
January 2019 Relieving pain…….Improving lives Exhibit 99.1

Forward Looking Statements This presentation includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements, among other things, relate to the deficiencies identified by the FDA in the complete response letter for IV meloxicam, our resubmission of an amended NDA for IV meloxicam and whether the FDA will approve the amended NDA and the labeling under any such approval, whether the FDA will require additional clinical studies to support the approval of IV meloxicam and the time and cost of such studies, our ability to resolve the deficiencies identified by the FDA in the complete response letter for IV meloxicam, development, launch and commercialization strategy and goals and expectations for IV meloxicam, if approved, future operations, prospects, plans and objectives of management. The words "anticipate", "believe", "could", "estimate", "expect", "intend", "may", "plan", "predict", "project", "will" and similar terms and phrases may be used to identify forward-looking statements in this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or a combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. These forward-looking statements should be considered together with the risks and uncertainties that may affect our business and future results included in our filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to us, and we assume no obligation to update any forward-looking statements except as required by applicable law. Non-Promotion: This presentation is intended to be non-promotional and for investor discussion purposes only. The information provided herein contains references to IV meloxicam, an investigational product. Use of IV meloxicam has not been approved by the FDA. The safety and efficacy of the investigational use of IV meloxicam has not been determined. There is no guarantee that IV meloxicam will be approved for marketing by any regulatory agency. IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.

Company Highlights Specialty pharmaceutical company focused on hospital and related settings with late stage investigational product, IV Meloxicam, targeting management of moderate to severe pain NDA resubmitted end of September 2018; PDUFA date March 24, 2019 Expanded IV Meloxicam IP Portfolio Multiple therapeutics in clinical development for hospital and related settings Revenue and cash flow positive contract development and manufacturing (CDMO) business Increased 2018 Full Year Financial Guidance to Revenue of $75M and EBITDA* of $32M 2018Q3YTD Revenue- $59.6M; EBITDA*- $26.3M 2017 Full Year Revenue- $71.8M; EBITDA*- $32.8M Cash position – $37.0M @ 9/30/18 Amended Alkermes IV Meloxicam license agreement; reduces 2019 cash requirements by $30 million, extends approval milestone payments over seven years Amended $100 million credit facility, restores $40 million in debt funding; $70 million drawn as of 12/31/18 Experienced management team with significant development, regulatory and commercial experience *EBITDA is a non-GAAP financial measure. See reconciliation on last page of presentation. IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.
