- SCTL Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Societal CDMO (SCTL) 8-KOther Events
Filed: 28 Nov 17, 12:00am

November 2017 Relieving pain…….Improving lives Exhibit 99.1

Special Note Regarding Forward-Looking Statements This presentation includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements, among other things, relate to our business strategy, goals and expectations concerning our product candidates, future operations, prospects, plans and objectives of management. The words "anticipate", "believe", "could", "estimate", "expect", "intend", "may", "plan", "predict", "project", "will" and similar terms and phrases are used to identify forward-looking statements in this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or a combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. This presentation is intended to be nonpromotional and for investor discussion purposes only. The information provided herein contains references to investigational products and use of these products has not been approved by the FDA. The safety and efficacy of the investigational use of these development products has not been determined. There is no guarantee that the investigational use listed will be filed with and/or approved for marketing by any regulatory agency. These forward-looking statements should be considered together with the risks and uncertainties that may affect our business and future results included in our filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to us, and we assume no obligation to update any forward-looking statements except as required by applicable law.

Company Highlights Specialty pharmaceutical company focused on hospital and related settings with late stage investigational product, IV Meloxicam, targeting management of moderate to severe pain Filed New Drug Application for IV Meloxicam in July 2017; Received FDA acceptance for review in September 2017 (PDUFA Date- May 26, 2018) Multiple therapeutics in clinical development for hospital and related settings Non-opioid, alpha 2 agonist for peri-procedural use; Recent acquisition of Neuromuscular Blockers (NMB) and related reversal agents from Cornell University Used for rapid induction and reversal of neuromuscular blockade Revenue and cash flow positive contract development and manufacturing (CDMO) business Cash position-$41.3 million @ 9/30/17 $100 million credit facility secured on November 17, 2017 Three tranches- $60M at closing; $20M at NDA approval; $20M after demonstrating early commercial traction Substantial reduction of our cost of capital after full repayment of existing debt; Provides funding for IV Meloxicam approval milestone Interest only structure allows for reinvestment of CDMO’s cash flows in value accretive commercial activities Experienced management team with significant development, regulatory and commercial experience

Experienced Management and Board Gerri Henwood – President and CEO Founded Recro Pharma (REPH, NASDAQ), Auxilium Pharmaceuticals (AUXL, NASDAQ) and IBAH (former NASDAQ Co. – acquired 1998); GSK Michael Celano – CFO Over 35 years of financial leadership experience – Kensey Nash, BioRexis, Orasure, Arthur Andersen/KPMG Randy Mack – SVP, Development Over 25 years of clinical development experience – Adolor, Auxilium, Abbott Labs and Harris Labs Stewart McCallum, MD – CMO Over 9 years of GSK Clinical experience – Development experience; past clinical Investigator, KOL and Stanford U. Fred