Exhibit (a)(5)(x)
IN THE COURT OF CHANCERY OF THE STATE OF DELAWARE
BRIAN COHEN, on Behalf of Himself and All Others Similarly Situated,
Plaintiff, v.
VIROPHARMA INCORPORATED, VINCENT J. MILANO, PAUL A. BROOKE, WILLIAM D. CLAYPOOL, MICHAEL R. DOUGHERTY, ROBERT J. GLASER, JOHN R. LEONE, HOWARD H. PIEN, JULIE H. MCHUGH, SHIRE PHARMACEUTICAL HOLDINGS IRELAND LIMITED, VENUS NEWCO, INC. AND SHIRE PLC,
Defendants. | ) | C.A. No. | |
| ) | | |
| ) | | |
| ) | CLASS ACTION | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
| ) | | |
VERIFIED CLASS ACTION COMPLAINT
Plaintiff Brian Cohen (“Plaintiff”), by his attorneys, alleges upon information and belief, except for his own acts, which are alleged on knowledge as follows:
SUMMARY OF THE ACTION
1. This is a stockholder class action brought by plaintiff, Brian Cohen (“Plaintiff”), a holder of common stock of ViroPharma Incorporated (“ViroPharma” or the “Company”), against ViroPharma’s Board of Directors (the “Board”), Shire Pharmaceutical Holdings Ireland Limited, Venus Newco, Inc., and Shire plc (collectively, “Shire”). This action seeks to enjoin a proposed transaction announced on November 11, 2013, pursuant to which Shire will acquire the outstanding shares of ViroPharma in a deal valued at approximately $4.2 billion, with each ViroPharma stockholder receiving $50.00 per share of Company stock (the “Proposed Transaction”) upon consummation of the merger. In pursuing the Proposed Transaction, each member of the Board violated applicable law by directly breaching and/or aiding breaches of
fiduciary duties of loyalty, good faith, due care, and candor owed to plaintiff and the proposed class.
2. On November 25, 2013, Shire commenced the tender offer (“Tender Offer”) with the filing of its Tender Offer Statement on Schedule TO (“TO”) with the Securities and Exchange Commission (“SEC”). ViroPharma filed its Recommendation Statement in connection with the Tender Offer on Schedule 14D-9 with the SEC on November 25, 2013 (the “Recommendation Statement”) and on that same date, also filed a Preliminary Proxy Statement on Schedule 14A with the SEC (the “Proxy” and collectively with the Recommendation Statement and TO, the “Disclosure Documents”). The Tender Offer is currently scheduled to expire at 6:00 p.m., New York City time, on Thursday, December 26, 2013 unless it is extended or earlier terminated.
3. In particular, in the Agreement and Plan of Merger the Company entered into on July 30, 2013 (the “Merger Agreement”), provides that:
[Shire] has agreed to cause [Venus Newco] to commence a cash tender offer (as promptly as practicable, but in no event later than November 25, 2013) for all of the Shares [of ViroPharma] at a purchase price of $50.00 per Share (the “Offer Price”), net to the seller in cash, without interest and less any required withholding taxes. As soon as practicable following the consummation of the Offer, and subject to the satisfaction or waiver of certain conditions set forth in the Merger Agreement, Merger Sub will merge with and into the Company (the “Merger”) pursuant to the provisions of section 251(h) of the Delaware General Corporation Law (the “DGCL”), with no stockholder vote required to consummate the Merger, and the Company will survive as a wholly owned subsidiary of [Shire]. At the effective time of the Merger, any Shares not purchased pursuant to the Offer (other than Shares owned by Parent or Merger Sub, or the Company or any of its subsidiaries, and Shares owned by stockholders who have properly exercised any available rights of appraisal under section 262 of the DGCL) will be automatically converted into the right to receive cash in an amount equal to the Offer Price.
4. As described herein, the Proposed Transaction, which has been approved by unanimous vote of the Board, is the product of a flawed process that is designed to ensure the
sale of ViroPharma to Shire on terms preferential to Shire, but detrimental to plaintiff and the other public stockholders of ViroPharma.
5. ViroPharma “is an international biotechnology company dedicated to the development and commercialization of novel solutions for physician specialists to address unmet medical needs of patients living with serious diseases that have few if any clinical therapeutic options, including therapeutics for rare and orphan diseases.” In its most recent 10-Q issued before the Proposed Transaction was announced, the Company stated that it expects future sales growth to be driven by developing its product pipeline, expanding its territories beyond the U.S. boarders, and through potential acquisitions. Indeed, ViroPharma has successfully acquired, developed and expanded its primary drug Cinryze - a drug developed to treat hereditary angioedema (HAE) - after acquiring the rights to sell it in the U.S. in October 2008. In 2012, ViroPharma acquired the rights to market and sell Cinryze in Europe which is expected to increase the company’s sales significantly. ViroPharma also has several drugs in its pipeline that investors have waited on for years to develop into sources of revenue for the Company. Yet, if the Proposed Transaction closes, ViroPharma investors such as Plaintiff will be denied the growth opportunity the Company has been touting. As described further herein, the claimed 27% premium fails to adequately value Company or compensate the Company’s stockholders.
6. The Proposed Transaction is also subject to unfair deal protection provisions which have been agreed to by the Board that deny stockholders the opportunity of a better price. Specifically, the Merger Agreement provides for: (i) a “no-solicitation” provision prohibiting the Company from properly shopping itself; (ii) a four business-day “matching rights” period during which Shire can fully evaluate and match any superior proposal received by the Company; and (iii) a termination fee of $127.14 million payable to Shire if terminates the
Merger Agreement to pursue another offer. In agreeing to provisions, each of member of ViroPharma’s Board violated applicable law by directly breaching and/or aiding the other defendants’ breaches of their fiduciary duties of loyalty, good faith, due care, and candor.
