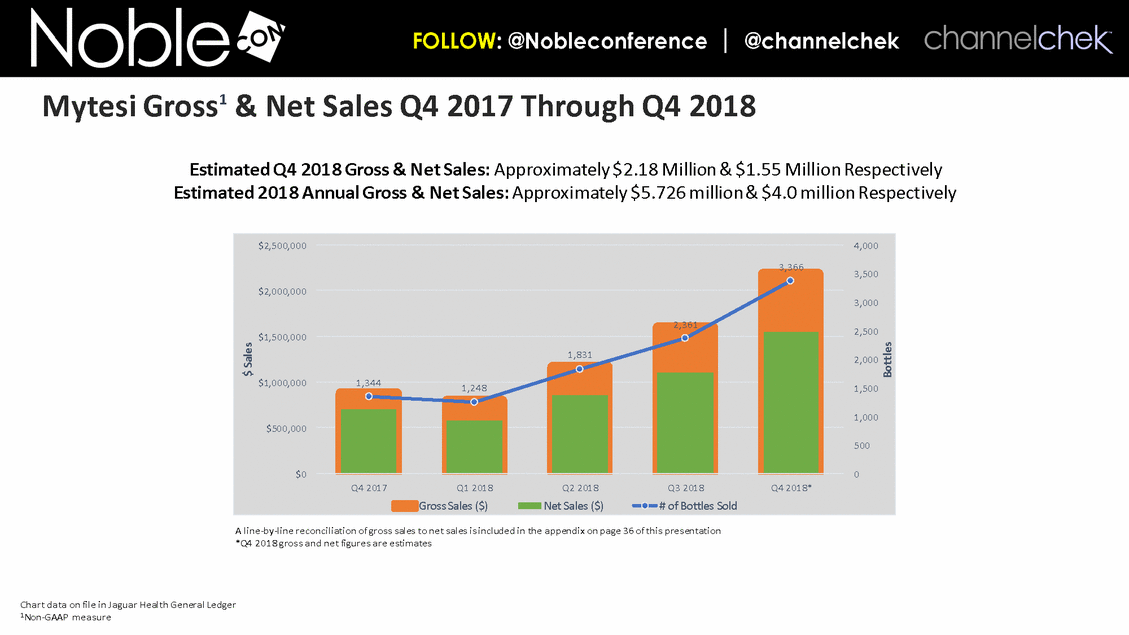
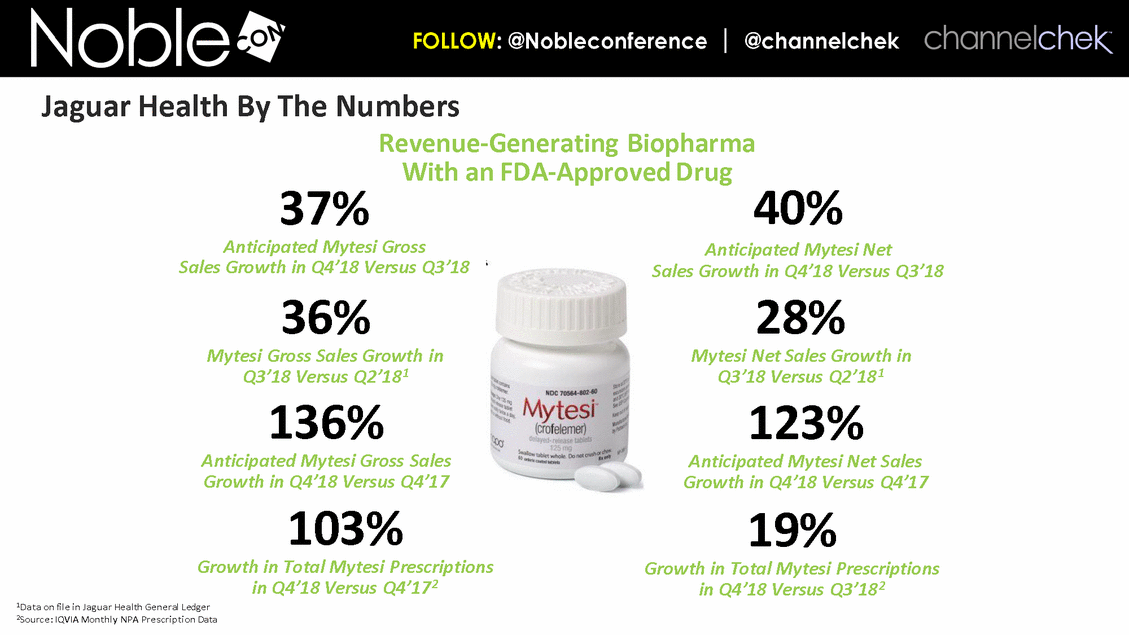
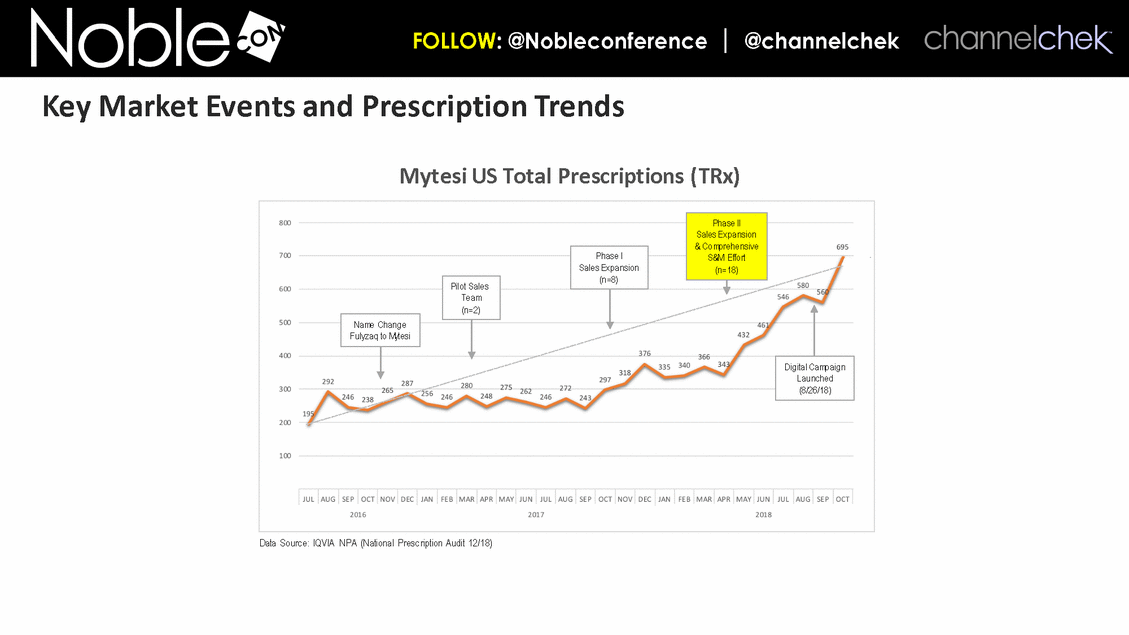
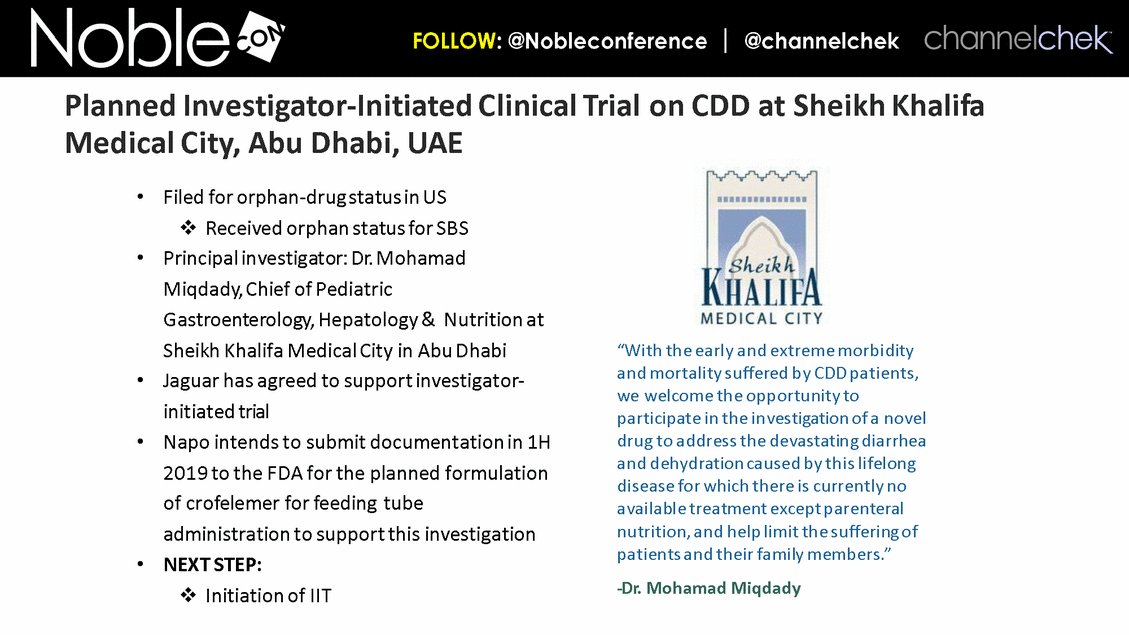
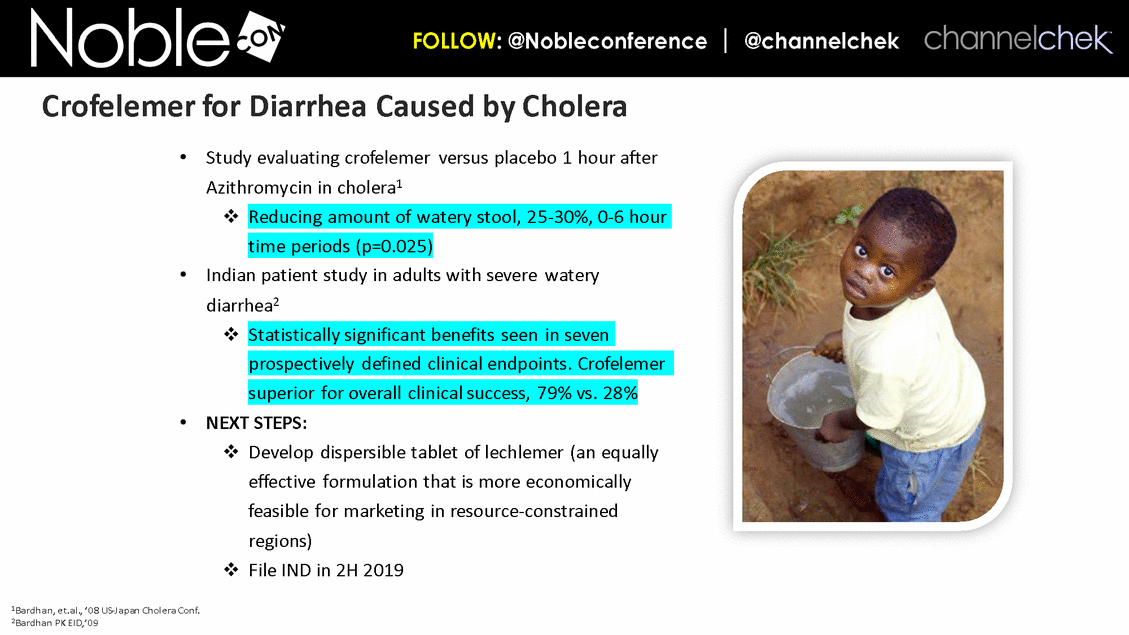
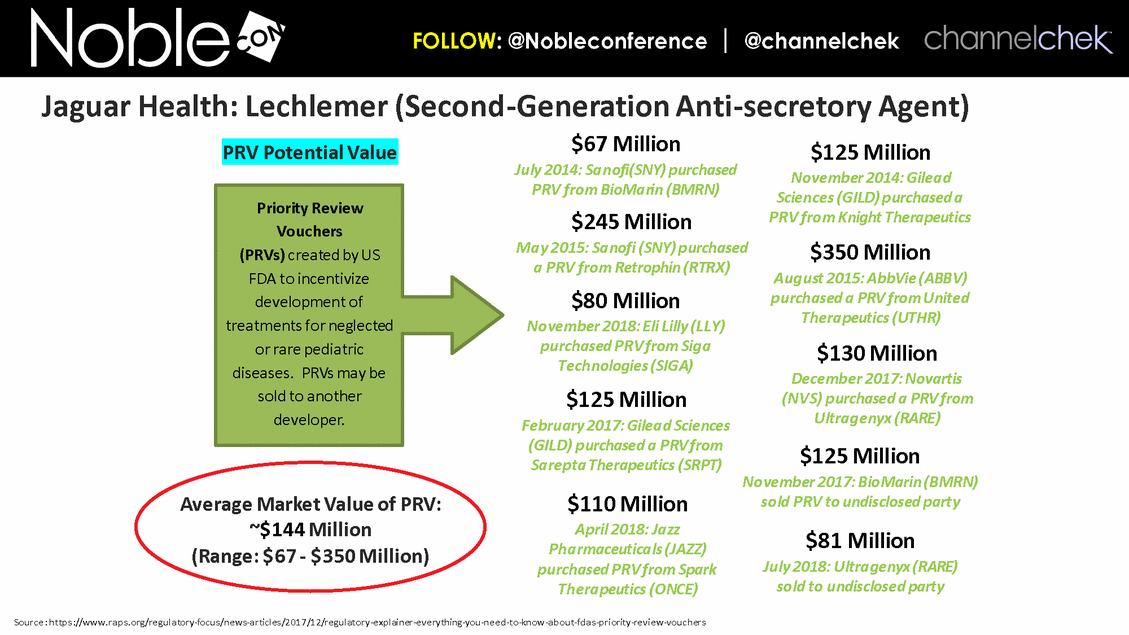
Cancer Therapy-Related Diarrhea SAB Lee Schwartzberg, MD, FACP: Executive Director of the West Cancer Center, a multispecialty oncology practice affiliated with the University of Tennessee; Chief, Division of Hematology/Oncology, the University of Tennessee Health Science Center Eric Roeland, MD: Attending Physician, Center for Palliative Care, Harvard Medical School Hope Rugo, MD: Clinical Professor of Medicine, Director Breast Oncology and Clinical Trials Education, Division of Hematology and Oncology, University of California San Francisco IBD SAB Corey Siegel, MD, MS: Associate Professor of Medicine; Associate Professor of The Dartmouth Institute; Director of the Inflammatory Bowel Disease Center at the Dartmouth-Hitchcock Medical Center Pediatric Indications (SBS and CDD) SAB Mohammed Miqdady, MD: Chief of Pediatric Gastroenterology, Hepatology & Nutrition