Graff – CCO Over 30 years of successful commercial experience, including sales and marketing leadership roles at Sepracor, RPR, and MAP Pharmaceuticals Scott Rizzo- General Manager, Gainesville CDMO Over 25 years of operations experience – Roche Pharmaceuticals, Barrier Therapeutics, PwC, E.I. DuPont Board of Directors Wayne B. Weisman – Chairman SCP VitaLife Partners Alfred Altomari CEO, Agile Therapeutics; former J&J William L. Ashton Harrison Consulting Group; frmly Amgen Michael Berelowitz, M.D. Former SVP, Specialty Care Business Unit, Pfizer Winston J. Churchill SCP VitaLife Partners Karen Flynn Senior Vice President and Chief Commercial Officer of West Pharmaceutical Services, Inc. Gerri Henwood – CEO Bryan Reasons SVP and CFO, Impax Laboratories

Recro Pharma: An Acute Care Company (REPH:NASDAQ)
Contract Manufacturing Acute Care Division Our Two Divisions
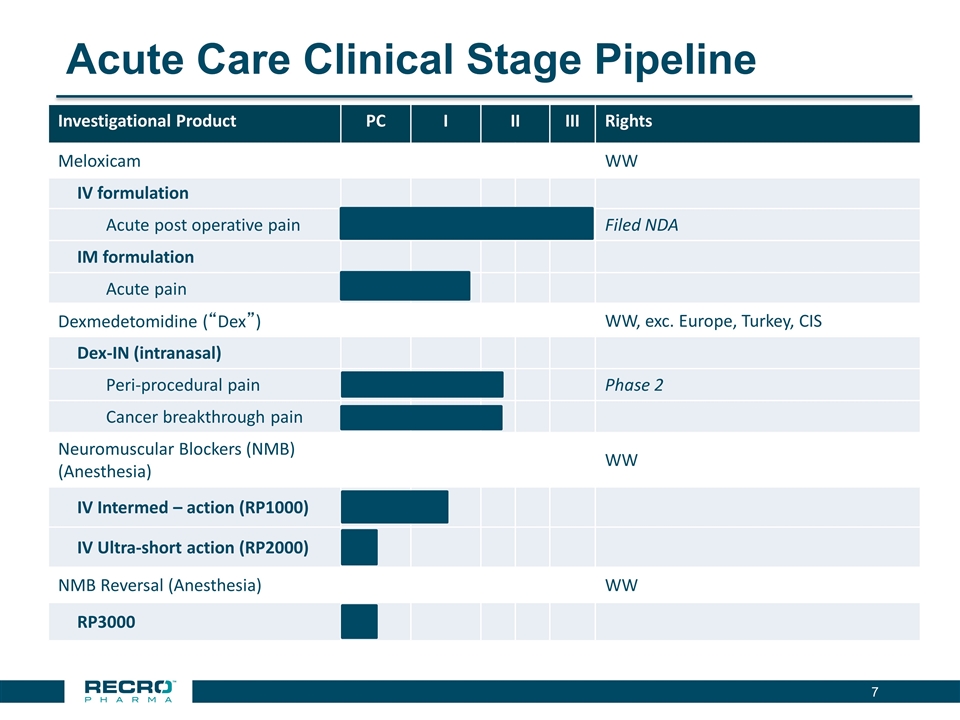
Acute Care Clinical Stage Pipeline Investigational Product PC I II III Rights Meloxicam WW IV formulation Acute post operative pain Filed NDA IM formulation Acute pain Dexmedetomidine (“Dex”) WW, exc. Europe, Turkey, CIS Dex-IN (intranasal) Peri-procedural pain Phase 2 Cancer breakthrough pain Neuromuscular Blockers (NMB) (Anesthesia) WW IV Intermed – action (RP1000) IV Ultra-short action (RP2000) NMB Reversal (Anesthesia) WW RP3000
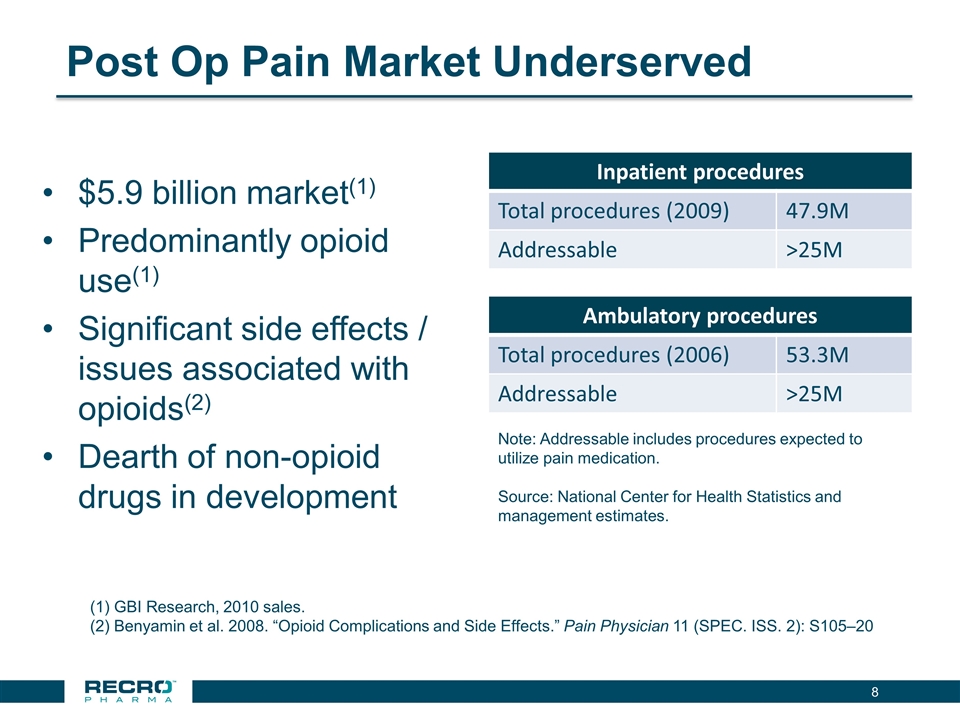
Post Op Pain Market Underserved $5.9 billion market(1) Predominantly opioid use(1) Significant side effects / issues associated with opioids(2) Dearth of non-opioid drugs in development Inpatient procedures Total procedures (2009) 47.9M Addressable >25M Ambulatory procedures Total procedures (2006) 53.3M Addressable >25M Note: Addressable includes procedures expected to utilize pain medication. Source: National Center for Health Statistics and management estimates. GBI Research, 2010 sales. Benyamin et al. 2008. “Opioid Complications and Side Effects.” Pain Physician 11 (SPEC. ISS. 2): S105–20
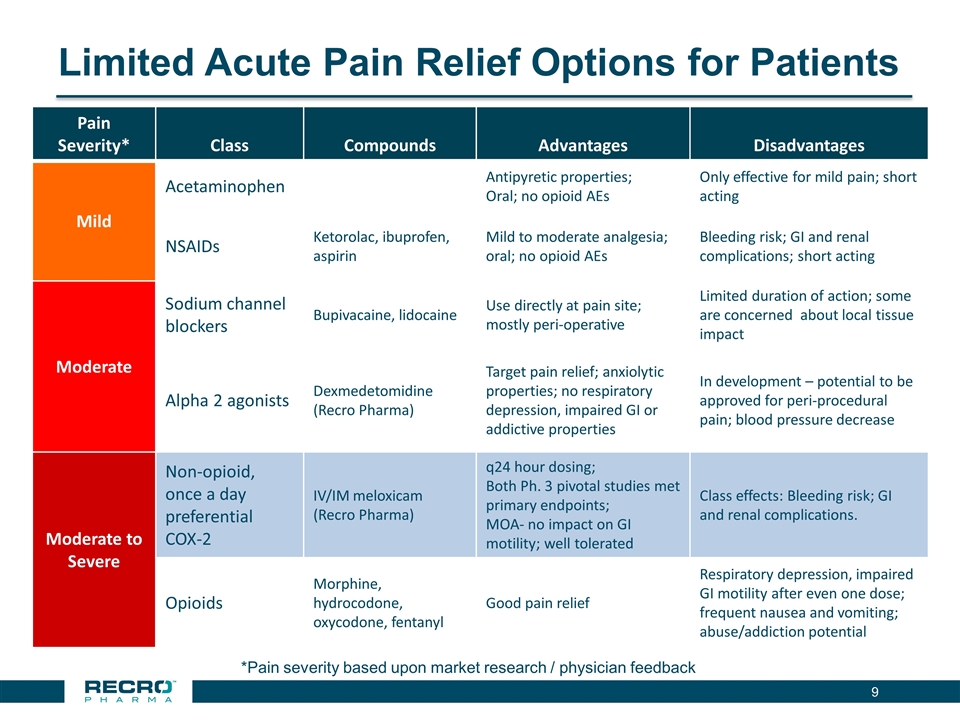
Limited Acute Pain Relief Options for Patients *Pain severity based upon market research / physician feedback Pain Severity* Class Compounds Advantages Disadvantages Mild Acetaminophen Antipyretic properties; Oral; no opioid AEs Only effective for mild pain; short acting NSAIDs Ketorolac, ibuprofen, aspirin Mild to moderate analgesia; oral; no opioid AEs Bleeding risk; GI and renal complications; short acting Moderate Sodium channel blockers Bupivacaine, lidocaine Use directly at pain site; mostly peri-operative Limited duration of action; some are concerned about local tissue impact Alpha 2 agonists Dexmedetomidine (Recro Pharma) Target pain relief; anxiolytic properties; no respiratory depression, impaired GI or addictive properties In development – potential to be approved for peri-procedural pain; blood pressure decrease Moderate to Severe Non-opioid, once a day preferential COX-2 IV/IM meloxicam (Recro Pharma) q24 hour dosing; Both Ph. 3 pivotal studies met primary endpoints; MOA- no impact on GI motility; well tolerated Class effects: Bleeding risk; GI and renal complications. Opioids Morphine, hydrocodone, oxycodone, fentanyl Good pain relief Respiratory depression, impaired GI motility after even one dose; frequent nausea and vomiting; abuse/addiction potential

IV Meloxicam
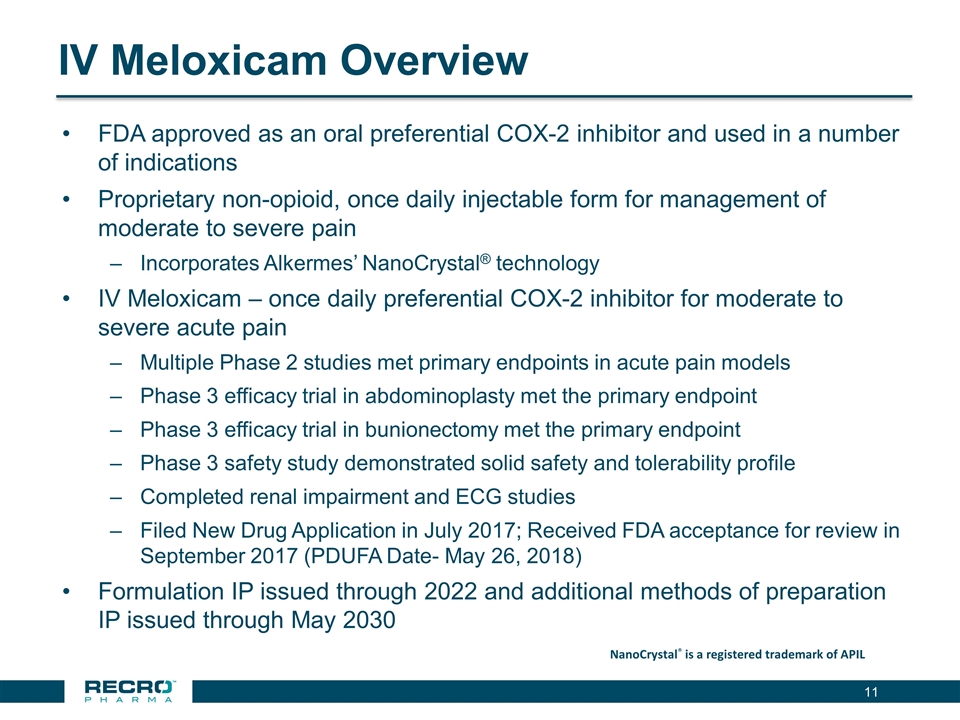