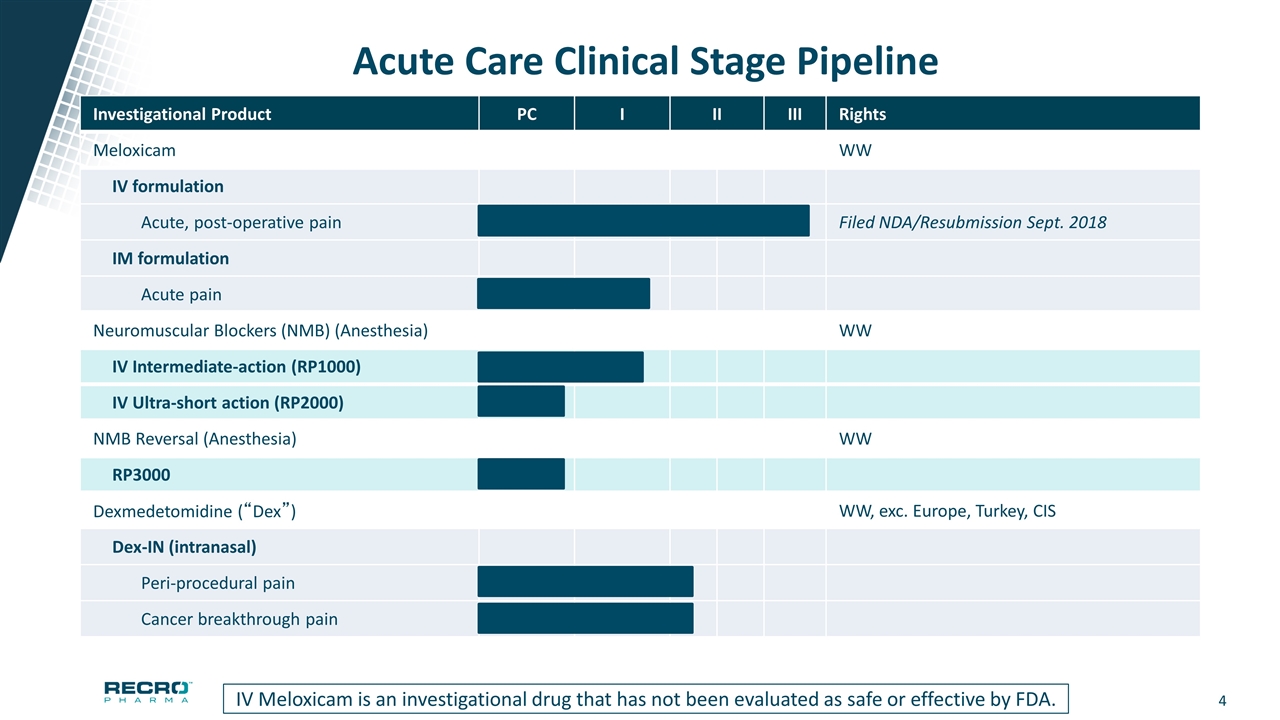
Acute Care Clinical Stage Pipeline Investigational Product PC I II III Rights Meloxicam WW IV formulation Acute, post-operative pain Filed NDA/Resubmission Sept. 2018 IM formulation Acute pain Neuromuscular Blockers (NMB) (Anesthesia) WW IV Intermediate-action (RP1000) IV Ultra-short action (RP2000) NMB Reversal (Anesthesia) WW RP3000 Dexmedetomidine (“Dex”) WW, exc. Europe, Turkey, CIS Dex-IN (intranasal) Peri-procedural pain Cancer breakthrough pain IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.
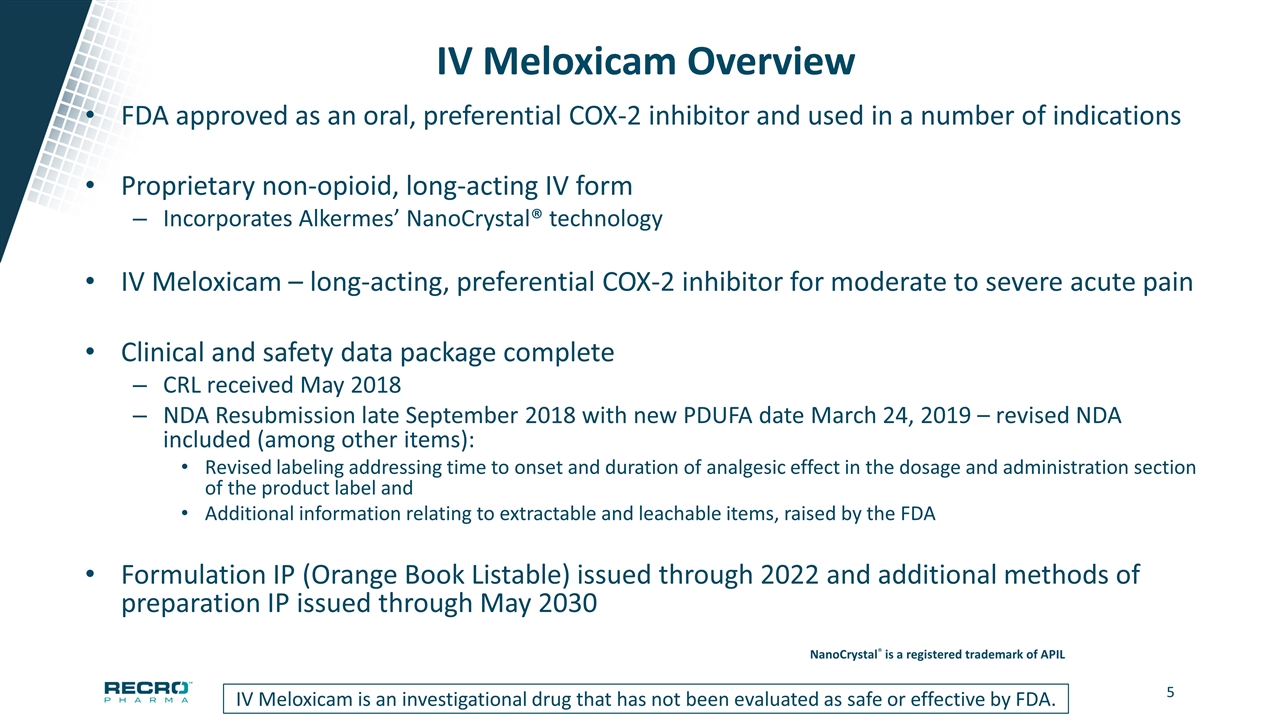
IV Meloxicam Overview FDA approved as an oral, preferential COX-2 inhibitor and used in a number of indications Proprietary non-opioid, long-acting IV form Incorporates Alkermes’ NanoCrystal® technology IV Meloxicam – long-acting, preferential COX-2 inhibitor for moderate to severe acute pain Clinical and safety data package complete CRL received May 2018 NDA Resubmission late September 2018 with new PDUFA date March 24, 2019 – revised NDA included (among other items): Revised labeling addressing time to onset and duration of analgesic effect in the dosage and administration section of the product label and Additional information relating to extractable and leachable items, raised by the FDA Formulation IP (Orange Book Listable) issued through 2022 and additional methods of preparation IP issued through May 2030 IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. NanoCrystal® is a registered trademark of APIL
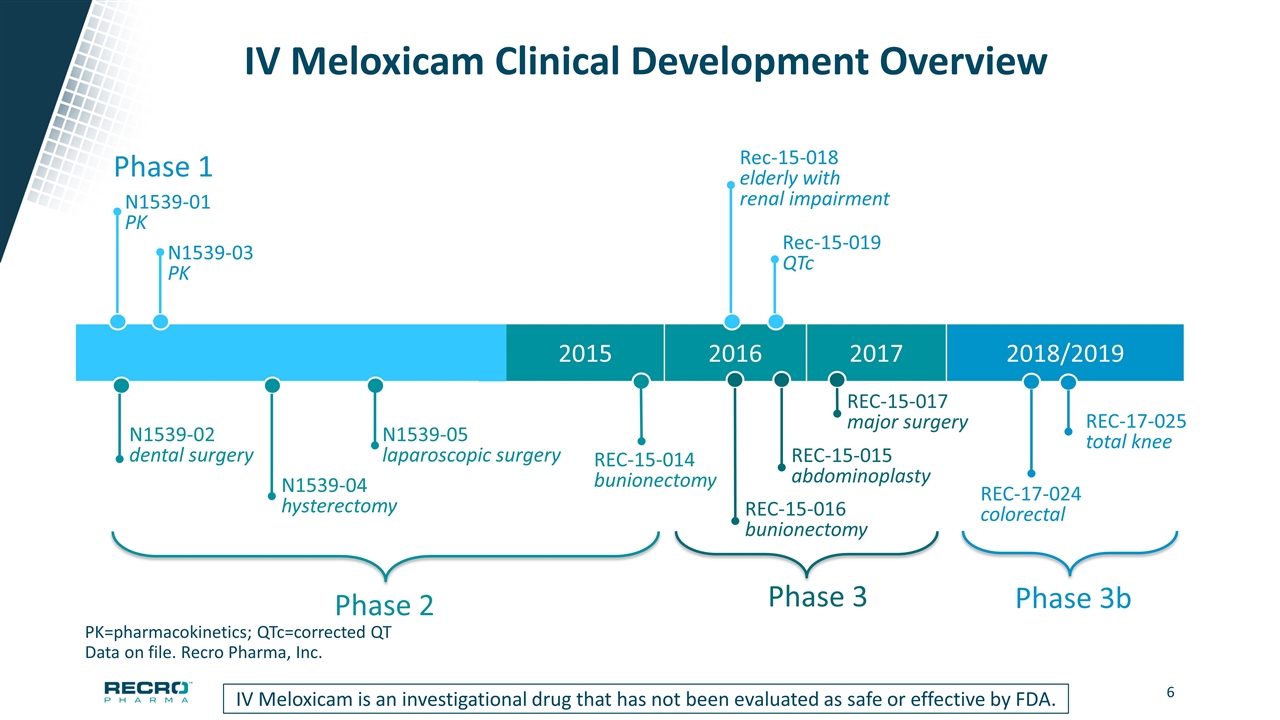
2015 2016 2017 2018/2019 IV Meloxicam Clinical Development Overview PK=pharmacokinetics; QTc=corrected QT Data on file. Recro Pharma, Inc. Phase 1 N1539-01 PK N1539-03 PK Rec-15-018 elderly with renal impairment Rec-15-019 QTc Phase 2 N1539-02 dental surgery N1539-04 hysterectomy N1539-05 laparoscopic surgery REC-15-014 bunionectomy Phase 3 REC-15-015 abdominoplasty REC-15-016 bunionectomy REC-15-017 major surgery IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Phase 3b REC-17-024 colorectal REC-17-025 total knee
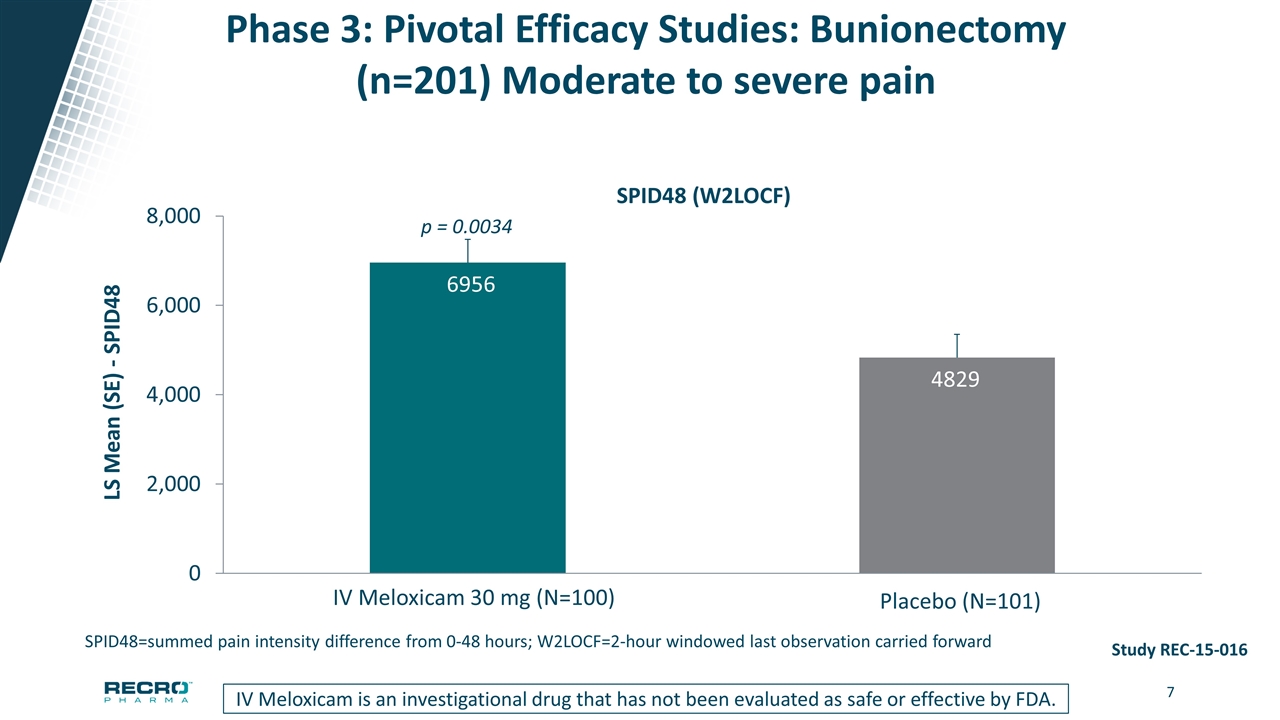
Phase 3: Pivotal Efficacy Studies: Bunionectomy (n=201) Moderate to severe pain p = 0.0034 Study REC-15-016 Meloxicam IV is an investigational drug that has not been evaluated as safe or effective by FDA SPID48 (W2LOCF) SPID48=summed pain intensity difference from 0-48 hours; W2LOCF=2-hour windowed last observation carried forward IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.