7. Further, on November 25, 2013, ViroPharma filed a recommendation statement on Schedule 14D-9 (the “Recommendation Statement”) with the Securities and Exchange Commission (the “SEC”) voicing the Board’s support for the Tender Offer. The Recommendation Statement however misrepresents and fails to disclose material information necessary for the Company’s stockholders to make an informed decision as to whether to tender their shares in the Tender Offer. Specifically, the Recommendation Statement fails to disclose, in violation of the Board’s duty of candor under state law, including: (i) certain information regarding the financial analysis performed by Goldman Sachs & Co. (“Goldman Sachs”), ViroPharma’s adviser on the fairness of the Merger Agreement and (ii) several important details regarding the process leading up to the signing of the Merger Agreement.
8. To remedy defendants’ breaches of fiduciary duty and other misconduct, Plaintiff seeks, inter alia: (i) injunctive relief preventing consummation of the Proposed Transaction; (ii) a directive to the members of the Board exercise their fiduciary duties to obtain a transaction which is in the best interests of ViroPharma stockholders; and (iii) rescission of, to the extent already implemented, the Merger Agreement, or any of the terms thereof.
PARTIES
9. Plaintiff has been a stockholder at all times relevant hereto and is a stockholder of ViroPharma.
10. Defendant ViroPharma is a Delaware corporation with principal executive offices located at 730 Stockton Drive, Exton, Pennsylvania 19341. Upon completion of the Proposed Transaction, ViroPharma will become a wholly owned subsidiary of Shire.
11. Defendant Vincent J. Milano (“Milano”) is ViroPharma’s Chief Executive Officer, President and Chairman of the Board, and he has served in those roles since March 2008.
12. Defendant Paul A. Brooke (“Brooke”) is a member of ViroPharma’s Board and has been since February 2001. Brook is a member of the Company’s Audit Committee.
13. Defendant William D. Claypool (“Claypool”) is a member of ViroPharma’s Board and has been since December 2003. Claypool is a member of the Company’s Compensation Committee.
14. Defendant Michael R. Dougherty (“Dougherty”) is a member of ViroPharma’s Board and has been since January 2004. Dougherty is Chairman of the Company’s Audit Committee and a member of its Nominating and Governance Committee.
15. Defendant Robert J. Glaser (“Glaser”) is a member of ViroPharma’s Board and has been since August 1997. Glaser is Chairman of the Company’s Compensation Committee.
16. Defendant John R. Leone (“Leone”) is a member of ViroPharma’s Board and has been since January 2006. Leone is a member of the Company’s Audit Committee.
17. Defendant Howard H. Pien (“Pien”) is a member of ViroPharma’s Board and has been since May 2006. Pien is Chairman of the Company’s Nominating and Governance Committee.
18. Defendant Julie H. McHugh (“McHugh”) is a member of ViroPharma’s Board and has been since January 2012.
19. Defendant Shire Pharmaceutical Holding Ireland Limited is incorporated in Ireland and develops and markets “innovative specialty medicines for symptomatic conditions to meet significant unmet patient needs.”
20. Defendant Venus Newco, Inc. is a Delaware corporation and a direct, wholly owned subsidiary of defendant Shire Pharmaceutical. Upon completion of the Proposed Transaction, Venus Newco will merge with and into ViroPharma and cease its separate corporate existence.
21. Defendant Shire plc is incorporated in New Jersey and, for the purposes of the Proposed Transaction, is a parent company of Venus Newco. Shire plc “has guaranteed the performance by Parent and Merger Sub of their obligations under the Merger Agreement.”
INDIVIDUAL DEFENDANTS’ FIDUCIARY DUTIES
22. Under Delaware law, the officers and directors of a publicly traded corporation have fiduciary duties of loyalty and care to stockholders. To diligently comply with these duties, neither the officers nor the directors may take any action that:
(a) adversely affects the value provided to the corporation’s stockholders;
(b) will discourage, inhibit, or deter alternative offers to purchase control of the corporation or its assets;
(c) contractually prohibits themselves from complying with their fiduciary duties;
(d) will otherwise adversely affect their duty to secure the best value reasonably available under the circumstances for the corporation’s stockholders; and/or
(e) will provide the officers and/or directors with preferential treatment at the expense of, or separate from, the public stockholders.
23. In accordance with the Board’s duties of loyalty, the Individual Defendants, as officers and/or directors of ViroPharma, are obligated under Delaware law to refrain from:
(a) participating in any transaction where the officers’ or directors’ loyalties are divided;
(b) participating in any transaction where the officers or directors receive, or are entitled to receive, a personal financial benefit not equally shared by the public stockholders of the corporation; and/or
(c) unjustly enriching themselves at the expense or to the detriment of the public stockholders.
24. Defendants, separately and together, in connection with the Proposed Transaction, are knowingly or recklessly violating their fiduciary duties and aiding and abetting such breaches, including their duties of loyalty, due care, and candor owed to plaintiff and other public stockholders of ViroPharma.
FACTUAL ALLEGATIONS
ViroPharma’s Background and Growth Potential
25. ViroPharma was founded in 1994 and trades on the Nasdaq Global Market under the ticker symbol “VPHM.”
26. ViroPharma is “is an international biotechnology company dedicated to the development and commercialization of novel solutions for physician specialists to address unmet medical needs of patients living with serious diseases that have few if any clinical therapeutic options, including therapeutics for rare and orphan diseases.”
27. Viropharma’s products include Cinryze, which accounted for 76 percent of Viropharma’s total sales in 2012 and is designed for “routine prophylaxis against angioedema attacks in adolescent and adult patients with HAE,” Buccolam, which is being commercialized in Europe for “treatment of prolonged, acute, convulsive seizures in infants, toddlers, children and adolescents, from 3 months to less than 18 years of age,” and Plenadren, which is designed to treat adrenal insufficiency (“AI”). Plenadren has been approved in Europe and the Company is considering expanding its markets to the United States.
