Exhibit 99.2

Jaguar Health, Inc. (NASDAQ: JAGX) Napo EU Overview – November 2020

Forward - Looking Statements 2 This presentation contains forward - looking statements . All statements other than statements of historical facts contained in this presentation, including statements regarding the Company’s plan to establish a subsidiary in the EU with exclusive rights to develop and commercialize crofelemer/Mytesi in European Territory for HIV and inflammatory diarrhea indications, the Company’s expectation that a Napo subsidiary in the EU will be the target company for the Post Pandemic Recovery SPAC, the anticipated terms of the proposed acquisition of such Napo subsidiary by the Post Pandemic Recovery SPAC and potential range of ownership by the Post Pandemic Recovery SPAC in such Napo subsidiary post - acquisition, the expectation that the Post Pandemic Recovery SPAC will be listed on the Italian AIM, the Company’s plan to pursue a possible indication of prophylaxis and/or symptomatic relief of post acute COVID - 19 syndrome, the endpoints that the Company intends to explore in a study for such indication, the Company’s plans to pursue a possible indication of symptomatic relief of diarrhea from cholera, the Company’s plans to pursue additional business development deals, plans to expand the geography for commercialization of Mytesi, and the timing of data results from planned proof of concept studies, field studies, investigator - initiated trials, sponsored studies, and other studies are forward - looking statements . These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions . The forward - looking statements in this presentation are only predictions . We have based these forward - looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations . These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control . Please see the risk factors identified in our Annual Report on Form 10 - K and our other filings with the SEC . The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements . Readers are also advised that our projected sales do not take into account the royalties and other payments we will need to make to our licensors and strategic partners . Moreover, we operate in a dynamic industry and economy . New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise . NASDAQ:JAGX

Jaguar Health (NASDAQ: JAGX) Wholly Owned Subsidiary Napo Pharmaceuticals, Inc. 3 NASDAQ:JAGX From Tree to Bottle: New ways and novel plant - based medicine to treat gastrointestinal disorders Post Pandemic Recovery SPAC To be l isted on Italian AIM Target company Prophylaxis and/or symptomatic relief of post acute COVID - 19 syndrome
NASDAQ:JAGX 4 » “Long - hauler” or “Chronic COVID” or “Post acute COVID syndrome” refers to patients infected with COVID - 19 who suffer with symptoms which may include gastrointestinal distress (i.e. diarrhea, constipation, nausea, pain), fatigue, brain fog, forgetfulness, cardiovascular effects, arthritis, etc. » While this emerging syndrome is under study around the world, there seems to be a vast whole - body inflammation » Theory is their immune systems continue to overreact even though the infection has passed » Predominant in younger patients and those with mild/asymptomatic disease » Inflammation in the GI track often manifests as diarrhea □ An “observable” symptom that can provide for early diagnosis □ Early diagnosis of Chronic COVID could prevent burden of long - term chronic illness □ Symptomatic relief important for patient comfort, dignity, and reduction in potential contagion from watery stools Post acute COVID - 19 Syndrome: A collection of chronic symptoms in a persisting in COVID - 19 recovered patient Center for Post - COVID Care at Mount Sinai, New York City Similar centers in U.S. & Europe: • Post - COVID Recovery Clinic at Penn Medicine (University of Pennsylvania Health System) • Post - COVID rehab institute in Genoa, Italy • NHS Seacole Centre at Headley Court, Surrey, UK • COVID - 19 rehabilitation centre at Bradford Teaching Hospitals, UK

