
Corporate Presentation August 2023

We anticipate raising funds from real estate asset sales to reduce our outstanding debt principal. There are a number of risks and uncertainties that could impact real estate values and or our ability, if any, to successfully monetize the sale of any non-core real-estate assets including, but not limited to, market forces, economic conditions, revenue concentration, debt levels, geographic location, interest rates, results of engineering plans, geotechnical surveys, coverage density, physical characteristics of the land (e.g. rock, wetlands delineation, streams, powerlines, topography, zoning), ability to reach acceptable contractual terms and obtaining the required approvals and release(s) from our senior secured lender. Any historical or projected financial information contained in this presentation are not intended to be indicative of future financial results. The events and circumstances reflected in these forward-looking statements, may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Undue reliance should not be placed on the forward-looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors could emerge from time to time, and it is not possible for our management to predict all uncertainties that the Company may face. Non-GAAP Measures To supplement our financial results determined by U.S. generally accepted accounting principles (“GAAP”), we have included certain non-GAAP information for our business. We believe that non-GAAP financial measures are helpful in understanding our business as it is useful to investors in allowing for greater transparency of supplemental information used by management. Non-GAAP financial measures should be considered in addition to, but not as a substitute for, reported GAAP results. Please see the “Reconciliation of GAAP to Non-GAAP Financial Measures” at the end of this presentation for a reconciliation of non-GAAP financial measures to their most directly comparable GAAP measures. This presentation includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements, among other things, relate to the Company’s growth drivers and expected levels of our organic growth; the impact of our investment in development and commercial initiatives; the anticipated impact of real estate transactions, debt repayment and contract renegotiations; financial guidance, including timing of revenues and EBITDA; our ability to manage costs and to achieve our financial goals; our ability to operate under lending covenants; our ability to maintain sufficient liquidity to operate the business; our ability to pay our debt under our credit agreement and to maintain relationships with CDMO commercial partners and develop additional commercial and development partnerships. The words "anticipate", "believe", "could", "estimate", “upcoming”, "expect", "intend", "may", "plan", "predict", "project", "will" and similar terms and phrases may be used to identify forward-looking statements in this presentation. The forward-looking statements in this presentation are only predictions. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or a combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that could cause the company’s actual outcomes to differ materially from those expressed in or underlying these forward-looking statements include, but are not limited to, unstable market and macroeconomic conditions, including any adverse impact on the customer ordering patterns or inventory rebalancing or disruption in raw materials or supply chain; demand for the company’s services, which depends in part on customers’ research and development funding, their clinical plans and the market success of their products; customers' changing inventory requirements and manufacturing plans; customers and prospective customers decisions to move forward with the company’s manufacturing services; the average profitability, or mix, of the products the company manufactures; the company’s ability to enhance existing or introduce new services in a timely manner; the Company’s ability to close its previously announced land sale transaction on the anticipated timeline; fluctuations in the costs, availability, and suitability of the components