28. The long term prospects for ViroPharma’s finances are good. In June 2011, the European Commission granted ViroPharma “Centralized Marketing Authorization for Cinryze in adults and adolescents with HAE for routine prevention, pre-procedure prevention and acute treatment of angioedema attacks.” On August 6, 2012, the U.S. Food and Drug Administration approved the Company’s “supplement to the Cinryze Biologics License Application (BLA) for industrial scale manufacturing which increases [its] manufacturing capacity of Cinryze.” The Company also has several efforts underway to develop alternative uses for Cinryze.
29. Sales of Cinryze have increased substantially in the past year and are expected to continue to grow as the Company expands its sales area. In the third quarter of 2013, net sales of Cinryze were $106.5 million, up from $85.3 million for the same quarter in 2012.
30. ViroPharma also maintains several pipeline drugs under development, including Maribavir, which is being developed for use in transplant procedures, VP 20621/NTCD to treat recurrent clostridium difficile, VP 20629/IPA, for the treatment of Friedreich’s Ataxia.
31. ViroPharma has spoken frequently to investors about its expectations that the Company would grow dramatically as revenue from new drugs and new applications began to flow into the company. On January 7, 2013, ViroPharma announced sales guidance for 2013. The Company said at the time that it expected revenue of between $450 million and $475 million, with sales of Cinryze to account for between $390 million and $400 from North America alone. Following the announcement of this news, the Company’s stock price rose $1.01, or 4.3 percent.
32. On February 27, 2013, ViroPhrama announced its fourth (“4Q”) quarter 2012 and full-year 2012 results. The Company generated net sales of $427.9 million for the year, which marked a decline from 2011 that ViroPhrama explained was the result of “the loss of Vancocin
revenues partially offset by Cinryze growth.” In commenting on the Company’s position moving forward, Defendant Milano said:
ViroPharma enters 2013 in a strong position to generate significant growth for years to come. Cinryze in the U.S. continues to exceed our expectations, our products in Europe are beginning to demonstrate traction as we continue to expand those launches throughout the EU, the pipeline is more robust than it has ever been in our company’s history and financially our company is in a very good position to remain opportunistic for the right business development assets. Additionally, we’ve very aggressively improved our capital structure through our stock repurchase program. In 2013, our focus will remain on execution and continuing to deliver positive results to all of our key stakeholders and most importantly those that are at the core of our mission, the patients (emphasis added).
33. Further, the Company specifically addressed the growth in Cinryze net sales and its impact on the Company, stating:
Cinryze net sales during the three months and year ended December 31, 2012 were $97.0 million and $327.1 million, respectively, a 45 percent and 30 percent increase over the respective periods in 2011 due to demand growth. Vancocin net sales during the three and twelve months ended December 31, 2012 were $5.0 million and $90.8 million, respectively, compared to $77.8 million and $288.9 million during the three months and year ended December 31, 2011, respectively. The decrease is due to the introduction of generic vancomycin (emphasis added).
34. On May 1, 2013, ViroPharma announced its financial results for the first quarter (“1Q”) of 2013. The Company again saw results that declined year-over-year, but noted that the increasing sales of Cinryze were offsetting the introduction of generic vancomycin. Specifically, the press release stated:
Net sales were $107 million for the first quarter ended March 31, 2013 as compared to $136 million in the comparative period of 2012. The decline in net sales quarter over quarter was driven by the decrease in Vancocin revenues partially offset by commercial product growth for Cinryze. The first quarter 2013 U.S. Cinryze net sales which grew by 44 percent over the first quarter of 2012 to $97 million, including approximately $91 million of patient demand (emphasis added).
35. In the same release, Defendant Milano commented on the Company’s pipeline:
The early part of 2013 has seen great progress both in our commercial business as well as our development pipeline. In addition to the virologic response data we will share during our conference call today from subjects enrolled into our two maribavir studies, we also expect results from several key programs for Cinryze in the coming quarters such as subcutaneous Cinryze administration, antibody-mediated rejection (AMR) in kidney transplant, new uses for C1 INH, as well as additional progress updates with maribavir.
36. The release also highlighted the Company’s transition to Cinryze as the product driving its results. Specifically, the Company noted:
Cinryze net sales during the first quarter of 2013 were $99.5 million, a 46 percent increase over the same period in 2012 driven by demand growth, rebuilding channel inventories and net realized price growth. Vancocin net sales during the three months were $4 million compared to $66 million for the same period in 2012. The decrease is due to the impact of the launch of generic versions of vancomycin in April of 2012. During the first quarter of 2013, we generated net sales of approximately $7 million from our European operations.
37. On June 11, 2013, ViroPharma commented on its experimental drug Maribavir and revealed that:
the European Commission has granted orphan drug designation for maribavir for treatment of cytomegaloviral (CMV) disease in patients with impaired cell mediated immunity. The “Orphan Medicinal Product Designation” is designed to encourage the development of drugs which may provide significant benefit to patients suffering from rare diseases identified as “life-threatening or chronically debilitating” conditions. ViroPharma has previously received orphan drug designation for maribavir in the United States for treatment of clinically significant cytomegalovirus viremia and disease in at-risk patients.
Under EMA guidelines, Orphan Medicinal Product Designation provides 10 years of potential market exclusivity if the product candidate is approved for marketing in the European Union and the orphan designation is maintained.
38. On August 1, 2013, ViroPharma announced its operating results for the second quarter of 2013. The Company revealed increased net sales driven by Cinryze. Specifically the Company stated:
Net sales were $104 million for the second quarter ended June 30, 2013 as compared to $95 million in the comparative period of 2012. The increase in net sales quarter over quarter was driven by the commercial product growth of both Cinryze in the U.S. and higher European product sales. During the second
quarter of 2013 U.S. Cinryze net sales grew by 22 percent over the second quarter of 2012 to $91 million.
