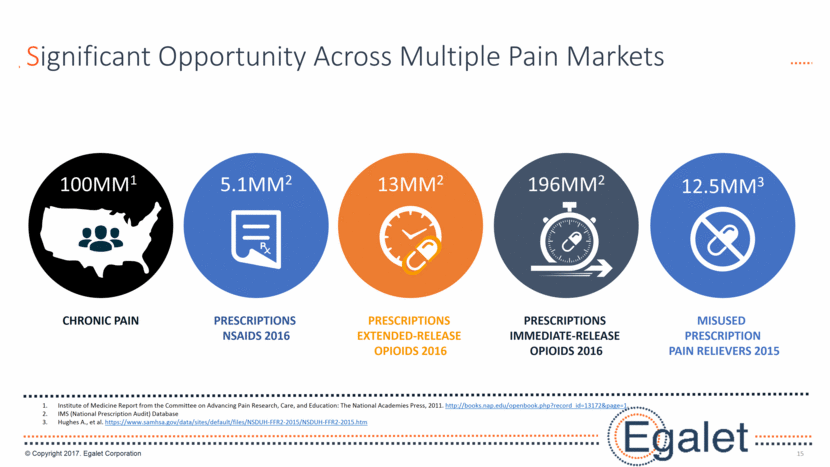
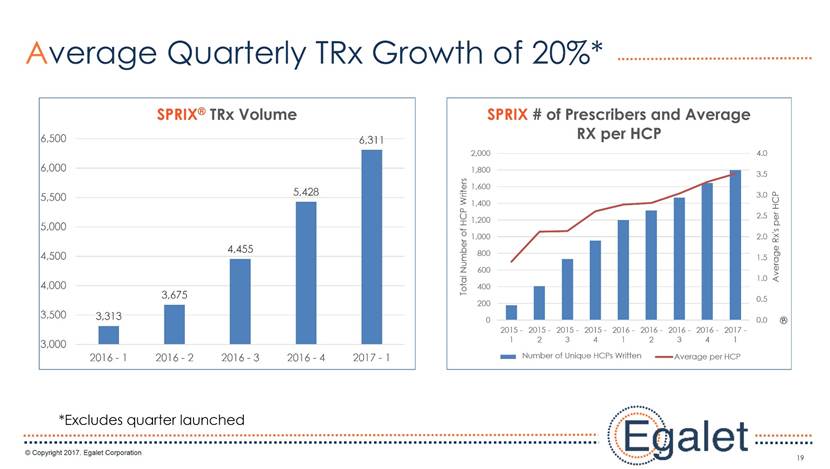
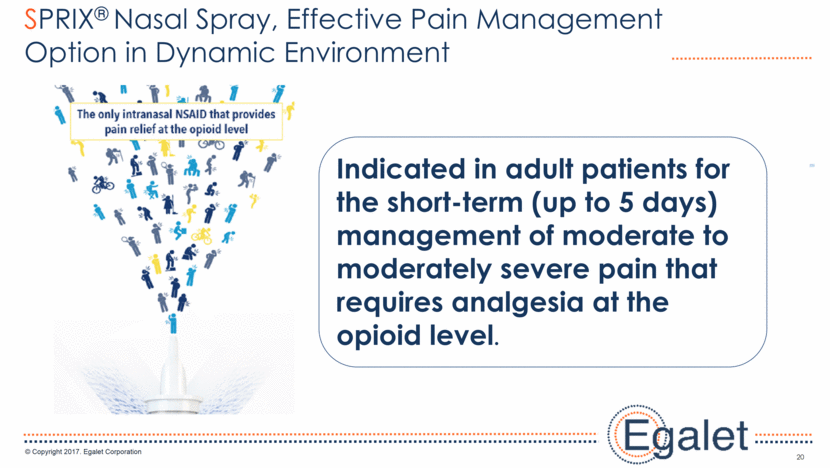
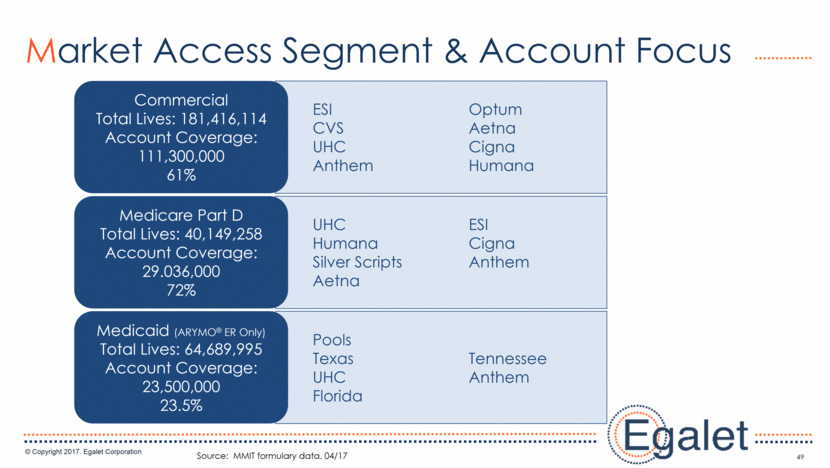
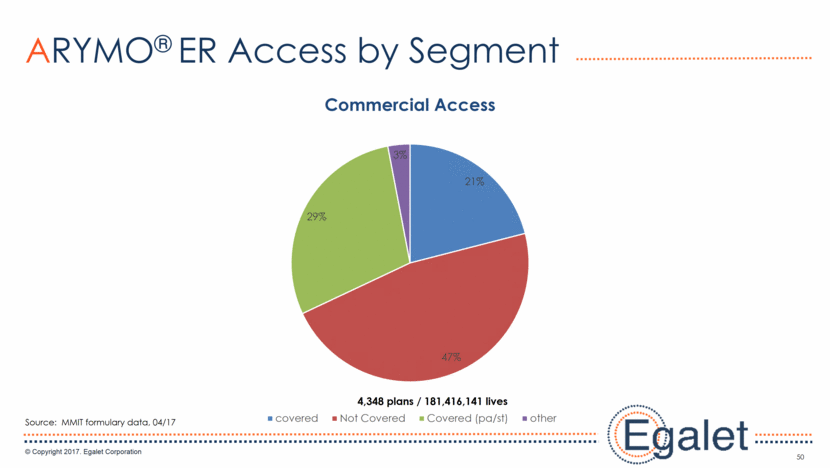
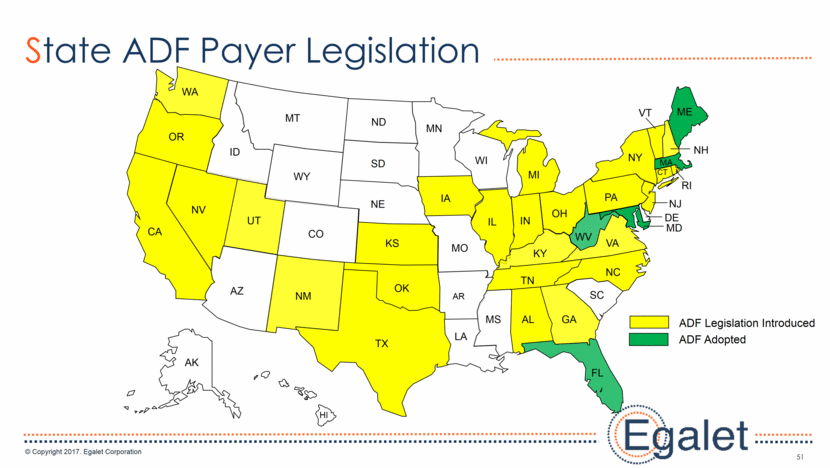
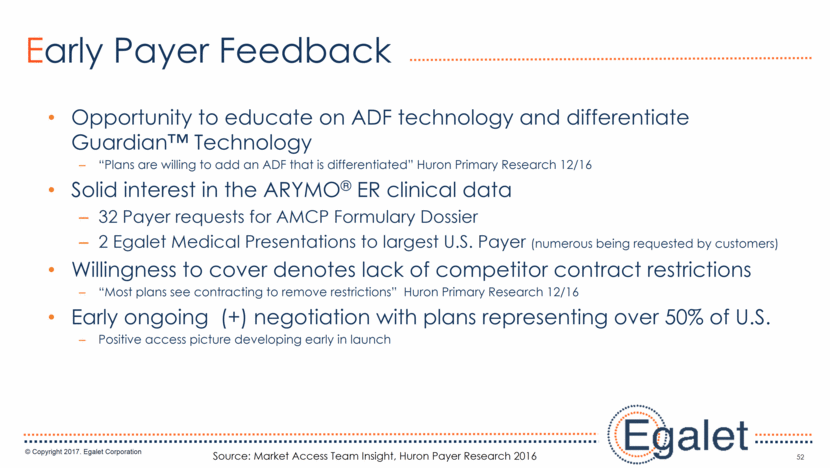
3 Forward Looking Statements Statements included in this presentation that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties, risks and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “could,” “plans,” “future,” “expects,” “goal,” “intends,” “assess,” “continue to,” “potential,” “anticipates,” “believes,” “estimates,” or “predicts,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the potential market size for our products, (ii) the timing or likelihood of regulatory filings and approvals for our products and product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the impact of our existing commercial presence on the commercialization of our new products; (v) the impact of the addition of our new products on our market presence; (vi) statements regarding the expansion of new prescribers and prescriptions for our products; (vii) the timing of the expansion of dosage levels for our products; (viii) the implications for the success of our products based on our current demand experience; (ix) our expectations regarding our path to sustainability and growth, including our business development plans; (x) the strategic imperatives with regard to our products, including our goals with regard to market access; (xi) the eventual outcome of the FDA’s actions relating to abuse-deterrent products; and (xii) our expectations regarding our finances, including our expenses, and our funding sources, our use of funds and potential payments under our notes and our royalty rights agreements. In addition, we, through our senior management, from time to time make forward-looking public statements concerning our expected future operations and performance and other developments. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX®, ARYMO® ER and OXAYDO®; our ability to execute on our sales and marketing strategy, including developing relationships with customers, physicians, payors and other constituencies; the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates and our ability to serve those markets; unexpected safety or efficacy data; competitive factors; changes in the regulatory environment for our products; any further FDA action relating to abuse-deterrent products; general market conditions; our need for future capital; our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; and other risk factors described in our filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. See Sprix.com, Oxaydo.com and Arymoer.com for full prescribing information including boxed warning and medication guide.