of the products the company manufactures, including active pharmaceutical ingredients, excipients, purchased components and raw materials, or the company’s customers facing increasing or new competition; the Company’s ability to collect on customers’ receivable balances; the extent to which health epidemics and other outbreaks of communicable diseases could disrupt our operations; and other risks and uncertainties discussed in our filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to us, and we assume no obligation to update any forward-looking statements except as required by applicable law. Forward Looking Statements

Investment Highlights Re-Organized, Rebranded Company Poised for Growth and Diversification Success State-of-the-Art, Newly Upgraded Facilities, Available Capacity in U.S. 30+ Years of Successful Commercial Manufacturing for Multiple Global Customers Solid Base of Development and Commercial Customers Highly Experienced Management Team and Talented Workforce to Drive Future Growth Strong Regulatory Track Record Spanning Multiple Countries and Agencies NDA Ownership and Profit-Sharing Structure for Certain Drug Assets End-to-End Capabilities with Unique Expertise Solving a Wide Array of Complex Dosage Formulation & Development Challenges 3
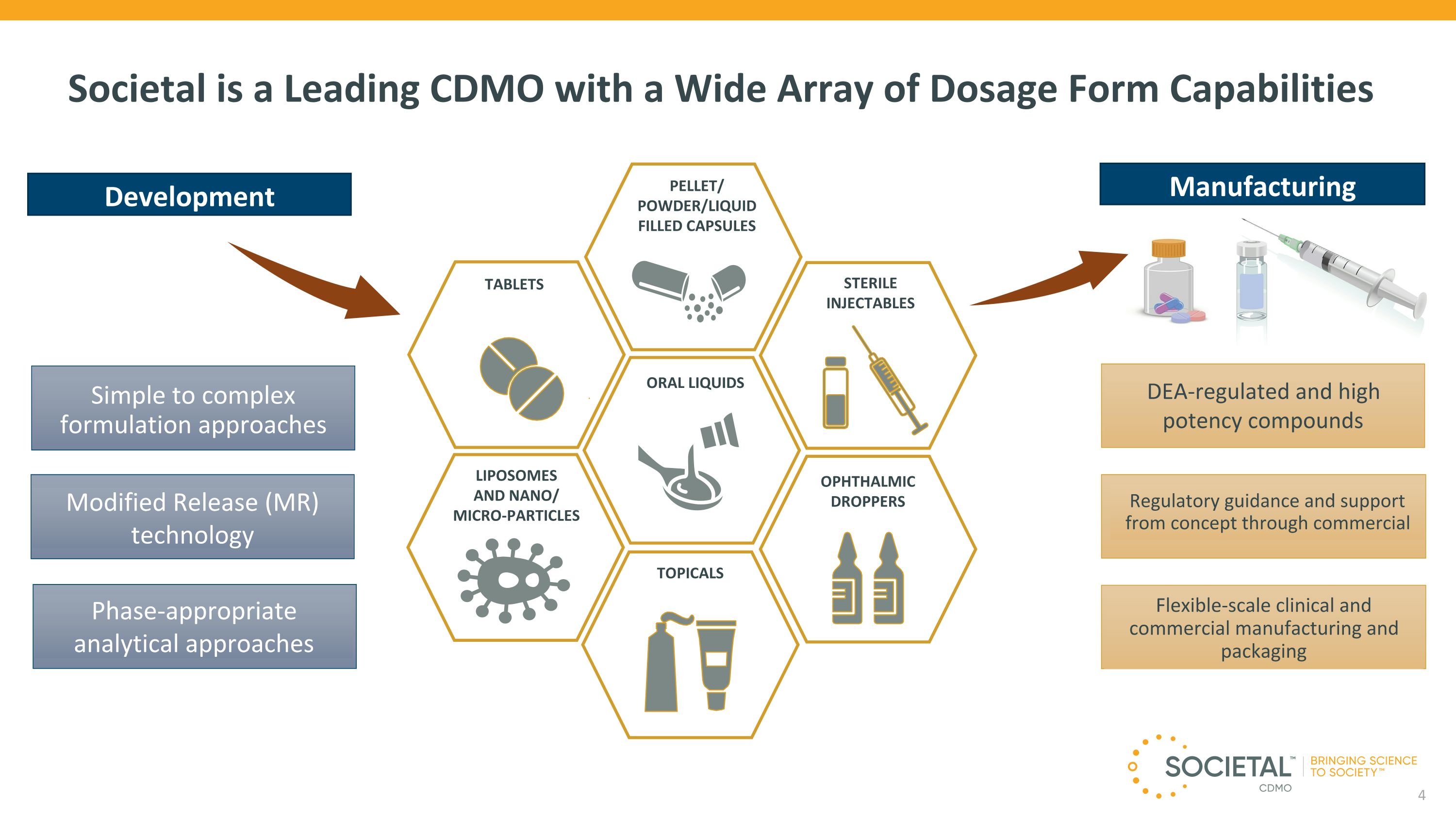
Societal is a Leading CDMO with a Wide Array of Dosage Form Capabilities DEA-regulated and high �potency compounds Regulatory guidance and support �from concept through commercial Flexible-scale clinical and commercial manufacturing and packaging Simple to complex formulation approaches Modified Release (MR) technology Phase-appropriate analytical approaches LIPOSOMES �AND NANO/�MICRO-PARTICLES PELLET/ POWDER/LIQUID �FILLED CAPSULES ORAL LIQUIDS TABLETS OPHTHALMIC DROPPERS STERILE INJECTABLES TOPICALS Manufacturing Development 4
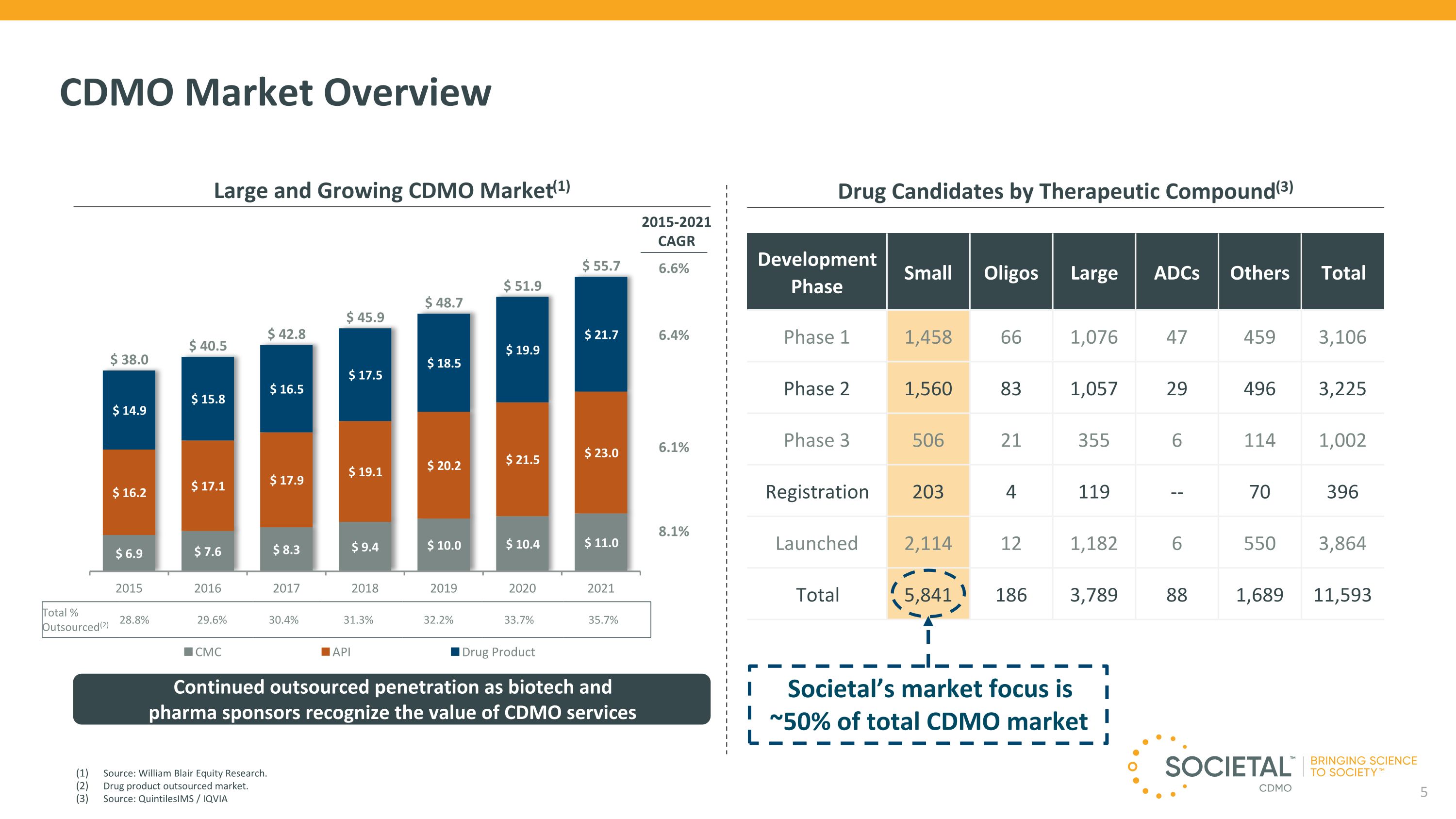
14 CDMO Market Overview Drug Candidates by Therapeutic Compound(3) Total % Outsourced(2) 28.8% 29.6% 30.4% 31.3% 32.2% 33.7% 35.7% Continued outsourced penetration as biotech and �pharma sponsors recognize the value of CDMO services 6.6% 6.4% 6.1% 8.1% 2015-2021 CAGR Large and Growing CDMO Market(1) Source: William Blair Equity Research. Drug product outsourced market. Source: QuintilesIMS / IQVIA Societal’s market focus is ~50% of total CDMO market Development Phase Small Oligos Large ADCs Others Total Phase 1 1,458 66 1,076 47 459 3,106 Phase 2 1,560 83 1,057 29 496 3,225 Phase 3 506 21 355 6 114 1,002 Registration 203 4 119 -- 70 396 Launched 2,114 12 1,182 6 550 3,864 Total 5,841 186 3,789 88 1,689 11,593 5
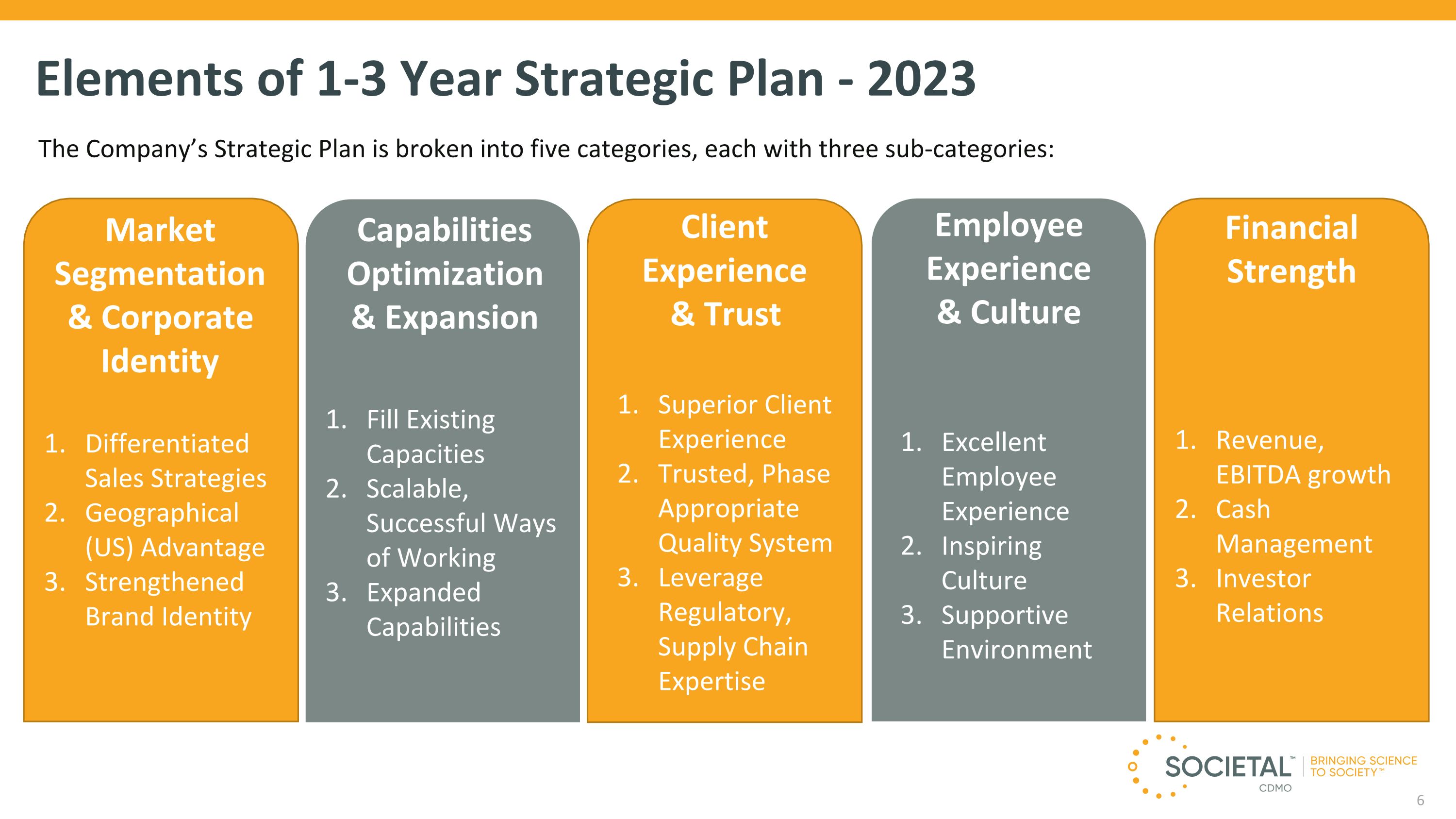
Elements of 1-3 Year Strategic Plan - 2023 The Company’s Strategic Plan is broken into five categories, each with three sub-categories: Market Segmentation & Corporate Identity Differentiated Sales Strategies Geographical (US) Advantage Strengthened Brand Identity Capabilities Optimization & Expansion Fill Existing Capacities Scalable, Successful Ways of Working Expanded Capabilities Client Experience & Trust Superior Client Experience Trusted, Phase Appropriate Quality System Leverage Regulatory, Supply Chain Expertise Employee Experience & Culture Excellent Employee Experience Inspiring Culture Supportive Environment Financial Strength Revenue, EBITDA growth Cash Management Investor Relations
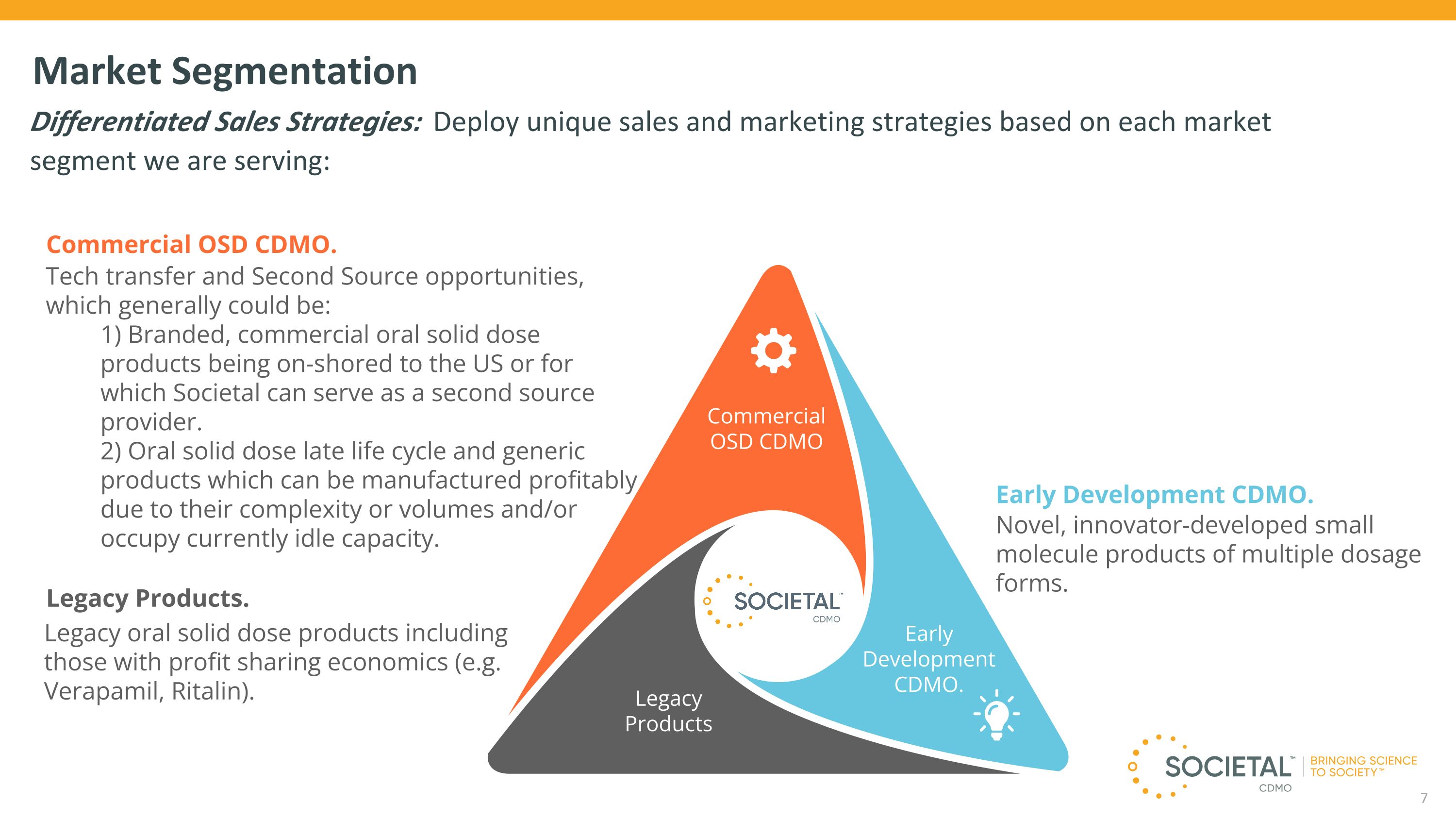
Market Segmentation Differentiated Sales Strategies: Deploy unique sales and marketing strategies based on each market segment we are serving: 7 Legacy oral solid dose products including those with profit sharing economics (e.g. Verapamil, Ritalin). Commercial OSD CDMO. Tech transfer and Second Source opportunities, which generally could be: 1) Branded, commercial oral solid dose products being on-shored to the US or for which Societal can serve as a second source provider. 2) Oral solid dose late life cycle and generic products which can be manufactured profitably due to their complexity or volumes and/or occupy currently idle capacity. Legacy Products. Legacy Products Early Development CDMO. Commercial OSD CDMO Novel, innovator-developed small molecule products of multiple dosage forms. Early Development CDMO.