39. Defendant Milano was quoted in the press release as stating:
The second quarter of 2013 represented a period of strong commercial execution coupled with significant progress in our clinical development pipeline, despite this morning’s news related to our combination efforts with Halozyme. In addition to the positive maribavir interim data update we shared last month and the continued advancement of enrollment in the two studies, we also are approaching critical data milestones heading into the second half of 2013, including our antibody mediated rejection study with Cinryze in kidney transplant patients and analysis of the completed subjects from the Cinryze subcutaneous administration study which we updated this morning. On the commercial front, the continued growth trajectory of U.S. Cinryze remains strong, with early signs of positive impact from our sales force optimization and continued progress with our European operations. Overall, we believe ViroPharma is well positioned to continue generating growth and strong momentum.
40. In describing the continuing transition from Vancocin to Cinryze, the Company disclosed in the August 1, 2013 press release that:
Cinryze global net sales during the second quarter of 2013 were $95 million, a 23 percent increase over the same period in 2012 driven by demand growth and net realized price growth, partially offset by a reduction in channel inventories. Vancocin net sales during the second quarter of 2013 were $4 million compared to $16 million for the same period in 2012. During the second quarter of 2013, we generated net sales of approximately $8 million from our European operations compared to $4 million during the second quarter of 2012.
41. On October 31, 2013, ViroPharma reported its third quarter 2013 results, which showed continued growth. Specifically, the Company stated in its press release that:
Our U.S. net sales of Cinryze during the three and nine months ended September 30, 2013 increased to $102 million and $290 million, from sales of $84 million and $226 million, respectively, during the same periods in the prior year due to demand growth.
42. In the same press release, Defendant Milano is quoted as saying:
The third quarter of 2013 represented another extremely strong period of growth and positive momentum across our entire organization. We continue to generate strong growth in our commercial business, notably led by the acceleration of Cinryze demand here in the United States. Our pipeline also
continues to advance, establishing a number of important data points over the next several months.
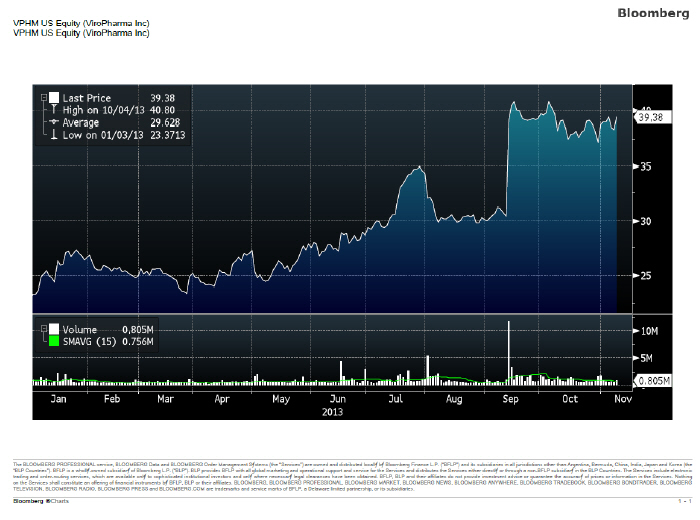
43. ViroPharma’s financial results and pipeline development drove its stock price from an opening price of $23.25 on January 2, 2013 to a closing price of $39.67 on November 8, 2013 – the last day before the deal was announced as seen in the chart below:
44. Interestingly, on November 12, 2013, the day after the Proposed Transaction was announced, ViroPharma announced the results of “a new quality data analysis from the randomized, placebo-controlled pivotal prophylaxis study of Cinryze.” This release marked the first prospective evaluation of the impact of routine preventative treatment on the quality of life
of patients with HAE. The tests results, according to the Company, showed “significantly better quality of life” for patients using Cinryze compared to those who received a placebo.
The Proposed Transaction
45. On November 11, 2013, before the U.S. markets opened, Shire and ViroPharma announced that they entered into the Merger Agreement, pursuant to which Shire would acquire all the outstanding shares of ViroPharma for $50 in cash, for a total value of $4.2 billion.
46. The press release stated in relevant part that:
Shire plc (LSE: SHP, NASDAQ: SHPG) and ViroPharma Incorporated (NASDAQ: VPHM) today announce that their Boards of Directors have unanimously approved, and the companies have entered into, a merger agreement pursuant to which Shire will acquire all the outstanding shares of the rare disease company ViroPharma for $50 per share in cash, for a total consideration of approximately $4.2 billion. The $50 per share price in the transaction represents a 27% premium to ViroPharma's closing share price on Friday, November 8, 2013, the last trading day prior to announcement, and a 64% premium to ViroPharma's unaffected share price of $30.47 on September 12, 2013.
ViroPharma is a high growth, rare disease biopharmaceutical company, whose commercial product CINRYZE® (C1 esterase inhibitor [human]), is a leading brand for the prophylactic treatment of Hereditary Angioedema (HAE).