Mytesi® relevance to inflammatory diarrhea 5 NASDAQ:JAGX Long - term survivor enteropathy: Inflammatory chronic syndrome typically affecting long - term HIV/AIDS survivors — for which they may be prescribed Mytesi Mytesi’s FDA - approved Indication in the U.S.: Mytesi (crofelemer 125mg delayed - release tablets) is FDA - approved for symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy (ART).
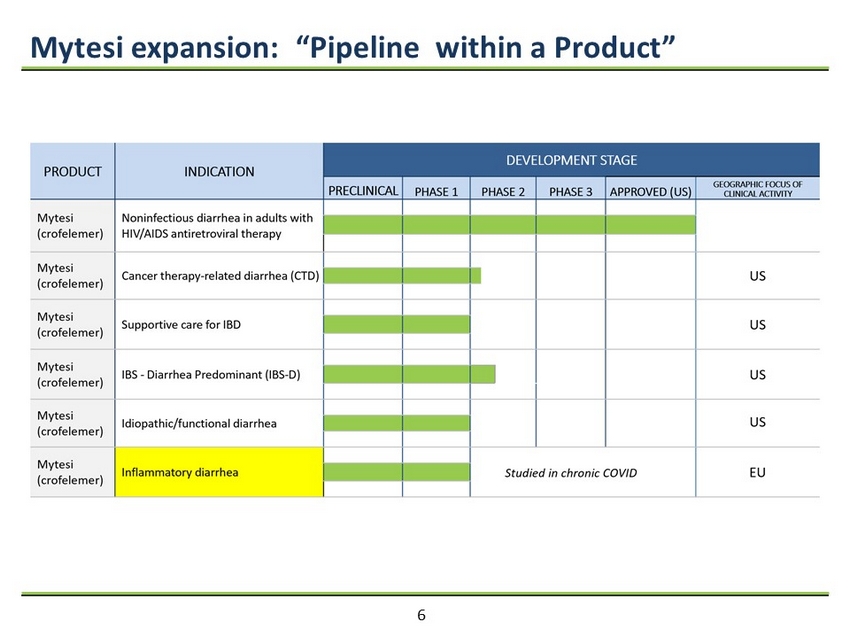
Mytesi expansion: “Pipeline within a Product” 6

NASDAQ:JAGX 7 “Tip of the iceberg” – Dr. Paul Whitaker, Organized Post - COVID Patient Clinic, Bradford, England » "Recurrent fevers, persistent constipation or diarrhea , intense bouts of fatigue, debilitating brain fog and vivid hallucinations — some people who catch COVID - 19 experience symptoms like these for months on end, and we're still learning why that is," reports Live Science. Source: Link » "Some less common symptoms included high fevers and severe gastrointestinal issues, such as constipation lasting for weeks, bowel obstructions and diarrhea leading to rapid weight loss. Source: Link » “But a positive result for the virus was only the beginning of Nichols’ health struggles. By mid - April, the previously healthy 120 pound 32 - year - old with no pre - existing conditions developed walking pneumonia, experienced continued gastrointestinal symptoms, and developed hand tremors in her left hand and numbness in her left foot that lasted two months. After four straight months of nausea, vertigo, and constant diarrhea , she’d lost 12 pounds.” Source: Link » “My life as a Covid Long - Hauler has been nothing short of hell. I’ve had unexplained daily nausea, vomiting and diarrhea for 212 days .” Source: Link » “….. only the tip of the iceberg in terms of patients we needed to see and a lot of patients never actually presented to hospital in the first place.“

