2 | |||||||
| Other (income) expense to non-guarantors | $ | 53.7 | $ | (19.2) | |||||||
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have a material current effect or that are reasonably likely to have a material future effect on our financial condition, changes in financial condition, net sales or expenses, results of operations, liquidity, capital expenditures, or capital resources. We acquire and collaborate on potential products still in development and enter into R&D arrangements with third parties that often require milestone payments to the third-party contingent upon the occurrence of certain future events linked to the success of the asset in development. Milestone payments may be required contingent upon the successful achievement of an important point in the development life cycle of the product. Because of the contingent nature of these payments, they are not included in our table of contractual obligations.
Contractual Obligations and Commitments
There were no material changes in contractual obligations as of July 2, 2022 from those provided in our 2021 Form 10-K, other than as described below.
As a result of changes in our debt structure from the New Senior Secured Credit Facilities, below is a revised schedule of our enforceable and legally binding obligations as of July 2, 2022 related to our short and long-term debt arrangements.
| Payment Due | |||||||||||||||||||||||||||||
2022 (2) | 2023-2024 | 2025-2026 | After 2026 | Total | |||||||||||||||||||||||||
Short and long-term debt (1) | $ | 90.2 | $ | 1,082.5 | $ | 1,006.8 | $ | 3,128.0 | $ | 5,307.5 | |||||||||||||||||||
(1) Short-term and long-term debt includes interest payments, which were calculated using the effective interest rate at July 2, 2022.
(2) Reflects remaining six months of 2022.
Significant Accounting Policies
There have been no other material changes to the significant accounting policies as disclosed in our 2021 Form 10-K, other than as described below.
On March 21, 2022, the SEC issued a proposed rule that would enhance and standardize the climate-related disclosures provided by public companies. Under the proposed rule, we would be required to provide quantitative and qualitative disclosures in registration statements and annual reports that include climate-related financial impact and expenditure metrics as well as a discussion of climate-related impacts on financial estimates and assumptions, all of which would be presented in a footnote to the financial statements. Such disclosures would also be subject to management's internal control over financial reporting and external audit. We will continue to monitor developments around this proposed rule, which once finalized, is expected to allow for a multi-year phased transition to achieve compliance.
70
Perrigo Company plc - Item 2
Unallocated, Interest, Other, and Taxes
Critical Accounting Estimates
The determination of certain amounts in our financial statements requires the use of estimates. These estimates are based upon our historical experiences combined with management’s understanding of current facts and circumstances. Although the estimates are considered reasonable based on the currently available information, actual results could differ from the estimates we have used. There have been no material changes to the critical accounting estimates as disclosed in our 2021 Form 10-K.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
There have been no material changes to our quantitative or qualitative disclosures found in Item 7A, "Quantitative and Qualitative Disclosures about Market Risk," of our 2021 Form 10-K.
ITEM 4. CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) of the Exchange Act) as of July 2, 2022. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective in ensuring that all material information relating to us and our consolidated subsidiaries required to be included in our periodic SEC filings would be made known to them by others within those entities in a timely manner and that no changes are required at this time.
Evaluation of the Effectiveness of Internal Control over Financial Reporting
Our management assessed the effectiveness of our internal control over financial reporting as of July 2, 2022. The framework used in carrying out our evaluation was the 2013 Internal Control - Integrated Framework published by the Committee of Sponsoring Organizations ("COSO") of the Treadway Commission. In evaluating our information technology controls, we also used components of the framework contained in the Control Objectives for Information and related Technology, which was developed by the Information Systems Audit and Control Association’s IT Governance Institute, as a complement to the COSO internal control framework. Management has concluded that our internal control over financial reporting was effective as of July 2, 2022. The results of management’s assessment have been reviewed with our Audit Committee.
Changes in Internal Control over Financial Reporting
Other than described below, there have been no changes in our internal control over financial reporting during the three months ended July 2, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
We acquired HRA Pharma during the second quarter of 2022 (refer to Item 1. Note 3). As permitted by Securities and Exchange Commission Staff interpretive guidance for newly acquired businesses, management excluded HRA Pharma from its evaluation of internal control over financial reporting as of July 2, 2022. We are in the process of documenting and testing HRA Pharma's internal controls over financial reporting. We will incorporate HRA Pharma into our annual report on internal control over financial reporting for our year ending December 31, 2023. As of July 2, 2022, HRA Pharma net assets excluded from management’s assessment totaled $1.9 billion. HRA Pharma contributed $57.8 million of Net sales and $13.1 million of Net Loss, inclusive of $6.8 million of cost of goods sold related to the acquisition step up to fair value on inventories sold and $16.4 million of amortization related to intangible assets recognized on acquisition, in our Condensed Consolidated Statements of Operations for the six months ended July 2, 2022.
PART II. OTHER INFORMATION
71
ITEM 1. LEGAL PROCEEDINGS
Refer to Item 1. Note 14 and Item 1. Note 15 of the Notes to the Condensed Consolidated Financial Statements.