IV Meloxicam Overview FDA approved as an oral preferential COX-2 inhibitor and used in a number of indications Proprietary non-opioid, once daily injectable form for management of moderate to severe pain Incorporates Alkermes’ NanoCrystal® technology IV Meloxicam – once daily preferential COX-2 inhibitor for moderate to severe acute pain Multiple Phase 2 studies met primary endpoints in acute pain models Phase 3 efficacy trial in abdominoplasty met the primary endpoint Phase 3 efficacy trial in bunionectomy met the primary endpoint Phase 3 safety study demonstrated solid safety and tolerability profile Completed renal impairment and ECG studies Filed New Drug Application in July 2017; Received FDA acceptance for review in September 2017 (PDUFA Date- May 26, 2018) Formulation IP issued through 2022 and additional methods of preparation IP issued through May 2030 NanoCrystal® is a registered trademark of APIL

Phase 3 Safety Study Reported Q2 2017 Randomized, Double-blind, Placebo-controlled Safety Study Subjects requiring major surgery that was expected to result in inpatient hospitalization for at least 24-48 hours 721 subjects at 31 centers in 4 countries treated with: Placebo IV Meloxicam 30 mg Study drug administration Added to existing pain management protocol after surgery (No minimum pain score required for randomization) Once daily for up to 7 doses Additional dose at discharge was at the discretion of subject and investigator.
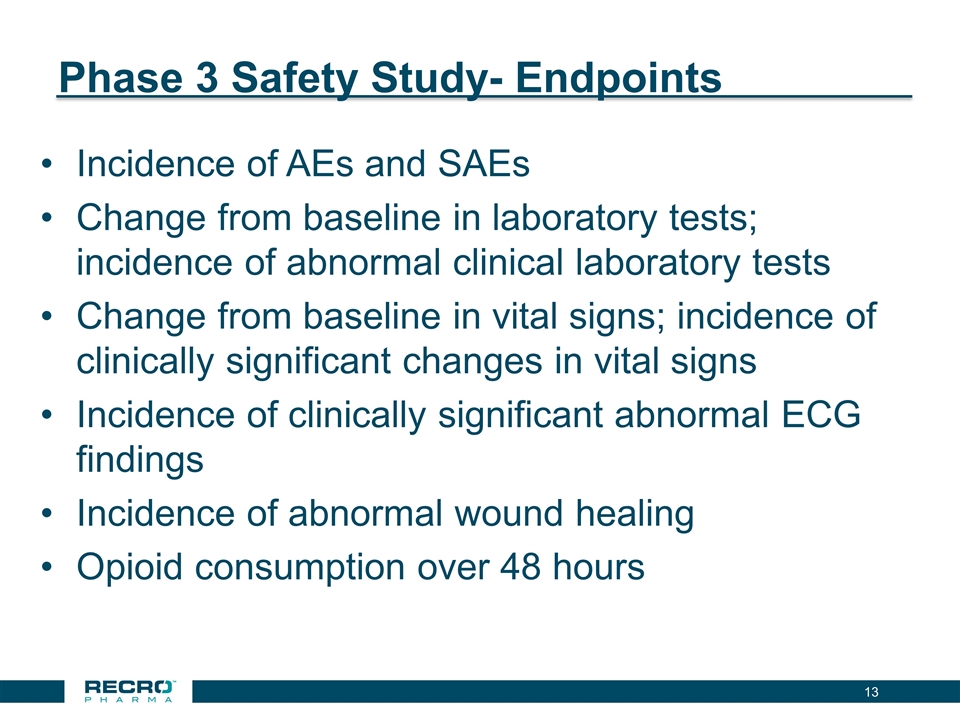
Phase 3 Safety Study- Endpoints Incidence of AEs and SAEs Change from baseline in laboratory tests; incidence of abnormal clinical laboratory tests Change from baseline in vital signs; incidence of clinically significant changes in vital signs Incidence of clinically significant abnormal ECG findings Incidence of abnormal wound healing Opioid consumption over 48 hours
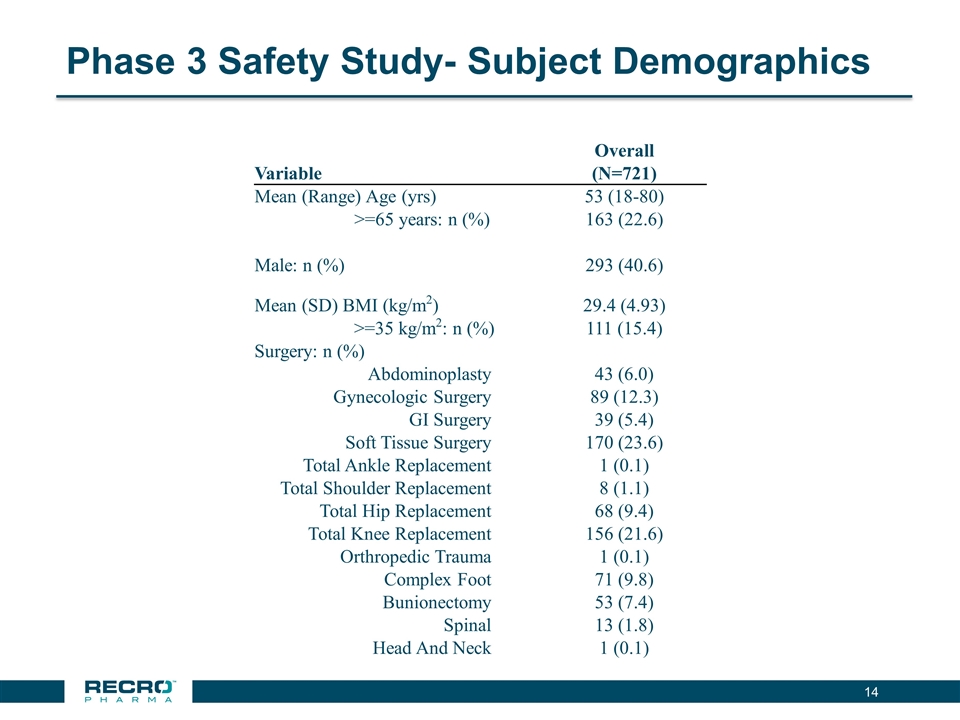
Phase 3 Safety Study- Subject Demographics Overall Variable (N=721) Mean (Range) Age (yrs) 53 (18-80) >=65 years: n (%) 163 (22.6) Male: n (%) 293 (40.6) Mean (SD) BMI (kg/m2) 29.4 (4.93) >=35 kg/m2: n (%) 111 (15.4) Surgery: n (%) Abdominoplasty 43 (6.0) Gynecologic Surgery 89 (12.3) GI Surgery 39 (5.4) Soft Tissue Surgery 170 (23.6) Total Ankle Replacement 1 (0.1) Total Shoulder Replacement 8 (1.1) Total Hip Replacement 68 (9.4) Total Knee Replacement 156 (21.6) Orthropedic Trauma 1 (0.1) Complex Foot 71 (9.8) Bunionectomy 53 (7.4) Spinal 13 (1.8) Head And Neck 1 (0.1)
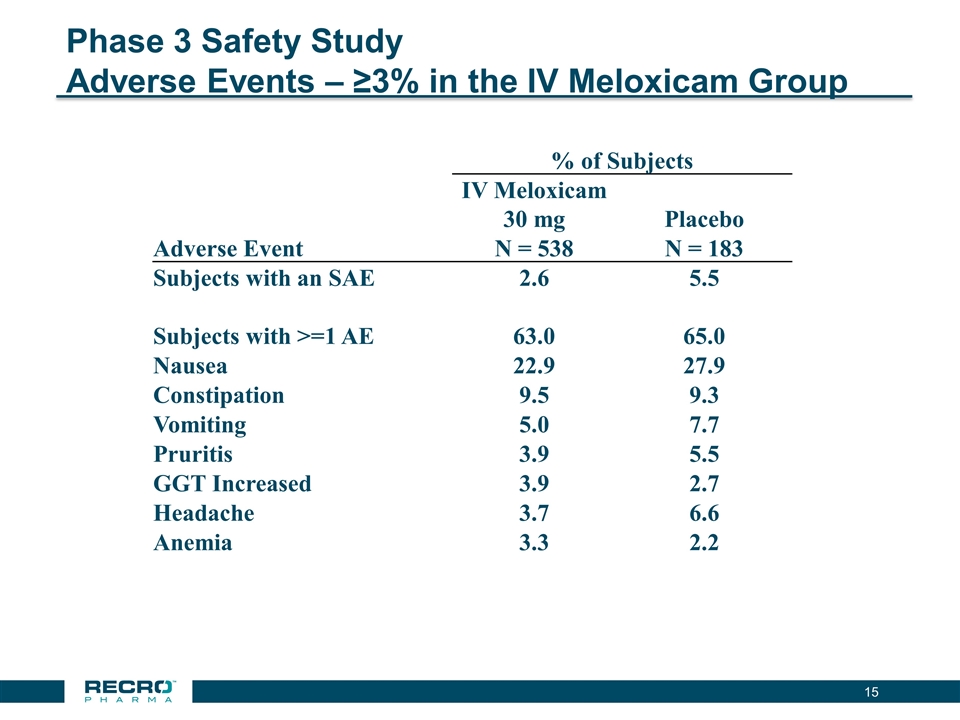
Phase 3 Safety Study Adverse Events – ≥3% in the IV Meloxicam Group % of Subjects IV Meloxicam 30 mg Placebo Adverse Event N = 538 N = 183 Subjects with an SAE 2.6 5.5 Subjects with >=1 AE 63.0 65.0 Nausea 22.9 27.9 Constipation 9.5 9.3 Vomiting 5.0 7.7 Pruritis 3.9 5.5 GGT Increased 3.9 2.7 Headache 3.7 6.6 Anemia 3.3 2.2
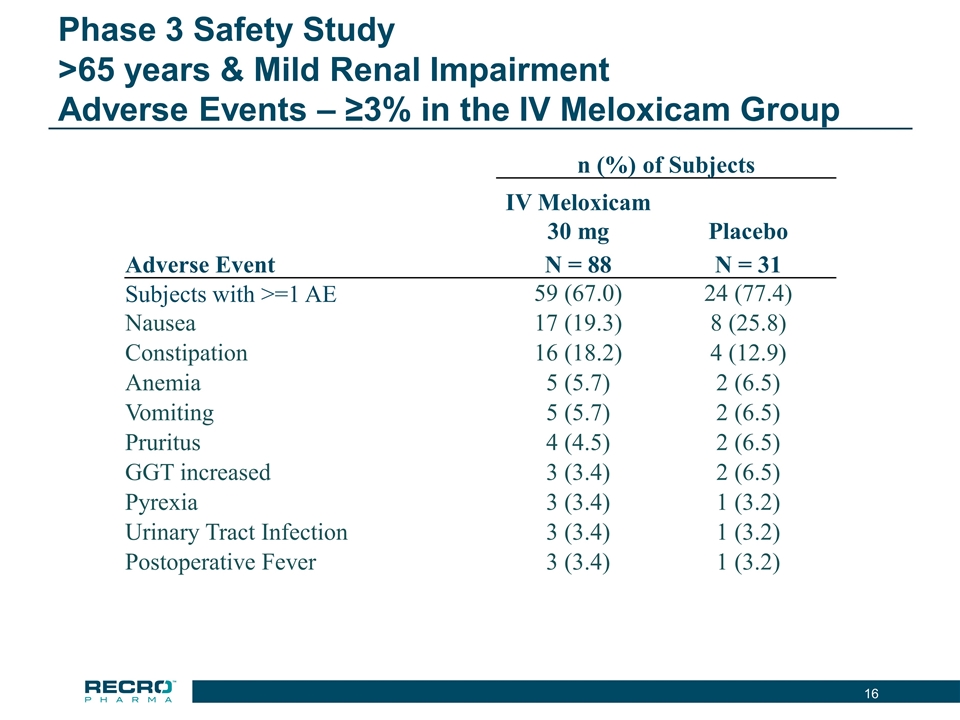
Phase 3 Safety Study >65 years & Mild Renal Impairment Adverse Events – ≥3% in the IV Meloxicam Group n (%) of Subjects IV Meloxicam 30 mg Placebo Adverse Event N = 88 N = 31 Subjects with >=1 AE 59 (67.0) 24 (77.4) Nausea 17 (19.3) 8 (25.8) Constipation 16 (18.2) 4 (12.9) Anemia 5 (5.7) 2 (6.5) Vomiting 5 (5.7) 2 (6.5) Pruritus 4 (4.5) 2 (6.5) GGT increased 3 (3.4) 2 (6.5) Pyrexia 3 (3.4) 1 (3.2) Urinary Tract Infection 3 (3.4) 1 (3.2) Postoperative Fever 3 (3.4) 1 (3.2)
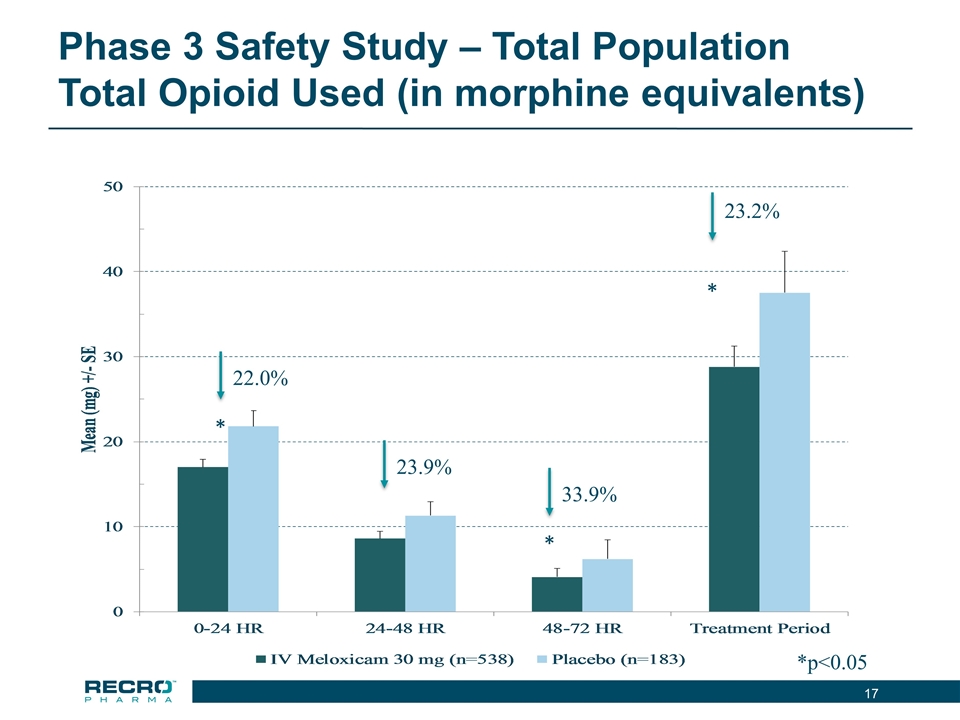
Phase 3 Safety Study – Total Population Total Opioid Used (in morphine equivalents) *p<0.05 22.0% * 23.9% 33.9% * 23.2% *
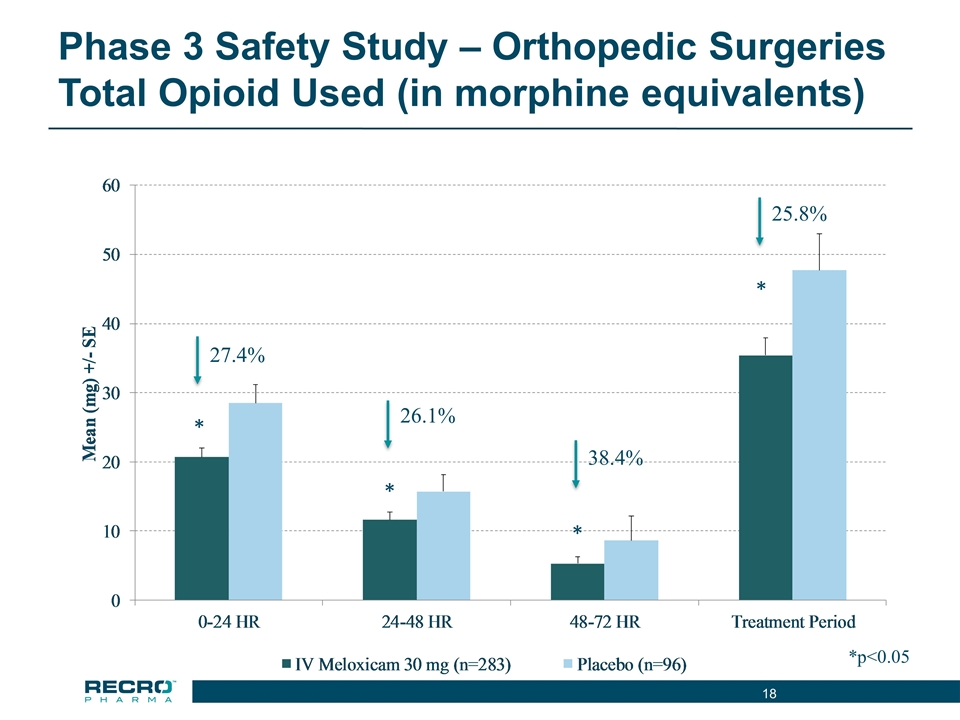