NASDAQ:JAGX 8 Target Population in Europe Could Exceed 25 Million People; More Than 100 Million People Worldwide » Over 25% of the U.K. likely to have had COVID - 19 already: A team of researchers from The University of Manchester, Salford Royal and Res Consortium, have shown that a significant proportion of people in the UK — over 25% — is likely to have been infected already by the COVID - 19 virus. Source: Link » “In fact, nearly ⅓ of COVID sufferers, dubbed "long haulers," may experience similar long - lasting effects, according to a new study. (Study published week of Nov 16, 2020 in the journal Annals of Internal Medicine). Source: Link » “Dr. Anthony Fauci, the nation's leading infectious disease expert, called the issue ‘post - COVID 19 syndrome’ at a seminar held by the American Society of Tropical Medicine and Hygiene. ‘Virologically a certain percentage, sometimes as high as one third , experienced lingering symptoms for weeks to months,’ he said.” Source: Link » “The Daily Mail reported that people of all ages , including children and teenagers, the elderly, and pregnant women, have been struck with lingering symptoms months after they’ve cleared the virus - some finding themselves periodically breathless, while others experience draining fatigue, skin rashes or diarrhea .” Source: Link » The Daily Mail reports the number of coronavirus survivors suffering ‘long - covid’ is ‘ever - growing.’ The report noted one study found that three months after a group of 110 hospitalized UK COVID - 19 patients had been discharged, 81 - about 74% - of them were still suffering lingering symptoms. However, other studies are more conservative, estimating the figure to be one in 10 , the report said.” Source: Link
NASDAQ:JAGX 9 » Single payer health care system has great incentive to focus on mitigation of burden of long - term chronic illness □ Expense □ Wellness of a “younger” patient population » Identification of the problem facilitates clinical enrollment » The European Medicines Agency (EMA) is interacting with developers of potential COVID - 19 treatments and vaccines to enable promising medicines to reach patients as soon as possible. It is also making use of real - world data to monitor the safety and effectiveness of medicines used in patients with COVID - 19. » Target label, Mytesi follow - on indication: Prophylaxis and/ or symptomatic relief of diarrhea due to inflammation [studied in post - COVID infection recovery patient] » Expected Endpoints: □ Relief of diarrhea [prophylactic and symptomatic] □ Reduction in inflammatory gut markers □ Gut biome restoration □ Reduction in viral fecal shedding Why a European focus for this long - hauler post recovery patient indication?

NASDAQ:JAGX 10 » Napo EU to have exclusive licensed rights to develop and commercialize crofelemer/Mytesi in European Territory for HIV and inflammatory diarrhea indications □ Access to all regulatory information and IP » Napo EU obligation: To conduct clinical trials to support registration in European territory for inflammatory diarrhea □ Dedicated operational employees and management in Europe » JAGX obligation: Provide business and scientific leadership, service agreement as necessary with JAGX staff and management □ Provide contract manufacturing service to Napo EU [benefit of scale] Proposed Merger of Napo European Operation [Napo EU] into SPAC Napo EU to be incorporated in Italy, operations located in Switzerland
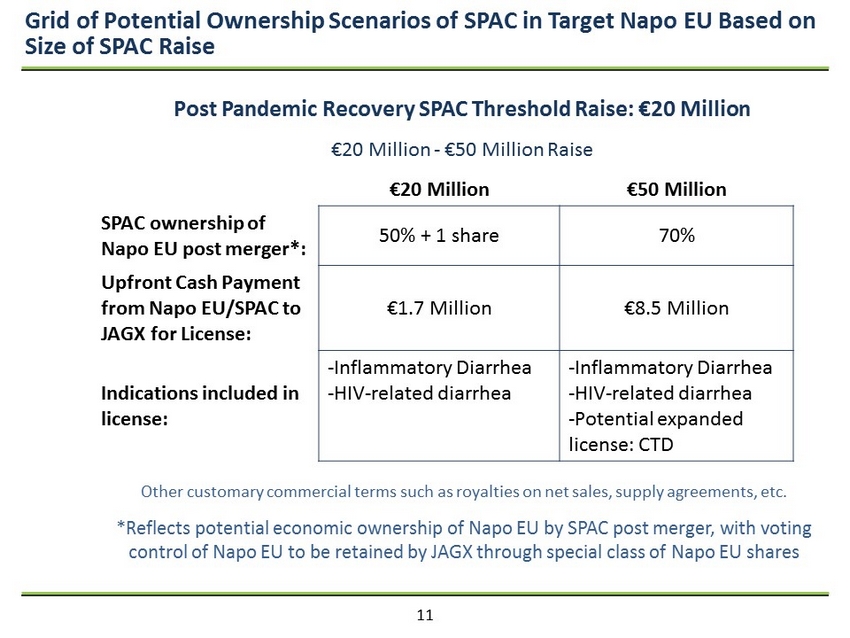
11 Grid of Potential Ownership Scenarios of SPAC in Target Napo EU Based on Size of SPAC Raise €20 Million €50 Million SPAC ownership of Napo EU post merger*: 50% + 1 share 70% Upfront Cash Payment from Napo EU/SPAC to JAGX for License: €1.7 Million €8.5 Million Indications included in license: - Inflammatory Diarrhea - HIV - related diarrhea - Inflammatory Diarrhea - HIV - related diarrhea - Potential expanded license: CTD Post Pandemic Recovery SPAC Threshold Raise: €20 Million €20 Million - €50 Million Raise Other customary commercial terms such as royalties on net sales, supply agreements, etc. *Reflects potential economic ownership of Napo EU by SPAC post merger, with voting control of Napo EU to be retained by JAGX through special class of Napo EU shares