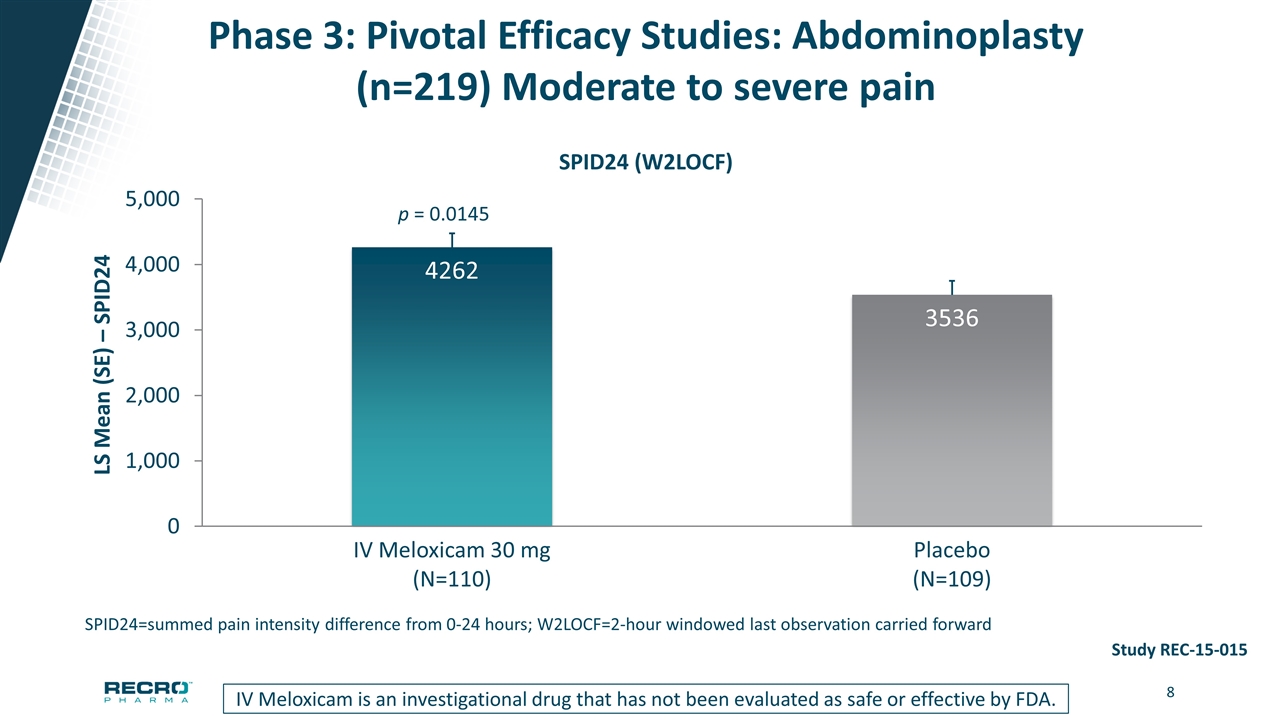
Phase 3: Pivotal Efficacy Studies: Abdominoplasty (n=219) Moderate to severe pain SPID24=summed pain intensity difference from 0-24 hours; W2LOCF=2-hour windowed last observation carried forward IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Study REC-15-015
Phase 3 Safety Study in Major Surgery [IV Meloxicam or Placebo] + Standard of Care Design 722 subjects undergoing major surgery with inpatient hospitalization for at least 24-48 hours Randomized, double-blind, placebo-controlled trial; IV meloxicam 30mg Evaluated the safety of IV Meloxicam in the setting of major surgery 31 centers Study drug administered as a bolus Once daily dosing for up to 7 doses Additional dose at time of discharge at the discretion of subject and primary investigator IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Study REC-15-017
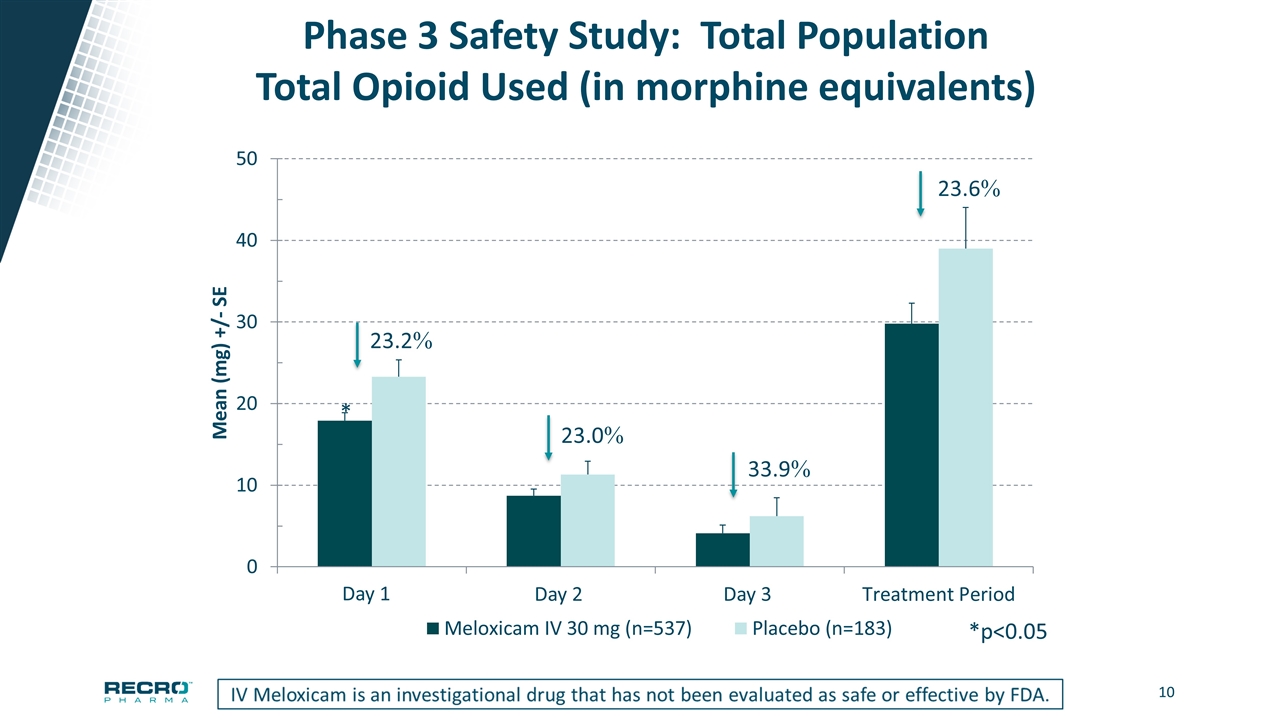
Phase 3 Safety Study: Total Population Total Opioid Used (in morphine equivalents) * *p<0.05 Day 1
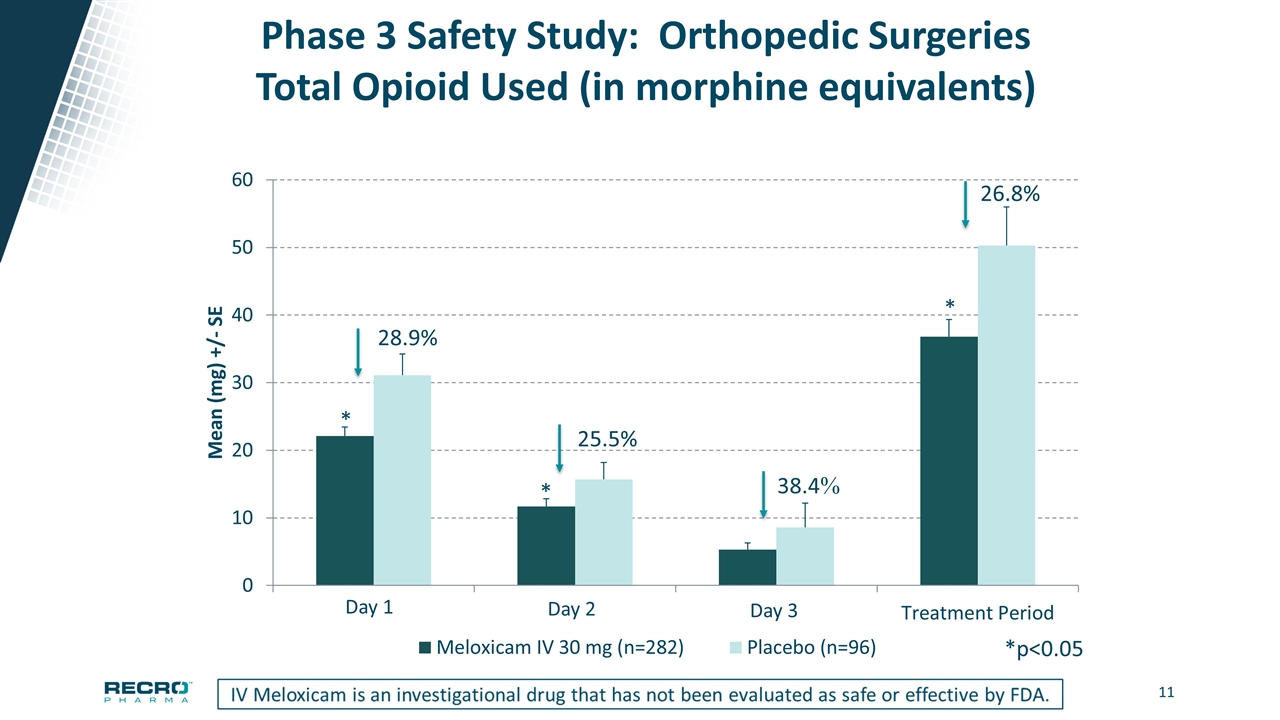
Phase 3 Safety Study: Orthopedic Surgeries Total Opioid Used (in morphine equivalents) 28.9% 25.5% *p<0.05 * * * Day 2 Day 3
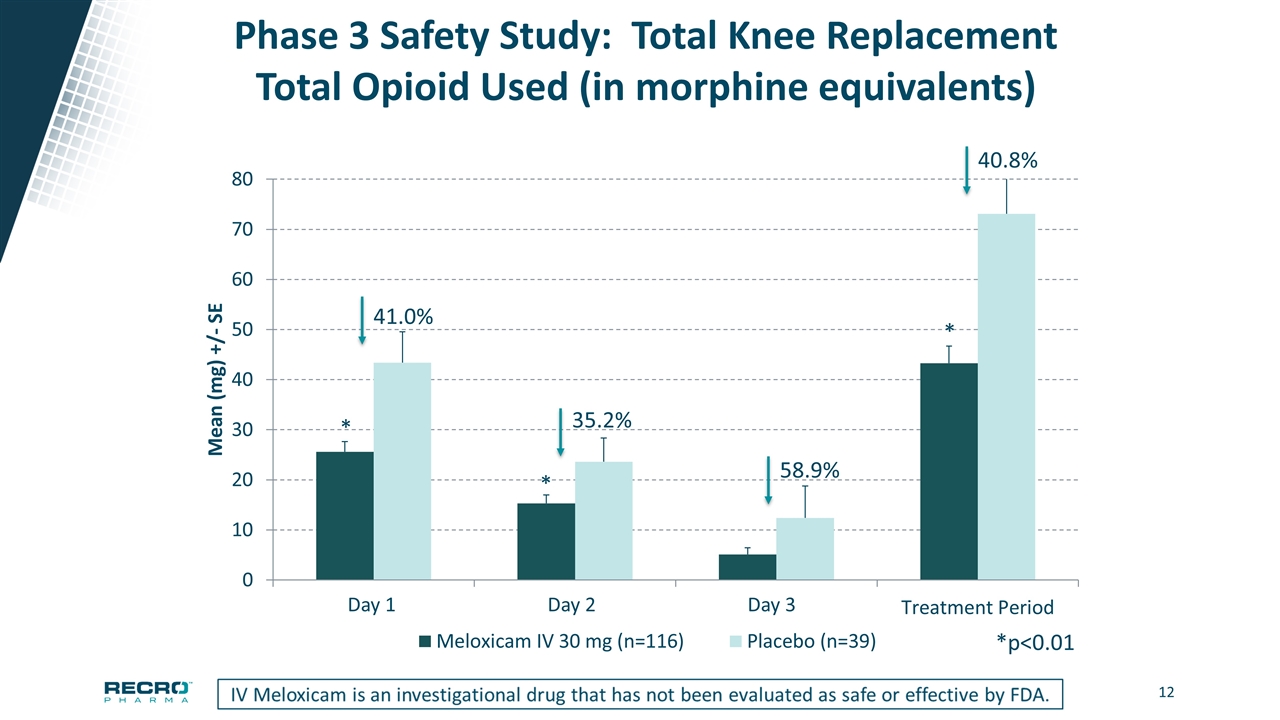
Phase 3 Safety Study: Total Knee Replacement Total Opioid Used (in morphine equivalents) 41.0% 35.2% *p<0.01 * * * 58.9% 40.8% Day 1 Day 2 Day 3