Branded Commercial Tablet Product Exclusive U.S. based Manufacturer Long term Contractual Master Services & Supply Agreement Annual minimum purchase requirements Expanding Base of Commercial Customers End-to-end solutions for customers from early-stage development to scaled commercial production Verapamil PM/Verelan™ SR/PM Societal owns NDA and DMF In event of termination Societal can switch distributors within a few months Branded & authorized generic sustained release capsules Complex formulation and manufacturing – proprietary know-how Exclusive sole supplier Mature single player market Verapamil SR Societal owns NDA and DMF Authorized generic sustained release capsules, including an exclusive dosage form Complex formulation and manufacturing–proprietary ‘know-how’ Exclusive sole supplier Mature two player market – Teva maintains ~70% market share Ritalin LA ™/Focalin XR Societal owns DMF Branded & authorized generic sustained release capsules – sold US/OUS Complex formulation and manufacturing Exclusive sole supplier Mature multi-player market Regulatory & tech transfer risk and cost given Societal quality track record and lifecycle of product Donnatal® Elixir and Tablets Exclusive sole supplier, 5yr agreement through beginning of 2025 4 APIs and multi-step manufacturing process Annual purchase requirements 8 Strong commercial customer base stabilizes business and minimizes fluctuations in revenues Long-term relationships (20+ years) with key commercial partners and fully contracted through 2024 (Verapamil) – 2025 (Ritalin/Focalin) Commercial customer forecasts (generally 12-to-24-month projections) with binding PO’s typically for first three months, provides demand visibility and helps optimize supply chain execution Tech Transfers in Process Two unnamed Oral Solid Dose Tech Transfers Two development programs Three proposals for additional commercial programs Ritalin® IR tablets
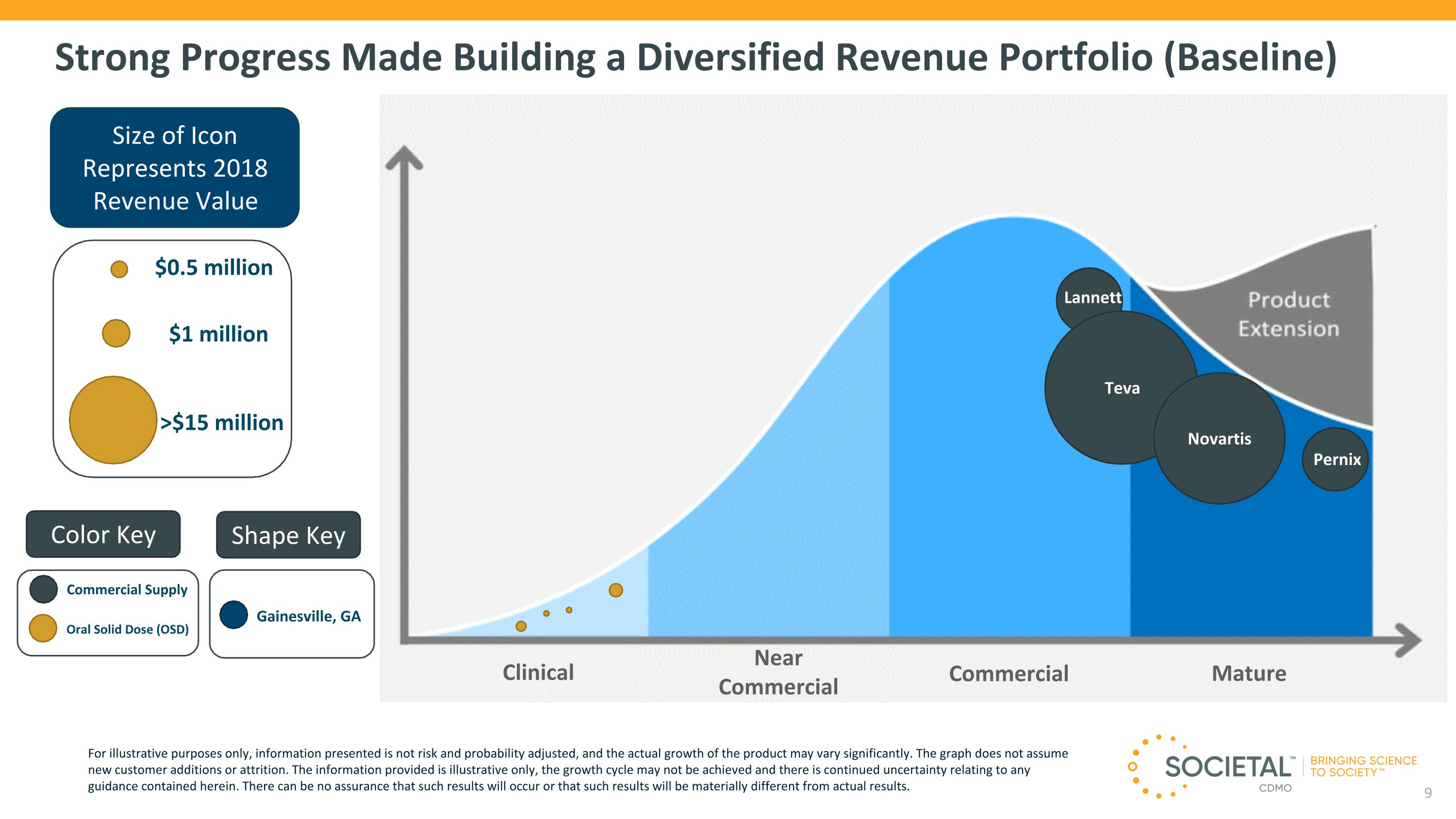
Strong Progress Made Building a Diversified Revenue Portfolio (Baseline) Size of Icon Represents 2018 Revenue Value $1 million >$15 million $0.5 million Teva Novartis Clinical Color Key Shape Key Gainesville, GA Oral Solid Dose (OSD) Near Commercial Commercial Mature Commercial Supply Lannett Pernix For illustrative purposes only, information presented is not risk and probability adjusted, and the actual growth of the product may vary significantly. The graph does not assume new customer additions or attrition. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results will be materially different from actual results.