NASDAQ:JAGX 12 » Swiss Growth Forum - Andreea Porcelli: CEO, Swiss Growth Forum. 25 years of experience as the founder of the first female owned investment bank and Investment Forum in the EU with an unmatched institutional investor network in the US, EU and the GCC. » Joseph Konowiecki : Chairman of Alignment Healthcare ( $4 Billion IPO in Q1 2021 with Morgan Stanley as lead underwriter). Former GC of United Health Group with extensive C - level experience at the helm of public companies for over 30 years. » Vittorio Grimaldi: Head of CGM Family Office and Asset Management in Monaco. Former Head of Asset Management at San Paolo Funds Italy managing over $40 billion in equities. Over 30 years of experience managing publicly traded funds. » Escrow agent: Banca Finnat » Law firm [prospectus and listing activity]: Tonucci and Partners SPAC Promoters
How Mytesi Works NASDAQ:JAGX

How Mytesi Works NASDAQ:JAGX 14 » Mytesi is a non - opioid that works differently from other treatments for diarrhea With Mytesi, it’s about waterflow Mytesi normalizes waterflow in the GI tract Less water flowing into your GI tract = less watery diarrhea Mytesi acts locally in the GI tract Most other diarrhea medicines work by slowing down your GI tract, i.e. opioids cause constipation Mytesi is a non - opioid, non - antibiotic, non - addictive drug approved for chronic use
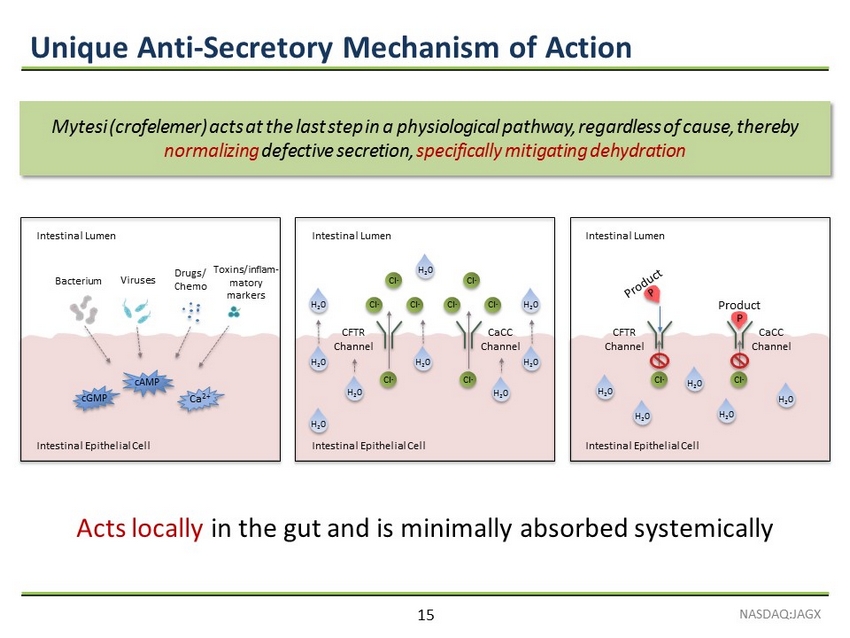
Unique Anti - Secretory Mechanism of Action NASDAQ:JAGX 15 P Intestinal Lumen Intestinal Epithelial Cell Cl - CFTR Channel CaCC Channel Cl - H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 Cl - Cl - Cl - Cl - Cl - Cl - Intestinal Lumen Intestinal Epithelial Cell Cl - CFTR Channel CaCC Channel Cl - H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 H 2 0 Bacterium Viruses Drugs/ Chemo Toxins/inflam - matory markers cAMP cGMP Ca 2+ Intestinal Epithelial Cell Intestinal Lumen Product Acts locally in the gut and is minimally absorbed systemically Mytesi (crofelemer) acts at the last step in a physiological pathway, regardless of cause, thereby normalizing defective secretion, specifically mitigating dehydration