Shire transaction highlights
| • | Excellent strategic fit |
| • | Expands rare disease portfolio which Shire is strategically committed to strengthen |
| • | Adds CINRYZE, with growing sales in the prophylactic treatment of HAE, which complements Shire’s FIRAZYR® (icatibant injection) |
| • | Enhances Shire’s short and long term revenue growth profile |
| • | Expected annual cost synergies of approximately $150 million by 2015, over and above the improved operating leverage already being driven by the ongoing One Shire reorganization |
| • | Immediately accretive to Shire’s Non GAAP EPS following completion and enhances earnings growth profile |
| • | Shire expects transaction to deliver ROIC in excess of its weighted average cost of capital |
| • | Acquisition to be effected by a tender offer and funded from Shire’s cash resources and existing and new bank facilities |
| • | Conference call for investors today (details below) |
Shire Chief Executive Officer, Flemming Ornskov MD comments:
“The acquisition of ViroPharma will immediately benefit Shire and is entirely consistent with our clear strategic objective of strengthening our rare disease portfolio. It brings us a new growth driving product which augments our already strong growth prospects. Shire is uniquely positioned to drive the continued success of CINRYZE for the benefit of patients through our knowledge of the rare disease space, our international infrastructure and our biologics manufacturing expertise. Shire is also excited by the prospect of being able to offer two complementary treatments, FIRAZYR for the treatment of acute HAE attacks and CINRYZE for prophylactic treatment of patients suffering from HAE. Shire’s priority will be to ensure CINRYZE patients continue to enjoy high standards of service. Shire has conducted a thorough and collaborative due diligence process over the last few months and, following completion of the transaction, the integration process will be focused on delivering value to all stakeholders. This acquisition is expected to create a $2 billion(1) rare disease revenue base and delivers further strong growth prospects.”
Vincent J. Milano, ViroPharma’s Chief Executive Officer stated:
“We are pleased to announce our merger with Shire, which like ViroPharma, is focused on developing products for patients suffering from rare diseases. After thoroughly evaluating our strategic options we determined that this transaction is in the best interests of ViroPharma, our shareholders and our patients.
By joining with Shire, ViroPharma will become part of a larger, more diverse biopharmaceutical company and will benefit from Shire’s innovation, scale and global reach. We will have access to resources to expand product distribution, giving us a platform to provide our crucial therapies, such as CINRYZE, to more patients than ever before. We look forward to working with Shire’s team and to being part of an even stronger, more geographically diverse organization.”
47. This price undervalues ViroPharma and harms its stockholders. As noted by the companies in their press release announcing the Proposed Transaction, if the Proposed Transaction closes, “Shire expects the addition of CINRYZE to its Rare Disease Business Unit to
create a growing $2 billion revenue business in 2014 which will represent approximately 40% of Shire’s total product sales on a pro forma basis. The acquisition of ViroPharma is expected to enhance Shire’s revenue growth profile in both the short and long term.” Further, “Shire estimates that it will realize approximately $150 million of annual cost synergies across the business by 2015, over and above the improved operating leverage already being driven by the ongoing One Shire reorganization.”
48. The impact for Shire will be immediate, as it “expects that the acquisition of ViroPharma will be accretive to Shire’s Non GAAP EPS immediately and in the longer term. Shire also expects that the transaction will deliver ROIC in excess of its weighted average cost of capital.”
49. On September 14, 2013, approximately one month before the Proposed Transaction was announced, Deutsche’s analyst Robyn Karnauskas projected that ViroPharma would be worth $52 per share to Shire specifically due to the synergies created by the merger. Ms. Karnauskas even identified other companies that could be interested in purchasing ViroPharma in the short term.
50. Having failed to maximize the sale price for the Company, members of the Board breached the fiduciary duties they owe to the Company’s public stockholders because the Company has been improperly valued and stockholders will not likely receive adequate or fair value for their ViroPharma common stock in the Proposed Transaction.
The Merger Agreement Is Improperly Structured
51. Furthermore, and in violation of the duty of the members of ViroPharma’s Board to maximize stockholder value, the Merger Agreement contains terms designed to favor the Proposed Transaction and deter alternative bids.
52. For example, §6.2 of the Merger Agreement includes a “no solicitation” provision which states that the Company may not:
directly or indirectly, (i) solicit, initiate, propose or knowingly encourage, or take any other action to knowingly facilitate, any Takeover Proposal or any inquiries or offers or the making of any proposal or any other efforts or attempt that could reasonably be expected to lead to a Takeover Proposal or (ii) enter into, continue or otherwise knowingly participate in any communications or negotiations regarding, or furnish to any Person any information with respect to, or otherwise knowingly cooperate in any way with any Person with respect to, any Takeover Proposal or any inquiries or offers or the making of any proposal or any other efforts or attempt that could reasonably be expected to lead to a Takeover Proposal. The Company shall, and shall cause its Subsidiaries and direct its Representatives to, immediately cease and cause to be terminated all existing communications and negotiations with any Person conducted heretofore with respect to any Takeover Proposal and shall request the prompt return or destruction of all confidential information previously furnished in connection therewith.
53. In addition, pursuant to §6.2 of the Merger Agreement, should an unsolicited bidder appear, the Company must notify Shire of the competing offer promptly, but in no event later than within forty-eight (48) hours after receiving the alternative proposal.
54. Further, §6.1(d) of the Merger Agreement severally limits the Board’s ability to recommend stockholders approve any competing bid and provides that Shire has four (4) businesses days to submit a superior offer. Shire is thus able to match the unsolicited offer because it is granted unfettered access to the unsolicited offer, in its entirety, including the identity of the third-party bidder, eliminating any leverage that the Company has in receiving the unsolicited offer, and significantly deterring an alternative offer from coming forward.
55. The Merger Agreement gives Shire access to any rival bidder’s information and provides Shire with a superior bargaining position to any competitive bidder. Accordingly, no rival bidder is likely to emerge because the Merger Agreement unfairly ensures that any “auction” will favor Shire and piggy-back upon the due diligence (and financial outlay) of the foreclosed second bidder.
56. Moreover, pursuant to §9.3(b) of the Merger Agreement, the Company has agreed to pay an improper termination fee of $127.14 million payable to Shire in certain circumstances, including if the Company terminates the Merger Agreement because the Board has determined to pursue another alternative superior offer.
57. Finally, the Individual Defendants chose to structure the deal as a Tender Offer. This structure greatly increases the chances of consummating the Proposed Transaction and leaves stockholders with minimal time to effectively challenge the deal.