ITEM 1A. RISK FACTORS
Our Annual Report on Form 10-K for the year ended December 31, 2021 includes a detailed discussion of our risk factors. At the time of this filing, there have been no material changes to the risk factors that were included in the Form 10-K, other than as described below.
The synergies and benefits expected from acquiring HRA Pharma may not be realized in the amounts anticipated or at all and integrating HRA Pharma's business may be more difficult, time consuming or costly than expected.
We may experience challenges integrating the HRA Pharma business and managing our expanded operations. Our ability to realize the benefits expected from the HRA Pharma acquisition will depend, in part, on our ability to successfully integrate the business, control costs and maintain growth. Integrations can be complex and time consuming, and the integration may result in temporarily depressed sales while integration of supply chain and distribution channels take place. Any delays, additional unexpected costs, or other difficulties encountered in the integration process could have a material adverse effect on the Company’s revenues, expenses, operating results and/or financial condition.
Even if integration occurs successfully, we may not achieve projected synergies or level of anticipated sales growth in new products, brands, or geographic markets within the anticipated timeframe, or at all. There are inherent uncertainties involved in identifying and assessing the profit potential, value, strengths, weaknesses, risks, and contingent and other liabilities of acquisitions, such as HRA Pharma, some of which can be affected by changes in the business, the industry, competition, consumer trends or general economic conditions.
Our indebtedness could adversely affect our ability to implement our strategic initiatives.
Our business requires continuous capital investments, and there can be no assurance that financial capital will always be available on favorable terms or at all. Additionally, our leverage and debt service obligations could adversely affect the business. At July 2, 2022, our total indebtedness outstanding was $4.1 billion. The agreements governing our New Senior Secured Credit Facilities impose material operating and financial restrictions that limit our operating flexibility, including the following:
•Our New Senior Secured Credit Facilities contain and agreements governing our other indebtedness may contain a number of restrictions and covenants that limit our ability to make distributions or other payments to our investors and creditors, or repurchase our shares, unless certain financial tests or other criteria are satisfied. These covenants include specified financial ratios and tests, which could affect our ability to operate our business or limit our ability to take advantage of potential business opportunities, such as acquisitions. If we do not comply with the covenants and restrictions contained in the agreements governing our indebtedness, we could be in default under those agreements, and the debt, together with accrued interest, could then be declared immediately due and payable.
•A default under certain indebtedness could lead to an acceleration of debt under other debt instruments that contain cross-acceleration or cross-default provisions. If our indebtedness is accelerated, there can be no assurance that we would be able to repay or refinance our debt or obtain sufficient new financing.
•In addition, an event of default under the Credit Agreement may permit the lenders to refuse to permit additional borrowings under the 2022 Revolver or to terminate all commitments to extend further credit under the 2022 Revolver. Furthermore, if we are unable to repay the amounts due and payable under the Credit Agreement, the lenders may be able to proceed against the collateral granted to them to secure that indebtedness.
72
Perrigo Company plc - Item 5
Risk Factors
•Downgrades to our credit ratings may limit our access to capital and materially increase borrowing costs on current or future financing, including via trade payables with vendors. Customers' inclination to purchase goods from us may also be affected by the publicity associated with deterioration of our credit ratings.
•There are various maturity dates associated with our New Senior Secured Credit Facilities, senior notes, and other debt facilities. There is no assurance that cash, future borrowings or equity financing will be available for the payment or refinancing of our indebtedness. Further, there is no assurance that any future refinancing or renegotiation of our New Senior Secured Credit Facilities, senior notes or other debt facilities, or additional agreements will not have materially different or more stringent terms. Refer to Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
.
73
Perrigo Company plc - Item 6
Exhibits
ITEM 6. EXHIBITS
Exhibit Number | Description | |||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
| 10.1* | Term Loan and Revolving Credit Agreement by and among Perrigo Company plc, as parent, Perrigo Investments, LLC, as a borrower, the Designated Borrowers, the Lenders, the Issuing Banks, and the Swing Line Lenders from time to time party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent, and as Collateral Agent, dated as of April 20, 2022* (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 20, 2022). | |||||||
| 10.2** | ||||||||
| 22 | List of Guarantor Subsidiaries (filed herewith). | |||||||
| 31.1 | ||||||||
| 31.2 | ||||||||
| 32 | ||||||||
| 101. INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document. | |||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | |||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | |||||||
| 104 | Cover Page Interactive Date File, formatted in Inline XBRL (contained in Exhibit 101). | |||||||
| * | Certain schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon its request. | |||||||
| ** | Denotes management contract or compensatory plan or arrangement. | |||||||
74
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| PERRIGO COMPANY PLC | |||||||||||
| (Registrant) | |||||||||||
| Date: | August 9, 2022 | /s/ Murray S. Kessler | |||||||||
| Murray S. Kessler | |||||||||||
| Chief Executive Officer and President | |||||||||||
| (Principal Executive Officer) | |||||||||||
| Date: | August 9, 2022 | /s/ Eduardo Bezerra | |||||||||
| Eduardo Bezerra | |||||||||||
| Chief Financial Officer | |||||||||||
| (Principal Accounting and Financial Officer) | |||||||||||
75