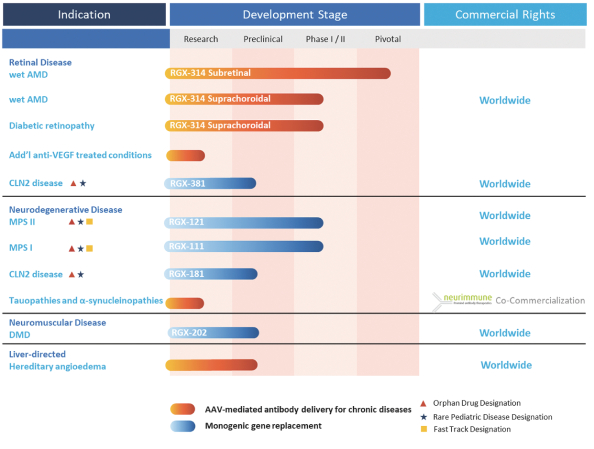
We completed an End of Phase 2 meeting with the U.S. Food and Drug Administration, or FDA, to discuss the details of a pivotal program to support a Biologics License Application, or BLA, for RGX-314. Based on discussions with the FDA, we plan to conduct two randomized, well-controlled clinical trials to evaluate the efficacy and safety of RGX-314 in patients with wet AMD, and expect to enroll approximately 700 patients total. In addition, based on our discussions with the FDA, we believe we have a clear path to support current Good Manufacturing Practice, or cGMP, commercial-ready manufacturing plans in the pivotal program. We expect to submit a BLA based on these trials in 2024.
ATMOSPHERETM, the first planned pivotal trial, will evaluate the efficacy and safety of RGX-314 in patients with wet AMD. The trial will enroll approximately 300 patients across two RGX-314 dose arms versus ranibizumab. The primary endpoint of the trial is non-inferiority to ranibizumab based on change from baseline in Best Corrected Visual Acuity (BCVA) at one year. Site activation and patient screening are ongoing and we expect to begin dosing patients in this trial in the first quarter of 2021.
The second planned pivotal trial is expected to be similar in design to ATMOSPHERE, and we plan to initiate the trial in the second half of 2021. The trial is expected to have two RGX-314 dose arms versus aflibercept and the planned primary endpoint is non-inferiority to aflibercept based on the change from baseline in BCVA at one year.
We have completed enrollment of patients in Cohort 1 of AAVIATETM, a Phase II trial to evaluate the suprachoroidal delivery of RGX-314 using the SCS Microinjector for the treatment of wet AMD. We expect to report interim data from Cohort 1 of this trial in the third quarter of 2021, and enrollment of patients in Cohort 2 is expected to begin in the first quarter of 2021.
Enrollment of patients continues in Cohort 1 for ALTITUDETM, a Phase II trial to evaluate the suprachoroidal delivery of RGX-314 using the SCS Microinjector for the treatment of DR. We expect to report initial data from this trial in 2021.
As of December 31, 2020, suprachoroidal delivery of RGX-314 in AAVIATE and ALTITUDE has been reported to be generally well-tolerated, with no evidence of ocular inflammation.
RGX-202
We are developing RGX-202 for the treatment of Duchenne muscular dystrophy, or DMD, a severe, progressive, degenerative muscle disease. DMD is caused by mutations in the DMD gene which encodes for dystrophin, a protein involved in muscle cell structure and signaling pathways. Without dystrophin, muscles throughout the body degenerate and become weak, eventually leading to loss of movement and independence, required support for breathing, cardiomyopathy and premature death.
RGX-202 is designed to deliver a novel microdystrophin transgene which includes an extended coding region of the C-Terminal, or CT, domain found in naturally occurring dystrophin, as well as other fundamental improvements. Presence of the CT domain has been shown to recruit several key proteins to the muscle cell membrane, leading to improved muscle resistance to contraction-induced muscle damage in dystrophic mice. Additional design features, including codon optimization and reduction of CpG content, may potentially improve gene expression, increase translational efficiency and reduce immunogenicity.
RGX-202 is designed to use the NAV AAV8 vector, a vector used in numerous clinical trials, and a well-characterized muscle specific promoter, Spc5-12, to support the delivery and targeted expression of genes throughout skeletal and heart muscle.