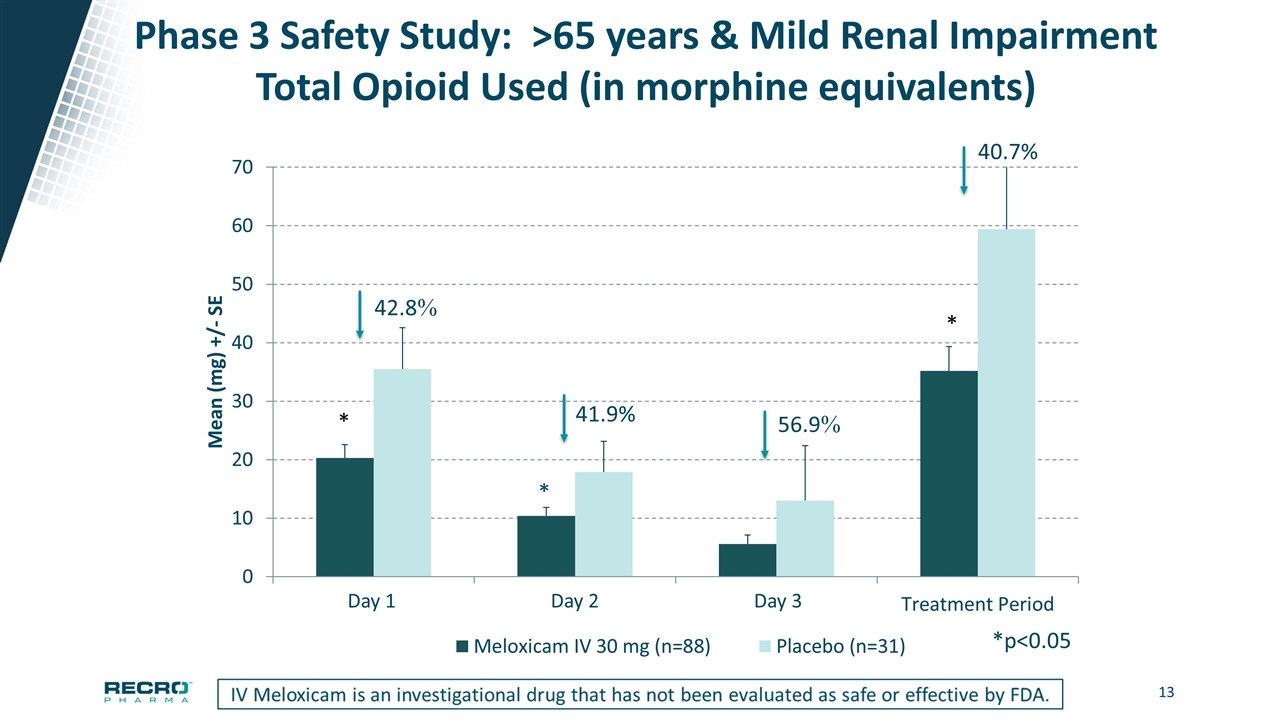
Phase 3 Safety Study: >65 years & Mild Renal Impairment Total Opioid Used (in morphine equivalents) *p<0.05 Day 1 Day 2 Day 3
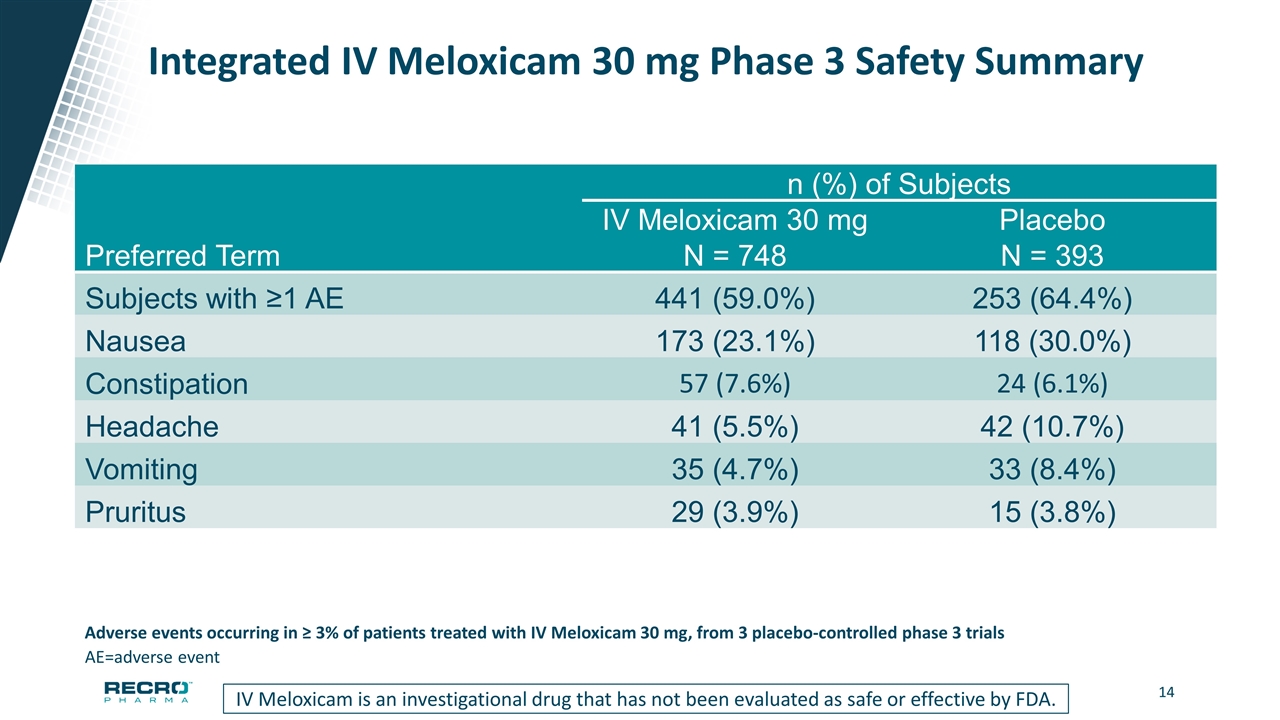
Integrated IV Meloxicam 30 mg Phase 3 Safety Summary n (%) of Subjects IV Meloxicam 30 mg Placebo Preferred Term N = 748 N = 393 Subjects with ≥1 AE 441 (59.0%) 253 (64.4%) Nausea 173 (23.1%) 118 (30.0%) Constipation 57 (7.6%) 24 (6.1%) Headache 41 (5.5%) 42 (10.7%) Vomiting 35 (4.7%) 33 (8.4%) Pruritus 29 (3.9%) 15 (3.8%) AE=adverse event Adverse events occurring in ≥ 3% of patients treated with IV Meloxicam 30 mg, from 3 placebo-controlled phase 3 trials IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.
Next Steps for IV Meloxicam NDA resubmitted September 2018 NDA Resubmission included* (among other items): Label changes to address the concerns that the FDA has expressed regarding time to onset and duration of analgesic effect Additional information with respect to extractables and leachables to address FDA concerns New PDUFA Date Set for March 24, 2019 Execute commercialization and launch plan following potential FDA approval Further expansion of IP portfolio * There can be no assurance that this approach will be acceptable to the FDA IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.