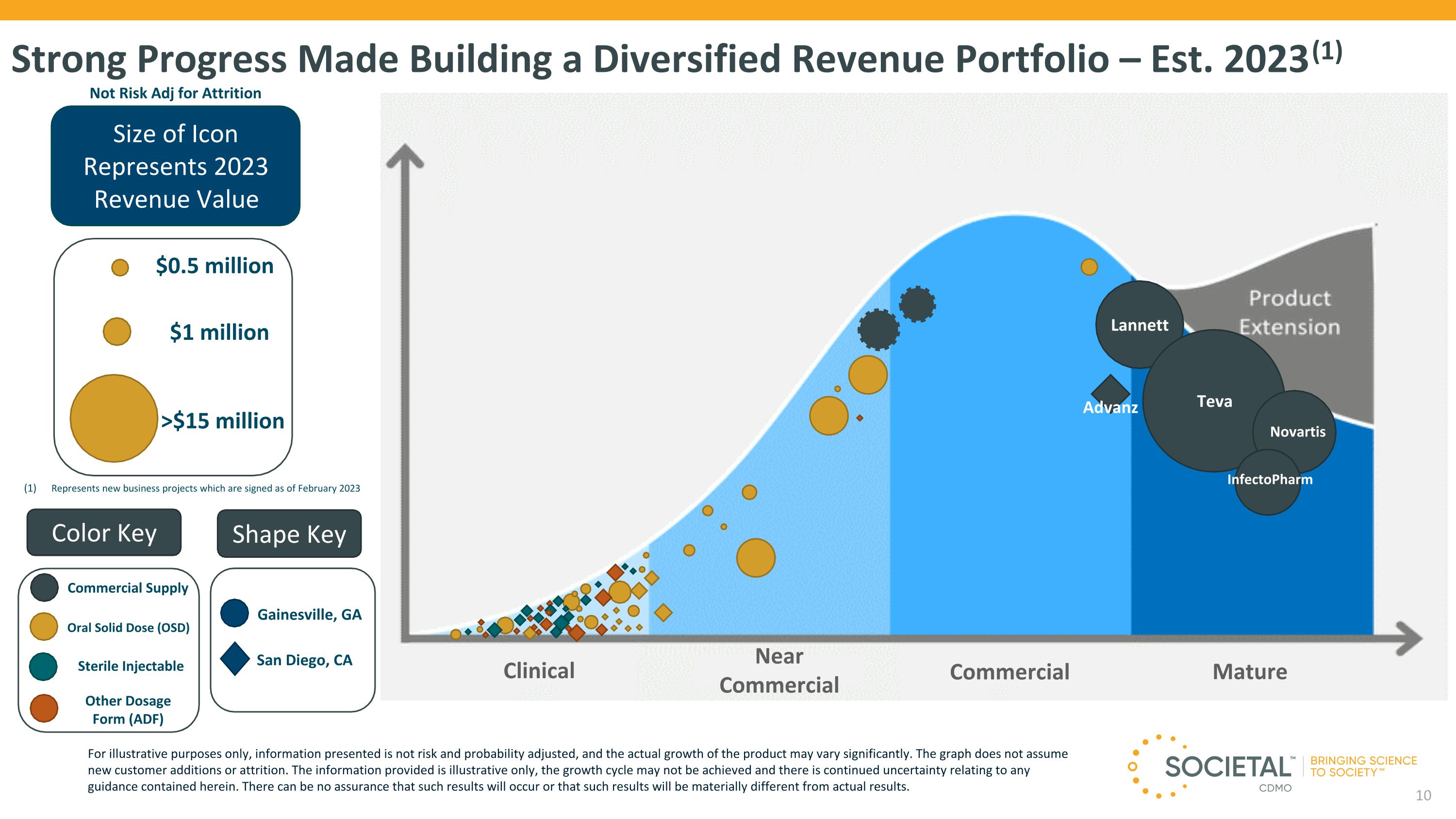
Strong Progress Made Building a Diversified Revenue Portfolio – Est. 2023 (1) Size of Icon Represents 2023 Revenue Value $1 million >$15 million $0.5 million Not Risk Adj for Attrition Lannett Teva Advanz Clinical Color Key Shape Key Gainesville, GA Sterile Injectable Oral Solid Dose (OSD) Other Dosage Form (ADF) San Diego, CA Near Commercial Commercial Mature Commercial Supply For illustrative purposes only, information presented is not risk and probability adjusted, and the actual growth of the product may vary significantly. The graph does not assume new customer additions or attrition. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results will be materially different from actual results. InfectoPharm Represents new business projects which are signed as of February 2023 Novartis
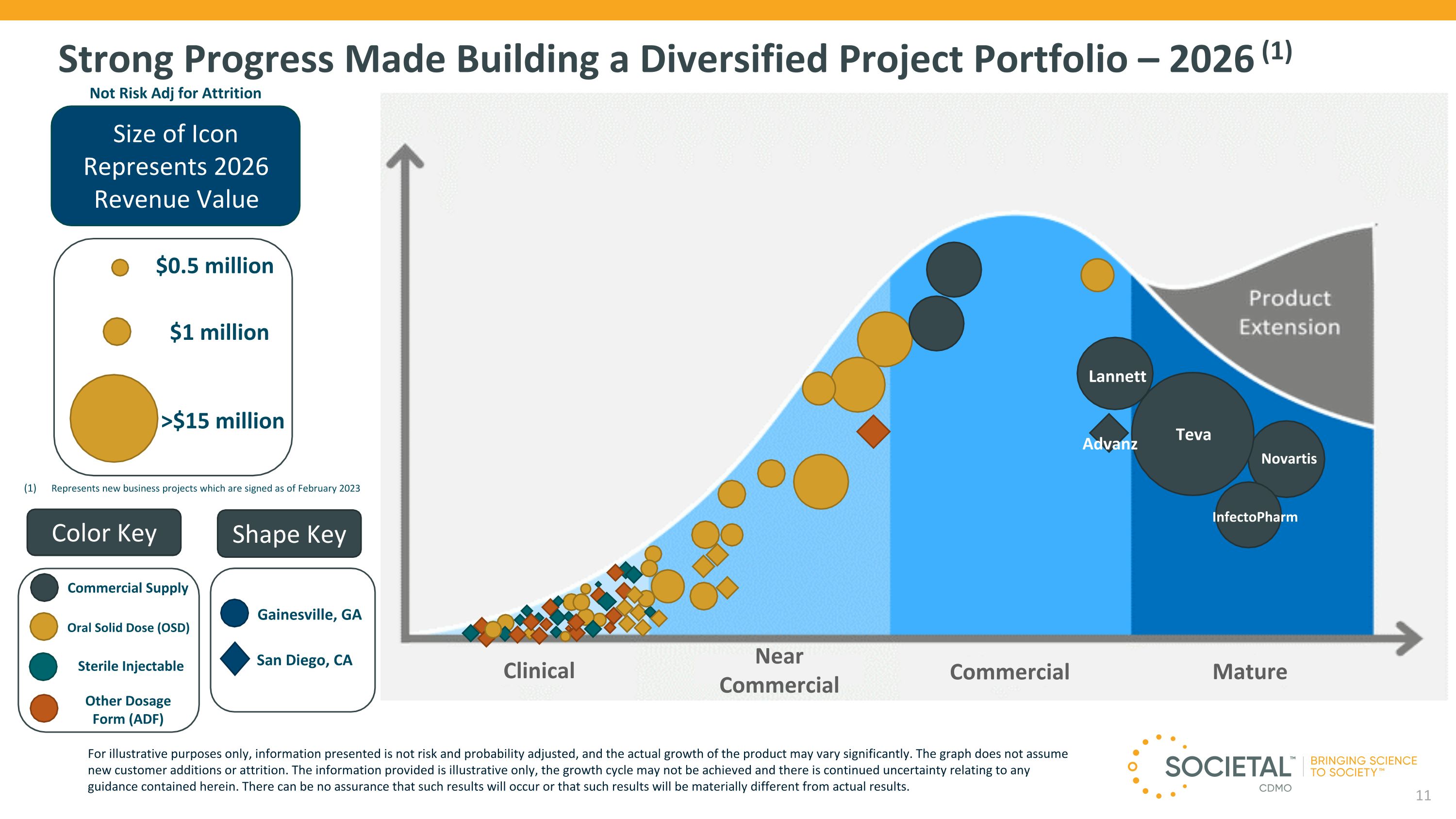
Strong Progress Made Building a Diversified Project Portfolio – 2026 (1) Size of Icon Represents 2026 Revenue Value $1 million >$15 million $0.5 million Not Risk Adj for Attrition Clinical Color Key Shape Key Gainesville, GA Sterile Injectable Oral Solid Dose (OSD) Other Dosage Form (ADF) San Diego, CA Near Commercial Commercial Mature Commercial Supply For illustrative purposes only, information presented is not risk and probability adjusted, and the actual growth of the product may vary significantly. The graph does not assume new customer additions or attrition. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results will be materially different from actual results. Represents new business projects which are signed as of February 2023 Teva InfectoPharm Advanz Lannett Novartis
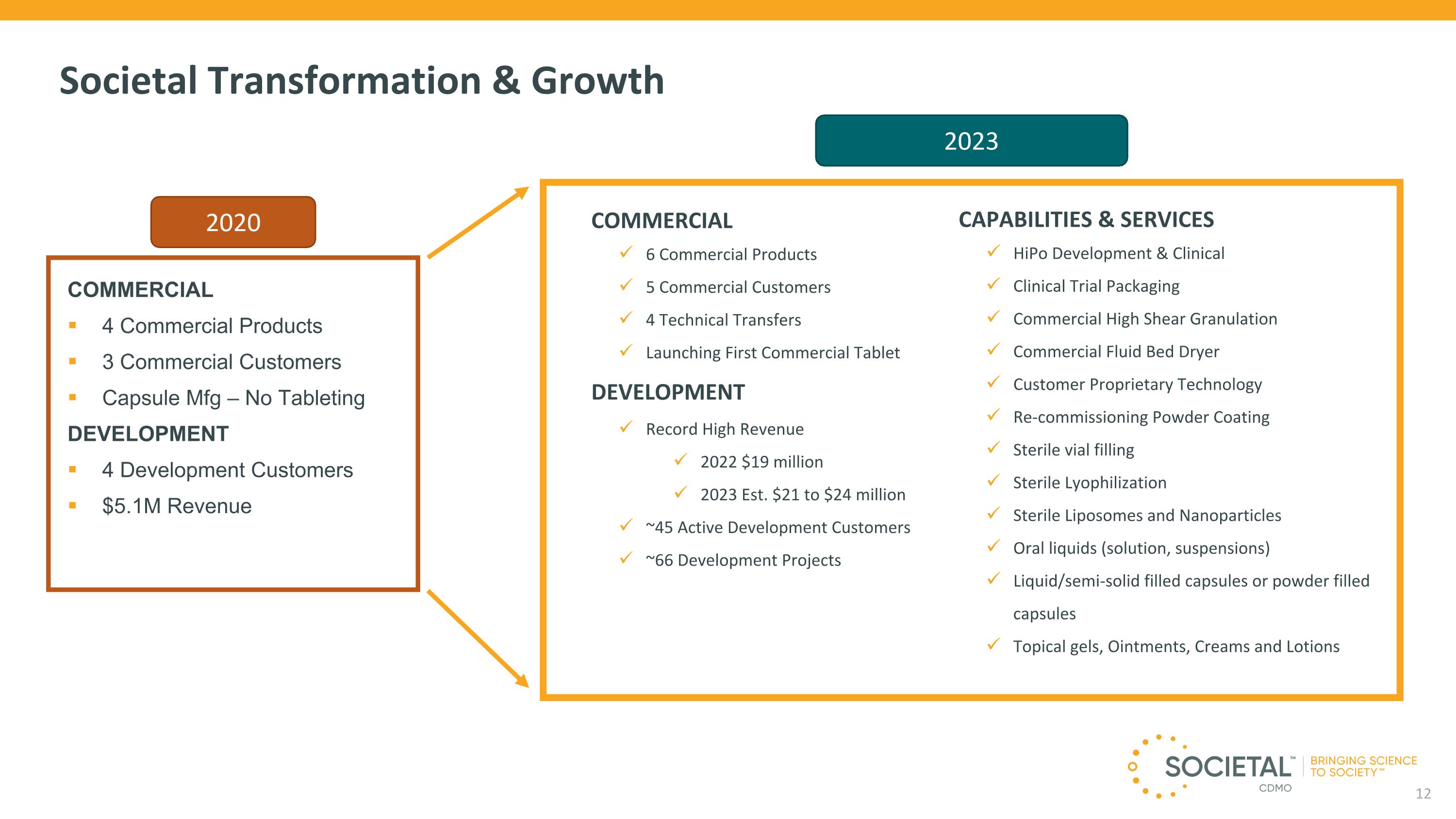