The Materially Misleading And/Or Incomplete Recommendation Statement
58. The Recommendation Statement discloses certain information regarding Goldman Sachs’ financial analyses used to support its fairness opinion. These disclosures concerning Goldman Sachs’ financial analyses are materially incomplete and misleading in several ways.
Materially Incomplete and Misleading Disclosures Concerning Goldman Sachs’ Financial Analysis
59. First, the Recommendation Statement, on pages 29 to 30, fails to disclose material information relating to Goldman Sachs’ Illustrative Discounted Cash Flow (“DCF”) Analysis, in particular, the Recommendation Statement must disclose: (i) the definitions for present value and enterprise value used in this analysis; (ii) the rationale for applying discount rates ranging from 11.0% to 13.0%; (iii) whether the weighted average cost of capital (“WACC”) was derived through the capital asset pricing model (“CAPM”), and if so, the Recommendation Statement must disclose the market assumptions (i.e., risk free rate, two year beta, and equity risk premium); and (iv) the range of terminal values applied in this analysis and the source from which the terminal values were obtained. These omissions are material because without this information, the Company’s stockholders are unable to fully understand Goldman Sachs’ analysis and, thus, are unable to determine what weight, if any, to place on the fairness opinion
in determining whether to tender their shares in the Tender Offer.
60. Second, the Recommendation Statement, on pages 30 to 31, fails to disclose material information relating to Goldman Sachs’ Relative Trading Multiples Analysis, in particular, the Recommendation Statement must disclose: (i) the source and/or sources from which Goldman Sachs obtained the data and metrics analyzed in this analysis; (ii) the metrics and multiples observed by Goldman Sachs in this analysis (i.e., Equity Market Value, Enterprise Value (“EV”), EV to 2014 Revenue, EV to 2014 EBITDA, PE Multiple to 2013 EPS, and PE Multiple to 2014 EPS; (iii) whether historical multiples were considered (i.e., EV to LTM Revenue and/or EV to LTM EBITDA), and if observed the Recommendation Statement must disclose them, and if historical multiples were not considered, then Goldman Sachs’ rationale for not observing such multiples; and (iv) whether certain companies were considered in this analysis, but ultimately excluded from consideration. These omissions are material because without this information, the Company’s stockholders are unable to fully understand Goldman Sachs’ analysis and, thus, are unable to determine what weight, if any, to place on the fairness opinion in determining whether to tender their shares in Tender Offer.
61. Third, the Recommendation Statement fails to disclose whether Goldman Sachs performed a Selected Transactions Analysis, and if such analysis was performed, the Recommendation Statement must disclose of fair summary of this analysis, or if this analysis was not performed, Goldman Sachs’ rationale for not performing this analysis as part of its Fairness Presentation to the Board. These omissions are material because without this information, the Company’s stockholders are unable to fully understand Goldman Sachs’ analysis and, thus, are unable to determine what weight, if any, to place on the fairness opinion in determining whether to tender their shares in the Tender Offer.
62. Fourth, the Recommendation Statement, on pages 31 to 32, fails to disclose material information relating to Goldman Sachs’ Premia Paid Analysis, in particular, the Recommendation Statement must disclose the 1-Day, 1-Month, 3-Month, and 52-week high for each transaction observed in this analysis. These omissions are material because without this information, the Company’s stockholders are unable to fully understand Goldman Sachs’ analysis and, thus, are unable to determine what weight, if any, to place on the fairness opinion in determining whether to tender their shares in the Tender Offer.
63. Fifth, the Recommendation Statement, on pages 36 to 38, fails to disclose material information relating to the Non-Risk-Adjusted Projections and Risk-Adjusted Projections, in particular, the Recommendation Statement must disclose: (i) the net effective tax rate utilized by the Company’s management; (ii) change-in working capital; and (iii) for the Non-Risk-Adjusted Projections, the unlevered free cash flows as discussed on page 16 of the Recommendation Statement. Stockholders are entitled to this material information in order to make a fully informed decision whether to tender their shares in the Tender Offer.
Materially Incomplete and Misleading Disclosures Regarding the Flawed Process
64. The Recommendation Statement also omits several important details regarding the process leading up to the signing of the Merger Agreement.
65. For example, on page 16, the Recommendation Statement discloses that during the May 17, 2013 Board meeting, the Board discussed, among other things, “… updates to cash flow projections as a result of, among other items, the timing of milestone payments.” The Recommendation Statement however fails to disclose what the Company’s management considered material regarding the milestone payments to begin “refining” the non-risk adjusted projections. And the Recommendation Statement must disclose the anticipated milestone
payments anticipated to be received by the Company under the Non-Risk-Adjusted Projections and Risk-Adjusted Projections, respectively. This information is material to ensure that the Company’s management did not alter the projections in an effort to effectuate the Merger Agreement with Shire.
66. On page 16, the Recommendation Statement discloses that “Mathew Emmens, the Chairman of Shire’s board of directors, placed a call to Board member Robert J. Glaser to discuss the Company’s July 12, 2013 letter.” The Recommendation Statement must disclose whether director Glaser has a pre-existing business relationship with Mathew Emmens of Shire, and/or whether director Glaser has sat on any boards with Mathew Emmens in the two-years preceding the announcement of the Merger Agreement. This information is material because it evinces if director Glaser had any conflict of interest when evaluating and voting in favor of the Merger Agreement.
67. On page 18, the Recommendation Statement discloses that “Goldman Sachs representatives, together with members of the Company’s management, reviewed a broad universe of potential acquirors of the Company and reviewed with the Board nine (9) strategic potential acquirors who Goldman Sachs and the Company’s management believed could have an interest in exploring a potential transaction with the Company . . .” The Recommendation Statement must disclose the rationale for Goldman Sachs’ and the Company’s management’s decision not to solicit financial buyers as alternative potential suitors for the Company. This information is material because it evinces the process the Board took to maximize stockholder value, or whether the Board merely sought to improperly sell the Company to Shire.