Go To Market Strategy
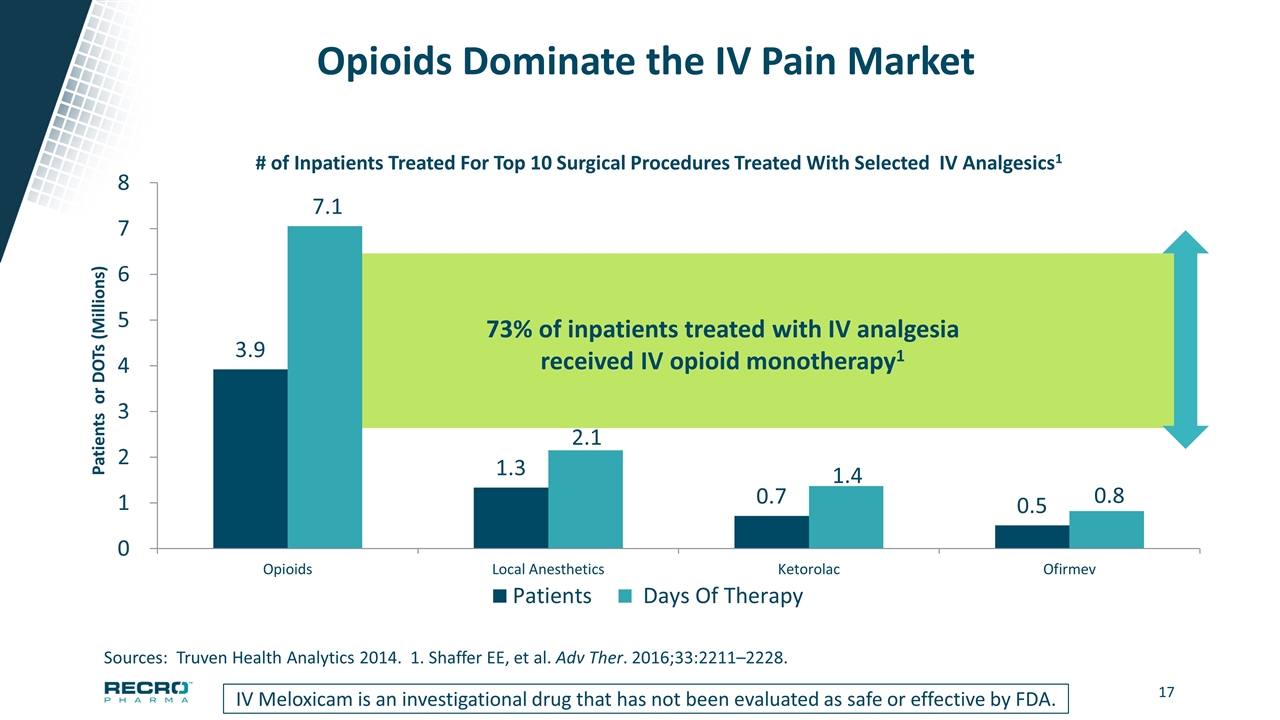
Opioids Dominate the IV Pain Market IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Sources: Truven Health Analytics 2014. 1. Shaffer EE, et al. Adv Ther. 2016;33:2211–2228. 73% of inpatients treated with IV analgesia received IV opioid monotherapy1

Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Post-operative Period In opioid-naive patients, many surgical procedures are associated with an increased risk of chronic opioid use in the post-operative period1 6%-10% of opioid naïve surgical patients will use opioids persistently2 Incidence of new persistent opioid use was similar between minor and major surgery, 5.9% and 6.5% respectively Incidence of new persistent opioid use in the nonoperative control group was only 0.4% IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. 1. Sun EC, et al: JAMA Intern Med. Published online July 11, 2016. 2. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;e170504. doi:10.1001/jamasurg.2017.0504
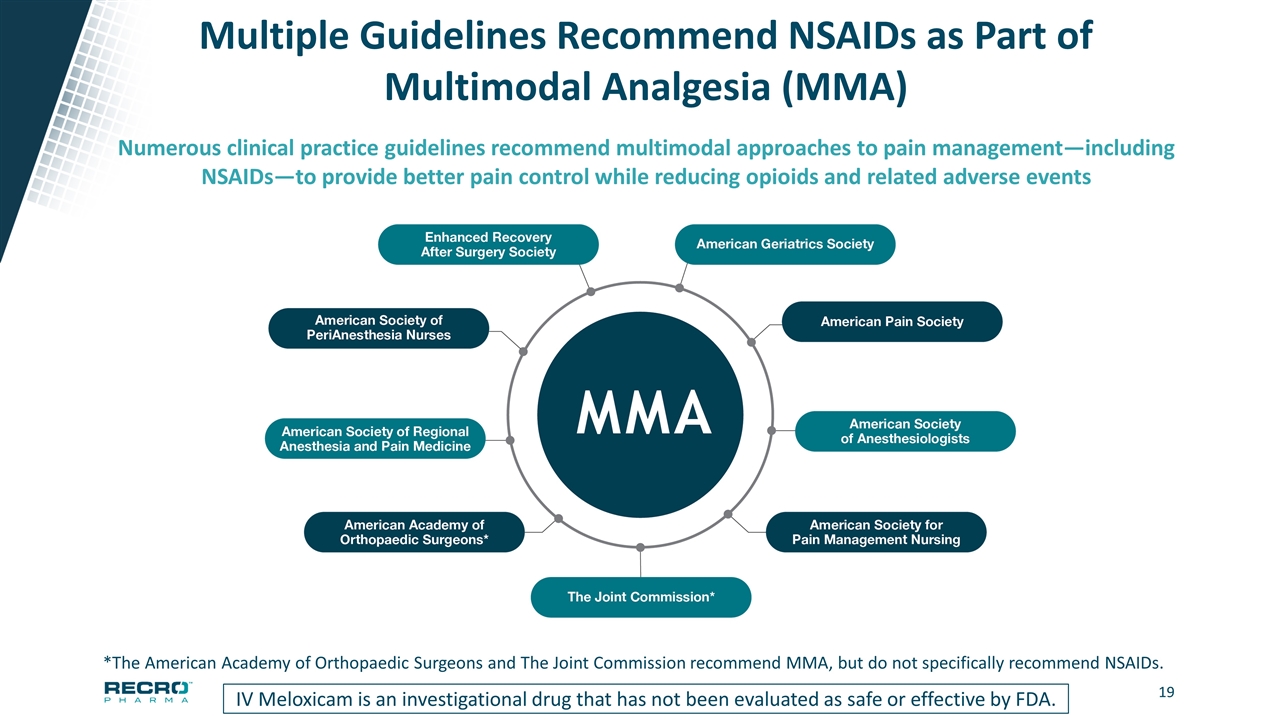
Multiple Guidelines Recommend NSAIDs as Part of Multimodal Analgesia (MMA) IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Numerous clinical practice guidelines recommend multimodal approaches to pain management—including NSAIDs—to provide better pain control while reducing opioids and related adverse events *The American Academy of Orthopaedic Surgeons and The Joint Commission recommend MMA, but do not specifically recommend NSAIDs.
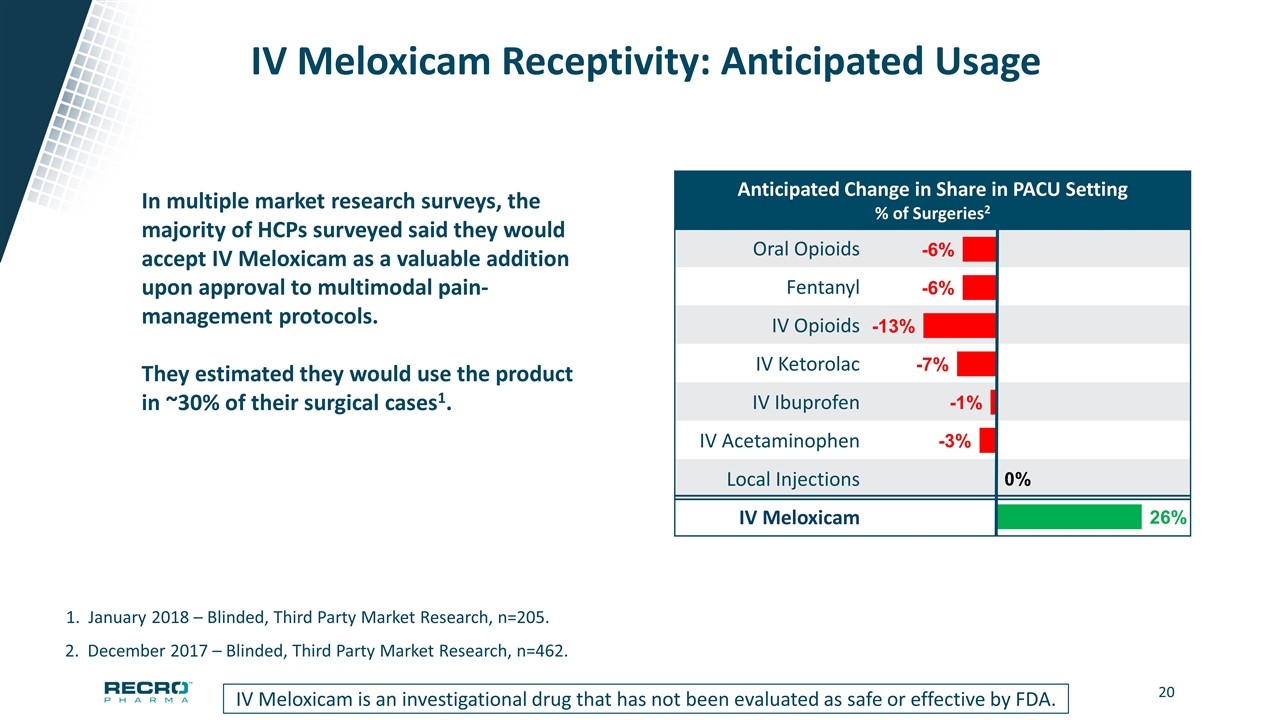
IV Meloxicam Receptivity: Anticipated Usage IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. 2. December 2017 – Blinded, Third Party Market Research, n=462. 1. January 2018 – Blinded, Third Party Market Research, n=205. In multiple market research surveys, the majority of HCPs surveyed said they would accept IV Meloxicam as a valuable addition upon approval to multimodal pain-management protocols. They estimated they would use the product in ~30% of their surgical cases1. Anticipated Change in Share in PACU Setting % of Surgeries2 Oral Opioids Fentanyl IV Opioids IV Ketorolac IV Ibuprofen IV Acetaminophen Local Injections IV Meloxicam
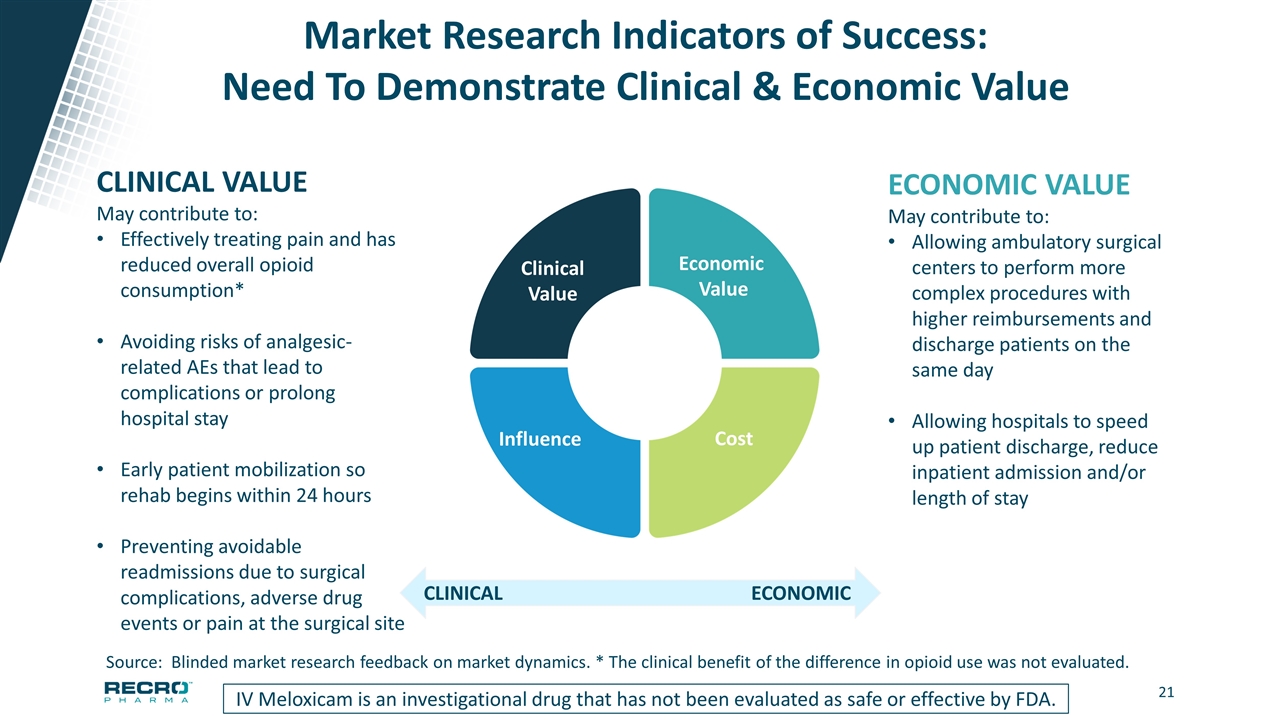
Market Research Indicators of Success: Need To Demonstrate Clinical & Economic Value IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. CLINICAL VALUE May contribute to: Effectively treating pain and has reduced overall opioid consumption* Avoiding risks of analgesic-related AEs that lead to complications or prolong hospital stay Early patient mobilization so rehab begins within 24 hours Preventing