Phase 3 Safety Study – Orthopedic Surgeries Total Opioid Used (in morphine equivalents) *p<0.05 27.4% * 26.1% * 38.4% * 25.8% *
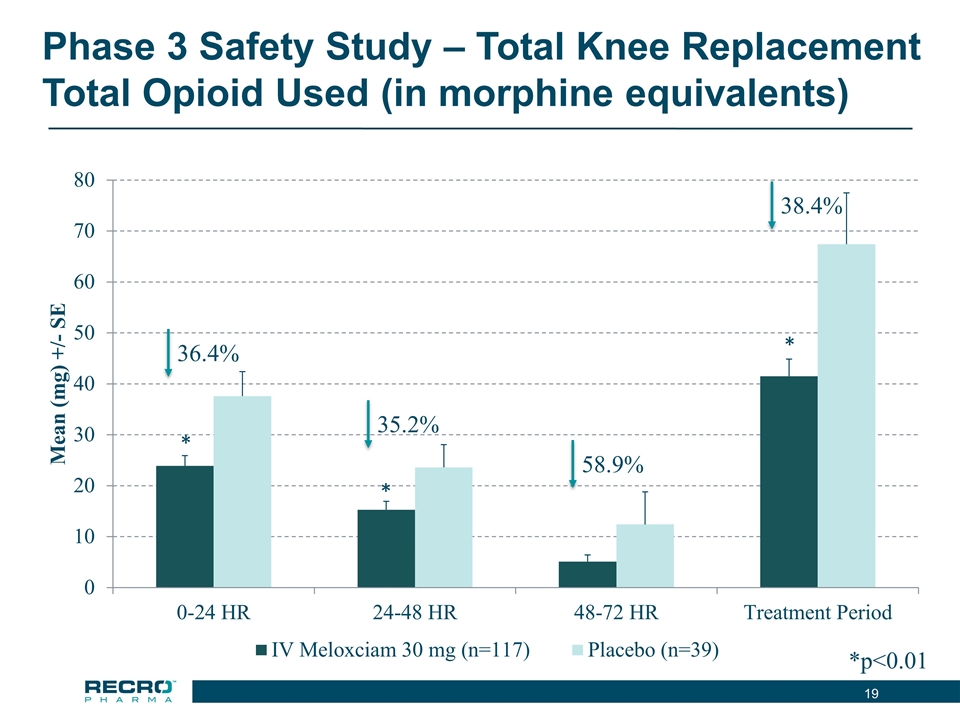
Phase 3 Safety Study – Total Knee Replacement Total Opioid Used (in morphine equivalents) 36.4% 35.2% *p<0.01 * * * 58.9% 38.4%
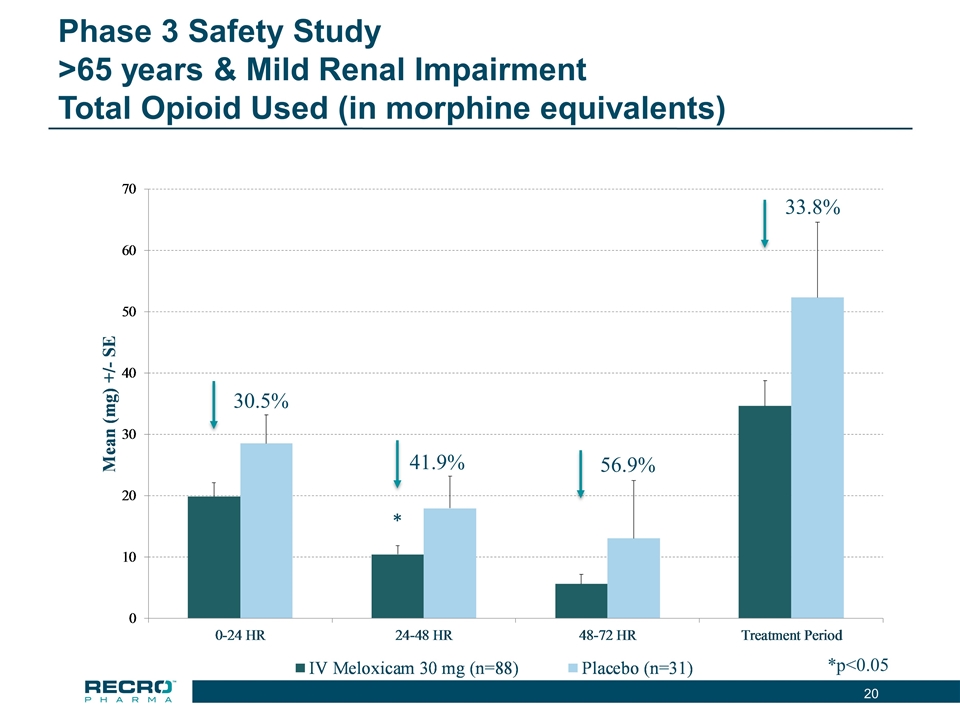
Phase 3 Safety Study >65 years & Mild Renal Impairment Total Opioid Used (in morphine equivalents) *p<0.05 30.5% 41.9% * 56.9% 33.8%
Phase 3 Abdominoplasty Study Multicenter, Multi-dose, Randomized, Double-blind, Placebo-controlled 219 subjects randomized to IV Meloxicam 30mg or Placebo Study medication administered once daily up to 3 doses 98% of subjects completed the 48 hour assessments Standard analgesia design Pain Intensity assessments (SPID24 = Primary Endpoint) Use of rescue medication Time to onset Patient Global Assessment of Pain Control
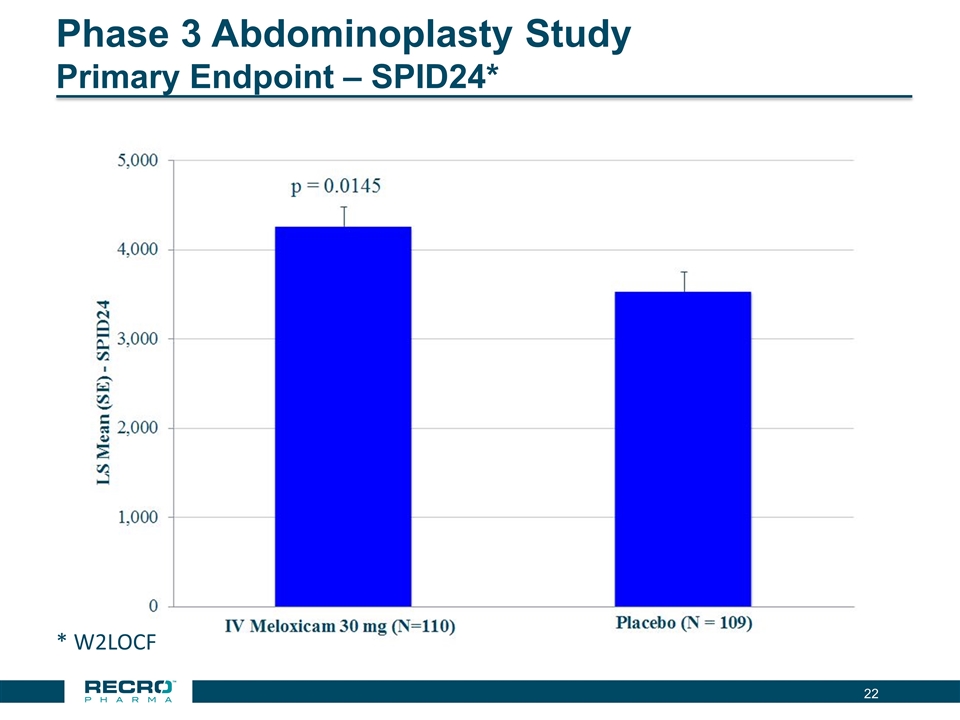
Phase 3 Abdominoplasty Study Primary Endpoint – SPID24* * W2LOCF
Phase 3 Abdominoplasty Study Summary of Secondary Endpoints SPID6, Times to Meaningful Pain Relief and First Rescue, Number of Subjects rescued 0-24 and 0-48 hours, % Subjects with >30 and >50% Improvement within 6 Hours and >50% within 24 hours, PGA of Pain Control at 24 hours were not significantly different between treatment groups. Parameter Met criteria for Statistical Significance: SPID12 SPID48 SPID24-48 Number of Subjects Rescued 24-48 Hours Number of Times Rescued 0-24 Hours Number of Times Rescued 24-48 Hours Number of Times Rescued 0-48 Hours Time to Perceptible Pain Relief % Subjects with >30% Improvement - 24 Hours PGA of Pain Control at 48 hours
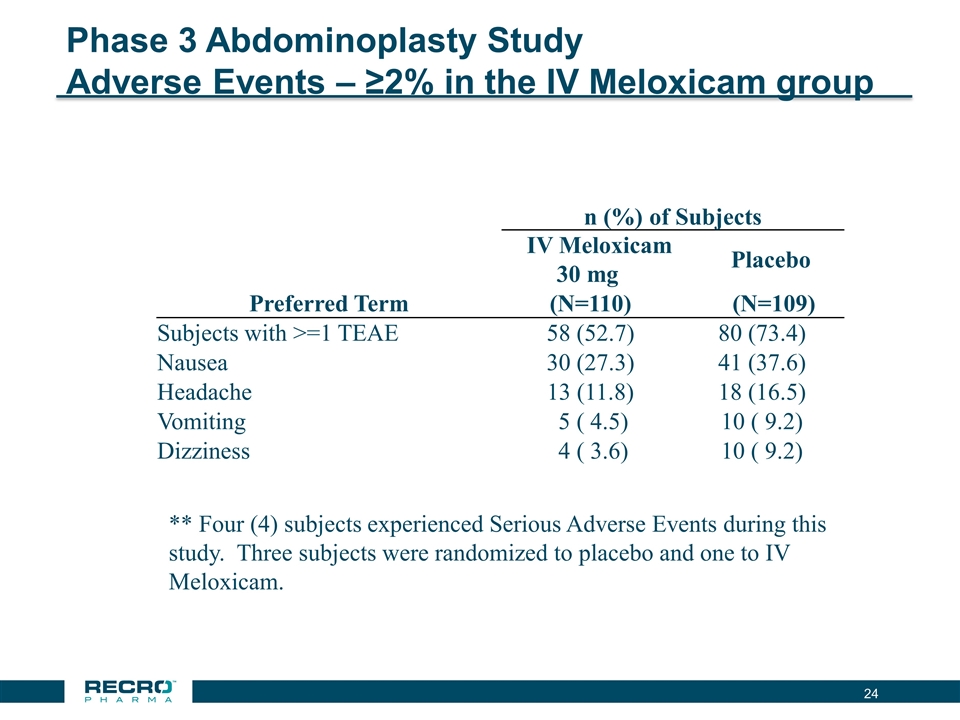
Phase 3 Abdominoplasty Study Adverse Events – ≥2% in the IV Meloxicam group n (%) of Subjects IV Meloxicam 30 mg Placebo Preferred Term (N=110) (N=109) Subjects with >=1 TEAE 58 (52.7) 80 (73.4) Nausea 30 (27.3) 41 (37.6) Headache 13 (11.8) 18 (16.5) Vomiting 5 ( 4.5) 10 ( 9.2) Dizziness 4 ( 3.6) 10 ( 9.2) ** Four (4) subjects experienced Serious Adverse Events during this study. Three subjects were randomized to placebo and one to IV Meloxicam.
Phase 3 Bunionectomy Study Multicenter, Multi-dose, Randomized, Double-blind, Placebo-controlled 201 subjects randomized to either IV Meloxicam 30 mg or Placebo Study medication administered once daily up to 3 doses 95% of subjects completed the 48 hour assessments Standard analgesia design Pain Intensity assessments (SPID48 = Primary Endpoint) Use of rescue medication Time to onset Patient Global Assessment of Pain Control
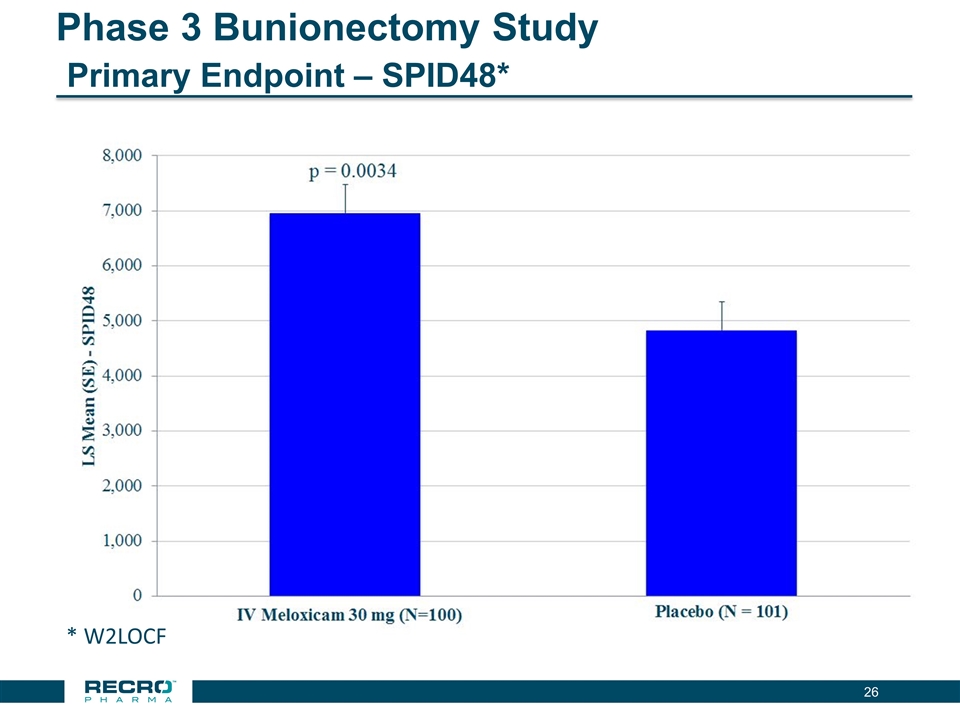
Phase 3 Bunionectomy Study Primary Endpoint – SPID48* * W2LOCF
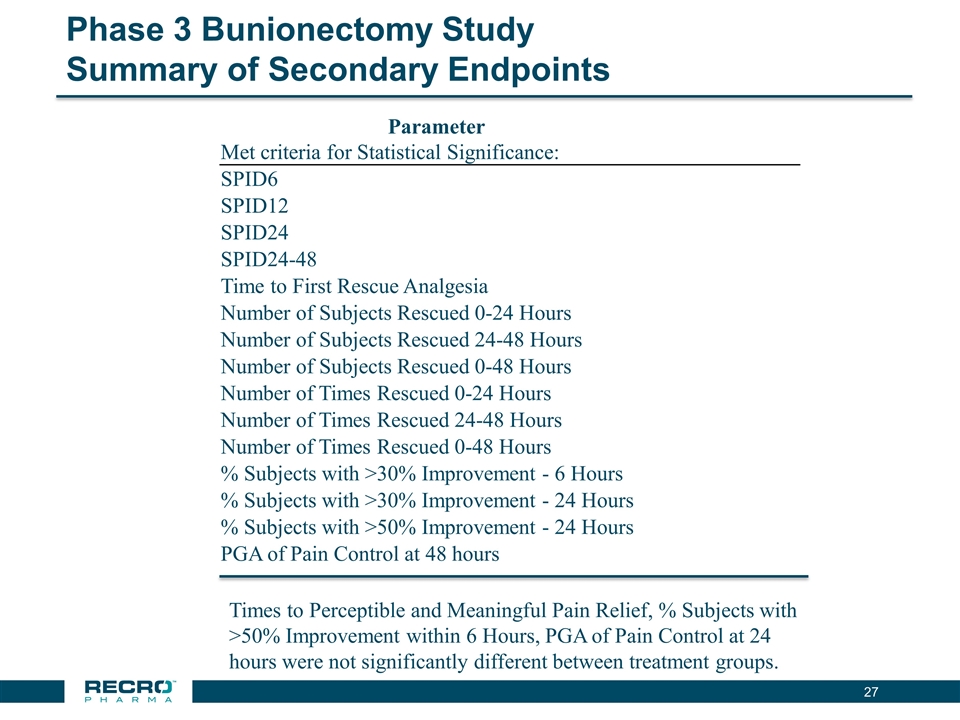
Phase 3 Bunionectomy Study Summary of Secondary Endpoints Parameter Met criteria for Statistical Significance: SPID6 SPID12 SPID24 SPID24-48 Time to First Rescue Analgesia Number of Subjects Rescued 0-24 Hours Number of Subjects Rescued 24-48 Hours Number of Subjects Rescued 0-48 Hours Number of Times Rescued 0-24 Hours Number of Times Rescued 24-48 Hours Number of Times Rescued 0-48 Hours % Subjects with >30% Improvement - 6 Hours % Subjects with >30% Improvement - 24 Hours % Subjects with >50% Improvement - 24 Hours PGA of Pain Control at 48 hours Times to Perceptible and Meaningful Pain Relief, % Subjects with >50% Improvement within 6 Hours, PGA of Pain Control at 24 hours were not significantly different between treatment groups.