at Sheikh Khalifa Medical City in Abu Dhabi Martin Martin, MD: Professor, Department of Pediatrics, David Geffen School of Medicine at UCLA Sue Rhee, MD: Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition Pediatric gastroenterologist and liver specialist, UCSF Benioff Children’s Hospital KOLs: Diarrhea Related to HIV and Other Infectious Diseases Patrick Clay, PharmD: Consultant Herbert DuPont, MD: Professor and Director, Center for Infectious Diseases, University of Texas Houston School of Public Health Pradip Bardhan, MBBS, MD: Chief Physician at ICDDR,B, Bangladesh Paulo Pacheco, MD: Clinical Assistant Professor, Department of Medicine, New York University Langone Health Elie Schochet, MD, FACS: Colorectal surgeon, Holy Cross Medical Group KOLs: Cancer Therapy-Related Diarrhea Herbert DuPont, MD: Professor and Director, Center for Infectious Diseases, University of Texas Houston School of Public Health Pablo C. Okhuysen, M.D: Department of Infectious Diseases, Infection Control, and Employee Health, Division of Internal Medicine, MD Anderson Napo Scientific Advisory Board (SAB) Members & Key Opinion Leader (KOL) Advisors to Napo Pravin Chaturvedi, PhD: Chair of Napo’s SABs. Pravin brings 25+ years drug development experience in pharmaceutical/biotech field; Successfully developed crofelemer (Mytesi) (first pivotal adaptive design) KOLs: Diarrhea Related to IBS Anthony Lembo, MD: Director of the GI Motility and Functional Bowel Disorders Program at Beth Israel Deaconess Medical Center and Associate Professor of Medicine at Harvard Medical School Doug Drossman, MD: Co-Director Emeritus, UNC Center for Functional GI and Motility Disorders Adjunct Professor of Medicine and Psychiatry, University of North Carolina School of Medicine William Chey, MD: Professor of Internal Medicine and Professor of Nutritional Sciences, University of Michigan School of Public Health KOLs: Diarrhea Related to IBD Brooks D. Cash, MD, AGAF, FACG, FACP, FASGE: Division Director, Gastroenterology, Hepatology, and Nutrition Visiting Professor of Medicine, The University of Texas McGovern Medical School David Rubin, MD: Joseph B. Kirsner Professor of Medicine Section Chief, Gastroenterology, Hepatology and Nutrition Co-Director, Digestive Diseases Center, University of Chicago Medicine Charles Bernstein, MD: Distinguished Professor of Medicine and Bingham Chair in Gastroenterology Research, University of Manitoba William Sandborn, MD: Director, Inflammatory Bowel Disease Center Chief, Division of Gastroenterology Professor of Medicine, US San Diego Health Scott Lee, MD: Associate Professor of Medicine, Digestive Health Center, University of Washington Medical Center Edward Loftus, Jr., MD: Consultant, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mayo Clinic Douglas Wolf, MD: Medical Director of IBD Research at Atlanta Gastroenterology Associates. Clinical Assistant Professor of Medicine, Emory University School of Medicine KOLs: Pediatric Indications (SBS and CDD) Jay Thiagarajah, MD, PhD: Attending Physician, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital. Instructor of Pediatrics, Harvard Medical School James Goldenring, M.D., PhD: Professor of Surgery, Vanderbilt University School of Medicine. Paul W. Sanger Chair in Experimental Surgery. Professor of Cell and Developmental Biology