Mytesi Performance NASDAQ:JAGX

17 Adults Living with HIV/AIDS & Take ARTs NASDAQ:JAGX MacArthur RD, Clay P, Blick G, et al. Long - Term Crofelemer Provides Clinically Relevant Reductions in HIV - Related Diarrhea. Post er presented at: 9th IAS Conference on HIV Science (IAS 2017); 2017 July 23 - 26; Paris, France.
18 Mytesi Current Indication NASDAQ:JAGX Mytesi (crofelemer 125mg delayed - release tablets) is FDA - approved for symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy
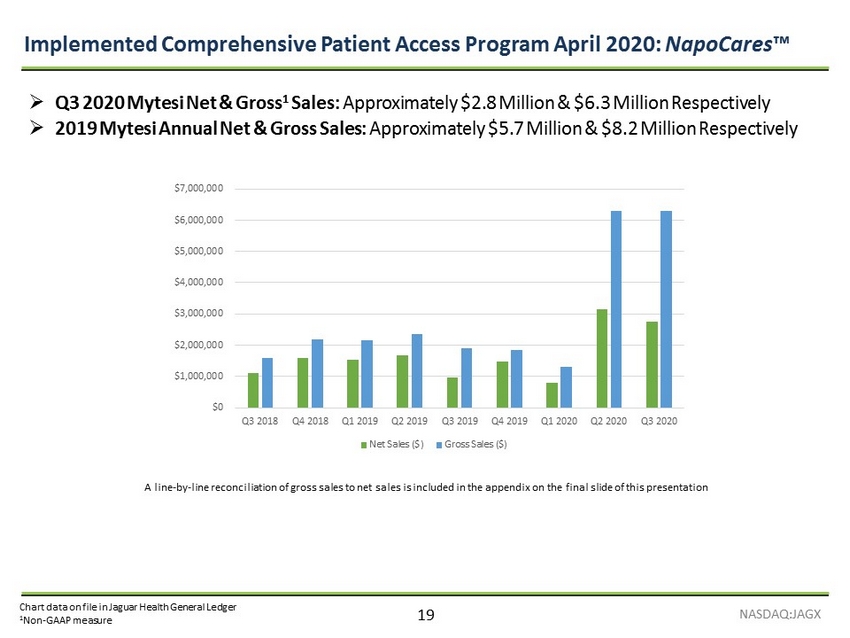
19 Chart data on file in Jaguar Health General Ledger 1 Non - GAAP measure Implemented Comprehensive Patient Access Program April 2020: NapoCares ™ NASDAQ:JAGX A line - by - line reconciliation of gross sales to net sales is included in the appendix on the final slide of this presentation » Q3 2020 Mytesi Net & Gross 1 Sales: Approximately $2.8 Million & $6.3 Million Respectively » 2019 Mytesi Annual Net & Gross Sales: Approximately $5.7 Million & $8.2 Million Respectively $0 $1,000,000 $2,000,000 $3,000,000 $4,000,000 $5,000,000 $6,000,000 $7,000,000 Q3 2018 Q4 2018 Q1 2019 Q2 2019 Q3 2019 Q4 2019 Q1 2020 Q2 2020 Q3 2020 Net Sales ($) Gross Sales ($)