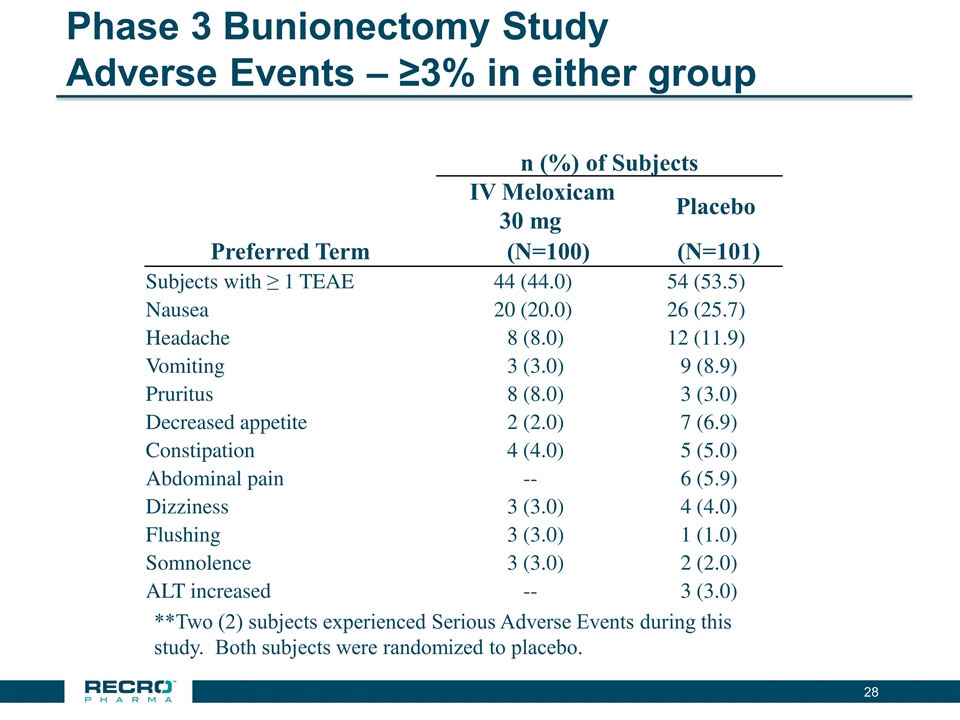
Phase 3 Bunionectomy Study Adverse Events – ≥3% in either group n (%) of Subjects IV Meloxicam 30 mg Placebo Preferred Term (N=100) (N=101) Subjects with ≥ 1 TEAE 44 (44.0) 54 (53.5) Nausea 20 (20.0) 26 (25.7) Headache 8 (8.0) 12 (11.9) Vomiting 3 (3.0) 9 (8.9) Pruritus 8 (8.0) 3 (3.0) Decreased appetite 2 (2.0) 7 (6.9) Constipation 4 (4.0) 5 (5.0) Abdominal pain -- 6 (5.9) Dizziness 3 (3.0) 4 (4.0) Flushing 3 (3.0) 1 (1.0) Somnolence 3 (3.0) 2 (2.0) ALT increased -- 3 (3.0) **Two (2) subjects experienced Serious Adverse Events during this study. Both subjects were randomized to placebo.
Next Steps for IV Meloxicam Filed NDA for IV Meloxicam in July 2017; Received FDA acceptance for review in September 2017 (PDUFA Date- May 26, 2018) Total across NDA: approximately 1400 meloxicam patients Largest Phase 3 double blind placebo controlled non-opioid study evaluating post operative IV pain product in a post-operative setting Finalize and initiate Phase 3B studies Orthopedic surgery (total knee)- opioid consumption/pain intensity Colorectal surgery-opioid consumption; length of stay Execute Commercialization and Launch Plan Strategic Segmentation, Prioritization and Targeting to Accelerate Formulary Adoption
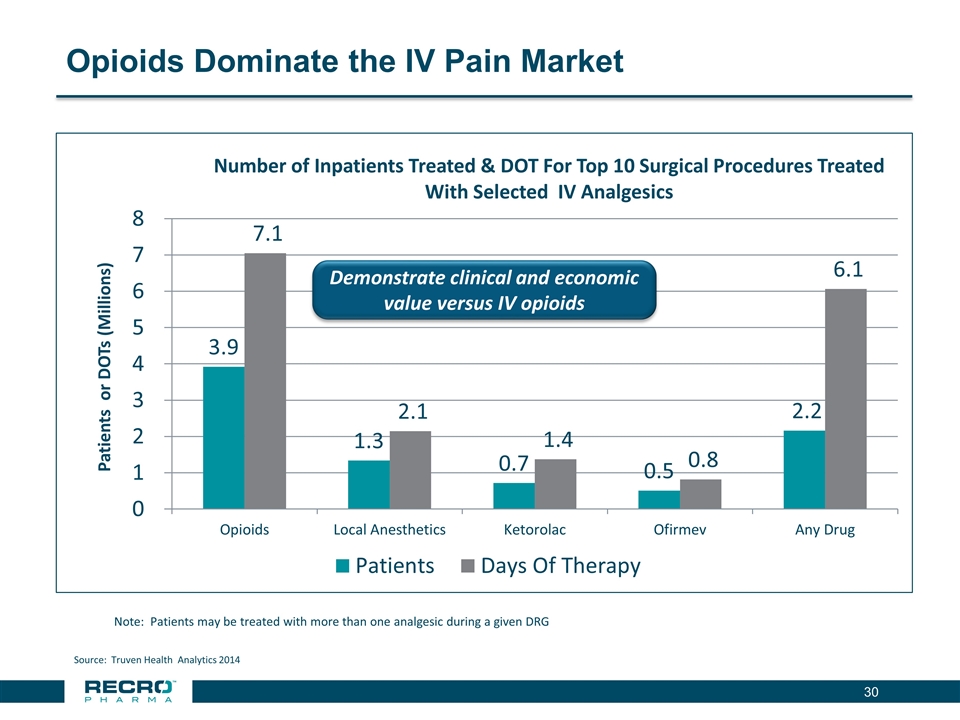
Opioids Dominate the IV Pain Market Note: Patients may be treated with more than one analgesic during a given DRG Source: Truven Health Analytics 2014 Demonstrate clinical and economic value versus IV opioids

Efficacy in Double Blind Laparoscopic Abdominal Surgery compared to Ketorolac and to Placebo *** p < 0.001 vs. Placebo CONFIDENTIAL
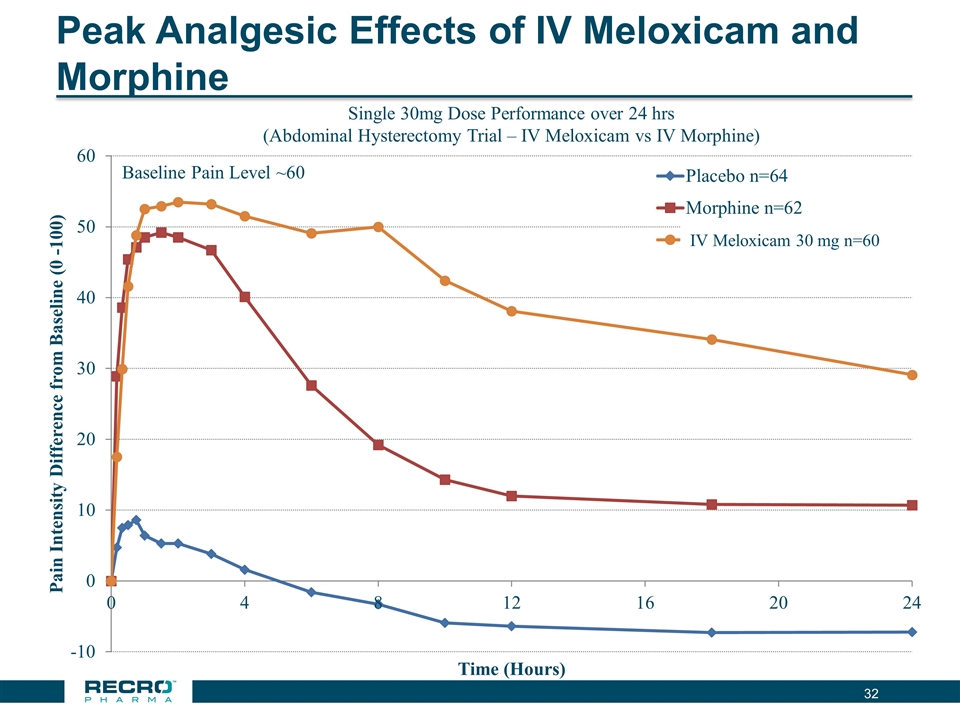
Peak Analgesic Effects of IV Meloxicam and Morphine Single 30mg Dose Performance over 24 hrs (Abdominal Hysterectomy Trial – IV Meloxicam vs IV Morphine) Baseline Pain Level ~60
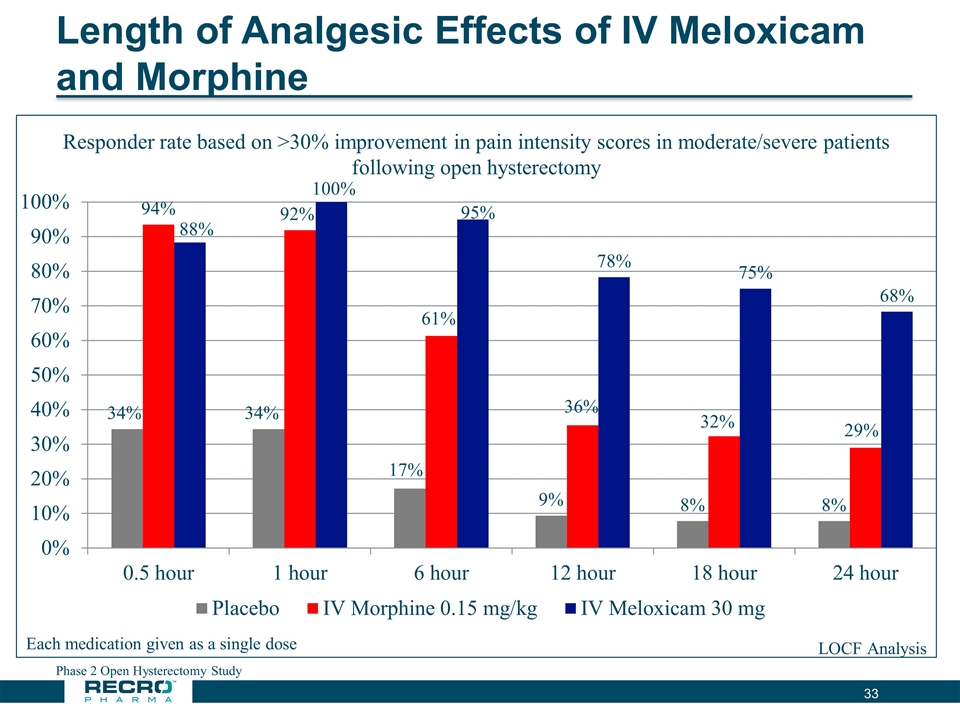
Length of Analgesic Effects of IV Meloxicam and Morphine Phase 2 Open Hysterectomy Study
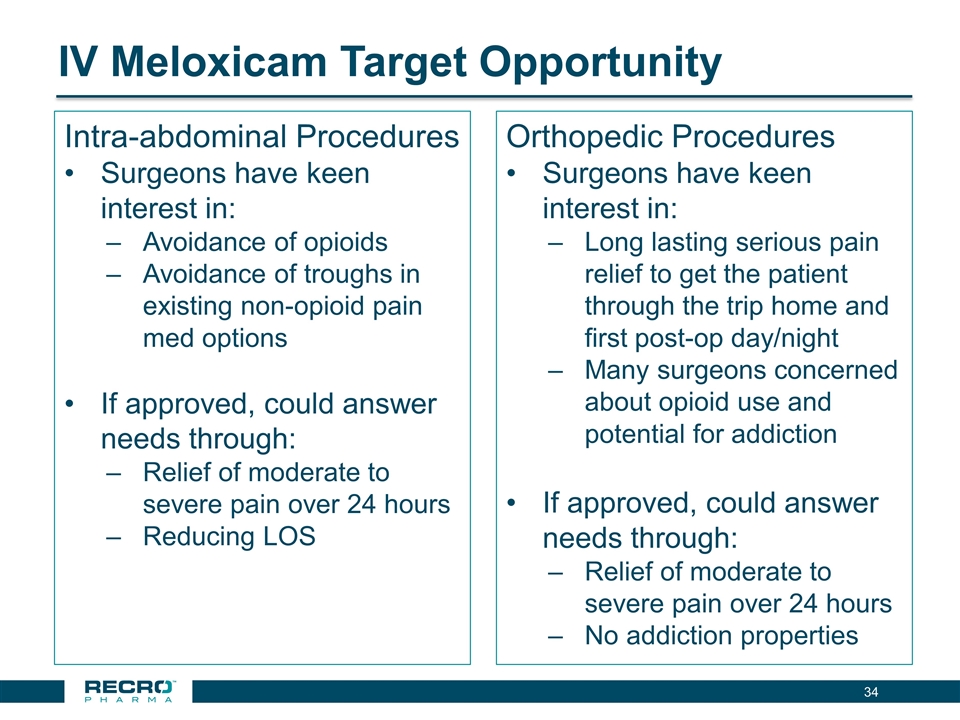
IV Meloxicam Target Opportunity Intra-abdominal Procedures Surgeons have keen interest in: Avoidance of opioids Avoidance of troughs in existing non-opioid pain med options If approved, could answer needs through: Relief of moderate to severe pain over 24 hours Reducing LOS Orthopedic Procedures Surgeons have keen interest in: Long lasting serious pain relief to get the patient through the trip home and first post-op day/night Many surgeons concerned about opioid use and potential for addiction If approved, could answer needs through: Relief of moderate to severe pain over 24 hours No addiction properties
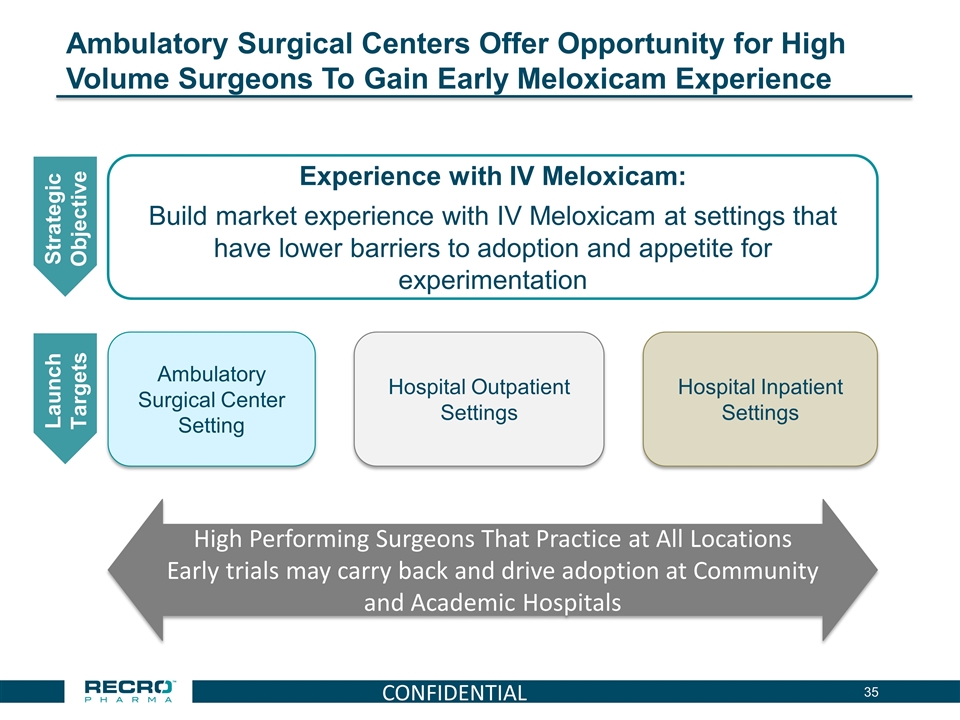
Ambulatory Surgical Centers Offer Opportunity for High Volume Surgeons To Gain Early Meloxicam Experience Strategic Objective Experience with IV Meloxicam: Build market experience with IV Meloxicam at settings that have lower barriers to adoption and appetite for experimentation Launch Targets Hospital Outpatient Settings Hospital Inpatient Settings Ambulatory Surgical Center Setting High Performing Surgeons That Practice at All Locations Early trials may carry back and drive adoption at Community and Academic Hospitals CONFIDENTIAL
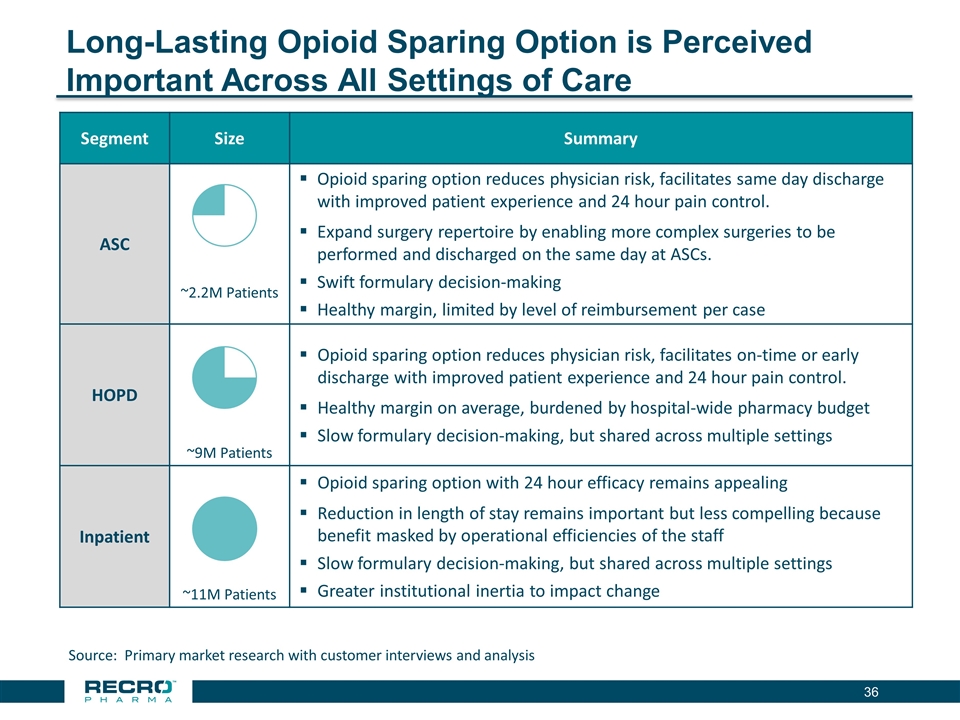
Long-Lasting Opioid Sparing Option is Perceived Important Across All Settings of Care Segment Size Summary ASC ~2.2M Patients Opioid sparing option reduces physician risk, facilitates same day discharge with improved patient experience and 24 hour pain control. Expand surgery repertoire by enabling more complex surgeries to be performed and discharged on the same day at ASCs. Swift formulary decision-making Healthy margin, limited by level of reimbursement per case HOPD ~9M Patients Opioid sparing option reduces physician risk, facilitates on-time or early discharge with improved patient experience and 24 hour pain control. Healthy margin on average, burdened by hospital-wide pharmacy budget Slow formulary decision-making, but shared across multiple settings Inpatient ~11M Patients Opioid sparing option with 24 hour efficacy remains appealing Reduction in length of stay remains important but less compelling because benefit masked by operational efficiencies of the staff Slow formulary decision-making, but shared across multiple settings Greater institutional inertia to impact change Source: Primary market research with customer interviews and analysis