COMMERCIAL 4 Commercial Products 3 Commercial Customers Capsule Mfg – No Tableting DEVELOPMENT 4 Development Customers $5.1M Revenue Societal Transformation & Growth CAPABILITIES & SERVICES HiPo Development & Clinical Clinical Trial Packaging Commercial High Shear Granulation Commercial Fluid Bed Dryer Customer Proprietary Technology Re-commissioning Powder Coating Sterile vial filling Sterile Lyophilization Sterile Liposomes and Nanoparticles Oral liquids (solution, suspensions) Liquid/semi-solid filled capsules or powder filled capsules Topical gels, Ointments, Creams and Lotions COMMERCIAL 6 Commercial Products 5 Commercial Customers 4 Technical Transfers Launching First Commercial Tablet DEVELOPMENT Record High Revenue 2022 $19 million 2023 Est. $21 to $24 million ~45 Active Development Customers ~66 Development Projects 2020 2023 12
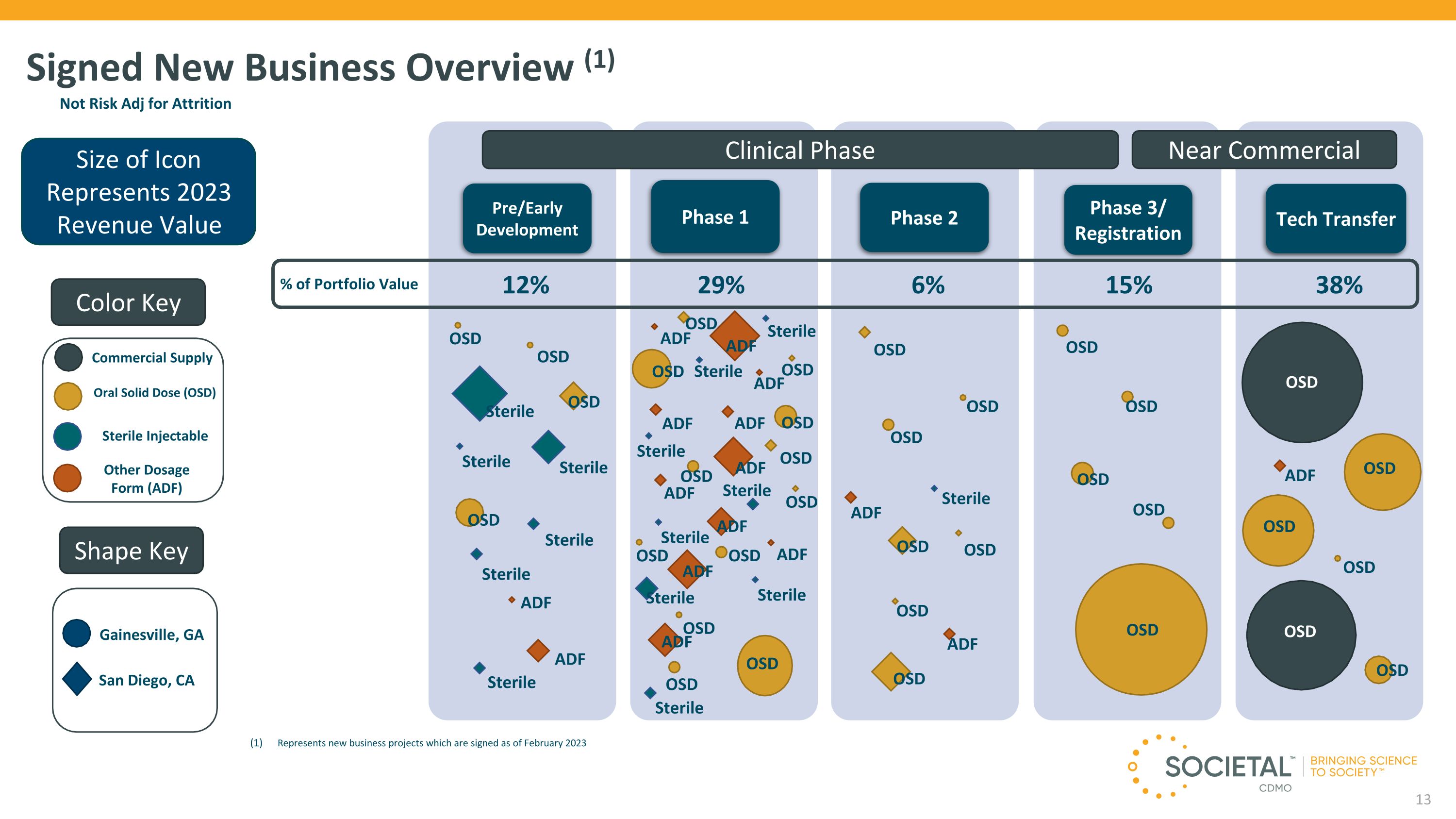
Signed New Business Overview (1) Size of Icon Represents 2023 Revenue Value Pre/Early Development Phase 1 Phase 2 Phase 3/ Registration Tech Transfer OSD Sterile OSD OSD OSD ADF ADF ADF OSD OSD OSD OSD Sterile Sterile Color Key Shape Key Gainesville, GA Sterile Injectable Oral Solid Dose (OSD) Other Dosage Form (ADF) San Diego, CA Commercial Supply Clinical Phase Near Commercial 12% 29% 6% 15% 38% % of Portfolio Value Represents new business projects which are signed as of February 2023 OSD Sterile 13 OSD OSD OSD OSD ADF Sterile Sterile OSD OSD OSD OSD OSD OSD OSD Sterile ADF OSD ADF ADF ADF ADF ADF ADF ADF ADF ADF ADF ADF Sterile Sterile Sterile Sterile Sterile Sterile Sterile Sterile OSD OSD OSD OSD OSD OSD OSD OSD OSD OSD OSD OSD OSD Not Risk Adj for Attrition
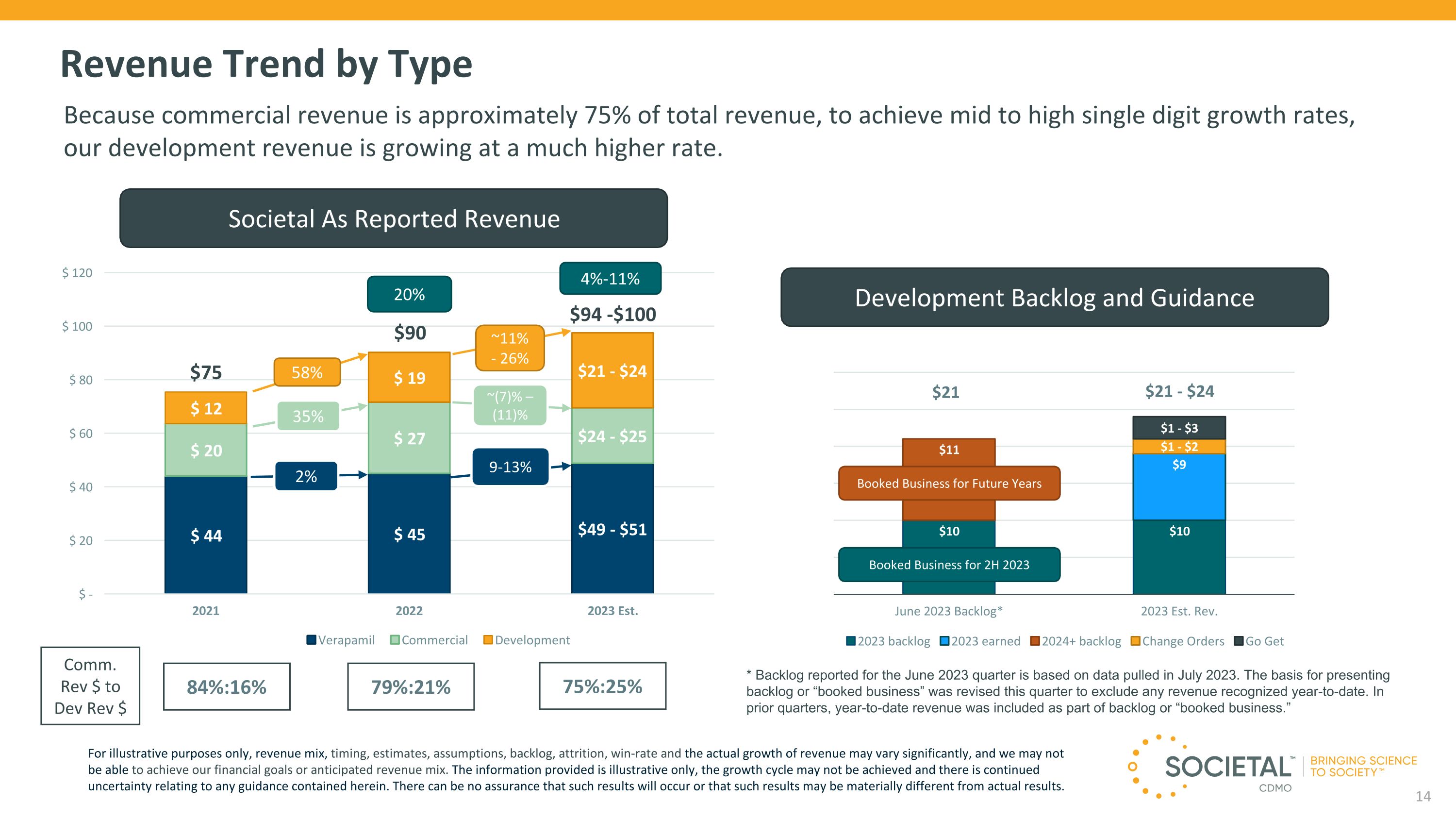
$94 -$100 $75 Revenue Trend by Type Societal As Reported Revenue 58% ~11%- 26% ~(7)% – (11)% 35% 84%:16% 79%:21% 75%:25% Comm. Rev $ to Dev Rev $ 20% 4%-11% Because commercial revenue is approximately 75% of total revenue, to achieve mid to high single digit growth rates, our development revenue is growing at a much higher rate. For illustrative purposes only, revenue mix, timing, estimates, assumptions, backlog, attrition, win-rate and the actual growth of revenue may vary significantly, and we may not be able to achieve our financial goals or anticipated revenue mix. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results may be materially different from actual results. Development Backlog and Guidance $21 Booked Business for Future Years Booked Business for 2H 2023 $21 - $24 * Backlog reported for the June 2023 quarter is based on data pulled in July 2023. The basis for presenting backlog or “booked business” was revised this quarter to exclude any revenue recognized year-to-date. In prior quarters, year-to-date revenue was included as part of backlog or “booked business.” 2% 9-13% $90
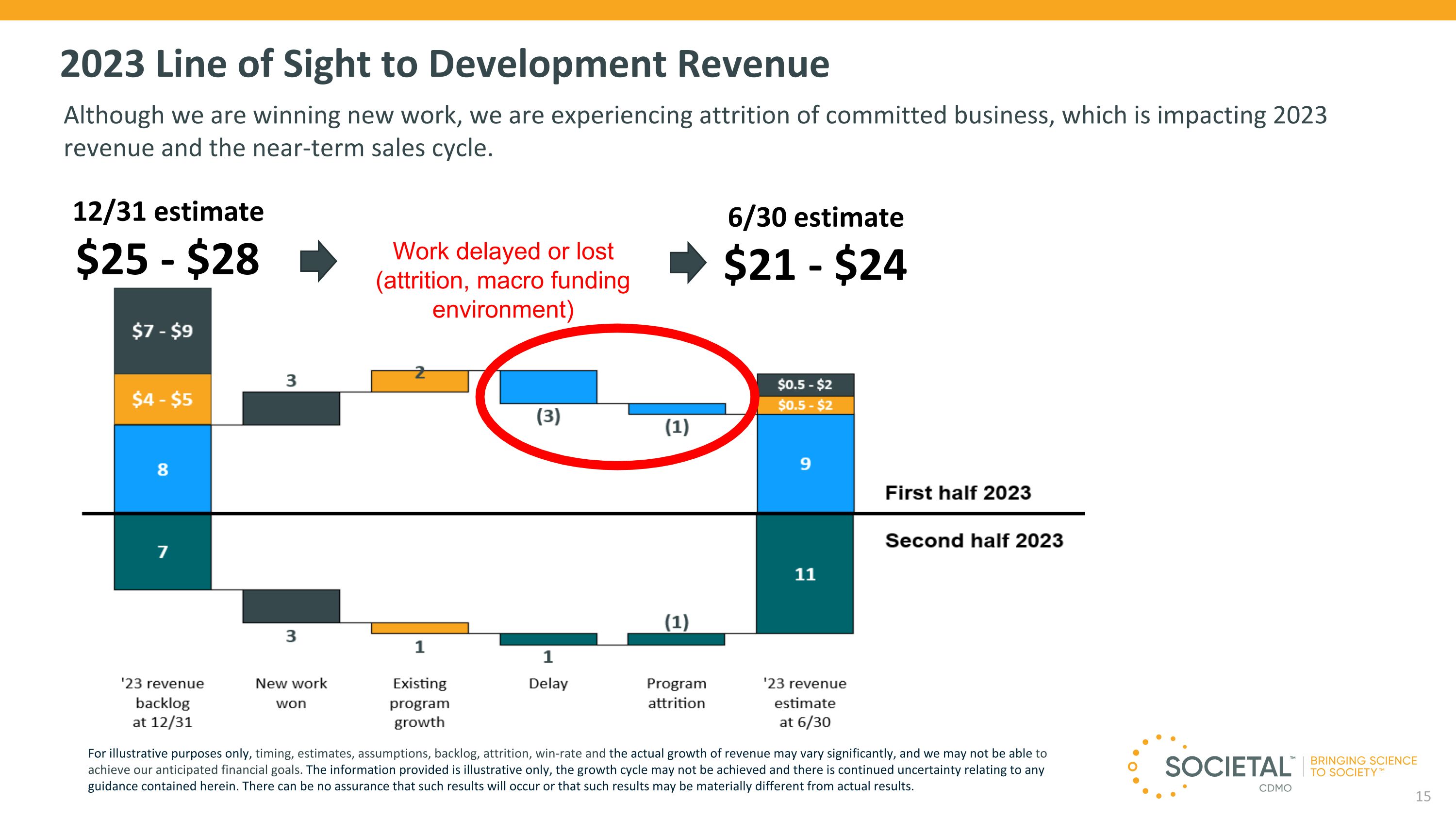
2023 Line of Sight to Development Revenue Although we are winning new work, we are experiencing attrition of committed business, which is impacting 2023 revenue and the near-term sales cycle. Work delayed or lost (attrition, macro funding environment) 12/31 estimate $25 - $28 6/30 estimate $21 - $24 For illustrative purposes only, timing, estimates, assumptions, backlog, attrition, win-rate and the actual growth of revenue may vary significantly, and we may not be able to achieve our anticipated financial goals. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results may be materially different from actual results.
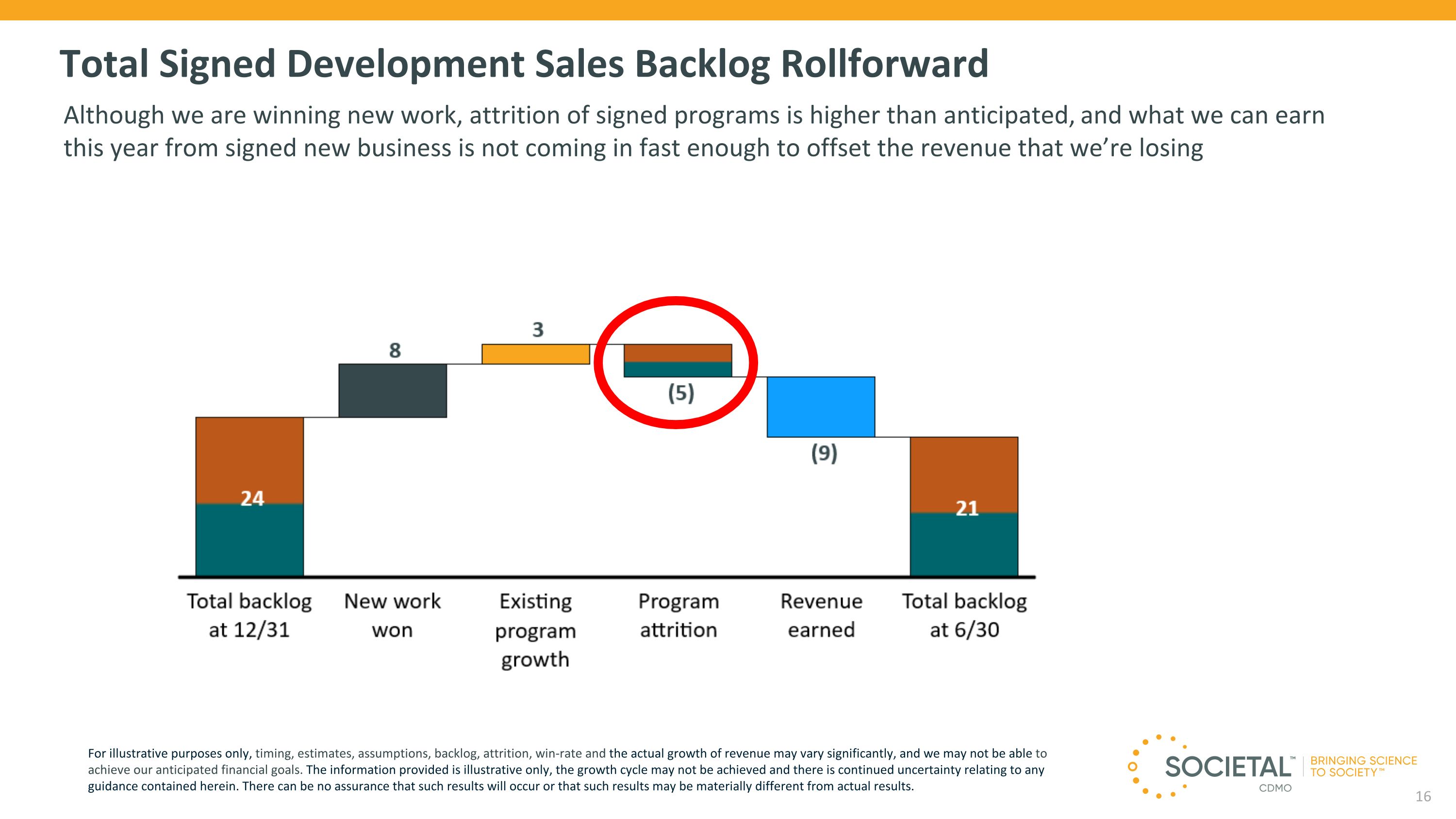
Total Signed Development Sales Backlog Rollforward Although we are winning new work, attrition of signed programs is higher than anticipated, and what we can earn this year from signed new business is not coming in fast enough to offset the revenue that we’re losing For illustrative purposes only, timing, estimates, assumptions, backlog, attrition, win-rate and the actual growth of revenue may vary significantly, and we may not be able to achieve our anticipated financial goals. The information provided is illustrative only, the growth cycle may not be achieved and there is continued uncertainty relating to any guidance contained herein. There can be no assurance that such results will occur or that such results may be materially different from actual results.