avoidable readmissions due to surgical complications, adverse drug events or pain at the surgical site ECONOMIC VALUE May contribute to: Allowing ambulatory surgical centers to perform more complex procedures with higher reimbursements and discharge patients on the same day Allowing hospitals to speed up patient discharge, reduce inpatient admission and/or length of stay ECONOMIC CLINICAL Source: Blinded market research feedback on market dynamics. * The clinical benefit of the difference in opioid use was not evaluated. Influence Clinical Value Cost Economic Value
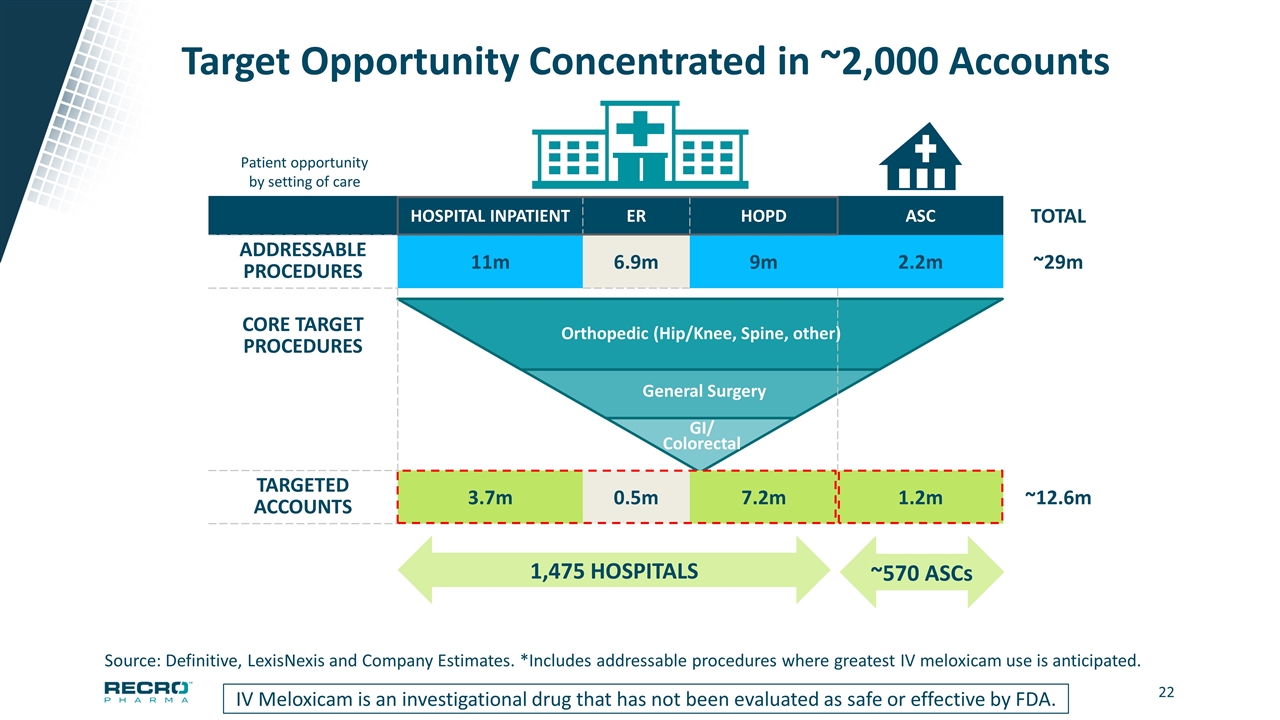
Target Opportunity Concentrated in ~2,000 Accounts IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. HOSPITAL INPATIENT ER HOPD ASC TOTAL ADDRESSABLE PROCEDURES 11m 6.9m 9m 2.2m ~29m CORE TARGET PROCEDURES TARGETED ACCOUNTS 3.7m 0.5m 7.2m 1.2m ~12.6m 1,475 HOSPITALS ~570 ASCs Source: Definitive, LexisNexis and Company Estimates. *Includes addressable procedures where greatest IV meloxicam use is anticipated. GI/ Colorectal General Surgery Orthopedic (Hip/Knee, Spine, other) Patient opportunity by setting of care
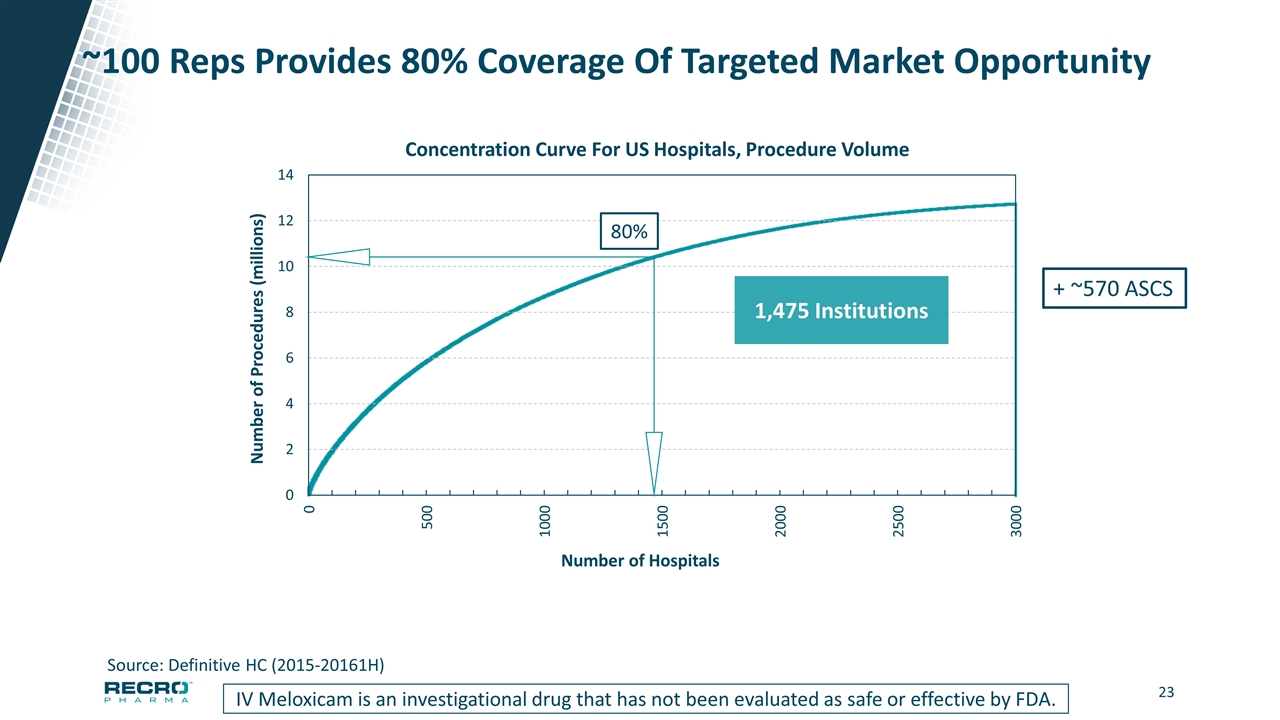
~100 Reps Provides 80% Coverage Of Targeted Market Opportunity IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. Source: Definitive HC (2015-20161H) Number of Procedures (millions) Number of Hospitals 80% 1,475 Institutions + ~570 ASCS
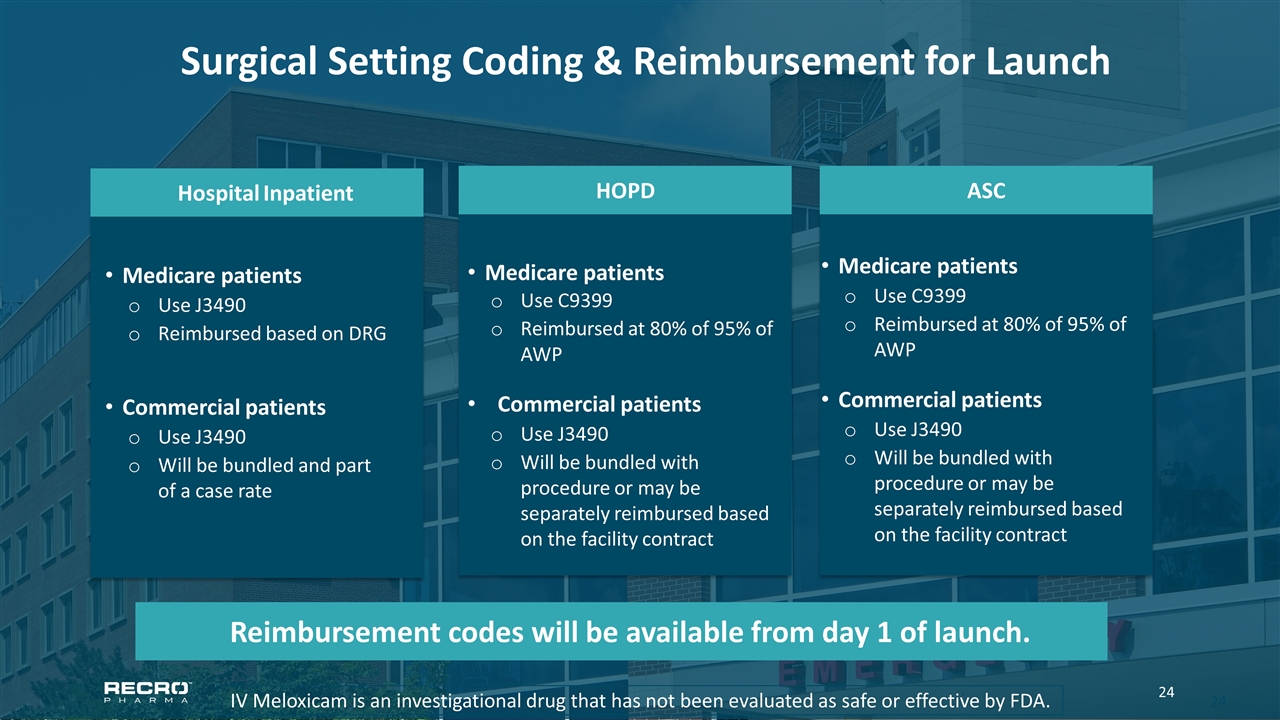
Surgical Setting Coding & Reimbursement for Launch IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA. ASC Medicare patients Use C9399 Reimbursed at 80% of 95% of AWP Commercial patients Use J3490 Will be bundled with procedure or may be separately reimbursed based on the facility contract HOPD Medicare patients Use C9399 Reimbursed at 80% of 95% of AWP Commercial patients Use J3490 Will be bundled with procedure or may be separately reimbursed based on the facility contract Hospital Inpatient Medicare patients Use J3490 Reimbursed based on DRG Commercial patients Use J3490 Will be bundled and part of a case rate Reimbursement codes will be available from day 1 of launch.

Contract Development and Manufacturing (CDMO) Business Overview Gainesville

Gainesville CDMO Facility
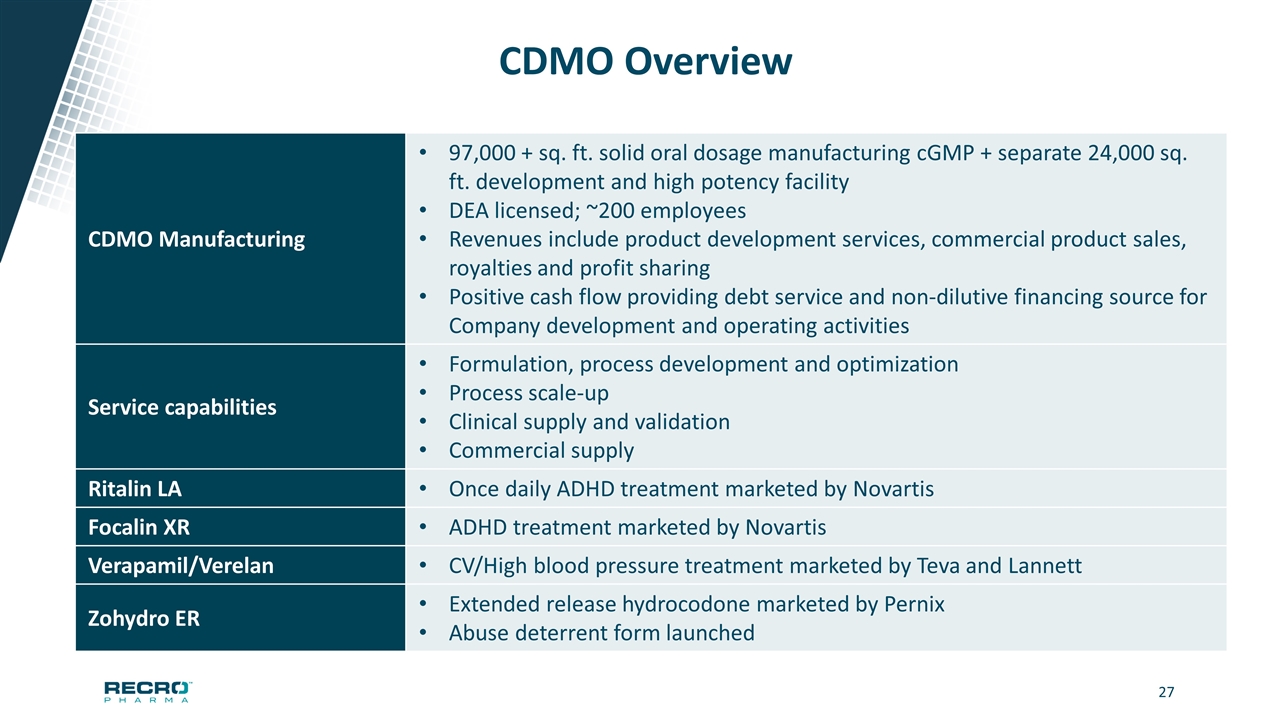