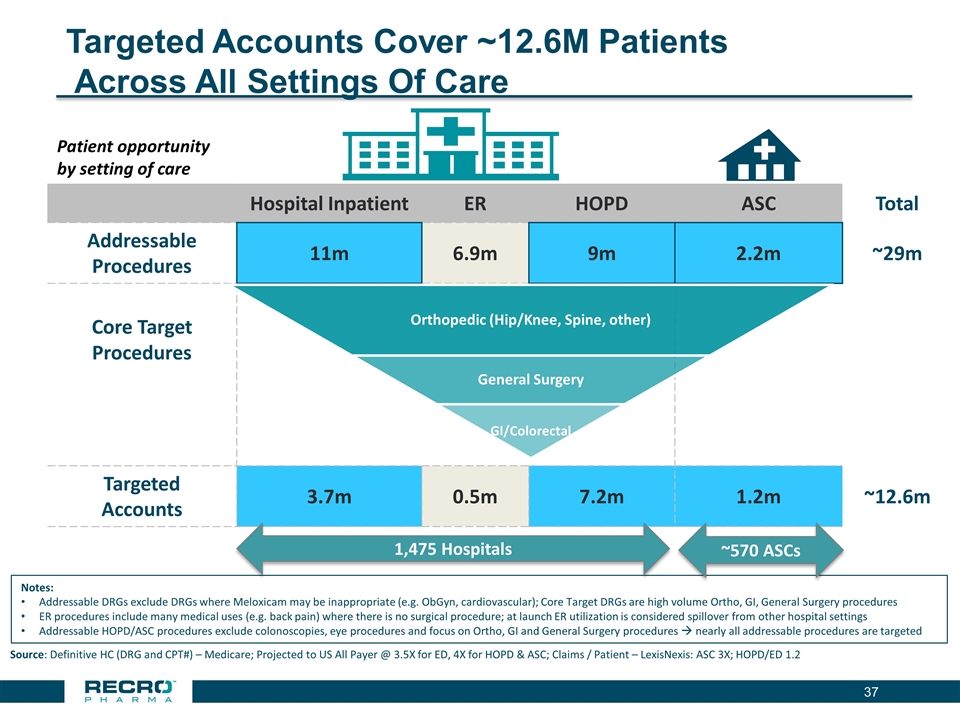
Targeted Accounts Cover ~12.6M Patients Across All Settings Of Care Hospital Inpatient ER HOPD ASC Total Addressable Procedures 11m 6.9m 9m 2.2m ~29m Core Target Procedures General Surgery Targeted Accounts 3.7m 0.5m 7.2m 1.2m ~12.6m Patient opportunity by setting of care Source: Definitive HC (DRG and CPT#) – Medicare; Projected to US All Payer @ 3.5X for ED, 4X for HOPD & ASC; Claims / Patient – LexisNexis: ASC 3X; HOPD/ED 1.2 Notes: Addressable DRGs exclude DRGs where Meloxicam may be inappropriate (e.g. ObGyn, cardiovascular); Core Target DRGs are high volume Ortho, GI, General Surgery procedures ER procedures include many medical uses (e.g. back pain) where there is no surgical procedure; at launch ER utilization is considered spillover from other hospital settings Addressable HOPD/ASC procedures exclude colonoscopies, eye procedures and focus on Ortho, GI and General Surgery procedures à nearly all addressable procedures are targeted 1,475 Hospitals ~570 ASCs Orthopedic (Hip/Knee, Spine, other) GI/Colorectal General Surgery
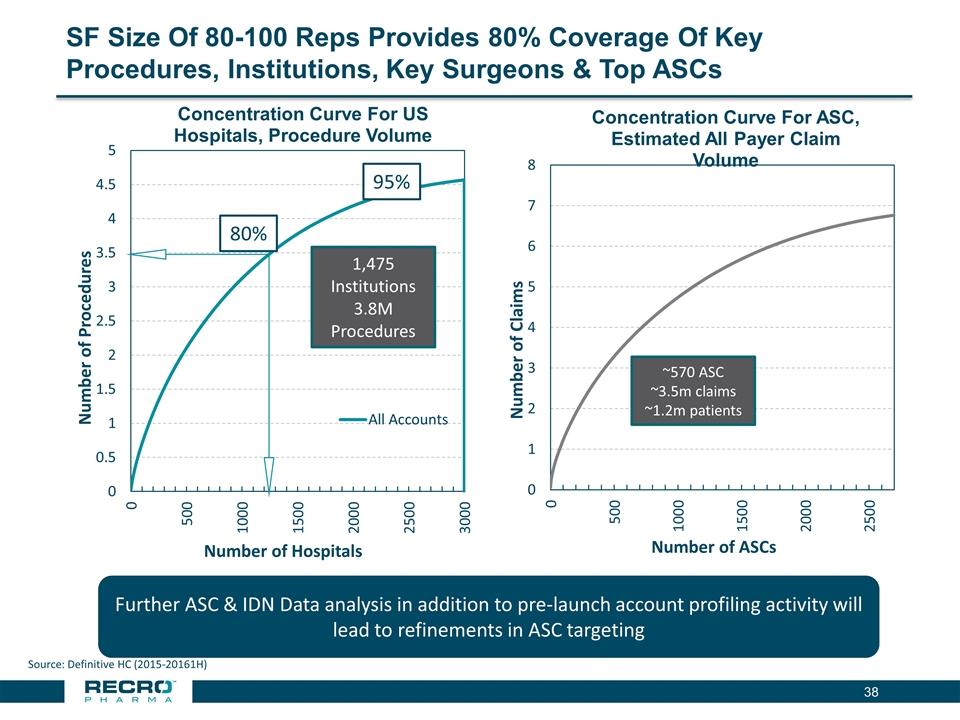
SF Size Of 80-100 Reps Provides 80% Coverage Of Key Procedures, Institutions, Key Surgeons & Top ASCs Source: Definitive HC (2015-20161H) Number of Procedures Number of Hospitals 80% 95% 1,475 Institutions 3.8M Procedures ~570 ASC ~3.5m claims ~1.2m patients Number of Claims Number of ASCs Further ASC & IDN Data analysis in addition to pre-launch account profiling activity will lead to refinements in ASC targeting
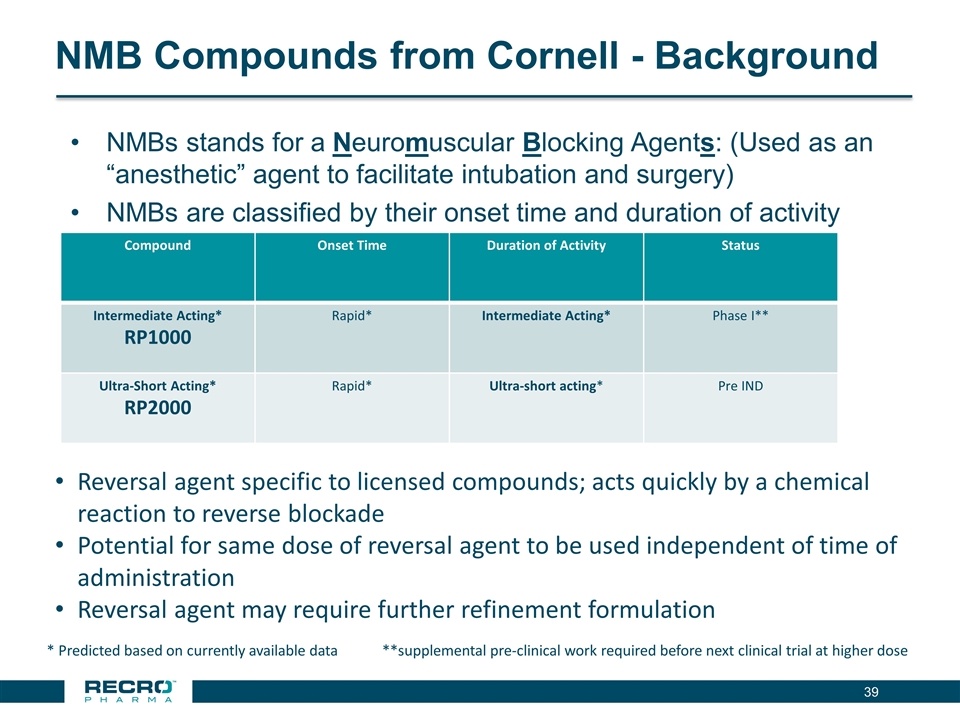
NMB Compounds from Cornell - Background NMBs stands for a Neuromuscular Blocking Agents: (Used as an “anesthetic” agent to facilitate intubation and surgery) NMBs are classified by their onset time and duration of activity * Predicted based on currently available data **supplemental pre-clinical work required before next clinical trial at higher dose Compound Onset Time Duration of Activity Status Intermediate Acting* RP1000 Rapid* Intermediate Acting* Phase I** Ultra-Short Acting* RP2000 Rapid* Ultra-short acting* Pre IND Reversal agent specific to licensed compounds; acts quickly by a chemical reaction to reverse blockade Potential for same dose of reversal agent to be used independent of time of administration Reversal agent may require further refinement formulation
NMB – What is the clinical need? Novel NMBs and related agents with the potential for rapid onset with ultra-short and intermediate durations of action, along with the potential to be rapidly reversed Clinical potential: reducing the time required for induction of anesthesia and the time needed to recover from NMB that patients require post procedure (in the OR and in the PACU) while potentially enhancing patient safety. Current alternatives when patients need “reversing” from neuromuscular blockade: encapsulating/binding reversal agents (Sugammadex) enzymatic reversal agents (Neostigmine) Both pathways may require higher doses and therefore incur greater expense if used earlier in a procedure compared to late in a procedure (i.e., when more NMB agent is remaining in the patient’s system) However, the proposed reversal agent used to reverse RP1000 and RP2000, may more quickly reverse neuromuscular blockade via a chemical reaction This outcome could be beneficial to the patient and result in meaningful cost savings for the hospital/ASC