Revenue - Generating Biopharma With an FDA - Approved Drug 1 Data on file in Jaguar Health General Ledger 151% The average of the total number of Mytesi bottles sold in Q2 & Q3 2020 represents 151% of the number sold in Q1 2020 280% Mytesi Q3 2020 net sales represent 280% of Q3 2019 net sales, or an increase of approximately $1.8 million quarter over quarter 1 332% Mytesi Q3 2020 gross sales represent 332% of Q3 2019 gross sales, or an increase of approximately $4.4 million quarter over quarter 1 20 Jaguar Health by the Numbers NASDAQ:JAGX
Expansion of Crofelemer Indications NASDAQ:JAGX
» Early October 2020: Napo’s Pivotal Phase 3 clinical trial initiated and funded with non - dilutive royalty deal (first $6 million tranche of funding received in October 2020 with additional royalty financings of $5 million and $6 million available in February 2021 and July 2021 with mutual agreement of the parties) » Agreements with FDA: □ Crofelemer safety studies acceptable and no new nonclinical toxicity studies required □ Chemistry, manufacturing and controls (CMC) data acceptable □ No additional requirements for drug interaction studies for the CTD program NASDAQ:JAGX 22 Cancer Therapy - Related Diarrhea (CTD) PHASE 3 » Features of single Phase 3 pivotal trial : □ Planned Label: Symptomatic relief of diarrhea in adult patients with solid tumors receiving targeted cancer therapies with or without cycle chemotherapy □ Principal investigator & co - investigators identified: MD Anderson
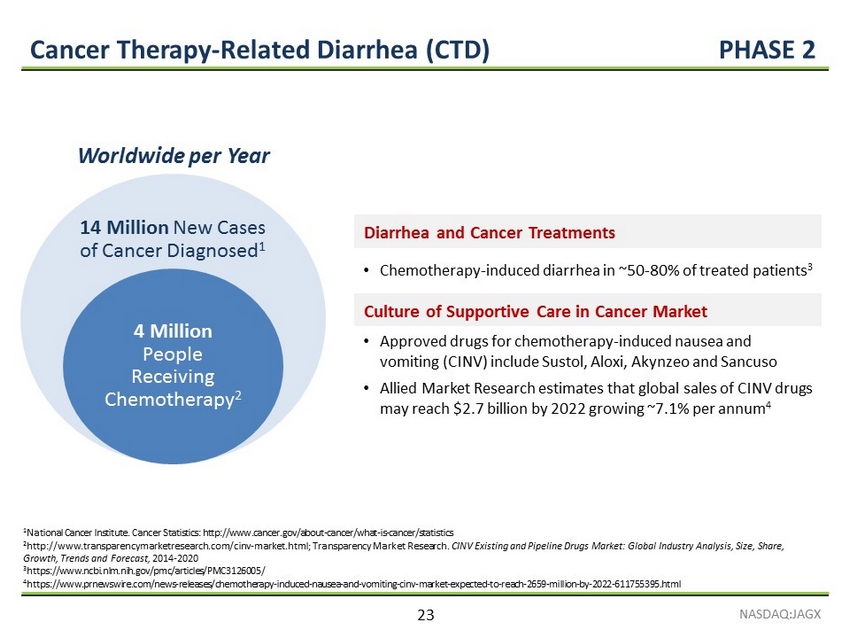
Cancer Therapy - Related Diarrhea (CTD) PHASE 2 Worldwide per Year 1 National Cancer Institute. Cancer Statistics: http://www.cancer.gov/about - cancer/what - is - cancer/statistics 2 http://www.transparencymarketresearch.com/cinv - market.html; Transparency Market Research. CINV Existing and Pipeline Drugs Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2014 - 2020 3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3126005/ 4 https://www.prnewswire.com/news - releases/chemotherapy - induced - nausea - and - vomiting - cinv - market - expected - to - reach - 2659 - million - by - 2022 - 611755395.html 14 Million New Cases of Cancer Diagnosed 1 4 Million People Receiving Chemotherapy 2 Diarrhea and Cancer Treatments • Chemotherapy - induced diarrhea in ~50 - 80% of treated patients 3 Culture of Supportive Care in Cancer Market • Approved drugs for chemotherapy - induced nausea and vomiting (CINV) include Sustol, Aloxi, Akynzeo and Sancuso • Allied Market Research estimates that global sales of CINV drugs may reach $2.7 billion by 2022 growing ~7.1% per annum 4 NASDAQ:JAGX 23