68. On page 18, the Recommendation Statement also discloses that “Thomas Doyle, the Company’s Vice President of Strategic Initiatives, reviewed and discussed with the Board
certain other matters relating to the recent developments. The Board requested that management consider refinements to its probability assumptions to account for additional potential upside and downside scenarios.” The Recommendation Statement fails to disclose details regarding the events relating to Cinryze that would potentially affect the Company’s Non-Risk-Adjusted Projections. This information is material to ensure that the Company’s management did not alter the projections in an effort to effectuate the Merger Agreement with Shire.
69. On page 19, the Recommendation Statement discloses that “[o]n September 8, 2013, the Company provided Shire with the Non-Risk-Adjusted Projections described in more detail in ‘(e) Certain Financial Projections—The Risk-Adjusted Projections.’” The Recommendation Statement fails to disclose whether Parties A, B, and C, who executed non-disclosure agreements with the Company, were provided a copy of the Risk-Adjusted Projections, and if not, the rationale for not providing these revised projections to these parties. This information is material because it evinces the process the Board took to maximize stockholder value and whether the Board steered the process toward a sale with Shire.
70. On page 19, the Recommendation Statement discloses that “[t]he Company did not seek to enter into a confidentiality agreement with Party F because it determined that Party F would not be a viable participant in the Company’s process.” The Recommendation Statement fails to disclose the Board’s rationale for determining that Party F was not “a viable participant in the Company’s process.” This information is material because it evinces the process the Board took to maximize stockholder value and whether the Board steered the process toward a sale of Shire.
71. On pages 19, 20, and 21, the Recommendation Statement discloses that Parties A, B, C, D, and E terminated their review of the Company. The Recommendation Statement fails to
disclose the rationale for these parties decision to terminate their review of the Company. This information is material because it evinces the process the Board took to maximize stockholder value.
CLASS ACTION ALLEGATIONS
72. Plaintiff brings this action for himself and on behalf of all holders of ViroPharma common stock which have been or will be harmed by the conduct described herein (the “Class”). Excluded from the Class are defendants and any individual or entity affiliated with any defendant.
73. This action is properly maintainable as a class action.
74. The Class is so numerous that joinder of all members is impracticable. According to the Company’s U.S. Securities and Exchange Commission (“SEC”) filings, there were more than 65 million shares of ViroPharma common stock outstanding as of February 15, 2013.
75. There are questions of law and fact which are common to the Class and which predominate over questions affecting any individual Class member. The common questions include the following:
(a) whether the Individual Defendants have breached their fiduciary duties of undivided loyalty, due care, good faith, and candor with respect to plaintiff and the other members of the Class in connection with the Proposed Transaction;
(b) whether the Individual Defendants misrepresented and omitted material facts in violation of the fiduciary duty of candor owed by them to Plaintiff and the other members of the Class;
(c) whether the Individual Defendants have breached any of their other fiduciary duties owed to plaintiff and the other members of the Class in connection with the Proposed Transaction, including their duty of candor;
(d) whether the Individual Defendants, in bad faith and for improper motives, impeded or erected barriers designed to discourage other potentially interested parties from making an offer to acquire the Company or its assets;
(e) whether ViroPharma aided and abetted any of the Individual Defendants’ breaches of fiduciary duty owed to plaintiff and the other members of the Class in connection with the Proposed Transaction;
(f) whether Shire aided and abetted any of the Individual Defendants’ breaches of fiduciary duty owed to plaintiff and the other members of the Class in connection with the Proposed Transaction; and
(g) whether plaintiff and the other members of the Class would suffer irreparable injury were the Proposed Transaction consummated without the actions complained of herein being corrected.
76. Plaintiff’s claims are typical of the claims of the other members of the Class and plaintiff does not have any interests adverse to the Class.
77. Plaintiff has retained competent counsel experienced in litigation of this nature and will fairly and adequately represent and protect the interests of the Class.
78. The prosecution of separate actions by individual members of the Class would create a risk of inconsistent or varying adjudications with respect to individual members of the Class which would establish incompatible standards of conduct for the party opposing the Class.
79. Plaintiff anticipates that there will be no difficulty in the management of this litigation. A class action is superior to other available methods for the fair and efficient adjudication of this controversy.
80. Defendants have acted on grounds generally applicable to the Class with respect to the matters complained of herein, thereby making appropriate the relief sought herein with respect to the Class as a whole.
FIRST CAUSE OF ACTION
Claim for Breach of Fiduciary Duties Against the Individual Defendants and Does 1-15
81. Plaintiff incorporates by reference and realleges each and every allegation contained above as though fully set forth herein.
82. The Individual Defendants have violated the fiduciary duties of care, loyalty, and independence owed to the public stockholders of ViroPharma and have acted to put their personal interests ahead of the interests of ViroPharma stockholders.
83. By the acts, transactions, and course of conduct alleged herein, defendants, individually and acting as a part of a common plan, are attempting to unfairly deprive plaintiff and other members of the Class of the true value inherent in and arising from ViroPharma.
84. The Individual Defendants have violated their fiduciary duties by entering ViroPharma into the Proposed Transaction without regard to the effect of the proposed transaction on ViroPharma stockholders.
85. As demonstrated by the allegations above, the Individual Defendants to exercise the care required, and breached their duties of loyalty and care owed to the stockholders of ViroPharma by entering into the merger through the unfair process exemplified by the Merger Agreement.
86. By reason of the foregoing acts, practices, and course of conduct, the Individual Defendants have failed to exercise ordinary care and diligence in the exercise of their fiduciary obligations toward plaintiff and the other members of the Class.