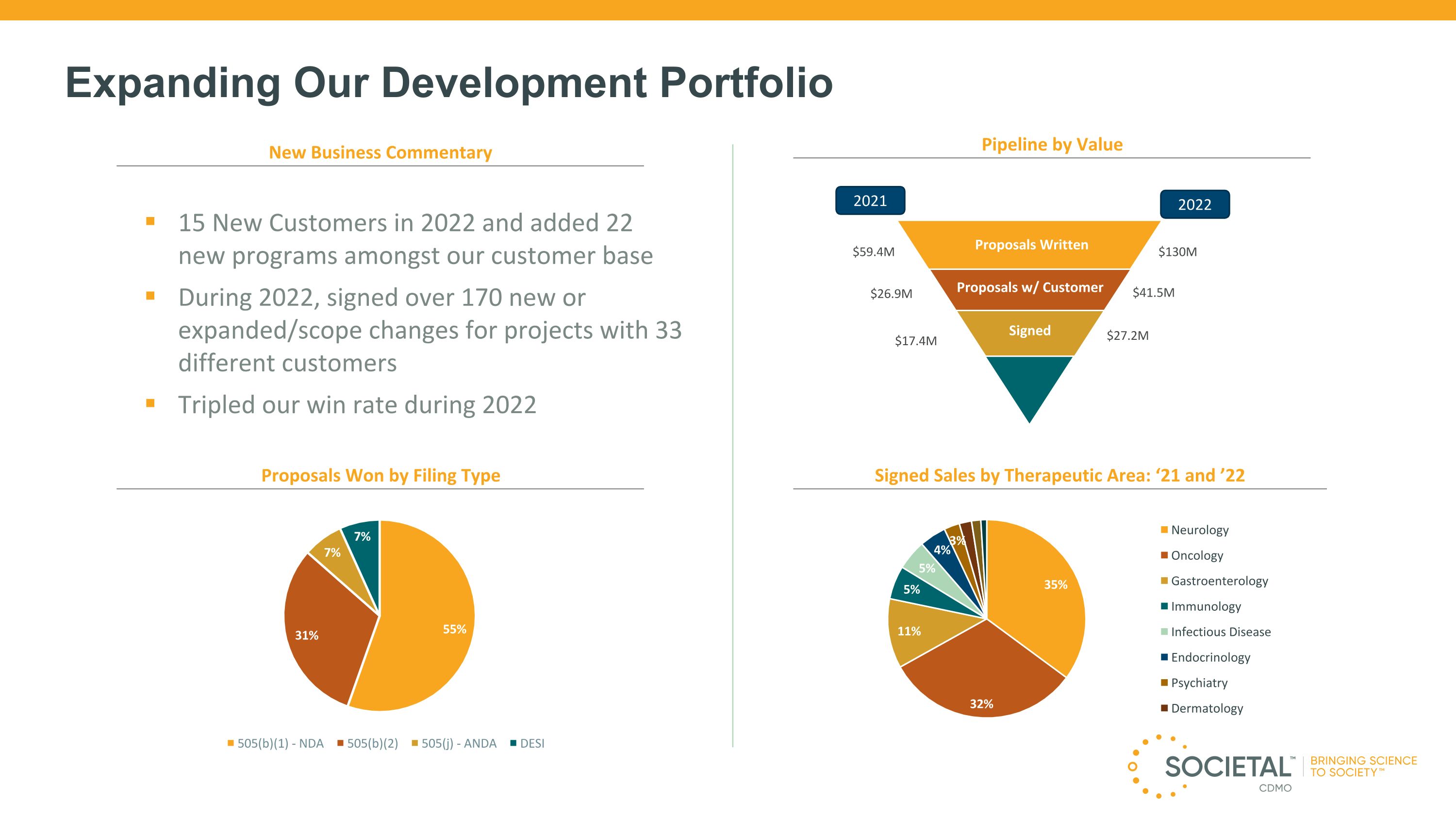
New Business Commentary Pipeline by Value Proposals Won by Filing Type Signed Sales by Therapeutic Area: ‘21 and ’22 15 New Customers in 2022 and added 22 new programs amongst our customer base During 2022, signed over 170 new or expanded/scope changes for projects with 33 different customers Tripled our win rate during 2022 Proposals w/ Customer Signed Proposals Written $27.2M $41.5M $130M 71 Expanding Our Development Portfolio $17.4M $26.9M $59.4M 2021 2022

State-of-the-Art Facilities Societal™ CDMO – Gould Facility Located in Gainesville, GA Size: 24,000 ft2 ~35 FTEs Opened 2018 Current capacity (single shift): ~30-40% Leased through 2025 with renewal options Located in Gainesville, GA Size: 97,000 ft2 ~190 FTEs Opened ~1985 Current capacity (single shift): ~60% Leased through 2042 with renewal options Chestnut performs development and cGMP (pre-commercial) development manufacturing before tech transfer to Gould site. High potency commercial production remains at Chestnut Significant experience transitioning projects from �late-phase development to robust, long-term �commercial production Societal™ CDMO – Chestnut Facility Societal™ CDMO – San Diego Located in San Diego, CA Size: 24,500 ft2 ~60 FTEs Opened 2014 Current capacity (single shift): ~30-40%(1) State of the art facility, FDA and FDB (CA) inspected San Diego performs development work, focusing on Advanced Dosage Forms – Development Services (aseptic fill / finish, inhalation, etc.) Commercial Development California is the #1 state for life sciences VC investment(2) Excludes new vial filler and lyophilizer services. Source: California Life Science Association and PWC’s California Life Sciences Report 2020. 18
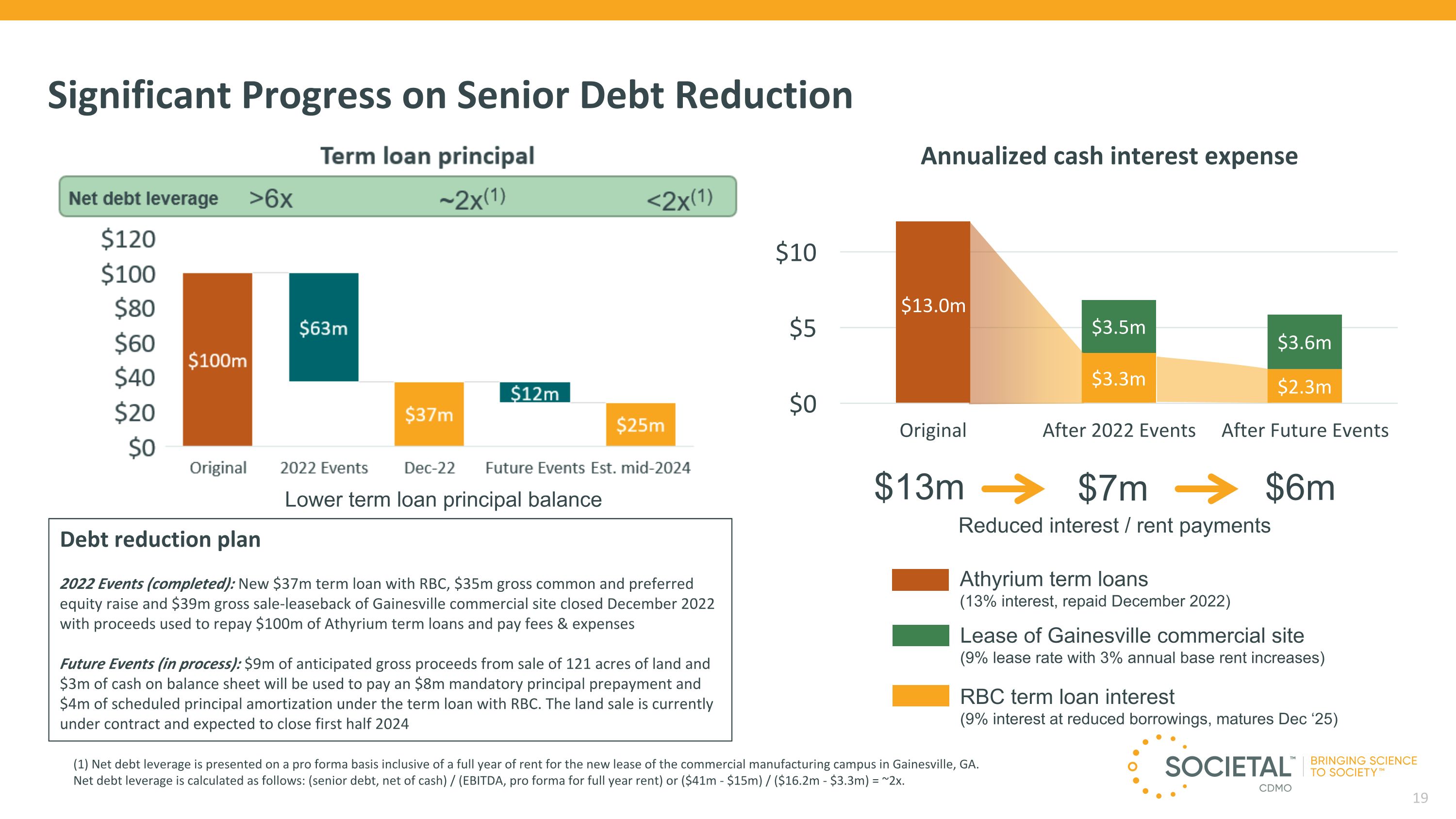
Significant Progress on Senior Debt Reduction Reduced interest / rent payments $13m $7m $6m Term loan principal Annualized cash interest expense Lower term loan principal balance Debt reduction plan 2022 Events (completed): New $37m term loan with RBC, $35m gross common and preferred equity raise and $39m gross sale-leaseback of Gainesville commercial site closed December 2022 with proceeds used to repay $100m of Athyrium term loans and pay fees & expenses Future Events (in process): $9m of anticipated gross proceeds from sale of 121 acres of land and $3m of cash on balance sheet will be used to pay an $8m mandatory principal prepayment and $4m of scheduled principal amortization under the term loan with RBC. The land sale is currently under contract and expected to close first half 2024 Lease of Gainesville commercial site (9% lease rate with 3% annual base rent increases) RBC term loan interest (9% interest at reduced borrowings, matures Dec ‘25) Athyrium term loans (13% interest, repaid December 2022) Net debt leverage >6x ~2x(1) <2x(1) (1) Net debt leverage is presented on a pro forma basis inclusive of a full year of rent for the new lease of the commercial manufacturing campus in Gainesville, GA. Net debt leverage is calculated as follows: (senior debt, net of cash) / (EBITDA, pro forma for full year rent) or ($41m - $15m) / ($16.2m - $3.3m) = ~2x.