Name / Title Experience Lisa Cont e Founder & CEO • 30 + years of industry experience • Obtained first anti - secretory human product FDA approval • Board of directors of Healing Forest Conservancy • Raised over $400 mm Carol Lizak, MBA SVP Finance, Chief Accounting Officer • 20+ years of corporate controllership, financial planning & analysis • 10+ years with public companies including foreign subs (5 years in biopharma) • Prior to joining Jaguar, raised $14M in capital lease and supported $100M follow - on raise Steven King, PhD Chief Sustainable Supply, Ethnobotanical Research & IP Officer • Served as head of sustainable supply, ethnobotanical research & IP: 1989 - 2020 • Board of Directors of Healing Forest Conservancy Pravin Chaturvedi, PhD Chief Scientific Officer Chair of Scientific Advisory Board • 25+ years drug development experience • Co - Founded Scion, IndUS and Oceanyx Pharmaceuticals • Successfully developed Mytesi® (first pivotal adaptive design) and 7 pharmaceutical products David Sesin, PhD Chief Manufacturing Officer • Pharmaceutical scientist with experience from drug discovery through manufacturing • Developed crofelemer manufacturing process Jonathan Wolin, JD, MBA, CPA Chief of Staff, Chief Compliance Officer & General Counsel • Extensive experience providing legal advice and guidance to public and private companies in the healthcare and biotechnology industries Ian H. Wendt, MBA Vice President Commercial Strategy • Has held commercial leadership roles across sales, marketing and operations at some of the largest brands in the pharmaceutical industry over past 25 years Melissa Yaeger, JD Sr. VP, Regulatory Affairs & Quality Assurance • Leadership supporting the approval of multiple products • International regulatory leadership • Gilead, Becton Dickinson, several specialized biotechnology companies Michael K. Guy, DVM, MS, PhD VP, Preclinical & Nonclinical Studies • 20+ years experience in animal and human pharmaceutical development, including clinical development, manufacturing, regulatory and pre - clinical drug discovery Executive Management Team 24 NASDAQ:JAGX
Name / Title Experience James Bochnowski Chairman • Founder of Delphi Ventures, one of the first VC firms to focus exclusively on investing in life sciences companies • Co - founded Technology Venture Investors Lisa Conte Founder, CEO & President • 2 8 + years of industry experience • Obtained first anti - secretory human product FDA approval John Micek III Director • Managing Partner of Verdant Ventures • Former Managing Director of Silicon Prairie Partners, LP Jonathan B. Siegel Director • Founded JBS Healthcare Ventures with a focus on public and private healthcare investments • 18+ years of investment experience Greg Divis Director • CEO of Avadel Pharmaceuticals • 28+ years of direct operating and global leadership experience in specialty pharmaceuticals Board of Directors NASDAQ:JAGX 25

» Q4 2020 - 2021: Additional business development activity » Q4 2020 : Non - dilutive financing » Q1 2021: Year - end Mytesi financial performance » 2H 2021: Top line results expected for investigator - initiated Phase 2 CTD trial » 2H 2021: Initiate CDD phase 1/2 study (orphan indication) – US and Middle East » Mid - 2021: Results of clinical study evaluating effect of Mytesi on the microbiome » Q3 2021: Launch Canalevia for CID & EID in dogs » Q4 2021: Initiate study of lechlemer for symptomatic relief of diarrhea from cholera (subject to funding) Upcoming Milestones NASDAQ:JAGX 26
Capitalization Table NASDAQ:JAGX