87. As a result of the Individual Defendants’ unlawful actions, plaintiff and the other members of the Class will be irreparably harmed in that they will not receive their fair portion of the value of ViroPharma’s assets and operations. Unless the Tender Offer is enjoined by the Court, the Individual Defendants will continue to breach their fiduciary duties owed to plaintiff and the members of the Class, will not engage in arm’s-length negotiations on the Merger Agreement terms, and may consummate the Tender Offer, all to the irreparable harm of the members of the Class. Moreover, the Individual Defendants breached their duty of candor by failing to disclose to Plaintiff and the Class all material information necessary to make an informed decision regarding whether to tender their shares in the Tender Offer.
88. Plaintiff and the members of the Class have no adequate remedy at law. Only through the exercise of this Court’s equitable powers can plaintiff and the Class be fully protected from the immediate and irreparable injury which defendants’ actions threaten to inflict.
SECOND CAUSE OF ACTION
Claim for Aiding and Abetting Breaches of Fiduciary Duty Against ViroPharma
89. Plaintiff incorporates by reference and realleges each and every allegation contained above as though fully set forth herein.
90. The Individual Defendants owed to plaintiff and the members of the Class certain fiduciary duties as fully set out herein.
91. By committing the acts alleged herein, the Individual Defendants breached their fiduciary duties owed to plaintiff and the members of the Class.
92. ViroPharma colluded in or aided and abetted the Individual Defendants’ breaches of fiduciary duties, and was an active and knowing participant in the Individual Defendants’ breaches of fiduciary duties owed to plaintiff and the members of the Class.
93. Plaintiff and the members of the Class have no adequate remedy at law. Only through the exercise of this Court’s equitable powers can plaintiff and the Class be fully protected from the immediate and irreparable injury which defendants’ actions threaten to inflict.
THIRD CAUSE OF ACTION
Claim for Aiding and Abetting Breaches of Fiduciary Duty Against Shire
94. Plaintiff incorporates by reference and realleges each and every allegation contained above as though fully set forth herein.
95. The Individual Defendants owed to plaintiff and the members of the Class certain fiduciary duties as fully set out herein.
96. By committing the acts alleged herein, the Individual Defendants breached their fiduciary duties owed to plaintiff and the members of the Class.
97. Defendants Shire Pharmaceutical Holdings Irleand Limited, Venus Newco, Inc. and Shire plc colluded in or aided and abetted the Individual Defendants’ breaches of fiduciary duties, and were active and knowing participants in the Individual Defendants’ breaches of fiduciary duties owed to plaintiff and the members of the Class.
98. Defendants Shire Pharmaceutical Holdings Irleand Limited, Venus Newco, Inc. and Shire plc participated in the breach of the fiduciary duties by the Individual Defendants for the purpose of advancing their own interests. Defendants Shire Pharmaceutical Holdings Irleand Limited, Venus Newco, Inc. and Shire plc obtained and will obtain both direct and indirect benefits from colluding in or aiding and abetting the Individual Defendants’ breaches. Defendants Shire Pharmaceutical Holdings Irleand Limited, Venus Newco, Inc. and Shire plc will benefit from the acquisition of the Company at an inadequate and unfair price if the Proposed Transaction is consummated.
99. Plaintiff and the members of the Class have no adequate remedy at law. Only through the exercise of this Court’s equitable powers can plaintiff and the Class be fully protected from the immediate and irreparable injury which defendants’ actions threaten to inflict.
PRAYER FOR RELIEF
WHEREFORE, plaintiff demands injunctive relief, in his favor and in favor of the Class and against defendants as follows:
A. Declaring that this action is properly maintainable as a class action;
B. Declaring and decreeing that the Merger Agreement was agreed to in breach of the fiduciary duties of the Individual Defendants and is therefore unlawful and unenforceable;
C. Rescinding, to the extent already implemented, the Merger Agreement;
D. Enjoining defendants, their agents, counsel, employees, and all persons acting in concert with them from consummating the Proposed Transaction, unless and until the Company adopts and implements a procedure or process reasonably designed to enter into a merger agreement providing the best possible value for stockholders;
E. Enjoining the Individual Defendants unless or until they fully disclose to Plaintiff and the Class all material information necessary to make an informed decision whether to tender their shares in the Tender Offer;
F. Directing the Individual Defendants to exercise their fiduciary duties to commence a sale process that is reasonably designed to secure the best possible consideration for ViroPharma and obtain a transaction which is in the best interests of ViroPharma stockholders;
G. Imposition of a constructive trust, in favor of plaintiff and members of the Class, upon any benefits improperly received by defendants as a result of their wrongful conduct;
H. Awarding plaintiff the costs and disbursements of this action, including reasonable attorneys’ and experts’ fees; and
I. Granting such other and further equitable relief as this Court may deem just and proper.
| Dated: December 4, 2013 | Respectfully submitted,
FARUQI & FARUQI, LLP
By: /s/ Peter B. Andrews
Peter B. Andrews (Del. Bar No. 4623) Craig J. Springer (Del. Bar No. 5529) 20 Montchanin Road, Suite 145 Wilmington, DE 19807 Tel: (302) 482-3182
E-mail: pandrews@faruqilaw.com E-mail: cspringer@faruqilaw.com
Attorneys for Plaintiff |
OF COUNSEL:
FARUQI & FARUQI, LLP
Juan E. Monteverde
David M. Sborz
369 Lexington Avenue, 10th Fl.
New York, NY10017
Tel.: (212) 983-9330
HOLZER HOLZER & FISTEL LLC
Corey D. Holzer
Marshall P. Dees
200 Ashford Cir.,
Atlanta, GA 30338
Tel.: (770) 392-0090
Attorneys for Plaintiff
28