Contract Development and Manufacturing (CDMO) Business Overview Gainesville

Gainesville CDMO Facility Gainesville, GA, Facility Located on 148 acres of land, the facility has approximately 97,000 square feet of space Plant began operations early 90’s Includes 288 pallet vault built in accordance with DEA specifications Operates under cGMP standards Two level facility FDA/EMA/DEA Licensed Development, Scale-up and full scale manufacturing Manufactures product for U.S., European and Asian markets Solid oral-dosage forms capacity Capsule capacity- 500-700 million capsules Tablet capacity- 1.5 billion tablets
CDMO Overview CDMO facility 97,000 + sq. ft. solid oral dosage manufacturing cGMP DEA licensed; ~185 employees Revenues include product sales, royalties and profit sharing Positive cash flow providing debt service and non-dilutive financing source for Company development and operating activities Service capabilities Formulation, process development and optimization Process scale-up Clinical supply and validation Commercial supply Ritalin LA Once daily ADHD treatment marketed by Novartis Focalin XR ADHD treatment marketed by Novartis Verapamil/Verelan CV/High blood pressure treatment marketed by Teva and Lannett Zohydro ER Extended release hydrocodone marketed by Pernix Launched in 2014 Abuse deterrent form launched
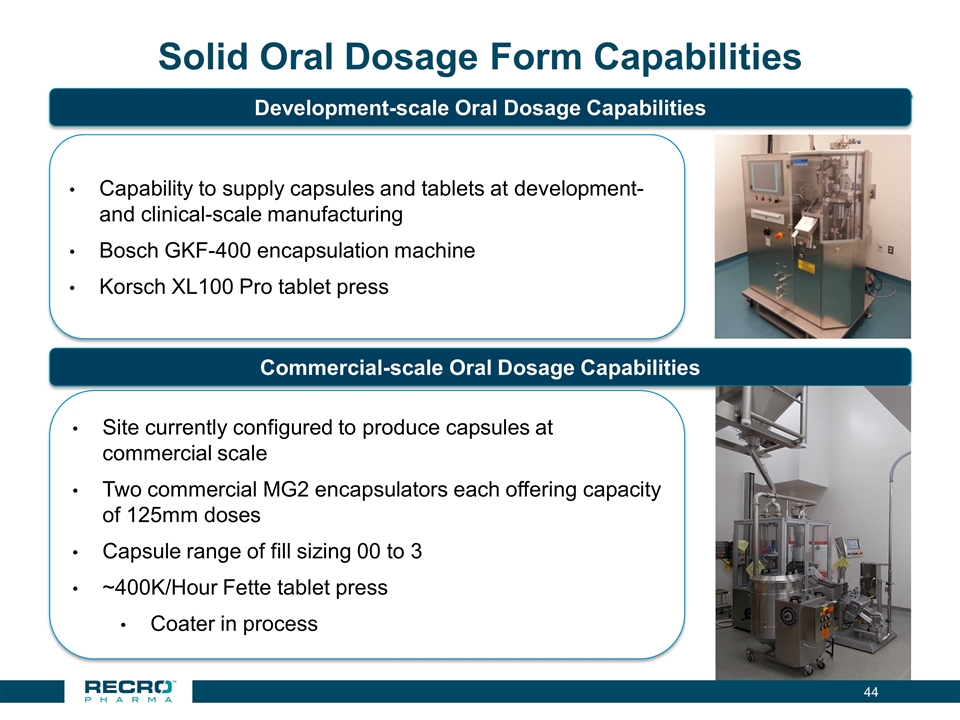
Solid Oral Dosage Form Capabilities Site currently configured to produce capsules at commercial scale Two commercial MG2 encapsulators each offering capacity of 125mm doses Capsule range of fill sizing 00 to 3 ~400K/Hour Fette tablet press Coater in process Commercial-scale Oral Dosage Capabilities Development-scale Oral Dosage Capabilities Capability to supply capsules and tablets at development- and clinical-scale manufacturing Bosch GKF-400 encapsulation machine Korsch XL100 Pro tablet press

2017 Additional Capabilities-Commercial Tablet Press and Film Coating Equipment Fette FE55 LBB Bohle BTC 100

Packaging Operations One line operation with annual maximum capacity of 2.5 million bottles per shift Capability of packaging various size capsules and tablets Can handle round or square bottles 40cc – 500cc, and counts of up to 500 units/bottle Met serialization requirements guidance early
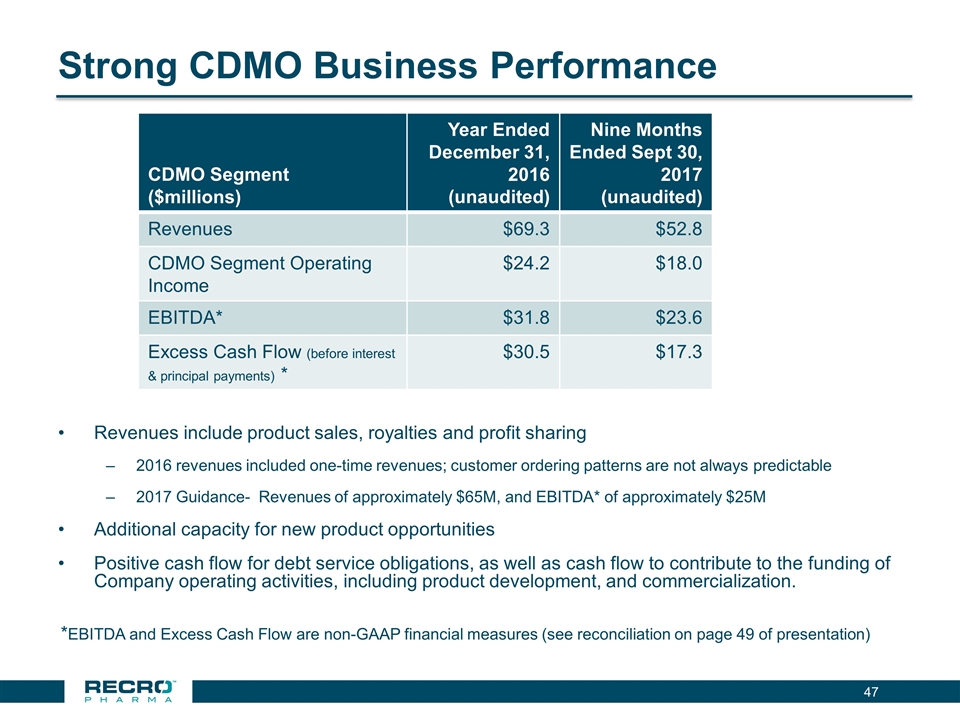
Strong CDMO Business Performance Revenues include product sales, royalties and profit sharing 2016 revenues included one-time revenues; customer ordering patterns are not always predictable 2017 Guidance- Revenues of approximately $65M, and EBITDA* of approximately $25M Additional capacity for new product opportunities Positive cash flow for debt service obligations, as well as cash flow to contribute to the funding of Company operating activities, including product development, and commercialization. *EBITDA and Excess Cash Flow are non-GAAP financial measures (see reconciliation on page 49 of presentation) CDMO Segment ($millions) Year Ended December 31, 2016 (unaudited) Nine Months Ended Sept 30, 2017 (unaudited) Revenues $69.3 $52.8 CDMO Segment Operating Income $24.2 $18.0 EBITDA* $31.8 $23.6 Excess Cash Flow (before interest & principal payments) * $30.5 $17.3

$100 Million November 2017 Credit Facility Five-year, interest only term loan at LIBOR +9.75%; Issuance of 348,664 warrants at $8.60/share Three Tranches $60 million at closing $20 million upon FDA approval of IV meloxicam subject to certain financial liquidity conditions $20 million available after Recro demonstrates early IV meloxicam commercial traction Financial flexibility to aid our transformation into a commercial-stage enterprise Substantial reduction of our cost of capital Provides funding for IV Meloxicam FDA approval $45 million milestone Interest only structure allows for reinvestment of CDMO’s cash flows in value accretive commercial activities Repaid existing OrbiMed credit facility with payment of $31.7 million, plus transaction fees.