NASDAQ:JAGX Capitalization as of November 19, 2020 Common Shares Outstanding, voting 77,260,687 Non - Voting Common 1 2,020 Convertible Preferred (Series B - 2 as converted at 6,559 per Common Stock) 2 1,246,210 Options Outstanding 3 4,535,140 Options available for grant (includes New Employee Inducement Plan) 3 615,705 RSUs 3 5,613 Warrants – Jaguar 4 2,032,415 Warrants – Other (weighted average exercise price $90.00) 1,029 Warrants – Series 1 (net of conversion) 1,840,865 Warrants – Series 2 (July 2019 offering) 1,940,865 Warrants – Series 3 (May 2020 inducement offering) 214,500 Warrants – PIPE Dec 2019 1,250,000 Warrants – Other 100,780 Total Warrants 4 7,380,454 Fully Diluted Shares 5 91,045,829 1 Represents 2,120,785 shares of our non - voting common stock that are convertible into 2,020 shares of voting common stock. 2 Represents 6,559 shares of Series B - 2 Preferred Stock that are convertible into 1,246,210 shares of voting common stock. 3 Includes 4,535,140 options granted to officers, directors, employees, and 3 part - time/consultants (34,175 options are above $30 .50 strike price), 615,705 options available for grant, and 5,613 RSUs.) 4 Bridge warrants from July 2019 offering 1,953,125, 45,750 warrants from letter of credit, and 33,863 warrants above $2.50 exe rci se price per share. 5 Excludes 557,500 shares of Series C Perpetual Preferred Stock and 842,500 shares of Series D Perpetual Preferred Stock that a re entitled to receive, upon a liquidation event, a payment per share equal to $8.00 that is payable before any payment is made to any common stock. Except for this payment, the Series C Pe rpe tual Preferred Stock and Series D Perpetual Preferred Stock are not entitled to receive any further payments upon a liquidation event. . 28

Chronic COVID Investment Highlights 29 NASDAQ:JAGX • Only U.S. FDA - approved diarrhea treatment that’s been studied specifically in adults with HIV/AIDS • Product already approved in U.S. with chronic safety for current HIV diarrhea indication • Utilized in inflammatory diarrhea/enteropathy for current HIV diarrhea indication • International supply chain in place Mytesi (Crofelemer): FDA - Approved Human Drug • Napo EU license to study, develop and commercialize crofelemer for an indication of prophylaxis and/or symptomatic relief of inflammatory diarrhea, initially in Europe • Company believes chronic COVID may be analogous to enteropathy, an inflammatory chronic syndrome typically affecting long - term HIV/AIDS survivors. S. Crofelemer Expansion • A significant number of COVID - 19 survivors experience many symptoms, including diarrhea and abdominal discomfort, for several months after recovery • It is estimated that ~25% of people in the U.K. have already been infected with SARS - CoV - 2, the virus that causes COVID - 19, and it appears to be the general consensus that ~30% of COVID - 19 patients end up suffering from long - hauler syndrome Growing Population of COVID Recovery Patient Long - haulers • Napo EU intends to focus clinical exploration in Europe, where single - payer healthcare systems focus on preventative measures to diagnose long - hauler syndrome, for which chronic diarrhea can be an observable symptom • Mitigation of chronic disease, particularly for a younger population • EMA focus on therapeutics and real world experience Europe is a Logical Target Market • Prophylaxis and/or symptomatic relief of long - term diarrhea or other gastrointestinal disfunctions in post acute COVID - 19 syndrome patients • Early diagnosis and mitigation of enormous burden of chronic disease, particularly in younger population Potential Benefits for COVID Recovery Patients, if Crofelemer Approved for Chronic COVID Indication • Napo has conducted IP filings in support of the development of crofelemer for potential inflammatory diarrhea indication, including in a long - hauler post - COVID recovery situation • Napo holds ~144 patents (majority do not expire until 2027 - 2031) and ~39 patents pending • Botanical guidance protection – no generic pathway Proprietary Position

Jaguar Health, Inc. (NASDAQ: JAGX) Investor Relations Contact Peter Hodge phodge@jaguar.health NASDAQ:JAGX





