CDMO Overview CDMO Manufacturing 97,000 + sq. ft. solid oral dosage manufacturing cGMP + separate 24,000 sq. ft. development and high potency facility DEA licensed; ~200 employees Revenues include product development services, commercial product sales, royalties and profit sharing Positive cash flow providing debt service and non-dilutive financing source for Company development and operating activities Service capabilities Formulation, process development and optimization Process scale-up Clinical supply and validation Commercial supply Ritalin LA Once daily ADHD treatment marketed by Novartis Focalin XR ADHD treatment marketed by Novartis Verapamil/Verelan CV/High blood pressure treatment marketed by Teva and Lannett Zohydro ER Extended release hydrocodone marketed by Pernix Abuse deterrent form launched
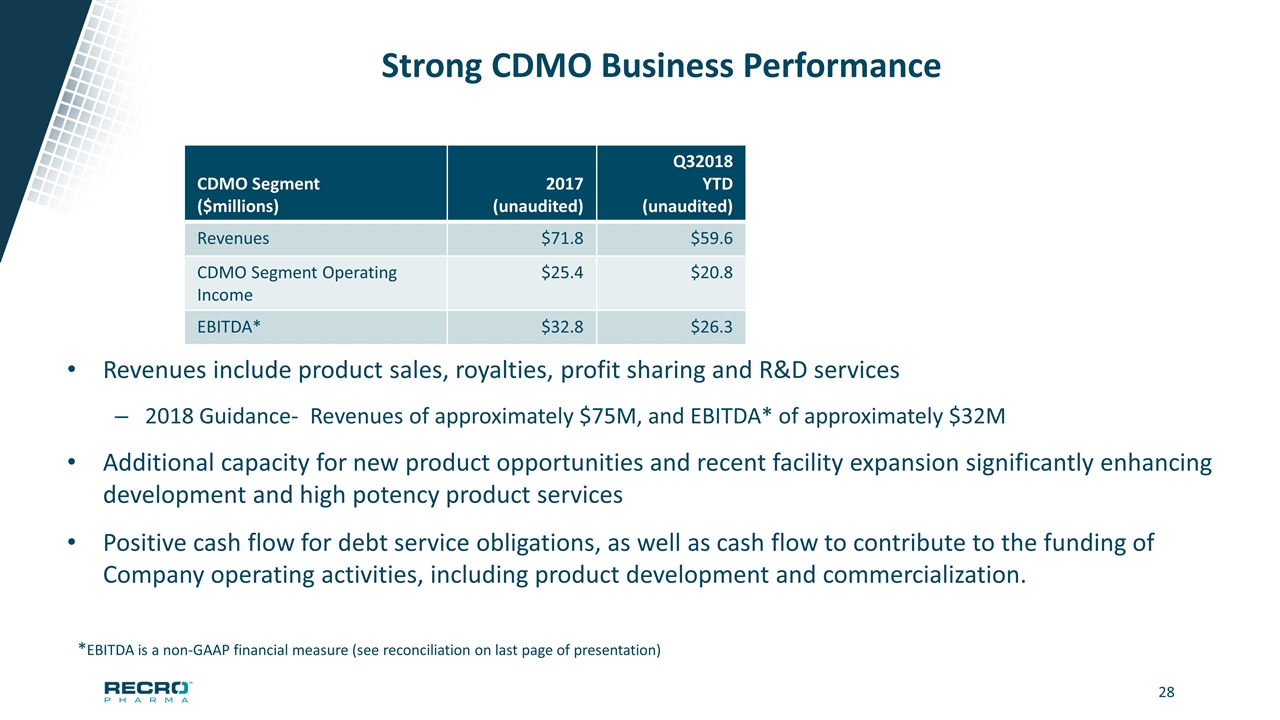
Strong CDMO Business Performance Revenues include product sales, royalties, profit sharing and R&D services 2018 Guidance- Revenues of approximately $75M, and EBITDA* of approximately $32M Additional capacity for new product opportunities and recent facility expansion significantly enhancing development and high potency product services Positive cash flow for debt service obligations, as well as cash flow to contribute to the funding of Company operating activities, including product development and commercialization. *EBITDA is a non-GAAP financial measure (see reconciliation on last page of presentation) CDMO Segment ($millions) 2017 (unaudited) Q32018 YTD (unaudited) Revenues $71.8 $59.6 CDMO Segment Operating Income $25.4 $20.8 EBITDA* $32.8 $26.3

License/Financing Activities IV Meloxicam License Agreement (Amended December 2018) Reduces 2019 cash requirements by $30 million, extends approval milestone payments over seven years, repriced existing 350,000 warrants at market pricing plus a 20% premium to $8.26 per share. The combined revised consideration for the amended milestone payment results in a net present value of $45 million utilizing an approximate discount rate of 11%. Milestone payment due upon approval of IV Meloxicam restructured as follows: $5 million within 30 days of amendment; $5 million in April 2019; $5 million within 180 days of approval of IV meloxicam $45 million in seven equal annual payment of $6.4 million each, commencing on the first anniversary following FDA approval $100 Million November 2017 Credit Facility (Amended December 2018) Amended credit facility to restore $40 million in debt funding, repriced existing 348,664 warrants to market price of $6.84 per share. Restructured in three tranches $10 million at closing (Total drawn as of December 31, 2018 = $70 million) $15 million upon FDA approval of IV Meloxicam and $20 million in unrestricted cash proforma for certain milestone payments $15 million available after Recro demonstrates early IV Meloxicam commercial traction – original agreement Financial flexibility to aid our transformation into a commercial-stage enterprise; Contributes toward funding for IV Meloxicam milestone payments under the Company’s licensing agreement amended in December 2018 IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.