Company Highlights Specialty pharmaceutical company focused on hospital and related settings with late stage investigational product, IV Meloxicam, targeting management of moderate to severe pain Filed New Drug Application for IV Meloxicam in July 2017; Received FDA acceptance for review in September 2017 (PDUFA Date- May 26, 2018) Multiple therapeutics in clinical development for hospital and related settings Non-opioid, alpha 2 agonist for peri-procedural use; Recent acquisition of Neuromuscular Blockers (NMB) and related reversal agents from Cornell University Used for rapid induction and reversal of neuromuscular blockade Revenue and cash flow positive contract development and manufacturing (CDMO) business Cash position-$41.3 million @ 9/30/17 $100 million credit facility secured on November 17, 2017 Substantial reduction of our cost of capital after full repayment of existing debt; Provides funding for IV Meloxicam approval milestone Interest only structure allows for reinvestment of CDMO’s cash flows in value accretive commercial activities Experienced management team with significant development, regulatory and commercial experience
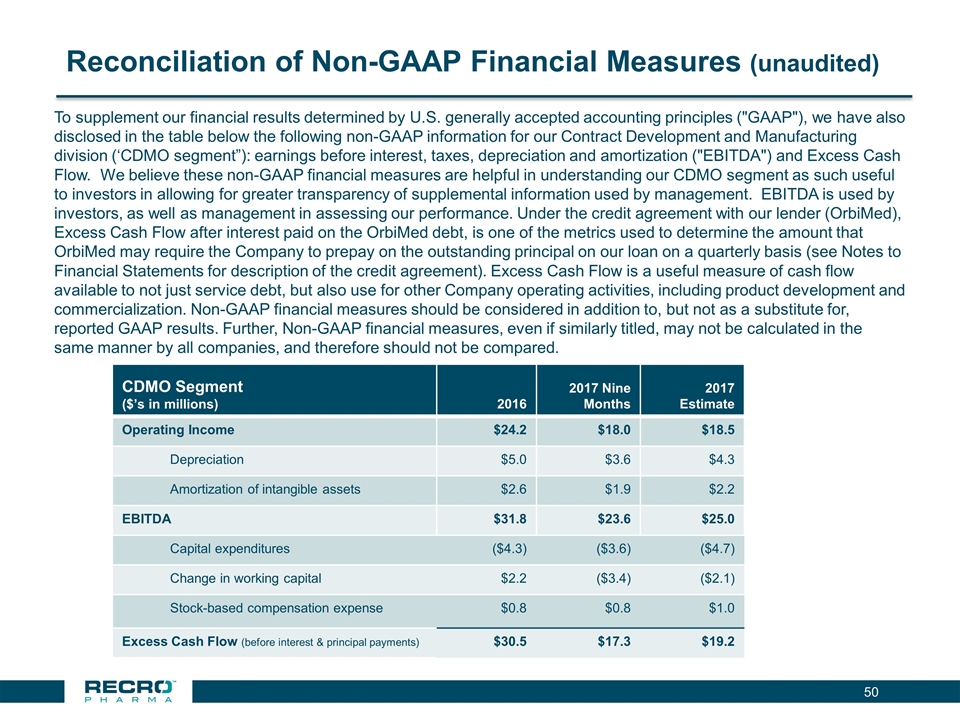
Reconciliation of Non-GAAP Financial Measures (unaudited) CDMO Segment ($’s in millions) 2016 2017 Nine Months 2017 Estimate Operating Income $24.2 $18.0 $18.5 Depreciation $5.0 $3.6 $4.3 Amortization of intangible assets $2.6 $1.9 $2.2 EBITDA $31.8 $23.6 $25.0 Capital expenditures ($4.3) ($3.6) ($4.7) Change in working capital $2.2 ($3.4) ($2.1) Stock-based compensation expense $0.8 $0.8 $1.0 Excess Cash Flow (before interest & principal payments) $30.5 $17.3 $19.2 To supplement our financial results determined by U.S. generally accepted accounting principles ("GAAP"), we have also disclosed in the table below the following non-GAAP information for our Contract Development and Manufacturing division (‘CDMO segment”): earnings before interest, taxes, depreciation and amortization ("EBITDA") and Excess Cash Flow. We believe these non-GAAP financial measures are helpful in understanding our CDMO segment as such useful to investors in allowing for greater transparency of supplemental information used by management. EBITDA is used by investors, as well as management in assessing our performance. Under the credit agreement with our lender (OrbiMed), Excess Cash Flow after interest paid on the OrbiMed debt, is one of the metrics used to determine the amount that OrbiMed may require the Company to prepay on the outstanding principal on our loan on a quarterly basis (see Notes to Financial Statements for description of the credit agreement). Excess Cash Flow is a useful measure of cash flow available to not just service debt, but also use for other Company operating activities, including product development and commercialization. Non-GAAP financial measures should be considered in addition to, but not as a substitute for, reported GAAP results. Further, Non-GAAP financial measures, even if similarly titled, may not be calculated in the same manner by all companies, and therefore should not be compared.

Appendix
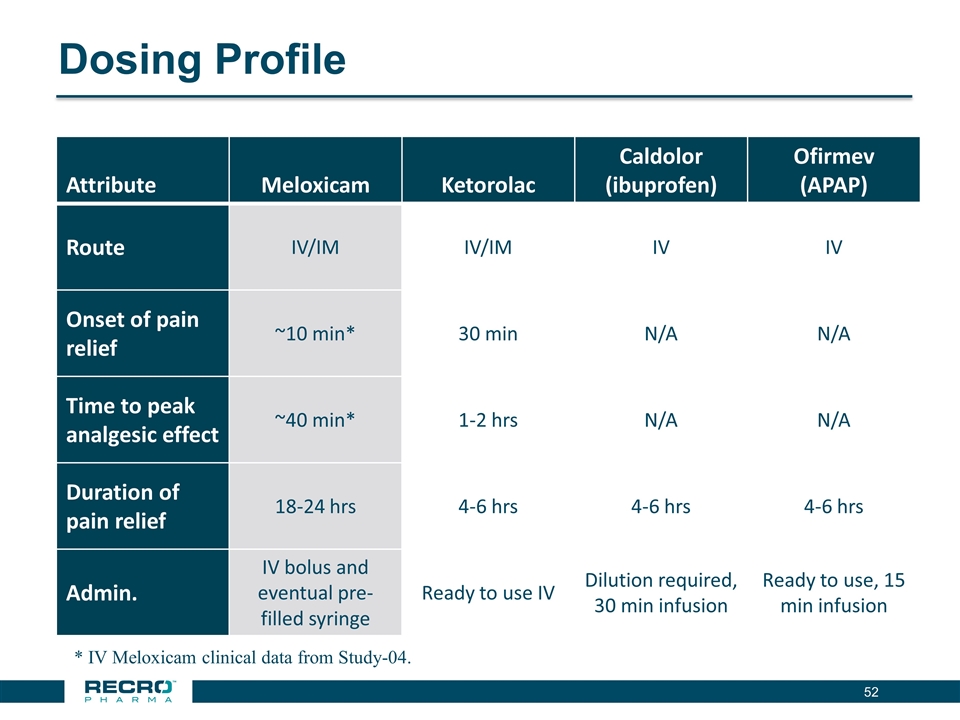
Dosing Profile Attribute Meloxicam Ketorolac Caldolor (ibuprofen) Ofirmev (APAP) Route IV/IM IV/IM IV IV Onset of pain relief ~10 min* 30 min N/A N/A Time to peak analgesic effect ~40 min* 1-2 hrs N/A N/A Duration of pain relief 18-24 hrs 4-6 hrs 4-6 hrs 4-6 hrs Admin. IV bolus and eventual pre-filled syringe Ready to use IV Dilution required, 30 min infusion Ready to use, 15 min infusion * IV Meloxicam clinical data from Study-04.
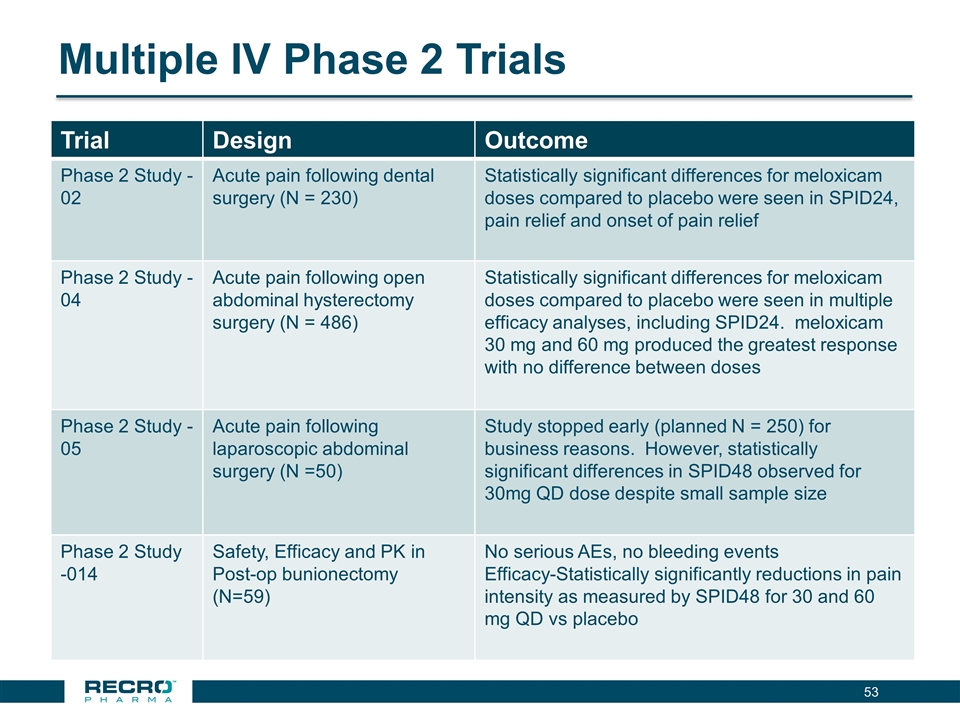
Multiple IV Phase 2 Trials Trial Design Outcome Phase 2 Study -02 Acute pain following dental surgery (N = 230) Statistically significant differences for meloxicam doses compared to placebo were seen in SPID24, pain relief and onset of pain relief Phase 2 Study -04 Acute pain following open abdominal hysterectomy surgery (N = 486) Statistically significant differences for meloxicam doses compared to placebo were seen in multiple efficacy analyses, including SPID24. meloxicam 30 mg and 60 mg produced the greatest response with no difference between doses Phase 2 Study -05 Acute pain following laparoscopic abdominal surgery (N =50) Study stopped early (planned N = 250) for business reasons. However, statistically significant differences in SPID48 observed for 30mg QD dose despite small sample size Phase 2 Study -014 Safety, Efficacy and PK in Post-op bunionectomy (N=59) No serious AEs, no bleeding events Efficacy-Statistically significantly reductions in pain intensity as measured by SPID48 for 30 and 60 mg QD vs placebo