Recent Developments: Debt Restructuring We successfully restructured our debt and certain covenants with our creditors to provide additional financial flexibility to the company during this time of market uncertainty and to align to the expected updated timing of the land sale. Restructuring Highlights RBC Debt Covenants: Minimum Liquidity: Maintain $4.0M; step down to $3.5M at 6/30/2024; step up to $4.5M at 9/30/2024; step up to $5.0M at 12/31/2024 Monthly tests at month end of $1.5M Leverage Ratio: Maintain at 3.75x; step down to 2.75x at earlier of (i) 6/30/2024 or (ii) Gainesville, GA land sale Fixed Charge Coverage Ratio Lowered to 1.00x at 9/30/2024; 1.05x thereafter Debt Repayment In the event of the Gainesville, GA land sale, $7.5M of RBC principal is repaid (was $10.0M) IRISYS Sellers Note Principal of ~$2.1M is deferred until earlier of land sale or June 2024

Financial highlights Full year 2023 guidance Revenue: $94 to $100 million, an increase of 4% - 11% over 2022 Net loss: $(10.7) to $(7.7) million EBITDA, as adjusted: $12 to $15 million Revenue and operating cash flow positive contract development and manufacturing (CDMO) business Second quarter 2023 financial results Revenues were $21.8 million, a decrease of 6% from Q2 2022 Net loss: $3.2 million EBITDA, as adjusted, was $2.8 million, down $1.2 million from Q2 2022 Second quarter 2023 highlights Signed Multiple New Business Agreements with New and Existing Customers Secured Schedule 1 Controlled Substance Manufacturing License from Drug Enforcement Agency; Allows Expansion into Manufacture of Psychedelic Drug Products
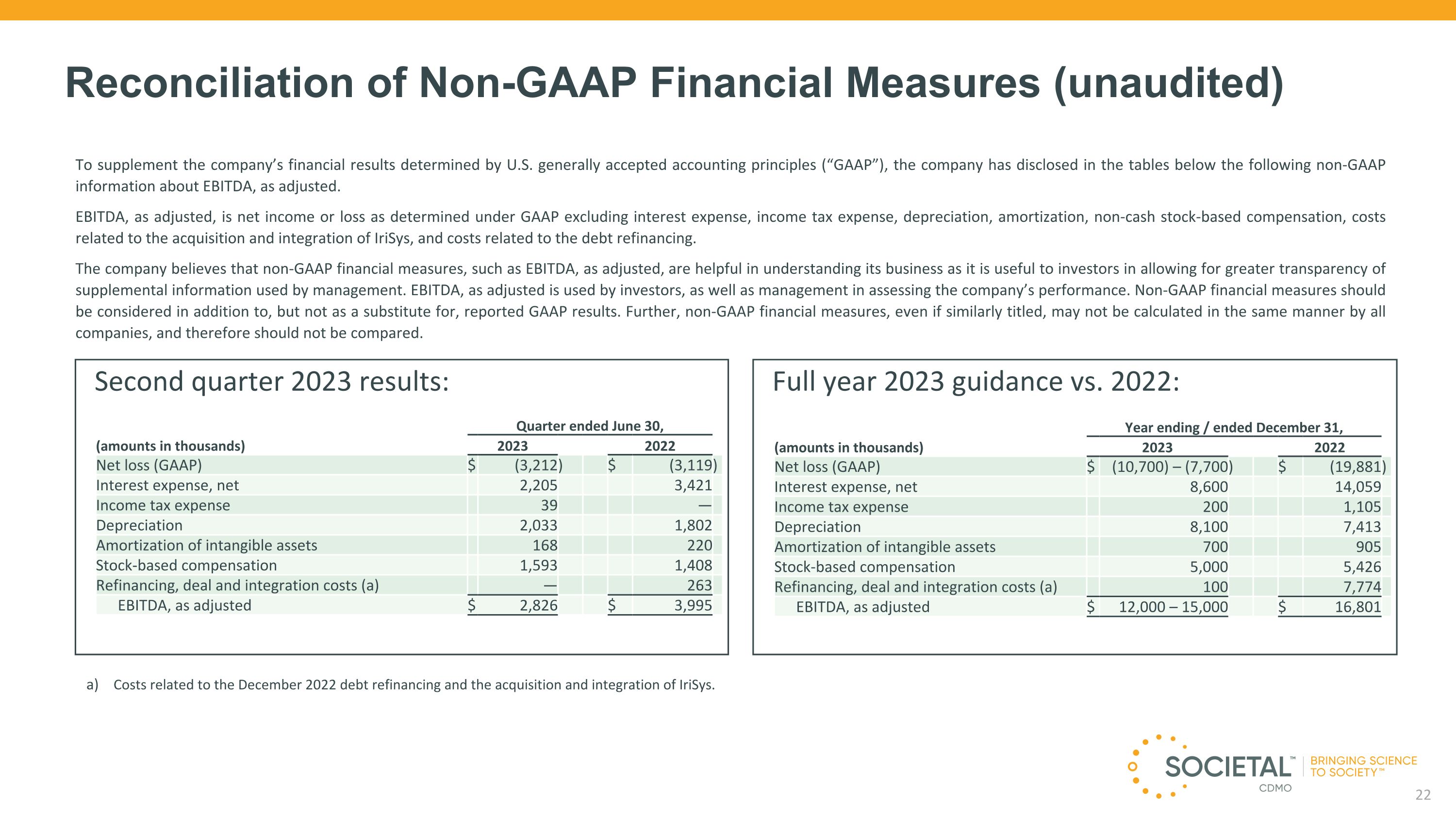
Second quarter 2023 results: Quarter ended June 30, (amounts in thousands) 2023 2022 Net loss (GAAP) $ (3,212 ) $ (3,119 ) Interest expense, net 2,205 3,421 Income tax expense 39 — Depreciation 2,033 1,802 Amortization of intangible assets 168 220 Stock-based compensation 1,593 1,408 Refinancing, deal and integration costs (a) — 263 EBITDA, as adjusted $ 2,826 $ 3,995 Reconciliation of Non-GAAP Financial Measures (unaudited) To supplement the company’s financial results determined by U.S. generally accepted accounting principles (“GAAP”), the company has disclosed in the tables below the following non-GAAP information about EBITDA, as adjusted. EBITDA, as adjusted, is net income or loss as determined under GAAP excluding interest expense, income tax expense, depreciation, amortization, non-cash stock-based compensation, costs related to the acquisition and integration of IriSys, and costs related to the debt refinancing. The company believes that non-GAAP financial measures, such as EBITDA, as adjusted, are helpful in understanding its business as it is useful to investors in allowing for greater transparency of supplemental information used by management. EBITDA, as adjusted is used by investors, as well as management in assessing the company’s performance. Non-GAAP financial measures should be considered in addition to, but not as a substitute for, reported GAAP results. Further, non-GAAP financial measures, even if similarly titled, may not be calculated in the same manner by all companies, and therefore should not be compared. Costs related to the December 2022 debt refinancing and the acquisition and integration of IriSys. Full year 2023 guidance vs. 2022: Year ending / ended December 31, (amounts in thousands) 2023 2022 Net loss (GAAP) $ (10,700) – (7,700 ) $ (19,881 ) Interest expense, net 8,600 14,059 Income tax expense 200 1,105 Depreciation 8,100 7,413 Amortization of intangible assets 700 905 Stock-based compensation 5,000 5,426 Refinancing, deal and integration costs (a) 100 7,774 EBITDA, as adjusted $ 12,000 – 15,000 $ 16,801