Company Highlights Specialty pharmaceutical company focused on hospital and related settings with late stage investigational product, IV Meloxicam, targeting management of moderate to severe pain NDA resubmitted end of September 2018; PDUFA date March 24, 2019 Expanded IV Meloxicam IP Portfolio Multiple therapeutics in clinical development for hospital and related settings Revenue and cash flow positive contract development and manufacturing (CDMO) business Increased 2018 Full Year Financial Guidance to Revenue of $75M and EBITDA* of $32M 2018Q3YTD Revenue- $59.6M; EBITDA*- $26.3M 2017 Full Year Revenue- $71.8M; EBITDA*- $32.8M Cash position – $37.0M @ 9/30/18 Amended Alkermes IV Meloxicam license agreement; reduces 2019 cash requirements by $30 million, extends approval milestone payments over seven years Amended $100 million credit facility, restores $40 million in debt funding; $70 million drawn as of 12/31/18 Experienced management team with significant development, regulatory and commercial experience *EBITDA is a non-GAAP financial measure. See reconciliation on last page of presentation. IV Meloxicam is an investigational drug that has not been evaluated as safe or effective by FDA.
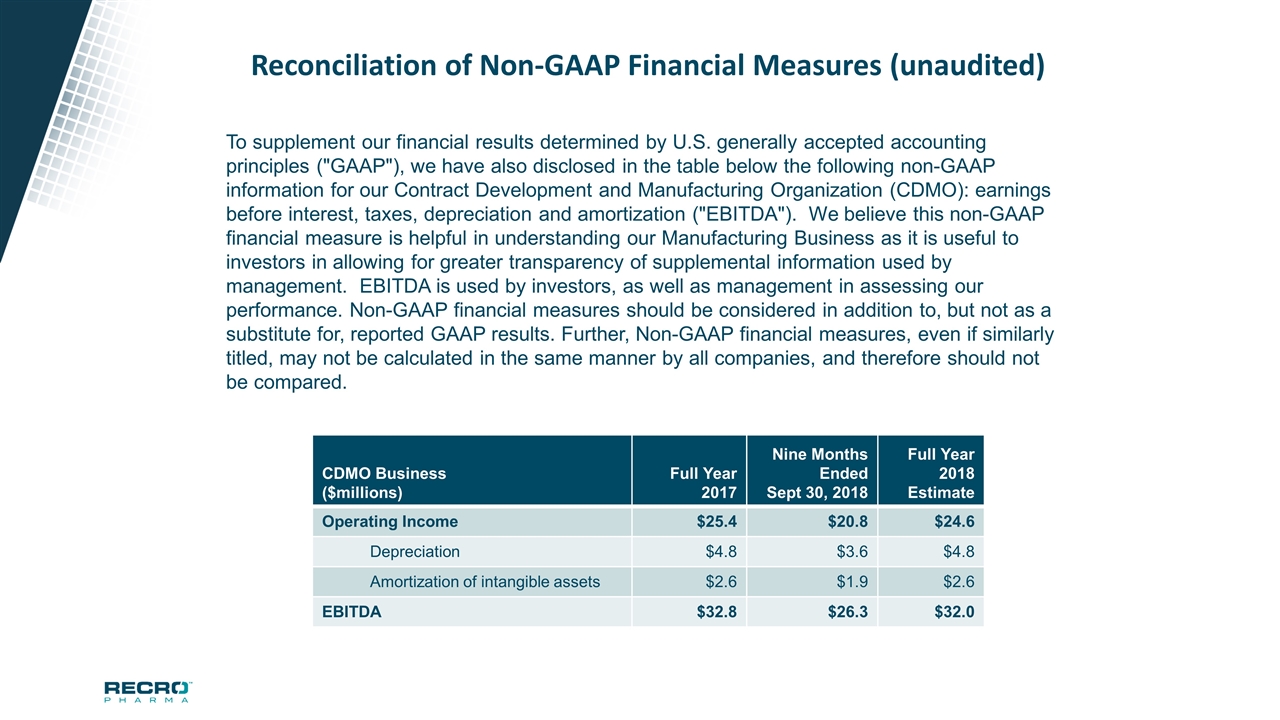
Reconciliation of Non-GAAP Financial Measures (unaudited) CDMO Business ($millions) Full Year 2017 Nine Months Ended Sept 30, 2018 Full Year 2018 Estimate Operating Income $25.4 $20.8 $24.6 Depreciation $4.8 $3.6 $4.8 Amortization of intangible assets $2.6 $1.9 $2.6 EBITDA $32.8 $26.3 $32.0 To supplement our financial results determined by U.S. generally accepted accounting principles ("GAAP"), we have also disclosed in the table below the following non-GAAP information for our Contract Development and Manufacturing Organization (CDMO): earnings before interest, taxes, depreciation and amortization ("EBITDA"). We believe this non-GAAP financial measure is helpful in understanding our Manufacturing Business as it is useful to investors in allowing for greater transparency of supplemental information used by management. EBITDA is used by investors, as well as management in assessing our performance. Non-GAAP financial measures should be considered in addition to, but not as a substitute for, reported GAAP results. Further, Non-GAAP financial measures, even if similarly titled, may not be calculated in the same manner by all companies, and therefore should not be compared.






























